Abstract
Purpose
When rescue artificial oocyte activation (ROA) is performed on the day after intracytoplasmic sperm injection (ICSI) or later, embryonic development is poor and seldom results in live births. The efficacy of an early ROA after ICSI is unclear. Is early ROA effective in rescuing unfertilized oocytes that have not undergone second polar body extrusion several hours after ICSI?
Methods
We performed retrospective cohort study between October 2016 and September 2019, targeting 2891 oocytes in 843 cycles when ICSI was performed. We performed ROA with calcium ionophore on 395 of the 475 oocytes with no second polar extrusion 2.5–6 h after ICSI.
Results
The normal fertilization rate of ROA oocytes was significantly higher than non-ROA oocytes (65.8% vs 6.7%, P < 0.001). The blastocyst development rate in ROA oocytes was significantly lower than spontaneously activated oocytes (48.9% vs 67.2%, P < 0.001). The ROA oocyte implantation rate did not significantly differ from the spontaneously activated oocytes (36.0% vs 41.2%). We observed no differences in the implantation rates and blastocyst development rates over the 2.5–6 h from ICSI until ROA.
Conclusion
Early ROA is effective, and the optimal timing appears to be 2.5–6 h after ICSI.
Keywords: Artificial oocyte activation, Inactivated oocytes, Calcium ionophore, Intracytoplasmic sperm injection, Second polar body
Introduction
It is well known that performing artificial oocyte activation (AOA) with calcium ionophore directly after intracytoplasmic sperm injection (ICSI) at the subsequent treatment session for cases that exhibited complete fertility impairment and low fertilization rates after ICSI improves fertilization rates [1], and it is widely used clinically. However, AOA is unnecessary if fertilization of oocytes progresses normally after ICSI without any additional treatment. As the safety of this procedure, particularly with respect to the future child, is unknown, unnecessary performing AOA needs to be avoided.
When performing rescue artificial oocyte activation (ROA) for oocytes in which second polar body extrusion cannot be directly confirmed after a certain length of time has passed after ICSI, performing ROA on the day following ICSI or later may enable oocyte fertilization; however, blastocyst development is extremely poor [2, 3]; only a very small number of births have been reported to result from this [4]. Factors affecting such poor development resulting from ROA performed long after ICSI include oocyte aging or forced progression of fertilization in oocytes that have undergone premature chromosome condensation (PCC) of the sperm nucleus [2, 3, 5, 6]. Monitoring whether second polar body extrusion has occurred 4.5–5 h after ICSI and performing early ROA on activation-impaired oocytes that did not exhibit second polar body extrusion resulted in more fertilized oocytes than in oocytes that did not undergo ROA [7]. However, the efficacy of ROA is yet to be established. Performing ROA too early will lead to unnecessary procedures, whereas performing it too late could result in poor blastocyst development. To the best of our knowledge, no study has investigated the optimal ROA timing considering all factors, including blastocyst development.
We performed ROA on some oocytes with no second polar body extrusion following ICSI and investigated the efficacy of performing early ROA within 6 h after ICSI until second polar body extrusion, fertilization, and embryonic development occurred.
Materials and methods
We retrospectively investigated all cases of ICSI between October 2016 and September 2019. Among the metaphase (M) II oocytes at the time of retrieval, we performed ROA on the oocytes following ICSI between 12:00 and 15:00. We performed ROA on the retrieved oocytes in which second polar body extrusion of ≤70% was achieved (ROA oocytes). We did not perform ROA on oocytes in which >70% exhibited second polar body extrusion (non-ROA oocytes). Even if ≤70% oocytes exhibited second polar body extrusion, ROA was not performed if the spindle body could not be confirmed during ICSI, if the oocyte exhibited significant cytoplasm twisting when ROA was to be performed or if the oocyte was of very poor quality (non-ROA oocytes). Patients who underwent AOA immediately after ICSI due to low fertilization rate in the previous cycle were excluded from ROA. Therefore, our analysis targeted 2891 oocytes from 843 cycles.
Ethical approval
We obtained informed consent after providing sufficient explanations that ROA could result in a large number of fertilized oocytes and the fact that the effects of ROA on children born after this procedure are unknown. This study was conducted in accordance with the Declaration of Helsinki, with the approval of the Ethics Committee of the XXXXXXX XXXXXX XXXXXX. We obtained permission to access the database.
Oocyte retrieval and ICSI
Controlled ovarian hyper-stimulation was performed using the agonist/hMG, antagonist/hMG, and clomiphene/antagonist/hMG methods. Oocyte retrieval was performed using transvaginal ultrasound guidance 36–38 h after a trigger was applied with hCG or GnRHa agonist. We performed oocyte retrieval between 7:00 and 10:00. At the time of egg inspection, using an inverted microscope (IX70, Olympus, Tokyo, Japan), we confirmed the presence of the first polar body by extending and observing the egg cumulus cell complex. We then classified the oocytes as either MII or MI oocytes. After placing the retrieved oocytes in a preculture (HTF medium; Irvine Scientific, Santa Ana, USA) for at least 3 h, they were denudated by placing them in a modified HTF medium (Irvine Scientific, Santa Ana, USA) containing 0.025% hyaluronidase. We observed the spindle body using a spindle visualization system (Oosight Imaging system, Hamilton Thorne, Beverly, USA) and performed ICSI. Directly after ICSI, the oocytes were transferred to a global medium (Life Global, Brussels, Belgium) and cultured in a time-lapse incubator (CCM-iBIS, Astec, Fukuoka, Japan). Images at 15-min intervals were taken to observe second polar body extrusion. The time at which second polar body extrusion was considered to have occurred was when the entire second polar body had emerged from the cytoplasm.
Performing ROA
We performed ROA between 17:30 and 18:30 (≥2.5 h and <6 h after ICSI) (ROA oocytes). ROA was performed by soaking the oocytes in a culture medium (modified HTF medium, Irvine Scientific, Santa Ana, USA) containing 10 μg calcium ionophore (A23187, Sigma-Aldrich, St. Louis, USA) added for 15 min at 37°C while shielded from the light outside the incubator. Before performing ROA, we confirmed if no second polar body extrusion had occurred using an inverted microscope. For oocytes in which there were signs of second polar body extrusion, such as elevation of the cell surface and oocytes in which the second polar body was unclear, the spindle body was observed as an aid for evaluation before performing ROA.
Blastocyst culture and transfer
Fertilization was considered to occur if we observed an appearance and disappearance of the pronucleus using a time-lapse incubator.
Apart from 12 embryos that underwent fresh cleavage stage embryo transfer, all embryos were cultured until the blastocyst stage and then frozen. Blastocysts that had increased in size to at least the Gardner classification [8] 2BB on the afternoon of day 4 and to at least stage 3 on days 5 or 6 were frozen. The 2PN embryos were considered to be of good quality if they had matured to at least 2BB on day 4, to at least 3BB on day 5 and to at least 4BB on day 6.
After thawing the blastocyst for 4–20 h, we transferred them into the uterus of the subjects during the oocyte retrieval cycle or a hormone-regulated cycle and compared the single-blastocyst transfer cases.
Statistical analyses
We compared the fertilization and development of spontaneously activated oocytes, ROA oocytes, and non-ROA oocytes using chi-squared test and revised multiple comparison with the Bonferroni method. In addition, we compared implantation and miscarriage rates for the spontaneously activated oocytes and ROA oocytes using chi-squared test. Furthermore, we investigated the age at oocyte retrieval using Mann–Whitney’s U test. We used Kruskal–Wallis test and multiple comparisons with the Bonferroni method to investigate a possible relationship between the time from ICSI to ROA and fertilization rates. We investigated the blastocyst development rates using Mann–Whitney’s U test. We used Spearman’s correlation coefficient to compare the ICSI–ROA times and time from ROA to second polar body extrusion in each case. P values of <0.05 were considered to indicate statistically significance. The overall statistical analyses were performed using Software R.
Results
Comparison of spontaneously activated oocytes, ROA oocytes, and non-ROA oocytes
Among the 2891 oocytes that underwent ICSI, we excluded 104 that directly degenerated after ICSI and 22 that were unable to undergo clear analysis with time-lapse imaging. By 17:30–18:30, when the decision was made on whether to perform ROA, 2290 oocytes had spontaneously activated and released the second polar body (spontaneously activated oocytes). The second polar body extrusion could not be confirmed during the final assessment of 475 oocytes. Of these, 395 oocytes from 241 cycles were subjected to ROA (Fig. 1).
Fig. 1.
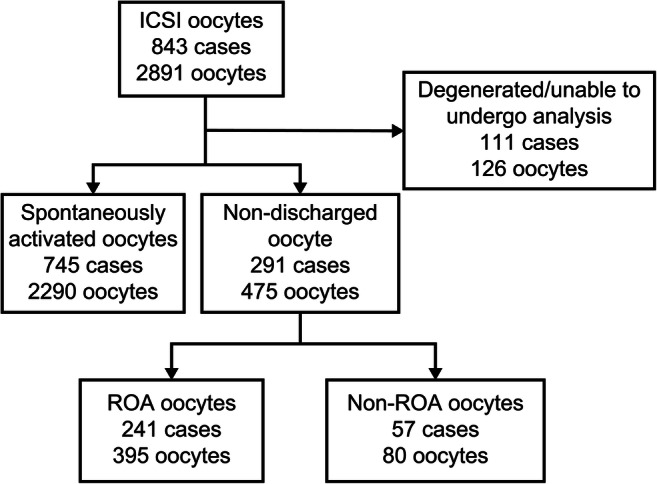
Study flow chart. ICSI, intracytoplasmic sperm injection; ROA, rescue artificial oocyte activation
For spontaneously activated oocytes, the 2PN, 1PN, ≥3PN, 0PN, blastocyst development, and good quality blastocyst development rates were 90.7%, 3.0%, 2.6%, 3.8%, 67.2%, and 37.8%, respectively, whereas for ROA oocytes, these rates were 65.8%, 10.6%, 5.3%, 18.2%, 48.9%, and 20.5%, respectively. Therefore, when compared with spontaneously activated oocytes, ROA oocytes exhibited significantly lower 2PN, blastocyst development, and good quality blastocyst development rates (P < 0.001) and significantly higher 1PN (P < 0.001), ≥3PN (P < 0.0036), and 0PN rates (P < 0.001).
For the non-ROA oocytes, the 2PN, 1PN, ≥3PN, 0PN, blastocyst development, and good quality blastocyst development rates were 6.7%, 5.3%, 13.3%, 74.7%, 40.0%,and 0%, respectively. Therefore, when compared with the non-ROA oocytes, ROA oocytes exhibited significantly higher 2PN rates (P < 0.001) and significantly lower ≥3PN (P = 0.0093) and 0PN rates (P < 0.001; Table 1).
Table 1.
Fertilization and blastocyst development rates of embryos derived from oocytes with second polar body extrusion either by spontaneous activation or rescue artificial activation (ROA) and non-ROA that did not exhibit extrusion
| Spontaneously activated oocytes | ROA oocytes | Non-ROA oocytes | P value | ||
|---|---|---|---|---|---|
| Spontaneously activated oocytes vs. ROA oocytes | ROA oocytes vs. non-ROA oocytes | ||||
| Number of oocytes | 2290 | 395 | 75 | ||
| 1PN rate, n (%) | 68 (3.0%) | 42 (10.6%) | 4 (5.3%) | <0.001 | 0.15 |
| 2PN rate, n (%) | 2077 (90.7%) | 260 (65.8%) | 5 (6.7%) | <0.001 | <0.001 |
| 3PN or higher rate, n (%) | 59 (2.6%) | 21 (5.3%) | 10 (13.3%) | 0.0036 | 0.0093 |
| 0PN rate, n (%) | 87 (3.8%) | 72 (18.2%) | 56 (74.7%) | <0.001 | <0.001 |
| Blastocyst development rate, n (% per 2PN) | 1396 (67.2%) | 127 (48.9%) | 5 (40.0%) | <0.001 | 1.00 |
| Good quality blastocyst development rate, n (% per 2PN) | 785 (37.8%) | 53 (20.5%) | 0 (0.0%) | <0.001 | 0.59 |
The spontaneously activated oocytes-blastocyst implantation and miscarriage rates were 41.4% and 20.5%, respectively, whereas the good quality blastocyst implantation and miscarriage rates were 45.9% and 18.4%, respectively. The ROA oocyte-blastocyst implantation and miscarriage rates were 36.0% and 44.4%, respectively, whereas the good quality blastocyst implantation and miscarriage rates were 41.2% and 28.6%, respectively (Table 2).
Table 2.
Clinical outcome of embryos from spontaneously and rescued artificially activated oocytes (ROA), respectively
| Spontaneously activated oocytes | ROA oocytes | P value | |
|---|---|---|---|
| Single-blastocyst transfer number | 425 | 25 | |
| Age at oocyte retrieval | 35.7 ± 4.8 | 36.7 ± 3.9 | 0.38 |
| Implantation rate, n (% per blastocyst transfer) | 176 (41.4%) | 9 (36.0%) | 0.74 |
| Miscarriage rate, n (% per clinical pregnancy) | 36 (20.5%) | 4 (44.4%) | 0.20 |
| Live birth rate, n (% per blastocyst transfer) | 140 (32.9%) | 5 (20%) | 0.20 |
| Good quality single-blastocyst transfer number | 344 | 17 | |
| Implantation rate, n (% per good quality blastocyst transfer) | 158 (45.9%) | 7 (41.2%) | 0.89 |
| Miscarriage rate, n (% per clinical pregnancy) | 29 (18.4%) | 2 (28.6%) | 0.86 |
| Live birth rate, n (% per good quality blastocyst transfer) | 129 (37.5%) | 5 (29.4%) | 0.68 |
Time from ICSI until second polar body extrusion
Cumulative second polar body extrusion rates between ICSI and second polar body extrusion for spontaneously activated oocyte were 2.5% for <1 h after ICSI, 52.4% at <2 h, 89.7% at <3 h, and 98.6% at <4 h. The mean time until second polar body extrusion was 119 ± 44 min.
Second polar body extrusion occurred in 310 of the 395 oocytes that underwent ROA. Cumulative second polar body extrusion rates between ICSI and second polar body extrusion for ROA oocytes that exhibited second polar body extrusion were 4.2% at <4 h after ICSI, 25.4% at <5 h, 71.2% at <6 h, and 93.0% at <7 h. The mean time until second polar body extrusion was 332 ± 60 min (Fig. 2). Isolated bimodality was observed in the frequency distribution of time between ICSI and second polar body extrusion for spontaneously activated and ROA oocytes.
Fig. 2.
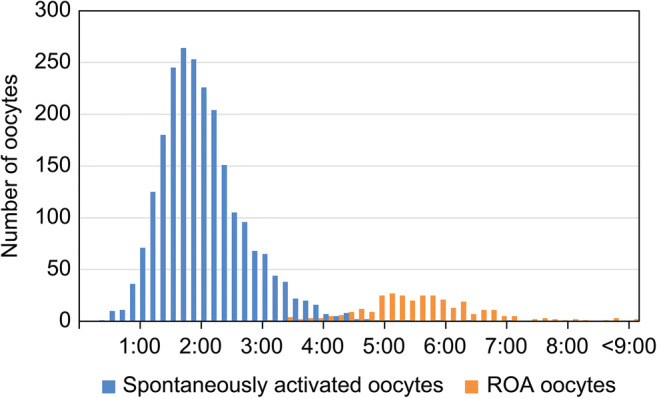
Frequency comparison of time from ICSI until second polar body extrusion between spontaneously activated and ROA oocytes. ROA, rescue artificial oocyte activation
Fertilization rates and blastocyst development rates for good- and poor-quality non-ROA oocytes
Although there was no second polar body extrusion, ROA was not performed in 75 oocytes. These comprised 54 good quality oocytes that did not undergo ROA because another oocyte with sufficient second polar body extrusion were obtained and 21 poor-quality oocytes that did not undergo ROA due to the poor quality of oocytes (Table 3).
Table 3.
Time between intracytoplasmic sperm injection (ICSI) and second polar body extrusion, fertilization, and blastocyst development rates of embryos from good- and poor-quality rescued non-artificially activated (ROA) oocytes, respectively
| Extrusion | No extrusion | |
|---|---|---|
| Number of good quality non-ROA oocytes | 2 | 52 |
| Mean time until extrusion | 4:45 ± 21 | |
| 1PN rate, n (%) | 0 (0.0%) | 1 (1.9%) |
| 2PN rate, n (%) | 2 (100.0%) | 1 (1.9%) |
| 3PN or higher rate, n (%) | 0 (0.0%) | 2 (3.9%) |
| 0PN rate, n (%) | 0 (0.0%) | 48 (92.3%) |
| Blastocyst development rate, n (% per 2PN) | 1 (50.0%) | 1 (100.0%) |
| Good quality blastocyst development rate, n (% per 2PN) | 0 (0.0%) | 1 (0.0%) |
| Number of poor-quality non-ROA oocytes | 2 | 19 |
| Mean time until extrusion | 6:30 ± 42 | |
| 1PN rate, n (%) | 1 (50.0%) | 2 (10.5%) |
| 2PN rate, n (%) | 0 (0.0%) | 2 (10.5%) |
| 3PN or higher rate, n (%) | 1 (50.0%) | 7 (36.8%) |
| 0PN rate, n (%) | 0 (0.0%) | 8 (42.1%) |
| Blastocyst development rate, n (% per 2PN) | - | 0 (0.0%) |
| Good quality blastocyst development rate, n (% per 2PN) | - | 0 (0.0%) |
ICSI intracytoplasmic sperm injection, ROA rescue artificial oocyte activation
After the final observation to determine second polar body extrusion, 2 (3.7%) of the 54 good quality oocytes released the second polar body. We observed second polar body extrusion 285 ± 21 min after ICSI and classified both oocytes as 2PN. Among the 52 good quality oocytes that did not exhibit second polar body extrusion, pronuclei were confirmed in four (7.7%), and 2PN was confirmed in one oocyte (1.9%).
After the final observation to determine second polar body extrusion, 2 (9.5%) of the 21 poor-quality oocytes released the second polar body. Second polar body extrusion was observed at 390 ± 42 min after ICSI, with one oocyte becoming 1PN and the other 3PN. Among the 19 poor-quality oocytes that did not exhibit second polar body extrusion, pronuclei were confirmed in 10 oocytes (58.8%), but only two (10.5%) were 2PN. Thus, poor-quality oocytes took a longer time than the good quality ones for second polar body extrusion. Moreover, many oocytes exhibited pronuclei without any second polar body extrusion, with a larger rate of abnormal fertilization of ≥3PN.
Time from ICSI to ROA and time from ROA to second polar body extrusion
We performed ROA on 286 oocytes between 2.5 and 6 h after ICSI. It was performed at <3 h after ICSI for 13 oocytes (4.2%), ≥3 and <4 h for 156 (50.3%), ≥4 and <5 h for 121 (39.0%), and ≥5 and <6 h for 20 (6.5%; Table 4).
Table 4.
Relationship between time from ICSI to ROA and time from ROA to second polar body extrusion in ROA oocytes that exhibited second polar body extrusion
| Time from ROA to second polar body extrusion (h) | ||||||
|---|---|---|---|---|---|---|
| <0.5 | <1 | <2 | <3 | ≥3 | ||
| Time from ICSI to ROA (h) | <3 | 2 (15.3%) | 2 (30.8%) | 6 (76.9%) | 3 (100%) | |
| 3 to <4 | 5 (3.2%) | 17 (14.1%) | 94 (74.4%) | 30 (93.6%) | 10 (100%) | |
| 4 to <5 | 5 (4.1%) | 13 (14.9%) | 70 (72.7%) | 24 (92.6. %) | 9 (100%) | |
| ≥5 | 1 (5.0%) | 1 (10.0%) | 15 (85.0%) | 2 (95.0%) | 1 (100%) | |
Actual numbers refer to the number of oocytes, % values refer to cumulative second polar body extrusion rates by time from ICSI to ROA; r = 0.071, P = 0.21
ICSI intracytoplasmic sperm injection, ROA rescue artificial oocyte activation
The cumulative second polar body extrusion rates between ROA and second polar body extrusion for ROA oocytes that exhibited second polar body extrusion were 4.2% at <30 min after ROA, 14.8% at <1 h, 74.5% at <2 h, and 93.5% at <3 h (mean time: 98 ± 59 min). We observed no relationships between the time from ICSI to ROA and time from ROA to second polar body extrusion (P = 0.071). However, a shorter time from ICSI to ROA was associated with a larger percentage of oocytes that exhibited second polar body extrusion within an hour after ROA.
The comparison of relative frequency distribution between ICSI and second polar body extrusion in spontaneously activated oocytes and the time from ROA to second polar body extrusion in ROA oocytes revealed that the result was approximately 20 min shorter for ROA oocytes. Moreover, the percentage of oocytes that exhibited second polar body extrusion within 30 min was slightly higher for ROA oocytes (Fig. 3). We observed no relationships among ICSI to ROA time with fertilization, blastocyst development, good quality blastocyst development, implantation, or miscarriage rates (Table 5).
Fig. 3.
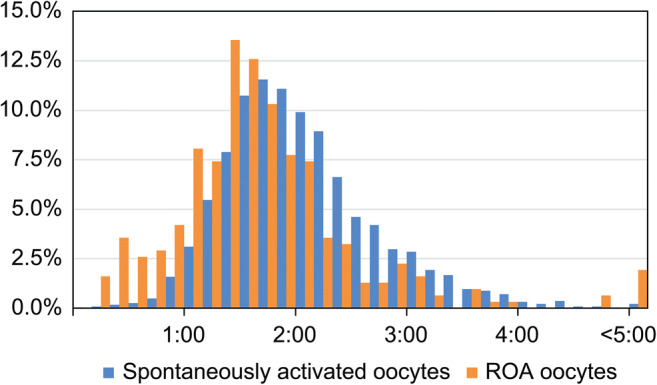
Relative frequency comparison of time from ICSI until second polar body extrusion in spontaneously activated oocytes and time from ROA until second polar body extrusion in ROA oocytes. ROA, rescue artificial oocyte activation
Table 5.
Fertilization rates, blastocyst development rates, and clinical outcome by time from ICSI to ROA
| <4 h | ≥4 h | P value | |
|---|---|---|---|
| ROA oocytes | 169 | 141 | |
| 1PN rate, n (%) | 23 (13.6%) | 17 (12.1%) | 0.46 |
| 2PN rate, n (%) | 132 (78.1%) | 118 (83.7%) | |
| 3PN or higher rate, n (%) | 7 (4.1%) | 4 (2.8%) | |
| 0PN rate, n (%) | 7 (4.1%) | 2 (1.4%) | |
| Blastocyst development rate, n (% per 2PN) | 67 (50.7%) | 57 (48.3%) | 0.98 |
| Good quality blastocyst development rate, n (% per 2PN) | 28 (21.2%) | 23 (19.5%) | 1.00 |
| Single-blastocyst transfer number | 15 | 10 | |
| implantation rate, n (% per blastocyst transfer) | 6 (40.0%) | 3 (30.0%) | 0.93 |
| Miscarriage rate, n (% per clinical pregnancy) | 2 (33.3%) | 2 (66.7%) | 0.81 |
| Live birth rate, n (% per blastocyst transfer) | 4 (26.7%) | 1 (10.0%) | 0.61 |
ICSI intracytoplasmic sperm injection, ROA rescue artificial oocyte activation
Discussion
Frequency distribution for the time between ICSI and second polar body extrusion exhibited isolated bimodality for the spontaneously activated and ROA oocytes. In the ROA oocytes, fertilization progressed and second polar body extrusion occurred as a result of ROA. The fertilization rate of the ROA oocytes was significantly higher than the non-ROA oocytes, thereby proving the efficacy of performing ROA within 6 h following ICSI. We observed sperm nuclei exhibiting PCC in many oocytes unfertilized on the day following ICSI, indicating that many cases of unfertilized oocytes were caused by activation failure [9–11]. The second polar body extrusion rate for ROA oocytes was high (78.5%), indicating efficient activation even in oocytes that exhibited activation failure. Thus, performing ROA could rescue many activation-impaired oocytes.
Even if the second polar body was not released, some eggs did not undergo ROA, but the second polar body extrusion occurred after the final determination of the second polar body release. However, this percentage was low in our study (5.3%). Enjoji et al. [7] reported that after observing the second polar body for 4.5–5 h after ICSI, ROA was performed on some oocytes that did not exhibit second polar body extrusion, resulting in fertilization of 73% and 41% oocytes that did and did not undergo ROA, respectively. The lower fertilization rates for non-ROA oocytes in our study may be due to the fact that a different method was used to assess whether second polar body extrusion had occurred. In the present study, we used a time-lapse incubator to observe second polar body extrusion, and we observed the spindle body of oocytes for which the second polar body was unclear. Therefore, we were able to accurately determine whether second polar body extrusion had occurred. This may have resulted in a lower fertilization rate for the non-ROA oocytes.
AOA for cases that have exhibited low fertilization rates in the past is usually performed immediately after to 30 min after ICSI [12]. Performing activation at a later time point is likely to result in poorer development. We performed ROA 2.5 to <6 h after ICSI, and our results suggested that performing ROA within this range does not greatly affect fertilization and development. However, the development of ROA oocytes is poorer than that of spontaneously activated oocytes. This may be due to oocyte aging because fertilization does not progress or due to sperm changes within the ovum. Although reports reveal that longer times from oocyte retrieval to ICSI and from denudation to ICSI result in lower fertilization and pregnancy rates [13–15], other reports indicate that there are no effects until 9 [16] or 12 h [17] following ICSI. Thus, no consensus has been reached regarding how delayed ICSI timing affects embryo development. The development of ROA oocytes may be impaired as activation is more delayed for these oocytes than spontaneously activated oocytes that undergo ICSI. Moreover, it takes a significant amount of time after sperm injection before activation is stimulated in ROA oocytes. During this time, more oocytes may exhibit PCC of the sperm nucleus, which could also impair development. The 1PN rate for ROA oocytes may have been higher than that for spontaneously activated oocytes because oocytes into which sperm had not entered were activated, resulting in female 1PN oocytes. Moreover, the sperm nucleus already exhibited PCC, making it impossible for some oocytes to form male PN. When performing ROA on the day after ICSI or later, development is extremely poor even after achieving a fertilized oocyte. Therefore, it is important to perform ROA as quickly as possible, before the sperm exhibits PCC.
Oocytes that underwent ROA within 3 h following ICSI tended to exhibit second polar body extrusion within a short period of time. Thus, it considered that more oocytes that underwent ROA within 3 h after ICSI underwent fertilization without ROA being performed compared to the oocytes that underwent ROA 3 h after ICSI and thereafter. The oocytes for which second polar body extrusion was observed within 0.5 h after ROA appeared to have included a large number of oocytes that would fertilize even if ROA was not performed. This accounts for 15% of the oocytes that underwent ROA within 3 h following ICSI and approximately 4% of the oocytes that underwent ROA ≥3 h following ICSI. When we compared the time between ICSI and second polar body extrusion in spontaneously activated oocytes with that between ROA and second polar body extrusion in ROA oocytes, it was found to be approximately 20 min earlier for ROA oocytes. This is possibly because in a certain number of ROA oocytes, while the sperm was injected into the cytoplasm and decondensation of the sperm nucleus occurred, metaphase-promoting factor activation did not decrease and fertilization progression had stopped. Thus, when performing ROA at least 3 h following ICSI, when using a time-lapse incubator to observe polar body dynamics to accurately determine second polar body extrusion based on observation of the spindle body, if second polar body extrusion is unclear, the rate of unnecessary ROA procedures could be kept to ≤4%.
Although we observed no differences in embryo development of ROA oocytes at 2.5–6 h following ICSI, as few oocytes underwent ROA at least 5 h following ICSI, there is a possibility that development was impaired in such embryos. Thus, our results suggest optimal timing for performing ROA to maximize ROA procedures while minimizing the effects on embryo development 3–5 h following ICSI. To the best of our knowledge, this report is the first to prove the efficacy of ROA including the evaluation of embryo development.
As approximately 10% oocytes that undergo ICSI also undergo ROA, performing ROA will not necessarily greatly increase the overall ICSI fertilization rate. However, if good quality oocytes with no second polar body extrusion at least 4 h following ICSI are left, >95% will not fertilize. It appears that the likelihood of a live birth could increase by performing ROA, achieving a large number of fertilized oocytes for cases in which only a small number of oocytes can be retrieved and cases in which many oocytes do not exhibit second polar body extrusion.
Reports have confirmed the safety of AOA indicating that performing AOA using calcium ionophore does not increase the number of abnormal embryos or fetuses [5, 18, 19]. If AOA was performed on all oocytes directly after ICSI, ROA will not be required. However, due to the ineffectiveness of AOA for cases that do not exhibit a low fertilization rate in the previous cycle [20, 21], performing ROA only on oocytes that require the procedure appears to be effective.
Although we observed no significant differences in terms of implantation and miscarriage rates of ROA and spontaneously activated oocytes, the implantation rate was low for ROA oocytes and miscarriage rate tended to be high; the effects of delayed activation on DNA are unknown. Therefore, we need to carefully select the cases eligible for ROA.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yuu Fukasaku, Naoko Hayashi, Nozomi Miyazaki, Hiroaki Kawato, and Hiroyuki Minoura. The first draft of the manuscript was written by Takashi Shibahara, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Availability of data and material
Not applicable
Code availability
Not applicable
Declarations
Ethics approval
Approval was obtained from the ethics committee of Minoura Ladies Clinic. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained verbally from all individual participants included in the study.
Consent for publication
The participants verbally consented to the submission to the journal.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murugesu S, Saso S, Jones BP, Bracewell-Milnes T, Athanasiou T, Mania A, et al. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertil Steril. 2017;108:468–82.e3. doi: 10.1016/j.fertnstert.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Lu Q, Zhao Y, Gao X, Li Y, Ma S, Mullen S, Critser JK, Chen ZJ. Combination of calcium ionophore A23187 with puromycin salvages human unfertilized oocytes after ICSI. Eur J Obstet Gynecol Reprod Biol. 2006;126:72–76. doi: 10.1016/j.ejogrb.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Sakurai M, Watanabe S, Tanaka T, Matsunaga R, Yamanaka N, Kamihata M, Kani C, Kuwahata A, Ochi M, Horiuchi T. Effect of artificial oocyte activation by calcium ionophore on one-day-old unfertilized oocytes after ICSI. J Mamm Ova Res. 2015;32:115–120. doi: 10.1274/jmor.32.115. [DOI] [Google Scholar]
- 4.Lu Q, Chen X, Li Y, Zhang XH, Liang R, Zhao YP, et al. A live birth of activated one-day-old unfertilized oocyte for a patient who experienced repeatedly near-total fertilization failure after intracytoplasmic sperm injection. Chin Med J. 2012;125:546–548. [PubMed] [Google Scholar]
- 5.Liu Y, Han XJ, Liu MH, Wang SY, Jia CW, Yu L, Ren G, Wang L, Li W. Three-day-old human unfertilized oocytes after in vitro fertilization/intracytoplasmic sperm injection can be activated by calcium ionophore A23187 or strontium chloride and develop to blastocysts. Cell Rep. 2014;16:276–280. doi: 10.1089/cell.2013.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capalbo A, Ottolini CS, Griffin DK, Ubaldi FM, Handyside AH, Rienzi L. Artificial oocyte activation with calcium ionophore does not cause a widespread increase in chromosome segregation errors in the second meiotic division of the oocyte. Fertil Steril. 2016;105:807–14.e2. doi: 10.1016/j.fertnstert.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Enjoji M, Muroi M, Takamizawa S. Yanagida K Clinical application of calcium ionophore (A23187) oocyte activation in fertilization failure after ICSI. J Mamm Ova Res. 2015;32:29–35. doi: 10.1274/jmor.32.29. [DOI] [Google Scholar]
- 8.Gardner DK, Lane M, Stevens J, Schlenker T. Schoolcraft WB Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty SP, Payne D, Swann NJ. Mattews CD Aetiology of failed and abnormal fertilization after intracytoplasmic sperm injection. Hum Reprod. 1995;10:2623–2629. doi: 10.1093/oxfordjournals.humrep.a135757. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbusch BE. Frequency and patterns of premature sperm chromosome condensation in oocytes failing to fertilize after intracytoplasmic sperm injection. J Assist Reprod Genet. 2000;17:253–259. doi: 10.1023/A:1009454231659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmiady H, Tandler-Schneider A. Kentenich H Premature chromosome condensation of the sperm nucleus after intracytoplasmic sperm injection. Hum Reprod. 1996;11:2239–2245. doi: 10.1093/oxfordjournals.humrep.a019083. [DOI] [PubMed] [Google Scholar]
- 12.Vanden Meerschaut F, Nikiforaki D, Heindryckx B. De Sutter P Assisted oocyte activation following ICSI fertilization failure. Reprod BioMed Online. 2014;28:560–571. doi: 10.1016/j.rbmo.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Andrews MM, Fishel SB, Rowe PH, Berry JA, Lisi F. Rinaldi L Analysis of intracytoplasmic sperm injection procedures related to delayed insemination and ejaculated, epididymal and testicular spermatozoa. Reprod BioMed Online. 2001;2:89–97. doi: 10.1016/S1472-6483(10)62231-6. [DOI] [PubMed] [Google Scholar]
- 14.Falcone P, Gambera L, Pisoni M, Lofiego V, De Leo V, Mencaglia L, et al. Correlation between oocyte preincubation time and pregnancy rate after intracytoplasmic sperm injection. Gynecol Endocrinol. 2008;24:295–299. doi: 10.1080/09513590802095613. [DOI] [PubMed] [Google Scholar]
- 15.Patrat C, Kaffel A, Delaroche L, Guibert J, Jouannet P, Epelboin S, et al. Optimal timing for oocyte denudation and intracytoplasmic sperm injection. Obstet Gynecol Int. 2012;2012:403531. doi: 10.1155/2012/403531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanagida K, Yazawa H, Katayose H, Suzuki K, Hoshi K. Sato A Influence of oocyte preincubation time on fertilization after intracytoplasmic sperm injection. Hum Reprod. 1998;13:2223–2226. doi: 10.1093/humrep/13.8.2223. [DOI] [PubMed] [Google Scholar]
- 17.Rienzi L, Ubaldi F, Anniballo R, Cerulo G. Greco E Preincubation of human oocytes may improve fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13:1014–1019. doi: 10.1093/humrep/13.4.1014. [DOI] [PubMed] [Google Scholar]
- 18.Deemeh MR, Tavalaee M. Nasr-Esfahani MH Health of children born through artificial oocyte activation: a pilot study. Reprod Sci. 2015;22:322–328. doi: 10.1177/1933719114542017. [DOI] [PubMed] [Google Scholar]
- 19.Miller N, Biron-Shental T, Sukenik-Halevy R, Klement AH, Sharony R, Berkovitz A. Oocyte activation by calcium ionophore and congenital birth defects: a retrospective cohort study. Fertil Steril. 2016;106:590–6.e2. doi: 10.1016/j.fertnstert.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Caglar Aytac P, Kilicdag EB, Haydardedeoglu B, Simsek E, Cok T. Parlakgumus HA Can calcium ionophore "use" in patients with diminished ovarian reserve increase fertilization and pregnancy rates? A randomized, controlled study. Fertil Steril. 2015;104:1168–1174. doi: 10.1016/j.fertnstert.2015.07.1163. [DOI] [PubMed] [Google Scholar]
- 21.Montag M, Köster M, van der Ven K. Bohlen U, van der Ven H The benefit of artificial oocyte activation is dependent on the fertilization rate in a previous treatment cycle. Reprod BioMed Online. 2012;24:521–526. doi: 10.1016/j.rbmo.2012.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable
Not applicable


