Abstract
Background
Brain metastases (BM) are a rare complication in colorectal cancer (CRC) patients and associated with an unfavorable survival prognosis. Primary tumor side (PTS) was shown to act as a prognostic and predictive biomarker in several trials including metastatic CRC (mCRC) patients. Here, we aim to investigate whether PTS is also associated with the outcome of CRC patients with BM.
Methods
Patients treated for CRC BM between 1988 and 2017 at an academic care center were included. Right-sided CRC was defined as located in the appendix, cecum and ascending colon and left-sided CRC was defined as located in the descending colon, sigma and rectum.
Results
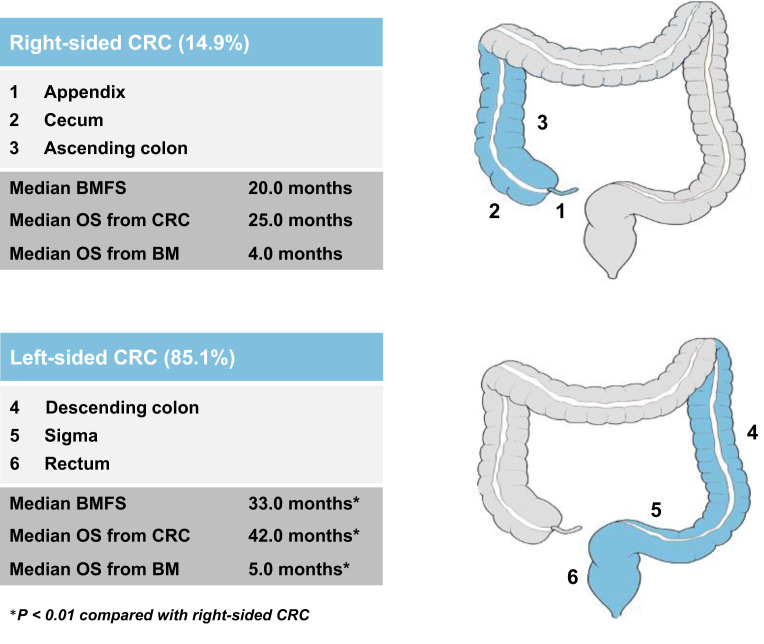
Two hundred and eighty-one CRC BM patients were available for this analysis with 239/281 patients (85.1%) presenting with a left-sided and 42/281 patients (14.9%) with a right-sided primary CRC. BM-free survival (BMFS) was significantly longer in left-sided compared with right-sided CRC patients (33 versus 20 months, P = 0.009). Overall survival from CRC diagnosis as well as from diagnosis of BM was significantly longer in patients with a left-sided primary (42 versus 25 months, P = 0.002 and 5 versus 4 months, P = 0.005, respectively). In a multivariate analysis including graded prognostic assessment, PTS remained significantly associated with prognosis after BM (hazard ratio 0.65; 95% confidence interval: 0.46-0.92 months, P = 0.0016).
Conclusions
PTS was associated with survival times after the rare event of BM development in CRC patients. Therefore, its prognostic value remains significant even thereafter.
Key words: colorectal cancer, brain metastases, primary tumor side, sidedness
Highlights
-
•
Primary tumor side is a relevant and independent prognostic factor in mCRC.
-
•
Left-sided CRC was associated with a significantly longer BMFS compared with right-sided CRC.
-
•
OS from initial diagnosis of CRC as well as from BM was significantly longer in patients with left-sided primaries.
Introduction
Recent data strongly support the biological heterogeneity of colorectal cancer (CRC), arguing that CRCs are actually several different diseases originating at the same location. In addition to molecular biomarkers such as KRAS, NRAS, BRAF, mismatch repair (MMR) deficiency and HER2, primary tumor side (PTS) of CRC has been recently described to act as a prognostic and predictive surrogate parameter in metastatic CRC (mCRC) patients. One-third of colorectal tumors are right-sided and originate from the embryonic midgut, whereas two-thirds are left-sided and derive from the embryonic hindgut.1,2 Right-sided tumors are associated with a generally worse prognosis compared with left-sided colorectal tumors, as reflected by a higher incidence of mucinous, undifferentiated and signet-ring cell tumors and a usually more advanced stage of disease at initial diagnosis.3, 4, 5, 6 Significant underlying molecular differences could be identified, since right-sided tumors are highly immunogenic characterized by higher rates of MMR deficiency as well as BRAF mutations and exhibit a higher incidence of activated RAS and PIK3CA mutations.7,8 Moreover, the microbial richness was shown to increase from the proximal to the distal colon.9 In line with these differences in biological behavior, PTS was only recently incorporated in treatment guidelines as a predictive surrogate parameter for the selection of targeted therapies in the metastatic setting.10 As observed in several retrospective analyses of phase II and III randomized trials, overall survival (OS) benefit with anti-epidermal growth factor receptor (EGFR) antibodies such as cetuximab and panitumumab was only evident in patients with left-sided RAS wild-type mCRC, whereas patients with right-sided RAS wild-type mCRC may rather benefit from anti-vascular endothelial growth factor receptor (VEGFR) antibodies such as bevacizumab.11, 12, 13, 14, 15, 16 So far, this impact might be especially relevant in metastatic disease, since studies of CRC patients with early stages suggested no significant outcome differences with regards to PTS.17
Only little is known about differences in metastatic behavior between patients with left-sided and right-sided CRC. Whereas liver and lung metastases are more often observed in left-sided CRC patients, peritoneal metastases may be more common in right-sided CRC. However, the incidence of brain metastases (BM) seems to be comparable, although evidence in this distinct patient population remains scarce due to the rare occurrence of BM in CRC patients.17 Only 6% of BM patients present with gastrointestinal primaries most frequently located in the rectum and esophagus.18 Small series so far suggested that BM from CRC are associated with a particularly poor prognosis between 3 and 11 months. Performance status was thereby shown to be significantly worse compared with other entities of primary tumors at BM diagnosis.19,20
Within this study, we aim to investigate the influence of PTS on the clinical course and prognosis in a uniquely large cohort of CRC BM patients.
Material and methods
Patients
Overall, 323 patients treated between 1988 and 2017 for CRC BM at the Medical University of Vienna were identified from the Vienna Brain Metastasis Registry. Seven patients had to be excluded due to incomplete information regarding the clinical course of disease, 10 patients due to incomplete information regarding PTS and 12 patients due to diagnosis of a second primary tumor. Furthermore, 13 patients had to be excluded due to non-exact localization of the primary tumor in the transverse colon and the splenic flexure, respectively, to avoid a potential classification bias in terms of sidedness. Therefore, 281 patients were available for this retrospective analysis (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100168). If leptomeningeal carcinomatosis (LC) was present concomitantly to diagnosis of parenchymal BM, patients were also eligible for inclusion. Information relating to patient demographics, case history and survival were collected by retrospective chart review. This study was conducted in accordance with the Declaration of Helsinki and approval by the institutional review board (IRB) was obtained (ethics committee of the Medical University of Vienna, 1167/2019). All authors had access to the study data and reviewed and approved the final manuscript.
All patients were managed by a dedicated team of CRC BM specialists. Treatment decisions were taken in an interdisciplinary tumor conference. Treatment was carried out according to best clinical evidence and according to current standard of care.
Localization of primary tumor and classification of sidedness
Information about PTS was retrieved according to surgery protocols and histology reports. Patients with primaries in the transverse colon were excluded to avoid a potential classification bias in terms of tumor side allocation. Sidedness of the primary tumor was categorized according to recent international standards11: tumors of the appendix, cecum and ascending colon were categorized as right-sided and tumors of the descending colon, sigma and rectum as left-sided tumors (Figure 1).
Figure 1.
Graphical abstract of the study.
BM, brain metastases; BMFS, brain metastases-free survival; CRC, colorectal cancer; OS, overall survival.
Statistical analysis
For comparisons patients were grouped in two groups based on the PTS: left-sided and right-sided CRC. OS was defined as the interval from first diagnosis of CRC, diagnosis of mCRC and diagnosis of BM, respectively, until death or last date of follow-up and estimated with the Kaplan–Meier product limit method. To test for differences between two parameters, the chi-square test was used for binary variables and the Mann–Whitney U test for differences in mean ranks between two variables. To test for differences between OS curves, the log-rank test was used. BM-free survival (BMFS) was defined as the interval from diagnosis of CRC until diagnosis of BM. Two-tailed P values <0.05 were considered to indicate statistical significance. The association of PTS with OS from diagnosis of CRC BM was the main point of interest of the present study.
The graded prognostic assessment (GPA) including Karnofsky performance status (KPS) (<70, 70-89, 90-100), age (<50, 50-59, ≥60 years), extracranial metastases (present, absent) and number of BM (1, 2-3, >3) and the recently updated GPA for gastrointestinal cancers (GI-GPA), respectively, including KPS (<80, 80, 90-100), age (<60, ≥60 years), extracranial metastases (present, absent) and number of BM (1, 2-3, >3) are the best established prognosticators of outcome in CRC BM patients.19 Therefore, we predefined a priori the inclusion of the PTS together with either the GPA or the GI-GPA into the multivariate model, depending on their significance in the univariate analysis. A multivariate analysis was carried out using the Cox regression model. Due to the exploratory and hypothesis-generating design of the present study, no adjustment for multiple testing was applied and no formal sample-size calculation was conducted.21 All statistics were calculated using statistical package for the social sciences (SPSS®) 26.0 software (SPSS Inc., Chicago, IL).
Results
Patient characteristics
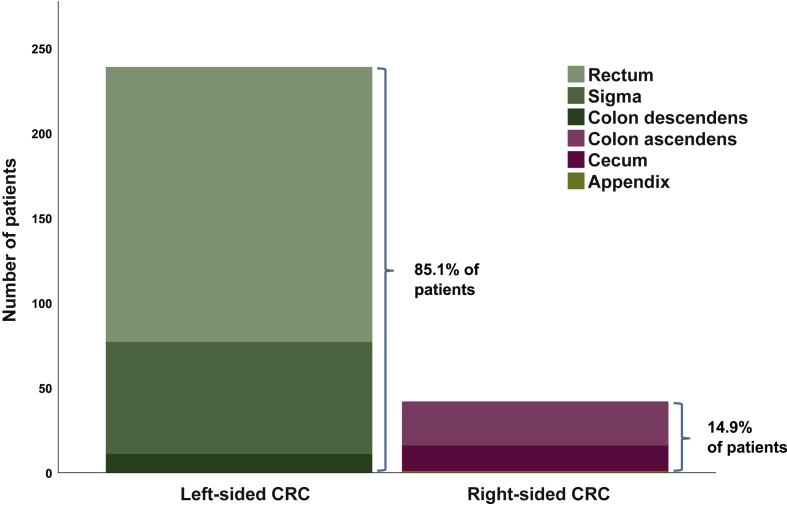
A total of 281 patients with CRC BM were available for this analysis. Median age at initial diagnosis of CRC was 61 years (range 33-89 years) and at diagnosis of CRC BM 65 years (range 34-89 years). Some 109/281 patients (38.8%) were female and 172/281 male (61.2%). A total of 92/281 patients (32.7%) had stage IV disease at initial diagnosis of CRC. The primary colorectal tumor was located in the left-sided colon in 239/281 patients (85.1%) including 11/281 patients (3.9%) with a primary in the descending colon, 66/281 patients (23.5%) with a primary in the sigma and 162/281 patients (57.7%) with a primary in the rectum. A total of 42/281 patients (14.9%) presented with a right-sided tumor including 1/281 patients (0.4%) with a primary of the appendix, 15/281 patients (5.3%) with a primary of the cecum and 26/281 patients (9.3%) with a primary in the ascending colon (Figure 2). Median BMFS was 23 months (range 1-135 months) among the overall population. Median OS from diagnosis of the primary tumor was 40 months (range 0-182 months), from diagnosis of mCRC 22 months (range 0-143 months) and from diagnosis of CRC BM 5 months (range 0-76 months).
Figure 2.
Bar diagram of contribution of patients according to primary tumor side (PTS).
CRC, colorectal cancer.
Association of PTS with clinical characteristics of CRC BM patients
Baseline characteristics were well balanced between left- and right-sided tumors. Median age at diagnosis of BM and median KPS were not statistically different between right-sided and left-sided CRC patients (64 versus 69 years and 70%; P > 0.05; Mann–Whitney U test). At diagnosis of BM, 55.2% with left-sided CRC and 56.8% patients with right-sided CRC presented with progressive extracranial disease (P > 0.05; chi-square test). Median number of BM at initial diagnosis of BM was one in left-sided as well as right-sided CRC patients (P > 0.05; Mann–Whitney U test). Incidence of concomitant LC diagnosis to solid BM was not significantly different between left- and right-sided CRC patients (2.9% versus 0%, P > 0.05; chi-square test) as well as incidence of intracranial recurrence after initial BM therapy (26.5% versus 42.3%, P > 0.05; chi-square test). Detailed patient characteristics according to PTS are listed in Table 1.
Table 1.
Patient characteristics according to primary tumor side (PTS)
| Patient characteristics | Overall ptx population |
ptx with LS CRC |
ptx with RS CRC |
P value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| 281 | 100 | 239 | 85.1 | 42 | 14.9 | ||
| Sex | |||||||
| Female | 109 | 38.8 | 90 | 37.7 | 19 | 45.2 | n.s. |
| Male | 172 | 61.2 | 149 | 62.3 | 23 | 54.8 | |
| Median age at diagnosis of CRC (years) | 61 | 61 | 66 | 0.024 | |||
| Range | 33-89 | 33-89 | 38-79 | ||||
| Stage IV at diagnosis of CRC | |||||||
| Yes | 92 | 34.1 | 76 | 33.0 | 16 | 40.0 | n.s. |
| No | 178 | 65.9 | 154 | 67.0 | 24 | 60.0 | |
| Unknown | 11 (3.9%) | ||||||
| RAS mutation (KRAS or NRAS) | |||||||
| Yes | 39 | 79.6 | 33 | 76.7 | 6 | 100.0 | n.s. |
| No | 10 | 20.4 | 10 | 23.3 | 0 | 0.0 | |
| Unknown | 232 (82.6%) | ||||||
| Visceral metastases before BM | |||||||
| Yes | 206 | 78 | 178 | 78.4 | 28 | 75.7 | n.s. |
| No | 58 | 22 | 49 | 21.6 | 9 | 24.3 | |
| Unknown | 17 (6.0%) | ||||||
| Liver metastases before BM | |||||||
| Yes | 122 | 43.4 | 103 | 43.1 | 19 | 45.2 | n.s. |
| No | 142 | 50.5 | 125 | 52.3 | 17 | 40.5 | |
| Unknown | 17 (6.0%) | ||||||
| Lung metastases before BM | |||||||
| Yes | 177 | 66.5 | 156 | 68.4 | 21 | 55.3 | n.s. |
| No | 89 | 33.5 | 72 | 31.6 | 17 | 44.7 | |
| Unknown | 15 (5.3%) | ||||||
| Systemic disease at diagnosis of BM | |||||||
| No evidence of extracranial disease and complete remission | 43 | 16.1 | 36 | 15.7 | 7 | 18.9 | n.s. |
| Partial remission | 6 | 2.2 | 4 | 1.7 | 2 | 5.4 | |
| Stable disease | 70 | 26.2 | 63 | 27.4 | 7 | 18.9 | |
| Progressive disease | 126 | 47.2 | 110 | 47.8 | 16 | 43.2 | |
| Synchronous diagnosis of CRC and BM | 22 | 8.2 | 17 | 7.4 | 5 | 13.5 | |
| Unknown | 14 (5.0%) | ||||||
| Progressive systemic disease at diagnosis of BM | |||||||
| Yes | 148 | 55.4 | 127 | 55.2 | 21 | 56.8 | n.s. |
| No | 119 | 44.6 | 103 | 44.8 | 16 | 43.2 | |
| Unknown | 14 (5.0%) | ||||||
| Median age at diagnosis of BM (years) | 65 | 64 | 69 | n.s. | |||
| Range | 34-89 | 34-89 | 39-81 | ||||
| Median KPS at diagnosis of BM | 70 | 70 | 70 | n.s. | |||
| Range | 20-100 | 20-100 | 40-100 | ||||
| Median number of BM at initial BM diagnosis | 1 | 1 | 1 | n.s. | |||
| Range | 1-3 | 1-8 | 1-5 | ||||
| Concomitant LC at diagnosis of BM | |||||||
| Yes | 7 | 2.5 | 7 | 2.9 | 0 | 0 | n.s. |
| No | 274 | 97.5 | 232 | 97.1 | 42 | 100.0 | |
| First line therapy for BM | |||||||
| WBRT | 41 | 14.9 | 36 | 15.5 | 5 | 11.9 | n.s. |
| Stereotactic radiosurgery | 118 | 42.9 | 98 | 42.1 | 20 | 47.6 | |
| Resection | 109 | 39.6 | 93 | 39.9 | 16 | 38.1 | |
| Best supportive care | 7 | 2.5 | 6 | 2.6 | 1 | 2.4 | |
| Unknown | 6 (2.1%) | ||||||
| BM recurrence after initial therapy | |||||||
| Yes | 100 | 40.2 | 91 | 42.3 | 9 | 26.5 | n.s. |
| No | 149 | 59.8 | 124 | 57.7 | 25 | 73.5 | |
| Unknown | 32 (11.4%) | ||||||
BM, brain metastases; CRC, colorectal cancer; KPS, Karnofsky performance status; LC, leptomeningeal carcinomatosis; LS, left-sided; n.s., non-significant; ptx, patients; RS, right-sided; WBRT, whole brain radiotherapy.
Bold value indicates difference in age at diagnosis between right and left-sided CRC.
Association of PTS with survival times in CRC BM patients
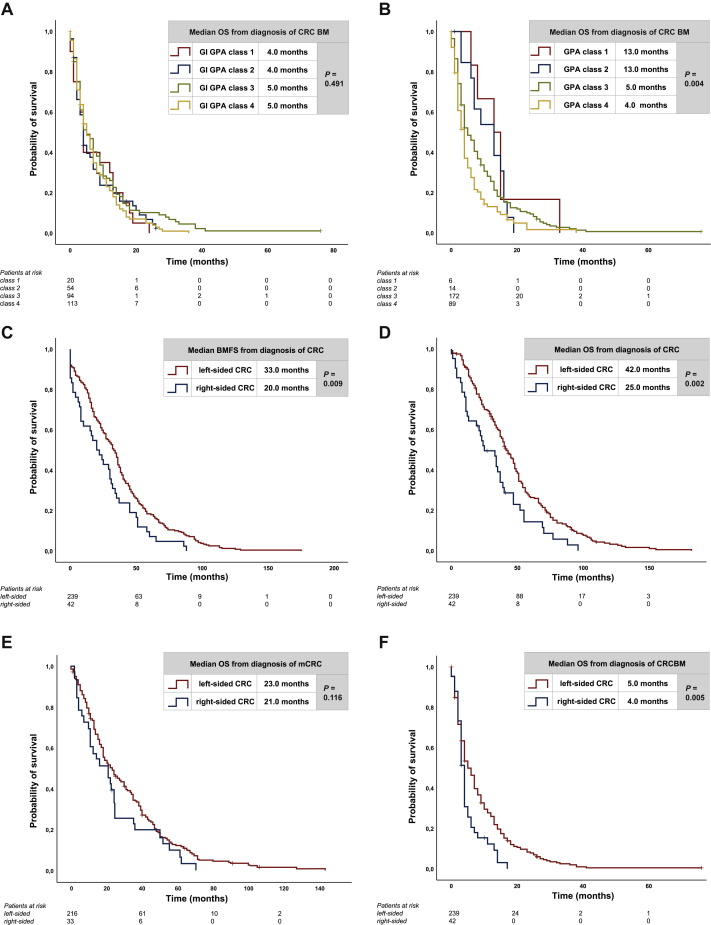
Median OS from diagnosis of CRC BM according to the time period of initial CRC diagnosis was not significantly different between patients diagnosed before the year 2000, between 2000 and 2010 and after the year 2010 (5 versus 4 versus 4 months, P > 0.05; log-rank test). Median OS from BM diagnosis was numerically, but not significantly different between GI-GPA classes (4 months with class 1 versus 4 months with class 2 versus 5 months with class 3 versus 5 months with class 4, P > 0.05; log-rank test) (Figure 3A). Therefore, we also carried out survival analysis according to GPA classes, which was significantly associated with OS from diagnosis of BM (13 months with class 1 versus 13 months with class 2 versus 5 months with class 3 versus 4 months with class 4, P = 0.004; log-rank test) (Figure 3B). Patients with left-sided tumors had a significantly longer BMFS compared with patients with right-sided tumors (33 versus 20 months, P = 0.009; log-rank test) (Figure 3C). Median OS from first diagnosis of CRC was significantly longer in patients with left-sided tumors compared with right-sided tumors (42 versus 25 months, P = 0.002, log-rank test) (Figure 3D). Median OS from diagnosis of mCRC was not statistically different between left- and right-sided tumors (23 versus 21 months, P > 0.05; log-rank test) (Figure 3E). Median OS from diagnosis of BM was significantly longer in patients with left-sided tumors compared with right-sided tumors (5 versus 4 months, P = 0.005, log-rank test) (Figure 3F).
Figure 3.
Kaplan–Meier estimates for (A) median OS after diagnosis of BM according to GI-GPA, (B) median OS after diagnosis of BM according to GPA, (C) median BMFS according to primary tumor side (PTS), (D) median OS from diagnosis of CRC according to PTS, (E) median OS from diagnosis of metastatic CRC (mCRC) according to PTS, (F) median OS from diagnosis of BM according to PTS.
BM, brain metastases; BMFS, brain metastases-free survival; CRC, colorectal cancer; GI-GPA, graded prognostic assessment of gastrointestinal cancer; GPA, graded prognostic assessment; OS, overall survival.
To evaluate the independent association of sidedness of the primary tumor on prognosis of CRC BM patients, we carried out a multivariate analysis including significantly associated parameters from univariate analyses: PTS and GPA. Within this analysis, GPA [hazard ratio 1.37 (95% confidence interval: 1.12-1.67; P = 0.002, Cox proportional hazards model)] as well as PTS [hazard ratio 0.65 (95% confidence interval: 0.46-0.92; P = 0.016, Cox proportional hazards model)] were shown to be independent prognosticators of OS (Table 2).
Table 2.
Influence of primary tumor side (PTS) on overall survival (OS) after diagnosis of brain metastases (BM). Univariable and multivariable Cox proportional hazard models
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| PTS | 0.628 (0.444-0.889) | 0.009 | 0.651 (0.460-0.923) | 0.016 |
| GPA | 1.389 (1.140-1.693) | 0.001 | 1.370 (1.124-1.669) | 0.002 |
| GI-GPA | 1.008 (0.883-1.152) | n.s. | — | — |
CI, confidence interval; GI-GPA, graded prognostic assessment of gastrointestinal cancer; GPA, graded prognostic assessment; n.s., non-significant; PTS, primary tumor side.
Discussion
Sidedness of the primary tumor was significantly associated with the clinical course in the present series of CRC BM patients. Time from diagnosis of the primary tumor to BM development, as well as survival from BM diagnosis was significantly shorter in patients with a right-sided primary tumor than in patients with a left-sided primary tumor. The present observation suggests that the biological metastatic drivers differing between right- and left-sided CRC might even impact the disease course in the rare event of BM. Therefore, our data further support the theory that CRCs comprise several molecular diverse diseases with differing metastatic behavior originating in the same organ.
In the present cohort of CRC BM patients, left-sided primary was with 85.1% more frequently observed than right-sided primary tumor. This 4 : 1 side distribution is well in line with the one previously observed for mCRC without BM.12,22,23 Therefore, as previously postulated in rather small series, PTS per se might not influence the development of BM.24 Survival prognosis of CRC patients in our study was more than 1.5-fold better with a left-sided compared with a right-sided primary. An underlying reason, therefore, might display profound differences in molecular and biological characteristics as well as resulting targeted treatment approaches. Differences in embryological origins lead to distinct gene expression patterns with different methylation and mutation profiles, as well as distinctions in the microbiome of patients.6,9,25 Recent next generation sequencing studies revealed higher rates of KRAS, NRAS, BRAF, PIK3CA, CTNNBI and SMAD mutations as well as CpG island methylator phenotype (CIMP) and MMR defects in right-sided CRC, whereas left-sided tumors presented more TP53 mutations.26 Based on that, the consensus molecular subtypes (CMS) of CRC originally described in 2015 by Guinney et al.27 and defined by gene-expression arrays had been analyzed with regards to prognostic relevance of PTS. Here, CMS2 indicating a rather favorable prognosis was more common in left-sided and CMS1 indicating a rather poor prognosis in right-sided tumors.27,28 These differences in molecular profiles might impact the brain-specific metastatic behavior, resulting in an easier colonization of right-sided CRC cells in the brain parenchyma. Indeed, RAS mutant CRC was previously shown to present with a significantly higher cumulative incidence of lung, bone and brain metastasis.29 Further, PIK3CA mutations were postulated to increase the brain metastatic behavior in breast cancer.30 Preclinical and clinical data further support that PIK3CA inhibitors have clinical efficacy in BM.31, 32, 33 Further, molecular research focusing specifically on molecular drivers could reveal targets for targeted treatment approaches.
Here, we were able to report a unique large cohort of CRC BM patients to gain further insight into the correlation of PTS and prognosis in the specific setting of BM.
Clearly, our study comprises some limitations, which have to be considered. First, due to the retrospective nature, our results need to be interpreted with caution. Second, unfortunately only limited information on the molecular profile of tumors was available, which would have been indeed of great interest to further investigate characteristics of PTS. Since the majority of patients (80%) were diagnosed and treated before the year 2014 when RAS testing was implemented into clinical routine, RAS status only was only available in a small patient subgroup. Despite small sample sizes, the proportion of RAS mutations in left-sided CRC within this study was distinctively pronounced compared with larger randomized trials representing a rather aggressive subgroup population. Nevertheless, right-sided CRC clearly was shown to be a negative prognosticator.
We applied the standard prognostic assessment scores in our population including GPA as well as the disease-specific form of the GI-GPA. Only the GPA and not the later updated GI-GPA remained significantly associated with OS after BM diagnosis. A potential reason could be that only half of the patients of the validation study for the GI-GPA had a primary within the colon, while the rest presented mainly upper and other gastrointestinal primaries. CRC might therefore display a distinct subgroup of gastrointestinal malignancies. Moreover, the GPA distinguishes more precisely with regards to age and KPS compared with the GI-GPA, which may have allowed for a better discrimination of our patient population.
Conclusion
To our best knowledge, our study represents the largest single-center analysis of CRC BM patients to date. We could determine a clear association between PTS and BMFS as well as OS, since patients with right-sided CRC develop BM significantly earlier and exhibit a significantly impaired prognosis compared with left-sided CR CBM patients. Further investigation of the underlying molecular drivers is warranted to identify potential future treatment targets.
Acknowledgments
Funding
None declared.
Disclosure
GP has received honoraria for lectures or advisories from Bayer, Servier, Taiho, Roche, Merck, Amgen, Lilly, Bristol-Myers Squibb (BMS), Merck Sharp & Dome (MSD), MedMedia/MedAhead and Terumo. MP has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, BMS, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, AstraZeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, MSD, Tocagen, Adastra. The following for-profit companies have supported clinical trials and contracted research conducted by MP with payments made to his institution: Boehringer-Ingelheim, BMS, Roche, Daiichi Sankyo, MSD, Novocure, GlaxoSmithKline, AbbVie. ASB has received research support from Daiichi Sankyo (≤€10 000), Roche (>€10 000) and honoraria for lectures, consultation or advisory board participation from Roche, BMS, Merck, Daiichi Sankyo (all <€5000) as well as travel support from Roche, Amgen and AbbVie. All other authors have declared no conflicts of interest.
Data sharing
All data generated or analyzed during this study are included in this published article. Data, analytic methods and study materials will not be made available to other researchers. Individual participant data will not be shared.
Supplementary data
Consort diagram of patient allocation. BM, brain metastases; mCRC, metastatic colorectal cancer; PTS, primary tumor side.
References
- 1.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101(5):403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 2.LaPointe L.C., Dunne R., Brown G.S. Map of differential transcript expression in the normal human large intestine. Physiol Genomics. 2008;33(1):50–64. doi: 10.1152/physiolgenomics.00185.2006. [DOI] [PubMed] [Google Scholar]
- 3.Zheng C., Jiang F., Lin H., Li S. Clinical characteristics and prognosis of different primary tumor location in colorectal cancer: a population-based cohort study. Clin Transl Oncol. 2019;21(11):1524–1531. doi: 10.1007/s12094-019-02083-1. [DOI] [PubMed] [Google Scholar]
- 4.De Renzi G., Gaballo G., Gazzaniga P., Nicolazzo C. Molecular biomarkers according to primary tumor location in colorectal cancer: current standard and new insights. Oncology. 2021;99(3):135–143. doi: 10.1159/000510944. [DOI] [PubMed] [Google Scholar]
- 5.Benedix F., Schmidt U., Mroczkowski P., Gastinger I., Lippert H., Kube R. Colon carcinoma--classification into right and left sided cancer or according to colonic subsite?--Analysis of 29,568 patients. Eur J Surg Oncol. 2011;37(2):134–139. doi: 10.1016/j.ejso.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Missiaglia E., Jacobs B., D'Ario G. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25(10):1995–2001. doi: 10.1093/annonc/mdu275. [DOI] [PubMed] [Google Scholar]
- 7.Yang S.Y., Cho M.S., Kim N.K. Difference between right-sided and left-sided colorectal cancers: from embryology to molecular subtype. Expert Rev Anticancer Ther. 2018;18(4):351–358. doi: 10.1080/14737140.2018.1442217. [DOI] [PubMed] [Google Scholar]
- 8.Baran B., Mert Ozupek N., Yerli Tetik N., Acar E., Bekcioglu O., Baskin Y. Difference between left-sided and right-sided colorectal cancer: a focused review of literature. Gastroenterol Res. 2018;11(4):264–273. doi: 10.14740/gr1062w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Z., Guo B., Gao R., Zhu Q., Qin H. Microbiota disbiosis is associated with colorectal cancer. Front Microbiol. 2015;6:20. doi: 10.3389/fmicb.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) www.nccn.org Available at. Accessed February 1, 2021.
- 11.Venook A.P., Niedzwiecki D., Innocenti F. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance) J Clin Oncol. 2016;34(suppl 15):3504. [Google Scholar]
- 12.Boeckx N., Koukakis R., Op de Beeck K. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: results from two randomized first-line panitumumab studies. Ann Oncol. 2017;28(8):1862–1868. doi: 10.1093/annonc/mdx119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tejpar S., Stintzing S., Ciardiello F. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3(2):194–201. doi: 10.1001/jamaoncol.2016.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold D., Lueza B., Douillard J.-Y. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials†. Ann Oncol. 2017;28(8):1713–1729. doi: 10.1093/annonc/mdx175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holch J.W., Ricard I., Stintzing S., Modest D.P., Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98. doi: 10.1016/j.ejca.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Morano F., Corallo S., Lonardi S. Negative hyperselection of patients with RAS and BRAF wild-type metastatic colorectal cancer who received panitumumab-based maintenance therapy. J Clin Oncol. 2019;37(33):3099–3110. doi: 10.1200/JCO.19.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benedix F., Kube R., Meyer F., Schmidt U., Gastinger I., Lippert H. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53(1):57–64. doi: 10.1007/DCR.0b013e3181c703a4. [DOI] [PubMed] [Google Scholar]
- 18.Bartelt S., Momm F., Weissenberger C., Lutterbach J. Patients with brain metastases from gastrointestinal tract cancer treated with whole brain radiation therapy: prognostic factors and survival. World J Gastroenterol. 2004;10(22):3345–3348. doi: 10.3748/wjg.v10.i22.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sperduto P.W., Fang P., Li J. Estimating survival in patients with gastrointestinal cancers and brain metastases: an update of the graded prognostic assessment for gastrointestinal cancers (GI-GPA) Clin Transl Radiat Oncol. 2019;18:39–45. doi: 10.1016/j.ctro.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperduto P.W., Fang P., Li J. Survival and prognostic factors in patients with gastrointestinal cancers and brain metastases: have we made progress? Transl Res. 2019;208:63–72. doi: 10.1016/j.trsl.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bender R., Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol. 2001;54(4):343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 22.Taieb J., Tabernero J., Mini E. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(8):862–873. doi: 10.1016/S1470-2045(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 23.André T., Vernerey D., Mineur L. Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized, open-label, international duration evaluation of adjuvant (IDEA) France, phase III trial. J Clin Oncol. 2018;36(15):1469–1477. doi: 10.1200/JCO.2017.76.0355. [DOI] [PubMed] [Google Scholar]
- 24.Lei S., Ge Y., Tian S. Colorectal cancer metastases to brain or bone and the relationship to primary tumor location: a population-based study. J Gastrointest Surg. 2020;24(8):1833–1842. doi: 10.1007/s11605-019-04308-8. [DOI] [PubMed] [Google Scholar]
- 25.Glebov O.K., Rodriguez L.M., Nakahara K. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev. 2003;12(8):755–762. [PubMed] [Google Scholar]
- 26.Salem M.E., Weinberg B.A., Xiu J. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget. 2017;8(49):86356–86368. doi: 10.18632/oncotarget.21169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guinney J., Dienstmann R., Wang X. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loree J.M., Pereira A.A.L., Lam M. Classifying colorectal cancer by tumor location rather than sidedness highlights a continuum in mutation profiles and consensus molecular subtypes. Clin Cancer Res. 2018;24(5):1062–1072. doi: 10.1158/1078-0432.CCR-17-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaeger R., Cowell E., Chou J.F. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer. 2015;121(8):1195–1203. doi: 10.1002/cncr.29196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Da Silva L., Simpson P.T., Smart C.E. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res. 2010;12(4):R46. doi: 10.1186/bcr2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ippen F.M., Grosch J.K., Subramanian M. Targeting the PI3K/Akt/mTOR pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases. Neuro Oncol. 2019;21(11):1401–1411. doi: 10.1093/neuonc/noz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batalini F., Moulder S.L., Winer E.P., Rugo H.S., Lin N.U., Wulf G.M. Response of brain metastases from PIK3CA-mutant breast cancer to alpelisib. JCO Precis Oncol. 2020;4 doi: 10.1200/PO.19.00403. PO.19.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ippen F.M., Alvarez-Breckenridge C.A., Kuter B.M. The dual PI3K/mTOR pathway inhibitor GDC-0084 achieves antitumor activity in PIK3CA-mutant breast cancer brain metastases. Clin Cancer Res. 2019;25(11):3374–3383. doi: 10.1158/1078-0432.CCR-18-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consort diagram of patient allocation. BM, brain metastases; mCRC, metastatic colorectal cancer; PTS, primary tumor side.