Abstract
The impairment of mitochondrial metabolism is a hallmark of aging. Mitonuclear imbalance and the mitochondrial unfolded protein response (UPRmt) are two conserved mitochondrial mechanisms that play critical roles in ensuring mitochondrial proteostasis and function. Here, we combined bioinformatics, physiological, and molecular analyses to examine the role of mitonuclear imbalance and UPRmt in the skeletal muscle of aged rodents and humans. The analysis of transcripts from the skeletal muscle of aged humans (60–70 years old) revealed that individuals with higher levels of UPRmt-related genes displayed a consistent increase in several mitochondrial-related genes, including the OXPHOS-associated genes. Interestingly, high-intensity interval training (HIIT) was effective in stimulating the mitonuclear imbalance and UPRmt in the skeletal muscle of aged mice. Furthermore, these results were accompanied by higher levels of several mitochondrial markers and improvements in physiological parameters and physical performance. These data indicate that the maintenance or stimulation of the mitonuclear imbalance and UPRmt in the skeletal muscle could ensure mitochondrial proteostasis during aging, revealing new insights into targeting mitochondrial metabolism by using physical exercise.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00246-5) contains supplementary material, which is available to authorized users.
Keywords: Aging, UPRmt, Skeletal muscle, Exercise, Mitonuclear imbalance
Introduction
Aging is understood as a phenomenon that is marked by the progressive loss of cell functions. Mitochondrial dysfunction is often observed in several cell types in response to aging. It has been previously evidenced that losses of proteostasis, mitochondrial dysfunctions, and changes in intercellular communication are some of the major hallmarks of aging [8]. The association between mitochondrial dysfunctions and aging occurs because mitochondria are complex architecture organelles which require highly orchestrated cellular communication mechanisms to maintain their functions [13]. The loss of mitochondrial proteostasis or the imbalance between mitochondrial and nuclear protein synthesis elicit at least two specialized cellular mechanisms responsible for restoring mitochondrial homeostasis, called mitonuclear imbalance and mitochondrial unfolded protein response (UPRmt). Mitonuclear imbalance rises due to the stoichiometric imbalance between mitochondrial-encoded proteins (such as MTCO1, an mtDNA-encoded ETC protein) and nuclear-encoded proteins (such as ATP5a or SDHA, nDNA-encoded ETC components). The MTCO1/SDHA ratio has been well described as a marker of mitonuclear imbalance, which culminates in greater UPRmt activation [6]. UPRmt protects the mitochondrial structure against proteotoxic damage, guaranteeing optimal functions, such as the assembly of the oxidative phosphorylation (OXPHOS) machinery [10]. In response to misfolded proteins or dysfunctional OXPHOS machinery, UPRmt is activated to recover mitochondrial functions [11, 16]. However, it is important to mention that UPRmt could be activated in other circumstances, independently of a mitonuclear imbalance, including during cellular apoptosis [18] and carcinogenesis [7].
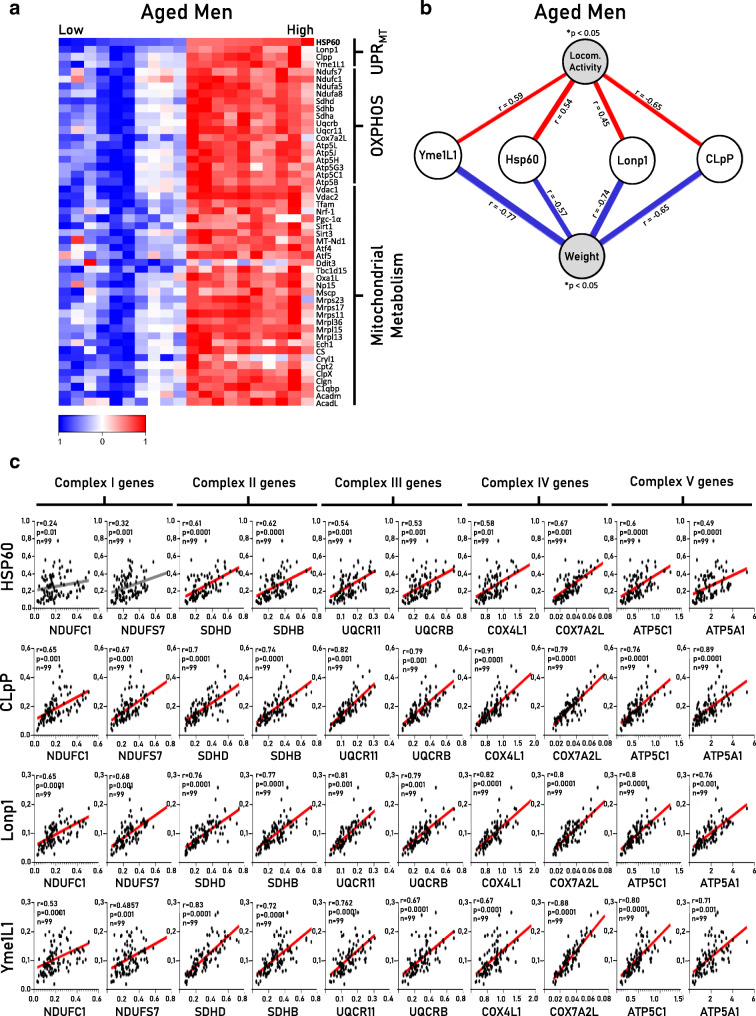
Curiously, mtDNA and nDNA communication are affected during aging in worms and flies, downregulating the activity of UPRmt components, mainly the mitochondrial chaperones and proteases [10, 12]. Aiming to identify markers of mitonuclear imbalance and UPRmt in the skeletal muscle of rodents and humans, we first screened a comprehensive library database of gene expression in the skeletal muscle of aged humans (for details, see Appendix 1). The heatmap analysis clearly revealed that aged men (60–70 years old) with a preserved level of UPRmt genes (Yme1L1, Hsp60, Lonp1, and CLpP) displayed greater levels of several mitochondrial-related genes in their skeletal muscle (Fig. 1a), while elderly subjects with a lower UPRmt displayed lower levels of mitochondria-related genes. Also, UPRmt markers (Yme1L1, Hsp60, Lonp1, and CLpP) in the skeletal muscle were positively correlated with locomotor activity (red lines) and negatively correlated with body weight (blue lines) (Fig. 1b). Furthermore, we found a strong positive association between the UPRmt genes and OXPHOS complexes I–V (Fig. 1c). Our initial data are in accordance with a previous study that demonstrates that UPRmt is a conserved mechanism across species, including worms [10], flies [12], and rodents [6], and it is highly associated with OXPHOS integrity and mitochondrial metabolism during the aging process.
Fig. 1.
Bioinformatic analysis revealed positive association between markers of UPRmt and mitochondrial metabolism in skeletal muscle of aged humans. a The heatmap displays the association between UPRmt markers and OXPHOS-related genes and mitochondrial metabolism. b The association analyses between markers of UPRmt and whole-body physiological parameters. c Spearman’s correlation analyses between UPRmt genes and OXPHOS components in skeletal muscle of aged humans. Each point represents a sample. Statistical significance was fixed at r > 0.4 and *p < 0.05
It has been demonstrated that boosting nicotinamide adenine dinucleotide (NAD+) levels could trigger mitonuclear imbalance and UPRmt to improve mitochondrial function and metabolism, increasing physical performance [5, 14]. Also, targeting UPRmt in the skeletal muscle by boosting NAD+ levels could improve whole-body metabolism and inhibit mitochondrial dysfunctions during pathological conditions such as obesity, in rodents [1] and in humans with type 2 diabetes [17]. Interestingly, physical exercise indirectly increases NAD+ accumulation [3]. Furthermore, electrical stimulation induces UPRmt markers in the skeletal muscle cells [9]. Recently, we demonstrated that aerobic training induced the mitonuclear imbalance and UPRmt markers, including the heat shock protein HSP60 and the proteases Lonp1 and Yem1L1, in the skeletal muscle of old mice [4]. Collectively, these studies lead us to hypothesize that high-intensity interval training could be an interesting non-pharmacological strategy to activate the mitonuclear imbalance and UPRmt in the skeletal muscle, counteracting the mitochondrial impairments that occur during aging.
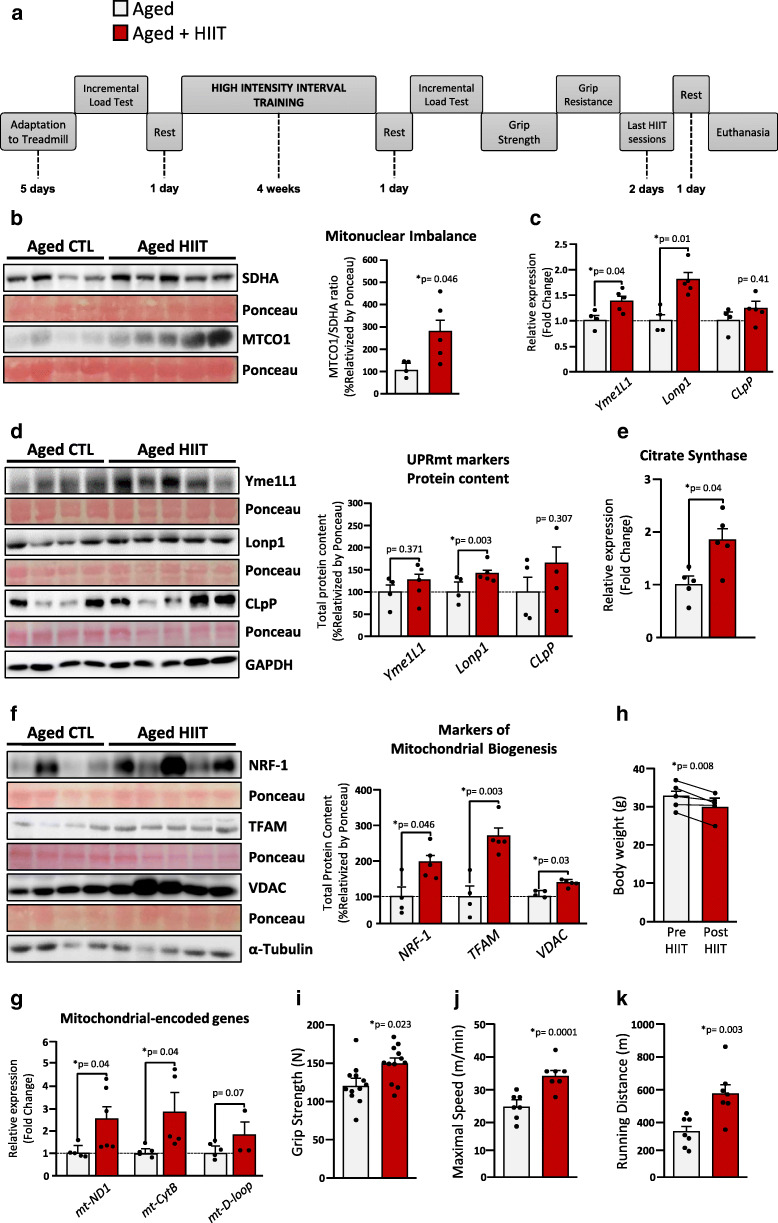
To test our hypothesis, male C57BL/6 J mice aged 24 months old were subjected to 4 weeks of high-intensity interval training (HIIT) (for details, see Appendix 1 and Supplementary Table 1). Initially, to guarantee the high intensity of the physical exercise training, we monitored and confirmed the hyperlactatemia after each training session (see Appendix 3). Next, we found that HIIT robustly induced the mitonuclear imbalance (increased MTCO1/SDHA ratio) in the skeletal muscle of aged mice (Fig. 2b). In addition, the HIIT-exercised mice displayed higher levels of gene expression of the UPRmt markers ATP-dependent metalloprotease-1 (Yme1L1) and the Lon protease homolog-1 (Lonp1) (Fig. 2c), and Lonp1 protein levels (Fig. 2d). We also observed a discrete but nonsignificant increase in Yme1L1 CLpP protein content in the skeletal muscle of trained group (Fig. 2d).
Fig. 2.
The effects of high-intensity interval training (HIIT) on mitonuclear imbalance, UPRmt, and mitochondrial metabolism in skeletal muscle of aged mice. a Figure represents the 4-week HIIT protocol designed for aged animals. b Exercise induces mitonuclear imbalance and improves the gene expression (c) and protein content (d) of UPRmt markers. RT-qPCR and western blot analyses were also performed to evaluate: e citrate synthase and (f–g) markers of mitochondrial biogenesis. h Total body mass (n = 9). Results achieved during grip strength (i) and treadmill running tests (j–k). Each point represents a sample, varying according to the experiment performed. Statistical significance was fixed at *p < 0.05
Next, we sought to evaluate the key players in mitochondrial metabolism. Exercise training increased citrate synthase expression (Fig. 2e), suggesting enhancements in mitochondrial function. Furthermore, exercise training also increased the protein content of mitochondrial biogenesis markers, such as the voltage-dependent anion channels (VDAC), the nuclear respiratory factor-1 (NRF-1), and the mitochondrial transcription factor A (Tfam), when compared with the old sedentary group (Fig. 2f).
Thereafter, we monitored the mitochondrial DNA copy number in the gastrocnemius of aged animals after exercise training. It has been previously evidenced that HIIT improves mitochondrial metabolism and content in skeletal muscle [2, 15]. Our results evidenced that HIIT-exercised aged animals presented higher expression of mitochondrial-encoded genes mt-ND1, mt-CytB, and mt-D-loop (Fig. 2g), suggesting increased mtDNA in the skeletal muscle of these animals. Lastly, our results suggest that 4 weeks of HIIT was able to modify the whole-body metabolism of aged animals. Comparing the sedentary with the exercised animals, the HIIT protocol reduced total body weight (Fig. 2h) without any differences in food intake (data not shown). In addition, the HIIT-exercised animals presented greater physical performance in grip strength (Fig. 2i) and treadmill running tests compared with aged sedentary animals (Fig. 2j–k).
To confirm that mitochondrial alterations are specifically associated with physical exercise but not with the morphological alterations caused by a long-term protocol, we subjected a group of male C57BL/6 J aged mice to a single bout of high-intensity interval exercise (HIIE). As expected, a single session of exercise did not change the total body weight or adiposity content (data not shown). Interestingly, a single session of HIIE induced the mitonuclear imbalance (MTCO1/ATP5a ratio) in the skeletal muscle of aged animals, which was accompanied by discrete changes in the protein levels of UPRmt markers (results are present in Appendix 2). These data confirm that physical exercise induces mitonuclear imbalance in the skeletal muscle of old mice independently of morphological alterations.
Taken together, our data provide consistent evidence that aged humans with preserved UPRmt markers display greater mitochondrial-related genes in the skeletal muscle, in particular, the OXPHOS-related genes. Importantly, we propose that a physiological stimulus such as regular physical exercise or even a single bout of exercise could activate the mitonuclear imbalance and UPRmt in the skeletal muscle of aged mice. These findings suggest that the maintenance or the stimulation of mitonuclear imbalance and UPRmt could be critical for mitochondrial proteostasis in the skeletal muscle during the aging process, reinforcing the idea that HIIT is an efficient non-pharmacological therapeutic strategy to counteract age-related dysfunctions in mitochondrial metabolism.
Electronic supplementary material
Appendix 1. A detailed description of the materials and methods utilized in this study. (PDF 315 kb)
Appendix 2. Schematic design and results from a single session of HIIE. (PDF 509 kb)
Appendix 3. Lactate analysis after HIIT and treadmill test in aged mice. (PDF 534 kb)
Appendix 4. Detailed information regarding all Western blot analyses. (PDF 1178 kb)
(PDF 493 kb)
(PDF 386 kb)
(PDF 369 kb)
Authors’ contributions
All authors contributed to the study conception and design. AVC and ERR designed the manuscript and the figures. GFP performed the bioinformatic analyses. LL, CKK, and VRRS monitored and took care of the aged animals. AVC performed the HIIT protocol. BMC and LTB performed the acute exercise experiments. AVC, RSB, CPA, RRB, and LL performed the physical tests and participated in the in vivo experiments. FMS and AVC performed the PCR analyses. AVC, BMC, LTB, and RRB performed the western blot analyses. LPM, ASRS, DEC, and JRP provided the laboratory and intellectual support for the present study.
Funding information
The National Council for Scientific and Technological Development (CNPq) (case numbers 304771/2017-1 and 401189/2016-3), the Coordination for the Improvement of Higher Education Personnel (CAPES) (finance code 001), and the São Paulo Research Foundation (FAPESP) (grant numbers 2011/09656-0 and 2018/07634-9) all financed this study.
Data availability
Additional information can be found online in the supporting information of this study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavanelle V, Boisseau N, Otero YF, Combaret L, Dardevet D, Montaurier C, et al. Effects of high-intensity interval training and moderate-intensity continuous training on glycaemic control and skeletal muscle mitochondrial function in db/db mice. Sci Rep. 2017;7(1):204. doi: 10.1038/s41598-017-00276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connell NJ, Houtkooper RH, Schrauwen P. NAD+ metabolism as a target for metabolic health: have we found the silver bullet? Diabetologia. 2019;62(6):888–899. doi: 10.1007/s00125-019-4831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordeiro AV, Brícola RS, Braga RR, Lenhare L, Silva VRR, Anaruma CP, et al. Aerobic exercise training induces the mitonuclear imbalance and UPRmt in the skeletal muscle of aged mice. J Gerontol Ser A, Biol Sci Med Sci. 2020. 10.1093/gerona/glaa059. [DOI] [PubMed]
- 5.Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology (Baltimore, Md.) 2016;63(4):1190–1204. doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497(7450):451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenny TC, Gomez ML, Germain D. Mitohormesis, UPR mt, and the complexity of mitochondrial DNA landscapes in cancer. Cancer Res. 2019;79(24):6057–6066. doi: 10.1158/0008-5472.CAN-19-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memme JM, Oliveira AN, Hood DA. Chronology of UPR activation in skeletal muscle adaptations to chronic contractile activity. American Journal of Physiology. Cell Physiol. 2016;310(11):C1024–C1036. doi: 10.1152/ajpcell.00009.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, et al. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154(2):430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nargund AM, Fiorese CJ, Pellegrino MW, Deng P, Haynes CM. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt) Mol Cell. 2015;58(1):123–133. doi: 10.1016/j.molcel.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owusu-Ansah E, Song W, Perrimon N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell. 2013;155(3):699–712. doi: 10.1016/j.cell.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellegrino MW, Nargund AM, Haynes CM. Signaling the mitochondrial unfolded protein response. Biochim Biophys Acta. 2013;1833(2):410–416. doi: 10.1016/j.bbamcr.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirinen E, Cantó C, Jo YS, Morato L, Zhang H, Menzies KJ, et al. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014;19(6):1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos-Filho D, Chicaybam G, De-Souza-Ferreira E, Guerra Martinez C, Kurtenbach E, Casimiro-Lopes G, Galina A. High intensity interval training (HIIT) induces specific changes in respiration and electron leakage in the mitochondria of different rat skeletal muscles. PLoS One. 2015;10(6):e0131766. doi: 10.1371/journal.pone.0131766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian Y, Merkwirth C, Dillin A. Mitochondrial UPR: a double-edged sword. Trends Cell Biol. 2016;26(8):563–565. doi: 10.1016/j.tcb.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 17.van de Weijer T, Phielix E, Bilet L, Williams EG, Ropelle ER, Bierwagen A, et al. Evidence for a direct effect of the NAD + precursor acipimox on muscle mitochondrial function in humans. Diabetes. 2015;64(4):1193–1201. doi: 10.2337/db14-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu M, Bi X, He X, Yu X, Zhao M, Zang W. Inhibition of the mitochondrial unfolded protein response by acetylcholine alleviated hypoxia/reoxygenation-induced apoptosis of endothelial cells. Cell Cycle. 2016;15(10):1331–1343. doi: 10.1080/15384101.2016.1160985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. A detailed description of the materials and methods utilized in this study. (PDF 315 kb)
Appendix 2. Schematic design and results from a single session of HIIE. (PDF 509 kb)
Appendix 3. Lactate analysis after HIIT and treadmill test in aged mice. (PDF 534 kb)
Appendix 4. Detailed information regarding all Western blot analyses. (PDF 1178 kb)
(PDF 493 kb)
(PDF 386 kb)
(PDF 369 kb)
Data Availability Statement
Additional information can be found online in the supporting information of this study.