Abstract
The impacts of chemical fertilizers and pesticides have raised public concerns regarding the sustainability and security of food supplies, prompting the investigation of alternative methods that have combinations of both agricultural and environmental benefits, such as the use of biofertilizers involving microbes. These types of microbial inoculants are living microorganisms that colonize the soil or plant tissues when applied to the soil, seeds, or plant surfaces, facilitating plant nutrient acquisition. They can enhance plant growth by transforming nutrients into a form assimilable by plants and by acting as biological control agents, known as plant growth-promoting bacteria. The potential use of bacteria as biofertilizers in agriculture constitutes an economical and eco-friendly way to reduce the use of chemical fertilizers and pesticides. In this context, nanotechnology has emerged as a new source of quality enrichment for the agricultural sector. The use of nanoparticles can be an effective method to meet the challenges regarding the effectiveness of biofertilizers in natural environments. Given the novel sustainable strategies applied in agricultural systems, this review addresses the effects of nanoparticles on beneficial plant bacteria for promoting plant growth.
Keywords: Endophytes, Nanobiofertilizer, Nanoparticles, Plant growth, Rhizobacteria
Introduction
Population growth associated with economic development has led to increased demand for food (Ma et al. 2018). For this reason, the indiscriminate use of chemical fertilizers and pesticides to achieve high crop yields has caused depletion of soil microbial communities, contamination of water and soil, loss of biodiversity, and an imbalance in ecosystem function (Vance 2001; Vessey 2003; Gurikar et al. 2016). Considering environmental degradation and limited land resources, alternatives that can partially or even wholly replace chemical inputs in agriculture have never been more critical.
Sustainable agriculture demands addressing the concerns of agriculture and the environment (Tilman et al. 2002). High crop yields with fewer environmental degradation can be achieved through the effective use of biofertilizers, biopesticides, and ecologically conscientious water use, and soil maintenance. For these purposes, the application of plant growth-promoting bacteria (PGPB) is a sustainable practice to benefit plants. Rhizospheric bacteria are symbiotic free-living soil PGPB associated with plant roots, while endophytic PGPB colonize leaves, flowers, or inner tissues of the host plant (Glick 2020). Rhizospheric and endophytic bacteria promote plant growth through different mechanisms, including those involving nutrient uptake, plant stress resistance, and protection against phytopathogens (Redman et al. 2002; Waller et al. 2005; Ryan et al. 2008; Castro et al. 2018; Batista et al. 2018). Therefore, biofertilizers consisting of microorganisms have increasingly shown economic potential for use in organic farming. The global plant biostimulant market is estimated to grow by 12% per year and worth USD 3.0 million by 2022 (Meticulous Market Research 2017).
Nevertheless, many factors cause destabilization and inconsistency of microbial inoculants in the field, hindering biofertilizer efficacy. In recent years, nanotechnology has been widely used for precision agriculture. Due to their small sizes and unique properties compared to those of their bulk materials, nanoparticles (NPs) are being studied for use in addressing some obstacles of biofertilizers, such as reproducibility, storage stability, dehydration, and temperature sensitivity (Bansal et al. 2014; Duhan et al. 2017).
Nanostructured materials have been indicated to increase the potential of PGPB as inoculants—so-called nanobiofertilizers. Silicon, zinc, titanium, and gold NPs have been reported to increase the number of bacterial cells and improve the beneficial properties of PGPB in plants (Dimkpa et al. 2012a; Karunakaran et al. 2013; Rangaraj et al. 2014; Palmqvist et al. 2015; Shukla et al. 2015; Zand et al. 2020). The use of nanomaterials is broadly considered the next technological and scientific step to support humankind's development (Singla et al. 2020).
In this context, nanotechnology applied to beneficial microorganisms constitutes a promising approach to revolutionizing the agricultural sector by optimizing crop systems and providing economic and environmental benefits. Therefore, this article aims to provide an overview of the potential advantages of formulations using inorganic NPs associated with PGPB for plant development.
Nanotechnology for sustainable agriculture
Nanotechnology involves materials with unusual properties that appear either due to the quantum confinement effects or the existence of exceptionally reactive surfaces, which occur only at a nanometric scale. Compared with the macroscopic level, the nanoscale level brings about material properties that are different and novel due to the reduced size, greater surface area-to-weight ratio, and shape of nanomaterials (Roduner 2006; Gutiérrez et al. 2011). In terms of their advantageous properties, nanomaterials have high reactivity and improved bioavailability/bioactivity, adherence, and surface effects (Gutiérrez et al. 2011). NPs can be formed by only a single element (such as silver) or by a mixture of elements, such as those composing oxides (TiO2, SiO2, ZnO) (Dinesh et al. 2012).
Innovative strategies are increasingly necessary for sustainable agriculture (Prasad et al. 2017). Approaches based on nanotechnology constitute efficient tools to overcome challenges in the agricultural and food industries, such as the growing demand for food, food safety, plant disease, and climate change (Biswal et al. 2012). Recently, researchers have started testing nanomaterials with relatively few negative environmental impacts to improve crop production. Within the agricultural field, nanotechnology is applied in several areas: seed science, nanofertilizers, nanoherbicides, water management, nanoscale carriers, biosensors, agricultural engineering, and animal science (Koul 2019). For example, smart agricultural systems developed with nanomaterials enable high nutrient absorption by plants, delivery and controlled release of molecules in target tissues, early detection of diseases, and protection from the environment (Suman et al. 2010; Tarafdar et al. 2013; Bhattacharyya et al. 2016). Nanostructured materials have been used in association with agricultural inputs (nanofertilizers and nanopesticides) to promote targeted delivery and release in plants at relatively low doses per application, consequently decreasing the byproducts that could remain in the soil and disrupt the ecosystem (Varma et al. 2017).
Nanoencapsulations with specific bacterial strains can be inoculated into seeds, which are referred to as smart seeds, to decrease seeding rates, ensure correct field stands, and improve crop performance. Smart seeds can also be dispersed over the field and scheduled to germinate under appropriate temperature, moisture, and soil pH conditions (Chinnamuthu and Boopathi 2009; El-Ramady et al. 2018).
Nanomaterials in biofertilizer formulations
Plant survival depends on multiple aspects of the community to which it belongs (Lundberg et al. 2012). It is well known that PGPB support plant health by several direct and indirect mechanisms. The direct mechanisms include the uptake of essential nutrients from the soil, including nitrogen, phosphorous, iron, and the synthesis or regulation of plant growth-related hormones, such as auxin, cytokinin, or gibberellin (Glick 2012; Gond et al. 2015; White et al. 2015). Indirectly, PGPB help plants endure biotic stress by producing antibiotics, antioxidant enzymes, and other molecules; balancing reactive oxygen species; lowering nutrient availability for pathogens; synthesizing pathogen-inhibiting volatile compounds; and promoting induced systemic resistance in the plant (Santoyo et al. 2012; Glick 2020; Li et al. 2016; Srivastava et al. 2016).
Since crop plants can take up only 30–50% of chemical fertilizers, a substantial amount of input remains in the soil, polluting groundwater. Consequently, fertilizer efficiency has decreased over the years due to saturation (Mózner et al. 2012). Biofertilizers consist of live or latent microorganisms (inoculants) in a formulation that provides easy handling and extended storage, acting as a delivery instrument of microbes to increase nutrient availability for plants (Sahu and Brahmaprakash 2016; Kour et al. 2020). PGPB, such as nitrogen fixers, phosphorus solubilizers, potassium solubilizers, and biocontrol agents, are used worldwide in agriculture as inoculants (Mohammadi and Sohrabi 2012; Sahu and Brahmaprakash 2016). N-fixing inoculants dominate the global biofertilizer market, as plants cannot convert atmospheric nitrogen into usable nitrogen, an essential nutrient for plant survival (GVR 2020). The genera Azospirillum, Acetobacter, Azotobacter, and Pseudomonas include species most commonly used as inoculants. Moreover, Pseudomonas and Bacillus species are potent biocontrol agents and plant growth promoters under stress conditions (Praveen Kumar et al. 2014; Kumar and Verma 2018). The advantages of biofertilizers over chemical fertilizers include increased sustainability, low environmental impact, improved soil fertility, and increased accessibility to marginal and small farmers (Thomas and Singh 2019). Therefore, microbial biofertilizers can reduce or replace chemical fertilizers in agriculture and alleviate adverse impacts.
Although PGPB are promising and are already being used commercially as inoculants in cropping systems, their practical application may not coincide with the expected performance in plants due to problems concerning their stability in the soil, field applications, and delivery systems (Koul 2019). As many factors influence bacterial colonization in plants, generally, bacterial populations decline quickly after inoculation, decreasing bacterial activity in the rhizosphere. To prevent this decline in the field, PGPB need either an appropriate microenvironment or physical protection for a prolonged period (Bashan et al. 2014; Timmusk et al. 2017). Furthermore, a minimum number of bacterial cells is a critical factor for obtaining a positive plant response. For example, the bacterium Azospirillum brasiliense needs to be at 106–107 cells/plant (Bashan 1986). For these reasons, the application of PGPB in crop systems requires peat or liquid carriers to stabilize and support the bacteria during storage and transport (Namasivayam et al. 2014). The most crucial portion of the biofertilizer is the carrier, which originates from organic or inorganic compounds or is synthesized from specific molecules (Smith 1992; Malusá et al. 2012).
Alternative materials must be explored to preserve bacterial inoculum quality and efficiency, as well as reduce production costs and adverse impacts (Herrmann and Lesueur 2013). For these purposes, nanostructured materials may be a modern and efficient approach that may enhance the stability and targeting of beneficial microorganisms in agriculture. The incorporation of nanoformulations can provide stable and reproducible biofertilizers by increasing their resistance to desiccation, heat, and UV radiation (Vandergheynst et al. 2007; Ghormade et al. 2011). This approach involves integrating microbial cells into inorganic or organic NPs, delivering the bacteria under regulated conditions to target tissues at specific times (Timmusk et al. 2018).
The large specific surface area of NPs with a negative charge and different groups mostly determines the specificities and interactions between microorganisms and NPs, independent of the NP material. Despite the total negative charge, there may be positive charges and hydrophobic sites on the surface of cells and solid particles, promoting adhesion between microorganisms and nanomaterials (Breznak et al. 2012; Kurdish 2019). Aggregation and binding patterns can occur because NPs induce hydrophobic regions on the bacterial cell membrane or because NPs can attach to hydrophobic spots already present on the bacteria (Hayden et al. 2012). However, interactions of particles with bacteria occur not only by electrostatic attraction but also by bacterial surface chemical reactions, such as those associated with phospholipid membrane exposure (Palmqvist et al. 2015). The lipopolysaccharide (LPS), lipoteichoic acid (LTA), proteins, and phospholipids of bacterial cells are perhaps the most important biomolecules interacting with NPs (Jiang et al. 2010). The method of NP transport into bacterial cells depends on the size of the compounds, and there is still no specific model to predict PGPB and NP interactions (Shukla et al. 2015).
It has been suggested that NPs affect the plant-PGPB system through direct and indirect mechanisms. In the direct mode, the NPs enhance nutrient availability, while in the indirect one, NPs stimulate the PGPB and consequently improve the growth-promoting effects (Kalia and Kaur 2019). The mechanisms of which NPs indirectly benefit the plant–microbe association include increased cell viability and bacterial growth rate, help plants to endure adverse conditions by inducing the secretion of specific compounds in microbes, support microbial interaction by providing a high surface area, enhanced plant beneficial features of microbes, and protection of inoculants in the field when applied as carriers (Ghalamboran and Ramsden 2012; Karunakaran et al. 2013; Abdel Latef et al. 2018; Gudadhe et al. 2018).
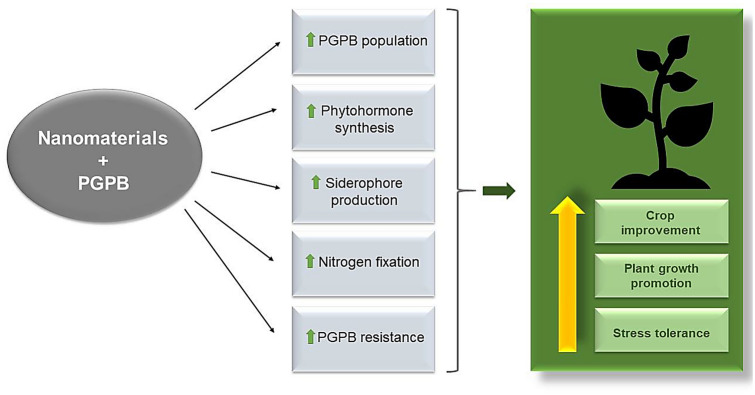
Nanomaterials can positively impact beneficial bacterial traits, such as the production of secondary metabolites and nitrogen fixation (Fig. 1). For example, copper oxide NPs (CuO NPs) increased IAA synthesis in the plant growth-promoting bacterium Pseudomonas chlororaphis, and zinc oxide NPs (ZnO NPs) increased siderophore production, probably induced by ion release in the bacterium (Dimkpa et al. 2012a, b). Silver NPs (AgNPs) increased the nitrogen fixation potential in Nitrosomonas europaea by upregulating the expression of the nitrification-related genes amoA1 and amoC2 (Yang et al. 2013). However, these studies also showed negative impacts of NPs on beneficial bacterial traits, indicating that their effects on bacteria are unpredictable and depend on several factors. Indeed, there are various mechanisms through which nanomaterials influence bacterial metabolism, although they are not well understood. These mechanisms apparently include ion interactions, changes in gene expression, and modification of cell membranes.
Fig. 1.
Positive effects of NPs on beneficial bacteria and plant growth
The association between NPs and PGPB can also improve the suppression of phytopathogens. Since NPs increase the number of bacterial cells, PGPB have an advantage over phytopathogens in the competition for niches and nutrients. Additionally, by increasing the production of secondary metabolites of PGPB and nutrient uptake, plants are more resistant to phytopathogen attack. Silica NPs, in particular, increase the thickness and mechanical resistance of plant cell walls, providing extra protection against herbivory and entry of phytopathogens (Djamin and Pathak 1967; Kaufman et al. 1979; Cui et al. 2020). It is well documented that several beneficial bacteria exert antagonistic activity against microorganisms harmful to plants in their natural environments, either by competing or by synthesizing antimicrobial biomolecules (Sturz et al. 2000; Berg et al. 2005; Ryan et al. 2008; Quecine et al. 2014). Therefore, it is crucial to conduct experiments testing bacteria under laboratory, greenhouse, and field conditions to confirm their antagonistic properties. In this way, NPs improve the bacteria already selected for biocontrol. In fact, it is difficult to predict whether NPs also stimulate phytopathogens. Thus, large-scale studies on target bacteria, NPs, phytopathogens, and plants are necessary to ensure the safety and effectiveness of nanobiofertilizers.
There is a lack of studies regarding plant growth-promoting bacterium-NP-plant interactions at the molecular level. Thus, the plant molecular activity triggered by nanomaterials associated with PGPB is still poorly understood. Physiological studies involving NPs and plants have demonstrated that small-size NPs can move through the symplast, such as through plasmodesmata, whereas larger NPs accumulate in the apoplastic space (Jha and Pudake 2016). These studies did not include bacteria, so the effects might differ when introducing PGPB into the system.
Rhizophagy is a mechanism in which plant roots internalize and break down microbes to extract nutrients (Paugnfoo-Lonhienne et al. 2013). White et al. (2012, 2014) found that plant roots produce reactive oxygen, which degrades cell walls, membranes, proteins, and other molecules of the symbiotic bacteria in the periplasmic spaces of cells to extract nitrogen from the bacteria. These findings indicated that plants absorb nutrients from microbes as part of a microbivory process (White et al. 2019). In the rhizophagy cycle, as proposed by White et al. (2018), free-living microorganisms obtain nutrients from the soil and become intracellular/endophytic after entering the plant through its root tips; the host plant then extracts those nutrients through an oxidative process. After the nutrients are exhausted, the surviving microbes leave the plants through root hairs and recharge in the rhizosphere, starting the cycle again (White et al. 2018). Following this logic, we hypothesize that some of the NPs attached to the bacterial surface remain within plant tissues in this process.
TiO2 nanoparticles
Titanium dioxide (TiO2) is an oxide formed from a transition metal and is one of the most commonly used semiconductors for photocatalysis (Gupta and Tripathi 2011). It is also present in many everyday products, such as paint, paper, plastics, ceramics, cosmetics, and food packaging (Jameel 2015). Some of the reasons for its widespread use include its chemical stability, handling safety, and biocompatibility (Shi et al. 2013). TiO2 exists as three different crystalline phases: anatase, rutile, and brookite. The first two involve tetragonal structures and are the most common (Noman et al. 2019). Considering the photocatalytic activity of TiO2, anatase is preferred over rutile because the former adsorbs less oxygen and has a higher Fermi level. However, due to its bandgap being close to 3.0 eV, which varies for each phase, this material can be activated only under UV radiation (Tanaka et al. 1991). Because TiO2 nanoparticles have a larger surface area than bulk titanium dioxide does, they have a greater photocatalytic capacity.
There are different synthesis methods for obtaining titania nanoparticles: deposition methods, oxidation methods, hydrothermal methods, and sol–gel methods (Baetzold 1981; Seifried et al. 2000; Feng et al. 2005; Chen 2009). Hydrothermal and sol–gel methods are the most commonly used for controlling the size and shape of nanoparticles. Hydrothermal synthesis is defined as crystallization above room temperature and under high pressure within a solvent that can be aqueous or nonaqueous. With this process, the final material properties can be controlled by adjusting the pH, temperature, duration of synthesis, type of solvent, or the amount of each reagent added (Liu et al. 2014). The synthesis is usually carried out in an autoclave that can withstand the extreme conditions used. Among the advantages, the robust control of size, purity, and low agglomeration predominate (Chen et al. 2015; Mamaghani et al. 2019). The sol–gel synthesis method is a wet chemical method and allows the synthesis of high-purity nanoparticles at a relatively low temperature and allows increased stoichiometry control of doping. This method involves the conversion of a solution with a titanium precursor into an inorganic solid formed by nanoparticles. The solution (sol), consisting of colloidal particles dispersed in a liquid, is formed by hydrolysis or solubilization of the precursor, and condensation leads to the formation of a gel. Typical precursors include metal oxides and metal chlorides (Akpan and Hameed 2010; Macwan et al. 2011).
Evidence that titania NPs have adhesive effects on bacteria was reported by Park et al. (2008), who found that Pseudomonas spp. presented adhesion and bioluminescence rates that were higher on surfaces with nanostructured titania than with conventional titania. Thus, it can be hypothesized that nanotitania could be used to guide bacteria to a specific site (Palmqvist et al. 2015). TiO2 nanoparticles synthesized by the sol–gel method have been demonstrated to help plant growth-promoting rhizobacteria (PGPR) attach to plant roots (Table 1). Bacteria in the presence of nanoparticles form stable and thicker layers than do those grown within a self-produced biofilm (Timmusk et al. 2018). Additionally, the biomass of wheat seedlings increased when double inoculations of PGPR were performed with titanium NPs in peat soil under adverse conditions caused by drought, salt, and fungal pathogens. These findings can be explained by the increased bacterial colonization in the presence of NPs (Timmusk et al. 2018). Likewise, Palmqvist et al. (2015) reported that titania NPs helped the plant growth-promoting bacterium Bacillus amyloliquefaciens attach to oilseed rape (Brassica napus) roots and protected plants against the fungal pathogen Alternaria brassicae (Palmqvist et al. 2015). Zand et al. (2020) tested TiO2 nanoparticles in association with the rhizobacterium Pseudomonas fluorescens to promote phytoremediation in soils contaminated with cadmium (Cd). The nanoparticles were synthesized by the sol–gel method in which TiCl4 was used as a precursor and ranged in size between 10 and 40 nm. White clover (Trifolium repens) seedlings were exposed to PGPR and different doses of TiO2; the results showed that the coapplication of bacteria and NPs at concentrations up to 500 mg/kg improved T. repens plant growth and increased plant biomass. The Cd uptake and accumulation capacity also increased under the same conditions in both the roots and the shoots. However, at a concentration of 1000 mg/kg TiO2, the effect was inhibitory (Zand et al. 2020). Furthermore, titanium NPs at a concentration of 0.01% benefited broad bean plants in saline soil by increasing either enzymatic activity or the solubility of sugars and amino acids. These findings show that TiO2 nanoparticles can also be useful for growing crops in contaminated soils (Abdel Latef et al. 2018).
Table 1.
Published studies on the use of nanoparticles with PGPB in plants
| Nanoparticles | Synthesis | Concentration | PGPB | Plant Species | Findings | Reference |
|---|---|---|---|---|---|---|
| CuO | Commercial | 50 mg/kg | Soil microbiota | Wheat (Triticum aestivum) |
Increased N2 fixation Increased nitrification Increased plant growth |
Guan et al. (2020) |
| SiO2 | Extracted from Equisetum telmateia |
0.05 ppm 0.07 ppm |
Pseudomonas stutzeri Mesorhizobium spp. |
Land cress (Barbarea verna) |
Increased shoot and root dry weight Increased nitrogen and phosphate in the soil |
Boroumand et al. (2020) |
| TiO2 | Sol–gel, with TiCl4 used as a precursor |
100 mg/kg 250 mg/kg 500 mg/kg 500 mg/kg 1000 mg/kg |
Pseudomonas fluorescens | Trifolium repens |
Coapplication of bacteria and NPs promoted the growth of T. repens and increased its biomass Enhanced Cd uptake and Cd accumulation capacity, promoted soil phytoremediation 1000 mg/kg TiO2 caused inhibitory effects on plant growth |
Zand et al. (2020) |
|
Alginate-SiO2 Carbon nanotubes |
Method of Tu et al. (2015) | – |
P. fluorescens Bacillus subtilis |
UCB-1 pistachio | Increased plant biomass and bud length | Pour et al. (2019) |
| TiO2 | Captigel Sol–gel approach | 50 µg/mL | Bacillus thuringiensis, Paenibacillus polymyxa, and Alcaligenes faecalis | Wheat (Triticum aestivum) |
Double inoculants with NPs stabilized bacterial attachment to the roots Increased biomass of plant in peat soil under adverse conditions |
Timmusk et al. (2018) |
| TiO2 | Commercial | 50–400 µg/mL |
Pseudomonas aeruginosa P. fluorescens Bacillus amyloliquefaciens |
– |
200 µg/mL decreased phosphate solubilization and production of iron-binding siderophore molecules 200 µg/mL reduced the production antibiotics by P. aeruginosa and P. fluorescens All the effects were dependent on the concentration |
Haris and Ahmad (2017) |
| ZnO | Commercial | 200 µg/mL |
P. aeruginosa P. fluorescens B. amyloliquefaciens |
– |
Inhibited IAA production and phosphate solubilization in the three bacteria Reduced the production of antibiotics by P. aeruginosa and P. fluorescens The three bacterial species produced increased amounts of siderophore All the effects depended on the concentration |
Haris and Ahmad (2017) |
| TiO2 | Captigel Sol–gel approach | 50 µg/mL | B. amyloliquefaciens | Brassica napus |
The NPs promoted bacterial attachment to plant roots Improved colonization and protected the oilseed rape from a pathogen |
Palmqvist et al. (2015) |
| Au | Citrate reduction method, with HAuCl4 used as a precursor | 6.25 µg/mL | P. fluorescens, B. subtilis, Paenibacillus elgii, and Pseudomonas putida | – |
Increased the growth of P. fluorescens, B. subtilis, and P. elgii No impact on P. putida |
Shukla et al. (2015) |
| Fe, Zn, Mn | Commercial |
4 L/ha 8 L/ha |
Azotobacter spp. Pseudomonas spp. |
Spring wheat (Triticum aestivum) | Increased spike length, spike number, seed number, seed number per spike, seed weight, and number of days until physiological maturity | Mardalipour et al. (2014) |
| SiO2 | Rice husk using alkaline treatment followed by acid precipitation | 0.5 g/kg |
Phosphate solubilizers Nitrogen fixers Silicate solubilizers |
Maize (Zea mays L.) | Increased bacterial populations, total biomass, and soil nutrient contents | Rangaraj et al. (2014) |
| SiO2 | Rice husk using alkaline treatment followed by acid precipitation | 10 mg/L |
Bacillus megaterium Bacillus brevis P. fluorescens Azotobacter vinelandii |
Maize (Zea mays L.) |
Increased bacterial viability and the total soil bacterial population Increased N, P, and K contents Caused 100% seed germination |
Karunakaran et al. (2013) |
| ZnO | Commercial | 500 mg/L | Pseudomonas chlororaphis | – | Inhibited IAA production by the bacteria | Dimkpa et al. (2012b) |
| CuO | Commercial | 200 mg/L | P. chlororaphis | – | Enhanced IAA production by the bacteria | Dimkpa et al. (2012b) |
SiO2 nanoparticles
Silicon is the second most common element on Earth after oxygen and is very abundant in the soil (Wainwright et al. 1997). Plants naturally demand silica to help respond to biotic and abiotic stresses (Ma 2004); these responses include enhancing water-use efficiency and the photosynthetic potential of plants and increasing both the mechanical strength and the rigidity of leaves, thus preventing them from falling over and preventing pathogen attack (Datnoff et al. 1997; Jian et al. 2006; Namaganda et al. 2009). Plants produce silica bodies by absorbing soluble silica from the groundwater and transporting it to different tissues through the vascular system.
Silicon oxide (SiO2) nanoparticles, also known collectively as silica, are among the most widely used nanoparticles (Neethirajan et al. 2009). They can be either extracted from the environment or synthesized. When extracted, they are in a mineral silica form with a crystalline phase (such as quartz), differing from synthetic nanoparticles, which are amorphous. The advantage of synthesizing these NPs is the purity of the final material, as the extracted NPs have impurities from other metals (Rahman and Padavettan 2012). Silica NPs are extensively used in various fields due to their ease and low cost of synthesis; large amounts of silica can be obtained, and their morphology and size, as well as their biocompatibility, chemical inertness, and large surface area can be controlled (Rao et al. 2005; Jeelani et al. 2020). One of the main applications is their use in controlled-release systems, allowing the loading of various compounds, such as drugs, DNA, RNA, proteins, fertilizer components, and pesticide ingredients. In addition to allowing the controlled output of molecules, silica NPs can improve the efficiency, specificity, bioactivity, and biocompatibility of molecules (Hom et al. 2009; Xu et al. 2019). One way to better control release is by synthesizing mesoporous silica NPs, as the pores hinder molecules from exiting (Narayan et al. 2018; Karaman and Kettiger 2018). The use of silica NPs in agriculture has also been reported to increase maize seed viability when present in smart pesticide formulations and augment bioremediation (Yuvakkumar et al. 2011; Kumari and Singh 2016; Liang et al. 2020).
The most commonly used methods to synthesize SiO2 nanoparticles include reverse microemulsion, flame synthesis, and sol–gel methods. In reverse microemulsion synthesis, spherical micelles are formed by a surfactant dissolved in an organic solvent. When in contact with water, the polar heads organize themselves to form reverse micelles, which are microcavities containing water. The nanoparticles then infiltrate these microwells after the addition of the silicon precursor. This method's disadvantages include high costs and difficulty removing the surfactant from the final material (Yoo and Pak 2013). Flame synthesis, also known as chemical vapor condensation, is based on the flame decomposition of metal–organic precursors. The most commonly used process involves reacting oxygen and hydrogen with a precursor of silicon, silicon tetrachloride (SiCl4). Disadvantages of this method include the difficulty in controlling the NP size and morphology (Shekar et al. 2012). Finally, the sol–gel method is based on the hydrolysis and condensation of a silicon precursor, an alkoxide, in the presence of a catalyst, either an acidic or basic one. The most widely used sol–gel method is the Stöber method. The precursor used is tetraethylorthosilicate (TEOS, Si(OC2H5)4), which is added to a solution containing water and ethanol. Hydrolysis, catalyzed by an ammonium hydroxide base, occurs with the nucleophilic attack of water on the alkoxide, forming silanol groups (Si(OH)4). The subsequent condensation of these groups results in Si–O–Si chains, creating the three-dimensional structure of silica (Stöber et al. 1968). The great advantage of the Stöber method is the production of dispersed NPs with a spherical shape and the ability to control their size. In the sol–gel synthesis method, the NP sizes depend on the concentration of the reagents and temperature. Their final sizes are based on the TEOS/NH3 molar ratio; the higher the ratio is, the smaller the diameter (Bailey and Mecartney 1992). Arantes et al. (2012) conducted a study using chemometrics to investigate the influence of each reagent on the final size of NPs. In all experiments where the ammonia amount was increased, the NPs obtained also increased in size (Arantes et al. 2012). In mesoporous silica, a surfactant is added to the reaction medium, and the solution is heated before adding TEOS. Cetrimonium bromide (CTAB) is the most commonly used surfactant, and the temperatures used are close to 80 °C (Parangi et al. 2014).
An experiment conducted with maize (Zea mays) seeds demonstrated that 50 nm silica NPs increased the viability of PGPB and their population in the soil. The NPs did not show a toxic effect, maintaining an optimal pH for the bacteria. Moreover, they increased the amount of nitrogen, phosphorus, and potassium (NPK), resulting in the germination of all maize seeds (Karunakaran et al. 2013). Rangaraj et al. (2014) also studied the effects of nanosilica on PGPB in maize. The results showed that the nanoparticles increased the bacterial population, total biomass, and soil nutrient contents. In both studies, silica NPs proved to be better than other sources of silicon. Nanosilica was also reported to benefit tomato (Lycopersicum esculentum) plants by increasing the germination rate, average germination time, seedling fresh weight, and dry weight (Siddiqui and Al-Whaibi 2014). In all these studies, the germination was better, indicating the potential of silica NPs for crop improvement.
Another way NPs can be used in agriculture is based on their encapsulating capacity. Pour et al. (2019) encapsulated Pseudomonas fluorescens and Bacillus subtilis in alginate-silica NPs and carbon nanotubes. This method helped to improve the root length and micropropagation of UCB-1 pistachio. Inoculation of explants with encapsulated bacteria increased plant biomass and bud length compared to those of the controls (Pour et al. 2019). Boroumand et al. (2020) extracted silica NPs from Equisetum telmateia; the researchers sprayed them with the phosphate-solubilizing rhizobacterium Pseudomonas stutzeri and Mesorhizobium spp. to test their effects on land cress (Barbarea verna) plant growth. The NPs improved bacterial growth when they were applied at concentrations of 0.05 and 0.07 ppm. When NPs were used simultaneously with both bacterial species, the dry weights of the shoots and roots were the highest recorded, and the nitrogen and phosphate contents increased in the soil, improving plant growth (Boroumand et al. 2020). Silica NPs (5–20 nm) have also shown a positive effect on B. subtilis, inducing an 85% increase in cytokinin synthesis (Kurdish et al. 2018).
The interaction between nanosilica and bacteria can be explained by the hydration properties of silica NP surfaces, facilitating their attraction to bacteria and consequently improving bacterial acid resistance (Gordienko and Kurdish 2007; Hirota et al. 2010). Furthermore, studies have shown an increase in the negative surface charge of some gram-negative bacteria when silicon dioxide particles were added to the media. It has been proposed that the increase in charge density is due to a change in porin conformation resulting from the adsorption of the particles by cell surfaces (Gordienko et al. 1993, 1999; Gordienko and Kurdish 2005, 2007).
Studies show that SiO2 NPs can enhance the ability of bacteria to promote plant growth. The stimulating effect of mineral NPs on bacterial growth may be explained by the improved oxygen mass transfer and ion exchange processes in the media (Kurdish 2019). We hypothesized that the same effect might happen with other nanomaterials. Furthermore, NP attachment to the bacterial surface may alter the shape and size of cells, increasing growth (Phenrat et al. 2009).
ZnO nanoparticles
Zinc oxide (ZnO) is an n-type semiconductor; its nanoparticle properties include high chemical stability, nontoxicity, biocompatibility, and photothermal stability, and these particles can be produced at relatively low costs (Weldegebrieal 2020). The high capacity to absorb ultraviolet radiation makes zinc oxide a promising photocatalyst. This compound is considered an alternative to TiO2 because it has a similar bandgap (3.37 eV) (Kołodziejczak-Radzimska and Jesionowski 2014). Zinc oxide NPs are very useful in medicine and food packing due to their biocompatibility, biodegradability, and antimicrobial properties (Akbar et al. 2019). Additionally, ZnO has piezoelectric properties, generating electrical tension under mechanical pressure, making it a viable material for sensors (Bhatia et al. 2016). These nanoparticles are classified as one-, two-, or three-dimensional based on their structure; one-dimensional examples include nanorods, needles, tubes, and wires. An example of a two-dimensional ZnO particle is a nanosheet, and a three-dimensional model is a flower (Wang 2004).
ZnO NPs can be synthesized by both physical and chemical methods. The most commonly used physical methods include laser ablation, characterized by the removal of atoms from a solid with an intense laser beam, and physical vapor deposition, in which the material in a vapor state is transferred to a substrate (Thareja and Shukla 2007; Laurenti et al. 2015). Among the chemical methods, the precipitation, sol–gel, and hydrothermal synthesis methods are most common. In precipitation synthesis, a reduction in zinc salt in solution occurs using a reducing agent that controls the size of NPs, followed by thermal treatment (Kołodziejczak-Radzimska et al. 2010). The zinc precursors used are zinc chloride and zinc acetate, while the reducing agent used is ammonium carbonate (López et al. 2017). The sol–gel method makes it possible to obtain ZnO nanoparticles repeatability and at low cost, in addition to allowing surface modifications (Al Abdullah et al. 2017). The hydrothermal method can be used for synthesizing nanoparticles of different shapes and sizes by varying the composition of the reaction mixture, resulting in materials with high crystallinity (Bharti et al. 2017).
ZnO NPs have been used in agriculture to improve food crop growth and increase yields. For example, ZnO NPs at a 1000 ppm concentration promoted seed germination and seedling vigor and increased root and stem growth in peanut (Arachis hypogaea) plants (Prasad et al. 2012). When these NPs interact with PGPB, various effects have been reported. The bacterium Pseudomonas chlororaphis and 500 mg/L ZnO NPs produced higher amounts of siderophores than did the controls in 24 h. An explanation for this would be the release of Zn2+ ions by NPs, which bind to siderophores. However, ZnO was shown to inhibit the production of IAA at 48 h. Nevertheless, this reduction cannot be explained by the release of cations since IAA production was shown not to be affected in a Zn2+ solution (Dimkpa et al. 2012a, b). Zinc oxide NPs also inhibited IAA production in the ZnS plant growth-promoting bacterial species P. aeruginosa, P. fluorescens, and B. amyloliquefaciens. The concentrations of NPs used ranged from 100 to 400 µg/mL, and as the concentration increased, the production decreased. The NPs also inhibited the phosphate solubilization of the three bacteria, which again proved to depend on zinc oxide concentration. Regardless, all the bacteria produced more siderophores in the presence of ZnO NPs than in the control conditions, and the increase in NP concentration led to increased production of this compound (Haris and Ahmad 2017).
In another study, ZnO NPs inoculated with PGPR in soybean plants promoted increased plant height, number of nodules, and grain weight. When the concentration of zinc oxide was increased, the dry weight of nodules per plant, pod number per plant, and grain number per plant also increased (Seyed Sharifi and Khoramdel 2016). Similarly, Gudadhe et al. (2018) reported that ZnSO4 NPs, in association with the Pseudomonas spp. PGPB, increased nitrogen, phosphorus, potassium, and zinc contents in rice plants, promoting increased grain yield and seed nutrient contents (Gudadhe et al. 2018).
Other nanoparticles
Soils have a rich amount of natural mineral nanoparticles, which play an essential role in the physiological and biochemical processes of microorganisms due to their close interactions (Kurdish and Kigel 1997; Mishra and Kumar 2009; Kurdish 2010). Therefore, the effects of nanomaterials on PGPB have been explored to improve bacterial efficiency in plants. Kurdish et al. (2015) investigated the impact of soil nanomaterials on N fixation and P mobilization bacterial activity. The authors found that NPs of vermiculite and saponite promoted bacterial growth and that vermiculite NPs increased both the dehydrogenase activity in Azotobacter vinelandii and the peroxidase activity in B. subtilis at concentrations of 1.5 and 2.5 g/L. The formulation consisting of bacteria and nanomaterials enhanced grain yield and protein content and reduced lesions caused by diseases in wheat and barley plants. Furthermore, Chobotarov et al. (2017) reported that vermiculite NPs increased the accumulation of abscisic acid (ABA) in A. vinelandii and B. subtilis and 3-indoleacetic acid (IAA) synthesis in B. subtilis. The phytohormone ABA is a key regulator of plant tolerance to biotic and abiotic stresses (Zhu 2002; Fujita et al. 2006), while IAA is essential for plant growth and development (Waseem et al. 2018). Since mineral NPs are present in nature, as well as silica, their use can be considered a safer approach with vast prospective potential along with PGPB to enhance plant growth and tolerance to stressful environments and phytopathogens. However, it is necessary to investigate the interactions between different bacterial species and NPs of various sizes and concentrations under natural conditions. An increase in IAA synthesis was also shown in the plant growth-promoting bacterium Pseudomonas chlororaphis by CuO NPs at a concentration of 200 mg/L, possibly explained by ion release (Dimkpa et al. 2012b). Depending on their dose, these NPs are harmful to beneficial plant bacteria (Baek and An 2011). Hence, this finding highlights the dose-dependent effect of nanomaterials in bacteria. The application of CuO NPs to the soil of wheat (Triticum aestivum) increased microbial community health, enhanced nitrogen fixation, and decreased denitrification processes, which led to plant growth. A correlation was observed between increased nitrate concentration in the rhizosphere and an increase in the expression of genes related to N fixation (Guan et al. 2020). These findings suggest that CuO NPs can be involved in N fixation-related gene expression.
Gold NPs (AuNPs) are valuable materials due to their chemical inertness, resistance to surface oxidation, and low toxicity in natural environments (Shankar et al. 2004; Shukla et al. 2015). Shukla et al. (2015) endorsed the potential of these NPs in nanobiofertilizer formulations due to their growth-promoting effects on the beneficial bacteria P. fluorescens and B. subtilis as the AuNP concentration increased.
A successful nanobiofertilizer can also be achieved by mixing more than one bacterial species with various nanomaterials. Mardalipour et al. (2014) reported the benefits of different NPs associated with two PGPB in spring wheat plants. The nanobiofertilizer (Biozar®), which includes Azotobacter, Pseudomonas, and NPs of Fe, Zn, and Mn, improved agronomic traits and increased crop growth and yield.
AgNPs are effective at controlling plant diseases (Jo et al. 2009; Aguilar-Méndez et al. 2011; Abdellatif et al. 2016). These particles act as growth stimulants and enable plants to inhibit senescence induced by reactive oxygen species (ROS) under stress conditions (Karuppanapandian et al. 2011). Pallavi-Mehta et al. (2016) examined the effects of silver nanoparticles on the bacterial rhizosphere diversity and growth of wheat (Triticum aestivum), cowpea (Vigna sinensis), and Brassica (Brassica juncea) plants. While wheat showed only a negative effect, the cowpea showed a positive response in growth parameters and root nodulation, and Brassica exhibited a positive response for shoot parameters. Regarding the bacterial diversity in cowpea and wheat, the 75 ppm concentration reduced the population of N fixers and siderophore producers, while 50 ppm positively affected P solubilizers. In the case of Brassica, there was no change in diversity when either concentration was used (Pallavi-Mehta et al. 2016).
Chavan and Nadanathangam (2019) reported that, at low concentrations, AgNPs presented bactericidal activity against N-fixing, phosphate-solubilizing, and biofilm-forming bacteria (2–22 µg/mL). However, there was an increase in the abundance of the bacteria Stenotrophomonas spp. and Pseudomonas spp., which have a great capacity to promote plant growth (Chavan and Nadanathangam 2019). Khan and Bano (tested the influence of AgNPs and Pseudomonas spp. and Bacillus cereus PGPR on maize growth. The NPs attached to the bacteria improved the root area and length. Nanosilver particles also improved rhizobacterial bioremediation ability for Pb, Cd, and Ni (Khan and Bano 2016). AgNPs with Pseudomonas putida were also reported to benefit cucumber (Cucumis sativus L.) plants. These particles enhanced the activities of phenylalanine ammonia-lyase (PAL), superoxide dismutase (SOD), and catalase (CAT) and increased the flavonoid contents of cucumber leaves, thus improving plant tolerance to disease and stress (Nawaz and Bano 2019).
It is worth mentioning that even though these findings show the potential of AgNPs to promote plant growth, they also warrant attention because of their harmful effects on some bacterial populations. Several studies have reported on the bactericidal activity of AgNPs and their adverse impacts on soil microbial diversity. Nevertheless, their toxicity seems to rely on bacterial species and synthesis methods of NPs (Dimkpa et al. 2011).
Industrial-scale production of nanoparticles
Two main approaches can be used to produce nanoparticles on an industrial scale, bottom–up and top–down. Bottom–up methods involve forming a complex and more structured arrangement of atoms, resulting in structures such as clusters and nanoparticles. On the other hand, top–down methods imply that the nanostructure is synthesized by removing atoms or crystalline planes. Both have advantages and limitations. The most common top–down approach for producing nanoparticles is the ball milling process. This method can produce large amounts of nanoparticles from cheap raw materials but with the disadvantage of presenting broad particle size distributions and irregular nanoparticles. Several oxides can be processed by ball milling, including several phosphates (Danelon et al. 2015). Other top-down routes, such as electron beam induce etching, produce only a few milligrams or less material, which is only feasible for exceptional (and expensive) applications. Bottom–up routes are simple and often use water as a solvent, which represents an environmental advantage. Metallic nanoparticles, such as silver and gold, can be easily synthesized on a large scale from water-soluble salts reduced by cheaper molecules, like citrates or alcohols (Gorup et al. 2011). Nowadays, natural extracts have emerged as an environmentally friendly method to produce metallic nanoparticles (Fernandes et al. 2018). Nonaqueous solvents have opened a new route for obtaining nanoparticles in cases where they can act as solvent and reactant. Silica and other oxides can be synthesized on an industrial scale from the hydrolysis of some alkoxides, usually using several small reactors instead of a large reactor.
Toxicological effects of nanoparticles on plant growth-promoting bacteria
Even though nanomaterials have the potential to increase the abundance of PGPB, they might negatively affect soil bacteria. As mentioned before, the shape of NPs seems to be one of the factors influencing their biological activity. For example, Dimkpa et al. (2011) found that spherical AgNPs did not damage the integrity of Pseudomonas chlororaphis membranes, while Pal et al. (2007) found that triangular AgNPs exhibited the strongest biocidal action in Escherichia coli. Thus, it has been proposed that AgNPs with different shapes may also have other modes of action regardless of having the same surface areas (Pal et al. 2007). In another study, Dimkpa et al. (2012b) reported that CuO NPs enhanced bacterial IAA production, while ZnO NPs reduced the synthesis of this phytohormone in the same bacteria. The authors suggested that NPs with different shapes (CuO NPs have a round shape, and ZnO NPs have elongated structures) were critical in the production of bacterial secondary metabolites (Dimkpa et al. 2012b).
Bacterial toxicity also seems to be dependent on the nature of the metal (Sinha et al. 2011) and the concentration of NPs (Hayden et al. 2012). Chavan et al. (2020) reported that TiO2 NPs were more inhibitory to gram-positive bacteria, suggesting that the bacterial surface is primarily responsible for TiO2 NP toxicity. Rousk et al. (2012) proved that Cu and Zn at more soluble forms were more toxic to soil bacteria than the metal oxide forms or nanoforms. Therefore, these NPs can be more harmful than the bulk material only when the metal dissolution is higher in this form, depending on the soil (Rousk et al. 2012).
The overall conclusion regarding the toxicity of nanomaterials is that the NP reactivity, toxicity, and uptake increase as the particle size decreases; positively charged NPs are more toxic to most living systems; NP toxicity is correlated with ionic dissolution; and rod-shaped and anisotropic NPs are more toxic even with less absorption (Singh et al. 2019).
Regarding phytotoxicity, NPs have either positive or negative impacts on plants. The toxic effects depend on plant species and NP composition (their nature, size, concentration, and exposure time) (Jha and Pudake 2016).
The introduction of nanomaterials into the environment might hinder biological organisms at many levels. The major obstacle involves several variables associated with nanomaterials, such as their material composition, size, shape, concentration, and interactions.
It is important to emphasize the evidence of agricultural improvements caused by the association of NPs and PGPB. Thus, there is an urgent need for in-depth investigations concerning lethal and sublethal doses on a case-to-case basis.
Conclusion and future perspectives
There is no doubt that the use of PGPB is a promising viable eco-friendly alternative to the use of chemicals in agriculture. However, knowledge of specific plant–microbe interactions and innovative approaches must be explored for the ability to use them in the field successfully.
Nanotechnology serves as an effective tool to overcome obstacles associated with microbial biofertilizers. Many nanomaterials have shown positive effects either on PGPB or on the growth of various plant species, making these materials promising components of biofertilizer formulations. The approach of using nanomaterials in association with beneficial bacteria in plants can increase bacterial viability and resistance in the environment, improving their field stability and consequently promoting plant growth. However, there are different effects of NPs on bacterial metabolism and concerns about the possible hazardous consequences of nanomaterials on the environment, food safety, and human health, so these effects must be investigated.
Further experimentation involving plant growth-promoting bacterium-NP-plant is necessary to evaluate the effectiveness of this complex system at the molecular level. Several factors are involved: the nature of bacteria and their metabolism, nanomaterials (their substance, synthesis method, shape, size, and concentration), plant species, and environmental interactions. In addition, nanomaterial characterization is also of great importance, since there are many combinations in which NP characteristics interact in different ways with the natural environment and cells.
Most of the experiments cited in this review were performed under controlled conditions, thus excluding abiotic and biotic interactions found in the field. Therefore, field experiments must be conducted to better understand and validate the previous findings.
The use of nanomaterials associated with PGPB can potentially increase yields in agricultural systems and address global food demands. Thus, any possible hazard needs to be investigated with sensitive risk assessment methods.
Acknowledgements
This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP n. 2015/10974-8 and n. 2018/12871-0); and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) (Proc. no 88882.426494/2019-01; Finance Code 001).
Author contributions
ACPM term, conceptualization, investigation, writing—original draft, writing—review and editing, project administration, visualization, supervision. LSR conceptualization, writing—original draft, writing—review and editing. ERC conceptualization, writing—original draft, writing—review and editing. PTL term, conceptualization, investigation, writing—original draft, writing—review and editing, project administration, visualization, supervision, funding acquisition.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Contributor Information
Amanda Carolina Prado de Moraes, Email: amandacpmoraes@yahoo.com.br.
Lucas da Silva Ribeiro, Email: lucas.silva.ribeiro03@gmail.com.
Emerson Rodrigues de Camargo, Email: camargo@ufscar.br.
Paulo Teixeira Lacava, Email: ptlacava@ufscar.br.
References
- Abdel Latef AAH, Srivastava AK, El-sadek MSA, et al. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad Dev. 2018;29:1065–1073. doi: 10.1002/ldr.2780. [DOI] [Google Scholar]
- Abdellatif KF, Abdelfattah RH, El-Ansary MSM. Green nanoparticles engineering on root-knot nematode infecting eggplants and their effect on plant DNA modification. Iran J Biotechnol. 2016;14(4):250–259. doi: 10.15171/ijb.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Méndez MA, Martín-Martínez ES, Ortega-Arroyo L, Cobián-Portillo G, Sánchez Espíndola E. Synthesis and characterization of silver nanoparticles: effect on phytopathogen Colletotrichum gloesporioides. J Nanopart Res. 2011;13(6):2525–2532. doi: 10.1007/s11051-010-0145-6. [DOI] [Google Scholar]
- Akbar A, Sadiq MB, Ali I, et al. Synthesis and antimicrobial activity of zinc oxide nanoparticles against foodborne pathogens Salmonella typhimurium and Staphylococcus aureus. Biocatal Agric Biotechnol. 2019;17:36–42. doi: 10.1016/j.bcab.2018.11.005. [DOI] [Google Scholar]
- Akpan UG, Hameed BH. The advancements in sol–gel method of doped-TiO2 photocatalysts. Appl Catal A Gen. 2010;375:1–11. doi: 10.1016/j.apcata.2009.12.023. [DOI] [Google Scholar]
- al Abdullah K, Awad S, Zaraket J, Salame C. Synthesis of ZnO nanopowders by using sol-gel and studying their structural and electrical properties at different temperature. Energy Proc. 2017;119:565–570. doi: 10.1016/j.egypro.2017.07.080. [DOI] [Google Scholar]
- Arantes TM, Pinto AH, Leite ER, et al. Synthesis and optimization of colloidal silica nanoparticles and their functionalization with methacrylic acid. Colloids Surf A. 2012;415:209–217. doi: 10.1016/j.colsurfa.2012.09.041. [DOI] [Google Scholar]
- Baek YW, An YJ. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci Total Environ. 2011;409:160–1608. doi: 10.1016/j.scitotenv.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Baetzold RC. Chemisorption of halogen on copper and silver clusters. J Am Chem Soc. 1981;103:6116–6120. doi: 10.1021/ja00410a022. [DOI] [Google Scholar]
- Bailey JK, Mecartney ML. Formation of colloidal silica particles from alkoxides. Colloids Surf. 1992;63:151–161. doi: 10.1016/0166-6622(92)80081-C. [DOI] [Google Scholar]
- Bansal P, Duhan JS, Gahlawat SK. Biogenesis of nanoparticles: a review. Afr J Biotechnol. 2014;13:2778–2785. doi: 10.5817/AJB2013.13458. [DOI] [Google Scholar]
- Bashan Y. Significance of timing and level of inoculation with rhizosphere bacteria on wheat plants. Soil Biol Biochem. 1986;18:297–301. doi: 10.1016/0038-0717(86)90064-7. [DOI] [Google Scholar]
- Bashan Y, de-Bashan LE, Prabhu SR, Hernandez JP. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013) Plant Soil. 2014;378:1–33. doi: 10.1007/s11104-013-1956-x. [DOI] [Google Scholar]
- Batista BD, Lacava PT, Ferrari A, et al. Screening of tropically derived, multi-trait plant growth-promoting rhizobacteria and evaluation of corn and soybean colonization ability. Microbiol Res. 2018;206:33–42. doi: 10.1016/j.micres.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Berg G, Eberl L, Hartmann A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ Microbiol. 2005;7:1673–1685. doi: 10.1111/j.1462-2920.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- Bharti DB, Bharati A. Synthesis of ZnO nanoparticles using a hydrothermal method and a study its optical activity. Luminescence. 2017;32:317–320. doi: 10.1002/bio.3180. [DOI] [PubMed] [Google Scholar]
- Bhatia D, Sharma H, Meena RS, Palkar VR. A novel ZnO piezoelectric microcantilever energy scavenger: fabrication and characterization. Sens Bio-Sens Res. 2016;9:45–52. doi: 10.1016/j.sbsr.2016.05.008. [DOI] [Google Scholar]
- Bhattacharyya A, Duraisamy P, Govindarajan M, Buhroo AA, Prasad R. Nanobiofungicides: emerging trend in insect pest control. In: Prasad R, editor. Advances and applications through fungal nanobiotechnology. Cham: Springer International Publishing; 2016. pp. 307–319. [Google Scholar]
- Biswal SK, Nayak AK, Parida UK, Nayak PL. Applications of nanotechnology in agriculture and food sciences. Int J Sci Innov Discov. 2012;2:21–36. [Google Scholar]
- Boroumand N, Behbahani M, Dini G. Combined effects of phosphate solubilizing bacteria and nanosilica on the growth of land cress plant. J Soil Sci Plant Nutr. 2020 doi: 10.1007/s42729-019-00126-8. [DOI] [Google Scholar]
- Breznak JA, McFeters GA, Rutter PR (2012) Microbial adhesion and aggregation: report of the dahlem workshop on microbial adhesion and aggregation, Berlin, 1984, vol 31. Springer Science & Business Media
- Castro RA, Dourado MN, de Almeida JR, et al. Mangrove endophyte promotes reforestation tree (Acacia polyphylla) growth. Braz J Microbiol. 2018;49:59–66. doi: 10.1016/j.bjm.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan S, Nadanathangam V. Effects of nanoparticles on plant growth-promoting bacteria in Indian agricultural soil. Agronomy. 2019;9:140. doi: 10.3390/agronomy9030140. [DOI] [Google Scholar]
- Chavan S, Sarangdhar V, Nadanathangam V. Toxicological effects of TiO2 nanoparticles on plant growth promoting soil bacteria. Emerg Contam. 2020;6:87–92. doi: 10.1016/j.emcon.2020.01.003. [DOI] [Google Scholar]
- Chen X. Titanium dioxide nanomaterials and their energy applications. Chin J Catal. 2009;30:839–851. doi: 10.1016/s1872-2067(08)60126-6. [DOI] [Google Scholar]
- Chen K, Zhu L, Yang K. Tricrystalline TiO2 with enhanced photocatalytic activity and durability for removing volatile organic compounds from indoor air. J Environ Sci (china) 2015 doi: 10.1016/j.jes.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Chinnamuthu C, Boopathi P. Nanotechnology and Agroecosystem Madras Agric J. 2009;96:17–31. [Google Scholar]
- Chobotarov A, Volkogon M, Voytenko L, Kurdish I. Accumulation of phytohormones by soil bacteria Azotobacter vinelandii and Bacillus subtilis under the influence of nanomaterials. J Microbiol Biotechnol Food Sci. 2017;7:271–274. doi: 10.15414/jmbfs.2017/18.7.3.271-274. [DOI] [Google Scholar]
- Cui J, Li Y, Jin Q, Li F. Silica nanoparticles inhibit arsenic uptake into rice suspension cells via improving pectin synthesis and the mechanical force of the cell wall. Environ Sci Nano. 2020;7:162–171. doi: 10.1039/C9EN01035A. [DOI] [Google Scholar]
- Danelon M, Pessan JP, Souza Neto FN, Camargo ER, Delbem ACB. Effect of toothpaste with nano-sized trimetaphosphate on dental caries. In sity study. Colloids Surf A. 2015;415:806–813. doi: 10.1016/j.jdent.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Datnoff LE, Deren CW, Snyder GH. Silicon fertilization for disease management of rice in Florida. Crop Prot. 1997;16:525–531. doi: 10.1016/S0261-2194(97)00033-1. [DOI] [Google Scholar]
- Dimkpa CO, Calder A, Gajjar P, et al. Interaction of silver nanoparticles with an environmentally beneficial bacterium, Pseudomonas chlororaphis. J Hazard Mater. 2011;188:428–435. doi: 10.1016/j.jhazmat.2011.01.118. [DOI] [PubMed] [Google Scholar]
- Dimkpa CO, Mclean JE, Britt DW, Anderson AJ. CuO and ZnO nanoparticles differently affect the secretion of fluorescent siderophores in the beneficial root colonizer, Pseudomonas chlororaphis O6. Nanotoxicology. 2012;6:635–642. doi: 10.3109/17435390.2011.598246. [DOI] [PubMed] [Google Scholar]
- Dimkpa CO, Zeng J, McLean JE, et al. Production of indole-3-acetic acid via the indole-3-acetamide pathway in the plant-beneficial bacterium pseudomonas chlororaphis O6 is inhibited by ZnO nanoparticles but enhanced by CuO nanoparticles. Appl Environ Microbiol. 2012;78:1404–1410. doi: 10.1128/AEM.07424-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh R, Anandaraj M, Srinivasan V, Hamza S. Engineered nanoparticles in the soil and their potential implications to microbial activity. Geoderma. 2012 doi: 10.1016/j.geoderma.2011.12.018. [DOI] [Google Scholar]
- Djamin A, Pathak MD. Role of silica in resistance to asiatic rice borer, Chilo suppressalis (walker), in rice varieties1. J Econ Entomol. 1967;60:347–350. doi: 10.1093/jee/60.2.347. [DOI] [Google Scholar]
- Duhan JS, Kumar R, Kumar N, et al. Nanotechnology: the new perspective in precision agriculture. Biotechnol Rep. 2017;15:11–23. doi: 10.1016/j.btre.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ramady H, El-Ghamry A, Mosa A, Alshaal T. Nanofertilizers vs. biofertilizers: new insights. Environ Biodivers Soil Secur. 2018;2:51–72. doi: 10.21608/jenvbs.2018.3880.1029. [DOI] [Google Scholar]
- Feng X, Zhai J, Jiang L. The fabrication and switchable superhydrophobicity of TiO2 nanorod films. Angewandte Chemie Int Ed. 2005;44:5115–5118. doi: 10.1002/anie.200501337. [DOI] [PubMed] [Google Scholar]
- Fernandes RA, Beretta AA, Torres EC, Buszinski AFM, Fernandes GL, Mendes-Gouveia CC, Souza Neto FN, Gorup LF, Camargo ER, Barbosa DB. Antimicrobial potential of silver nanoparticles phytosynthesized by pormegratate peel extract. Antibiotics. 2018;7:51. doi: 10.3390/antibiotics7030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signalling networks. Curr Opin Plant Biol. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Ghalamboran MR, Ramsden JJ. Viability of Bradyrhizobium japanicum on soybean seeds enhanced by magnetite nanoparticles during desiccation. Int J Biol Life Sci. 2012;8:228–233. doi: 10.7150/ijbs.8.228. [DOI] [Google Scholar]
- Ghormade V, Deshpande M, Paknikar KM. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv. 2011;29:792–803. doi: 10.1016/j.biotechadv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;9:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BR. Beneficial plant-bacterial interactions. Heidelberg: Springer; 2020. [Google Scholar]
- Gond SK, Torres MS, Bergen MS, Helsel Z, White JFJR. Induction of salt tolerance and upregulation of aquaporin genes in tropical corn by rhizobacterium Pantoea agglomerans. Lett Appl Microbiol. 2015;60(392):399. doi: 10.1111/lam.12385. [DOI] [PubMed] [Google Scholar]
- Gordienko AS, Kurdish IK. Interaction of some Pseudomonas genus representatives with high-dispersed silicon dioxide particles. Mikrobiolohichnyi Zhurnal (Kiev, Ukraine: 1993) 2005;5:89–96. [PubMed] [Google Scholar]
- Gordienko AS, Kurdish IK. Surface electrical properties of bacillus subtilis cells and the effect of interaction with silicon dioxide particles. Biophysics. 2007;52:217–219. doi: 10.1134/S0006350907020121. [DOI] [PubMed] [Google Scholar]
- Gordienko AS, Zbanatskaya I, Kurdish IK. Change in electrosurface properties of Methylomonas rubra cells at contact interaction with particles of silicon dioxide. Can J Microbiol. 1993;39:902–905. doi: 10.1139/m93-136. [DOI] [Google Scholar]
- Gordienko AS, Zbanatskaya IV, Kurdish IK. Increase in the negative surface charge of Methylomonas rubra cells at contact interaction with the particles of silicon dioxide. Mikrobiologičeskij Žurnal. 1999;61:48–55. [Google Scholar]
- Gorup LF, Longo E, Leite ER, Camargo ER. Moderating effect of ammonia on particle growth and stability of quasi-monodisperse silver nanoparticles synthesized by the Turkevich method. J Colloid Interface Sci. 2011;360:355–358. doi: 10.1016/j.jcis.2011.04.099. [DOI] [PubMed] [Google Scholar]
- Grand View Research (2020) Biofertilizers market size, share & growth report. Market Analysis Report. https://www.grandviewresearch.com/industry-analysis/biofertilizers-industry. Accessed 30 Sept 2020
- Guan X, Gao X, Avellan A, et al. CuO nanoparticles alter the rhizospheric bacterial community and local nitrogen cycling for wheat grown in a calcareous soil. Environ Sci Technol. 2020;54:8699–8709. doi: 10.1021/acs.est.0c00036. [DOI] [PubMed] [Google Scholar]
- Gudadhe NN, Suthar KP, Thanki JD. Synthesis and evaluation of ZnO nanoparticles through green and chemical methods and its effect on rice. Udaipur: XXI Biennial National Symposium of Indian Society of Agronomy. MPUAT; 2018. pp. 24–26. [Google Scholar]
- Gupta SM, Tripathi M. A review of TiO2 nanoparticles. Chin Sci Bull. 2011;56:1639. doi: 10.1007/s11434-011-4476-1. [DOI] [Google Scholar]
- Gurikar C, Naik MK, Sreenivasa MY. Azotobacter: PGPR activities with special reference to effect of pesticides and biodegradation. In: Singh D, Singh H, Prabha R, editors. Microbial inoculants in sustainable agricultural productivity. New Delhi: Springer; 2016. pp. 229–244. [Google Scholar]
- Gutiérrez FJ, Mussons ML, Gatón P, Rojo R (2011) Nanotechnology and food industry. Scientific, health and social aspects of the food industry. In: Scientific, health and social aspects of the food industry. Tech, Croatia Book Chapter, pp 95–128
- Haris Z, Ahmad I. Impact of metal oxide nanoparticles on beneficial soil microorganisms and their secondary metabolites. Int J Life Sci Sci Res. 2017;3:1020–1030. doi: 10.21276/ijlssr.2017.3.3.10. [DOI] [Google Scholar]
- Hayden SC, Zhao G, Saha K, et al. Aggregation and interaction of cationic nanoparticles on bacterial surfaces. J Am Chem Soc. 2012;134:6920–6923. doi: 10.1021/ja301167y. [DOI] [PubMed] [Google Scholar]
- Herrmann L, Lesueur D. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl Microbiol Biotechnol. 2013;9:78859–78873. doi: 10.1007/s00253-013-5228-8. [DOI] [PubMed] [Google Scholar]
- Hirota R, Hata Y, Ikeda T, et al. The silicon layer supports acid resistance of Bacillus cereus spores. J Bacteriol. 2010;192:111–116. doi: 10.1128/JB.00954-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom C, Lu J, Tamanoi F. Silica nanoparticles as a delivery system for nucleic acid-based reagents. J Mater Chem. 2009;19:6308–6316. doi: 10.1039/b904197d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameel NZ. Synthesis of TiO2 nanoparticles by sol-gel method using laser ablation for nano paint application. Dissertation, University of Baghdad; 2015. [Google Scholar]
- Jeelani PG, Mulay P, Venkat R, Ramalingam C. Multifaceted application of silica nanoparticles. A review. SILICON. 2020;12:1337–1354. doi: 10.1007/s12633-019-00229-y. [DOI] [Google Scholar]
- Jha S, Pudake RN. Molecular mechanism of plant-nanoparticle interactions. Plant Nanotechnol Princ Pract. 2016;12:1337–1354. doi: 10.1007/978-3-319-42154-4_7. [DOI] [Google Scholar]
- Jian FM, Tamai K, Yamaji N, et al. A silicon transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- Jiang W, Yang K, Vachet RW, Xing B. Interaction between oxide nanoparticles and biomolecules of the bacterial cell envelope as examined by infrared spectroscopy. Langmuir. 2010;26:18071–18077. doi: 10.1021/la103738e. [DOI] [PubMed] [Google Scholar]
- Jo YK, Kim BH, Jung G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 2009;93(10):1037–1043. doi: 10.1094/PDIS-93-10-1037. [DOI] [PubMed] [Google Scholar]
- Kalia A, Kaur H. Nano-biofertilizers: harnessing dual benefits of nano-nutrient and bio-fertilizers for enhanced nutrient use efficiency and sustainable productivity. In: Pudake R, Chauhan N, Kole C, editors. Nanoscience for sustainable agriculture. Cham: Springer; 2019. pp. 51–73. [Google Scholar]
- Karaman DŞ, Kettiger H. Silica-based nanoparticles as drug delivery systems: chances and challenges. In: Grumezescu AM, editor. Inorganic frameworks as smart nanomedicines. William Andrew Publishing; 2018. pp. 1–40. [Google Scholar]
- Karunakaran G, Suriyaprabha R, Manivasakan P, et al. Effect of nanosilica and silicon sources on plant growth promoting rhizobacteria, soil nutrients and maize seed germination. IET Nanobiotechnol. 2013;7:70–77. doi: 10.1049/iet-nbt.2012.0048. [DOI] [PubMed] [Google Scholar]
- Karuppanapandian T, Moon JC, Kim C, et al. Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust J Crop Sci. 2011;5:709. [Google Scholar]
- Kaufman PB, Takeoka Y, Carlson TE, Bigelow WC, Jones JD, Moore PH, Ghosheh NS. Studies on silica deposition in sugarcane (Saccharum spp.) using scanning electron microscopy, energy-dispersive X-ray analysis, neutron activation analysis, and light microscopy. Phytomorphology. 1979;29:185–193. [Google Scholar]
- Khan N, Bano A. Role of plant growth promoting rhizobacteria and Ag-nano particle in the bioremediation of heavy metals and maize growth under municipal wastewater irrigation. Int J Phytorem. 2016;18:211–221. doi: 10.1080/15226514.2015.1064352. [DOI] [PubMed] [Google Scholar]
- Kołodziejczak-Radzimska A, Jesionowski T. Zinc oxide-from synthesis to application: a review. Materials (Basel) 2014;7:2833–2881. doi: 10.3390/ma7042833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kołodziejczak-Radzimska A, Jesionowski T, Krysztafkiewicz A. Obtaining zinc oxide from aqueous solutions of KOH and Zn(CH3COO)2. Physicochem Probl Miner Process. 2010;44:93–102. [Google Scholar]
- Koul O. Nano-biopesticides today and future perspectives. Academic Press; 2019. [Google Scholar]
- Kour D, Rana KL, Yadav AN, et al. Microbial biofertilizers: bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal Agric Biotechnol. 2020;23:101487. doi: 10.1016/j.bcab.2019.101487. [DOI] [Google Scholar]
- Kumar A, Verma JP. Does plant—microbe interaction confer stress tolerance in plants: a review? Microbiol Res. 2018;207:41–52. doi: 10.1016/j.micres.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Kumari B, Singh DP. A review on multifaceted application of nanoparticles in the field of bioremediation of petroleum hydrocarbons. Ecol Eng. 2016;97:98–105. doi: 10.1016/j.ecoleng.2016.08.006. [DOI] [Google Scholar]
- Kurdish IK. Introduction of microorganisms in agroecosystems. Kyiv: Naukova Dumka; 2010. p. 253. [Google Scholar]
- Kurdish IK. Interaction of microorganisms with nanomaterials as a basis for creation of high-efficiency biotechnological preparations. In: Prasad R, Kumar V, Kumar M, Choudhary D, editors. Nanotechnology in Bioformulations. Nanotechnology in the Life Sciences. Cham: Springer; 2019. pp. 259–287. [Google Scholar]
- Kurdish IK, Kigel NF. Effect of high-dispersed materials on physiological activity of methanotrophic bacteria. Mikrobiol Z. 1997;59:35–42. [Google Scholar]
- Kurdish I, Roy A, Chobotarov A, et al. Free-flowing complex bacterial preparation for crop and efficiency of its use in agroecosystems. J Microbiol Biotechnol Food Sci. 2018;4:527–531. [Google Scholar]
- Kurdish I, Roy A, Chobotarov A, Herasimenko I, Plotnikov V, Gylchuk V, Korniychuk A. Free-flowing complex bacterialpreparation for crop and efficiency of its use in agroecosystems. J Microbiol Biotechnol Food Sci. 2015;04(06):527–531. doi: 10.15414/jmbfs.2015.4.6.527-531. [DOI] [Google Scholar]
- Laurenti M, Garino N, Porro S, et al. Zinc oxide nanostructures by chemical vapour deposition as anodes for Li-ion batteries. J Alloy Compd. 2015;640:321–326. doi: 10.1016/j.jallcom.2015.03.222. [DOI] [Google Scholar]
- Li H, Ding X, Wang C, et al. Control of tomato yellow leaf curl virus disease by Enterobacter asburiae BQ9 as a result of priming plant resistance in tomatoes. Turk J Biol. 2016;40(150):159. doi: 10.3906/biy-1502-12. [DOI] [Google Scholar]
- Liang Y, Gao Y, Wang W, et al. Fabrication of smart stimuli-responsive mesoporous organosilica nano-vehicles for targeted pesticide delivery. J Hazard Mater. 2020;389:122075. doi: 10.1016/j.jhazmat.2020.122075. [DOI] [PubMed] [Google Scholar]
- Liu N, Chen X, Zhang J, Schwank JW. A review on TiO2-based nanotubes synthesized via hydrothermal method: formation mechanism, structure modification, and photocatalytic applications. Catal Today. 2014;225:34–51. doi: 10.1016/j.cattod.2013.10.090. [DOI] [Google Scholar]
- López FA, Cebriano T, García-Díaz I, et al. Synthesis and microstructural properties of zinc oxide nanoparticles prepared by selective leaching of zinc from spent alkaline batteries using ammoniacal ammonium carbonate. J Clean Prod. 2017;148:795–803. doi: 10.1016/j.jclepro.2017.02.031. [DOI] [Google Scholar]
- Lundberg DS, Lebeis SL, Paredes SH, et al. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012;488:86–90. doi: 10.1038/nature11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr. 2004;50:11–18. doi: 10.1080/00380768.2004.10408447. [DOI] [Google Scholar]
- Ma M, Jiang X, Wang Q, et al. Isolation and identification of PGPR strain and its effect on soybean growth and soil bacterial community composition. Int J Agric Biol. 2018;20:1289–1297. doi: 10.17957/IJAB/15.0627. [DOI] [Google Scholar]
- MacWan DP, Dave PN, Chaturvedi S. A review on nano-TiO2 sol-gel type syntheses and its applications. J Mater Sci. 2011;46:3669–3686. doi: 10.1007/s10853-011-5378-y. [DOI] [Google Scholar]
- Malusá E, Sas-Paszt L, Ciesielska J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci World J. 2012 doi: 10.1100/2012/491206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamaghani AH, Haghighat F, Lee CS. Hydrothermal/solvothermal synthesis and treatment of TiO2 for photocatalytic degradation of air pollutants: preparation, characterization, properties, and performance. Chemosphere. 2019;219:804–825. doi: 10.1016/j.chemosphere.2018.12.029. [DOI] [PubMed] [Google Scholar]
- Mardalipour M, Zahedi H, Sharghi Y. Evaluation of nano biofertilizer efficiency on agronomic traits of spring wheat at different sowing date. Biol Forum Int J. 2014;6:349. [Google Scholar]
- Meticulous Market Research Pvt. Ltd. (2017) Bioestimulants market - global opportunity analysis and industry forecasts to 2022. Research and Markets. https://www.researchandmarkets.com/research/tk76h2/biostimulants. Accessed 10 Sept 2020
- Mohammadi K, Sohrabi Y. Bacterial biofertilizers for sustainable crop production: a review. J Agric Biol Sci. 2012;7:307–316. [Google Scholar]
- Mózner Z, Tabi A, Csutora M. Modifying the yield factor based on more efficient use of fertilizer —the environmental impacts of intensive and extensive agricultural practices. Ecol Ind. 2012;16:58–66. doi: 10.1016/j.ecolind.2011.06.034. [DOI] [Google Scholar]
- Namaganda M, Krekling T, Lye KA. Leaf anatomical characteristics of Ugandan species of Festuca L. (Poaceae) S Afr J Bot. 2009;75:52–59. doi: 10.1016/j.sajb.2008.07.004. [DOI] [Google Scholar]
- Namasivayam SKR, Saikia SL, Bharani RA. Evaluation of persistence and plant growth promoting effect of bioencapsulated formulation of suitable bacterial bio-fertilizers. Biosci Biotechnol Res Asia. 2014;11:407–415. doi: 10.13005/bbra/1289. [DOI] [Google Scholar]
- Narayan R, Nayak UY, Raichur AM, Garg S. Mesoporous silica nanoparticles: a comprehensive review on synthesis and recent advances. Pharmaceutics. 2018;10:118. doi: 10.3390/pharmaceutics10030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz S, Bano A. Effects of PGPR (Pseudomonas sp.) and Ag-nanoparticles on enzymatic activity and physiology of cucumber. Recent Pat Food Nutr Agric. 2019;11:124–136. doi: 10.2174/2212798410666190716162340. [DOI] [PubMed] [Google Scholar]
- Neethirajan S, Gordon R, Wang L. Potential of silica bodies (phytoliths) for nanotechnology. Trends Biotechnol. 2009;27:461–467. doi: 10.1016/j.tibtech.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Noman MT, Ashraf MA, Ali A. Synthesis and applications of nano-TiO2: a review. Environ Sci Pollut Res. 2019;26:3262–3291. doi: 10.1007/s11356-018-3884-z. [DOI] [PubMed] [Google Scholar]
- Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallavi-Mehta CM, Srivastava R, et al. Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech. 2016;6:254. doi: 10.1007/s13205-016-0567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist NGM, Bejai S, Meijer J, et al. Nano titania aided clustering and adhesion of beneficial bacteria to plant roots to enhance crop growth and stress management. Sci Rep. 2015;5:10146. doi: 10.1038/srep10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parangi TF, Patel RM, Chudasama U. Synthesis and characterization of mesoporous Si-MCM-41 materials and their application as solid acid catalysts in some esterification reactions. Bull Mater Sci. 2014;37:609–615. doi: 10.1007/s12034-014-0709-7. [DOI] [Google Scholar]
- Park MR, Banks MK, Applegate B, Webster TJ. Influence of nanophase titania topography on bacterial attachment and metabolism. Int J Nanomed. 2008;3:497–504. doi: 10.2147/ijn.s4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paungfoo-Lonhienne C, Schmidt S, Webb RI, Lonhienne TGA. Rhizophagy—a new dimension of plant-microbe interactions. Mol Microb Ecol Rhizosphere. 2013;1:1199–1207. doi: 10.1002/9781118297674.ch115. [DOI] [Google Scholar]
- Phenrat T, Long TC, Lowry GV, Veronesi B. Partial oxidation ("aging") and surface modification decrease the toxicity of nanosized zerovalent iron. Environ Sci Technol. 2009;43:195–200. doi: 10.1021/es801955n. [DOI] [PubMed] [Google Scholar]
- Pour MM, Saberi-Riseh R, Mohammadinejad R, Hosseini A. Nano-encapsulation of plant growth-promoting rhizobacteria and their metabolites using alginate-silica nanoparticles and carbon nanotube improves UCB1 pistachio micropropagation. J Microbiol Biotechnol. 2019;29:1096–1103. doi: 10.4014/jmb.1903.03022. [DOI] [PubMed] [Google Scholar]
- Prasad TNVKV, Sudhakar P, Sreenivasulu Y, et al. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr. 2012;35:905–927. doi: 10.1080/01904167.2012.663443. [DOI] [Google Scholar]
- Prasad R, Bhattacharyya A, Nguyen QD. Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front Microbiol. 2017;8:1014. doi: 10.3389/fmicb.2017.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praveen Kumar G, Mir Hassan Ahmed SK, Desai S, et al. In vitro screening for abiotic stress tolerance in potent biocontrol and plant growth promoting strains of Pseudomonas and Bacillus spp. Int J Bacteriol. 2014 doi: 10.1155/2014/195946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quecine M, Batista B, Lacava P. Diversity and biotechnological potential of plant-associated endophytic bacteria. In: Kumar PA, editor. Biotechnology: plant biotechnology. Houston: Studium Press LLC; 2014. pp. 377–423. [Google Scholar]
- Rahman IA, Padavettan V. Synthesis of Silica nanoparticles by Sol-Gel: size-dependent properties, surface modification, and applications in silica-polymer nanocomposites a review. J Nanomater. 2012 doi: 10.1155/2012/132424. [DOI] [Google Scholar]
- Rangaraj S, Gopalu K, Rathinam Y, et al. Effect of silica nanoparticles on microbial biomass and silica availability in maize rhizosphere. Biotechnol Appl Biochem. 2014;61:668–675. doi: 10.1002/bab.1191. [DOI] [PubMed] [Google Scholar]
- Rao KS, El-Hami K, Kodaki T, et al. A novel method for synthesis of silica nanoparticles. J Colloid Interface Sci. 2005;289:125–131. doi: 10.1016/j.jcis.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM. Thermotolerance generated by plant/fungal symbiosis. Science. 2002;298:1581. doi: 10.1126/science.1072191. [DOI] [PubMed] [Google Scholar]
- Roduner E. Size matters: why nanomaterials are different. Chem Soc Rev. 2006;35:583–592. doi: 10.1039/b502142c. [DOI] [PubMed] [Google Scholar]
- Rousk J, Ackermann K, Curling SF, Jones DL. Comparative toxicity of nanoparticulate CuO and ZnO to soil bacterial communities. PLoS ONE. 2012;7:e34197. doi: 10.1371/journal.pone.0034197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RP, Germaine K, Franks A, et al. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett. 2008;278:1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- Sahu PK, Brahmaprakash GP. Formulations of biofertilizers - approaches and advances. In: Singh D, Singh H, Prabha R, editors. Microbial inoculants in sustainable agricultural productivity. New Delhi: Springer; 2016. pp. 179–198. [Google Scholar]
- Santoyo G, del Orozco-Mosqueda MC, Govindappa M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Biocontrol Sci Technol. 2012;22:855–872. doi: 10.1080/09583157.2012.694413. [DOI] [Google Scholar]
- Seifried S, Winterer M, Hahn H. Nanocrystalline titania films and particles by chemical vapor synthesis. Chem Vap Depos. 2000;6:239–244. doi: 10.1002/1521-3862(200010)6:5<239::AID-CVDE239>3.0.CO;2-Q. [DOI] [Google Scholar]
- Seyed Sharifi R, Khoramdel S. Effects of nano-zinc oxide and seed inoculation by plant growth promoting rhizobacteria (PGPR) on yield, yield components and grain filling period of soybean (Glycine max L.) Iran J Field Crops Res. 2016;13:738–753. [Google Scholar]
- Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275:496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Shekar S, Sander M, Riehl RC, et al. Modelling the flame synthesis of silica nanoparticles from tetraethoxysilane. Chem Eng Sci. 2012;70:54–66. doi: 10.1016/j.ces.2011.06.010. [DOI] [Google Scholar]
- Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla SK, Kumar R, Mishra RK, et al. Prediction and validation of gold nanoparticles (GNPs) on plant growth promoting rhizobacteria (PGPR): a step toward development of nano-biofertilizers. Nanotechnol Rev. 2015;4:439–448. doi: 10.1515/ntrev-2015-0036. [DOI] [Google Scholar]
- Siddiqui MH, Al-Whaibi MH. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.) Saudi J Biol Sci. 2014;21:13–17. doi: 10.1016/j.sjbs.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AV, Laux P, Luch A, et al. Review of emerging concepts in nanotoxicology: opportunities and challenges for safer nanomaterial design. Toxicol Mech Methods. 2019;29:378–387. doi: 10.1080/15376516.2019.1566425. [DOI] [PubMed] [Google Scholar]
- Singla A, Sankar KM, Singh Y. Ecotoxicology: methods and risks. In: Kharissova O, Martínez L, Kharisov B, editors. Handbook of nanomaterials and nanocomposites for energy and environmental applications. Cham: Springer; 2020. pp. 1–19. [Google Scholar]
- Sinha R, Karan R, Sinha A, Khare SK. Interaction and nanotoxic effect of ZnO and Ag nanoparticles on mesophilic and halophilic bacterial cells. Bioresour Technol. 2011;102:1516–1520. doi: 10.1016/j.biortech.2010.07.117. [DOI] [PubMed] [Google Scholar]
- Smith RS. Legume inoculant formulation and application. Can J Microbiol. 1992;38:485–492. doi: 10.1139/m92-080. [DOI] [Google Scholar]
- Srivastava S, Bist V, Srivastava S, et al. Unraveling aspects of Bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Front Plant Sci. 2016;7:587. doi: 10.3389/fpls.2016.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöber W, Fink A, Bohn E. Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci. 1968;26:62–69. doi: 10.1016/0021-9797(68)90272-5. [DOI] [Google Scholar]
- Sturz A, Christie BR, Nowak J. Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit Rev Plant Sci. 2000;19:1–30. doi: 10.1080/07352680091139169. [DOI] [Google Scholar]
- Suman V, Prasad R, Jain VK, Varma A. Role of nanomaterials in symbiotic fungus growth enhancement. Curr Sci. 2010;99:1189–1191. [Google Scholar]
- Tanaka K, Capule MFV, Hisanaga T. Effect of crystallinity of TiO2 on its photocatalytic action. Chem Phys Lett. 1991;187:73–76. doi: 10.1016/0009-2614(91)90486-S. [DOI] [Google Scholar]
- Tarafdar JC, Sharma S, Raliya R. Nanotechnology: interdisciplinary science of applications. Afr J Biotechnol. 2013;12:219–226. doi: 10.5897/AJB12. [DOI] [Google Scholar]
- Thareja RK, Shukla S. Synthesis and characterization of zinc oxide nanoparticles by laser ablation of zinc in liquid. Appl Surf Sci. 2007;253:8889–8895. doi: 10.1016/j.apsusc.2007.04.088. [DOI] [Google Scholar]
- Thomas L, Singh I. Microbial biofertilizers: types and applications. In: Giri B, Prasad R, Wu QS, Varma A, editors. Biofertilizers for Sustainable Agriculture and Environment. Cham: Springer; 2019. pp. 1–19. [Google Scholar]
- Tilman D, Cassman KG, Matson PA, et al. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- Timmusk S, Behers L, Muthoni J, et al. Perspectives and challenges of microbial application for crop improvement. Front Plant Sci. 2017;8:49. doi: 10.3389/fpls.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk S, Seisenbaeva G, Behers L. Titania (TiO2) nanoparticles enhance the performance of growth-promoting rhizobacteria. Sci Rep. 2018;8:617. doi: 10.1038/s41598-017-18939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable sources. Plant Physiol. 2001;127:390–397. doi: 10.1104/pp.010331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandergheynst J, Scher H, Guo HY, Schultz D. Water-in-oil emulsions that improve the storage and delivery of the biolarvacide Lagenidium giganteum. Biocontrol. 2007;52:207–229. doi: 10.1007/s10526-006-9021-9. [DOI] [Google Scholar]
- Varma A, Uma, Khanuja M. Role of nanoparticles on plant growth with special emphasis on Piriformospora indica: a review. In: Ghorbanpour M, Manika K, Varma A, editors. Nanoscience and plant-soil systems. Cham: Springer; 2017. pp. 387–403. [Google Scholar]
- Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- Wainwright M, Al-Wajeeh K, Grayston SJ. Effect of silicic acid and other silicon compounds on fungal growth in oligotrophic and nutrient-rich media. Mycol Res. 1997;101:933–938. doi: 10.1017/S0953756297003560. [DOI] [Google Scholar]
- Waller F, Achatz B, Baltruschat H, et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci USA. 2005;102:13386–13391. doi: 10.1073/pnas.0504423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZL. Nanostructures of zinc oxide. Mater Today. 2004;7:26–33. doi: 10.1016/S1369-7021(04)00286-X. [DOI] [Google Scholar]
- Waseem M, Ahmad F, Habib S, Li Z. Genome-wide identification of the auxin/indole-3-acetic acid (Aux/IAA) gene family in pepper, its characterisation, and comprehensive expression profiling under environmental and phytohormones stress. Sci Rep. 2018;8:1–12. doi: 10.1038/s41598-018-30468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldegebrieal GK. Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: a review. Inorg Chem Commun. 2020;120:108140. doi: 10.1016/j.inoche.2020.108140. [DOI] [Google Scholar]
- White JF, Crawford H, Torres MS, et al. A proposed mechanism for nitrogen acquisition by grass seedlings through oxidation of symbiotic bacteria. Symbiosis. 2012;57:161–171. doi: 10.1007/s13199-012-0189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JF, Torres MS, Somu MP, et al. Hydrogen peroxide staining to visualize intracellular bacterial infections of seedling root cells. Microsc Res Tech. 2014;77:566–573. doi: 10.1002/jemt.22375. [DOI] [PubMed] [Google Scholar]
- White JF, Chen Q, Torres MS, et al. Collaboration between grass seedlings and rhizobacteria to scavenge organic nitrogen in soils. AoB Plants. 2015;7:plu093. doi: 10.1093/aobpla/plu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Kingsley K, Verma S, Kowalski K. Rhizophagy cycle: an oxidative process in plants for nutrient extraction from symbiotic microbes. Microorganisms. 2018;6:95. doi: 10.3390/microorganisms6030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JF, Torres MS, Verma SK, Elmore MT, Kowalski KP, Kingsley KL. Evidence for widespread microbivory of endophytic bacteria in roots of vascular plants through oxidative degradation in root cell periplasmic spaces. In: Singh AK, Kumar A, Singh PK, editors. PGPR amelioration in sustainable agriculture. Woodhead Publishing; 2019. pp. 167–193. [Google Scholar]
- Xu C, Lei C, Yu C. Mesoporous silica nanoparticles for protein protection and delivery. Front Chem. 2019;7:290. doi: 10.3389/fchem.2019.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang J, Xiu Z, Alvarez PJJ. Impacts of silver nanoparticles on cellular and transcriptional activity of nitrogen-cycling bacteria. Environ Toxicol Chem. 2013;32:1488–1494. doi: 10.1002/etc.2230. [DOI] [PubMed] [Google Scholar]
- Yoo H, Pak J. Synthesis of highly fluorescent silica nanoparticles in a reverse microemulsion through double-layered doping of organic fluorophores. J Nanopart Res. 2013;15:1609. doi: 10.1007/s11051-013-1609-2. [DOI] [Google Scholar]
- Yuvakkumar R, Elango V, Rajendran V, et al. Influence of nanosilica powder on the growth of maize crop (Zea Mays L.) Int J Green Nanotechnol Biomed. 2011;3:180–190. doi: 10.1080/19430892.2011.628581. [DOI] [Google Scholar]
- Zand AD, Mikaeili Tabrizi A, Vaezi Heir A. Application of titanium dioxide nanoparticles to promote phytoremediation of Cd-polluted soil: contribution of PGPR inoculation. Bioremediat J. 2020;24:171–189. doi: 10.1080/10889868.2020.1799929. [DOI] [Google Scholar]
- Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]