Abstract
Successful human reproduction requires gamete maturation, fertilization, and early embryonic development. Human oocyte maturation includes nuclear and cytoplasmic maturation, and abnormalities in the process will lead to infertility and recurrent failure of IVF/ICSI attempts. In addition, the quality of oocytes/embryos in the clinic can only be determined by morphological markers, and there is currently a lack of molecular markers for determining oocyte quality. As the number of patients undergoing IVF/ICSI has increased, many patients have been identified with recurrent IVF/ICSI failure. However, the genetic basis behind this phenotype remains largely unknown. In recent years, a few mutant genes have been identified by us and others, which provide potential molecular markers for determining the quality of oocytes/embryos. In this review, we outline the genetic determinants of abnormalities in the processes of oocyte maturation, fertilization, and early embryonic development. Currently, 16 genes (PATL2, TUBB8, TRIP13, ZP1, ZP2, ZP3, PANX1, TLE6, WEE2, CDC20, BTG4, PADI6, NLRP2, NLRP5, KHDC3L, and REC114) have been reported to be the causes of oocyte maturation arrest, fertilization failure, embryonic arrest, and preimplantation embryonic lethality. These abnormalities mainly have Mendelian inheritance patterns, including both dominant inheritance and recessive inheritance, although in some cases de novo mutations have also appeared. In this review, we will introduce the effects of each gene in the specific processes of human early reproduction and will summarize all known variants in these genes and their corresponding phenotypes. Variants in some genes have specific effects on certain steps in the early human reproductive processes, while other variants result in a spectrum of phenotypes. These variants and genetic markers will lay the foundation for individualized genetic counseling and potential treatments for patients and will be the target for precision treatments in reproductive medicine.
Keywords: Human oocyte maturation, Gene mutations, Fertilization, Embryonic development
Introduction
The first IVF procedure was performed in 1978, and over the past four decades, the number of patients undergoing IVF/ICSI attempts has increased worldwide and it is now estimated that more than 6 million babies have been born through IVF/ICSI [1, 2]. However, the success rate of IVF/ICSI is still only around 30–40%. There are two main factors preventing improvement in the success rate. First, there is a lack of good molecular markers for evaluating the quality of oocytes/embryos. Morphological markers are currently used in the clinic for choosing oocytes to be fertilized and embryos to be implanted. However, being morphologically normal does not mean that the oocytes/embryos are normal on the molecular level, which is the key to becoming functional oocytes and viable embryos. Second, a number of patients have experienced recurrent failure of IVF/ICSI attempts for unknown genetic reasons, including oocyte maturation arrest, fertilization failure, embryonic arrest, and implantation failure.
Successful mammalian reproduction requires gamete development, fertilization, and early embryonic development, and defects in any of these processes will result in infertility, recurrent miscarriage, or even birth defects [3–7]. It is well known that maternal factors such as age, sperm factors such as severe oligoasthenozoospermia, and environmental factors such as smoking can influence oocyte and embryo quality. However, there are still a number of patients with failed IVF/ICSI attempts for unknown reasons. In this review, we focus on the maternal genetic factors that can contribute to the failure of IVF/ICSI attempts. Human oocytes pass through the germinal vesicle (GV) stage, metaphase I (MI) stage, and finally reach metaphase II (MII), which is the stage at which oocytes can be fertilized [8, 9]. Human oocyte maturation includes nuclear maturation and cytoplasmic maturation. Nuclear maturation refers to the oocyte developing normally into the MII stage and producing haploid cells by extruding the first polar body. Cytoplasmic maturation refers to the series of changes in the oocyte cytoplasm that are required for fertilization, activation, and embryonic development [9]. After an oocyte is ovulated from the ovary, it waits for the sperm to start a new life. Under physiological conditions, sperms travel through the vagina and uterus, finally encountering the oocyte in the oviduct. The sperm pass through the corona radiata and zona pellucida and fuse with the oocyte and begin fertilization. During fertilization, the oocyte completes meiosis, extrudes the second polar body, and forms both the maternal and paternal pronucleus. After fertilization, the zygote undergoes several cleavages to form the blastocyst. Finally, the blastocyst is implanted in the uterus [10–13].
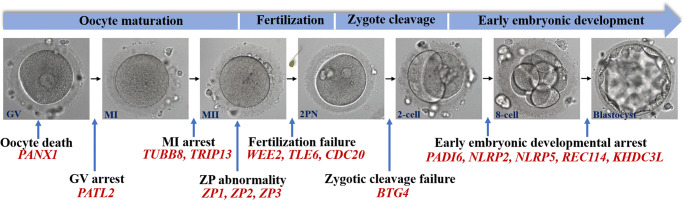
In a study from 1990, four infertile women who had undergone failed IVF attempts and who had three kinds of anomalies in human oocyte development were described. These included failure of oocyte maturation from the GV stage (oocyte GV arrest), failure of polar body formation (oocyte MI arrest), and the absence of oocytes in mature follicular aspirates (empty follicle syndrome) [14]. A few subsequent reports described additional cases with similar phenotypes and other defects in fertilization and early embryonic arrest [15–19]. Although such cases represent a very low percentage of IVF failures, these patients undergo several rounds of stimulations always showing the same outcome, thus suggesting a genetic predisposition. Therefore, it is of importance to uncover the underlying genetic determinants contributing to patients with failure of IVF/ICSI attempts. However, for a long period of time, there have only been a few reports on the genetic causes behind these defects. Recently, more and more causative genes involved in oocyte and embryo development have been identified. In this review, we summarize these recently discovered genetic factors that are responsible for oocyte and embryo developmental defects (Fig. 1).
Fig. 1.
Recently discovered genetic factors that are responsible for oocyte and embryo developmental defects
Methods
A literature search was conducted for all publications in PubMed/Medline until December 2020 using the following search terms: “oocyte maturation arrest”, “oocyte maturation defects”, “fertilization failure”, “early embryonic arrest”, “implantation failure”, and “human embryonic lethality” with possible combinations with the keywords “genetics”, “gene mutations”, “subfertility”, and “infertility”. Any additionally relevant articles identified from the bibliographies of the initially retrieved articles and reviews were also included. Only English-language publications or articles in other languages but with an abstract in English were included. In addition, we focused on studies containing several individuals with mutations in a specific gene because this reduced false positive results and provided strong and convincing evidence for roles of the genes in the corresponding phenotypes. Finally, we did not include studies describing the association between single nucleotide polymorphisms and a particular phenotype because these kinds of studies cannot provide strong genetic evidence.
Results
Oocyte GV arrest and mutations in PATL2
Oocytes are initially arrested at the diplotene stage of prophase I until puberty, and these are referred to as GV oocytes. Upon exposure to surging luteinizing hormone, GV oocytes resume meiosis followed by chromatin condensation and breakdown of the nuclear envelope [8]. However, a few infertile patients have been found who suffer recurrent failure IVF/ICSI attempts because their oocytes are always arrested at the GV stage and cannot undergo subsequent maturation. In 2017, we and others identified patients with GV arrest showing a recessive inheritance pattern [20, 21]. Mutations in PATL2 were identified in some consanguineous families with the GV arrest phenotype, and other phenotypes were subsequently observed. Some families had oocytes arrested at the MI stage or had PB1 oocytes with abnormally large first polar bodies [21]. Most of these PB1 oocytes could not be fertilized or could be fertilized but then underwent subsequent embryonic arrest [21]. We concluded that the phenotypic variability depended on the extent of the effects of the mutations on the PATL2 protein, with greater impairment of the PATL2 protein resulting in oocytes arrested at earlier stages. The role of PATL2 mutations in oocyte maturation defects was soon confirmed by two other groups [22, 23]. A group from France found that 26% of their patients of North African descent with oocytes arrested at either the GV or MI stage or with atretic cells presented with PATL2 homozygous mutations, indicating that PATL2 mutations are a major cause of oocyte maturation defects in this population (n = 23) [22]. In another group from China, mutations in PATL2 were found to account for 44.4% of the individuals with oocyte GV arrest (n = 9), suggesting that PATL2 mutations are the major cause for GV arrest in different ethnic populations [23]. Following these studies, other groups have identified several novel mutations in PATL2 that are responsible for different phenotypes, including oocyte maturation, fertilization failure, and embryo developmental arrest [24, 25]. The mutations in PATL2 and resulting phenotypes are listed in Table 1.
Table 1.
Summary of gene mutations involved in human oocyte and embryo defects
| Gene | Mode of inheritance | Phenotype in mutants | Refs |
|---|---|---|---|
| PATL2 | AR | Oocyte maturation arrest | [20–25] |
| Fertilization failure | [21, 25] | ||
| Early embryonic arrest | [21, 25] | ||
| TUBB8 | AD, AR, de novo, incomplete dominance, unknown | Oocyte maturation arrest | [26–35] |
| Oocytes with large polar body | [27] | ||
| Abnormal fertilization | [30] | ||
| Fertilization failure | [27–29, 31] | ||
| No cleavage | [27, 28, 30, 36] | ||
| Early embryonic arrest | [27–31] | ||
| Embryonic implantation failure | [27, 28] | ||
| Multiple pronuclei formation | [31, 37] | ||
| TRIP13 | AR | Oocyte maturation arrest | [38] |
| ZP1 | AD, AR | Oocytes without a zona pellucida | [39–42] |
| Empty follicle syndrome | [39, 40, 43–49] | ||
| ZP2 | AD, AR | Oocytes without a zona pellucida | [40] |
| Oocytes with a thin zona pellucida | [40, 45, 50] | ||
| Fertilization failure | [50] | ||
| Empty follicle syndrome | [43] | ||
| ZP3 | AD | Oocytes without a zona pellucida | [40, 41, 51] |
| Empty follicle syndrome | [41, 43, 51, 52] | ||
| PANX1 | AD | Oocyte death | [53] |
| TLE6 | AR | Early embryonic arrest | [47, 54–57] |
| Fertilization failure | [54, 55] | ||
| Embryonic implantation failure | [55] | ||
| WEE2 | AR | Fertilization failure | [58–64] |
| Poor fertilization | [64] | ||
| CDC20 | AR | Oocyte maturation arrest | [65] |
| Fertilization failure | [65] | ||
| Early embryonic arrest | [65] | ||
| BTG4 | AR | Zygotic cleavage failure | [66] |
| PADI6 | AR | Early embryonic arrest | [56, 57, 67, 68] |
| Zygotic cleavage failure | [69] | ||
| Recurrent hydatidiform moles | [70] | ||
| NLRP2 | AR | Early embryonic arrest | [57, 71] |
| NLRP5 | AR | Early embryonic arrest | [57, 71, 72] |
| Fertilization failure | [47, 73] | ||
| KHDC3L | AR | Early embryonic arrest | [56] |
| Recurrent hydatidiform moles | [74, 75] | ||
| Recurrent pregnancy loss | [76] | ||
| REC114 | AR | Multiple pronuclei formation | [77] |
| Early embryonic arrest | [77] | ||
| Recurrent hydatidiform moles | [78] |
AD, autosomal dominant; AR, autosomal recessive
Oocyte MI arrest and mutations in TUBB8 and TRIP13
The chromosomes begin to condense following oocyte GV breakdown, and bipolar spindles are formed when oocytes reach metaphase I. Meiosis I is completed by extruding the first polar body [8]. In the clinic, some infertile patients have been diagnosed with oocyte MI arrest, and oocytes retrieved from some of these patients do not have the first polar body [14–18, 79]. In 2016, we identified patients with oocyte MI arrest with a dominant inheritance pattern, and we identified mutations in TUBB8 as responsible for the disease [26]. These mutations were either inherited from the patients’ fathers or occurred de novo. Further studies showed that mutations in TUBB8 account for around 30% of cases of oocyte MI arrest, indicating the dominant role of TUBB8 in the disease [27–31]. The human β-tubulin family consists of nine β-tubulin isotypes [80], but TUBB8 is the only one to be specifically expressed in human oocytes and early embryos [26], thus indicating that it plays a role in human oocyte spindle assembly and likely contributes to the uniqueness of primate oocyte maturation.
Further studies have shown that recessive inheritance pattern of TUBB8 is also pathogenic [27–30, 36]. These patients either have homozygous mutations or compound heterozygous mutations, with one rare variant coming from both parents. Mutations in TUBB8 have been shown to result in the following five phenotypes: (1) oocyte MI arrest [26–35], (2) PB1 oocytes that fail to be fertilized [27–31], (3) PB1 oocytes that can be fertilized, but the embryos fail to cleave [27, 28, 30, 36], (4) PB1 oocytes that can be fertilized and embryos that can undergo cleavage, but ensuing embryonic development is arrested [27–31, 37], and (5) some usable embryos with implantation potential can be obtained but fail to result in pregnancy after implantation [27, 28]. Table 1 includes the published mutations in TUBB8 and their corresponding phenotypes. Considering the expanding spectrum of TUBB8 mutations in human oocyte development, fertilization, and early embryonic development, screening for TUBB8 mutations has potential value for evaluating the functionality of PB1 oocytes and for providing precise diagnoses for infertile patients with recurrent failure of IVF/ICSI attempts.
We further identified bi-allelic missense mutations in TRIP13, a new causative gene responsible for oocyte MI arrest [38]. All mutations reduce the protein abundance of TRIP13 and lead to the accumulation of HORMAD2. More importantly, injecting TRIP13 cRNA into oocytes from one affected individual was able to rescue the phenotype of oocyte MI arrest, which has implications for future therapeutic treatments.
Defects in the zona pellucida and mutations in the ZP protein family
Mature oocytes are surrounded by the extracellular zona pellucida, which mediates sperm binding and penetration and is essential for fertilization [81]. In mice, the zona pellucida is composed of three glycoproteins — ZP1, ZP2, and ZP3 — while in humans it is composed of four glycoproteins — ZP1, ZP2, ZP3, and ZP4 [82, 83]. In mice, knockout of either ZP2 or ZP3 causes sterility by destroying the zona pellucida [84, 85]. In 2013, a form of infertility was identified in a family in which the patients had undergone several failed IVF/ICSI attempts, and these patients presented with an autosomal recessive inheritance pattern characterized by abnormal eggs lacking a zona pellucida [39]. A homozygous frameshift mutation in ZP1 was identified in these patients. In 2017, another form of infertility caused by empty follicle syndrome was found to follow an autosomal dominant inheritance pattern [51]. A recurrent heterozygous missense mutation in ZP3 was identified in two inherited and in two sporadic cases. These patients had difficulties in retrieving oocytes after hormone stimulation, and an in vitro functional study demonstrated that the mutation impairs the assembly of the ZP proteins and might therefore result in oocyte degeneration. An additional infertile patient was identified after failure of IVF attempts, and this patient’s oocytes either had no zona pellucida or only a very thin zona pellucida [86]. The genetic study indicated that the patient carried both a heterozygous missense mutation in ZP2 and a heterozygous frameshift mutation in ZP3. The two mutations were transmitted from her parents, indicating a recessive inheritance pattern. The patient was treated by ICSI with an improved culture system and successfully delivered a healthy baby. In another recent study, homozygous mutations in ZP2 were identified in oocytes from patients in two independent consanguineous families [50]. All of these oocytes were surrounded by a thin zona pellucida that was defective in sperm binding. These oocytes failed to be fertilized by IVF, but they could be fertilized by ICSI and could result in live birth. Mutations in human ZP1, ZP2, and ZP3 and their corresponding phenotypes are summarized in Table 1 [39–52].
Discovery of “oocyte death” and mutations in PANX1
In 2019, a previously unreported phenotype named “oocyte death” was identified [53]. All the retrieved oocytes showed cytoplasmic shrinkage and darkening before or after fertilization, and heterozygous mutations in PANX1 were found to be responsible for the phenotype. PANX1 is one of three members of the Pannexin1 family of proteins, which play important roles in cellular communication [87]. Functional studies in HeLa cells and Xenopus laevis oocytes indicated that mutations altered the PANX1 glycosylation pattern, thus affecting the subcellular localization of PANX1 in cultured cells and resulting in aberrant PANX1 channel activity [53]. Unexpectedly, knock-in mice with the four different mutations were all healthy and fertile. Further experiments demonstrated that expression of PANX1 in human oocytes is significantly higher than it is in mouse oocytes, which might provide an explanation for the absence of a phenotype in the knock-in mice, and oocyte-specific overexpression of mutant Panx1 can recapitulate the oocyte death phenotype [53]. These findings indicate that abnormal glycosylation and channelopathy are causes of female infertility.
Fertilization failure and mutations in TLE6, WEE2, and CDC20
After extruding the first polar body, oocytes enter meiosis II and arrest at the MII stage until fertilization [8]. Fertilization involves the transition from meiosis to mitosis and the transition from egg to embryo and thus involves several biological events, including sperm-egg binding, the release of cortical granules, the extrusion of the second polar body, and pronucleus formation [88]. Knockout of a few genes in mice — including Juno and CD9 in oocytes and Izumo, Adam2, Adam3, and Calmegin in sperm — causes sterility through fertilization failure [89–94]. However, the genetic factors behind fertilization failure in humans have remained largely unknown. In 2015, three infertile patients in two consanguineous families were reported to have the phenotype of fertilization failure in which several morphologically normal oocytes could be retrieved from the patients during IVF and ICSI, but no zygotes could be formed [54]. A homozygous single mutation in TLE6 was identified in these patients, and a functional study suggested that the mutation impaired PKA-mediated phosphorylation of TLE6 and prevented the interaction between TLE6 and other subcortical maternal complex (SCMC) proteins [54]. Additional mutations in TLE6 have been found to be responsible for fertilization failure, early embryonic arrest, and embryonic implantation failure (Table 1) [47, 55–57].
In 2018, we identified homozygous mutations in WEE2 in patients from four families with fertilization failure [58]. Oocytes from these patients were morphologically normal. After injection of sperm, these oocytes could extrude the second polar body, but they failed to form zygotes. In vitro and in vivo evidence suggests that mutations in WEE2 significantly decrease the amount of WEE2 protein, leading to abnormal serine phosphorylation of WEE2 and reduced tyrosine 15 phosphorylation of Cdc2, which results in MII exit and subsequent fertilization failure. By injecting WEE2 cRNA into a patient’s oocytes, the phenotype of fertilization failure could be rescued and blastocysts could form in vitro on day 6. Preimplantation screening analysis showed that these blastocysts had normal numbers of chromosomes and did not have large deletions or repetitions in the genome. This provides a potential therapeutic strategy for the treatment of these patients with WEE2 mutations. Three subsequent studies identified additional mutations in WEE2 (Table 1), and together these studies suggest that mutations in WEE2 are the major cause of human fertilization failure [59–64].
Recently, we identified five sporadic cases carrying homozygous mutation or compound heterozygous mutations in CDC20 [65]. Two of the patients showed the typical phenotype of fertilization failure. Injecting wild type CDC20 cRNA into patients’ oocytes could recuse the phenotype of MI arrest, similar to what was seen in patients with WEE2 mutations [58]. The other three patients harboring CDC20 mutations had the phenotype of oocyte MI arrest or early embryonic arrest [65]. This phenotypic variability might be the result of different degrees of impairment resulting from different CDC20 mutations.
Apart from complete fertilization failure, other fertilization problems are also seen in the clinic, such as multiple pronuclei formation. Until now, the genetic factors for multiple pronuclei formation have been largely unknown. A few studies have reported that some patients carrying mutations in TUBB8 or REC114 present with multiple pronuclei, and other genetic causes for multiple pronuclei should be investigated further [31, 37, 77].
Zygotic cleavage failure and mutations in BTG4
An oocyte and a sperm will first form a zygote after fertilization and then undergo zygotic cleavage to begin embryonic development [88]. For some individuals, morphologically normal oocytes can be retrieved and successfully fertilized, but they fail to undergo cleavage, thus showing a unique early embryonic phenotype that we named “zygotic cleavage failure (ZCF)” [66]. By whole-exome sequencing, four homozygous mutations in BTG4 were identified in four independent families with the phenotype of ZCF, and they followed a Mendelian recessive inheritance pattern [66]. BTG4 is a key adaptor of the CCR4-NOT deadenylase complex, which bridges CNOT7 to EIF4E and facilitates the decay of maternal mRNAs in early embryonic development [95]. Functional studies in HeLa cells indicated that BTG4 mutations altered the protein level of BTG4 or the interaction between BTG4 and CNOT7. In vivo studies further demonstrated that the process of maternal mRNA decay was disrupted in the zygotes of the affected individuals, which provides a mechanistic explanation for the phenotype of ZCF [66]. To date, BTG4 is the only identified gene responsible for the phenotype of human ZCF.
Early embryonic arrest and mutations in PADI6, NLRP2, NLRP5, KHDC3L, and REC114
Following normal fertilization and zygotic cleavage, mitosis is initiated in the embryo [88]. With the degradation of maternal RNAs and proteins and the activation of the embryonic genome, embryos undergo further cleavage, differentiation, and development [96, 97]. Studies have shown that the expression of several proteins control specific parts of the process. The SCMC is essential for embryonic activation and subsequent progression past the 2-cell stage [97]. The SCMC consists of FLOPED, PADI6, TLE6, FILIA, NLRP2, and NLRP5, and knockout of the corresponding genes in female mice results in sterility due to embryonic arrest prior to the 4-cell stage [98–102]. In the clinic, early embryonic arrest is commonly observed and is one of the major reasons for failed IVF/ICSI attempts [19]. However, the genetic causes for the phenotype are largely unknown. In 2016, we identified PADI6 as the first mutant gene responsible for patients with early embryonic arrest [67]. The amount of phosphorylated RNA polymerase II and the expression levels of a few genes involved in zygotic genome activation were reduced in the affected individuals’ embryos. Importantly, although oocytes from the patients were morphologically normal, the PADI6 protein was lacking in patients’ oocytes, indicating that the phenotype of early embryonic arrest results from defects in oocyte cytoplasmic maturation. In addition to the phenotype of early embryonic arrest, PADI6 mutations were also shown to be responsible for zygotic cleavage failure and recurrent hydatidiform moles [56, 57, 67–70].
Other mutant genes were further identified. Homozygous and compound heterozygous mutations in NLRP2 and NLRP5 were found to be responsible for human embryonic arrest or fertilization failure [47, 57, 71–73]. Nlrp2 knockout female mice are subfertile and show age-associated maternal fertility [102], while human patients with mutations in NLRP2 exhibit phenotypic variability (Table 1). Patients carrying homozygous truncating mutations or compound heterozygous truncating or missense mutations in NLRP2 produce very few viable embryos after IVF/ICSI, while some patients with compound heterozygous missense mutations have a limited number of viable embryos and are able to eventually give birth to live full-term infants after several transplantation attempts [71]. This variability can be explained by the fact that different mutations impair the function of the NLRP2 protein to different extents. Additional novel mutations in genes encoding proteins of the SCMC, including TLE6, PADI6, and KHDC3L, have been identified as being responsible for embryonic arrest at the cleavage stage or morula stage (Table 1). It is worth noting that mutations in KHDC3L were previously shown to cause recurrent hydatidiform mole [74, 75]. A study has shown that a novel homozygous frameshift mutation in KHDC3L causes early embryonic arrest in which embryos formed after ICSI are fertilized normally but arrest at the morula stage [76]. These studies provide further evidence for the important role of the SCMC in human embryonic development and in recurrent IVF/ICSI failure. In 2019, we identified a new pathogenic gene REC114 responsible for early embryonic arrest from two consanguineous families [77]. The two patients underwent recurrent IVF/ICSI failure due to early embryonic arrest and carry homozygous splicing or missense mutation in REC114. Moreover, it is reported that a homozygous splicing mutation in REC114, which is located directly before exon 4 in the splicing region, might cause recurrent hydatidiform moles in humans [78]. REC114 is an essential factor in meiosis via the formation of a complex with MEI4 and IHO1 at sites of double-strand breaks [103, 104], suggesting that REC114 is essential for both oocyte meiosis and early embryonic development.
Discussion and conclusion
A number of patients have been identified with recurrent failure of IVF/ICSI attempts caused by abnormalities in gamete and embryo development as well as defects in oocyte maturation. The increasing number of IVF/ICSI cycles being performed provides an unprecedented opportunity for systematically evaluating the phenotypes of oocyte maturation, fertilization, and early embryonic defects. Such work will help identify more novel genes and uncover new signaling pathways, functions, and genetic mechanisms involved in early human reproduction. In the clinic, the quality of oocytes is key for successful IVF/ICSI and for the health of the neonate, but there is currently a lack of molecular markers for evaluating the quality of oocytes. In recent years, a series of genetic factors have been identified as potential markers for evaluating oocyte quality (Table 1). In these studies, women carrying mutations rarely gave birth, and it was reported that only two mothers carrying NLPR2 mutations gave birth after several rounds of transplantation [71]. The similar situation was observed in the women with recurrent hydatidiform mole. Elie et al. showed that only 1% of patients carrying biallelic mutations of NLRP7 could give birth from spontaneous conceptions [105], and for most patients, oocyte donation may be a way to having their own babies. These findings can thus provide some guidance for clinicians to provide genetic counseling. We expect that by whole-exome sequencing and whole-genome sequencing, more and more genetic factors responsible for oocyte quality will likely be identified in the future, which might benefit patients who suffer from these conditions. Such discoveries will help improve the success rate of IVF/ICSI attempts, optimize clinical therapeutic treatment, and provide genetic diagnoses for patients.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFC1003800, 2017YFC1001500 and 2016YFC1000600), the National Natural Science Foundation of China (81725006, 81822019, 81771581, 81971450, and 81971382), the project supported by Shanghai Municipal Science and Technology Major Project (2017SHZDZX01), Project of Shanghai Municipal Science and Technology Commission (19JC1411001), the Natural Science Foundation of Shanghai (19ZR1444500), Shuguang Program of Shanghai Education Development Foundation and Shanghai Municipal Education Commission (18SG03), the Foundation of Shanghai Health and Family Planning Commission (20154Y0162), the Capacity Building Planning Program for Shanghai Women and Children’s Health Service, the collaborative innovation center project construction for Shanghai Women and Children’s Health.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qing Sang, Email: sangqing@fudan.edu.cn.
Lei Wang, Email: wangleiwanglei@fudan.edu.cn.
References
- 1.Adamson GD, Tabangin M, Macaluso M, de Mouzon J. The number of babies born globally after treatment with the assisted reproductive technologies (ART) Fertil Steril. 2013;100(3):S42. doi: 10.1016/j.fertnstert.2013.07.1807. [DOI] [Google Scholar]
- 2.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 3.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14(11):1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shamseldin HE, Tulbah M, Kurdi W, Nemer M, Alsahan N, Al Mardawi E, et al. Identification of embryonic lethal genes in humans by autozygosity mapping and exome sequencing in consanguineous families. Genome Biol. 2015;16(1):116. doi: 10.1186/s13059-015-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webster A, Schuh M. Mechanisms of aneuploidy in human eggs. Trends Cell Biol. 2017;27(1):55–68. doi: 10.1016/j.tcb.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Hart RJ. Physiological aspects of female fertility: role of the environment, modern lifestyle, and genetics. Physiol Rev. 2016;96(3):873–909. doi: 10.1152/physrev.00023.2015. [DOI] [PubMed] [Google Scholar]
- 7.Yatsenko SA, Rajkovic A. Genetics of human female infertility†. Biol Reprod. 2019;101(3):549–566. doi: 10.1093/biolre/ioz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130(6):791–799. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- 9.Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8(4):485–489. doi: 10.1071/rd9960485. [DOI] [PubMed] [Google Scholar]
- 10.Adhikari D, Liu K. The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol Cell Endocrinol. 2014;382(1):480–487. doi: 10.1016/j.mce.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Georgadaki K, Khoury N, Spandidos DA, Zoumpourlis V. The molecular basis of fertilization (Review) Int J Mol Med. 2016;38(4):979–986. doi: 10.3892/ijmm.2016.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niakan KK, Han J, Pedersen RA, Simon C, Pera RA. Human pre-implantation embryo development. Development. 2012;139(5):829–841. doi: 10.1242/dev.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiao SY, Yang YH, Chen SR. Molecular genetics of infertility: loss-of-function mutations in humans and corresponding knockout/mutated mice. Hum Reprod Update. 2021;27(1):154–189. doi: 10.1093/humupd/dmaa034. [DOI] [PubMed] [Google Scholar]
- 14.Rudak E, Dor J, Kimchi M, Goldman B, Levran D, Mashiach S. Anomalies of human oocytes from infertile women undergoing treatment by in vitro fertilization. Fertil Steril. 1990;54(2):292–296. doi: 10.1016/s0015-0282(16)53706-6. [DOI] [PubMed] [Google Scholar]
- 15.Hartshorne G, Montgomery S, Klentzeris L. A case of failed oocyte maturation in vivo and in vitro. Fertil Steril. 1999;71(3):567–570. doi: 10.1016/s0015-0282(98)00505-6. [DOI] [PubMed] [Google Scholar]
- 16.Eichenlaub-Ritter U, Schmiady H, Kentenich H, Soewarto D. Recurrent failure in polar body formation and premature chromosome condensation in oocytes from a human patient: indicators of asynchrony in nuclear and cytoplasmic maturation. Hum Reprod. 1995;10(9):2343–2349. doi: 10.1093/oxfordjournals.humrep.a136297. [DOI] [PubMed] [Google Scholar]
- 17.Bergère M, Lombroso R, Gombault M, Wainer R, Selva J. An idiopathic infertility with oocytes metaphase I maturation block: case report. Hum Reprod. 2001;16(10):2136–2138. doi: 10.1093/humrep/16.10.2136. [DOI] [PubMed] [Google Scholar]
- 18.Levran D, Farhi J, Nahum H, Glezerman M, Weissman A. Maturation arrest of human oocytes as a cause of infertility: case report. Hum Reprod. 2002;17(6):1604–1609. doi: 10.1093/humrep/17.6.1604. [DOI] [PubMed] [Google Scholar]
- 19.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3(4):367–382. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 20.Maddirevula S, Coskun S, Alhassan S, Elnour A, Alsaif HS, Ibrahim N, Abdulwahab F, Arold ST, Alkuraya FS. Female infertility caused by mutations in the oocyte-specific translational repressor PATL2. Am J Hum Genet. 2017;101(4):603–608. doi: 10.1016/j.ajhg.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen B, Zhang Z, Sun X, Kuang Y, Mao X, Wang X, Yan Z, Li B, Xu Y, Yu M, Fu J, Mu J, Zhou Z, Li Q, Jin L, He L, Sang Q, Wang L. Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet. 2017;101(4):609–615. doi: 10.1016/j.ajhg.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christou-Kent M, Kherraf ZE, Amiri-Yekta A, Le Blévec E, Karaouzène T, Conne B, et al. PATL2 is a key actor of oocyte maturation whose invalidation causes infertility in women and mice. EMBO Mol Med. 2018;10(5). 10.15252/emmm.201708515. [DOI] [PMC free article] [PubMed]
- 23.Huang L, Tong X, Wang F, Luo L, Jin R, Fu Y, Zhou G, Li D, Song G, Liu Y, Zhu F. Novel mutations in PATL2 cause female infertility with oocyte germinal vesicle arrest. Hum Reprod. 2018;33(6):1183–1190. doi: 10.1093/humrep/dey100. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Zhu L, Wang J, Luo G, Xi Q, Zhou X, Li Z, Yang X, Duan J, Jin L, Zhang X. Novel homozygous mutations in PATL2 lead to female infertility with oocyte maturation arrest. J Assist Reprod Genet. 2020;37(4):841–847. doi: 10.1007/s10815-020-01698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L, Chen H, Li D, Song D, Chen B, Yan Z, lyu Q, Wang L, Kuang Y, Li B, Sang Q. Novel mutations in PATL2: expanding the mutational spectrum and corresponding phenotypic variability associated with female infertility. J Hum Genet. 2019;64(5):379–385. doi: 10.1038/s10038-019-0568-6. [DOI] [PubMed] [Google Scholar]
- 26.Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, Shi J, Tian G, Luchniak A, Fukuda Y, Li B, Yu M, Chen J, Xu Y, Guo L, Qu R, Wang X, Sun Z, Liu M, Shi H, Wang H, Feng Y, Shao R, Chai R, Li Q, Xing Q, Zhang R, Nogales E, Jin L, He L, Gupta ML, Jr, Cowan NJ, Wang L. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med. 2016;374(3):223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L, Guan Y, Wang W, Chen B, Xu S, Wu L, Yan Z, Li B, Fu J, Shi R, Shi J, du J, Li Q, Zhang Z, Mu J, Zhou Z, Dong J, Jin L, He L, Sun X, Kuang Y, Wang L, Sang Q. Identification novel mutations in TUBB8 in female infertility and a novel phenotype of large polar body in oocytes with TUBB8 mutations. J Assist Reprod Genet. 2020;37(8):1837–1847. doi: 10.1007/s10815-020-01830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen B, Wang W, Peng X, Jiang H, Zhang S, Li D, Li B, Fu J, Kuang Y, Sun X, Wang X, Zhang Z, Wu L, Zhou Z, Lyu Q, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Sang Q, Wang L. The comprehensive mutational and phenotypic spectrum of TUBB8 in female infertility. Eur J Hum Genet. 2019;27(2):300–307. doi: 10.1038/s41431-018-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen B, Li B, Li D, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Kuang Y, Sang Q, Wang L. Novel mutations and structural deletions in TUBB8: expanding mutational and phenotypic spectrum of patients with arrest in oocyte maturation, fertilization or early embryonic development. Hum Reprod. 2017;32(2):457–464. doi: 10.1093/humrep/dew322. [DOI] [PubMed] [Google Scholar]
- 30.Feng R, Yan Z, Li B, Yu M, Sang Q, Tian G, Xu Y, Chen B, Qu R, Sun Z, Sun X, Jin L, He L, Kuang Y, Cowan NJ, Wang L. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J Med Genet. 2016;53(10):662–671. doi: 10.1136/jmedgenet-2016-103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang P, Yin C, Li M, Ma S, Cao Y, Zhang C, Chen T, Zhao H. Mutation analysis of tubulin beta 8 class VIII in infertile females with oocyte or embryonic defects. Clin Genet. 2021;99(1):208–214. doi: 10.1111/cge.13855. [DOI] [PubMed] [Google Scholar]
- 32.Xing Q, Wang R, Chen B, Li L, Pan H, Li T, Ma X, Cao Y, Wang B. Rare homozygous mutation in TUBB8 associated with oocyte maturation defect-2 in a consanguineous mating family. J Ovarian Res. 2020;13(1):42. doi: 10.1186/s13048-020-00637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang J, Wang W, Qian C, Xue J, Wang T, Li H, Li H. Human oocyte maturation arrest caused by a novel missense mutation in TUBB8. J Int Med Res. 2018;46(9):3759–3764. doi: 10.1177/0300060518778638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang AC, Zhang YS, Wang BS, Zhao XY, Wu FX, Zhai XH, Sun JX, Mei SY. Mutation analysis of the TUBB8 gene in primary infertile women with arrest in oocyte maturation. Gynecol Endocrinol. 2018;34(10):900–904. doi: 10.1080/09513590.2018.1464138. [DOI] [PubMed] [Google Scholar]
- 35.Huang L, Tong X, Luo L, Zheng S, Jin R, Fu Y, Zhou G, Li D, Liu Y. Mutation analysis of the TUBB8 gene in nine infertile women with oocyte maturation arrest. Reprod BioMed Online. 2017;35(3):305–310. doi: 10.1016/j.rbmo.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Yuan P, Zheng L, Liang H, Li Y, Zhao H, Li R, Lai L, Zhang Q, Wang W. A novel mutation in the TUBB8 gene is associated with complete cleavage failure in fertilized eggs. J Assist Reprod Genet. 2018;35(7):1349–1356. doi: 10.1007/s10815-018-1188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sha Q, Zheng W, Feng X, Yuan R, Hu H, Gong F, Hu L, Lin G, Ou X. Novel mutations in TUBB8 expand the mutational and phenotypic spectrum of patients with zygotes containing multiple pronuclei. Gene. 2020;145227:145227. doi: 10.1016/j.gene.2020.145227. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Li B, Fu J, Li R, Diao F, Li C, Chen B, du J, Zhou Z, Mu J, Yan Z, Wu L, Liu S, Wang W, Zhao L, Dong J, He L, Liang X, Kuang Y, Sun X, Sang Q, Wang L. Bi-allelic missense pathogenic variants in TRIP13 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet. 2020;107(1):15–23. doi: 10.1016/j.ajhg.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang HL, Lv C, Zhao YC, Li W, He XM, Li P, Sha AG, Tian X, Papasian CJ, Deng HW, Lu GX, Xiao HM. Mutant ZP1 in familial infertility. N Engl J Med. 2014;370(13):1220–1226. doi: 10.1056/NEJMoa1308851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Z, Ni C, Wu L, Chen B, Xu Y, Zhang Z, Mu J, Li B, Yan Z, Fu J, Wang W, Zhao L, Dong J, Sun X, Kuang Y, Sang Q, Wang L. Novel mutations in ZP1, ZP2, and ZP3 cause female infertility due to abnormal zona pellucida formation. Hum Genet. 2019;138(4):327–337. doi: 10.1007/s00439-019-01990-1. [DOI] [PubMed] [Google Scholar]
- 41.Cao Q, Zhao C, Zhang X, Zhang H, Lu Q, Wang C, Hu Y, Ling X, Zhang J, Huo R. Heterozygous mutations in ZP1 and ZP3 cause formation disorder of ZP and female infertility in human. J Cell Mol Med. 2020;24(15):8557–8566. doi: 10.1111/jcmm.15482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okutman Ö, Demirel C, Tülek F, Pfister V, Büyük U, Muller J, et al. Homozygous splice site mutation in ZP1 causes familial oocyte maturation defect. Genes (Basel). 2020;11(4). 10.3390/genes11040382. [DOI] [PMC free article] [PubMed]
- 43.Yang P, Chen T, Liu Y, Hou Z, Wu K, Cao Y, et al. The critical role of ZP genes in female infertility characterized by empty follicle syndrome and oocyte degeneration. Fertil Steril. 2020. 10.1016/j.fertnstert.2020.11.003. [DOI] [PubMed]
- 44.Xu Q, Zhu X, Maqsood M, Li W, Tong X, Kong S, Wang F, Liu X, Wei Z, Zhang Z, Zhu F, Cao Y, Bao J. A novel homozygous nonsense ZP1 variant causes human female infertility associated with empty follicle syndrome (EFS) Mol Genet Genomic Med. 2020;8(7):e1269. doi: 10.1002/mgg3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo G, Zhu L, Liu Z, Yang X, Xi Q, Li Z, Duan J, Jin L, Zhang X. Novel mutations in ZP1 and ZP2 cause primary infertility due to empty follicle syndrome and abnormal zona pellucida. J Assist Reprod Genet. 2020;37(11):2853–2860. doi: 10.1007/s10815-020-01926-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai C, Chen Y, Hu L, Du J, Gong F, Dai J, et al. ZP1 mutations are associated with empty follicle syndrome: evidence for the existence of an intact oocyte and a zona pellucida in follicles up to the early antral stage. A case report. Hum Reprod. 2019;34(11):2201–2207. doi: 10.1093/humrep/dez174. [DOI] [PubMed] [Google Scholar]
- 47.Maddirevula S, Awartani K, Coskun S, AlNaim LF, Ibrahim N, Abdulwahab F, et al. A genomics approach to females with infertility and recurrent pregnancy loss. Hum Genet. 2020;139(5):605–613. doi: 10.1007/s00439-020-02143-5. [DOI] [PubMed] [Google Scholar]
- 48.Liu M, Shen Y, Zhang X, Wang X, Li D, Wang Y. Novel biallelic loss-of-function variants in ZP1 identified in an infertile female with empty follicle syndrome. J Assist Reprod Genet. 2020;37(9):2151–2157. doi: 10.1007/s10815-020-01855-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun L, Fang X, Chen Z, Zhang H, Zhang Z, Zhou P, Xue T, Peng X, Zhu Q, Yin M, Liu C, Deng Y, Hu H, Li N. Compound heterozygous ZP1 mutations cause empty follicle syndrome in infertile sisters. Hum Mutat. 2019;40(11):2001–2006. doi: 10.1002/humu.23864. [DOI] [PubMed] [Google Scholar]
- 50.Dai C, Hu L, Gong F, Tan Y, Cai S, Zhang S, Dai J, Lu C, Chen J, Chen Y, Lu G, du J, Lin G. ZP2 pathogenic variants cause in vitro fertilization failure and female infertility. Genet Med. 2019;21(2):431–440. doi: 10.1038/s41436-018-0064-y. [DOI] [PubMed] [Google Scholar]
- 51.Chen T, Bian Y, Liu X, Zhao S, Wu K, Yan L, Li M, Yang Z, Liu H, Zhao H, Chen ZJ. A recurrent missense mutation in ZP3 causes empty follicle syndrome and female infertility. Am J Hum Genet. 2017;101(3):459–465. doi: 10.1016/j.ajhg.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang D, Zhu L, Liu Z, Ren X, Yang X, Li D, Luo Y, Peng X, Zhou X, Jia W, Hou M, Li Z, Jin L, Zhang X. A novel mutation in ZP3 causes empty follicle syndrome and abnormal zona pellucida formation. J Assist Reprod Genet. 2020;38:251–259. doi: 10.1007/s10815-020-01995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sang Q, Zhang Z, Shi J, Sun X, Li B, Yan Z, et al. A pannexin 1 channelopathy causes human oocyte death. Sci Transl Med. 2019;11(485). 10.1126/scitranslmed.aav8731. [DOI] [PubMed]
- 54.Alazami AM, Awad SM, Coskun S, Al-Hassan S, Hijazi H, Abdulwahab FM, et al. TLE6 mutation causes the earliest known human embryonic lethality. Genome Biol. 2015;16:240. doi: 10.1186/s13059-015-0792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin J, Xu H, Chen B, Wang W, Wang L, Sun X, Sang Q. Expanding the genetic and phenotypic spectrum of female infertility caused by TLE6 mutations. J Assist Reprod Genet. 2020;37(2):437–442. doi: 10.1007/s10815-019-01653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Song D, Mykytenko D, Kuang Y, Lv Q, Li B, Chen B, Mao X, Xu Y, Zukin V, Mazur P, Mu J, Yan Z, Zhou Z, Li Q, Liu S, Jin L, He L, Sang Q, Sun Z, Dong X, Wang L. Novel mutations in genes encoding subcortical maternal complex proteins may cause human embryonic developmental arrest. Reprod BioMed Online. 2018;36(6):698–704. doi: 10.1016/j.rbmo.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Zheng W, Hu H, Dai J, Zhang S, Gu Y, Dai C, Guo J, Xu X, Li Y, Zhang S, Hu L, Gong F, Lu G, Lin G. Expanding the genetic and phenotypic spectrum of the subcortical maternal complex genes in recurrent preimplantation embryonic arrest. Clin Genet. 2020;99:286–291. doi: 10.1111/cge.13858. [DOI] [PubMed] [Google Scholar]
- 58.Sang Q, Li B, Kuang Y, Wang X, Zhang Z, Chen B, Wu L, Lyu Q, Fu Y, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Wang L. Homozygous mutations in WEE2 cause fertilization failure and female infertility. Am J Hum Genet. 2018;102(4):649–657. doi: 10.1016/j.ajhg.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian Y, Wang G, Wang J, Mu X, Chen H, Song X, Bai X. Novel compound heterozygous mutation in WEE2 is associated with fertilization failure: case report of an infertile woman and literature review. BMC Womens Health. 2020;20(1):246. doi: 10.1186/s12905-020-01111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou X, Zhu L, Hou M, Wu Y, Li Z, Wang J, Liu Z, Zhang D, Jin L, Zhang X. Novel compound heterozygous mutations in WEE2 causes female infertility and fertilization failure. J Assist Reprod Genet. 2019;36(9):1957–1962. doi: 10.1007/s10815-019-01553-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao S, Chen T, Yu M, Bian Y, Cao Y, Ning Y, Su S, Zhang J, Zhao S. Novel WEE2 gene variants identified in patients with fertilization failure and female infertility. Fertil Steril. 2019;111(3):519–526. doi: 10.1016/j.fertnstert.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Z, Mu J, Zhao J, Zhou Z, Chen B, Wu L, Yan Z, Wang W, Zhao L, Dong J, Sun X, Kuang Y, Li B, Wang L, Sang Q. Novel mutations in WEE2: expanding the spectrum of mutations responsible for human fertilization failure. Clin Genet. 2019;95(4):520–524. doi: 10.1111/cge.13505. [DOI] [PubMed] [Google Scholar]
- 63.Yang X, Shu L, Cai L, Sun X, Cui Y, Liu J. Homozygous missense mutation Arg207Cys in the WEE2 gene causes female infertility and fertilization failure. J Assist Reprod Genet. 2019;36(5):965–971. doi: 10.1007/s10815-019-01418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dai J, Zheng W, Dai C, Guo J, Lu C, Gong F, Li Y, Zhou Q, Lu G, Lin G. New biallelic mutations in WEE2: expanding the spectrum of mutations that cause fertilization failure or poor fertilization. Fertil Steril. 2019;111(3):510–518. doi: 10.1016/j.fertnstert.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 65.Zhao L, Xue S, Yao Z, Shi J, Chen B, Wu L, Sun L, Xu Y, Yan Z, Li B, Mao X, Fu J, Zhang Z, Mu J, Wang W, du J, Liu S, Dong J, Wang W, Li Q, He L, Jin L, Liang X, Kuang Y, Sun X, Wang L, Sang Q. Biallelic mutations in CDC20 cause female infertility characterized by abnormalities in oocyte maturation and early embryonic development. Protein Cell. 2020;11:921–927. doi: 10.1007/s13238-020-00756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng W, Zhou Z, Sha Q, Niu X, Sun X, Shi J, Zhao L, Zhang S, Dai J, Cai S, Meng F, Hu L, Gong F, Li X, Fu J, Shi R, Lu G, Chen B, Fan H, Wang L, Lin G, Sang Q. Homozygous mutations in BTG4 cause zygotic cleavage failure and female infertility. Am J Hum Genet. 2020;107(1):24–33. doi: 10.1016/j.ajhg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Y, Shi Y, Fu J, Yu M, Feng R, Sang Q, Liang B, Chen B, Qu R, Li B, Yan Z, Mao X, Kuang Y, Jin L, He L, Sun X, Wang L. Mutations in PADI6 cause female infertility characterized by early embryonic arrest. Am J Hum Genet. 2016;99(3):744–752. doi: 10.1016/j.ajhg.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng W, Chen L, Dai J, Dai C, Guo J, Lu C, Gong F, Lu G, Lin G. New biallelic mutations in PADI6 cause recurrent preimplantation embryonic arrest characterized by direct cleavage. J Assist Reprod Genet. 2020;37(1):205–212. doi: 10.1007/s10815-019-01606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maddirevula S, Coskun S, Awartani K, Alsaif H, Abdulwahab FM, Alkuraya FS. The human knockout phenotype of PADI6 is female sterility caused by cleavage failure of their fertilized eggs. Clin Genet. 2017;91(2):344–345. doi: 10.1111/cge.12866. [DOI] [PubMed] [Google Scholar]
- 70.Qian J, Nguyen NMP, Rezaei M, Huang B, Tao Y, Zhang X, Cheng Q, Yang HJ, Asangla A, Majewski J, Slim R. Biallelic PADI6 variants linking infertility, miscarriages, and hydatidiform moles. Eur J Hum Genet. 2018;26(7):1007–1013. doi: 10.1038/s41431-018-0141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mu J, Wang W, Chen B, Wu L, Li B, Mao X, Zhang Z, Fu J, Kuang Y, Sun X, Li Q, Jin L, He L, Sang Q, Wang L. Mutations in NLRP2 and NLRP5 cause female infertility characterised by early embryonic arrest. J Med Genet. 2019;56(7):471–480. doi: 10.1136/jmedgenet-2018-105936. [DOI] [PubMed] [Google Scholar]
- 72.Xu Y, Qian Y, Liu Y, Wang Q, Wang R, Zhou Y, Zhang C, Pang Z, Ye H, Xue S, Sun L. A novel homozygous variant in NLRP5 is associate with human early embryonic arrest in a consanguineous Chinese family. Clin Genet. 2020;98(1):69–73. doi: 10.1111/cge.13744. [DOI] [PubMed] [Google Scholar]
- 73.Li M, Jia M, Zhao X, Shi R, Xue X. A new NLRP5 mutation causes female infertility and total fertilization failure. Gynecol Endocrinol. 2020;37:1–2. doi: 10.1080/09513590.2020.1832069. [DOI] [PubMed] [Google Scholar]
- 74.Parry DA, Logan CV, Hayward BE, Shires M, Landolsi H, Diggle C, Carr I, Rittore C, Touitou I, Philibert L, Fisher RA, Fallahian M, Huntriss JD, Picton HM, Malik S, Taylor GR, Johnson CA, Bonthron DT, Sheridan EG. Mutations causing familial biparental hydatidiform mole implicate c6orf221 as a possible regulator of genomic imprinting in the human oocyte. Am J Hum Genet. 2011;89(3):451–458. doi: 10.1016/j.ajhg.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fallahi J, Anvar Z, Razban V, Momtahan M, Namavar-Jahromi B, Fardaei M. Founder effect of KHDC3L, p.M1V mutation, on Iranian patients with recurrent hydatidiform moles. Iran J Med Sci. 2020;45(2):118–124. doi: 10.30476/ijms.2019.45335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang W, Chen Z, Zhang D, Zhao B, Liu L, Xie Z, Yao Y, Zheng P. KHDC3L mutation causes recurrent pregnancy loss by inducing genomic instability of human early embryonic cells. PLoS Biol. 2019;17(10):e3000468. doi: 10.1371/journal.pbio.3000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang W, Dong J, Chen B, Du J, Kuang Y, Sun X, et al. Homozygous mutations in REC114 cause female infertility characterised by multiple pronuclei formation and early embryonic arrest. J Med Genet. 2020;57(3):187–194. doi: 10.1136/jmedgenet-2019-106379. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen NMP, Ge ZJ, Reddy R, Fahiminiya S, Sauthier P, Bagga R, Sahin FI, Mahadevan S, Osmond M, Breguet M, Rahimi K, Lapensee L, Hovanes K, Srinivasan R, van den Veyver IB, Sahoo T, Ao A, Majewski J, Taketo T, Slim R. Causative mutations and mechanism of androgenetic hydatidiform moles. Am J Hum Genet. 2018;103(5):740–751. doi: 10.1016/j.ajhg.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmiady H, Neitzel H. Arrest of human oocytes during meiosis I in two sisters of consanguineous parents: first evidence for an autosomal recessive trait in human infertility: Case report. Hum Reprod. 2002;17(10):2556–2559. doi: 10.1093/humrep/17.10.2556. [DOI] [PubMed] [Google Scholar]
- 80.Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol. 2014;16(4):335–344. doi: 10.1038/ncb2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abou-Haila A, Bendahmane M, Tulsiani DR. Significance of egg's zona pellucida glycoproteins in sperm-egg interaction and fertilization. Minerva Ginecol. 2014;66(4):409–419. [PubMed] [Google Scholar]
- 82.Lefièvre L, Conner SJ, Salpekar A, Olufowobi O, Ashton P, Pavlovic B, Lenton W, Afnan M, Brewis IA, Monk M, Hughes DC, Barratt CL. Four zona pellucida glycoproteins are expressed in the human. Hum Reprod. 2004;19(7):1580–1586. doi: 10.1093/humrep/deh301. [DOI] [PubMed] [Google Scholar]
- 83.Wassarman PM, Litscher ES. The mouse egg's zona pellucida. Curr Top Dev Biol. 2018;130:331–356. doi: 10.1016/bs.ctdb.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 84.Rankin TL, O'Brien M, Lee E, Wigglesworth K, Eppig J, Dean J. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development. 2001;128(7):1119–1126. doi: 10.1242/dev.128.7.1119. [DOI] [PubMed] [Google Scholar]
- 85.Rankin T, Familari M, Lee E, Ginsberg A, Dwyer N, Blanchette-Mackie J, Drago J, Westphal H, Dean J. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development. 1996;122(9):2903–2910. doi: 10.1242/dev.122.9.2903. [DOI] [PubMed] [Google Scholar]
- 86.Liu W, Li K, Bai D, Yin J, Tang Y, Chi F, Zhang L, Wang Y, Pan J, Liang S, Guo Y, Ruan J, Kou X, Zhao Y, Wang H, Chen J, Teng X, Gao S. Dosage effects of ZP2 and ZP3 heterozygous mutations cause human infertility. Hum Genet. 2017;136(8):975–985. doi: 10.1007/s00439-017-1822-7. [DOI] [PubMed] [Google Scholar]
- 87.Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013;1828(1):15–22. doi: 10.1016/j.bbamem.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 88.Clift D, Schuh M. Restarting life: fertilization and the transition from meiosis to mitosis. Nat Rev Mol Cell Biol. 2013;14(9):549–562. doi: 10.1038/nrm3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508(7497):483–487. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science. 2000;287(5451):319–321. doi: 10.1126/science.287.5451.319. [DOI] [PubMed] [Google Scholar]
- 91.Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434(7030):234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 92.Cho C, Bunch DO, Faure JE, Goulding EH, Eddy EM, Primakoff P, et al. Fertilization defects in sperm from mice lacking fertilin beta. Science. 1998;281(5384):1857–1859. doi: 10.1126/science.281.5384.1857. [DOI] [PubMed] [Google Scholar]
- 93.Fujihara Y, Oji A, Kojima-Kita K, Larasati T, Ikawa M. Co-expression of sperm membrane proteins CMTM2A and CMTM2B is essential for ADAM3 localization and male fertility in mice. J Cell Sci. 2018;131(19). 10.1242/jcs.221481. [DOI] [PMC free article] [PubMed]
- 94.Ikawa M, Wada I, Kominami K, Watanabe D, Toshimori K, Nishimune Y, Okabe M. The putative chaperone calmegin is required for sperm fertility. Nature. 1997;387(6633):607–611. doi: 10.1038/42484. [DOI] [PubMed] [Google Scholar]
- 95.Yu C, Ji SY, Sha QQ, Dang Y, Zhou JJ, Zhang YL, Liu Y, Wang ZW, Hu B, Sun QY, Sun SC, Tang F, Fan HY. BTG4 is a meiotic cell cycle-coupled maternal-zygotic-transition licensing factor in oocytes. Nat Struct Mol Biol. 2016;23(5):387–394. doi: 10.1038/nsmb.3204. [DOI] [PubMed] [Google Scholar]
- 96.Bianchi E, Sette C. Post-transcriptional control of gene expression in mouse early embryo development: a view from the tip of the iceberg. Genes (Basel) 2011;2(2):345–359. doi: 10.3390/genes2020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li L, Zheng P, Dean J. Maternal control of early mouse development. Development. 2010;137(6):859–870. doi: 10.1242/dev.039487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, Dean J, Nelson LM. Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet. 2000;26(3):267–268. doi: 10.1038/81547. [DOI] [PubMed] [Google Scholar]
- 99.Li L, Baibakov B, Dean J. A subcortical maternal complex essential for preimplantation mouse embryogenesis. Dev Cell. 2008;15(3):416–425. doi: 10.1016/j.devcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Esposito G, Vitale AM, Leijten FP, Strik AM, Koonen-Reemst AM, Yurttas P, et al. Peptidylarginine deiminase (PAD) 6 is essential for oocyte cytoskeletal sheet formation and female fertility. Mol Cell Endocrinol. 2007;273(1-2):25–31. doi: 10.1016/j.mce.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 101.Zheng P, Dean J. Role of Filia, a maternal effect gene, in maintaining euploidy during cleavage-stage mouse embryogenesis. Proc Natl Acad Sci U S A. 2009;106(18):7473–7478. doi: 10.1073/pnas.0900519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mahadevan S, Sathappan V, Utama B, Lorenzo I, Kaskar K, Van den Veyver IB. Maternally expressed NLRP2 links the subcortical maternal complex (SCMC) to fertility, embryogenesis and epigenetic reprogramming. Sci Rep. 2017;7:44667. doi: 10.1038/srep44667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kumar R, Oliver C, Brun C, Juarez-Martinez AB, Tarabay Y, Kadlec J, et al. Mouse REC114 is essential for meiotic DNA double-strand break formation and forms a complex with MEI4. Life Sci Alliance. 2018;1(6):e201800259. doi: 10.26508/lsa.201800259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stanzione M, Baumann M, Papanikos F, Dereli I, Lange J, Ramlal A, Tränkner D, Shibuya H, de Massy B, Watanabe Y, Jasin M, Keeney S, Tóth A. Meiotic DNA break formation requires the unsynapsed chromosome axis-binding protein IHO1 (CCDC36) in mice. Nat Cell Biol. 2016;18(11):1208–1220. doi: 10.1038/ncb3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Akoury E, Gupta N, Bagga R, Brown S, Déry C, Kabra M, Srinivasan R, Slim R. Live births in women with recurrent hydatidiform mole and two NLRP7 mutations. Reprod BioMed Online. 2015;31(1):120–124. doi: 10.1016/j.rbmo.2015.03.011. [DOI] [PubMed] [Google Scholar]