Abstract
AMP-activated protein kinase (AMPK) is a central regulator of both lifespan and health across multiple model organisms. β-Guanidinopropionic acid (GPA) is an endogenous AMPK activator previously shown to improve metabolic function in young and obese mice. In this study, we tested whether age of administration significantly affects the physiological outcomes of GPA administration in mice. We report that intervention starting at 7–8 months (young) results in activation of AMPK signaling and a phenotype consisting of lower body mass, improved glucose control, enhanced exercise tolerance, and altered mitochondrial electron transport chain flux similar to previous reports. When GPA treatment is started at 18–19 months (old), the effect of GPA on AMPK signaling is blunted compared to younger mice despite similar accumulation of GPA in skeletal muscle. Even so, GPA administration in older animals delayed age-related declines in lean mass, improved measures of gait performance and circadian rhythm, and increased fat metabolism as measured by respiratory exchange ratio. These results are likely partially driven by the relative difference in basal function and metabolic plasticity between young and old mice. Our results suggest that age-related declines in AMPK sensitivity may limit potential strategies targeting AMPK signaling in older subjects and suggest that further research and development is required for AMPK activators to realize their full potential.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00372-8.
Keywords: Aging, Beta-guanidinopropionic acid, Healthspan, AMPK
Introduction
The aging process promotes deterioration of physiological function at all levels of biological organization ultimately leading to increased morbidity and mortality. Developments in public health and medicine have led to a steady increase in life expectancy over the last several decades. However, the aging of the global population continues to increase and prolonging the functional disease-free period of life known as healthspan will significantly impact overall health. While there is still some question as to whether comprehensive markers of healthy aging can be assessed [1], it is clear that many age-related pathologies are linked to increased overall mortality. Interventions capable of modifying the development of such pathologies are likely to be of benefit in improving healthspan and healthy aging overall.
AMP-activated protein kinase (AMPK) is a highly conserved sensor of cellular energy status that has been shown to regulate the aging process [2]. Under conditions of energetic stress, such as exercise, cytosolic ATP is consumed by biochemical processes resulting in increased AMP:ATP ratio [3–5]. This increase in the ratio of AMP:ATP activates AMPK and a subsequent downstream signaling cascade responsible for stimulating catabolic pathways and inhibiting anabolic pathways in order to recover ATP levels [3–5]. At the cellular level, activation of AMPK has been demonstrated to regulate mitochondrial quality and function by promoting mitochondrial biogenesis and mitophagy [3, 5]. At the organismal level, overexpression of orthologs of AMPK has been reported to increase lifespan in both C. elegans [6] and Drosophila [7]. Similarly, pharmaceutical activation of AMPK by the antidiabetic drug metformin [8] has also been reported to extend the lifespan of C. elegans [9] though results in mice have been equivocal [10, 11].
β-Guanidinopropionic acid (GPA) is produced endogenously as part of creatine synthesis as the transamidation product of arginine and β-alanine — a reaction catalyzed by L-arginine:glycine amidinotransferase (AGAT) [12, 13]. Endogenous GPA concentrations in mouse tissue have been reported for muscle (~0.3 ng/mg), serum (< 0.013 μmol), and liver (0.218 ng/mg) with urinary excretion reported as 6.30 μmol/g creatinine [14, 15]. GPA has been reported to compete with creatine for uptake and act as a competitive inhibitor of the cytosolic creatine kinase (CK) [16, 17]. Under energetic demand, the creatine phosphokinase system serves to buffer ATP pools in the cell by transfer of high energy phosphate groups from phosphocreatine to ADP and AMP [17]. Inhibition of this system by GPA has been shown to result in an increase in the cellular AMP:ATP ratio resulting in AMPK activation [18–21].
In previous reports, GPA has been shown to modulate metabolic function including glucose metabolism and mitochondrial biogenesis [18, 21–24]. GPA has been reported to increase glucose disposal rate in rhesus macaques and reduce plasma glucose levels in multiple murine models of hyperglycemia including ob/ob and KKAy mice [22]. Previous work in young rodents has shown that oral supplementation of GPA reduces body weight, increases exercise endurance, and produces a shift towards oxidative muscle fibers in vivo [19]. Increased mtDNA content has also been reported with GPA treatment in young rodents [18]. Notably, it has been reported that sensitivity of AMPK to energetic stress becomes blunted with age. Old (28 month) male rats were reported to exhibit reduced skeletal muscle AMPK activation relative to young (3 month) rats in response to exercise or AMPK activators including AICAR and GPA [25]. Similarly, aging is also associated with accumulation of mitochondrial genome deletions in skeletal muscle that may contribute to loss of muscle fibers [26]. Likewise, GPA has been reported to increase the rate of ETC abnormal fibers leading to reductions in skeletal muscle fiber number in 30-month-old male rats [26]. These results highlight the asymmetry in outcomes mediated by GPA in the context of aging and serve to highlight the need for additional aging studies to better understand the many molecular and physiological consequences of AMPK activating therapies across age. In this study, we tested whether the effects of GPA on physiological function were similar when administered to young or old mice. Moreover, we asked whether GPA-mediated activation of AMPK may be a way to compress morbidity and ultimately improve health and function in old age.
Methods
Animals
All studies used male and female HET3 mice bred using dams of CByB6F1/J, JAX stock #100009 (dams, BALB/cByJ; sires, C57BL/6J) and sires of C3D2F1/J, JAX stock #100004 (dams, C3H/HeJ; sires, DBA/2J). The breeding plan to generate these mice has been described in detail elsewhere [27]. Young male (7 months, 21–24 weeks treatment, n = 10 Ctrl and 15 GPA to start), young female (8 months, 24–27 weeks treatment, n = 15 per group to start), old male (18–19 months, 17–22 weeks treatment, n = 13 Ctrl and 11 GPA to start), and old female (19 months, 25–26 weeks treatment, n = 10 per group to start) mice were assigned to either 1% w/w GPA (TestDiet; 5LG6/300 ppm GPA) or control (Lab Supply; 5LG6) diet ad libitum and housed at up to 5 mice per cage. Quantitative MRI was performed monthly during the treatment period using an EchoMRI (Echo Medical Systems). Food consumption was calculated weekly on a per cage basis. All animal experiments were approved by the Institutional Animal Care and Use Committees at UTHSCSA. During the course of treatment, 2 young male GPA, 1 old female control, 4 old female GPA, 6 old male control, and 4 old male GPA mice died and were not included in subsequent measures. Data presented in this paper represent the total number of animals alive and tested at each assessment point.
Liquid chromatography-tandem mass spectrometry
Reference standards (GPA, Nicotinamide) and all other reagents were purchased from Sigma. The HPLC system consisted of a Shimadzu SIL 20A HT autosampler, LC-20AD pumps (2), and an AB Sciex API 3200 tandem mass spectrometer with turbo ion spray. The LC analytical column was a C18 Excel 3 ACE (3 × 75 mm, 3 micron) purchased from MacMod (Chadds Ford, PA, USA) and was maintained at 25 °C during the chromatographic runs using a Shimadzu CT-20A column oven. Mobile phase A contained 99.9% H2O with 0.1% formic acid. Mobile phase B contained 99.9% acetonitrile with 0.1% formic acid. The flow rate of the mobile phase was 0.5 mL/min. GPA was eluted isocratically with 70% mobile phase A and 30% mobile phase B. The GPA transition was detected in positive mode at 132.016 Da (precursor ion) and the daughter ion was detected at 72.1 Da. The internal standard (Nicotinamide) transition was detected at 122.9 Da (precursor ion) and the daughter ion was detected at 79.9 Da. GPA and Nicotinamide stock solutions were prepared in Millipore water at a concentration of 1 mg/mL and stored in aliquots at – 80 °C. Working stock solution were prepared each day from the stock solutions at concentrations of 100 and 10 μg/mL and used to spike the calibrators. GPA was quantified in homogenate prepared from mouse quadriceps. Calibrator samples were prepared at the time of sample analysis by spiking untreated muscle tissue samples to achieve final concentrations of 0, 5, 10, 20, 100, 200, 600, and 3000 ng/mg. All muscle samples were homogenized and mixed with 10 μL of 100 μg/mL internal standard (Nicotinamide) and 300 μL of mobile phase A in 1.5 mL microcentrifuge tubes. Samples were vortexed vigorously and centrifuged at 13,000×g for 5 min at 25 °C. Supernatants were filtered in microfilterfuge tubes and 10 μL of the final extracts were injected into the LC/MS/MS. The ratios of GPA:Nicotinamide peak areas for each unknown sample were compared against a linear regression of the ratios obtained from calibration samples for quantification. The lower limit of detection was established at 5 ng/mg muscle.
Protein expression
Skeletal muscle protein was extracted from snap frozen gastrocnemius with RIPA (11 μL/mg) and homogenized at 30 Hz for 2 min by ball homogenizer (Qiagen). Total protein was quantified using Pierce BCA assay (Bio-Rad). Electrophoresis was performed using Criterion TGX precast gels (Bio-Rad). Blots were transferred to PVDF membranes (Bio-Rad). Total protein was quantified by Ponceau S staining (Sigma) and imaged on a Perfection V39 flatbed scanner (Epson). Blots were then blocked with 2% BSA in TBST and stained at with primary antibody overnight at 4 °C [pAMPK (Cell Signaling CS2535 [40H9]), AMPK (Cell Signaling CS5831), pACC (Cell Signaling CS3661), ACC (Cell Signaling CS3676)]. Secondary staining was performed using goat anti-rabbit (Santa Cruz SC-2004) at room temperature for 1 h. Imaging was performed using Pierce ECL Plus Western Blotting Substrate (ThermoFisher) and a Typhoon FLA 7000 (Amersham). All quantifications were performed in ImageStudioLite (LI-Cor).
qPCR
DNA and RNA were extracted from snap frozen gastrocnemius using the Allprep DNA/RNA Mini Kit (Qiagen) and addition of 20 mg/mL Proteinase K (Sigma) in a buffer of 20 mM Tris-HCL, 5% Tween-20, 0.5% Triton-X100, and 30 mM EDTA. Quantification of 12s and 18s DNA was performed using RT2 SYBR Green Mastermix with ROX (Qiagen) on a 7900HT Fast Real-Time PCR System (ThermoFisher). Quantification of PGC1ɑ and Actin mRNA was performed using Luna Universal One-Step RT-qPCR mastermix (NEB) on a 7900HT Fast Real-Time PCR System (ThermoFisher). All samples were run in triplicate and quantified using the standard curve method. Primer sequences and cycling parameters are given in Supplemental Table 1.
Glucose metabolism
Glycated hemoglobin A1c (HbA1c) was measured using whole blood obtained at time of sacrifice on a DCA Vantage Analyzer (Siemens). Fasting (16 h overnight) blood glucose was measured in whole blood taken from tail vein using AimStrip Plus digital glucose meter. Glucose tolerance test was performed following 16 h overnight fast. Mice were administered 1.5 mg glucose/g body weight (Sigma) by intraperitoneal injection. Blood glucose was measured in blood from the tail vein at 15–30 min intervals after IP injection using AimStrip Plus digital glucose meter.
Plasma biomarkers
Plasma LBP was measured using Mouse LBP PikoKine ELISA Kit (BOSTER). Plasma C-peptide, insulin, active ghrelin, leptin, GIP, IL-6, MCP-1, and TNFα were measured using the MILLIPLEX MAP Mouse Metabolic Hormone Magnetic Bead Panel (Millipore-Sigma MMHMAG-44K) according to manufacturer’s instructions.
Performance measures
Gait, exercise tolerance, rotarod, grip, 24-h activity, and RER were assessed by the Integrated Physiology of Aging Core at the San Antonio Nathan Shock Center (San Antonio, TX, USA). Gait was analyzed using an ExerGait Treadmill (Columbus Instruments) and Treadscan Software (Clever Sys) at 10 cm/s after 2 days of training. Rotarod performance was measured using a 4–40 RPM increase in speed over 300 s on a Rotamex Rotarod (Columbus Instruments) following 4 days of training and averaged over eight consecutive trials. Grip strength was determined using a force measurement meter (Chatillon) with a mesh pull bar and Columbus Instruments Grip Strength Meter software (Columbus Instruments) and averaged over five consecutive trials. Exercise tolerance was determined by treadmill running using an Exer-3/6 treadmill (Columbus Instruments) following 2 days of training; 24-h activity and RER were recorded for individually housed animals using an Opto-M4 Activity meter (Columbus Instruments) and Oxymax-CLAMS monitoring system (Columbus Instruments), respectively.
High-resolution respirometry
High-resolution respirometry was carried out on using the OROBOROS O2K Oxygraph system (Oroboros Inst.). Soleus was carefully excised and stored in ice cold BIOPS buffer. Each muscle was cut in half perpendicular to fiber bundles and treated as biological replicates. Permeabilization was performed with saponin. O2 flux was measured in 2 mL Miro6-CRE medium at 37 °C; 1 mM L-malic acid and 0.25 mM DL-octanoylcarnitine are added to elicit ETF_L, 2.5 mM ADP w/MgCl2 for ETF_P, 10 mM L-glutamic acid for complex 1_P, and 10 mM succinate for complex 1&2_P. Additional ADP w/MgCl2 is added followed by 10 μM cytochrome C to evaluate mitochondrial membrane integrity. FCCP is then titrated in 1 μM steps to achieve complex 1&2_E, 1 μM rotenone for complex 2_E, and finally 5 mM malonic acid and 2.5 μM antimycin A to evaluate residual oxygen consumption. All solutions were prepared according to published protocols and using recommended suppliers [28, 29].
Electron microscopy
Snap frozen quadriceps were fixed in phosphate buffered 4% formaldehyde and 1% glutaraldehyde. Longitudinal 90 nm sections were prepared for transmission electron microscopy (TEM) by University of Texas Health San Antonio Electron Microscopy Laboratory using 1% Zetterquist’s buffered osmium tetroxide and embedded in PolyBed 812 resin. Samples were imaged on a JEOL 1400 TEM (JEOL). Three random fields were selected as biological replicates for each sample and cross-sectional area was manually evaluated using Fiji [30].
Citrate synthase activity
Skeletal muscle protein prepared as above was diluted to a concentration of 1 μg/μL in 0.1 M Tris-HCl pH 8.1; 10 μL of each sample was plated into a 96 well clear bottom plate (Corning) followed by 150 μL 83.3 μM Ellman’s reagent (DNTB), 0.25 mg/mL acetyl-CoA in 0.1 M Tris-HCl pH 8.1, and 10 μL of 10 mM oxaloacetate. Reactions were monitored at 412 nm and 5 s intervals for 5 min.
Statistical analysis
All statistical calculations were performed with GraphPad Prism 8 (GraphPad). Two-way ANOVAs were carried out as Sex*Treatment, with multiple comparisons corrected by Sidak’s method. Mixed effects analysis was carried out as either Time*Sex*Treatment or Time*Treatment as necessary. A full table of statistical results is available in the supplemental material (Table S2–S4).
Results
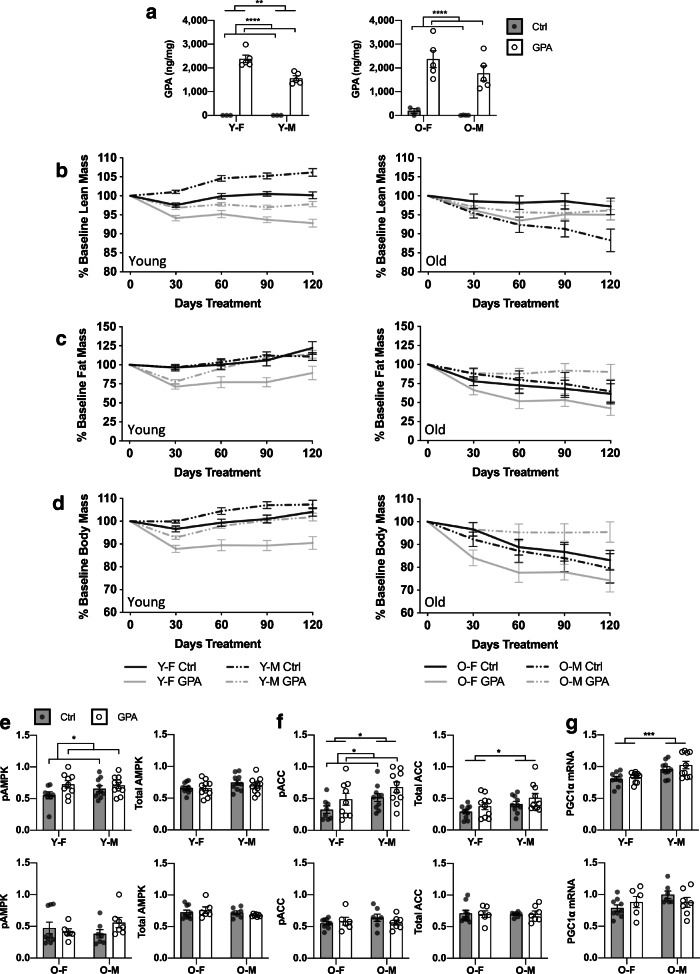
Previous studies in young rodents have reported that GPA treatment reduces body mass and increases the predominance of smaller oxidative muscle fibers [19, 21, 31]. In our study, we tested the effects of chronic (~5–6 month) GPA administration in either young (7–8 month) or old (18–19 month) HET3 mice. Skeletal muscle GPA concentrations were increased by supplementation to similar degrees in both young and old mice (Fig. 1a). In young mice, overall lean mass as determined by QMR was reduced by GPA treatment to a similar extent in both males and females. GPA also reduced fat mass in young mice, though males exhibited an initial decline and subsequent recovery suggesting duration of treatment may be particularly relevant to this measure. In contrast, fat mass was unaltered by GPA treatment in old animals. Lean mass was also unchanged in old females, though GPA treatment prevented a progressive loss in lean mass in old males (Fig. 1b–c). Fat and muscle wet weights collected at time of sacrifice were similar to in vivo measurements (Fig. S1B–H). These changes were also reflected in total body mass with GPA generally lowering body mass in young animals and while preserving body mass in old males relative to controls (Fig. 1d, S1A). Total food consumption was reduced with GPA when raw values were compared (Fig. S2A); however, when normalized to body weight, there was little effect of GPA on relative food consumption (Fig. S2B).
Fig. 1.
GPA concentration in quadriceps was determined by LC-MS-MS (a). Lean (b) and fat (c) mass were determined by QMR in for young and old HET3 mice treated with control or 1% GPA. Body mass as percentage of baseline (d) was also collected at time of QMR. Analysis was performed by mixed effects model. AMPK signaling was evaluated by measuring expression phospho- and total-AMPK (e) and ACC (f) in gastrocnemius. PGC1ɑ mRNA (g) was also measured in gastrocnemius. All data displayed as mean ± SEM. Data in (a, e–g) analyzed by 2-way ANOVA. n = 3–5/group (a), n = 7–10 old, 10–15 young (b–d), n = 6–9 old, 8–10 young (e–g). Selected statistical results for (b–d) as follows: b [(young: Treatment = ****, Time × Treatment = ****, Time × Sex × Treatment = ns) and (old: Treatment = ns, Time × Treatment = ns, Time × Sex × Treatment = *)]. c [(young: Treatment = **, Time × Treatment = **, Time × Sex × Treatment = **) and (old: Treatment = ns, Time × Treatment = ns, Time × Sex × Treatment = ns)]. d [(young: Treatment = ****, Time × Treatment = ****, Time × Sex × Treatment = ns) and (old: Treatment = ns, Time × Treatment = ns, Time × Sex × Treatment = *)]. * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, **** = p ≤ 0.0001. Y-F, young female; Y-M, young male; O-F, old female; O-M, old male
AMPK activation is considered to be the canonical mechanism of action for GPA [19, 21]. AMPK phosphorylation was increased in gastrocnemius of young but not old animals (Fig. 1e, Fig. S3A–B). Acetyl-CoA carboxylase (ACC) phosphorylation, a direct effector of AMPK signaling, was also increased in young but not old animals as a result of treatment (Fig. 1f, Fig. S3A–B). However, mRNA expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1a), a downstream target of AMPK, was unchanged by treatment at either age (Fig. 1g). Given that the concentration of GPA was similar in young and old skeletal muscle, these results suggest that the observed differences in GPA-mediated AMPK activation arise as a result of age-related changes in the sensitivity of AMPK to stimuli not differences in GPA uptake.
AMPK activation is associated with improved glucose uptake and decreased gluconeogenesis [3, 5, 32]. GPA treatment did not significantly alter glucose clearance or fasting blood glucose in either young or old animals (Fig. 2a–c). However, glycated hemoglobin A1c (HbA1c) was reduced with GPA in young but not old animals with a greater effect observed in males (♂p = 0.006 vs. ♀p = 0.3600) (Fig. 2d). Plasma concentrations of C-peptide and insulin were increased with GPA in old, but not young mice (Fig. 2e–f). Similarly, active ghrelin, leptin, and gastric inhibitory polypeptide (GIP) were also elevated following GPA treatment in old animals only (Fig. 2g–i). Notable sex effects were also observed in response to GPA intervention in old animals with old males exhibiting a larger increase in C-peptide levels (♂p = 0.0253 vs. ♀p = 0.5246) (Fig. 2e), and old females displaying increased active ghrelin (p = 0.0463) (Fig. 2g). Together, these results indicate that GPA mediates dissimilar metabolic alterations in young and old animals.
Fig. 2.
Results of glucose tolerance test (GTT) (a), with quantification (b), and fasting blood glucose (c) in young and old HET3 mice treated with control or 1% GPA (n = 3–9 old, 9–10 young). %HbA1C (d) (n = 6–9 old, 10–15 young) obtained from whole blood at time of sacrifice. C-peptide (e), insulin (f), active ghrelin (g), leptin (h), and GIP (i) were measured in plasma using the Milliplex Metabolic Profile Panel (n = 3–9 old, 10–11 young). All data is displayed as mean ± SEM and analyzed by 2-way ANOVA. * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, **** = p ≤ 0.0001. Y-F, young female; Y-M, young male; O-F, old female; O-M, old male
To test whether GPA had effects on musculoskeletal function, we assessed gait, exercise tolerance, rotarod performance, and grip strength. Age-related decline in gait speed has been linked to increased morbidity and risk of mortality in humans [33]. In young mice treated with GPA, we found no significant alterations in gait (Fig. 3a–b, S4A–D). However, in old mice, GPA treatment significantly reduced forepaw swing time while leaving stride length unaltered indicating better overall gait parameters compared to untreated mice (Fig. 3a–b, S4E–H). Exercise tolerance, as measured by treadmill running, was improved in young mice treated with GPA; in contrast, GPA had no effect on exercise tolerance in old animals (Fig. 3c). Grip strength was not significantly altered by treatment at either age (Fig. 3d). GPA treatment reduced rotarod latency in old animals, particularly in old males (♂p = 0.0070 vs. ♀p = 0.9602) (Fig. 3e). However, GPA-treated old males were heavier than controls at this stage and increased body mass is known to be associated with reduced rotarod performance. Together, these results indicate that GPA mediates potentially beneficial but separate alterations to musculoskeletal function in young and old animals.
Fig. 3.
Average swing time and stride length for front (a) and rear (b) paws as determined by Treadscan (n = 5–9 old, 10 young per group). Exercise tolerance including distance run and total work done (c) were determined by treadmill running (n = 3–9 old, 10 per group young). Grip strength (d) (n = 6–9 old, 10 per group young), rotarod latency (e) (n = 5–9 old, 10 per group young), and activity, including dark/light ratio (f) (n = 4–9 per group), were also assessed. All data is displayed as mean ± SEM and analyzed by 2-way ANOVA. * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, **** = p ≤ 0.0001. Y-F, young female; Y-M, young male; O-F, old female; O-M, old male
Circadian rhythm has been reported to deteriorate with age and may play an important role in promoting overall health and longevity [34]. Mice were evaluated for 24-h activity using beam breaks. GPA-treated old males and females exhibited reduced light cycle activity without significant alteration to dark cycle activity resulting in a significant increase in the dark/light ratio (Fig. 3f).
Development of chronic inflammation has been identified as a potential mediator of health and function in aging [35, 36]. In an effort to determine if GPA alters the development of systemic inflammation, we evaluated multiple markers of inflammation in old mice. mRNA expression of markers of senescence and senescence-associated secretory phenotype (SASP) were generally unchanged with GPA in gastrocnemius of old mice (Fig. S5A–I). Similarly, concentrations of Il-6, MCP-1, and TNFα in plasma were also unaltered by GPA treatment in old animals (Fig. S5J–L). Intestinal barrier function has been reported to decline with age leading to increased systemic inflammation and declines in overall health and function [37–39]. Plasma lipopolysaccharide-binding protein (LBP), a marker of intestinal permeability, was also not altered by GPA intervention at either age group (Fig. S5M). These results suggest that GPA does not generally influence the development of chronic inflammation in aged animals.
AMPK signaling is a key regulatory mechanism controlling mitochondrial bioenergetics including regulation of mitochondrial fuel preference and ATP production [3, 5, 32]. Notably, fatty acid oxidation (FAO) is regulated by AMPK via phosphorylation of ACC [3, 5, 32, 40]. To evaluate the effect of GPA on mitochondrial metabolism, we utilized the OROBOROS Oxygraph O2K system to measure flux capacity in permeabilized soleus. Electron-transferring flavoprotein complex (ETF) is localized to the inner mitochondrial membrane and forms a crucial link between FAO and ATP production. ETF-mediated respiration was reduced in the presence of ADP, but not absence, in muscles isolated from young animals treated with GPA (Fig. 4a–b). Similarly, complex 1-mediated respiration was also lower in young mice following GPA treatment, particularly in young males (♂p = 0.0141 vs. ♀p = 0.4476) (Fig. 4c). Glutamate flux control factor (FCF), an internally normalized measure of respiration in response to the complex 1 substrate glutamate, was unchanged in either age group with treatment while succinate FCF, an internally normalized measure of respiration in response to the complex 2 substrate succinate, was increased in young but not old mice (Fig. 4d–e). As an in vivo surrogate of mitochondria function, we also measured whole-animal respiration in GPA-treated and control mice. In vivo respiratory exchange ratio (RER) was significantly elevated in GPA-treated young females, but not males consistent with a predominance of carbohydrate metabolism, particularly during their light cycle (Fig. 4f). Conversely, both old males and females treated with GPA exhibited lower RER during the dark cycle, indicative of increased fat metabolism (Fig. 4g). Together, these results suggest that GPA mediates dissimilar adaptations in young and old mice in terms of ETC flux and mitochondrial fuel source. These results also highlight the effect of sex and circadian rhythm on these outcomes suggesting that GPA-mediated metabolic adaptations may be more complex than previously believed.
Fig. 4.
High-resolution respirometry (HRR) data were obtained from permeabilized soleus using the OROBOROS O2K system. Data is normalized to percent maximum respiration with baseline subtracted. Values are given for ETF-Leak (a), ETF-coupled (b), and complex 1-coupled (c) respiration. Internally normalized flux control ratios (FCF) for glutamate (d) and succinate (e) are also shown. HRR values (a–e) represent the mean of two biological replicates. Data in (a–e) displayed as mean ± SEM and analyzed by 2-way ANOVA (n = 6–9 old, 10 per group young). In vivo respirometry for respiratory exchange ratio (RER) (f–g) was analyzed by mixed effects model [(Y-F: Time = ****, Treatment = **, Time × Treatment = ns), (Y-M: Time = ****, Treatment = ns, Time × Treatment = ns), (O-F: Time = ****, Treatment = ns, Time × Treatment = *), (O-M: Time = ***, Treatment = *, Time × Treatment = ns)]. Shaded area = SEM, line = mean, (n = 4–9 old, 10 per group young). * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, **** = p ≤ 0.0001. Y-F, young female; Y-M, young male; O-F, old female; O-M, old male
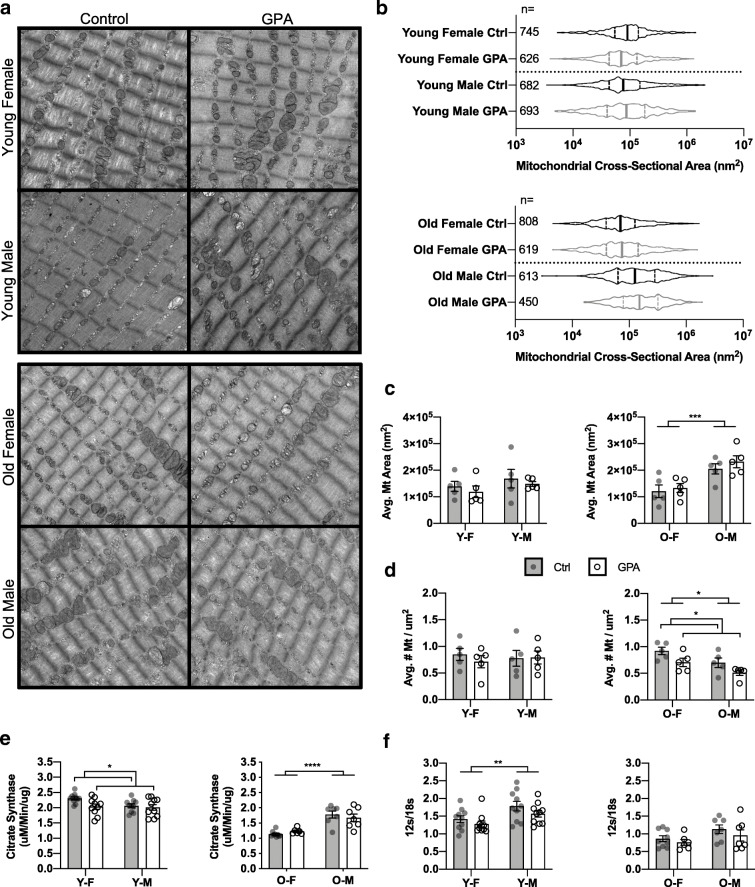
Skeletal muscle aging is associated with declines in mitochondrial quantity leading to reduced functional capacity [41]. Electron micrographs of quadriceps revealed no statistically significant alterations to average mitochondrial cross-sectional area at any age (Fig. 5a–c) though average number of mitochondria per μM2 was lower in old animals after GPA (Fig. 5d). Citrate synthase activity, a marker of mitochondrial content and key enzyme in the citric acid cycle, was unchanged in old animals but was lower in young with the strongest effect observed in young females (♂p = 0.6347 vs. ♀p = 0.0182) (Fig. 5e). The ratio of 12s/18s rRNA, a proxy for mitochondrial genome copy number, was unchanged in either age group (Fig. 5f). Together, these data indicate that in vivo mitochondrial content was relatively unchanged with treatment in either age group.
Fig. 5.
Example electron micrographs (a) were obtained from quadriceps of young and old HET3 mice treated with control or 1% GPA. Distribution of mitochondrial cross-sectional area (b) and quantification of average cross-sectional area (c) with average number of mitochondria per μM2 (d) are also shown (n = 5 per group). Values in (c–d) represent the mean of 3 random fields per animal. Citrate synthase activity (e) and 12s/18s rRNA ratio (f) were determined using snap frozen gastrocnemius (n = 6–9 old, 10 per group young). Data displayed as mean ± SEM (c–f). Comparisons by 2-way ANOVA. * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, **** = p ≤ 0.0001. Y-F, young female; Y-M, young male; O-F, old female; O-M, old male
Discussion
In this study, we expand upon the existing literature and evaluate the result of early and late-life supplementation with GPA on a subset of clinically relevant markers of health and function. The results presented here demonstrate that GPA leads to distinct outcomes that depend, in part, on the age at which treatment is initiated. The mechanism of action for GPA is reported to be akin to energetic stress and we hypothesize that the disparate outcomes reported here might be partially driven by the relative difference in basal function and metabolic plasticity between young and old mice. In particular, activation of AMPK by various stimuli has been reported to decline with age [25]. In our study, GPA supplementation resulted in similar increases in skeletal muscle GPA content in both young and old animals. However, increased AMPK activation was only apparent in young animals. These results are consistent with previous reports of age-related declines in AMPK sensitivity and may suggest that old animals require higher dosages of GPA, or AMPK activators in general, to achieve similar levels of AMPK activation compared to young animals.
Young animals treated with GPA exhibited altered body composition including reductions in both lean and fat mass despite similar food intake. These findings are largely consistent with previous reports [19]. In contrast to early-life administration, we report that GPA does not reduce lean mass in old mice, but in fact appears to preserve maintenance of basal body composition in old males. Several measures of physiological function also showed disparate responses to GPA that seem dependent on age of administration. For example, early-life intervention was found to result in enhanced exercise tolerance while late-life intervention leads to better gait performance and potential preservation of circadian rhythm — both notable for their correlation with overall morbidity and mortality in humans. These findings highlight the need to consider age of administration when evaluating translational application of GPA or other AMPK activators.
In our study, we observed no change in either fasting blood glucose or glucose tolerance as a result of either early- or late-life intervention. However, HbA1c levels were reduced in young mice treated with GPA suggesting improved overall glucose metabolism. GPA has been previously reported to increase GLUT4-mediated glucose uptake leading to lower fasting glucose and plasma insulin levels in young animals [19, 22, 24]. Interestingly, we found that plasma concentrations of C-peptide and insulin were increased in older mice treated with GPA. While increased insulin secretion is not necessarily indicative of insulin resistance, this result does suggest that greater concentrations of insulin are required to maintain normative glucose homeostasis in older mice treated with GPA. Further investigation of the potentially negative effects of chronic GPA intervention in old animals would be necessary to clarify these results.
Of significant interest to this study is the effect of GPA on mitochondrial function and energetics. Age-associated declines in fat oxidation have been previously reported [42] and are thought to result in lipid imbalance and adiposity in the elderly. Here, young mice treated with GPA had an RER suggestive of preference for carbohydrate-mediated metabolism. Moreover, young mice exhibited reduced ETC flux with respect to ETF-coupled and complex 1-coupled respiration in permeabilized muscle fibers though succinate FCF was increased. These results are similar to previous reports suggesting that GPA increases oxidative metabolism in young animals, particularly in muscle. In contrast, old mice treated with GPA showed no change in ETC flux in permeabilized muscle fibers though changes in RER are consistent with enhancement of fat oxidation. These results would seem to suggest that while GPA leads to changes in oxidative flux in skeletal muscle of young animals, in the context of aging, GPA may instead function to modify mitochondrial fuel preference and may be of interest in rescuing age-related losses in fat oxidation.
AMPK activation is capable of increasing mitochondrial biogenesis, and GPA has been previously reported to increase expression of mitochondrial oxidative enzymes [5, 18, 21, 32]. However, in our study, mitochondrial cross-sectional area and genome copy number were not altered by treatment in either young or old mice. Indeed, citrate synthase activity was lower, in young mice following GPA intervention. This suggests that the primary effect of GPA on mitochondria may be to alter fuel preference rather than increase overall mitochondrial content; an effect which may rely on the degree of AMPK activation.
In summary, we found that early-life intervention with GPA improved exercise tolerance, increased oxidative metabolism, and improved glucose metabolism consistent with previous reports. However, when delivered late in life, GPA supplementation mediates distinct outcomes consistent with delaying age-related declines in body composition, mitochondrial energetics, gait performance, and potentially circadian rhythm. We hypothesize that these results are potentially driven by the loss of basal function and metabolic plasticity that has been reported to occur with age. More broadly, our findings suggest that age-related declines in AMPK sensitivity may limit potential strategies targeting AMPK signaling in older subjects and suggest that further research and development is required for AMPK activators to realize their full potential.
Supplementary Information
(PDF 4145 kb)
Acknowledgements
Milliplex analyte panels were performed by Bioanalytics and Single-Cell Core at UT-Health San Antonio. Preparation of TEM images was performed by the Electron Microscopy Laboratory at UT-Health San Antonio. Gait, exercise tolerance, rotarod, grip, 24-h activity, and RER were performed by the Integrated Physiology of Aging Core at the San Antonio Nathan Shock Center (P30 AG013319). GPA concentrations were measured by Greg Friesenhahn of the Biological Psychiatry Analytic Laboratory at UT-Health San Antonio and the Analytical Pharmacology and Drug Evaluation Core of the San Antonio Nathan Shock Center. This material is the result of work supported with resources and the use of facilities at South Texas Veterans Health Care System, San Antonio, Texas. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Code availability
Not applicable.
Author contribution
JDD formulated the study. JDD, KMT, and BCG collected the data. JDD analyzed the results, created the figures, and drafted the manuscript. JDD and ABS edited the manuscript. JDD, KMT, BCG, and ABS reviewed and approved the manuscript.
Funding
This research was funded in part by R01 AG050797, R01 AG057431, and the Geriatric Research, Education and Clinical Center of the South Texas Veterans Health Care System and by the American Heart Association 19PRE34450033.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
All animal experiments were approved by the Institutional Animal Care and Use Committees at UTHSCSA.
Consent to participate
Not applicable.
Consent to publication
The authors consent to publication of the attached manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaeberlein M. How healthy is the healthspan concept? Geroscience. 2018;40(4):361–364. doi: 10.1007/s11357-018-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11(2):230–241. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418(2):261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332(6036):1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 5.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18(24):3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulgherait M, Rana A, Rera M, Graniel J, Walker DW. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014;8(6):1767–1780. doi: 10.1016/j.celrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5(1):e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, Magalhães JP, Martinez PA, McCord JM, Miller BF, Müller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15(5):872–884. doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tachikawa M, Hosoya K. Transport characteristics of guanidino compounds at the blood-brain barrier and blood-cerebrospinal fluid barrier: relevance to neural disorders. Fluids Barriers CNS. 2011;8(1):13. doi: 10.1186/2045-8118-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taes YE, Marescau B, De Vriese A, De Deyn PP, Schepers E, Vanholder R, et al. Guanidino compounds after creatine supplementation in renal failure patients and their relation to inflammatory status. Nephrol Dial Transplant. 2008;23(4):1330–1335. doi: 10.1093/ndt/gfm793. [DOI] [PubMed] [Google Scholar]
- 14.Marescau B, Deshmukh DR, Kockx M, Possemiers I, Qureshi IA, Wiechert P, de Deyn PP. Guanidino compounds in serum, urine, liver, kidney, and brain of man and some ureotelic animals. Metabolism. 1992;41(5):526–532. doi: 10.1016/0026-0495(92)90213-t. [DOI] [PubMed] [Google Scholar]
- 15.de Jonge WJ, Marescau B, D’Hooge R, De Deyn PP, Hallemeesch MM, Deutz NE, et al. Overexpression of arginase alters circulating and tissue amino acids and guanidino compounds and affects neuromotor behavior in mice. J Nutr. 2001;131(10):2732–2740. doi: 10.1093/jn/131.10.2732. [DOI] [PubMed] [Google Scholar]
- 16.Fitch CD, Shields RP, Payne WF, Dacus JM. Creatine metabolism in skeletal muscle. 3. Specificity of the creatine entry process. J Biol Chem. 1968;243(8):2024–2027. doi: 10.1016/S0021-9258(18)93544-1. [DOI] [PubMed] [Google Scholar]
- 17.Boehm EA, Radda GK, Tomlin H, Clark JF. The utilisation of creatine and its analogues by cytosolic and mitochondrial creatine kinase. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1996;1274(3):119–128. doi: 10.1016/0005-2728(96)00018-7. [DOI] [PubMed] [Google Scholar]
- 18.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99(25):15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oudman I, Clark JF, Brewster LM. The effect of the creatine analogue beta-guanidinopropionic acid on energy metabolism: a systematic review. PLoS One. 2013;8(1):e52879. doi: 10.1371/journal.pone.0052879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitch CD, Jellinek M, Fitts RH, Baldwin KM, Holloszy JO. Phosphorylated beta-guanidinopropionate as a substitute for phosphocreatine in rat muscle. Am J Physiol. 1975;228(4):1123–1125. doi: 10.1152/ajplegacy.1975.228.4.1123. [DOI] [PubMed] [Google Scholar]
- 21.Williams DB, Sutherland LN, Bomhof MR, Basaraba SA, Thrush AB, Dyck DJ, et al. Muscle-specific differences in the response of mitochondrial proteins to beta-GPA feeding: an evaluation of potential mechanisms. Am J Physiol Endocrinol Metab. 2009;296(6):E1400–E1408. doi: 10.1152/ajpendo.90913.2008. [DOI] [PubMed] [Google Scholar]
- 22.Meglasson MD, Wilson JM, Yu JH, Robinson DD, Wyse BM, de Souza CJ. Antihyperglycemic action of guanidinoalkanoic acids: 3-guanidinopropionic acid ameliorates hyperglycemia in diabetic KKAy and C57BL6Job/ob mice and increases glucose disappearance in rhesus monkeys. J Pharmacol Exp Ther. 1993;266(3):1454–1462. [PubMed] [Google Scholar]
- 23.Ohira Y, Ishine S, Tabata I, Kurata H, Wakatsuki T, Sugawara S, et al. Non-insulin and non-exercise related increase of glucose utilization in rats and mice. Jpn J Physiol. 1994;44(4):391–402. doi: 10.2170/jjphysiol.44.391. [DOI] [PubMed] [Google Scholar]
- 24.Pandke KE, Mullen KL, Snook LA, Bonen A, Dyck DJ. Decreasing intramuscular phosphagen content simultaneously increases plasma membrane FAT/CD36 and GLUT4 transporter abundance. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R806–R813. doi: 10.1152/ajpregu.90540.2008. [DOI] [PubMed] [Google Scholar]
- 25.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5(2):151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbst A, Wanagat J, Cheema N, Widjaja K, McKenzie D, Aiken JM. Latent mitochondrial DNA deletion mutations drive muscle fiber loss at old age. Aging Cell. 2016;15(6):1132–1139. doi: 10.1111/acel.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Strong R. An aging interventions testing program: study design and interim report. Aging Cell. 2007;6(4):565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 28.Gnaiger E, Fasching M, Fontana-Ayoub M. Selected media and chemicals for respirometry with mitochondrial preparations. Mitochondrial Physiol Netw. 2016 https://bioblast.at/index.php/MiPNet03.02_Chemicals-Media.
- 29.Gnaiger E, Fontana-Ayoub M, Fasching M. Mitochondrial respiration medium-MiR06. Mitochondrial Physiol Netw. 2018; https://wiki.oroboros.at/index.php/MiPNet14.13_Medium-MiR06.
- 30.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams GR, Haddad F, Baldwin KM. Interaction of chronic creatine depletion and muscle unloading: effects on postural locomotor muscles. J Appl Physiol. 1994;77(3):1198–1205. doi: 10.1152/jappl.1994.77.3.1198. [DOI] [PubMed] [Google Scholar]
- 32.Garcia D, Shaw RJ. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell. 2017;66(6):789–800. doi: 10.1016/j.molcel.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Froy O. Circadian rhythms, aging, and life span in mammals. Physiology (Bethesda). 2011;26(4):225–235. doi: 10.1152/physiol.00012.2011. [DOI] [PubMed] [Google Scholar]
- 35.Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Sanada F, Taniyama Y, Muratsu J, Otsu R, Shimizu H, Rakugi H, Morishita R. Source of chronic inflammation in aging. Front Cardiovasc Med. 2018;5:12. doi: 10.3389/fcvm.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 38.Schinaman JM, Rana A, Ja WW, Clark RI, Walker DW. Rapamycin modulates tissue aging and lifespan independently of the gut microbiota in Drosophila. Sci Rep. 2019;9(1):7824. doi: 10.1038/s41598-019-44106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng J, Zeng L, Lai X, Li J, Liu L, Lin Q, Chen Y. Metformin protects against intestinal barrier dysfunction via AMPKalpha1-dependent inhibition of JNK signalling activation. J Cell Mol Med. 2018;22(1):546–557. doi: 10.1111/jcmm.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50(Suppl):S138–S143. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seo DY, Lee SR, Kim N, Ko KS, Rhee BD, Han J. Age-related changes in skeletal muscle mitochondria: the role of exercise. Integr Med Res. 2016;5(3):182–186. doi: 10.1016/j.imr.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toth MJ, Tchernof A. Lipid metabolism in the elderly. Eur J Clin Nutr. 2000;54(Suppl 3):S121–S125. doi: 10.1038/sj.ejcn.1601033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 4145 kb)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.