Abstract
The capacity to move is essential for independence and declines with age. Slow movement speed, in particular, is strongly associated with negative health outcomes. Prior research on mobility (herein defined as movement slowness) and aging has largely focused on musculoskeletal mechanisms and processes. More recent work has provided growing evidence for a significant role of the nervous system in contributing to reduced mobility in older adults. In this article, we report four pieces of complementary evidence from behavioral, genetic, and neuroimaging experiments that, we believe, provide theoretical support for the assertion that the basal ganglia and its dopaminergic function are responsible, in part, for age-related reductions in mobility. We report four a posteriori findings from an existing dataset: (1) slower central activation of ballistic force development is associated with worse mobility among older adults; (2) older adults with the Val/Met intermediate catecholamine-O-methyl-transferase (COMT) genotype involved in dopamine degradation exhibit greater mobility than their homozygous counterparts; (3) there are moderate relationships between performance times from a series of lower and upper extremity tasks supporting the notion that movement speed in older adults is a trait-like attribute; and (4) there is a relationship of functional connectivity within the medial orbofrontal (mOFC) cortico-striatal network and measures of mobility, suggesting that a potential neural mechanism for impaired mobility with aging is the deterioration of the integrity of key regions within the mOFC cortico-striatal network. These findings align with recent basic and clinical science work suggesting that the basal ganglia and its dopaminergic function are mechanistically linked to age-related reductions in mobility capacity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-020-00303-z.
Keywords: Mobility, Neural control, Dopamine, Basal ganglia, Aging, Sarcopenia
Introduction
By 2050, the world’s population aged 60 years and older (the World Health Organization’s cut point for an older adult) is expected to total 2 billion (22% of the total population) up from 900 million (12% of the total population) in 2015 [1]. Accordingly, all countries face major challenges to ensure that their health and social systems are ready to make the most of this demographic shift. The capacity to move is essential for independence and declines with age. Mobility limitations are predominately characterized by slowness when walking and performing physical tasks [2, 3]. Mobility limitations affect ~ 35% of adults over 70 and the majority of adults over 85 [3–5] and are highly associated with fall risk, disability, increased dependency, hospitalization, and mortality [2, 6–10]. Slow gait speed, in particular, is strongly associated with survival rate [2]. Accordingly, age-related mobility limitations are a significant public health burden, with annual US healthcare costs of $42 billion [11]. Unfortunately, our understanding of the underlying mechanisms of, and our prevention and intervention strategies for, mobility limitations remains very limited in scope.
Prior research on mobility and aging has largely focused on factors related to musculoskeletal mechanisms and processes. This work has found that muscle weakness and low lean mass to a lesser extent are contributors to mobility limitations (e.g., [12–15]). However, work conducted over the past 15 years has also provided accumulating evidence for the role of the aging central nervous system (CNS) in contributing to mobility limitations [16–19] potentially via slower and less “automated” walking, requiring greater engagement of attention and cognitive resources [17, 19]. Unfortunately, the precise central neural mechanisms associated with aging that lead to decreased mobility are poorly understood.
Within the CNS, the basal ganglia may be theoretically linked to age-related reductions in mobility capacity via its associated dopaminergic function. Animal studies indicate that the basal ganglia circuit is critical for motivated, instrumental behavior [20]. This circuit consists of the striatum as its input and the internal globus pallidus and substantia nigra pars reticulate (SNr) as its output [21]. The outputs of the basal ganglia shape activity in the spinal-targeting pathways that regulate voluntary movement [22, 23]. A major modulator of the state of the striatum is dopamine (DA). Progressive degeneration of midbrain dopaminergic neurons has been associated with deficits in the initiation, speed, and fluidity of voluntary movement [24–26]. Slower movement initiation and speed, as well as a reduction in movement amplitude, are some of the most profound effects of either acute or chronic disruption of DA signaling [27]. A series of recent studies have indicated that tonic activity [27, 28], as well as phasic activity before action initiation [27, 28], in the dorsal striatum is critical for the regulation of movement vigor (i.e., combination of reaction time and velocity). Specifically, Panigrahi and colleagues (2015) reported that restoration of dopaminergic tone with a synthetic precursor in mice ameliorated deficits in movement vigor and its neural representation, while suppression of striatal activity during movement was sufficient to reduce vigor [27]. Subsequently, da Silva et al. (2018) showed that a large proportion of substantia nigra pars compacta (SNc) DA neurons transiently increased their activity before self-paced movement initiation in mice, and this activity was related to the vigor of future movements [28].
Studies of the aging human brain have shown that regulation of DA action is significantly reduced in old age via structural degradation, including neuronal loss, fewer neuroreceptor sites, and a lack of transporter molecules [29]. For instance, age-dependent declines of brain DA levels have been reported in the basal ganglia, specifically the dorsal striatum post-mortem [30]. In vivo imaging studies have since confirmed these findings [29]. Moreover, after the age of 20, the availability of DA D1-like receptors declines in the human striatum at a rate of ~ 7% per decade [31, 32], with the D2-like family demonstrating a similar decrease in receptor density (~ 5–10%/decade) [33, 34] and receptor binding potential (~ 6–8%/decade) [33, 35, 36]. Despite a large number of studies documenting decreased striatal DA activity with aging, little is known about its functional significance in relation to age-related mobility, which is somewhat surprising when considering its involvement in the pathophysiology of movement disorders (e.g., Parkinson’s disease) [37]. However, a very recent pilot study that treated a small number of older adults (n = 16; mean age 72.5 years) who suffered from depression with L-DOPA for 3-weeks reported a 16% and 28% significant increase in single task and dual task usual gait speed, respectively (note that the mean single task usual gait speed at baseline was 0.77 m/s, which indicates that, on average, this population was likely mobility limited) [38]. Findings of this nature clearly indicate the need for further work examining the role of dopaminergic function and age-related mobility capacity.
In this manuscript, we report four pieces of complementary evidence from behavioral, genetic, and neuroimaging experiments that, we believe, support a theoretical construct wherein the basal ganglia, and its dopaminergic function, contribute to age-related reductions in mobility capacity. It should be noted that these experiments were not designed and executed a priori, but instead involve secondary analyses of an existing dataset. The premise, purpose, and hypotheses for each of these experiments are as follows:
Analysis 1. Reduced dopaminergic function has long been suggested to underlie slowing of brief, ballistic contractions (i.e., contractions that exhibit maximum velocities and accelerations over a very short period of time) [39–44]. There are a number of neurophysiological mechanisms that could contribute to the nervous systems’ ability to rapidly generate force. The most obvious are impairments in motor unit recruitment and discharge rates [45]. As originally described by Liddell and Sherrington in 1925 [46], the motor unit provides the primary output for the central nervous system, converting sensory and descending inputs into forces to generate muscle contraction and, hence, movement. The motor unit consists of the α-motoneuron and the muscle fibers that its axon innervates (the latter commonly referred to as the muscle unit). The recruitment and rate modulation of motor units, and hence the force exerted by a muscle, depend on the interaction between the synaptic input received by the motoneuron pool and the intrinsic properties of the motoneurons. Accordingly, a healthy motor unit is critical for optimal skeletal muscle function. Numerous studies have reported evidence suggesting that aging is associated with reductions in neuronal excitability in a number of neuron types [47–53], which could explain the reports of age-related reductions in maximal motor unit discharge rates and incidence of doublet discharge rates [52–57]. In addition, one study showed that 12 weeks of dynamic exercise training resulted in an enhancement in the speed of voluntary ballistic contraction, which was associated with an increased incidence of doublets [58]. However, motor unit activation and firing rates preceding force generation are also likely contributors [45]. Of note, dopaminergic function could influence any or all of these factors in rapid force generation. Accordingly, we examined the nervous systems’ contribution to the ability to rapidly drive muscle force production. We then tested the hypothesis that slower neural rate of force development is associated with worse mobility among older adults. Mobility was assessed via the mobility battery assessment (MBA) score, which is a compositive measure of mobility that is based on movement speed/time to complete locomotor and non-locomotor tasks [59].
Analysis 2. Catecholamine-O-methyl-transferase (COMT) initiates dopamine degradation [60, 61]. Its activity is mainly determined by a single nucleotide polymorphism in the COMT gene (Val158Met, rs4680) separating high (Val/Val), intermediate (Val/Met), and low metabolizers (Met/Met), which results in the Val/Val genotype having low tonic DA, the Val/Met genotype having intermediate tonic DA, and the Met/Met genotype having high tonic DA (Table 1) [60, 61]. The intermediate Val/Met genotype has been suggested to be most optimal based on it theoretically balancing the roles of tonic and phasic DA (i.e., tonic–phasic regulation of DA transmission) [61–63]. Accordingly, we examined the effect of COMT-rs4680 genotype polymorphisms on mobility capacity in older adults. We hypothesized that older adults with the Val/Met intermediate genotype would exhibit better mobility (i.e., MBA score) than their homozygous counterparts, after statistically controlling for leg extensor muscle strength and appendicular lean mass.
Table 1.
Catechol-O-methyltransferase (COMT) enzyme and its three phenotypes activities
| Homozygous COMT G/G | Homozygous Val158Val | Enzyme with high activity | Low level of tonic dopamine |
|---|---|---|---|
| Heterozygous COMT A/G | Heterozygous Val158Met | Enzyme with medium activity | Medium level of tonic dopamine |
| Homozygous COMT A/A | Homozygous Met158Met | Enzyme with low activity | High level of tonic dopamine |
Analysis 3. It has been suggested that movement speed (commonly referred to as “movement vigor” in the field of neuroeconomics) is partly associated with the amount of dopamine, particularly via a transient increase, in the basal ganglia [27, 64]. Among healthy individuals, there is well-recognized diversity in movement vigor, with some people tending to consistently move rapidly, whereas others tend to move slowly, as evidenced by their saccades (i.e., rapid, ballistic eye movements) [64–66] and their reaching movements [64]. Consequently, in recent years, the speed at which people move has been conceptualized as a trait-like attribute [64, 67]. Accordingly, we examined the relationship between performance times (as a proxy for speed) among a series of lower and upper extremity tasks that, conceptually, challenge different physiological systems (e.g., some challenge muscle strength, while others challenge dexterity/motor coordination). We hypothesized that we would observe moderate relationships among performance times, providing preliminary support for the notion that movement speed in older adults is a trait-like attribute of individuality.
Analysis 4. Although the speed that one volitionally moves may generally be consistent across situations consistent with trait, it may also be affected by other factors, such as motivation and willingness to exert effort. The mesolimbic DA system facilitates motivated behavior [68]. Cortico-striatal circuits through the orbitofrontal cortex (OFC) in particular play key roles in complex human behaviors such as evaluation, affect regulation, and reward-based decision-making [69]. The OFC is an important node of multiple networks involving both visceral and motor systems, and it is thought to serve as a nexus for sensory integration, particularly in the context of value-guided behavior [69]. A recently described medial OFC (mOFC) cortico-striatal loop network has been suggested to be involved in reward processing, motivation, and reward-guided learning (Fig. 1) [69–71]. The orbitofrontal cortex is uniquely placed to integrate sensory and visceral motor information to modulate behavior through both visceral and motor systems and has been suggested to play a role in goal-directed behavior [70]. This area has also been suggested to encode the subjective valuation of prospective effort [72], and this network likely encodes the relative value of rewarding stimuli [70, 71]. As such, it may be associated with movement speed. Within the regions of this network and across whole-brain studies, degraded structural properties, specifically reduced gray matter volume and increased white matter hyperintensity, have been associated with impaired mobility [16, 73]. Accordingly, in this experiment, we utilized resting state functional connectivity and structural MRI-based analyses to examine the relationship between the strength of coherence in the mOFC cortico-striatal loop network with the MBA score. We hypothesized that we would observe an association between resting state functional connectivity of this network and mobility.
Fig. 1.
Structure of the medial orbofrontal cortex (mOFC) cortico-striatal loop, originating from the mOFC shown in red proposed by Fettes et al. [69]. Reprinted under the terms of the Creative Commons Attribution License. mOFC, medial orbitofrontal cortex; VM caudate, ventromedial caudate; NAcc, nucleus accumbens; GPi, globus pallidus interna; SN, substantia nigra; DM Thalamus, dorsomedial thalamic nuclei; VA Thalamus, ventroanterior thalamic nuclei; VL Thalamus, ventrolaterial thalamic nuclei
Methods
Data for this report were derived from part of a larger study/dataset (The UNCODE Study; NCT02505529). The participants in each analysis were the same, but the sample sizes differ. The total number of participants in the broader dataset was 89, of which 3 were consented and enrolled but withdrew prior to completing a testing session. Thus, there were 86 participants with data in the overall dataset. For this manuscript, participants were included in the respective analyses only if they had completed data for each analysis, which passed quality control and assurance measures across all variables of interest for each individual experiment. The primary reasons for sample size attrition for the various experiments were due to some participants choosing to not undergo or being unable to tolerate certain procedures (e.g., genetic testing, supramaximal electrical stimulation, magnetic resonance imaging, etc.), close examination of data indicating poor quality data (e.g., a countermovement prior to performing the ballistic contractions in experiment 1), or the MRI procedures only being performed on a subset of the study participants due to scheduling or budgetary reasons (only applies to experiment 4). We should note that the experiments with the larger sample sizes (e.g., experiment 2) do not necessarily contain all of the participants that participated in the experiments with smaller sample sizes (e.g., experiment 1). For instance, experiment 1 had a sample size of 53, and experiment 2 had a sample size of 72. Two of the participants who were in experiment 1, however, chose not to undergo genetic testing and as such are not represented in the data analysis for experiment 2.
To participate in the broader study, participants had to be 60+ years of age and have a body mass index (BMI) between 18 and 40 kg/m2. Study participants had to be living independently, could not demonstrate disabilities in their activities of daily living (ADLs), and had to be free of major musculoskeletal, neurological, cardiac, pulmonary, renal, psychiatric, and cognitive disease or disorders. Supplementary Table 1 provides a complete list of the study inclusion and exclusion criteria. Participants were instructed to abstain from drinking caffeinated beverages for 4 h prior and alcohol for 24 h prior to all testing sessions. The Ohio University Institutional Review Board approved the study procedures, and all study participants provided written informed consent prior to their participation. The participant data for each of the four experiments is presented as the mean ± standard deviation unless otherwise indicated.
Analysis 1. The nervous systems’ ability to rapidly drive muscle force production
Study participants
Fifty-three older adults (75.2 ± 6.7 (range: 63–92) years; 68% women; height: 163.7 ± 10.4 cm; weight: 71.2 ± 13.8 kg; BMI: 26.5 ± 4.6 kg/m2) participated in the experiment. This sample represents 62% of the original database.
Quantification of the nervous systems’ ability to rapidly drive muscle force production
We quantified the nervous systems’ ability to rapidly drive muscle force production by comparing the rate of force development during maximal voluntary isometric ballistic contractions to the rate of force development during electrically stimulated contractions. Theoretically, expressing the rate of voluntary force development (RFDVOLUNTARY), which is comprised of both neural and musculoskeletal contributors, to the peak rate of force development (i.e., yank [56]) of a post-activation potentiated electrically-stimulated contraction (YankEVOKED), which is largely independent of neural contributions and primary reflects the muscular contributions, yields a value representing the nervous system’s capability to rapidly produce ballistic force (i.e., Central Activation Ballistic Force Ratio = RFDVOLUNTARY / YankEVOKED), with a smaller value indicative of a greater neural deficit in rapid activation. To quantify the CAB ratio of the leg extensors, volitional and electrically evoked doublet torque production were measured. This was done using a Biodex System 4 torque motor (Biodex Medical Systems Inc., Shirley, NY) and a Digitimer DS7AH stimulator (Digitimer, Hertfordshire, UK), as we have previously described [74, 75]. Provided with strong verbal encouragement, participants first performed three maximal voluntary isometric contractions (MVC) (60-s rest between trials) where they were instructed to “push out as hard as you can for 5-seconds easing into the contraction over the first one second.” Next, for the ballistic contractions, participants were instructed to “kick out as fast and hard as possible” three times (60-s rest between trials) with emphasis placed on “fast” as per prior suggestions [76]. Subsequently, we quantified post-activation potentiated electrically evoked leg extensor contractile properties similar to our prior descriptions [74].
The CAB ratio was quantified using measurements from the leg extensor isometric ballistic contraction and the electrically evoked doublet contraction recordings. The RFDVOLUNTARY of the extensor isometric ballistic contraction was quantified between contraction onset and 100 ms. Onset of the contraction was determined manually, using a two-point differential, identifying where the torque/time tracing last left baseline (i.e., the data point showing the last positive deflection in force production). The muscular component of ballistic force generation (i.e., YankEVOKED) was quantified using the peak torque/time of the doublet contraction recording. By normalizing RFDVOLUNTARY by the musculoskeletal component (YankEVOKED) of rapid force production, we can theoretically derive a value representative of the nervous system’s ability to produce ballistic force (i.e., CAB ratio).
Assessments of mobility
On a separate visit, study participants were asked to perform a variety of physical function/mobility tasks that were used to quantify our primary measure of mobility: the MBA score [59]. The MBA was developed to serve as a composite measure of physical function that comprises locomotor and non-locomotor tests. It has been shown to better assess lower extremity self-reported mobility function and discriminate community dwelling older adults with and without mobility limitations than singular, standard tests of mobility [59]. The MBA score was derived from a combination of (1) 6-min walk gait speed, (2) time to complete the four square step test, (3) 5x chair rise time, (4) stair climb power, and (5) time to complete a complex functional task involving rising from the floor and then lifting and carrying a laundry basket to a table [59]. Based on the performance of all of these tasks, we calculated the MBA score using principal component analysis that yields a dimensionless composite score in which all five tasks are loaded onto one component. The MBA scores have a distribution of a mean of 0 and a standard deviation of 1.0. A more positive MBA score indicates better mobility.
Statistical analyses
Pearson’s correlation coefficients were calculated to examine associations between variables (e.g., MBA and YNI). Additionally, these correlations were calculated after controlling for age. A pre-set alpha 0.05 or less was required for statistical significance, and all analyses were conducted using IBM SPSS Statistics version 25.
Analysis 2. COMT genotype, dopamine degradation, and mobility
Study participants
Seventy-two older adults (74.9 ± 6.5 (range: 63–92) years; 68.1% women; height: 164.3 ± 10.2 cm; weight: 72.7 ± 15.7 kg; BMI: 26.9 ± 4.9 kg/m2) participated in the experiment. This sample represents 84% of the original database.
COMT genotyping
For each subject, a buccal swab was obtained with an Isohelix SK1 buccal swabs (Isohelix DNA Sampling and Purification, Unit 2 Roebuck Business Park, Ashford Road, Harrietsham, Kent, UK; Cat. # SK-1S) and stored with an Isohelix Dri-Capsules (Cat. # SGC-50) at the time of specimen collection. DNA was isolated using Maxwell® RSC Automated Nucleic Acid Extraction instrument (Promega Corporation 2800 Woods Hollow Road · Madison, WI; Cat. # AS4500) and Maxwell® RSC Buccal Swab DNA Kit (Cat. # AS1640). In brief, 330 ul of lysis buffer/proteinase K (PK) solution previously prepared was added to the buccal swab tube. The lysate was incubated 20 min at 56 °C and transferred to the clearing column and assembled on ClickFit microtube. Clearing column and ClickFit microtube were spun 2 min at 14,000 rpm, and the clearing column was then discarded. Maxwell® RSC buccal swab DNA cartridge was placed in the deck tray. The flowthrough liquid was added to cartridge well #1, plunger was placed on well #8, 5 μl of RNase A solution (Cat. # A7973) was added to well #3, and an elution tube with 50 ul of elution buffer was installed on the deck tray. The cartridge was transferred to Maxwell® RSC automated nucleic acid extraction instrument, and the protocol for DNA isolation was performed.
The genotyping was performed using a predesigned TaqMan Drug Metabolism Enzyme (DME) Genotyping Assay (Applied Biosystems 850 Lincoln Centre Drive, Foster City, CA; SNP ID: rs4680, Assay ID: C__25746809_50, Cat. # 4362691), according to TaqMan® SNP Genotyping Assay User Guide (Pub. No. MAN0009593). In brief, 10 ng of gDNA were analyzed using 20X TaqMan® DME assay and 2X TaqMan® Genotyping Master Mix (Cat. # 4381656). Fifty cycles of amplification were then performed, and a post-PCR step (60 °C for 30 s) was run to measure the dyes fluorescence intensity. The post-PCR data were imported to TaqMan Genotyper Software Version 1.5.0 to perform the genotyping analysis.
Statistical analyses and covariates
An analysis of variance (ANOVA) was initially performed to examine differences in MBA score (for details on the MBA score, see description in experiment 1) between the three respective COMT-rs4680 genotype polymorphisms (Table 1) followed by an LSD post hoc test. Subsequently, we examined the effect of adjusting for leg extensor isometric muscle strength normalized to body weight similar to our prior descriptions [75] and appendicular lean mass normalized to height2 using dual-energy X-ray absorptiometry similar to our prior descriptions [77] by entering these as covariates in an ANCOVA. The rationale for adjusting for strength and mass is based on prior work suggesting that these variables contribute, in part, to mobility [12–15]. Secondarily, we also (1) added age as a covariate to the model and (2) disaggregated the data by sex and repeated the analyses. The sex-specific analyses should be considered exploratory due to the small sample size. A pre-set alpha of 0.05 or less was required for statistical significance, and all analyses were conducted using IBM SPSS Statistics version 25.
Analysis 3. Movement speed in older adults as a trait-like attribute of individuality
Study participants
Seventy-two older adults participated in the experiment (73.5 ± 5.2 (range: 63–85) years; 65% women; height: 165.5 ± 9.6 cm; weight: 73.5 ± 16.1 kg; BMI: 27.1 ± 4.7 kg/m2). This sample represents 84% of the original database.
Performance times/speeds
We quantified performance time (or speed in some cases) for the tasks associated with the MBA, which have been described in detail elsewhere [59]. Additionally, we quantified time to complete a modified Purdue Pegboard Test and the Trail Making Test A.
The Purdue Pegboard (Model 32,020; Lafayette Instrument Co., Lafayette, Indiana) consisted of 50 holes arranged in two parallel columns of 25 and pegs located in two cups at the top of the board (one cup on the right and one on the left of midline of the board) and could be reached conveniently by both hands. The pegboard was located on a table, and participants sat comfortably in front of it. Participants were instructed to start the test on a verbal cue, while an examiner timed the test with a stopwatch. Participants were instructed to fill the 25 holes with the pegs on the dominant hand side with the dominant hand (the hand used for writing) as fast as possible. Two trials were completed. Then, participants were instructed to fill the 25 holes with the pegs on the non-dominant hand side with the non-dominant hand as fast as possible, and again, two trials were completed. An average time was subsequently calculated across all trials.
The Trail Making Test A requires an individual to draw lines sequentially connecting 25 encircled numbers distributed on a sheet of paper. Participants were instructed to complete the test as quickly and accurately as possible. When an error was made, the participant was instructed to return to the “circle” where the error originated and continue. Time to complete the test was recorded.
Statistical analyses and covariates
Pearson’s correlation coefficients were calculated to examine associations between performance times. These correlations were also calculated when adjusting for (i.e., partial correlations) (1) leg extensor isometric muscle strength normalized to body weight similar to our prior descriptions [75] and appendicular lean mass normalized to height2 using dual-energy X-ray absorptiometry similar to our prior descriptions [77] and (2) age. The rationale for adjusting for strength and mass is based on prior work suggesting that these variables contribute, in part, to mobility [12–15]. A pre-set alpha 0.05 or less was required for statistical significance, and all analyses were conducted using IBM SPSS Statistics version 25.
Analysis 4. Mobility and the mOFC cortico-striatal loop network functional connectivity
Study participants
Fifty-two older adults participated in the experiment (74.1 ± 7.0 (range: 63–92) years; 71% women; height: 164.4 ± 10.4 cm; weight: 73.7 ± 16.8 kg; BMI: 27.2 ± 5.4 kg/m2). This sample represents 60% of the original database.
Neuroimaging
A 3.0 T Philips Acheiva scanner with a 16-channel head coil was used to acquire T1 structural, fluid-attenuated inversion recovery imaging (FLAIR), and resting state fMRI (rsfMRI). High-resolution whole-brain axial gradient-echo MPRAGE 3-D T1-weighted images were acquired for volumetric gray and white matter, and cortical surface area analyses from FreeSurfer ROIs [78–83] and rsfMRI registration (TE/TR = 3.4/7.4 ms, flip angle = 8, slice thickness = 1 mm (contiguous slices), Field of View (FOV) = 250 × 250 × 200 mm,1 mm3 resolution). A high-resolution 3D FLAIR sequence (TE/TR = 359/8.0 ms, 1 mm3 resolution, gap = 0, FOV = 240 × 240 × 160 mm) was used along with semi-automated analysis tools for determining the distribution of pixel values, measuring ROIs, and segmentation of images [79, 80, 82–86]. rsfMRI was collected with echoplanar BOLD imaging (EPI) methods, with a TR of 3000 ms, TE 35 ms, flip angle of 90 degrees, FOV = 240 × 240 × 160 mm, voxel size of 2.5 mm3, and number of slices of 53. Participants were instructed to close their eyes during the scans. Wakefulness was monitored by the MRI technologist before and after the scan to confirm a consistent participant state for all resting state fMRIs.
Functional connectivity analysis and statistical analysis
The rsfMRI processing and analyses were completed with the CONN toolbox version 18b and SPM12. Prior to fMRI preprocessing, the first three volumes of the time series were removed due to consistent global signal instability. Data were then preprocessed which included realignment and unwarping (motion correction), structural segmentation and normalization to MNI space, functional normalization to MNI space guided by the structural segmentation, and spatial smoothing of the fMRI time series with a Gaussian smoothing kernel of 8 mm FWHM. To further correct for subject motion, the artifact rejection toolbox was used to identify outlier volumes based upon a normative 97th percentile setting (global signal deviation greater than 5 standard deviations from mean or participant framewise motion of greater than 0.9 mm) [87]. These outlier volumes were used to generate covariates included in first level analysis. A band-pass filter was applied in the range of 0.008–0.09 Hz to reduce non-physiological BOLD signal (such as low-frequency scanner drift). Further denoising was done using the aCompCor strategy as implemented in CONN 18b, which aims to reduce BOLD signal noise by the extraction of signal principal components from noise regions of interest such as cerebral spinal fluid [88]. Upon completion of preprocessing steps, ROI to ROI connectivity was estimated using CONN 18b. The average framewise displacement was 0.333 mm.
To define the mOFC cortico-striatal network, several atlases were used to obtain representative regions of the network. The mOFC cortico-striatal network is defined in the literature as the following ROIs: mOFC, ventromedial caudate, nucleus accumbens, globus pallidus interna, substantia nigra, dorsomedial thalamic nuclei, ventroanterior thalamic nuclei, and ventrolaterial thalamic nuclei [69]. To the authors’ knowledge, a comprehensive mask of this network broken into regional components is not readily available; therefore, the network was operationally defined as the following ROIs: thalamus, caudate, accumbens, and orbitofrontal cortex from the CONN default atlas; substania nigra from the PD25 atlas [89]; and globus pallidus interna from the BGHAT atlas [90]. Functional connectivity between each region within the network was quantified via Fisher transformed Pearson correlation coefficients between the average residual BOLD time series of pairs of ROIs. Network coherence of the mOFC cortico-striatal network was determined by the average connectivity of all ROI pairs within the network. The mOFC cortico-striatal network connectivity was extracted for correlation analysis with the MBA score. These correlations were also calculated when adjusting for (i.e., partial correlations) (1) leg extensor isometric muscle strength normalized to body weight similar to our prior descriptions [75] and appendicular lean mass normalized to height2 using dual-energy X-ray absorptiometry similar to our prior descriptions [77] and (2) age.
Structural analysis and statistical analysis
WMH volume was extracted from the FLAIR imaging for the mOFC. The subcortical regions were not included due to lack of FLAIR resolution and lack of white matter presence. Lesions were segmented by the lesion prediction algorithm of Schmidt et al. (2017) [91] as implemented in the LST toolbox version 2.0.15 (www.statistical-modelling.de/lst.html) for SPM. This algorithm consists of a binary classifier in the form of a logistic regression model trained on the data of 53 multiple sclerosis patients with severe lesion patterns. As covariates for this model, a similar lesion belief map as for the lesion growth algorithm was used as well as a spatial covariate that takes into account voxel-specific changes in lesion probability [92]. Parameters of this model fit are used to segment lesions in new images by providing an estimate for the lesion probability for each voxel. A 50% probability threshold was used to binarize predicted lesion voxels. We subsequently obtained regional values by extracting the voxels from the FreeSurfer white matter parcellation.
Gray matter volume was extracted from the T1 structural images for the mOFC, caudate, accumbens, pallidum, and thalamus. The precise sub-regions of the network could not be extracted due to technical limitations associated with image resolution. The white matter lesion and gray matter volume data were extracted for correlation analysis with the MBA score. For these exploratory associations, we used Spearman rank correlation coefficients for the WMH data (as they were non-normally distributed) and a Pearson’s correlation coefficients for the GM data. All volumetric measures were adjusted for total intracranial volume. These correlations were also calculated when adjusting for (i.e., partial correlations) (1) leg extensor isometric muscle strength normalized to body weight similar to our prior descriptions [76] and appendicular lean mass normalized to height2 using dual-energy X-ray absorptiometry similar to our prior descriptions [78] and (2) age. p values were corrected using the Benjamini–Hochberg method, which is a procedure for the case of multiple comparisons [93]. Corrected p values (i.e., those adjusted for alpha inflation) ≤ 0.05 were considered statistically significant.
Results
Analysis 1. The nervous systems’ ability to rapidly drive muscle force production
The CAB ratio was significantly associated with the MBA score when men and women were combined (r = 0.58, p < 0.001), as well as when correlations were calculated for men and women separately (men r = 0.66, p < 0.01; women r = 0.57, p < 0.001). Controlling for age resulted in non-substantial changes in these associations (combined: r = 0.58, p < 0.001; men r = 0.67, p < 0.01; women r = 0.54, p < 0.001). The CAB ratio was significantly associated with the MBA score when data from men and women were combined (r = 0.38, p < 0.01), as well as when correlations were calculated for men and women separately (men r = 0.60, p = 0.01; women r = 0.37, p = 0.028) (Fig. 2). Controlling for age resulted non-substantial changes in these associations (combined: r = 0.45, p < 0.01; men r = 0.53, p = 0.04; women r = 0.54, p < 0.001). With regard to the musculoskeletal component of rapid force development, YankEVOKED was significantly associated with the MBA score when data from men and women were combined (r = 0.27, p = 0.05), but not if correlations were calculated for men and women separately (men r = −0.04, p = 0.87; women r = 0.22, p = 0.21). Controlling for age resulted in the combined association no longer being statistically significant (r = 0.27, p = 0.06) and had non-substantial effects on the sex-specific associations (men r = 0.20, p = 0.46; women r = 0.01, p = 0.96).
Fig. 2.

The central activation ballistic force ratio (CAB Ratio) is associated with mobility in older adults. The CAB ratio, which is derived from comparing the rates of rapid voluntary and electrically-stimulated force development to represent a measure indicative of the nervous systems ability to generate rapid, ballistic force, was associated with mobility (r = 0.38, p = 0.006). The figure illustrates sex-specific lines of best fit, which are significant for both men (filled circles/solid line; r = 0.60, p = 0.12) and women (open circles/dashed line; r = 0.37, p = 0.028)
Analysis 2. COMT genotype, dopamine degradation, and mobility
Among women, 24 had the Val158Met genotype, 14 had the Met158Met genotype, and 11 had the Val158Val genotype. Among men, 10 had the Val158Met genotype, 2 had the Met158Met genotype, and 11 had the Val158Val genotype.
As expected, there was a main effect for COMT-rs4680 genotype on MBA score (F = 3.76; p = 0.028). Post hoc testing indicated that individuals with the Val158Met genotype exhibited a significantly higher MBA score in comparison to individuals with the Met158Met genotype (p = 0.013) (Table 2). The Val158Met genotype and the Val158Val were not statistically different (p = 0.067) nor were the Met158Met and the Val158Val genotypes (p = 0.429) (Table 2). After adjusting for normalized muscle strength and lean mass, a main effect for COMT-rs4680 genotype on MBA score was observed (F = 3.80; p = 0.027) (Fig. 3 and Table 2). Post hoc testing indicated that individuals with the Val158Met genotype exhibited a significantly higher MBA score in comparison to individuals with the Met158Met (p = 0.031) and Val158Val genotype (p = 0.024) (Fig. 3 and Table 2). The Met158Met and the Val158Val MBA scores were not statistically different (p = 0.936) (Fig. 3 and Table 2). When age was added as an additional covariate to the model, the main effect for COMT-rs4680 genotype on MBA score was no longer significant (F = 1.68; p = 0.19).
Table 2.
Mean mobility battery assessment (MBA) scores for COMT-rs4680 genotype polymorphisms. Estimated marginal means ± standard error of the mean
| Model | Met158Met | Val158Met | Val158Val |
|---|---|---|---|
| Men and women combined: unadjusted | − 0.42 ± 0.96 | 0.32 ± 0.65* | − 0.17 ± 1.34 |
| Men and women combined: covarying for normalized strength & lean mass | − 0.25 ± 0.20 | 0.28 ± 0.14* | − 0.23 ± 0.17 |
| Men and women combined: covarying for normalized strength, lean mass, & age | − 0.12 ± 0.18 | 0.18 ± 0.12 | − 0.16 ± 0.15 |
| Women only: unadjusted | − 0.48 ± 1.0 | 0.31 ± 0.72* | − 0.83 ± 1.12 |
| Women only: covarying for normalized strength &lean mass | − 0.48 ± 0.23 | 0.18 ± 0.18* | − 0.54 ± 0.27 |
| Women only: covarying for normalized strength, lean mass, & age | − 0.30 ± 0.20 | 0.01 ± 0.16 | − 0.37 ± 0.23 |
| Men only: unadjusted | − 0.04 ± 0.54 | 0.36 ± 0.45 | 0.48 ± 0.67 |
| Men only: covarying for normalized strength & lean mass | 0.24 ± 0.35 | 0.41 ± 0.15 | 0.39 ± 0.15 |
| Women only: covarying for normalized strength, lean mass, & age | 0.46 ± 0.35 | 0.42 ± 0.14 | 0.34 ± 0.14 |
Note that the MBA score is dimensionless with a more positive value representing greater mobility
Lean mass: appendicular lean mass normalized to height2
*Val158Met genotype > both Met158Met and Val158Val genotype p < 0.05
Fig. 3.
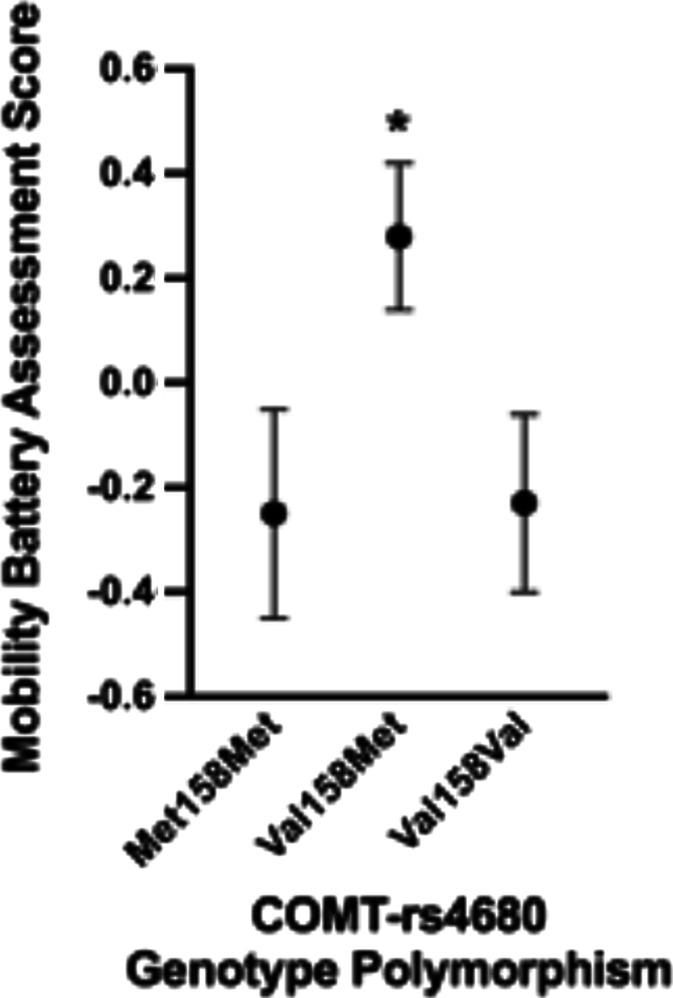
Mobility battery assessment (MBA) scores for COMT-rs4680 genotype polymorphisms when adjusted for appendicular lean mass/height2 and isometric leg extensor strength/body weight. As noted by the asterisk, the estimated marginal means for the Val158Met genotype were significantly greater in comparison to the Met158Met (p = 0.031) and Val158Val genotypes (p = 0.024)
When we disaggregated data by sex and ran separate models, we observed a main effect for COMT-rs4680 genotype on MBA score for women, but not for men (women: F = 5.5, p < 0.001; men: F = 0.72; p = 0.50). Post hoc testing indicated that women with the Val158Met genotype exhibited a significantly higher MBA score in comparison to individuals with the Met158Met (p = 0.027) and Val158Val genotype (p = 0.004) (Table 2). The Met158Met and the Val158Val MBA scores were not statistically different (p = 0.407) (Table 2). After adjusting for normalized muscle strength and lean mass, a main effect for COMT-rs4680 genotype on MBA score was observed for women, but not for men (women: F = 3.84, p = 0.03; men: F = 0.10; p = 0.90). Data from the men, in particular, should be interpreted with caution due to the small sample size. Post hoc testing indicated that women with the Val158Met genotype exhibited a significantly higher MBA score in comparison to individuals with the Met158Met (p = 0.025) and Val158Val genotype (p = 0.030) (Table 2). The Met158Met and the Val158Val MBA scores were not statistically different (p = 0.88) (Table 2). When age was added as an additional covariate to these sex-specific model, the main effect for COMT-rs4680 genotype on MBA score was no longer significant (women: F = 1.06, p = 0.36; men: F = 0.10; p = 0.91).
Analysis 3. Movement speed in older adults as a trait-like attribute of individuality
Table 3 presents the unadjusted and adjusted correlations between performance time (or speed) for three lower extremity and two upper extremity tasks, and Fig. 4 illustrates the unadjusted associations. Nine out of ten of these tasks were significantly associated with each other when no adjustments were made (Table 3; average absolute value of the correlations were ~ 0.42). Even after adjusting for normalized muscle strength and lean mass, all tasks were associated (Table 3).
Table 3.
Correlations between performance time (or speed) for three lower extremity and two upper extremity tasks (unadjusted (UA) and when adjusted for leg extensor muscle strength normalized to body weight and appendicular lean mass normalized to height (S&LM), as well as S&LM along with age (S&LM + Age)
| 6MGS | 4SST | PPT | TMTA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U | S&LM | S&LM + Age | U | S&LM | S&LM + Age | U | S&LM | S&LM + Age | U | S&LM | S&LM + Age | |
| 5xCR | − 0.53* | − 0.32* | − 0.32* | 0.63* | 0.53* | 0.53* | 0.41* | 0.36* | 0.36* | 0.37* | 0.47* | 0.46* |
| 6MGS | − 0.62* | − 0.52* | − 0.49* | − 0.36* | − 0.33* | − 0.20 | − 0.22 | − 0.32* | − 0.29 | |||
| 4SST | 0.60* | 0.59* | 0.57* | 0.49* | 0.57* | 0.56* | ||||||
| PPT | 0.51* | 0.57* | 0.56* | |||||||||
| TMTA | ||||||||||||
5xCR 5x chair rise, 6MGS 6-min walk gait speed, 4SST four square step test, PPT Purdue pegboard test, TMTA trails making task A, U unadjusted, S&LM covaried for leg extension Strength normalized to body weight and appendicular Lean Mass normalized to height2, S&LM + Age covaried for leg extension Strength normalized to body weight, appendicular Lean Mass normalized to height2, and age
*denotes p < 0.01
Fig. 4.
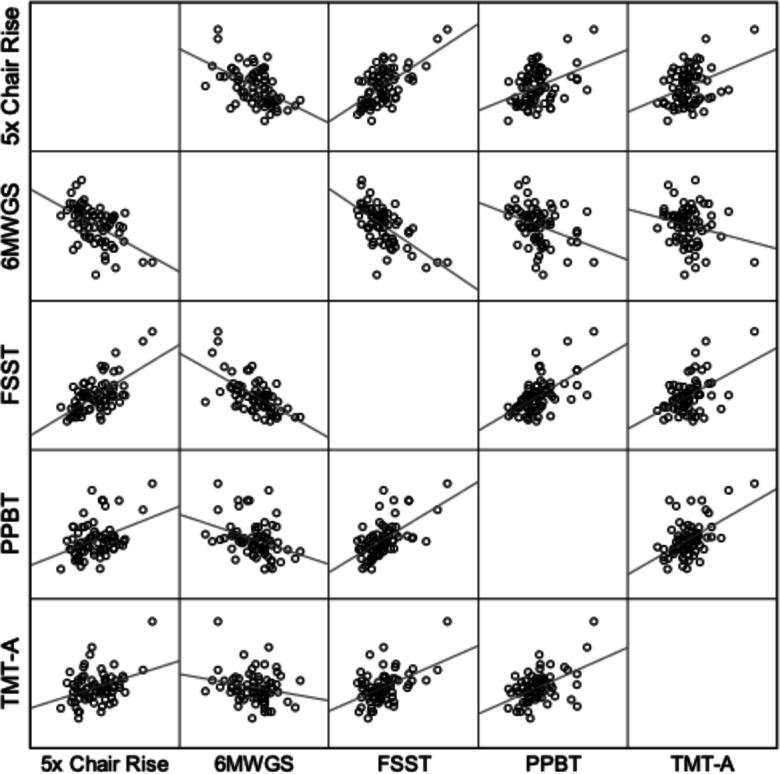
Scatterplots illustrating the associations between performance time (or speed) for three lower extremity and two upper extremity tasks. Note that the 6-min walk data is expressed as gait speed (m/s) and that a lower value indicates slower speed, which explains the observed negative association with this variable. All correlations except for one are significant at the p < 0.01 level (see Table 2 for r values). These data provide general support for the concept that movement speed is a trait-like attribute of individuality in older adults (OAs)
Analysis 4. Mobility and the mOFC cortico-striatal loop network functional connectivity
As expected, the mOFC cortico-striatal network functional connectivity was correlated with MBA score (r = − 0.28, p = 0.03; Fig. 5), indicating increased functional connectivity of the network as mobility becomes more impaired. This correlation increases further when we examine older adults with the poorest mobility (i.e., lower quantile) (n = 23, r = −0.37, p = 0.02; Fig. 5), indicating that this network may substantially increase in connectivity when sufficient mobility loss has occurred. MBA score was also highly correlated with increased white matter lesion volume (loss of white matter structural integrity) in the right mOFC and left mOFC and less gray matter volume in selected regions of the basal ganglia (i.e., right and left accumbens) and the thalamus (Table 4). There was no relationship between MBA score and gray matter volume of the mOFC or the caudate (Table 4).
Fig. 5.
Scatterplots illustrating the associations between the mOFC cortico-striatal network functional connectivity and the mobility battery assessment (MBA) score for the entire sample (a) and the older adults whose MBA score was in the lower quantile (i.e., lower half) (b). The open circles represent females and closed circles represent males
Table 4.
Medial orbitofrontal cortico-striatal network region gray matter and white matter hyperintensity correlates with the mobility battery assessment (MBA) score
| Area-parameter | Hemisphere | Correlation coefficient (corrected p value) | Correlation coefficient (corrected p value) | Correlation coefficient (corrected p value) |
|---|---|---|---|---|
| Covarying for TIV | Covarying for TIV, normalized strength, & LM | Covarying for TIV, normalized strength, LM, & age | ||
| mOFC-WMH volume | Left | − 0.45 (p = 0.003)* | − 0.34 (p = 0.032)* | − 0.28 (p = 0.253) |
| Right | − 0.55 (p = 0.003)* | − 0.42 (p = 0.013)* | − 0.24 (p = 0.386) | |
| mOFC-GM volume | Left | 0.18 (p = 0.263) | 0.25 (p = 0.116) | − 0.08 (p = 0.830) |
| Right | 0.09 (p = 0.585) | 0.17 (p = 0.271) | 0.01 (p = 0.967) | |
| Caudate-GM volume | Left | − 0.25 (p = 0.123) | − 0.38 (p = 0.020)* | − 0.06 (p = 0.830) |
| Right | 0.075 (p = 0.606) | − 0.16 (p = 0.278) | − 0.05 (p = 0.830) | |
| Accumbens-GM volume | Left | 0.35 (p = 0.033)* | 0.33 (p = 0.044)* | 0.06 (p = 0.830) |
| Right | 0.42 (p = 0.010)* | 0.46 (p = 0.003)* | 0.23 (p = 0.295) | |
| Pallidum-GM volume | Left | − 0.25 (p = 0.123) | − 0.29 (p = 0.070) | − 0.15 (p = 0.640) |
| Right | 0.31 (p = 0.056) | 0.31 (p = 0.062) | 0.28 (p = 0.253) | |
| Thalamus-GM volume | Left | 0.61 (p = 0.010)* | 0.55 (p = 0.003)* | 0.35 (p = 0.170) |
| Right | 0.40 (p = 0.013)* | 0.471 (p = 0.003)* | 0.26 (p = 0.253) |
mOFC medial orbofrontal cortex, WMH white matter hyperintensity, GM gray matter, TIV total intracranial volume; normalized strength: isokinetic strength/body weight; LM: appendicular lean mass normalized to height2
*denotes corrected p value < 0.05
Correlation coefficient for WMH volume data are Spearman’s rank correlation coefficients (56% and 66% of participants had no WMH volumes in the left and right hemispheres, respectively), and coefficients for GM volume represent Pearson correlation coefficients
The correlation between mOFC cortico-striatal network functional connectivity and MBA score remained significant and similar after adjusting for normalized muscle strength and lean mass (r = − 0.27, p = 0.05). When age was added as an additional covariate to the model, the relationship was no longer significant (r = − 0.07, p = 0.66). Table 4 displays the associations between MBA score and the GM volumes after statistically adjusting for normalized muscle strength, lean mass, and age. Key findings here were that after adjusting for normalized muscle strength and lean mass, the associations remained significant, but when age was added to the model, they were no longer significant.
Discussion
In this manuscript, we report four pieces of indirect, though complementary evidence that we believe provide theoretical support for the assertion that the basal ganglia and its dopaminergic function are responsible, in part, for age-related reductions in mobility capacity. The four findings are (1) slower nervous system rate of force development is associated with worse mobility among older adults; (2) older adults with the Val/Met intermediate genotype exhibit significantly greater mobility than their homozygous counterparts after statistically controlling for relative muscle strength and lean mass; (3) older adults show moderate relationships between performance times from a series of lower and upper extremity tasks, which supports the notion that movement speed in older adults is a trait-like attribute of individuality; and (4) there is a relationship of functional connectivity within the MOFC cortico-striatal network and measures of mobility, suggesting that a potential neural mechanism for impaired mobility with aging is deterioration of the integrity of key regions within the mOFC cortico-striatal network. We first interpret and discuss the findings from each experiment and then more broadly discuss the theoretical implications for the findings collectively.
Analysis 1. The nervous systems’ ability to rapidly drive muscle force production
Our finding that the slower nervous system rate of force development is associated with worsened mobility among older adults is in agreement with prior work reporting that the rate of rise in force and the rate of rise of the voluntary electromyogram amplitude during rapid contractions are reduced in mobility-limited older adults [94] and also associated with reduced gait speed [95]. Our work advances these prior findings by leveraging data from evoked contractile properties to theoretically remove the contribution of the musculoskeletal system and other extraneous factors (e.g., the non-physiological influences, such as adipose tissue and signal cancelation on the EMG amplitude [96, 97]) to derive an index that is more specific to the nervous systems’ ability to rapidly generate force.
There are a number of neurophysiological mechanisms that could contribute to the nervous systems’ ability to rapidly generate force. The most obvious are impairments in motor unit recruitment and discharge rates [45]. As noted in the introduction, numerous studies have reported evidence suggesting that aging is associated with reductions in neuronal excitability in a number of neuron types [47–53], which could explain the reports of age-related reductions in maximal motor unit discharge rates and incidence of doublet discharge rates [52–57]. In addition, one study showed that 12 weeks of dynamic exercise training resulted in an enhancement in the speed of voluntary ballistic contraction, which was associated with an increased incidence of doublets [58]. These findings suggest that our results are likely due to the older adults, particularly those with poor mobility, having a reduced incidence of doublets. However, delayed motor unit activation and slower firing rates preceding force generation are also likely contributors [45]. Of note, dopaminergic function could influence any or all of these factors in rapid force generation.
Reduced dopaminergic function is not generally discussed as a potential explanation for the impairments in the rate of neuromuscular activation. However, it should be noted that dopaminergic function has long been suggested to underlie slowing of brief, ballistic contractions [39–44]. For instance, rodent studies have demonstrated that DA D1 and D2 receptor antagonists result in delays in movement initiation and slowing in movement execution [44]. Additionally, patient’s with Parkinson’s disease—whose motor signs are generally thought to be the result of a reduction in DA within the basal ganglia [98]—exhibit dramatic slowing in movement speed [40–43]. Moreover, L-DOPA treatment has been shown to increase the mean velocity of an initial ballistic movement towards a target without loss of accuracy in patients with Parkinson’s disease [99]. Similarly, the delivery of transcranial pulsed electromagnetic fields, which arguably modulate striatal dopaminergic function [100–106], has been shown to improve the rate of force development in Parkinson’s disease [41]. Accordingly, we speculate that our findings are, in part, explained by deficits in dopaminergic function in the basal ganglia.
Analysis 2. COMT genotype, dopamine degradation, and mobility
To our knowledge, there are only three prior studies that have examined the association between genetic markers of dopaminergic transmission and indices of physical function in older adults [107–109]. Our findings are consistent with these prior reports, which—generally speaking—found that older individuals with genotypes that allow more effective DA transmission performed better on various motor tasks and had faster gait speeds [107–109]. For instance, Metti and colleagues reported a U-shaped association between the COMT Val158Met genotypes and decline in 6-min walk gait speed over a 10-year period, such that the Val/Val and Met/Met genotypes slowed more over a 10-year period than those with the intermediate DA Val/Met genotype [109]. Our study complements these prior findings and expands them by demonstrating that COMT Val158Met genotypes are broadly associated with mobility in older adults (as we used a composite battery score of mobility comprised of performance in both locomotor and non-locomotor tasks). Interestingly, some of the prior work suggests that the association between COMT genotype and motor and cognitive function was most pronounced in men [107, 108]. While our sex-specific findings must be interpreted cautiously due to the sample size, they do suggest that there is an interrelationship between COMT genotype and motor function in women. Below we further interpret and discuss our findings.
Biosynthesis of DA commences with the hydroxylation of tyrosine by cytoplasmatic tyrosine hydroxylase to produce L-3,4-dihydroxyphenylalanine (L-DOPA) [110, 111]. Upon excitation of dopaminergic neurons, the synaptic vesicles are emptied into the synaptic cleft (degranulation) to interact with the postsynaptic DA receptors or regulatory presynaptic DA autoreceptors [112, 113]. To stop signaling, extracellular DA has to be removed from the synaptic cleft. It can either be recycled after reuptake by dopaminergic neurons or be degraded after uptake by glial cells. Neuronal reuptake by the DA transporter (DAT) [114] is followed by sequestration into the synaptic storage vesicles by the vesicular monoamine transporter 2. DA still accumulating in the cytosol, as a consequence of leakage from synaptic vesicles, is degraded by monoamine oxidase (MAO). Synaptic cleft DA is also taken up by surrounding glial cells. These cells readily degrade DA by MAO and also by COMT. COMT transfers methyl groups from S-adenosylmethionine (SAM) to hydroxyl groups of various catecholic compounds [115, 116]. COMT is expressed in the frontal cortex and striatum [117]. In the striatum, DATs are highly abundant and responsible for rapid dopamine uptake into dopaminergic terminals [118] where it is either packaged into storage vesicles or metabolized by MAO [119]. In contrast, DATs are both less abundant and located farther from synaptic sites in prefrontal cortex (PFC) neurons [120], where uptake by the norepinephrine transporter and subsequent metabolism by COMT predominates [121].
Thus, in general, it is believed that the increase in COMT activity conferred by the Val158 allele selectively influences dopamine signaling in the pre-frontal cortex more than the striatum. As such, our finding that COMT Val158Met polymorphisms are differentially associated with mobility may be interpreted to be due to COMT expression altering DA levels in regions of the frontal cortex that are involved in both cognitive and physical performance. This polymorphism has been linked to changes in executive function and visuospatial ability over a 5-year period in middle-aged and older adults (carriers of the Val allele (higher enzyme activity) compared with carriers of the Met158Met genotype (low enzyme activity) performed worse on executive functioning and visuospatial tasks, and individuals with the Val158Val genotype declined in executive functioning over the 5-year period, whereas carriers of the Met allele remained stable in performance) [122]. Our interpretation would also be consistent with (1) a previously suggested hypothetical inverted-U-shaped relationship between cortical DA state and PFC-dependent cognitive function [61] that is predicated on the intermediate Val/Met genotype being most optimal based on it theoretically balancing the roles of tonic and phasic DA (i.e., tonic–phasic regulation of DA transmission) [61–63] and (2) recent findings that executive function predicts decline in mobility after a fall [123].
We should note, however, that the function and effects of DAT (i.e., regulating striatal DA signaling) and COMT (contributing to regulation of DA in the cortex) are likely not so neatly separable as described above and commonly interpreted. For instance, reward anticipation and receipt drive result in increases in DA levels in both striatum and frontal cortex [124–126], and COMT influences the extent of this effect in the prefrontal cortex [127]. DA levels and COMT have also been shown to mediate the balance between habitual (model-free) and goal-directed (model-based) reinforcement learning [128–130]. Thus, while DAT predominates in the striatum and COMT in the prefrontal cortex, both are expressed to some extent in both regions [117, 120, 131], and reciprocal interactions between cortical and striatal dopamine [132, 133] suggest indirect effects likely exist. Accordingly, whether the actions of DAT and COMT are truly separable is questionable. Moreover, it has been suggested that in certain neurodegenerative disorders of the striatum, where dopaminergic axons are decreased in number, the lower affinity systems (e.g., COMT) become more important [111]. It is therefore difficult to know whether our findings can be attributed to dopaminergic influences arising from the prefrontal cortex, the striatum, or a combination of both. There are a few studies that have directly examined the striatal DA contribution to age-related changes in gait, balance, and other parameters of motor function using PET-imaging to assay striatal DA activity and denervation [134–138]. For instance, in 29 adults between 24 and 86 years, Volkow and colleagues noted that DA D2 receptor availability in the striatum was associated with a finger-tapping speed (r = 0.56 in both the caudate and putamen, p < 0.01) [138]. Additionally, in a study of 40 individuals 21–85 years old, it was found that, after accounting for age and binding potential, lower striatal DA transporter activity explained ~ 23% and 35% of the between-subject variance in “comfortable pace” gait speed and cadence, respectively [137]. Certainly, further work is warranted that parses out the relative contribution of dopaminergic function in the cortex and striatum, as well as the role of reward processing regions and mechanisms, in contributing to mobility limitations in older adults.
Analysis 3. Movement speed in older adults as a trait-like attribute of individuality
Movement vigor, a term that has largely arisen from the field of neuroeconomics, is commonly used in the context of describing elementary, stimulus-driven movements, such as saccades and reaching [67]. The operational definition is typically the inverse of the time from stimulus onset to movement completion, conditioned on distance (i.e., combination of reaction time and velocity) [67, 139]. While classic theories in motor control suggest that differences in movement vigor reflect a speed accuracy trade-off [140], recent data examining these competing hypotheses provided strong evidence indicating that movement vigor has no impact on end-point accuracy [141]. In recent years, neuroscientists have begun to conceptualize movement vigor as a trait-like attribute of individuality [67]. For instance, Reppert and colleagues recently reported a strong positive relationship between vigor of arm and head movements in young adults (r = 0.83, p < 0.01), with movement vigor not altering end-point accuracy [64]. Interestingly, peak horizontal saccade velocity—which theoretically should not be impacted by musculoskeletal mechanisms and processes—has been reported to decrease with age, with older adults demonstrating saccade velocity about half that of young adults [142].
We observed moderate associations in movement speeds across a broad variety of tasks, which supports the notion that movement speed is a trait-like attribute. These associations largely remained after adjusting for lean mass, relative strength, and age. However, based on the strength of the associations, it is clear there is room for other factors to influence movement speed. One potential explanation of movement speed being a trait-like attribute is that it is dependent on the subjective evaluation of reward and effort [64, 67]. A common aspect of individuality is our subjective preference in evaluation of reward and effort [143], and this prior work suggests that the degree to which people are willing to exert effort varies. Interestingly, these differences are associated with between-subject differences in the neural circuits that evaluate reward and effort, particularly circuits that regulate DA transmission [141]. Pathologic and pharmacologic changes in these circuits have been shown to alter patterns of decision-making (e.g., depression: less willing to exert effort; amphetamines: more willing to exert effort), and they have also been shown to affect patterns of elementary movements (e.g., saccades are slower in the case of depression and faster in the case of amphetamine [141]). These findings suggest overlap between the neural circuits that evaluate reward/effort and influence the decision of action selection with circuits that control movement vigor. Unfortunately, this interpretation is not testable with the current study design and should only be considered speculative.
Analysis 4. Mobility and the mOFC cortico-striatal loop network functional connectivity
The findings from experiment four may provide additional evidence supporting the speculation above related to the association between evaluation of reward/effort and vigor. Cortico-striatal circuits through the OFC, in particular, play key roles in complex human behaviors, such as evaluation, affect regulation, and reward-based decision-making [69]. In this experiment, we focused our attention on the mOFC cortico-striatal loop network, which is a specific basal ganglia circuit suggested to be involved in reward processing, motivation, and reward-guided learning [69–71]. In light of this, we hypothesized that its connectivity would be associated with movement speed. Our data indicate that increased mOFC cortico-striatal network connectivity and regional declines in structural integrity (increased WMH volume of the mOFC and decreased gray matter volume in selected regions of the basal ganglia) within the network are correlated with reduced mobility. While the network connectivity r values across the cohort and within the impaired mobility group are modest-to-moderate, this is relatively consistent with prior work examining the neural correlates of mobility with less variable neural metrics (gray matter and white matter integrity vs. the more variable network connectivity) and less variable behavioral metrics (gait speed vs. the more variable and holistic MBA score) [144, 145]. Specifically, mOFC cortico-striatal functional and structural neural correlates of the MBA score are in line with the prior established neural correlates of mobility decline with aging (white matter lesion, gray matter density, ventricular volume) (r = 0.2–0.4) [73, 146], suggesting that these neural correlates of mobility are meaningful but may have largely gone overlooked. Interestingly, we observed that, when adjusting for age, the associations were no longer significant. When there is a significant correlation between variables and the correlation disappears when adding a control variable, this indicates that both of those variables are correlated with the control variable. Thus, removing age takes away most of the apparent relationship between the two variables and suggests that many of the neuroimaging variables as well as MBA are associated with chronological age.
Our results are consistent with the “less wiring, more firing” hypothesis of neural activity compensation for aging-related structural integrity decline in those with reduced cognitive function [147]. The key difference being our study examined mobility instead of cognitive function, finding that increased functional connectivity and decreased structural integrity of key regions in the mOFC cortico-striatal loop network were correlated to reduced mobility. While our study design is cross-sectional and unable to attribute causality, it is possible that the increased network connectivity is secondary to the aging-related structural decline in an attempt to preserve functionality in those with poor mobility as has been previously hypothesized as white matter degrades or afferent fibers are reduced, synaptic responsiveness may increase [147]. There has been much debate over the precise causal contribution of various brain regions and networks to the mesolimbic DA system and thus reward evaluation. While our data provide a reasonable preliminary footing that the mOFC cortico-striatal network plays a role in mobility, and, thus, likely DA modulation, our mobility and imaging paradigm did not directly perturbate the dynamic attribution of incentive salience to reward-related stimuli. Future work examining the neural correlates of aging may consider paradigms that not only examine motor or cognitive function but effort-reward processes as well to determine their relative contribution to mobility decline in line with prior established mechanisms. Additionally, we should note that future studies using enhanced methods for acquisition of BOLD signal in the mOFC (an area that has been traditionally difficult to acquire) would help to expand our understanding of the role of this region in this network and processes.
Synthesis, integration, limitations, and conclusions
The majority of the prior work on mobility and aging has been focused on factors related to musculoskeletal mechanisms and processes. In this article, we present findings from a series of experiments/analyses that we believe provide theoretical support for the assertion that the basal ganglia, and its dopaminergic function, are responsible, in part, for age-related reductions in mobility capacity. In this context, we also discuss prior related work that supports this assertion. Because the data presented represent secondary analyses from a larger dataset, and the experiments were not directly designed to a priori test the respective hypotheses, many of the experiments have nuanced limitations. For instance, in experiment 2, our sample size is quite small for a genetics-based comparison, and in experiment 3, we used time to complete a task as a proxy for speed. Thus, we recommend cautious interpretation of these findings. However, collectively, these findings do align with recent basic and clinical science work suggesting that the basal ganglia, and its dopaminergic function, are mechanistically linked to age-related reductions in mobility capacity. Specifically, we report four findings: (1) slower nervous system rate of force development is associated with worse mobility among older adults; (2) older adults with the Val/Met intermediate genotype exhibit markedly greater mobility than their homozygous counterparts after statistically controlling for relative muscle strength and lean mass; (3) there are moderate relationships between performance times from a series of lower and upper extremity tasks, which supports the notion that movement speed in older adults is a trait-like attribute of individuality; and (4) there is a relationship of functional connectivity within the MOFC cortico-striatal network and measures of mobility, suggesting a potential neural mechanism for impaired mobility with aging is the deterioration of the integrity of key regions within the mOFC cortico-striatal network. Accordingly, we call for increased attention to the central neural mechanisms of age-related mobility limitations by the fields of gerontology and geriatric medicine.
Supplementary information
(DOCX 16 kb)
Acknowledgments
We thank the Genomics Shared Resource at the Ohio State University Comprehensive Cancer Center, Columbus, OH, for conducting the COMT genomics analyses.
Funding
This work was supported in part by grants from the National Institutes of Health (R01AG044424 to BC Clark and P30CA16058 to the Ohio State University’s Comprehensive Cancer Center).
Compliance with ethical standards
This study was reviewed and approved by the Ohio University Institutional Review Board and all study participants provided written informed consent.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Organization), W.W.H. Ageing and health. 2018 [cited 2020 September 15]; Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- 2.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility--giving mobility clinical visibility: a mobility working group recommendation. JAMA. 2014;311(20):2061–2062. doi: 10.1001/jama.2014.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musich S, Wang SS, Ruiz J, Hawkins K, Wicker E. The impact of mobility limitations on health outcomes among older adults. Geriatr Nurs. 2018;39(2):162–169. doi: 10.1016/j.gerinurse.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Shumway-Cook A, Ciol MA, Yorkston KM, Hoffman JM, Chan L. Mobility limitations in the Medicare population: prevalence and sociodemographic and clinical correlates. J Am Geriatr Soc. 2005;53(7):1217–1221. doi: 10.1111/j.1532-5415.2005.53372.x. [DOI] [PubMed] [Google Scholar]
- 6.Vermeulen J, Neyens JCL, van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr. 2011;11:33. doi: 10.1186/1471-2318-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, Sewall A, Goodpaster B, Satterfield S, Cummings SR, Harris TB, for the Health, Aging and Body Composition Study Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57(8):1411–1419. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 9.Gill TM, Allore HG, Hardy SE, Guo Z. The dynamic nature of mobility disability in older persons. J Am Geriatr Soc. 2006;54(2):248–254. doi: 10.1111/j.1532-5415.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 10.Simonsick EM, Newman AB, Visser M, Goodpaster B, Kritchevsky SB, Rubin S, Nevitt MC, Harris TB, for the Health, Aging and Body Composition Study Mobility limitation in self-described well-functioning older adults: importance of endurance walk testing. J Gerontol A Biol Sci Med Sci. 2008;63(8):841–847. doi: 10.1093/gerona/63.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26(2):130–135. doi: 10.1007/s11606-010-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manini TM, Visser M, Won-Park S, Patel KV, Strotmeyer ES, Chen H, Goodpaster B, de Rekeneire N, Newman AB, Simonsick EM, Kritchevsky SB, Ryder K, Schwartz AV, Harris TB. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55(3):451–457. doi: 10.1111/j.1532-5415.2007.01087.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhasin S et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc, 2020. [DOI] [PubMed]
- 14.Reid KF, Naumova EN, Carabello RJ, Phillips EM, Fielding RA. Lower extremity muscle mass predicts functional performance in mobility-limited elders. J Nutr Health Aging. 2008;12(7):493–498. doi: 10.1007/BF02982711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wages NP, Simon JE, Clark LA, Amano S, Russ DW, Manini TM, Clark BC. Relative contribution of muscle strength, lean mass, and lower extremity motor function in explaining between-person variance in mobility in older adults. BMC Geriatr. 2020;20(1):255. doi: 10.1186/s12877-020-01656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69(11):1375–1388. doi: 10.1093/gerona/glu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorond FA, Cruz-Almeida Y, Clark DJ, Viswanathan A, Scherzer CR, de Jager P, Csiszar A, Laurienti PJ, Hausdorff JM, Chen WG, Ferrucci L, Rosano C, Studenski SA, Black SE, Lipsitz LA. Aging, the central nervous system, and mobility in older adults: neural mechanisms of mobility impairment. J Gerontol A Biol Sci Med Sci. 2015;70(12):1526–1532. doi: 10.1093/gerona/glv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varma VR, Hausdorff JM, Studenski SA, Rosano C, Camicioli R, Alexander NB, Chen WG, Lipsitz LA, Carlson MC. Aging, the central nervous system, and mobility in older adults: interventions. J Gerontol A Biol Sci Med Sci. 2016;71(11):1451–1458. doi: 10.1093/gerona/glw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, Bennett DA, Black SE, Camicioli R, Carlson MC, Ferrucci L, Guralnik JM, Hausdorff JM, Kaye J, Launer LJ, Lipsitz LA, Verghese J, Rosano C. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68(11):1379–1386. doi: 10.1093/gerona/glt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199(1):43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 21.Dudman JT, Krakauer JW. The basal ganglia: from motor commands to the control of vigor. Curr Opin Neurobiol. 2016;37:158–166. doi: 10.1016/j.conb.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Deniau JM, et al. The pars reticulata of the substantia nigra: a window to basal ganglia output. Prog Brain Res. 2007;160:151–172. doi: 10.1016/S0079-6123(06)60009-5. [DOI] [PubMed] [Google Scholar]
- 23.Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990;13(7):254–258. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- 24.Berardelli A, et al. Pathophysiology of bradykinesia in Parkinson's disease. Brain. 2001;124(Pt 11):2131–2146. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- 25.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6(10):755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 26.Turner RS, Desmurget M. Basal ganglia contributions to motor control: a vigorous tutor. Curr Opin Neurobiol. 2010;20(6):704–716. doi: 10.1016/j.conb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panigrahi B, Martin KA, Li Y, Graves AR, Vollmer A, Olson L, Mensh BD, Karpova AY, Dudman JT. Dopamine is required for the neural representation and control of movement vigor. Cell. 2015;162(6):1418–1430. doi: 10.1016/j.cell.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 28.da Silva JA, Tecuapetla F, Paixão V, Costa RM. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature. 2018;554(7691):244–248. doi: 10.1038/nature25457. [DOI] [PubMed] [Google Scholar]
- 29.Kaasinen V, Rinne JO. Functional imaging studies of dopamine system and cognition in normal aging and Parkinson's disease. Neurosci Biobehav Rev. 2002;26(7):785–793. doi: 10.1016/s0149-7634(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 30.Carlsson A, Winblad B. Influence of age and time interval between death and autopsy on dopamine and 3-methoxytyramine levels in human basal ganglia. J Neural Transm. 1976;38(3–4):271–276. doi: 10.1007/BF01249444. [DOI] [PubMed] [Google Scholar]
- 31.Suhara T, Fukuda H, Inoue O, Itoh T, Suzuki K, Yamasaki T, Tateno Y. Age-related changes in human D1 dopamine receptors measured by positron emission tomography. Psychopharmacology. 1991;103(1):41–45. doi: 10.1007/BF02244071. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Chan GLY, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB, Stoessl AJ. Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse. 1998;30(1):56–61. doi: 10.1002/(SICI)1098-2396(199809)30:1<56::AID-SYN7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 33.Rinne JO, Hietala J, Ruotsalainen U, Säkö E, Laihinen A, Någren K, Lehikoinen P, Oikonen V, Syvälahti E. Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab. 1993;13(2):310–314. doi: 10.1038/jcbfm.1993.39. [DOI] [PubMed] [Google Scholar]
- 34.Wong DF, Young D, Wilson PD, Meltzer CC, Gjedde A. Quantification of neuroreceptors in the living human brain: III. D2-like dopamine receptors: theory, validation, and changes during normal aging. J Cereb Blood Flow Metab. 1997;17(3):316–330. doi: 10.1097/00004647-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Antonini A, Leenders KL. Dopamine D2 receptors in normal human brain: effect of age measured by positron emission tomography (PET) and [11C]-raclopride. Ann N Y Acad Sci. 1993;695:81–85. doi: 10.1111/j.1749-6632.1993.tb23033.x. [DOI] [PubMed] [Google Scholar]
- 36.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, MacGregor RR, Schlyer DJ, Hitzemann R, Wolf AP. Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18F-N-methylspiroperidol. Psychiatry Res. 1996;67(1):11–16. doi: 10.1016/0925-4927(96)02809-0. [DOI] [PubMed] [Google Scholar]
- 37.Stoessl AJ, Lehericy S, Strafella AP. Imaging insights into basal ganglia function, Parkinson's disease, and dystonia. Lancet. 2014;384(9942):532–544. doi: 10.1016/S0140-6736(14)60041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutherford BR, Choi J, Slifstein M, O'Boyle K, Abi-Dargham A, Brown PJ, Wall MW, Vanegas-Arroyave N, Sakhardande J, Stern Y, Roose SP. Neuroanatomical predictors of L-DOPA response in older adults with psychomotor slowing and depression: a pilot study. J Affect Disord. 2020;265:439–444. doi: 10.1016/j.jad.2020.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Fahn S, Jankovic J, Hallett M. Principles and Practice of Movement Disorders. 2nd ed. 2011. New York: Elsevier Saunders.
- 40.Pahwa R, Lyons KE, and Koller WC, Handbook of Parkinson’s Disease. 3rd ed. 2003, Boca Raton, Fl: Taylor and Francis Group.
- 41.Malling ASB, Morberg BM, Wermuth L, Gredal O, Bech P, Jensen BR. Effect of transcranial pulsed electromagnetic fields (T-PEMF) on functional rate of force development and movement speed in persons with Parkinson's disease: a randomized clinical trial. PLoS One. 2018;13(9):e0204478. doi: 10.1371/journal.pone.0204478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazzoni P, Hristova A, Krakauer JW. Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation. J Neurosci. 2007;27(27):7105–7116. doi: 10.1523/JNEUROSCI.0264-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose MH, Løkkegaard A, Sonne-Holm S, Jensen BR. Tremor irregularity, torque steadiness and rate of force development in Parkinson's disease. Mot Control. 2013;17(2):203–216. doi: 10.1123/mcj.17.2.203. [DOI] [PubMed] [Google Scholar]
- 44.Hauber W. Impairments of movement initiation and execution induced by a blockade of dopamine D1 or D2 receptors are reversed by a blockade of N-methyl-D-aspartate receptors. Neuroscience. 1996;73(1):121–130. doi: 10.1016/0306-4522(96)00036-x. [DOI] [PubMed] [Google Scholar]
- 45.Del Vecchio A, et al. You are as fast as your motor neurons: speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans. J Physiol. 2019;597(9):2445–2456. doi: 10.1113/JP277396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liddell EGT and Sherrington CS, Recruitment and some factors of reflex inhibition. Proceedings of the Royal Society of London. B., 1925. 97: p. 488–518.
- 47.Clark BC, Taylor JL, Hong SL, Law TD, Russ DW. Weaker seniors exhibit motor cortex hypoexcitability and impairments in voluntary activation. J Gerontol A Biol Sci Med Sci. 2015;70(9):1112–9. doi: 10.1093/gerona/glv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh MM, Disterhoft JF. Learning and aging affect neuronal excitability and learning. Neurobiol Learn Mem. 2020;167:107133. doi: 10.1016/j.nlm.2019.107133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landfield PW, Pitler TA. Prolonged Ca2+−dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226(4678):1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- 50.Moyer JR, Jr, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol. 1992;68(6):2100–2109. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- 51.Murphy GG, Fedorov NB, Giese KP, Ohno M, Friedman E, Chen R, Silva AJ. Increased neuronal excitability, synaptic plasticity, and learning in aged Kvbeta1.1 knockout mice. Curr Biol. 2004;14(21):1907–1915. doi: 10.1016/j.cub.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 52.Christie A, Kamen G. Short-term training adaptations in maximal motor unit firing rates and after hyperpolarization duration. Muscle Nerve. 2010;41(5):651–660. doi: 10.1002/mus.21539. [DOI] [PubMed] [Google Scholar]
- 53.Kalmar JM, Button DC, Gardiner K, Cahill F, Gardiner PF. Caloric restriction does not offset age-associated changes in the biophysical properties of motoneurons. J Neurophysiol. 2009;101(2):548–557. doi: 10.1152/jn.90617.2008. [DOI] [PubMed] [Google Scholar]
- 54.Christie A, Kamen G. Doublet discharges in motoneurons of young and older adults. J Neurophysiol. 2006;95(5):2787–2795. doi: 10.1152/jn.00685.2005. [DOI] [PubMed] [Google Scholar]
- 55.Duchateau J, Baudry S. Maximal discharge rate of motor units determines the maximal rate of force development during ballistic contractions in human. Front Hum Neurosci. 2014;8:234. doi: 10.3389/fnhum.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamen G, Sison SV, du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol. 1995;79(6):1908–1913. doi: 10.1152/jappl.1995.79.6.1908. [DOI] [PubMed] [Google Scholar]
- 57.Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci. 2004;59(12):1334–1338. doi: 10.1093/gerona/59.12.1334. [DOI] [PubMed] [Google Scholar]
- 58.Van Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol. 1998;513(Pt 1):295–305. doi: 10.1111/j.1469-7793.1998.295by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riwniak C, et al., Comparison of a multi-component physical function battery to usual walking speed for assessing lower extremity function and mobility limitation in older adults. J Nutr Health Aging, In Press. [DOI] [PMC free article] [PubMed]
- 60.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Schacht JP. COMT val158met moderation of dopaminergic drug effects on cognitive function: a critical review. Pharm J. 2016;16(5):430–438. doi: 10.1038/tpj.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 63.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41(1):1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 64.Reppert TR, Rigas I, Herzfeld DJ, Sedaghat-Nejad E, Komogortsev O, Shadmehr R. Movement vigor as a traitlike attribute of individuality. J Neurophysiol. 2018;120(2):741–757. doi: 10.1152/jn.00033.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi JE, Vaswani PA, Shadmehr R. Vigor of movements and the cost of time in decision making. J Neurosci. 2014;34(4):1212–1223. doi: 10.1523/JNEUROSCI.2798-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bargary G, Bosten JM, Goodbourn PT, Lawrance-Owen AJ, Hogg RE, Mollon JD. Individual differences in human eye movements: an oculomotor signature? Vis Res. 2017;141:157–169. doi: 10.1016/j.visres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Shadmehr R, Reppert TR, Summerside EM, Yoon T, Ahmed AA. Movement vigor as a reflection of subjective economic utility. Trends Neurosci. 2019;42:323–336. doi: 10.1016/j.tins.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salamone JD, Pardo M, Yohn SE, López-Cruz L, SanMiguel N, Correa M. Mesolimbic dopamine and the regulation of motivated behavior. Curr Top Behav Neurosci. 2016;27:231–257. doi: 10.1007/7854_2015_383. [DOI] [PubMed] [Google Scholar]
- 69.Fettes P, Schulze L, Downar J. Cortico-striatal-thalamic loop circuits of the orbitofrontal cortex: promising therapeutic targets in psychiatric illness. Front Syst Neurosci. 2017;11:25. doi: 10.3389/fnsys.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 71.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Hogan PS, Galaro JK, Chib VS. Roles of ventromedial prefrontal cortex and anterior cingulate in subjective valuation of prospective effort. Cereb Cortex. 2018. [DOI] [PMC free article] [PubMed]
- 73.Wilson J, Allcock L, Mc Ardle R, Taylor JP, Rochester L. The neural correlates of discrete gait characteristics in ageing: a structured review. Neurosci Biobehav Rev. 2019;100:344–369. doi: 10.1016/j.neubiorev.2018.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russ DW, et al. Development of a neuromuscular electrical stimulation protocol for sprint training. Med Sci Sports Exerc. 2012;44:1810–1819. doi: 10.1249/MSS.0b013e31825423f1. [DOI] [PubMed] [Google Scholar]
- 75.Clark BC, Manini TM, Wages NP, Simon JE, Clark LA. Voluntary vs electrically stimulated activation in age-related muscle weakness. JAMA Netw Open. 2019;2(9):e1912052. doi: 10.1001/jamanetworkopen.2019.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J. Rate of force development: physiological and methodological considerations. Eur J Appl Physiol. 2016;116(6):1091–1116. doi: 10.1007/s00421-016-3346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tavoian D, Ampomah K, Amano S, Law TD, Clark BC. Changes in DXA-derived lean mass and MRI-derived cross-sectional area of the thigh are modestly associated. Sci Rep. 2019;9(1):10028. doi: 10.1038/s41598-019-46428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szymkowicz SM, et al. Associations between subclinical depressive symptoms and reduced brain volume in middle-aged to older adults. Aging Ment Health. 2018. [DOI] [PMC free article] [PubMed]
- 79.Szymkowicz SM, Dotson VM, McLaren ME, de Wit L, O'Shea DM, Talty FT, O'Shea A, Porges EC, Cohen RA, Woods AJ. Precuneus abnormalities in middle-aged to older adults with depressive symptoms: an analysis of BDI-II symptom dimensions. Psychiarty Research: Neuroimaging. 2017;268:9–14. doi: 10.1016/j.pscychresns.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nissim, N.R., et al., Frontal structural neural correlates of working memory performance in older adults. Front Aging Neurosci, 2017 [DOI] [PMC free article] [PubMed]
- 81.McLaren ME, Szymkowicz SM, O'Shea A, Woods AJ, Anton SD, Dotson VM. Vertex-wise examination of symptom dimensions of subthreshold depression and brain volumes. Psychiatry Res. 2017;260:70–75. doi: 10.1016/j.pscychresns.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Shea A, et al. Cognitive aging and the hippocampus in older adults. Front Aging Neurosci. 2016;8:298. doi: 10.3389/fnagi.2016.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seider T, et al. Cognitively engaging activity is associated with greater cortical and subcortical volume. Front Aging Neurosci. 2016;8:1–10. doi: 10.3389/fnagi.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seider T, et al. Age exacerbates HIV associated white matter abnormalities. J Neuro-Oncol. 2016;22(2):201–212. doi: 10.1007/s13365-015-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szymkowicz SM, McLaren ME, O'Shea A, Woods AJ, Anton SD, Dotson VM. Depressive symptoms modify age effects on hippocampal subfields in older adults. Geriatr Gerontol Int. 2017;17(10):1494–1500. doi: 10.1111/ggi.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woods AJ, Hamilton RH, Kranjec A, Minhaus P, Bikson M, Yu J, Chatterjee A. Space, time, and causality in the human brain. Neuroimage. 2014;92:285–297. doi: 10.1016/j.neuroimage.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 88.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiao Y, Fonov V, Chakravarty MM, Beriault S, al Subaie F, Sadikot A, Pike GB, Bertrand G, Collins DL. A dataset of multi-contrast population-averaged brain MRI atlases of a Parkinsons disease cohort. Data Brief. 2017;12:370–379. doi: 10.1016/j.dib.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prodoehl J, Yu H, Little DM, Abraham I, Vaillancourt DE. Region of interest template for the human basal ganglia: comparing EPI and standardized space approaches. Neuroimage. 2008;39(3):956–965. doi: 10.1016/j.neuroimage.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmidt P. Bayesian inference for structured additive regression models for large-scale problems with applications to medical imaging. , in LMU München: Fakultät für Mathematik, Informatik und Statistik. 2017.
- 92.Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, Hoshi M, Ilg R, Schmid VJ, Zimmer C, Hemmer B, Mühlau M. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage. 2012;59(4):3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 93.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 94.Clark DJ, et al. Muscle performance and physical function are associated with voluntary rate of neuromuscular activation in older adults. J Gerontol A Biol Sci Med Sci. 2011;66(1):115–121. doi: 10.1093/gerona/glq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clark DJ, Manini TM, Fielding RA, Patten C. Neuromuscular determinants of maximum walking speed in well-functioning older adults. Exp Gerontol. 2013;48(3):358–363. doi: 10.1016/j.exger.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96(4):1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 97.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG: an update. J Appl Physiol (1985) 2014;117(11):1215–1230. doi: 10.1152/japplphysiol.00162.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119(7):1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baroni A, Benvenuti F, Fantini L, Pantaleo T, Urbani F. Human ballistic arm abduction movements: effects of L-dopa treatment in Parkinson's disease. Neurology. 1984;34(7):868–876. doi: 10.1212/wnl.34.7.868. [DOI] [PubMed] [Google Scholar]
- 100.Cacace F, Mineo D, Viscomi MT, Latagliata EC, Mancini M, Sasso V, Vannelli A, Pascucci T, Pendolino V, Marcello E, Pelucchi S, Puglisi-Allegra S, Molinari M, Picconi B, Calabresi P, Ghiglieri V. Intermittent theta-burst stimulation rescues dopamine-dependent corticostriatal synaptic plasticity and motor behavior in experimental parkinsonism: possible role of glial activity. Mov Disord. 2017;32(7):1035–1046. doi: 10.1002/mds.26982. [DOI] [PubMed] [Google Scholar]
- 101.Hsieh TH, Huang YZ, Rotenberg A, Pascual-Leone A, Chiang YH, Wang JY, Chen JJJ. Functional dopaminergic neurons in substantia nigra are required for transcranial magnetic stimulation-induced motor plasticity. Cereb Cortex. 2015;25(7):1806–1814. doi: 10.1093/cercor/bht421. [DOI] [PubMed] [Google Scholar]
- 102.Ghiglieri V, Pendolino V, Sgobio C, Bagetta V, Picconi B, Calabresi P. Theta-burst stimulation and striatal plasticity in experimental parkinsonism. Exp Neurol. 2012;236(2):395–398. doi: 10.1016/j.expneurol.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 103.Ko JH, Strafella AP. Dopaminergic neurotransmission in the human brain: new lessons from perturbation and imaging. Neuroscientist. 2012;18(2):149–168. doi: 10.1177/1073858411401413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Strafella AP, et al. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126(Pt 12):2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- 105.Monte-Silva K, Ruge D, Teo JT, Paulus W, Rothwell JC, Nitsche MA. D2 receptor block abolishes theta burst stimulation-induced neuroplasticity in the human motor cortex. Neuropsychopharmacology. 2011;36(10):2097–2102. doi: 10.1038/npp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Di Lazzaro V, et al. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol. 2008;586(16):3871–3879. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holtzer R, Ozelius L, Xue X, Wang T, Lipton RB, Verghese J. Differential effects of COMT on gait and executive control in aging. Neurobiol Aging. 2010;31(3):523–531. doi: 10.1016/j.neurobiolaging.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hupfeld KE, Vaillancourt DE, Seidler RD. Genetic markers of dopaminergic transmission predict performance for older males but not females. Neurobiol Aging. 2018;66:180 e11–180 e21. doi: 10.1016/j.neurobiolaging.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Metti AL, Rosano C, Boudreau R, Massa R, Yaffe K, Satterfield S, Harris T, Rosso AL. Catechol-O-methyltransferase genotype and gait speed changes over 10 years in older adults. J Am Geriatr Soc. 2017;65(9):2016–2022. doi: 10.1111/jgs.14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal. 2013;11(1):34. doi: 10.1186/1478-811X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Finberg JPM. Inhibitors of MAO-B and COMT: their effects on brain dopamine levels and uses in Parkinson's disease. J Neural Transm (Vienna) 2019;126(4):433–448. doi: 10.1007/s00702-018-1952-7. [DOI] [PubMed] [Google Scholar]
- 112.Werkman TR, Glennon JC, Wadman WJ, McCreary A. Dopamine receptor pharmacology: interactions with serotonin receptors and significance for the aetiology and treatment of schizophrenia. CNS Neurol Disord Drug Targets. 2006;5(1):3–23. doi: 10.2174/187152706784111614. [DOI] [PubMed] [Google Scholar]
- 113.Zhang H, Sulzer D. Regulation of striatal dopamine release by presynaptic auto- and heteroreceptors. Basal Ganglia. 2012;2(1):5–13. doi: 10.1016/j.baga.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eriksen J, Jorgensen TN, Gether U. Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges. J Neurochem. 2010;113(1):27–41. doi: 10.1111/j.1471-4159.2010.06599.x. [DOI] [PubMed] [Google Scholar]
- 115.Mannisto PT, et al. Characteristics of catechol O-methyl-transferase (COMT) and properties of selective COMT inhibitors. Prog Drug Res. 1992;39:291–350. doi: 10.1007/978-3-0348-7144-0_9. [DOI] [PubMed] [Google Scholar]
- 116.Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51(4):593–628. [PubMed] [Google Scholar]
- 117.Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116(1):127–137. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- 118.Cass WA, Zahniser NR, Flach KA, Gerhardt GA. Clearance of exogenous dopamine in rat dorsal striatum and nucleus accumbens: role of metabolism and effects of locally applied uptake inhibitors. J Neurochem. 1993;61(6):2269–2278. doi: 10.1111/j.1471-4159.1993.tb07469.x. [DOI] [PubMed] [Google Scholar]
- 119.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56(3):331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 120.Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18(7):2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kaenmaki M, et al. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J Neurochem. 2010;114(6):1745–1755. doi: 10.1111/j.1471-4159.2010.06889.x. [DOI] [PubMed] [Google Scholar]
- 122.de Frias CM, et al. Catechol O-methyltransferase Val158Met polymorphism is associated with cognitive performance in nondemented adults. J Cogn Neurosci. 2005;17(7):1018–1025. doi: 10.1162/0898929054475136. [DOI] [PubMed] [Google Scholar]
- 123.Hughes TF, Beer JC, Jacobsen E, Ganguli M, Chang CCH, Rosano C. Executive function predicts decline in mobility after a fall: The MYHAT study. Exp Gerontol. 2020;137:110948. doi: 10.1016/j.exger.2020.110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bassareo V, De Luca MA, Di Chiara G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci. 2002;22(11):4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bassareo V, De Luca MA, Di Chiara G. Differential impact of pavlovian drug conditioned stimuli on in vivo dopamine transmission in the rat accumbens shell and core and in the prefrontal cortex. Psychopharmacology. 2007;191(3):689–703. doi: 10.1007/s00213-006-0560-7. [DOI] [PubMed] [Google Scholar]
- 126.Ellwood IT, Patel T, Wadia V, Lee AT, Liptak AT, Bender KJ, Sohal VS. Tonic or phasic stimulation of dopaminergic projections to prefrontal cortex causes mice to maintain or deviate from previously learned behavioral strategies. J Neurosci. 2017;37(35):8315–8329. doi: 10.1523/JNEUROSCI.1221-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lapish CC, Ahn S, Evangelista LM, So K, Seamans JK, Phillips AG. Tolcapone enhances food-evoked dopamine efflux and executive memory processes mediated by the rat prefrontal cortex. Psychopharmacology. 2009;202(1–3):521–530. doi: 10.1007/s00213-008-1342-1. [DOI] [PubMed] [Google Scholar]
- 128.Wunderlich K, Smittenaar P, Dolan RJ. Dopamine enhances model-based over model-free choice behavior. Neuron. 2012;75(3):418–424. doi: 10.1016/j.neuron.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sharp ME, Foerde K, Daw ND, Shohamy D. Dopamine selectively remediates 'model-based' reward learning: a computational approach. Brain. 2016;139(Pt 2):355–364. doi: 10.1093/brain/awv347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Doll BB, Bath KG, Daw ND, Frank MJ. Variability in dopamine genes dissociates model-based and model-free reinforcement learning. J Neurosci. 2016;36(4):1211–1222. doi: 10.1523/JNEUROSCI.1901-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Laatikainen LM, Sharp T, Harrison PJ, Tunbridge EM. Sexually dimorphic effects of catechol-O-methyltransferase (COMT) inhibition on dopamine metabolism in multiple brain regions. PLoS One. 2013;8(4):e61839. doi: 10.1371/journal.pone.0061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clarke HF, Cardinal RN, Rygula R, Hong YT, Fryer TD, Sawiak SJ, Ferrari V, Cockcroft G, Aigbirhio FI, Robbins TW, Roberts AC. Orbitofrontal dopamine depletion upregulates caudate dopamine and alters behavior via changes in reinforcement sensitivity. J Neurosci. 2014;34(22):7663–7676. doi: 10.1523/JNEUROSCI.0718-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49(4):603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 134.Bohnen NI, Muller MLTM, Kuwabara H, Cham R, Constantine GM, Studenski SA. Age-associated striatal dopaminergic denervation and falls in community-dwelling subjects. J Rehabil Res Dev. 2009;46(8):1045–1052. doi: 10.1682/jrrd.2009.03.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cham R, Perera S, Studenski SA, Bohnen NI. Striatal dopamine denervation and sensory integration for balance in middle-aged and older adults. Gait Posture. 2007;26(4):516–525. doi: 10.1016/j.gaitpost.2006.11.204. [DOI] [PubMed] [Google Scholar]
- 136.Cham R, et al. Age-related striatal dopaminergic denervation and severity of a slip perturbation. J Gerontol A Biol Sci Med Sci. 2011;66(9):980–985. doi: 10.1093/gerona/glr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cham R, Studenski SA, Perera S, Bohnen NI. Striatal dopaminergic denervation and gait in healthy adults. Exp Brain Res. 2008;185(3):391–398. doi: 10.1007/s00221-007-1161-3. [DOI] [PubMed] [Google Scholar]
- 138.Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155(3):344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- 139.Sedaghat-Nejad E, Herzfeld DJ, Shadmehr R. Reward prediction error modulates saccade vigor. J Neurosci. 2019;39(25):5010–5017. doi: 10.1523/JNEUROSCI.0432-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394(6695):780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- 141.Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32(18):6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Irving EL, Steinbach MJ, Lillakas L, Babu RJ, Hutchings N. Horizontal saccade dynamics across the human life span. Invest Ophthalmol Vis Sci. 2006;47(6):2478–2484. doi: 10.1167/iovs.05-1311. [DOI] [PubMed] [Google Scholar]
- 143.Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the 'EEfRT'? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009;4(8):e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ewers M, Teipel SJ, Dietrich O, Schönberg SO, Jessen F, Heun R, Scheltens P, Pol L, Freymann NR, Moeller HJ, Hampel H. Multicenter assessment of reliability of cranial MRI. Neurobiol Aging. 2006;27(8):1051–1059. doi: 10.1016/j.neurobiolaging.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 145.McGonigle DJ, Howseman AM, Athwal BS, Friston KJ, Frackowiak RSJ, Holmes AP. Variability in fMRI: an examination of intersession differences. Neuroimage. 2000;11(6 Pt 1):708–734. doi: 10.1006/nimg.2000.0562. [DOI] [PubMed] [Google Scholar]
- 146.Zheng JJ, et al. Impact of white matter lesions on physical functioning and fall risk in older people: a systematic review. Stroke. 2011;42(7):2086–2090. doi: 10.1161/STROKEAHA.110.610360. [DOI] [PubMed] [Google Scholar]
- 147.Daselaar SM, Iyengar V, Davis SW, Eklund K, Hayes SM, Cabeza RE. Less wiring, more firing: low-performing older adults compensate for impaired white matter with greater neural activity. Cereb Cortex. 2015;25(4):983–990. doi: 10.1093/cercor/bht289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16 kb)




