Graphical abstract
Datumetine binds with NMDAR altering its calcium current. Acute exposure downregulates NMDAR signalling.

Keywords: Datumetine, NMDAR, Datura, Docking, Hippocampus
Highlights
-
•
Datura intoxication induces an amnesic experience.
-
•
N-methyl-d-aspartate receptor (NMDAR) is implicated in-memory processing.
-
•
Flexible docking showed datumetine binds with NMDAR.
-
•
Datumetine alters hippocampal NMDAR electrical activity.
-
•
Acute datumetine exposure alters NMDAR biological activity.
Abstract
The usage (abuse) of Datura metel is becoming increasingly worrisome among the Nigerian populace especially among the youth considering its side effects such as hallucination. This work was designed to identify the phytochemicals in datura plant that potentially interact with NMDAR as it affects the electrical and memory activities of the brain.
Ligand-protein interaction was assessed using autodock vina to identify phytochemicals that can interact with NMDAR. Datumetine was found to have the best interaction fit with NMDAR at both allosteric and orthosteric binding sites. Furthermore, using electrophysiological, behavioural and western blotting techniques, it was observed that the administration of datumetine positively modulates the NMDAR current by prolonging burst duration and interspike interval, induces seizures in C57BL/6 mice. Acute exposure leads to memory deficit on NOR and Y-maze test while immunoblotting results showed increased expression of GluN1 and CamKIIα while pCamKIIα-T286, CREB and BDNF were downregulated. The results showed that the memory deficit seen in datura intoxication is possibly the effects of datumetine on NMDAR.
1. Introduction
Drug or substance abuse is a global concern, posing serious mental, social and economic burden across all nations, with no exception to race, age or economic status. According to the United Nations Office of Drugs and Crime (UNODC), it is estimated that about 50 % of the global drug users reside in Africa [1], it was reported that this value might be more considering the difficulty in reaching some part of Africa and lack of proper documentation on drug usage within the continent [2]. Because of strict legislation on the usage of cannabis, heroin, and amphetamines, new psychoactive substances (NPS), especially from plants, are increasingly been used as alternatives by drug abusers. These new substances consumed are called ‘herbal high’ among its users [2,3]. The usage of these plants has become worrisome because no legislations are preventing their use [2,3].
Africa alone saw the emergence of 3 NPS, out of which 2 are plants that grown widely within the continent, one of the plants is Datura metel [3]. D. metel also refered to angel’s trumpet belongs to the Solonaceae family [[4], [5], [6]]. The plant usually grows to a height of 1.5 m with dark violet stem, the leaf is usually ovate and/or broadly ovate asymmetrical with erect flowers while the fruits are round with spiny bark [4]. The plant grows widely in the bush and mostly around dumpsites. The plant has been reported to be a good source of bio-fuels with rich phenolics, aromantic, nitrogenated and oxygenated compounds with high promising calorific value [7]. The plant has been showed to be a good potential of biofuel [8].
Despite its potential usefulness, the plant has gained psychoactive use among the adolescent especially secondary school students in Nigeria [9,10]. In Nigeria, D. metel accounts for 0.08% of drug abuse [11]. The recreational use of datura is connected to its hallucinogenic property [12]. The plant has been reported to contain tropane alkaloid which acts by blocking acetylcholine through muscarinic receptors [[13], [14], [15]]. Due to its anticholinergic property, the plant is very toxic and high dose can result in death [16].
Widely reports indicated that abusers of the plant do not remember any event(s) during the intoxication phase, and as such characterized as mind-altering and date rape drug [10,17]. A report showed that thieves used this plant to dispossess their victims of their belongings as they do not remember anything during the intoxication [18]. Most research works have focused on the tropane alkaloids of the plant elucidating its hallucinogenic properties and anticholinergic properties and further attributed to atropine, scopolamine, and hyoscine [17,19,20]. However, there is a dearth of information on the biological activity of other components of the plant.
The acute and short amnesia experienced by the users of the plant suggests the probability of interactions between phytochemicals in the plant and NMDAR in the brain, especially in the hippocampus which usually results in the intoxication as experienced by the users. Hence, the present study was designed to identify the basic mechanisms leading to the short-term memory deficit following exposure.
2. Materials and methods
2.1. Ethical clearance
Ethical approval was obtained from the University of Ilorin Ethical Review Committee (UERC) with approval number UERC/ASN/2018/1277 and protocol identification code UERC/BMS/108, together with the approval of Louisiana State University IACUC.
2.2. Ligand docking
The structure of compounds that have been isolated from Datura plants [21] was simulated against NMDAR. The simulation was performed using Autodock Vina version 4.2.6 (USA) [22]. The simulation was achieved using a flexible docking to get the compounds that bind to the binding sites of NMDAR. Structure of the NMDAR and compounds were retrieved from the protein data bank (PDB) and PubChem respectively. The compound with the lowest binding energy was then used for biological activity testing to evaluate its biological implication of binding with NMDAR. Different intermolecular forces that contribute to the free energy of some of the stable binding compounds were further evaluated using autodock4.2 [23], and the results are supplied as supplementary materials.
2.3. Purchase and preparation of datumetine
Datumetine (CAS No.: 67078-20-0 and Catalog No.: CFN00214) was purchased from ChemFaces Biochemical Company China. The compound was dissolved in dimethyl sulfoxide (DMSO) to achieve 1 mg/mL stock solution and stored at 4 °C. It was then serially diluted based on the dosage of animals required for working solution (kept at room temperature) to administer to animals.
2.4. Animal care
Thirty (30) adult C57BL/6 mice procured from Jackson’s Laboratory (Bar Harbor, ME, US) were used for the experiment. They were housed under standard laboratory condition of 12 h alternating light and dark cycle. They were kept in standard cages of five animals/cage. Feed and water were given ad libitum. The handling of animals was approved by Louisiana State University- Institutional Animal Care and Use Committee.
2.5. Drug treatment
The animals were divided into three groups of 10 mice each. Animals were administered 0.25 mg/kg (0.25 Datumetine) and 1.0 mg/kg (1.0 Datumetine) body weight of datumetine while the control mice received 0.1 mL of DMSO (DMSO-control). All treatments were done intraperitoneally for 14 days.
2.6. Electrophysiology
The electrical activity of the brain was recorded on four anaesthetize (100 mg/kg of ketamine +10 mg/kg xylazine) mice to evaluate the datumetine effect on NMDAR linked Ca2+ current. When the animals were deeply sedated, they were tested for toe and tail pinch reflex. They were then fixed on a stereotaxic frame. A 7 mm by 7 mm craniotomy was done to expose the dura and drops of artificial cerebrospinal fluid (aCSF) was applied to the exposed area to prevent dryness. A 10 mm long and 50 μm thick shank of the acute neural probe was used (Neuronexus, Michigan, USA). The probe shank with four electrodes arranged as a tetrode, with an inter-electrode distance of 25 μm was used. The electrodes were connected to a pre-amplifier head stage (Intantech, California, USA), tethered to a recording controller and amplifier system (Intantech, California, USA) (see Fig. 1). The electrode was gently lowered down into the brain tissue using an ultrafine (μm range) hydraulic micromanipulator (Narishige, Japan) to reach the CA1-DG field at stereotaxic coordinates (AP: 1.94 mm, ML: 1.0 mm, DV: 1.5 mm) relative to the Bregma. Stainless steel ground wires soldered onto the head stage-electrode adapter (Neuronexus; A4 to Omnetics CM32 adapter) were tied to ground screw that was fixed on the occipital bone. The stereotaxic apparatus, micromanipulator, electrode and subject mouse were kept in a Faraday cage and connected to the amplifier ground.
Fig. 1.
Schematic diagram showing the placement and connection of electrode in the mice brain. The electrode was inserted into the CA1 region of the hippocampus.
At the onset of each recording procedure, the tested impedance of the electrodes was at 1 kHz. For all recording sessions in this experiment, impedance measurement for the tetrodes ranged between 0.6–3.1 megΩ. Single unit activity was recorded by setting the cut-off frequency at 250 Hz and 7.5 kHz for proper and lower limit respectively, sampled at 30 kHz/s. Neural activity was monitored for 20 min to ensure the stability of the animal’s vitals before the commencement of recordings. Baseline recording was done for 30 min for each animal, after which 0.1 mL of 0.07 μM of datumetine was injected to the animals and another recording was taken for 30 min.
The continuously recorded action potential spike trains were analysed in an Offline Spike Sorting Software (OFSS) Version 4 (Plexon Inc., Dallas, USA). Neural spikes were extracted from the continuous data through threshold crossing in the OFSS. The extracted spikes were sorted into clusters using a combination of Valley seeking and K-means clustering methods. Spikes were assigned to single unit clusters through a 3-dimensional space principal component analysis (PCA) projection. Further analysis of the sorted spikes was done in Neuroexplorer Version 5 (Nex Technologies USA). Sorted neural spike waveforms, clustered units, and upsampled continuous data were exported into the Neuroexplorer software for analysis of spike properties.
2.7. Behavioural studies
Memory function of the animals was assessed using Y-maze and Novel object recognition (NOR) test. Animals were familiarised with the behavioural room and apparatus three days before the experimental days. The behavioural protocol was captured and analyzed using EthoVision version 13 tracking software (Noldus Information Tech. the Netherlands).
2.8. Y-maze
This was done to ensure the hippocampal-dependent spatial working memory of the mice. The mice were placed facing the edge where two arms meet and left to make their own decisions of the arms to follow. The duration of the test was for 5 min. Visiting three arms consecutively was termed right decision (right) while visiting one arm twice in three alternations was termed a wrong decision (wrong). Percentage alternation (index of memory) was calculated for the mice using the formula:
2.9. Novel Object Recognition (NOR) Test
This was to assess the cortical dependent non-spatial working memory. The mice were exposed in an open chamber to 2 identical objects to acclimatize with them for 5 min which is termed as trial 1 (T1). The mice were taken back to their cage with food and water. One hour later i.e. intertrial interval (ITI), the mice were put back in the chamber with one of the objects being replaced by a novel one for five minutes. The time used in rearing the old (old time) and a new object (new time) was recorded. Animals were considered rearing the object when the centre body of the animal was less than 2 cm from the object while sitting on the object was not considered. The memory index was calculated using the formula:
2.10. Animal sacrifice
The animals were sacrificed 24 h after the last administration. The animals were deeply anaesthetized in an isoflurane gas chamber. They were then perfused transcardially with 10 mM phosphate-buffered solution (1X PBS pH 7.4), brains excised, and the hippocampus dissected out on a cold flat surface, kept inside 1X PBS containing specimen bottle and stored at −20 °C till further processing.
2.11. Western blotting
Samples for western blotting were homogenized in 10 times volume of radioimmunoprecipitation assay buffer (RIPA) buffer (Lot 2912060 EMD Millipore Corp. Billerica USA) to the weight of the specimen, using hand-held homogenizer and kept on ice for 30 min and vortexed every 10 min. They were then saponified (using PRO200 Bio-Gen series homogenizer Oxford USA) on ice for 5 min, centrifuged (using accuspin Micro 17R Fisher Scientific USA) at 12,000 rpm for 15 min at 4 °C. The supernatant was collected in another tube for further processing.
Total protein concentration was assayed using Pierce BCA assay kit (Lot 23225 Thermo scientific Rockford USA) and the protein concentration was evaluated based on the standards using microplate reader (Tecan Spark® Männedorf, Switzerland) at 562 nm wavelength. Proportional dilution was made based on the estimated protein concentration and 10 μg/μL solution was made and half volume of laemmli SDS buffer (Lot Y18C501) was added for loading on the gel well.
Gel Electrophoresis was done using 8% gel (for protein >100 kDa) and 12% gel (for protein <100 kDa) (see supplementary materials for details of the gel composition). Samples were loaded in the well starting with the ladder (loading standard) and the samples (10 μL each well). Electrophoresis was done at 140 mA, 300 V for 80 min (current was constant) using PS300B 300 V supply (AA Hoefer Holliston, USA) [24].
At the end of electrophoresis, the protein was transferred to polyvinylidene difluoride (PVDF) membrane. The PVDF membrane was placed in methanol for 1 min for activation and later transferred to transfer buffer (Tris base, glycine, methanol, and distilled water) and rock for 10 min. The stacking gel was released from the electrophoresis machine and quickly transferred to the transfer buffer. The transfer sandwich was made by taking the cassettes and the black part facing down inside the transfer buffer. Foam, blotting-pad, gel, membrane, blotting-pad and foam were arranged in the cassette in the order mentioned. The cassettes were closed and clipped before placing in the transfer machine with all-black part facing back. The transfer machine was filled with transfer buffer and ran at 100 V, 400 mA (current constant) for 70 min.
When completed, the membrane was transferred to 10 mM Tris-buffered saline (1X TBS) and stored at 4 °C until staining.
Staining of the membrane was done by washing twice in 1X TBS containing 2% triton-X (1X TBST) for 10 min each. Membranes were blocked in 3% bovine serum albumin (BSA) (Cat: BP671-10, Fisher Scientific USA) prepared in 1X TBST for 55 min at room temperature on a gentle rocker. Membranes were then incubated in primary antibodies diluted in 3 % BSA; anti-CREB (9197S 1:1000, 43 kDa), anti-Trk/BDNF (92991S 1:1000, 120−140 kDa), anti-CamKII-α (3357S 1:1000, 50 kDa), anti-pCamKII-αT286 (12716S 1:1000, 60 kDa) and anti-NMDAR (GluN1) (5704S 1:1000, 120 kDa) all from Cell Signaling Tech. Danvas MA, the USA at 4 °C overnight.
The next day, the membranes were rinsed twice in 1X TBST followed by washing twice in 1X TBST for 10 min each. Membranes were then incubated in HRP conjugated Chicken anti-rabbit antibody (3:10,000 A15987 Invitrogen Fisher Scientific USA) for 60 min at room temperature. Membranes were then washed in 1X TBST twice for 10 min and stored in 1X TBS for viewing.
Stained membranes were viewed by developing the substrate using ECL WB substrate (TF 268244 Thermoscientific Rockford USA). 2 mL of solution A and B were mixed. Stained membranes were incubated in the solution for 70 s. They were then placed inside a plastic film and rolled to sleek. These were then viewed using the Bio-Rad Chemidoc Touch Imaging machine (V3 Bio-Rad USA) at 100 s exposure time.
House-keeping protein (beta-actin) was later stained for by stripping off antibodies and staining molecules using 10 mL of stripping buffer (Restore plus WB stripping buffer 46430 Thermoscientific USA). They were then rinsed 6 times in 1X PBST and washed twice in 1X PBST for 10 min each. Later they were washed in 1X TBS and 1X TBST twice for 10 min each. They were blocked in 3 % BSA for 55 min and incubated in horseradish peroxidase (HRP) conjugated ß-actin antibody (3:10000, 12262S Cell Signaling Tech. Danvas MA, USA) for 60 min at room temperature. The steps after antibody incubation to imaging was repeated to capture the expression of ß-actin.
Quantification of the blotting volume/density was done using Image Lab version 6.0.1 Standard edition Bio-Rad Laboratories Inc. The USA.
2.12. Statistical analysis
All statistical analysis was done using GraphPad Prism Version 7.0 (San Diego, USA) Quantitative data were expressed as mean ± SEM in bar charts. Electrophysiology data were analysed using unpaired t-test with Welch’s correction if variance showed a significant difference. Other data were analysed using one- way analysis of variance (ANOVA) and Tukey post-hoc test was done when ANOVA showed significant with the p-value set at 0.05.
3. Results
3.1. Datumetine showed the best binding fits to NMDA receptor
Simulation of structures of compounds found in Datura plants against NMDAR at its orthostatic and allosteric binding site revealed that datumetine (an alkaloid) possess the most negative ΔG value which indicates that it will form a stable binding complex with the receptor at both sites (see Fig. 2). The free energy was found to be -8.3 kcal/mol at the allosteric binding site and -8.6 kcal/mol at the orthostatic binding site (See supplementary file for the docking score).
Fig. 2.
Representative diagram showing the orientation of datumetine binding to NMDAR generated from the simulation software. Datumetine interacts with NMDAR at Tyr144.
3.2. Datumetine induces epileptic episodes in mice
Animals treated with datumetine showed signs of epilepsy immediately after the treatment (See video 1a–c). This persists for a while and reverses back later. Animals treated with 1 mg/kg showed longer seizure time than those treated with 0.25 mg/kg. This symptom later reduces with time as the treatment progresses and animals showed little to no sign of epilepsy towards the end of treatment. However, this persists in animals treated with 1 mg/kg of datumetine.
3.3. Datumetine prolonged firing activity of bursty hippocampal neurons
We recorded multiple single units from the CA1 region of the hippocampus in 4 mice. Infusion of datumetine to anaesthetized animals under-recording prolonged the firing activity of the hippocampal neurons (see Fig. 3). Before the infusion of the drug, the burst activity occurred in a few milliseconds, but after the datumetine was infused to the animals the burst activities increased to a few seconds. Increase in a burst duration could indicate datumetine as a positive modulator of the NMDAR by increasing the time the pore of NMDAR is kept open for Ca2+ movement. The interspike interval histogram (ISIH) for single units was used to classify the burst firing into bursty, tonic and irregular based on the shape of their waveforms and firing properties (see supplementary materials). Burst firing is the characteristics of the hippocampal glutamate neurons. These were then analysed to get the burst properties.
Fig. 3.
Representative diagram showing the spike train of experimental animals during recording. Both groups of animals showed burst activity with different characteristics.
3.4. Datumetine alters hippocampal action potentials
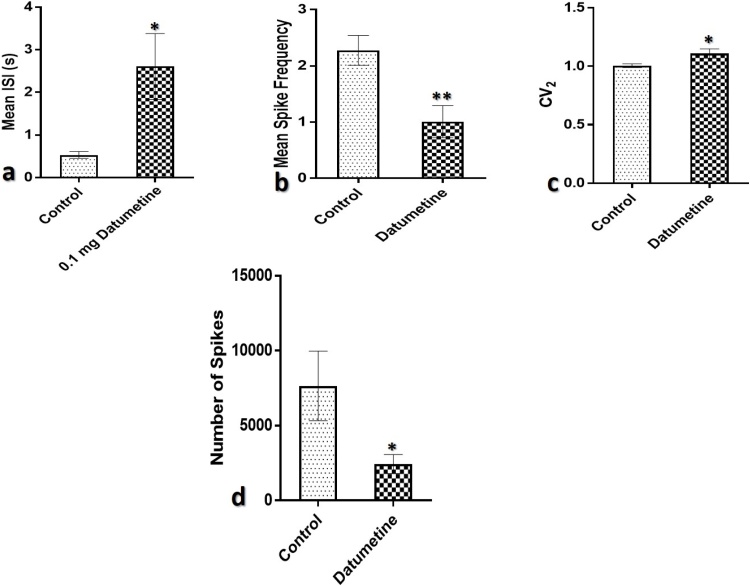
Spontaneous evoked neural spikes in the CA1-DG field were analysed to determine the impact of datumetine on NMDAR electric current. The time interval between spikes (ISI) which represents neural refractoriness was significantly increased in animals infused with datumetine compared to control (Fig. 4a). The degree of distribution of spikes was measured using CV2 analysis. Values less than 1 showed increase regularity in firing while values greater than 1 indicates irregular firing activity. Bursts neurons from the experimental animals showed that the spikes were of Poisson process in the datumetine group as they have values greater than 1 while that of control is less than 1 (Fig. 4c). Statistical analysis showed that exposure to datumetine significantly increased the irregularity of spikes distribution compared to the control.
Fig. 4.
Graphical representation of spike properties of hippocampal neurons from electrophysiology recordings.
(a) represents the inter-spike interval which is significantly higher in datumetine infused animals.
(b) represents the number of spikes in a second (mean spike frequency) which is significantly reduced in datumetine exposed animals.
(c) represents the CV2 which showed regularity of spikes, datumetine induce irregularity of spikes.
(d) represents the number of spikes which is significantly reduced in datumetine exposed animals (*p < 0.05, **p < 0.01).
Individual action potential activity in the representation of spikes was significantly reduced in datumetine infused animals (*p < 0.05) compared to control (Fig. 4d). The number of spikes in a second termed as the frequency of spikes was significantly reduced (**p < 0.01) compared to control (Fig. 4b) indicating spike adaptation.
3.5. Datumetine increased burst activities of bursts neurons
Burst is defined as repeated action potential with 0.5 ms interval. The number of bursts was found to increase in animals infused with datumetine than the control (Fig. 5a) during the recording session. This increase was not statistically significant. The number of bursts activity per minute was significantly reduced (*p < 0.05) in animals infused with datumetine (Fig. 5b) than control.
Fig. 5.
Graphical representation of the burst properties of hippocampal neurons from electrophysiology recording.
(a) represents the number of bursts, no significant difference is observed between groups.
(b) represents the bursts per minute, datumetine significantly reduce the number of bursts in a minute.
(c) represents the mean interburst interval which is significantly increased in datumetine exposed animals.
(d) represents the mean burst duration which is prolonged in datumetine exposed animals but not statistically significant (*p < 0.05).
The average time interval between each burst termed as the mean inter-burst interval (IBI) was found to be significantly increased in datumetine infused animals (*p < 0.05) than the control (Fig. 5c). Burst duration was increased in datumetine infused animals than the control (Fig. 5d). No statistical significance was observed in the duration of the burst.
3.6. Acute datumetine exposure induces memory deficit in mice
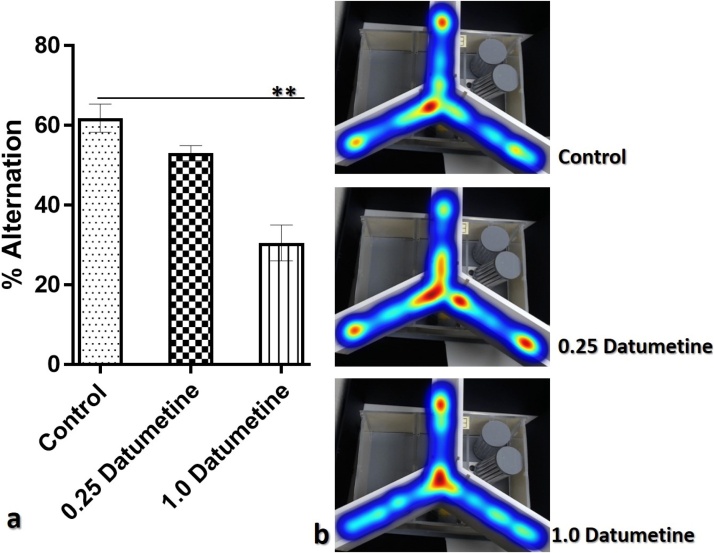
The result from the Y-maze experiment showed that animals treated with 1 mg/kg showed a significant decline in memory index (% alternation) compared to control (**p < 0.01). No significant difference was observed in % alternation in animals treated with 0.25mg/kg compared to control, and 1mg/kg treated mice (Fig. 6).
Fig. 6.
Results of the Y-maze protocol.
(a): graphical representation of memory index (% alternation) of experimental animals. 1.0 Datumetine is significantly lower than the control (**p < 0.01).
(b): Representative heatmap visualization of animal activity during the Y-maze test. 1.0 Datumetine spends more time at the centre than other groups.
Result of the novel object recognition test showed a significant decline in the memory index of animals exposed to datumetine compared to control (*p < 0.05, **p < 0.01) (Fig. 7). No significant difference was observed between the datumetine exposed groups.
Fig. 7.
Results of NOR test.
(a): graphical representation of memory index of experimental animals. All animals exposed to datumetine showed significant memory deficit compared to control (*p < 0.05, **p < 0.01).
(b): representative heatmap visualization of animal activity during NOR test.
3.7. Datumetine increases the expression of NMDAR with downregulation of downstream signalling molecules
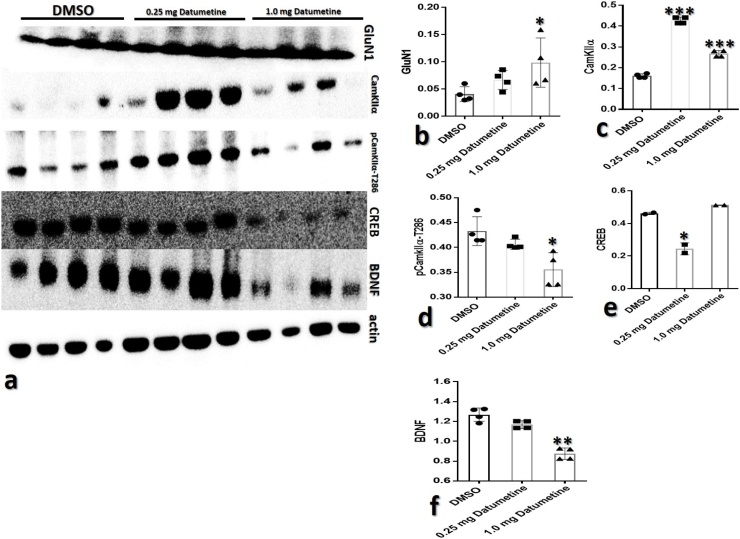
Fig. 8a showed the representative immunoblots of NMDAR and downstream signalling molecules studied. Expression of NMDAR was assessed using GluN1, mice exposed to datumetine showed a steady rise in the relative expression of NMDAR with 1.0 Datumetine mice showing the highest significant (*p < 0.005) increase compared to the control (Fig. 8b).
Fig. 8.
Datumetine effects on NMDAR signalling.
(a): representative immunoblots of GluN1, CamKIIα, pCamKIIα-T286, CREB, BDNF and actin expression in the cell lysates of the hippocampus of experimental animals;
(b): quantification of relative GluN1 expression;
(c): quantification of relative CamKIIα expression;
(d): quantification of relative pCamKIIα-T286 expression;
(e): quantification of relative CREB expression; and
(f): quantification of relative BDNF expression.
(n = 4 per group, *p < 0.05, **p < 0.01, ***p < 0.001 one-way ANOVA with Tukey post-hoc test).
Cam KII-α which is NMDAR C-terminus associated molecule important in information processing and storage was evaluated. Datumetine exposure significantly increased (***p < 0.0001) the relative expression of CamKII-α compared to the control. The level of increase was inversely correlated to the dose of datumetine exposure as animals exposed to 1.0 mg/kg showed a reduction in the level of CamKII-α compared to 0.25 Datumetine mice (Fig. 8c). CamKII-α is auto-phosphorylated at Threonine-286 upon NMDAR activation. The level of phosphorylated CamKIIα (pCam-T286) was reduced in mice exposed to datumetine, this reduced expression was significant in mice exposed to 1.0 mg/kg of datumetine compared with the control (Fig. 8d).
Two downstream NMDAR signalling molecules expression were assessed. CREB a major signalling molecule of NMDA receptor activation was significantly reduced in 0.25 Datumetine mice when compared to the control with no significant difference between the 1.0 Datumetine mice and the control (Fig. 8e). BDNF, another NMDAR signalling molecule which also serves as neuroprotective protein was significantly reduced in mice exposed to datumetine when compared to control (Fig. 8f).
4. Discussion
Datura plant has been reported to possess other compounds aside the major alkaloid that has been previously identified [[25], [26], [27], [28], [29], [30], [31], [32], [33]]. Recent studies have reported other biological effects of the plant which has not been attributed to any specific compound in the plant [6,34]. Since recreative users of the plant experience amnesic-like behaviour during its intoxication process, the study hypothesizes that the plant may contain compounds that affect hippocampal functioning.
4.1. Datumetine docks with NMDAR
Docking has been a veritable tool in screening a large library of compounds against the protein of interest to show their interaction [35,36]. Our docking result revealed that datumetine forms a best binding fit to NMDAR when simulated.
NMDAR has been identified as a major ionic receptor in hippocampal functioning [[37], [38], [39]]. Interesting our results for the first time showed that D. metel not only to have anticholinergic properties [13,14], yet there are other compounds present in the plant that can interact with various ionic receptors in the brain. Datumetine was simulated against other ionic receptors in the brain and it showed no binding fits with others indicating that it is specific for NMDA receptor.
4.2. Datumetine alters electrical activities of hippocampal neurons
Docking result only shows the possibility of binding/interaction between a ligand and a protein but the biological activity of this interaction cannot be evaluated. This led to the further investigation of the functional effects of NMDA receptor Ca2+ current in the hippocampus (CA-DG field) when datumetine was added. Based on the firing properties of the neurons, burst neurons activities were further characterized. Datumetine prolongs the duration of the firing rate between spikes. This showed that the opening of NMDAR channels was extended indicating prolong refractory period. Synaptic NMDAR binds with glutamate in the presence of glycine allow Ca2+ influx into the neuron and K+ efflux leading to depolarization in few milliseconds [40]. We hypothesize that the prolong ISI occurs when datumetine binds to NMDAR to keep the channel opened for more duration of time allowing for more ion exchange until either the receptor is desensitized or datumetine is metabolized. The prolong refractory period of datumetine is further evident by the reduction in the number of spikes and frequency of spikes recorded in the burst firing neuron population. Sabatier et al. [41], reported that prolong ISI is due to Ca2+ rush leading to hyperpolarising after potential (HAP) effect. Reduced mean spike frequency has been reported to be an adaptation changes of the membrane to overstimulation [42]. This change is reported to be due to either HAP [43], excitation and inhibitory interaction [44] or receptor fatigue [42]. The regularity of firing which was represented by the CV2 analysis showed that datumetine alters the regularity of firing of hippocampal neurons. Hippocampal neurons fire in synchrony correlating input with output [45,39], while datumetine altering this synchrony may increase error in the hippocampal behavioural task as reported in other literature [[46], [47], [48], [49]].
Burst neuron showed an increase in the number of burst activities together with the duration of burst after datumetine treatment. This showed that datumetine act as a positive potentiator of the NMDAR by increasing the NMDAR’s activity. Datumetine prolongs the interval between bursts and reduces the rate of burst occurrence. This is in line with the prolong refractory period induced by datumetine binding.
4.3. Datumetine induces memory deficit
Administration of datumetine induces a seizure in animals. This showed that datumetine has a positive modulatory effect on the NMDAR. Epilepsy usually arises as a result of hyperactivity of excitatory neurotransmission or reduced activity of inhibitory neurotransmission [50]. This showed that datumetine acts by enhancing NMDAR conductance since it will readily bind to it. The memory effect of datumetine-NMDAR interaction was later assessed by evaluating the animal’s behaviour on NOR and Y-maze after long term exposure. The hippocampal-dependent task of Y-maze was significantly impaired in animals exposed to a high dose of datumetine. This could have resulted because of the erroneous synchrony of hippocampal firing that was observed in the electrophysiology recordings. NOR result indicates memory decline in all animals exposed to datumetine. This indicates the memory effect of NMDAR electrical activity. Memory decline has been attributed to datura use [51], although some authors attributed this effect to the anticholinergic properties of the plant [[52], [53], [54], [55], [56]]. Our results have shown that tropane alkaloids are not only phytochemical in D. metel that induce memory deficit and that datumetine can also play a major role.
4.4. Datumetine alters NMDAR signalling
NMDAR activation allows for Ca2+ influx to mediate diverse intracellular signalling [57]. Since datumetine is suspected to enhance NMDAR activity, signalling pathway of NMDAR is expected to be favoured. Long term exposure to datumetine increased the expression of GluN1. This increment has been attributed to response in altered excitatory neurotransmission [58]. Increase GluN1 expression has been reported to be caused by an increase in NMDAR activity [58]. This observation is similar to what is seen in chronic alcohol exposure [59]. Although alcohol has been reported to enhance inhibitory transmission in the brain [60], chronic alcohol exposure has shown to enhance NMDAR activity [61,62]. Similarly, neuropsychiatry disorders such as stroke, ischaemia and mood disorders that are characterised with hyperactivity of NMDAR showed increased expression of NMDAR in post mortem brain samples [[63], [64], [65]]. Preclinical studies have reported that NMDAR antagonist alleviates the symptoms [66] alluding to the fact that hyperactivation of NMDAR is part of the aetiology of these disorders. This result is different from the report of Cristino et al. [67], who showed that indirectly increasing NMDAR activity by knocking out gene coding for D-aspartate oxidase reduces GluN1 expression in the hippocampus with increased GluN1/AMPAR ratio. This difference may be due to the overall altered glutamate homeostasis observed in their study.
CamKII-α is implicated in NMDAR signal and memory encoding [68]. It is a holoenzyme that is autophosphorylated by NMDAR activation [69], reported being responsible for the bidirectional effects of NMDAR signalling [70]. Datumetine significantly increased the expression of CamKII-α similar to the report that chronic activity of CamKII-α is neurotoxic [70]. Reports from psychosocial stress research caused by the hyperfunction of NMDAR [71] showed reduced basal CamKII and phosphorylated form [72] which can be attributed to the dysregulation of NMDAR activity and modification of downstream molecules [73]. Increased NMDAR activity is also implicated in opioid-induced hyperalgesia (OIH) [74] which is also associated with increased Cam\KII activity as reported by Qi et al. [75] that blocking CamKII activity reverses OIH in rodents. Increased CamKII expression due to enhanced NMDAR activity is also reported in chronic alcohol consumption [76] and is well correlated with alcohol-drinking behaviours [77].
Although CamKII-α is tightly regulated by autophosphorylation upon NMDAR activation through Ca2+ [78]. Level of pCamKII-α-T286 was reduced in animals exposed to datumetine. CamKII-α is associated with GluN2A subunit of NMDAR [79], upon NMDAR activation influx of Ca2+ ions lead to its phosphorylation at Thr286 [68,80,81]. This phosphorylated state is independent of Ca2+ [81,82], and is associated more to GluN2B (another subunit of NMDAR) [83]. With increased activity of NMDAR, it is be expected that CamKIIα will be autophosphorylated at Threonine286 which will further affect NMDAR signalling independent of Ca2+ influx [84]. Our results showed reduced expression of pCamKIIα-T286 similar to what is reported in chronic stress state where dysregulation of NMDAR-CamKII signalling is implicated [85]. One possible mechanism for this scenario is the activation of calcineurin another calcium buffer protein which is reported to have more affinity for Ca2+ than CamKIIα [86,87]. Activated calcineurin activates protein phosphatase-1 (PP1) to desensitise phosphorylated CamKIIα [88]. In a model of chronic pain reported by Qi et al. [75] remifentanil-induced postoperative hyperalgesia (RIPH) enhances NMDAR activity elevating the level of pCamKIIα-T286. Although the authors reported no changes in the level of basal CamKIIα the disparity in the observation may be due to the method of evaluating the level of expression as the authors normalise the level of pCamKIIα-T286 immunoblot with that of CamKIIα immunoblot, unlike our studies where all immunoblots were normalised with actin. Reduced expression of pCasmKIIα-T286 has been reported in compounds that indirectly modulate NMDAR activity through serotonin and opioid pathways [89]. The author suggested this is necessary to reduce the overactivation of NMDAR. Increased expression of pCamKIIα-T286 together with increased NMDAR is of short term and well correlated to LTP induction [70,90,91]. Our study involves prolonging datumetine exposure which overstimulates NMDAR for a long time. This overactivation may have stimulated the activity of calcineurin seen in chronic stress [72,85] thereby shutting off the NMDAR/CamKIIα signalling pathway.
Activation of NMDAR downstream signalling pathway is the activation of CREB [[92], [93], [94]]. CREB is also activated by way of phosphorylation by pCamKIIα-T286 [95]. Consistent with the observation seen in the expression of pCamKIIα-T286, datumetine reduced the expression of CREB. Activation states of NMDAR have been reported to regulate CREB activity [96]. Moderate activation of NMDAR increased CREB activity while overactivation reduced it [97]. Different subunit of NMDAR has also been reported to differentially regulate the activity of CREB [96]. GluN2A activation enhances CREB activity [[98], [99], [100]] while GluN2B transiently phosphorylates CREB, prolong activation reduces CREB activity [101]. Reduction in CREB expression observed is also related to what was reported in the state of both synaptic and extrasynaptic NMDAR activation [100]. Consistent with our earlier observations reduced CREB expression is also implicated in state of NMDAR overactivation [100].
In normal NMDAR signalling, activation of CREB translocates to the nucleus to increase the expression of some genes responsible for synaptic plasticity and cell survival [[102], [103], [104], [105]], out of which BDNF is an example of gene regulated by NMDAR signalling [100]. Consistent with the previous signalling pathway molecule, datumetine reduced BDNF expression in exposed animals compared to control. NMDAR subunit differentially regulates BDNF activity [96]. Chen et al. [106] reported that GluN2A upregulate BDNF activity in moderate and overactivation state while, GluN2B upregulate BDNF activity in moderate activation state and downregulate BDNF activity in overactivation state [100]. Most significant reduction of BDNF is seen in 1.0 Datumetine animals, this may be due to the overactivation of NMDAR caused by datumetine as reported by Zhou et al. [100] that activation of both synaptic and extrasynaptic NMDAR regulates BDNF expression differentially based on activation state. Impaired BDNF has been implicated as part of the pathophysiology of stress [73], in a study of acute restraint stress, mRNA of BDNF was reduced in the hippocampus [107] and chronic restraint stress reduced BDNF protein expression [108]. As earlier reported that chronic stress enhances NMDAR activity with altered signalling, our results mimic what is observed in the chronic state of stress as reported by Li et al. [109]. Although increased BDNF expression has also been reported in some chronic stress model when applied for 10 days [110] and mild stress at two weeks [111]. Gronli et al. [112] and Allaman et al. [113] reported no changes in their studies. Most reports that recorded low expression of BDNF in chronic stress estimate total hippocampal BDNF [73] such as the current study contrary to studies that estimate BDNF expression at different regions of the hippocampus reported different pattern of expression in each region [112,114].
BDNF has also been implicated in excitotoxicity observed in hypoxia [115]. Exogenous supplements of BDNF protects the neuron against hypoxia-induced death [116]. Similar to our results, exposure to hypoxia reduced BDNF expression and increase the expression of NMDAR [115]. The authors attributed the reduced expression of BDNF observed to increase in activity of GluN2B subunit of NMDAR as they have earlier reported increase activity of NMDAR in hypoxic condition [117,118]. Although the specific subunit of datumetine binding to NMDAR is not known, whether it requires the presence of glutamate at the synapse for its activity. One possible explanation for the effect of datumetine seen could be that datumetine is competing with glutamate for binding at the synapse leading to lateral diffusion of either glutamate or datumetine to activate extrasynaptic NMDAR. Extrasynaptic NMDAR activation has been associated with NMDAR-induced cell death [101].
5. Conclusion
Datura is one of the most abused plants for intoxication purpose, thus its role in memory deficit and hallucination needs further investigation. Although most of the investigation had focussed on the tropane alkaloids; datumetine, one of the primary phytochemicals needs to be further investigated. Since several studies have shown NMDAR to be responsible for LTP and synaptic plasticity for encoding memory, the present study shows the best binding of datumetine to NMDAR. Altogether, the current work shows that datumetine positively potentiates NMDAR by prolonging its electrical activity with acute exposure altering NMDAR signalling leading to memory deficit.
Author statement
All authors participated in the design and plan of the work. IAO carried out the lab work. All authors prepare and revised the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The authors acknowledge Dr Olubiyi O.O. of Department of Pharmaceutical Chemistry Obafemi Awolowo University Nigeria for the docking simulation, Amita Shrestha and Dr Olalekan M. Ogundele of Comparative Biomedical Sciences Louisiana State University USA for hosting Azeez Ishola to carry out the research study in their laboratory. This study was supported by ISN CAEN-1A award to IAO to visit OOM lab in the Louisiana State University, USA.
Edited by Dr. A.M. Tsatsakis
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2021.05.009.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Ndinda L. DW Made for Minds. 2018. Illegal drug use on the rise in Africa.https://p.dw.com/p/17i47 [Google Scholar]
- 2.United Nations Office on Drugs and Crime UNODC . UNODC Research; 2018. World Drug Report: Executive Summary Conclusions and Policy Implications. ISBN: 978-92-1-148304-148308. [Google Scholar]
- 3.United Nations Office on Drugs and Crime UNODC . UNODC Research; 2017. Global Synthetic Drugs Assessment: Amphetamine-Type Stimulants and New Psychoactive Substances. [Google Scholar]
- 4.Jamdhade M.S., Survase S.A., Kare M.A., Bhuktar A.S. Phytochemical studies on Datura metel Linn. in Marathwada region, Maharashtra. J. Phytol. 2010;2(12):46–48. [Google Scholar]
- 5.Ganesh S., Radha R., Jayshree N. A review on phytochemical and pharmacological status of Datura fastuosa Linn. Int. J. Multidiscip. Res. Dev. 2015;2(4):602–605. [Google Scholar]
- 6.Al-Snafi A.E. Medical importance of Datura fastuosa (syn: Datura metel) and Datura stramonium – a review. IOSR J. Pharm. 2017;7(2):43–58. [Google Scholar]
- 7.Aysu T., Durak H. Thermochemical conversion of Datura stramonium L. by supercritical liquefaction and pyrolysis processes. J. Supercrit. Fluids. 2015;102:98–114. [Google Scholar]
- 8.Durak H., Aysu T. Structural analysis of bio-oils from subcritical and supercritical hydrothermal liquefaction of Datura stramonium L. J. Supercrit. Fluids. 2016;108:123–135. [Google Scholar]
- 9.Stella L., Vitelli M.R., Palazzo E., Oliva P., De Novellis V., Capuano A., Scafuro M.A., Berrino L., Rossi F., Maione S. Datura stramonium intake: a report on three cases. J. Psychoactive Drugs. 2010;42(4):507–512. doi: 10.1080/02791072.2010.10400713. [DOI] [PubMed] [Google Scholar]
- 10.Arefi M., Barzegari N., Asgari M., Soltani S., Farhidnia N., Fallah F. Datura poisoning, clinical and laboratory findings. Report of five cases. Rom. J. Leg. Med. 2016;24(4):308–311. [Google Scholar]
- 11.Moses A. 2010. Drug Abuse and Drug Dependence Treatment Situation. Activity Report. GL0/J71. [Google Scholar]
- 12.Soni P., Siddiqui A.A., Dwivedi J., Soni V. Pharmacological properties of Datura stramonium L. as a potential medicinal tree: an overview. Asian Pac. J. Trop. Biomed. 2012;2(12):1002–1008. doi: 10.1016/S2221-1691(13)60014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yussuf A. Phytochemical and antimicrobial studies. Int. J. Pharmacogn. 1991;29:252–258. [Google Scholar]
- 14.Donatus E.O., Ephraim C.I. Isolation, characterization and antibacterial activity of alkaloid from Datura metel Linn leaves. Afr. J. Pharm. Pharmacol. 2009;3(5):277–281. [Google Scholar]
- 15.Katzung G.B., Masters B.S., Trevor J.A. 11th ed. 2009. Basic and Clinical Pharmacology. Pg 113. [Google Scholar]
- 16.Prasad C.S., Gowda N.K.S. Dietary level and plasma concentration of micronutrients in crossbred dairy cows fed finger millet and rice straw as dry roughage source. Indian J. Dairy Sci. 2005;58(2):109–112. [Google Scholar]
- 17.Devi M.R., Bawari M., Paul S.B., Sharma G.D. Neurotoxic and medicinal properties of Datura stramonium l. – review. Assam Univ. J. Sci. Technol. 2011;7(1):139–144. [Google Scholar]
- 18.Kanchan T., Atreya A. Datura: the roadside poison. Wilderness Environ. Med. 2016;27:442–443. doi: 10.1016/j.wem.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Hilal T., Alabri A., Hamood A., Musalami S.Al, Hossain M.A., Weli A.M., Al-Riyami Q. Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J. King Saud Univ. - Sci. 2014;26:237–243. [Google Scholar]
- 20.Malami I., Alhassan M.A. Phytochemical evaluation and investigations in to sedative properties of Datura stramonium (Linn) seeds in experimental mice. IOSR J. Pharm. Biol. Sci. 2014;9(1):1–3. [Google Scholar]
- 21.Maheshwari N.O., Khan A., Chopade B.A. Rediscovering the medicinal properties of Datura sp.: a review. J. Med. Plants Res. 2013;7(39):2885–2897. [Google Scholar]
- 22.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comp. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. Autodock4 and AutoDockTools4: automated docking with selective receptor flexiblity. J. Comp. Chem. 2009;16:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shresta A., Sultana R., Lee C., Ogundele O. SK channel modulates synaptic plasticity by tuning CaMKIIα/β dynamics. Front. Cell. Neurosci. 2019;11:18. doi: 10.3389/fnsyn.2019.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khaleque A., Akm M.R., Ismail K.M., Sadrul Amin M., Kiamuddin M. Investigations on Datura fastuosa Solanaceae part 5: isolation of fastusine scopolamine and beta sitosterol from the pericarp fruit shell. Bangladesh J. Sci. Ind. Res. 1974;9(1/2):79–81. [Google Scholar]
- 26.Manickam M., Sinha-Bagchi A., Sinha Sc, Gupta M., Ray A.B. Withanolides of Datura fastuosa leaves. Phytocemistry. 1993;34(3):868–870. [Google Scholar]
- 27.Pan Y., Wang X., Hu X. Cytotoxic withanolides from the flowers of Datura metel. J. Nat. Prod. 2007;70(7):1127–1132. doi: 10.1021/np070096b. [DOI] [PubMed] [Google Scholar]
- 28.Yang B., Wang Q., Xia Y., Feng W., Kuang H. Withanolide compounds from the flower of Datura metel L. Helv. Chim. Acta. 2007;90(8):1522–1528. [Google Scholar]
- 29.Kuang H.X., Yang B.Y., Xia Y.G., Feng W.S. Chemical constituents from the flower of Datura metel L. Arch. Pharm. Res. 2008;31(9):1094–1097. doi: 10.1007/s12272-001-1274-6. [DOI] [PubMed] [Google Scholar]
- 30.Kiruthika K.A., Sornaraj R. Screening of bioactive components of the flower Datura metel using the GC-MS technology. Int. J. PharTech Res. 2011;3(4):2025–2028. [Google Scholar]
- 31.Yang B.Y., Guo R., Li T., Lin Y., Wang C., Shu Z., Wang Z., Zhang J., Xia Y., Jiang H., Wang Q., Kuang H. Five withanolides from the Leaves of Datura metel L. and their inhibitory effects on nitric oxide production. Molecules. 2014;19:4548–4559. doi: 10.3390/molecules19044548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang B.Y., Guo R., Li T., Wu J.J., Zhang J., Liu Y., Wang Q.H., Kuang H.X. New anti-inflammatory withanolides from the leaves of Datura metel L. Steroids. 2014;87:26–34. doi: 10.1016/j.steroids.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Han X.L., Wang H., Zhang Z.H., Tan Y., Wang J.H. Study on chemical constituents in seeds of Datura metel from Xinjiang. Hong Yao Cai. 2015;38(8):1646–1648. [PubMed] [Google Scholar]
- 34.Murthy B.K., Nammi S., Kota M.K., Krishna Rao R.V., Koteswara N., Annapurna A. Evaluation of hypoglycemic and antihyperglycemic effects of Datura metel (Linn.) seeds in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2004;91:95–98. doi: 10.1016/j.jep.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Kitchen D.B., Decornez H., Furr J.R., Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat. Rev. Drug Discov. 2004;3(11):935. doi: 10.1038/nrd1549. [DOI] [PubMed] [Google Scholar]
- 36.Gupta M., Sharma R., Kumar A. Docking techniques in pharmacology: How much promising? Comput. Biol. Chem. 2018;76:210–217. doi: 10.1016/j.compbiolchem.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Lambert J.D., Jones R.S. Activation of N-methyl-D-aspartate receptors contributes to the EPSP at perforant path synapses in the rat dentate gyrus in vitro. Neurosci. Lett. 1989;97:323–328. doi: 10.1016/0304-3940(89)90618-6. [DOI] [PubMed] [Google Scholar]
- 38.Lambert J.D., Jones R.S. A reevaluation of excitatory amino acid-mediated synaptic transmission in rat dentate gyrus. J. Neurophysiol. 1990;64:119–132. doi: 10.1152/jn.1990.64.1.119. [DOI] [PubMed] [Google Scholar]
- 39.Tόth K., Cutsuridis V. Hippocampal Microcircuits A Computational Modeler’s Resource Book. Springer press; 2010. Glutamatergic neurotransmission in the hippocampus. pp 99. [Google Scholar]
- 40.Song Q., Feng G., Zhang J., Xia X., Ji M., Lv L., Ping Y. NMDA receptor-mediated Ca2+ influx in the absence of Mg2+ block disrupts rest: activity rhythms in drosophila. Sleep. 2017;40(12):166. doi: 10.1093/sleep/zsx166. [DOI] [PubMed] [Google Scholar]
- 41.Sabatier N., Brown C.H., Ludwig M., Leng G. Spike pattering in rat supraoptic neurons in vivo and invitro. J. Physiol. 2004;558(1):161–180. doi: 10.1113/jphysiol.2004.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benda J., Tabak J. Spike-frequency adaptation. Encycl. Comp. Neurosci. 2014 doi: 10.1007/978-4614-7320-6_339-1. [DOI] [Google Scholar]
- 43.Sah P. Ca2+ -activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland C., Doiron B., Longtin A. Feedback-induced gain control in stochastic spiking networks. Biol. Cybern. 2009;100:475–489. doi: 10.1007/s00422-009-0298-5. [DOI] [PubMed] [Google Scholar]
- 45.McNaughton B.L. Evidence for two physiologically distinct perforant pathways to the fascia dentata. Brain Res. 1980;199:1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- 46.Lu W.Y., Man H.Y., Ju W., Trimble W.S., MacDonald J.F., Wang Y.T. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29(1):243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 47.Lau C.G., Takeuchi K., Rodenas-Ruano A., Takayasu Y., Murphy J., Bennett M.V.L., Zukin R.S. Regulation of NMDA receptor Ca2+ signaling and synaptic plasticity. Biochem. Soc. Trans. 2009;37(Pt6):1369–1374. doi: 10.1042/BST0371369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lűscher C., Malenka R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb. Perspect. Biol. 2012;4(6):5710. doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park P., Volianskis A., Sanderson T.M., Bortolotto Z.A., Jane D.E., Zhuo M., Kaang B.K., Collingridge G.L. NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369(1633) doi: 10.1098/rstb.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghasemi M., Schacter S.C. The NMDA receptor complex as a therapeutic target in epilepsy: a review. Epilepsy Behav. 2011;22:617–640. doi: 10.1016/j.yebeh.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 51.Olawepo A., Ishola A.O., Ajao M.S., Olayemi O.J., Olayaki L.A. Atropine exposure in adolescence predispose to adult memory loss in Wistar rats. Int. J. Biol. Chem. Sci. 2017;11(5):1937–1947. [Google Scholar]
- 52.Abena A.A., Miguel L.M., Mouanga A., Ouamba J.M., Sianard D.F., Thiebolt M.H., Hondi-Assah T.C., Diatewa M. Neuropsychopharmacological effects of leaves and seeds extracts of Datura fastuosa. Biotechnology. 2004;3(2):109–113. [Google Scholar]
- 53.Babalola S.A., Sulaiman M.M., Hassan A.Z., Adawa D.A.Y. Evaluation of crude methanolic seed extract of Datura metel L: as a potential oral anaesthetic in dogs. Vet. Res. 2013;a6(5–6):115–119. [Google Scholar]
- 54.Deepa M., Sugitha N., Mythili S., Sathiavelu A. Antioxidant activity and phytochemical analysis of Datura metel. Int. J. Drug Dev. Res. 2014;6(4):280–285. [Google Scholar]
- 55.Babalola S.A., Suleiman M.M., Hassan A.Z., Adawa D.A.Y. Evaluation of Datura metel l seed extract as a sedative/hypnotic: a preliminary study. J. Vet. Adv. 2015;5(4):857–862. [Google Scholar]
- 56.Etibor T.A., Ajibola M.I., Buhari M.O., Safiriyu A.A., Akinola O.B., Caxton-Martins E.A. Datura metel administration distorts medial prefrontal cortex histology of Wistar rats. World J. Neurosci. 2015;5:282–291. [Google Scholar]
- 57.Traynelis S.F., Wollmuth L.P., McBain C.J., Menniti F.S., Vance K.M., Ogden K.K., Hansen K.B., Yuan H., Myers S.J., Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gan J., Qi C., Mao L., Liu Z. Changes in surface expression of N-methyl-D-aspartate receptors in the striatum in a rat model of Parkinson’s disease. Drug Des. Dev. Ther. 2013;8:165–173. doi: 10.2147/DDDT.S51559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberto M., Siggins G.R. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase GABA release in central anygdala. PNAS. 2006;103:9715–9720. doi: 10.1073/pnas.0601899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goodwani S., Saternos H., Alasmari F., Sari Y. Metabotropic and ionotropic glutamate 5receptors as potential targets for the treatment of alcohol use disorder. Neurosci. Behav. Rev. 2017;77:14–31. doi: 10.1016/j.neubiorev.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen W.Y., Rosner B., Hankinson S.E., Colditz G.A., Willett W.C. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alasmari F., Goodwari S., McCullumsmith R.E., Sari Y. Role of glutamatergic system and mesocorticolimbic circuits inn alcohol dependence. Prog. Neurobiol. 2018;171:32–49. doi: 10.1016/j.pneurobio.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashimoto K., Sawa A., Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol. Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 64.Lan M.J., McLoughlin G.A., Griffin J.L., Tsang T.M., Huang J.T., Yuan P., Manji H., Holmes E., Bahn S. Metabonomic analysis identifies molecular changes associated with the pathophysiology and drug treatment of bipolar disorder. Mol. Psychiatry. 2009;14:269–279. doi: 10.1038/sj.mp.4002130. [DOI] [PubMed] [Google Scholar]
- 65.Sanacora G., Treccani G., Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paul I.A., Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann. N. Y. Acad. Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- 67.Cristino L., Luongo L., Squillace M., Paolone G., Mango D., Piccinin S., Zianni E., Imperatore R., Iannotta M., Longo F., Errico F., Vescovi A.L., Morari M., Maione S., Gardoni F., Nistico R., Usiello A. D-Aspartate oxidase influences glutamatergic system homeostasis in mammalian brain. Neurobiol. Aging. 2015;36(5):1890–1902. doi: 10.1016/j.neurobiolaging.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Coultrap S.J., Bayer K.U. CaMKII regulation in information processing and storage. Trends Neurosci. 2012;35(10):607–618. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malenka R.C., Bear M.F. LTP and LTD: an embarassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Barlett T.E., Wang Y.T. The intersections of NMDAR-dependent synaptic plasticity and cell survival. Neuropharmacology. 2013;74:59–68. doi: 10.1016/j.neuropharm.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 71.Fontella F.U., Vendite D.A., Tabajara A.S., Porciuncula L.O., da Silva Torres I.L., Jardim F.M. Repeated restraint stress alters hippocampal glutamate uptake and releases in the rat. Neurochem. Res. 2004;29(9):1703–1709. doi: 10.1023/b:nere.0000035805.46592.6c. [DOI] [PubMed] [Google Scholar]
- 72.Gerges N.Z., Aleisa A.M., Schwarz L.A., Alkadhi K.A. Reduced basal CamKII levels in hippocampal CA1 region: a possible cause of stress-induced impairment of LTP in chronically stressed rats. Hippocampus. 2004;14(3):402–410. doi: 10.1002/hipo.10193. [DOI] [PubMed] [Google Scholar]
- 73.Vasquez C.E., Riener R., Reynolds E., Britton G.B. NMDA receptor dysregulation in chronic state: a possible mechanism underlying depression with BDNF downregulation. Neurochem. Int. 2014;79:88–97. doi: 10.1016/j.neuint.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 74.Lee M., Silverman S.M., Hansen H., Patel V.B., Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Phys. 2011;14(2):154–161. [PubMed] [Google Scholar]
- 75.Qi F., Liu T., Zhang X., Gao X., Li Z., Chen L., Lin C., Wang L., Wang Z.J., Tang H., Chen Z. Ketamine reduces remifentanil-induced postoperative hyperalgesia mediated by CaMKII-NMDAR in the primary somatosensory cerebral cortex region in mice. Neuropharmacology. 2020;162:107783. doi: 10.1016/j.neuropharm.2019.107783. [DOI] [PubMed] [Google Scholar]
- 76.Renteria R., Maier E.Y., Buske T.R., Morrisett R.A. Selective alterations of NMDAR function and plasticity in D1 and D2 medium spiny neurons in the nucleus accumbens shell following chronic intermittent ethanol exposure. Neuropharmacology. 2017;112:164–171. doi: 10.1016/j.neuropharm.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Easton A.C., Lucchesi W., Lourdusamy A., Lenz B., Solati J., Golub Y., Lewczuk P., Fernandes C., Desriveres S., Dawirs R.R., Moll G.H., Kornhuber J., Frank J., Hoffmann P., Soyka M., Kiefer F., The GESGA Consortium, Schumann G., Giese K.P., Muller C.P. αCaMKII autophosphorylation controls the establishment of alcohol drinking behaviour. Neuropsychopharmacology. 2013;38(9):1636–1647. doi: 10.1038/npp.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gardoni F., Schrama L.H., van Dalen J.J., Gispen W.H., Cattabeni F., Di Luca M. AlphaCaMKII binding to the C-terminal tail of NMDA receptor subunit NR2A and its modulation by autophosphorylation. FEBS Lett. 1999;456(3):394–398. doi: 10.1016/s0014-5793(99)00985-0. [DOI] [PubMed] [Google Scholar]
- 79.Gardoni F., Schrama L.H., Kamal A., Gispen W.H., Cattabeni F., Di Luca M. Hippocampal synaptic plasticity involves competition between Ca2+/calmodulin-dependent protein kinase II and postsynaptic density 95 for binding to the NR2A subunit of the NMDA receptor. J. Neurosci. 2001;21(5):1501–1509. doi: 10.1523/JNEUROSCI.21-05-01501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lisman J., Yasuda R., Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2014;13(3):169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coultrap S.J., Freund R.K., O’Leary H., Sanderson J.L., Roche K.W., Dell’Acqua M.L., Bayer K.U. Autonomous CaMKII mediates both LTP and LTD using a mechanism for differential substrate site selection. Cell Rep. 2014;6:431–437. doi: 10.1016/j.celrep.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kennedy M.B. 2nd ed. vol. 3. Elsevier Inc; USA: 2010. Calcium/calmodulin-dependent protein kinase II. (Handbook of Cell Signaling). [Google Scholar]
- 83.Barria A., Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48(2):289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 84.Miller S.G., Kennedy M.B. Regulation of brain type II Ca2+/calmodulin dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44(60):861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 85.Calabrese F., Guidotti G., Molteni R., Racagni G., Mancini M., Riva M.A. Stress-induced changes of hippocampal NMDA receptors: modulation by duloxetine treatment. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li L., Stefan M.I., Le Nove N. Calcium input frequency, duration and amplitude differentially modulate the relative activation of calcineurin and CaMKII. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rumi-Masante J., Rusinga F.I., Lester T.E., Dunlap T.B., Williams T.D., Dunker A.K. Structural basis for activation of calcineurin by calmodulin. J. Mol. Biol. 2012;415(12):307–317. doi: 10.1016/j.jmb.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bradshaw J.M., Kubota Y., Meyer T., Schulman H. An ultrasensitive Ca2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signalling. PNAS. 2003;100(18):10512–10517. doi: 10.1073/pnas.1932759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodriguez-Munoz M., Sanchez-Blazquez P., Garzon J. Fenfluramine diminishes NMDA receptor-mediated seizures via its mixed activity at serotonin 5HT2A and type 1 sigma receptors. Oncotarget. 2018;9(34):23373–233895. doi: 10.18632/oncotarget.25169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buard I., Coultrap S.J., Freund R.K., Lee Y.S., Dell’Acqua M.L., Silva A.J., Bayer K.U. CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J. Neurosci. 2010;30:8214–8220. doi: 10.1523/JNEUROSCI.1469-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ashpole N.M., Hudmon A. Excitotoxic neuroprotection and vulnerability with CaMKII inhibition. Mol. Cell. Neurosci. 2011;46:720–730. doi: 10.1016/j.mcn.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 92.Leveille F., Papadia S., Fricker M., Bell K.F., Soriano F.X., Martel M.A., Puddifoot C., Habel M., Wyllie D.J., Ikonomidou C., Tolkovsky A.M., Hardingham G.E. Suppression of the intrinsic apoptosis pathway by synaptic activity. J. Neurosci. 2010;30:2623–2635. doi: 10.1523/JNEUROSCI.5115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hardingham G.E., Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang S.J., Buchthal B., Lau D., Hayer S., Dick O., Schwaninger M., Veltkamp R., Zou M., Weiss U., Bading H. A signaling cascade of nuclear calcium-CREB-ATF3 activated by synaptic NMDA receptors defines a gene repression module that protects against extrasynaptic NMDA receptor-induced neuronal cell death and ischemic brain damage. J. Neurosci. 2011;31:4978–4990. doi: 10.1523/JNEUROSCI.2672-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deisseroth K., Heist E.K., Tsien R.W. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392(6672):198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- 96.Sun Y., Xu Y., Cheng X., Chen X., Xie Y., Zhang L., Wang L., Hu J., Gao Z. The differences between GluN2A and GluN2B signaling in the brain. J. Neurosci. Res. 2018;96:1430–1443. doi: 10.1002/jnr.24251. [DOI] [PubMed] [Google Scholar]
- 97.Valera E., Sanchez-Martin F.J., Ferrer-Montiel A.V., Messeguer A., Merino J.M. NMDA-induced neuroprotection in hippocampal neurons is mediated through the protein kinase A and CREB (cAMP-response element-binding protein) pathway. Neurochem. Int. 2008;53(5):148–154. doi: 10.1016/j.neuint.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 98.Terasaki Y., Sasaki T., Yagita Y., Okazaki S., Sugiyama Y., Oyama N., Kitagawa K. Activation of NR2A receptors induces ischemic tolerance through CREB signaling. J. Cereb. Blood Flow Metab. 2010;30(8):1441–1449. doi: 10.1038/jcbfm.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sasaki T., Takemori H., Yagita Y., Terasaki Y., Uebi T., Horike N., Takagi H., Susumu T., Teraoka H., Kusano K.I., Hatano O., Oyama N., Sugiyama Y., Sakoda S., Kitagawa K. SIK2 is a key regulator for neuronal survival after ischemia via TORC1-CREB. Neuron. 2011;69(1):106–119. doi: 10.1016/j.neuron.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 100.Zhou X., Ding Q., Chen Z., Yun H., Wang H. Involvement of the GluN2A and GluN2B subunits in synaptic and extrasynaptic N-methyl-D-aspartate receptor function and neuronal excitotoxicity. J. Biol. Chem. 2013;288(33):24151–24159. doi: 10.1074/jbc.M113.482000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luo T., Wu W.H., Chen B.S. NMDA receptor signaling: death or survival? Front. Biol. 2011;6(6):468–476. doi: 10.1007/s11515-011-1187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tao X., West A.E., Chen W.G., Corfas G., Greenberg M.E. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002;33(3):383–395. doi: 10.1016/s0896-6273(01)00561-x. [DOI] [PubMed] [Google Scholar]
- 103.Chen W.G., Chang Q., Lin Y., Meissner A., West A.E., Griffith E.C. Depression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302(5646):885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 104.Martinowich K., Hattori D., Wu H., Fouse S., He F., Hu Y. DNA methylation-related chromatin remodelling in activity-dependent BDNF gene regulation. Science. 2003;302(5646):890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 105.Tian F., Marini A.M., Lipsky R.H. NMDA receptor activation induces differential epigenetic modification of Bdnf promoters in hippocampal neurons. Amino Acids. 2010;38(4):1067–1074. doi: 10.1007/s00726-009-0315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Q., He S., Hu X.L., Yu J., Zhou Y., Zheng J. Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J. Neurosci. 2007;27(3):542–552. doi: 10.1523/JNEUROSCI.3607-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murakami S., Imbe H., Morikawa Y., Kubo C., Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci. Res. 2005;53(2):129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 108.Xu H., Luo C., Richardson J.S., Li X.M. Recovery of hippocampal cell proliferation and BDNF levels, both of which are reduced by repeated restraint stress, is accelerated by chronic venlafaxine. Pharmacogenomics J. 2004;4(5):322–331. doi: 10.1038/sj.tpj.6500265. [DOI] [PubMed] [Google Scholar]
- 109.Li J., Zhou Y., Liu B.B., Liu Q., Geng D., Weng L.J. Nobiletin ameliorates the deficits in hippocampal BDNF, TrkB, and synapsin I induced by chronic unpredictable mild stress. Evid. Complement. Alternat. Med. 2013;2013 doi: 10.1155/2013/359682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nair A., Vadodaria K.C., Banerjee S.B., Benekareddy M., Dias B.G., Duman R.S. Stressor-specific regulation of distinct brain-derived neurotrophic factor transcripts and cyclic AMP response element-binding protein expression in the postnatal and adult rat hippocampus. Neuropsychopharmacology. 2007;32(7):1504–1519. doi: 10.1038/sj.npp.1301276. [DOI] [PubMed] [Google Scholar]
- 111.Xiao L., Shu C., Tang J., Wang H., Liu Z., Wang G. Effects of different CMS on behaviors, BDNF/CREB/Bcl-2 expression in rat hippocampus. Biomed. Aging Pathol. 2011;1(3):138–146. [Google Scholar]
- 112.Gronli J., Bramham C., Murison R., Kanhema T., Fiske E., Bjorvatn B. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol. Biochem. Behav. 2006;85(4):842–849. doi: 10.1016/j.pbb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 113.Allaman I., Papp M., Kraftsik R., Fiumelli H., Magistretti P.J., Martin J.L. Expression of brain-derived neurotrophic factor is not modulated by chronic mild stress in the rat hippocampus and amygdala. Pharmacol. Rep. 2008;60(6):1001–1007. [PubMed] [Google Scholar]
- 114.Park S.W., Lee S.K., Kim J.M., Yoon J.S., Kim Y.H. Effects of quetiapine on the brain derived neurotrophic factor expression in the hippocampus and neocortex of rats. Neurosci. Lett. 2006;402(1–2):25–29. doi: 10.1016/j.neulet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 115.Das D., Biswal S., Barhwal K.K., Chaurasia O.P., Hota S.K. Kaempferol inhibits extrasynaptic NMDAR mediated downregulation of TRKβ in rat hippocampus during hypoxia. Neuroscience. 2018;39:77–91. doi: 10.1016/j.neuroscience.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 116.Jain V., Baitharu I., Prasad D., Ilavazhagan G. Enriched environment prevents hypobaric hypoxia induced memory impairment and neurodegeneration: role of BDNF/PI3K/GSK3β pathway coupled with CREB activation. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0062235. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Hota S.K., Barhwal K., Singh S.B., Ilavazhagan G. Chronic hypobaric hypoxia-induced apoptosis in the CA1 region of the hippocampus: a possible role of NMDAR mediated p75NTR upregulation. Exp. Neurol. 2008;212(1):5–13. doi: 10.1016/j.expneurol.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 118.Hota S.K., Barhwal K., Singh S.B., Sairam M., Ilavazhagan G. NR1 and GluR2 expression mediate excitotoxicity in chronic hypobaric hypoxia. J. Neurosci. Res. 2008;86(5):1142–1152. doi: 10.1002/jnr.21554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.