Abstract
The human brain requires adequate cerebral blood flow to meet the high demand for nutrients and to clear waste products. With age, there is a chronic reduction in cerebral blood flow in small resistance arteries that can eventually limit proper brain function. The endothelin system is a key mediator in the regulation of cerebral blood flow, but the contributions of its constituent receptors in the endothelial and vascular smooth muscle layers of cerebral arteries have not been well defined in the context of aging. We isolated posterior cerebral arteries from young and aged Fischer 344 rats, as well as ETB receptor knock-out rats and mounted the vessels in plexiglass pressure myograph chambers to measure myogenic tone in response to increasing pressure and targeted pharmacological treatments. We used an ETA receptor antagonist (BQ-123), an ETB receptor antagonist (BQ-788), endothelin-1, an endothelin-1 synthesis inhibitor (phosphoramidon), and vessel denudation to dissect the roles of each receptor in aging vasculature. Aged rats exhibited a higher myogenic tone than young rats, and the tone was sensitive to the ETA antagonist, BQ-123, but insensitive to the ETB antagonist, BQ-788. By contrast, the tone in the vessels from young rats was raised by BQ-788 but unaffected by BQ-123. When the endothelial layer that is normally enriched with ETB1 receptors was removed from young vessels, myogenic tone increased. However, denudation of the endothelial layer did not influence vessels from aged animals. This indicated that endothelial ETB1 receptors were not functional in the vessels from aged rats. There was also an increase in ETA receptor expression with age, whereas ETB receptor expression remained constant between young and aged animals. These results demonstrate that in young vessels, ETB1 receptors maintain a lower myogenic tone, but in aged vessels, a loss of ETB receptor activity allows ETA receptors in vascular smooth muscle cells to raise myogenic tone. Our findings have potentially important clinical implications for treatments to improve cerebral perfusion in older adults with diseases characterized by reduced cerebral blood flow.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-020-00309-7.
Keywords: ETA receptors, ETB receptors, Fischer 344 rats, Myogenic tone, Cerebral artery
Introduction
Despite its relatively small size, the human brain consumes large quantities of oxygen and glucose, and accounts for 20% of human caloric expenditure [5, 61]. Functional activity in the brain requires adequate cerebral blood flow to meet the high demand for nutrients and to clear carbon dioxide and metabolic waste products [29, 86]. The use of radiolabeled oxygen with positron emission tomography, pulsed arterial spin labeling, and transcranial Doppler ultrasonography has revealed that with age, there is a chronic reduction in cerebral blood flow in humans that leads to hypoperfusion [9, 47, 50, 78, 90]. Chen et al. observed that rates of cerebral blood flow in the cortical gray matter drop nearly 20% from ages 30 to 70 years. This age-related decline in cerebral blood flow can eventually be accompanied by deficits in oxygen and glucose consumption that limit proper brain function (reviewed in [37]). The dynamic regulation of cerebral blood flow is mediated in part by the endothelin (ET) system which controls the myogenic tone of cerebral vessels [33, 59]. Myogenic tone refers to a state of sustained contraction in the vascular smooth muscle cell layer that controls the luminal diameter of the vessel, and in turn, the volume of blood that travels through it [36]. Thus, contributions of the ET system to a heightened myogenic tone may be associated with reduced cerebral blood flow in the setting of advanced age.
The ET system comprises two ET receptors, ETA and ETB, which respond to endogenous endothelins to regulate blood vessel diameter [25]. There are three endogenous ET peptides (ET-1, ET-2, and ET-3) that activate the ET receptors. ET-1 is the predominant isoform in the human vasculature, but each isoform has similar effects on vascular tone and they primarily differ in terms of binding affinity and tissue distribution (reviewed in [14]). In endothelial cells, ET-1 is biosynthesized from big ET-1 by endothelin-converting enzymes (ECEs) and then stored in Weibel-Palade bodies to be released upon stimulation [51]. ET-1 is also stored in secretory vesicles and is released constitutively through a cyclic AMP-independent mechanism to maintain basal vascular tone (for review [19, 63]). The predominant ECE, ECE-1, is a membrane-bound phosphoramidon (PPA)-sensitive metalloprotease that catalyzes the conversion of big ET-1 to ET-1 in humans [19]. ETB receptors are subdivided into two splice variants, ETB1 and ETB2, which are not known to differ in ligand binding, but do differ in tissue localization as well as ET-1 response [22, 70]. ETA and ETB2 receptors are expressed in the vascular smooth muscle cell layer and cause vasoconstriction through elevation of intracellular calcium in response to ET-1 released from the endothelial cell layer [14, 15, 25, 60, 89, 92]. ETA and ETB2 receptors generally mediate the normal increases in myogenic tone, fibrogenesis, and pro-inflammatory processes associated with ET-1 [21, 65]. In healthy vessels, these effects are balanced by the effects of ETB1 receptors in the endothelial cell layer which facilitate the release of nitric oxide (NO) and prostaglandins to stimulate vasodilation in response to ET-1 [14, 57, 73]. Although each ET receptor is important to the maintenance of proper vascular function, the baseline myogenic tone and response to ET peptides is thought to be a function of the balance between vascular smooth muscle and endothelial ET receptors within the vascular bed [70]. Thus, an imbalance between ET receptor function in the vascular smooth muscle cell layer and endothelium could contribute to cerebral artery dysfunction in aging.
Although a decline in cerebral blood flow with age has been well characterized, the role of the ET system in this process has not been established. The primary objective of this study was to examine how the contributions of the ET system to myogenic tone change with age using a common rodent model of aging, the Fischer 344 rats. Posterior cerebral arteries (PCAs) were isolated from young and aged male Fischer 344 rats and subjected to a controlled range of intraluminal pressures. We used selective ET receptor antagonists, ET-1 synthesis inhibitors, removal of the endothelium, and transgenic ETB knock-out rats to dissect the role of each ET receptor in the maintenance of myogenic tone in the PCAs of young and aged rats.
Methods
Animals
The animal protocols in this study conformed to the guidelines of the Canadian Council on Animal Care and were approved by the Dalhousie University Committee on Laboratory Animals. The care and experimental use of animals was planned with respect to the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines [42]. Animals were maintained on a 12-h light/dark cycle with ad libitum access to food and water in the Animal Care Facility at Dalhousie University. Three-month-old male Fischer 344 rats were purchased from Charles River Laboratories (St. Constant, QC) and were aged in the animal care facility at Dalhousie University.
The transgenic rats, commonly known as spotted lethal rats, were of Wistar-Kyoto background [43]. These rats were used in experiments when they were 3-6 months of age, comparable to the young male Fischer 344 rats. Spotted lethal rats have a 300 base pair deletion of the EDNRB gene and do not produce functional ETB1 or ETB2 receptors [27]. Herein, rats with one functional copy of the EDNRB gene are referred to as heterozygous and those without a functional EDNRB gene are referred to as ETB knock-out (ETB-KO) rats. Heterozygous rats were obtained as a generous gift from Dr. Thomas Yorio (University of North Texas) and were subsequently bred in-house to produce wild-type and ETB-KO rats. Rats were genotyped using DNA isolated from ear punches with the KAPA2G hot-start mouse genotyping kit (Sigma-Aldrich; Burlington, ON). The EDNRB allele was genotyped using custom PCR primers (forward: 5′-AGCCGGTGCGGACGCGCCTT-3′; reverse: 5′-CACGACTTAGAAAGCTACAC-3′). In this work, only male rats were included. We recognize this as a limitation for generalization of the observations between sexes.
Vessel perfusion experiments
Young (3-6 months) and aged (14-22 months) rats were injected with heparin (3000 U/kg) via intraperitoneal (i.p.) injection to inhibit blood coagulation and deeply anaesthetized with sodium pentobarbital (IP, 220 mg/kg). The brains were immediately removed from the cranial cavity after decapitation and placed in cold HEPES-physiological saline solution (HEPES-PSS; in mM: 145 NaCl; 5 KCl; 5 D-glucose, 10 HEPES; 1 MgCl2) at pH 7.3 and 37 °C. The posterior cerebral arteries (PCAs) were carefully cleared from surrounding connective tissue and second or third-order branches of 100-150 μm were prepared as per Shipley and Muller-Delp and Mandalà et al. PCAs were used as they are an example of cerebral resistance arteries with relatively few branches and can be easily cannulated and pressurized without leaks. The PCA also has been used previously to investigate pressurized cerebral arteries in the rat model and to examine cerebral artery smooth muscle-endothelium interactions [49, 58, 84, 85]. Middle cerebral arteries were overly branched and not amenable to these experiments.
Vessels were mounted in a plexiglass pressure myograph chamber (Living Systems Instrumentation, Burlington, VT) filled with HEPES-PSS. The proximal ends of arterial segments were mounted to the inflow cannula and perfused with HEPES-PSS at 25 mmHg for 10 min to rinse out intraluminal blood. After the 10-min rinse, the distal end of each vessel segment was tied. The entire pressure chamber was placed on the stage of a Nikon Eclipse TS100 inverted microscope (Nikon Instruments Inc.; Melville, NY) equipped with a Panasonic GP-CD60 camera (Panasonic Canada Inc.; Mississauga, ON) and the pClamp version 8.1 analysis software (Molecular Devices; Sunnyvale, CA) to monitor intraluminal diameter. The PCAs were pressurized to a baseline intraluminal pressure of 20 mmHg using a pressure servo-control unit (Living Systems Instrumentation, Burlington, USA). The PCAs were constantly superfused at 4 mL/min with HEPES-PSS. To facilitate active resistance to increased pressure, the physiological salt solution was appropriately supplemented with 2.8 mM CaCl2. To establish pressure curves, intraluminal pressure was increased from 20 to 200 mmHg in increments of 20 mmHg and held for approximately 5 min at each pressure point. PCA diameter was monitored at each pressure point and the measurement was taken once the diameter was stable. Pressure curves were constructed for vessels in the absence of drug and these served as the control group. In separate vessels, drugs were added to the perfusate and vessels were equilibrated before a new pressure curve was produced. Myogenic tone was calculated at each pressure point as (∆(diameter in 0 mM CaCl2 buffer−diameter in 2.8 mM CaCl2 buffer)/diameter in 0 mM CaCl2 buffer) * 100%. Each vessel was derived from one animal and each vessel was only used once in either a control or a drug-treatment experiment.
Drug treatments
For experiments involving treatments with BQ-123 [40] and BQ-788 [38] (Sigma-Aldrich; Burlington, ON), the drugs were added to the superfusion buffer and vessels were equilibrated for 30 min before the pressure was altered. We selected the concentrations of the ETA antagonist (BQ123) and the ETB antagonist (BQ788) based on concentrations of these drugs used to block the effects of ET-1 in previous studies of resistance arteries [2, 3, 24, 48, 80]. For experiments that involved PPA (Sigma-Aldrich; Burlington, ON), the drug was also added 30 min prior to any change in pressure. PPA binds to the catalytic domain of ECE-1 and inhibits the synthesis and secretion of ET-1 [51, 87]. PPA is not selective for ECE-1, as it efficiently blocks the neutral endopeptidase (NEP) activity, which increase vasodilators such as atrial natriuretic peptide and bradykinin [19].
For experiments where arteries were denuded to remove the endothelial cell layer, the vessels were perfused with 0.3% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) detergent for 5 min at 25 mmHg and rinsed with HEPES-PSS for approximately 10 min. Successful denudation for each vessel with CHAPS perfusion was confirmed by examining carbachol-induced vasodilation as per Ho and Hiley [34]. Carbachol-induced vasodilation was abolished in successfully denuded vessels but maintained in sham perfused vessels (data not shown).
Reverse transcription quantitative PCR
Reverse transcription quantitative PCR (RT-qPCR) was used to measure the expression of ETA receptors (forward: 5′-GGCCCTTGGAGACCTTATCTAC-3′, reverse: 5′-TGCTCTGTACCTGTCCACACT-3′), ETB receptors (forward: 5′-CCCTTCACCTCAGCAGGATT-3′, reverse: 5′-CAGCAGCACAAACACGACTTA-3′), and von Willebrand factor (VWF; forward: 5′-TTTGCTCAGGGACATGGCTTA-3′, reverse: 5′-AGGTGAGGGCCAGAACTAACA-3′). Βeta-actin was used as a reference gene (forward: 5′-CCCGCGAGTACAACCTTCTT-3′, reverse: 5′-GACCCATACCCACCATCACA-3′). The mRNA abundance of each gene was compared between the PCAs of young and aged Fischer 344 rats. VWF mRNA was used as a surrogate marker for endothelial number as VWF mRNA abundance is tightly correlated with the number of endothelial cells in each sample [93].
To perform RT-qPCR, PCAs were first carefully dissected from the rat brains, blotted dry, and weighed prior to homogenization in 600 μL RLT buffer (Qiagen) supplemented with 40 mM dithiothreitol (DTT). The tissues were subsequently passed several times through a 23-gauge needle. Total RNA was isolated from the homogenate using the RNeasy® mini kit (Qiagen). RNA was eluted in 30 μL RNase-free water. To produce cDNA, the total RNA was supplemented with 4 μM oligodT (12-18 nt) and 0.8 mM dNTP mix and incubated at 65 °C for 5 min. The mixture was then added to the first strand buffer containing 5 mM DTT, 2 U/μL RNaseOUT RNase inhibitor, in the presence (+RT) or absence (−RT) of 10 U/μL SuperScript III reverse-transcriptase (Invitrogen), incubated at 50 °C for 1 h and inactivated at 70 °C for 15 min. For each qPCR reaction, 1 μL of cDNA was combined with 2 μL of 10X LightCycler® FastStart DNA Master SYBR Green I (Roche), supplemented to contain 1 mM MgCl2 and 0.5 μM forward and reverse oligomers, and topped to 20 μL with nuclease free water. The reaction parameters consisted of 95 °C for 10 min followed by 50 cycles of 95-62-72 °C for 10 s each, with fluorescent capture after every cycle. A melt curve was produced for every reaction to verify that the amplification was of a single product and consistent among all samples.
Statistical analyses
Statistical analyses were performed using GraphPad Prism Version 6.0 (GraphPad Software Inc., USA). All data are presented as the mean ± standard error of the mean. The repeated t test with Holm-Sidak correction for multiple comparisons was used to analyze experiments with two datasets. The repeated-measures two-way ANOVA with Tukey’s multiple comparisons test was used for experiments that included three datasets to be compared. For all analyses, the threshold for significance was P < 0.05.
Results
Basal myogenic tone is elevated in aged Fischer 344 rats
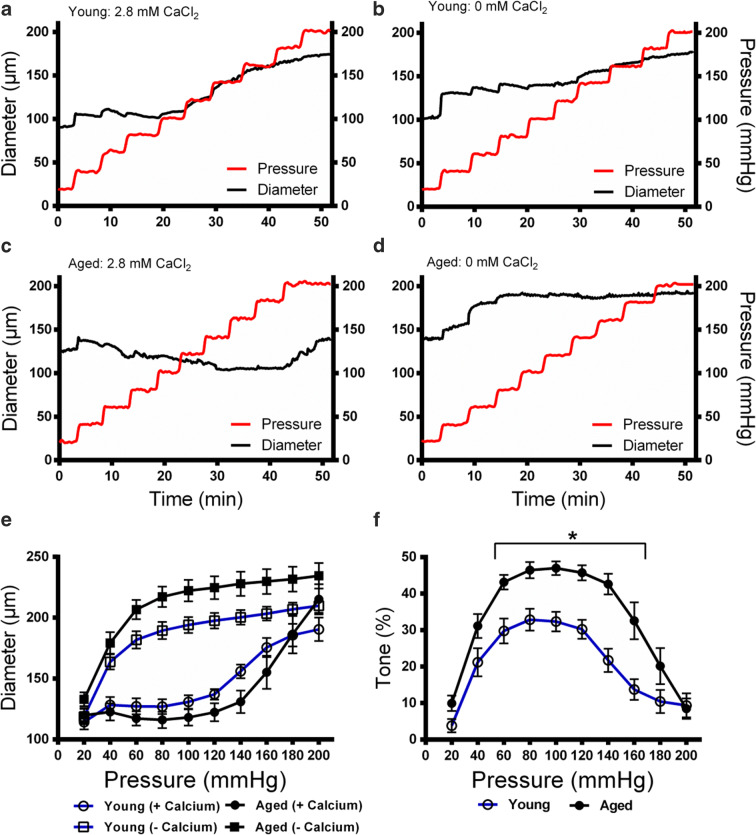
To define the baseline myogenic tone in the young and aged groups of Fischer 344 rats, PCAs were isolated from the rats and subjected to intraluminal pressures ranging from 20 to 200 mmHg. In the presence of calcium, the PCAs were capable of autonomous contraction which allowed the vessels to maintain a constant diameter and resist an initial increase in pressure. However, PCAs were eventually overwhelmed by pressure and forced to dilate. In the absence of calcium, autonomous contraction was inhibited, and the vessel diameter was dictated by the intraluminal pressure. Diameters of the PCAs were measured throughout the full range of intraluminal pressures over a period of 50 min in the presence or absence of 2.8 mM CaCl2 to control the potential for autonomous contraction (Fig. 1a-d). This yielded a paired dataset, with and without calcium that was used to calculate myogenic tone (Fig. 1e). Myogenic tone was calculated as the absolute difference between diameter of the PCAs between these conditions as a fraction of the diameter in the absence of calcium at each pressure point. In young rats, the baseline myogenic tone peaked at 32.9% at 80 mmHg (Fig. 1f). In aged rats, the myogenic tone peaked at 47.0% at 100 mmHg. The difference in tone remained significant between 60 and 160 mmHg. Thus, the baseline myogenic tone was higher in aged rats compared to the young rats.
Fig. 1.
Myogenic tone in PCAs isolated from young and aged Fischer 344 rats. Intraluminal pressure was increased from 20 to 200 mmHg in young (n = 10) and aged (n = 9) rat PCAs incubated in 2.8 mM CaCl2 or 0 mM CaCl2 with 4 mM EGTA. Vessel diameter was recorded at each pressure point over a period of 50 min. a Young vessel diameter measured with increased pressures in 2.8 mM CaCl2. b Young vessel diameter measured with increased pressures in 0 mM CaCl2. c Aged vessel diameter measured with increased pressures in 2.8 mM CaCl2. d Aged vessel diameter measured with increased pressures in 0 mM CaCl2. e Integrated information on pressure and diameter from young and aged vessels that were monitored in the presence and absence of 2.8 mM CaCl2. f Myogenic tone for young and old vessels. Tone was calculated as the difference in diameter in the presence and absence of calcium, as a fraction of the diameter in the absence of calcium. * P < 0.05 for young vs. aged animals at each pressure point. Significance was determined using repeated unpaired t tests with Holm-Sidak correction for multiple comparisons at each pressure point
Contributions of ETA and ETB receptors to myogenic tone in young and aged Fischer 344 rats
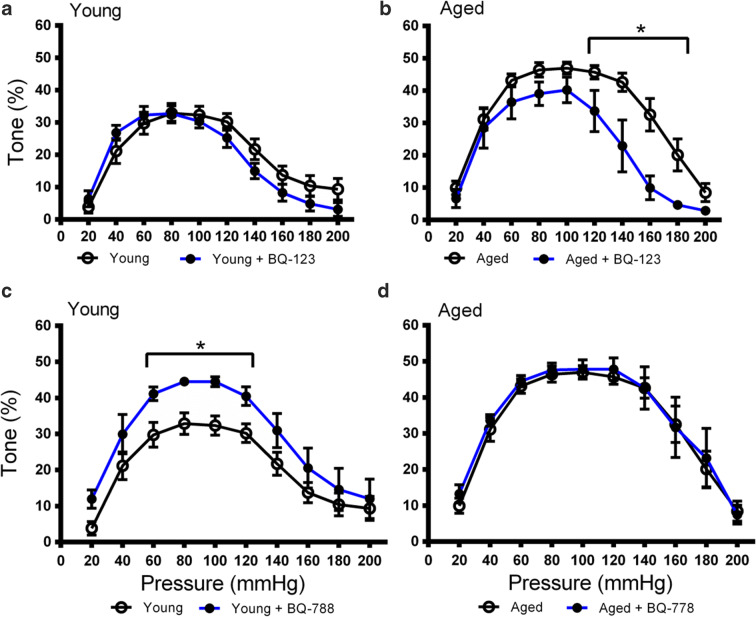
To determine if the increased myogenic tone in aged rats was linked to ETA receptor-dependent signaling, the myogenic tone of PCAs from young and aged rats was calculated in the presence and absence of the selective ETA receptor antagonist BQ-123 (1 μM). BQ-123 did not affect the tone of PCAs from young rats (Fig. 2a, Fig. S1A). In contrast, the peak myogenic tone decreased from 47.0 to 40.2% at 100 mmHg in the presence of BQ-123 in PCAs from aged rates, and the difference was significant from 120 to 180 mmHg relative to the untreated control (Fig. 2b, Fig. S1B). This indicated that the aged vasculature was more sensitive to ETA receptor blockade and suggested that ETA receptor-dependent signaling in the PCA may be enhanced with age. Furthermore, the blockade of ETA receptor-dependent effects on myogenic tone was more pronounced at high intraluminal pressures.
Fig. 2.
Influence of ETA and ETB inhibition on myogenic tone in PCAs from young and aged Fischer 344 rats. a Myogenic tone in the PCAs of young rats in the presence (n = 7) or absence (n = 10) of the ETA antagonist BQ-123 (1 μM). b Myogenic tone in the PCAs of aged rats in the presence (n = 5) or absence (n = 9) of the ETA antagonist BQ-123 (1 μM). c Myogenic tone in the PCAs of young rats in the presence (n = 6) or absence (n = 9) of the ETB antagonist BQ-778 (1 μM). d Myogenic tone in the PCAs of aged rats in the presence (n = 6) or absence (n = 9) of the ETB antagonist BQ-778 (1 μM). * P < 0.05 vs. antagonist treatment at each pressure point. Significance was determined using repeated unpaired t tests with Holm-Sidak correction for multiple comparisons at each pressure point
The role of ETB receptor-dependent signaling in the myogenic tone of young and aged PCAs was probed using an ETB receptor-selective antagonist. The myogenic tone of the PCAs was determined in the presence of the selective ETB receptor antagonist BQ-788 (1 μM) to block ETB receptor-dependent effects on myogenic tone. Consistent with the role of endothelial ETB1 receptor signaling in vasodilation, inhibition of ETB receptor-dependent signaling caused an increase in peak myogenic tone from 32.9 to 44.5% at 80 mmHg in young vasculature and the difference remained significant between 60 and 120 mmHg (Fig. 2c, Fig. S1C). This pattern of increased tone following ETB receptor blockade was not observed in the aged PCA preparations (Fig. 2d, Fig. S1D). This indicated that only the young vasculature was sensitive to the effects of ETB receptor inhibition and that ETB receptor-dependent mechanisms may contribute more to myogenic tone in young rats. Also, of note, the effects of ETB receptor inhibition in young vasculature were more pronounced in the middle portion of the pressure range as opposed to the high end observed with ETA receptor inhibition in aged PCAs.
To determine if there were differences in endothelin receptor expression between young and aged animals, RT-qPCR was used to measure the mRNA abundance of ETA and ETB receptors in the PCAs of young and aged Fischer 344 rats. The qPCR results indicated that ETA receptor expression increased with age (from ~ 6 to ~ 18 months of age) (Fig. S2A). In contrast, there was no change in ETB receptor expression between the young and aged groups of Fischer 344 rats (Fig. S2B). We also found a reduction in VWF mRNA abundance in aged rats which was indicative of a reduced number of endothelial cells in the aged vessels (Fig. S2C). Taken together, these results demonstrate that the vascular smooth muscle cells in aged vessels express higher levels of ETA receptors. However, there was no difference in overall quantities of ETB1 and ETB2 receptors between young and aged vessels despite a potential decrease in endothelial cell number.
Influence of the endothelium and ET-1-dependent signaling on myogenic tone in young and aged Fischer 344 rats
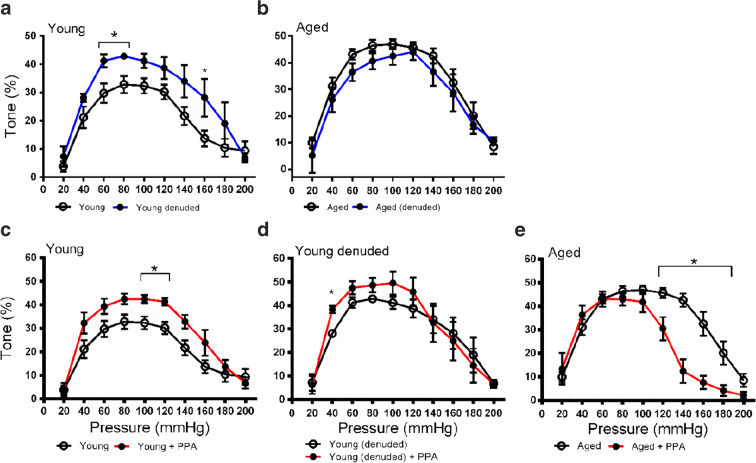
To determine the influence of the endothelium on the myogenic tone in cerebral vessels of young and aged rats, we denuded vessels to remove only the endothelium which expresses the ETB1 receptors responsible for vasodilation. Denudation of PCA endothelial cells by CHAPS detergent perfusion increased the myogenic tone in the young vasculature and the difference was significant at 60, 80, and 160 mmHg (Fig. 3a, Fig. S3A). The peak myogenic tone in the young PCAs was raised from 32.9 to 42.9% at 80 mmHg. In the aged rats, there were no differences observed following removal of the endothelial cell layer (Fig. 3b, Fig. S3B). The peak myogenic tone in the aged PCAs was shifted from 47.0 at 100 mmHg to 44.0% at 120 mmHg. These data indicate that the removal of the endothelial cell layer affected myogenic tone in PCAs from young, but not aged, rats.
Fig. 3.
Influence of vessel denudation and inhibition of ET-1 biosynthesis on myogenic tone in PCAs from young and aged Fischer 344 rats. Endogenous ET-1 biosynthesis was inhibited using phosphoramidon (PPA; 3 μM) to inhibit endogenous endothelin-converting enzyme-1 (ECE-1). a Myogenic tone in the PCAs of young rats before (n = 10) and after (n = 5) denudation. b Myogenic tone in the PCAs of aged rats before (n = 9) and after (n = 3) denudation. c Myogenic tone in the PCAs of young rats before (n = 10) and after (n = 4) treatment with PPA. d Myogenic tone in the denuded PCAs of young rats before (n = 5) and after (n = 4) treatment with PPA. e Myogenic tone in the PCAs of aged rats before (n = 9) and after (n = 5) treatment with PPA. * P < 0.05 vs. treatment with PPA and/or denudation at each pressure point. Significance was determined using repeated unpaired t tests with Holm-Sidak correction for multiple comparisons at each pressure point
As ET-1 acts on ETA and ETB2 receptors in vascular smooth muscle cells to increase myogenic tone, we hypothesized that a blockade of ET-1-dependent signaling would reduce the myogenic tone in young and aged rats. Endogenous production of ET-1 was indirectly blocked by the direct inhibition of endothelin-converting enzyme-1 (ECE-1) using 3-μM PPA in the perfusion buffer for 30 min prior to the experiment. Surprisingly, in the PCAs from young animals, the PPA treatment raised the peak tone from 32.9 to 42.5% at 80 mmHg and produced an increase in the myogenic tone at 100 and 120 mmHg (Fig. 3c, Fig. S3C). This increase in tone was only observed in young denuded vessels at 40 mmHg (Fig. 3d, Fig. S3D). As expected, in aged PCAs, the PPA treatment produced a significant decrease in tone at the high end of the pressure range, from 120 to 180 mmHg (Fig. 3e, Fig. S3E). This would be consistent with a pattern of ET-1 release acting on ETA and ETB2 receptors to maintain myogenic tone in response to increased pressure. These data indicate that ET-1 maintains a raised myogenic tone in the microvasculature of aged animals but may depress myogenic tone in young animals.
ETB knock-out rats recapitulate the dysfunction of aging vessels
To further dissect the role of ETB receptors in regulating myogenic tone in the rat PCA, we employed wild-type, heterozygous, and ETB-KO rats. The baseline myogenic tone of wild-type rats was no different from the tone of the young Fischer 344 rats (Fig. S4). In wild-type rats, the myogenic tone peaked at 29.9% at 80 and 100 mmHg (Fig. 4, Fig. S5). In ETB-KO rats, the myogenic tone was comparable to the aged Fischer 344 rats (Fig. S6). The myogenic tone in vessels from these animals peaked at 44.5% at 120 mmHg and was elevated relative to wild-type rats from 40 to 200 mmHg. The myogenic tone in heterozygous rats peaked at 41.8% at 100 mmHg but was only significantly elevated from 120 to 160 mmHg relative to wild-type rats. From 180 to 200 mmHg, the myogenic tone of wild-type and heterozygous rats was depressed while the tone in ETB-KO rats remained elevated. This may highlight a role of ETB2 receptors in vasorelaxation at extremely high pressures.
Fig. 4.
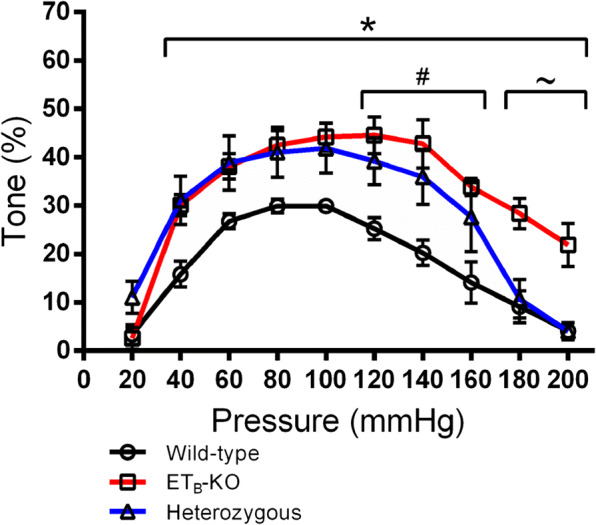
Effect of ETB knockout (ETB-KO) on myogenic tone in wild-type and genetically modified heterozygous and ETB-KO Wistar-Kyoto rats. Myogenic tone was determined for vessels from wild-type (n = 5), heterozygous (n = 4), and ETB-KO rats (n = 6). Tone was calculated as the difference in diameter in the presence and absence of calcium, as a fraction of the diameter in the absence of calcium. * P < 0.05 wild-type vs. ETB-KO. # P < 0.05 wild-type vs. heterozygous. ~ P < 0.05 ETB-KO vs. heterozygous. Significance was determined by repeated-measures two-way ANOVA with Tukey’s multiple comparisons test to determine the effect of genotype at each pressure point
We used young vessels as an example of functional ETB receptors with young denuded vessels to represent the loss of only ETB1 receptors, and vessels from ETB-KO rats to demonstrate the loss of both receptors. We observed that the aged vessels were most like the ETB-KO vessels from 60 to 160 mmHg but were most like denuded young vessels at the extreme ends of the pressure range. We also found that the vessels from ETB-KO rats did not significantly differ in myogenic tone from young denuded vessels until 200 mmHg intraluminal pressure (Fig. S7). This indicated that removal of the endothelium, containing only ETB1 receptors, achieved nearly the same effect as a knock-down of all ETB receptors through most of the pressure range.
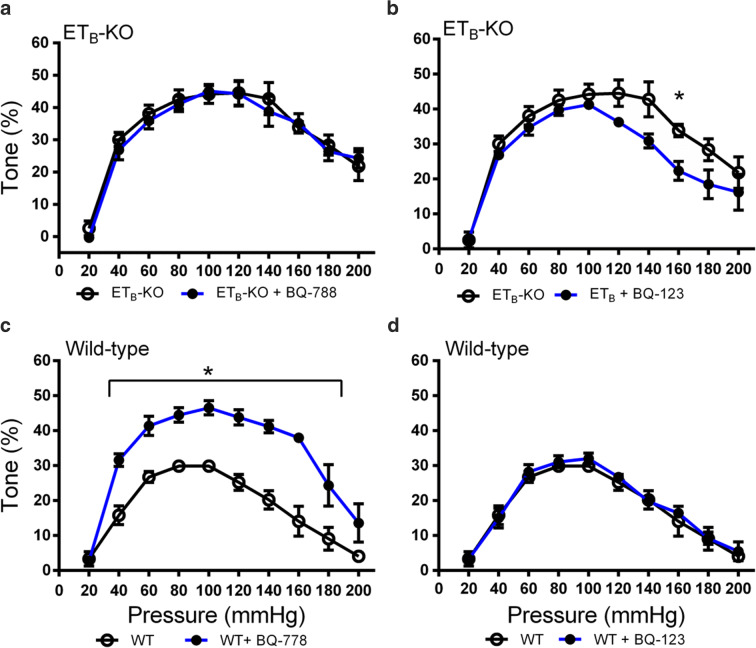
To confirm that the altered myogenic tone was due to the EDNRB genotype, the experiments in ETB-KO and wild-type rats were conducted in new vessels treated with either BQ-123 or BQ-788. The selective ETB receptor antagonist, BQ-788, had no effect on the myogenic tone in ETB-KO rats but significantly enhanced the tone from 40 to 160 mmHg in wild-type rats (Fig. 5, Fig. S8). This confirmed that the PCAs from ETB-KO rats exhibited a complete loss of sensitivity to BQ-788. The selective ETA antagonist, BQ-123, had no effect on the myogenic tone in wild-type rats but had a small effect in ETB-KO rats, although the effect was only statistically significant at 160 mmHg.
Fig. 5.
Influence of ETA and ETB inhibition of myogenic tone in PCAs from wild-type and ETB-KO rats. Experiments were conducted after vessels were treated with either the ETA selective antagonist BQ-123 (1 μM) or the ETB selective antagonist BQ-788 (1 μM). a Myogenic tone in ETB-KO rat PCAs in the presence (n = 5) or absence (n = 6) of BQ-788. b Myogenic tone in ETB-KO rat PCAs in the presence (n = 5) or absence (n = 6) of BQ-123. c Myogenic tone in wild-type rat PCAs in the presence (n = 3) or absence (n = 5) of BQ-788. d Myogenic tone in wild-type rat PCAs in the presence (n = 4) or absence (n = 5) of BQ-123. * P < 0.05 vs. antagonist treatment at each pressure point. Significance was determined using repeated unpaired t tests with Holm-Sidak correction for multiple comparisons at each pressure point
Discussion
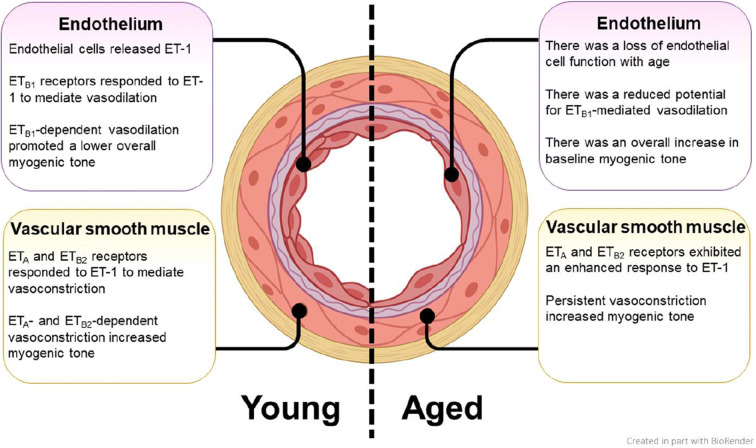
The purpose of this study was to define the contributions of ETA and ETB receptors to myogenic tone in the cerebral microvasculature of young and aged Fischer 344 rats. Our findings are summarized in Fig. 6. We found that aged rats exhibited a higher baseline myogenic tone relative to young rats, and that the tone in these aged vessels was lowered with the ETA receptor antagonist, BQ-123, but was insensitive to the ETB1 and ETB2 receptor antagonist, BQ-788. In contrast, the myogenic tone in the vessels from young rats was raised by BQ-788 but unaffected by BQ-123. When vessels from the young rats were denuded by CHAPS perfusion to remove the endothelial layer that is normally enriched with ETB1 receptors, the myogenic tone became elevated, but denudation had no effect on the vessels from aged animals. This indicated that ETB1 receptors in the endothelium, which normally lower myogenic tone, were not functional in the vessels from aged rats. Furthermore, inhibition of endogenous ET-1 biosynthesis raised the myogenic tone in vessels from young rats. However, the lack of ET-1 biosynthesis reduced the tone in the aged rat preparations. These results suggest that in young vessels, ETB1 receptors in the endothelium predominately mediate the response to ET-1 and maintain a lower myogenic tone, but in aged vessels, a loss of ETB receptor activity allows ETA receptors in vascular smooth muscle cells to raise the myogenic tone. Taken together, the results indicate that there is a loss of ETB receptor function in cerebral arteries of aged Fischer 344 rats. This appears to result in a failure of ETB1 receptors to balance the effects of ETA receptors, causing elevation of the myogenic tone.
Fig. 6.
Summary of the contributions of ETA, ETB1, and ETB2 receptors to myogenic tone in PCAs of young and aged rats. ETA and ETB2 are expressed in the vascular smooth muscle and ETB1 receptors are expressed in the endothelium. Figure created in part with http://biorender.com
We found that myogenic tone was significantly higher in PCA preparations from aged Fischer 344 rats relative to the younger adult rats. These findings agree with the results of previous studies in a variety of different aged animal models [7, 45, 68]. Thus, our findings concur with existing knowledge with respect to differences in myogenic tone between young and aged animals. The mechanisms responsible for this age-related rise in myogenic tone are multifactorial and not fully understood (reviewed in [88]). It was believed that the endothelium plays a major role; however, the specific contributions of the ET receptors, including ETB isoforms, had not previously been examined with respect to cerebral vessels.
There is some evidence that ETA receptors make greater relative contributions to vascular tone with age [6, 79], and that the constrictor activity of ETA receptors can mask that of ETB2 receptors [53]. Van Guilder et al. observed that aged men, roughly 60 years of age, had greater endogenous ET-1 bioavailability and continuous ETA receptor activation which led to increased vascular tone. Donato et al. also reported that muscle arterioles from aged rats exhibited an augmented vasoconstrictor response to ET-1. This response was also insensitive to ETB receptor blockade via BQ-788 which indicated that it was mediated primarily by ETA receptors. In contrast, MacIntyre et al. observed reduced responsiveness of retinal arterioles to ET-1 with age. We measured increased levels of ETA receptor mRNA in aged PCAs, whereas MacIntyre et al. reported no difference in ETA receptor protein between the retinas of young and aged Fischer 344 rats. Thus, changes in ETA receptor abundance could contribute to these effects. McCulloch et al. previously found that ETB2 receptors predominantly regulate vascular tone in the presence of low concentrations of ET-1 (< 1 nM), but ETA receptors mediate the vascular smooth muscle response to higher concentrations of ET-1. A major finding of the present work is that the myogenic tone of PCA preparations from aged animals was depressed following treatment with the ETA antagonist, BQ-123, but antagonism of ETB receptors had no effect on the myogenic tone. In contrast to the aged animals, myogenic tone in PCA preparations from young Fischer 344 rats was elevated upon treatment with the ETB antagonist, BQ-788, but antagonism of ETA receptors had no influence on myogenic tone. These data strongly suggest that ETA receptors contribute more toward the maintenance of myogenic tone than ETB receptors in aged PCAs. In previous work in vivo, it was difficult to determine if the ETB2 receptors became dysfunctional in the aged rats, or if those animals may have had higher local concentrations of ET-1 in the cerebral vascular beds which allowed the ETA receptors to control the myogenic tone in the PCAs [52, 83]. In our study, vessels were rinsed thoroughly prior to experimentation to rule out the influence of enhanced ET-1 production. However, ETA receptors exhibit long-lasting vasoconstrictive activity, whereas ETB receptors are rapidly deactivated following activation [12]. Thus, increased myogenic tone as a function of extended activity from ETA receptors cannot be ruled out.
Endothelium-dependent vasodilation has been shown to decline with age [55, 56]. [4] observed that aged monkeys (Macaca fascicularis) exhibited enhanced vasoconstriction in response to ET-1 due to impaired NO-mediated vasodilation. This indicated that aged M. fascicularis had dysfunctional endothelial cell layers with ETB1 receptors that failed to stimulate vasodilation in response to ET-1. In the present study, when PCAs from young animals were denuded, myogenic tone increased to nearly match the tone of the aged vessels that were unaffected by denudation. This suggests that the specific removal of endothelial ETB1 receptors elevated myogenic tone only in young vessels because this cell layer was already dysfunctional in aged vessels. As we measured decreased VWF mRNA in the vessels from aged animals, a reduction in the number of healthy endothelial cells in the aged vessels could be a contributing mechanism to this observation. However, we measured consistent levels of ETB receptor mRNA between young and aged vessels which supports the previous observations of MacIntyre et al. This suggests that there is likely a loss of ETB receptor function in aged vessels rather than a loss of receptor abundance. When young vessels were treated with PPA to inhibit ET-1 biosynthesis, we observed an increase in myogenic tone that was not recapitulated in denuded vessels. However, there was a sharp loss in myogenic tone in the aged vessels when ET-1 biosynthesis was inhibited. Thus, it appears that ET-1 functions to depress myogenic tone in young vessels via ETB1 receptors in the endothelium. However, ET-1 elevated the tone via ETA receptors in the vascular smooth muscle of PCAs from aged rats, consistent with observations made in extracerebral vessels [17, 79, 83].
We employed heterozygous and ETB-KO rats to further probe the contribution of the ETB receptors to myogenic tone with age. Endothelial ETB1 receptors are known to respond to ET-1 to induce vasodilation [26, 62]. In contrast, ETB2 receptors in the vascular smooth muscle share a function with ETA receptors and mediate vascular contraction [52]. We found that the young Fischer 344 rats had a comparable myogenic tone to the young wild-type spotted lethal rats, and that the ETB-KO rats were comparable to the aged Fischer 344 rats. Thus, a complete knockout of ETB1 and ETB2 receptors seemed to account for the baseline differences observed between young and aged Fischer 344 rats. Furthermore, the vessels from ETB-KO rats had a myogenic tone that was not significantly different from the denuded young Fischer 344 rats up to 200 mmHg of intraluminal pressure. This indicated that the removal of only ETB1 receptors could account for most of the rise in myogenic tone. However, ETB-KO rats and aged rats had a significantly raised myogenic tone relative to young rats at pressure points where young and denuded young vessels were not significantly different from each other. The implications of this are twofold: This indicates that removal of ETB2 receptors in the vascular smooth muscle cells may have a small effect on myogenic tone, and that ETB2 receptors in the young vessels may play a role to depress myogenic tone. In the case of the young vessels, this would be contradictory to the known vasoconstrictive role of ETB2 receptors [72].
We observed pressure-specific differences in the actions of ETA and ETB receptor antagonists as well as PPA. BQ-788 had the most pronounced effect to increase myogenic tone from 60 to 120 mmHg which indicated that the ETB receptors contributed less to the myogenic tone outside of this range. There is evidence that increased intraluminal pressure impairs endothelial function and inhibits the NO-mediated vasodilation that would normally be facilitated by ETB1 receptors [20]. This would also explain the reduced effect of denudation on the young vessels with high intraluminal pressure. We also found that the myogenic tone of vessels from ETB-KO rats remained elevated through the extreme high end of the pressure range, something not observed in heterozygous or aged rat vessels, or in the denuded young vessels. This would indicate that ETB2 receptors contribute to vasorelaxation in response to high intraluminal pressures. This response has not been reported elsewhere to our knowledge. Further work will be required to identify these potential additional roles for ETB2 receptors in young and aged cerebral vascular beds. In contrast with ETB antagonism, BQ-123 had the most pronounced effect to reduce tone in the upper end of the pressure range (> 120 mmHg). There is also evidence that high intraluminal pressure elicits a myogenic vasoconstrictor response in some vessels. To our knowledge, it has not yet been documented if ETA receptors are known to play a role in this phenomenon in PCAs. However, our data indicates that this may be the case. Finally, as PPA inhibits the biosynthesis of the endogenous ligand for these receptors, the same explanations may apply for the pressure-specific actions of PPA as compared to the receptor antagonists.
Our findings have potentially important clinical implications. Cerebral artery dysfunction in aging is thought to be a contributing factor in cerebrovascular diseases, which are key causes of morbidity and mortality in older people [32]. Aged mouse PCAs exhibit ultrastructural changes including increased stiffness and wall thickness [16]. Rat PCAs also have decreased luminal diameters and reduced wall stress because of thicker vessel walls, although there are no differences in distensibility [49]. Additionally, vessels in aging brains are more likely to exhibit signs of atheromatous plaque formation and chronic inflammation which compound the reduction in vessel elasticity [44, 66, 91]. This age-dependent cerebral artery remodeling may set the stage for the development of acquired cerebrovascular diseases of aging including dementia and stroke [32]. We have shown pharmacologically that dysfunctional ETB receptors in the cerebral microvasculature of aged male Fischer 344 rats led to enhanced tone in these vascular beds. Although both ETB receptor subtypes became dysfunctional and insensitive to receptor antagonists in the aged vessels, data from denuded and ETB-KO rat vessels indicated that the loss of endothelial ETB1 receptors was primarily responsible for the rise in myogenic tone. Although ETB1 receptor-selective agonists [75] and antagonists [39, 76] do exist, it is currently unknown whether ETB1 receptors are a viable target to treat age-related endothelial dysfunction in vivo. The ETB receptor agonist, IRL-1620, has recently been effective in a rat model of pediatric ischemic stroke to improve cerebral blood flow and reduce infarct volume [10]. The same drug has also recently completed a phase II human trial to treat cerebral ischemia in patients with mild to moderate Alzheimer disease (ClinicalTrials.gov Identifier: NCT04046484; [30]). Thus, there is demonstrated potential of ETB1 receptor-targeted treatments to improve disease outcomes characterized in part by reduced cerebral blood flow. However, additional experiments are now warranted to further define the dysregulation of ETB receptor subtypes in aging vessels. This future work will be important to determine if ETB1 receptors represent a viable target to maintain healthy cerebral blood flow in aging adults.
Although we observed a loss of ETB receptor function in the aged PCAs, further work will be required to understand the mechanistic underpinnings of these effects. It is currently unclear whether these PCAs exhibit a net loss of endothelial cells, or if the ETB receptors become dysfunctional within the same number of individual cells. We measured a reduction in VWF mRNA with no change in ETB receptor mRNA abundance which was indicative of a reduced number of endothelial cells with potentially more ETB receptors per cell, although a more complete histological analysis would be required to confirm this. It is known that plasma ET-1 is elevated with age and ET-1-mediated vasoconstriction is augmented in older people [79, 83]. Also, an increase in ET-1 synthesis was observed in cultured aortic endothelial cells obtained older than young adult donors [81]. There is evidence that ET-1 contributes to vascular endothelial dysfunction with aging. ET-1 expression is increased in vascular endothelial cells obtained from brachial arteries and antecubital veins of older donors compared to young adults [18]. There is also evidence that there is an age-related loss in the number of endothelial cells in large elastic arteries and coronary arteries, as increased oxidative stress with age can compromise endothelial cell viability [82]. This ultimately can lead to reduced endothelial NO production and enhanced vascular tone [13]. Reduction in NO production was observed with aging in both humans and experimental animals [67, 74]. The endothelial NO synthase (eNOS) expression in vascular endothelial cells obtained from the brachial artery of older adults is greater compared with young adults [18]. The increased eNOS expression with aging may be a compensatory mechanism to overcome low NO bioavailability. Furthermore, a reduction in prostanoid-mediated vasodilation was reported in older humans and animal models [69]. This effect is mediated through several mechanisms including increased expression of prostanoid vasoconstrictors, altered cyclo-oxygenase, and prostaglandin H synthase activities [28, 77]. The reduction in NO and prostanoid-mediated vasodilation might further contribute to the increased myogenic tone in aged rat observed in the current study. However, as only aged vessel preparations were insensitive to ETB receptor antagonism, there also appears to be some receptor-dependent dysfunction. These ETB receptor-specific mechanisms include several possibilities such as disruption of oxidation-reduction-sensitive transcription factors or alterations in receptor trafficking that reduce cell surface expression and diminish drug sensitivity. Future experiments will be required to determine a loss of endothelial cells specifically in the PCAs of Fischer 344 rats, and to identify the cellular mechanisms responsible for the loss of ETB receptor function.
A limitation of this work was the choice to focus only on male Fischer 344 and spotted lethal rats. Gender differences in the onset and rate of vascular aging have been reported in human [54, 71]. Such differences are mainly attributed to differences in sex hormones in women and men with aging. Studies suggest that decreasing testosterone levels in men contribute to accelerated vascular aging [54, 71]. Men exhibit patterns of endothelial dysfunction in the brachial artery up to 10 years before women [8] and experience different changes in cerebral blood flow with age [1, 31]. There are also known differences between the sexes with respect to cerebral blood flow and endothelial function [1, 23, 31, 64]. Furthermore, low testosterone levels in men have been associated with increased risk of cardiovascular diseases such as hypertension and coronary artery disease [35, 41, 46]. In an 18-year follow-up study conducted of men aged 30 and older, higher mortality rates were observed in men who demonstrated the most pronounced age-related decline in total testosterone [11]. Given the known differences between male and female animals, further work will be required to determine if the conclusions of this work will be applicable to females as well. A further limitation was the ability to focus only on PCAs. Medium cerebral arteries and smaller resistance vessels were either too branched or too small to be used with the pressure curve experiments and could not be used to serve as a comparison. Thus, further work would be required to verify that these findings apply to other portions of the cerebral vasculature.
Conclusion
Our data indicate that there is an age-related loss of endothelial function in the cerebral microvasculature of aged Fischer 344 rats. Specifically, experiments with antagonists indicate that there is a decrease in the functional contributions of ETB1 and ETB2 receptor subtypes in the endothelial and vascular smooth muscle layers, respectively. This appears to lead to an imbalance in the activity of ETA and ETB receptors in favor of ETA receptors. Thus, the vasodilatory effects of ET-1 at ETB1 receptors fail to counterbalance its vasoconstrictive effects at ETA receptors. Although the specific mechanism is undetermined, this ultimately results in elevated myogenic tone in the aging cerebral vasculature. For future work, we have also highlighted some potential features of ETB receptor subtypes that warrant further attention in both young and aging vasculature.
Supplementary Information
(DOCX 1309 kb)
Acknowledgments
The authors acknowledge Adel Zrein and Peter Nicholl for technical assistance.
Author contributions
MEMK, EMDW, and SEH designed the experiments. JZ and AMB performed the experiments. APY analyzed the data and made the figures. APY wrote the manuscript. All authors commented on and approved the final manuscript.
Funding
This work was funded by a Heart and Stroke Foundation grant to SEH (grant number: G-19-002626), and a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (grant number: 05162-2014) to MEMK.
Compliance with ethical standards
The animal protocols in this study conformed to the guidelines of the Canadian Council on Animal Care and were approved by the Dalhousie University Committee on Laboratory Animals.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aanerud J, Borghammer P, Rodell A, Jónsdottir KY, Gjedde A. Sex differences of human cortical blood flow and energy metabolism. J Cereb Blood Flow Metab. 2017;37:2433–2440. doi: 10.1177/0271678X16668536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus JA, Hughes RJA, Wright CE. Distortion of KB estimates of endothelin-1 ETA and ETB receptor antagonists in pulmonary arteries: possible role of an endothelin-1 clearance mechanism. Pharmacol Res Perspect. 2017;5(6):e00374. doi: 10.1002/prp2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstead WM. Endothelin-1 contributes to normocapnic hyperoxic pial artery vasoconstriction. Brain Res. 1999;842(1):252–255. doi: 10.1016/s0006-8993(99)01825-9. [DOI] [PubMed] [Google Scholar]
- 4.Asai K, Kudej RK, Takagi G, Kudej AB, Natividad F, Shen YT, Vatner DE, Vatner SF. Paradoxically enhanced endothelin-B receptor-mediated vasoconstriction in conscious old monkeys. Circulation. 2001;103:2382–2386. doi: 10.1161/01.cir.103.19.2382. [DOI] [PubMed] [Google Scholar]
- 5.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Barrett-O’Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Endothelin-A-mediated vasoconstriction during exercise with advancing age. J Gerontol A Biol Sci Med Sci. 2015;70:554–565. doi: 10.1093/gerona/glu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287:H2448–H2453. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- 8.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 9.Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage. 2011;55:468–478. doi: 10.1016/j.neuroimage.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cifuentes EG, Hornick MG, Havalad S, Donovan RL, Gulati A. Neuroprotective effect of IRL-1620, an endothelin B receptor agonist, on a pediatric rat model of middle cerebral artery occlusion. Front Pediatr. 2018;6. 10.3389/fped.2018.00310. [DOI] [PMC free article] [PubMed]
- 11.Corona G, Rastrelli G, Monami M, Guay A, Buvat J, Sforza A, Forti G, Mannucci E, Maggi M. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165(5):687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 12.Cramer H, Müller-Esterl W, Schroeder C. Subtype-specific desensitization of human endothelin ETA and ETB receptors reflects differential receptor phosphorylation. Biochemistry. 1997;36:13325–13332. doi: 10.1021/bi9708848. [DOI] [PubMed] [Google Scholar]
- 13.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 14.Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. Endothelin. Pharmacol Rev. 2016;68:357–418. doi: 10.1124/pr.115.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davenport AP, O’Reilly G, Kuc RE. Endothelin ETA and ETB mRNA and receptors expressed by smooth muscle in the human vasculature: majority of the ETA sub-type. Br J Pharmacol. 1995;114:1110–1116. doi: 10.1111/j.1476-5381.1995.tb13322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz-Otero JM, Garver H, Fink GD, Jackson WF, Dorrance AM. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am J Physiol Heart Circ Physiol. 2016;310:H365–H375. doi: 10.1152/ajpheart.00562.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res. 2005;66:393–401. doi: 10.1016/j.cardiores.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Orléans-Juste P, Plante M, Honoré JC, Carrier E, Labonté J. Synthesis and degradation of endothelin-1. Can J Physiol Pharmacol. 2003;81(6):503–510. doi: 10.1139/y03-032. [DOI] [PubMed] [Google Scholar]
- 20.Durand MJ, Phillips SA, Widlansky ME, Otterson MF, Gutterman DD. The vascular renin-angiotensin system contributes to blunted vasodilation induced by transient high pressure in human adipose microvessels. Am J Physiol Heart Circ Physiol. 2014;307(1):H25–H32. doi: 10.1152/ajpheart.00055.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elisa T, Antonio P, Giuseppe P, Alessandro B, Giuseppe A, Federico C, Marzia D, Ruggero B, Giacomo M, Andrea O, Daniela R, Mariaelisa R, Claudio L. Endothelin receptors expressed by immune cells are involved in modulation of inflammation and in fibrosis: relevance to the pathogenesis of systemic sclerosis. 2015. pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elshourbagy NA, Adamou JE, Gagnon AW, Wu H-L, Pullen M, Nambi P. Molecular characterization of a novel human endothelin receptor splice variant. J Biol Chem. 1996;271:25300–25307. doi: 10.1074/jbc.271.41.25300. [DOI] [PubMed] [Google Scholar]
- 23.Esposito G, Van Horn JD, Weinberger DR, Berman KF. Gender differences in cerebral blood flow as a function of cognitive state with PET. J Nucl Med. 1996;37:559–64. [PubMed]
- 24.Feger GI, Schilling L, Ehrenreich H, Wahl M. Endothelium-dependent relaxation counteracting the contractile action of endothelin-1 is partly due to ETB receptor activation. Res Exp Med (Berl) 1997;196(6):327–337. doi: 10.1007/BF02576857. [DOI] [PubMed] [Google Scholar]
- 25.Frommer KW, Müller-Ladner U. Expression and function of ETA and ETB receptors in SSc. Rheumatology. 2008;47:v27–v28. doi: 10.1093/rheumatology/ken274. [DOI] [PubMed] [Google Scholar]
- 26.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 27.Gariepy CE, Cass DT, Yanagisawa M. Null mutation of endothelin receptor type B gene in spotting lethal rats causes aganglionic megacolon and white coat color. Proc Natl Acad Sci U S A. 1996;93:867–872. doi: 10.1073/pnas.93.2.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gendron ME, Thorin E. A change in the redox environment and thromboxane A2 production precede endothelial dysfunction in mice. Am J Physiol Heart Circ Physiol. 2007;293:H2508–H2515. doi: 10.1152/ajpheart.00352.2007. [DOI] [PubMed] [Google Scholar]
- 29.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 30.Gulati A, Hornick MG, Briyal S, Lavhale MS. A novel neuroregenerative approach using ET(B) receptor agonist, IRL-1620, to treat CNS disorders. Physiol Res. 2018;67:S95–S113. doi: 10.33549/physiolres.933859. [DOI] [PubMed] [Google Scholar]
- 31.Gur RE, Gur RC. Gender differences in regional cerebral blood flow. Schizophr Bull. 1990;16:247–254. doi: 10.1093/schbul/16.2.247. [DOI] [PubMed] [Google Scholar]
- 32.Hachinski V. Dementia: paradigm shifting into high gear. Alzheimers Dement. 2019;15:985–994. doi: 10.1016/j.jalz.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Hamner JW, Tan CO. Relative contributions of sympathetic, cholinergic, and myogenic mechanisms to cerebral autoregulation. Stroke. 2014;45:1771–1777. doi: 10.1161/STROKEAHA.114.005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho W-SV, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmboe SA, Skakkebæk NE, Juul A, Scheike T, Jensen TK, Linneberg A, Thuesen BH, Andersson AM. Individual testosterone decline and future mortality risk in men. Eur J Endocrinol. 2018;178(1):123–130. doi: 10.1530/EJE-17-0280. [DOI] [PubMed] [Google Scholar]
- 36.Johansson B. Myogenic tone and reactivity: definitions based on muscle physiology. J Hypertens. 1989;7:S5–S8. doi: 10.1097/00004872-198901000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev. 2010;68:S74–S87. doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karaki H, Sudjarwo SA, Hori M. Novel antagonist of endothelin ETB1 and ETB2 receptors, BQ-788: effects on blood vessel and small intestine. Biochem Biophys Res Commun. 1994;205:168–173. doi: 10.1006/bbrc.1994.2645. [DOI] [PubMed] [Google Scholar]
- 39.Karaki H, Sudjarwo SA, Hori M, Sakata K, Urade Y, Takai M, Okada T. ETB receptor antagonist, IRL 1038, selectively inhibits the endothelin-induced endothelium-dependent vascular relaxation. Eur J Pharmacol. 1993;231:371–374. doi: 10.1016/0014-2999(93)90112-u. [DOI] [PubMed] [Google Scholar]
- 40.Karet FE, Kuc RE, Davenport AP. Novel ligands BQ123 and BQ3020 characterize endothelin receptor subtypes ETA and ETB in human kidney. Kidney Int. 1993;44:36–42. doi: 10.1038/ki.1993.210. [DOI] [PubMed] [Google Scholar]
- 41.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116(23):2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 42.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnamoorthy RR, Rao VR, Dauphin R, Prasanna G, Johnson C, Yorio T. Role of the ETB receptor in retinal ganglion cell death in glaucoma. Can J Physiol Pharmacol. 2008;86:380–393. doi: 10.1139/Y08-040. [DOI] [PubMed] [Google Scholar]
- 44.Kukull WA, Ganguli M. Epidemiology of dementia: concepts and overview. Neurol Clin. 2000;18:923–950. doi: 10.1016/s0733-8619(05)70233-4. [DOI] [PubMed] [Google Scholar]
- 45.Küng CF, Lüscher TF. Different mechanisms of endothelial dysfunction with aging and hypertension in rat aorta. Hypertension. 1995;25:194–200. doi: 10.1161/01.hyp.25.2.194. [DOI] [PubMed] [Google Scholar]
- 46.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113(Pt 1):27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- 48.MacIntyre JN, Slusar JE, Zhu J, Dong AX, Howlett SE, Kelly ME. Age-associated alterations in retinal arteriole reactivity to endothelin-1 differ between the sexes. Mech Ageing Dev. 2012;133(9-10):611–619. doi: 10.1016/j.mad.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Mandalà M, Pedatella AL, Morales Palomares S, Cipolla MJ, Osol G. Maturation is associated with changes in rat cerebral artery structure, biomechanical properties and tone. Acta Physiol. 2012;205:363–371. doi: 10.1111/j.1748-1716.2011.02406.x. [DOI] [PubMed] [Google Scholar]
- 50.Marchal G, Rioux P, Petit-Taboué MC, Sette G, Travère JM, Le Poec C, Courtheoux P, Derlon JM, Baron JC. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol. 1992;49:1013–1020. doi: 10.1001/archneur.1992.00530340029014. [DOI] [PubMed] [Google Scholar]
- 51.Matsumura Y, Ikegawa R, Tsukahara Y, Takaoka M, Morimoto S. Conversion of big endothelin-1 to endothelin-1 by two types of metalloproteinases derived from porcine aortic endothelial cells. FEBS Lett. 1990;272(1-2):166–170. doi: 10.1016/0014-5793(90)80475-x. [DOI] [PubMed] [Google Scholar]
- 52.McCulloch KM, Docherty CC, Morecroft I, MacLean MR. Endothelin B receptor-mediated contraction in human pulmonary resistance arteries. Br J Pharmacol. 1996;119:1125–1130. doi: 10.1111/j.1476-5381.1996.tb16013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mickley EJ, Gray GA, Webb DJ. Activation of endothelin ETA receptors masks the constrictor role of endothelin ETB receptors in rat isolated small mesenteric arteries. Br J Pharmacol. 1997;120:1376–1382. doi: 10.1038/sj.bjp.0701036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreau KM, Babcock MC, Hildreth KL. Sex differences in vascular aging in response to testosterone. Biol Sex Differ. 2020;11(1):18. doi: 10.1186/s13293-020-00294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- 56.Muller-Delp JM, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;282:H1843–H1854. doi: 10.1152/ajpheart.00666.2001. [DOI] [PubMed] [Google Scholar]
- 57.Nilsson T, Cantera L, Adner M, Edvinsson L. Presence of contractile endothelin-A and dilatory endothelin-B receptors in human cerebral arteries. Neurosurgery. 1997;40:346–353. doi: 10.1097/0006123-199702000-00023. [DOI] [PubMed] [Google Scholar]
- 58.Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res. 1991;68(2):359–367. doi: 10.1161/01.res.68.2.359. [DOI] [PubMed] [Google Scholar]
- 59.Ozturk ED, Tan CO. Human cerebrovascular function in health and disease: insights from integrative approaches. J Physiol Anthropol. 2018;37:4. doi: 10.1186/s40101-018-0164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pierre LN, Davenport AP. Blockade and reversal of endothelin-induced constriction in pial arteries from human brain. Stroke. 1999;30:638–643. doi: 10.1161/01.str.30.3.638. [DOI] [PubMed] [Google Scholar]
- 61.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 62.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Phys. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- 63.Russell FD, Davenport AP. Secretory pathways in endothelin synthesis. Br J Pharmacol. 1999;126(2):391–398. doi: 10.1038/sj.bjp.0702315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sader MA, Celermajer DS. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53:597–604. 10.1016/S0008-6363(01)00473-4 [DOI] [PubMed]
- 65.Schiffrin EL, Turgeon A, Deng LY. Effect of chronic ETA-selective endothelin receptor antagonism on blood pressure in experimental and genetic hypertension in rats. Br J Pharmacol. 1997;121:935–940. doi: 10.1038/sj.bjp.0701224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia aging study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 67.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120(9):357–375. doi: 10.1042/CS30100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shipley RD, Muller-Delp JM. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res. 2005;66:374–383. doi: 10.1016/j.cardiores.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 69.Singh N, Prasad S, Singer DRJ, MacAllister RJ. Ageing is associated with impairment of nitric oxide and prostanoid dilator pathways in the human forearm. Clin Sci (Lond). 2002;102:595–600. [PubMed]
- 70.Soma S, Takahashi H, Muramatsu M, Oka M, Fukuchi Y. Localization and distribution of endothelin receptor subtypes in pulmonary vasculature of normal and hypoxia-exposed rats. Am J Respir Cell Mol Biol. 1999;20:620–630. doi: 10.1165/ajrcmb.20.4.3356. [DOI] [PubMed] [Google Scholar]
- 71.Stanhewicz AE, Wenner MM, Stachenfeld NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol. 2018;315(6):H1569–H1588. doi: 10.1152/ajpheart.00396.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sudjarwo SA, Hori M, Tanaka T, Matsuda Y, Okada T, Karaki H. Subtypes of endothelin ETA and ETB receptors mediating venous smooth muscle contraction. Biochem Biophys Res Commun. 1994;200:627–633. doi: 10.1006/bbrc.1994.1494. [DOI] [PubMed] [Google Scholar]
- 73.Szok D, Hansen-Schwartz J, Edvinsson L. In depth pharmacological characterization of endothelin B receptors in the rat middle cerebral artery. Neurosci Lett. 2001;314:69–72. doi: 10.1016/s0304-3940(01)02293-5. [DOI] [PubMed] [Google Scholar]
- 74.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 75.Takai M, Umemura I, Yamasaki K, Watakabe T, Fujitani Y, Oda K, Urade Y, Inui T, Yamamura T, Okada T. A potent and specific agonist, Suc-[Glu9,Ala11,15]-endothelin-1(8-21), IRL 1620, for the ETB receptor. Biochem Biophys Res Commun. 1992;184:953–959. doi: 10.1016/0006-291x(92)90683-c. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka T, Tsukuda E, Nozawa M, Nonaka H, Ohno T, Kase H, et al. RES-701-1, a novel, potent, endothelin type B receptor-selective antagonist of microbial origin. Mol Pharmacol. 1994:45724–30. [PubMed]
- 77.Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- 78.Tarumi T, Zhang R. Cerebral blood flow in normal aging adults: cardiovascular determinants, clinical implications, and aerobic fitness. J Neurochem. 2018;144:595–608. doi: 10.1111/jnc.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thijssen DHJ, Rongen GA, van Dijk A, Smits P, Hopman MTE. Enhanced endothelin-1-mediated leg vascular tone in healthy older subjects. J Appl Physiol. 2007;103:852–857. doi: 10.1152/japplphysiol.00357.2007. [DOI] [PubMed] [Google Scholar]
- 80.Thorin E, Cernacek P, Dupuis J. Endothelin-1 regulates tone of isolated small arteries in the rat: effect of hyperendothelinemia. Hypertension. 1998;31(4):1035–1041. doi: 10.1161/01.hyp.31.4.1035. [DOI] [PubMed] [Google Scholar]
- 81.Tokunaga O, Fan J, Watanabe T, Kobayashi M, Kumazaki T, Mitsui Y. Endothelin. Immunohistologic localization in aorta and biosynthesis by cultured human aortic endothelial cells. Lab Investig. 1992;67:210–217. [PubMed] [Google Scholar]
- 82.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension. 2007;50:403–409. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- 84.Ward ME, Yan L, Kelly S, Angle MR. Flow modulation of pressure-sensitive tone in rat pial arterioles: role of the endothelium. Anesthesiology. 2000;93(6):1456–1464. doi: 10.1097/00000542-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 85.Ward ME, Yan L, Angle MR. Modulation of rat pial arteriolar responses to flow by glucose. Anesthesiology. 2002;97(2):471–477. doi: 10.1097/00000542-200208000-00026. [DOI] [PubMed] [Google Scholar]
- 86.Williams LR, Leggett RW. Reference values for resting blood flow to organs of man. Clin Phys Physiol Meas. 1989;10:187–217. doi: 10.1088/0143-0815/10/3/001. [DOI] [PubMed] [Google Scholar]
- 87.Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78(3):473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- 88.Xu X, Wang B, Ren C, Hu J, Greenberg DA, Chen T, Xie L, Jin K. Age-related impairment of vascular structure and functions. Aging Dis. 2017;8:590–610. doi: 10.14336/AD.2017.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 90.Yao H, Sadoshima S, Ooboshi H, Sato Y, Uchimura H, Fujishima M. Age-related vulnerability to cerebral ischemia in spontaneously hypertensive rats. Stroke. 1991;22:1414–1418. doi: 10.1161/01.str.22.11.1414. [DOI] [PubMed] [Google Scholar]
- 91.Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VM-Y, Trojanowski JQ, Arnold SE. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135:3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu JC, Pickard JD, Davenport AP. Endothelin ETA receptor expression in human cerebrovascular smooth muscle cells. Br J Pharmacol. 1995;116:2441–2446. doi: 10.1111/j.1476-5381.1995.tb15093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zanetta L, Marcus SG, Vasile J, Dobryansky M, Cohen H, Eng K, Shamamian P, Mignatti P. Expression of Von Willebrand factor, an endothelial cell marker, is up-regulated by angiogenesis factors: a potential method for objective assessment of tumor angiogenesis. Int J Cancer. 2000;85(2):281–288. doi: 10.1002/(sici)1097-0215(20000115)85:2<281::aid-ijc21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1309 kb)