Abstract
Both glucose tolerance and adaptive immune function exhibit significant age-related alterations. The influence of the immune system on obesity-associated glucose intolerance is well characterized; however, whether the immune system contributes to age-related glucose intolerance is not as well understood. Here, we report that advancing age results in an increase in T cell infiltration in the epididymal white adipose tissue (eWAT), liver, and skeletal muscle. Subtype analyses show that both CD4+, CD8+ T cells are greater with advancing age in each of these tissues and that aging results in a blunted CD4 to CD8 ratio. Anti-CD3 F(ab’)2 fragments depleted CD4+ and CD8+ cells in eWAT, CD4+ cells only in the liver, and did not deplete quadriceps T cells. In old mice, T cells producing both interferon-γ and tumor necrosis factor-α are accumulated in the eWAT and liver, and a greater proportion of skeletal muscle T cells produced interferon-γ. Aging resulted in increased proportion and numbers of T regulatory cells in eWAT, but not in the liver or muscle. Aging also resulted in greater numbers of eWAT and quadriceps CD206- macrophages and eWAT, liver and quadriceps B cells; neither cell type was altered by anti-CD3 treatment. Anti-CD3 treatment improved glucose tolerance in old mice and was accompanied by improved signaling related to liver and skeletal muscle insulin utilization and decreased gluconeogenesis-related gene expression in the liver. Our findings indicate a critical role of the adaptive immune system in the age-related metabolic dysfunction.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00368-4.
Keywords: Adipose tissue, T lymphocyte, Macrophages, Glucose intolerance, Liver, Skeletal muscle
Introduction
Glucose metabolism plays a central role in metabolic homeostasis, and an impairment in this homeostasis leads to numerous diseases [1]. Aging is an independent risk factor of glucose intolerance that manifests as impaired tissue glucose uptake, peripheral insulin resistance, decreased insulin signaling, and elevated hepatic gluconeogenesis [2–4]. Adipose tissue per se is a major regulator of systemic glucose metabolism, and different adipose tissue depots play independent and critical roles. For example, epididymal white adipose tissue (eWAT) regulates systemic glucose metabolism via the metabolic hormones leptin, adiponectin, and resistin, as well as many other active small molecules [5, 6]. Subcutaneous white adipose tissue (scWAT) and brown adipose tissue (BAT) are considered protective adipose depots, and, unlike eWAT, an increase in scWAT and BAT mass is accompanied by improved metabolic function [6]. Results from a series of epidemiological and experimental studies demonstrate that the age-related decline in metabolic function is directly related to altered adipose tissue phenotype [2, 4].
Obesity is associated with T cell and macrophage infiltration in eWAT, and this immune cell infiltration orchestrates an inflammatory response that contributes to glucose intolerance [7, 8]. The role of adipose tissue macrophages and inflammatory cytokines in obesity-associated metabolic dysfunction has been widely studied in murine models using genetic or pharmacological approaches to reduce or inhibit macrophages or different inflammatory cytokines [9–11]. However, the role of adipose tissue T cells on systemic glucose intolerance in aging is incompletely understood.
Recent studies show that genetic or pharmacological deletion of T cells improves obesity-induced glucose intolerance by reducing adiposity [8, 12]. However, in the context of healthy aging, where mice are not overtly obese, the distribution of immune cells in adipose tissue and the underlying mechanisms of insulin resistance differ from that observed in obesity [13]. As an example, it has been demonstrated that different subpopulations of T cells contribute to metabolic dysfunction in diet-induced obese mice vs. middle-aged mice [8, 14, 15]. Collectively, these studies suggest a distinct role for T cells in aged vs. obese adipose tissue.
Compared to eWAT, the immune cell profile in scWAT and BAT have received less attention. Although both scWAT and BAT harbor T cells [16, 17], whether aging alters T cell infiltration in these adipose depots is largely unknown. Likewise, little is known about the contribution of the immune system to glucose homeostasis with advancing age in the two other major sites of glucose disposal, the liver and skeletal muscle. A recent report has shown that hepatic T cells contribute to impairments in glucose tolerance in high-fat diet-fed mice [18]. Furthermore, in both humans and mice, acute inflammation in response to a viral infection can induce glucose intolerance in the skeletal muscle [19]. Whether T cells from the aged immune system induce dysfunction in the liver and skeletal muscle remains to be elucidated.
In this study, we sought to test the hypothesis that T cells mediate glucose intolerance in old mice. We assessed systemic glucose tolerance and fasted plasma insulin concentrations before and after treatment with anti-CD3 F(ab’)2 fragments. This regimen depletes T cells both systemically and in adipose tissue [12, 20]. We also assessed adipose, liver, and skeletal muscle immune cell infiltration and insulin signaling in these tissues following anti-CD3 treatment.
Methods and materials
Animals
Young (4–6 month) and old (22–24 month) male C57BL/6 mice were used for this study. Young mice were purchased from Charles River, and old mice were obtained from the National Institute on Aging rodent colony maintained at Charles River Inc. All mice were maintained in the Salt Lake City VA Medical Center’s Animal Facility in the standard shoe box cages on a 12:12 light:dark cycle with water and food ad libitum. All animal studies were in compliance with the Guide for the Care and Use of Laboratory Animals (2011) and were approved by the University of Utah and Salt Lake City VA Medical Center Animal Care and Use Committee.
Anti-CD3 and isotype control treatments
To deplete T cells, mice were treated with anti-CD3 F(ab’)2 fragments (BioXCell) 150 μg ip once every 7 days for 28 days, a treatment regimen shown to systemically deplete T cells [12]. Control mice were treated with isotype control F(ab’)2 fragments. In a subset of old mice, ~50 μl of blood was obtained from a tail nick at baseline, day 10, and day 20, and blood T cell proportions were assessed using flow cytometry as described below. On day 28, the mice were euthanized for tissue collection. Some mice were fasted for 3 h prior to euthanasia.
Flow cytometry
A terminal blood draw was performed via cardiac puncture, while mice were maintained under isoflurane anesthesia. Following euthanasia, the chest cavity was opened, and the right atrium was nicked. To remove circulating leukocytes, a cannula was placed in the left ventricle, and the animals were perfused with saline + 10 U/ml of heparin at physiological pressure until the effluent was cleared of blood.
Spleens, eWAT, scWAT, BAT, quadriceps, and a lobe of liver were excised, weighed, and digested using collagenase type I (2mg/ml) and DNAse (0.1mg/ml) dissolved in phosphate-buffered saline containing calcium and magnesium for 30–60 min at 37 °C. The tissues were further dispersed using repeated pipetting, and the resultant homogenate was passed through a 70-μm sterile filter, yielding single-cell suspensions. Red blood cells were lysed using RBC lysis buffer (Tonbo Biosciences). Single-cell suspensions were labeled with the following anti-mouse antibodies at a 1:100 concentration: violetfluor450-CD45, Tonbo #75-0454 (total leukocytes); APC-CD3, Tonbo #20-0032 (pan T cells); FITC-CD4, Tonbo #30-0041 (T helper cells); PE-Cy7-CD8, Tonbo #60-0081 (cytotoxic T cells); PerCP Cy5.5-CD44, Tonbo #65-00441 (naïve vs memory); and APC-Cy7-CD62L Tonbo #25-0621 (central vs effector). The T cell gating strategy is shown in the Online Supplemental (OS) Fig. 1. In separate experiments, B cells and macrophages were assessed as described [21] with violetfluor450-CD45 (total leukocytes); APC Cy7-CD19, Biolegend #115529 (B cells); PE-CD64, Biolegend #139303 (macrophages); FITC-CD11c, Tonbo #35-0114 (exclusion of dendritic cells); and APC-CD206, Biolegend #141707 (macrophage phenotype). The macrophage and B cell gating strategies are shown in OS Fig. 2. Cell subpopulations were assessed on a BD FACS Canto.
To assess T cell cytokine production, single-cell suspensions from the eWAT, liver, and pooled gastrocnemius (muscles from both legs of each mouse) were stimulated with phorbol 12-myristate 13-acetate (PMA, 10 ng/ml) and ionomycin (1 μg/ml) for 6 h. During this incubation, protein transport out of the Golgi apparatus was blocked with brefeldin A (10 μg/ml). Following stimulation cells were labeled with the following anti-mouse antibodies: PE/Dazzle-CD45, Biolegend #109846; APC-CD3, Tonbo #20-0032; FITC-CD4, Tonbo #30-0041; and PE-Cy5-CD8, Tonbo #55-0081. Following cell surface staining cells were fixed in 2% paraformaldehyde and permeablized with intracellular staining permeabilization wash buffer (Biolegend). Cells were then labeled with PE-Cy7-interferon (IFN)-γ, Tonbo #60-7311, and PerCPCy5.5- tumor necrosis factor (TNF)-α, Biolegend #506321. Gating strategy is shown in OS Fig. 1.
To assess the proportion of T regulatory cells (Tregs), eWAT, liver, and pooled quadriceps were stained with the following mouse antibodies: Alexa Fluor 532-CD45 Thermo #58-0454-82, violetfluor 500-CD3 Tonbo #85-0032, BV570-CD4 Biolegend #100542, PerCp-Cy5.5-CD8 Tonbo #65-1886, and BV421-CD25 Biolegend #102043. Following cell surface staining cells were fixed and permeablized using True-Nuclear Transcription Factor buffer (Biolegend) and stained with anti-mouse PE-Foxp3 Biolegend #126404. Gating strategy is shown in OS Fig. 1.
For all flow cytometry assays, dead cells were labeled (depending on antibody panel) with violetFluor450, violetFluor510, or Red 780 Ghost Dye (Tonbo) and excluded from analysis. The “fluorescence minus one” technique was used to establish gating, as described previously (22).
Plasma cytokines
Heparinized blood collected at exsanguination was centrifuged for 15 min at 2000× g and stored at −80 °C. Plasma cytokine concentrations were assessed using the LEGENDPLEX Mouse Inflammation cytometric bead array kit (Biolegend) according to manufacturer’s directions.
Metabolic testing
Glucose tolerance was assessed prior to and 21–26 days after initiation of anti-CD3 treatment by intraperitoneal glucose tolerance test (GTT) as described previously [22]. Briefly, the mice were fasted for 6 h in the morning, and baseline blood glucose was measured using a Precision Xceed Pro Glucometer in blood collected via a tail nick. Mice were injected with glucose (2g/kg body mass, ip), and blood glucose level was measured again 15, 30, 60, 90, and 120 min after the injection. Additionally, 70-μl blood was collected before the injection and 5 min after the injection to measure baseline and glucose-stimulated insulin secretion, respectively. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the following equation—HOMA-IR = (Fasting blood glucose (mg/dL) × Fasting plasma insulin (ng/mL))/405.
Enzyme-linked immunosorbent assay
Fasted (6h) and glucose-stimulated (2g/kg body mass, 5 min) plasma insulin was measured using commercially available rat/mouse insulin ELISA kit (Millipore Sigma) according to the manufacturer’s protocol. Fasted plasma adiponectin was measured using mouse adiponectin kit (Millipore Sigma) according to the manufacturer’s protocol.
Western blots
After euthanasia, tissues from fasted mice were dissected and snap frozen in liquid nitrogen and stored at −80 °C. Protein lysates were prepared from muscle and liver tissues using ice cold RIPA buffer (Sigma Aldrich). Protein expression was measured by standard western blot procedures using anti-mouse primary antibodies against total Akt (pan-Akt; 1:1000; 60 kDa; cell signaling), phosphorylated Akt (s-473 p-Akt; 1:1000; 60 kDa; cell signaling), beta actin (anti-beta actin; 1:1000; 40 kDa; Abcam), and vinculin (hVIN-1, monoclonal; 1:1000; 116 kDa, Sigma Aldrich). Goat Anti-Rabbit IgG (H+L)-HRP Conjugate (Bio-Rad) was used as the secondary antibody. Images were visualized and quantified using Bio-Rad ChemiDocTM XRS+ with Image LabTM Software.
Quantitative PCR
Total mRNA was isolated using RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. mRNA was converted into cDNA using QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer’s protocol. Quantitative PCR was performed on 96-well plates using RT2 SYBR Green qPCR Master Mix (Qiagen) with the Bio-Rad CFXTM Real-Time System. Expression of the genes were normalized to 18 s and fold change was calculated using the ΔΔCt method. Primer sequences are provided in the Online Supplement table.
Hepatic triglycerides
We quantified hepatic total triglycerides using a commercially available kit (Abcam) following the manufacturer’s protocol. Briefly, we washed ~50-mg tissue sample in ice cold PBS and homogenized in 1-mL ddH2O. The samples were heated for 5 min at 100 °C, cooled down at room temperature, and centrifuged for 2 min at max speed. The supernatants were used to measure the triglycerides by a colorimetric assay.
Statistical analysis
Most group differences were determined by two-way ANOVA using age and anti-CD3 treatment as factors with Tukey post hoc tests. Group differences in serial measures of circulating T cells were assessed with a repeated measured ANOVA with time and group as factors. Group differences in the glucose tolerance tests group were assessed by repeated measures ANOVA. Because many mice of both ages exhibited plasma cytokine levels at or below the levels of assay detection data, the non-parametric Kruskal–Wallis test with multiple comparisons was employed to assess group differences in these data.
Results
Animal characteristics
Body mass of the old mice was higher compared to young mice. Young mice exhibited increased body mass over the treatment period, and old mice exhibited decreased mass; however, these changes were not altered by anti-CD3 treatment (Table 1). Old mice had lower quadriceps mass compared to young mice, and anti-CD3 treatment did not alter quadriceps mass either in young or old mice (Table 1). We did not observe any age-related or treatment-dependent differences in eWAT fat pad mass (Table 1).
Table 1.
Animal Characteristics
| Group | Pre-treatment body mass (g) | Post-treatment body mass (g) | Δ in body mass over the treatment period (g) | eWAT fat pad mass (g) | Quadriceps mass (g) |
|---|---|---|---|---|---|
| Young isotype | 26.26 ± 0.71 | 28.41 ± 1.04 | 4.30 ± 2.21 | 0.29 ± 0.02 | 0.24 ± 0.01 |
| Young anti-CD3 | 25.88 ± 0.72 | 27.10 ± 0.81 | 2.65 ± 1.52 | 0.30 ± 0.02 | 0.24 ± 0.01 |
| Old isotype | 31.11 ± 0.72* | 29.96 ± 0.73 | −1.49 ± 1.45* | 0.35 ± 0.03 | 0.21 ± 0.02* |
| Old anti-CD3 | 32.03 ± 0.55* | 30.03 ± 0.69 | −1.84 ± 1.03* | 0.28 ± 0.03 | 0.19 ± 0.02* |
Data are shown as mean ± SEM, *p < 0.05 vs. young. N = 6–8/group
We sought to determine whether anti-CD3 treatment altered plasma cytokine concentrations using a multiplex cytokine array. Of the 12 cytokines assessed, we found significant group differences in IFN-β and IL-1β. Plasma IFN-β was greater in old isotype control mice compared to either young isotype or young anti-CD3-treated mice. IFN-β in old anti-CD3-treated mice was not significantly different from any other group (OS Fig. 3). Plasma IL-1β concentration was greater in old anti-CD3-treated mice when compared to young isotype or young anti-CD3-treated mice. Plasma IL-1β in old isotype controls was not significantly different from any other group (OS Fig. 3).
Immune cell populations and phenotype with age and anti-CD3 treatment
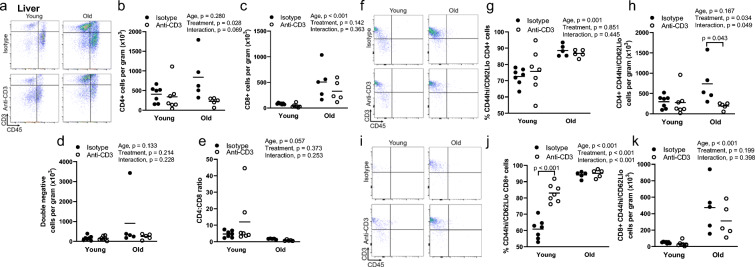
In an initial subset of old mice, we sought to determine the efficacy of the anti-CD3 antibody fragments. There was no difference in the proportion of circulating CD3+ cells at baseline (OS Fig. 4a). At day 10 and day 20 of the treatment period, mice treated with anti-CD3 fragments exhibited blunted proportions of circulating total (CD3+) T cells (OS Fig. 6a). Both young and old mice exhibited blunted proportions of circulating CD3+ cells at the time of euthanasia (OS Fig. 4b). In the spleens of young and old control mice, total T cell numbers were not altered with advancing age (Fig. 1a, b). Splenic T cell counts were > 50% lower following 28 days of anti-CD3 treatment in both young and old mice compared to age-matched controls (Fig. 1a, b). We next sought to determine whether T cells infiltrate metabolically active tissues with age and whether anti-CD3 treatment could ameliorate this infiltration. In eWAT, we found that old mice exhibited significantly higher T cell numbers compared to young mice (Fig. 1c, d). Anti-CD3 treatment resulted in lower T cell numbers in the eWAT (Fig. 1c, d). Old mice did not exhibit greater T cell numbers in scWAT or BAT compared to young mice (Fig. 1e–h). The liver of old mice exhibited greater T cell infiltration compared to young mice, and anti-CD3 treatment resulted in ameliorated T cell infiltration (Fig. 1i, j). In quadriceps, aging resulted in greater T cell infiltration, but this infiltration was not altered by anti-CD3 treatment (Fig. 1k, l).
Fig. 6.
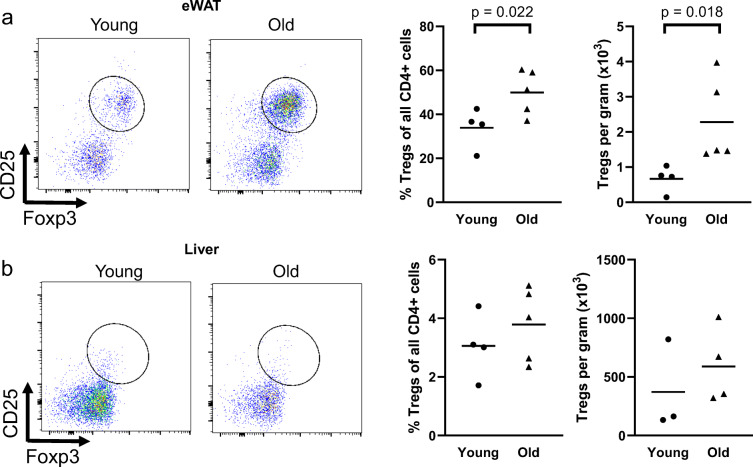
Effects of age on Treg proportion and accumulation in the eWAT and liver. Epididymal white adipose tissue (eWAT) and livers from young and old mice were dissected, mechanically disrupted, enzymatically digested activated in vitro, and stained for CD45 (total leukocytes), CD3 (pan T cells), CD4, CD8, CD25, and Foxp3. CD25+, Foxp3+ cells are considered Tregs. a Treg proportion and b accumulation in eWAT. c Treg proportion and d accumulation in liver. Group differences were assessed with an independent sample T test; p values are included on the panel with a line indicating the group comparison
Fig. 1.
Effects of age and anti-CD3 treatment on tissue T cell infiltration. The spleen (a and b), epididymal white adipose tissue (eWAT) (c and d), subcutaneous white adipose (scWAT) (e and f), brown adipose (BAT) (g and h), liver (i and j), and quadriceps (k and l) were dissected from young and old, isotype control and anti-CD3 F(ab’)2-treated mice. Tissues were mechanically disrupted, enzymatically digested, and stained with anti-CD45 (leukocytes), and anti-CD3 (T cells) dead cells were excluded with violetFluor510 Ghost Dye. Group differences were assessed by two-way ANOVA and Tukey’s post hoc test
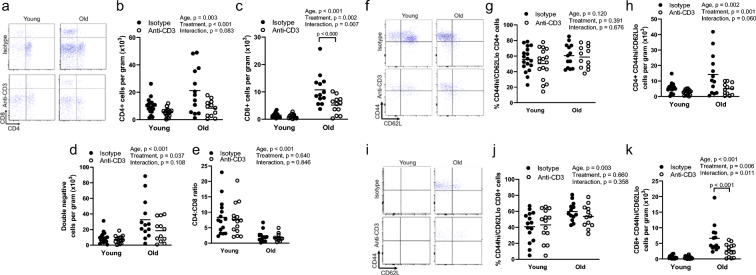
As we found age-related increases in T cell infiltration in the eWAT, liver, and quadriceps, we further assessed T cell phenotype in these tissues. We found that old mice exhibited greater CD4+, CD8+, and double-negative T cell numbers in the eWAT compared to young (Fig. 2a–d). Anti-CD3 treatment resulted in lower CD4+ and double-negative T cell counts in young and old mice and lower CD8+ T cell counts in old mice only (Fig. 2a–d). Old mice exhibited significantly blunted CD4-to-CD8 ratios independent of anti-CD3 treatment (Fig. 2a, e); this is similar to the CD4-to-CD8 ratios observed in the spleen (young control 4.4 ± 1.8; young anti-CD3 3.5 ± 1.0; old control 0.7 ± 0.1; old anti-CD3 1.5 ± 0.9). Collectively, these results indicate that age-related increases in eWAT T cell infiltration are driven by increases in multiple T cell subtypes.
Fig. 2.
Effects of age and anti-CD3 treatment on epididymal white adipose tissue T cell phenotype. Epididymal white adipose tissue (eWAT) from young and old, isotype control and anti-CD3 F(ab’)2-treated mice was dissected, mechanically disrupted, enzymatically digested, and stained with anti-CD45 (leukocytes), anti-CD3 (pan T cells), anti-CD4 (helper T cells), anti-CD8 (cytotoxic T cells), anti-CD44 (memory T cells), anti-CD62L (central/effector), and violetFluor510 Ghost Dye (exclusion of dead cells). a Sample flow cytometry plot assessing eWAT CD4+ and CD8+ distribution, b CD4+ T cell counts, c CD8+ T cell counts, d double-negative (DN) T cell counts, and e CD4-to-CD8 ratio. f Sample flow cytometry plot for CD4+ naïve/memory phenotype. g Proportion of CD4+ effector memory cells and h cell counts. i Sample flow cytometry plot for CD8+ naïve/memory phenotype. j Proportion of CD8+ effector memory cells and (K) cell counts. Group differences were assessed by two-way ANOVA and Tukey’s post hoc test
We next assessed the memory phenotype of eWAT-infiltrating T cells. Among CD4+ cells, we found that the proportion of effector memory (CD44hi/CD62Llo) cells was not altered with either age or anti-CD3 treatment (Fig. 2f, g) but that the total number of CD4+ effector memory cells in eWAT was greater in old mice (Fig. 2f, h). Numbers of CD4+ effector memory cells were blunted in mice treated with anti-CD3 fragments (Fig. 2f, h). Among CD8+ cells from the eWAT of old animals, there was both a greater proportion and a greater number of effector memory cells (Fig. 2i–k). Treatment of old mice with anti-CD3 fragments resulted in lower numbers of eWAT CD8+ effector memory cells (Fig. 2i–k).
In the liver, we found that aging resulted in greater infiltration of CD8+, but not CD4+ or double-negative T cells (Fig. 3a–d). The CD4-to-CD8 ratio tended to be lower with age (p = 0.057) (Fig. 3e). Aging resulted in a greater proportion of CD4+ cells exhibiting an effector memory phenotype that was not altered by anti-CD3 treatment (Fig. 3f, g). Total numbers of CD4+ effector memory cells were not altered by age (p = 0.167), but this cell population was lower in the livers of old anti-CD3-treated mice (Fig. 3f, h). Similar to CD4+ cells, aging resulted in a greater proportion of liver CD8+ cells exhibiting an effector memory phenotype (Fig. 3i, j). In addition, aging resulted in a greater number of liver-infiltrating CD8+ effector memory cells (Fig. 3i, k).
Fig. 3.
Effects of age and anti-CD3 treatment on liver T cell phenotype. The livers from young and old, isotype control and anti-CD3 F(ab’)2-treated mice were dissected, mechanically disrupted, enzymatically digested, and stained with anti-CD45 (leukocytes), anti-CD3 (pan T cells), anti-CD4 (helper T cells), anti-CD8 (cytotoxic T cells), anti-CD44 (memory T cells), anti-CD62L (central/effector), and violetFluor510 Ghost Dye (exclusion of dead cells). a Sample flow cytometry plot assessing liver CD4+ and CD8+ distribution, b CD4+ T cell counts, c CD8+ T cell counts, d double-negative (DN) T cell counts, and e CD4-to-CD8 ratio. f Sample flow cytometry plot for CD4+ naïve/memory phenotype. g Proportion of CD4+ effector memory cells and h cell counts. i Sample flow cytometry plot for CD8+ naïve/memory phenotype. j Proportion of CD8+ effector memory cells and k cell counts. Group differences were assessed by two-way ANOVA and Tukey’s post hoc test
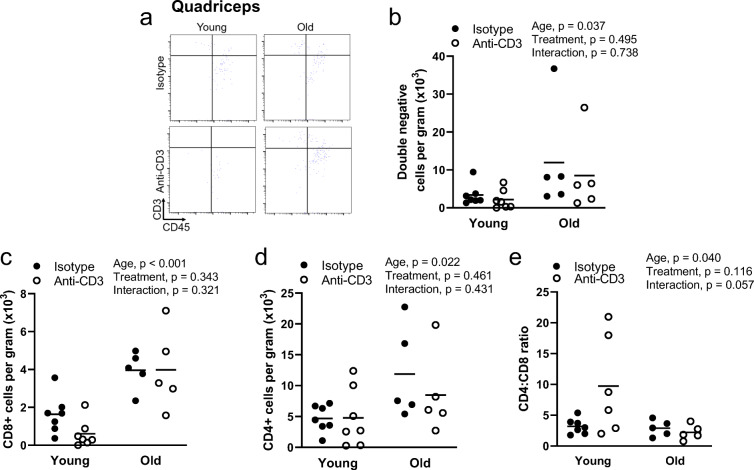
In quadriceps, aging resulted in a greater infiltration of CD4+, CD8+, and double-negative T cells (Fig. 4a–d). Anti-CD3 treatment did not alter T cell infiltration in quadriceps (Fig. 4a–d). The CD4-to-CD8 ratio was blunted in quadriceps with age but was not altered by anti-CD3 treatment (Fig. 4a, d). Because quadriceps exhibited markedly less total T cell infiltration compared to other tissues, regardless of age, we were not able to reliably determine the proportions of memory T cells due to low subpopulation counts.
Fig. 4.
Effects of age and anti-CD3 treatment on quadriceps T cell phenotype. Quadriceps from young and old, isotype control and anti-CD3 F(ab’)2-treated mice were dissected, mechanically disrupted, enzymatically digested, and stained with anti-CD45 (leukocytes), anti-CD3 (pan T cells), anti-CD4 (helper T cells), anti-CD8 (cytotoxic T cells), anti-CD44 (memory T cells), anti-CD62L (central/effector), and violetFluor510 Ghost Dye (exclusion of dead cells). a Sample flow cytometry plot assessing quadriceps CD4+ and CD8+ distribution, b CD4+ T cell counts, c CD8+ T cell counts, d double-negative (DN) T cell counts, and e CD4-to-CD8 ratio. Group differences were assessed by two-way ANOVA and Tukey’s post hoc test
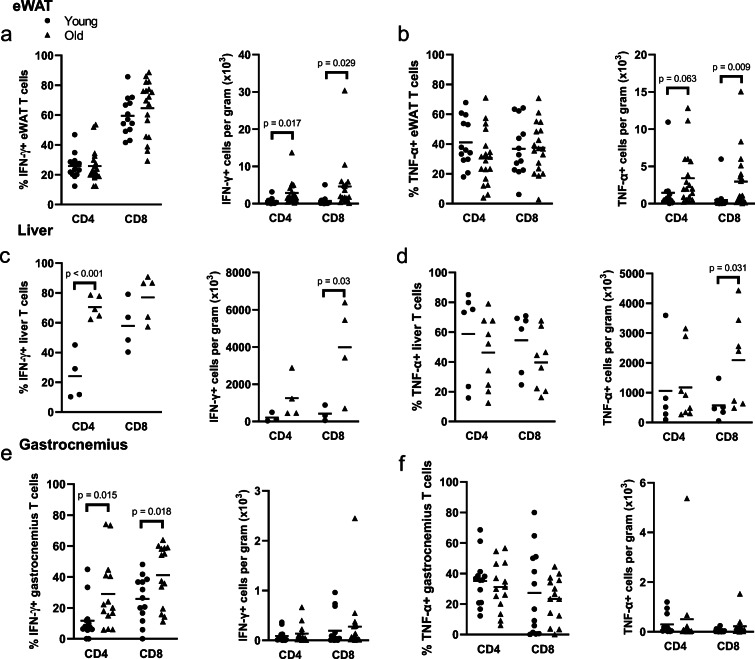
To determine whether aging is associated with and increased inflammatory phenotype of tissue-infiltrating T cells, we assessed interferon (IFN)-γ and tumor necrosis factor (TNF)-α production in T cells from the eWAT, liver, and gastrocnemius. In eWAT, the proportion of both CD4+ and CD8+ T cells that produce IFN-γ and TNF-α were not altered with age; however, we found that there was a significant accumulation of the total numbers of both CD4+ and CD8+ cells that produce IFN-γ and TNF-α (Fig. 5a, b). In the liver, we found that a greater proportion of CD4+, but not CD8+ cells, produced IFN-γ, but there was a significant accumulation of total IFN-γ-producing CD8+ cells in liver (Fig. 5c). The proportion of TNF-α-producing CD4+ and CD8+ cells was not altered in the liver, but there was a significant accumulation of TNF-α-producing CD8+ cells (Fig. 5d). In gastrocnemius, we found a greater proportion of CD4- and CD8-producing IFN-γ cells, but neither proportions of TNF-α nor accumulation of cells producing either cytokine was altered with age (Fig. 5e, f).
Fig. 5.
Effects of age on IFN-γ and TNF-α production and tissue infiltration. Epididymal white adipose tissue (eWAT), livers, and gastrocnemius from young and old mice were dissected, mechanically disrupted, enzymatically digested activated in vitro, and stained for CD45 (total leukocytes), CD3 (pan T cells), CD4, CD8, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α. a Proportion and number of IFN-γ-producing cells in the eWAT. b Proportion and number of TNF-α-producing cells in the eWAT. c Proportion and number of IFN-γ-producing cells in the liver. d Proportion and number of TNF-α-producing cells in the liver. e Proportion and number of TNF-α-producing cells in gastrocnemius, f Proportion and number of IFN-γ-producing cells in gastrocnemius. Group differences were assessed with an independent sample T test; p values are included on the panel with a line indicating the group comparison
As eWAT Tregs have been implicated in metabolic dysfunction in middle-aged mice [14, 15], we assessed Treg accumulation in the eWAT, liver, and quadriceps with age. We found that aging resulted in both a greater proportion and accumulation of eWAT Tregs (Fig. 6a). In the liver, we found that neither the proportion, nor accumulation, of Tregs were altered with age (Fig. 6b); further, we found that the proportion of liver Tregs was approximately tenfold lower (3–4% of all CD4+ T cells) in the liver when compared to the eWAT (35–50% of all CD4+ cells). Tregs were almost completely absent in quadriceps (< 50 cells per muscle) regardless of age.
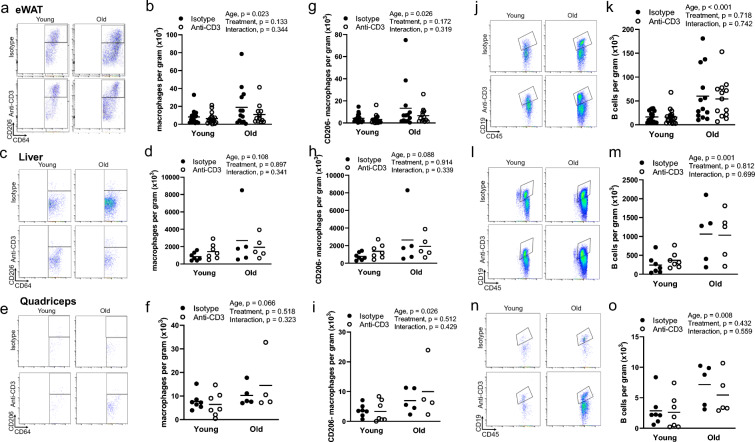
We next sought to determine whether alterations in tissue-infiltrating T cells influenced macrophage and B cell infiltration. We found that macrophage infiltration was greater in the eWAT but not the liver or quadriceps of old animals (Fig. 7a–f). Anti-CD3 treatment had no effect on macrophage infiltration (Fig. 7a–f). To assess macrophage phenotype, we examined the cell surface marker CD206; macrophages that lack CD206 are generally associated with a proinflammatory phenotype [23]. We found greater numbers of CD206- macrophages with age in the eWAT and quadriceps but not the liver, with no effect of anti-CD3 treatment (Fig. 7g–h). We also examined B cell infiltration and found that the eWAT, liver, and quadriceps exhibited greater B cell infiltration with age (Fig. 7j–o). B cell infiltration was not altered by anti-CD3 treatment (Fig. 7j–o).
Fig. 7.
Effects of age and anti-CD3 treatment on macrophage and B cell infiltration and phenotype. Epididymal white adipose tissue (eWAT), livers, and quadriceps from young and old, isotype control and anti-CD3 F(ab’)2-treated mice were dissected, mechanically disrupted, enzymatically digested, and stained with anti-CD45 (leukocytes), anti-CD64 (macrophages), anti-CD11c (exclusion of dendritic cells), anti-CD206 (macrophage phenotype), and anti-CD19 (B cells). Sample flow cytometry plots and summary data showing eWAT (a, b), liver (c, d), and quadriceps (e, f) macrophage counts. CD206- macrophage counts in eWAT (g), liver (h), and quadriceps (i). k Sample flow cytometry plots and j quantification of eWAT B cell infiltration. l Sample flow cytometry plots and m quantification of liver B cell infiltration. n Sample flow cytometry plots and o quantification of quadriceps B cell infiltration. Group differences were assessed by two-way ANOVA and Tukey’s post hoc test
T cell depletion improves age-related metabolic dysfunction
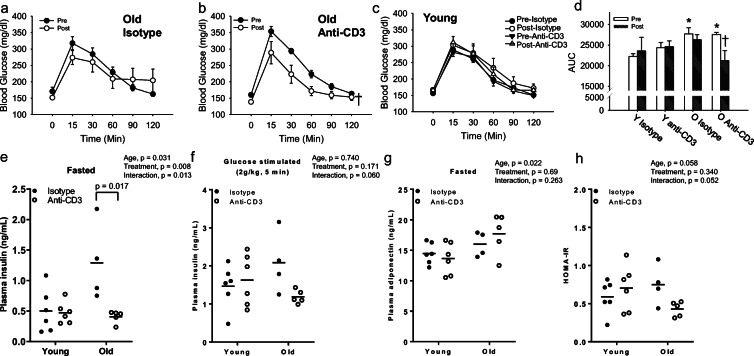
To gain insight into the role of T cells in age-related metabolic dysfunction, intraperitoneal GTTs were performed before and after anti-CD3 treatment. Aging was associated with glucose intolerance as evidenced by higher blood glucose concentrations during the GTT (Fig. 8a–d). As expected, we did not find any difference in pre-treatment glucose tolerance between treatment groups regardless of age (Fig. 8a–c). Anti-CD3 treatment improved glucose tolerance in old mice (Fig. 8b, d), but this treatment regimen did not alter glucose tolerance in young mice (Fig. 8c, d). Isotype control treatment did not impact glucose tolerance in either young or old mice (Fig. 8a, c, d). The improvement in glucose tolerance in old anti-CD3-treated mice occurred in the absence of changes in either body mass or eWAT mass (Table 1).
Fig. 8.
Effects of T cell depletion on metabolic parameters. a, b, and c Blood glucose response curves during glucose tolerance tests (GTTs) of young (Y) and old (O) isotype control and anti-CD3 F(ab’)2-treated mice shown pre- and post-treatment period. d GTT area under the curve in young and old isotype control and anti-CD3 F(ab’)2-treated mice. Data are shown as mean ± SEM, *p < 0.05 vs. young. †p < 0.05 vs. isotype old control (N, young = 12/group, old = 11/group). e, f Plasma insulin after 6-h fasting and 5 min after intraperitoneal injection of glucose. g Fasting plasma fasting adiponectin. h HOMA-IR, calculated from fasted blood glucose and plasma insulin concentrations. N = 5–8/group. Group differences in GTT data were assessed by repeated measures ANOVA. Other group differences were assessed by two-way ANOVA test and Tukey’s post hoc test
Plasma insulin concentrations under fasted and glucose-stimulated conditions and the insulin-sensitizing hormone, adiponectin, were measured as an indicator of insulin sensitivity. Fasted plasma insulin was greater in old mice, suggestive of insulin resistance (Fig. 8e). Anti-CD3 treatment reduced fasted plasma insulin in old but not young mice (Fig. 8e). Likewise, although anti-CD3 treatment did not impact the insulin response to glucose in young mice, glucose-stimulated plasma insulin tended to be lower in anti-CD3-treated old mice (p = 0.060) compared to isotype control-treated age-matched mice (Fig. 8f). Plasma adiponectin was not altered by either age or anti-CD3 treatment (Fig. 8g). Lastly, we calculated the HOMA-IR, a measure of peripheral insulin resistance, and we found no difference in insulin resistance between young T cell-depleted mice and isotype control, whereas T cell-depleted old mice demonstrated a reduction (p = 0.052) in insulin resistance compared to isotype control (Fig. 8h). Taken together, these findings suggest that anti-CD3 treatment reverses age-related glucose intolerance and peripheral insulin resistance.
Improved glucose homeostasis is associated with reduced gluconeogenic gene expression and enhanced insulin signaling
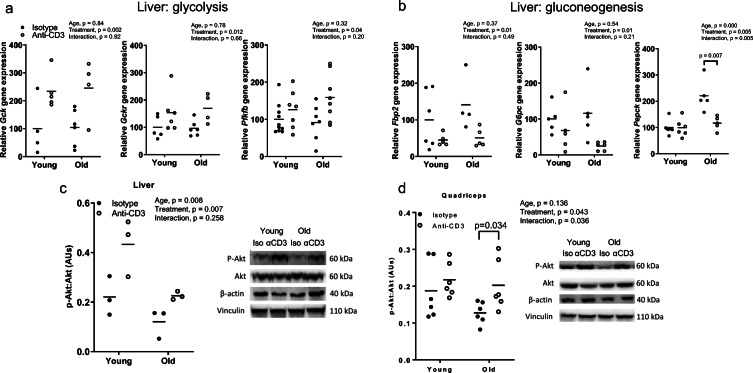
To begin to assess the mechanisms by which anti-CD3 treatment improves glucose tolerance, we assessed gene expression in the eWAT, liver, and quadriceps. Transcript-level expression of glycolytic genes (Gck, Gckr, Pfkfb), but not insulin receptor substrates (Irs1, Irs2), were higher in the liver from anti-CD3-treated mice for both age groups (Fig. 9a and OS Fig. 5b). Expression of gluconeogenic genes (Pepck Fbp2, G6pc) was significantly lower in the liver from old anti-CD3-treated mice; whereas there was no significant effect of treatment in young mice (Fig. 9c). To assess the impact of reduced Pepck expression on hepatic lipid deposition, we measured hepatic total triglycerides content. Hepatic triglycerides did not exhibit any age-related or anti-CD3-induced alterations (OS Fig. 5a). Expression of Irs1 and Irs2 were significantly higher in quadriceps, but no effect was found on glycolytic gene (Hk2, Pfkfb) expression, in anti-CD3-treated mice for both age groups (OS Fig. 5c). Expression of Pfkfb, Hk2, Irs1, and Irs2 in eWAT were not different with age or anti-CD3 treatment (OS Fig. 5d).
Fig. 9.
Expression of metabolic genes and Akt phosphorylation in the liver and muscle. Glycolytic (a) and gluconeogenic (b) gene expression in the liver. Summary data and representative images of Akt phosphorylation in the (c) liver and (d) quadriceps. Phosphorylation of Akt was normalized to total Akt. Beta actin and vinculin were used as additional control. AU, arbitrary units. Group differences were assessed by two-way ANOVA test and Tukey’s post hoc test
We next assessed serine-473 phosphorylation of Akt in the liver and quadriceps by western blotting, as a measure of peripheral insulin signaling. Aging resulted in a blunted ratio of phosphorylated to total Akt in the liver but not quadriceps (p = 0.136, Fig. 9c, d); anti-CD3 treatment increased this ratio in both of these tissues in old mice (Fig. 9c, d), suggesting an improvement in insulin sensitivity.
Discussion
The primary findings of this study are as follows. (1) Aging is associated with increased T cell infiltration in the eWAT, liver, and skeletal muscle but not scWAT or BAT. (2) Age-related T cell infiltration of the eWAT and liver are primarily driven by T cells of an effector memory and proinflammatory phenotype. (3) Treatment of old mice with anti-CD3 F(ab’)2 fragments resulted in the depletion of T cells in the eWAT and liver but not skeletal muscle. (4) Anti-CD3 treatment did not alter the eWAT, liver, or skeletal muscle B cell or macrophage infiltration in old mice. (5) Anti-CD3 treatment improved glucose tolerance as well as reduced plasma insulin in old mice. (6) Anti-CD3 treatment resulted in gene expression changes consistent with decreased gluconeogenesis in the liver and improved insulin action in the skeletal muscle. (7) Anti-CD3 treatment resulted in increased Akt signaling in the liver and skeletal muscle of old mice. Collectively, these results suggest that T cells play a major role in age-associated metabolic impairments in multiple metabolically active tissues.
Inflammation and immune cell infiltration of the adipose and liver (although less well described) are key contributors to obesity-associated insulin resistance and metabolic dysfunction [10, 12, 18, 24, 25]. Both macrophages and T cells have been implicated in driving obesity-associated inflammation and the associated insulin resistance [12, 25]. Insulin resistance also occurs with advanced age [26, 27], but it is less clear whether immune cells play a role in this dysfunction.
In this investigation, we found that the eWAT, liver, and skeletal muscle, but not scWAT and BAT, of old animals exhibited greater numbers of T cells and a blunted CD4-to-CD8 ratio compared to young controls. This observation appears to be at least partially driven by the systemic, age-associated, increase in the proportion of CD8+ cells and the resultant lower CD4-to-CD8 ratio observed here and by others [28]. In mesenteric visceral adipose tissue, we have also found a similar age-related increase in T cell counts and lower CD4-to-CD8 ratio [29]. The increase in T cell numbers with age in the eWAT and liver is in accordance with previous observations [14, 23, 30]. In contrast to our observation here, that aging is associated with increased infiltration of eWAT by CD8+ T cells, these studies found an increase in total CD4+ and CD4+ Tregs cells without an increase in CD8+ T cells in the eWAT [14, 23]. In the eWAT, we also found a large population of CD4- and CD8- DN cells largely absent in the eWAT from young animals and the mesenteric adipose of old mice [29]; these DN T cells may represent γδ T cells which may also participate in adipose inflammation [31]. In addition, we found that aging is associated with greater numbers of CD4+ effector memory cells and a greater proportion and number of CD8+ effector memory cells in the eWAT. These effector memory cells play a critical role in obesity-associated metabolic dysfunction [32]. Interestingly, we found that aging was not associated with an alteration in the proportion of IFN-γ- and TNF-α-producing cells in the eWAT; however, we found significant accumulation of IFN-γ- and TNF-α-producing cells of both CD4+ and CD8+ subsets. This observation suggests that bulk recruitment of T cells rather alterations in cytokine production of T cells drives age-related inflammation of the adipose tissue. We also found an increased proportion and accumulation of Tregs in the eWAT. This is in accordance with observations of others in middle-aged mice [14, 15].
Our observation that the liver exhibits age-related immune cell infiltration is in agreement with others [30]. We found increases in both CD4+ and CD8+ but not double-negative T cells in the liver. Both the proportion and numbers of CD4+ and CD8+ effector memory T cells were greater in the livers from old mice. In addition to adipose-resident effector memory cells, hepatic CD8+ effector memory T cells play an important role in glucose intolerance in diet-induced obesity [18]. We also found that a greater proportion of liver CD4+ cells produced IFN-γ and that there was an accumulation of total CD8+ cells that produce IFN-γ and TNF-α in the livers of old mice. In contrast to adipose, we did not observe an age-related increase in Treg proportion or total cell number in the liver. We also found that liver Tregs were substantially less abundant when compared to eWAT regardless of age. Collectively, these observations suggest that the phenotype of T cells that mediate age-related metabolic dysfunction may differ between tissues.
We also observed greater CD4+ and CD8+ infiltration in the quadriceps of old mice compared to young. The majority of investigations in the interaction of muscle and immune system with age have focused on the regenerative capacity of the muscle and sarcopenia [33–35] rather than metabolic function. Here we show that independent of injury, aging is associated with greater skeletal muscle T cell infiltration.
To determine whether T cells directly mediate age-related glucose intolerance, we treated old mice with anti-CD3 F(ab’)2 fragments with a similar regimen shown to decrease T cells systemically [20]. We observed that anti-CD3 treatment resulted in lower total, CD4+, CD8+, and DN T cells in the eWAT; lower total and CD4+ counts in the liver; and no alteration in quadriceps compared to controls. Likewise, anti-CD3 treatment resulted in decreased counts of effector memory CD4+ and CD8+ T cells in eWAT and CD4+ effector memory counts in the liver. Notably, effector memory cells, particularly CD8+ effector memory cells, were almost completely absent in both the eWAT and liver of young animals. This observation combined with the role of effector memory cells in diet-induced metabolic dysfunction [18, 32] suggests that effector memory cells may play a key role in age-related visceral adipose inflammation. Although reports have implicated eWAT Tregs in metabolic abnormalities in middle-aged mice [14, 15], our observations that (a) there are greater eWAT effector memory and inflammatory cytokine-producing CD8+ cells with age and (b) that pan T cell depletion improved glucose tolerance in old mice suggest that multiple T cell subtypes may play a role in age-related metabolic dysfunction. In contrast to the eWAT, in the liver, anti-CD3 treatment primarily decreased CD4+, rather than both T cell subsets. These observations suggest that T cell subtype that mediates age-related metabolic dysfunction may differ between tissues. The mechanistic roles of these individual cell types in the adipose and liver in age-related metabolic dysfunction requires further elucidation.
Contrary to our expectations, we did not observe a difference in quadriceps T cell infiltration with anti-CD3 treatment. This may be due to relatively low skeletal muscle T cell numbers at baseline (approximately 100-fold lower than the liver and 2-fold lower than WAT per gram of tissue), limiting the ability to detect small changes. Alternatively, as the best described role of T cells in the skeletal muscle is injury repair [33], absent traumatic injury, the turnover from tissue to circulation of skeletal muscle-resident T cells may be low, limiting the exposure of skeletal muscle-resident T cells to circulating anti-CD3 antibody fragments and preserving their numbers compared to other tissues. We also found that although a greater proportion of gastrocnemius-infiltrating T cells produce IFN-γ with age, this did not lead to increased total numbers of IFN-γ-producing T cells. It should be noted that due to limited amounts of tissue, we assessed naïve/memory phenotype and Tregs in the quadriceps, and T cell cytokine production in the gastrocnemius and heterogeneity between these two muscles cannot be ruled out. Overall, with relatively lower T cell accumulation and a lack of a depletion effect with anti-CD3 treatment in the muscle, the mechanism by which aged T cell might alter skeletal muscle metabolic function remains to be determined.
An anti-CD3 treatment regimen similar to that employed here has been shown to improve glucose tolerance in a diet-induced obesity model [12]. In that study, the authors found that anti-CD3 treatment increased the proportion of eWAT CD4+ Tregs and anti-inflammatory macrophages [12]. In this investigation, we found that improvements in glucose tolerance in old mice are independent of changes in the eWAT, liver, or skeletal muscle B cell or macrophage number or phenotype. It should be noted that others have demonstrated a role for macrophages and B cells in age-related metabolic dysfunction [36, 37], so a role for these cells cannot be completely ruled out. Interestingly, age-related adipose tissue B cell infiltration mediates metabolic dysfunction in female mice, and old female mice exhibit approximately 10-fold greater adipose tissue B cell infiltration compared to that observed in males [37]. In the current investigation, male mice exhibited improvements in glucose tolerance independent of tissue B cell infiltration. Together these findings suggest that immune-mediated alterations in metabolic function may be sex-specific and underscores the need for future study. We also found that anti-CD3 treatment did not alter age-related increases in plasma IL-1β and IFN-β, both of which have been shown to play a role in glucose intolerance [18, 38, 39]. This observation suggests that T cells affect tissues locally to drive age-related glucose intolerance.
Despite the fact that multiple biochemical and physiological processes are involved in the etiology of impaired glucose tolerance, reduced glucose uptake by the liver and skeletal muscle as well as elevated gluconeogenesis in the liver are key features [3, 40]. To begin to assess how T cells may act in metabolic tissues to impair glucose tolerance with age, we assessed gene expression in the eWAT, liver, and skeletal muscle. Neither aging nor anti-CD3 treatment altered the gene expression of glycolytic genes in the eWAT. In contrast, quadriceps of old anti-CD3-treated mice exhibited greater glycolytic gene expression when compared to old controls. Further, expression of genes related to gluconeogenesis were blunted in the liver of old anti-CD3-treated mice; this is in agreement with studies demonstrating that genetic deletion of T cells results in similar alterations of gluconeogenic gene expression in the livers of obese mice [18]. In addition to its role in gluconeogenesis, phosphoenolpyruvate carboxykinase has been shown to be a critical regulator of hepatic triglyceride metabolism [41]. However, we did not find any age-related or anti-CD3-dependent changes in hepatic triglyceride content, suggesting that reduced Pepck expression observed in anti-CD3-treated old mice contributes to improved glucose tolerance through blunted gluconeogenesis and that hepatic lipid metabolism is not altered by anti-CD3 treatment.
We also found that anti-CD3 treatment altered serine-473 phosphorylation of Akt in the liver and skeletal muscle, two tissues primarily responsible for maintaining systemic glucose homeostasis. We found that Akt phosphorylation was greater in both the liver and skeletal muscle in old anti-CD3-treated mice compared to controls, suggesting improved insulin signaling with anti-CD3 treatment. In the quadriceps, improvements in insulin signaling with anti-CD3 treatment were independent of muscle T cell infiltration. It is intriguing that anti-CD3 treatment did not result in lower T cell number in quadriceps despite lower cell numbers in most other tissues. The finding that insulin signaling was improved in the skeletal muscle independent of muscle T cell infiltration suggests that there is important cross-talk between tissues that might mediate this improvement. This concept is supported by a recent investigation that found that T cell-derived cytokines can directly blunt skeletal muscle insulin sensitivity during acute infection [19].
There are some limitations to the present study. The assay we employed to measure adiponectin measures total adiponectin, whereas the high-molecular-weight isoform of adiponectin has been shown to a better predictor of insulin sensitivity [42]. However, circulating plasma levels of high-molecular-weight and total adiponectin parallel each other in mice [43]. Because total adiponectin levels were not altered with age or anti-CD3 treatment in our study, it is unlikely that changes in the high-molecular-weight adiponectin mediate the improvements in glucose tolerance observed in old mice with T cell depletion, although we cannot completely exclude this possibility. We examined serine-473 phosphorylation of Akt, as a measure of tissue insulin sensitivity; this is an indirect index of insulin signaling when compared to threonine-308 phosphorylation or phosphorylation of the insulin receptor itself. Therefore, future studies are required to further elucidate the role of T cells on the insulin signaling cascade in aging tissues.
The major finding of this investigation, that anti-CD3 treatment improves glucose tolerance in old mice, demonstrates that T cells play a critical role in age-related metabolic dysfunction. This improvement was associated with reduced eWAT and liver, but not skeletal muscle and T cell infiltration, and was independent of changes in the eWAT, liver, and skeletal muscle B cell or macrophage number. Lastly, anti-CD3 treatment altered pathways that regulate glucose metabolism in the liver and skeletal muscle but not eWAT. Future studies aimed at determining the precise T cell subtype involved in age-related glucose intolerance and the nature of immune cell/tissue interaction of key metabolic tissues will further elucidate this novel mechanism of age-related glucose intolerance.
Supplementary Information
(PDF 205 kb)
Funding
This work was supported by the National Institutes of Health grants, K01 AG061271 (DWT), R01 AG060395 (AJD), and R01 AG048366 (LAL), and the Veteran’s Affairs Merit Review Award I01 BX004492 (LAL) from the US Department of Veterans Affairs Biomedical Laboratory Research and Development Service.
Declarations
Disclosures
None.
Disclaimer
The contents do not represent the views of the U.S. Department of Veterans Affairs, the National Institutes of Health, or the US government.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Daniel W. Trott and Md Torikul Islam contributed equally to this work.
References
- 1.Brewer RA, Gibbs VK, Smith DL., Jr Targeting glucose metabolism for healthy aging. Nutrition and Healthy Aging. 2016;4(1):31–46. doi: 10.3233/NHA-160007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chia CW, Egan JM, Ferrucci L. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circ Res. 2018;123(7):886–904. doi: 10.1161/CIRCRESAHA.118.312806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23(7):804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rines AK, Sharabi K, Tavares CD, Puigserver P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat Rev Drug Discov. 2016;15(11):786–804. doi: 10.1038/nrd.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, et al. Endotext. 2000.
- 6.Palmer AK, Kirkland JL. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp Gerontol. 2016;86:97–105. doi: 10.1016/j.exger.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frasca D, Blomberg BB, Paganelli R. Aging, obesity, and inflammatory age-related diseases. Front Immunol. 2017;8:1745. doi: 10.3389/fimmu.2017.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 9.Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13(6):465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- 10.Lesniewski LA, Hosch SE, Neels JG, de Luca C, Pashmforoush M, Lumeng CN, Chiang SH, Scadeng M, Saltiel AR, Olefsky JM. Bone marrow-specific Cap gene deletion protects against high-fat diet-induced insulin resistance. Nat Med. 2007;13(4):455–462. doi: 10.1038/nm1550. [DOI] [PubMed] [Google Scholar]
- 11.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 12.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15(8):921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: Links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda, Md) 2017;32(1):9–19. doi: 10.1152/physiol.00012.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bapat SP, Myoung Suh J, Fang S, Liu S, Zhang Y, Cheng A, Zhou C, Liang Y, LeBlanc M, Liddle C, Atkins AR, Yu RT, Downes M, Evans RM, Zheng Y. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528(7580):137–141. doi: 10.1038/nature16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu D, Wong CK, Han JM, Orban PC, Huang Q, Gillies J, et al. T reg-specific insulin receptor deletion prevents diet-induced and age-associated metabolic syndrome. J Exp Med. 2020;217(8). doi: 10.1084/jem.20191542. [DOI] [PMC free article] [PubMed]
- 16.McLaughlin T, Liu LF, Lamendola C, Shen L, Morton J, Rivas H, Winer D, Tolentino L, Choi O, Zhang H, Hui Yen Chng M, Engleman E. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34(12):2637–2643. doi: 10.1161/ATVBAHA.114.304636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medrikova D, Sijmonsma TP, Sowodniok K, Richards DM, Delacher M, Sticht C, Gretz N, Schafmeier T, Feuerer M, Herzig S. Brown adipose tissue harbors a distinct sub-population of regulatory T cells. PLoS One. 2015;10(2):e0118534. doi: 10.1371/journal.pone.0118534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghazarian M, Revelo XS, Nøhr MK, Luck H, Zeng K, Lei H, et al. Type I interferon responses drive intrahepatic T cells to promote metabolic syndrome. Sci Immunol. 2017;2(10). doi: 10.1126/sciimmunol.aai7616. [DOI] [PMC free article] [PubMed]
- 19.Šestan M, Marinović S, Kavazović I, Cekinović Đ, Wueest S, Turk Wensveen T, et al. Virus-induced interferon-γ causes insulin resistance in skeletal muscle and derails glycemic control in obesity. Immunity. 2018;49(1):164-77.e6. doi: 10.1016/j.immuni.2018.05.005. [DOI] [PubMed]
- 20.Hirsch R, Bluestone JA, DeNenno L, Gress RE. Anti-CD3 F(ab')2 fragments are immunosuppressive in vivo without evoking either the strong humoral response or morbidity associated with whole mAb. Transplantation. 1990;49(6):1117–1123. doi: 10.1097/00007890-199006000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Zamarron BF, Mergian TA, Cho KW, Martinez-Santibanez G, Luan D, Singer K, DelProposto JL, Geletka LM, Muir LA, Lumeng CN. Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes. 2017;66(2):392–406. doi: 10.2337/db16-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donato AJ, Henson GD, Morgan RG, Enz RA, Walker AE, Lesniewski LA. TNF-alpha impairs endothelial function in adipose tissue resistance arteries of mice with diet-induced obesity. Am J Phys Heart Circ Phys. 2012;303(6):H672–H679. doi: 10.1152/ajpheart.00271.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lumeng CN, Liu J, Geletka L, Delaney C, Delproposto J, Desai A, Oatmen K, Martinez-Santibanez G, Julius A, Garg S, Yung RL. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187(12):6208–6216. doi: 10.4049/jimmunol.1102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zamboni M, Armellini F, Harris T, Turcato E, Micciolo R, Bergamo-Andreis IA, Bosello O. Effects of age on body fat distribution and cardiovascular risk factors in women. Am J Clin Nutr. 1997;66(1):111–115. doi: 10.1093/ajcn/66.1.111. [DOI] [PubMed] [Google Scholar]
- 27.Pascot A, Lemieux S, Lemieux I, Prud'homme D, Tremblay A, Bouchard C, Nadeau A, Couillard C, Tchernof A, Bergeron J, Despres JP. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care. 1999;22(9):1471–1478. doi: 10.2337/diacare.22.9.1471. [DOI] [PubMed] [Google Scholar]
- 28.Callahan JE, Kappler JW, Marrack P. Unexpected expansions of CD8-bearing cells in old mice. J Immunol. 1993;151(12):6657–6669. [PubMed] [Google Scholar]
- 29.Trott DW, Henson GD, Ho MHT, Allison SA, Lesniewski LA, Donato AJ. Age-related arterial immune cell infiltration in mice is attenuated by caloric restriction or voluntary exercise. Exp Gerontol. 2018;109:99–107. doi: 10.1016/j.exger.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh P, Coskun ZZ, Goode C, Dean A, Thompson-Snipes L, Darlington G. Lymphoid neogenesis and immune infiltration in aged liver. Hepatology. 2008;47(5):1680–1690. doi: 10.1002/hep.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta P, Nuotio-Antar AM, Smith CW. γδ T cells promote inflammation and insulin resistance during high fat diet-induced obesity in mice. J Leukoc Biol. 2015;97(1):121–134. doi: 10.1189/jlb.3A0414-211RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y, et al. Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest. 2016;126:4626–4639. doi: 10.1172/JCI8860610.1172/JCI88606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deyhle MR, Hyldahl RD. The role of T lymphocytes in skeletal muscle repair from traumatic and contraction-induced injury. Front Physiol. 2018;9:768. doi: 10.3389/fphys.2018.00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, Mathis D. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity. 2016;44(2):355–367. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Wehling-Henricks M, Welc SS, Fisher AL, Zuo Q, Tidball JG. Aging of the immune system causes reductions in muscle stem cell populations, promotes their shift to a fibrogenic phenotype, and modulates sarcopenia. FASEB J. 2019;33(1):1415–1427. doi: 10.1096/fj.201800973R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim IH, Xu J, Liu X, Koyama Y, Ma HY, Diggle K, You YH, Schilling JM, Jeste D, Sharma K, Brenner DA, Kisseleva T. Aging increases the susceptibility of hepatic inflammation, liver fibrosis and aging in response to high-fat diet in mice. Age (Dordr) 2016;38(4):291–302. doi: 10.1007/s11357-016-9938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camell CD, Günther P, Lee A, Goldberg EL, Spadaro O, Youm YH, et al. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab. 2019;30(6):1024-39.e6. doi: 10.1016/j.cmet.2019.10.006. [DOI] [PMC free article] [PubMed]
- 38.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, van den Berg S, Romijn J, Rensen PCN, Joosten LAB, Netea MG, Kanneganti TD. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108(37):15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol. 2018;19(10):654–672. doi: 10.1038/s41580-018-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hakimi P, Johnson MT, Yang J, Lepage DF, Conlon RA, Kalhan SC, et al. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr Metab (Lond) 2005;2:33. doi: 10.1186/1743-7075-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, Mantzoros CS, Hu FB. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149(5):307–316. doi: 10.7326/0003-4819-149-5-200809020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueno D, Masaki T, Gotoh K, Chiba S, Kakuma T, Yoshimatsu H. Cilnidipine regulates glucose metabolism and levels of high-molecular adiponectin in diet-induced obese mice. Hypertens Res. 2013;36(3):196–201. doi: 10.1038/hr.2012.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 205 kb)