Abstract
Pathological age-related loss of skeletal muscle strength and mass contribute to impaired physical function in older adults. Factors that promote the development of these conditions remain incompletely understood, impeding development of effective and specific diagnostic and therapeutic approaches. Inconclusive evidence across species suggests disruption of action potential signal transmission at the neuromuscular junction (NMJ), the crucial connection between the nervous and muscular systems, as a possible contributor to age-related muscle dysfunction. Here we investigated age-related loss of NMJ function using clinically relevant, electrophysiological measures (single-fiber electromyography (SFEMG) and repetitive nerve stimulation (RNS)) in aged (26 months) versus young (6 months) F344 rats. Measures of muscle function (e.g., grip strength, peak plantarflexion contractility torque) and mass were assessed for correlations with physiological measures (e.g., indices of NMJ transmission). Other outcomes also included plantarflexion muscle contractility tetanic torque fade during 1-s trains of stimulation as well as gastrocnemius motor unit size and number. Profiling NMJ function in aged rats identified significant declines in NMJ transmission stability and reliability. Further, NMJ deficits were tightly correlated with hindlimb grip strength, gastrocnemius muscle weight, loss of peak contractility torque, degree of tetanic fade, and motor unit loss. Thus, these findings provide direct evidence for NMJ dysfunction as a potential mechanism of age-related muscle dysfunction pathogenesis and severity. These findings also suggest that NMJ transmission modulation may serve as a target for therapeutic development for age-related loss of physical function.
Keywords: Weakness, Grip strength, Dynapenia, Sarcopenia, Neuromuscular junction, Aging, Synapse
Introduction
Physical function declines with aging, beginning as early as the fifth decade of life in humans [1]. Reduced physical functioning affects the ability to maintain independence and predicts increased risk for future cardiovascular disease, mobility decline, and mortality [2]. Population aging has prompted increased interest in identifying mechanisms that contribute to, and interventions for, this loss of function.
Two important contributors to physical function decline are pathological age-related losses of skeletal muscle function and size [3–5]. These are associated with increased risk for falls, reduced quality of life, and increased risk for mortality in older adults [6–10]. “Sarcopenia” was coined by Rosenberg in 1989 to describe age-related muscle wasting; roughly two decades later, Clark and Manini proposed the term “dynapenia” to describe age-related loss of muscle strength and power [9, 11]. The term sarcopenia is now commonly used to describe the geriatric syndrome of pathological skeletal muscle size and function deficit [12], but specific criteria for the clinical diagnosis of sarcopenia continue to evolve. Sarcopenia arises through multifactorial pathophysiological mechanisms, but risk factors that contribute to the pathophysiology are incompletely understood and likely individually variable [13, 14]. Interestingly, while inadequate maintenance of muscle mass exerts clear negative consequences during aging, the negative consequences of skeletal muscle function deficit on older adults are more severe [9, 15].
Existing studies of the pathophysiology of age-related skeletal muscle dysfunction implicate a combination of neurologic and muscular factors [16–18]. Of particular interest is the intersection between the nervous and muscular systems: the neuromuscular junction (NMJ) [19–30]. The NMJ couples these systems for motor output and control and forms a link for bidirectional trophic signaling [19, 24, 26]. Importantly, in rodent models, age consistently alters NMJ morphological integrity [31–36]; whether such changes have functional implications is less clear (i.e., whether these alterations in NMJ morphology are associated with reduced physiological and physical function is poorly understood). In fact, some studies in aged mice have found no correlation between NMJ morphological changes and synaptic function, as measured by isolated ex vivo recordings in the diaphragm [37]. Further, while aging might be expected to diminish function at the NMJ, isolated recordings of endplate potentials or endplate currents (i.e., measuring NMJ synaptic transmission) are enhanced in older versus younger mice [37–39]. Ex vivo NMJ recordings, however, do not directly test whether endplate responses are adequate to reach action potential threshold and generate action potentials [40, 41].
To surmount this limitation, we have recently applied single-fiber electromyography (SFEMG), a clinical electrophysiological method for in vivo assessment of NMJ transmission, in mouse models of aging [40]. Our recent work using SFEMG demonstrated that aged (27-month-old) mice show NMJ transmission failure in the gastrocnemius muscle [40, 42]. SFEMG is an extracellular technique that uses a selective electrode (i.e., a specially constructed needle electrode with a very small recording surface (25 micrometers)) to record action potentials from individual muscle fibers and allows sensitive detection of action potential generation failure [43, 44]. SFEMG is the most sensitive measure of in vivo NMJ function [44]. Whether NMJ transmission failure is associated with changes of muscle strength and function during aging is unknown. As such, we aimed to determine how NMJ transmission defects contribute to loss of muscle strength and function in an aged rat model. Our findings provide key new insight to guide further preclinical and clinical studies of NMJ function with aging.
Methods
Overview of study design and animal care
All procedures were approved and performed in accordance with the Institutional Animal Care and Use Committee of The Ohio State University. A total of 8 young F344 rats (6 months old, 3 females, 5 males) and 8 aged rats (26 months old, 3 female, 5 male) were obtained from the National Institutes of Health aging rat colony. In rats, 26 months of age roughly corresponds to ~70 years of age in humans [45]. Rats underwent in vivo assessments of grip strength, muscle contractility, NMJ transmission, motor unit number, and terminal/endpoint assessment of gastrocnemius muscle mass, as detailed below.
Procedure for anesthesia
Rats were anesthetized during electrophysiological recordings and muscle contractility assessment via inhaled isoflurane delivered at 3–5% for induction and 2–3% for maintenance anesthesia using a SomnoSuite low-flow anesthesia system (Kent Scientific, Torrington, CT). Body temperature was maintained at 37°C with an infrared heating pad (Kent Scientific). A petroleum-based eye lubricant (Dechra, Northwich, UK) was applied to avoid corneal dryness and irritation. Hair of the left forelimb and right hindlimb was shaved (model VPG 6530, Remington, DeForest, WI).
Electrophysiological assessment of NMJ transmission
Repetitive nerve stimulation (RNS) and SFEMG were performed to investigate sufficiency of NMJ transmission to elicit action potential. Decrement on RNS reflects reduction of the summated excitation of a muscle following repetitive supramaximal stimulation of a peripheral nerve [46]. During RNS, placement of electrodes for stimulation, grounding, and recording were the same as described below for compound muscle action potential (CMAP). Repetitive CMAP amplitudes were recorded following a train of 10 supramaximal stimuli delivered to the sciatic nerve at 10, 20, 30, 40, and 50 Hz. Change of CMAP amplitude between the first stimulation and the 10th stimulation (decrement) was calculated at each frequency (10, 20, 30, 40, 50) with the following equation: % Amplitude decrement = [(Amplitude of 10th response − Amplitude 1st response)/Amplitude of 1st response] * 100%.
Stimulated SFEMG was performed similar to our prior studies in mouse models using a clinical electrodiagnostic system with Viking software (Natus Neurology Inc., Middleton, WI, USA) [47]. SFEMG is the most sensitive technique for interrogation of the fidelity of NMJ transmission to generate an action potential and is routinely used clinically [44, 46]. Individual muscle fiber action potentials are recorded using a specialized electrode with a selective recording surface and narrow filter settings during peripheral nerve stimulation [44, 46]. Two isolated 28-gauge monopolar needle electrodes (Natus Neurology Inc.) were inserted subcutaneously at the proximal edge of the gastrocnemius muscle to stimulate the right tibial nerve at a frequency of 10 Hz (constant current intensity 0.3–10 mA with a pulse duration of 0.1 ms). Filter settings were 1–10 kHz. Gain was adjusted from 200 to 1000 μV per division to visualize potentials. Sweep speed was set at 500 μs, and a disposable strip ground electrode (CareFusion, Middleton, WI, USA) was placed on the left foot. The SFEMG recording electrode (Natus Neurology Inc.) was inserted into the right gastrocnemius muscle parallel to the long axis of the muscle fibers to record single-fiber action potentials (SFAPs) and minimize muscle trauma. Stimulus was adjusted to isolate SFAP for analysis. To consider a response as an SFAP, the following criteria were required: rise time from baseline peak to negative of <500 μs, minimum amplitude (baseline peak to negative) of 200 μV, and a stable all-or-nothing response. Jitter, or variability of SFAP latency between discharges at a synapse, was calculated from 50 to 100 consecutive shocks using peak detection. Potentials with jitter <4 μs were not included in the analyses to avoid potentials elicited by direct muscle stimulation [48]. On average, 7 synapses were assessed from each animal. A total of 50–100 consecutive discharges were assessed from each synapse to calculate jitter, and values were averaged by animal. Blocking was calculated as the percentage of junctions within each animal with evidence of NMJ transmission failure to generate action potentials.
Assessment of grip strength and muscle mass
Bilateral forelimb and hindlimb grip strength were assessed using a force transducer (Model GT3, Bioseb SAS BP32025-F-13845 Vitrolles Cedex, Pinellas Park, FL, USA). A researcher grasped the rat’s body, and once the rat clasped the transducer bar, the researcher tugged with a steady and constant motion until the rat lost grip. Three trials of forelimb and hindlimb grip strength were completed, and the average of the three trials was calculated for forelimb and hindlimb grip and used for analyses. Following completion of all in vivo assessments, rats were euthanized with CO2 and decapitated, and gastrocnemius muscles were removed and weighed.
Assessment of muscle contractile torque and maintenance of contractile during stimulus trains
In vivo plantar flexion muscle contractility was assessed using methods similar to those previously described in mouse and rat models [49–51]. Briefly, an anesthetized rat was placed in the supine position, and the right hindlimb paw was affixed to a force plate using Transpore medical tape (3M, Maplewood, MN). The knee/distal femoral condyles were fixed to the platform using blunt clamps. The right hindlimb and foot were positioned with the tibia and foot at a 90° angle. A pair of disposable monopolar electrodes (Natus Neurology Inc.) was placed subcutaneously in the region of the medial posterior leg near the tibial branch of the sciatic nerve. Peak twitch torque was visualized during single stimulations (0.20-ms square wave pulse), and stimulus intensity was adjusted until eliciting maximal twitch response. Subsequently, maximum torque production was assessed by delivering trains of supramaximal stimuli (~120–150% of maximal stimulus level) at 5, 15, 30, 45, 90, and 150 Hz. For frequencies 5, 15, 30, 45, and 90, a 1-s train of stimuli was delivered, and for 150 Hz, a 900-ms train was delivered. The order of stimulation trains was 150, 5, 15, 30, 45, and then 90 Hz. Thirty-second rest periods were given between each stimulus train. For comparisons between groups, we used peak absolute maximal torque values and peak torque values normalized to body mass and muscle mass. Additionally, the % decline of plantar flexion torque during the 1-s train at 90 Hz and the 900-ms train at 150 Hz (tetanic fade: % change between maximum and minimum during tetanic stimuli) was compared between young and aged rats [52].
Other neuromuscular outcomes
In vivo electrophysiological measures of CMAP amplitude, average single motor unit potential (SMUP) amplitude, and motor unit number estimation (MUNE) were acquired from the hindlimb and forelimb as previously described using a Sierra Summit clinical electrodiagnostic system (Cadwell, Kennewick, WA) [49]. For forelimb recordings, rats were positioned supine, and the left paw was affixed to the stage with Transpore medical tape (3M). A pair of TECA 6030-TP surface disk electrodes (Natus Neurology) was lightly coated with electrode gel (Parker Labs, Fairfield, NJ) and placed on the skin overlying the ventromedial forelimb muscles (E1) and the left paw (E2). An adhesive electrode (Natus Neurology) was attached to the tail. A pair of 28-gauge monopolar needle electrodes was used as the cathode and anode for nerve stimulation and placed subcutaneously over the mid-left pectoralis muscle and just rostral to the left pectoralis muscle, respectively, separated by approximately 1 cm. For hindlimb recordings, the rat was placed in the prone position. The right hindlimb was affixed to the stage with Transpore medical tape (3M). A pair of TECA 6030-TP surface disc electrodes (Natus Neurology) was lightly coated with electrode gel and placed over the bulk of the gastrocnemius muscle (E1) and on the calcaneus (E2). A pair of 28-gauge monopolar needle electrodes was used as the cathode and anode for nerve stimulation and placed subcutaneously in the region of the sciatic nerve at the proximal thigh.
During CMAP recordings, a supramaximal stimulus (120% of maximum) was delivered to the left brachial plexus and right sciatic nerves. Screen sensitivity and duration were set as sensitivity of 200 mV, 20 mV per division, and duration of 10 ms, 1 ms per division. Peak-to-peak CMAP values were recorded in mV. Incremental stimulation was used to obtain 10 incremental CMAP responses, which were averaged to determine average SMUP size. MUNE values for the forelimb and hindlimb were calculated as MUNE = CMAP / Average SMUP.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8.4.3 (GraphPad Software Inc., San Diego, CA, USA). All data were analyzed for normality with the Shapiro-Wilk test. SFEMG jitter, grip strength, CMAP, SMUP, and MUNE data were normally distributed and were compared using unpaired t-tests. Body mass was compared for age and sex using two-way ANOVA. Muscle contractility and RNS were compared using repeated measures, two-way ANOVA to investigate effects of stimulation frequency and age. Sidak’s multiple comparisons were used to compare RNS at each frequency between young and aged rats. The Mann-Whitney test was used to compare % blocking on SFEMG and tetanic fade because these data were not normally distributed. The Pearson correlation coefficients were calculated between data with normal distribution and data with non-normal distribution The Spearman correlation coefficients were calculated. Simple linear regression lines were added to all X-Y plots for correlations between variables with normal distributions. A p-value of <0.05 was considered statistically significant.
Results
Aged rats show overt NMJ transmission defects
We assessed NMJ transmission in two ways: SFEMG and RNS. The RNS data demonstrated significantly greater reduction of CMAP amplitudes during trains of stimuli in aged versus young rats (~13% at 50 Hz) (Fig. 1a,b). Of note, RNS in young rats typically showed a slight incremental response as is expected with stimulus rates that do not allow interstimulus muscle relaxation and thus muscle shortening [53]. Therefore, we examined the relative CMAP amplitude reduction in aged rats (Fig. 1b). SFEMG was also performed to investigate for increased jitter and blocking. In Fig. 1c–d, SFEMG recordings from a normal and a failing synapse are shown. SFEMG jitter, a measure of NMJ transmission variability, was significantly higher (~2-fold) in aged rats (Fig. 1c). Similarly, NMJ blocking on SFEMG was significantly higher in aged animals (~27% of synapses showed blocking in aged rats versus only 1 synapse in young rats (<2%)) (Fig. 1d).
Fig. 1.
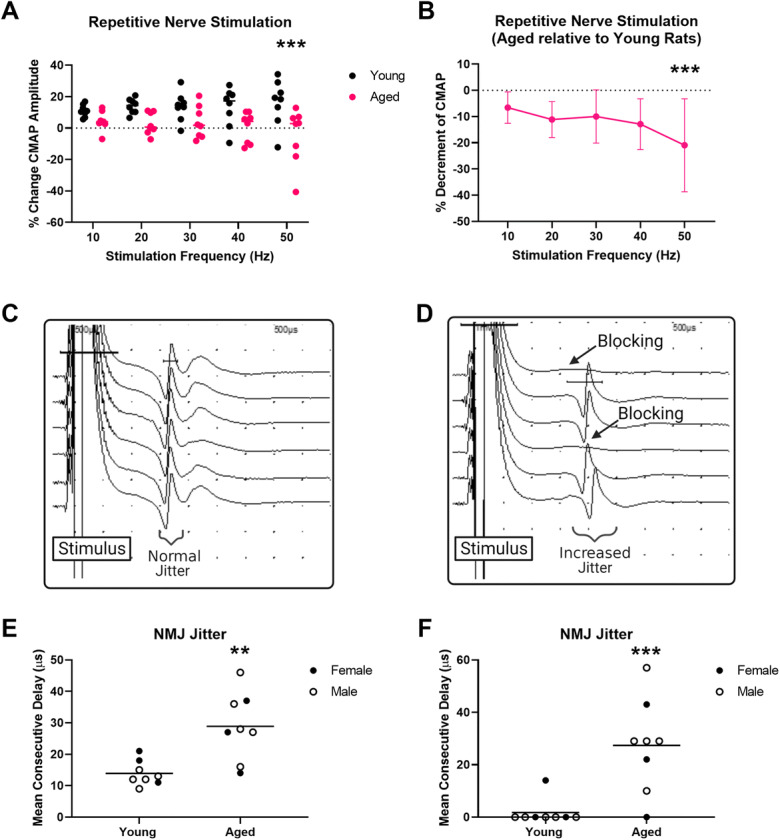
Aged rats demonstrate significant deficits in neuromuscular junction transmission. a–b Repetitive nerve stimulation demonstrated significant reduction of compound muscle action potential (CMAP) amplitudes in aged versus young rats during trains of 10 stimuli delivered at 10, 20, 30, 40, and 50 Hz. (a Absolute in aged and young rats. b Aged shown relative to young.) (Repeat measure two-way ANOVA: frequency, p=0.6070; age, p=0.0099; interaction, age × frequency, p=0.0695; Sidak’s multiple comparisons demonstrate significantly reduced amplitudes at 50-Hz stimulation in aged versus young rats, adjusted p=0.0007.) c Representative single-fiber electromyography traces showing single muscle fiber action potentials recorded from a healthy synapse with no blocking and normal jitter (scale: amplitude, 0.5 mV/division and 500 μs/division). d Representative single-fiber electromyography traces showing single muscle fiber action potentials recorded from a failing synapse with evidence of action potential blocking and increased jitter (scale: amplitude, 1 mV/division and 500 μs/division). e NMJ jitter on single-fiber electromyography showed increased variability of transmission (jitter) in aged versus young rats (t-test: p=0.0023). e NMJ % blocking on single-fiber electromyography was increased in aged versus young rats (Mann-Whitney test: p=0.0025). Young rats, n=8 (3 females, 5 males), and aged rats, n=8 (3 females, 5 males). ** p<0.01, *** p<0.001
Age-related increases of body mass and age-related losses of muscle strength, mass, and contractility torque
Body mass was compared between aged rats ((aged females, n=3, 259±22 grams and aged males, n=5, 444±22 grams) and young rats (females, n=3, 197±23 grams; males, n=5, 349±12 grams) and showed significant differences for age (p<0.0001) and sex (p<0.0001), but no interaction between age and sex (p=0.1253) (two-way ANOVA). Hindlimb grip testing revealed significant loss of muscle strength (both absolute and normalized to body mass) (Fig. 2a–b). Forelimb grip testing revealed no difference between young and aged rats when comparing absolute values (young, n=8 (5 males/3 females), 435±109 gram versus aged, n=8 (5 males, 3 females) 455±126 grams, p=0.7350) or normalized values (young, n=8 (5 males/3 females), 1.6±0.7 grams/gram of body mass versus aged, n=8 (5 males, 3 females) 1.3±0.4 grams/gram of body mass, p=0.2291) (data not shown).
Fig. 2.
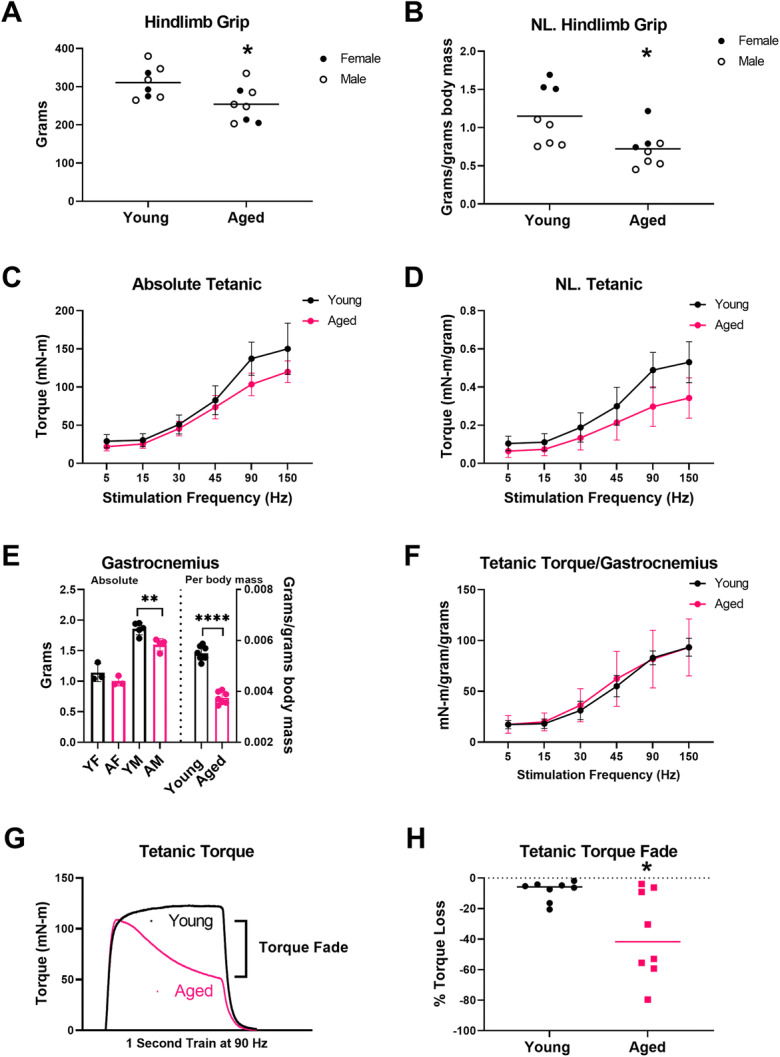
Hindlimb grip strength, plantarflexion muscle contractility, and muscle mass in aged rats. Absolute hindlimb (a) and normalized hindlimb (b) showed reduced muscle strength in aged rats (p=0.0228 and p=0.0168, respectively). Grip: young rats, n=8 (3 females, 5 males), and aged rats, n=8 (3 females, 5 males) (females filled circles, males empty circles). c During stimulation at 5, 15, 30, 45, 90, and 150 Hz, absolute plantar flexion torque was lower in aged rats compared to young rats (stimulation rate p<0.0001, age p=0.0260, and stimulation rate × age p=0.0004). d Similarly, plantar flexion torque normalized to body mass was lower in aged rats (stimulation rate p<0.0001, age p=0.0135, and stimulation rate × age: p<0.0001). e Right: Absolute gastrocnemius muscle mass was not significantly reduced in aged female (AF) versus young female (YF) rats (unpaired t-test, p=0.2209, young females n=3, aged females n=3) but was significantly reduced in aged male (AM) rats compared with young males (YM) (t-test, p=0.0061, young males n=5 and aged males n=4). e Left: Gastrocnemius mass normalized to body mass was significantly reduced in aged versus young rats (t-test, p<0.0001). Muscle weights: young rats, n=8 (3 females, 5 males), and aged rats, n=8 (3 females, 4 males). f Plantarflexion contractility normalized to gastrocnemius mass demonstrated no difference between young and aged rats (stimulation rate p<0.0001, age p=0.0.1018, and stimulation rate × age p=0.2054). g Representative superimposed tetanic torque tracings during a 1-s train of stimuli at 90 Hz from an aged and a young rat, showing loss of torque (fade) in an aged rat versus stable torque production in the young rat. h Aged rats demonstrated significantly greater fade of torque production during a 1-s train of tetanic stimulations at 90 Hz (Mann-Whitney, p=0.0499). * p<0.05, ** p<0.01, **** p<0.0001
In vivo muscle contractility assessment demonstrated significantly reduced peak plantarflexion torque, both absolute and normalized to body mass (Fig. 2c–d). At endpoint, absolute muscle mass was significantly lower in aged males versus young males but not in aged females versus young females, and there were significant reductions of muscle mass when normalized to body mass and comparing across all sexes (Fig. 2e). In contrast to peak absolute torque and peak torque normalized to body mass, in vivo plantarflexion torque normalized to gastrocnemius mass demonstrated no significant differences between aged and young rats (Fig. 2f).
In addition to peak torque production, we also investigated the ability of hindlimb plantarflexion muscles to maintain torque during a single 1-s train of stimuli at 90 Hz. Similar to observations by Fogerty et al. in the diaphragm muscle of aged rats, we observed increased loss of torque production in aged rats during trains of tetanic stimuli (i.e., tetanic fade) [52]. Therefore, we compared the ability to maintain torque production during 1-s train of 90-Hz stimulation between age groups by calculating the % decline between peak torque and minimum torque during the 1-s train of stimuli. Figure 2g shows an example of an aged rat with features of tetanic fade versus a young rat with stable torque production. When examining % torque decline during a 1-s train of stimuli at 90 Hz, aged rats demonstrated a four-fold greater loss of torque compared with young rats (Fig. 4h). Similarly, tetanic fade at 150 Hz was also increased by roughly two-fold in aged rats (−55±32%) compared with young rats (−26±19%) (unpaired t-test, p=0.0485) (data not shown).
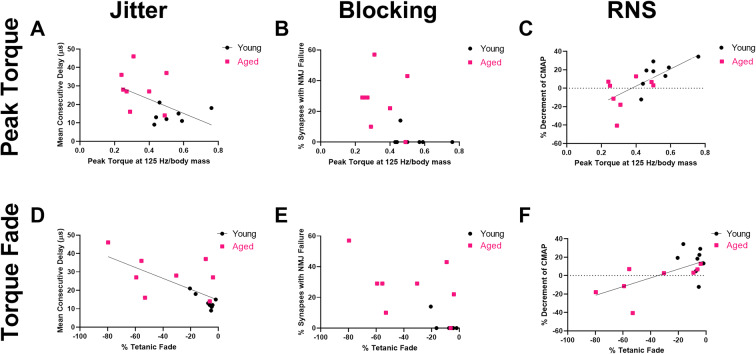
Fig. 4.
Peak plantarflexion torque and torque fade correlate with measures of NMJ transmission defects. Peak plantarflexion torque (normalized to body mass) demonstrated no significant correlation with a single-fiber electromyography (SFEMG) jitter (Spearman rs = −0.4948, p=0.0532) but significant correlations with b SFEMG NMJ blocking (Spearman rs = −0.6418, p=0.0091) and c CMAP amplitude decrement on RNS (Pearson r=0.6941, p=0.0029). Young rats, n=8 (3 females, 5 males), and aged rats, n=8 (3 females, 5 males). Tetanic fade % demonstrated significant correlations with d. SFEMG jitter (Spearman rs=−0.6406, p=0.0089), e blocking % (Spearman rs=−0.6777, p=0.0053), and f RNS decrement (Spearman rs=0.5088, p=0.0464). Young rats, n=8 (3 females, 5 males), and aged rats, n=8 (3 females, 5 males)
NMJ transmission defects correlate with muscle mass and strength
To understand the relationship between NMJ defects and muscle size and function, jitter and blocking on SFEMG and CMAP amplitude decrement on RNS were analyzed for correlations with gastrocnemius muscle mass and normalized hindlimb grip strength. SFEMG jitter and blocking significantly correlated with muscle weight but showed only a trend for normalized hindlimb grip strength (Fig. 3a–d). RNS was significantly correlated with both muscle weight and normalized hindlimb grip strength (Fig. 3e–f).
Fig. 3.
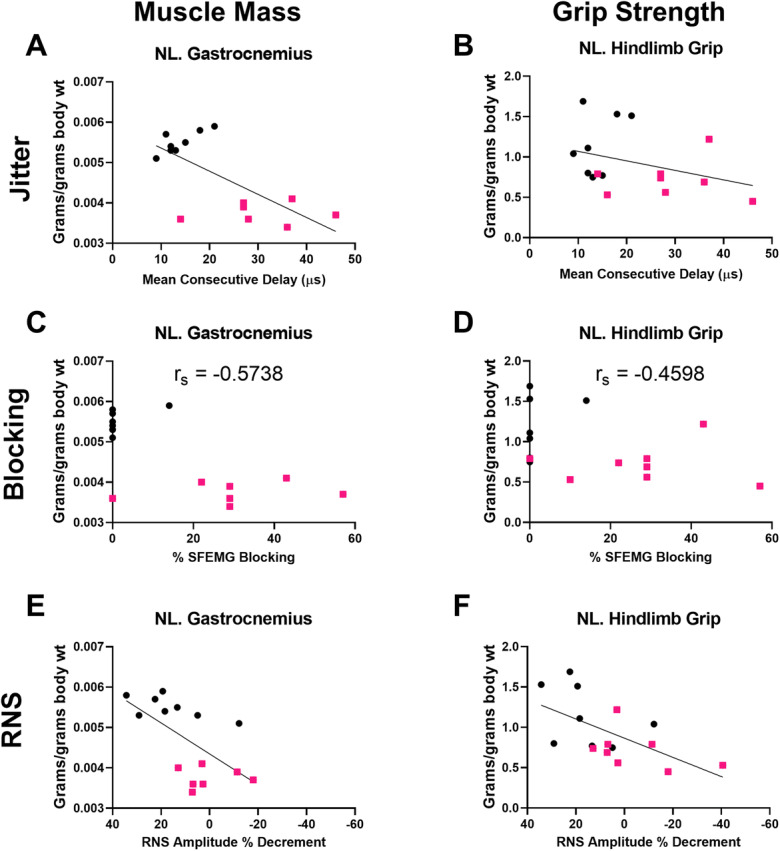
NMJ transmission defects correlate with normalized grip strength. a–b Increased single-fiber electromyography jitter (NMJ variability, mean consecutive delay) was correlated with lower normalized gastrocnemius weight (a Pearson r=−0.6924, p=0.0042) but not normalized grip (b Pearson r=−0.3430, p=0.1934). c–d Similarly, increased single-fiber electromyography blocking (NMJ failure) correlated with lower muscle weight (c Spearman rs=−0.5738, p=0.0278) but not normalized grip (d Spearman rs= −0.4598, p=0.0745). e–f Greater RNS CMAP amplitude decrement (loss) correlated with lower gastrocnemius muscle weight normalized to body weight (e Pearson r=0.6147, p=0.0148) and lower grip strength normalized to body weight (f Pearson r=0.6009, p=0.0138). Muscle weights: young rats, n=8 (3 females, 5 males), and aged rats, n=7 (3 females, 4 males). Grip: young rats, n=8 (3 females, 5 males), and aged rats, n=8 (3 females, 5 males)
Peak contractile torque and torque fade correlate with NMJ transmission defects
Associations between plantarflexion contractile torque (peak and tetanic fade) were analyzed versus jitter and blocking on SFEMG and CMAP amplitude decrement on RNS. Peak plantarflexion torque (125 Hz stimulation, normalized to body mass) did not correlate significantly with SFEMG jitter but was significantly correlated with both SFEMG NMJ blocking and CMAP amplitude decrement on RNS (Fig. 4a–c). In contrast, associations with peak tetanic torque at 125 Hz normalized to gastrocnemius mass did not show significant correlation with mean jitter (Spearman, rs= −0.3363, p=0.2191), NMJ blocking (Spearman, rs= −0.4902, p=0.0656), or CMAP amplitude decrement on RNS (Pearson, r=0.4208, p=0.1183) (data not shown). Tetanic fade at 90 Hz was significantly correlated with SFEMG jitter and blocking and CMAP amplitude decrement on RNS (Fig. 4e–f).
Age-related motor unit defects correlate with NMJ transmission defects
NMJ dysfunction is one of many possible defects in the neuromuscular system that may contribute to age-related decline. We explored other potential pathophysiological mechanisms of muscle decline including loss of muscle excitation, motor unit number, and motor unit size to investigate the relationship to NMJ dysfunction. CMAP responses recorded from the gastrocnemius and forelimb flexor muscles did not significantly differ between aged and young rats; there was, however, a trend for lower amplitude in the hindlimb (Fig. 5a and d). In contrast, MUNE was significantly lower in both muscles in aged versus young rats (22% in the forelimb and 32% in the hindlimb) (Fig. 5c and f). SMUPs also differed significantly between groups in the hindlimb (Fig. 5e), but not the forelimb (Fig. 5b).
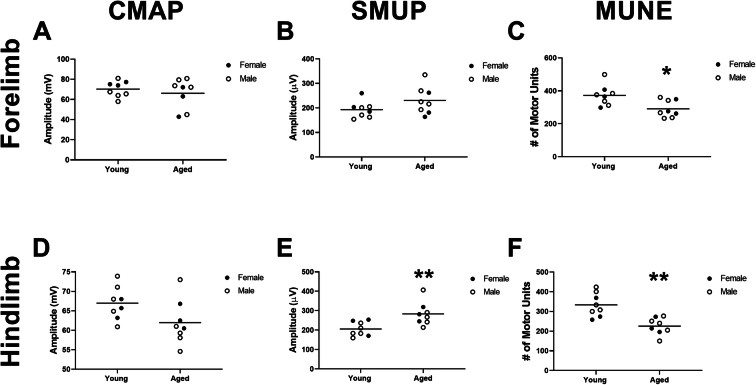
Fig. 5.
Motor unit connectivity is reduced in forelimb and hindlimb muscles of aged rats. In the forelimb, a compound muscle action potential (CMAP) and b single motor unit (SMUP) amplitudes were not significantly altered (p=0.4917 and p=0.1216, respectively), but c motor unit number estimation (MUNE) was significantly lower in aged versus young rats (p=0.0134). In the hindlimb, d CMAP was not significantly altered (p=0.0655), but both e SMUP amplitudes and f MUNE were significantly lower in aged versus young rats (p=0.0074 and p=0.0010, respectively). Young rats, n=8 (3 females, 5 males), and aged rats, n=8 (3 females, 5 males). Unpaired t-test. *p<0.05, **p<0.05
To investigate potential interrelationships between NMJ transmission and motor unit changes, we explored the correlations between SFEMG measures of jitter and blocking and RNS CMAP amplitude decrement versus CMAP amplitude, average motor unit size (SMUP), and motor unit number estimation. SFEMG jitter and blocking showed significant inverse correlations with CMAP amplitude and MUNE but not SMUP (Fig. 6a–f). RNS showed correlation with SMUP and MUNE but not CMAP (Fig. 6g–i).
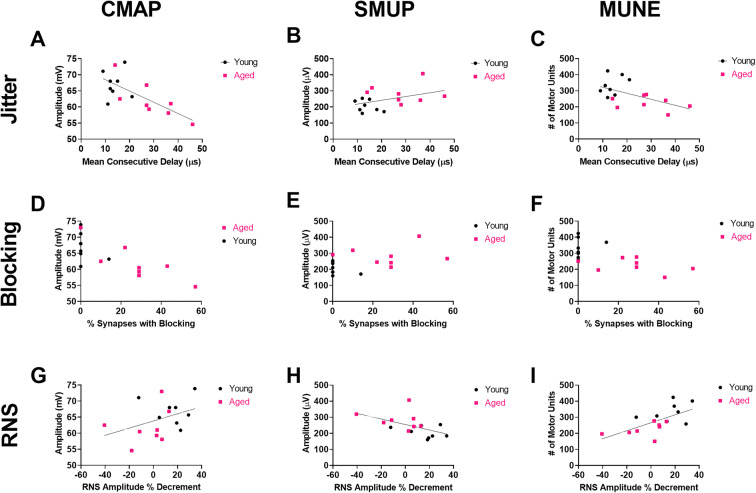
Fig. 6.
NMJ transmission defects correlate with motor unit connectivity (a–c). Jitter showed significant inverse correlation with CMAP amplitude (Pearson r=−0.7022, p=0.0024), nonsignificant direct correlation with SMUP (Pearson r=0.4134, p=0.1114), and significant indirect correlation with MUNE (Pearson r=−0.5507, p=0.0271). d–f Similarly, blocking showed significant inverse correlation with CMAP amplitude (Spearman rs = −0.7760, p=0.0008), nonsignificant direct correlation with SMUP (Spearman rs = 0.4051, p= 0.1200), and significant indirect correlation with MUNE (Spearman rs = −0.6289, p=0.0110). g–i RNS showed no significant correlation with CMAP amplitude (Pearson r=3896, p=0.1357) but significant indirect correlation with SMUP (Pearson r=−0.5241, p=0.0372) and significant direct correlation with MUNE (Pearson r=0.6284, p=0.0091). Young rats, n=8 (3 females, 5 males), and aged rats, n=8 (3 females, 5 males)
Discussion
While loss of muscle mass and strength confer risk for adverse health outcomes in aging populations [54–59], key mechanistic underpinnings and major pathophysiological drivers of these have remained elusive. Here we report novel findings that aging results in NMJ transmission defects in rat hindlimb muscles, specifically the gastrocnemius, and perhaps more importantly, these NMJ functional defects are correlated with grip strength, muscle mass, peak muscle contractile torque production, and tetanic contractile torque fade.
Aged rats demonstrate striking NMJ defects
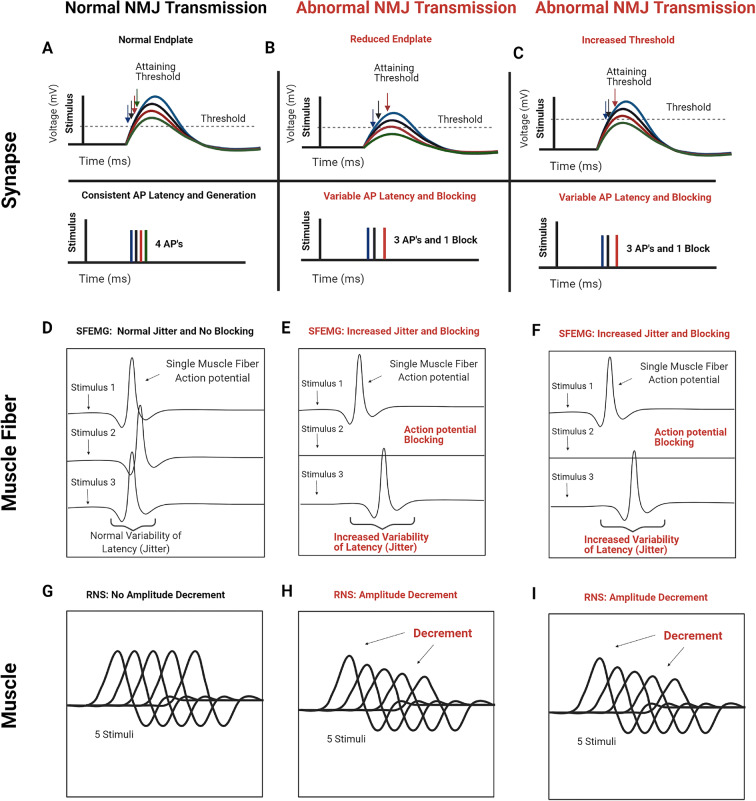
Using different techniques, NMJ transmission can be assessed on the level of an isolated synapse, a single muscle fiber, and a muscle or group of muscles (Fig. 7). In the current study, we used two in vivo methods, SFEMG and RNS, to demonstrate that reliability of NMJ transmission to trigger action potentials is reduced in hindlimb muscles of aged rats. SFEMG and RNS are standard clinical electrophysiological methods that assess NMJ transmission at the level of individual muscle fibers and muscle, respectively [44, 46]. SFEMG assesses two parameters of NMJ reliability, jitter and blocking, and RNS assessed CMAP amplitude change or decrement. In Fig. 7, the physiological underpinnings of increased jitter and blocking on SFEMG and CMAP amplitude decrement on RNS are shown. The safety factor at the NMJ describes sufficiency of the endplate response to reach threshold for action potential generation (Fig. 7a). Loss of the safety factor (i.e., insufficiency of synaptic function to trigger muscle fiber action potential) can be related to reduction of the endplate response (Fig. 7b) as well as altered muscle fiber threshold (Fig. 7c). Interestingly, the majority of the available data support that endplate responses are maintained or even accentuated in aged rodents [37–39, 60]. These prior findings of endplate response maintenance in aged rodents could suggest no loss of NMJ transmission reliability in aged rodents, but this possibility is based on the assumption that muscle fiber threshold for action potential generation is not altered as shown in Fig. 7c.
Fig. 7.
Abnormal NMJ transmission and single-fiber electromyography (SFEMG) and repetitive nerve stimulation (RNS) correlates of synaptic alterations. Synaptic responses: a normal NMJ with normal endplate potential and action potential threshold (dotted line) in the top trace and action potential (AP) responses in the bottom trace. Since the endplate potential is large, the variability in endplate potential amplitude normally present at the NMJ has little effect on the timing of the muscle fiber action potential. b Abnormal NMJ with reduced endplate potential amplitude. Because the endplate potentials are smaller (top trace), their variability in amplitude introduces variability in muscle AP latency and failure (bottom trace). c NMJ with normal synaptic function, but reduced muscle fiber excitability, which causes increased threshold. The increase in muscle fiber action potential threshold causes the same increase in muscle AP latency and failure (bottom trace) as seen with reduced endplate potential amplitude. Single muscle fiber responses: d normal single-fiber electromyography (SFEMG) showing normal jitter and no action potential blocking. e–f Abnormal SFEMG showing increased jitter and blocking. Muscle responses: g normal repetitive nerve stimulation (RNS) showing no compound muscle action potential (CMAP) amplitude decrement. h–i Abnormal RNS showing CMAP amplitude decrement. (Created with BioRender.com)
NMJ transmission failure maps to muscle size, strength, and contractility
We investigated the relationships between NMJ transmission defects and muscle mass, grip strength, and muscle contractile torque production to understand how NMJ defects may be involved in age-related skeletal muscle dysfunction. Perhaps one of the most striking findings we noted was that aged rats not only demonstrated losses of peak torque but also demonstrated rapid fade of torque production Hz (tetanic torque fade) during trains of stimuli at 90 Hz and 150 compared with young rats. These findings are consistent with inability of aged rats to maintain force production during trains of stimuli akin to sustained muscular tasks and power output. Tetanic torque fade was strongly correlated with RNS and SFEMG findings, suggesting that NMJ transmission defects likely contribute to this maintenance of contractility dysfunction. Similar results were reported by Fogarty and colleagues in the diaphragm of aged F344 rats [52]. A limitation of the current study, unlike the study by Fogarty and colleagues, is that muscle contraction following nerve stimulation was not compared to contraction following direct muscle stimulation [52]. Additionally, here we only tested isometric contractions with the hindlimb at a fixed 90° position, and therefore investigating different types of contractions and muscle lengths and positions with indirect and direct stimulation approaches could provide important and novel insights in regard to neuromuscular function deficits in aging.
We found that hindlimb muscle strength was reduced in aged rats and also showed significant associations with measures of NMJ transmission defects, but perhaps as expected, as grip strength only assesses peak strength, these relationships were moderate. Future work could potentially explore these relationships by using more comprehensive assessment that includes measures of muscle power. Importantly, muscle power has been shown to be a strong predictor of physical function in older adults [61–63]. The fact that SFEMG assesses individual muscle fibers rather than summated muscle fiber action potentials (CMAP) makes SFEMG more sensitive as compared with RNS [44, 46, 64]. Yet, SFEMG interrogates only a subpopulation of synapses in a muscle and thus may not provide an accurate summation of functional status of the muscle assessed, and the findings in some instances of stronger correlations with RNS as compared with SFEMG in our studies may be related to this difference between the techniques. An important point worth mentioning regarding SFEMG is that it cannot be performed to selectively record from selected muscle fiber types, and thus the SFEMG recordings will likely be from a variable mix of muscle fiber types. This may be important as older adults have been shown to be predisposed to greater losses of type II fibers [65]. In this study we focused on the gastrocnemius, and in future studies investigating different muscles with differing compositions of muscle fiber types should be considered. It is also important to point out that SFEMG or RNS cannot ascertain the mechanism underlying a defect in neuromuscular transmission (Fig. 7b–c).
In addition to muscle contractile torque and strength, we showed that NMJ defects on SFEMG and RNS also correlated with wet muscle mass. The link between altered NMJ transmission and muscle weight may be related to changes in trophic signaling or possibly improved muscle activation and therefore improved muscle size maintenance integrity [66, 67]. Together, these findings provide preliminary evidence that NMJ transmission loss may be an important contributor to age-related physical function decline due to a link to muscle weakness and wasting.
Other age-related neuromuscular defects and the relationship with NMJ defects
Defining the cellular mechanisms of sarcopenia is necessary to improve technologies for diagnosis and monitoring and to inform the development of effective therapeutic interventions. Here, similar to prior rodent studies, we identified age-related loss of motor unit numbers in the gastrocnemius of aged rats [35, 50, 68–70]. There are various MUNE techniques employed preclinically and clinically with different limitations, but spike-triggered approaches are often considered a standard in aging research [71, 72]. The spike-triggered approaches require voluntary contraction which preclude preclinical application, and therefore here we used a variation of the incremental technique [72]. We have shown this approach to have good test-retest reliability in the hindlimb and forelimb with low mean coefficient of variation (~11%), correlate with retrograde labeled motor neuron counts, and detect motor unit losses in a genetic model of motor neuron degeneration, the G93A SOD1 rat [49]. Similar to a prior study in aged rats, we showed that functional deficits appear more severe in the hindlimb as compared with the forelimb [35]. In contrast to forelimb and hindlimb MUNE, CMAP amplitudes recording from the forelimb and hindlimb were not significantly reduced in aged rats, but the hindlimb CMAP did show a trend toward reduction. These CMAP amplitude findings combined with the findings on SMUP amplitude in the forelimb and hindlimb suggest that age-related motor unit losses were relatively well compensated for by collateral sprouting. This was indicated by a significant 38% increases in SMUP amplitude in the hindlimb and nonsignificant 20% increase in the forelimb. CMAP amplitude reduction during aging is expected. Yet, nonsignificant changes of CMAP amplitude have been observed in prior preclinical studies and clinical studies, and this is possibly related to variability in the sufficiency of collateral sprouting [73, 74].
We were also interested in the relationships between CMAP, SMUP, and MUNE versus SFEMG jitter and blocking and RNS. These correlation analyses showed that rats with the highest CMAP amplitudes and MUNE values and smallest SMUP tended to show the most preservation of NMJ function. Whether NMJ dysfunction during aging is related to secondary process of motor unit loss and ineffective NMJ remodeling versus a primary age-related NMJ defect remains unclear. Recent clinical work has suggested that sarcopenia phenotypes may be related to inadequate NMJ remodeling and transmission instability and have also suggested that exercise may promote improvements in collateral sprouting [75–77]. Full understanding of the interrelationships between motor unit loss, NMJ transmission defects, and muscle function characteristics requires additional study, particularly in larger preclinical and clinical cohorts. Future studies using the in vivo methodologies described here could be performed longitudinally to investigate the onset and progression of motor unit losses (e.g., MUNE reduction), NMJ remodeling (e.g., SMUP increases), and NMJ transmission reliability and to interrogate the impact of interventions on these changes.
NMJ transmission and the possible link to age-related muscle dysfunction across species
In a recent study of aged mice, assessed with SFEMG, we demonstrated NMJ transmission failure in aged mice, but associations with strength were not assessed [40]. Thus, here we sought to identify similar NMJ transmission defects in an aged rat model and determine whether associations with strength were evident. Indeed, we acquired electrophysiological evidence via RNS and SFEMG for NMJ transmission failure in aged rats at an age equivalent to approximately 65–70 years in humans [78]. NMJ transmission failure in limb muscles is therefore a feature of aging conserved between mice and rats. However, the human age equivalent of affected rats in the current study (~65–70 years) is earlier than that of affected mice in our prior study (80+ years) which included ages from across the lifespan of mice; thus, NMJ defects appear to emerge at younger ages in rat [40, 78, 79]. Interestingly, our prior studies investigating SFEMG in aged mice did not consistently show tetanic torque fade (unpublished observation), and SFEMG findings were not significantly correlated with peak muscle contractility torque or wet muscle mass [40]. NMJ defects may therefore play a greater role in age-related muscle dysfunction in rats than in mice. As rats are sometimes considered a closer model to human neuromuscular physiology, this may be a particularly important finding [80–82]. Here we only investigated a single muscle (the gastrocnemius) and two ages of rats, and thus further studies are needed to better understand NMJ transmission defect onset and progression with aging.
Potential translational implications of NMJ defects
Our findings highlight NMJ transmission failure as a candidate mechanism for age-related loss of physical function. Modulation of NMJ transmission could therefore represent a potential method to reduce age-related muscle dysfunction in older adults. SFEMG is routinely used clinically for the diagnosis of NMJ disorders such as myasthenia gravis [46]. There exist some limited clinical SFEMG data, measured during voluntary contractions, that show increased jitter in older adults; blocking has not been described in older adults except in the context of clinical neuromuscular disorders such as motor neuron disease and myasthenia gravis [83]. Importantly, voluntary SFEMG can only interrogate low-threshold motor units, while stimulated SFEMG bypasses this limitation [44, 84]. Aged-related changes in the neuromuscular system of humans disproportionately involve type 2 fibers (type 2>1 atrophy in muscle biopsies of sarcopenic older adults) [85]. Thus, it remains possible, or perhaps likely, that inadequate NMJ transmission to trigger action potential generation could disproportionately affect higher-threshold motor units. We are not aware of any clinical studies that have specifically investigated blocking on stimulated SFEMG in the context of aging and sarcopenia. Some recent evidence using a less selective technique (near-fiber electromyography) supports instability of NMJ transmission in older adults on the basis of increased jitter, which would be akin to blocking on SFEMG [76, 86–89]. One caveat of this technique is that it does not specifically isolate individual muscle fiber potentials to distinguish failure. Future studies leveraging the SFEMG approach to interrogate NMJ failure in older adults with sarcopenia are needed.
Summary
This study provides support that age-related loss of muscle function involves NMJ transmission failure. Such defects could be variable between species, and similarly, individual patients may exhibit variable drivers of sarcopenia phenotypes. Clinical studies will be important to delineate the role of NMJ transmission failure in older adults.
Abbreviations
- CMAP
Compound muscle action potential
- MUNE
Motor unit number estimation
- NMJ
Neuromuscular junction
- RNS
Repetitive nerve stimulation
- SFAP
Single-fiber action potential
- SFEMG
Single-fiber electromyography
- SMUP
Single motor unit potential
Author contribution
Study Design: CP, MH, HH, SR, BC, and WA. Data acquisition: CP, MH, HH, WA. Data analyses and interpretation: CP, MH, JS, SR, MR, BC, WA. Draft of the manuscript: CP, BC, WA. Editing of manuscript: CP, MH, HH, JS, SR, MR, BC, WA. Acquisition of funding: JS, MR, BC, WA.
Funding
WDA was funded by the National Institutes of Health (NIH) R56AG055795 (NIA). BCC was funded by NIH R01AG044424 (NIA). JMS is supported by the National Institute of Disability, Independent Living and Rehabilitation Research Grant 90SI5020, NIH R01 NS118200-01 (NINDS), the European Union Era Net – Neuron Program, SILENCE Grant 01EW170A, the Craig H Neilsen Foundation Grant 596764, the Wings for Life Spinal Cord Research Foundation, and the William E. Hunt and Charlotte M. Curtis endowment. JMS is also a Discovery Theme Initiative Scholar (Chronic Brain Injury) of The Ohio State University. MRR was funded by NIH R01 AR074985
Data availability
Data is available upon request from the corresponding author.
Declarations
Ethics approval
All procedures were approved and performed in accordance with the Institutional Animal Care and Use Committee of The Ohio State University.
Competing interests
SBR has equity in, and serves a consultant and scientific advisor to, Myolex Inc., a company that designs impedance devices for clinical and research use; he is also a member of the company’s Board of Directors. The company also has an option to license patented impedance technology for which SBR is named as an inventor. In the past 3 years, BCC and WDA have received research funding from NMD Pharma for aging muscle research; BCC has received funding from Astellas Pharma Global Development, Inc. for aging muscle research; and BCC has served as a consultant to Regeneron Pharmaceuticals for topics pertinent to aging muscle research.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/13/2021
A Correction to this paper has been published: 10.1007/s11357-021-00464-5
References
- 1.Hall KS, Cohen HJ, Pieper CF, Fillenbaum GG, Kraus WE, Huffman KM, Cornish MA, Shiloh A, Flynn C, Sloane R, Newby LK, Morey MC. Physical performance across the adult life span: correlates with Age and physical activity. J Gerontol A Biol Sci Med Sci. 2017;72(4):572–578. doi: 10.1093/gerona/glw120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. Jama. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 3.Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58(11):2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 4.Robinson S, Granic A, Sayer AA. Nutrition and muscle strength, as the key component of sarcopenia: an overview of current evidence. Nutrients. 2019;11(12). 10.3390/nu11122942. [DOI] [PMC free article] [PubMed]
- 5.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601. doi: 10.1093/ageing/afz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 7.Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med. 2011;27(3):387–399. doi: 10.1016/j.cger.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57(10):B359–B365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 9.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63(8):829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 10.Park KH. Mechanisms of muscle denervation in aging: insights from a mouse model of amyotrophic lateral sclerosis. Aging Dis. 2015;6(5):380–389. doi: 10.14336/ad.2015.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg IH. Sarcopenia: origins and clinical relevance. Clin Geriatr Med. 2011;27(3):337–339. doi: 10.1016/j.cger.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Correa-de-Araujo R, Hadley E. Skeletal muscle function deficit: a new terminology to embrace the evolving concepts of sarcopenia and age-related muscle dysfunction. J Gerontol A Biol Sci Med Sci. 2014;69(5):591–594. doi: 10.1093/gerona/glt208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhillon RJ, Hasni S. Pathogenesis and management of sarcopenia. Clin Geriatr Med. 2017;33(1):17–26. doi: 10.1016/j.cger.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold WD, Padilla Colón CJ. Maintaining muscle function across the lifespan: the state of science. Am J Phys Med Rehab. 2020;Publish Ahead of Print. [DOI] [PMC free article] [PubMed]
- 15.Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, Magaziner JM, Newman AB, Kiel DP, Cooper C, Guralnik JM, Cauley JA, Arai H, Clark BC, Landi F, Schaap LA, Pereira SL, Rooks D, Woo J, Woodhouse LJ, Binder E, Brown T, Shardell M, Xue QL, DʼAgostino RB, Sr, Orwig D, Gorsicki G, Correa-de-Araujo R, Cawthon PM. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriatr Soc. 2020;68:1410–1418. doi: 10.1111/jgs.16372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13(3):271–276. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisleder N, Brotto M, Komazaki S, Pan Z, Zhao X, Nosek T, Parness J, Takeshima H, Ma J. Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release. J Cell Biol. 2006;174(5):639–645. doi: 10.1083/jcb.200604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seene T, Kaasik P. Muscle weakness in the elderly: role of sarcopenia, dynapenia, and possibilities for rehabilitation. Eur Rev Aging Phys Act. 2012;9(2):109–117. doi: 10.1007/s11556-012-0102-8. [DOI] [Google Scholar]
- 19.Taetzsch T, Valdez G. NMJ maintenance and repair in aging. Curr Opin Physiol. 2018;4:57–64. doi: 10.1016/j.cophys.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon YN, Yoon SS. Sarcopenia: neurological point of view. J Bone Metab. 2017;24(2):83–89. doi: 10.11005/jbm.2017.24.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol. 2016;594(8):1965–1978. doi: 10.1113/JP270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badawi Y, Nishimune H. Impairment mechanisms and intervention approaches for aged human neuromuscular junctions. Front Mol Neurosci. 2020;13:568426. doi: 10.3389/fnmol.2020.568426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castets P, Ham DJ, Rüegg MA. The TOR pathway at the neuromuscular junction: more than a metabolic player? Front Mol Neurosci. 2020;13:162. doi: 10.3389/fnmol.2020.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepore E, Casola I, Dobrowolny G, Musarò A. Neuromuscular Junction as an Entity of Nerve-Muscle Communication. Cells. 2019;8(8). 10.3390/cells8080906. [DOI] [PMC free article] [PubMed]
- 25.Khosa S, Trikamji B, Khosa GS, Khanli HM, Mishra SK. An overview of neuromuscular junction aging findings in human and animal studies. Curr Aging Sci. 2019;12(1):28–34. doi: 10.2174/1874609812666190603165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Xiong WC, Mei L. Neuromuscular junction formation, aging, and disorders. Annu Rev Physiol. 2018;80:159–188. doi: 10.1146/annurev-physiol-022516-034255. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci. 2014;6:208. doi: 10.3389/fnagi.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudolf R, Khan MM, Labeit S, Deschenes MR. Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci. 2014;6:99. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deschenes MR. Motor unit and neuromuscular junction remodeling with aging. Curr Aging Sci. 2011;4(3):209–220. doi: 10.2174/1874609811104030209. [DOI] [PubMed] [Google Scholar]
- 30.Willadt S, Nash M, Slater C. Age-related changes in the structure and function of mammalian neuromuscular junctions. Ann N Y Acad Sci. 2018;1412(1):41–53. doi: 10.1111/nyas.13521. [DOI] [PubMed] [Google Scholar]
- 31.Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS One. 2011;6(12):e28090. doi: 10.1371/journal.pone.0028090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS One. 2012;7(4):e34640. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valdez G, Tapia JC, Kang H, Clemenson GD, Jr, Gage FH, Lichtman JW, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A. 2010;107(33):14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deschenes MR. Adaptations of the neuromuscular junction to exercise training. Curr Opin Physiol. 2019;10:10–16. doi: 10.1016/j.cophys.2019.02.004. [DOI] [Google Scholar]
- 35.Pannérec A, Springer M, Migliavacca E, Ireland A, Piasecki M, Karaz S et al. A robust neuromuscular system protects rat and human skeletal muscle from sarcopenia. Aging (Albany NY). 2016;8(4):712-29. 10.18632/aging.100926. [DOI] [PMC free article] [PubMed]
- 36.Gutmann E, Hanzlíková V. Age changes of motor endplates in muscle fibres of the rat. Gerontology. 1965;11(1-2):12–24. doi: 10.1159/000211462. [DOI] [Google Scholar]
- 37.Willadt S, Nash M, Slater CR. Age-related fragmentation of the motor endplate is not associated with impaired neuromuscular transmission in the mouse diaphragm. Sci Rep. 2016;6:24849. doi: 10.1038/srep24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banker BQ, Kelly SS, Robbins N. Neuromuscular transmission and correlative morphology in young and old mice. J Physiol. 1983;339:355–377. doi: 10.1113/jphysiol.1983.sp014721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fahim MA. Morphological correlates of physiological responses in partially denervated mouse muscle during aging. Int J Dev Neurosci. 1993;11(3):303–310. doi: 10.1016/0736-5748(93)90002-U. [DOI] [PubMed] [Google Scholar]
- 40.Chugh D, Iyer CC, Wang XY, Bobbili P, Rich MM, Arnold WD. Neuromuscular junction transmission failure is a late phenotype in aging mice. Neurobiol Aging. 2020;86:182–190. doi: 10.1016/j.neurobiolaging.2019.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metzger S, Dupont C, Voss AA, Rich MM. Central role of subthreshold currents in myotonia. Ann Neurol. 2020;87(2):175–183. doi: 10.1002/ana.25646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chugh D, Iyer CC, Bobbili P, Blatnik III AJ, Kaspar BK, Meyer K, Burghes A HM, Clark BC, Arnold WD Voluntary wheel running with and without follistatin overexpression improves NMJ transmission but not motor unit loss in late life of C57BL/6J mice. Neurobiol Aging. 2020;(Submitted for review). [DOI] [PMC free article] [PubMed]
- 43.Selvan VA. Single-fiber EMG: a review. Ann Indian Acad Neurol. 2011;14(1):64–67. doi: 10.4103/0972-2327.78058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stalberg E, Trontelj JV. The study of normal and abnormal neuromuscular transmission with single fibre electromyography. J Neurosci Methods. 1997;74(2):145–154. doi: 10.1016/S0165-0270(97)02245-0. [DOI] [PubMed] [Google Scholar]
- 45.Andreollo N, Santos EF, Arajo MR, Lopes L. Rat's age versus human's age: what is the relationship? Arq Bras Cir Dig: ABCD Braz Arch Dig Surg. 2012;25(1):49–51. doi: 10.1590/S0102-67202012000100011. [DOI] [PubMed] [Google Scholar]
- 46.Juel VC. Evaluation of neuromuscular junction disorders in the electromyography laboratory. Neurol Clin. 2012;30(2):621–639. doi: 10.1016/j.ncl.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Chugh D, Iyer CC, Wang X, Bobbili P, Rich MM, Arnold WD. Neuromuscular junction transmission failure is a late phenotype in aging mice. Neurobiol Aging. 2019;86:182–190. doi: 10.1016/j.neurobiolaging.2019.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meekins GD, Carter GT, Emery MJ, Weiss MD. Axonal degeneration in the Trembler-j mouse demonstrated by stimulated single-fiber electromyography. Muscle Nerve. 2007;36(1):81–86. doi: 10.1002/mus.20786. [DOI] [PubMed] [Google Scholar]
- 49.Harrigan ME, Filous AR, Tosolini AP, Morris R, Schwab JM, Arnold WD. Assessing rat forelimb and hindlimb motor unit connectivity as objective and robust biomarkers of spinal motor neuron function. Sci Rep. 2019;9(1):16699. doi: 10.1038/s41598-019-53235-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheth KA, Iyer CC, Wier CG, Crum AE, Bratasz A, Kolb SJ, Clark BC, Burghes AHM, Arnold WD. Muscle strength and size are associated with motor unit connectivity in aged mice. Neurobiol Aging. 2018;67:128–136. doi: 10.1016/j.neurobiolaging.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wier CG, Crum AE, Reynolds AB, Iyer CC, Chugh D, Palettas MS, Heilman PL, Kline DM, Arnold WD, Kolb SJ. Muscle contractility dysfunction precedes loss of motor unit connectivity in SOD1(G93A) mice. Muscle Nerve. 2019;59(2):254–262. doi: 10.1002/mus.26365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fogarty MJ, Porras MAG, Mantilla CB, Sieck GC. Diaphragm neuromuscular transmission failure in aged rats. J Neurophysiol. 2019;122(1):93–104. doi: 10.1152/jn.00061.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howard JF., Jr Electrodiagnosis of disorders of neuromuscular transmission. Phys Med Rehabil Clin N Am. 2013;24(1):169–192. doi: 10.1016/j.pmr.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 54.Manini TM, Visser M, Won-Park S, Patel KV, Strotmeyer ES, Chen H, Goodpaster B, de Rekeneire N, Newman AB, Simonsick EM, Kritchevsky SB, Ryder K, Schwartz AV, Harris TB. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55(3):451–457. doi: 10.1111/j.1532-5415.2007.01087.x. [DOI] [PubMed] [Google Scholar]
- 55.McGrath R, Erlandson KM, Vincent BM, Hackney KJ, Herrmann SD, Clark BC. Decreased handgrip strength is associated with impairments in each autonomous living task for aging adults in the United States. J Frailty Aging. 2019;8(3):141–145. doi: 10.14283/jfa.2018.47. [DOI] [PubMed] [Google Scholar]
- 56.Duchowny K. Do nationally representative cutpoints for clinical muscle weakness predict mortality? Results from 9 years of follow-up in the health and retirement study. J Gerontol A Biol Sci Med Sci. 2019;74(7):1070–1075. doi: 10.1093/gerona/gly169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Jr, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R, Rahman O, Swaminathan S, Iqbal R, Gupta R, Lear SA, Oguz A, Yusoff K, Zatonska K, Chifamba J, Igumbor E, Mohan V, Anjana RM, Gu H, Li W, Yusuf S. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 58.Syddall H, Cooper C, Martin F, Briggs R, Aihie SA. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32(6):650–656. doi: 10.1093/ageing/afg111. [DOI] [PubMed] [Google Scholar]
- 59.Cawthon PM, Manini T, Patel SM, Newman A, Travison T, Kiel DP, Santanasto AJ, Ensrud KE, Xue QL, Shardell M, Duchowny K, Erlandson KM, Pencina KM, Fielding RA, Magaziner J, Kwok T, Karlsson M, Ohlsson C, Mellström D, Hirani V, Ribom E, Correa-de-Araujo R, Bhasin S. Putative cut-points in sarcopenia components and incident adverse health outcomes: an SDOC analysis. J Am Geriatr Soc. 2020;68(7):1429–1437. doi: 10.1111/jgs.16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith DO. Acetylcholine storage, release and leakage at the neuromuscular junction of mature adult and aged rats. J Physiol. 1984;347(1):161–176. doi: 10.1113/jphysiol.1984.sp015059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40(1):4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, Pu CT, Hausdorff JM, Fielding RA, Singh MAF. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol Ser A. 2000;55(4):M192–M1M9. doi: 10.1093/gerona/55.4.M192. [DOI] [PubMed] [Google Scholar]
- 63.Bean JF, Kiely DK, LaRose S, Goldstein R, Frontera WR, Leveille SG. Are changes in leg power responsible for clinically meaningful improvements in mobility in older adults? J Am Geriatr Soc. 2010;58(12):2363–2368. doi: 10.1111/j.1532-5415.2010.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rich MM. The control of neuromuscular transmission in health and disease. Neuroscientist. 2006;12(2):134–142. doi: 10.1177/1073858405281898. [DOI] [PubMed] [Google Scholar]
- 65.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84(2-3):275–294. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 66.Messi ML, Delbono O. Target-derived trophic effect on skeletal muscle innervation in senescent mice. J Neurosci. 2003;23(4):1351–1359. doi: 10.1523/JNEUROSCI.23-04-01351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2(1):21–29. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 68.Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:91-5. [DOI] [PubMed]
- 69.Kung TA, Cederna PS, van der Meulen JH, Urbanchek MG, Kuzon WM, Faulkner JA. Motor unit changes seen with skeletal muscle sarcopenia in oldest Old rats. J Gerontol Ser A Biol Med Sci. 2013;69:657–665. doi: 10.1093/gerona/glt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tavoian D, Arnold WD, Mort SC, de Lacalle S. Sex differences in body composition but not neuromuscular function following long-term, doxycycline-induced reduction in circulating levels of myostatin in mice. PLoS One. 2019;14(11):e0225283. doi: 10.1371/journal.pone.0225283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gooch CL, Doherty TJ, Chan KM, Bromberg MB, Lewis RA, Stashuk DW, Berger MJ, Andary MT, Daube JR. Motor unit number estimation: a technology and literature review. Muscle Nerve. 2014;50(6):884–893. doi: 10.1002/mus.24442. [DOI] [PubMed] [Google Scholar]
- 72.Doherty TJ, Stashuk DW. Decomposition-based quantitative electromyography: methods and initial normative data in five muscles. Muscle Nerve. 2003;28(2):204–211. doi: 10.1002/mus.10427. [DOI] [PubMed] [Google Scholar]
- 73.Chung T, Tian Y, Walston J, Hoke A. Increased single fiber jitter level is associated with reduction in motor function with aging. Am J Phys Med Rehab / Assoc Acad Phys. 2018;97:551–556. doi: 10.1097/PHM.0000000000000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilmore KJ, Morat T, Doherty TJ, Rice CL. Motor unit number estimation and neuromuscular fidelity in 3 stages of sarcopenia. Muscle Nerve. 2017;55(5):676–684. doi: 10.1002/mus.25394. [DOI] [PubMed] [Google Scholar]
- 75.Mosole S, Carraro U, Kern H, Loefler S, Fruhmann H, Vogelauer M, Burggraf S, Mayr W, Krenn M, Paternostro-Sluga T, Hamar D, Cvecka J, Sedliak M, Tirpakova V, Sarabon N, Musarò A, Sandri M, Protasi F, Nori A, Pond A, Zampieri S. Long-term high-level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol. 2014;73(4):284–294. doi: 10.1097/nen.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 76.Piasecki M, Ireland A, Piasecki J, Degens H, Stashuk DW, Swiecicka A et al. Long-term endurance and power training may facilitate motor unit size expansion to compensate for declining motor unit numbers in older Age. Front Physiol. 2019;10(449). 10.3389/fphys.2019.00449. [DOI] [PMC free article] [PubMed]
- 77.Sonjak V, Jacob K, Morais JA, Rivera-Zengotita M, Spendiff S, Spake C, Taivassalo T, Chevalier S, Hepple RT. Fidelity of muscle fibre reinnervation modulates ageing muscle impact in elderly women. J Physiol. 2019;597(19):5009–5023. doi: 10.1113/jp278261. [DOI] [PubMed] [Google Scholar]
- 78.Sengupta P. The laboratory rat: relating its age with human's. Int J Prev Med. 2013;4(6):624–630. [PMC free article] [PubMed] [Google Scholar]
- 79.Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci. 2016;152(Supplement C):244–248. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 80.Ellenbroek B, Youn J. Rodent models in neuroscience research: is it a rat race? Dis Model Mech. 2016;9(10):1079–1087. doi: 10.1242/dmm.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baek K-W, Jung Y-K, Kim J-S, Park JS, Hah Y-S, Kim S-J, et al. Rodent model of muscular atrophy for sarcopenia study. J Bone Metab. 2020;27(2):97–110. doi: 10.11005/jbm.2020.27.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91(4):1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 83.Ad Hoc Committee of The ASIGoSFEMG, Gilchrist JM Single fiber EMG reference values: a collaborative effort. Muscle Nerve. 1992;15(2):151–161. doi: 10.1002/mus.880150205. [DOI] [PubMed] [Google Scholar]
- 84.Sanders DB, Arimura K, Cui L, Ertaş M, Farrugia ME, Gilchrist J, Kouyoumdjian JA, Padua L, Pitt M, Stålberg E. Guidelines for single fiber EMG. Clin Neurophysiol. 2019;130(8):1417–1439. doi: 10.1016/j.clinph.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 85.McPhee JS, Cameron J, Maden-Wilkinson T, Piasecki M, Yap MH, Jones DA, et al. The contributions of fiber atrophy, fiber loss, in situ specific force, and voluntary activation to weakness in sarcopenia. J Gerontol Ser A. 2018;73(10):1287–1294. doi: 10.1093/gerona/gly040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Power GA, Allen MD, Gilmore KJ, Stashuk DW, Doherty TJ, Hepple RT, Taivassalo T, Rice CL. Motor unit number and transmission stability in octogenarian world class athletes: Can age-related deficits be outrun? J Appl Physiol. 2016;121(4):1013–1020. doi: 10.1152/japplphysiol.00149.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piasecki M, Ireland A, Jones DA, McPhee JS. Age-dependent motor unit remodelling in human limb muscles. Biogerontology. 2016;17(3):485–496. doi: 10.1007/s10522-015-9627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piasecki M, Ireland A, Piasecki J, Stashuk DW, Swiecicka A, Rutter MK, Jones DA, McPhee JS. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J Physiol. 2018;596(9):1627–1637. doi: 10.1113/jp275520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piasecki M, Ireland A, Coulson J, Stashuk DW, Hamilton-Wright A, Swiecicka A et al. Motor unit number estimates and neuromuscular transmission in the tibialis anterior of master athletes: evidence that athletic older people are not spared from age-related motor unit remodeling. Physiol Rep. 2016;4(19). 10.14814/phy2.12987. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request from the corresponding author.