Abstract
Spinal manipulative therapy (SMT) is a common nonpharmacologic treatment for low back pain (LBP). Although generally supported by systematic reviews and practice guidelines, clinical trials evaluating SMT have been characterized by small effect sizes. This study adopts a multiphase optimization strategy (MOST) framework to examine individual components of an SMT delivery protocol using a single-blind trial with the goal of identifying and optimizing a multi-component SMT protocol. We enrolled 241 participants with LBP. All participants received 2 SMT treatment sessions in the first week then were randomly assigned additional treatment based on a fully factorial design. The three randomized treatment components provided in twice weekly sessions over 3 weeks were multifidus activating exercise, spinal mobilizing exercise and additional SMT dose. Primary outcomes included clinical (Oswestry Disability index, numeric pain intensity rating) and mechanistic (spinal stiffness, multifidus muscle activation) measures assessed at baseline, 1-, 4- and 12-weeks. Significant differences were found for the Oswestry index after 12 weeks for participants receiving multifidus activating exercise (mean difference = −3.62, 97.5% CI: −6.89, −0.35; p=0.01). There were no additional significant main or interaction effects for other treatment components or different outcome measures. The optimized SMT protocol identified in this study included SMT sessions followed by multifidus activating exercises.
Keywords: low back pain, spinal manipulative therapy, exercise, factorial design
INTRODUCTION
Low back pain (LBP) is among the most common health conditions and a leading cause of disability globally.1, 38 The adverse societal impacts of ineffective LBP management are increasingly recognized. Back pain is the most costly health condition in the United States and is the most common diagnosis for which opioids are prescribed.22, 70 Practice guidelines recommend nonpharmacological treatment (NPT) for pain conditions including LBP.18, 23, 58 While many NPTs are effective for LBP, the magnitude of effects are small and highly heterogeneous within groups of patients.10 Optimizing the effectiveness of NPTs for LBP is a priority for reducing costs and over-reliance on pharmacological management.
Improving the effectiveness of NPTs for LBP faces two fundamental challenges. First, mechanisms of action for most NPTs are largely unknown.5, 7, 64 Understanding the mechanisms through which an NPT provides therapeutic benefit would facilitate development of more effective and targeted treatment protocols. Second, NPTs are typically provided as complex, multicomponent interventions; though research studies and systematic reviews often examine isolated treatments modalities.11 Understanding which components of an NPT contribute to its benefits, and how components may interact to synergize or lessen overall effectiveness would help develop optimally effective treatment protocols.
Spinal manipulative therapy (SMT) is an NPT for LBP supported by many evidence-based reviews and guidelines.2, 11, 56, 63 However support for SMT is not universal, and even supportive reviews find that SMT, like other NPTs, is associated with small effect sizes.11, 60, 63 Efforts to optimize the effectiveness of SMT must confront the barriers common to NPTs; uncertainty regarding mechanisms and the complexity of the intervention. Several physiologic responses to SMT have been documented, but few have been associated with clinical outcomes in LBP patients and the mechanisms through which SMT provides therapeutic benefit remains unknown.4 Many studies evaluate SMT as a unimodal intervention, while others include SMT within a multi-component package.56, 68 including a diverse array of co-interventions such as exercise, massage, thermal modalities or other interventions.6 In practice, providers use various co-interventions with SMT.3, 48 The choice of co-interventions in research and practice has not been guided by evidence. Research has not evaluated individual SMT co-interventions or their interactions in an effort to develop multi-component SMT protocols designed to optimize outcomes.
If the mechanisms through which SMT exerts clinical benefit were understood, co-interventions designed to impact these mechanisms may lead to optimized protocols. Prior work has reported associations between post-SMT changes in spinal stiffness and trunk muscle activation, specifically the lumbar multifidus muscle, and improvement in pain and function.16, 30, 39, 43, 47, 69 If these associations represent mechanisms of SMT, incorporating co-interventions presumed to impact stiffness and trunk muscle activation may help optimize a multicomponent SMT protocol, but this hypothesis has not been rigorously examined.
The Multiphase Optimization Strategy (MOST) framework outlines a step-wise process to identify and evaluate components of a multi-component treatment package prior to evaluation in a randomized clinical trial (RCT).14 The RCT is the preferred design to evaluate effectiveness of a multicomponent treatment, but cannot clarify the specific components contributing or detracting from overall effects.34 The MOST framework recommends factorial designs to simultaneously evaluate multiple components in order to identify the most promising based on main effects and interactions. This strategy has not been applied to the development of optimized SMT treatment protocol.
The purpose of this study was to apply a MOST framework towards the development of an optimized, multi-component, SMT treatment protocol. We examined three individual treatment components (SMT dose, spine mobilizing exercise, and lumbar multifidus activating exercises) with the goal of identifying a protocol that may be able to yield larger treatment effects in subsequent clinical trials. We evaluated the effects of these components on mechanistic and patient-centered outcomes. Both main effects of the individual components and interaction effects were evaluated.
METHODS
Study Design
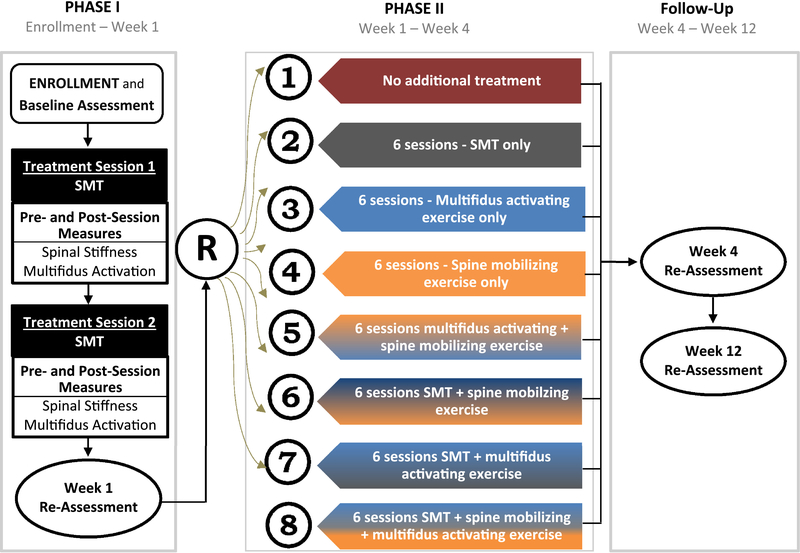
A full description of the study design and rationale can be found in the protocol publication.31 This report focuses on the project’s primary aims of examining the impact of different treatment components on mechanistic and clinical outcomes. The study used a phased, factorial design. During treatment phase I all participants received 2 SMT sessions and were re-assessed after 1 week (Fig. 1). At the 1-week assessment all participants were evaluated and randomized using a balanced factorial design with 3 treatments components (Phase II). The components tested were additional SMT dose, multifidus muscle activation exercises (ACTex) and spine mobilizing exercises (MOBex). Phase II treatment was provided over the next 3 weeks with 2 sessions per week for a total of 6 additional treatment sessions. Consistent with a factorial design, one group received no additional treatment. Additional assessments occurred 4-weeks (after treatment phase II) and 12-weeks after enrollment. Follow-up assessments were conducted by a researcher blinded to the participant’s randomized treatment allocation. The study was registered prior to participant enrollment (clinicaltrials.gov NCT02868034).
Figure 1.
Study Design
Randomization
Random assignment to one of eight possible Phase II treatment groups occurred after the completion of two Phase I SMT sessions. Randomization schemes were generated by a study statistician prior to enrollment with random number generation software using a random permuted blocked procedure in differing block sizes (4 or 6). Randomization was stratified by study site. The Phase II randomization assignment was concealed to the researchers and was delivered through the randomization module of the Research Electronic Data Capture (REDCap) site following completion of all assessment procedures.37
Study Participants
Participants were recruited at the University of Utah in Salt Lake City Utah, USA and the University of Alberta, Edmonton, Alberta, Canada. Eligibility criteria were intended to recruit a heterogeneous cohort with non-specific LBP without any contraindication to receive any of the study interventions or assessments. Eligibility criteria required the presence of LBP, at least moderate LBP-related disability operationalized as Oswestry score ≥20% and age 18–60 years old. Individuals were excluded if they had any prior surgery to the lumbosacral spine, were pregnant, were currently receiving mind-body or exercise treatment for LBP with a healthcare provider (e.g., chiropractor, physical therapist, massage therapist, etc.), or had signs of neurogenic LBP arising from the clinical examination (e.g., positive straight leg raise test, diminished muscle stretch reflex, etc.) or evidence of significant spinal pathology based solely on the clinical examination (e.g., spinal fracture, infection, etc.). Eligible individuals who chose to participate in the study provided informed consent using documentation approved by the Institutional Review Board at the respective institution (University of Utah or University of Alberta).
Study Assessments
Following informed consent participants completed a baseline assessment. Participant demographics were collected including age, gender, race/ethnicity, body mass index (BMI), marital status, employment status, highest education level and current and past LBP interventions. Participants were categorized as having chronic LBP or not based on the criteria recommended by the NIH Task Force on Research Standards for Chronic LBP.19 Baseline assessment also included participant self-report measures of fear-avoidance beliefs using the Fear Avoidance Beliefs Questionnaire physical activity (FABQPA) and work (FABQW) subscales.67
Patient-Centered Outcome Measures
Co-primary patient-centered outcomes, pain intensity and LBP-related disability, were assessed at baseline and each follow-up. A numeric pain rating scale (NPRS) was used to evaluate pain intensity. Participants were asked to make separate ratings of current pain intensity and the best and worst intensity over the past 24 hours on a 0–10 scale (“0” no pain and “10” worst imaginable pain). The mean of the three ratings was used to represent pain intensity.9 The Oswestry Disability Index (ODI) was used to evaluated LBP-related disability. The ODI results in a score ranging from 0–100 with higher numbers indicating greater disability.29
Mechanistic Outcome Measures
Lumbar multifidus muscle activation and lumbar spine stiffness were co-primary mechanistic outcomes. Details of these assessments are provided in the study protocol.31 Multifidus activation was measured with brightness-mode ultrasound images using a Sonosite MicroMaxx (Sonosite Inc. Bothell, WA, USA) and a 60-mm, 2–5 MHz curvilinear array using a validated protocol.44 Measures were taken with the participant prone. Images were obtained at the L4-L5 and L5-S1 levels with the multifidus muscle at rest and during submaximal contraction in response to the participant lifting a small weight in with the contralateral arm. Offline measures of multifidus thickness were obtained for both the resting and contracted states. Multifidus muscle activation was calculated as: (Thicknesscontract – Thicknessrest) / Thicknessrest). Spinal stiffness was assessed with the VerteTrack™ (VibeDx Corporation, Canada) which uses a dual-wheel roller to apply a vertical load along the lumbar spine of a prone participant.36 The indenter houses a sensor to provide continuous, real-time quantification of spinal deformation in response to a defined load. Stiffness was determined at each lumbar level as the ratio of the maximum applied force to the resultant displacement in N/mm. Within- and between-session reliability for spinal stiffness measures taken with this protocol are high.36
Study Interventions
All interventions were provided by licensed providers with experience providing SMT (chiropractor or physical therapist). The study involved three intervention components provided across the two phases of the study (Fig. 1). The SMT component was provided using the same protocol in both phases. The exercise treatment components were used only during phase II.
SMT Treatment Component
The SMT component began with a brief assessment by the provider to ensure the participant remained appropriate to receive SMT. The preferred SMT technique was provided using a protocol developed in our prior work.8, 28, 30 The technique is performed with the participant supine. The provider side bends and rotates the participant then applies a high-velocity, low-amplitude thrust through the anterior, superior iliac spine. If a cavitation occurs the SMT treatment is complete. If no cavitation occurs, the participant is repositioned and SMT is repeated. If no cavitation occurs on the second attempt, the provider will manipulate the opposite side. A maximum of two attempts per side is permitted. The initial SMT application is provided to the side identified by the patient as more painful. An alternate side-lying SMT technique may be used based on participant comfort. We have found equivalent outcomes with either the supine or side-lying technique.12
Multifidus Muscle Activating Exercise (ACTex) Treatment Component
The ACTex protocol is described in more detail in the protocol publication.31 The protocol begins with isometric contractions of the multifidus muscle with clinician feedback and performed in different positions (prone, standing, quadruped, etc.) which have been found to enhance recruitment of the multifidus muscle.42,41 A progression of lumbar extensor strengthening exercises shown to activate the multifidus muscle was also used for participants receiving this treatment component.24, 53
Spine Mobilizing Exercise (MOBex) Treatment Component
The MOBex protocol is described in more detail in the protocol publication.31 The protocol used a progression of repeated active movements into the end-ranges of spinal flexion and extension with the goal of improving spinal motion and reducing stiffness.26, 27 Using principles described by McKenzie,52 participants were assessed to determine if a movement in a particular direction decreased LBP symptoms or caused symptoms to centralize towards the spinal midline (i.e., a directional preference). If a directional preference was present the participant was instructed to perform movements towards end-range in the direction of preference along with mid-range motion exercises. In the absence of a directional preference participants were instructed in a progression of flexion or extension exercises based on provider’s judgment.
Data Analysis
Analyses in this report focus on the effects of the randomized treatment components (additional SMT; ACTex; MOBex) on mechanistic and patient-centered outcomes. We estimated the effects of the randomized interventions on the NPRS and ODI co-primary outcomes and other numeric outcomes by linear mixed models to relate mean levels of each outcome at 1-, 4- and-12 weeks to indicator variables to represent the main effects, pairwise interactions and the three-way interaction between treatment components. The 1-week assessment, which occurred after Phase I treatment and just prior to Phase II randomization, served as the baseline for these analyses. Consistent with the randomized design, the mean values for the outcomes at week 1 were constrained to be equal to a common overall mean without regard to treatment assignment.13 An unstructured covariance matrix was used to account for correlation of serially measured outcome scores.
Restricted maximum likelihood estimation were used for estimation of parameters and associated confidence intervals (CI) 66 for the following quantities for each outcome at 4- and 12-week assessments: a) main effects evaluating effects of each of treatment component averaged over the levels of the other 2 components, b) three pairwise interactions evaluating if the effect of a component differs between levels of one of the other components while averaging over the levels of the third component. Pairwise interactions inform whether the effects of each component pair are additive, synergistic or antagonistic; and c) 3-way interaction evaluating if each pairwise interaction differs depending on the third component. To account for two co-primary outcomes, each hypothesis test was performed with 2-sided α=0.025 and CIs constructed using a confidence coefficient of 0.975.
To examine which multi-modal combination of treatment components optimized outcomes based on the ODI, we first simplified the fully saturated factorial analysis of variance model by comparing the Bayes Information Criteria (BIC) among all possible models composed of main effects, pair-wise and the 3-way interaction which satisfied the hierarchical consistency constraint that the main effects corresponding to each term in a pair-wise interaction be retained in a model with pair-wise interaction terms; and pair-wise interactions along with component main effects were retained in a model with the 3-way interaction. The 12-week ODI was used as the dependent variable. Baseline (1-week) ODI scores and study site were included as covariates in all models. Models were ordered by BIC value with the lowest BIC value considered the optimized model. Models with BIC values greater than the optimized model but less than the intercept only (null model) were considered as possible optimal combinations that explained more variance in outcome than the null model.
Because analyses of the longitudinal models are based on restricted maximum likelihood estimation, statistical inferences remain valid if missing data follow a missing at random structure.66 Of participants providing data at 1-week, we retained 87.6% at the 4-week follow-up. Based on this rate of completion we did not employ multiple imputation.62
Power and Sample Size Justification
We estimated standard deviations and pre-post correlations for each outcome based on our prior work.30 Using these estimates, a sample size of 280 participants and presuming 92% retention to 4 weeks size provided at least 80% power to detect the minimum important differences for the patient-centered outcomes or hypothesized effect sizes for mechanistic outcomes for the analyses of main effects of each component for all 4 outcomes with a conservative 2-sided α=0.025 to account for two co-primary outcomes. Detectable effects for pairwise interaction terms were at or near minimally important differences or hypothesized effects with 80% power with the exception of spinal stiffness measures. The original sample size of 280 in the trial registration was increased to 315 because of a higher-then-anticipated number of individuals determined to be ineligible after providing consent based on post-consent assessments. A more detailed explanation of sample size assumptions is provided in the protocol publication.31 Statistical power was limited for detection of pairwise or 3-way interactions; accordingly, these comparisons were interpreted as exploratory.
RESULTS
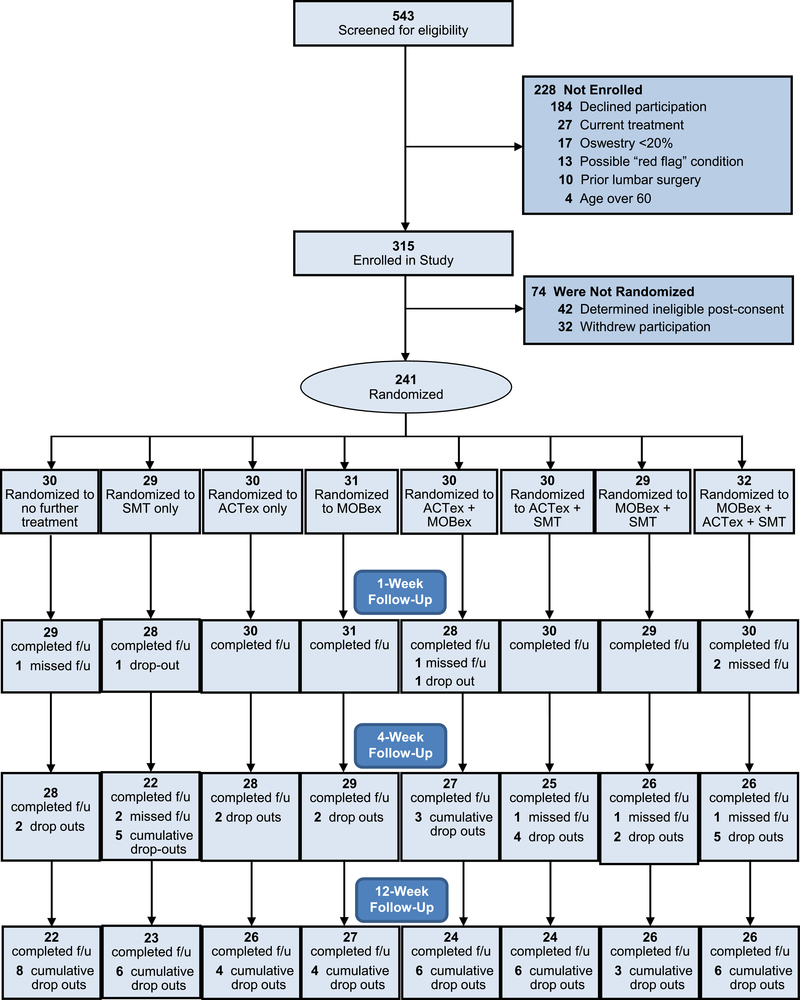
From February 2017 thru January 2019, 315 individuals provided consent for the study. Overall, 273 individuals met all eligibility criteria and enrolled in the study. Of these 32 (11.7%) withdrew during treatment phase I, leaving 241 participants who completed the 1-week assessment and were randomized to phase II treatment. Among those randomized to a phase II treatment, 211 (87.6%) and 198 (82.2%) completed the 4- and 12-week assessments respectively (Figure 2).
Figure 2.
Recruitment and Retention in the Study
Participants in the study reflected the demographics of Salt Lake City, Utah and Edmonton, Alberta. Across sites, 198 participants (73.1%) identified their race as Caucasian, 26 (9.6%) as Asian, and 8 (3.0%) as Black or African American. Twenty participants (7.4%) were of Hispanic or Latino ethnicity. Mean age was 39.6 (sd=11.9) years, 59.7% were female and 75.5% met the NIH definition for chronic LBP. Participants randomized to a phase II treatment (n=241) did not differ in meaningful or statistically significant ways from the overall sample. Few differences were noted between participants based on randomized assignment to phase II treatment components. Exceptions were BMI and educational level among those randomized to receive SMT (Table 1). Similarly, participants did not differ across treatment components for baseline scores on the mechanistic measures and patient-centered outcomes (Table 1). Of the 241 participants randomized to Phase II treatment, 30 were assigned no additional treatment and 211 received additional care in the ensuing 3 week period. Overall, 196 of these participants (92.9%) began their assigned treatment, 178 (84.4%) completed at least 4 of the 6 assigned treatment sessions and 153 (72.5%) completed all 6 assigned sessions (Table 2).
Table 1.
Baseline participant characteristics for the entire sample and based on randomized treatment group assigned at 1-week follow-up.
| Total Sample (n=271) | Randomized Treatment Components (n=241) |
||||||
|---|---|---|---|---|---|---|---|
| SMT | ACTEx | MOBEx | |||||
| Received (n=120) | Not received (n=121) | Received (n=122) | Not received (n=119) | Received (n=122) | Not received (n=119) | ||
| Gender (male) | 112 (41.3) | 49 (40.8) | 48 (39.7) | 50 (41.0) | 47 (39.5) | 54 (44.3) | 43 (36.1) |
| Age (Mean ± SD) | 39.8 ± 11.9 | 39.2 ± 12.0 | 40.5 ± 11.6 | 40.4 ± 12.2 | 39.4 ± 11.5 | 39.4 ± 11.6 | 40.3 ± 12.1 |
| BMI (Mean ± SD) | 28.3 ± 6.8 | 29.4 ± 7.8* | 27.6 ± 6.1* | 29.1 ± 7.7 | 27.7 ± 6.2 | 27.9 ± 5.8 | 29.0 ± 8.0 |
| Chronic LBP (yes) | 167(61.6) | 90 (75) | 93 (76.9) | 92 (75.4) | 91 (76.5) | 88 (72.1) | 95 (79.8) |
| LBP and leg pain (yes) | 57 (21.2) | 25 (20.8) | 27 (22.3) | 25 (20.5) | 27 (22.7) | 20 (16.4) | 32 (26.9) |
| Smoker╬ (yes) | 40 (14.9) | 19 (16.0) | 15 (12.4) | 20 (16.4) | 14 (11.9) | 17 (14.0) | 17 (14.3) |
| Education╬ (college degree) | 164 (61.0) | 67 (56.3)* | 83 (68.6)* | 74 (60.7) | 76 (64.4) | 80 (66.1) | 70 (58.8) |
| Living Situation╬ (married or live with significant other) | 169 (62.8) | 70 (58.8) | 81 (66.9) | 76 (62.3) | 75 (63.0) | 74 (61.2) | 77 (64.7) |
| Employment╬ (employed full-time) | 157 (58.4) | 73 (61.3) | 68 (56.2) | 72 (59.0) | 69 (58.5) | 73 (60.3) | 68 (57.1) |
| Depression╬ (yes) | 56 (21.2) | 24 (20.2) | 28 (23.5) | 26 (21.5) | 26 (22.2) | 24 (20.0) | 28 (23.7) |
| Anxiety╬ (yes) | 63 (23.7) | 31 (26.1) | 28 (23.3) | 26 (21.3) | 33 (28.2) | 28 (23.3) | 31 (26.1) |
| Upper Back / Neck Pain╬ (yes) | 69 (26.0) | 33 (27.7) | 30 (25.2) | 28 (23.1) | 35 (29.9) | 29 (24.4) | 34 (28.6) |
| Treatment Used for LBP Episode (yes) | |||||||
| Chiropractic | 129 (47.6) | 52 (43.3) | 62 (51.2) | 57 (46.7) | 57 (47.9) | 51 (41.8) | 63 (52.9) |
| Physical Therapy | 108 (39.9) | 46 (38.3) | 54 (44.6) | 53 (43.4) | 47 (39.5) | 56 (45.9) | 44 (37.0) |
| Opioid Medication | 56 (20.7) | 25 (20.8) | 25 (20.7) | 26 (21.3) | 24 (20.2) | 28 (23.0) | 22 (18.5) |
| Fear-Avoidance Beliefs╬ (Mean ± SD) | |||||||
| Physical Activity | 14.4 ± 5.0 | 14.8 ± 4.8 | 14.2 ± 5.0 | 14.8 ± 4.9 | 14.2 ± 4.9 | 14.7 ± 5.0 | 14.2 ± 4.7 |
| Work | 15.6 ± 10.1 | 14.8 ± 10.4 | 16.4 ± 9.4 | 15.7 ± 9.8 | 15.6 ± 10.1 | 15.0 ± 10.4 | 16.3 ± 9.4 |
| Oswestry (Mean ± SD) | 34.4 ± 11.8 | 35.2 ± 13.3 | 33.2 ± 10.0 | 35.4 ± 10.9 | 32.9 ± 12.6 | 32.9 ± 10.5 | 35.5 ± 12.9 |
| Pain Intensity (Mean ± SD) | 4.6 ± 1.7 | 4.7 ± 1.7 | 4.5 ± 1.6 | 4.7 ± 1.6 | 4.5 ± 1.7 | 4.6 ± 1.7 | 4.6 ± 1.6 |
| Multifidus Activation╬ (Mean ± SD) | 11.2% ± 9.3 | 11.4% ± 9.4 | 11.6% ± 9.6 | 12.3% ± 9.9 | 10.8% ± 9.0 | 11.0% ± 8.6 | 12.1% ± 10.3 |
| Spinal Stiffness (N/mm)╬ (Mean ± SD) | 6.0 ± 1.4 | 5.8 ± 1.2 | 6.0 ± 1.8 | 5.8 ± 1.1 | 6.0 ± 1.3 | 6.0 ± 1.2 | 5.9 ± 1.3 |
Values represent number of participants (%) unless otherwise indicated.
(╬ missing values at baseline (n=269 for education, living situation; n=264 for depression, anxiety, upper back/neck pain, and Fear-Avoidance baseline score, n=255 for spinal stiffness and n=260 for multifidus activation)
p<0.05
Table 2.
Compliance with randomized treatment sessions.
| Randomized Treatment Groups (n=241) | ||||||||
|---|---|---|---|---|---|---|---|---|
| No further treatment (n=30) | SMT only (n=29) | ACTex only (n=30) | MOBex only (n=31) | SMT + ACTex (n=30) | SMT + MOBex (n=29) | ACTex + MOBex (n=30) | SMT + ACTex + MOBex (n=32) | |
| Began Treatment (n, %) | - - - - | 27 (93.1) | 29 (96.7) | 31 (100) | 29 (96.7) | 29 (100) | 29 (96.7) | 32 (100) |
| Attended ≥ 4 sessions (n, %) | - - - - | 22 (75.9) | 27 (90.0) | 30 (96.8) | 28 (93.3) | 27 (93.1) | 26 (86.7) | 27 (84.4) |
| Attended 6 sessions (n, %) | - - - - | 21 (72.4) | 20 (66.7) | 23 (74.2) | 26 (86.7) | 25 (86.2) | 23 (76.7) | 21 (65.6) |
| Mean (sd) sessions attended | - - - - | 5.0 (1.9) | 5.4 (1.2) | 5.5 (1.0) | 5.6 (1.2) | 5.7 (1.0) | 5.6 (1.1) | 5.1 (1.6) |
Patient-Centered Outcomes
Among the 241 randomized participants, LBP-related disability improved across the 1-week Phase I period from a baseline mean ODI score of 34.2 (±11.8) to 1-week mean of 27.8 (±13.5); mean difference=6.3; CI: 4.9, 7.8, p<0.001. Pain intensity values improved from a baseline mean of 4.61 (±1.64) to a 1-week mean of 4.06 (±1.87); (mean difference = 0.56; CI: 0.35, 0.77, p<0.001; n=240 with complete pain intensity data).
In the phase II period a main effect for receiving ACTex was observed for the ODI (Table 3), exceeding the p<0.025 threshold at the 12-week assessment with a mean difference = 3.62 (97.5% CI: 0.50, 6.89) favoring patients receiving ACTex (p=0.01). There were no statistically significant 2- or 3-way interaction effects for the ODI outcome. For the outcome of pain intensity (Table 4), the main effect for MOBex approached the significance threshold at the 4-week assessment with a mean difference = 0.46 (97.5% CI: 0.0, 0.92) favoring patients receiving spinal MOBex (p=0.030). The 2-way interaction between MOBex and additional SMT also approached the significance threshold at the 4-week assessment with the group not receiving SMT showing greater mean improvement when MOBex was received compared to when it was not received. In the group receiving SMT there was little difference in the means for groups receiving or not receiving MOBex (p=0.030). Other 2- and 3-way interactions for the outcome of pain intensity were not significant. Results are provided in Appendix.
Table 3.
Main effect estimates for LBP-related disability outcome measure (Oswestry).
| Main Effects for Randomized Treatment Components | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention Not Used | Intervention Used | Relative Difference between Groups | |||||||
| Treatment Component | Assessment | Adjusted Mean (97.5% CI) | Change from 1-week | p value | Adjusted Mean (97.5% CI) | Change from 1-week | p value | Estimated relative difference 97.5% CI | p value |
| SMT | 4 weeks | 22.90 (20.47, 25.34) | −4.78 (−6.80, −2.75) | <.001 | 21.74 (19.23, 24.25) | −5.94 (−8.04, −3.83) | <.001 | −1.16 (−4.0, 1.68) | 0.36 |
| 12 weeks | 19.93 (17.29, 22.57) | −7.75 (−10.11, −5.38) | <.001 | 19.73 (17.05, 22.42) | −7.95 (−10.36, −5.54) | <.001 | −0.20 (−3.46, 3.07) | 0.89 | |
| ACTex | 4 weeks | 23.49 (21.01, 25.98) | −4.19 (−6.27, −2.10) | <.001 | 21.15 (18.7, 23.61) | −6.53 (−8.57, −4.48) | <.001 | −2.34 (−5.18, 0.50) | 0.06 |
| 12 weeks | 21.64 (18.96, 24.33) | −6.04 (−8.45, −3.63) | <.001 | 18.02 (15.38, 20.66) | −9.66 (−12.02, −7.29) | <.001 | −3.62 (−6.89, −0.35) | 0.01 | |
| MOBex | 4 weeks | 23.0 (20.52, 25.49) | −4.67 (−6.76, −2.59) | <.001 | 21.64 (19.18, 24.1) | −6.04 (−8.09, −3.99) | <0.01 | −1.36 (−4.2, 1.48) | 0.28 |
| 12 weeks | 20.69 (18.02, 23.37) | −6.99 (−9.39, −4.58) | <.001 | 18.97 (16.33, 21.62) | −8.71 (−11.08, −6.34) | <0.01 | −1.72 (−4.99, 1.55) | 0.24 | |
Mean values are adjusted for 1-week Oswestry score and study site. P values refer to change from 1-week values.
Table 4.
Main effect estimates for pain intensity outcome measure.
| Main Effects for Randomized Treatment Components | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention Not Used | Intervention Used | Relative Difference between Groups | |||||||
| Treatment Component | Assessment | Adjusted Mean (97.5% CI) | Change from 1-week | p value | Adjusted Mean (97.5% CI) | Change from 1-week | p value | Estimated relative difference 97.5% CI | p value |
| SMT | 4 weeks | 3.28 (2.91, 3.64) | −0.75 (−1.09, −0.42) | <.001 | 3.13 (2.76, 3.51) | −0.90 (−1.24, −0.56) | <.001 | −0.14 (−0.60, 0.32) | 0.48 |
| 12 weeks | 3.02 (2.61, 3.42) | −1.02 (−1.39, −0.64) | <.001 | 3.10 (2.69, 3.51) | −0.93 (−1.32, −0.55) | <.001 | 0.08 (−0.44, 0.61) | 0.72 | |
| ACTex | 4 weeks | 3.29 (2.92, 3.66) | −0.75 (−1.08, −0.41) | <.001 | 3.13 (2.76, 3.49) | −0.91 (−1.24, −0.57) | <.001 | −0.16 (−0.62, 0.30) | 0.43 |
| 12 weeks | 3.15 (2.74, 3.56) | −0.88 (−1.27, −0.5) | <.001 | 2.96 (2.56, 3.37) | −1.07 (−1.44, −0.69) | <.001 | −0.19 (−0.71, 0.33) | 0.42 | |
| MOBex | 4 weeks | 3.43 (3.06, 3.81) | −0.60 (−0.94, −0.26) | <.001 | 2.98 (2.61, 3.34) | −1.05 (−1.39, −0.72) | <0.01 | −0.46 (−0.92, 0.0) | 0.03 |
| 12 weeks | 3.15 (2.74, 3.56) | −0.88 (−1.27, −0.5) | <.001 | 2.97 (2.56, 3.37) | −1.07 (−1.44, −0.69) | <0.01 | −0.18 (−0.70, 0.34) | 0.44 | |
Mean values are adjusted for 1-week value of the pain intensity outcome measure and study site. P values refer to change from 1-week values.
Mechanistic Outcomes
For participants completing Phase I (n=241), mean multifidus activation was 11.55% (±9.48) at baseline and 11.92% (±10.34) at 1-week. Spinal stiffness means were 5.91 N/mm (±1.21) at baseline and 5.98 N/mm (±1.23) after 1-week. The change across Phase I was not significant for multifidus activation (p=0.39) or spinal stiffness measures (p=0.28). There were no significant main effects for any treatment component for multifidus activation (Table 5) or spinal stiffness (Table 6) outcomes. At 12 weeks, the 2-way interactions between SMT and ACTex (p=0.031) approached significance, in which patients assigned to SMT intervention had a greater reduction in stiffness without the addition of ACTex than with the ACTex. Other 2- and 3-way interaction results for multifidus activation and spinal stiffness are provided in Appendix.
Table 5.
Main effect estimates for multifidus activation outcome measure.
| Main Effects for Randomized Treatment Components | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention Not Used | Intervention Used | Relative Difference between Groups | |||||||
| Treatment Component | Assessment | Adjusted Mean (97.5% CI) | Change from 1-week | p value | Adjusted Mean (97.5% CI) | Change from 1-week | p value | Estimated relative difference 97.5% CI | p value |
| SMT | 4 weeks | 12.67 (10.83, 14.52) | 0.94 (−0.52, 2.41) | 0.15 | 12.10 (10.20, 14.0) | 0.37 (−1.17, 1.91) | 0.59 | −0.57 (−2.67, 1.52) | 0.54 |
| 12 weeks | 12.57 (10.69, 14.45) | 0.84 (−0.91, 2.59) | 0.28 | 12.20 (10.28, 14.12) | 0.47 (−1.33, 2.26) | 0.56 | −0.37 (−2.76, 2.02) | 0.73 | |
| ACTex | 4 weeks | 12.81 (10.94, 14.69) | 1.08 (−0.42, 2.58) | 0.11 | 12.0 (10.09, 13.84) | 0.23 (−1.27, 1.73) | 0.73 | −0.85 (−2.94, 1.25) | 0.36 |
| 12 weeks | 12.67 (10.75, 14.59) | 0.94 (−0.85, 2.73) | 0.24 | 12.10 (10.22, 13.97) | 0.37 (−1.39, 2.12) | 0.64 | −0.57 (−2.97, 1.82) | 0.59 | |
| MOBex | 4 weeks | 12.36 (10.47, 14.25) | 0.63 (−0.9, 2.15) | 0.36 | 12.42 (10.56, 14.28) | 0.69 (−0.79, 2.16) | 0.30 | 0.06 (−2.03, 2.16) | 0.95 |
| 12 weeks | 12.03 (10.1, 13.95) | 0.3 (−1.5, 2.1) | 0.71 | 12.74 (10.87, 14.62) | 1.01 (−0.74, 2.76) | 0.19 | 0.71 (−1.68, 3.11) | 0.50 | |
Mean values represent percent activation adjusted for 1-week value of the multifidus activation measure and study site. P values refer to change from 1-week values.
Table 6.
Main effect estimates for spinal stiffness outcome measure.
| Main Effects for Randomized Treatment Components | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention Not Used | Intervention Used | Relative Difference between Groups | |||||||
| Treatment Component | Assessment | Adjusted Mean (97.5% CI) | Change from 1-week | p value | Adjusted Mean (97.5% CI) | Change from 1-week | p value | Estimated relative difference 97.5% CI | p value |
| SMT | 4 weeks | 6.22 (6.01, 6.42) | 0.24 (0.06, 0.41) | 0.003 | 6.2 (5.98, 6.41) | 0.21 (0.03, 0.40) | 0.011 | −0.02 (−0.26, 0.22) | 0.85 |
| 12 weeks | 6.23 (6.03, 6.44) | 0.25 (0.06, 0.45) | 0.004 | 6.07 (5.85, 6.28) | 0.08 (−0.12, 0.28) | 0.35 | −0.17 (−0.43, 0.09) | 0.14 | |
| ACTex | 4 weeks | 6.31 (6.10, 6.52) | 0.33 (0.14, 0.51) | <.001 | 6.1 (5.90, 6.31) | 0.12 (−0.06, 0.30) | 0.13 | −0.2 (−0.45, 0.04) | 0.06 |
| 12 weeks | 6.24 (6.03, 6.46) | 0.26 (0.06, 0.46) | 0.004 | 6.06 (5.85, 6.27) | 0.07 (−0.12, 0.27) | 0.39 | −0.19 (−0.45, 0.07) | 0.11 | |
| MOBex | 4 weeks | 6.14 (5.93, 6.35) | 0.16 (−0.03, 0.34) | 0.06 | 6.28 (6.07, 6.48) | 0.29 (0.11, 0.47) | <.001 | 0.14 (−0.11, 0.38) | 0.20 |
| 12 weeks | 6.14 (5.92, 6.35) | 0.15 (−0.05, 0.36) | 0.09 | 6.16 (5.96, 6.37) | 0.18 (−0.01, 0.38) | 0.04 | 0.03 (−0.23, 0.29) | 0.81 | |
Mean values represent N/mm adjusted for 1-week value of the spinal stiffness measure and study site. P values refer to change from 1-week values.
Determination of Optimized Multi-Modal Protocol
The BIC comparison of all possible models composed of main effects, pair-wise and the 3-way interaction with 12-week ODI as the outcome variable identified the model with a single main effect for ACTex as the optimum model (BIC = 995.5). Five additional models explained more variance than the null model and represent possible optimal treatment combinations.(Table 7).
Table 7.
Multi-modal combinations of treatment components that optimized 12-week Oswestry outcome beyond the intercept-only model.
| Effects in Model | Bayesian Information Criteria (BIC) |
|---|---|
| ACTex | 995.45 |
| MOBex, ACTex | 996.05 |
| ACTex, MOBex, ACTex x MOBex | 996.06 |
| SMT, ACTex | 997.51 |
| SMT, ACTex, MOBex | 998.13 |
| SMT, ACTex, MOBex, ACTex x MOBex | 998.16 |
| INTERCEPT ONLY | 999.06 |
All models included the 1-week Oswestry score and study site as covariates. Lower BIC values indicate better fit relative to model complexity. The lowest BIC was achieved by the model that included ACTex as a main effect. ACTex x MOBex denotes the pairwise interaction between the ACTex and MOBex interventions.
DISCUSSION
This study examined the effects of different co-intervention components provided following a brief (2 session) SMT protocol on mechanistic (spinal stiffness and multifidus muscle activation) and patient-centered outcomes (pain intensity and LBP-related disability) in participants with LBP. Consistent with the MOST framework guiding the study, co-intervention components were selected based on preliminary work and theoretical models of mechanisms underlying SMT.30, 47, 69 The goal of the project within the MOST framework was to identify an SMT protocol that optimized outcomes by examining three co-intervention components (multifidus activating exercise, stiffness exercise and additional SMT dose) in a factorial experiment. We used a conservative significance level due to multiple outcome measures and the number of comparisons. Differences meeting the significance threshold were only identified for outcome of disability assessed with the ODI. Specifically, our results support inclusion of multifidus activating exercises as a component of an optimized SMT protocol based on its main effect on disability at the 12-week follow-up and its inclusion in all multi-modal models selected based on BIC values. Multi-modal models including additional SMT dose and range of motion exercises were identified as possible optimized models.
Implications of these finding for future clinical trials evaluating the effectiveness of SMT may consider multifidus activating exercise as a core component of an optimized protocol for the outcome of disability. The effect size after 12 weeks was modest however. Participants receiving ACTex experienced a mean 3.6 points greater change on the ODI from the 1-weeks assessment, a value below thresholds for clinically important difference estimated from 6–10 points on the ODI. 29, 54 Additional SMT dose and range of motion exercises did not have a negative impact on outcomes and were included in some optimized multi-modal models. We interpret these finding to indicate these components may be considered as adaptable aspects of an optimized SMT protocol with inclusion based on local circumstances, patient preferences or shared decisions made between patient and clinician. Multi-component interventions are composed of both core and adaptable elements, and the distinction is an important consideration for future clinical trials and implementation efforts.17
Selection of multifidus activating exercise as a component of an optimized SMT protocol is consistent with current models emphasizing the SMT’s neurophysiologic impact as an important mechanism underlying its therapeutic effects.4,30 The multifidus is an important stabilizing muscle of the lumbar spine45 that is susceptible to morphologic changes32 and reduced force output with LBP.21, 49 Some studies report that failure to regain pre-LBP multifidus morphology and activation is related to worse recovery and increased risk of recurrence.42, 49 Collectively these findings provide rationale to support our finding of an optimized protocol including SMT with activating exercise as a core co-intervention.
Although multifidus activating exercise was identified as a core component in an optimized protocol, we did not find that these exercises resulted in changes in the ability to activate the multifidus muscle. Thus while our results support multifidus activating exercise as a core component of an optimized SMT protocol, we did not identify the anticipated mechanistic explanation for this result. There are several possible explanations. First, exercise programs designed to strengthen trunk muscles including the multifidus are evidence-based in their own right for LBP.50, 61 Thus our findings may represent the additive benefit of providing two distinct interventions, each of which has small, positive effects on outcome, but without a common mechanistic pathway. Second, the measurement procedures used to quantify change in multifidus activation may have been insufficiently reliable. While multifidus thickness measured with ultrasound has high reliability between and within raters,40 measuring change in thickness measures longitudinally may compound measurement error and diminish reliability to the point of obscuring the ability to detect change.46 Finally, SMT and multifidus activating exercise may work along a shared pathway, but not one specifically related to the function or morphology of the muscle itself. For example, recent research suggests SMT’s effects may be at least partially explained through effects on maladaptive behavioral and cognitive responses to fear of movement or activity.25 Fear of movement and other adverse pain cognitions are known to mediate of the effects of various nonpharmacologic pain treatments.51 It may be the case that both SMT and multifidus exercise impact similar psychological factors to improve outcomes. We did not test this hypothesis.
Neither the spine mobilizing exercise component nor additional SMT dose beyond two sessions showed a consistent, significant impact on patient-centered or mechanistic outcomes. Our finding that SMT dose did not impact clinical outcomes is generally consistent with the small body of literature investigating SMT dose-response that has not found strong relationships between outcomes and increasing number of SMT sessions.35, 57 Reduction of spinal stiffness has historically been considered a mechanism underlying the effects of SMT,65 though few studies have reported relationships between SMT and changes in stiffness, or relationship of stiffness changes with clinical outcomes. We had previously reported a relationship between immediate change in spinal stiffness and disability outcomes after 1 week, leading to our inclusion of spinal mobilizing exercises in this study.30 In this study spinal stiffness exercise did not produce greater change in spinal stiffness and was generally not associated with superior clinical outcomes. These results may indicate that spinal stiffness changes are not be a mechanism underlying the effects of SMT, or could reflect the lack of evidence for stretching or range of motion exercises as effective for LBP.55 Alternatively, our results may reflect the need match mobilizing exercise specifically to patients with greater degrees of baseline stiffness, a hypothesis we did not evaluate.
Although SMT has been studied in numerous clinical trials, it has been difficult to determine how its effectiveness can be improved from this body of literature. Clinical trials typically either provide SMT as a unimodal intervention contradicting clinical practice, or provide SMT as part of a treatment package without systematic preliminary evaluation of the individual components within the package.14 Clinical trials with multi-modal SMT treatment packages that do not investigate the component parts are unable to provide evidence on strategies to optimize the package for future studies, leading to a collection of SMT trials testing various, disconnected protocols. The traditional treatment package testing approach is costly and inefficient as it prevents researchers from learning which components contribute to outcomes and which do not, then applying these lessons in subsequent studies to iteratively build more effective protocols.15 The MOST framework identifies the need for optimization of a treatment protocol as a preliminary step before a trial evaluating effectiveness. Optimization of a protocol can also facilitate future efforts at implementation by clarifying the core components that need to be delivered with high fidelity, components that can be adapted to local context, and those components that can or should be discarded.33
We embedded this study within a MOST framework. Future studies will consider additional optimization elements or modifications to the elements examined in this study, moving towards an SMT protocol for evaluation in subsequent effectiveness trials. In this study we provided 1 week of treatment with SMT alone before examining co-interventions. Additional optimization around the timing, progression and dosage of SMT and exercise components may provide strategies to further enhance efficiency and clinical outcomes. Optimization of contextual factors around the delivery of SMT is another important consideration. Contextual factors are the physical, psychological and social elements characterizing a therapeutic encounter,20 which can elicit a pronounced impact on outcomes.59 Future studies should examine strategies to enhance the working alliance between clinician and patient and build patient self-efficacy as an effort to optimize SMT protocols.
This study has important limitations. First, although our selection of co-intervention components was based on preliminary work and mechanistic hypotheses, other co-intervention components could be examined (e.g., different types of exercise, etc.). Second, our longest term outcome was 12 weeks. Third, we provided 2 sessions of SMT alone prior to factorial randomization which may not reflect typical practice patterns. Fourth, although we sought to recruit a heterogeneous group of persons with LBP, we excluded individuals with a history or surgery or signs of neurogenic LBP which limits generalizability to these groups. Finally, we did not tailor intervention components to any aspect of the patient’s baseline characteristics. It is possible that a component such as mobilizing exercise may be beneficial for patients with impaired range of motion or other moderating factors. Additional research is needed to examine hypotheses related to personalizing SMT protocol components to particular examination findings.
Supplementary Material
HIGHLIGHTS.
Spinal manipulative therapy (SMT) research is characterized by small effect sizes.
Research has typically studied SMT as a unimodal treatment
Clinicians usually provide SMT as part of a multimodal treatment package.
We used a factorial randomized trial to develop a multimodal SMT protocol.
Disability was improved by combining SMT and multifidus activating exercises.
Future research using optimized SMT protocols may increase treatment effects.
PERSPECTIVE.
Optimizing the effects of nonpharmacological treatments such as spinal manipulative therapy (SMT) for low back pain is challenging due to uncertainty regarding mechanisms and the complexity of multi-component protocols. This factorial randomized trial examined SMT protocols provided with differing co-interventions with mechanistic and patient-centered outcomes. Patient-centered outcomes were optimized by inclusion of lumbar multifidus strengthening exercises.
Acknowledgments
DISCLOSURES
Research reported in this publication was supported by the National Center for Complementary & Integrative Health of the National Institutes of Health under Award UH3AT009293–01). The study was supported by the University of Utah Study Design and Biostatistics Center with funding in part through grant 5UL1TR001067–02 (formerly 8UL1TR000105 and UL1RR025764) from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health. The authors report no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Julie M. Fritz, College of Health, University of Utah, 383 Colorow Drive, Salt Lake City, UT, USA 84108.
Jason Sharpe, Department of Physical Therapy & Athletic Training, University of Utah, 520 Wakara Way, Salt Lake City, UT, USA 84108.
Tom Greene, Department of Internal Medicine and Director, Population Health Research Study Design and Biostatistics Center, School of Medicine, University of Utah, 295 Chipeta Way, Salt Lake City, UT, USA 84132.
Elizabeth Lane, Department of Physical Therapy & Athletic Training, University of Utah, 520 Wakara Way, Salt Lake City, UT, USA 84108.
Maliheh Hadizadeh, Department of Physical Therapy, Faculty of Rehabilitation Medicine, University of Alberta, 3-44 Corbett Hall, Edmonton, Alberta, Canada T6G 2G4.
Molly McFadden, Division of Epidemiology, School of Medicine, University of Utah, 295 Chipeta Way, Salt Lake City, UT, USA 84132.
Douglas Santillo, U.S. Army-Baylor Physical Therapy Program, 3630 Stanley Road, Bldg 2841, Fort Sam Houston, TX, USA 78234.
Jedidiah Farley, Department of Physical Therapy & Athletic Training, University of Utah, 520 Wakara Way, Salt Lake City, UT, USA 84108.
Jake Magel, Department of Physical Therapy and Athletic Training, University of Utah, 520 Wakara Way, Salt Lake City, UT, USA 84108.
Anne Thackeray, Department of Physical Therapy & Athletic Training, University of Utah, 520 Wakara Way, Salt Lake City, UT, USA 84108.
Gregory Kawchuk, Department of Physical Therapy, Faculty of Rehabilitation Medicine, University of Alberta, 3-44 Corbett Hall, Edmonton, Alberta, Canada T6G 2G4.
REFERENCES
- 1.Global Burden of Disease Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1545–1602, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andronis L, Kinghorn P, Qiao S, Whitehurst DG, Durrell S, McLeod H. Cost-effectiveness of noninvasive and non-pharmacological interventions for low back pain: a systematic literature review. Appl Health Econ Health Policy 15:173–201, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Beliveau PJH, Wong JJ, Sutton DA, Simon NB, Bussieres AE, Mior SA, French SD. The chiropractic profession: a scoping review of utilization rates, reasons for seeking care, patient profiles, and care provided. Chiropr Manual Therap 25:35, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bialosky JE, Beneciuk JM, Bishop MD, Coronado RA, Penza CW, Simon CB, George SZ. Unraveling the mechanisms of manual therapy: modeling an approach. J Orthop Sports Phys Ther 48:8–18, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther 14:531–538, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronfort G, Haas M, Evans R, Kawchuk G, Dagenais S. Evidence-informed management of chronic low back pain with spinal manipulation and mobilization Spine J 8:213–225, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Burns JW, Nielson WR, Jensen MP, Heapy AA, Czlapinski RA, Kerns RD. Does change occur for the reasons we think it does? A test of specific therapeutic operations during cognitive-behavioral treatment of chronic pain. Clin J Pain 31:603–611, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Childs JD, Fritz JM, Flynn TW, Irrgang JJ, Johnson KK, Majkowski GR, Delitto A. Validation of a clinical prediction rule to identify patients with low back pain likely to benefit from spinal manipulation. Ann Intern Med 141:920–928, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine 30:1331–1335, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, Fu R, Dana T, Kraegel P, Griffin J, Grusing S, Brodt E: Noninvasive Treatments for Low Back Pain. Comparative Effectiveness Review No. 169. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290–2012-00014-I.). In: AHRQ Publication No. 16-EHC004-EF, Agency for Healthcare Research and Quality; Rockville, MD, February 2016. [PubMed] [Google Scholar]
- 11.Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, Fu R, Dana T, Kraegel P, Griffin J, Grusing S, Brodt ED. Nonpharmacologic therapies for low back pain: Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 166:493–505, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Cleland JA, Fritz JM, Kulig K, Davenport TE, Eberhart S, Magel JS, Childs JD. Comparison of the effectiveness of three manual physical therapy techniques in a subgroup of patients with low back pain who satisfy a clinical prediction rule: a randomized clinical trial. Spine 34:2720–2729, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Coffman CJ, Edelman D, Woolson RF. To condition or not condition? Analysing ‘change’ in longitudinal randomised controlled trials. BMJ Open 6:e013096, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins LM, Murphy SA, Nair VN. A strategy for optimizing and evaluating behavioral interventions. Ann Behav Med 30:65–73, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med 32:S112–S118, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colloca CJ, Keller TS. Stiffness and neuromuscular reflex response of the human spine to to posteroanterior manipulative thrusts in patients with low back pain J Manipulative Physiol Ther 24:489–500, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 4:50, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Veterans Affairs: VA/DoD Clinical Practice Guideline for Diagnosis and Treatment of Low Back Pain, Veterans Affairs Dept; Office of Quality, Safety and Value, Washington, D.C., 2018. [Google Scholar]
- 19.Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, Carrino J, Chou R, Cook K, DeLitto A, Goertz C, Khalsa P, Loeser J, Mackey S, Panagis J, Rainville J, Tosteson T, Turk D, Von Korff M, Weiner DK. Focus article report of the NIH task force on research standards for chronic low back pain. Clin J Pain 30:701–712, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Di Blasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. Influence of context effects on health outcomes: a systematic review. Lancet 357:757–762, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Dickx N, Cagnie B, Parlevliet T, Lavens A, Danneels L. The effect of unilateral muscle pain on recruitment of the lumbar multifidus during automatic contraction. An experimental pain study. Man Ther 15:364–369, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Dieleman JL, Cao J, Chapin A, Chen C, Li Z, Liu A, Horst C, Kaldjian A, Matyasz T, Scott KW, Bui AL, Campbell M, Duber HC, Dunn AC, Flaxman AD, Fitzmaurice C, Naghavi M, Sadat N, Shieh P, Squires E, Yeung K, Murray CJL. US health care spending by payer and health condition, 1996–2016. JAMA 323:863–884, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowell D, Haegerich TM, Chou R. CDC guidelines for prescribing opioids for chronic pain - United States, 2016. JAMA 315:1624–1645, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekstrom RA, Osborn RW, Hauer PL. Surface electromyographic analysis of the low back muscles during rehabilitation exercises. J Orthop Sports Phys Ther 38:736–745, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Ellingsen DM, Napadow V, Protsenko E, Mawla I, Kowalski MH, Swensen D, O’Dwyer-Swensen D, Edwards RR, Kettner N, Loggia ML. Brain mechanisms of anticipated painful movements and their modulation by manual therapy in chronic low back pain. J Pain 19:1352–1365, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elnaggar IM, Nordin M, Sheikhzadeh A, Parnianpour M, Kahanovitz N. Effects of spinal flexion and extension exercises on low-back pain and spinal mobility in chronic mechanical low-back pain patients. Spine 16:967–972, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Filiz MB, Firat SC. Effects of physical therapy on pain, functional status, sagittal spinal alignment, and spinal mobility in chronic non-specific low back pain. Eurasian J Med 51:22–26, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flynn T, Fritz J, Whitman J, Wainner R, Magel J, Rendeiro D, Butler B, Garber M, Allison S. A clinical prediction rule for classifying patients with low back pain who demonstrate short term improvement with spinal manipulation. Spine 27 2835–2843, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Fritz JM, Irrgang JJ. A comparison of a modified Oswestry disability questionnaire and the Quebec back pain disability scale. Phys Ther 81:776–788, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Fritz JM, Koppenhaver SL, Kawchuk GN, Teyhen DS, Hebert JJ, Childs JD. Preliminary investigation of the mechanisms underlying the effects of manipulation: exploration of a multivariate model including spinal stiffness, multifidus recruitment, and clinical findings. Spine 36:1772–1781, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fritz JM, Sharpe JA, Lane E, Santillo D, Greene T, Kawchuk GN. Optimizing treatment protocols for spinal manipulative therapy: study protocol for a randomized trial. Trials 19, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural changes of lumbar muscles in non-specific low back pain: a systematic review. Pain Physician 19:E985–e1000, 2016 [PubMed] [Google Scholar]
- 33.Guastaferro K, Collins LM. Achieving the goals of translational science in public health intervention research: The Multiphase Optimization Strategy (MOST). Am J Public Health 109:S128–s129, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gwadz MV, Collins LM, Cleland CM, Leonard NR, Wilton L, Gandhi M, Scott Braithwaite R, Perlman DC, Kutnick A, Ritchie AS. Using the multiphase optimization strategy (MOST) to optimize an HIV care continuum intervention for vulnerable populations: a study protocol. BMC Public Health 17:383, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas M, Vavrek D, Peterson D, Polissar N, Neradilek MB. Dose-response and efficacy of spinal manipulation for care of chronic low back pain: a randomized controlled trial. Spine J 14:1106–1116, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hadizadeh M, Kawchuk GN, Parent E. Reliability of a new loaded rolling wheel system for measuring spinal stiffness in asymptomatic participants. BMC Musculoskelet Disord. 20:176, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Informat 42:377–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J, Pransky G, Sieper J, Smeets RJ, Underwood M. What low back pain is and why we need to pay attention. Lancet 391:2356–2367, 2018 [DOI] [PubMed] [Google Scholar]
- 39.Harvey MP, Descarreaux M. Short term modulation of trunk neuromuscular responses following spinal manipulation: a control group study. BMC Musculoskelet Disord. 14:92, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hebert JJ, Koppenhaver SL, Parent EC, Fritz JM. A systematic review of the reliability of rehabilitative ultrasound imaging for the quantitative assessment of the abdominal and lumbar trunk muscles. Spine 34:E848–856, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Herbert WJ, Heiss DG, Basso M. Influence of feedback schedule in motor performance and learning of a lumbar multifidus muscle task using rehabilitative ultrasound imaging: a randomized clinical trial. Phys Ther 88:261–269, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine 21:2763–2769, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Keller TS, Colloca CJ. Mechanical force spinal manipulation increases trunk muscle strength assessed by electromyography: a comparative clinical trial. J Manipulative Physiol Ther 23:585–595, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Kiesel KB, Uhl TL, Underwood FB, Rodd DW, Nitz AJ. Measurement of lumbar multifidus muscle contraction with rehabilitative ultrasound imaging. Man Ther. 12:161–166, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Ward SR, Kim CW, Eng CM, Gottschalk LJ, Tomiya A, Garfin SR, Lieber RL. Architectural analysis and intraoperative measurements demonstrate the unique design of the multifidus muscle for lumbar spine stability. J Bone Joint Surg Am 91:176–185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koppenhaver SL, Fritz JM, Hebert JJ, Kawchuk GN, Childs JD, Parent EC, Gill NW, Teyhen DS. Association between changes in abdominal and lumbar multifidus muscle thickness and clinical improvement after spinal manipulation. J Orthop Sports Phys Ther 41:389–399, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Koppenhaver SL, Fritz JM, Hebert JJ, Kawchuk GN, Parent EC, Gill NW, Childs JD, Teyhen DS. Association between history and physical examination factors and change in lumbar multifidus muscle thickness after spinal manipulation in patients with low back pain. J Electromyogr Kinesiol 22:724–731, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ladeira CE, Cheng M, Hill CJ. Physical therapists’ treatment choices for non-specific low back pain in Florida: an electronic survey. J Man Manipulative Therap 23:109–118, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacDonald DA, Moseley GL, Hodges PL. Why do some patients keep hurting their back? Evidence of ongoing back muscle dysfunction during remission from recurrent back pain. Pain 142:183–188, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Macedo LG, Saragiotto BT, Yamato TP, Costa LO, Menezes Costa LC, Ostelo RW, Maher CG. Motor control exercise for acute non-specific low back pain. Cochrane Database Syst Rev. 2:Cd012085, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marshall PWM, Schabrun S, Knox MF. Physical activity and the mediating effect of fear, depression, anxiety, and catastrophizing on pain related disability in people with chronic low back pain. PLoS One 12:e0180788, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKenzie RA, May S: The Lumbar Spine: Mechanical Diagnosis and Therapy. 2 edition, Orthopedic Physical Therapy Products, Minneapolis, MN, 2003. [Google Scholar]
- 53.Okubo Y, Kaneoka K, Imai A, Shiina I, Tatsumura M, Izumi S, Miyakawa S. Electromyographic analysis of transversus abdominis and lumbar multifidus using wire electrodes during lumbar stabilization exercises. J Orthop Sports Phys Ther 40:743–750, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Ostelo RWJ, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, Bouter LM, De Vet H. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine 33:90–94, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Owen PJ, Miller CT, Mundell NL, Verswijveren SJ, Tagliaferri SD, Brisby H, Bowe SJ, Belavy DL. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. Br J Sports Med. 2019, October 30, e-pub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paige NM, Miake-Lye IM, Booth MS, Beroes JM, Mardian AS, Dougherty P, Branson R, Tang B, Morton SC, Shekelle PG. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain: systemtatic review and meta-analysis. JAMA 317:1451–1460, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pasquier M, Daneau C, Marchand AA, Lardon A, Descarreaux M. Spinal manipulation frequency and dosage effects on clinical and physiological outcomes: a scoping review. Chiropr Man Therap 27:23, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med 166:514–530, 2017 [DOI] [PubMed] [Google Scholar]
- 59.Rossettini G, Carlino E, Testa M. Clinical relevance of contextual factors as triggers of placebo and nocebo effects in musculoskeletal pain. BMC Musculoskel Dis 19, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubinstein SM, Terwee CB, Assendelft WJ, de Boer MR, van Tulder MW. Spinal manipulative therapy for acute low back pain: an update of the cochrane review. Spine 38:E158–177, 2013 [DOI] [PubMed] [Google Scholar]
- 61.Saragiotto BT, Maher CG, Yamato TP, Costa LO, Menezes Costa LC, Ostelo RW, Macedo LG. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst Rev.Cd012004, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 8:3–15, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Skelly AC, Chou R, Dettori JR, Turner JA, Friedly JL, Rundell SD, Fu R, Brodt ED, Wasson N, Winter C: Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review. Comparative Effectiveness Review No. 209. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290–2015-00009-I.) AHRQ Publication No 18-EHC013-EF, Agency for Healthcare Research and Quality, Rockville, MD, 2018. [PubMed] [Google Scholar]
- 64.Thorn BE, Burns JW. Common and specific treatment mechanisms in psychosocial pain interventions: the need for a new research agenda. Pain 152:705–706, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Triano JJ. Biomechanics of spinal manipulative therapy. Spine J 1:121–130, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Verbeke G, Molenberghs G: Linear mixed models for longitudinal data, Springer Verlag, Heidelberg, Germany, 2000. [Google Scholar]
- 67.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 52:157–168, 1993 [DOI] [PubMed] [Google Scholar]
- 68.Walker BF, French SD, Grant W, Green S. Combined chiropractic interventions for low-back pain. Cochrane Database Syst Rev.Cd005427, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong AY, Parent EC, Dhillon SS, Prasad N, Kawchuk GN. Do participants with low back pain who respond to spinal manipulative therapy differ biomechanically from nonresponders, untreated controls or asymptomatic controls? Spine 40:1329–1337, 2015 [DOI] [PubMed] [Google Scholar]
- 70.Young JC, Jonsson Funk M, Dasgupta N. Medical use of long-term extended-release opioid analgesics in commercially insured adults in the United States. Pain Med 21:724–735, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.