Abstract
Background
Phosphoenolpyruvate carboxykinase (PCK) has been almost exclusively recognized as a critical enzyme in gluconeogenesis, especially in the liver and kidney. Accumulating evidence has shown that the enhanced activity of PCK leads to increased glucose output and exacerbation of diabetes, whereas the defects of PCK result in lethal hypoglycemia. Genetic mutations or polymorphisms are reported to be related to the onset and progression of diabetes in humans.
Scope of review
Recent studies revealed that the PCK pathway is more complex than just gluconeogenesis, depending on the health or disease condition. Dysregulation of PCK may contribute to the development of obesity, cardiac hypertrophy, stroke, and cancer. Moreover, a regulatory network with multiple layers, from epigenetic regulation, transcription regulation, to posttranscription regulation, precisely tunes the expression of PCK. Deciphering the molecular basis that regulates PCK may pave the way for developing practical strategies to treat metabolic dysfunction.
Major conclusions
In this review, we summarize the metabolic and non-metabolic roles of the PCK enzyme in cells, especially beyond gluconeogenesis. We highlight the distinct functions of PCK isoforms (PCK1 and PCK2), depict a detailed network regulating PCK's expression, and discuss its clinical relevance. We also discuss the therapeutic potential targeting PCK and the future direction that is highly in need to better understand PCK-mediated signaling under diverse conditions.
Keywords: Phosphoenolpyruvate carboxykinase (PCK), Gluconeogenesis, Glyceroneogenesis, Transcription regulation, Posttranscription regulation, Epigenetic regulation, Protein kinase
Abbreviations
- AP-1
Activator protein 1
- C/EBPα
CAAT/enhance binding protein α
- CRE
cAMP response elements
- CREB
cAMP Response Element-Binding Protein
- F1,6BP
Fructose 1,6-bisphosphate
- F6P
Fructose 6-phosphate
- G6P
Glucose-6-phosphate
- G6PC
Glucose-6-phosphatase
- GRE
Glucocorticoid response element
- GSIS
glucose-stimulated biphasic insulin secretion
- HCC
Hepatocellular carcinoma
- INSIGs
Insulin induced genes
- IRS
Insulin response sequence
- OAA
Oxaloacetate
- PCK
Phosphoenolpyruvate carboxykinase
- PEP
Phosphoenolpyruvate
- PGC-1
Peroxisome proliferative activated receptor-γ co-activator 1
- PKA
Protein kinase A
- SCAP
SREBP cleavage-activating protein
- SREBPs
Sterol regulatory element-binding proteins
- TCA
Tricarboxylic acid cycle
- TG
Triglycerides
1. Introduction
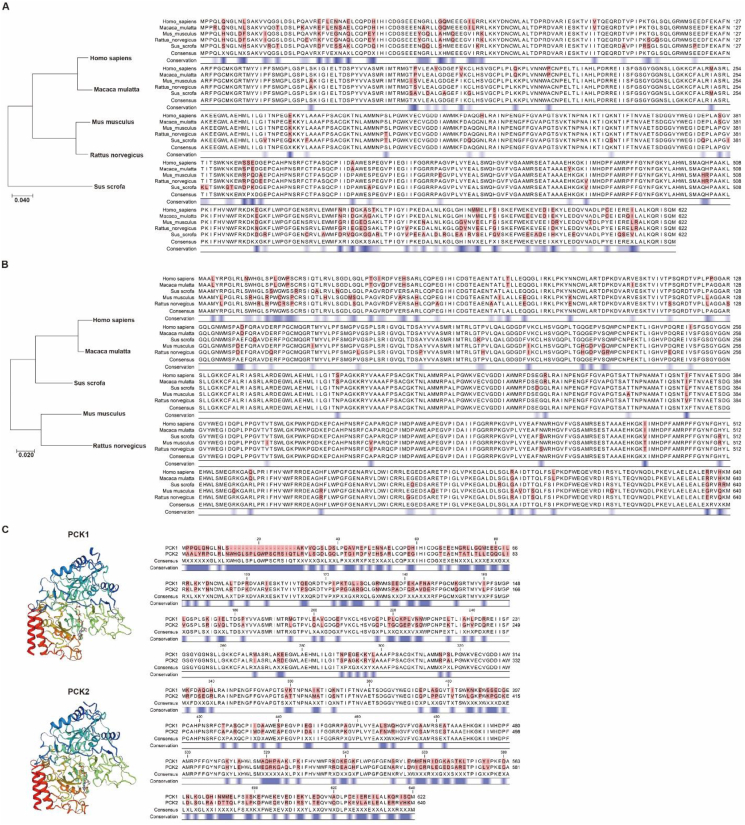
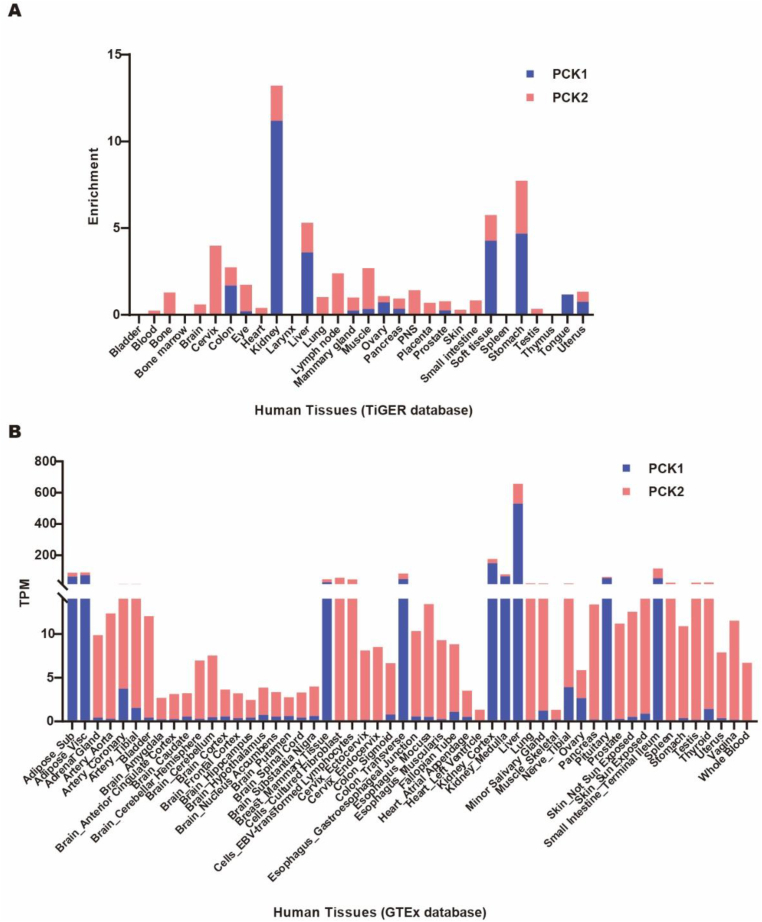
Phosphoenolpyruvate carboxykinase (PCK) was first discovered in the 1950s. It is highly conserved among species (Figure 1A–B) and generally recognized as the key enzyme regulating gluconeogenesis. There are two PCK isoforms in all vertebrates, the mitochondrial form (PCK2) and cytosolic form (PCK1), encoded by separate genes. In 1953, PCK2, also called PEPCK2 or PEPCK-M, was first isolated from chicken liver. PCK1, also called PEPCK1 or PEPCK-C, was discovered in the murine liver in 1963. Both PCK1 and PCK2 contain 10 exons and 9 introns in humans, mice, and rats (Figure 1C). However, they have differential activity among species and organs (Table 1) [[1], [2], [3], [4]]. Generally, PCK1 is highly enriched in gluconeogenic organs such as the kidney, liver, and intestine. Adipose tissue also expresses a high level of PCK1. PCK2 is widely expressed and could be induced by diverse stress [5]. Table 1 shows that PCK1 is the majority form of enzyme in murine hepatocytes, constituting as much as 95% of the total PCK, whereas conversely PCK2 constitutes nearly 100% of the total PCK in birds, and PCK1 and PCK2 are almost equally abundant in the human liver [6]. By analyzing the expression data from the GTEx and TiGER databases, we provide a more detailed graph showing the relative expression of PCK1 and PCK2 in multiple human organs (Figure 2A–B). PCK and PCK-mediated reactions have recently received increasing attention, as this is the only pathway linking the TCA metabolite pool with glycolytic intermediates upstream of PEP and plays a unique role in diverse pathophysiological processes.
Figure 1.
Comparison of PCK conservation. (A) Amino acid alignment of PCK1 among different species. Different amino acids are highlighted in red. Conservation is scaled at the bottom, in which blue stands for less conserved. The Genome Workbench was applied to calculate the conservation score. (B) Amino acid alignment of PCK2 among different species. (C) 3D protein structure (SWISS-MODEL) and amino acid alignment of human PCK1 and PCK2.
Table 1.
The relative enzyme activity of PCK isoforms in different organs of certain species.
| Organ/Species | % of Total Activity |
||
|---|---|---|---|
| PCK1 | PCK1 | ||
| Liver | Human | 50 | 50 |
| Pig | 50 | 50 | |
| Guinea Pig | 50 | 50 | |
| Dog | 50 | 50 | |
| Cat | 50 | 50 | |
| Cow | 40 | 60 | |
| Sheep | 40 | 60 | |
| Rabbit | 10 | 90 | |
| Pigeon | 0 | 100 | |
| Chicken | 0 | 100 | |
| Mouse | 95 | 5 | |
| Rat | 90 | 10 | |
| Hamster | 90 | 10 | |
| Adipose | Human | 85 | 15 |
| Kidney | Chicken | 50 | 50 |
Figure 2.
Relative expression of PCK1 and PCK2 in multiple human organs. Raw data were retrieved from the TiGER (A) and GTEx (B) databases.
2. The metabolic function of PCK
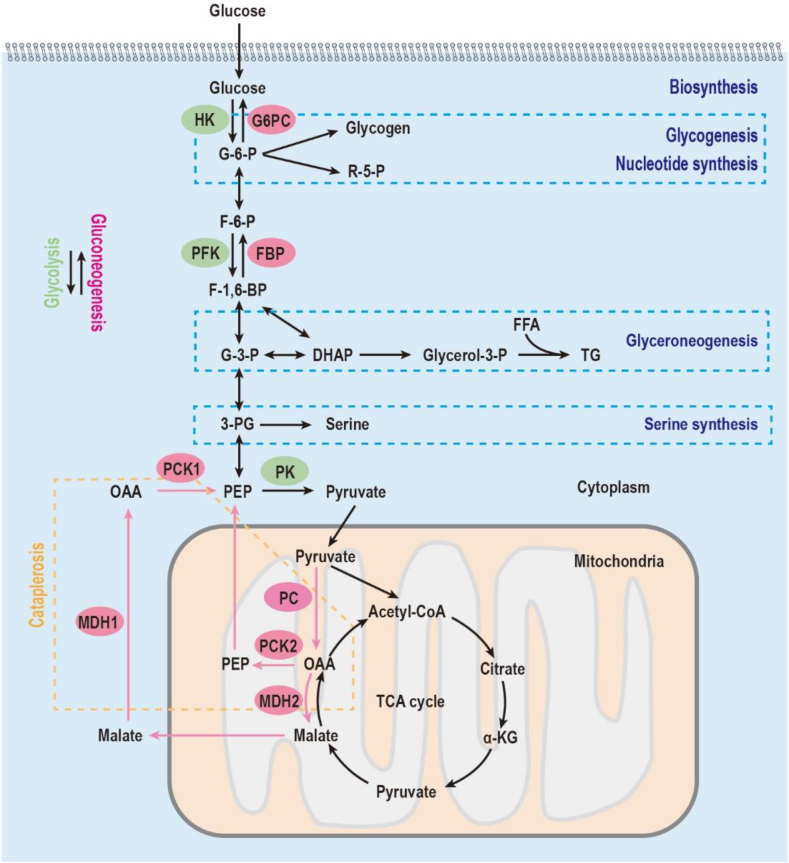
Most of the current knowledge on PCK comes from studies of mice and rats, of which PCK1 is the major component of the enzyme in the liver. The cytosolic form, PCK1, constitutes almost 95% of the total PCK in the murine liver, whereas PCK2 is barely detectable [7]. As illustrated in Figure 3, PCK1 catalyzes the reaction of oxaloacetate (OAA) and GTP into phosphoenolpyruvate (PEP), GDP, and CO2. As the mitochondrial membrane is impermeable to OAA, OAA is first reduced to malate and shuttled to the cytoplasm via SLC25A11, where malate is re-oxidized into OAA again. OAA is then fueled into the gluconeogenic pathway through the conversion to PEP mediated by PCK1. This is the first rate-limiting step of gluconeogenesis, which couples the TCA cycle with glycolysis [8]. This feature leads to the common understanding that PCK exclusively regulates gluconeogenesis. However, mice without PCK1 suffer from severe hypoglycemia right after birth and then die [8]. More importantly, supplementation of glucose increases blood glucose but fails to improve survival, implicating a critical role other than gluconeogenesis [8]. More evidence is provided showing the essential cataplerotic role of PCK in the following 4 metabolic processes: (1) glucose biosynthesis (gluconeogenesis), (2) glycerol biosynthesis (glyceroneogenesis), (3) serine biosynthesis, and (4) amino acid metabolism. These processes depend on the central role of PCK converting OAA into PEP but diverge based on the origin of OAA.
Figure 3.
Schematic representation of metabolic pathways. Glycolysis requires the three rate-limiting enzymes shown in the green ovals. Gluconeogenesis needs the four key enzymes shown in the red ovals. Glyceroneogenesis is a truncated version of gluconeogenesis sharing the same enzymes. Abbreviations: 3-PG, 3-phosphoglycerate; DHAP, dihydroxyacetone phosphate; F-1,6-P, fructose-1,6-biphosphate; F-6-P, fructose-6-phosphate; FBP, fructose-1,6-bisphosphatase; FFA, free fatty acid; G-3-P, glyceraldehyde-3-phosphate; G-6-P, glucose-6-phosphate; G6PC, glucose-6-phosphatase; HK, hexokinase; MDH, malate dehydrogenase; OAA, oxaloacetate; PC, pyruvate carboxylase; PCK, phosphoenolpyruvate carboxykinase; PEP, phosphoenolpyruvate; PFK, phosphofructokinase; PK, pyruvate kinase; R-5-P, ribose-5-phosphate; TG, triglyceride.
2.1. PCK and gluconeogenesis
Glucose is the primary fuel for the brain, blood cells, and fetal heart in mammals. Thus, the maintenance of glucose levels is crucial for the normal function of these organs and fetal development. Gluconeogenesis plays an essential role in glucose homeostasis to maintain its blood level within a relatively narrow range [9]. In this process, lactate, amino acids, glycerol, and other non-carbohydrate substrates are transformed into glucose to meet energy demands under prolonged starvation, stress, or increased workload. PCK regulates the first rate-limiting step in hepatic gluconeogenesis, which catalyzes OAA into PEP. Notably, this conversion begins either in the cytoplasm or mitochondria mediated by PCK1 and PCK2, respectively. In detail, mitochondrial OAA can also be reduced to malate and enter the cytoplasm, where malate is oxidized into OAA again. Cytoplasmic OAA is then converted into PEP via PCK1. In the gluconeogenic cycle, PEP is dephosphorylated to yield fructose 1,6-bisphosphate (F1,6BP), fructose 6-phosphate (F6P), glucose-6-phosphate (G6P), and glucose as the final product. Alternatively, OAA is carboxylated from two pyruvate molecules and then decarboxylated and phosphorylated into PEP by PCK2 in the mitochondria. PEP is then shuttled to the cytoplasm as the critical substrate of gluconeogenesis. Gluconeogenesis is fine-tuned by cAMP, insulin, and glucocorticoids. Thus, overactivation of PCK in the liver leads to hyperglycemia and exacerbation of diabetes, whereas global PCK1 deficiency causes severe hypoglycemia, which may result in embryonic lethality [5,6]. Given the liver's major role in blood glucose homeostasis, efforts were also made to examine the role of hepatic PCK1 in gluconeogenesis. Interestingly, mice with liver-specific knockout of PCK1 exhibited euglycemia after fasting due to compromised glucose utilization, but developed steatosis [10], which highly implies an organ-specific role of PCK in gluconeogenesis.
2.2. PCK-regulated glyceroneogenesis
Triglycerides (TG) represent a major lipid storage and energy source [11]. TG is broken down into one glycerol molecule and three fatty acid molecules through lipolysis [12]. The fatty acid is then oxidized into acetyl-CoA to fuel the Krebs cycle and generate ATP. Up to 40% of fatty acid, together with glycerol 3-phosphate, is recycled to form TG again, also known as the triglyceride/fatty acid cycle [13]. Triglyceride metabolism disorder can lead to atherosclerosis, obesity, non-alcoholic fatty liver disease, life-threatening pancreatitis, and cardiovascular diseases [14,15]. As a required precursor of TG, glycerol 3-phosphate can be derived from pyruvate, termed glyceroneogenesis [16]. Glyceroneogenesis is critical to maintain the triglyceride/fatty acid cycle. It contributes more than expected to human plasma TG, which is up to 10–60% using isotope tracers and deuterium labeling [17]. Figure 3 shows that glyceroneogenesis is a truncated gluconeogenesis sharing many gluconeogenic enzymes [18]. PCK1 is the rate-limiting enzyme in both gluconeogenesis and glyceroneogenesis that catalyzes OAA into PEP, which is then converted into glycerol 3-phosphate in both the adipose tissue and liver. This process is highly enhanced in the fasted state, facilitating the development of insulin resistance [19]. Adipose-specific deletion of PCK1 leads to decreased glyceroneogenesis and lipodystrophy [20], whereas adipose-specific overexpression of PCK1 results in obesity [[21], [22], [23]]. Mice with liver-specific knockout of PCK1 show unesterified fatty acid accumulation without lipogenesis in the liver [10,24]. These phenotypes generated by perturbation of PCK1 largely depend on the differential expression of PCK1 itself and the key enzymes of metabolic pathways in distinct organs. Glucose-6-phosphatase (G6PC), the final step regulating gluconeogenesis, is highly expressed in the liver and barely detectable in fat tissue [25]. Thus, PCK1 in adipose tissue plays an essential role in glyceroneogenesis compared to that in gluconeogenesis.
2.3. PCK and amino acids
PCK diverts TCA intermediate metabolites into cytosol, thus probably participating in anaplerosis and cataplerosis involving amino acids. PCK2-overexpressing cells exhibit high anabolic activity with higher amino acid consumption from culture media, especially for glycine and proline [26]. After breaking down and entering TCA, amino acid products are further converted into PEP catalyzed by PCK and fed into the serine biosynthetic pathway [27]. Serine is generally regarded as a nutritionally dispensable but metabolically indispensable amino acid, which is the major substrate for one carbon metabolism and critical for nucleotide synthesis, methylation, and anti-oxidation [28]. Therefore, serine biosynthesis is important for cell growth and proliferation [28]. Intriguingly, PCK2 and key enzymes for serine biosynthesis share similar expression patterns during cell growth [29]. By applying isotope labeling, PCK2 was found to promote oxaloacetate-derived carbons into de novo synthesized serine [30]. This strongly implies that PCK-dependent diversion of TCA intermediates plays a critical role in serine biosynthesis.
3. A novel role of PCK1 as a protein kinase
PCK is a vital metabolic enzyme in gluconeogenesis and glyceroneogenesis as previously discussed. Several lines of evidence show that metabolic enzymes could function as kinases to regulate cellular activities [31,32]. Surprisingly, PCK may also have kinase activity to regulate the transcription of lipogenesis genes [33]. Tumor cells have a high level of lipid metabolism to synthesize intermediates for membrane biosynthesis, signaling molecules, and energy required for rapid tumor cell proliferation [31,34]. Vital genes required for lipid metabolism are controlled by sterol regulatory element-binding proteins (SREBPs) [35,36], which are escorted by SREBP cleavage-activating protein (SCAP) shuttling from the endoplasmic reticulum to the Golgi apparatus [37]. In the Golgi apparatus, SREBPs are processed to generate active fragments and translocate into the nucleus as transcriptional factors. For the first time, Xu et al. reported that phosphorylated PCK1 by AKT translocated into the endoplasmic reticulum and phosphorylated insulin-induced genes (INSIG1/2) to disrupt the interaction between INSIG and SCAP. Free SCAP escorts SREBPs to the Golgi apparatus and the activation of SREBPs leads to the transcription of lipogenesis genes, which promotes hepatocellular carcinoma (HCC) development [33]. PCK1-regulated SREBP activation is also noted in primary HCC compared to that in the adjacent normal tissue from patients, implying an important clinical relevance and a potential for cancer therapy [33].
4. Regulatory network of PCK expression
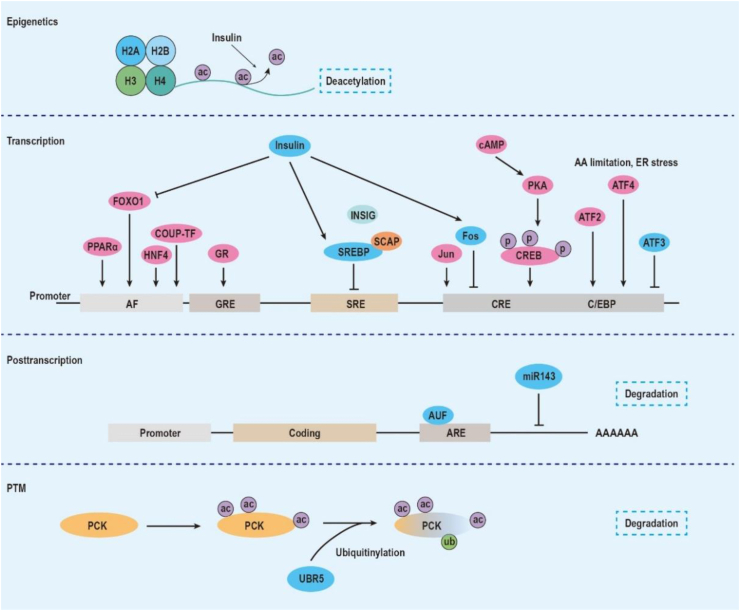
4.1. Transcription regulation of PCK
PCK1 is tightly regulated at multiple levels, from transcription to enzymatic activity. After PCK1 cDNA was isolated and cloned, efforts have been made to characterize its regulation. By applying “run-off” analysis, researchers successfully determined that Pck1's mRNA half-life is considerably short, only ∼30 min [38,39]. Moreover, the rate of PCK1 transcription shows a good linear correlation with the level of Pck1 mRNA. Thus, transcriptional regulation of PCK1 has an impact on its function [40]. To date, numerous regulatory elements in the PCK1 promoter have been identified (summarized in Figure 4). Hanson and Reshef et al. characterized hormonal regulatory elements and cAMP response elements (CRE) within the PCK1 promoter using a series of deletion mutations [[41], [42], [43]]. Roesler et al. further identified a number of protein-binding sites within the PCK1 promoter by DNase I footprinting [44]. Following this work, several important hormonal regulators, transcriptional factors, and their corresponding binding elements were identified and characterized, including the cAMP response element [39,42], CAAT/enhancer-binding protein α (C/EBPα) element [41,44,45], c-Jun/c-Fos-binding element [46], glucocorticoid response region [47,48], insulin response sequence [49,50], thyroid hormone response element [51,52], PPARγ-binding element [[53], [54], [55]], nuclear factor 1 [56,57], insulin/SREBP-1 response element [58,59], and retinoic acid response element [60]. Transcriptional factors and their accessory factors orchestrate to regulate the expression of Pck1 mRNA.
Figure 4.
Illustrative representation of PCK's regulatory mechanism at different levels, from histone modification at the epigenetic level, interaction of transcriptional factors and promoter elements at the transcription level, ARE and miR-mediated mRNA degradation, and ubiquitinylation-mediated protein degradation. Abbreviations: AF, accessory factor; ARE, adenine-uridine rich elements; ATF, activating transcription factor; AUF, AU-rich element RNA-binding protein; C/EBP, CAAT/enhancer-binding protein; COUP-TF, chicken ovalbumin upstream promoter transcription factor; CRE, cAMP regulatory element; CREB, cAMP regulatory element-binding protein; FOXO1, forkhead transcription factor; GR, glucocorticoid receptor; GRE, glucocorticoid regulatory element; HNF, hepatic nuclear factor; PKA, protein kinase A; PPAR, peroxisome proliferator-activated receptor coactivator; SRE, SREBP regulatory element; SREBP, sterol regulatory element-binding protein; UBR5, E3 ubiquitin-protein ligase.
Most knowledge on Pck1 regulatory elements initially has come from studies on nutritional stress, which is closely related to cell growth and development. Under starvation, cAMP or protein kinase A (PKA) is activated [61]. Supplementation of cAMP or ectopic expression of PKA highly induces Pck1 mRNA expression by ∼5 fold and ∼30 fold, respectively [39,41]. A CRE element located at −260 to −82 bp is involved in the binding of cAMP response element-binding protein (CREB) and C/EBPα. The activating transcription factor (ATF) family shares the basic region leucine zipper domain with CREB and C/EBPα. ATF proteins dimerize and bind to a common core sequence “TGACGT” to initiate transcription, which is identical to the consensus of CRE [62]. Stress-inducible mitogen-activated protein kinase p38β instead of ERK augments ATF2 transcriptional activity on PCK1 [63]. Conversely, ATF3 functions as a stress signaling hub of endoplasmic reticulum stress, cytokines, and toxins. Liver-specific overexpression of ATF3 in mice leads to liver dysfunction including hypoglycemia and reduced adipose tissue mass. Further research showed that ATF3 binds to CRE and represses the transcription of PCK1 [64]. c-Jun and c-Fos, together with ATF, belong to the activator protein 1 (AP-1) superfamily and may share the similar consensus sequence site [65]. Gurney et al. showed that c-Jun:c-Jun and c-Fos:c-Jun dimers mainly bind to two distinct regions: CRE (−82 to −90 bp) and P3(II)/P4 (−252 to −258 bp and −268 to −285 bp, respectively) [46]. Ectopic expression of c-Jun upregulates PCK1 expression by over 10 fold, whereas c-Fos blocks PCK1 promoter activity. Moreover, the inhibitory role of c-Fos on PCK1 expression requires additional cofactors bound to the P3(I) region (Figure 4).
Glucocorticoids are necessary to facilitate responses in biological processes such as starvation and pathological processes such as diabetes. Glucocorticoids, similar to cAMP, have been reported to exhibit a predominant positive control of Pck1 mRNA synthesis [43,66]. Generally, glucocorticoid-activated receptor dimer binds to the glucocorticoid response element (GRE) upstream from the transcription initiation site [67]. The PCK1 promoter contains an element spanning ∼110 bp responsible for glucocorticoid effects (−467 to −402 bp) and is bound by glucocorticoid receptor (GR) (−395 to −349 bp). Accessory factor 1 (AF1) and accessory factor 2 (AF2) are located in regions −455 to −431 bp and −420 to −403 bp, respectively. These two elements are required for full glucocorticoid responsiveness. The orphan receptors COUP-TF (also NR2F2) and HNF-4 (also NR2A1) were later identified to function as accessory factors that bind to the AF1 element for glucocorticoid response [47].
In contrast, insulin is a main inhibitory regulator of PCK1 expression [49]. A 15-base pair sequence, designated insulin response sequence (IRS) located at −416 to −402 bp, was described and exhibited a strong negative control of PCK1 promoter activity even in the presence of cAMP supplement or dexamethasone administration [49]. Insulin can also stimulate the expression of negative regulator c-Fos in a rapid manner [68], which may participate in the insulin-dependent inhibition of PCK1. Forkhead transcription factor FOXO1 and peroxisome proliferator-activated receptor-γ co-activator 1 (PGC-1) collaborate to induce PCK1 expression upon starvation, glucagon, or glucocorticoid treatment [50]. Insulin greatly suppresses FOXO1-PGC-1-mediated upregulation of PCK1 through phosphorylating FOXO1 by AKT. Insulin can also increase the level of the precursor form of SREBP-1c through PI3K [69]. The cleaved SREBP-1c subsequently binds to two SREBP regulatory elements (SREs) in PCK1 promoter at −322 to −313 bp and −590 to −581 bp, respectively [59]. Mutation of these SREs increases basal transcription of PCK1 by 5 fold and enhances PKA-induced PCK1 expression as much as 27 fold. Characterizing insulin-mediated PCK expression may provide potential therapeutic targets for diabetes.
Unlike PCK1, the molecular mechanisms underlying PCK2 transcription regulation have not been elucidated. Given that PCK2 is preferentially expressed in various cancer cells, Méndez-Lucas et al. pinpointed that PCK2 was a vital pro-survival regulator against amino acid starvation and endoplasmic reticulum stress [70]. ATF4, not ATF3, binds to the amino acid response element (AARE) site (GTTACATCA) in PCK2 promoter located at position −917 bp and induces its transcription via GCN2-eIF2α-ATF4 and PERK-eIF2α-ATF4 signaling. It remains unclear how PCK2 is regulated.
4.2. Posttranscriptional and posttranslational regulation of PCK
There is no evidence showing that PCK has covalent modifications or allosteric regulation [5]. Therefore, regulation of PCK largely depends on mRNA transcription and its degradation. mRNA stability and miRNA-based degradation are the two main mechanisms regulating PCK abundance. Adenine-uridine rich elements (AREs) are identified in the 3′-untranslated region (3′-UTR) and direct mRNA degradation [71]. Interestingly, PCK1 without 3′-UTR is extremely stable, with a half-life of 5 days [72]. However, PCK1 containing intact 3′-UTR degrades very rapidly (t 1/2 = 1.2 h). AUF1 binds to AU-rich regions in 3′-UTR and promotes the rapid degradation of Pck1 mRNA. 3′-UTR may also be targeted by miRNAs to facilitate mRNA degradation. miR-143 has been reported to target PCK2 and decreases its expression in fibroblasts [73]. Further efforts to identify potential miRNAs targeting PCK are still needed. We recently identified that RNA-binding protein Lin28 binds to Pck2 mRNA and enhances its ability to exert a gluconeogenic role [74]. It was also reported that PCK1 can be acetylated by three lysine residues (70, 71, and 594) and degraded by ubiquitinylation [75,76]. The acetylation of PCK1 controls enzyme activity to regulate gluconeogenesis and anaplerosis [77,78]. Thus, posttranscriptional regulation and protein modification are essential to maintain the homeostasis of Pck mRNA.
4.3. Epigenetic regulation of PCK
Gene expression depends on epigenetic regulation, including histone modification, DNA methylation, chromatin remodeling, and non-coding RNA regulation [79]. New evidence shows that PCK may be under fine-tuned epigenetic regulation as well. As previously discussed, insulin is a vital regulator of PCK1 expression on the transcriptional layer, which affects H3K4me3, H3ac, and H4ac levels over the PCK1 region to suppress its expression [80,81]. A dynamic pattern of DNA methylation has been documented in multiple developing organs of rats [82], in which hypomethylation is accompanied by enhanced expression of PCK1. A similar pattern is also found in baboon fetal liver exposed to reduced nutrients [83]. This epigenetic signature on PCK1 can be transmitted to offspring [84].
5. PCK and clinical relevance
5.1. Diabetes and obesity
The main function of PCK is associated with glucose homeostasis [5]. In 1976, Vidnes et al. reported a deficiency of cytosolic PCK activity caused persistent hypoglycemia in a boy who died at the age of 2 years and 10 months [85]. A later genetic analysis showed a correlation between PCK1 SNP and type 2 diabetes [86]. These human observations create considerable interest in elucidating the molecular mechanisms of PCK in metabolic syndrome using multiple lines of transgenic mice. Of note, PCK's function largely depends on the origin of organs. PCK1 is the key enzyme for hepatic glucose output and glyceroneogenesis in adipose tissue. Whole-body deletion of PCK1 leads to postnatal death within 3 days due to severe hypoglycemia and impaired Krebs cycle in mice [87]. However, mice with liver-specific knockout of PCK1 maintain euglycemia after fasting by coupling impaired hepatic gluconeogenesis to compromised glucose utilization, but develop fatty liver [10]. Beyond regulating hepatic glucose output, an adipose-specific deletion of PCK1 shows lipodystrophy in mice resulting from decreased rates of glyceroneogenesis in adipose tissue [20]. Conversely, global or adipose-specific overexpression of PCK1 results in insulin-independent diabetes and obesity under a high-fat diet [[21], [22], [23]]. The clinical significance of adipose-derived PCK1 largely relies on the key fact that PCK1 dominates glyceroneogenesis in adipose tissue and maintains insulin sensitivity via affecting the concentration of FFA in the blood [[88], [89], [90]]. Unlike PCK1, PCK2 mainly regulates glucose homeostasis by stimulating insulin release. Pancreatic cells sense the glucose, integrate neuroendocrine signals originating from the brain and gut, and secrete insulin and other digestive enzymes [91]. PCK2 is the dominant form in the pancreas, almost exclusively expressed in β cells compared to PCK1 (Figure 2A–B) [92,93]. PCK2 utilizes mitochondrial GTP (mtGTP) as a phosphate donor to catalyze OAA into PEP. It serves as a critical mtGTP recycling mechanism generated from succinyl-CoA synthase reactions [93]. Given mtGTP's fundamental role in promoting glucose-stimulated biphasic insulin secretion (GSIS) [94], this is an important K+-ATP-independent mechanism for insulin secretion, together with classical K+-ATP-dependent insulin secretion [95]. In particular, PCK2 enhances PEP production as much as 3 fold and thereby stimulates insulin release upon glucose stimulation, while silencing PCK2 could totally inhibit GSIS [93]. Correspondingly, single-nucleotide polymorphisms (SNPs) in PCK2, rs4982856 SNP, were identified to positively correlate with new-onset diabetes after kidney transplantation in a Japanese population [96].
5.2. Cancer
Reprogramming cellular metabolism is regarded as a critical hallmark of cancer. Tumor cells rely on aerobic glycolysis to sustain energy, redox balance, and biomass for uncontrolled rapid proliferating demands even with fully functional mitochondria, known as the Warburg effect [97,98]. Over the past decade, a surge in publications rejuvenated this concept, but left even more unsolved questions, particularly regarding its molecular mechanisms, metabolite fluxes, and functions. Intriguingly, PCK is emerging as a novel regulator that certain types of cancer cells use for a specific metabolic route for biosynthesis under nutrient-restrictive conditions [30,99]. Both PCK1 and PCK2 function to channel TCA intermediate OAA into PEP for biomass synthesis in cancer cells. They facilitate the utilization of noncarbohydrate sources, including amino acids such as glutamine, catalyzed into lipids and nucleic acids. Montal et al. found that PCK1 was highly expressed in colon-derived tumors. Inhibition of PCK1 markedly reduced TCA activity and suppressed the cell cycle, which was mainly dependent on glutamine. Most interestingly, PCK1 enhanced glutaminolysis but did not promote the conversion of glutamine-derived PEP into glucose in colon cancer cells, apparently different from PCK1-mediated gluconeogenesis in non-tumorigenic cells [99]. Because G6PC was almost undetectable in these cells, colon cancer cells seem to rewire the anabolic metabolism to fully use intermediates for biosynthesis instead of glucose secretion. During this process, mTORC1 instead of mTORC2 was activated by newly formed PEP-derived amino acids from anabolic metabolism and promoted tumor cell growth [99]. Beyond the metabolic demands for rapid growth, cancer cells also encounter nutrient-poor and hypoxic environments due to their rapid nutrient consumption and inadequate vasculature [100]. Under these conditions, glutamine generally functions as major noncarbohydrate substrate in cancer cells [101]. Vincent et al. identified PCK2 as a key growth regulator in non-small cell lung cancer cells in glucose-free microenvironments using metabolomics [30]. Mechanistically, PCK2 was induced by hypoxia-inducible factor-1 (HIF-1) and reversed glycolysis to synthesize purine nucleotides required for rapid cell proliferation [30]. PEP generated by PCK2 could further increase intracellular calcium, which provokes the calmodulin (CaM)-dependent activation of c-Myc and NFAT signaling [102]. Thereafter, PEP is utilized in anabolism to produce amino acids required for growth, especially serine, glycine, and proline. Silencing or inhibiting PCK2 shuts down the TCA cycle and increases ROS, leading to impaired proteostasis and limited growth of cancer cells [26]. Vincent et al. showed that this PCK2-mediated metabolic plasticity was common in tumors of the thyroid, bladder, breast, and kidney [30]. In all, these studies revealed an important regulatory mechanism of how tumor cells handle metabolic reprogramming with fluctuating microenvironments and bioenergetic demands through PCK-mediated metabolic reprogramming.
A more complicated issue is that the role of PCK is highly dependent on the origin of cancer cells, and PCK may exert an inhibitory role in the development of certain types of cancer [103,104]. Different from the increased expression of PCK in cancer of the colon, lungs, and skin, both PCK1 and PCK2 are simultaneously downregulated in primary HCC. This low expression of PCK predicts a poor prognosis in those patients [103]. More importantly, re-expression of PCK1 or PCK2 results in excessive cataplerosis and oxidative stress, which lead to TCA collapse and apoptosis [103]. The divergent role of PCK in cancer of different organ origins may be due to the much higher activity of TCA intermediate cycling (anaplerosis and cataplerosis) in liver cells compared to other cell types [105]. As discussed in Section 3, a recent study added another regulatory layer to PCK1-mediated HCC development in which PCK1 functions as a protein kinase exclusively in the endoplasmic reticulum to phosphorylate INSIGs to activate lipogenesis and promote HCC growth [33]. Huh7 human HCC cells carrying a PCK1 S90A mutation, which abolishes the activity of phosphorylating INSIGs, fails to grow after in vivo xenografting [33]. Except for compartment-specific activity, this may also depend on glucose availability, since neither PCK1 overexpression nor depletion promotes HCC growth under high-glucose conditions [106]. Although much remains to be explored, these studies, in concert, demonstrate the metabolic vulnerability of cancer cells and indicate that both PCK1 and PCK2 may be promising therapeutic targets in clinic practice.
5.3. Cardiovascular diseases
The heart is a central organ that continuously pumps blood and fulfills the circulatory needs of peripheral organs. As terminally differentiated cells that lack proliferative capacity, cardiomyocytes develop hypertrophic growth in response to increased workloads. In a myriad of conditions such as hypertension, myocardial infarction, valvular diseases, and cardiomyopathy, cardiac hypertrophy helps normalize increased wall tension and temporarily maintains cardiac output. However, pathological cardiac hypertrophy largely exacerbates the risk of heart failure and sudden death [107,108]. A hypertrophic heart undergoes extensive metabolic reprogramming along with structural changes, featured by a shift from fatty acid oxidation to glycolysis [109,110]. Considerable research has been devoted to addressing whether and how such metabolic reprogramming contributes to cardiac hypertrophy and whether it could be a next potential therapeutic target in cardiovascular diseases. By applying genetic screening and stable isotope-labeled metabolic flux, we could pinpoint that PCK2, instead of PCK1, is markedly induced in the heart under pressure overload or treatment with adrenergic agonist, which causes cardiac hypertrophic growth. Suppression of PCK2 attenuated cardiac hypertrophic growth without significantly impairing cardiomyocyte function. Mechanistically, PCK2 was vital to initiating cardiac fetal gene programming and enhancing anabolism. Ectopic expression of PCK2 diverted glucose-derived OAA into PEP and thereafter enhanced the serine biosynthetic pathway. PCK2 was under fine posttranscriptional regulation of RNA-binding protein LIN28 [74]. Strikingly, cardiac metabolic reprogramming featuring a reliance on glycolysis precedes ventricular hypertrophy [74,111,112]. PCK2-mediated hypertrophic response demonstrates that metabolic repatterning could be an initiator that is instrumental in ventricular structural remodeling. More importantly, PCK2 could be a common key regulator in muscle cells beyond cardiomyocytes. Brown et al. demonstrated that growth hormone (GH) or β-adrenergic agonist greatly coordinately increased Pck2 mRNA and serine biosynthetic pathway gene expression to promote the growth of porcine skeletal muscle [29]. Inhibition of PCK2 activity could reduce serine synthetic pathway genes but induce myogenic differentiation [113]. Further study of PCK2 regulation could inspire a novel treatment for heart and muscle protection.
5.4. Neurologic diseases
Glucose is the primary energy source for the brain. Perturbation of glucose homeostasis may influence the progression and prognosis of diverse neurologic diseases. Stroke is one of the most common neurologic disorders and the leading cause of death, 87% of which is ischemic stroke [114]. During cerebral ischemia, hyperglycemia is a general sign of adverse clinical outcomes [115]. However, targeting hyperglycemia did not show promising protection for those patients with stroke in clinical trials [116]. Geng et al. identified that PCK may be the missing link in this context [117]. PCK and its gluconeogenic product PEP dramatically increased after ischemia, suggesting that PCK-mediated metabolic diversion may participate in this process. By applying the PCK-specific inhibitor 3-mercaptopropionic acid (3-MPA) or knockdown shRNA, cerebral ischemic damage was markedly alleviated with reduced lactate production, acidosis, and oxidative stress. Most interestingly, as the metabolic product of PCK, more PEP was further catalyzed into lactate compared to that converted into glucose. This highly implies that either defects of PEP-utilizing processes or PCK-mediated byproducts may evoke detrimental signaling in the ischemic brain. Together, these results identify a novel role of PCK in ischemic stroke, suggesting more efforts should be made to illustrate the therapeutic potential of PCK in neurologic disorders.
6. Conclusions
This review summarizes the current knowledge of the multifaceted roles of PCK, including glyceroneogenesis, anabolism, and protein kinase, beyond its vital function in gluconeogenesis. This review also highlights a comprehensive regulatory network covering epigenetic, transcription, and posttranscription regulation and posttranslational modification of PCK isoforms. In the future, distinct regulatory elements of PCK's isoforms should be further studied considering the complex structure of their promoters. By applying state-of-the-art metabolomics and flux analyses, we may gain deeper insights into its metabolic functions, especially in tissue or context-specific manners. All this information may facilitate a better understanding of the clinical relevance of PCK and its therapeutic potential.
Author contributions
M.X. and H.M. conceived of the manuscript. S.Y. wrote the manuscript. S.M. prepared the figures. All the authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82070251 and 81870203 to M.X. and 82070252 to H.M.) and the Zhejiang Provincial Natural Science Foundation (LR21H020001 to H.M.).
Data availability statement
All the data are included in the article.
Contributor Information
Meixiang Xiang, Email: xiangmx@zju.edu.cn.
Hong Ma, Email: hong_ma@zju.edu.cn.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Hanson R.W., Garber A.J. Phosphoenolpyruvate carboxykinase. I. Its role in gluconeogenesis. American Journal of Clinical Nutrition. 1972;25(10):1010–1021. doi: 10.1093/ajcn/25.10.1010. [DOI] [PubMed] [Google Scholar]
- 2.Watford M., Hod Y., Chiao Y.B., Utter M.F., Hanson R.W. The unique role of the kidney in gluconeogenesis in the chicken. The significance of a cytosolic form of phosphoenolpyruvate carboxykinase. Journal of Biological Chemistry. 1981;256(19):10023–10027. [PubMed] [Google Scholar]
- 3.Ballard F.J., Hanson R.W., Kronfeld D.S. Gluconeogenesis and lipogenesis in tissue from ruminant and nonruminant animals. Federation Proceedings. 1969;28(1):218–231. [PubMed] [Google Scholar]
- 4.Savon S., Hakimi P., Hanson R.W. Expression of the genes for the mitochondrial and cytosolic forms of phosphoenolpyruvate carboxykinase in avian liver during development. Biology of the Neonate. 1993;64(1):62–68. doi: 10.1159/000243972. [DOI] [PubMed] [Google Scholar]
- 5.Beale E.G., Harvey B.J., Forest C. PCK1 and PCK2 as candidate diabetes and obesity genes. Cell Biochemistry and Biophysics. 2007;48(2–3):89–95. doi: 10.1007/s12013-007-0025-6. [DOI] [PubMed] [Google Scholar]
- 6.Yang J., Kalhan S.C., Hanson R.W. What is the metabolic role of phosphoenolpyruvate carboxykinase? Journal of Biological Chemistry. 2009;284(40):27025–27029. doi: 10.1074/jbc.R109.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soling H.D., Kleineke J., Willms B., Janson G., Kuhn A. Relationship between intracellular distribution of phosphoenolpyruvate carboxykinase, regulation of gluconeogenesis, and energy cost of glucose formation. European Journal of Biochemistry. 1973;37(2):233–243. doi: 10.1111/j.1432-1033.1973.tb02980.x. [DOI] [PubMed] [Google Scholar]
- 8.Hakimi P., Johnson M.T., Yang Y., Lepage D.F., Conlon R.A., Kalhan S.C. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutrition and Metabolism. 2005;2:33. doi: 10.1186/1743-7075-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen M.C., Vatner D.F., Shulman G.I. Regulation of hepatic glucose metabolism in health and disease. Nature Reviews Endocrinology. 2017;13(10):572–587. doi: 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.She P., Burgess S.C., Shiota M., Flakoll P., Donahue E.P., Malloy C.R. Mechanisms by which liver-specific PEPCK knockout mice preserve euglycemia during starvation. Diabetes. 2003;52(7):1649–1654. doi: 10.2337/diabetes.52.7.1649. [DOI] [PubMed] [Google Scholar]
- 11.Reshef L., Olswang Y., Cassuto H., Blum B., Croniger C.M., Kalhan S.C. Glyceroneogenesis and the triglyceride/fatty acid cycle. Journal of Biological Chemistry. 2003;278(33):30413–30416. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- 12.Viecili P.R.N., da Silva B., Hirsch G.E., Porto F.G., Parisi M.M., Castanho A.R. Triglycerides revisited to the serial. Advances in Clinical Chemistry. 2017;80:1–44. doi: 10.1016/bs.acc.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Jensen M.D., Ekberg K., Landau B.R. Lipid metabolism during fasting. American Journal of Physiology. Endocrinology and Metabolism. 2001;281(4):E789–E793. doi: 10.1152/ajpendo.2001.281.4.E789. [DOI] [PubMed] [Google Scholar]
- 14.AbouRjaili G., Shtaynberg N., Wetz R., Costantino T., Abela G.S. Current concepts in triglyceride metabolism, pathophysiology, and treatment. Metabolism. 2010;59(8):1210–1220. doi: 10.1016/j.metabol.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Alves-Bezerra M., Cohen D.E. Triglyceride metabolism in the liver. Comparative Physiology. 2017;8(1):1–8. doi: 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson R.W., Ballard F.J., Reshef L. Glyceroneogenesis, the pathway that almost wasn't. Biochemistry and Molecular Biology Education. 2006;34(5):317–323. doi: 10.1002/bmb.2006.494034052637. [DOI] [PubMed] [Google Scholar]
- 17.Kalhan S.C., Mahajan S., Burkett E., Reshef L., Hanson R.W. Glyceroneogenesis and the source of glycerol for hepatic triacylglycerol synthesis in humans. Journal of Biological Chemistry. 2001;276(16):12928–12931. doi: 10.1074/jbc.M006186200. [DOI] [PubMed] [Google Scholar]
- 18.Beale E.G., Hammer R.E., Antoine B., Forest C. Glyceroneogenesis comes of age. The FASEB Journal. 2002;16(13):1695–1696. doi: 10.1096/fj.02-0407rev. [DOI] [PubMed] [Google Scholar]
- 19.Millward C.A., Desantis D., Hsieh C.W., Heaney J.D., Pisano S., Olswang Y. Phosphoenolpyruvate carboxykinase (Pck1) helps regulate the triglyceride/fatty acid cycle and development of insulin resistance in mice. The Journal of Lipid Research. 2010;51(6):1452–1463. doi: 10.1194/jlr.M005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olswang Y., Cohen H., Papo O., Cassuto H., Croniger C.M., Hakimi P. A mutation in the peroxisome proliferator-activated receptor gamma-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proceedings of the National Academy of Sciences of the U S A. 2002;99(2):625–630. doi: 10.1073/pnas.022616299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franckhauser S., Munoz S., Elias I., Ferre T., Bosch F. Adipose overexpression of phosphoenolpyruvate carboxykinase leads to high susceptibility to diet-induced insulin resistance and obesity. Diabetes. 2006;55(2):273–280. doi: 10.2337/diabetes.55.02.06.db05-0482. [DOI] [PubMed] [Google Scholar]
- 22.Franckhauser S., Munoz S., Pujol A., Casellas A., Riu E., Otaegui P. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes. 2002;51(3):624–630. doi: 10.2337/diabetes.51.3.624. [DOI] [PubMed] [Google Scholar]
- 23.Valera A., Pujol A., Pelegrin M., Bosch F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proceedings of the National Academy of Sciences of the U S A. 1994;91(19):9151–9154. doi: 10.1073/pnas.91.19.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez-Valades A.G., Mendez-Lucas A., Vidal-Alabro A., Blasco F.X., Chillon M., Bartrons R. Pck1 gene silencing in the liver improves glycemia control, insulin sensitivity, and dyslipidemia in db/db mice. Diabetes. 2008;57(8):2199–2210. doi: 10.2337/db07-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue F., Cheng Y., Breschi A., Vierstra J., Wu W., Ryba T. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515(7527):355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyrossova P., Arago M., Moreno-Felici J., Fu X., Mendez-Lucas A., Garcia-Roves P.M. PEPCK-M recoups tumor cell anabolic potential in a PKC-zeta-dependent manner. Cancer & Metabolism. 2021;9(1):1. doi: 10.1186/s40170-020-00236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owen O.E., Kalhan S.C., Hanson R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. Journal of Biological Chemistry. 2002;277(34):30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 28.Yang M., Vousden K.H. Serine and one-carbon metabolism in cancer. Nature Reviews Cancer. 2016;16(10):650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 29.Brown D.M., Williams H., Ryan K.J., Wilson T.L., Daniel Z.C., Mareko M.H. Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) and serine biosynthetic pathway genes are co-ordinately increased during anabolic agent-induced skeletal muscle growth. Scientific Reports. 2016;6:28693. doi: 10.1038/srep28693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent E.E., Sergushichev A., Griss T., Gingras M.C., Samborska B., Ntimbane T. Mitochondrial phosphoenolpyruvate carboxykinase regulates metabolic adaptation and enables glucose-independent tumor growth. Molecules and Cells. 2015;60(2):195–207. doi: 10.1016/j.molcel.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Egervari G., Wang Y., Berger S.L., Lu Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nature Reviews Molecular Cell Biology. 2018;19(9):563–578. doi: 10.1038/s41580-018-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu S., Wang Y. Nonmetabolic functions of metabolic enzymes in cancer development. Cancer Communications. 2018;38(1):63. doi: 10.1186/s40880-018-0336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu D., Wang Z., Xia Y., Shao F., Xia W., Wei Y. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature. 2020;580(7804):530–535. doi: 10.1038/s41586-020-2183-2. [DOI] [PubMed] [Google Scholar]
- 34.Rohrig F., Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nature Reviews Cancer. 2016;16(11):732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 35.Brown M.S., Goldstein J.L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 36.Horton J.D., Goldstein J.L., Brown M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. Journal of Clinical Investigation. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nohturfft A., Yabe D., Goldstein J.L., Brown M.S., Espenshade P.J. Regulated step in cholesterol feedback localized to budding of SCAP from ER membranes. Cell. 2000;102(3):315–323. doi: 10.1016/s0092-8674(00)00037-4. [DOI] [PubMed] [Google Scholar]
- 38.Beale E.G., Katzen C.S., Granner D.K. Regulation of rat liver phosphoenolpyruvate carboxykinase (GTP) messenger ribonucleic acid activity by N6, O2'-dibutyryladenosine 3',5'-phosphate. Biochemistry. 1981;20(17):4878–4883. doi: 10.1021/bi00520a012. [DOI] [PubMed] [Google Scholar]
- 39.Lamers W.H., Hanson R.W., Meisner H.M. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proceedings of the National Academy of Sciences of the U S A. 1982;79(17):5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatting M., Tavares C.D.J., Sharabi K., Rines A.K., Puigserver P. Insulin regulation of gluconeogenesis. Annals of the New York Academy of Sciences. 2018;1411(1):21–35. doi: 10.1111/nyas.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J.S., Park E.A., Gurney A.L., Roesler W.J., Hanson R.W. Cyclic AMP induction of phosphoenolpyruvate carboxykinase (GTP) gene transcription is mediated by multiple promoter elements. Journal of Biological Chemistry. 1991;266(28):19095–19102. [PubMed] [Google Scholar]
- 42.Short J.M., Wynshaw-Boris A., Short H.P., Hanson R.W. Characterization of the phosphoenolpyruvate carboxykinase (GTP) promoter-regulatory region. II. Identification of cAMP and glucocorticoid regulatory domains. Journal of Biological Chemistry. 1986;261(21):9721–9726. [PubMed] [Google Scholar]
- 43.Wynshaw-Boris A., Short J.M., Loose D.S., Hanson R.W. Characterization of the phosphoenolpyruvate carboxykinase (GTP) promoter-regulatory region. I. Multiple hormone regulatory elements and the effects of enhancers. Journal of Biological Chemistry. 1986;261(21):9714–9720. [PubMed] [Google Scholar]
- 44.Roesler W.J., Vandenbark G.R., Hanson R.W. Identification of multiple protein binding domains in the promoter-regulatory region of the phosphoenolpyruvate carboxykinase (GTP) gene. Journal of Biological Chemistry. 1989;264(16):9657–9664. [PubMed] [Google Scholar]
- 45.Park E.A., Roesler W.J., Liu J., Klemm D.J., Gurney A.L., Thatcher J.D. The role of the CCAAT/enhancer-binding protein in the transcriptional regulation of the gene for phosphoenolpyruvate carboxykinase (GTP) Molecular and Cellular Biology. 1990;10(12):6264–6272. doi: 10.1128/mcb.10.12.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurney A.L., Park E.A., Giralt M., Liu J., Hanson R.W. Opposing actions of Fos and Jun on transcription of the phosphoenolpyruvate carboxykinase (GTP) gene. Dominant negative regulation by Fos. Journal of Biological Chemistry. 1992;267(25):18133–18139. [PubMed] [Google Scholar]
- 47.Hall R.K., Sladek F.M., Granner D.K. The orphan receptors COUP-TF and HNF-4 serve as accessory factors required for induction of phosphoenolpyruvate carboxykinase gene transcription by glucocorticoids. Proceedings of the National Academy of Sciences of the U S A. 1995;92(2):412–416. doi: 10.1073/pnas.92.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imai E., Stromstedt P.E., Quinn P.G., Carlstedt-Duke J., Gustafsson J.A., Granner D.K. Characterization of a complex glucocorticoid response unit in the phosphoenolpyruvate carboxykinase gene. Molecular and Cellular Biology. 1990;10(9):4712–4719. doi: 10.1128/mcb.10.9.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Brien R.M., Lucas P.C., Forest C.D., Magnuson M.A., Granner D.K. Identification of a sequence in the PEPCK gene that mediates a negative effect of insulin on transcription. Science. 1990;249(4968):533–537. doi: 10.1126/science.2166335. [DOI] [PubMed] [Google Scholar]
- 50.Puigserver P., Rhee J., Donovan J., Walkey C.J., Yoon J.C., Oriente F. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423(6939):550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 51.Giralt M., Park E.A., Gurney A.L., Liu J.S., Hakimi P., Hanson R.W. Identification of a thyroid hormone response element in the phosphoenolpyruvate carboxykinase (GTP) gene. Evidence for synergistic interaction between thyroid hormone and cAMP cis-regulatory elements. Journal of Biological Chemistry. 1991;266(32):21991–21996. [PubMed] [Google Scholar]
- 52.Park E.A., Jerden D.C., Bahouth S.W. Regulation of phosphoenolpyruvate carboxykinase gene transcription by thyroid hormone involves two distinct binding sites in the promoter. Biochemical Journal. 1995;309(Pt 3):913–919. doi: 10.1042/bj3090913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bogacka I., Xie H., Bray G.A., Smith S.R. The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivo. Diabetes Care. 2004;27(7):1660–1667. doi: 10.2337/diacare.27.7.1660. [DOI] [PubMed] [Google Scholar]
- 54.Devine J.H., Eubank D.W., Clouthier D.H., Tontonoz P., Spiegelman B.M., Hammer R.M. Adipose expression of the phosphoenolpyruvate carboxykinase promoter requires peroxisome proliferator-activated receptor gamma and 9-cis-retinoic acid receptor binding to an adipocyte-specific enhancer in vivo. Journal of Biological Chemistry. 1999;274(19):13604–13612. doi: 10.1074/jbc.274.19.13604. [DOI] [PubMed] [Google Scholar]
- 55.Hommes F.A., Bendien K., Elema J.D., Bremer H.J., Lombeck I. Two cases of phosphoenolpyruvate carboxykinase deficiency. Acta Paediatrica Scandinavica. 1976;65(2):233–240. doi: 10.1111/j.1651-2227.1976.tb16543.x. [DOI] [PubMed] [Google Scholar]
- 56.Crawford D.R., Leahy P., Hu C.Y., Chaudhry A., Gronostajski R., Grossman G. Nuclear factor I regulates expression of the gene for phosphoenolpyruvate carboxykinase (GTP) Journal of Biological Chemistry. 1998;273(22):13387–13390. doi: 10.1074/jbc.273.22.13387. [DOI] [PubMed] [Google Scholar]
- 57.Leahy P., Crawford D.R., Grossman G., Gronostajski R.M., Hanson R.W. CREB binding protein coordinates the function of multiple transcription factors including nuclear factor I to regulate phosphoenolpyruvate carboxykinase (GTP) gene transcription. Journal of Biological Chemistry. 1999;274(13):8813–8822. doi: 10.1074/jbc.274.13.8813. [DOI] [PubMed] [Google Scholar]
- 58.Chakravarty K., Leahy P., Becard D., Hakimi P., Foretz M., Ferre P. Sterol regulatory element-binding protein-1c mimics the negative effect of insulin on phosphoenolpyruvate carboxykinase (GTP) gene transcription. Journal of Biological Chemistry. 2001;276(37):34816–34823. doi: 10.1074/jbc.M103310200. [DOI] [PubMed] [Google Scholar]
- 59.Chakravarty K., Wu S.Y., Chiang C.M., Samols D., Hanson R.W. SREBP-1c and Sp1 interact to regulate transcription of the gene for phosphoenolpyruvate carboxykinase (GTP) in the liver. Journal of Biological Chemistry. 2004;279(15):15385–15395. doi: 10.1074/jbc.M309905200. [DOI] [PubMed] [Google Scholar]
- 60.Hall R.K., Scott D.K., Noisin E.L., Lucas P.C., Granner D.K. Activation of the phosphoenolpyruvate carboxykinase gene retinoic acid response element is dependent on a retinoic acid receptor/coregulator complex. Molecular and Cellular Biology. 1992;12(12):5527–5535. doi: 10.1128/mcb.12.12.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan K., Gao L.N., Cui Y.L., Zhang Y., Zhou X. The cyclic AMP signaling pathway: exploring targets for successful drug discovery (Review) Molecular Medicine Reports. 2016;13(5):3715–3723. doi: 10.3892/mmr.2016.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hai T., Hartman M.G. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene. 2001;273(1):1–11. doi: 10.1016/s0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- 63.Cheong J., Coligan J.E., Shuman J.D. Activating transcription factor-2 regulates phosphoenolpyruvate carboxykinase transcription through a stress-inducible mitogen-activated protein kinase pathway. Journal of Biological Chemistry. 1998;273(35):22714–22718. doi: 10.1074/jbc.273.35.22714. [DOI] [PubMed] [Google Scholar]
- 64.Allen-Jennings A.E., Hartman M.G., Kociba G.J., Hai T. The roles of ATF3 in liver dysfunction and the regulation of phosphoenolpyruvate carboxykinase gene expression. Journal of Biological Chemistry. 2002;277(22):20020–20025. doi: 10.1074/jbc.M200727200. [DOI] [PubMed] [Google Scholar]
- 65.van Dam H., Castellazzi M. Distinct roles of jun : fos and jun : ATF dimers in oncogenesis. Oncogene. 2001;20(19):2453–2464. doi: 10.1038/sj.onc.1204239. [DOI] [PubMed] [Google Scholar]
- 66.Petersen D.D., Koch S.R., Granner D.K. 3' noncoding region of phosphoenolpyruvate carboxykinase mRNA contains a glucocorticoid-responsive mRNA-stabilizing element. Proceedings of the National Academy of Sciences of the U S A. 1989;86(20):7800–7804. doi: 10.1073/pnas.86.20.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vegiopoulos A., Herzig S. Glucocorticoids, metabolism and metabolic diseases. Molecular and Cellular Endocrinology. 2007;275(1–2):43–61. doi: 10.1016/j.mce.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 68.Stumpo D.J., Blackshear P.J. Insulin and growth factor effects on c-fos expression in normal and protein kinase C-deficient 3T3-L1 fibroblasts and adipocytes. Proceedings of the National Academy of Sciences of the U S A. 1986;83(24):9453–9457. doi: 10.1073/pnas.83.24.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azzout-Marniche D., Becard D., Guichard C., Foretz M., Ferre P., Foufelle F. Insulin effects on sterol regulatory-element-binding protein-1c (SREBP-1c) transcriptional activity in rat hepatocytes. Biochemical Journal. 2000;350 Pt 2:389–393. [PMC free article] [PubMed] [Google Scholar]
- 70.Mendez-Lucas A., Hyrossova P., Novellasdemunt L., Vinals F., Perales J.C. Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) is a pro-survival, endoplasmic reticulum (ER) stress response gene involved in tumor cell adaptation to nutrient availability. Journal of Biological Chemistry. 2014;289(32):22090–22102. doi: 10.1074/jbc.M114.566927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barreau C., Paillard L., Osborne H.B. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Research. 2005;33(22):7138–7150. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hajarnis S., Schroeder J.M., Curthoys N.P. 3'-Untranslated region of phosphoenolpyruvate carboxykinase mRNA contains multiple instability elements that bind AUF1. Journal of Biological Chemistry. 2005;280(31):28272–28280. doi: 10.1074/jbc.M501204200. [DOI] [PubMed] [Google Scholar]
- 73.Trakooljul N., Hicks J.A., Liu H.C. Identification of target genes and pathways associated with chicken microRNA miR-143. Animal Genetics. 2010;41(4):357–364. doi: 10.1111/j.1365-2052.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 74.Ma H., Yu S., Liu X., Zhang Y., Fakadej T., Liu Z. Lin28a regulates pathological cardiac hypertrophic growth through pck2-mediated enhancement of anabolic synthesis. Circulation. 2019;139(14):1725–1740. doi: 10.1161/CIRCULATIONAHA.118.037803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang W., Wang S., Xiao M., Lin Y., Zhou L., Lei Q. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Molecules and Cells. 2011;43(1):33–44. doi: 10.1016/j.molcel.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiong Y., Lei Q.Y., Zhao S., Guan K.L. Regulation of glycolysis and gluconeogenesis by acetylation of PKM and PEPCK. Cold Spring Harbor Symposia on Quantitative Biology. 2011;76:285–289. doi: 10.1101/sqb.2011.76.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Latorre-Muro P., Baeza J., Hurtado-Guerrero R., Hicks T., Delso I., Hernandez-Ruiz C. Self-acetylation at the active site of phosphoenolpyruvate carboxykinase (PCK1) controls enzyme activity. Journal of Biological Chemistry. 2020 doi: 10.1074/jbc.RA120.015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Latorre-Muro P., Baeza J., Armstrong E.A., Hurtado-Guerrero R., Corzana F., Wu L.E. Dynamic acetylation of phosphoenolpyruvate carboxykinase toggles enzyme activity between gluconeogenic and anaplerotic reactions. Molecules and Cells. 2018;71(5):718–732 e9. doi: 10.1016/j.molcel.2018.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Z., Li S., Subramaniam S., Shyy J.Y., Chien S. Epigenetic regulation: a new frontier for biomedical engineers. Annual Review of Biomedical Engineering. 2017;19:195–219. doi: 10.1146/annurev-bioeng-071516-044720. [DOI] [PubMed] [Google Scholar]
- 80.Honma K., Kamikubo M., Mochizuki K., Goda T. Insulin-induced inhibition of gluconeogenesis genes, including glutamic pyruvic transaminase 2, is associated with reduced histone acetylation in a human liver cell line. Metabolism. 2017;71:118–124. doi: 10.1016/j.metabol.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Strakovsky R.S., Zhang X., Zhou D., Pan Y.X. Gestational high fat diet programs hepatic phosphoenolpyruvate carboxykinase gene expression and histone modification in neonatal offspring rats. The Journal of Physiology. 2011;589(Pt 11):2707–2717. doi: 10.1113/jphysiol.2010.203950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benvenisty N., Mencher D., Meyuhas O., Razin A., Reshef L. Sequential changes in DNA methylation patterns of the rat phosphoenolpyruvate carboxykinase gene during development. Proceedings of the National Academy of Sciences of the U S A. 1985;82(2):267–271. doi: 10.1073/pnas.82.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nijland M.J., Mitsuya K., Li C., Ford S., McDonald T.J., Nathanielsz P.W. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. The Journal of Physiology. 2010;588(Pt 8):1349–1359. doi: 10.1113/jphysiol.2009.184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoile S.P., Lillycrop K.A., Thomas N.A., Hanson M.A., Burdge G.C. Dietary protein restriction during F0 pregnancy in rats induces transgenerational changes in the hepatic transcriptome in female offspring. PloS One. 2011;6(7) doi: 10.1371/journal.pone.0021668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vidnes J., Sovik O. Gluconeogenesis in infancy and childhood. III. Deficiency of the extramitochondrial form of hepatic phosphoenolpyruvate carboxykinase in a case of persistent neonatal hypoglycaemia. Acta Paediatrica Scandinavica. 1976;65(3):307–312. doi: 10.1111/j.1651-2227.1976.tb04890.x. [DOI] [PubMed] [Google Scholar]
- 86.Duplus E., Benelli C., Reis A.F., Fouque F., Velho G., Forest C. Expression of phosphoenolpyruvate carboxykinase gene in human adipose tissue: induction by rosiglitazone and genetic analyses of the adipocyte-specific region of the promoter in type 2 diabetes. Biochimie. 2003;85(12):1257–1264. doi: 10.1016/j.biochi.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 87.She P., Shiota M., Shelton K.D., Chalkley R., Postic C., Magnuson M.A. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Molecular and Cellular Biology. 2000;20(17):6508–6517. doi: 10.1128/mcb.20.17.6508-6517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glorian M., Duplus E., Beale E.G., Scott D.K., Granner D.K., Forest C. A single element in the phosphoenolpyruvate carboxykinase gene mediates thiazolidinedione action specifically in adipocytes. Biochimie. 2001;83(10):933–943. doi: 10.1016/s0300-9084(01)01343-8. [DOI] [PubMed] [Google Scholar]
- 89.McGarry J.D. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258(5083):766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 90.Tordjman J., Chauvet G., Quette J., Beale E.G., Forest C., Antoine B. Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells. Journal of Biological Chemistry. 2003;278(21):18785–18790. doi: 10.1074/jbc.M206999200. [DOI] [PubMed] [Google Scholar]
- 91.Schuit F.C., Huypens P., Heimberg H., Pipeleers D.G. Glucose sensing in pancreatic beta-cells: a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus. Diabetes. 2001;50(1):1–11. doi: 10.2337/diabetes.50.1.1. [DOI] [PubMed] [Google Scholar]
- 92.MacDonald M.J., McKenzie D.I., Walker T.M., Kaysen J.H. Lack of glyconeogenesis in pancreatic islets: expression of gluconeogenic enzyme genes in islets. Hormone and Metabolic Research. 1992;24(4):158–160. doi: 10.1055/s-2007-1003284. [DOI] [PubMed] [Google Scholar]
- 93.Stark R., Pasquel F., Turcu A., Pongratz R.L., Roden M., Cline G.W. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. Journal of Biological Chemistry. 2009;284(39):26578–26590. doi: 10.1074/jbc.M109.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kibbey R.G., Pongratz R.L., Romanelli A.J., Wollheim C.B., Cline G.W., Shulman G.I. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metabolism. 2007;5(4):253–264. doi: 10.1016/j.cmet.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Straub S.G., Sharp G.W. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metabolism Research Review. 2002;18(6):451–463. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 96.Yokoyama N., Ishimura T., Oda T., Ogawa S., Yamamoto K., Fujisawa M. Association of the PCK2 gene polymorphism with new-onset glucose intolerance in Japanese kidney transplant recipients. Transplantation Proceedings. 2018;50(4):1045–1049. doi: 10.1016/j.transproceed.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 97.Balsa-Martinez E., Puigserver P. Cancer cells hijack gluconeogenic enzymes to fuel cell growth. Molecules and Cells. 2015;60(4):509–511. doi: 10.1016/j.molcel.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 98.Liberti M.V., Locasale J.W. The Warburg effect: how does it benefit cancer cells? Trends in Biochemical Sciences. 2016;41(3):211–218. doi: 10.1016/j.tibs.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 99.Montal E.D., Dewi R., Bhalla K., Ou L., Hwang B.J., Ropell A.E. PEPCK coordinates the regulation of central carbon metabolism to promote cancer cell growth. Molecules and Cells. 2015;60(4):571–583. doi: 10.1016/j.molcel.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Research. 1989;49(23):6449–6465. [PubMed] [Google Scholar]
- 101.Cluntun A.A., Lukey M.J., Cerione R.A., Locasale J.W. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer. 2017;3(3):169–180. doi: 10.1016/j.trecan.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moreno-Felici J., Hyrossova P., Arago M., Rodriguez-Arevalo S., Garcia-Roves P.M., Escolano C. Phosphoenolpyruvate from glycolysis and PEPCK regulate cancer cell fate by altering cytosolic Ca(2) Cells. 2019;9(1) doi: 10.3390/cells9010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu M.X., Jin L., Sun S.J., Liu P., Feng X., Cheng Z.L. Metabolic reprogramming by PCK1 promotes TCA cataplerosis, oxidative stress and apoptosis in liver cancer cells and suppresses hepatocellular carcinoma. Oncogene. 2018;37(12):1637–1653. doi: 10.1038/s41388-017-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tang Y., Zhang Y., Wang C., Sun Z., Li L., Cheng S. Overexpression of PCK1 gene antagonizes hepatocellular carcinoma through the activation of gluconeogenesis and suppression of glycolysis pathways. Cellular Physiology and Biochemistry. 2018;47(1):344–355. doi: 10.1159/000489811. [DOI] [PubMed] [Google Scholar]
- 105.Sunny N.E., Parks E.J., Browning J.D., Burgess S.C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metabolism. 2011;14(6):804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiang J., Chen C., Liu R., Gou D., Chang L., Deng H. Gluconeogenic enzyme PCK1 deficiency promotes CHK2 O-GlcNAcylation and hepatocellular carcinoma growth upon glucose deprivation. Journal of Clinical Investigation. 2021;131(8) doi: 10.1172/JCI144703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Frey N., Katus H.A., Olson E.N., Hill J.A. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109(13):1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 108.Nakamura M., Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nature Reviews Cardiology. 2018;15(7):387–407. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 109.Kolwicz S.C., Jr., Tian R. Glucose metabolism and cardiac hypertrophy. Cardiovascular Research. 2011;90(2):194–201. doi: 10.1093/cvr/cvr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tran D.H., Wang Z.V. Glucose metabolism in cardiac hypertrophy and heart failure. Journal of American Heart Association. 2019;8(12) doi: 10.1161/JAHA.119.012673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doenst T., Pytel G., Schrepper A., Amorim P., Farber G., Shingu Y. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovascular Research. 2010;86(3):461–470. doi: 10.1093/cvr/cvp414. [DOI] [PubMed] [Google Scholar]
- 112.Zhang L., Jaswal J.S., Ussher J.R., Sankaralingam S., Wagg C., Zaugg M. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circulation Heart Fail. 2013;6(5):1039–1048. doi: 10.1161/CIRCHEARTFAILURE.112.000228. [DOI] [PubMed] [Google Scholar]
- 113.Brearley M.C., Daniel Z., Loughna P.T., Parr T., Brameld J.M. The phosphoenolpyruvate carboxykinase (PEPCK) inhibitor, 3-mercaptopicolinic acid (3-MPA), induces myogenic differentiation in C2C12 cells. Scientific Reports. 2020;10(1):22177. doi: 10.1038/s41598-020-79324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 115.Piironen K., Putaala J., Rosso C., Samson Y. Glucose and acute stroke: evidence for an interlude. Stroke. 2012;43(3):898–902. doi: 10.1161/STROKEAHA.111.631218. [DOI] [PubMed] [Google Scholar]
- 116.Li W.A., Moore-Langston S., Chakraborty T., Rafols J.A., Conti A.C., Ding Y. Hyperglycemia in stroke and possible treatments. Neurological Research. 2013;35(5):479–491. doi: 10.1179/1743132813Y.0000000209. [DOI] [PubMed] [Google Scholar]
- 117.Geng X., Shen J., Li F., Yip J., Guan L., Rajah G. Phosphoenolpyruvate carboxykinase (PCK) in the brain gluconeogenic pathway contributes to oxidative and lactic injury after stroke. Molecular Neurobiology. 2021;58(5):2309–2321. doi: 10.1007/s12035-020-02251-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are included in the article.