Abstract
Purpose
The purpose of this study is to explore the reproductive outcomes of women with Turner syndrome (TS) in preimplantation genetic testing (PGT) cycles.
Methods
A retrospective study of 100 controlled ovarian stimulating cycles, 68 TS (sixty-four mosaic Turner syndrome (MTS) and four pure Turner syndrome (PTS)) women underwent PGT was conducted from 2013 to 2018.
Results
Embryo X chromosome abnormal rates of TS women were significantly higher than women with normal karyotype (7.04 vs 1.61%, P<0.01). Cumulative live birth rates (CLBR) after PGT-NGS treatment were lower in TS than control (31.15 vs 45.59%, P<0.05). Clinical pregnancy rates per transfer (CPR), miscarriage rates (MR) and live birth rates per transfer (LBR) remained comparable between TS and control group. Reproductive outcomes (X chromosome abnormal rates, CPR, MR, LBR and CLBR) among low (<10%), medium (10–50%) and high (>50%) level 45,X mosaicism groups were not statistically different.
Conclusions
To avoid high risk of embryo X chromosome abnormalities, prenatal or preimplantation genetic testing should be recommended to mosaic or pure TS patients.
Keywords: Aneuploidy,; Live birth rate,; Mosaicism,; Preimplantation genetic screening,; Turner syndrome (TS)
Introduction
Turner syndrome (TS) is a chromosomal aberration with a total or partial loss of one X chromosome, affecting 1/2000 to 1/2500 newborn girls [1]. About 40–50% of the patients are classical TS carrying monosomy X (45,X); the remaining are mosaic cases in whom normal and abnormal cell lines exist together. Of the patients, 15–25% are monosomy X mosaicism with normal cell lines, 3% with triple X and 10–12% mixed with 46,XY [2]. Other than mosaicism, there are patients with X chromosome partial abnormality such as isochromosome Xq or isodicentric Xp (10%), ring X chromosome and deletion of Xp22.3 [2].
Due to the absence of total or partial X chromosome, TS patients show a series of symptoms, such as growth failure, short stature, lymphedema sequence (web neck), cardiac and renal anomalies [3, 4]. The most common features of TS in reproduction are pubertal delay or failure, infertility and premature ovarian insufficiency (POI) [5]. Reproductive treatment strategies for these patients include fertility preservation in teenage girls with ovarian follicles remaining [6] or receiving oocyte donation while suffering POI [7, 8]. Although correlation between genotype and phenotype is not well researched, mosaic case presents milder phenotypic abnormalities compared with those with 45,X karyotype [9–11]. Patients show milder symptoms would maintain proper ovarian function and may be able to get pregnant with their own oocytes. Research showed spontaneous pregnancies occurred in 4.8–7.6% of women with TS, but miscarriage rates were higher (30.8–45.1%), probably due to higher chances of aneuploidy and lower maternal endometrium receptivity [12]. Considering the higher risk of embryo chromosomal abnormalities, the 2016 Cincinnati guidelines recommended either prenatal genetic or preimplantation genetic testing in TS patients undergoing fertility treatments [2]. However, as far as we know, there is little information about the impact of preimplantation genetic testing in fertility treatments to TS patients.
The purpose of this study was to evaluate reproductive outcomes of PGT treatment with autologous oocytes in different level mosaicism TS patients, which may provide reliable data for therapeutic strategy in clinical assisted reproductive treatment.
Materials and methods
Study population
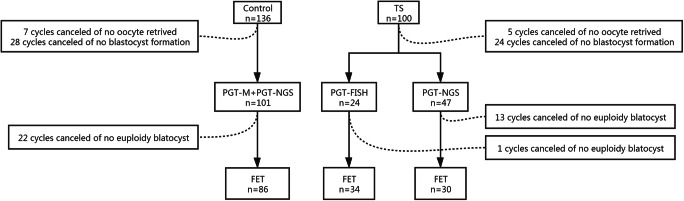
A retrospective comparative study was conducted. Anonymous data were obtained from the reproductive centre of Reproductive and Genetic Hospital of CITIC-Xiangya. Between January 2013 and April 2018, 68 women diagnosed with TS who received PGT-A treatments were taken into analysis. In order to compare the reproductive outcomes between Turner syndrome patients and normal counterparts, we collected an age-matched group of 136 women with normal karyotype underwent PGT-NGS due to monogenic disease during 2017–2018 as control. X chromosome aneuploid rates, clinical pregnancy rates per transfer, miscarriage rates, live birth rates per transfer and cumulative live birth rates were compared between these two groups (Fig. 1). Then, TS patients were divided into three groups according to the different levels of 45,X mosaic rate: low (mosaic rate less than 10%); medium (10% to 50%); and high (more than 50%). Age, miscarriage times, primary infertility, infertile years, BMI and ovarian reserve markers (AFC and AMH) were compared among groups. Reproductive outcomes including embryo X chromosome aneuploid rates, clinical pregnancy rates per transfer, miscarriage rates, live birth rates per transfer and cumulative live birth rates were also contrasted.
Fig. 1.
Schematic process of the enrolled patients
Karyotype analysis
Before IVF treatment, a conventional karyotype analysis was performed on cultured peripheral blood lymphocytes for all couples. Sixty to one hundred metaphase GTG of lymphocytes karyotype analyses were examined for each patient. Mosaic rates of TS cases were defined as proportions of 45,X monosomy cells in all examined lymphocytes. Generally, embryo X chromosome abnormalities include number and structure aspects.
Treatment protocol
AMH, basal FSH and basal LH were evaluated on day 3 of menstruation. There were three stimulation protocols: GnRH agonist, antagonist and mild stimulation protocol. The doses of recombinant FSH (Gonal-f, Merck Serono, Germany; or Puregon, MSD, USA) and urinary human menopausal gonadotropin (Menopur, Ferring, Switzerland) were adjusted according to the ovarian response, such as the measurement of serum sex steroids and AFC under ultrasonography. When at least three follicles reached 18 mm or more, HCG at a dose of 5000–10000 IU was used to trigger oocyte maturation. All cases underwent appropriate ovarian stimulation protocols.
Oocyte retrieval was performed 34–36 h after the trigger. Oocytes were inseminated by intracytoplasmic sperm injection and embryos were cultured to blastocyst stage; 3 to 5 trophoblast cells were biopsied and then sent for further analyses. Some of the patients received fluorescence in situ hybridization for diagnosing abnormality of chromosome 18,X,Y; others received comprehensive chromosomal screening by NGS as described before [13]. Euploid blastocysts were chosen to be transferred.
Clinical pregnancy was defined as an intrauterine gestational sac at 28 days after the blastocyst transfer, as detected on ultrasonography. Miscarriage was defined as pregnancy loss before 24 weeks of gestation. Live birth was defined as delivery of a viable infant at 28 weeks of gestation or more after the blastocyst transfer.
Statistical analysis
Chi-squared analyses were performed for the comparison of the embryonic aneuploidy rates (number of aneuploidy blastocysts/total number of blastocysts biopsied) and pregnancy rates (CPR, MR, LBR, CLBR) in different groups. For continuous variables, the Kruskal–Wallis H test was performed to assess the differences within groups. Multiple logistic regression was performed to evaluate the relationship between the confounding factors, the maternal mosaic rates and the embryo X chromosome abnormality rates. The confounding factors included female age, BMI, AMH and FSH dose.
Results
A total of 68 patients diagnosed with TS who underwent PGT from 2013–2018 were recruited. Karyotypes of all patients are shown in Table 1 (Fig. 2). There were 4 women with 45,X monosomy and the rest were mosaic cases. One case with karyotype of 46,XX,del(q24) was excluded according to 2016 Cincinnati guidelines for TS [2]. There were 12 cases of recurrent pregnancy loss, and one pure TS patient had suffered 4 miscarriages. The average miscarriage times were 0.65±0.89. Twenty-one TS women were primary infertility. There were 100 controlled ovary stimulation cycles, and 213 blastocysts were biopsied, among them 92 blastocysts received FISH test for chromosomes 18 and X,Y abnormality, and 121 received NGS test checking for 46 chromosomes.
Table 1.
Karyotype of 68 patients
| Karyotype | Number | Proportion |
|---|---|---|
| 45,X | 4 | 5.89% |
| Mos,45,X/46XX | 51 | 75% |
| Mos,45,X/46,XX/47,XXX | 11 | 16.17% |
| Mos,45,X/46,XY/47,XXX | 1 | 1.47% |
| Mos,45,X/47,XXX | 1 | 1.47% |
Fig. 2.
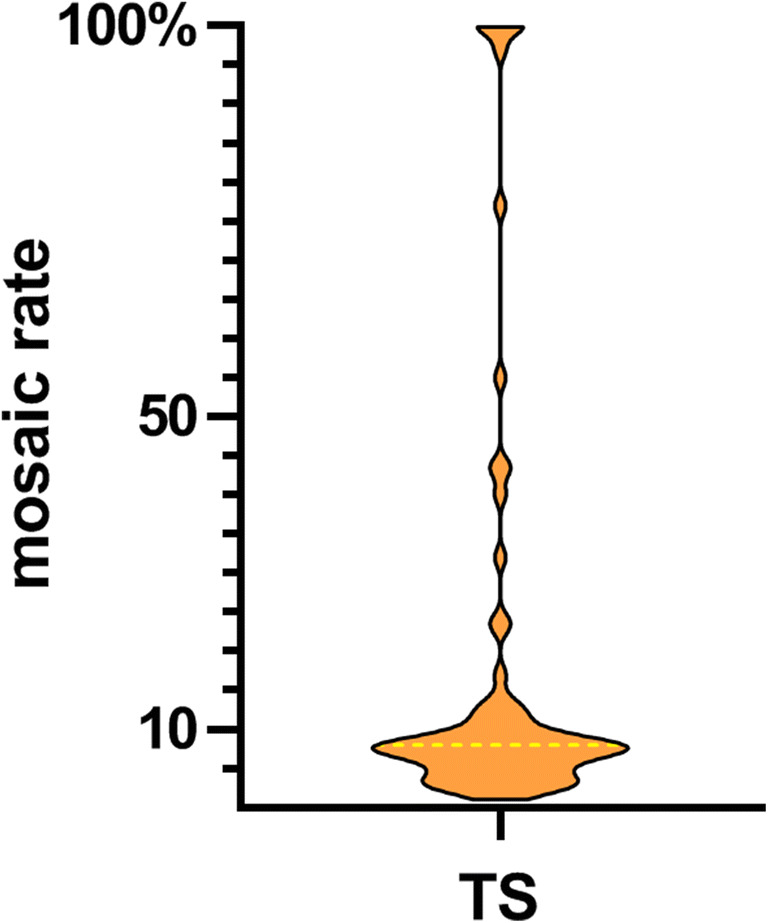
Distribution violin plot of mosaic rates in 68 TS patients. The median number of mosaic rates was drawn with a dotted yellow line
The average age was 35.41±5.84 in TS group and 35.8±5.54 in control (P>0.05). None of infertile years, BMI, AFC, or AMH reached any statistical difference between TS and control group. For treatment protocols, GnRH antagonist was used more frequently in TS group (P<0.05). Chances of cycle cancellation due to no euploidy blastocyst formation were statistically comparable between two groups, while embryo sex chromosome aneuploid rate presented noticeably higher in TS women than normal controls (7.04 vs 1.61%, P<0.01) (Table 2).
Table 2.
Basal characteristics and embryo X chromosome abnormality rate in TS and control group
| TS n=100 | Control n=136 | P value | |
|---|---|---|---|
| Age | 35.41±5.84 | 35.8±5.54 | NS |
| Infertile years | 4.61±4.16 | 4.13±3.85 | NS |
| BMI (kg/m2) | 22.63±2.66 | 22.05±2.14 | NS |
| 45,X mosaic rate | 0.17±0.26 | 0 | |
| AFC | 13.54±9.47 | 15.25±10.48 | NS |
| AMH(ng/ml) | 3.23±3.37 | 3.64±3.30 | NS |
| Treatment protocol | |||
| GnRH agonist | 25 | 64 | <0.05 |
| GnRH antagonist | 66 | 67 | |
| Other | 9 | 5 | |
| Cancel rate | 43% (43/100) | 42.65% (58/136) | NS |
| X chromosome aneuploid rate | 7.04% (15/213) | 1.61% (4/249) | <0.01 |
NS, not significantly different; BMI, body mass index; AFC, antral follicle count
Considering that different embryo testing methods may affect the reproductive outcomes, we next compared the outcomes between TS patients underwent PGT-NGS testing (n=61) and control group (n=136). We found that cumulative live birth rate was lower in TS women than control (31.15 vs 45.59%, P<0.05), while clinical pregnancy rate per transfer, miscarriage rate and live birth rate per transfer remained comparable (Table 3).
Table 3.
Reproductive outcomes after PGT-NGS treatment in TS women and control group
| TS-NGS n=61 |
Control NGS n=136 |
P value | |
|---|---|---|---|
| CPR/per transfer | 73.33% (22/30) | 81.49% (70/86) | NS |
| Miscarriage rate | 13.63% (3/22) | 8.57% (6/70) | NS |
| LBR/per transfer | 63.33% (19/30) | 72.09% (62/86) | NS |
| CLBR | 31.15% (19/61) | 45.59% (62/136) | <0.05 |
NS, not significantly different; CPR, clinical pregnancy rate; MR, miscarriage rate; LBR, live birth rate; CLBR: cumulative live birth rate
In order to investigate relationship between X monosomy mosaic rates and reproductive outcomes, TS patients receiving PGT-A were further divided into three groups according to their peripheral blood karyotype of monosomy X mosaic rates: low (mosaic rate less than 10%); medium (10% to 50%); and high (more than 50%). Characteristic data showed that the high mosaic rate group was younger, but their ovarian reserve was worse. The average counts of AFC in high group were 5.56±3.94 and 14.47±9.67 in low and 13.91±9.15 in medium (P<0.05). AMH were also low in high mosaic rate group, though not statistical different (Table 4).
Table 4.
PGT outcomes of different mosaic rate groups
| Mosaic rate group | Low (<10%) n=68 | Medium (10–50%) n=23 | High (>50%) n=9 | P value |
|---|---|---|---|---|
| Age | 36.35±5.55 | 34.61±6.14 | 30.33±4.72 | <0.05 |
| Miscarriage times | 0.5±0.73 | 0.87±0.76 | 0.56±0.73 | NS |
| Primary infertility | 38.24% (26/68) | 17.39% (4/23) | 55.55% (5/9) | NS |
| Infertile years | 5.16±4.72 | 3.00±1.69 | 4.33±2.87 | NS |
| BMI (kg/m2) | 22.7±2.91 | 22.57±1.89 | 22.28±2.48 | NS |
| 45,X mosaic rate | 0.06±0.02 | 0.20±0.13 | 0.92±0.16 | |
| AFC | 14.47±9.67 | 13.91±9.15 | 5.56±3.94 | <0.05 |
| AMH (ng/ml) | 3.29±3.48 | 3.85±3.41 | 1.26±1.29 | NS |
| Treatment protocol | ||||
| GnRH agonist | 20 | 5 | 0 | |
| GnRH antagonist | 44 | 15 | 7 | |
| Other | 4 | 3 | 2 | |
| Cancel rate | 44.1% (30/68) | 34.78% (8/23) | 55.55% (5/9) | NS |
| PGT-FISH | 29.41% (15/51) | 40% (6/15) | 60% (3/5) | NS |
| X chromosome aneuploid rate | 7.19%% (11/153) | 5.88% (3/51) | 11.11% (1/9) | NS |
| CPR/per transfer | 57.1% (28/49) | 58.3% (7/12) | 33.33% (1/3) | NS |
| Miscarriage rate | 11% (3/28) | 14.29% (1/7) | 0 | NS |
| LBR/per transfer | 50.9% (25/49) | 50% (6/12) | 33.33% (1/3) | NS |
| CLBR | 37% (25/68) | 26.09% (6/23) | 11.11% (1/9) | NS |
NS, not significantly different between each two groups; CPR, clinical pregnancy rate; MR, miscarriage rate; LBR, live birth rate
As for reproductive outcomes, it showed no statistical difference in clinical pregnancy rates per transfer, miscarriage rates, live birth rates per transfer and cumulative live birth rates among low, medium and high mosaic group (Table 4). Considering that embryo X chromosome abnormality rates were obviously higher in TS women, we wondered if maternal 45,X mosaic rates correlate to embryo X chromosome abnormalities. Surprisingly, there was no difference in the abnormal rate of X chromosome among three groups (7.19 vs 5.88 vs 11.11%, P>0.05) (Table 4). Furthermore, to exclude the influence of maternal age on embryo aneuploidy, we conducted a logistic regression. After adjustment, neither maternal age nor 45,X mosaic rates had any impact on embryo X chromosome abnormality rates (Table 5).
Table 5.
Multiple logistic regression analysis of the association between the confounding variables, mosaic rate and embryonic sex chromosome abnormality
| P value | Odds ratio(95%CI) | |
|---|---|---|
| Maternal age | 0.68 | 0.97 (0.85–1.12) |
| Duration of infertility | 0.48 | 1.06 (0.90–1.26) |
| BMI | 0.42 | 1.10 (0.87–1.40) |
| AMH | 0.46 | 1.06 (0.92–1.22) |
| Maternal 45,X mosaic rate | 0.58 | 2.31 (0.12–44.69) |
| FSH dose | 0.86 | 1.00 (1.00–1.00) |
CI, 95% CI for the odds ratio
Discussion
During first meiosis, germ cells lacking X chromosome may result in sister chromosomes synapsis failure, which would accelerate oocytes atresia [14]. Thus, gonadal dysgenesis and premature ovarian failure are the most common clinical features in TS. According to the previous research, average menopause age of TS women was 29.3 years old [15]. However, the existence of normal cell lines in some mosaic cases will rescue oocytes from atresia, and some may survive during puberty or even reproductive years. Patients could show spontaneous menstruation and pregnancy, but with poor fertility outcomes [16].
Tarani et al. analysed 160 spontaneous pregnancies of 74 TS patients and found that 58% pregnancies resulted in a miscarriage or fetal malformation. Compared with normal people, newborn babies of TS women were more likely to suffer Down syndrome (4 vs 0.4%) or Turner syndrome (15 vs 0.5%) [17]. This was similar to our results found that the embryo X chromosome abnormal rates were significantly higher in TS patients (7.04 vs 1.61%, P<0.01).
According to previous reports, advanced maternal age is the major risk for embryo aneuploidy [18]. However, age could not explain the increased embryo X chromosome abnormal rates in TS women. We speculated that higher chance of embryo X chromosome abnormalities occurred due to abnormal maternal karyotypes. Though Ogata et al. thought aneuploid oogonium will atresia due to synapsis failure of homologous chromosomes[14], others believe that some XO oogonium may complete meiosis by coupling the heterologous X chromosome with the autosome or itself [19]. Banzai et al. had reported murine model with XO karyotype could produce normal oocytes, which confirmed this presumption [20].
Interestingly, instead of producing monosomic zygotes, we found that embryo sex chromosomes of TS women were mostly trisomic and only three blastocysts showed the same 45,X karyotype like their TS mothers. He Ren’s results were common with ours; they found that in mos,45,X/46,XY male cases, the XY disomy of testicular sperm was significantly higher than that of healthy controls [21]. These indicated that though surviving in the first meiosis, 45,X oogonium may suffer abnormal separation during the second meiosis. After all, we know very little about the meiosis of aneuploid germ cells.
Subsequently, Bryman et al. found miscarriage rate of spontaneous pregnancies in mos,45,X/46,XX TS cases was up to 45% [12]. Foudila et al. had announced that clinical pregnancy rates of donated oocytes in TS women were lower than that in normal population (28 vs 46%), suggesting that 45,X abnormal cell line in uterus may block endometrial decidualization, thereby affecting embryo development [22]. On the contrary, we found that after transferring euploid blastocysts, the embryo implantation potentials and the risk of pregnancy loss in mosaic TS cases were similar to normal contemporaries [23].
It was reported that mos,45,X/46,XX women showed milder phenotype and higher chance of conceive spontaneously [24, 25]. However, whether mosaic rates of monosomy have correlation with fertility stays unclear. We found that, after receiving PGT, maternal X monosomy mosaic rates did not affect neither embryo aneuploid rates nor reproductive outcomes (including clinical pregnancy rate, miscarriage rate, live birth rate). Similarly, Emek Doger’s study demonstrated that there was no statistical difference in miscarriage and live birth rate between patients with monosomy mosaic rates above 10% and below [24].
However, whether the maternal X chromosome mosaic rates are related to reproductive outcomes still needs to be carefully considered. The mosaic rates of peripheral blood lymphocytes may not reflect mosaicism in ovary, which could interrupt the analysis of true correlation between karyotype and phenotype. The research of Peek R studied the karyotypes of 10 adolescent ovary tissues which belonged to TS girls who went ovarian tissue cryopreservation. They found the mosaicism degrees of each follicle’s granular cells and oocytes were different [26]. In addition, this phenomenon was also appeared among different tissues, such as ovary, blood, oral and urinary epithelium [26, 27]. But so far, analysis of lymphocyte karyotype is the most common used diagnose method, which is especially suitable for patients with no indication of ovary biopsy [28].
Given the limitations of this study, most non-mosaic TS patients had received oocytes donation due to congenital malformation or premature ovarian insufficiency and thus, had been excluded. But four 45,X monosomic patients were included in our research, which could represent this karyotype.
Conclusion
Information about reproductive outcomes of preimplantation genetic testing in TS patients (PTS/MTS) is rare; our research enriched this field. We found that embryo X chromosome abnormalities were extremely higher in TS patients. To avoid high risk of embryo X chromosome abnormality, prenatal genetic or preimplantation genetic testing should be recommended to mosaic or pure TS patients. We strongly supported 2016 Cincinnati guidelines for the content of TS patients’ therapeutic regimen.
Abbreviations
- AFC
antral follicle count
- BMI
body mass index
- FISH
fluorescent in situ hybridization
- MTS
mosaic Turner syndrome
- NGS
next-generation sequencing
- PTS
pure Turner syndrome
- PGT
preimplantation genetic testing
- TS
Turner syndrome
Author’s contributions
Jingnan Liao conceived of the study, participated in the design, obtained the data, performed the statistical analysis and drafted the manuscript. Keli Luo performed the controlled ovarian stimulating and frozen embryo transfer treatment. Dehua Cheng, Pingyuan Xie, Yueqiu Tan and Liang Hu carried out the genetic analysis. Guangxiu Lu participated in the design. Fei Gong participated in the design and edited the manuscript. Ge Lin gave advice to the statistical analysis and edited the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Key Research and Development Program of China Project 2016YFC1000200, the National Natural Science Foundation of China (NO.81974230) and Hunan Provincial Grant for Innovative Province Construction (2019SK4012).
Data Availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
The study was approved by the ethical committee of the CITIC-Xiangya Reproductive and Genetic Hospital (LL-SC-2020-009).
Consent for publication
Written informed consent for publication was obtained from all authors.
Competing interests
The authors stated that they do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hook EB, Warburton D. Turner syndrome revisited: review of new data supports the hypothesis that all viable 45,X cases are cryptic mosaics with a rescue cell line, implying an origin by mitotic loss. Hum Genet. 2014;133:417–424. doi: 10.1007/s00439-014-1420-x. [DOI] [PubMed] [Google Scholar]
- 2.Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, Lin AE, Mauras N, Quigley CA, Rubin K, Sandberg DE, Sas TCJ, Silberbach M, Söderström-Anttila V, Stochholm K, van Alfen-van derVelden JA, Woelfle J, Backeljauw PF, International Turner Syndrome Consensus Group[Corporate Author] Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177:G1–70. doi: 10.1530/EJE-17-0430. [DOI] [PubMed] [Google Scholar]
- 3.Milbrandt T, Thomas E. Turner syndrome. Pediatr Rev. 2013;34:420–421. doi: 10.1542/pir.34-9-420. [DOI] [PubMed] [Google Scholar]
- 4.Morgan T. Turner syndrome: diagnosis and management. Am Fam Physician. 2007;76:405–410. [PubMed] [Google Scholar]
- 5.Sybert VP, McCauley E. Turner's syndrome. N Engl J Med. 2004;351:1227–1238. doi: 10.1056/NEJMra030360. [DOI] [PubMed] [Google Scholar]
- 6.Talaulikar VS, Conway GS, Pimblett A, Davies MC. Outcome of ovarian stimulation for oocyte cryopreservation in women with Turner syndrome. Fertil Steril. 2019;111:505–509. doi: 10.1016/j.fertnstert.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Hagman A, Loft A, Wennerholm UB, Pinborg A, Bergh C, Aittomaki K, Nygren KG, Bente Romundstad L, Hazekamp J, Soderstrom-Anttila V. Obstetric and neonatal outcome after oocyte donation in 106 women with Turner syndrome: a Nordic cohort study. Hum Reprod. 2013;28:1598–1609. doi: 10.1093/humrep/det082. [DOI] [PubMed] [Google Scholar]
- 8.Ohl J. Oocyte donation in Turner syndrome. Gynecol Obstet Fertil. 2008;36:886–890. doi: 10.1016/j.gyobfe.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Doger E, Cakiroglu Y, Ceylan Y, Ulak E, Ozdamar O, Caliskan E. Reproductive and obstetric outcomes in mosaic Turner's syndrome: a cross-sectional study and review of the literature. Reprod Biol Endocrinol. 2015;13:59. doi: 10.1186/s12958-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Mansoury M, Barrenas ML, Bryman I, et al. Chromosomal mosaicism mitigates stigmata and cardiovascular risk factors in Turner syndrome. Clin Endocrinol. 2007;66:744–751. doi: 10.1111/j.1365-2265.2007.02807.x. [DOI] [PubMed] [Google Scholar]
- 11.Snyder EA, San RA, Pina-Aguilar RE, et al. Genetic counseling for women with 45,X/46,XX mosaicism: Towards more personalized management. Eur J Med Genet. 2021;64:104140. doi: 10.1016/j.ejmg.2021.104140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryman I, Sylven L, Berntorp K, et al. Pregnancy rate and outcome in Swedish women with Turner syndrome. Fertil Steril. 2011;95:2507–2510. doi: 10.1016/j.fertnstert.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 13.Tan Y, Yin X, Zhang S, Jiang H, Tan K, Li J, Xiong B, Gong F, Zhang C, Pan X, Chen F, Chen S, Gong C, Lu C, Luo K, Gu Y, Zhang X, Wang W, Xu X, Vajta G, Bolund L, Yang H, Lu G, du Y, Lin G. Clinical outcome of preimplantation genetic diagnosis and screening using next generation sequencing. Gigascience. 2014;3:30. doi: 10.1186/2047-217X-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogata T, Matsuo N. Turner syndrome and female sex chromosome aberrations: deduction of the principal factors involved in the development of clinical features. Hum Genet. 1995;95:607–629. doi: 10.1007/BF00209476. [DOI] [PubMed] [Google Scholar]
- 15.Reindollar RH, Novak M, Tho SP, McDonough PG. Adult-onset amenorrhea: a study of 262 patients. Am J Obstet Gynecol. 1986;155:531–543. doi: 10.1016/0002-9378(86)90274-7. [DOI] [PubMed] [Google Scholar]
- 16.Martin CE, Roa LC. Spontaneous puberty and menarche in a patient with Turner syndrome and 45X monosomy. An Pediatr (Barc) 2009;70:200–202. doi: 10.1016/j.anpedi.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Tarani L, Lampariello S, Raguso G, Colloridi F, Pucarelli I, Pasquino AM, Bruni LA. Pregnancy in patients with Turner's syndrome: six new cases and review of literature. Gynecol Endocrinol. 1998;12:83–87. doi: 10.3109/09513599809024955. [DOI] [PubMed] [Google Scholar]
- 18.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT., Jr The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101:656–663. doi: 10.1016/j.fertnstert.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 19.MacLennan M, Crichton JH, Playfoot CJ, Adams IR. Oocyte development, meiosis and aneuploidy. Semin Cell Dev Biol. 2015;45:68–76. doi: 10.1016/j.semcdb.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banzai M, Omoe K, Ishikawa H, Endo A. Viability, development and incidence of chromosome anomalies of preimplantation embryos from XO mice. Cytogenet Cell Genet. 1995;70:273–277. doi: 10.1159/000134050. [DOI] [PubMed] [Google Scholar]
- 21.Ren H, Chow V, Ma S. Meiotic behaviour and sperm aneuploidy in an infertile man with a mosaic 45,X/46,XY karyotype. Reprod BioMed Online. 2015;31:783–789. doi: 10.1016/j.rbmo.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Foudila T, Soderstrom-Anttila V, Hovatta O. Turner's syndrome and pregnancies after oocyte donation. Hum Reprod. 1999;14:532–535. doi: 10.1093/humrep/14.2.532. [DOI] [PubMed] [Google Scholar]
- 23.Simpson JL, Kuliev A, Rechitsky S. Overview of Preimplantation Genetic Diagnosis (PGD): Historical Perspective and Future Direction. Methods Mol Biol. 1885;2019:23–43. doi: 10.1007/978-1-4939-8889-1_2. [DOI] [PubMed] [Google Scholar]
- 24.Denes AM, Landin-Wilhelmsen K, Wettergren Y, Bryman I, Hanson C. The proportion of diploid 46,XX cells increases with time in women with Turner syndrome--a 10-year follow-up study. Genet Test Mol Biomark. 2015;19:82–87. doi: 10.1089/gtmb.2014.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard V, Donadille B, Zenaty D, Courtillot C, Salenave S, Brac de la Perrière A, Albarel F, Fèvre A, Kerlan V, Brue T, Delemer B, Borson-Chazot F, Carel JC, Chanson P, Léger J, Touraine P, Christin-Maitre S, CMERC Center for Rare Disease Spontaneous fertility and pregnancy outcomes amongst 480 women with Turner syndrome. Hum Reprod. 2016;31:782–788. doi: 10.1093/humrep/dew012. [DOI] [PubMed] [Google Scholar]
- 26.Peek R, Schleedoorn M, Smeets D, van de Zande G, Groenman F, Braat D, van der Velden J, Fleischer K. Ovarian follicles of young patients with Turner's syndrome contain normal oocytes but monosomic 45,X granulosa cells. Hum Reprod. 2019;34:1686–1696. doi: 10.1093/humrep/dez135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surico N, Messina M, Ponzio G, Libanori E, Chiodo F, Milani P, Folpini E. Limited diagnostic value of lymphocytic karyotype in primary amenorrhea with streak gonads. Eur J Obstet Gynecol Reprod Biol. 1987;26:145–150. doi: 10.1016/0028-2243(87)90049-9. [DOI] [PubMed] [Google Scholar]
- 28.Noordman ID, van der Velden JA, Timmers HJ, Pienkowski C, Köhler B, Kempers M, Reisch N, Richter-Unruh A, Arlt W, Nordenström A, Webb EA, Roeleveld N, Claahsen-van der Grinten H. Karyotype - Phenotype Associations in Patients with Turner Syndrome. Pediatr Endocrinol Rev. 2019;16:431–440. doi: 10.17458/per.vol16.2019.nvt.karyotypeturnersyndrome. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.