Abstract
The increasingly older population in most developed countries will likely experience aging-related chronic diseases such as diabetes, metabolic syndrome, heart and lung diseases, osteoporosis, arthritis, dementia, and/or cancer. Genetic and environmental factors, but also lifestyle choices including physical activity and dietary habits, play essential roles in disease onset and progression. Sixty-five percent of Americans diagnosed with cancer now survive more than 5 years, making the need for informed lifestyle choices particularly important to successfully complete their treatment, increase the recovery from the cytotoxic therapy options, and improve cancer-free survival. This review will discuss the findings on the use of prolonged fasting, as well as fasting-mimicking diets to augment cancer treatment. Preclinical studies in rodents strongly support the implementation of these dietary interventions and a small number of clinical trials begin to provide encouraging results for cancer patients and cancer survivors.
Keywords: Cancer, Chemotherapy, Fasting, Fasting-mimicking diet
Introduction
Disease incidence increases with age and significantly contributes to mortality, and thus improved lifespan most likely reflects a reduced or delayed disease burden. Evidence that mammalian longevity can be prolonged emerged during the early 1900s, but it was not until the 1990s that caloric or dietary restriction became extensively used to delay aging and the onset of age-related diseases. Today, a plethora of studies in model organisms has demonstrated that modifications in dietary composition (e.g., protein content), caloric intake, or feeding patterns can have a major impact on the development of aging-related diseases, including cancer. In humans, diet-related overweight and obesity are well-established risk factors for some of the most prominent cancers, including breast cancer in post-menopausal women and colorectal, hepatic, pancreatic, or advanced prostate cancer [1]. Prostate cancer, the second most common malignancy in men, has an approximately sixfold higher incidence in Western than in non-Western countries, most likely due to differences in the consumed food. Furthermore, a poor prognostic outcome, higher risk for cancer recurrence, comorbidity, and disease-specific or overall mortality has been reported for the overweight. For underweight or malnourished cancer survivors (e.g., as a result of aggressive treatment modalities) on the other hand, maintaining a healthy body weight during and after treatment may prevent delayed wound healing, increase quality of life impairments, and further reduce the risk of treatment complications. Advice from healthcare professionals is often conflicting and evidence-based nutritional advice after cancer treatment remains vague. We hypothesize that cancer-preventive dietary recommendations most likely help to avoid the development of secondary malignancies.
Dietary restriction mediates longevity and stress resistance
In mammals, various forms of partial or complete food deprivation have been investigated, ranging from daily 20–40% reduced calorie intake (calorie restriction, CR), restriction of specific nutrients without affecting daily calorie intake (e.g., protein restriction), intermittent fasting (IF, including alternate day fasting, ADF), periodic fasting (PF), time-restricted feeding/eating (TRF, TRE), as well as fasting-mimicking diets (FMD). The health benefits of these interventions include an extension in the lifespan of rodents which is accompanied with a lower incidence of nearly all chronic diseases and a more youthful metabolic state [2, 3]. Notably, almost all these dietary interventions promote stress resistance which is an often accompanying feature of longevity extension in various model organisms ranging from unicellular yeast to mammals, indicating that the underlying molecular mechanisms are at least partially conserved in many species [2, 4, 5]. The varying level of efficacy in inducing stress resistance however depends on the form and severity of the implemented dietary intervention.
Caloric restriction is currently the most robust environmental intervention known to increase healthy life and prolong lifespan in several models, including rodents and monkeys [6–8]. Nearly a century of work has established that chronic 20–40% CR has profound impacts on age-related diseases including neurodegenerative disorders, autoimmune disease, cardiovascular disease, and type II diabetes mellitus and that CR reduces cancer incidence and progression in various rodent strains [2, 7, 9]. CR attenuates aging-associated shrinkage of telomeres in many mouse tissues and reduces the incidence of tumors in mice that overexpress telomerase [10]. Similarly to humans, cancer incidence increases with age in rhesus monkeys and intestinal adenocarcinoma is the most diagnosed cancer [11]. Whereas the results on lifespan extension in rhesus monkeys remain inconclusive, two different studies (one at the National Institute on Aging (NIA) and one at the Wisconsin National Primate Research Center (WNPRC)) demonstrate a reduced incidence of neoplasia in the CR-fed monkey cohorts. In an adult-onset WNPRC study, cancer incidence was reduced by 50%, whereas no neoplasia could be identified in the young-onset CR cohort in the NIA study (compared to the ~ 85% incidence in the NIA control cohort) [12, 13]. However, no significant difference in cancer incidence in an old-onset CR and control cohort was apparent, thus suggesting that early-onset CR interventions might be necessary to reduce the risk of developing cancer, at least in rhesus monkeys. Whether other old-onset fasting-based interventions may reduce cancer incidence in non-human primates has not been tested and it remains unclear if they will demonstrate differences in effectiveness. CR as a lifelong intervention might be effective in cancer prevention, but has not been explored as a treatment modality in humans. CR as a clinically relevant intervention has been largely unexplored because the long-term adherence necessary to gain these health benefits makes CR less likely feasible as a rapidly deployable approach [14–17].
It is hard to assign all the health benefits of CR to calorie reduction alone. A major component of most animal CR studies is the phenomenon that calorically restricted animals tend to consume all the food that is presented rather rapidly, thus restricting the overall time window that animals actively consume food in. Expanding research in the past decade has established that this time-restricted feeding (TRF) contributes to some of the CR benefits but also that TRF has benefits without the need for CR. In other words, providing a limited window of time in which rodents consume an otherwise unlimited amount of food is associated with improvements in health and longevity [18, 19].
Intermittent fasting (IF) is among the most studied fasting methods in rodents and consistently has been shown to promote protection against multiple diseases and lifespan extension [20–22]. IF is an eating pattern that cycles between periods of eating and fasting. There are several different IF methods, all of which split the day or week into eating periods and fasting periods. IF consists of eating a calorie-restricted diet for 1–3 days/week; an example is the popular 5:2 diet [16, 23, 24]. Alternate-day fasting (ADF) is a sub-category of IF, where days of low-calorie consumption or complete fasting alternate with days of either ad libitum food consumption or feasting (> 100% caloric intake) [23, 24].
Periodic water-only fasting (PF) consists of total food abstinence for several days, in mouse models generally 48–72 h, without limiting hydration. Benefits of PF in preclinical studies include weight loss (during the fasting period but regained after refeeding), improved insulin sensitivity, autophagy activation, cell renewal, and anticancer effects, but safety and compliance concerns may be responsible for the limited contribution of PF to standard medical practice [4, 18, 23, 25–31]. The differences between IF and PF in mice include the duration and/or frequency: IF cycles usually last up to 24 h and are separated by 24 h of food intake (caloric intake may be higher on the refeeding days to compensate for the reduced calories on the fast days). PF instead lasts 2 or more days and is followed by 1 week of normal food intake to regain normal weight [4]. Owing to this difference, the molecular changes of a variety of growth factors (including IGF-1) and metabolic markers (such as ketone bodies, serum glucose, etc.) differ significantly in their response.
Fasting-mimicking diets (FMDs) were developed to address the safety concerns associated with PF, such as low rates of compliance and malnourishment. FMDs—developed to be a short-term intervention that is low in protein, high in healthy fats, and containing complex carbohydrates, as well as essential vitamins and minerals—mimic the effects of fasting by decreasing insulin/glucose signaling pathway activation, while avoiding the potential side effects of lack of essential nutrients [32, 33]. In mice, the FMD extends median lifespan, reduces inflammation and cancer incidence, enhances cognitive performance, and improves overall health [33–36]. In a randomized crossover clinical trial, the FMD reduced risk factors for metabolic syndrome, cardiovascular diseases, cancer, and aging [32]. Additionally, and similar to other periodic fasting and meal-timing interventions, the consumption periods between the interventions can be modulated based on specific nutrition requirements, for example, an increase in protein intake to reduce the aging-associated loss of lean mass in the elderly [37].
It is important to highlight that (i) an increase in health span (induced by fasting or other anti-aging interventions) does not always associate with increased longevity [38] and (ii) it remains to be fully established if increased longevity is associated with all forms of fasting. Conversely, increased lifespan is not always associated with increased health span or delayed anti-aging symptoms [39, 40].
The underlying feature of all these dietary approaches, despite significant differences and with varying degrees, is that reduced calories, the time of food intake, the composition of the diet, or the complete abstinence of food all affect the organism’s nutrient availability. Sufficient levels of nutrients are of fundamental importance to enable organismal growth and cellular proliferation. Periods of low food availability suppress growth and instead activate evolutionary-conserved protective metabolic pathways to ameliorate the accumulation of cellular damage and to ensure reproductive fitness [41, 42]. Notably, the response to these opposing environmental conditions is regulated by overlapping pathways: nutrient abundance activates nutrient-sensing signaling cascades that promote cellular growth while nutrient scarcity downregulates these signaling pathways, thereby blocking cellular proliferation and activating stress resistance transcription factors which negatively regulate pro-aging pathways [2, 6, 43–45]. Evolutionary-conserved orthologs of the genes that regulate lifespan and stress resistance in lower eukaryotes, including S. cerevisiae and C. elegans, also regulate stress resistance and/or lifespan in mammals [46–50].
For example, deleterious mutations in the insulin/growth hormone (GH)/insulin-like growth factor 1 (IGF-1) axis that decrease intracellular nutrient-sensing activity result in an increase in lifespan by up to 150% in laboratory mice [51, 52] and cells derived from these long-lived mice have a higher resistance against H2O2-induced oxidative stress, ultraviolet light, genotoxins, and other stressors including heat and cadmium [52, 53]. Vice versa, the overexpression of GH decreases activities of the antioxidant enzymes superoxide dismutases and catalase and shortens the lifespan compared to wild-type control mice [54–56].
Fasting in cancer treatment
Aging is the major risk factor for the development of most cancers which requires the exposure to changes in the environmental niche and the combination of cellular damage and DNA mutations over time [57–59]. Thus, changing the likelihood of acquiring these mutations, or increasing the body’s ability to effectively reduce cellular damage, also decreases the incidence rates of many cancers. Numerous studies have shown that CR reduces the incidence and progression of spontaneous or induced tumors in various animal models [13, 60–66]. CR causes potent anti-growth effects in various auto- and xenograft mouse tumor models, although resistance has been observed in some cells with mutations that cause a constitutive activation of the phosphatidylinositol-3-kinase (PI3K) pathway [67]. A 20-year longitudinal adult-onset 30% CR study in rhesus monkeys decreased cancer incidence by 50% which may indicate that similar interventions could potentially be effective as a cancer-preventive intervention in humans [12]. Furthermore, CR, when combined with protein restriction, can reduce clinical markers associated with increased cancer risk [7, 68]. However, despite data indicating benefits in cancer prevention in most animal models, CR as a feasible therapy modality for humans is expected to be problematic because chronically restricting the calorie intake of cancer patients would be likely very difficult to include into existing treatment paradigms. Even more problematic, CR only delays, but does not stop, the progression of established cancers [69, 70]. The biggest concern is that chronic CR might not be feasible for cancer patients receiving chemotherapy, surgery, or immunity-based treatments, or who are at risk for losing weight, as well as frail and/or cachectic patients, because CR induces long-term weight loss, may delay wound healing, and impairs immune function [2, 71–73].
To overcome the disadvantage of requiring long-term interventions, various laboratories deployed variations of fasting bouts. Unlike CR, the PF approach utilizes relatively short (up to 4 days) periods of food restriction that can be easily adopted to comply with existing chemotherapy regimen and without chronic weight loss (since body weight is usually regained during the refeeding period). Additionally, due to the short time required to achieve the desired metabolic effects which delay the incidence and growth of cancer cells, fasting is expected to allow for higher rates of adherence during the intervention period. A 3-day-long PF decreases blood glucose levels in mice by up to 75% compared to the 15% reduction accomplished by long-term CR or IF [4, 74]; considering the glucose dependence of many malignant cells, this is an important advantage of PF over CR or IF [75, 76]. In addition, the pro-proliferative growth factor IGF-1 is reduced by up to 75% following PF [77, 78], while CR causes a 25% IGF-1 reduction in mice [79] and only when CR is combined with protein restriction are IGF-1 levels in humans reduced [68]. In rats, fasting for 8 days reduces pre-neoplastic liver masses by 20–30%, lowers DNA replication, but increases apoptosis and thus reduced the number and volume of putative pre-neoplastic liver foci by 85% throughout the following 17 months in rats [80]. Despite these benefits, safety and compliance concerns associated with PF (usually voiced by the treating medical professionals) are limiting the use contribution of PF in the clinic [4, 18, 23, 25–31]. The FMD has been developed to overcome the PF-associated side effects while maintaining its benefits and consists of a 4–5-day lasting plant-based meal plan that provides between 10% and 50% of the recommended daily calories and is low in protein and sugar, but relatively high in fat content [32, 33].
Prospectively, the role of dietary restriction against cancer initiation, progression, or development of metastasis in mouse models has been studied extensively and generally supports an anticancer role [81, 82]. However, the field should also continue to pay close attention to those studies that did not report any protection, as, for example, seen in spontaneous breast and prostate tumor development [83–86], or colorectal cancer [87]. It is paramount to understand how to time a PF relative to any possible intervention since the refeeding phase following a fast is associated with increased cellular proliferation in the liver and colorectal epithelium [33, 88]. Therefore, exposure to carcinogens during the refeeding period can enhance the growth of mammary tumors or aberrant crypt foci in the colorectal mucosa even with otherwise non-carcinogenic doses [89–91]. It is important that food intake after any PF cycle should be initiated only after the half-life of potential carcinogens. Whether the same is true for FMD remains currently unknown but, given the similar effects to PF, the same recommendations should be considered.
Differential stress resistance
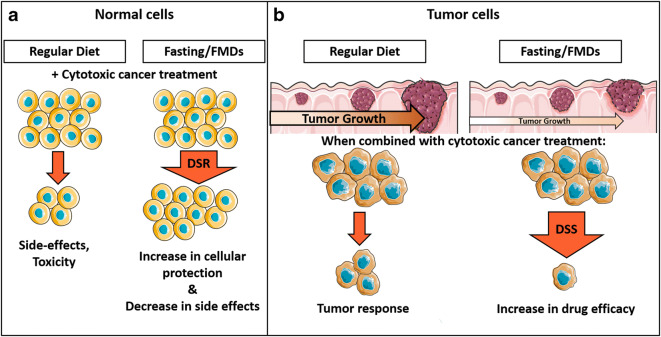
The nutrient-modulated connection between cellular proliferation and stress resistance is the foundation for a differential protection of normal cells from toxicity under fasting conditions. PF reduces circulating IGF-1 and glucose levels and inactivates evolutionary-conserved nutrient-sensing signaling cascades, including mTOR and PKA, in normal cells and thereby induces the resistance to oxidative and other stresses (Fig. 1A). Studies indicate that bouts of fasting have potent protective effects against many cytotoxic insults, ranging from chemotherapeutics to multifactorial surgical stress. For example, fasting has protective effects against oxidative stress-induced ischemia reperfusion injury in rodents [92–95]. Fasting ameliorates traumatic brain injury by inducing neuroprotective effects, reduced oxidative damage, and improved cognitive function [96].
Fig. 1.
Fasting or fasting-mimicking diets improve chemotherapy efficacy and protect normal cells against cytotoxic side effects. (A) A nutrient-rich environment promotes cellular growth of normal cells, making them susceptible to the cytotoxic effects of many chemotherapeutic agents. Fasting or fasting-mimicking diets (FMDs) decrease IGF-I and glucose and result in reduced signaling through the intracellular nutrient-sensing cascade, thereby halting cellular growth but promoting a differential stress resistance (DSR). The activation of intracellular stress resistance pathways decreases cytotoxicity and drug treatment–related side effects. (B) In a well-nourished environment, nutritional requirements for unregulated cellular proliferation are met and tumor growth is supported. Treating the cancer with chemotherapeutic drugs yields the expected response and the tumor mass shrinks. Fasting or FMDs greatly limit the availability of metabolites required to sustain the unregulated growth of malignant cells, resulting in reduced cancer growth or shrinking of the tumor. Combining chemotherapy with fasting/FMDs is associated with two beneficial scenarios: (1) fasting/FMDs induces the activation of protective response in normal cells, whereas mutations in cancer cells prohibit the activation of the cellular stress response; (2) fasting/FMDs sensitizes malignant cells to chemotherapy treatment and thereby increases treatment efficacy, referred to as differential stress sensitization (DSS)
The differential stress resistance (DSR, Fig. 1 A) hypothesis is based on the fact that in response to nutrient-scarce environmental conditions, normal cells reduce proliferation; optimize their metabolism to utilize metabolites generated from the breakdown of fats, proteins, and organelles; and induce stress response pathways [97, 98]. Due to the acquired self-sufficiency caused by mutations in oncogenes (e.g., Ras, Akt, mTOR) and tumor suppressor genes (e.g., Rb, p53, PTEN) that enable proliferation even in an environment that does not support growth, cancer cells do not enter a non-dividing state [99]. Thus, DSR is a dietary intervention strategy that increases the cellular resistance against cytotoxic insults, such as chemotherapy or other drugs, in normal cells without affecting the efficacy of these agents against malignant cells [98]. In vivo, 60–72 h of fasting prior to chemotherapy protects various mouse strains from a lethal dose of etoposide [98, 100]. Similarly, DSR by fasting protects CD-1 mice from high-dose doxorubicin [78] and reduces the delayed-type chemotherapy-induced nausea and vomiting in cancer-bearing dogs receiving doxorubicin [101]. Fasting protects FabplCre;Apc15lox/+ mice against irinotecan-induced weight loss, reduced activity, diarrhea, and leukopenia [102]. Thus, fasting prior to chemotherapy allows for the use of higher doses of chemotherapeutic agents which may increase treatment efficacy in otherwise hard-to-treat situations. Examples of this may include advanced metastatic tumors that are extremely difficult to cure once tumor masses have spread to different organs or a change in limitations of drugs that have a lifetime cumulative dosage. For example, doxorubicin is limited to a cumulative dose of < 550 mg/m2 to reduce the risk of cardiotoxicity [103]. Analogously to the fasting-induced reduction in circulating IGF-1 levels, a conditional liver-specific igf-1 gene deletion (LID) in transgenic mice results in a 70–80% reduction in circulating IGF-1 levels and these mice are protected against high-dose cytotoxic chemotherapy drugs such as cyclophosphamide, doxorubicin, and 5-fluorouracil [78]. In particular, the hearts of doxorubicin-treated control mice showed loss of myofibrils and infiltration of immune cells, whereas DXR-dependent cardiac myopathy was not observed in LID mice [78]. On the contrary, increased levels of IGF-1 reverse cancer prevention by stimulating cell proliferation and inhibiting apoptosis and restoring IGF-1 to normal levels during fasting reverse the protection against lethal doses of doxorubicin [78]. In addition to the reduction in IGF-1 levels, serum glucose levels also play an important role in the fasting-mediated DSR effects and reducing glucose levels protecting mice against chemotoxicity [104]. Yet drugs that promote hyperglycemia, including dexamethasone and rapamycin, are commonly administered to help with the management of chemotherapy-related adverse effects in cancer patients even though they have been shown to sensitize cardiomyocytes and mice to doxorubicin [104]. This increase in toxicity can be reversed by combining dexamethasone or rapamycin treatment with fasting or insulin. Glucose injections alone reverse the fasting-dependent protection against DXR in mice, indicating that elevated glucose mediates, at least in part, the sensitizing effects of rapamycin and dexamethasone [104]. The effects of fasting or glucose restriction are mediated by PKA and AMP-activated protein kinase (AMPK) by activating the mammalian Msn2/4 ortholog early growth response protein 1 (EGR1).
The protective effect of fasting is partly mediated by changes to the stem cell population. Fasting before high-dose etoposide treatment preserves the architecture and barrier function of the small intestine by maintaining the viability of crypt stem cells [100]. PF reduces the immunosuppression and mortality of the chemotherapeutic drug cyclophosphamide through changes in the signal transduction pathways of long-term hematopoietic stem cells and niche cells to promote self-renewal and lineage-balanced regeneration [105], in agreement with preliminary findings on the PF-induced protection of lymphocytes from chemotoxicity in cancer patients [106]. PF also promotes regenerative effects in the blood, liver, muscle, and nervous system, indicating that fasting stimulates the regeneration of healthy cells and tissue following tissue damage after chemotherapy [33, 105]. Chemotherapy combined with radiation therapy is a commonly used treatment approach for locally advanced pancreatic cancer, yet curative radiation doses in this disease setting are limited due to the close proximity of the pancreas to the duodenum. Fasting in mice improves intestinal stem cell regeneration, as revealed by microcolony assay, and improved host survival of lethal doses of total abdominal irradiation compared with fed controls [107].
Differential stress sensitization
The constitutive activation of pro-proliferative pathways plays a central role in promoting cancer growth and survival. The Ras and Akt protein are central mediators within the nutrient-sensing pathways and are frequently constitutively activated in many cancerous cells [99, 108, 109]. Cancer cells develop in a highly nourished environment and usually experience an almost unlimited nutrient supply; thus the reduced availability of glucose, amino acids, and other metabolites during fasting presents a significant disadvantage to tumor cells. Malignant cells are commonly characterized by high rates of glucose uptake and rely on glycolysis (Warburg effect) to provide them with energy and biosynthetic precursors essential for proliferation [75] and allow tumor cell survival and apoptosis evasion through decreased respiration and the restriction of cytochrome c–induced apoptosis [110, 111]. Glucose metabolism alone only insufficiently supplies the necessary building blocks for cellular proliferation and malignant cells, therefore requiring amino acid uptake as a nitrogen source [75]. Consequently, protein/amino acid restriction delays the onset and progression of various cancer types and increase longevity [37, 112, 113].
Importantly, the acquired mutations that allow unregulated cellular growth in malignant cells can be viewed as their Achilles heel since gain-of/loss-of-function mutations prohibit a response to a changing environment. For example, relying on high levels of glucose uptake in the normally nutrient-rich environment would not be disadvantageous but this scenario changes quickly when glucose is restricted. In another example, expression of the oncogene-like RAS2val19 in yeast reverses the fasting-induced DSR protection against hydrogen peroxide. Even more importantly, RAS2val19 expression sensitizes yeast cells to cytotoxic stressors compared to wild-type cells [114]. Similar results were observed in human and rodent cancer cell lines treated with doxorubicin and/or cyclophosphamide, or temozolomide in an in vitro model of fasting by reducing glucose and/or serum availability in the growth medium: compared to the unrestricted conditions, fasted cancer cells were more susceptible to chemotherapy treatment, a phenomenon termed differential stress sensitization (DSS; Fig. 1B) [114, 115]. In human mesothelioma and lung carcinoma cells, serum starvation alone sensitizes to cisplatin treatment [116]. HepG2 and Huh-7 human hepatocellular carcinoma cell lines show additive cytotoxic effects in both cell lines when combining the kinase inhibitor sorafenib with fasting-like conditions [117].
In vivo, water-only fasting cycles (lasting 48–72 h) can be as effective as chemotherapy in reducing the progression of subcutaneous melanoma and breast cancer models, whereas the combination of fasting with chemotherapy improves treatment efficacy [114]. Fasting combined with gemcitabine decreases pancreatic tumor progression by ≥ 40%, partially by increasing gemcitabine uptake [118]. Cisplatin in combination with fasting reduces mesothelioma progression ≥ 60% compared to the control and a complete remission is observed in 60% of the mice treated with PF/cisplatin [116]. Forty-eight hours of water-only fasting sensitizes mouse glioma models to radio- and chemotherapy [115], an effect not observed by dietary protein restriction alone [119]. Similarly, fasting when combined with radiotherapy delays the growth of an orthotopic KPC pancreatic tumor model after radiation and increases the median survival to 43 days compared to 7 days in non-fasted, irradiated tumor-bearing mice [107]. Water-only fasting alone or when combined with oxaliplatin has been shown to reduce the progression of the CT26 colorectal tumor [120]. Mechanistically, this effect was caused by downregulating aerobic glycolysis and glutaminolysis, and increasing oxidative phosphorylation in the mitochondrial electron transport chain which limits ATP production in the malignant cells, but instead, increases oxidative stress and apoptosis [120]. In murine 4T1 breast cancer cells, water-only fasting increases oxidative stress, caspase-3 cleavage, DNA damage accumulation, and apoptosis [114]. The fasting-induced sensitization of mesothelioma cells to cisplatin is mediated by the AMPK-dependent activation of the ATM/Chk2/p53 signaling cascade [116]. In 4T1 breast cancer and B16 melanoma cells, fasting causes the SUMO2/3-dependent sumoylation of the DNA polymerase REV1, resulting in the release of p53 and pro-apoptotic gene expression and the induction of apoptosis in these cells [121]. Many oncogenes are tyrosine kinases and thereby provide a target for cancer treatment. In in vitro and xenograft models, PF potentiates the growth-inhibiting efficacy of tyrosine kinase inhibitors by inhibiting MAPK signaling and the E2F-dependent transcription [122]. In a subcutaneous non-small cell lung cancer xenograft model, crizotinib or fasting effectively reduced tumor progression but the combination of PF with crizotinib was more efficient than both singular treatment options. Similar results have been demonstrated in a colorectal cancer xenograft model for the tyrosine kinase inhibitor regorafenib [122]. In a metastatic mouse neuroblastoma model, 48 h of water-only fasting followed by the single administration of a high-dose chemotherapy cocktail (doxorubicin and cisplatin) successfully reduces drug toxicity and metastases and results in long-term cancer-free survival [114], thus showing that dose escalation approaches which utilize both DSR and DSS might be a feasible intervention to treat otherwise hard-to-manage metastasized cancers.
Fasting and fasting-mimicking diets induce similar changes in the body’s metabolic environment [33] and thus are predicted to have similar effects in cancer treatment. This was confirmed by a study showing that the FMD, alone or in combination with the chemotherapeutic drugs doxorubicin or cyclophosphamide, is as effective as fasting in reducing the tumor progression in murine breast cancer (4T1) or melanoma (B16) models [123]. Notably, in the 4T1 breast cancer model, the combination of FMD with doxorubicin increases cytotoxic CD8+ tumor-infiltrating lymphocyte numbers which is partially mediated by the stress-inducible protein heme oxygenase-1 (HO-1) and a reduced number of regulatory T cells in the tumor bed [123]. KRAS-mutant cancers may exhibit a high susceptibility to pharmacological doses of vitamin C through the formation of hydrogen peroxide and hydroxyl radicals which, in turn, cause macromolecular damage and cell death, thus, making the generally non-toxic vitamin C a potential treatment against this aggressive tumor type [124–126]. However, vitamin C’s anticancer activity is limited by the upregulation of HO-1. The FMD reverses the vitamin C–induced HO-1 upregulation, resulting in increasing concentration of reactive iron and oxygen species and subsequent cell death [127]. These effects can be even further potentiated by combining vitamin C and FMD with the chemotherapy drug oxaliplatin [127].
In MCF7 mouse models of hormone-receptor-positive breast cancer, periodic fasting as well as the FMD enhances the activity of the endocrine therapeutics tamoxifen and fulvestrant while preventing tamoxifen-induced endometrial hyperplasia. Combining fulvestrant with the cyclin-dependent kinase 4/6 inhibitor palbociclib and FMD promotes long-lasting tumor regression and even reverts the acquired resistance to drug treatment [128]. Of note, many preclinical mouse models generally utilize relatively young to middle-aged mice ranging from 4 weeks old to 6 months old and only rarely utilize old animals.
Fasting and FMDs in human cancer trials
Although numerous preclinical studies emphasize the benefits of combining fasting or FMDs with commonly used chemotherapeutic drugs or other emerging therapeutic approaches, only a limited number of clinical trials on fasting and FMDs in patients undergoing chemotherapy have been published [106, 128–134]. These limited trials support the safety and feasibility of fasting/FMDs when combined with chemotherapy (see Table 1).
Table 1.
Water-only fasting and fasting-mimicking diets in clinical trials
| Type of study | Intervention | N= | Demographic | Type of malignancy | Other treatment | Outcome | Reference | |
|---|---|---|---|---|---|---|---|---|
| Water-only fasting | Case series report | Fasting (48–140 h) prior to and/or following (5–56 h) CHX | 10 | 7 female, 3 male; median age of 61 years (range 44–78 years) | Breast (4), prostate (2), ovarian, uterine, non-small cell lung carcinoma, and esophageal adenocarcinoma (1 each) | Docetaxel, cyclophosphamide, carboplatin, 5FU, carboplatin, paclitaxel, gemcitabine, doxorubicin | Reduction in chemotherapy-associated adverse events (CTCAE) | Safdie et al. 2009 [106] |
| Randomized intervention (NCT01304251) | Fast 24 h before and after CHX | 13 | 13 female; median age of 52 years (range 44–69 years) | HER2-negative, stage II/III breast cancer (13) | (Neo)-adjuvant docetaxel, doxorubicin, cyclophosphamide | Fasting was well tolerated and reduced hematological toxicity of CHX in HER2-negative BC patients; faster recovery of DNA damage in PBMCs | de Groot et al. 2015 [130] | |
| Randomized dose escalation (NCT00966364) | Fasting before CHX for 24, 48, and 72 h (divided as 48 pre-chemo and 24 post-chemo) | 20 | 17 female, 3 male; median age of 61 years (range 31–75 years) | Urothelial (6), breast (5), non-small cell lung carcinoma (1), ovarian (6), uterine (2) | Gemcitabine, cisplatin, carboplatin, paclitaxel, trastuzumab | Safety and feasibility criteria met; reduced DNA damage in leukocytes from subjects who fasted for ≥ 48 h | Dorff et al. 2016 [129] | |
| Fasting-mimicking diet | Randomized crossover (NCT02158897) | 5-day FMD | 100 | 63 female, 37 male; median age of 42 years | - | - | Safety and feasibility; reduced markers/risk factors for cancer | Wei et al. 2017 [33] |
| Randomized crossover (NCT02158897) | < 400 kcal 36–48 h before and 24 h after the end CHX | 34 | 34 female; median age of 51 years (range 28–69 years) | Breast (30), ovarian (4) | Docetaxel, paclitaxel, carboplatin, cyclophosphamide, epirubicin, doxorubicin, methotrexate, fluorouracil + IgG1 antibody bevacizumab, pertuzumab, or trastuzumab | Tolerability; improved quality of life and fatigue during CHX | Bauersfeld et al. 2018 [132] | |
| Randomized phase II (NCT03595540, NCT03340935) | 5-day FMD | 36 | 36 female; median age of 51 years (range 37–73 years) | Metastatic HR+ breast cancer (36). | Fulvestrant, palbociclib, exemestane, trastuzumab, pertuzumab, letrozole, GnRH agonist, abemaciclib, tamoxifen, everolimus | Tolerability; improved quality of life; partial clinical response | Caffa et al. 2020 [128] | |
| Multi-center, randomized phase II (NCT02126449) | 4-day FMD (3 days prior to and on the day of CHX) | 131 | 131 female; median age of 50 years (range 27–71 years) | HER2-negative stage II/III breast cancer (131) | Doxorubicin, cyclophosphamide, docetaxel, 5FU, epirubicin + dexamethasone | Radiologically complete or partial response, Miller and Payne 4/5 pathological response occurs more often in patients using the FMD | de Groot et al. 2020 [134] |
In a case series, 10 cancer patients (4 breast, 2 prostate, 1 ovarian, 1 uterus, 1 lung, and 1 esophageal cancer) with a median age of 61 years fasted for up to 140 h before and/or up to 56 h following chemotherapy [106]. The patients, who received an average of 4 cycles of various chemotherapy drugs in combination with fasting, self-reported no major side effects caused by fasting other than hunger and lightheadedness. Six patients who underwent chemotherapy with and without fasting reported a significant reduction in fatigue, weakness, and gastrointestinal adverse events while fasting on chemotherapy compared to chemotherapy alone. Additionally, fasting did not prevent chemotherapy-induced reductions in tumor volume or in tumor markers in those patients in which cancer progression could be assessed [106]. In a Dutch study, 7 women with HER2-negative, stage II/III breast cancer receiving neoadjuvant (taxotere, adriamycin, and cyclophosphamide) chemotherapy water-only fasted for 24 h before and after beginning chemotherapy compared to 6 women who were advised to eat according to the guidelines for healthy nutrition [130]. The short-term fast was well tolerated, and the mean erythrocyte and thrombocyte counts were higher in the fasted cohort than in the non-fasted group 7 days after chemotherapy. γ-H2AX, a marker of DNA damage, levels in leukocytes were increased 30 min after chemotherapy in non-fasted patients but not in fasted patients [130]. Twenty patients primarily treated for either urothelial, ovarian, or breast cancer with platinum-based chemotherapy were randomized to fast for 24, 48, or 72 h (divided as 48 h before chemotherapy and 24 h after chemotherapy) in a dose escalation study [129]. Clinical feasibility, defined as three or more out of six subjects in each cohort consuming ≤ 200 kcal per day during the fast period without excess toxicity, was met. The most common side effects were fatigue, headache, and dizziness but were ≤ grade 2 following CTC adverse event v4.0 grading. In patients who fasted for at least 48 h, versus a 24-h fast, a trend towards less grade 3 or grade 4 neutropenia was also documented. As in the Dutch study, reduced DNA damage in leukocytes from subjects who fasted for at least 48 h compared with subjects who fasted for only 24 h could also be detected [129].
The feasibility and potential impact of the FMD in humans was first tested a pilot clinical trial in generally healthy adults [33] and then extended in a randomized clinical crossover trial in 100 healthy volunteers [32]. Subjects who followed 3 months of an unrestricted diet were compared to subjects who consumed the FMD for 5 consecutive days per month for 3 months. The FMD resulted in reduced body weight, as well as trunk and total body fat, lowered blood pressure, and decreased IGF-1 and blood glucose levels. In a post hoc analysis in those subjects that completed 3 FMD cycles, body mass index, fasting glucose, IGF-1, triglycerides, total and low-density lipoprotein cholesterol, and C-reactive protein were more beneficially affected in participants at risk for disease than in subjects who were not at risk [32]. Blood glucose did not change in participants with baseline levels ≤ 99 mg/dl but was significantly reduced in prediabetic participants with baseline fasting glucose > 99 mg/dl; notably, this reduction resulted in glucose levels within the normal range in these subjects. Although for serum IGF-1 levels no clinically relevant risk level has been established, several epidemiological studies have associated IGF-1 levels ≥ 200 ng/ml with an increased risk for various cancers [37, 135]. IGF-1 levels in subjects with baseline levels ≥ 225 ng/ml were reduced nearly four times more than the reduction observed in participants with IGF-1 concentrations below 225 ng/ml [32].
In a randomized crossover clinical trial of 34 patients with breast or ovarian cancer, the FMD effects on the quality of life and side effects in combination with chemotherapy (including taxanes (docetaxel, paclitaxel), platinum agents (carboplatin, cyclophosphamide), anthracyclines (epirubicin, doxorubicin, methotrexatate, fluorouracil), the IgG1 antibody bevacizumab, and pertuzumab or trastuzumab for patients with HER2/neu overexpression) were assessed [132]. The FMD consisted of a daily caloric intake of < 400 kcal primarily stemming from vegetable juice and small standardized quantities of light vegetable broth starting 36–48 h before the beginning of chemotherapy and lasting until 24 h after the end of chemotherapy. The FMD prevented the chemotherapy-induced reduction in quality of life without serious adverse events and it also reduced fatigue. The combination of periodic FMD and endocrine therapeutics in 36 patients with hormone-receptor-positive/HER2 breast cancer (clinical trials NCT03595540 and NCT03340935) indicates promising results: 3 patients treated in the second-line treatment setting received a total of 10 (one patient) and 8 (two patients) FMD cycles. Two of those patients demonstrated a clinically controlled disease (at time of the data publication in July 2020), whereas one of the patients that completed 8 FMD cycles progressed after 11 months; the median progression-free survival (PFS) in this clinical setting is 9 months [128]. In these trials, the FMD lowered blood glucose, serum IGF-1, leptin, and C-peptide, while increasing circulating ketone bodies, results that are in line with the mechanistic understanding obtained from preclinical studies. In the randomized, multi-center phase 2 “DIetary REstriction as an Adjunct to Neoadjuvant ChemoTherapy for HER2 Negative Breast Cancer (DIRECT)” trial, 131 patients with HER2-negative stage II/III breast cancer received either a FMD or their regular diet for 3 days prior to and during neoadjuvant chemotherapy [134]. The authors report no difference in toxicity between both study arms, despite the fact that the corticosteroid dexamethasone was omitted in the FMD group, suggesting that the FMD may obviate the need for dexamethasone in the prevention of the side effects of chemotherapy. In the FMD arm, a radiologically complete or partial response occurred more often and the per-protocol analysis reveals that the Miller and Payne 4/5 pathological response (indicating 90–100% tumor cell loss) was more likely to occur in patients using the FMD [134]. Per-protocol analyses also indicates better emotional, physical, cognitive, and social functioning scores as well as lower fatigue, nausea, and insomnia symptom scores for patients adhering to the FMD in comparison with non-adherent patients and patients on their regular diet [133].
However, the use of periodic fasting or of FMDs in cancer treatment is not free of concerns, particularly in light of the possibility that these dietary regimens may precipitate malnutrition, sarcopenia, and cachexia in predisposed or frail patients (e.g., patients who develop anorexia as a consequence of chemotherapy). Additionally, general exclusion criteria (incl. age < 18 years; BMI < 20 kg/m2; pregnant or nursing, prior diagnosis of eating disorders; severe heart, liver, kidney, or lung diseases; known allergy to FMD components) as well as disease-specific disqualifying criteria (i.e., previous chemotherapy treatment, baseline peripheral blood cell counts, metastasis, diabetes mellitus treated with insulin or insulin secretagogues, etc.) must be considered before the FMD, or other diet-based interventions can be explored.
Considering the reported trials above, no instances of severe (above grade 3) weight loss or of malnutrition were reported in the clinical studies of fasting in combination with chemotherapy, and those patients who did experience weight loss during the fasting period typically recovered their weight before the next fast. Nevertheless, regular anorexia and nutritional status assessments should be included in fasting studies to correct any ensuing nutritional impairments. Most importantly, the effectiveness of fasting/FMD interventions on reducing tumor growth with/without the combination of chemotherapy, endocrine therapy, etc. remains largely undocumented and limited to very few cancer types (i.e., breast cancer) in phase I/II clinical trials. Therefore, the effectiveness, safety, and practicability of different forms of fasting to fight cancer should still be carefully approached.
Conclusions
In summary, these findings above convincingly show that restricting glucose, amino acids, and growth factors by fasting and FMDs induces the protection of the organism, organs, and cells while simultaneously reducing tumor progression in a nutrient-restricted challenging environment, particularly in combination with commonly used chemotherapeutic drugs or other emerging therapeutic approaches. FMD cycles are expected to be more feasible than chronic dietary regimens because patients consume food during the FMD, but it has to be considered that almost all fasting-based interventions might be difficult to adhere to for a majority of people. The FMD approach may increase compliance because patients can maintain their regular diet between cycles or may choose alternative, potentially complementary dietary approaches during the refeeding period. Most importantly, FMDs do not result in severe weight loss and have shown no detrimental effects on the immune and endocrine systems. Several clinical trials of FMDs in combination with chemotherapy or with other types of active treatments are currently ongoing at US and European hospitals, primarily in patients who are diagnosed with breast cancer, with single-arm clinical studies designed to assess the feasibility and safety of the FMD or randomized clinical studies to evaluate FMD effects on the toxicity of chemotherapy or on the quality of life in patients undergoing chemotherapy. At the time of cancer diagnosis, patients may inquire about which lifestyle modifications they can initiate that may slow tumor progression. Notably, identifying a “personalized dietary strategy” that benefits a subject most meets the need for an active engagement of patients in their care process (“patient empowerment”), which, in the perspective of promoting patient participation in the therapeutic process, is strongly recommended by the oncology community.
Authors’ contributions
SB wrote the article.
Data availability
Not applicable.
Compliance with ethical standards
Conflict of interest
USC has a licensed intellectual property related to a commercial fasting-mimicking diet to L-Nutra that is discussed in this review. As part of this license agreement, the University has the potential to receive royalty payments from L-Nutra.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rock CL, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245–271. doi: 10.3322/caac.21591. [DOI] [PubMed] [Google Scholar]
- 2.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 4.Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson LT, Mitchell JR. Benefits of short-term dietary restriction in mammals. Exp Gerontol. 2013;48(10):1043–1048. doi: 10.1016/j.exger.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299(5611):1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 7.Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31(2):89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124(6):952–965. doi: 10.1161/CIRCRESAHA.118.313352. [DOI] [PubMed] [Google Scholar]
- 10.Vera E, Bernardes de Jesus B, Foronda M, Flores JM, Blasco MA. Telomerase reverse transcriptase synergizes with calorie restriction to increase health span and extend mouse longevity. PLoS One. 2013;8(1):e53760. doi: 10.1371/journal.pone.0053760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez NA, Garcia KD, Fortman JD, Hewett TA, Bunte RM, Bennett BT. Clinical and histopathological evaluation of 13 cases of adenocarcinoma in aged rhesus macaques (Macaca mulatta) J Med Primatol. 2002;31(2):74–83. doi: 10.1034/j.1600-0684.2002.01001.x. [DOI] [PubMed] [Google Scholar]
- 12.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontana L, Villareal DT, Das SK, Smith SR, Meydani SN, Pittas AG, Klein S, Bhapkar M, Rochon J, Ravussin E, Holloszy JO, the CALERIE Study Group Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell. 2016;15(1):22–27. doi: 10.1111/acel.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J, Ravussin E, Varady KA. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177(7):930–938. doi: 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, Cutler RG, Evans G, Whiteside S, Maudsley S, Camandola S, Wang R, Carlson OD, Egan JM, Mattson MP, Howell A. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110(8):1534–1547. doi: 10.1017/S0007114513000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes. 2011;35(5):714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manoogian ENC, Panda S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res Rev. 2017;39:59–67. doi: 10.1016/j.arr.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990;55(1):69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- 21.Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16(6):706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16(3):129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 23.de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541–2551. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 24.Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. 2017;37:371–393. doi: 10.1146/annurev-nutr-071816-064634. [DOI] [PubMed] [Google Scholar]
- 25.Brandhorst S, Longo VD. Fasting and caloric restriction in cancer prevention and treatment. Recent Results Cancer Res. 2016;207:241–266. doi: 10.1007/978-3-319-42118-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nencioni A, Caffa I, Cortellino S, Longo VD. Fasting and cancer: molecular mechanisms and clinical application. Nat Rev Cancer. 2018;18(11):707–719. doi: 10.1038/s41568-018-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aloui A, Baklouti H, Souissi N, Chtourou H. Effects of Ramadan fasting on body composition in athletes: a systematic review. Tunis Med. 2019;97(10):1087–1094. [PubMed] [Google Scholar]
- 28.Michalsen A, Li C. Fasting therapy for treating and preventing disease - current state of evidence. Forsch Komplementmed. 2013;20(6):444–453. doi: 10.1159/000357765. [DOI] [PubMed] [Google Scholar]
- 29.Zantar A, Azzoug S, Belhimer F, Chentli F. Diabetes and Ramadan. Presse Med. 2012;41(11):1084–1088. doi: 10.1016/j.lpm.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Hutcheon DA. Malnutrition-induced Wernicke’s encephalopathy following a water-only fasting diet. Nutr Clin Pract. 2015;30(1):92–99. doi: 10.1177/0884533614561793. [DOI] [PubMed] [Google Scholar]
- 31.Finnell JS, Saul BC, Goldhamer AC, Myers TR. Is fasting safe? A chart review of adverse events during medically supervised, water-only fasting. BMC Complement Altern Med. 2018;18(1):67. doi: 10.1186/s12906-018-2136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei M, et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med. 2017;9(377):eaai8700. doi: 10.1126/scitranslmed.aai8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB, Longo VD. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and health span. Cell Metab. 2015;22(1):86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng CW, Villani V, Buono R, Wei M, Kumar S, Yilmaz OH, Cohen P, Sneddon JB, Perin L, Longo VD. Fasting-mimicking diet promotes Ngn3-driven beta-cell regeneration to reverse diabetes. Cell. 2017;168(5):775–788. doi: 10.1016/j.cell.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rangan P, Choi I, Wei M, Navarrete G, Guen E, Brandhorst S, Enyati N, Pasia G, Maesincee D, Ocon V, Abdulridha M, Longo VD. Fasting-mimicking diet modulates microbiota and promotes intestinal regeneration to reduce inflammatory bowel disease pathology. Cell Rep. 2019;26(10):2704–2719. doi: 10.1016/j.celrep.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, Suarez J, Michalsen A, Cross AH, Morgan TE, Wei M, Paul F, Bock M, Longo VD. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 2016;15(10):2136–2146. doi: 10.1016/j.celrep.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM, Longo VD. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19(3):407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, González-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, Wahl D, Ali A, Calvo-Rubio M, Burón MI, Guiterrez V, Ward TM, Palacios HH, Cai H, Frederick DW, Hine C, Broeskamp F, Habering L, Dawson J, Beasley TM, Wan J, Ikeno Y, Hubbard G, Becker KG, Zhang Y, Bohr VA, Longo DL, Navas P, Ferrucci L, Sinclair DA, Cohen P, Egan JM, Mitchell JR, Baur JA, Allison DB, Anson RM, Villalba JM, Madeo F, Cuervo AM, Pearson KJ, Ingram DK, Bernier M, de Cabo R. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 2016;23(6):1093–1112. doi: 10.1016/j.cmet.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schröder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, Hans W, Hettich MM, Holtmeier R, Hölter SM, Moreth K, Prehn C, Puk O, Rácz I, Rathkolb B, Rozman J, Naton B, Ordemann R, Adamski J, Beckers J, Bekeredjian R, Busch DH, Ehninger G, Graw J, Höfler H, Klingenspor M, Klopstock T, Ollert M, Stypmann J, Wolf E, Wurst W, Zimmer A, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Ehninger D. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123(8):3272–3291. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie K, Neff F, Markert A, Rozman J, Aguilar-Pimentel JA, Amarie OV, Becker L, Brommage R, Garrett L, Henzel KS, Hölter SM, Janik D, Lehmann I, Moreth K, Pearson BL, Racz I, Rathkolb B, Ryan DP, Schröder S, Treise I, Bekeredjian R, Busch DH, Graw J, Ehninger G, Klingenspor M, Klopstock T, Ollert M, Sandholzer M, Schmidt-Weber C, Weiergräber M, Wolf E, Wurst W, Zimmer A, Gailus-Durner V, Fuchs H, Hrabě de Angelis M, Ehninger D. Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nat Commun. 2017;8(1):155. doi: 10.1038/s41467-017-00178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madia F, Wei M, Yuan V, Hu J, Gattazzo C, Pham P, Goodman MF, Longo VD. Oncogene homologue Sch9 promotes age-dependent mutations by a superoxide and Rev1/Polzeta-dependent mechanism. J Cell Biol. 2009;186(4):509–523. doi: 10.1083/jcb.200906011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrison DE, Archer JR. Natural selection for extended longevity from food restriction. Growth Dev Aging. 1989;53(1-2):3. [PubMed] [Google Scholar]
- 43.Longo VD. Mutations in signal transduction proteins increase stress resistance and longevity in yeast, nematodes, fruit flies, and mammalian neuronal cells. Neurobiol Aging. 1999;20(5):479–486. doi: 10.1016/s0197-4580(99)00089-5. [DOI] [PubMed] [Google Scholar]
- 44.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408(6809):255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 45.Kenyon C. A conserved regulatory system for aging. Cell. 2001;105(2):165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 46.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144(9):3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 47.Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 48.Bonkowski MS, Dominici FP, Arum O, Rocha JS, al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4(2):e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Selman C, Tullet JMA, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson ICA, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949):140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown-Borg HM. Hormonal control of aging in rodents: the somatotropic axis. Mol Cell Endocrinol. 2009;299(1):64–71. doi: 10.1016/j.mce.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 52.Murakami S. Stress resistance in long-lived mouse models. Exp Gerontol. 2006;41(10):1014–1019. doi: 10.1016/j.exger.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 53.Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289(1):E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 54.Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp Gerontol. 2000;35(2):199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- 55.Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes. Exp Biol Med (Maywood) 2002;227(2):94–104. doi: 10.1177/153537020222700203. [DOI] [PubMed] [Google Scholar]
- 56.Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides. 2002;36(2-3):201–208. doi: 10.1054/npep.2002.0889. [DOI] [PubMed] [Google Scholar]
- 57.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 58.DePinho RA. The age of cancer. Nature. 2000;408(6809):248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 59.Campisi J, Andersen JK, Kapahi P, Melov S. Cellular senescence: a link between cancer and age-related degenerative disease? Semin Cancer Biol. 2011;21(6):354–359. doi: 10.1016/j.semcancer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tannenbaum A. The dependence of tumor formation on the composition of the calorie-restricted diet as well as on the degree of restriction. 1945. Nutrition. 1996;12(9):653–654. [PubMed] [Google Scholar]
- 61.Tannenbaum A. The initiation and growth of tumours. Introduction. 1. Effects of underfeeding. Am J Cancer. 1940;38:335–350. [Google Scholar]
- 62.Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23(5):817–822. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- 63.Hursting SD, Perkins SN, Phang JM. Calorie restriction delays spontaneous tumorigenesis in p53-knockout transgenic mice. Proc Natl Acad Sci U S A. 1994;91(15):7036–7040. doi: 10.1073/pnas.91.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215(4538):1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 65.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 66.Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458(7239):725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7(5):681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mukherjee P, Abate LE, Seyfried TN. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin Cancer Res. 2004;10(16):5622–5629. doi: 10.1158/1078-0432.CCR-04-0308. [DOI] [PubMed] [Google Scholar]
- 70.Bonorden MJ, et al. Intermittent calorie restriction delays prostate tumor detection and increases survival time in TRAMP mice. Nutr Cancer. 2009;61(2):265–275. doi: 10.1080/01635580802419798. [DOI] [PubMed] [Google Scholar]
- 71.Kristan DM. Calorie restriction and susceptibility to intact pathogens. Age (Dordr) 2008;30(2-3):147–156. doi: 10.1007/s11357-008-9056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reed MJ, Penn PE, Li Y, Birnbaum R, Vernon RB, Johnson TS, Pendergrass WR, Sage EH, Abrass IB, Wolf NS. Enhanced cell proliferation and biosynthesis mediate improved wound repair in refed, caloric-restricted mice. Mech Ageing Dev. 1996;89(1):21–43. doi: 10.1016/0047-6374(96)01737-x. [DOI] [PubMed] [Google Scholar]
- 73.Kim SK, Demetri GD. Chemotherapy and neutropenia. Hematol Oncol Clin North Am. 1996;10(2):377–395. doi: 10.1016/s0889-8588(05)70344-0. [DOI] [PubMed] [Google Scholar]
- 74.Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30(30):3305–3316. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- 75.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. [PubMed] [Google Scholar]
- 77.Underwood LE, Thissen JP, Lemozy S, Ketelslegers JM, Clemmons DR. Hormonal and nutritional regulation of IGF-I and its binding proteins. Horm Res. 1994;42(4-5):145–151. doi: 10.1159/000184187. [DOI] [PubMed] [Google Scholar]
- 78.Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70(4):1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3(6):e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grasl-Kraupp B, Bursch W, Ruttkay-Nedecky B, Wagner A, Lauer B, Schulte-Hermann R. Food restriction eliminates preneoplastic cells through apoptosis and antagonizes carcinogenesis in rat liver. Proc Natl Acad Sci U S A. 1994;91(21):9995–9999. doi: 10.1073/pnas.91.21.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lv M, Zhu X, Wang H, Wang F, Guan W. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: a systematic review and meta-analysis. PLoS One. 2014;9(12):e115147. doi: 10.1371/journal.pone.0115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kanarek N, Petrova B, Sabatini DM. Dietary modifications for enhanced cancer therapy. Nature. 2020;579(7800):507–517. doi: 10.1038/s41586-020-2124-0. [DOI] [PubMed] [Google Scholar]
- 83.Pape-Ansorge KA, Grande, Christensen TA, Maihle NJ, Cleary MP. Effect of moderate caloric restriction and/or weight cycling on mammary tumor incidence and latency in MMTV-Neu female mice. Nutr Cancer. 2002;44(2):162–168. doi: 10.1207/S15327914NC4402_07. [DOI] [PubMed] [Google Scholar]
- 84.Mehta RS, Harris SR, Gunnett CA, Bunce OR, Hartle DK. The effects of patterned calorie-restricted diets on mammary tumor incidence and plasma endothelin levels in DMBA-treated rats. Carcinogenesis. 1993;14(8):1693–1696. doi: 10.1093/carcin/14.8.1693. [DOI] [PubMed] [Google Scholar]
- 85.Thomas JA, 2nd, et al. Effect of intermittent fasting on prostate cancer tumor growth in a mouse model. Prostate Cancer Prostatic Dis. 2010;13(4):350–355. doi: 10.1038/pcan.2010.24. [DOI] [PubMed] [Google Scholar]
- 86.Buschemeyer WC, 3rd, et al. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate. 2010;70(10):1037–1043. doi: 10.1002/pros.21136. [DOI] [PubMed] [Google Scholar]
- 87.Castejon M, et al. Energy restriction and colorectal cancer: a call for additional research. Nutrients. 2020;12(1):114. doi: 10.3390/nu12010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cuervo AM, Bergamini E, Brunk UT, Dröge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1(3):131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 89.Premoselli F, Sesca E, Binasco V, Caderni G, Tessitore L. Fasting/re-feeding before initiation enhances the growth of aberrant crypt foci induced by azoxymethane in rat colon and rectum. Int J Cancer. 1998;77(2):286–294. doi: 10.1002/(sici)1097-0215(19980717)77:2<286::aid-ijc19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 90.Sesca E, Premoselli F, Binasco V, Bollito E, Tessitore L. Fasting-refeeding stimulates the development of mammary tumors induced by 7,12-dimethylbenz[a]anthracene. Nutr Cancer. 1998;30(1):25–30. doi: 10.1080/01635589809514636. [DOI] [PubMed] [Google Scholar]
- 91.Tessitore L, Tomasi C, Greco M. Fasting-induced apoptosis in rat liver is blocked by cycloheximide. Eur J Cell Biol. 1999;78(8):573–579. doi: 10.1016/S0171-9335(99)80023-5. [DOI] [PubMed] [Google Scholar]
- 92.Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Müller C, de Jong M, van IJcken W, IJzermans JN, Hoeijmakers JH, de Bruin RW. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2009;9(1):40–53. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Ginhoven TM, Mitchell JR, Verweij M, Hoeijmakers JHJ, Ijzermans JNM, de Bruin RWF. The use of preoperative nutritional interventions to protect against hepatic ischemia-reperfusion injury. Liver Transpl. 2009;15(10):1183–1191. doi: 10.1002/lt.21871. [DOI] [PubMed] [Google Scholar]
- 94.Verweij M, van Ginhoven TM, Mitchell JR, Sluiter W, den Engel S, Roest HP, Torabi E, IJzermans JNM, Hoeijmakers JHJ, de Bruin RWF. Preoperative fasting protects mice against hepatic ischemia/reperfusion injury: mechanisms and effects on liver regeneration. Liver Transpl. 2011;17(6):695–704. doi: 10.1002/lt.22243. [DOI] [PubMed] [Google Scholar]
- 95.Varendi K, Airavaara M, Anttila J, Vose S, Planken A, Saarma M, Mitchell JR, Andressoo JO. Short-term preoperative dietary restriction is neuroprotective in a rat focal stroke model. PLoS One. 2014;9(4):e93911. doi: 10.1371/journal.pone.0093911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davis LM, Pauly JR, Readnower RD, Rho JM, Sullivan PG. Fasting is neuroprotective following traumatic brain injury. J Neurosci Res. 2008;86(8):1812–1822. doi: 10.1002/jnr.21628. [DOI] [PubMed] [Google Scholar]
- 97.Longo VD, Ellerby LM, Bredesen DE, Valentine JS, Gralla EB. Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J Cell Biol. 1997;137(7):1581–1588. doi: 10.1083/jcb.137.7.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105(24):8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 100.Tinkum KL, Stemler KM, White LS, Loza AJ, Jeter-Jones S, Michalski BM, Kuzmicki C, Pless R, Stappenbeck TS, Piwnica-Worms D, Piwnica-Worms H. Fasting protects mice from lethal DNA damage by promoting small intestinal epithelial stem cell survival. Proc Natl Acad Sci U S A. 2015;112(51):E7148–E7154. doi: 10.1073/pnas.1509249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Withers SS, Kass PH, Rodriguez CO, Jr, Skorupski KA, O’Brien D, Guerrero TA, Sein KD, Rebhun RB. Fasting reduces the incidence of delayed-type vomiting associated with doxorubicin treatment in dogs with lymphoma. Transl Oncol. 2014;7:377–383. doi: 10.1016/j.tranon.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huisman SA, Bijman-Lagcher W, IJzermans JNM, Smits R, de Bruin RWF. Fasting protects against the side effects of irinotecan but preserves its anti-tumor effect in Apc15lox mutant mice. Cell Cycle. 2015;14(14):2333–2339. doi: 10.1080/15384101.2015.1044170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yildirim Y, Gultekin E, Avci ME, Inal MM, Yunus S, Tinar S. Cardiac safety profile of pegylated liposomal doxorubicin reaching or exceeding lifetime cumulative doses of 550 mg/m2 in patients with recurrent ovarian and peritoneal cancer. Int J Gynecol Cancer. 2008;18(2):223–227. doi: 10.1111/j.1525-1438.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 104.Di Biase S, et al. Fasting regulates EGR1 and protects from glucose- and dexamethasone-dependent sensitization to chemotherapy. PLoS Biol. 2017;15(3):e2001951. doi: 10.1371/journal.pbio.2001951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheng C-W, Adams GB, Perin L, Wei M, Zhou X, Lam BS, da Sacco S, Mirisola M, Quinn DI, Dorff TB, Kopchick JJ, Longo VD. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14(6):810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD. Fasting and cancer treatment in humans: a case series report. Aging (Albany NY) 2009;1(12):988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de la Cruz Bonilla M, Stemler KM, Jeter-Jones S, Fujimoto TN, Molkentine J, Asencio Torres GM, Zhang X, Broaddus RR, Taniguchi CM, Piwnica-Worms H. Fasting reduces intestinal radiotoxicity, enabling dose-escalated radiation therapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2019;105(3):537–547. doi: 10.1016/j.ijrobp.2019.06.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 109.Medema RH, Bos JL. The role of p21ras in receptor tyrosine kinase signaling. Crit Rev Oncog. 1993;4(6):615–661. [PubMed] [Google Scholar]
- 110.Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10(12):1477–1483. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruckenstuhl C, Büttner S, Carmona-Gutierrez D, Eisenberg T, Kroemer G, Sigrist SJ, Fröhlich KU, Madeo F. The Warburg effect suppresses oxidative stress induced apoptosis in a yeast model for cancer. PLoS One. 2009;4(2):e4592. doi: 10.1371/journal.pone.0004592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol Metab. 2014;25(11):558–566. doi: 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jeon H, Kim JH, Lee E, Jang YJ, Son JE, Kwon JY, Lim TG, Kim S, Park JHY, Kim JE, Lee KW. Methionine deprivation suppresses triple-negative breast cancer metastasis in vitro and in vivo. Oncotarget. 2016;7(41):67223–67234. doi: 10.18632/oncotarget.11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee C, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4(124):124ra27. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, Conti PS, Chen TC, Longo VD. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS One. 2012;7(9):e44603. doi: 10.1371/journal.pone.0044603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shi Y, Felley-Bosco E, Marti TM, Orlowski K, Pruschy M, Stahel RA. Starvation-induced activation of ATM/Chk2/p53 signaling sensitizes cancer cells to cisplatin. BMC Cancer. 2012;12:571. doi: 10.1186/1471-2407-12-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lo Re O, Panebianco C, Porto S, Cervi C, Rappa F, di Biase S, Caraglia M, Pazienza V, Vinciguerra M. Fasting inhibits hepatic stellate cells activation and potentiates anti-cancer activity of Sorafenib in hepatocellular cancer cells. J Cell Physiol. 2018;233(2):1202–1212. doi: 10.1002/jcp.25987. [DOI] [PubMed] [Google Scholar]
- 118.D'Aronzo M, et al. Fasting cycles potentiate the efficacy of gemcitabine treatment in in vitro and in vivo pancreatic cancer models. Oncotarget. 2015;6(21):18545–18557. doi: 10.18632/oncotarget.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brandhorst S, Wei M, Hwang S, Morgan TE, Longo VD. Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp Gerontol. 2013;48:1120–1128. doi: 10.1016/j.exger.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bianchi G, Martella R, Ravera S, Marini C, Capitanio S, Orengo A, Emionite L, Lavarello C, Amaro A, Petretto A, Pfeffer U, Sambuceti G, Pistoia V, Raffaghello L, Longo VD. Fasting induces anti-Warburg effect that increases respiration but reduces ATP-synthesis to promote apoptosis in colon cancer models. Oncotarget. 2015;6(14):11806–11819. doi: 10.18632/oncotarget.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shim HS, Wei M, Brandhorst S, Longo VD. Starvation promotes REV1 SUMOylation and p53-dependent sensitization of melanoma and breast cancer cells. Cancer Res. 2015;75(6):1056–1067. doi: 10.1158/0008-5472.CAN-14-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caffa I, D’Agostino V, Damonte P, Soncini D, Cea M, Monacelli F, Odetti P, Ballestrero A, Provenzani A, Longo VD, Nencioni A. Fasting potentiates the anticancer activity of tyrosine kinase inhibitors by strengthening MAPK signaling inhibition. Oncotarget. 2015;6(14):11820–11832. doi: 10.18632/oncotarget.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Di Biase S, et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell. 2016;30(1):136–146. doi: 10.1016/j.ccell.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ma Y, et al. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014;6(222):222ra18. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 125.Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio IIC, Giannopoulou EG, Rago C, Muley A, Asara JM, Paik J, Elemento O, Chen Z, Pappin DJ, Dow LE, Papadopoulos N, Gross SS, Cantley LC. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A. 2008;105(32):11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Di Tano M, et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat Commun. 2020;11(1):2332. doi: 10.1038/s41467-020-16243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caffa I, Spagnolo V, Vernieri C, Valdemarin F, Becherini P, Wei M, Brandhorst S, Zucal C, Driehuis E, Ferrando L, Piacente F, Tagliafico A, Cilli M, Mastracci L, Vellone VG, Piazza S, Cremonini AL, Gradaschi R, Mantero C, Passalacqua M, Ballestrero A, Zoppoli G, Cea M, Arrighi A, Odetti P, Monacelli F, Salvadori G, Cortellino S, Clevers H, de Braud F, Sukkar SG, Provenzani A, Longo VD, Nencioni A. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature. 2020;583(7817):620–624. doi: 10.1038/s41586-020-2502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dorff TB, Groshen S, Garcia A, Shah M, Tsao-Wei D, Pham H, Cheng CW, Brandhorst S, Cohen P, Wei M, Longo V, Quinn DI. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16(1):360. doi: 10.1186/s12885-016-2370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de Groot S, Vreeswijk MPG, Welters MJP, Gravesteijn G, Boei JJWA, Jochems A, Houtsma D, Putter H, van der Hoeven JJM, Nortier JWR, Pijl H, Kroep JR. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer. 2015;15:652. doi: 10.1186/s12885-015-1663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vernieri C, et al. Exploiting FAsting-mimicking Diet and MEtformin to improve the efficacy of platinum-pemetrexed chemotherapy in advanced LKB1-inactivated lung adenocarcinoma: the FAME trial. Clin Lung Cancer. 2018;20(3):e413–e417. doi: 10.1016/j.cllc.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 132.Bauersfeld SP, Kessler CS, Wischnewsky M, Jaensch A, Steckhan N, Stange R, Kunz B, Brückner B, Sehouli J, Michalsen A. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: a randomized cross-over pilot study. BMC Cancer. 2018;18(1):476. doi: 10.1186/s12885-018-4353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lugtenberg RT, et al. Quality of life and illness perceptions in patients with breast cancer using a fasting mimicking diet as an adjunct to neoadjuvant chemotherapy in the phase 2 DIRECT (BOOG 2013-14) trial. Breast Cancer Res Treat. 2020. [DOI] [PMC free article] [PubMed]
- 134.de Groot S, et al. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat Commun. 2020;11(1):3083. doi: 10.1038/s41467-020-16138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pollack MN. Insulin, insulin-like growth factors, insulin resistance, and neoplasia. Am J Clin Nutr. 2007;86(3):s820–s822. doi: 10.1093/ajcn/86.3.820S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.