Abstract
Ischemia reperfusion injury (IRI) is a common cause of acute kidney injury (AKI) in the aging population. A reduction of hydrogen sulfide (H2S) production in the old kidney and renal IRI contribute to renal pathology and injury. Recent studies suggest that microRNAs (miRs) play an important role in the pathophysiology of AKI and a significant crosstalk exists between H2S and miRs. Among the miRs, miR-21 is highly expressed in AKI and is reported to have both pathological and protective role. In the present study, we sought to determine the effects of age-induced reduction in H2S and mir-21 antagonism in AKI. Wild type (WT, C57BL/6J) mice aged 12–14 weeks and 75–78 weeks underwent bilateral renal ischemia (27 min) and reperfusion for 7 days and were treated with H2S donor, GYY4137 (GYY, 0.25 mg/kg/day, ip) or locked nucleic acid anti-miR-21 (20 mg/kg b.w., ip) for 7 days. Following IRI, old kidney showed increased macrophage polarization toward M1 inflammatory phenotype, cytokine upregulation, endothelial–mesenchymal transition, and fibrosis compared to young kidney. Treatment with GYY or anti-miR-21 reversed the changes and improved renal vascular density, blood flow, and renal function in the old kidney. Anti-miR-21 treatment in mouse glomerular endothelial cells showed upregulation of H2S-producing enzymes, cystathionine β-synthase (CBS), and cystathionineγ-lyase (CSE), and reduction of matrix metalloproteinase-9 and collagen IV expression. In conclusion, exogenous H2S and inhibition of miR-21 rescued the old kidney dysfunction due to IRI by increasing H2S levels, reduction of macrophage-mediated injury, and promoting reparative process suggesting a viable approach for aged patients sustaining AKI.
Keywords: MicroRNA-21, Hydrogen sulfide, IRI, Macrophage polarization
Introduction
In the aging population, renal ischemia reperfusion injury (IRI) is the most common cause of acute kidney injury (AKI) and a high risk for progression to end-stage renal disease (ESRD) [1–3]. Age-associated morphological and functional changes contribute to this process [4]. In addition, recent clinical studies suggest that aging plays an important role in modifying the risk for AKI [5–7]. The aging changes include vascular stiffness, glomerular sclerosis, reduced auto-regulation, declining glomerular filtration rate (GFR), and increased sensitivity to vasopressors and impaired response to vasodilators [4, 8, 9]. Due to the increased prevalence of AKI in elderly patients and limitations of current treatment, it is crucial to seek alternate approaches to reduce morbidity and mortality.
Hydrogen sulfide (H2S), a gaseous signaling molecule, has several physiological and pathophysiological roles in the body. H2S is synthesized in the transsulfuration pathway by cystathionine β- synthase (CBS), cystathionineγ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST), all of which are highly expressed by the kidney [10–12]. Recent studies reveal that endogenous H2S production is decreased in the old kidney and linked to aging-induced fibrosis [13, 14]. In other studies involving renal IRI, the expression of CBS and CSE and the generation of H2S are significantly decreased leading to oxidative stress and renal damage [15, 16]. However, the effects of aging and renal IRI in combination on the kidney and changes in H2S production have not been explored.
MicroRNAs (miRs) are small non-coding RNAs (~ 22 base pairs) that regulate gene expression by binding to target mRNA to facilitate degradation or inhibition of translation. Insights into the role of miRs have shown that they are involved in diverse cellular and metabolic pathways in normal and pathophysiological conditions. MicroRNA profiling in renal IRI has revealed differential expression of several miRs. In vitro and in vivo studies report the upregulation of miR-21 in hypoxia and renal IRI; however, the results are conflicting and indicate both pathological and protective role in the kidney [17–20]. Studies have reported that significant crosstalk exists between miR-21 and H2S. An upregulation of miR-21 was associated with inhibition of CSE expression, and H2S treatment was associated with downregulation of miR-21 [21, 22]. The beneficial effects of H2S following IRI has been demonstrated in several studies; however, the role of miR-21 and the link between dysregulation of miR-21 expression and H2S production in aging-associated renal IRI have not been studied.
In the present study, we report that IRI in old kidney is associated with reduced H2S levels and miR-21 upregulation. Exogenous H2S and in vivo treatment with locked nucleic acid anti-miR-21 ameliorated macrophage-mediated inflammation, fibrosis, and renal dysfunction.
Results
Hydrogen sulfide production is decreased and miR-21 increased in old mice after renal IRI
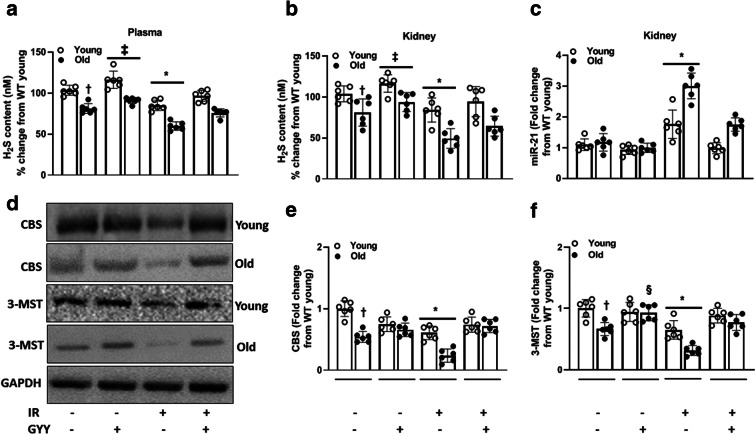
To determine the difference in H2S in young and old mice, we measured the levels in plasma and kidney. H2S levels in untreated young mice were considered baseline. In untreated mice, plasma H2S was lower in the old mice compared to young, and GYY treatment increased the levels in young and old mice without IRI (Fig. 1a). After IRI, H2S decreased significantly in old mice than in the young. In mice undergoing IRI, GYY treatment restored normal plasma H2S in young and old mice similar to their respective baseline values (Fig. 1a). In the kidney, H2S levels were similar to plasma in both untreated and treated mice (Fig. 1b). GYY treatment restored normal levels in young mice with IR. In old mice with IRI, GYY treatment increased H2S but did not return to its baseline value (Fig. 1b).
Fig. 1.
IRI reduces H2S levels and induces miR-21 in old kidneys. Mice underwent bilateral renal ischemia (27 min.) and 7-day reperfusion. GYY was given starting 1 day before ischemia and continued for 7 days. a Plasma and b kidney H2S were decreased in old mice and worsened following IRI. c Exogenous H2S treatment (GYY) blocked the expression of miR-21 in old kidney undergoing IRI. d, e GYY upregulated the expression of H2S-producing enzymes, CBS, and 3-MST. Data is mean ± SD, n = 6/group, *p < 0.05 vs. other groups, †p < 0.05 vs. untreated young mice, ‡p < 0.05 vs. untreated mice, §p < 0.05 vs. untreated old mice
To determine whether the expression of miR-21 is affected by aging and IRI, we quantified its expression. Following IRI, in the old kidneys, miR-21 upregulation was higher compared to young kidney and decreased with H2S treatment (Fig. 1c).
We next quantified protein expression of CBS and 3-MST enzymes that produce H2S in the kidney. The baseline expression of CBS and 3-MST in young kidneys was considered normal for comparing groups. The baseline CBS in old untreated kidney was decreased compared to young untreated kidney (Fig. 1d and e). IRI downregulated CBS expression in old kidney compared to young and increased with GYY treatment (Fig. 1d). In untreated mice, the expression of 3-MST was lower in old kidneys compared to young. H2S treatment alone increased 3-MST in old mice but not in young (Fig. 1d and f). IRI significantly decreased 3-MST in old mice compared to young and was normalized in both groups following H2S treatment (Fig. 1d and f).
H2S treatment increases macrophage M2 phenotype following renal IRI
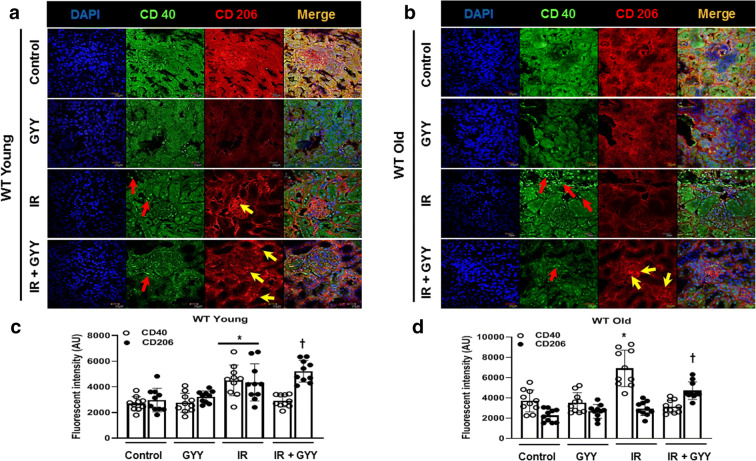
Ischemia and reperfusion release cytokines and chemokines from activated renal cells leading to inflammatory reaction. Infiltration of inflammatory and reparative macrophage is documented in the early and late phases of IRI respectively [23]. To determine whether H2S affects macrophage polarization, we performed immunohistochemistry. In young kidneys with IRI, increased CD40+ (inflammatory M1 phenotype) macrophages were observed in the tubular and interstitial region (red arrows) and CD206+ (anti-inflammatory M2 phenotype) macrophage in the glomerular and tubular regions (Fig. 2a and b, yellow arrows). Old kidneys sustaining IRI showed increased CD40+ macrophages compared to young kidney and were localized to the tubular region (Fig. 2a–d, red arrows). Old kidney showed low CD206+ cells after IRI (Fig. 6b and d, yellow arrows). In the IRI + H2S groups, CD206+ cells were significantly increased in young and old kidneys but to a greater extent in young kidneys (Fig. 2a–d, yellow arrows).
Fig. 2.
H2S ameliorates macrophage-mediated inflammation following IRI in old mice. a Cryosections (5 μm thick) were used for staining. Immunohistochemical localization of CD40 (marker of inflammatory M1 Macrophage) and CD206 (marker of anti-inflammatory M2 macrophage) in young kidneys. b CD40 and CD206 staining from old kidneys. CD40 is predominantly seen the tubular and peritubular regions of old kidney with IRI (red arrows). H2S increased CD206-positive cells following IRI (yellow arrows). c, d Fluorescence intensity of CD40 and CD206 from young and old kidneys respectively. Data was quantified using the ImageJ software (https://imagej.nih.gov/ij/). Data is mean ± SD, n = 5/group (10 data points for each), *p < 0.05 vs. other groups, †p < 0.05 vs. untreated mice. Scale bar: 20 μm; magnification × 60
Fig. 6.
IRI affected the renal cortical blood flow in old mice and H2S improved flow. a Representative images of untreated and treated WT young mice showing peak systolic velocity (PSV) and end diastolic velocity (EDV). b Representative images from old mice. c Renal cortical artery resistive index (RI) was increased in old mice following IRI and improved significantly with H2S. Data is mean ± SD, n = 6/group, *p < 0.05 vs. all other groups, †p < 0.05 vs. untreated young mice, ‡p < 0.05 vs. untreated old mice
Hydrogen sulfide reverses IR-induced overexpression of EMMPRIN, meprin A, and MMP-9 in old kidneys
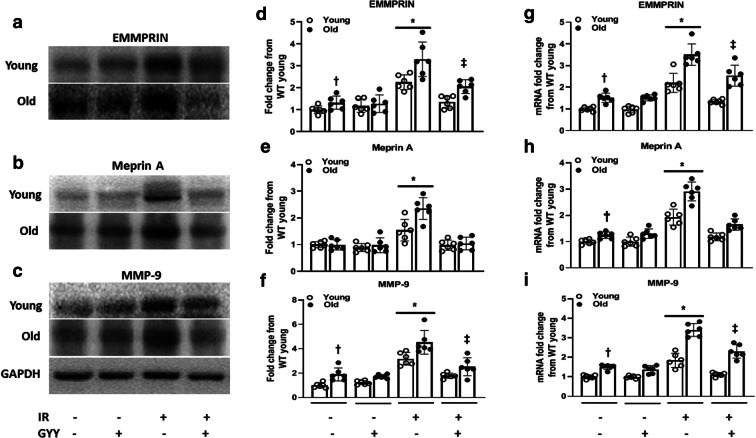
Renal IRI is associated with extracellular matrix (ECM) breakdown and remodeling. We therefore investigated the expression of enzymes involved in ECM metabolism. ECM metalloproteinase inducer (EMMPRIN) is widely expressed by the proximal tubular epithelial cells and is also an inducer of matrix metalloproteinases (MMPs) [24]. The baseline expression of EMMPRIN in old kidneys was increased compared to young kidney and increased further following IRI (Fig. 3a and d). The baseline expression of meprin A, a zinc-dependent metalloproteinase, was similar in young and old kidneys. Following IRI, meprin A was overexpressed in old kidney compared to young (Fig. 3b and e). The baseline expression of MMP-9 was increased in old kidney before and after IR compared to young kidney (Fig. 3c and f). In the groups sustaining IRI, H2S normalized the expression of EMMPRIN and meprin A to their respective baseline values but not MMP-9 (Fig. 3c and f). The mRNA levels of EMMPRIN, meprin A, and MMP-9 were increased in untreated old kidney compared to untreated young, and their levels increased in old kidneys following IR compared to young kidneys with IRI (Fig. 3g–i). H2S normalized the mRNA levels in young kidneys and decreased in old kidney sustaining IRI (Fig. 3g–i).
Fig. 3.
H2S reduces the expression of matrix metalloproteinases (MMPs) and its inducer in old mice following IRI. a EMMPRIN, an inducer of MMPs, is upregulated in old mice renal IRI and reduced with H2S treatment. b, c Effect of H2S on the expression of meprin A and MMP-9 in renal IRI of old mice. d, e, f Bar graphs for EMMPRIN, meprin A, and MMP-9 respectively. Band intensities were quantified by using the ImageJ software (https://imagej.nih.gov/ij/). g, h, i mRNA levels of EMMPRIN, meprin A, and MMP-9 were measured by qPCR. Data is mean ± SD, n = 6/group, *p < 0.05 vs. all groups, †p < 0.05 vs. untreated young mice, ‡p < 0.05 vs. untreated old mice
H2S reduces endothelial–mesenchymal transition after ischemia reperfusion injury
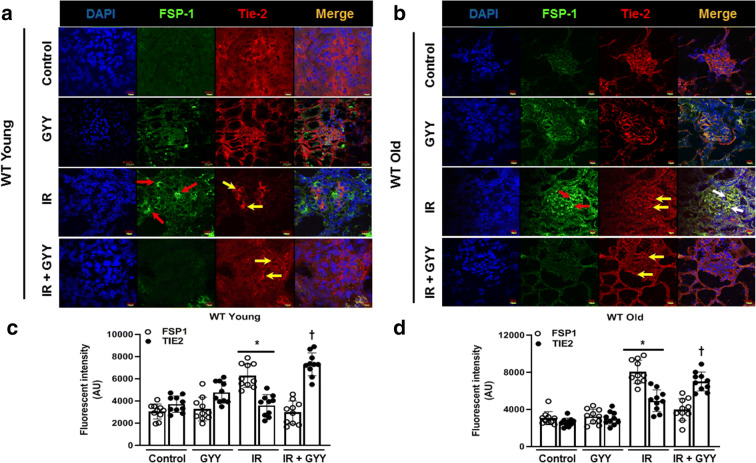
Endothelial–mesenchymal transition (EndoMT) is a contributing factor to renal fibrosis following IRI [25]. We therefore quantified the expression of endothelial marker, Tie-2, and fibroblast marker, FSP-1. There was no change in the expression of Tie-2 in the kidneys of untreated, GYY treated, and IR groups in young mice (Fig. 4a and c). In young kidney following IRI, FSP-1 expression was increased in the glomeruli and tubules (Fig. 4a, red arrows). There was no co-expression of Tie-2 and FSP-1 in young kidney with IR. In old kidney with IRI, there was increased co-expression of FSP-1 and Tie-2 markers in the glomeruli (Fig. 4b, white arrows). H2S reduced the expression of FSP-1 in young and old kidneys with IRI (Fig. 4a-d).
Fig. 4.
IRI induces endothelial–mesenchymal transition, and H2S reverses the changes. Cryosections (5 μm thick) were stained with mesenchymal marker (FSP-1, red arrow) and endothelial marker (Tie-2, yellow arrow), and colocalization was seen in the glomerular region of old kidney following IRI (white arrows) and reduced with H2S. ac Representative immunohistochemical image of FSP-1 and Tie-2 and fluorescence intensity in young kidney. bd Representative immunohistochemical image and fluorescence intensity from old kidneys. Data was quantified using the ImageJ software. Data is mean ± SD, n = 5/group (10 data points for each), *p < 0.05 vs. other groups, †p < 0.05 vs. untreated mice. Scale bar: 10 μm, WT + GYY: 20 μm; magnification × 60
Renal function, resistive index, and vascularity in old mice in response to IRI and H2S treatment
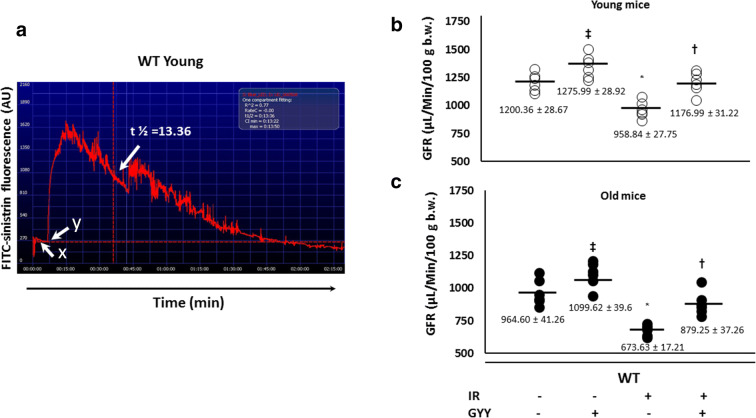
GFR in young mice was considered normal (Fig. 5a and b). In untreated old mice, GFR was decreased compared to untreated young mice (Fig. 5c). H2S treatment alone increased GFR in young and old mice. In young mice, IRI reduced GFR and improved with H2S treatment (Fig. 5b). In old mice, IRI decreased GFR significantly compared to IRI in young mice and improved with H2S (Fig. 5c).
Fig. 5.
H2S improves impaired renal function due to IRI in old mice. A Representative image showing transcutaneous measurement of FITC-sinistrin over 2 h. x- Baseline. y- Time point of IV FITC-sinistrin injection. Arrow shows the time point used to determine half-life (t1/2) and calculate the GFR as described in the “Materials and methods.” D, E Effect of IRI and H2S treatment on GFR in young and old mice respectively. Data is mean ± SD, n = 6/group, *p < 0.05 vs. other groups, †p < 0.05 vs. untreated respective groups, ‡p < 0.05 vs. untreated young and old mice respectively
Renal resistive index (RI) is a measure of vascular distensibility that is affected by aging and pathological process. In old mice, H2S alone reduced RI significantly than in young mice (Fig. 6a-c). In young mice, RI increased following IRI and normalized with H2S (Fig. 6a and c). In old mice, baseline RI was increased and worsened in response to IRI and decreased with H2S treatment (Fig. 6b and c).
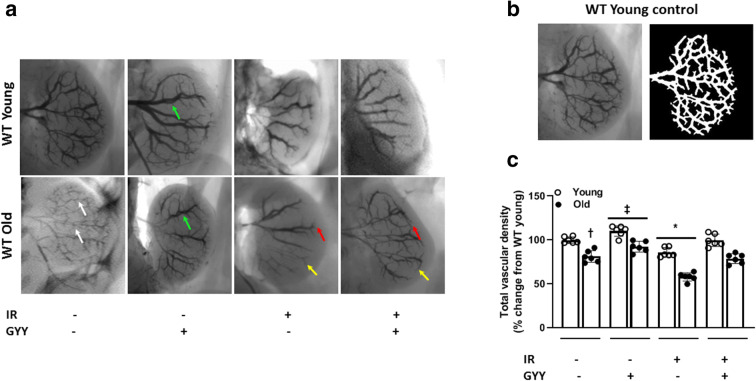
We next determined the renal vascular density by barium sulfate angiography. The total vascular density in young mice was considered normal (Fig. 7a and b). In untreated old mice, the renal vasculature was rarefied and decreased compared to young (Fig. 7a). H2S treatment alone increased the interlobar vessel diameter and coverage in young and old mice (Fig. 7a and c, green arrow). Following IRI, old mice showed significant reduction in vascular density compared to young and improved with H2S treatment (Fig. 7a and c). The interlobular arteries (red arrow) and arcuate arteries (yellow arrow) were decreased in old IRI mice compared to young and increased with H2S (Fig. 7a).
Fig. 7.
H2S increases renal vascular density in old mice sustaining IRI. Barium sulfate was infused via renal artery as described in “Materials and methods.” a Representative images of barium sulfate angiogram in all groups of young and old mice. Vascular density is reduced in old mice following IRI. White arrows show narrow renal vessels in untreated old kidney. Red and yellow arrows show loss of arcuate and interlobular arteries following IRI in old kidney and recovery following H2S treatment. Green arrows show dilated interlobar vessels following H2S treatment. b Representative image panel of young untreated mice (left) and analysis (right) using the Vessel Segmentation software developed by university of Luebeck (https://www.isip.uni-luebeck.de/downloads/vessel-segmentation-and-analysis.html). c Quantification of relative total vascular density and presented as percent change from untreated young mice. Data is mean ± SD, n = 6/group, *p < 0.05 vs. other groups, †p < 0.05 vs. untreated young mice, ‡p < 0.05 vs. untreated groups
LNA miR-21 inhibitor decreases miR-21 expression
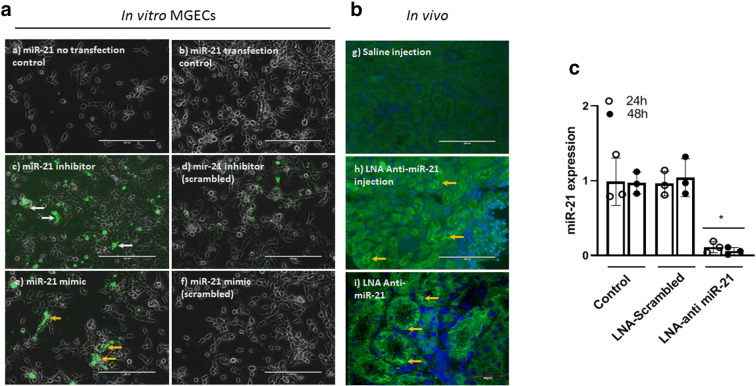
Mouse glomerular endothelial cells (MGECs) were transfected with FAM-labeled scrambled LNA or LNA anti-miR-21 for 24 h and 48 h as described in “Materials and methods.” Cells were imaged under EVOS FL microscope (Thermo Fisher Scientific, Carlsbad, CA) for transfection efficacy (Fig. 8a). To determine whether LNA anti-miR-21 intraperitoneal injection incorporated into the kidney, we did immunohistochemistry and confirmed fluorescence in kidney sections (Fig. 8b). The expression of miR-21 in MGECs was quantified by qPCR and was found significantly reduced at both 24 and 48 h time points compared to control and scrambled LNA-treated cells (Fig. 8c).
Fig. 8.
FAM-labeled locked nucleic acid (LNA) anti-miR-21 treatment in mouse glomerular endothelial cells (MGECs) and kidneys decreased the expression of miR-21. MGECs were transfected with miR-21 mimic and negative control (50 nM). Cells were transfected at 60% confluency using lipofectamine RNAiMAX transfection reagent for 24- and 48-h time points. a In vitro experiments in MGECs. a) miR-21 no transfection control, b) miR-21 transfection control, c) miR-21 inhibitor (white arrows), d) miR-21 inhibitor (scrambled), e) miR-21 mimic (yellow arrows), f) miR-21 mimic (scrambled). Scale bar: 200 μm, magnification × 20. b Kidneys show fluorescence of LNA anti-miR-21 in renal tubular region. LNA anti-miR-21 was given by intraperitoneal injections, g) saline injection, h) LNA anti-miR-21. Scale bar: 200 μm, magnification × 20. i) LNA anti-miR-21. Scale bar: 40 μm, magnification × 60. c Quantification of miR-21 in MGECs following LNA anti-miR-21 treatment by qPCR shows significant reduction. Data was normalized with RNU6 and presented as mean ± SD, n = 3 separate experiments, *p < 0.05 vs. other groups
LNA anti-miR-21 upregulates the expression of CBS and CSE, and reduces MMP-9 in vitro
To determine the effect of miR-21 inhibition on H2S-producing enzymes and MMP-9, we quantified the protein expression in MGECs treated without or with mineral oil and miR-21 mimic, inhibitor, and respective scrambled miR. In the cells treated without mineral oil and with miR-21 mimic, the expression of CBS and CSE was reduced and MMP-9 increased compared to untreated control (Fig. 9a and b). The miR-21 inhibitor increased CBS and CSE and decreased MMP-9 expression (Fig. 9a and b). The expression of all three enzymes remained unchanged in the cells treated with scrambled miRs compared to control.
Fig. 9.
LNA anti-miR-21 treatment in MGECs upregulates the expression of CBS and CSE and reduces MMP-9. Cells were transfected and treated as described in the “Materials and methods” section. Protein expression was quantified by western blot. a Expression of CBS, CSE, and MMP-9 to miR-21 mimic and inhibitor treatment. b Fold change quantification using the ImageJ software (https://imagej.nih.gov/ij/). c Panel showing the expression of CBS, CSE, and MMP-9 to miR-21 mimic, inhibitor, and hypoxia-reoxygenation (H-RO). d Fold change. Data is mean ± SD, n = 4 separate experiments, *p < 0.05 vs. respective controls, †p < 0.05 vs. miR-21 mimic and mimic + H-RO
In in vitro model of IR, MGECs treated with mineral oil only (hypoxia-reoxygenation, H-RO) showed significant reduction of CBS, more than CSE, and increased MMP-9 (Fig. 9c and d). Similar results were seen with miR-21 mimic + H-RO. In cells with H-RO + scrambled miR-21 mimic, CBS and CSE were reduced compared to control and MMP-9 was increased (Fig. 9c and d). In the cells treated with miR-21 inhibitor + H-RO, CBS and CSE were restored to normal and MMP-9 decreased significantly (Fig. 9c and d). Scrambled miR-21 inhibitor + H-RO treatment showed reduced CBS and CSE and similar MMP-9 as seen in the H-RO group (Fig. 9c and d).
LNA anti-miR-21 reduces inflammation and macrophage polarization following IRI
To determine whether anti-miR-21 suppressed renal inflammation in old kidneys, we quantified TNFα and iNOS, and arginase1 (ARG1) as indicators of M1 and M2 macrophages respectively by western blot. Macrophage markers, CD40, CD206, cytokines iNOS, and arginase1, were measured by qPCR. In untreated mice, ARG1 was decreased in old kidney compared to young (Fig. 10a and b). There was no change in TNFα and iNOS in untreated young and old mice. In old mice, anti-miR-21 alone did not affect TNFα and iNOS but ARG1 was increased (Fig. 10a and b). IRI increased TNFα and iNOS significantly in old kidneys compared to young and decreased with anti-miR-21 treatment (Fig. 10a and b). IRI and anti-miR-21 treatment increased ARG1 expression to a greater extent in young than old mice compared to respective IRI only groups (Fig. 10a and b).
Fig. 10.
LNA anti-miR-21 inhibits M1 macrophage polarization and increases M2 macrophages. a Representative western blot images for TNFα, iNOS, and ARG1 expression. b Protein quantification of TNFα, iNOS, and ARG1 using the ImageJ software (https://imagej.nih.gov/ij/). c Real-time PCR results of CD40, CD206, iNOS, and ARG1 normalized to GAPDH. Data are mean ± SD, n = 6/group. *p < 0.05 vs. other groups, †p < 0.05 vs. untreated young and old mice, ‡p < 0.05 vs. untreated young mice, §p < 0.05 vs. untreated old mice
The mRNA levels of CD40 and its secretory molecule iNOS increased significantly in old mice sustaining IRI compared to young and decreased to anti-miR-21 treatment (Fig. 10c). The CD206 levels were increased in young mice with IRI which increased further with anti-miR-21. There was no change in CD206 in the old mice sustaining IRI. In the IRI + anti-miR-21 groups, the CD206 response to anti-miR-21 was higher in the young mice compared to old (Fig. 10c).
Inhibition of miR-21 reduces IRI-induced renal fibrosis
IRI-induced AKI damages tubular epithelial cells leading to generation of pro-fibrotic factors. We quantified the expression of MMP-9, TGF-β, and collagen IV (Col IV) by western blot and qPCR. In untreated old mice, MMP-9 and Col IV were increased compared to young mice but TGF-β showed similar expression (Fig. 11a and b). In old mice, IRI increased MMP-9, TGF-β, and Col IV significantly compared to young mice and was mitigated with anti-miR-21 treatment (Fig. 11a and b). The mRNA levels correlated with the protein expression (Fig. 11c).
Fig. 11.
LNA anti-miR-21 reduces extracellular matrix remodeling. a Representative western blot images for MMP-9, TGF-ß, and collagen IV (Col IV) showing increased expression in old kidneys sustaining IRI and reduction following anti-miR-21 treatment. b Quantification of bands using the ImageJ software (https://imagej.nih.gov/ij/). c The mRNA levels were measured by qPCR and normalized to GAPDH. Data are mean ± SD, n = 5/group. *p < 0.05 vs. other groups, †p < 0.05 vs. untreated young and old mice, ‡p < 0.05 vs. IRI + anti-miR-21 young mice, §p < 0.05 vs. untreated young mice
LNA anti-miR-21 increases renal cortical blood flow following IRI
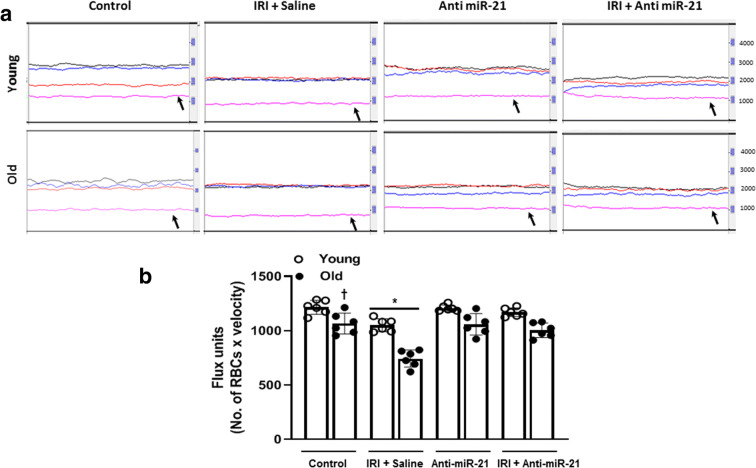
Renal cortical blood flow defect is an ongoing phenomenon after IRI. In real-time Doppler flowmetry, our data showed significant reduction in the baseline cortical perfusion of the old mice compared to untreated young mice (Fig. 12a and b). Anti-miR-21 inhibition alone did not affect renal cortical blood flow in young and old mice. IRI reduced cortical perfusion significantly in the old mice compared to young mice (Fig. 12a and b). Inhibition of miR-21 following IRI showed normalization of cortical perfusion in young mice similar to young untreated mice and significant improvement in old mice (Fig. 12a and b).
Fig. 12.
Renal cortical blood flow is increased with anti-miR-21 treatment. Laser speckle flowmetry was used to measure the blood flow. a Representative line traces of renal cortical blood flow in untreated and treated young and old kidneys. Black trace is from aorta, red from renal artery, blue from renal vein, and pink trace from renal cortex (black arrow). b Quantification of flux units (No. of RBCs × velocity). Data are mean ± SD, n = 6/group. *p < 0.05 vs. other groups, †p < 0.05 vs. untreated young mice
Discussion
The aging kidney is susceptible to ischemic and toxic insults leading to AKI, thus posing an increased risk for CKD/ESRD [26]. Inflammation and impaired vascular responses lead to dysfunction and maladaptive renovascular remodeling. Endogenous microRNAs can modulate the pathological processes to influence the disease course and functional outcomes after IRI. In the present study, we show that in the presence of H2S deficiency in the aged kidney, renal IRI is associated with upregulation of miR-21, macrophage polarization to inflammatory M1 phenotype, and expression of matrix metalloproteinase-9 resulting in fibrosis and functional impairment. Exogenous H2S therapy suppresses miR-21, inflammation, and endothelial–mesenchymal transition (EndoMT) and improves renal vascular density and function. Furthermore, we demonstrate that locked nucleic acid anti-miR-21 treatment in vitro upregulates the expression of H2S-producing enzymes, CBS, and CSE, and in in vivo inhibits TNFα, iNOS, TGF-β, and MMP-9 to promote reparative anti-inflammatory macrophage M2 phenotype and improves renal blood flow (RBF).
Recent clinical studies identify age as an independent risk factor that can modify the incidence and outcome of AKI. In patients older than 75 years, the presence of diabetes, hypertension, chronic obstructive pulmonary disease, and cardiac interventions increased the risk for AKI [5, 6]. In contrast, patients younger than 65 years showed higher risk for AKI in the presence of chronic liver disease, nephrotoxic medications, and intervention with contrast agents [7]. The findings above suggest that interaction between the age and risk factors can influence the incidence of AKI in the aging population. Our study is in agreement with the above and shows that IR insult in aging mice causes severe renal injury.
Global miRNA profiling in 10–12-week-old mice undergoing unilateral renal warm IRI revealed differential expression of miRs [27]. There was immediate upregulation of miR-21 and miR-20a, and on day 3 of miR-146, -199a-3p, and -214. Other miRs that were downregulated include miR-192, -194, and -197 [27]. The role of miR-21 in renal IRI is complex and reported to regulate mechanisms of injury and repair. In young mice, delayed ischemic preconditioning of kidneys was associated with increased miR-21 expression that protected the kidneys from subsequent IRI, whereas miR-21 silencing exacerbated renal injury [18, 28]. In other models of IRI and unilateral ureteral obstruction (UUO), miR-21 upregulation increased injury and fibrosis [19]. Our data shows that miR-21 is highly expressed in the old kidney than young following IRI, and H2S treatment suppresses its expression.
Renal IRI is followed by robust inflammatory response wherein macrophages play a key role as mediators of inflammation and repair [29]. The opposing functions are attributed to two subsets of macrophages where M1 is pro-inflammatory and M2 is a regulator of tissue repair [30]. Decreased immune surveillance is a contributing factor to age-related diseases including IRI [31, 32]. In a recent study, Kim et al. demonstrated that increased M1 infiltration and defective M2 polarization worsened renal IRI and fibrosis in old mice compared to young [33]. Other studies in young mice have reported that EndoMT contributes to renal fibrosis in different models of disease [34, 35]. Our results further supports the earlier findings. In addition, we show that IRI-induced inflammation in the old kidney is associated with excess matrix enzymes, EMMPRIN, meprin A, and MMP-9 and EndoMT suggesting increased ECM remodeling than in young kidney. Several studies including our own documented that H2S suppresses macrophage-mediated inflammation in diverse pathologies [11, 36, 37]. In the present study, H2S treatment in old mice reduced M1 cells and promoted M2 cell infiltration after IRI and reduced ECM remodeling to improve function.
Recovery of renal function after IRI is dependent on both the age of mice and duration of ischemia. In 1-year-old mice, renal function returned to normal by 4–5 days after IRI [32]. In contrast, in the present study, we found that although the renal function improved with H2S treatment after IRI in the old mice, it did not return to baseline values. This difference could be due to severe injury in our mice, which were considerably older than the mice in the previous study. Increased microvascular rarefaction and reduction in blood flow have been reported in old mice compared to young following IRI [32]. Furthermore, the loss of endothelial integrity and ECM changes together can increase vessel stiffness and reduce luminal diameter [38, 39]. Aging is associated with differential expression of miRs in the kidney that regulate gene expression directly or indirectly contributing to structural modifications in the kidney and vasculature [40–42]. In addition, vascular aging is also linked to reduction of nicotinamide adenine dinucleotide (NAD+), a co-substrate for sirtuins required for cell survival in the vasculature [43]. In a recent study, Kiss et al. demonstrated up- and downregulation of several miRs in the aorta of aged mice that had low levels of NAD+ compared to young mice [43]. Supplementation of nicotinamide mononucleotide (NMN), a NAD+ precursor, restored the age-related miR changes similar to the levels seen in the aorta of young mice suggesting NAD+ modulation of miRs to reverse vascular aging [43]. The mitigation of dysregulated miRs has the potential to increase nitric oxide bioavailability and reduce inflammation. In the present study, we found that old kidneys showed increased renal RI and reduced total vascular density compared to young, which worsened following IRI. This in part could be due to a combination of age- and AKI-induced miR changes leading to vascular remodeling. H2S is a potent vasodilator and anti-inflammatory agent, and by increasing its bioavailability, we observed a reduction of RI and improvement in renal vasculature of the interlobar, arcuate, and interlobular branches suggesting improved RBF.
Systemic administration of LNA anti-miR-21 causes target inhibition mainly in the kidney and liver. Our data showed high transfection efficiency in the MGECs and in in vivo experiments, anti-miR-21 localized to tubular epithelium. In an earlier study, overexpression of miR-21 was shown to inhibit CSE and its regulator, transcription factor specificity protein-1, thus reducing H2S production [21]. Our findings are in agreement with the study above. We found that LNA anti-miR-21 transfection in MGECs increased the expression of both CBS and CSE suggesting that miR-21 regulates H2S production in hypoxia-reoxygenation scenario similar to IRI. The exact signaling mechanisms however need to be investigated in future studies. Recent studies have also shown that miR-21 inhibition reduced inflammation, fibrosis, and tubular injury in a genetic mouse model of Alport nephropathy and streptozotocin-induced diabetes [44, 45]. We show that in the old mice following IRI, silencing miR-21 suppresses CD40 and increases CD206+ cells suggesting activation of repair processes in the kidney. The secretory molecules, iNOS and arginase 1, confirmed the cells as M1 and M2 macrophages respectively.
MMPs are responsible for degrading ECM and basement membrane that are important in organogenesis, tissue remodeling, and repair. Previously, we have shown that activation of MMP-9 is associated with diabetic ECM remodeling [46]. In a clinical study involving renal transplantation, excessive urinary MMP-9 in the immediate transplant period correlated with interstitial fibrosis and tubular atrophy at 3 months suggesting early activation of MMP-9 in the kidney [47]. Several studies have shown MMP-9 activation in renal IRI [48–50]. MMP-9 specifically cleaves collagen type IV that is predominant in the glomerulus and tubular basement membrane [51]. Furthermore, MMP-9 activates TGF-β in the tubular epithelial cells and contributes to epithelial mesenchymal transition [52, 53]. In our study, the upregulation of MMP-9, TGF-β, and increased Col IV lends further support to the earlier studies. In animal and clinical study involving UUO and renal transplantation respectively, the upregulation of circulating miR-21 correlated with increased renal fibrosis [54]. In miR-21−/− mice sustaining UUO and unilateral ischemia and reperfusion, reduced MMP activity was associated with decreased injury and fibrosis [19]. These findings are in line with the data from our study which showed that targeting miR-21 decreases MMP-9- and TGF-β-mediated fibrosis suggesting an important role for miR-21 in aged kidney IRI.
IRI results in reduction of blood flow (BF) that deprives the kidney of oxygen and nutrient supply. Therefore, restoration of BF is essential to reduce injury and aid functional recovery. Several studies have documented that H2S infusion to young healthy rats increased renal BF and urine flow rate, and when given before ischemia, it protected the kidney from IRI and improved renal function [55–57]. Similarly, our study showed increased renal function (GFR) and BF (data not presented) to H2S treatment following IRI. In a unilateral model of renal IRI, silencing miR-21 and diverting blood flow to injured kidney increased the kidney reserve to decrease albuminuria and protect the tubules [19]. In the present study, there was an increase in the renal BF in response to anti-miR-21 during IRI. Since miR-21 silencing upregulated the expression of CBS and CSE in MGECs, it is likely that H2S generation was increased in these mice leading to improvement in cortical BF as detected by laser Doppler signals.
Limitations
H2S is known to have multiple biological roles in the body that include signaling, vasodilation, angiogenesis, anti-inflammatory, and as an anti-oxidant [58]. Several clinical and experimental studies have documented age-related decline of H2S [59–61]. An earlier study reported that miR-21 suppresses transcription factor SP1, to downregulate CSE expression thus H2S production [21]. Other studies have reported that H2S suppresses miR-21 expression in alcohol-induced cardiomyopathy and thyroxine-induced cardiac insufficiency to reduce fibrosis [62, 63]. Taken together, these studies suggest the bidirectional nature of H2S and miR-21 regulation in pathophysiological conditions. Although the present study demonstrates a relationship between H2S and miR-21 in IRI of the aged kidney, the regulatory roles of one over the other and the other ways by which H2S offers renal protection require additional studies to decipher the underlying mechanisms.
In conclusion, we demonstrate that H2S deficiency aggravates renal IRI in the aged mice and upregulation of miR-21 plays an important role in persistent inflammation and activation of ECM enzymes and EndoMT leading to fibrosis. A biological role for anti-miR-21 in renal IRI of aged mice appears to be restoration of H2S levels suggesting a significant crosstalk between miR-21- and H2S-producing enzymes that together limit inflammation and initiate M2-mediated repair mechanisms. Furthermore, miR-21 blockade and H2S treatment resulted in the improvement of physiological function endpoints suggesting a potential option in patients sustaining ischemic insults in the intensive care unit or after transplantation. Finally, miR-21 targets multiple genes in the kidney that can cause pathology; its inhibition therefore has the ability to modulate the disease course leading to better outcomes.
Materials and methods
C57BL/6J mice (Stock no.: 000664) were purchased from Jackson Laboratory (Bar Harbor, ME) and mice aged 10–12 weeks (young) and 75–78 weeks (old) were used in this study. The animals were fed standard chow and tap water ad libitum. All animal protocols were performed in accordance with institutional animal care guidelines and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, 2011). This study was approved by Institutional Animal Care and Use Committee (IACUC) of the University Of Louisville School Of Medicine.
Antibodies and reagents
CBS (Cat.: SC67154), CD40 (Cat.: SC-13128), CD206 (Cat.: SC-34577), EMMPRIN (Cat.: SC-9757), meprin A (Cat.: SC-23491), arginase 1 (SC-20150), and HRP conjugated antibodies, anti-mouse (Cat.: sc-358920), anti-goat (Cat.: SC2768), anti-rabbit (Cat.: SC2004) were purchased from Santa Cruz Biotechnology (Dallas, Tx). iNOS was from BD Biosciences (Cat.: 610329, San Jose, CA); FSP1 (Cat.: Ab27957), Tie-2 (Cat.: Ab24859), TGF-β (Ab64715), and MST (Cat.: Ab85377) were from Abcam (Cambridge, MA). MMP-9 (Cat.: MA515886) and secondary antibodies, anti-mouse (Alexa Fluor 488, Cat.: A-11001), anti-goat (TriTc, Cat.: A15974), anti-rabbit (Alexa Fluor 488, Cat.: A-11008), and anti-mouse (Alexa Fluor 594, Cat.: A11005) were purchased from Thermo Fisher Scientific (Waltham, MA). Collagen IV antibody (NBP1-26549) was from Novus Biologicals (Centennial, CO). TRIzol reagent (Cat.: 15596-026) was from Invitrogen. EasyScript cDNA Synthesis kit (Cat.: G234) was from MidSci (St. Louis, MO). PCR primers for EMMPRIN, meprin A, and MMP-9 were from Invitrogen. mir-21 and RNU-6 primers were from IDT (Coralville, IA). mirVanaTM miRNA isolation kit was from Life Technologies (Cat.: AM1561, Carlsbad, CA). qScript™ microRNA cDNA Synthesis Kit, Quanta Biosciences, was from VWR (Cat.: 89168-788, Radnor, PA). Dichloromethane complex (GYY4137) was from Sigma-Aldrich (St. Louis, MO).
MicroRNA mimics and inhibitors
All LNA mimics and inhibitors were purchased from Exiqon (Woburn, MA). In in vivo studies, the sequence for custom-made LNA anti-mir-21 5′-3′ (FAM) was TCAGTCTGATAAGCT and for scrambled LNA was ACGTCTATACGCCCA. In cell transfection studies, the sequence for mmu-miR-21a-5p was uagcuuaucagacugauguuga (accession no.: MIMAT0000530).
Measurement of plasma and kidney hydrogen sulfide
The H2S levels in the plasma and kidney tissue was measured as described before [46].
Ischemia and reperfusion injury and LNA anti-miR-21 treatment
H2S donor, GYY4137 (GYY, 0.25 mg/kg/day), was given 1 day before IR by intraperitoneal injection and continued for 7 days. Under 2,2,2, tribromoethanol anesthesia (125 mg/kg, ip) and preemptive analgesia (meloxicam, 5 mg/kg SC), mice were placed prone and a midline dorsal incision was made. Both kidneys and their pedicles were exposed by retroperitoneal dissection and the vessels clamped for 27 min. After clamp removal, the kidneys were allowed reperfusion for 7 days. Young and old mice were randomly assigned to two groups and given locked nucleic acid (LNA) anti-mir-21 (20 mg/kg, ip) or scrambled LNA 1 day before renal ischemia and reperfusion. Mice were euthanized after 7 days, and tissues were collected.
Cell culture and transfection
MGECs were purchased from Cell Biologics (Chicago, IL) and cultured in complete mouse endothelial cell media (Cat. no.: 1168) with growth factor supplement. The cells were seeded at 2 × 105 in a 6-well plate and incubated for 24 h. After 24 h and washes, the wells were replaced with fresh culture medium without antibiotics. The cells were transfected with 50 nM of the miR-21 mimic/inhibitor, and 5 μl of the RNAiMAX transfection reagent per well. The inhibitors/mimics were incubated in 250 μl of Opti-MEM (Cat. no.: 31985070, Thermo Fisher, Carlsbad, CA), and the transfection reagent was incubated in a separate tube of 250-μl Opti-MEM for 20 min. The inhibitors/mimics were combined with the transfection reagent for a total of 500 μl of reagents in Opti-MEM. This mixture was added dropwise into the wells containing 2 ml of complete medium without antibiotics for 24 and 48 h. Cell images were acquired on EVOS FL microscope (Thermo Fisher Scientific, Carlsbad, CA).
In vitro ischemia reperfusion experiments were done on MGECs using the mineral oil method described before [64]. Briefly, after MGECs achieved 90% confluence, cells were washed and fresh complete media was added. Mineral oil was then added to create a thin layer on top of the media for 24 h. Cells were treated as described above.
Western blotting
Standard western blot was done as described before [65].
Immunohistochemistry
Kidney sections (5-μm thickness) were stained with CD40 (dil.: 1:100), CD206 (dil.1:100), FSP1 (dil.: 1:100), and Tie-2 (dil.: 1:25) at 4 °C overnight followed by appropriate secondary antibodies conjugated with Alexa Flour 488, 594, or TriTc. Images were captured on Olympus FluoView1000 (B&B Microscope Ltd., PA) and quantified using ImageJ (https://imagej.nih.gov/ij/).
Measurement of glomerular filtration rate
GFR was measured as described before with modification [66, 67]. Briefly, mice were anesthetized with isoflurane and the left dorsum was depilated with Nair Cream (Ewing, NJ). The NIC-Kidney device including battery (Mannheim Pharma and Diagnostics, GmbH, Mannheim, Germany) was fixed on the dorsum using an adhesive patch. The left groin was opened with a 3-mm incision and the femoral vein exposed. FITC-sinistrin (7 mg/100 g b.w.) was injected into the vein using SteriJect 32-g needle (TSK Laboratory, Japan). After ensuring hemostasis, the skin was closed with 5–0 nylon (Aros Surgical, Newport Beach, CA). The mice were allowed to recover and move around in the cage. After 2 h, the NIC-Kidney device was removed and data downloaded using the MPD software (Mannheim Pharma and Diagnostics, GmbH, Mannheim, Germany). The half-life data from the FITC-sinistrin elimination is used to calculate GFR using the formula GFR (μL/min/100 g b.w.) = conversion factor/t1/2 FITC-sinistrin (min). The conversion factor is 14,616.8 (μL/100 g b.w.) [67].
Renal ultrasound, laser Doppler flowmetry, and barium angiography
Renal ultrasound was done as described before [65]. Briefly, under isoflurane anesthesia, the kidney was imaged using transducer, MS550D (22–55 MHz), and the Vevo 2100 system (VisualSonics, Toronto, ON, Canada). The Vevo 2100 software was used to obtain renal cortical artery resistive index. The renal cortical blood flow was measured using Speckled Contrast Imager (Moor FLPI, Wilmington, DE) as described before [68]. Barium angiography was done as described before [69]. Data was analyzed using the software developed by University of Lubeck (http://www.isip.uni-luebeck.de/?id=150).
Quantitative real-time PCR
Total RNA was extracted using Trizol reagent (Invitrogen), and cDNA was synthesized using EasyScript cDNA Synthesis kit. PCR was done on Lightcycler® 96 system (Roche Diagnostics Corporation, Indianapolis, IN). The primers are listed in Table 1.
Table 1.
Primers used in the study
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| EMMPRIN | GATCCAGTGGTGGTTTGAA | ATCAACAGAGAGCGAACTG |
| Meprin A | CTTCAGCTACTACACTGACC | TTCACTCGCTTATTGGTTCA |
| MMP-9 | CACACGACATCTTCCAGTACCA | TCATTTTGGAAACTCACACGCC |
| miR-21 | UAGCUUAUCAGACUGAUGUUGA | N.A. |
| RNU6 | GUGCUCGCUUCGGCAGCACAUAUACUAAAAUUGGAAC GAUACAGAGAAGAUUAGCAUGGCCCCUGCGCAAGGAU GACACGCAAAUUCGUGAAGCGUUCCAUAUUUUU | N.A. |
| TGF-β | CTATTGCTTCAGCTCCACAG | GACAGAAGTTGGCATGGTAG |
| Col IV | GACCACTATGCTTGAAGTGA | ACAGAAGGCCTTAGTAGTCT |
| CD206 | TGTTGATTGTTGATTGCCAC | ACCAGTGTAGCAGTGTTAAG |
| ARG1 | TCGTGTACATTGGCTTGCGA | CTTCCTTCCCAGCAGGTAGC |
| iNOS | CTCTACAACATCCTGGAGCAAGTG | ACTATGGAGCACAGCCACATTGA |
Enriched miRNAs were extracted from the kidney using the mirVana™ miRNA isolation kit (Life Technologies, Carlsbad, CA). MicroRNA cDNA was synthesized using the qScript™ microRNA cDNA Synthesis Kit, Quanta Biosciences (VWR, Radnor, PA), following manufacturer’s protocol. The levels of miR-21 were detected by real-time PCR. The gene, RNU-6 (IDT, Coralville, IA), was used for normalization.
Statistical analysis
Data are presented as mean ± SD. n represents the number of animals. The difference between the groups was determined by ANOVA followed by the post hoc Tukey test. The Mann–Whitney U test was done for nonparametric data. Difference with a p value < 0.05 was considered significant.
Code availability
We used Microsoft excel, GraphPad Prism 8, and ImageJ for analysis.
Authors’ contributions
SP and US designed the study; SP did all in vivo experiments, acquired data, performed analysis, and wrote the manuscript. SK contributed to methods and data acquisition. GW did in vitro experiments and acquired data.
Funding
This study was supported, in part, by NIH grant DK 116591 to US and AHA grant: 15SDG25840013 to SP.
Data availability
Available on request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The funders had no role in study design, data collection, and analysis.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sathnur Pushpakumar, Email: sbpush01@louisville.edu.
Utpal Sen, Email: utpal.sen@louisville.edu.
References
- 1.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66(4):1613–21. 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed]
- 2.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. The Journal of clinical investigation. 2011;121(11):4210–21. 10.1172/JCI45161. PubMed PMID: 22045571; PubMed Central PMCID: PMCPMC3204829. [DOI] [PMC free article] [PubMed]
- 3.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, et al. Acute kidney injury increases risk of ESRD among elderly. Journal of the American Society of Nephrology : JASN. 2009;20(1):223–8. 10.1681/ASN.2007080837. PubMed PMID: 19020007; PubMed Central PMCID: PMCPMC2615732. [DOI] [PMC free article] [PubMed]
- 4.O'Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. Journal of the American Society of Nephrology : JASN. 2017;28(2):407–20. 10.1681/ASN.2015121308. PubMed PMID: 28143966; PubMed Central PMCID: PMCPMC5280008. [DOI] [PMC free article] [PubMed]
- 5.Pavkov ME, Harding JL, Burrows NR. Trends in hospitalizations for acute kidney injury - United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2018;67(10):289–93. 10.15585/mmwr.mm6710a2. PubMed PMID: 29543788; PubMed Central PMCID: PMCPMC5857198. [DOI] [PMC free article] [PubMed]
- 6.Kane-Gill SL, Sileanu FE, Murugan R, Trietley GS, Handler SM, Kellum JA. Risk factors for acute kidney injury in older adults with critical illness: a retrospective cohort study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;65(6):860–9. 10.1053/j.ajkd.2014.10.018. PubMed PMID: 25488106; PubMed Central PMCID: PMCPMC4442750. [DOI] [PMC free article] [PubMed]
- 7.Fuhrman DY, Kane-Gill S, Goldstein SL, Priyanka P, Kellum JA. Acute kidney injury epidemiology, risk factors, and outcomes in critically ill patients 16–25 years of age treated in an adult intensive care unit. Ann Intensive Care. 2018;8(1):26. doi: 10.1186/s13613-018-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. 2016;23(1):19–28. 10.1053/j.ackd.2015.08.004. PubMed PMID: 26709059; PubMed Central PMCID: PMCPMC4693148. [DOI] [PMC free article] [PubMed]
- 9.Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis. 2010;17(4):302–7. 10.1053/j.ackd.2010.05.002. PubMed PMID: 20610357; PubMed Central PMCID: PMCPMC2901622. [DOI] [PMC free article] [PubMed]
- 10.Sen U, Pushpakumar SB, Amin MA, Tyagi SC. Homocysteine in renovascular complications: hydrogen sulfide is a modulator and plausible anaerobic ATP generator. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2014;41:27–37. 10.1016/j.niox.2014.06.006. PubMed PMID: 24963795; PubMed Central PMCID: PMCPMC4156889. [DOI] [PMC free article] [PubMed]
- 11.Kasinath BS. Hydrogen sulfide to the rescue in obstructive kidney injury. Kidney international. 2014;85(6):1255–8. 10.1038/ki.2013.529. PubMed PMID: 24875544; PubMed Central PMCID: PMCPMC4048854. [DOI] [PMC free article] [PubMed]
- 12.Koning AM, Frenay AR, Leuvenink HG, van Goor H. Hydrogen sulfide in renal physiology, disease and transplantation-the smell of renal protection. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2015;46:37–49. doi: 10.1016/j.niox.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Lee HJ, Feliers D, Barnes JL, Oh S, Choudhury GG, Diaz V, et al. Hydrogen sulfide ameliorates aging-associated changes in the kidney. Geroscience. 2018;40(2):163–76. 10.1007/s11357-018-0018-y. PubMed PMID: 29717417; PubMed Central PMCID: PMCPMC5964063. [DOI] [PMC free article] [PubMed]
- 14.Hou CL, Wang MJ, Sun C, Huang Y, Jin S, Mu XP, et al. Protective effects of hydrogen sulfide in the ageing kidney. Oxid Med Cell Longev. 2016;2016:7570489. 10.1155/2016/7570489. PubMed PMID: 27882191; PubMed Central PMCID: PMCPMC5108860. [DOI] [PMC free article] [PubMed]
- 15.Xu Z, Prathapasinghe G, Wu N, Hwang SY, Siow YL, O K. Ischemia-reperfusion reduces cystathionine-beta-synthase-mediated hydrogen sulfide generation in the kidney. American journal of physiology Renal physiology. 2009;297(1):F27–F35. 10.1152/ajprenal.00096.2009. [DOI] [PubMed]
- 16.Wu N, Siow YL, O K. Ischemia/reperfusion reduces transcription factor Sp1-mediated cystathionine beta-synthase expression in the kidney. The Journal of biological chemistry. 2010;285(24):18225–33. 10.1074/jbc.M110.132142. PubMed PMID: 20392694; PubMed Central PMCID: PMCPMC2881747. [DOI] [PMC free article] [PubMed]
- 17.Xu X, Kriegel AJ, Jiao X, Liu H, Bai X, Olson J, et al. miR-21 in ischemia/reperfusion injury: a double-edged sword? Physiol Genomics. 2014;46(21):789–97. 10.1152/physiolgenomics.00020.2014. PubMed PMID: 25159851; PubMed Central PMCID: PMCPMC4280148. [DOI] [PMC free article] [PubMed]
- 18.Xu X, Kriegel AJ, Liu Y, Usa K, Mladinov D, Liu H, et al. Delayed ischemic preconditioning contributes to renal protection by upregulation of miR-21. Kidney international. 2012;82(11):1167–75. 10.1038/ki.2012.241. PubMed PMID: 22785173; PubMed Central PMCID: PMCPMC3777822. [DOI] [PMC free article] [PubMed]
- 19.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med. 2012;4(121):121ra18. 10.1126/scitranslmed.3003205. PubMed PMID: 22344686; PubMed Central PMCID: PMCPMC3672221. [DOI] [PMC free article] [PubMed]
- 20.Song N, Zhang T, Xu X, Lu Z, Yu X, Fang Y, et al. miR-21 protects against ischemia/reperfusion-induced acute kidney injury by preventing epithelial cell apoptosis and inhibiting dendritic cell maturation. Front Physiol. 2018;9:790. 10.3389/fphys.2018.00790. PubMed PMID: 30013485; PubMed Central PMCID: PMCPMC6036242. [DOI] [PMC free article] [PubMed]
- 21.Yang G, Pei Y, Cao Q, Wang R. MicroRNA-21 represses human cystathionine gamma-lyase expression by targeting at specificity protein-1 in smooth muscle cells. J Cell Physiol. 2012;227(9):3192–3200. doi: 10.1002/jcp.24006. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Hao DD, Zhang JS, Zhu YC. Hydrogen sulphide inhibits cardiomyocyte hypertrophy by up-regulating miR-133a. Biochem Biophys Res Commun. 2011;413(2):342–347. doi: 10.1016/j.bbrc.2011.08.101. [DOI] [PubMed] [Google Scholar]
- 23.Huen SC, Cantley LG. Macrophage-mediated injury and repair after ischemic kidney injury. Pediatr Nephrol. 2015;30(2):199–209. 10.1007/s00467-013-2726-y. PubMed PMID: 24442822; PubMed Central PMCID: PMCPMC5048744. [DOI] [PMC free article] [PubMed]
- 24.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, et al. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55(2):434–9. [PubMed]
- 25.Curci C, Castellano G, Stasi A, Divella C, Loverre A, Gigante M, et al. Endothelial-to-mesenchymal transition and renal fibrosis in ischaemia/reperfusion injury are mediated by complement anaphylatoxins and Akt pathway. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(4):799–808. 10.1093/ndt/gft516. [DOI] [PubMed]
- 26.Coca SG. Acute kidney injury in elderly persons. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;56(1):122–31. 10.1053/j.ajkd.2009.12.034. PubMed PMID: 20346560; PubMed Central PMCID: PMCPMC2902696. [DOI] [PMC free article] [PubMed]
- 27.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14339–44. 10.1073/pnas.0912701107. PubMed PMID: 20651252; PubMed Central PMCID: PMCPMC2922548. [DOI] [PMC free article] [PubMed]
- 28.Jiao X, Xu X, Fang Y, Zhang H, Liang M, Teng J, et al. miR-21 contributes to renal protection by targeting prolyl hydroxylase domain protein 2 in delayed ischaemic preconditioning. Nephrology (Carlton). 2017;22(5):366–73. 10.1111/nep.12787. [DOI] [PubMed]
- 29.Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123(1):7–13. 10.1016/j.clim.2006.09.008. PubMed PMID: 17064966; PubMed Central PMCID: PMCPMC1888143. [DOI] [PMC free article] [PubMed]
- 30.Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32(6):463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 31.Ferenbach DA, Nkejabega NC, McKay J, Choudhary AK, Vernon MA, Beesley MF, et al. The induction of macrophage hemeoxygenase-1 is protective during acute kidney injury in aging mice. Kidney Int. 2011;79(9):966–976. doi: 10.1038/ki.2010.535. [DOI] [PubMed] [Google Scholar]
- 32.Clements ME, Chaber CJ, Ledbetter SR, Zuk A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PloS one. 2013;8(8):e70464. 10.1371/journal.pone.0070464. PubMed PMID: 23940580; PubMed Central PMCID: PMCPMC3734312. [DOI] [PMC free article] [PubMed]
- 33.Kim MG, Yang J, Ko YS, Lee HY, Oh SW, Cho WY, et al. Impact of aging on transition of acute kidney injury to chronic kidney disease. Sci Rep. 2019;9(1):18445. 10.1038/s41598-019-54585-1. PubMed PMID: 31804508; PubMed Central PMCID: PMCPMC6895109. [DOI] [PMC free article] [PubMed]
- 34.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. Journal of the American Society of Nephrology : JASN. 2008;19(12):2282–7. 10.1681/ASN.2008050513. PubMed PMID: 18987304; PubMed Central PMCID: PMCPMC2588112. [DOI] [PMC free article] [PubMed]
- 35.Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol. 2009;175(4):1380–8. 10.2353/ajpath.2009.090096. PubMed PMID: 19729486; PubMed Central PMCID: PMCPMC2751535. [DOI] [PMC free article] [PubMed]
- 36.Zhou Y, Zhu X, Wang X, Peng Y, Du J, Yin H, et al. H2S alleviates renal injury and fibrosis in response to unilateral ureteral obstruction by regulating macrophage infiltration via inhibition of NLRP3 signaling. Exp Cell Res. 2020;387(1):111779. doi: 10.1016/j.yexcr.2019.111779. [DOI] [PubMed] [Google Scholar]
- 37.Weber GJ, Pushpakumar SB, Sen U. Hydrogen sulfide alleviates hypertensive kidney dysfunction through an epigenetic mechanism. American journal of physiology Heart and circulatory physiology. 2017;312(5):H874-H85. 10.1152/ajpheart.00637.2016. PubMed PMID: 28213404; PubMed Central PMCID: PMCPMC5451583. [DOI] [PMC free article] [PubMed]
- 38.Chade AR. Renal vascular structure and rarefaction. Compr Physiol. 2013;3(2):817–31. 10.1002/cphy.c120012. PubMed PMID: 23720331; PubMed Central PMCID: PMCPMC3936257. [DOI] [PMC free article] [PubMed]
- 39.Horbelt M, Lee SY, Mang HE, Knipe NL, Sado Y, Kribben A, et al. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. American journal of physiology Renal physiology. 2007;293(3):F688–F695. doi: 10.1152/ajprenal.00452.2006. [DOI] [PubMed] [Google Scholar]
- 40.Kwekel JC, Vijay V, Desai VG, Moland CL, Fuscoe JC. Age and sex differences in kidney microRNA expression during the life span of F344 rats. Biol Sex Differ. 2015;6(1):1. doi: 10.1186/s13293-014-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai XY, Ma Y, Ding R, Fu B, Shi S, Chen XM. miR-335 and miR-34a promote renal senescence by suppressing mitochondrial antioxidative enzymes. Journal of the American Society of Nephrology : JASN. 2011;22(7):1252–61. 10.1681/ASN.2010040367. PubMed PMID: 21719785; PubMed Central PMCID: PMCPMC3137573. [DOI] [PMC free article] [PubMed]
- 42.Lin X, Zhan JK, Wang YJ, Tan P, Chen YY, Deng HQ, et al. Function, role, and clinical application of microRNAs in vascular aging. Biomed Res Int. 2016;2016:6021394. 10.1155/2016/6021394. PubMed PMID: 28097140; PubMed Central PMCID: PMCPMC5209603 publication of this paper. [DOI] [PMC free article] [PubMed]
- 43.Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience. 2019;41(4):419–39. 10.1007/s11357-019-00095-x. PubMed PMID: 31463647; PubMed Central PMCID: PMCPMC6815288. [DOI] [PMC free article] [PubMed]
- 44.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. The Journal of clinical investigation. 2015;125(1):141–56. 10.1172/JCI75852. PubMed PMID: 25415439; PubMed Central PMCID: PMCPMC4382246. [DOI] [PMC free article] [PubMed]
- 45.Kolling M, Kaucsar T, Schauerte C, Hubner A, Dettling A, Park JK, et al. Therapeutic miR-21 silencing ameliorates diabetic kidney disease in mice. Mol Ther. 2017;25(1):165–80. 10.1016/j.ymthe.2016.08.001. PubMed PMID: 28129112; PubMed Central PMCID: PMCPMC5363308. [DOI] [PMC free article] [PubMed]
- 46.Kundu S, Pushpakumar S, Sen U. MMP-9- and NMDA receptor-mediated mechanism of diabetic renovascular remodeling and kidney dysfunction: hydrogen sulfide is a key modulator. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2015;46:172–85. 10.1016/j.niox.2015.02.003. PubMed PMID: 25659756; PubMed Central PMCID: PMCPMC4367483. [DOI] [PMC free article] [PubMed]
- 47.Kwiatkowska E, Domanski L, Bober J, Safranow K, Romanowski M, Pawlik A, et al. Urinary metalloproteinases-9 and -2 and their inhibitors TIMP-1 and TIMP-2 are markers of early and long-term graft function after renal transplantation. Kidney Blood Press Res. 2016;41(3):288–97. 10.1159/000443431. [DOI] [PubMed]
- 48.Ersan S, Tanrisev M, Cavdar Z, Celik A, Unlu M, Kocak A, et al. Pretreatment with nebivolol attenuates level and expression of matrix metalloproteinases in a rat model of renal ischaemia-reperfusion injury. Nephrology (Carlton) 2017;22(12):1023–1029. doi: 10.1111/nep.13007. [DOI] [PubMed] [Google Scholar]
- 49.Basile DP, Fredrich K, Weihrauch D, Hattan N, Chilian WM. Angiostatin and matrix metalloprotease expression following ischemic acute renal failure. American journal of physiology Renal physiology. 2004;286(5):F893–F902. doi: 10.1152/ajprenal.00328.2003. [DOI] [PubMed] [Google Scholar]
- 50.Caron A, Desrosiers RR, Beliveau R. Ischemia injury alters endothelial cell properties of kidney cortex: stimulation of MMP-9. Exp Cell Res. 2005;310(1):105–116. doi: 10.1016/j.yexcr.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Zhao H, Dong Y, Tian X, Tan TK, Liu Z, Zhao Y, et al. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World journal of nephrology. 2013;2(3):84–9. 10.5527/wjn.v2.i3.84. PubMed PMID: 24255890; PubMed Central PMCID: PMCPMC3832915. [DOI] [PMC free article] [PubMed]
- 52.Zheng G, Lyons JG, Tan TK, Wang Y, Hsu TT, Min D, et al. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta1 in renal tubular epithelial cells. Am J Pathol. 2009;175(2):580–91. 10.2353/ajpath.2009.080983. PubMed PMID: 19590041; PubMed Central PMCID: PMCPMC2716958. [DOI] [PMC free article] [PubMed]
- 53.Tan TK, Zheng G, Hsu TT, Wang Y, Lee VW, Tian X, et al. Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells. Am J Pathol. 2010;176(3):1256–70. 10.2353/ajpath.2010.090188. PubMed PMID: 20075196; PubMed Central PMCID: PMCPMC2832147. [DOI] [PMC free article] [PubMed]
- 54.Glowacki F, Savary G, Gnemmi V, Buob D, Van der Hauwaert C, Lo-Guidice JM, et al. Increased circulating miR-21 levels are associated with kidney fibrosis. PloS one. 2013;8(2):e58014. 10.1371/journal.pone.0058014. PubMed PMID: 23469132; PubMed Central PMCID: PMCPMC3585177. [DOI] [PMC free article] [PubMed]
- 55.Xia M, Chen L, Muh RW, Li PL, Li N. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J Pharmacol Exp Ther. 2009;329(3):1056–62. 10.1124/jpet.108.149963. PubMed PMID: 19246614; PubMed Central PMCID: PMCPMC2683781. [DOI] [PMC free article] [PubMed]
- 56.Morales-Loredo H, Barrera A, Garcia JM, Pace CE, Naik JS, Gonzalez Bosc LV, et al. Hydrogen sulfide regulation of renal and mesenteric blood flow. American journal of physiology Heart and circulatory physiology. 2019;317(5):H1157-H65. 10.1152/ajpheart.00303.2019. PubMed PMID: 31625777; PubMed Central PMCID: PMCPMC6879921. [DOI] [PMC free article] [PubMed]
- 57.Tripatara P, Patel NS, Collino M, Gallicchio M, Kieswich J, Castiglia S, et al. Generation of endogenous hydrogen sulfide by cystathionine gamma-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab Investig. 2008;88(10):1038–1048. doi: 10.1038/labinvest.2008.73. [DOI] [PubMed] [Google Scholar]
- 58.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92(2):791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 59.Chen YH, Yao WZ, Geng B, Ding YL, Lu M, Zhao MW, et al. Endogenous hydrogen sulfide in patients with COPD. Chest. 2005;128(5):3205–11. 10.1378/chest.128.5.3205. [DOI] [PubMed]
- 60.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, et al. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal. 2013;18(15):1906–19. 10.1089/ars.2012.4645. [DOI] [PubMed]
- 61.Wang WJ, Cai GY, Ning YC, Cui J, Hong Q, Bai XY, et al. Hydrogen sulfide mediates the protection of dietary restriction against renal senescence in aged F344 rats. Sci Rep. 2016;6:30292. 10.1038/srep30292. PubMed PMID: 27456368; PubMed Central PMCID: PMCPMC4960595. [DOI] [PMC free article] [PubMed]
- 62.Liang B, Xiao T, Long J, Liu M, Li Z, Liu S, et al. Hydrogen sulfide alleviates myocardial fibrosis in mice with alcoholic cardiomyopathy by downregulating autophagy. Int J Mol Med. 2017;40(6):1781–91. 10.3892/ijmm.2017.3191. PubMed PMID: 29039471; PubMed Central PMCID: PMCPMC5716447. [DOI] [PMC free article] [PubMed]
- 63.Liu M, Li Z, Liang B, Li L, Liu S, Tan W, et al. Hydrogen sulfide ameliorates rat myocardial fibrosis induced by thyroxine through PI3K/AKT signaling pathway. Endocr J. 2018;65(7):769–81. 10.1507/endocrj.EJ17-0445. [DOI] [PubMed]
- 64.Meldrum KK, Meldrum DR, Hile KL, Burnett AL, Harken AH. A novel model of ischemia in renal tubular cells which closely parallels in vivo injury. J Surg Res. 2001;99(2):288–293. doi: 10.1006/jsre.2001.6201. [DOI] [PubMed] [Google Scholar]
- 65.Pushpakumar S, Ren L, Kundu S, Gamon A, Tyagi SC, Sen U. Toll-like receptor 4 deficiency reduces oxidative stress and macrophage mediated inflammation in hypertensive kidney. Sci Rep. 2017;7(1):6349. 10.1038/s41598-017-06484-6. PubMed PMID: 28743964; PubMed Central PMCID: PMCPMC5526876. [DOI] [PMC free article] [PubMed]
- 66.Ellery SJ, Cai X, Walker DD, Dickinson H, Kett MM. Transcutaneous measurement of glomerular filtration rate in small rodents: through the skin for the win? Nephrology (Carlton) 2015;20(3):117–123. doi: 10.1111/nep.12363. [DOI] [PubMed] [Google Scholar]
- 67.Schreiber A, Shulhevich Y, Geraci S, Hesser J, Stsepankou D, Neudecker S, et al. Transcutaneous measurement of renal function in conscious mice. American journal of physiology Renal physiology. 2012;303(5):F783–8. 10.1152/ajprenal.00279.2012. [DOI] [PubMed]
- 68.Pushpakumar S, Kundu S, Pryor T, Givvimani S, Lederer E, Tyagi SC, et al. Angiotensin-II induced hypertension and renovascular remodelling in tissue inhibitor of metalloproteinase 2 knockout mice. J Hypertens. 2013;31(11):2270–81; discussion 81. 24077247, PMC4000563. 10.1097/HJH.0b013e3283649b33. [DOI] [PMC free article] [PubMed]
- 69.Pushpakumar SB, Kundu S, Metreveli N, Tyagi SC, Sen U. Matrix metalloproteinase inhibition mitigates renovascular remodeling in salt-sensitive hypertension. Physiological reports. 2013;1(3):e00063. 24159376, PMC3804376. 10.1002/phy2.63. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available on request.