Summary
Lipid droplets are endoplasmic reticulum-derived neutral lipid storage organelles that play critical roles in cellular lipid and energy homeostasis. Here, we present a protocol for the identification of high-confidence lipid droplet proteomes in a cell culture model. This approach overcomes limitations associated with standard biochemical fractionation techniques, employing an engineered ascorbate peroxidase (APEX2) to biotinylate endogenous lipid droplet proteins in living cells for subsequent purification and identification by proteomics.
For complete details on the use and execution of this protocol, please refer to Bersuker et al. (2018).
Subject areas: Cell Biology, Cell culture, Cell Membrane, Cell separation/fractionation, Cell-based Assays, Proteomics
Graphical abstract

Highlights
-
•
Protocol for the identification of high-confidence lipid droplet proteomes
-
•
Biotinylation of lipid droplet proteins using APEX2 targeted to lipid droplets
-
•
Purification of biotinylated lipid droplet proteins from buoyant fractions
-
•
Label-free quantitative proteomics to define lipid droplet proteomes
Lipid droplets are endoplasmic reticulum-derived neutral lipid storage organelles that play critical roles in cellular lipid and energy homeostasis. Here, we present a protocol for the identification of high-confidence lipid droplet proteomes in a cell culture model. This approach overcomes limitations associated with standard biochemical fractionation techniques, employing an engineered ascorbate peroxidase (APEX2) to biotinylate endogenous lipid droplet proteins in living cells for subsequent purification and identification by proteomics.
Before you begin
Lipid droplets (LDs) are neutral lipid (e.g., triacylglycerols and cholesterol esters) storage organelles that play important roles in cellular lipid and energy metabolism (Olzmann and Carvalho, 2019; Walther et al., 2017). While the canonical role of LDs is in the storage of energy as triacylglycerol, LDs have also been implicated in diverse cellular functions such as preventing lipotoxicity (Chitraju et al., 2017; Listenberger et al., 2003; Nguyen et al., 2017), regulating the metabolism of hydrophobic drugs (Dubey et al., 2020; Greenwood et al., 2019) and vitamins (Blaner et al., 2009; Haaker et al., 2020), acting as a platform during the life cycle of hepatitis C virus (Herker et al., 2010; Miyanari et al., 2007) and SARS-CoV2 (Dias et al., 2020), and mediating a protective anti-microbial host response (Bosch et al., 2020; Knight et al., 2018).
LDs are endoplasmic reticulum (ER)-derived organelles that form de novo through a process that involves the deposition of neutral lipids between the leaflets of the ER bilayer and the subsequent emergence of the LD from the outer phospholipid leaflet of the ER (Olzmann and Carvalho, 2019; Renne et al., 2020; Thiam and Ikonen, 2020). LDs have a unique ultrastructure and consist of a phospholipid monolayer that encircles a neutral lipid core. Proteins decorating the monolayer surface of LDs are targeted through two pathways: Class I LD proteins insert first into the ER and traffic to forming LDs, while Class II LD proteins insert directly into LDs (Kory et al., 2016; Roberts and Olzmann, 2020). LD proteins regulate the metabolism of stored lipids, LD biogenesis and stability, nutrient signaling responses, and LD associations with other organelles (e.g., mitochondria) (Bersuker and Olzmann, 2017; Roberts and Olzmann, 2020; Zhang and Liu, 2019).
Given the importance of LD proteins in the regulation of LDs and their functions, there has been considerable interest in defining the LD proteome in different cell types and under different metabolic conditions. Although LDs can be enriched due to their buoyancy on sucrose gradients (Brasaemle and Wolins, 2006), the interpretation of proteomic analyses of LD-enriched buoyant fractions is complicated due to co-fractionating membranes that result in the incorrect assignment of proteins as LD proteins (i.e., false positives). Common contaminants that are detected in buoyant fractions include abundant ER proteins such as protein disulfide isomerases and chaperones (e.g., GRP78/BiP). To overcome this obstacle, we developed a proximity labeling proteomics approach that exploits an engineered ascorbate peroxidase (APEX2) (Bersuker et al., 2018; Lam et al., 2015) (Figure 1). In the presence of H2O2 and biotin phenol, APEX2 generates short-lived biotin phenoxyl radicals that covalently modify electron-rich amino acids within ~10–20 nm (Bendayan, 2001; Hung et al., 2014; Lam et al., 2015). Expression of LD-resident proteins fused to APEX2 enables the biotinylation of endogenous LD proteins in living cells, allowing for affinity purification of the biotinylated proteins for proteomic analysis (Bersuker et al., 2018). This approach can be employed to generate high-confidence LD proteomes in cultured mammalian cell lines. Here, we provide an optimized step-by-step protocol for proximity labeling proteomic analysis of LD proteins in cultured Huh7 hepatocellular carcinoma cells (Figure 2).
Figure 1.
APEX2 is a genetically encoded engineered enzyme that can be employed for proximity biotinylation and proteomic identification of lipid droplet proteins
(A) Domain structure of APEX2 fusion proteins.
(B) Schematic of APEX2-mediated biotinylation of endogenous LD proteins. In the presence of biotin phenol and H2O2, APEX2 catalyzes the formation of a biotin phenoxyl radical (inset panel) that covalently modifies proteins in close proximity.
Figure 2.
Schematic illustrating the major protocol steps for proximity biotinylation and proteomic analysis of lipid droplet proteins
Cell lines expressing the APEX2 fusion proteins (i.e., Cyto-APEX2, PLIN2-APEX2, ATGL∗-APEX2) are treated to biotinylate endogenous LD proteins. LDs are then isolated by fractionation, biotinylated proteins are affinity purified, proteins are trypsinized, and peptides are analyzed by mass spectrometry.
Generate inducible APEX2 fusion protein cell lines
Timing: 4 weeks
Selective targeting of APEX2 to LDs is essential for specific labeling of LD proteomes. APEX2 fused to the C-terminus of LD-resident proteins PLIN2 and ATGL (Figure 1A) effectively targets APEX2 to LDs (Bersuker et al., 2018). If possible, biologically inactive forms should be used to avoid impacting LD metabolism. For example, an S47A mutant ATGL (ATGL∗) is used that lacks lipase activity, since overexpression of wild-type ATGL induces LD degradation. APEX2 is cytosolically oriented when targeted to LDs and will induce biotinylation of both LD and cytosolic proteins. Therefore, a cytosolic version of APEX2 (Cyto-APEX2) (Figure 1A) is necessary to control for non-specific labeling of cytosolic proteins.
Note: The selection of the LD protein for targeting of APEX2 to the LD is important. Ideally, the protein selected for tagging with APEX2 should be exclusively localized to LDs to minimize non-specific protein labeling and its expression should not influence LD dynamics. In Huh7 cells, we recommend PLIN2 and ATGL∗ for APEX2 tagging because they are endogenously expressed in this cell type and they exhibit a near-exclusive LD localization.
Control proteins tagged with APEX2 should be carefully chosen to exclude background labeling of non-LD proteins. In the case of a Class I LD protein with both LD and ER localization, an ER protein could be tagged with APEX2 oriented towards the cytosol as an appropriate control. Proteins labeled using this control (e.g., ER membrane and cytosolic proteins) may be counted as background and excluded from the LD proteome.
Note: If new APEX2 constructs are generated, the constructs should include a small epitope affinity tag (e.g., V5-tag) for visualization of the fusion proteins by western blotting and immunofluorescence microscopy. Attachment of the APEX2 enzyme to either the N- or C- terminus, the type of epitope tag used, as well as the addition of linkers and their amino acid composition may need to be individually optimized to ensure correct localization and biotinylation.
-
1.
Plate 100,000 Huh7 cells into two wells of a 6-well tissue culture plate in DMEM + 10% FBS such that cells are at ~50% confluence 24 h later.
-
2.
Introduce pLenti CMV TetR-containing viral medium to the cells in one well with 8 μg/mL polybrene. Incubate for 24 h.
-
3.
After 24 h, remove all media and replace with DMEM + 10% FBS containing selection antibiotic. Replace the selection media every 3–4 days until all control cells have died.
-
4.
Replace media with fresh DMEM + 10% FBS without antibiotic to allow for recovery. These are now your “TetR”cells, conferring the tetracycline-responsive repressor protein TetR.
-
5.
Plate 100,000 Huh7 TetR cells into four wells of a 6-well plate in DMEM + 10% FBS so that cells reach ~50% confluence 24 h later.
-
6.
To three of the wells, introduce viral media containing your APEX2 fusion protein (PLIN2-APEX2, ATGL∗-APEX2, or cyto-APEX) cloned into pLenti CMV/TO Puro Dest with 8 μg/mL polybrene. Incubate for 24 h.
-
7.
After 24 h, remove all media and replace with DMEM + 10% FBS containing 2 μg/mL puromycin. Replace the selection media every 3–4 days until all control cells have died.
-
8.
Replace media with fresh DMEM + 10% FBS without antibiotic to allow for recovery. These are now your doxycycline-inducible APEX2 cell lines.
Optimize expression and labeling activity of APEX2 fusion constructs
Timing: 1 week
Integration of LD-targeted fusion proteins under the control of an inducible promoter allows for gene expression to be fine-tuned to achieve optimal labeling of proteins. It is important to express the fusion proteins at levels low enough to avoid non-specific labeling, aberrant protein localization, and potential disruptions to LD biology.
-
9.
Seed each cell line to be analyzed into a 6-well plate and treat cells for 48 h with varying concentrations of doxycycline (typically ranging from 0–100 ng/mL) to induce expression of APEX2 fusion proteins. To increase LD abundance, incubate cells with 200 μM oleate (always complexed with 0.1% BSA) for 24 h prior to harvesting. Perform proximity labeling assay (see step-by-step method details, steps 1–6) and collect whole-cell lysate for western blot analysis.
-
10.
Evaluate the expression level of APEX2 fusion proteins by western blotting for the fusion construct’s V5-epitope tag (Figures 3A and 3B). In addition, the corresponding labeling activity can be evaluated by streptavidin blotting to identify the extent of biotinylation that occurs at each concentration of doxycycline (Figures 3A and 3B). Some labeling will be evident in the absence of APEX2 expression due to the presence of endogenously biotinylated proteins.
Note: When quantitatively comparing LD proteomes across different cell lines, it is important to establish a consistent expression profile that is similar for all APEX2 fusion proteins. The optimum concentration of doxycycline to select is one that yields robust biotinylation of endogenous LD proteins with minimal induction of fusion protein expression. The concentration of doxycycline may differ between cell lines.
Figure 3.
Characterization of APEX2 fusion protein expression, activity, and localization
(A and B) Huh7 cells stably expressing Cyto-APEX2 and PLIN2-APEX2 were treated with the indicated amounts of doxycycline (dox) for 48 h and treated with biotin phenol and H2O2 to induce biotinylation of endogenous proteins. The levels of protein expression and protein biotinylation were analyzed by western blotting with anti-V5 antibodies, anti-GAPDH antibodies, and streptavidin conjugated to IRDye 800CW.
(C) Huh7 cells stably expressing Cyto-APEX2 and PLIN2-APEX2 were treated for 48 h with 5 ng/mL and 1 ng/mL doxycycline, respectively. Cells were then treated for 24 h with 200 μM oleate and 1 μM BODIPY-C12-568 (red). Cells were fixed in 4% paraformaldehyde, immunostained with anti-V5 antibodies, and imaged by fluorescence microscopy using a Deltavision Elite widefield epifluoresence deconvolution microscope (GE Healthcare). Scale bars represent 10 μm.
Verify localization of APEX2 fusion constructs and biotinylated proteins
Timing: 1–2 weeks
It is essential to verify correct targeting of APEX2 fusion constructs to LDs by comparing their localization with fluorescent LD stains.
-
11.
Place microscope coverslips in the bottom of a 12-well tissue culture plate.
-
12.
Plate 10,000 APEX2-expressing cells directly on top of coverslips in DMEM + 10% FBS so that cells reach ~25% confluence 24 h later.
-
13.
Incubate cells with doxycycline for 48 h prior to fixation.
-
14.
Incubate cells with 200 μM oleate-BSA complex and 1 μM of the fluorescent fatty acid BODIPY 558/568 C12 for 24 h to metabolically label LDs prior to fixation.
Alternatives: An alternative to using BODIPY 558/568 C12 to label LDs is to employ a neutral lipid stain such as 10 μg/mL BODIPY 493/503.
-
15.
Treat cells with 500 μM biotin phenol for 30 min prior to fixation.
-
16.
To induce biotinylation, add 100 mM hydrogen peroxide stock solution directly to the media to achieve a final concentration of 1 mM. Swirl gently to mix and incubate at RT for 1 min prior to fixation.
-
17.
Quench the reaction by aspirating the media and washing cells three times with 1 mL quenching buffer.
-
18.
Wash cells once with 1 mL PBS.
-
19.
Fix cells by adding 500 μL of 4% paraformaldehyde and incubating for 15 min at RT in the dark.
CRITICAL: When using paraformaldehyde, work under a chemical flow hood to avoid inhalation of toxic fumes.
-
20.
Wash cells three times with 1 mL PBS.
-
21.
To permeabilize cell membranes, add 500 μL of PBS containing 1% BSA and 0.1% Triton X-100. Incubate for 15 min at RT in the dark.
-
22.
Wash cells three times with 1 mL PBS containing 1% BSA.
-
23.
Transfer coverslips from the 12-well plate to a sheet of parafilm using tweezers.
-
24.
Overlay the cells with 150 μL of anti-V5 primary antibody solution (1:500 in PBS with 1% BSA). Incubate for 2 h at RT or overnight at 4°C.
Note: Fluorescent reagents should be protected from the light when possible and incubations should be performed in the dark (e.g., under an opaque covering or placed in a drawer).
-
25.
Wash cells three times with 150 μL PBS containing 1% BSA.
-
26.
Overlay the cells with 150 μL of a solution containing goat anti-mouse Alexa FlourTM 488 secondary antibody (1:1000 in PBS with 1% BSA) to stain V5-APEX2 proteins. Alternatively, overlay the cells with 150 μL of a solution containing Streptavidin-Alexa FluorTM 488 (1:500 in PBS with 1% BSA) to stain biotinylated proteins. Incubate for 1 h at RT.
-
27.
Wash cells three times with 150 μL PBS containing 1% BSA.
-
28.
To mount the coverslips, add one drop of Fluoromount G to a glass microscope slide, being careful not to introduce any air bubbles. Place the coverslips cell-side down onto the drop of Fluoromount G using tweezers. Allow the slides to dry fully at RT overnight.
Note: To avoid introducing air bubbles when adding coverslips to Fluoromount G, hold the coverslip at a 45° angle and place the bottom edge immediately adjacent to the drop. Slowly set the coverslip down by allowing it to hinge over onto the drop. Minimize any movement of the coverslip once coverslip has been set down.
-
29.
Image stained cells using fluorescence microscopy.
Note: LD-targeted APEX2 fusion proteins should be visible as a ring encircling the outer edge of LDs (Figure 3C). The cytosolic control APEX2 construct should be diffuse throughout the cytosol and nucleoplasm and should not be enriched around LDs or other organelles.
-
30.
The subcellular distribution of the fusion constructs and biotinylated proteins can be further validated by performing a proximity labeling reaction and fractionating cell homogenates on a sucrose gradient. Analyze the full complement of fractions by western blotting. See step-by-step method details, steps 7–19.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-V5 tag | Invitrogen | Cat. # 46-0705; RRID: AB_2556564 |
| Rabbit polyclonal anti-PLIN2 | Abgent | Cat. # AP5118C; RRID: AB_10662954 |
| Mouse monoclonal anti-GAPDH | MilliporeSigma | Cat. # MAB374; RRID: AB_2107445 |
| Streptavidin, Alexa Fluor 488 Conjugate | Thermo Fisher Scientific | Cat. # S32354 |
| IRDye® 680LT Goat anti-Mouse IgG Secondary Antibody | LI-COR Biosciences | Cat. # 926-68020 |
| IRDye® 800CW Streptavidin | LI-COR Biosciences | Cat. # 926-32230 |
| IRDye® 800CW Goat anti-Rabbit IgG Secondary Antibody | LI-COR Biosciences | Cat. # 926-32211 |
| Chemicals, peptides, and recombinant proteins | ||
| Dulbecco's modified Eagle's medium | Corning | Cat. # 10-017-CV |
| Fetal bovine serum | Gemini Bio | Cat. # 100-500 |
| Hexadimethrine bromide (polybrene) | Sigma-Aldrich | Cat. # 107689 |
| Puromycin dihydrochloride | Thermo Fisher Scientific | Cat. # A1113803 |
| Blasticidin S HCl | Thermo Fisher Scientific | Cat. # A1113903 |
| Doxycycline | Sigma-Aldrich | Cat. # D9891 |
| Oleic acid | Sigma-Aldrich | Cat. # O1383 |
| Bovine serum albumin (fatty acid free, low endotoxin) | Sigma-Aldrich | Cat. # A8806 |
| Biotin-phenol | Iris Biotech GmbH | Cat. # LS-3500.0250 |
| Hydrogen peroxide (30%) | Fisher Scientific | Cat. # H325-100 |
| Sodium L-ascorbate | Sigma-Aldrich | Cat. # A4034 |
| Trolox | Sigma-Aldrich | Cat. # 238813 |
| Triton X-100 | Sigma-Aldrich | Cat. # T9284 |
| Pierce Protease Inhibitor Mini Tablets, EDTA-free | Thermo Fisher Scientific | Cat. # A32955 |
| Pierce Monomeric Avidin Agarose | Thermo Fisher Scientific | Cat. # 20228 |
| Gel Code Blue Stain Reagent | Thermo Fisher Scientific | Cat. # 24590 |
| Pierce Trypsin Protease, MS Grade | Thermo Fisher Scientific | Cat. # 90057 |
| BODIPY 493/503 (4,4-Difluoro-1,3,5,7,8-Pentamethyl-4-Bora-3a,4a-Diaza-s-Indacene) | Thermo Fisher Scientific | Cat. # D3922 |
| BODIPY 558/568 C12 (4,4-difluoro-5-(2-thienyl)-4-bora-3a,4a-diaza-s-indacene-3-dodecanoic acid) | Thermo Fisher Scientific | Cat. # D3835 |
| HCS LipidTOX™ Deep Red Neutral Lipid Stain | Thermo Fisher Scientific | Cat. #H34477 |
| Hemin | Sigma-Aldrich | Cat. # H9039 |
| Acetonitrile | Sigma-Aldrich | Cat. # 34998 |
| Iodoacetamide | Sigma-Aldrich | Cat. # I6125 |
| Dithiothreitol (DTT) | Thermo Fisher Scientific | Cat. # A39255 |
| Deposited data | ||
| Lipid droplet-targeted APEX proteome | https://www.ebi.ac.uk/pride/ | https://www.ebi.ac.uk/pride/archive/projects/PXD007695 |
| Experimental models: Cell lines | ||
| Huh7 Cyto-APEX2 | (Bersuker et al., 2018) | n/a |
| Huh7 PLIN2-APEX2 | (Bersuker et al., 2018) | n/a |
| Huh7 ATGL∗-APEX2 | (Bersuker et al., 2018) | n/a |
| Recombinant DNA | ||
| pLenti CMV TetR Blast | Addgene | Plasmid #17492 |
| pENTR1A no ccDB (w48-1) | Addgene | Plasmid #17398 |
| pLenti CMV/TO Puro DEST (670-1) | Addgene | Plasmid #17293 |
| Cyto-V5-APEX2, pLenti CMV/TO Puro DEST | Addgene | Plasmid #170572 |
| Plin2-V5-APEX2, pLenti CMV/TO Puro DEST | Addgene | Plasmid #170573 |
| ATGL∗-V5-APEX2, pLenti CMV/TO Puro DEST | Addgene | Plasmid #170574 |
| Software and algorithms | ||
| ImageJ software, version 1.53a | Schneider et al., 2012 | https://imagej.nih.gov/ij/ |
| MaxQuant | Max Planck Institute of Biochemistry | (Tyanova et al., 2016) |
| Adobe Illustrator, version 24.0.3 for Mac | Adobe | n/a |
| Other | ||
| Tube Slicer Assembly | Beckman Coulter | Cat. # 303811 |
| SW 41 TI rotor | Beckman Coulter | Cat. # 331336 |
| 13.2 mL, Open-Top Thinwall Ultra-Clear Tube | Beckman Coulter | Cat. # 344059 |
| Wheaton Dounce Tissue Grinder (7 mL size) | Spectrum Chemical | Cat. # 989-24610 |
| Branson Digital Sonifier SFX 150 | Emerson | Cat. # 101-063-962R |
Materials and equipment
10× oleate-BSA complex [100 mL]
| Reagent | Final concentration | Amount |
|---|---|---|
| 200 mM oleate in PBS | 2 mM | 1 mL |
| 10% BSA (fatty-acid free) in PBS | 0.1% | 10 mL |
| DMEM | 89 mL |
Prepare fresh using warm DMEM
Vortex before treating cells as oleate-BSA complex may settle
500× biotin-phenol [11 mL]
| Biotin-phenol (363.47 g/mol) | 250 mM | 1 g |
| DMSO | 11 mL |
Store at −80°C in single-use aliquots to avoid repeated freeze/thaw cycles
100× H2O2 [10 mL]
| H2O2, 30% | 100 mM | 1.133 mL |
| 1× PBS, pH 7.4 | 8.867 mL |
Prepare immediately prior to use
Store at RT
Quenching buffer [200 mL]
| Sodium ascorbate (198.1 g/mol) | 10 mM | 396.2 mg |
| Trolox (250.29 g/mol) | 5 mM | 250.3 mg |
| 1× PBS, pH 7.4 | 200 mL |
Prepare fresh
Store on ice
HLM buffer [500 mL]
| 1 M Tris-HCl, pH 7.4 | 20 mM | 10 mL |
| 0.5 M EDTA, pH 8.0 | 1 mM | 1 mL |
| Milli-Q water | 489 mL |
Store at RT
60% Sucrose [100 mL]
| Sucrose (324.3 g/mol) | 60% w/v | 60 g |
| HLM buffer | 50+ mL |
Dissolve sucrose in 50 mL HLM buffer
Add HLM buffer to a final volume of 100 mL
Store at 4°C
Step-by-step method details
Biotin phenol proximity labeling of endogenous proteins
Timing: 1 h
Biotinylation of LD proteins in oleate-treated APEX2 cell lines by incubating cells with biotin phenol and H2O2.
Note: Volumes listed are optimized for working with 150 mm plates. 15–20 plates were used in our studies.
-
1.
Incubate cells with 200 μM oleate complexed with 0.1% BSA for 24 h to increase LD abundance prior to harvesting.
-
2.
Add biotin phenol to growth medium at a final concentration of 500 μM and swirl gently to mix.
Note: To avoid using large amounts of biotin phenol, the volume of growth medium can be reduced from 20 mL to 10 mL prior to adding biotin phenol.
-
3.
Incubate cells with biotin phenol for 30 min at 37°C.
-
4.
Add H2O2 from 100× stock (1 mM final concentration) to medium for 1 min to induce biotinylation.
Note: To avoid labeling of undesired proteins, do not allow the biotinylation reaction to proceed longer than 1 min. It is recommended not to work with more than 2–3 plates at a time in order to avoid excess labeling time.
-
5.
Aspirate medium and immediately wash three times with 5 mL quenching buffer to stop the reaction.
-
6.
Wash once with 5 mL PBS to remove any residual quenching buffer.
Isolation of LD-enriched buoyant fractions
Timing: 4 h
Separation and isolation of the LD-enriched fractions containing the biotinylated LD proteins using sucrose gradient centrifugation and a tube slicer.
Note: Samples should be kept on ice throughout all steps of the fractionation.
-
7.
Harvest cells in 3 mL PBS using a cell scraper and transfer to a chilled 50 mL conical tube.
-
8.
Centrifuge at 500 × g for 10 min at 4°C to pellet cells and discard the supernatant.
-
9.
Resuspend cell pellet in 3 mL HLM buffer containing 1× protease inhibitor and incubate on ice for 10 min.
-
10.
Homogenize cells by applying 80× strokes using a tight-fitting dounce.
-
11.
Transfer the homogenate to a 15 mL conical tube and centrifuge at 1000 × g for 10 min at 4°C to pellet nuclei and any unbroken cells.
-
12.
Transfer the post-nuclear supernatant to a 13.2 mL ultracentrifuge tube, taking note of the approximate volume that was transferred for each sample (should be ~2–3 mL).
-
13.
Dilute the post-nuclear supernatant 1:3 with 60% sucrose HLM buffer (20% sucrose final concentration). Pipette up-and-down several times to ensure complete mixing.
CRITICAL: Inadequate mixing at this stage will prevent sufficient separation of the buoyant fraction during ultracentrifugation.
-
14.
Carefully layer 5 mL of 5% sucrose HLM buffer on top, being careful not to disturb the layer underneath. Layer an additional ~4.5 mL HLM buffer on top, leaving approximately 0.5 cm of space at the top of the tube.
Note: If the post-nuclear supernatant has a large volume, the amount of HLM buffer added to the top will be less than 4.5 mL. The slightly lower amount of HLM buffer does not alter the fractionation protocol or the purity of the buoyant fraction.
-
15.
Centrifuge at 28,000 × g in an SW-41TI rotor in an ultracentrifuge (e.g., Optima XPN-80) for 30 min at 4°C to pellet heavy organelles and float LDs.
Note: Be careful when handling the ultracentrifuge tubes after centrifugation to minimize mixing between the separated layers.
-
16.
Use a tube slicer to isolate the top 1 mL LD-enriched buoyant fraction (Figure 4 and Methods video S1). After pushing the blade through the tube underneath the LD-enriched buoyant fraction, pipette up-and-down several times to mix the fraction and then transfer to a 2 mL microcentrifuge tube.
Figure 4.
Isolation of the LD-enriched buoyant fraction using a tube slicer
(A) Illustration of fractions and their relative locations following ultracentrifugation of cellular homogenates.
(B) The LD-enriched buoyant fraction consists of a prominent white layer at the very top of the column (indicated by the red arrow). The amount of LDs is greatly enhanced by oleate treatment.
(C) Example of tube slicer apparatus setup. Following centrifugation and flotation of LDs, the tube is set in the tube slicer with the blade oriented just below the white LD fraction.
(D) Top view of tube slicer showing the white LD layer prior to isolation and the complete removal of the LDs following isolation. See also Methods video S1.
Note: This step is performed at RT.
Note: To maximize the amount of LDs collected, use an additional 200 μL of HLM buffer to wash the inner portion of the cut tube and tube slicer that contained the buoyant fraction. Pipette up-and-down paying special attention to the sides and corners and then combine together with the rest of the collected buoyant fraction.
-
17.
After removal of the LD-enriched fraction, collect the remaining fractions by pipetting 1 mL at a time from the top using a micropipette. Resuspend the pellet in 1 mL of HLM buffer.
-
18.
Remove 100 μL from each fraction and add 11.1 μL 10% SDS (1% SDS final) for analysis by western blot. Add 100 μL 10% SDS to the remainder of each fraction (1% SDS final) to be further analyzed by mass spectrometry.
-
19.
Sonicate all fractions once for 30 sec at 15% amplitude using a tip sonicator. To solubilize LD fractions, incubate at 37°C for 1 h with additional rounds of sonication every 20–30 min.
Note: To assess the purity of each fraction, separate 25 μL of the fraction by SDS-PAGE and western blot using antibodies against organelle markers such as the soluble protein GAPDH (enriched in the cytosolic fractions), the LD protein PLIN2 (enriched in the LD fraction), and the ER and LD protein UBXD8 (enriched in the pellet and LD fractions) (Bersuker et al., 2018; Brasaemle and Wolins, 2006; Olzmann et al., 2013).
Purification of biotinylated proteins from LD-enriched buoyant fractions
Timing: 6 h
Affinity purification and elution of biotinylated proteins from LD-enriched fractions using avidin-conjugated agarose beads and biotin-containing SDS elution buffer.
-
20.
Dilute 1 mL of buoyant fractions 1:10 with HLM buffer in a 15 mL conical tube to reduce SDS concentrations to 0.1%. The lower SDS concentration is required for compatibility with the affinity purification procedure.
Note: The entire buoyant fraction is typically used for the affinity purification and mass spectrometry, minus the samples that are removed where indicated for analysis of purity. Different cell types and metabolic conditions may require more or less amounts. We aim for ~1–2 μg of peptide for analysis by mass spectrometry.
-
21.
Prepare 200 μL of Agarose Monomeric Avidin bead slurry by washing twice with 1 mL of PBST (PBS with 0.1% Tween 20), followed by an additional wash with 1 mL of HLM buffer. For each wash, pellet beads by centrifugation at 1000 × g for 1 min, and discard the supernatant.
-
22.
Resuspend washed beads in 500 μL HLM buffer and add to the diluted buoyant fraction. Incubate at RT for 4 h on an end-over-end rotator to provide constant mixing.
-
23.
Pellet the beads by centrifugation at 1000 × g for 1 min and save the flow through for analysis if desired.
-
24.
Wash beads five times with 1 mL PBST followed by three times with 1 mL PBS.
-
25.
Elute biotinylated proteins from the beads by adding 500 μL of elution buffer containing 2% SDS with 3 mM biotin. Pipette up-and-down several times using a micropipette with a cut tip to decrease shearing of the beads. Rotate end-over-end for 15 min at RT followed by heating at 95°C for 15 min. Pellet beads by centrifugation at 1000 × g for 1 min and transfer the eluant to a new 1.5 mL microcentrifuge tube.
In-gel protein digestion and peptide extraction
Timing: 2 days
In-gel digestion of affinity purified proteins with trypsin, extraction of peptides, and preparation of peptides for mass spectrometry.
-
26.
Concentrate the eluant in a centrifugal vacuum concentrator on medium heat until approximately 50 μL is remaining. Mix the eluant with 5× Laemmli buffer and heat at 65°C for 5 min immediately prior to loading onto a polyacrylamide gel.
Note: Upon being concentrated, the eluant may become increasingly viscous and difficult to pipette. Moderately heating the samples increases the fluidity and allows for easier gel loading.
-
27.
Load half of each sample onto neighboring lanes of the gel and separate proteins by SDS-PAGE until the dye front is approximately halfway down the gel.
Note: If running multiple samples on the same gel, include an empty lane in between different samples to prevent any potential contact that could arise from bleed over into adjacent lanes.
-
28.
Wash gel three times with ddH2O for 5 min using an orbital shaker for constant agitation.
-
29.
Add enough of the gel-fixing buffer containing 50% ethanol and 10% acetic acid to cover the gel and incubate at RT for 1 h with gentle shaking.
-
30.
Remove gel-fixing buffer and add a gel-washing buffer containing 50% methanol and 10% acetic acid. Incubate at RT for 30 min with gentle shaking.
-
31.
Discard gel-washing buffer and wash gel three times with ddH2O.
-
32.
To visualize the proteins in each lane, add enough Gel Code Blue G-250 stain to fully cover the gel and incubate at RT overnight with gentle shaking.
-
33.
Wash gel with ddH2O for 4 h at RT with gentle shaking, replacing ddH2O every 45 min to remove the stain background for proper protein band visualization.
-
34.
Excise the proteins from the surrounding gel using a razor blade. Cut the pieces further into smaller fragments to increase surface area and transfer to a 2 mL microcentrifuge tube.
Note: To minimize keratin contamination, excise gel pieces in a laminar flow hood while wearing a long-sleeved laboratory coat. Spray down the working surface with 70% ethanol and clean all razor blades with an ethanol-sprayed Kimwipe prior to beginning.
-
35.
Overlay the gel pieces with 500 μL of a solution containing 50 mM ammonium bicarbonate in 50% acetonitrile. Incubate at RT for 40 min while briefly vortexing every 5–10 min. Remove as much of the solution as possible using a gel-loading pipette tip to prevent any accidental removal of gel pieces.
-
36.
Dehydrate the gel pieces by adding 1 mL of 100% acetonitrile and incubate at RT with occasional vortexing until the gel pieces turn white. Remove the acetonitrile once complete.
Note: This step may take several hours until the gel pieces are fully dehydrated. Replace the acetonitrile every hour until completion to ensure complete removal of any residual blue stain, as this can cause potential complications with mass spectrometers. Additional rounds of hydration and dehydration of the gel pieces with occasional vortexing may also be performed if necessary.
-
37.
Add a sufficient amount of 10 mM DTT in 50 mM ammonium bicarbonate to cover the gel pieces and incubate for 1 h at 56°C.
Note: This procedure for in-gel reduction and alkylation is optional and can also be done in solution prior to SDS-PAGE analysis (see step 26).
-
38.
Cool samples to RT and replace the DTT solution with roughly the same volume of 55 mM iodoacetamide in 50 mM ammonium bicarbonate. Incubate for 45 min at RT in the dark with occasional vortexing. Remove the iodoacetamide solution once complete.
Note: Iodoacetamide is light-sensitive and should be prepared fresh.
-
39.
Wash gel pieces with ~100 μL of 50 mM ammonium bicarbonate for 10 min while vortexing and dehydrate with ~100 μL of 50 mM ammonium bicarbonate in 50% acetonitrile.
-
40.
Remove the solution and dry the gel pieces in a vacuum centrifuge for 20 min.
-
41.
To digest proteins, add 0.5 μg of mass spectrometry-grade trypsin in 5 mM ammonium bicarbonate containing 5% acetonitrile to the gel pieces and incubate on ice for 30 min to allow the gel pieces to become slowly saturated. After 30 min, transfer samples to a 37°C incubator for overnight digestion.
CRITICAL: Enough trypsin should be added only to where the gel pieces become fully saturated with no more ~10–20 μL of extra solution remaining. Excess trypsin solution can result in disproportionately high levels of trypsin being detected by mass spectrometers and subsequently decreased detection of desired peptides. Use no more than 1 μg of trypsin per sample for MS analysis.
-
42.
Extract the digested peptides by adding enough 5% formic acid in acetonitrile to fully cover the gel pieces and incubating at 37°C for 15 min with constant agitation.
-
43.
Use a gel-loading pipette tip to transfer the supernatant to a new 1.5 mL microcentrifuge tube. Concentrate the sample using a centrifugal vacuum concentrator on medium heat until 20 μL is remaining.
-
44.
Repeat extraction steps 42–43 two additional times to ensure that all peptides are removed.
Note: To maximize peptide recovery, a bath sonicator can also be used in conjunction with constant agitation. Briefly after constant agitation for 15 min (step 42), sonicate the samples for 5 mins, pulse spin to collect the supernatant, and pool all peptides together.
Mass spectrometry and analysis
Timing: 1week
Identification of high confidence LD proteomes using mass spectrometry to analyze tryptic peptides, MaxQuant software to identity peptide identities and intensities for label-free quantification, and comparative analysis with controls to remove contaminant proteins.
-
45.
Analyze peptides using liquid chromatography coupled tandem mass spectrometry (LC-MS/MS). Our analyses were performed at the University of California, Davis Proteomics Core Facility with a Thermo Scientific Q Exactive Orbitrap Mass spectrometer connected to a Proxeon Easy-nLC II HPLC (Thermo Fisher Scientific) and Proxeon nanospray source. A flow rate of 300 nL/min was used and peptides were separated using a 90-min linear gradient of 5%–35% over B for 75 min, 35%–80% over B for 7 min, 80% hold over B for 3 min, and 5% hold over B for 5 mins (solvent A: 0.1% formic acid in water, solvent B: 0.1% formic acid in acetonitrile). Table 1 indicates key mass spectrometer parameters. All proteomic data files from two independent experimental replicates are available through the PRoteomics IDEntifications (PRIDE) database (Project PXD007695).
-
46.
Peptide identities, peptide intensities for label-free quantification, and MS/MS spectral counts can be determined by analyzing RAW output files using a variety of available proteomics software (e.g., SEQUEST and Mascot). For our analyses, we employ MaxQuant (current version v1.6.17.0) (Cox and Mann, 2008; Tyanova et al., 2016), an open source quantitative proteomics software package, using the reviewed human protein database obtained from Uniprot digested with trypsin/P and the indicated parameters (Table 2), including the addition of biotin phenol modification (C18H23N3O3S, +361.1460) to tyrosine.
-
47.
Export and open the protein groups file from MaxQuant. This file contains the relevant information regarding peptide identities, MS/MS spectral counts, and peptide intensities for label-free quantification. For label-free quantification, use the intensity-based absolute quantification (iBAQ) value, indicated as “intensity” in the MaxQuant protein groups file. iBAQ reports the sum of all peptide intensities divided by the theoretically observable peptides for that protein (Schwanhäusser et al., 2011).
-
48.
To identify a list of high confidence LD proteins, subtract the iBAQ intensity of the cyto-APEX2 from PLIN2-APEX2 and ATGL∗-APEX2 (averaged from two biological replicates) to provide a normalized value for proteins labeled by PLIN2-APEX2 (PLIN2-APEX2N) and ATGL∗-APEX2 (ATGL∗-APEX2N). This normalization controls for labeling of cytosolic proteins or non-specific binding of proteins to the beads.
Note: A higher PLIN2-APEX2N or ATGL∗-APEX2N value corresponds to higher levels of the biotinylated protein on LDs. Whereas low values or negative values indicate a labeled cytosolic protein or a protein that non-specifically binds to the beads. High PLIN2-APEX2N or ATGL∗-APEX2N values increases the confidence that a protein is an LD protein.
Note: We compared the method using iBAQ label-free quantification to our previous method (Bersuker et al., 2018) that used a normalized spectral abundance factor (SAF) based on spectral MS/MS counts (Figures 5A and 5B). There is strong agreement between the two approaches (Figures 5A and 5B), and between the proteins that are identified using PLIN2-APEX2 and ATGL∗-APEX2 for proximity labeling (Figure 5C).
Table 1.
Mass spectrometer settings
| Method parameter | Value |
|---|---|
| Polarity | Positive |
| Full MS | |
| Microscans | 1 |
| Resolution | 70,000 |
| Automatic gain control (AGC) target | 1 × 106 ion counts |
| Maximum ion time | 30 ms |
| Scan range | 350–1600 m/z |
| dd-MS2 | |
| Microscans | 1 |
| AGC target | 5 × 104 ion counts |
| Maximum ion time | 50 ms |
| TopN | 15 |
| Isolation window | 1.6 m/z |
| Normalized collision energy | 27 |
| dd settings | |
| Underfill ratio | 1% |
| Charge exclusion | unassigned, 1, 5–8, >8 |
| Peptide match | on |
| Exclude isotopes | on |
| Dynamic exclusion | 10 s |
Table 2.
MaxQuant parameter settings for analysis of MS data
| Parameter | Value |
|---|---|
| Variable modification | Acetyl (protein N-terminus) |
| Oxidation (M) | |
| biotin phenol-modified tyrosine | |
| Fixed modification | Carbamidomethyl (C) |
| Digest | Trypsin/P |
| Maximum number of modifications / peptide | 5 |
| Maximum missed cleavage | 2 |
| Minimum peptide length | 6 |
| Peptide FDR (%) | 1 |
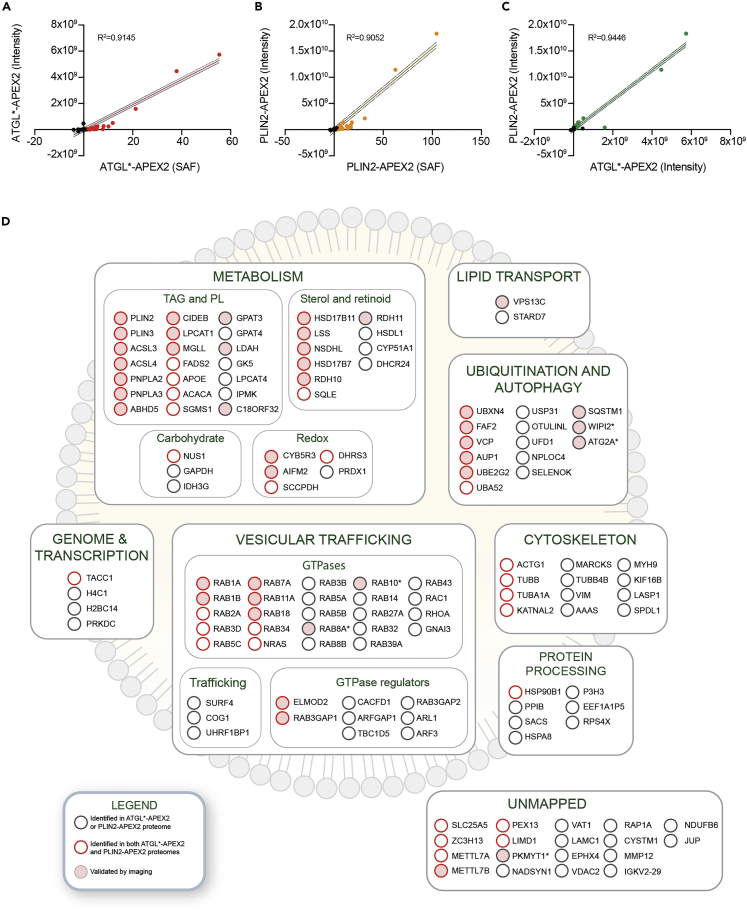
Figure 5.
Proximity biotinylation and label-free quantification to define high confidence LD proteomes in Huh7 cells
(A) Comparison of the iBAQ intensity and the previously determined spectral abundance factor (Bersuker et al., 2018) for proteins identified using ATGL∗-APEX2 proximity labeling proteomics. The line of best fit is shown and the dotted lines indicate the confidence interval.
(B) Comparison of the iBAQ intensity and the previously determined spectral abundance factor (Bersuker et al., 2018) for proteins identified using PLIN2-APEX2 proximity labeling proteomics. The line of best fit is shown and the dotted lines indicate the confidence interval.
(C) Comparison of the iBAQ intensity for proteins identified using PLIN2-APEX2 and ATGL∗-APEX2 proximity labeling proteomics. The line of best fit is shown and the dotted lines indicate the confidence interval.
(D) Illustration of the Huh7 high confidence LD proteome determined using the methods described in this protocol. An asterisk indicates an LD protein that has been validated by imaging and was identified in our studies but was below the threshold.
Expected outcomes
If the biotinylation is successful, a laddering of biotinylated proteins (visualized using streptavidin conjugated to a fluorescent molecule) should be observed by western blotting (Figures 3A and 3B). The biotinylation is likely stronger for Cyto-APEX2 than the LD-targeted APEX2 constructs (Figures 3A and 3B). The Cyto-APEX2 construct will appear diffuse through the cell, including the nucleoplasm (Figure 3C). By fluorescence microscopy, the LD-targeted APEX2 fusion proteins should mostly appear as cytoplasmic rings that encircle neutral lipid markers (Figure 3C). Following fractionation, a white LD-enriched layer should be apparent at the surface of the liquid (Figure 4). Isolation and processing of this fraction should yield ~1–2 μg of digested peptides to be analyzed by mass spectrometry.
The number and identity of the proteins as well as their abundance will differ depending upon the cell line and growth conditions. Employing oleate-treated Huh7 cells, PLIN2-APEX2N identified 183 proteins with values >0 and ATGL∗-APEX2N identified 110 proteins with values >0 (Tables S1, S2, and S3). Proteins such as PLIN2, ATGL, ACSL3, HSD17B11, and UBXD8 will likely be strongly detected and serve as positive controls. To define a high confidence LD proteome, we curated a gold standard list of known LD proteins that have been validated to localize to LDs using imaging approaches (e.g., immunofluorescence imaging of the endogenous or tagged protein) in published studies and were present in our proteomics datasets. Based on this gold standard list of proteins, we set a threshold that captured 85% of the validated LD proteins present in our list, yielding 115 proteins identified by PLIN2-APEX2N and 66 proteins identified by ATGL∗-APEX2N (Tables S1, S2, and S3). This threshold increases the stringency and confidence of the designated LD proteome. An illustration of the high confidence LD proteins determined using our proximity labeling proteomics approach with iBAQ label-free quantification was generated in Adobe Illustrator by manually curating proteins into functional groups (Figure 5D).
Limitations
There are several limitations to this APEX2 proximity biotinylation strategy. False negatives are possible when proteins are buried within protein complexes and surface residues for modification by biotin phenol are not readily accessible. Overexpression of LD proteins can potentially alter LD biology (e.g., biogenesis, turnover, organelle interactions) and the composition of the LD proteome (e.g., through protein crowding and competition for limited binding sites). The inducible expression of the APEX2 constructs is one way to reduce these potential problems, providing control of expression levels and timing. An alternative would be to introduce APEX2 directly into the genome through homologous recombination, fusing it to the endogenous copy of an LD protein (e.g., ATGL or PLIN2). In this scenario, it would be important to ensure that the APEX2 tag does not disrupt the function of the protein to which it is fused. Finally, the described method is limited to cultured mammalian cells due to the requirement for introduction of biotin phenol and a hydrogen peroxide treatment. Other proximity biotinylation strategies are suitable for in vivo applications, such as turbo-ID (Branon et al., 2018).
Troubleshooting
Problem 1
Low amount of biotinylation.
Potential solution 1
The timing of the hydrogen peroxide treatment can be increased to up to 5 min, though potential toxicity should be monitored. In addition, cells can also be pre-treated with 7 μM hemin chloride for 24 h to increase biotinylation. APEX2 is a heme-dependent enzyme and this treatment can increase its activity. Whether hemin chloride will be required to achieve sufficient biotinylation for proteomics may depend on the cell line, heme concentration, and APEX2 expression and activity.
Problem 2
Lipid droplet fraction is minimal after centrifugation of the sucrose gradient (step-by-step method details, step 15).
Potential solution 2a
LD amount is influenced by the cell line’s inherent proclivity towards forming LDs and can be impacted by the growth conditions (e.g., Fetal Bovine Serum lot). LD amount can be increased by longer treatments with oleate or by increasing the number of cells used.
Potential solution 2b
If a translucent, white layer is observed towards the bottom third of the ultracentrifuge tube rather than as expected at the top, it is likely that homogenization of the layers during step #13 was insufficient.
Problem 3
Low peptide counts despite strong peak in chromatogram.
Potential solution 3
In some cases, this strong peak can come from digested streptavidin that eluted with the proteins from the beads during harsh elution treatments. Include streptavidin in the MaxQuant peptide search to determine if digested streptativin peptides are abundant in the sample. We found that an elution buffer containing 2% SDS with 3 mM biotin gave the highest yield while producing minimal streptavidin contamination. If your sample contains large amounts of streptavidin peptides, consider trying alternative elution buffers as previously described (Rybak et al., 2004). In addition, create an exclusion list so that the mass spectrometer will ignore those peptides during the mass spectrometry run.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, James Olzmann (olzmann@berkeley.edu).
Materials availability
This study did not generate new unique reagents. Published plasmids are available from Addgene.
Data and code availability
LD proteomics data (Bersuker et al., 2018) can be downloaded from the PRoteomics IDEntification Database (https://www.ebi.ac.uk/pride/archive/projects/PXD007695).
Acknowledgments
This work was supported by a grant from the National Institutes of Health to J.A.O. (R01GM112948). J.A.O. is a Chan Zuckerberg Biohub investigator.
Author contributions
All authors contributed to the conceptualization, writing, and editing of the manuscript. C.W.H.P. and K.K.D. performed the experimental investigation and validation. All authors contributed to the generation of figures, tables, and video.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100579.
Supplemental information
Spectral counts and intensity values from MaxQuant. The mass spectrometry .RAW files that were analyzed, including ATGL∗-APEX2, PLIN2-APEX2, Cyto-APEX, and buoyant factions (BF), were previously described (Bersuker et al., 2018) and are available for download from the PRIDE database (Project PXD007695).
Proteins ranked by average iBAQ intensity for PLIN2-APEX2, normalized by subtraction of cyto-APEX2 intensities. References for validated LD proteins that exhibited an iBAQ intensity >0 are indicated.
Proteins ranked by average iBAQ intensity for ATGL∗-APEX2, normalized by subtraction of cyto-APEX2 intensities. References for validated LD proteins that exhibited an iBAQ intensity >0 are indicated.
References
- Bendayan M. Tech.Sight. Worth its weight in gold. Science. 2001;291:1363–1365. doi: 10.1126/science.291.5507.1363. [DOI] [PubMed] [Google Scholar]
- Bersuker K., Olzmann J.A. Establishing the lipid droplet proteome: Mechanisms of lipid droplet protein targeting and degradation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:1166–1177. doi: 10.1016/j.bbalip.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersuker K., Peterson C.W.H., To M., Sahl S.J., Savikhin V., Grossman E.A., Nomura D.K., Olzmann J.A. A proximity labeling strategy provides insights into the composition and dynamics of lipid droplet proteomes. Dev. Cell. 2018;44:97–112.e7. doi: 10.1016/j.devcel.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaner W.S., O’Byrne S.M., Wongsiriroj N., Kluwe J., D’Ambrosio D.M., Jiang H., Schwabe R.F., Hillman E.M.C., Piantedosi R., Libien J. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim. Biophys. Acta. 2009;1791:467–473. doi: 10.1016/j.bbalip.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M., Sánchez-Álvarez M., Fajardo A., Kapetanovic R., Steiner B., Dutra F., Moreira L., López J.A., Campo R., Marí M. Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science. 2020;370:eaay8085. doi: 10.1126/science.aay8085. [DOI] [PubMed] [Google Scholar]
- Branon T.C., Bosch J.A., Sanchez A.D., Udeshi N.D., Svinkina T., Carr S.A., Feldman J.L., Perrimon N., Ting A.Y. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 2018;36:880–887. doi: 10.1038/nbt.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasaemle D.L., Wolins N.E. Isolation of lipid droplets from cells by density gradient centrifugation. Curr. Protoc. Cell Biol. 2006;Chapter 3 doi: 10.1002/0471143030.cb0315s29. Unit 3.15. [DOI] [PubMed] [Google Scholar]
- Chitraju C., Mejhert N., Haas J.T., Diaz-Ramirez L.G., Grueter C.A., Imbriglio J.E., Pinto S., Koliwad S.K., Walther T.C., Farese R.V. Triglyceride synthesis by DGAT1 protects adipocytes from lipid-induced ER stress during lipolysis. Cell Metab. 2017;26:407–418.e3. doi: 10.1016/j.cmet.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- Dias S. da S.G., Soares V.C., Ferreira A.C., Sacramento C.Q., Fintelman-Rodrigues N., Temerozo J.R., Teixeira L., Nunes da Silva M.A., Barreto E., Mattos M. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 2020;16:e1009127. doi: 10.1371/journal.ppat.1009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey R., Stivala C.E., Nguyen H.Q., Goo Y.-H., Paul A., Carette J.E., Trost B.M., Rohatgi R. Lipid droplets can promote drug accumulation and activation. Nat. Chem. Biol. 2020;16:206–213. doi: 10.1038/s41589-019-0447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D.J., Dos Santos M.S., Huang S., Russell M.R.G., Collinson L.M., MacRae J.I., West A., Jiang H., Gutierrez M.G. Subcellular antibiotic visualization reveals a dynamic drug reservoir in infected macrophages. Science. 2019;364:1279–1282. doi: 10.1126/science.aat9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker M.W., Vaandrager A.B., Helms J.B. Retinoids in health and disease: A role for hepatic stellate cells in affecting retinoid levels. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865:158674. doi: 10.1016/j.bbalip.2020.158674. [DOI] [PubMed] [Google Scholar]
- Herker E., Harris C., Hernandez C., Carpentier A., Kaehlcke K., Rosenberg A.R., Farese R.V., Ott M. Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat. Med. 2010;16:1295–1298. doi: 10.1038/nm.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung V., Zou P., Rhee H.-W., Udeshi N.D., Cracan V., Svinkina T., Carr S.A., Mootha V.K., Ting A.Y. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol. Cell. 2014;55:332–341. doi: 10.1016/j.molcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., Braverman J., Asfaha K., Gronert K., Stanley S. Lipid droplet formation in Mycobacterium tuberculosis infected macrophages requires IFN-γ/HIF-1α signaling and supports host defense. PLoS Pathog. 2018;14:e1006874. doi: 10.1371/journal.ppat.1006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kory N., Farese R.V., Walther T.C. Targeting fat: mechanisms of protein localization to lipid droplets. Trends Cell Biol. 2016;26:535–546. doi: 10.1016/j.tcb.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S.S., Martell J.D., Kamer K.J., Deerinck T.J., Ellisman M.H., Mootha V.K., Ting A.Y. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods. 2015;12:51–54. doi: 10.1038/nmeth.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger L.L., Han X., Lewis S.E., Cases S., Farese R.V., Ory D.S., Schaffer J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanari Y., Atsuzawa K., Usuda N., Watashi K., Hishiki T., Zayas M., Bartenschlager R., Wakita T., Hijikata M., Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- Nguyen T.B., Louie S.M., Daniele J.R., Tran Q., Dillin A., Zoncu R., Nomura D.K., Olzmann J.A. DGAT1-Dependent Lipid Droplet Biogenesis Protects Mitochondrial Function during Starvation-Induced Autophagy. Dev. Cell. 2017;42:9–21.e5. doi: 10.1016/j.devcel.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzmann J.A., Carvalho P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019;20:137–155. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzmann J.A., Richter C.M., Kopito R.R. Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover. Proc. Natl. Acad. Sci. U S A. 2013;110:1345–1350. doi: 10.1073/pnas.1213738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renne M.F., Klug Y.A., Carvalho P. Lipid droplet biogenesis: A mystery “unmixing”? Semin. Cell Dev. Biol. 2020;108:14–23. doi: 10.1016/j.semcdb.2020.03.001. [DOI] [PubMed] [Google Scholar]
- Roberts M.A., Olzmann J.A. Protein quality control and lipid droplet metabolism. Annu. Rev. Cell Dev. Biol. 2020;36:115–139. doi: 10.1146/annurev-cellbio-031320-101827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak J.-N., Scheurer S.B., Neri D., Elia G. Purification of biotinylated proteins on streptavidin resin: a protocol for quantitative elution. Proteomics. 2004;4:2296–2299. doi: 10.1002/pmic.200300780. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanhäusser B., Busse D., Li N., Dittmar G., Schuchhardt J., Wolf J., Chen W., Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- Thiam A.R., Ikonen E. Lipid droplet nucleation. Trends Cell Biol. 2020;31:108–118. doi: 10.1016/j.tcb.2020.11.006. [DOI] [PubMed] [Google Scholar]
- Tyanova S., Temu T., Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016;11:2301–2319. doi: 10.1038/nprot.2016.136. [DOI] [PubMed] [Google Scholar]
- Walther T.C., Chung J., Farese R.V. Lipid Droplet Biogenesis. Annu. Rev. Cell Dev. Biol. 2017;33:491–510. doi: 10.1146/annurev-cellbio-100616-060608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Liu P. The new face of the lipid droplet: lipid droplet proteins. Proteomics. 2019;19:e1700223. doi: 10.1002/pmic.201700223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spectral counts and intensity values from MaxQuant. The mass spectrometry .RAW files that were analyzed, including ATGL∗-APEX2, PLIN2-APEX2, Cyto-APEX, and buoyant factions (BF), were previously described (Bersuker et al., 2018) and are available for download from the PRIDE database (Project PXD007695).
Proteins ranked by average iBAQ intensity for PLIN2-APEX2, normalized by subtraction of cyto-APEX2 intensities. References for validated LD proteins that exhibited an iBAQ intensity >0 are indicated.
Proteins ranked by average iBAQ intensity for ATGL∗-APEX2, normalized by subtraction of cyto-APEX2 intensities. References for validated LD proteins that exhibited an iBAQ intensity >0 are indicated.
Data Availability Statement
LD proteomics data (Bersuker et al., 2018) can be downloaded from the PRoteomics IDEntification Database (https://www.ebi.ac.uk/pride/archive/projects/PXD007695).