Abstract
Viral diseases have recently become a threat to human health and rapidly become a significant cause of mortality with a continually exacerbated unfavorable socio-economic impact. Coronaviruses, including severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome (MERS-CoV), and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), have threatened human life, with immense accompanying morbidity rates; the COVID-19 (caused by SARS-CoV-2) epidemic has become a severe threat to global public health. In addition, the design process of antiviral medications usually takes years before the treatments can be made readily available. Hence, it is necessary to invest scientifically and financially in a technology platform that can then be quickly repurposed on demand to be adequately positioned for this kind of pandemic situation through lessons learned from the previous pandemics. Nanomaterials/nanoformulations provide such platform technologies, and a proper investigation into their basic science and biological interactions would be of great benefit for potential vaccine and therapeutic development. In this respect, intelligent and advanced nano-based technologies provide specific physico-chemical properties, which can help fix the key issues related to the treatments of viral infections. This review aims to provide an overview of the latest research on the effective use of nanomaterials in the treatment of coronaviruses. Also raised are the problems, perspectives of antiviral nanoformulations, and the possibility of using nanomaterials effectively against current pandemic situations.
Keywords: COVID-19, Nanotechnology, Coronaviruses, Immunization, Virus-like particles, Vaccine, Nanoparticles, Antiviral
Graphical abstract
1. Introduction
Viral infections can pose a considerable threat to human health due to their troublesome widespread distribution and their ability to adapt through genetic mutations. The large number of fatalities caused by such infections has also become a huge concern for healthcare systems over the past few decades [1,2]. Despite tremendous scientific advancements, human beings frequently find themselves helpless when faced with new threats resulting from the emergence of new viruses. Viruses are the cause of about one-third of infectious disease deaths. Some old pathogens have reappeared in the past 20 years, and several new infectious pathogens, especially viruses, have emerged, among them, human immunodeficiency virus (HIV) and acute respiratory infections becoming the world's leading causes of mortality [3,4]. The lack of tools to tackle virulent diseases or a slow response to a pandemic outbreak may have significant social and economic effects.
The human respiratory mucosa seems to be the most significant conduit and the primary route for various viruses (e.g., influenza, respiratory syncytial, and parainfluenza viruses) to invade or infect. In the first case, these virulent pathogens first attack the upper respiratory tract and then enter the lower breathing airways, progressing to diseases [5]. They usually reach the host through airborne infections and transmit by direct contact or droplets/aerosols, replicate effectively in the respiratory tract, and often show clinical symptoms, including fever, dyspnea, cough, bronchiolitis and pneumonia [6,7]. The majority of respiratory tract diseases are caused by one of the numerous respiratory viruses, particularly influenza viruses, respiratory syncytial viruses, rhinoviruses, and the severe acute respiratory syndrome that infect the lower respiratory tract. In addition, adenovirus, parainfluenza virus, enterovirus, human bocavirus, and human metapneumovirus are common causes of respiratory viral infection, especially in young children [8,9]. SARS, the most recent infectious disease caused by a novel coronavirus, is typically an acute type of bronchopneumonia [10]. The family Coronaviridae, commonly known as coronaviruses, consists of two subfamilies: Torovirinae and Coronavirinae [11]. Coronavirus and coronavirus-like infections such as avian infectious bronchitis, transmissible gastroenteritis, porcine epidemic diarrhea, and feline infectious peritonitis have been identified in various wild and livestock animals, including swine, poultry, and cats [12]. Coronaviruses in humans include several viruses that cause the common cold and more serious respiratory diseases, including Middle East respiratory syndrome, severe acute respiratory syndrome, and coronavirus disease (COVID-19) [13]. All recent coronavirus epidemics have appeared unexpectedly and rapidly transmitted. Not only can they endanger public wellbeing, but they can also lead to disastrous consequences [14]. The first occurrence of “SARS coronavirus” infection resulted in a fatality rate of ~10%, with more than 8000 reported cases [15]. Ten years later, Middle East respiratory syndrome appeared in a global epidemic in 2012, spread nearly 27 countries/regions with 2494 individuals infected with a 34.4% mortality rate [16]. Many individual cases of new coronavirus infection, COVID-19, as a potentially deadly severe acute respiratory syndrome (SARS-CoV-2), were reported in late 2019 and early 2020. SARS-CoV-2 is notably more likely to appear in the elderly community, especially in people with hypertension, diabetes, cardiovascular, or coronary heart disease. On May 19, 2021, we are witnessing a worldwide spread of the novel corona virus with 163, 869, 893 positive cases, at least more than 3,398,302 deaths, and spread across 222 countries [13]. Various types of candidate vaccines and proposed antiviral drugs/agents are currently being evaluated in clinical trials on their potential effects against SARS-CoV-2 [17].
The design phase of antiviral medications generally takes years before the treatments can be made readily available since many legislative procedures are needed to evaluate vaccines and drugs' safety and effectiveness [18]. In particular, the repurposing of existing medications is actually at the forefront of study from a clinical perspective against COVID-19. The lack of time is one of the critical reasons for the current repurposing of medications and rapid preclinical and clinical testing of COVID-19 vaccine formulations [[19], [20], [21]]. The development, clinical trials, and new drug clearance typically take years to complete. Hence, it is necessary to invest scientifically and financially in the technology platform that can then be quickly repurposed on demand to be adequately positioned for this kind of pandemic situation through lessons learned from the previous pandemics. Nanodevices and nanoformulations provide such platform technologies, and a proper investigation into their basic science and biological interactions would be of great benefit for potential vaccine and therapeutic development [22].
For decades, nanomaterials have been used in various pharmaceutical applications, such as distributing nano-therapeutic molecules by crossing certain barriers (such as epithelial/endothelial, immune, or cell barriers) so that drugs can be targeted and delivered to specific sites without affecting healthy tissues or nano-vaccines targeting certain diseased organs or cell types, and communicate through biomolecules found in the blood or organ tissues [[23], [24], [25]]. In addition, the use of engineered nanomaterials to inactivate viruses or prevent virus binding to host cell surface receptors are some of the notable features of nanoscale antiviral therapies [26,27]. Furthermore, nanocarriers can transmit antigens effectively due to the simplicity of surface functionalization and the ability to carry multi-adjuvanted antigens [28]. Nanomaterials have been used in targeted therapies, namely active and passive targeting. Passive targeting could occur in a targeted nanoparticulate delivery system due to the increased permeability (due to inflammation or malignancy) of a vascular system of a particular site, which can result in the accumulation of more nanotherapeutic agents in the diseased site. Conversely, active targeting includes the incorporation of target ligands (e.g., antibodies, sugars, peptides, proteins, etc.) to steer the nanotherapeutics to a particular receptor, site, or epitope [[29], [30], [31]].
In this perspective review, we have considered the use of nano-based formulation approaches against previously known coronaviruses (middle east respiratory coronavirus, avian infectious bronchitis virus, severe acute respiratory syndrome coronavirus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus and feline coronavirus) and SARS-CoV-2 that are being developed as delivery carriers, including novel nanovaccine transfer platforms and effective nanodrugs to treat viral infection. Undoubtedly, these approaches used for previous epidemics of coronavirus would greatly benefit for potential vaccine and therapeutic development for the current pandemic and prevention of its explosive spread globally.
2. Application of nanomaterial in the treatment of middle east respiratory syndrome coronavirus
Since being first reported in a Saudi Arabic patient in 2012, Middle East respiratory syndrome coronavirus (MERS-CoV) has become a novel cause of severe acute respiratory disease [32]. A total of 2494 laboratory-confirmed cases of MERS were reported worldwide at the end of November 2019, including 858 related fatalities (case-fatality rate: 34.4%), according to a study from the World Health Organisation (WHO); most of these reported incidents from Saudi. MERS-CoV belongs to the order Nidovirales, the Coronaviridae family. It's among the newest zoonotic viruses identified. The Coronaviridae genus is classified into different genera (α, β, ÿ, and ÿ). Each category is broken down into lineages within subgroups. MERS-CoV belongs to the β-coronavirus family C. MERS-CoV is a positive-sense single-stranded RNA virus consisting of a 30-base pair genome that encodes RNA polymerase and four specific coronaviridae structural proteins. Spike (S), the virus' only surface glycoprotein that mediates virus attachment and fusion through the host cognate receptor, dipeptidyl peptidase 4 (DPP4), is the most immunogenic of these proteins [33,34]. The main objective of most current MERS-CoV vaccine research is to produce neutralizing antibodies to this unique spike protein of MERS-CoV, as the spike protein has been the most immunogenic structural protein. While several approaches have been reported to produce the MERS-CoV vaccine, no clinically approved MERS-CoV vaccine is available. Various forms of vaccines, including vector-based vaccines, subunit vaccines, and DNA vaccines, have been studied in previous research. Among these, neutralizing antibodies have been successfully developed and protected against infection by viral vector vaccine or immunization with DNA [12]. However, it is not possible to ignore health issues regarding DNA vaccines, their inadequate induction of neutralizing antibodies, and the possibility for decreased efficiency of viral vector vaccines due to pre-existing viral vector immunity triggered by repeated immunization. Although protein subunit vaccines can provoke the neutralizing antibodies, the induction of cellular immune responses is relatively poor. In addition, subunit vaccines have not been able to induce adequate host immune responses, which resulted in a lack of long-lasting memory to an antigen [35].
Not unsurprisingly, in the fields of vaccine research and manufacturing, emerging approaches that overcome such limits are a high priority. Virus-like particles (VLPs), nanoparticles (NPs), and multimeric peptide assemblies offer attractive platforms for the development of vaccines [36]. Attention should be paid to choosing a stable and reliable NP assembly system to produce NP vaccines that encourage cost-effective production and timely delivery of vaccines. In addition, most approaches consider the thermodynamic stability of the final assembled NPs without proper recognition of the kinetic complexities that govern regular assembly over spontaneous interactions leading to misfolded aggregations [[37], [38], [39]].
VLPs and NPs structurally mimic infectious virions but are nonpathogenic owing to a lack of viral genomes. The recombinant surface antigens of natural virions are arranged in closely organized structures as hollow particles depleted of genetic material. Antigenic epitopes can be seen on the NPs’ multivalent and highly repetitive surface, which contributing to B-cell receptors' crosslinking and the activation of long-term immune responses [[40], [41], [42]]. The regularly formed particles are highly immunogenic and are capable of diagnosing and prophylactic exploitation by imitating the presence of the actual infectious virions [43,44]. Matrix protein assemblies provide a molecular scaffold in the enveloped VLPs, and the lipid membrane encapsulates viral antigens. Various forms of glycoproteins can be incorporated as target antigens in the lipid membrane to produce immunological responses. However, several proteins (surface antigens and matrix proteins) are required in this process, and the enveloped VLPs are structurally incoherent and difficult to characterize. A possible solution is to incorporate the specific antigens to the surface of the self-assembled NPs that serve as the macromolecular scaffold for expressing the antigens of concern instead of the lipid membranes [45,46].
Lin et al. [47] prepared hollow polymeric (poly (lactic-co-glycolic acid)) nanoparticles to deliver subunit antigens (MERS-CoV) and adjuvant (STING agonists) in a virus-like manner to enhance the effectiveness of the vaccine (Fig. 1 A). The water-in-oil-in-water double emulsion method was used to prepare the hollow polymeric nanoparticles.
Fig. 1.
The schematic diagrams for the process of protein corona formation.
The free cyclic di-guanosinemonophosphate (cdGMP-STING agonists) rapidly diffused into the bloodstream and caused unfavorable systemic inflammatory responses. In comparison, NP(cdGMP) will approach the lymphatic system preferentially, minimizing systemic reactogenicity. With a negligible effect on cdGMP encapsulation performance, the surface linker incorporation efficiency was around 70% for NP(cdGMP). Notably, the antibody reaction in mice immunized with nanoparticle vaccine has been substantially enhanced by the amount of receptor binding domain-specific immunoglobulin G2a (RBD-specific IgG2a) antibodies, while free MF59 (immunologic adjuvant) and cdGMP-adjuvanted RBD antigens induced minimal levels of IgG2a. The hollow PLGA nanoparticles have offered tunable encapsulation efficiencies and controlled release of aqueous-soluble STING agonists compared to the low encapsulation efficiencies of PLGA-based nanoformulations for aqueous-soluble compounds in earlier studies [48,49]. This was due to the lower viscosity of the low molecular weight (10,000 Da) polymer dissolved in the organic solvent, relative to the high-molecular-weight polymer. Interfacial surface tensions were decreased in the double emulsion-based PLGA nanoparticles with the low-molecular-weight polymer solution, producing highly stable core-shell structures and high cdGMP solution encapsulation. Furthermore, there were combined advantages of biocompatibility, size consistency, colloidal stability, pH-responsive release, tunable adjuvant loading for the hollow polymeric nanoparticle. In order to modulate immune stimulation and functionalization of nanoparticles, this viromimetic nanoparticle can be further modified for particular vaccination requirements that could apply to the several viral infections that endanger public health.
Viral antigens from human pathogens are likely to misfold into aggregates, which necessitates the chemical restoration of these insoluble aggregates to restore solubility and allow normal antigen assembly. Kim et al. [45] used ferritin as a molecular scaffold to prepare novel bacterial NP of MERS-CoV antigen for self-assembly and prevent spike glycoprotein agglomeration into irregular conformations. Ferritin found in most biological species has 24 similar subunits that spontaneously attach and form nanoparticle complexes with 8 and 12 nm internal and external diameters. RNA molecules are capable of providing new molecular functions like molecular chaperons. In this study, the function of chaperna (chaperone + RNA) was exploited using a bacterial expression system for folding and hybrid ferritin monomer (HFM) assembly into nanoparticles. MERS-CoV spike glycoprotein (S) has been used to prepare MERS-CoV-like NPs. The protein folding efficiency and the development of NPs can be further increased by choosing suitable linkers. In addition, Fe2+ concentrations and salt concentrations have affected the efficiency and uniformity of the regular assembly into NPs of HFM. Furthermore, RNA binding avoided agglomeration into irregular conformations and steered the self-assembly of HFM into stable structure NPs. Immunization results exhibited that receptor-binding domain-SSG (linker)-bacterioferritin (RBD-[SSG]-FR) NPs induce a more robust local immune response compared to RBD. In addition, the antibody responses of IgG, IgG1 (Th1), IgG2a, and IgG2b (Th2) against MF59-adjuvanted antigens were reported to be greater than those of alum-adjuvanted antigens. Cellular immune responses of RBD-FR and RBD-[SSG]-FR immune groups were significantly increased relative to the RBD and FR immune groups.
Many MERS-CoV vaccines have been clinically unapproved due to lower cellular immune response levels and failure to develop long-lasting antigen memory. A heterologous prime-boost immunization technique was developed to address these limitations that combine recombinant adenovirus serotype 5, delivering the MERS-CoV spike protein gene (Ad5/MERS) and MERS spike protein nanoparticles [50]. The purified spike glycoprotein nanoparticles were obtained using combined processes of anion exchange and glucose affinity chromatography. Ad5/MERS was used to trigger the cellular immune response in the first (priming) vaccine, and then MERS spike protein nanoparticles were used to trigger the humoral immune response in the second and third vaccines (first and second booster). By priming with Ad5/MERS and by boosting with MERS spike protein nanoparticles, the cellular immune response can be effectively triggered and circumvent viral vectors' limitations. In addition, this prime-boost immunization demonstrated a balanced activation of T helper cell type 1 and 2 (Th1 and Th2) immune responses since it successfully incorporates the benefits of both a viral vector vaccine and a protein vaccine.
3. Application of nanomaterial in the treatment of infectious bronchitis virus
For the global poultry sector, avian infectious bronchitis (IB) is an emerging contagious respiratory infection with high economic importance. It was first identified by Schalk [51] in the late 1930s in North Dakota as a novel pathogen of acute respiratory disease in poultry and has since been identified in all countries with active poultry industries.
IBV is a coronavirus which only infects chickens of all ages but is severe at younger ages [52]. Infectious bronchitis is mainly a tropism for the respiratory tract's epithelial lining, characterized by respiratory symptoms [53]. IBV infection generally has three clinical symptoms: respiratory disease, reproductive diseases, and nephritis. The form of transmission is an airborne or mechanical transfer between birds, houses, and farms. Airborne transmission is through aerosol and occurs easily between birds kept at a distance of about 1, 5 m.
The pathogenic organism of infectious bronchitis is the infectious bronchitis virus (IBV) of the Nidovirales order, Coronaviridae family, in the sub-family Coronaviridae genus Gammacoronavirus [[54], [55], [56]]. The virus is enveloped and round/pleomorphic in shape. Virions are 120 nm in diameter with 20 nm club-shaped glycoprotein spikes [53]. IBV ′s primary immunogen is the S1 subunit protein, bound to the membrane by interaction with the S2 subunit, which is involved in viral entry and produces epitopes that induce the production of potent neutralizing antibodies as well as hemagglutination inhibiting antibodies [[57], [58], [59]].
Proper IBV vaccination may help in preventing the occurrence of clinical IB and associated poultry airsacculitis. However, in various parts of the world, variants of avian infectious bronchitis are continually emerging and can weaken the immunity provoked by commercial anti-IBV vaccines developed using classical viral strains. Therefore, to monitor the infection caused by new strains of IBV, live attenuated vaccines developed with regional specific variants have also been formulated in several countries. However, outbreaks of indigenous IBV genotype strains continuously occur and affect the poultry ‘s respiratory and urogenital tracts. For example, the Massachusetts serotype live-attenuated vaccine is widely used in Brazil, but the outbreak of the BR-I native strain continues to affect respiratory and genitourinary tracts of chickens. In addition, previous studies have shown that this vaccine against IBV BR-I variant strains offers only partial cross-protection in the experimental condition [[60], [61], [62], [63], [64], [65], [66]]. Moreover, there are several risks involved with adopting live attenuated vaccines, including reversion of virulence, recombination with virulent field strains, and mild tissue injuries that may gradually develop into significant secondary infections [67]. In addition to live attenuated vaccines, inactivated IBV vaccines have also been widely investigated; however, they provoked a weak mucosal immune response. High concentrations of antigens and multiple immunizations are typically needed for inactivated IBV vaccines to induce successful immune responses. Furthermore, local injuries are also detected when using specified administration routes (intramuscular or subcutaneous) [[67], [68], [69]].
New IB vaccine formulations are widely attempted to address the shortcomings described above. Considering the increasing need for improved vaccine technologies against emerging infectious threats, the delivery of antigens and adjuvants through synthetic nanoparticles has demonstrated immense potential for enhancing the vaccine's safety and efficacy. Particulate vaccines have been designed to mimic virus-like features, such as nanoscale morphology, multivalent antigen display, and antigen/adjuvant compartmentalization to facilitate the involvement of immune cells and the processing of antigens. In addition, particles at the nanoscale exhibited improved lymphatic transport than smaller subunit antigens. Displaying multiple antigens on a single nanoscale particle makes it much easier for the immune cells to present antigens more effectively. This excellent immune response potential of virus-like particles has led to synthetic nanomaterials to mimic virus properties to develop vaccines. Due to the high radii of curvature, synthetic nanoparticles usually possess high surface energies that cause biomolecules to adsorb in a process known as protein corona formation. A strong association of nanoparticles/proteins arises naturally as a means of passivating surface energies in protein-rich media, and these particles are contained in a protein layer that determines the particles' interactions with the environment. Due to its importance in biomedical applications, the production of protein corona is increasingly gaining scientific attention (Fig. 1). For example, sVLPs were synthesized by the association of spike glycoprotein on the gold nanoparticles' surfaces exploiting the nanoparticles' high passivate surface energies [70]. The full spike glycoprotein from the avian IB virus was obtained using the Bac-to-Bac baculovirus expression system. The spontaneous association of antigen-particle was obtained by mixing purified spike proteins with gold nanoparticles in 10% sucrose and optimized incubation conditions (Fig. 1B). The size of the nanoparticle increased from 100.6 nm to 139.2 nm with protein corona formation.
Results showed that antigen delivery increased by 6-fold with sVLPs compared to the free protein formulation. This was due to the strong adhesion of protein/particle on the nanoparticle surface and the facilitation of in vivo antigen transport by particle carrier. In addition, sVLPs exhibited noticeably higher antibody (IgG) levels, providing enhanced vaccination potency relative to free protein formulation. The improved immunogenicity was due to the increased antigen delivery to the lymph node and particulate nature inducing the other immune activation mechanisms. The sVLP sample indicated high levels of IFN-g mRNA relative to free protein and the whole inactivated vaccine samples, showing significant enhancement of antigen-specific cellular immunity. Finally, sVLPs immunized animal samples showed steadily decreased viral load due to the lowest relative viral mRNA expression.
Conventional inactivated IBV vaccinations normally induce decreased mucosal immune responses and local protection. In order to overcome these limitations, several nanoparticle-based vaccine delivery systems have been developed. For example, BR-I genotype strain encapsulated chitosan nanoparticle (IBV-CS), inactivated IBV vaccine, was developed using an ionic gelation technique to be administered via the oculo-nasal route to chickens [71]. The ionic gelation technique was used to produce the IBV-CS vaccine. Briefly, the embryo infectious dose obtained in the allantoic fluid was added dropwise in 0.05% chitosan, followed by adding sodium tripolyphosphate in the solution under magnetic stirring and incubation at room temperature. After that, the centrifugation step was followed to precipitate IBV-CS. The encapsulation efficiency was around 85%, and the size of the spherical-shaped nanoparticles was 286 nm. Results indicated that all vaccinated groups with IBV-CS formed a pronounced anti-IBV IgA and IgG antibody development memory at the upper respiratory tract mucosal sites. Also, cell-mediated immunity (CMI) memory responses showed early increases in tracheal IFNγ gene expression relative to non-vaccinated and challenged chickens. In addition, Zhao, et al. [72] has prepared N-2-hydroxypropyl ammonium chloride chitosan (N-2-HACC) and N,O-carboxymethyl chitosan (CMC) as an adjuvant and carrier for IBV vaccine antigens. The water solubility of quaternized chitosan exceeded that of the chitosan. It preserved the original characteristics of chitosan, including excellent bioavailability, low cost, biocompatibility, and the capability to open tight intracellular junctions. The water-soluble derivatives of chitosan, N-2-HACC, and CMC, have been prepared as nanoformulations for vaccine antigen delivery. The IBV antigen encapsulation into the N-2-HACC-CMC nanoparticles was achieved using a polyelectrolyte complex method. According to in vitro release assay, both NDV and IBV showed sustained release from nanoparticles after the initial burst release. Immunization studies revealed that IBV antigen encapsulated N-2-HACC-CMC nanoparticles induced the production of high levels of IgG and IgA antibodies intranasally, enhanced lymphocyte proliferation and higher amounts of interleukin-2 (IL-2), IL-4, and interferon-ÿ (IFN-π) production compared to commercially combined live attenuated vaccine.
4. Application of nanomaterial in the treatment of severe acute respiratory syndrome coronavirus
Severe acute respiratory syndrome (SARS) coronavirus was reported for the first time in 2002, followed by a series of fatal pneumonia cases accompanied by inflammatory cellular infiltration with diffuse alveolar damage [73]. It originated in Guangdong, China, at the end of 2002 and transmitted to other Asian countries and Canada via international air flight routes, resulting in 8450 total cases and 810 deaths in 33 countries and regions of 5 continents [74]. In comparison with most other coronaviruses that cause mild infection, the new SARS-CoV had a high mortality rate [75]. Increased age and comorbid conditions have been the risk factors for severe illness and death [76]. Public safety and socio-economic stability have been seriously impacted by the global SARS epidemic. While this epidemic was eventually brought under control in 2003, several isolated SARS-CoV outbreaks were caused due to accidental releases from laboratories in Taiwan, Singapore, and mainland China. New infections were found at the end of 2003 and early 2004 in people in contact with animals infected with SARS-CoV strains, which were substantially different from those predominant in Guangdong, China, during the 2002–2003 outbreak. Only sporadic cases have been reported since 2003; however, epidemics can recur at any time in the future, either through the virus leaking from laboratory samples or through SARS-CoV isolates arising from SARS-CoV-like viruses in animal hosts [74].
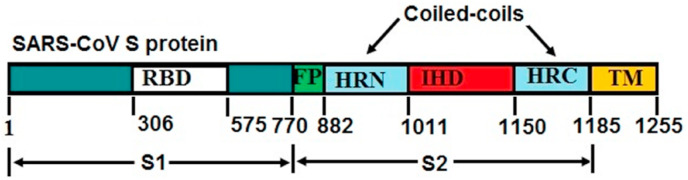
Spike (S) virion surface glycoprotein, the primary inducer of neutralizing antibodies, controls the attachment of SARS-CoV and subsequent entry into target cells [77]. Two subunits constitute the spike protein: S1 and S2. The human angiotensin-converting enzyme 2 of the host-cell receptor is recognized by the receptor-binding domain (RBD) in the S1 subunit. The S2 subunit is accountable for membrane fusion and has a fusion peptide (FP) sequence accompanied by two hydrophobic heptad repeat regions or coiled coils (HRN and HRC) [78] separated by a large interhelical domain or loop, a transmembrane (TM) domain and a cytoplasmic tail (Fig. 2 ). When the spike protein is attached to the host-cell receptor, a structural transition within the S2 heptad repeat regions will cause the FP and TM domains to pass adjacent to each other, thereby promoting the integration of the viral and cell membranes and enabling the nucleocapsid to reach the cell, the HRN and HRC structural transfer is a refolding of their trimeric states [79] to create a six-helix bundle [78], in which three HRN helices are formed in a central parallel, triple-stranded, α‐helical coiled coil, and the outer layer of three anti‐parallel HRC strands are stacked on the outside of the core [80].
Fig. 2.
Schematic illustration of full-length SARS‐CoV S protein.
Various methods have been used in previous efforts to develop a SARS-CoV vaccine, but none of them are currently licensed for use, and several trials have reported negative results [76]. Preliminary studies have shown that the whole inactivated SARS-CoV can be used as successful vaccination [[81], [82], [83]], but further studies have revealed that the extent of immunity provoked by inactivated SARS-CoV is insufficient to eliminate SARS-CoV symptoms while at the same time causing enhanced eosinophilia in vaccinated animals [76,84]. As a consequence, either spike subunits, recombinant viruses or DNA plasmids expressing SARS-CoV proteins, or VLP-based vaccines are the most potential candidates for coronavirus vaccine platforms, although all of these approaches have their own safety issues and approval procedures.
Nanoparticles-based vaccines have been developed to increase the vaccines' efficacy, immunization strategies and targeted delivery of immune response-promoting vaccines. Gold nanoparticle-adjuvanted S protein was prepared by Sekimukai et al. [85] to induce antigen-specific IgG response against SARS-CoV infection. A baculovirus expression system was used to prepare the coronavirus S protein. Gold nanoparticles (AuNPs) were obtained as a commercial gold colloid and coated with Bis(p-sulfonatophenyl)phenylphosphine dihydrate dipotassium salt (BSSP). After that, the BSPP-coated AuNPs were mixed with purified recombinant S protein and incubated for conjugation at room temperature for 1 h. The change of the diameter of AuNPs proved the conjugation of S protein. The levels of antigen-specific IgG in S-protein conjugated AuNP were considerably higher after animal immunization than in the S-protein immunized population. However, after the virus challenge, AuNP-adjuvanted S protein failed to provoke protective immune responses and limit eosinophilic infiltrations. This was due to the structural variations in S protein upon binding to the AuNP.
The drawbacks, such as insufficient protection and failure to prevent SARS-CoV symptoms of inactivated SARS-CoV vaccines, have led to spike subunit-based vaccines to treat SARS-CoV infection. For example, Coleman, et al. [86] showed that the purified coronavirus spike protein nanoparticles promoted the neutralizing coronavirus antibody response in mice. MERS-CoV and SARS-CoV S protein antigens were synthesized in specific recombinant baculovirus-infected Spodoptera frugiperda 9 (Sf9) cells. The S proteins were extracted from cell membranes using a non-ionic detergent, and the centrifugation step was followed to remove the insoluble materials. The combination of anion exchange, affinity, and size exclusion chromatography was used to purify the S proteins oligomers. The detergent's remainder is removed during the purification step, enabling the S trimers to assemble higher-ordered micellular protein-protein nanoparticles. According to the mice vaccination results, the nanoparticles of coronavirus S developed high levels of homologous virus-neutralizing antibodies. In addition, the use of adjuvant (alum) greatly improved responses to SARS-CoV S nanoparticles and also to MERS-CoV S nanoparticles in vaccinated mice. In addition, the production of neutralizing antibody levels was substantially increased by using alum or matrix M1 as adjuvants (15-fold with alum and 68-fold with matrix M1) relative to the neutralizing antibody levels generated due to S nanoparticles alone.
Given that most respiratory disorders arise through the mucosal membrane, it is clear and desirable that the vaccine can activate systemic and mucosal immune responses. Because of its effective transfection and buffering capacity, polyethyleneimine (PEI) is used as a non-viral vector in vitro and in vivo. Mucosal administration with PEI has been shown to act as a potent mucosal immunostimulant. Shim et al. [75] analyzed immune responses of intranasally immunized BALB/c mice (an albino, laboratory-bred strain of the house mouse) with the SARS DNA vaccine (proprotein convertase inhibitor-S/pci-S) in a dynamic pci-S/polyethylenimine nanoparticles complex. PEI/pci-S nanoparticles were synthesized in a solution form at PEI/pci-S ratio of 10. The nanoparticles were found to have a spherical shape of about 200 nm in size. PEI/pci-S complex intranasal immunization showed higher antigen-specific serum IgG responses than pci-S alone. Compared with a previous study (100 μg), their study used a small amount of DNA (20 μg) for immunization [87]. Despite the small amount of DNA (20 μg), it triggered systemic responses and mucosal immune responses. Furthermore, PEI/pci-S complex intranasal immunization also induced excellent cellular defense responses.
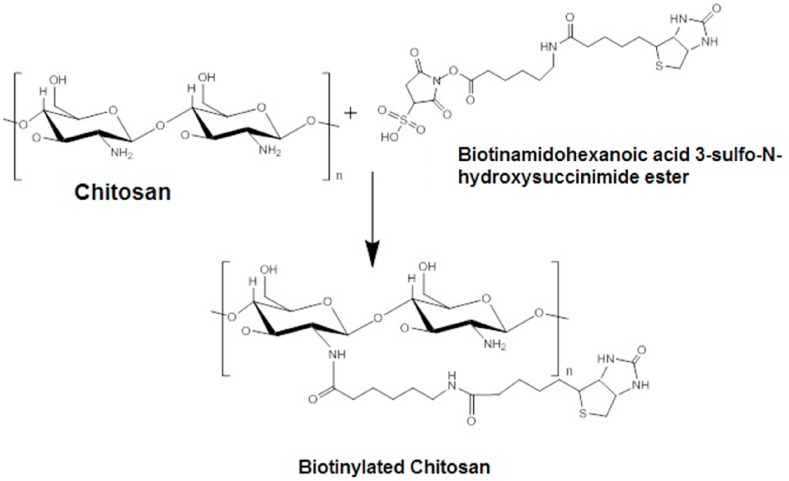
Owing to its outstanding biocompatibility, biodegradability and, non-toxicity, chitosan and its derivatives are excellent nucleic acid distribution carriers. The influence of chitosan's cationic charge density offers a high binding capacity with nucleic acids, enhancing the carrier properties of chitosan for gene delivery. It is expected that chitosan improves the nasal delivery potential in two ways. Firstly, chitosan's positive charge attaches to the negative sialic residues of nasal epithelial mucosa, thereby delaying clearance. Second, chitosan transiently opens tight junctions to facilitate enhanced paracellular transport through the nasal mucosa. Raghuwanshi et al. [88] developed a nasal route vaccine delivery system using dendritic cell (DC) targeting chitosan nanoparticles. To achieve specific DC targeting, the plasmid DNA (PDNA) loaded charged biotinylated chitosan nanoparticles were targeted with a bifunctional fusion protein (bfFp) vector. BfFp is a recombinant fusion protein composed of a single-chain anti-DEC-205 antibody (scFv) with truncated core-streptavidin. Fusion protein binds to biotinylated nanoparticles in its core streptavidin arm. The DC DEC-205 receptor targeting sensitivity is imparted by anti-Dec-205 scFv (Fig. 3 ). Biotinamidohexanoic acid 3-sulfo-N-hydroxysuccinimide ester sodium salt was used to biotinylate the chitosan hydrochloride (Fig. 4 ). The biotinylated chitosan nanoparticles (NPs) loaded with plasmid DNA (pVAXN) have been developed using a modified complex coacervation method.
Fig. 3.
Schematic illustrating the dendritic cell targeting chitosan nanoparticles.
Fig. 4.
Biotinylation of chitosan.
In the presence or absence of DC maturation stimuli, pVAXN as soluble NPs or bfFp targeted NPs were delivered by the intranasal or intramuscular route to BALB/c mice. The serum IgG profile showed that the combination of bfFp-targeted nanoparticle nasal administration and maturation stimuli (anti-CD40 mAb) resulted in increased rates of systemic IgG compared to naked DNA vaccine administered via the intranasal or intramuscular path. Nasal delivery of DC targeted nanoparticles in conjunction with anti-CD40 mAb demonstrated increased mucosal IgA levels in nasal washings compared to pVAXN, NP, and bfFp targeted NP intranasal delivery. Furthermore, for targeted formulations, a relatively increased level of IFN-ÿ was observed relative to the non-targeted formulation.
For humans, the SARS vaccine strategy using full-length S protein may not be the best choice. Therefore, the best approach will be to use small epitopes of S proteins, which are essential determinants of neutralization. The usage of the SARS-CoV S protein receptor-binding domain triggered the most potent neutralizing antibodies and extended defensive immunity. Pimentel et al. [89] prepared the SARS subunit vaccine using a modified version of the peptide nanoparticle. The modifications involved replacing cysteines with alanines and attaching the SARS HRC1 epitope to the C-terminus to be repeatedly shown on the nanoparticles' surface. A significant feature of peptide nanoparticles is that a repeated antigen display system is generated when the monomers self-assemble. Oligonucleotides coding for the SARS HRC1 B-cell epitope were annealed and ligated into a modified peptide transporter (pPEP-T) vector coding for the peptide monomer of the core particle. HRC peptides were obtained by transferring enveloped plasmid into the Escherichia Coli strain expression cells. The peptide nanoparticles consist of self-assembled monomers were obtained by a stepwise dialysis process. The nanoparticles exhibited spherical shape with different sizes ranging from 25 to 30 nm. According to immune responses in mice, the immunization using HRC1 nanoparticles demonstrated concentration-dependent neutralization of SARS-CoV infectivity; however, immunization with nanoparticles alone did not show significant neutralization activity. This system's immunogenic impact is due to the particle's nanometer scale, the repetitive epitope displays, and a strong mimicry of the native conformation.
5. Application of nanomaterial in the treatment of severe acute respiratory syndrome coronavirus 2
A novel coronavirus causing pneumonia was detected in Wuhan, China, at the end of December 2019 and spread rapidly in China and abroad. Later, on March 12, 2020, the WHO declared the outbreak of COVID-19 a pandemic [90]. On 11 February, the International Committee for Virus Taxonomy (ICTV)'s Coronavirus Research Group (CGS) named the virus as SARS-CoV-2 based on the study of the evolutionary past of the current coronavirus, and the pathogen is causing severe acute respiratory syndrome (SARS) [91]. The same day, the World Health Organisation (WHO) announced the “coronavirus outbreak” (COVID-19) caused by SARS-CoV-2. A total of >163 million coronavirus (COVID-19) cases and more than three million deaths were reported worldwide at the time of writing, with the US, India, and Brazil remaining the top three countries with the highest total number of confirmed cases [92]. It has been shown that the mortality from this pandemic varies from 1 to more than 7%. The main concern is the transmissibility of this virus, which contributes to increased infection rates as it spreads at a rate of 0.8–3% through the community, more significant than the average transmission rate of normal flu [93].
The COVID-19 symptoms can be relatively unspecific, and individuals affected may be asymptomatic. Fever is the most common symptom by far, with a percentage of 85.6%, followed by dry cough (68.7%) and fatigue (39.4%). Dyspnea, headache, loss of appetite, panting, sore throat, vomiting, diarrhea, rhinorrhea, and abdominal pain are less common symptoms [94]. The incubation time is estimated to be 5–14 days before the emergence of the disease. The latest studies reveal that certain COVID-19 patients experience back, liver, and eye conjunctivitis and brain injury (encephalitis) [92,[95], [96], [97]]. The SARS-CoV-2 genome has been successfully sequenced, showing that key genes are highly similar to other coronaviruses that cause respiratory illnesses such as SARS-CoV.
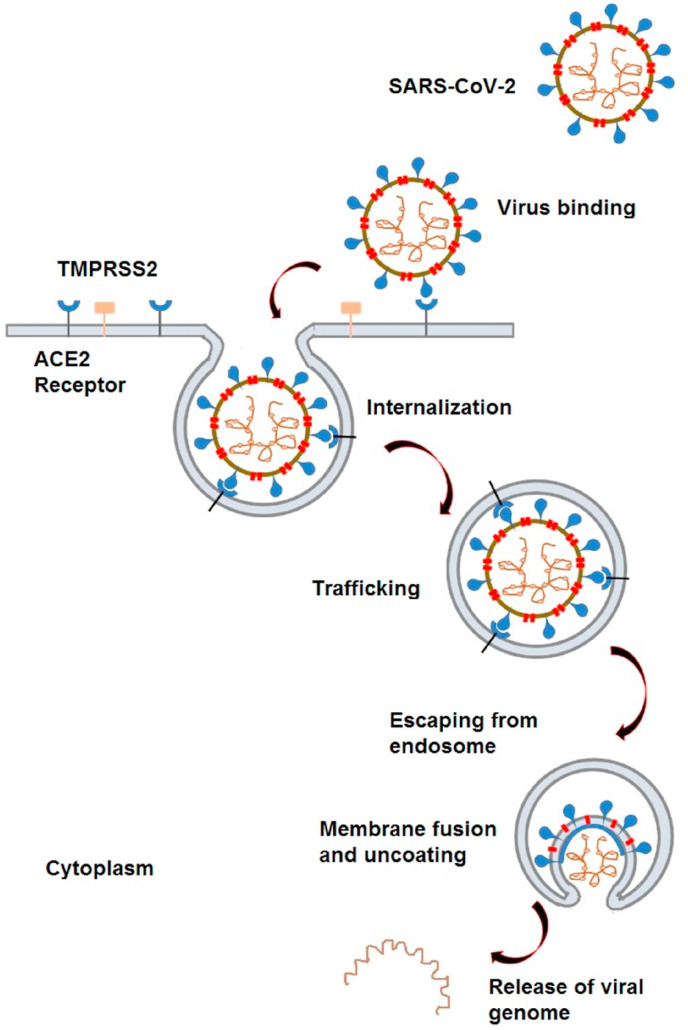
The receptor-binding domain of the envelope-embedded spike (S) 1 protein (C-terminal domain) of SARS-CoV-2 binds angiotensin-converting enzyme 2 (ACE2). The most popular treatment approaches focus on disrupting this critical event since binding the S protein to ACE2 is essential for the first infection stage. SARS-CoV-2 binds to the ACE2 receptors on the surface of host cells. The transmembrane serine protease 2 (TMPRSS2) allows for cellular entry of the virus by protease action. Later, viral particles are internalized into endosomes. After that, viral particles are uncoated due to low endosomal pH, and the virus genome is released for protein synthesis. Finally, viral RNA and protein are synthesized; new infectious particles are assembled and released. The first events of the infection are elucidated at the molecular level in Fig. 5 .
Fig. 5.
Schematic illustrating SARS-CoV-2 entry into cells.
It usually takes years before treatments can be made readily available for the design phase of antiviral medicines as there is a range of regulatory procedures that are needed to assess the safety and effectiveness of vaccines and drugs. Furthermore, when SARS-CoV-2 continues to mutate, highly specific viral targets may change, leading to drug resistance, as observed.
Nanotechnology provides a host of strategies, including new vaccines and medications, to combat viruses in a number of ways. Nanomaterials may also be used for the inhalation of medications in the respiratory system [27]. The respiratory tract (upper airways, lung) is the main target of SARS-CoV-2. Due to the comparatively high abundance of ACE2 and the permissive cell environment, the virus joins alveoli and meets alveolar epithelial type II cells (AECII). These cells serve as sources for the infection and gradually spread across the body, resulting in decreased lung function in extreme situations.
The use of nanoparticles targeting ACE2 receptors or viral S protein may block viral particles' cellular attachment to the alveoli. In order to systemically inactivate viral particles, different approaches, such as the use of neutralizing NPs or photocatalytic nanomaterials, can also be applied. Nanomaterial or immunomodulation-focused vaccines may be used to prevent COVID-19 infection or enhance the immune response. After the genetic sequence of SARS-CoV-2 was published on January 11, 2020, extensive scientific efforts have been made to create a vaccine against SARS-CoV-2. This excellent research mobilization led the first candidate vaccine to initiate the phase I human clinical trial on March 16, 2020 with unprecedented speed, and other innovative candidate vaccines are rapidly following. As of May 13th, 2021, there are 99 COVID-19 candidate vaccines in clinical trials and 184 in preclinical evaluation [98].
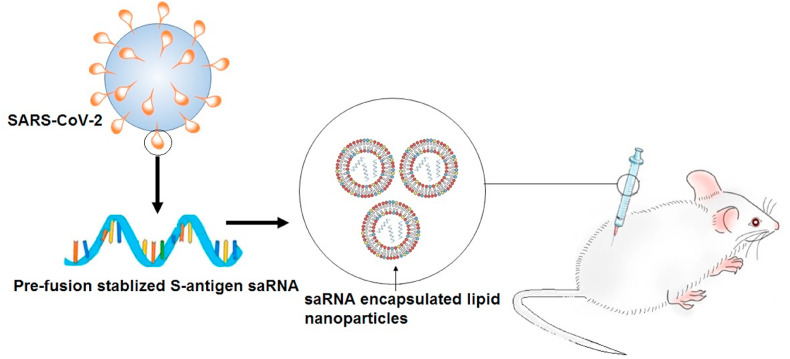
The vaccine's design would be based on either the direct delivery of viral antigens (such as recombinant proteins, vector-based vaccines, or whole inactivated or attenuated viruses) or the RNA or DNA encoding viral antigens. Although the cargo is mostly degraded, not bioavailable, or readily cleared, the drug, protein, or RNA delivery to the patient is associated with many complications. These complications have been resolved by encapsulating drug, protein, or RNA into nanoparticles in recent studies. For example, McKay, et al. [99] compared the immunogenicity of self-amplifying RNA (saRNA) encoding a pre-fusion stabilized (SARS-CoV-2) S protein encapsulated lipid nanoparticle (LNP)s to the body's immune response produced by a COVID-19 recovered patient (Fig. 6 ). A self-assembly method was used to encapsulate SaRNA in LNP. LNP is composed of ionizable lipid, phosphatidylcholine, cholesterol, and PEG-lipid with a mean hydrodynamic diameter of approximately 75 nm.
Fig. 6.
Schematic illustrating the immunization of BALB/c mice with saRNA encoding pre-fusion stabilized S protein encapsulated in lipid nanoparticles.
SaRNA lipid nanoparticle immunization in mice produced exceptionally high SARS-CoV-2 unique IgG antibodies in mice compared to both electroporated pDNA and normal human infections, and it was able to neutralize a pseudotyped virus effectively. In addition, the findings showed enhanced antibody titers, viral neutralization, and cellular response compared to electroporated pDNA, which was attributable to the use of potent LNP in this study.
In another study, Patra [100] suggests conjointly use: (i) an oligonucleotide against the 5′-UUUAAAC-3′heptanucleotide slippery sequence, and (ii) dismantling the pseudoknot that is preventing the pairing of matching sequences/RNA dimerization. The oligonucleotide of the antisense-type 3′-(N)x-AAAUUUG-(N)x-5′ [where N is any one of the A, U, G, C and x = 8 to 10] will block the slippery sequence. Antisense oligonucleotides designed to interfere with pseudoknot formation may stop the translation of the viral RNA by ribosome shifting. These polynucleotides propose to deliver using biodegradable nanoparticles. Ligands such as epidermal growth factor receptor (EGFR)/folate attached on the surface of nanoparticles (oligonucleotides loaded) could be easily transmitted to the affected tissues via ligand-receptor complex formation where cognate receptors are over-expressed, including EGFR/CD44 and subsequent endocytosis.
Furthermore, recent studies suggest using silver nanoparticles to treat COVID-19 early stage via inhalation delivery [101,102]. They suggested a model method and computation for achieving an antiviral minimum inhibitory concentration of silver particles at different respiratory system locations as a first-line approach to prevent infection progression. The dose depends mainly on the silver nanoparticles' size, and 3–7 nm is the optimal size. Efficient antibacterial minimum inhibitory concentration is estimated at 10 μg/ml, but 25 μg/ml is an appropriate target concentration for greater certainty to be obtained in the respiratory system's mucus fluid. The inhalation of aerosol droplets with a standard diameter of 5 μm is suggested, particularly for colloidal silver of 5 nm particles [101]. Similarly, Sarkar [102] proposed the nebulization of 10 nm size water dispersed Ag NPs with bronchodilators using a simple nebulizer machine or bi-level ventilation for corona patients. The antiviral effect of Ag NPs may be attributed to the attachment of Ag NPs to RNA virus surface glycoproteins, preventing the virus from integrating into host cells.
Aydemir and Ulusu [103] stated that angiotensin-converting enzyme 2 (ACE2) had a protective role in COVID-19-associated lung injury caused by infection due to enhanced synthesis of vasodilator angiotensin 1–7. In addition, enhanced catalytic function and enzyme stability were shown when those enzymes were coated on specified nanoparticles. Therefore, using nanomaterials, ACE2-coated/embedded stable quantum dots or nanoflowers can be synthesized with the improved catalytic activity of ACE2 and used to manufacture masks, gloves, chewing gums, nose filters, and garments. These facts propose that such viruses will be captured by utilizing nanotechnology in products such as masks, clothes, chewing gums, gloves, etc., before reaching the host. Allergens and pollens are caught by nose filters before they reach the host. Like nasal filters, ACE2-coated nanoparticle contained chewing gums, clothes, filters, and gloves may trap and hold viruses before they reach the host.
Graphene-based nanomaterials have been exquisitely explored in antiviral therapies owing to their exceptional physical and chemical modes of antimicrobial action on a broad range of pathogenic microorganisms. Graphene can modulate its electrical properties even though a single biomolecule is in contact with its surface, making it an ideal sensor surface. Oxygen in graphene offers an adsorption or functionalization site for proteins, enzymes, or nucleic acids. Graphene oxides (GOs) can specifically target analytes with chemically selective functionalization. In addition, targeted viral proteins can be efficiently identified by the antibody-conjugated GO sheets, and electronic nanomaterial properties can be coupled for signal amplification. Despite the low price of graphene products, it can be helpful not only in point-of-care or large population screening but also for the production of environmental sensors. The high specific surface area of graphene offers the best ligand contact surface for the adsorption of negatively charged sulphates. This, in turn will associate with virions’ positively charged residues and prevent the entry of viruses. After that, the light absorption property of graphene may be applied to destroy the captured viral particles [104]. This graphene material has identical negative load density, which is possibly the key factor impacting the inhibition of the virus [105]. It is reported that in combination with their aromatic plane, GO and reduced GO can adsorb charged lipids and degrade membranes [106].
Graphene derivatives have also been used as antiviral drug delivery platforms. For example, reverse transcriptase inhibitors conjugated with graphene quantum dots and hypericin-GO have been used to treat HIV and reovirus, respectively. Noticeably, hypericin is included as an antiviral agent in the list of computational identified alternative therapies for SARS-CoV-2 [107].
This nanomaterial is also applicable to regulating the disease's epidemiological propagation, apart from therapeutic approaches based on graphene capture. For example, specific influenza A nucleoprotein antibodies were conjugated on the surface of graphene oxide to create a textile-integrated sensor [108]. The detection limit of this sensor was 10 ng/ml and it was able to detect exposure to the virus before the symptoms appeared. Similarly, future research could also involve the creation of SARS-CoV-2 sensors for epidemiological control of virus transmission through protective clothing. GO films are also useful in clothing to create breathable barrier layers. Graphene coatings can be used with silver nitrate and titanium dioxide nanoparticles to capture pathogens, as previously mentioned. It is also reported that protective graphene face masks can be recycled through photocatalysis or heat [104].
It takes time to produce a safe and effective vaccine, but thanks to the unprecedented advancement of scientific research and global cooperation, scientists were able to develop COVID-19 vaccines in a short time while ensuring rigorous, scientifically appropriate and evidence-based regulatory standards. The acceptance of a vaccine abides by a series of well-established international regulations. Vaccines are tested extensively by scientists before being approved by the regulatory health authorities to ensure their efficacy, safety, and effectiveness. Vaccines, along with antibiotics, are the strongest protection against infectious diseases that we have to date; nevertheless, no vaccination is 100% secure or effective for everyone. This is due to the fact that each person's body responds differently to vaccinations. Vaccines must go through the appropriate phases of clinical trials on human subjects before being included in the general public, according to the US Food and Drug Administration (USFDA) and the National Institute of Health (NIH). As more caution and consideration are given to the market product's quality, this mechanism is becoming more complex [109,110].
A standard sequence of steps is followed in the development and testing of vaccines. Laboratory and animal studies are part of the preliminary stages, which are exploratory. Scientists identify natural or synthetic antigens that can help prevent or treat of disease at this time. Virus-like particles, weakened viruses or bacteria, weakened bacterial toxins, or other pathogen-derived compounds may be used as antigens [109].
Before a vaccine can be tested in people, scientists determine the candidate vaccine's safety and immunogenicity or the ability to induce an immune response using tissue culture, cell culture, or animal testing and is known as the pre-clinical stage. These experiments offer researchers an understanding of what cellular responses to expect in humans. They can also suggest a safe starting dosage and method of vaccine administration for the next step of the study. After that, the sponsor, usually a private company, submits an application describing the manufacturing and testing processes, laboratory reports and proposed study for an Investigational New Drug (IND) to the USFDA. Once the IND application has been approved, the candidate vaccine goes through three phases of testing (clinical studies with human subjects) under the oversight of FDA [109].
Phase I is the first attempt to assess the candidate vaccine in humans, emphasizes the vaccine's safety and generally involves 20–100 individuals who haven't been exposed to the disease being studied and are otherwise healthy. The goals of Phase 1 testing are to determine the safety of the vaccine (whether there are adverse reactions with increasing doses) and to learn how effective the vaccination performs in terms of the type and extent of immune response that the vaccine provokes in humans [109].
In the absence of safety issues from phase 1 trials, phase 2 testing involve more patients, with randomized-controlled studies testing multiple dosages on hundreds of participants of usually varying health statuses and across diverse demographic categories. These tests contribute to the safety details on typical short-term side effects and risks, investigate the interaction between the dosage given and the immune response, and include preliminary data on the effectiveness of the vaccine in its ability to provoke an immune response. Immune responses are assessed using standardized and validated procedures. People receiving the candidate vaccine are compared with a controlled group who may receive an FDA-approved vaccine, a placebo or another substance.
After a satisfactory phase III study, the vaccine manufacturer will submit a biologics license application to the FDA. The FDA will then inspect the vaccine's manufacturing facility and approve the vaccine's labelling [109].
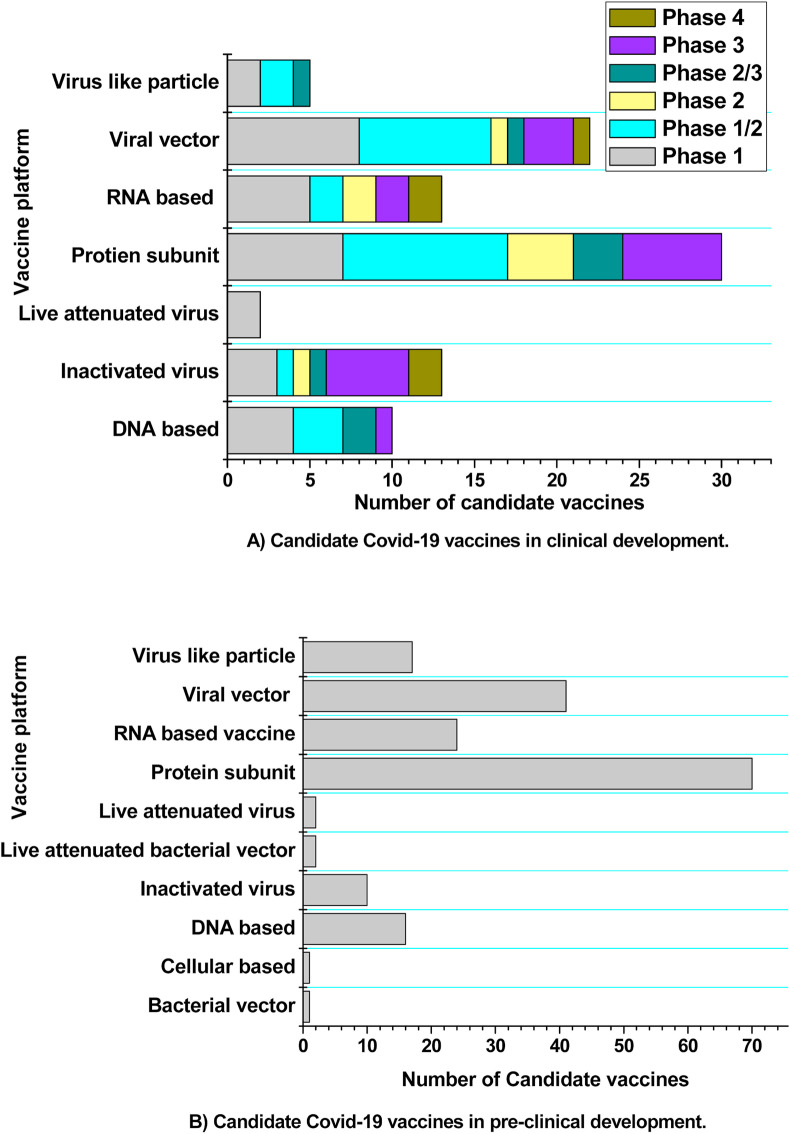
Vaccines are monitored using a variety of systems since they have been licensed. They are phase IV trials, the Vaccine Adverse Event Reporting System (VAERS), and the Vaccine Safety Datalink (VSD). Following the release of a vaccine, drug companies can perform optional phase IV trials. The vaccine's manufacturer may continue to evaluate it for safety, effectiveness, and other potential applications. VAERS is a voluntary reporting system that investigates the adverse effects and tries to determine whether the vaccination caused those effects. VSD compares rates of adverse events in newly vaccinated people to rates of unvaccinated people using real-time statistics [109,111]. Fig. 7 provides the details of COVID-19 vaccine candidates in clinical and pre-clinical development as of May 6, 2021.
Fig. 7.
COVID-19 vaccine candidates in clinical and pre-clinical development.
6. Application of nanomaterial in the treatment of transmissible gastroenteritis virus
Since acute infectious diarrhea poses a significant cause of severe piglet disease and mortality, porcine enteric coronaviruses (CoVs) are also among the main threats to the porcine industry worldwide. Transmissible gastroenteritis virus (TGEV) is one of the five enteric porcine coronaviruses identified to date. The main symptoms are vomiting, watery diarrhea, dehydration, and weight loss. The primary route of transmission of the virus is fecal-oral. Infected creatures are unable to digest food properly and die due to dehydration. The mortality rate can be greater than 90% and is inversely proportional to the pig's age [112]. Not like other porcine enteric CoVs, TGEV has both intestinal and respiratory tropisms, making it a useful model for studying the molecular determinants of coronavirus tissue tropism. TGEV is an RNA virus belonging to the family Coronaviride. The virus replicates in the cytoplasm of mature epithelial cells found at the tips of the small intestinal villi. TGE virions are circular, with an average diameter of 75–160 nm, comprising a single-stranded RNA and a large number of nucleocapsid protein molecules [113]. Traditional vaccines for this infection have several drawbacks, such as up to 99% ballast chemicals, making the vaccine reactogenic, the presence of toxic antimicrobial chemicals (e.g., phenol, formaldehyde), and reduced immunogenicity because empty virus particles seem unable to trigger cellular immunity. In addition, inactivated vaccines are also considered to have low immunogenicity since empty virus particles cannot induce cellular immunity. Different methodologies have been developed to solve these issues, such as vaccination with nanoparticles as antigens and therapeutic agent carriers [114].
For example, Lv, et al. [115] investigated the inhibitory effect of Ag nanoparticles, silver nanowires of 60 nm and 400 nm in diameter, and silver colloids on transmissible gastroenteritis virus (TGEV) induced cell infection in vitro. A mixture of ammonium nitrate, polyoxyethylene, glycerol, trioleate, and tween 20 has been used to stabilize Ag nanoparticles. For the nanowires and the colloids, polyvinylpyrrolidone (PVP) was used as the coating agent. Nanowires with higher coating agents showed less cytotoxicity on TGEV-susceptible porcine cell line (ST cells) than NPs with less coatings. Additionally, relative to NPs, the larger size of nanowires could be less absorbed by ST cells. Results revealed that the Ag nanoparticles and Ag nanowires have considerable anti-TGEV action; however, the Ag colloids do not affect the multiplication of TGEVs. This may be due to the higher PVP coating in the Ag colloids. PVP coatings reduce the free Ag surfaces which responsible for antiviral activity. The significant anti-TGEV action of Ag nanoparticles and Ag nanowires may be due to the direct interaction of Ag NPs and silver nanowires with the TGEV surface protein, including glycoprotein, thereby inhibiting the TGEV infection initiation. Ag nanoparticles and Ag nanowires may modify some TGEV surface proteins' composition and prevent their recognition and binding to the cellular receptor of porcine aminopeptidase.
Gold nanoparticles (GNPs) are nanomaterial with outstanding surface functional chemistry, excellent biocompatibility, stability, and zero toxicity. GNPs have been commonly used in human medicine, veterinary medicine, and biology as carriers of antigens and therapeutic agents. Staroverov et al. [116] studied the immunological response of guinea pigs following administration of GNP-conjugated transmissible gastroenteritis inactivated virus antigen as a nanocarrier. Spherical gold nanospheres were synthesized by reduction of aqueous tetrachloroauric acid under reflux condition (at 100 °C) followed by the addition of 1% aqueous sodium citrate. The average particle diameter was around 15 nm. Antigen–gold nanoparticle conjugates were synthesized by simple mixing. According to the results, the immunization of guinea pigs with TGEV antigen–GNPs induces antigen-presenting cells and increases the splenic lymphoid (antibody-forming) cells' proliferative ability. In addition, γ-IFN, IL-1β, and IL-6 levels were higher in animals immunized with antigen-gold nanoparticle conjugates relative to intact animals or in animals administered with antigen alone, suggesting that the GNP-antigen conjugates induce cellular immunity. Similarly, Mezhenny, et al. [117] investigated the immunogenicity of TGEV antigen conjugated colloidal gold nanoparticles (CG) against TGEV infection. GNPs were prepared using the redox reaction between chloroauric acid and sodium citrate. The diameter of the circular shaped gold nanoparticles was around 15 nm. Results showed that the TGE virus antigen and CG conjugate stimulates higher interferon and cytokine production levels compared to native antigen immunized in guinea pigs. In addition, the animals immunized with CG conjugate showed antibody titer twice as large as the control group animals. According to the colloidal particles' results, they promote the effective expression of viral peptides on cytotoxic T lymphocytes and natural killer cells.
Adjuvants are biomolecules introduced to vaccines or experimental antigens, which may boost the immune response via a variety of mechanisms. The popular clinically relevant vaccine adjuvants are alum, emulsions, liposomes, and silica. Alum is the adjuvant that has been most widely used since the 1920s. However, several drawbacks are associated with these adjuvants, such as local reactions to injection sites, causing IgE-mediated allergy, and the sensitivity of susceptible individuals. Overcoming these limitations, the development of nanoadjuvants has brought a new perspective to vaccine enhancement. For example, silicon nanoparticles have been used as an antigen carrier for promoting the uptake of co-administered antigens because of their immune compatibility, biocompatibility, particle size tunability, morphology, structure, and surface functionality. Jin et al. [118] investigated the immune enhancement of nano silicon adjuvanted TGEV vaccine and compared its results with the alum adjuvant. The purchased nanosilica adjuvant (70 nm) was mixed into the inactivated TGEV vaccine preparation at a ratio of 1:2. The results revealed that, relative to negative controls, the nanoadjuvant provided a significantly higher mucosal immune response in the early immune response. The nano silicon adjuvanted TGEV vaccine significantly enhanced cellular and humoral immunity in mice compared to the alum adjuvant. In addition, nanoparticles have narrow size distribution, greater surface area, and better adhesion that can boost adjuvant action, increase targeted antigen distribution, and significantly minimize side effects. Furthermore, the nano-silicon adjuvanted TGEV vaccine stimulates signaling channels for Toll-like receptors, controls the development and release of multiple inflammatory cytokines, and rapidly triggers the innate and adaptive immune systems to improve the immune response. These findings suggest that nano silicon may be used in the TGEV vaccine as an alternative for alum adjuvant and commercial adjuvant that offers an innovative approach for the development of the vaccine.
7. Application of nanomaterial in the treatment of porcine epidemic diarrhea virus
Porcine epidemic diarrhea virus (PEDV) is an enveloped single-stranded and positive-sense RNA virus of the genus Alphacoronavirus, Coronaviridae family, and Nidovirales order. PEDV induces large-scale diarrhea infections in pigs and a fatality risk of 80–100% in suckling piglets. The virus belongs to the genus α of the coronavirus family, which includes TGEV, bat coronavirus, and human coronavirus. Although both PEDV and TGEV infect pigs, PEDV is genetically more closely correlated with bat coronavirus than with TGEV; this leads to the possibility that PEDV might have originated from bats [119]. Apparently, there is no treatment for PEDV available. The existing techniques for controlling viral infectivity are based on identifying agents capable of interfering in particular steps such as viral entry and replication. For example, it was found that homoharringtonine (HHT), a natural compound inhibits the first step of the eukaryotic translation elongation process. HHT antagonizes the degree of endogenous and exogenous phosphorylation (p-eIF4E), which may control the selective translation of particular mRNA. Furthermore, a previous study reported that HHT had completely inhibited PEDV infection in cell cultures at a concentration of 500 nM [120,121]. Hydroxychloroquine (HCQ), an autophagy inhibitor, has also shown antiviral efficacy against a wide range of viruses. Hydroxychloroquine therapy at 50 μM and HHT treatment at 150 nM has been reported to minimize the virus titer in TCID50 (tissue culture infective dose) by 30 and 3.5 fold, respectively, and the combination has decreased the virus titer in TCID50 by 200 fold [122]. For disease control, live attenuated or killed vaccines have been introduced all around the world. However, all vaccines did not substantially decrease morbidity and virus shedding. Researchers are therefore working on next-generation PEDV vaccines. Among them, nanomaterial-based formulations, nanoparticle-based vaccine candidates, and nanoparticle-based carriers/delivery systems with various benefits have recently been investigated to treat PDEV.
The effects of metallic NPs have been evaluated against PEDV. For example, silver NPs demonstrated inhibition properties against host cell viral entry, as these NPs are able to interact with cell receptors. In a recent study, Du, et al. [123] prepared glutathione-capped Ag2S nanoclusters to prevent replication of porcine epidemic diarrhea virus by disrupting viral RNA synthesis and budding. For the synthesis of Ag2S nanoclusters, sulfur was dissolved in hydrazine hydrate (N2H4·H2O), and a supramolecular hydrogel was then synthesized by adding glutathione and Ag+ in a defined molar ratio under N2 atmosphere. Results demonstrated promising antiviral activity against PEDV infection by 3.0 log reduction of the virus titer at a 12 h post-infection (hpi) noncytotoxic concentration. In addition, the treatment with Ag2S nanoclusters prevents negative-strand RNA synthesis and viral budding. Moreover, the study reported that Ag2S nanoclusters triggered the IFN-stimulating gene development and Vero cell proinflammatory cytokine expression that could inhibit PEDV infection.
Though many metallic nanoparticles have shown excellent antiviral therapies, there are many reported metallic nanoparticle-induced cytotoxicity cases in human cells. Therefore, antiviral therapies focused on non-metallic nanoparticles have gained widespread interest due to the excellent biocompatibility and antiviral properties. For example, Ting, et al. [124] prepared a novel curcumin antiviral cationic carbon dot as a precursor that could enhance curcumin bioavailability and produce a synergistic antiviral effect to treat PEDV. Curcumin carbon dots (CCM-CDs) were prepared by grinding curcumin and citric uniformly, followed by hydrothermal processing. The average size of CCM-CDs was 1.5 ± 0.3 nm. CCM-CDs (125 μg/ml) inhibited virus entry by more than 50%. According to zeta potential studies, positive charge CCM-CDs have been shown to induce viral accumulation by electrostatic interactions, which results in decreased viral infectivity. Raman spectral analysis indicated that the viral protein structure has been altered by the incorporation of CCM-CDs. Furthermore, CCM-CDs showed inhibitory activity on negative-strand RNA synthesis, indicating that at the replication stage, CCM-CDs would effectively inhibit PEDV. CCM-CDs showed innate antiviral immunity by inducing the expressions of ISGs and proinflammatory cytokines. Finally, CCM-CDs could inhibit reactive oxygen species generation (ROS) (ROS can damage DNA by regulating apoptotic signaling pathways) induced by PEDV infection.
Biodegradable and biocompatible PLGA NPs have been used as a vaccine delivery system facilitated by particulate matter. The use of NPs prevents the trapped vaccine from protease degradation. Li et al. [125] tested immune responses from pregnant sows and suckling piglets induced by immunization with an emulsified killed porcine epidemic diarrhea (PEDV) vaccine or PLGA nanoparticle–trapped PEDV-killed vaccine antigens (KAg). PLGA-KAg was prepared using a standard double emulsion solvent evaporation technique. PLGA was dissolved in solvent dichloromethane, in which PEDV antigens were solubilized earlier. The solution was then homogenized and added to the 10% polyvinyl alcohol aqueous solution. After that, the formulation has been stirred, and nanoparticles have been washed and freeze-dried. In pregnant sows immunized with PLGA-KAg, PEDV-specific IgG and IgA specific antibody titers were enhanced in contrast with sows immunized with KAg. The passively immunized suckling piglets of those sows demonstrated similar outcomes. In pregnant sows vaccinated with PLGA-KAg, IFN-γ levels were induced in comparison to those vaccinated with KAg or emulsified killed PEDV, and lymphocyte proliferation responses were enhanced. Notably, the group vaccinated with PLGA-KAg had less piglet mortality than those vaccinated with KAg or emulsified killed PEDV. These findings suggest that PLGA-KAg is an effective and promising vaccine candidate in suckling piglets that can induce a protective immune response against PEDV infection.
8. Application of nanomaterial in the treatment of feline infectious peritonitis virus
Feline infectious peritonitis (FIP) is among the most severe and deadly viral diseases caused by feline coronavirus mutations in cats. Feline coronaviruses are pleomorphic, enveloped, single-stranded positive-sense RNA viruses with a non-segmented genome of approximately 30 kb and 11 putative open-reading frames. Feline coronaviruses exist as two distinct biotypes. They are feline infectious peritonitis virus and feline enteric virus [126]. The feline enteric coronavirus (FECV) biotype is prevalent, usually infecting the gut of cats and is normally disease-free, whereas the feline infectious peritonitis virus (FIPV) biotype is accountable for lethal systemic illness. In comparison, feline coronavirus also occurs as two serotypes: Feline coronavirus type I (FECV type I and FIPV type I) and: Feline coronavirus type II (FECV type II and FIPV type II). Both FIPV and FECV are represented within both feline coronavirus serotypes I and II [127]. Serological and genetic surveys have shown that the Type I feline coronavirus dominates in the world. FIP is one of Felidae's most devastating and deadly virus infections, not because of the high death rate but due to the lack of adequate diagnosis, prevention, and therapeutic options. Antibody-dependent enhancement (ADE) is further complicating the condition, which may worsen disease symptoms. Different vaccinations have been tested to prevent FIP, such as live virulence-attenuated or inactivated FIPV vaccine, but no vaccination has had an adequate impact. Those vaccinations instead enhanced the onset of FIP; in fact, the presence of non-neutralizing antibodies promoted the infection with FIP [128]. DNA vaccinations were unable to induce cell-mediated immunity against the FIPV, and the vaccines coadministered with interleukin-(IL-)12 yielded adverse effects in cats. In addition, the therapeutic efficiencies of different antiviral agents and immunomodulatory medicines against FIPV have been studied in previous studies, but most were to avail. Nevertheless, recent studies have shown that itraconazole, a common antifungal drug, GC376 3C-like protease inhibitor, and nucleoside analogue GS-441524, are effective in inhibiting infection in cats with naturally occurring FIP [129].
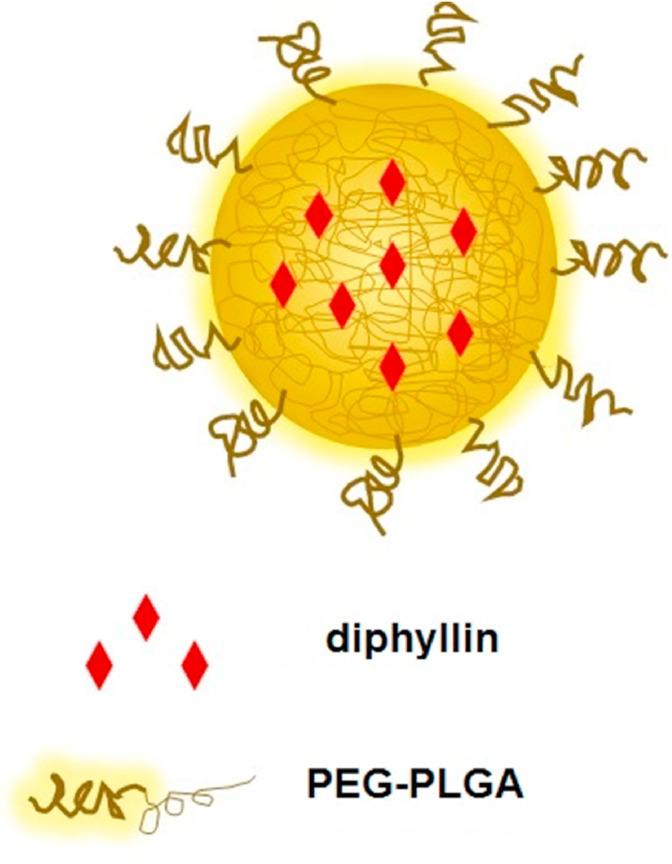
Among various attempts that have been taken to treat FIP, nanomaterials have gained considerable attention as nanodrug carriers or as antiviral agents. For example, Hu, et al. [130] prepared poly (ethylene glycol)-block-poly (lactide-coglycolide) (PEG-PLGA) polymeric nanoparticles to deliver diphyllin (vacuolar ATPase blocker) to treat feline coronavirus infection (Fig. 8 ). The biocompatible PEG-PLGA block-copolymer provides a hydrophobic core for the transport and delivery of hydrophobic diphyllin that offers solutions for compound's insolubility without organic solvents. Furthermore, intracellular ingestion of nanoparticles through the typical endocytosis process may boost the efficacy of diphyllin by promoting colocalization of the compound with an endosomal V-ATPase, thereby decreasing the off-target impact of the product and improving its antiviral activity. Nanoparticles were prepared by dissolving diphyllin in chloroform with PEG-PLGA. After that, the mixture was transferred to deionized water and then probe sonicated. Finally, the chloroform was evaporated by placing the mixture in a fume hood. The average particle diameter of the diphylin-loaded nanoparticle was 36.19 ± 1.15 nm. The nanoparticles have significantly enhanced the protection and effectiveness of diphyllin and have raised the therapeutic index in the investigated infection model by approximately 800. With nanoformulation, diphyllin nanoparticles showed a 13-fold decrease in cellular toxicity. In comparison, diphyllin NPs showed a 60-fold increase in antiviral activity relative to the free drug. In addition, diphyllin NPs were well tolerated after intravenous administration in an animal experiment.
Fig. 8.
Schematic illustrating the diphyllin-encapsulated PEG-PLGA nanoparticles.
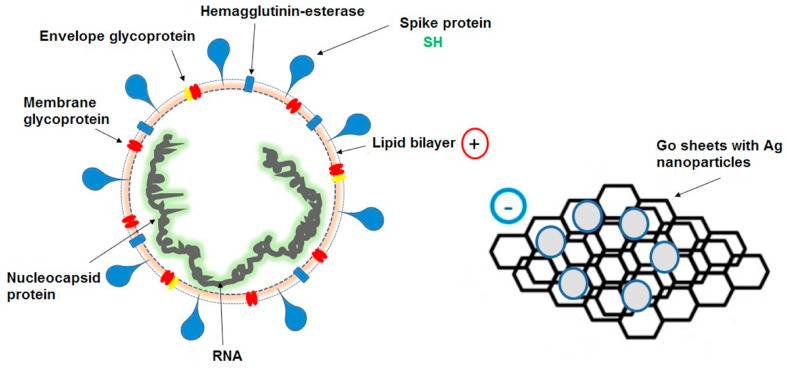
Researchers have discovered various antiviral materials due to the increasing number of virus diseases day by day. Among them, graphene has become a promising antibacterial substance owing to its large surface area, effective carrier mobility, and biocompatibility. In a recent study, Chen, et al. [131] studied the antiviral efficacy of graphene oxide (GO) sheets and GO sheets with silver particles (GO-Ag) against feline coronavirus (FCoV). The GO sheets were synthesized using the Hummers process. GO powders were dispersed in AgNO3 and ethylene glycol. The pulse microwave-assisted synthesis was carried out using microwave until silver seed growth and settled on the GO surface. Finally, the GO-Ag solution was placed in a vacuum oven at 60 °C and dried overnight. Both GO, and GO-Ag sheets displayed concentration-dependent inhibition behavior for FCoV infection in Felis catus whole fetus-4 (fcwf-4) cells. A higher percentage of FCoV infection inhibition could be found with lower FCoV concentration and higher GO and GO-Ag concentration. In comparison, GO-Ag could prevent FCoV infection more effectively than GO. GO sheets alone could prevent the infection at a non-cytotoxic concentration. The specific arrangement of GO sheets can lead to inhibition of FCoV infection by affecting the lipid envelope (Fig. 9 ). The results also indicated that negatively charged GO sheets could interact with positive charge viral lipid membrane and cause disruption of the lipid membrane. The tails of lipid extended from the disrupted lipid bilayer are closely connected to the aromatic ring plane of GO sheets. In addition, the conjugation of viral protein sulfur groups (SH) with silver ions on the graphene oxide wall was also important in the process of the inhibition of feline coronavirus.
Fig. 9.
Schematic illustrating the antiviral action of graphene-silver sheets (GO-Ag) against the enveloped virus.
Ng et al. [129] evaluated the therapeutic effects of synthesized chitosan-based curcumin NPs against FIP using in vivo and in vitro tests. The ionic-gelation method was used to synthesize the curcumin encapsulated chitosan nanoparticles (Cur-CS). The Cur-CS NPs were 330 nm in size, with a positive surface charge of 42.1 ± 3.0 mV. Cur-CS nanoparticles showed reduced cytotoxicity compared to curcumin. The viral inhibitory effects of Cur-CS nanoparticles were more significant than curcumin in FIPV-infected cells. In addition, Cur-CS nanoparticles showed enhanced anti-inflammatory activities and bioavailability properties compared to curcumin administration alone. The viral inhibitory activity of Cur-CS nanoparticle was demonstrated in ascites of cats infected with FIP by the decreased viral titre in macrophages. In addition, combination treatment of prednisolone and Cur-CS nanoparticles showed enhanced anti-inflammatory effects in cats diagnosed with FIP compared to Cur-CS nanoparticles and prednisolone alone.
Types of nanotechnology-based therapeutics used in different types of coronavirus infections, their synthesis methods, the purpose of use, and the outcome are mentioned in Table 1 .
Table 1.
Types of nanotechnology-based therapeutics used in different types of coronavirus infections, their synthesis methods, the purpose of use, and the outcome.
| Infection | Types of nanotechnology-based therapeutics | Process of the synthesis of nanotechnology-based therapeutics | The purpose of use | Results/outcome | References |
|---|---|---|---|---|---|
| Middle East respiratory syndrome | Hollow polymeric (poly (lactic-co-glycolic acid)) nanoparticles (synthetic virus like particle vaccine) | Water-in-oil-in-water double emulsion method | To deliver subunit antigens (MERS-CoV) and adjuvant (STING agonists) in a virus-like manner. | Minimized the systemic reactogenicity, substantially increased the RBD-specific IgG2a antibodies, offered tunable encapsulation efficiencies, offered the controlled release of aqueous-soluble STING agonists. | [47] |
| Middle East respiratory syndrome | Use of ferritin as a molecular scaffold to prepare novel bacterial NP of MERS-CoV antigen (recombinant vaccines) | Spontaneous attachment of ferritin to form MERS-CoV (S) nanoparticle complexes | For self-assembly and to prevent spike glycoprotein agglomeration into irregular conformations. | Induced more robust local immune response, significantly increased the cellular immune responses, increased the antibody (IgG, IgG1 (Th1), IgG2a, and IgG2b (Th2)) responses. | [45] |
| Middle East respiratory syndrome | MERS spike protein nanoparticles (heterologous prime–boost immunization) | Combined processes of anion exchange and glucose affinity chromatography. | To trigger the humoral immune response in the second and third vaccines (first and second booster). | The cellular immune response was effectively triggered, clearly demonstrated a balanced activation of T helper cell type 1 and 2 (Th1 and Th2) immune responses. | [50] |
| Avian infectious bronchitis | Spike glycoprotein on the surfaces of gold nanoparticles (synthetic virus like particle vaccine) | Spontaneous association of glycoprotein from avian IB virus and gold nanoparticles | For the synthesis of virus like particulate vaccine to mimic virus like features | Antigen delivery increased by 6-fold with sVLPs, exhibited noticeably higher antibody (IgG) levels, indicated high levels of IFN-g Mrna, viral load steadily decreased due to the lowest relative viral mRNA expression. | [70] |
| Avian infectious bronchitis | BR-I genotype strain encapsulated chitosan nanoparticle (inactivated IBV vaccine) | Ionic gelation technique | For administration of inactivated IBV vaccine via the oculo-nasal route to chickens. | 85% encapsulation efficiency was obtained, formed a pronounced anti-IBV IgA and IgG antibody development memory at the upper respiratory tract mucosal sites, showed early increases in tracheal IFNγ gene expression. | [71] |
| Avian infectious bronchitis | IBV antigen encapsulated N-2-hydroxypropyl ammonium chloride chitosan and N,O-carboxymethyl chitosan nanoparticles (adjuvanted IBV antigen vaccine) | Polyelectrolyte complex method | To act as an adjuvant and carrier for IBV vaccine antigens | Sustained release of antigen from nanoparticles, induced the production of high levels of IgG and IgA antibodies intranasally, enhanced lymphocyte proliferation, induced the production of higher amounts of interleukin-2 (IL-2), IL-4, and interferon-ÿ (IFN-π). | [72] |
| Severe acute respiratory syndrome coronavirus | Gold nanoparticle-adjuvanted S protein (adjuvanted S protein vaccine) | Gold NPs were mixed with purified recombinant S protein and incubated for conjugation. | To act as an adjuvant for S protein vaccine | Induced the production of high levels of antigen-specific IgG. | [85] |
| Severe acute respiratory syndrome coronavirus | Alum adjuvanted nanoparticles of coronavirus S protein (adjuvanted spike subunit-based vaccines) | S protein antigens synthesized in specific Sf9 cells were extracted from cell membranes using a non-ionic detergent, and the centrifugation. | To promote the neutralizing coronavirus antibody response in mice | Production of neutralizing antibody levels was substantially increased. | [86] |
| Severe acute respiratory syndrome coronavirus | SARS DNA in a dynamic pci-S/polyethylenimine nanoparticles complex (DNA vaccine) | PEI/pci-S nanoparticles were synthesized in a solution form at PEI/pci-S ratio of 10. | To act as a potent mucosal immunostimulant for intranasal immunization | Showed higher antigen-specific serum IgG responses, triggered mucosal immune responses, induced excellent cellular defense responses. | [75] |
| Severe acute respiratory syndrome coronavirus | Dendritic cell targeting chitosan nanoparticles (plasmid DNA vaccine) | Biotinylated chitosan nanoparticles were loaded with plasmid DNA using a modified complex coacervation method. | To developed nasal route vaccine delivery system using dendritic cell targeting | Resulted in increased rates of systemic IgG, demonstrated increased levels of mucosal IgA in nasal washings, observed the increased level of IFN-ÿ. | [88] |
| Severe acute respiratory syndrome coronavirus | Modified version of the peptide nanoparticle (subunit vaccine) | Peptides obtained by Eschericia Coli strain expression cells were processed into stepwise dialysis | To obtain repeated antigen display system | Showed concentration-dependent neutralization of SARS-CoV infectivity. | [89] |
| Severe acute respiratory syndrome coronavirus 2 | SaRNA encapsulated lipid nanoparticle (RNA vaccine) | A self-assembly method was used to encapsulate saRNA in lipid nanoparticle | To encapsulate SaRNA within lipid nanoparticle as a vaccine | Produced exceptionally high SARS-CoV-2 unique IgG antibodies, showed enhanced antibody titers, viral neutralization and cellular response. | [99] |
| Severe acute respiratory syndrome coronavirus 2 | Oligonucleotides loaded biodegradable nanoparticles (proposed vaccine) | Conjointly use: (i) An oligonucleotide against the 5′-UUUAAAC-3′heptanucleotide slippery sequence, and (ii) dismantling the pseudoknot. | To deliver Oligonucleotides | Will stop the translation of the viral RNA by ribosome shifting. | [100] |
| Severe acute respiratory syndrome coronavirus 2 | Silver nanoparticles (Proposed inhalation therapy) | Model method and computation | To achieve an antiviral minimum inhibitory concentration of silver particles at different locations of the respiratory system | The minimum inhibitory concentration is estimated at 10 μg/ml but 25 μg/ml, | [101] |
| Severe acute respiratory syndrome coronavirus 2 | Water dispersed silver nanoparticles (Proposed inhalation therapy) | Water dispersed silver nanoparticles (AgNP) size 10 nm with bronchodilators in lungs through nebuliza-tion with simple nebulizer machine or bi-level ventilation. | For nebulization with bronchodilators | The attachment of Ag NPs to RNA virus surface glycoproteins prevent the virus from integrating into host cells | [102] |
| Transmissible gastroenteritis | Silver nanoparticles, silver nanowires and silver colloids (Inhibitory activity) | For Ag nanoparticles, a mixture of ammonium nitrate, polyoxyethylene, glycerol, trioleate and tween 20 were used for the stabilization, For the nanowires and the colloids polyvinylpyrrolidone was used as the coating agent. | To investigate the inhibitory effect on TGEV | Nanowires with higher coating agents showed less cytotoxicity, Ag nanoparticles and Ag nanowires showed considerable anti-TGEV action, Ag nanoparticles and Ag nanowires prevent the recognition TGEV surface proteins and binding to the cellular receptor of porcine aminopeptidase. | [115] |
| Transmissible gastroenteritis | Gold nanoparticle conjugated inactivated TGEV antigen (inactivated antigen vaccine) | Antigen–gold nanoparticle conjugates were synthesized by simple mixing. | To be used as a nanocarrier for inactivated antigens | Increased the splenic lymphoid (antibody-forming) cells' proliferative ability, Obtained the higher levels of γ-IFN, IL-1β, and IL-6. | [116] |
| Transmissible gastroenteritis | Antigen conjugated colloidal gold nanoparticles (protein-based vaccine) | Gold nanoparticle were prepared using the redox reaction between chloroauric acid and sodium citrate. | To be used as a nanocarrier for TGEV antigen | Stimulated higher levels of interferon and cytokines production, showed higher levels of antibody titer, induced effective expression of viral peptides on cytotoxic T lymphocytes and natural killer cells. | [117] |
| Transmissible gastroenteritis | Nano silicon adjuvanted TGEV vaccine (inactivated antigen vaccine) | Nano silica was mixed into the inactivated TGEV vaccine preparation at a ratio of 1:2. | Nano silicon was used as an adjuvant | Provided significantly higher mucosal immune response, significantly enhanced cellular and humoral immunity, stimulated signaling channels for Toll-like receptors, controlled the development and release of multiple inflammatory cytokines, rapidly triggered the innate and adaptive immune systems. | [118] |
| Porcine epidemic diarrhea | Glutathione-capped Ag2S nanoclusters (antiviral activity) | Sulfur was dissolved in hydrazine hydrate (N2H4·H2O), and a supramolecular hydrogel was then synthesized by adding glutathione and Ag+ in a defined molar ratio under N2 atmosphere | To find antiviral activity | Showed antiviral activity against PEDV infection by 3.0 log reduction, prevented the negative-strand RNA synthesis and viral budding, triggered the IFN-stimulating gene development and Vero cell proinflammatory cytokine expression. | [123] |
| Porcine epidemic diarrhea | Curcumin cationic carbon dot (antiviral activity) | Grinding curcumin and citric uniformly, followed by hydrothermal processing. | To find antiviral activity | Induced viral accumulation by electrostatic interactions, viral protein structure was altered by the incorporation of CCM-CDs, showed inhibitory activity on negative-strand RNA synthesis, showed antiviral innate immunity. | [124] |
| Porcine epidemic diarrhea | PLGA nanoparticle–trapped PEDV-killed vaccine antigens (inactivated vaccine) | Standard double emulsion solvent evaporation technique | To prevent the trapped vaccine from protease degradation | Enhanced the PEDV-specific IgG and IgA specific antibody titers, induced IFN-γ levels, vaccinated groups showed less piglet mortality. | [125] |
| Feline infectious peritonitis | PEG-PLGA polymeric nanoparticles to deliver diphyllin (antiviral activity) | By dissolving diphyllin in chloroform with PEG-PLGA | To act as carrier for antiviral agent | Significantly enhanced the protection and effectiveness of diphyllin, raised the therapeutic index by approximately 800, showed a 13-fold decrease in cellular toxicity, showed a 60-fold increase in antiviral activity, well tolerated after intravenous administration. | [130] |
| Feline infectious peritonitis | Graphene oxide sheets with silver particles (antiviral activity) | The GO sheets were synthesized using Hummers process. GO powders were dispersed in AgNO3 and ethylene glycol. | To enhance antiviral action | Showed concentration-dependent inhibition behavior of FCoV | [131] |
| Feline infectious peritonitis | Chitosan-based curcumin nanoparticles (antiviral activity) | Ionic-gelation method | To enhance antiviral action | Showed reduced cytotoxicity, showed significantly enhanced viral inhibitory effects, showed enhanced anti-inflammatory activities and bioavailability properties. | [129] |
9. Problems and perspective
The application of nanoscience to manage infectious diseases offers innovative approaches to address current obstacles in conventional drug therapy. It provides an excellent opportunity for the improved mode of action of prevailing medicinal drugs. Also, nanoscience offers an excellent platform for developing modern nano-therapeutics and diagnostics, which are urgently required in the era of drug resistance. Nanoparticles/nanocarriers or nanovaccinations can be effectively designed by functionalizing the nanostructures with proper functional groups to incorporate conventional antiviral features into those modifications. Such modifications involve characteristics of advanced nanosystems with increased surface area to volume ratio to ensure large drug payloads, the ability to attach molecular targets to improve the specificity of desirable cell types, tissues or other compartments, improved pharmacokinetic, pharmacodynamics and solubility properties providing greater accumulation, controlled and sustained release, the ability to adjust the surface charge to promote the entrance of cells across the negatively charged cellular membrane [132].
Furthermore, nanoparticles can be designed as non-invasive and sustained delivery vaccine antigen carriers to a particular site. It will facilitate the development of single-dose vaccination formulations. E.g., Intranasal application of the nano vaccine against influenza [133], chitosan nanoparticles incorporated with hemagglutinin protein of H1N1 influenza virus [134], streptococcus equi proteins [135], and surface antigen (pRc/CMV-HBs) of hepatitis B virus [136].
Virus-like particle technology is a very effective vaccine delivery technology that helps to mimic the features of the virus in vaccine applications. Since they do not hold any genetic material, VLPs resemble the conformation of native viruses without being viral. Due to their high curvature radii, synthetic nanoparticles often have high surface energies that facilitate the adsorption of biomolecules, such as protein, in a process called protein corona formation. While the formulation of protein corona is of growing scientific importance due to its implications for biomedical applications, synthetic VLPs formed by protein corona formation allow successful vaccination [70]. Vaccines based on nanoparticles are supposed to boost the vaccine effectiveness, immunization strategies, and targeted delivery to enhance immune responses. Among various types of nanoparticles, gold nanoparticles seem to be the preferred nanomaterial for immunotherapy applications since their physicochemical characteristics prevent the development of antibodies toward platform material. Furthermore, several in vitro and in vivo studies have shown that gold nanoparticles stimulate different immune cells, including macrophages, lymphocytes, and dendritic cells, leading to the development of pro-inflammatory cytokines (i.e., IL‐1β and TNF‐α) and Th1 cytokines (IFN‐ÿ and IL‐2) [85]. In vaccine systems, one of the essential features of the usage of NPs is that they can distribute proteins/peptides to individual tissue cells to assist them in producing an effective immune response. In addition, the use of NPs enhances the uptake of antigen, shields the antigens from premature proteolytic degradation, regulates the antigen release, and makes it safe for human consumption [132].
The ongoing challenge of integrating NPs in biomedical applications is their possible toxicity. Certain materials that are usually considered harmless may become toxic when consumed as nanoparticulate form. For example, titanium dioxide is biologically inert in its naturally occurring mineral form, but it induces inflammatory responses in humans and animals when delivered as NPs smaller than 20 nm in diameter [137]. Likewise, gold is also widely accepted as a secure, inert carrier and is commonly seen in medical implants. However, gold nanoparticles of 1.4 nm in diameter have been shown to penetrate the cells and the nuclear membranes and are irreversibly attached to major DNA grooves, which result in instability. On the other hand, gold NPs with a relatively higher diameter (15 nm) are non-toxic even at a higher concentration of up to 60-fold [138].
Other NP-related toxicity concerns involve accumulation in cells, primarily due to prolonged exposure or long-term usage. For example, fluorescent quantum dots were detected in mice 2 years after injection [139]. In general, the approaches used to detect NP trafficking in cells are mostly based on pharmacological inhibitors or mutant cell lines. The concern with these analyses is that these techniques are not unique to one trafficking mechanism, and therefore findings may also be challenging to interpret or even contradict other literature. For example, while Rejman et al. [140] reported that the treatment of B16 cells with chlorpromazine strongly decreased the absorption of 50 nm size negatively charged polystyrene NPs compared with 200 nm size NPs, Dos Santos et al. [141] showed a contrary finding [140,141]. Therefore, it is ideal to obtain a combination of different inhibitors and mutant cell lines with carefully chosen controls.
Indeed, these nanoparticles play a major role as nanocarriers or nanovaccines in the treatment of viral infections; for certain viruses, such as SARS-CoVs, respiratory syncytial virus, and HIV/AIDS, diagnosis, safe and effective therapeutics are vital need. Today, three pathogenic viruses are present, notably MERS- and SARS-CoVs, and SARS-CoV-2 are the major outbreaks. Attributed to the prevalence of COVID-19, it is also important to discuss several details concerning virulence, pathogenesis, and the actual cause or mode of transmission of viral infections; such recommendations and potential alternatives for further studies, in particular on SARS-CoV-2, are including:
-
•
The influence of metallic NPs (for example, silver, gold, and copper NPs or hybrid NPs) can be assessed against SARS-CoV-2. Recent studies are suggesting the use of silver nanoparticles to treat COVID-19 at an early stage through inhalation. As a first-line treatment to inhibit the progression of the disease, a model and computation method for generating antiviral minimum inhibitory concentrations of silver particles in various respiratory system locations was proposed [101]. Besides the metal nanoparticles, bionanoparticles (e.g., nanocelluloses) carrying synthetic antiviral drugs or herbal antiviral drugs (e.g., curcumin) can also be suggested to treat COVID-19 at an early stage through inhalation.
-
•
Designing a noninvasive nanoparticle vaccine delivery system by targeting vaccine antigens to provide antigen-specific mucosal immunity can be used as an important approach to treat COVID-19. The respiratory tract holds a subset of local dendritic cells, which regularly sample and process innocuous agents. Therefore, dendritic cells of the respiratory tract could act as an ideal target for the absorption of specific NPs delivered along a non-invasive route to treat COVID-19. In previous studies, Raghuwanshi, et al. [88] developed a successful approach for enhancing the immunogenicity of low-dose DNA vaccine through targeted delivery to nasal resident dendritic cells. This vaccine approach provides a new insight for non-invasive ways of targeting vaccine antigens to dendritic cells.
-
•
Though there are many COVID-19 vaccines that have been produced all around the world; still, tens of thousands of people will need to be enrolled to confirm the effectiveness and to look for long-term side effects. Among several approaches to designing vaccines, one concern is that some vaccines can protect against disease but not against infection (the ability of the virus to get into the body). For example, there are many concerns regarding the usage of live attenuated vaccines, including reversion of virulence, recombination with virulent field strains, minor tissue injuries which may develop into severe secondary infections [67]. One of the latest developments in vaccine preparation, like virus-like particles, provides superior immune response capability that has led to synthetic nanomaterials to emulate viral features for vaccine development. The use of bionanoparticles in preparation of VLP provides a more effective approach concerning safety and biocompatibility [47]. In this regard, nanocellulose produced by acid hydrolysis can be suggested for the preparation of VLP in vaccination against COVID 19 as they carry negative surface charges and the available hydroxyl groups provide the potential of enzyme/protein immobilization (protein corona formation) and incorporation of adjuvants on the basis of its chemical conjunction and electrostatic adsorption [[142], [143], [144], [145], [146]].
-
•
The adjuvants are biomolecules introduced to vaccines through a number of pathways to enhance the immune response. Nano-adjuvants provide a new approach for developing vaccines. Owing to the immune-potentiating action, biocompatibility, particle size tunability, composition, shape, and surface functionality, polymeric nanoparticles and silicon nanoparticles have been widely used as adjuvants to facilitate the absorption of co-administered antigen [147]. These findings suggest using polymeric/silicon nanoparticles as adjuvants in the enhancement of the efficacy of COVID-19 vaccines.
-
•
Recently, it has been reported that carbon-based nanomaterials have effective antiviral properties. Graphene oxide derivatives showed antiviral activity by fighting for virus-cell binding. In addition, the surface-operated carbon nanodots could serve as entry inhibitors by interfering with the virus at the early stage of viral infection. Furthermore, carbon dots (CDs) have been shown to inhibit viral replication by positively regulating the antiviral type I interferon signaling. In addition, carbon nanotubes have also been proposed as carriers for antiviral agents in previous literature. According to these findings, carbon-based nanomaterials can be suggested to develop antiviral systems with reduced toxicity against SARS-CoV-2.
10. Conclusion
Today, the battle against coronavirus is becoming highly unpredictable because they have transformed our way of living, with the novel coronavirus pandemic (COVID-19) being a particularly troublesome example. Nano-based formulation approaches such as novel nanovaccine transfer platforms, delivery carriers, and nanodrugs have been developed against previously known coronaviruses. Nano-based approaches have been successfully used to deliver both antigens and adjuvants in a virus-like manner, prevent viral antigen agglomeration, and for prime-boost immunization, improving the immune response against MERS-CoV. It was observed that high surface energies of nanoparticles improved the antigen corona formation, and bionanoparticles enhanced the inactivated viral encapsulation in IBV vaccines. Due to the insufficient protection of inactivated viral vaccines against SARS-CoV, the plasmid DNA loaded nanoparticles, and immunostimulant/nanoparticle complexes have been developed to provoke the mucosal immune response. Furthermore, instead of using full-length S protein, the usage of SARS-CoV protein receptor-binding domains triggered the most promising neutralizing antibodies. There are about 283 candidate vaccines have been developed to provoke the immune response against SARS-CoV 2. Some SARS-CoV 2 vaccines use nanoencapsulation of viral proteins/RNA to prevent degradation of their genetic cargo. Ag nanoparticles with stabilizers have shown anti-TGEV action. Also, Ag2S nanoclusters have been used to prevent PEDV RNA synthesis and budding. In addition, Ag nanoparticles were suggested as inhalable nanotherapeutics against SARS-CoV 2. Furthermore, Au nanoparticles are a good candidate for antigen conjugation due to their stability, biocompatibility and surface functional chemistry. The use of non-metallic nanoparticles such as CCM-CDs and PLGA have shown excellent antiviral therapies against PEDV. CCM-CDs have shown inhibitory activity on negative-strand RNA synthesis. PLGA prevents the trapped vaccine from protease degradation. The development of nanoadjuvants has overcome the limitations of common vaccine adjuvants. The use of nanocarriers for antiviral drug delivery has shown excellent antiviral efficiencies compared to the free drug (e.g., diphyllin loaded PEG-PLGA nanoparticles and Cur-CS nanoparticles against FIPV). Indeed, these nanoparticles play a significant role in nanocarriers or nanovaccines; there are some concerns over the use of nanoparticles in biomedical applications, such as toxicity issues of some forms of nanoparticles and accumulation in cells.
In order to improve the identified existing therapeutic limitations, these nano-based systems have demonstrated versatile features. According to the studies mentioned above, researchers carried out high-level scientific investigations to discover novel and intelligent nano-based materials and matrices to design nanoformulations of antiviral drugs and nanovaccines for viral diseases, especially for the infections caused by human and animal coronaviruses. Various incidents of epidemics and pandemics have led to many causalities in the history of civilization. However, in the meantime, the use of smart and advanced technologies, particularly the field of evolving nanotechnologies, could have a significant influence on handling prevalent and potential outbreaks.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the financial support from the AUN/SEED-Net Special Program for Research Against COVID-19 (SPRAC): IF068-2020 for the success of this project.
References
- 1.Mourya D.T., Yadav P.D., Ullas P., Bhardwaj S.D., Sahay R.R., Chadha M.S., Shete A.M., Jadhav S., Gupta N., Gangakhedkar R.R. Emerging/re-emerging viral diseases & new viruses on the Indian horizon. Indian J. Med. Res. 2019;149:447. doi: 10.4103/ijmr.IJMR_1239_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindahl J.F., Grace D. The consequences of human actions on risks for infectious diseases: a review. Infect. Ecol. Epidemiol. 2015;5 doi: 10.3402/iee.v5.30048. 30048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brachman P.S. Oxford University Press; 2003. Infectious Diseases—Past, Present, and Future. [DOI] [PubMed] [Google Scholar]
- 4.Morens D.M., Folkers G.K., Fauci A.S. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Halifa S., Gauthier L., Arpin D., Bourgault S., Archambault D. Nanoparticle-based vaccines against respiratory viruses. Front. Immunol. 2019;10:22. doi: 10.3389/fimmu.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arruda E., Cintra O.A., Hayden F.G. Tropical Infectious Diseases; 2006. Respiratory Tract Viral Infections; p. 637. [Google Scholar]
- 7.Boncristiani H., Criado M., Arruda E. Encyclopedia of Microbiology; 2009. Respiratory Viruses; p. 500. [Google Scholar]
- 8.Nichols W.G., Campbell A.J.P., Boeckh M. Respiratory viruses other than influenza virus: impact and therapeutic advances. Clin. Microbiol. Rev. 2008;21:274–290. doi: 10.1128/CMR.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proença-Módena J.L., Acrani G.O., Snider G., Arruda E., Gerrant R., Walker D., Weller P. 2011. Respiratory Viruses: Influenza Virus, Respiratory Syncytial Virus, Human Metapneumovirus, Parainfluenza Viruses, Rhinovirus, Respiratory Adenoviruses, Coronaviruses (Unrelated to SARS) and Human Bocavirus, Tropical Infectious Diseasesprinciples, Pathogens & Practice. [Google Scholar]
- 10.Shan C., Yao Y.-F., Yang X.-L., Zhou Y.-W., Gao G., Peng Y., Yang L., Hu X., Xiong J., Jiang R.-D. Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques. Cell Res. 2020;30:670–677. doi: 10.1038/s41422-020-0364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrell C.J., Howard C.R., Murphy F.A. Academic Press; 2016. Fenner and White's Medical Virology. [Google Scholar]
- 12.Domańska-Blicharz K., Woźniakowski G., Konopka B., Niemczuk K., Welz M., Rola J., Socha W., Orłowska A., Antas M., Śmietanka K. Animal coronaviruses in the light of COVID-19. J. Vet. Res. 2020;64:333–345. doi: 10.2478/jvetres-2020-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal M., Berhanu G., Desalegn C., Kandi V. Severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020;12 doi: 10.7759/cureus.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan S., Siddique R., Li H., Ali A., Shereen M.A., Bashir N., Xue M. Impact of coronavirus outbreak on psychological health. J. Glob. Health. 2020;10 doi: 10.7189/jogh.10.010331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng V.C., Lau S.K., Woo P.C., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bchetnia M., Girard C., Duchaine C., Laprise C. The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a review of the current global status. J. Infect. Public Health. 2020;13(11):1601–1610. doi: 10.1016/j.jiph.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J., Xie B., Hashimoto K. Brain Behav. Immun.; 2020. Current Status of Potential Therapeutic Candidates for the COVID-19 Crisis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He H. InTech; 2013. Vaccines and Antiviral Agents, Current Issues in Molecular Virology: Viral Genetics and Biotechnological Applications; pp. 239–250. [Google Scholar]
- 19.Saha R.P., Singh M.K., Samanta S., Bhakta S., Mandal S., Bhattacharya M., Sharma A.R., Lee S.-S., Chakraborty C. Repurposing drugs, ongoing vaccine and new therapeutic development initiatives against COVID-19. Front. Pharmacol. 2020;11:1258. doi: 10.3389/fphar.2020.01258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deb B., Shah H., Goel S. Current global vaccine and drug efforts against COVID-19: pros and cons of bypassing animal trials. J. Biosci. 2020;45:1–10. doi: 10.1007/s12038-020-00053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saxena A. Drug targets for COVID-19 therapeutics: ongoing global efforts. J. Biosci. 2020;45:1–24. doi: 10.1007/s12038-020-00067-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nanotechnology versus coronavirus. Nat. Nanotechnol. 2020;15:617. doi: 10.1038/s41565-020-0757-7. 617. [DOI] [PubMed] [Google Scholar]
- 23.Valavanidis A., Vlachogianni T. Engineered nanomaterials for pharmaceutical and biomedical products new trends, benefits and opportunities. Pharm. Bioprocess. 2016;4:13–24. [Google Scholar]
- 24.Lombardo D., Kiselev M.A., Caccamo M.T. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019:2019. [Google Scholar]
- 25.Navya P., Kaphle A., Srinivas S., Bhargava S.K., Rotello V.M., Daima H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019;6:23. doi: 10.1186/s40580-019-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakravarty M., Vora A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2020:1–40. doi: 10.1007/s13346-020-00818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J.A., Pasquali M., Scott J.A., Vitale F., Unal M.A., Mattevi C. ACS Nano; 2020. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. [DOI] [PubMed] [Google Scholar]
- 28.Mohan T., Verma P., Rao D.N. Novel adjuvants & delivery vehicles for vaccines development: a road ahead. Indian J. Med. Res. 2013;138:779. [PMC free article] [PubMed] [Google Scholar]
- 29.Clemons T.D., Singh R., Sorolla A., Chaudhari N., Hubbard A., Iyer K.S. Distinction between active and passive targeting of nanoparticles dictate their overall therapeutic efficacy. Langmuir. 2018;34:15343–15349. doi: 10.1021/acs.langmuir.8b02946. [DOI] [PubMed] [Google Scholar]
- 30.Bazak R., Houri M., El Achy S., Kamel S., Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J. Cancer Res. Clin. 2015;141:769–784. doi: 10.1007/s00432-014-1767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bazak R., Houri M., El Achy S., Hussein W., Refaat T. Passive targeting of nanoparticles to cancer: a comprehensive review of the literature. Mol. Clin. Oncol. 2014;2:904–908. doi: 10.3892/mco.2014.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 33.Modjarrad K. Treatment strategies for Middle East respiratory syndrome coronavirus. J Virus Erad. 2016;2:1. doi: 10.1016/S2055-6640(20)30696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mubarak A., Alturaiki W., Hemida M.G. Middle east respiratory syndrome coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J. Immunol. Res. 2019:2019. doi: 10.1155/2019/6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naz R.K., Dabir P. Peptide vaccines against cancer, infectious diseases, and conception. Front. Biosci. 2007;12:1833–1844. doi: 10.2741/2191. [DOI] [PubMed] [Google Scholar]
- 36.Gregory A., Titball R., Williamson D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013;3:13. doi: 10.3389/fcimb.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb B., Sali A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinformatics. 2016;54 doi: 10.1002/cpbi.3. 5.6. 1-65.6. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raman S., Machaidze G., Lustig A., Aebi U., Burkhard P. Structure-based design of peptides that self-assemble into regular polyhedral nanoparticles. Nanomedicine: NBM (NMR Biomed.) 2006;2:95–102. doi: 10.1016/j.nano.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Martí-Renom M.A., Stuart A.C., Fiser A., Sánchez R., Melo F., Šali A. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- 40.Bachmann M.F., Jennings G.T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 41.Kushnir N., Streatfield S.J., Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31:58–83. doi: 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bachmann M.F., Rohrer U.H., Kundig T.M., Burki K., Hengartner H., Zinkernagel R.M. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 43.Guu T.S., Liu Z., Ye Q., Mata D.A., Li K., Yin C., Zhang J., Tao Y.J. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:12992–12997. doi: 10.1073/pnas.0904848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagensee M.E., Yaegashi N., Galloway D. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J. Virol. 1993;67:315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Y.-S., Son A., Kim J., Kwon S.B., Kim M.H., Kim P., Kim J., Byun Y.H., Sung J., Lee J. Chaperna-mediated assembly of ferritin-based Middle East respiratory syndrome-coronavirus nanoparticles. Front. Immunol. 2018;9:1093. doi: 10.3389/fimmu.2018.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang S.-M., Kim M.-C., Compans R.W. Virus-like particles as universal influenza vaccines. Expert Rev. Vaccines. 2012;11:995–1007. doi: 10.1586/erv.12.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin L.C.W., Huang C.Y., Yao B.Y., Lin J.C., Agrawal A., Algaissi A., Peng B.H., Liu Y.H., Huang P.H., Juang R.H. Viromimetic STING agonist‐loaded hollow polymeric nanoparticles for safe and effective vaccination against Middle East respiratory syndrome coronavirus. Adv. Funct. Mater. 2019;29 doi: 10.1002/adfm.201807616. 1807616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi H., Zhou H., Tang Q., Lee J.Y., Fan Z., Kim S., Staub M.C., Zhou T., Mei S., Han L. Block copolymer crystalsomes with an ultrathin shell to extend blood circulation time. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-018-05396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Discher B.M., Won Y.-Y., Ege D.S., Lee J.C., Bates F.S., Discher D.E., Hammer D.A. Polymersomes: tough vesicles made from diblock copolymers. Science. 1999;284:1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 50.Jung S.-Y., Kang K.W., Lee E.-Y., Seo D.-W., Kim H.-L., Kim H., Kwon T., Park H.-L., Kim H., Lee S.-M. Heterologous prime–boost vaccination with adenoviral vector and protein nanoparticles induces both Th1 and Th2 responses against Middle East respiratory syndrome coronavirus. Vaccine. 2018;36:3468–3476. doi: 10.1016/j.vaccine.2018.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schalk A. An apparently new respiratory disease of baby chicks. J. Am. Vet. Med. Assoc. 1931;78:413–423. [Google Scholar]
- 52.Glahn R.P., Wideman JR R.F., Cowen B.S. Order of exposure to high dietary calcium and Gray strain infectious bronchitis virus alters renal function and the incidence of urolithiasis. Poultry Sci. 1989;68:1193–1204. doi: 10.3382/ps.0681193. [DOI] [PubMed] [Google Scholar]
- 53.Cavanagh D., Naqi S. Infectious bronchitis, Diseases of poultry. 2003;11:101–119. [Google Scholar]
- 54.Cook J.K., Jackwood M., Jones R. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- 55.Ujike M., Taguchi F. Incorporation of spike and membrane glycoproteins into coronavirus virions. Viruses. 2015;7:1700–1725. doi: 10.3390/v7041700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khataby K., Fellahi S., Loutfi C., Mustapha E.M. Avian infectious bronchitis virus in Africa: a review. Vet. Q. 2016;36:71–75. doi: 10.1080/01652176.2015.1126869. [DOI] [PubMed] [Google Scholar]
- 57.Koch G., Hartog L., Kant A.v., Van Roozelaar D. Antigenic domains on the peplomer protein of avian infectious bronchitis virus: correlation with biological functions. J. Gen. Virol. 1990;71:1929–1935. doi: 10.1099/0022-1317-71-9-1929. [DOI] [PubMed] [Google Scholar]
- 58.Ignjatovic J., Galli L. The S1 glycoprotein but not the N or M proteins of avian infectious bronchitis virus induces protection in vaccinated chickens. Arch. Virol. 1994;138:117–134. doi: 10.1007/BF01310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khataby K., Kasmi Y., Souiri A., Loutfi C., Ennaji M.M. Emerging and Reemerging Viral Pathogens. Elsevier; 2020. Avian coronavirus: case of infectious bronchitis virus pathogenesis, diagnostic approaches, and phylogenetic relationship among emerging strains in Middle East and North africa regions; pp. 729–744. [Google Scholar]
- 60.Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56:634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- 61.De Wit J., Cook J.K., Van der Heijden H.M. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40:223–235. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jordan B. Vaccination against infectious bronchitis virus: a continuous challenge. Vet. Microbiol. 2017;206:137–143. doi: 10.1016/j.vetmic.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Balestrin E., Fraga A.P., Ikuta N., Canal C.W., Fonseca A.S., Lunge V.R. Infectious bronchitis virus in different avian physiological systems—a field study in Brazilian poultry flocks. Poultry Sci. 2014;93:1922–1929. doi: 10.3382/ps.2014-03875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernando F.S., da Silva Montassier M.d.F., Silva K.R., Okino C.H., de Oliveira E.S., Fernandes C.C., de Barros Bandarra M., Gonçalves M.C.M., Borzi M.M., dos Santos R.M. Nephritis associated with a S1 variant brazilian isolate of infectious bronchitis virus and vaccine protection test in experimentally infected chickens. Int. J. Poultry Sci. 2013;12:639. [Google Scholar]
- 65.Fernando F.S., Kasmanas T.C., Lopes P.D., da Silva Montassier M.d.F., Mores M.A.Z., Mariguela V.C., Pavani C., dos Santos R.M., Assayag M.S., Jr., Montassier H.J. Assessment of molecular and genetic evolution, antigenicity and virulence properties during the persistence of the infectious bronchitis virus in broiler breeders. J. Gen. Virol. 2017;98:2470–2481. doi: 10.1099/jgv.0.000893. [DOI] [PubMed] [Google Scholar]
- 66.De Wit J., Brandao P., Torres C., Koopman R., Villarreal L. Increased level of protection of respiratory tract and kidney by combining different infectious bronchitis virus vaccines against challenge with nephropathogenic Brazilian genotype subcluster 4 strains. Avian Pathol. 2015;44:352–357. doi: 10.1080/03079457.2015.1058916. [DOI] [PubMed] [Google Scholar]
- 67.Bande F., Arshad S.S., Hair Bejo M., Moeini H., Omar A.R. Progress and challenges toward the development of vaccines against avian infectious bronchitis. J. Immunol. Res. 2015:2015. doi: 10.1155/2015/424860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caron L. Etiology and immunology of infectious bronchitis virus. Braz. J. Poult. Sci. 2010;12:115–119. [Google Scholar]
- 69.Chhabra R., Chantrey J., Ganapathy K. Immune responses to virulent and vaccine strains of infectious bronchitis viruses in chickens. Viral Immunol. 2015;28:478–488. doi: 10.1089/vim.2015.0027. [DOI] [PubMed] [Google Scholar]
- 70.Chen H.-W., Huang C.-Y., Lin S.-Y., Fang Z.-S., Hsu C.-H., Lin J.-C., Chen Y.-I., Yao B.-Y., Hu C.-M.J. Synthetic virus-like particles prepared via protein corona formation enable effective vaccination in an avian model of coronavirus infection. Biomaterials. 2016;106:111–118. doi: 10.1016/j.biomaterials.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lopes P.D., Okino C.H., Fernando F.S., Pavani C., Casagrande V.M., Lopez R.F., Montassier M.d.F.S., Montassier H.J. Inactivated infectious bronchitis virus vaccine encapsulated in chitosan nanoparticles induces mucosal immune responses and effective protection against challenge. Vaccine. 2018;36:2630–2636. doi: 10.1016/j.vaccine.2018.03.065. [DOI] [PubMed] [Google Scholar]
- 72.Zhao K., Li S., Li W., Yu L., Duan X., Han J., Wang X., Jin Z. Quaternized chitosan nanoparticles loaded with the combined attenuated live vaccine against Newcastle disease and infectious bronchitis elicit immune response in chicken after intranasal administration. Drug Deliv. 2017;24:1574–1586. doi: 10.1080/10717544.2017.1388450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McPherson C., Chubet R., Holtz K., Honda-Okubo Y., Barnard D., Cox M., Petrovsky N. Springer; 2016. Development of a SARS Coronavirus Vaccine from Recombinant Spike Protein Plus Delta Inulin Adjuvant, in: Vaccine Design; pp. 269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang S., He Y., Liu S. SARS vaccine development. Emerg. Infect. Dis. 2005;11:1016. doi: 10.3201/eid1107.050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shim B.-S., Park S.-M., Quan J.-S., Jere D., Chu H., Song M.K., Kim D.W., Jang Y.-S., Yang M.-S., Han S.H. Intranasal immunization with plasmid DNA encoding spike protein of SARS-coronavirus/polyethylenimine nanoparticles elicits antigen-specific humoral and cellular immune responses. BMC Immunol. 2010;11:65. doi: 10.1186/1471-2172-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tseng C.-T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L., Peters C.J., Couch R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PloS One. 2012;7 doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou Z., Post P., Chubet R., Holtz K., McPherson C., Petric M., Cox M. A recombinant baculovirus-expressed S glycoprotein vaccine elicits high titers of SARS-associated coronavirus (SARS-CoV) neutralizing antibodies in mice. Vaccine. 2006;24:3624–3631. doi: 10.1016/j.vaccine.2006.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tripet B., Howard M.W., Jobling M., Holmes R.K., Holmes K.V., Hodges R.S. Structural characterization of the SARS-coronavirus spike S fusion protein core. J. Biol. Chem. 2004;279:20836–20849. doi: 10.1074/jbc.M400759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McReynolds S., Jiang S., Guo Y., Celigoy J., Schar C., Rong L., Caffrey M. Characterization of the prefusion and transition states of severe acute respiratory syndrome coronavirus S2-HR2. Biochemistry. 2008;47:6802–6808. doi: 10.1021/bi800622t. [DOI] [PubMed] [Google Scholar]
- 80.Xu Y., Lou Z., Liu Y., Pang H., Tien P., Gao G.F., Rao Z. Crystal structure of severe acute respiratory syndrome coronavirus spike protein fusion core. J. Biol. Chem. 2004;279:49414–49419. doi: 10.1074/jbc.M408782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spruth M., Kistner O., Savidis-Dacho H., Hitter E., Crowe B., Gerencer M., Brühl P., Grillberger L., Reiter M., Tauer C. A double-inactivated whole virus candidate SARS coronavirus vaccine stimulates neutralising and protective antibody responses. Vaccine. 2006;24:652–661. doi: 10.1016/j.vaccine.2005.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qin E., Shi H., Tang L., Wang C., Chang G., Ding Z., Zhao K., Wang J., Chen Z., Yu M. Immunogenicity and protective efficacy in monkeys of purified inactivated Vero-cell SARS vaccine. Vaccine. 2006;24:1028–1034. doi: 10.1016/j.vaccine.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin J., Zhang J.-S., Su N., Xu J.-G., Wang N., Chen J.-T., Chen X., Liu Y.-X., Gao H., Jia Y.-P. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir. Ther. 2007;12:1107. [PubMed] [Google Scholar]
- 84.Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., Funkhouser W., Gralinski L., Totura A., Heise M. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011;85:12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sekimukai H., Iwata‐Yoshikawa N., Fukushi S., Tani H., Kataoka M., Suzuki T., Hasegawa H., Niikura K., Arai K., Nagata N. Gold nanoparticle‐adjuvanted S protein induces a strong antigen‐specific IgG response against severe acute respiratory syndrome‐related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 2020;64:33–51. doi: 10.1111/1348-0421.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coleman C.M., Liu Y.V., Mu H., Taylor J.K., Massare M., Flyer D.C., Glenn G.M., Smith G.E., Frieman M.B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32:3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garzón M.R., Berraondo P., Crettaz J., Ochoa L., Vera M., Lasarte J.J., Vales A., Van Rooijen N., Ruiz J., Prieto J., Zulueta J. Induction of gp120-specific protective immune responses by genetic vaccination with linear polyethylenimine–plasmid complex. Vaccine. 2005;23:1384–1392. doi: 10.1016/j.vaccine.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 88.Raghuwanshi D., Mishra V., Das D., Kaur K., Suresh M.R. Dendritic cell targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Mol. Pharm. 2012;9:946–956. doi: 10.1021/mp200553x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pimentel T.A., Yan Z., Jeffers S.A., Holmes K.V., Hodges R.S., Burkhard P. Peptide nanoparticles as novel immunogens: design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem. Biol. Drug Des. 2009;73:53–61. doi: 10.1111/j.1747-0285.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jc L., Vt H., Scola L., Jm R. 2020. Hydroxychloroquine and Azithromycin as a Treatment of COVID-19: Preliminary Results of an Open-Label Non-randomized Clinical Trial. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.of the International C.S.G. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seah I., Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul. Immunol. Inflamm. 2020;28:391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atal S., Fatima Z. IL-6 inhibitors in the treatment of serious COVID-19: a promising therapy? Pharmaceut. Med. 2020:1–9. doi: 10.1007/s40290-020-00342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lovato A., de Filippis C. Ear Nose Throat J.; 2020. Clinical Presentation of COVID-19: a Systematic Review Focusing on Upper Airway Symptoms. 0145561320920762. [DOI] [PubMed] [Google Scholar]
- 95.Bansal M. Clinical Research & Reviews; 2020. Cardiovascular Disease and COVID-19, Diabetes & Metabolic Syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Brain Behav. Immun.; 2020. Nervous System Involvement after Infection with COVID-19 and Other Coronaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Organization W.H. World; 2020. DRAFT Landscape of COVID-19 Candidate Vaccines. [Google Scholar]
- 99.McKay P.F., Hu K., Blakney A.K., Samnuan K., Brown J.C., Penn R., Zhou J., Bouton C.R., Rogers P., Polra K. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020;11:1–7. doi: 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patra S.K. AIJR Preprints; 2020. Outfitting COVID-19: an Effective Therapeutic Approach: Targeted Therapy of COVID-19. [Google Scholar]
- 101.Zachar O. ScienceOpen Preprints; 2020. Formulations for COVID-19 Early Stage Treatment via Silver Nanoparticles Inhalation Delivery at Home and Hospital. [Google Scholar]
- 102.Sarkar S. Silver nanoparticles with bronchodilators through nebulisation to treat COVID 19 patients. Curr. Med. Res. 2020;3:449–450. 449–450. [Google Scholar]
- 103.Aydemir D., Ulusu N.N. Correspondence: angiotensin-converting enzyme 2 coated nanoparticles containing respiratory masks, chewing gums and nasal filters may be used for protection against COVID-19 infection. Trav. Med. Infect. Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Palmieri V., Papi M. Can graphene take part in the fight against COVID-19? Nano Today. 2020;33 doi: 10.1016/j.nantod.2020.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sametband M., Kalt I., Gedanken A., Sarid R. Herpes simplex virus type-1 attachment inhibition by functionalized graphene oxide. ACS Appl. Mater. Interfaces. 2014;6:1228–1235. doi: 10.1021/am405040z. [DOI] [PubMed] [Google Scholar]
- 106.Frost R., Jönsson G.E., Chakarov D., Svedhem S., Kasemo B. Graphene oxide and lipid membranes: interactions and nanocomposite structures. Nano Lett. 2012;12:3356–3362. doi: 10.1021/nl203107k. [DOI] [PubMed] [Google Scholar]
- 107.Smith M., Smith J.C. ChemRxiv.; 2020. Repurposing Therapeutics for COVID-19: Supercomputer-Based Docking to the SARS-CoV-2 Viral Spike Protein and Viral Spike Protein-Human ACE2 Interface. [Google Scholar]
- 108.Kinnamon D.S., Krishnan S., Brosler S., Sun E., Prasad S. Screen printed graphene oxide textile biosensor for applications in inexpensive and wearable point-of-exposure detection of influenza for at-risk populations. J. Electrochem. Soc. 2018;165 B3084. [Google Scholar]
- 109.U.S.F.a.D. Administration, U.S. Food and Drug Administration; 2020. Vaccine Development – 101. [Google Scholar]
- 110.Hajj Hussein I., Chams N., Chams S., El Sayegh S., Badran R., Raad M., Gerges-Geagea A., Leone A., Jurjus A. Vaccines through centuries: major cornerstones of global health. Frontiers in public health. 2015;3:269. doi: 10.3389/fpubh.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.World Health Organization . 2020. Coronavirus Disease (COVID-19) Pandemic. [Google Scholar]
- 112.Song X., Zhao X., Huang Y., Xiang H., Zhang W., Tong D. Transmissible gastroenteritis virus (TGEV) infection alters the expression of cellular microRNA species that affect transcription of TGEV gene 7. Int. J. Biol. Sci. 2015;11:913. doi: 10.7150/ijbs.11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fu Y., Li B., Liu G. Rapid and efficient detection methods of pathogenic swine enteric coronaviruses. Appl. Microbiol. Biotechnol. 2020:1. doi: 10.1007/s00253-020-10645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dhakal S., Renukaradhya G.J. Nanoparticle-based vaccine development and evaluation against viral infections in pigs. Vet. Res. 2019;50:90. doi: 10.1186/s13567-019-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lv X., Wang P., Bai R., Cong Y., Suo S., Ren X., Chen C. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials. 2014;35:4195–4203. doi: 10.1016/j.biomaterials.2014.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Staroverov S.A., Volkov A.A., Mezhenny P.V., Domnitsky I.Y., Fomin A.S., Kozlov S.V., Dykman L.A., Guliy O.I. Prospects for the use of spherical gold nanoparticles in immunization. Appl. Microbiol. Biotechnol. 2019;103:437–447. doi: 10.1007/s00253-018-9476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mezhenny P.V., Staroverov S.A., Fomin A.S., Volkov A.A., Domnitsky I.Y., Kozlov S.V., Laskavy V.N., Dykman L.A. Study of immunogenic properties of transmissible gastroenteritis virus antigen conjugated to gold nanoparticles. J. biomed. photonics eng. 2016:2. [Google Scholar]
- 118.Jin X.-H., Zheng L.-L., Song M.-R., Xu W.-S., Kou Y.-N., Zhou Y., Zhang L.-W., Zhu Y.-N., Wan B., Wei Z.-Y. A nano silicon adjuvant enhances inactivated transmissible gastroenteritis vaccine through activation the Toll-like receptors and promotes humoral and cellular immune responses. Nanomed. Nanotechnol. Biol. Med. 2018;14:1201–1212. doi: 10.1016/j.nano.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 119.Mole B. Deadly pig virus slips through US borders. Nature. 2013;499:388. doi: 10.1038/499388a. [DOI] [PubMed] [Google Scholar]
- 120.Zhou H., Xu R.Z., Gu Y., Shi P.F., Qian S. Targeting of phospho-eIF4E by homoharringtonine eradicates a distinct subset of human acute myeloid leukemia. Leuk. Lymphoma. 2020;61:1084–1096. doi: 10.1080/10428194.2017.1390229. [DOI] [PubMed] [Google Scholar]
- 121.Dong H.-J., Wang Z.-H., Meng W., Li C.-C., Hu Y.-X., Zhou L., Wang X.-J. The natural compound homoharringtonine presents broad antiviral activity in vitro and in vivo. Viruses. 2018;10:601. doi: 10.3390/v10110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li C.-C., Wang X.-J. Three kinds of treatment with Homoharringtonine, Hydroxychloroquine or shRNA and their combination against coronavirus PEDV in vitro. Virol. J. 2020;17:1–11. doi: 10.1186/s12985-020-01342-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Du T., Liang J., Dong N., Lu J., Fu Y., Fang L., Xiao S., Han H. Glutathione-capped Ag2S nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Appl. Mater. Interfaces. 2018;10:4369–4378. doi: 10.1021/acsami.7b13811. [DOI] [PubMed] [Google Scholar]
- 124.Ting D., Dong N., Fang L., Lu J., Bi J., Xiao S., Han H. Multisite inhibitors for enteric coronavirus: antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018;1:5451–5459. doi: 10.1021/acsanm.0c00970. [DOI] [PubMed] [Google Scholar]
- 125.Li B., Du L., Yu Z., Sun B., Xu X., Fan B., Guo R., Yuan W., He K. Poly (d, l-lactide-co-glycolide) nanoparticle-entrapped vaccine induces a protective immune response against porcine epidemic diarrhea virus infection in piglets. Vaccine. 2017;35:7010–7017. doi: 10.1016/j.vaccine.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 126.Payne S. 2017. Family Coronaviridae, Viruses; p. 149. [Google Scholar]
- 127.Jaimes J.A., Millet J.K., Stout A.E., André N.M., Whittaker G.R. A tale of two viruses: the distinct spike glycoproteins of feline coronaviruses. Viruses. 2020;12:83. doi: 10.3390/v12010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hohdatsu T., Yamato H., Ohkawa T., Kaneko M., Motokawa K., Kusuhara H., Kaneshima T., Arai S., Koyama H. Vaccine efficacy of a cell lysate with recombinant baculovirus-expressed feline infectious peritonitis (FIP) virus nucleocapsid protein against progression of FIP. Vet. Microbiol. 2003;97:31–44. doi: 10.1016/j.vetmic.2003.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ng S.W., Selvarajah G.T., Hussein M.Z., Yeap S.K., Omar A.R. Vitro evaluation of curcumin-encapsulated chitosan nanoparticles against feline infectious peritonitis virus and pharmacokinetics study in cats. BioMed Res. Int. 2020:2020. doi: 10.1155/2020/3012198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hu C.-M.J., Chang W.-S., Fang Z.-S., Chen Y.-T., Wang W.-L., Tsai H.-H., Chueh L.-L., Takano T., Hohdatsu T., Chen H.-W. Nanoparticulate vacuolar ATPase blocker exhibits potent host-targeted antiviral activity against feline coronavirus. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-13316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen Y.-N., Hsueh Y.-H., Hsieh C.-T., Tzou D.-Y., Chang P.-L. Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. Int. J. Environ. Res. Publ. Health. 2016;13:430. doi: 10.3390/ijerph13040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Singh L., Kruger H.G., Maguire G.E., Govender T., Parboosing R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. Dis. 2017;4:105–131. doi: 10.1177/2049936117713593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qi M., Zhang X.E., Sun X., Zhang X., Yao Y., Liu S., Chen Z., Li W., Zhang Z., Chen J. Intranasal nanovaccine confers homo‐and hetero‐subtypic influenza protection. Small. 2018;14 doi: 10.1002/smll.201703207. 1703207. [DOI] [PubMed] [Google Scholar]
- 134.Sawaengsak C., Mori Y., Yamanishi K., Mitrevej A., Sinchaipanid N. Chitosan nanoparticle encapsulated hemagglutinin-split influenza virus mucosal vaccine. AAPS PharmSciTech. 2014;15:317–325. doi: 10.1208/s12249-013-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Figueiredo L., Cadete A., Gonçalves L., Corvo M., Almeida A. Intranasal immunisation of mice against Streptococcus equi using positively charged nanoparticulate carrier systems. Vaccine. 2012;30:6551–6558. doi: 10.1016/j.vaccine.2012.08.050. [DOI] [PubMed] [Google Scholar]
- 136.Khatri K., Goyal A.K., Gupta P.N., Mishra N., Vyas S.P. Plasmid DNA loaded chitosan nanoparticles for nasal mucosal immunization against hepatitis B. Int. J. Pharm. 2008;354:235–241. doi: 10.1016/j.ijpharm.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 137.Musial J., Krakowiak R., Mlynarczyk D.T., Goslinski T., Stanisz B.J. Titanium dioxide nanoparticles in food and personal care products—what do we know about their safety? Nanomaterials. 2020;10:1110. doi: 10.3390/nano10061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Prabha S., Arya G., Chandra R., Ahmed B., Nimesh S. Effect of size on biological properties of nanoparticles employed in gene delivery. Artif. Cells Nanomed. Biotechnol. 2016;44:83–91. doi: 10.3109/21691401.2014.913054. [DOI] [PubMed] [Google Scholar]
- 139.Fitzpatrick J.A., Andreko S.K., Ernst L.A., Waggoner A.S., Ballou B., Bruchez M.P. Long-term persistence and spectral blue shifting of quantum dots in vivo. Nano Lett. 2009;9:2736–2741. doi: 10.1021/nl901534q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rejman J., Oberle V., Zuhorn I.S., Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dos Santos T., Varela J., Lynch I., Salvati A., Dawson K.A. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PloS One. 2011;6 doi: 10.1371/journal.pone.0024438. e24438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Edwards J.V., Prevost N.T., Condon B., French A., Wu Q. Immobilization of lysozyme-cellulose amide-linked conjugates on cellulose I and II cotton nanocrystalline preparations. Cellulose. 2012;19:495–506. [Google Scholar]
- 143.Barazzouk S., Daneault C. Tryptophan-based peptides grafted onto oxidized nanocellulose. Cellulose. 2012;19:481–493. [Google Scholar]
- 144.Arola S., Tammelin T., Setälä H., Tullila A., Linder M.B. Immobilization–stabilization of proteins on nanofibrillated cellulose derivatives and their bioactive film formation. Biomacromolecules. 2012;13:594–603. doi: 10.1021/bm201676q. [DOI] [PubMed] [Google Scholar]
- 145.Haghpanah J.S., Tu R., Da Silva S., Yan D., Mueller S., Weder C., Foster E.J., Sacui I., Gilman J.W., Montclare J.K. Bionanocomposites: differential effects of cellulose nanocrystals on protein diblock copolymers. Biomacromolecules. 2013;14:4360–4367. doi: 10.1021/bm401304w. [DOI] [PubMed] [Google Scholar]
- 146.Anirudhan T., Rejeena S. Adsorption and hydrolytic activity of trypsin on a carboxylate-functionalized cation exchanger prepared from nanocellulose. J. Colloid Interface Sci. 2012;381:125–136. doi: 10.1016/j.jcis.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 147.Zhu M., Wang R., Nie G. Applications of nanomaterials as vaccine adjuvants. Hum. Vaccines Immunother. 2014;10:2761–2774. doi: 10.4161/hv.29589. [DOI] [PMC free article] [PubMed] [Google Scholar]