Abstract
Introduction
In this nested case‐control study, we investigated if antiviral treatment given prior to onset of Alzheimer's disease (AD) could influence incident AD.
Methods
From a large population‐based cohort study in northern Sweden, 262 individuals that later developed AD were compared to a non‐AD matched control group with respect to prescriptions of herpes antiviral treatment. All included subjects were herpes simplex virus 1 (HSV1) carriers and the matching criteria were age, sex, apolipoprotein E genotype (ε4 allele carriership), and study sample start year.
Results
Among those who developed AD, 6 prescriptions of antivirals were found, compared to 20 among matched controls. Adjusted for length of follow‐up, a conditional logistic regression indicated a difference in the risk for AD development between groups (odds ratio for AD with an antiviral prescription 0.287, P = .018).
Discussion
Antiviral treatment might possibly reduce the risk for later development of HSV1‐associated AD.
Keywords: Alzheimer's disease, antiviral treatment, apolipoprotein E gene, dementia, herpes simplex, major neurocognitive disorder, nested case‐control study
1. BACKGROUND
Alzheimer's disease (AD) is a common cause of a major neurocognitive disorder that affects millions of people worldwide. 1 A growing body of evidence suggests that herpes simplex virus type 1 (HSV1) might play a considerable role in the development of AD. 2 , 3 HSV1 is common worldwide and its prevalence is estimated at 80% in the Swedish population. 4 , 5
Longitudinal cohort studies have shown that reactivation of HSV, measured as immunoglobulin (Ig) M‐positivity for HSV, increases the risk of developing AD approximately 8 to 10 years after HSV reactivation. Reactivation of HSV seems to have a stronger correlation to AD than HSV1 carriage alone. 6 , 7 , 8 Furthermore, population‐based cohort studies indicate that the genetic background, and allele 4 of the apolipoprotein E gene (APOE ε4) in particular, interact with HSV1 infections to increase the risk of AD. 9 , 10 , 11 APOE ε4 carriers have an increased risk of cold sores, 12 and animal studies have indicated that spread of HSV1 in a host is facilitated by the presence this allele. 13 , 14 Independently of APOE ε4, HSV1 carriage also seems to interact with other genes for AD development, 10 and a genetic variant affecting the development of efficient immunity toward HSV1, GM17/17, was recently shown to almost double the risk of later AD development. 15 Together these studies indicate that not so much HSV1 carriage alone, but HSV1, together with certain genetic susceptibility factors, increases the risk of AD, which might explain the relatively modest penetrance of HSV1 carriership itself.
HSV1 has the ability to establish lifelong latency in sensorial ganglia and can reactivate recurrently, giving rise to symptoms ranging from oral ulcers to severe encephalitis, providing clinical evidence for the ability of the virus to reach the brain. 16 Itzhaki et al. detected HSV1 DNA in brains of sporadic AD patients, 12 and later reported that most of the viral DNA was associated with amyloid plaques. 17 Likewise, signatures from herpes virus, including HSV1, were found when transcriptomes of brain samples from AD patients were analyzed. 18 Recent findings suggest that HSV1 also reactivates subclinically, and that migration to the brain without any symptoms of encephalitis is possible. 19 , 20 Advanced in vitro and in vivo studies have shown that repeated reactivation of HSV1 can trigger progressive accumulation of AD‐like neuropathological changes (amyloid plaque‐like formations, gliosis, neuroinflammationm and neuronal loss) and lead to declining cognitive function in mice, thus closely mimicking an AD‐like phenotype. 21 , 22 , 23 The accumulation of amyloid beta (Aβ) in response to HSV1 might be viewed in light of the possible antimicrobial peptide properties of Aβ. 24 , 25
While there is no cure for HSV1 infection, treatment with antiviral drugs can reduce viral replication and subsequent symptoms. Given the possible link between HSV1 reactivations and AD, an intriguing opportunity would be the potential prevention of the disease by using antiviral drugs to suppress HSV1 reactivations. A possible protective effect has been indicated in previous registry‐based studies, in which the risk of developing major neurocognitive disorder was reduced among individuals that had received treatment with herpes antivirals. 26 , 27 , 28 , 29 Registry‐based studies however lack in‐depth clinical information and could be sensitive to the risk of confounding by unknown factors. An important limitation with these previous studies is that HSV1 carriership and APOE genotype were not known. These results therefore need to be replicated in well‐defined population‐based cohorts. Here we evaluated the potential effects of herpes antiviral drugs on subsequent risk of AD in a smaller, but well‐defined, population‐based matched nested case‐control study with extensive background data including APOE ε4 and HSV1 carriage status.
2. METHODS
2.1. Study samples and data collection
The participants in this study were from the Betula cohort study. 30 , 31 , 32 The Betula study, initiated in 1988, is a prospective population‐based cohort study with the overall aim of investigating how memory function and health develop with age and to identify early signs of major neurocognitive disorders. 30 , 31 , 32 Five samples (S) from the general population in the municipality of Umeå, a city with 129,000 inhabitants in northern Sweden, were recruited at different time points with an interval of 5 years between every assessment. A total of 4425 individuals were included during the study. Ages at recruitment ranged from 35 to 95 years of age. All individuals were examined at inclusion, with the aim of collecting extensive information on health, lifestyle, and social variables, including individual testing with a broad battery of cognitive tests. Serum samples were drawn and stored frozen. Participants were longitudinally followed from sample baseline until 2015–2016, recording outcomes of major cognitive disorders, the event of death, or loss of follow‐up. The diagnoses of major neurocognitive disorder were based on the criteria of the Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition, and established by an experienced psychiatrist, specialized in psychiatry of the elderly, after reviewing all medical records from primary care, specialized outpatient care, and hospital inpatient care. Written consent was obtained from included participants. Selection and testing procedures have been described in detail previously. 6 , 32
RESEARCH IN CONTEXT
Systematic review: PubMed was used to search for previously published studies. Alzheimer's disease (AD) has a complex genesis, with the potential involvement of multiple factors, for example, environmental factors and genetic susceptibility. Herpes simplex virus type 1 carriage in combination with susceptibility genes has been associated with AD risk and antiviral treatment has been associated with lower risk of development of major neurocognitive disorders in registry‐based studies.
Interpretation: Our findings indicate that antiviral treatment indeed reduced the risk of later development of HSV1‐associated AD, providing evidence for the existence of a direct mechanistic link between HSV1 reactivations and a higher risk of later AD development.
Future directions: Further longitudinal studies are warranted to establish the protective effect of antiviral drugs on later AD development. Studies are also needed to establish the target patient group that will benefit from an antiviral prescription to reduce AD risk.
2.2. Serum analyses
The APOE genotype had been determined previously for most participants in Betula samples S1 through S3 (see Sundstrom et al. 33 for detailed methods). For individuals with the APOE genotype not previously determined, APOE ε4 carriership was established by analysis of protein isoform presence in sera due to unavailability of DNA‐containing samples. A commercially available enzyme‐linked immunosorbent assay (ELISA; Cat # K4699‐100; BioVision) was used to quantify the amount of APOE ε4. Sera were diluted 1:400 in assay diluent to fit a standard curve created with the provided recombinant APOE ε4 protein (10–168 ng/mL). Based on trials with serum samples of known APOE genotype, we concluded that sera with APOE ε4 concentrations within that range corresponded with presence of at least one APOE ε4 allele. Absorbance was read at 450 nm in a plate reader (model “Sunrise,” Tecan).
The earliest frozen serum samples from each participant were analyzed for anti‐HSV1 IgG. In short, an in‐house ELISA was used to detect antibodies toward HSV IgG antigen. 34 All HSV‐positive samples were analyzed for specific anti‐HSV1 IgG with the commercial ELISA kits (HerpeSelect‐assay, Focus Diagnostics). Results from the HerpeSelect assay were used to determine HSV1 positivity among the samples that were positive in the less specific, but sensitive, HSV assay. The serological testing protocols have been described previously. 6
All serological analyses were run according to routine procedures at the clinical diagnostics lab at the University Hospital of Umeå, Sweden. The methods are accredited by the Swedish Board for Accreditation and Conformity Assessment according to ISO 17025 standards.
2.3. Nested case‐control study design
From the full set of participants in the Betula cohort study (N = 4425), individuals were selected for whom HSV1 and APOE ε4 carriership were determined (N = 3384) and from them, those who were HSV1 carriers (anti‐HSV1 IgG‐positive individuals; N = 2839). Among these HSV1 carriers, 294 participants had developed AD during follow‐up. For each one of these participants who had received an AD diagnosis, that is, a case, a participant in the Betula study (likewise from the pool of HSV1 carriers) who had not developed AD up until the latest follow‐up in 2015–2016, that is, a control, was matched on age (±2 years), sex, study sample start year (i.e., S1: 1988; S2, S3: 1993; S4: 1998; S5: 2003; S6: 2013) and APOE ε4 carriage (genotypically or phenotypically determined). Only case‐control pairs for whom both cases and controls had consented to access to medical records were included in the final study group, resulting in a final set of 262 cases and their matched controls.
Matching was made with replacement, such that some of the controls were matched to more than one case. Twenty‐four controls were therefore matched twice, five controls were matched three times, and one control was matched five times. Thus, 224 unique individuals were used as controls for the 262 cases.
2.4. Drug data acquisition
A list of these participants, identified by their Swedish Tax Agency personal identity number, was generated. The list provided no information on whether a participant was a case or a control. A resident physician (E.H.) reviewed all prescriptions registered in the electronic medical record for each listed participant, which can be accessed using the Swedish Tax Agency personal identity number. Prescriptions have been made electronically since the mid‐1990s in the region of Västerbotten and medical records have been electronic since the mid‐1990s. For the purposes of the present study, antiviral drugs were defined as substances within the ATC‐code J05AB, that is, nucleoside analogues. Information on whether the individual had ever been prescribed an antiviral drug, what substance was prescribed, and the date of the first prescription was retrieved. Prescriptions written before a participant's inclusion in the Betula study were included but prescriptions written after the end of a person's follow‐up, for example, after AD diagnosis, were excluded.
2.5. Statistical analyses
Paired samples t‐test, Wilcoxon signed rank test, and McNemar's test without Yates correction were used, where applicable, for univariate analyses to compare cases and their matched controls. Conditional logistic regression was used to test whether antiviral herpes treatment was associated with the risk of developing AD. The conditional logistic regression model was adjusted for follow‐up time to control for its potential confounding effect. A Kaplan‐Meier plot was produced to visualize the cumulative risk of AD. Differences between the two groups were tested with a Mantel‐Cox log rank test. A P‐value of < .05 was regarded as statistically significant. The SPSS Statistics version 25.0 (IBM Corporation) software for Mac was used for statistical calculations.
2.6. Ethical approval
The Regional Ethical Review Board in Umeå, Sweden approved the study (2018‐92‐32 M). All participants in the present study have given their written informed consent for the study including medical record access.
3. RESULTS
Background characteristics of the 262 AD cases and their matched controls are presented in Table 1. Because the groups were matched on sex and APOE ε4 status, these prevalence values were identical among cases and controls with a predominance of women (74.8%) and almost half of the individuals were carriers of at least one APOE ε4 allele (48.9%, reflecting the proportion among AD cases). Neither mean age at inclusion nor mean length of formal education differed between the cases and controls (71.1 ± 8.8 years vs. 70.8 ± 9.4 years, P = .063; 7.8 ± 2.8 vs. 8.0 ± 3.0 years, P = .467, n = 450).
TABLE 1.
Background data for Alzheimer's disease cases and controls
| AD cases | Matched controls | P‐value | |
|---|---|---|---|
| N | 262 | 262 a | |
| Women, n (%) | 196 (74.8) | 196 (74.8) | |
| APOE ε4 carriers, n (%) | 128 (48.9) | 128 (48.9) | |
| HSV1 carriers, n (%) | 262 (100.0) | 262 (100.0) | |
| Age at inclusion, mean ± SD, (years) | 71.1 ± 8.8 | 70.8 ± 9.4 | .063 PST |
| Year of education, mean ± SD (years) | 7.8 ± 2.8 (N = 225) | 8.0 ± 3.0 (N = 225) | .467 PST |
| Cohort start year, median (range) | 1993 (1988–2003) | 1993 (1988–2003) | 1.000 WSRT |
| Follow‐up time, mean ± SD, (years) | 9.3 ± 6.1 | 13.5 ± 6.9 | <.001 PST |
| Antiviral drugb prescription during follow‐up, n (%) | 6 (2.3) | 20 (7.6) | 0.006 MN |
| Antiviral drug b prescribed/1000PY follow‐up, mean | 3.5 | 4.9 | .091 WSRT |
Abbreviations: AD, Alzheimer's disease; APOE ε4, allele 4 of the apolipoprotein E gene; HSV, herpes simplex virus type 1; MN, McNemar's test without Yates correction; PST, paired sample T‐test; PY, person‐year; SD, standard deviation; WSRT, Wilcoxon signed rank test.
224 unique individuals.
† Acyclovir analogue.
A total of 32 prescriptions of antivirals were found and of these, 7 prescriptions were excluded because they were written after the end of the follow‐up period (all these 7 prescriptions were made to AD cases after their diagnosis). Controls with early termination of follow‐up either had moved from the region (lost) or had died and recorded prescriptions after their follow‐up would be unknown. Antiviral prescription was significantly more common among controls compared to AD cases (7.6% vs. 2.3%, P = .006). The percentage of antiviral treatment among unique controls was also similar to the percentage calculated for controls as matched (19/224 = 8.5% compared to 20/262 = 7.6%).
The follow‐up time differed significantly between cases and controls (9.7 years ± 6.1 and 13.5 years ± 6.9, P < .001). A significant association between a longer follow‐up time and the probability of having received antiviral prescription was also seen (P = .001), hence, the follow‐up time variable was included in the regression analyses to adjust for its potential confounding effect.
A conditional logistic regression model, adjusted for follow‐up time, showed a significantly decreased risk of developing AD for those who had received at least one antiviral prescription compared to those who had not received any antiviral prescriptions (odds ratio [OR] 0.287, P = .018), as shown in Table 2.
TABLE 2.
Conditional logistic regression model with Alzheimer's disease as outcome
| Odds ratio | P‐value | Lower limit of 95% CI | Upper limit of 95% CI | |
|---|---|---|---|---|
| Antiviral drug a treatment | 0.287 | .018 | 0.102 | 0.809 |
| Follow‐up time | 0.861 | <.001 | 0.826 | 0.898 |
Abbreviation: CI, confidence interval.
Acyclovir analogue.
The risk of AD for persons who had, or had not, received antiviral prescriptions was illustrated using a Kaplan‐Meier plot (Mantel‐Cox log rank test: P = .032), in Figure 1. Note that only the relative proportions between groups could be interpreted, as the absolute risk is inflated by the fact that one‐half of the individuals in this nested case‐control sample developed AD, as per the study design.
FIGURE 1.
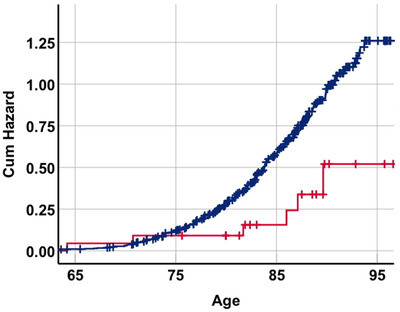
Kaplan‐Meier plot showing cumulative risk of Alzheimer's disease (AD) for persons who had received at least one antiviral prescription (acyclovir analogue) prior to censoring date (red) or no antiviral prescription prior to censoring date (blue). Mantel‐Cox log rank test P = .032. Note that the x‐axis depicts the age of the individual and the y‐axis is the cumulative risk of AD
A subgroup analysis based on APOE ε4 carriage indicated an association of antiviral treatment to AD risk to be present among both 134 non‐carriers (OR 0.242, P = .042) and 128 carriers (OR 0.370, P = .227); however, not significantly among the latter.
To check for reverse causation, that is, if individuals with subclinical major cognitive disorder could have a lower probability of receiving an antiviral prescription, the number of years between first prescription and end of follow‐up time was analyzed between cases and controls. There was no significant difference in this time for AD cases compared to controls (9.0 ± 6.2 years vs. 7.2 ± 4.6 years, P = .430).
4. DISCUSSION
In the present nested case‐control study, antiviral treatment was associated with an approximate 70% risk reduction for later AD development (OR 0.287, P = .018). A subgroup analysis indicated that both APOE ε4 non‐carriers and APOE ε4 carriers contributed to the observed association, although the association did not reach statistical significance among the APOE ε4 carriers. To the best of our knowledge, this is the first time this has been shown in a well‐investigated population‐based cohort with known HSV1‐ and APOE ε4 status. The results indicate that antiviral treatment may reduce the risk of developing AD, in line with recent registry‐based studies, and adds to the accumulating evidence for an association between HSV1 reactivation and the risk of later AD development. 3 , 6 , 7 , 26 , 27 , 28 , 29 However, because this is an observational study, associations should be interpreted with caution and not as causal relationships. Differences, other than herpes antiviral treatment, between the groups contributing to the observed associations cannot be excluded.
Primary strengths of our study include the prospective cohort design with extensive background data and long follow‐up time, as well as the nested case‐control method for close matching of AD cases with controls allowing an efficient handling of important confounding and interaction variables, including HSV1 and APOE ε4 carriage, age, and sex. An additional strength of our study is that the AD diagnoses were of high clinical quality, with the diagnoses reviewed by an experienced psychiatrist, specialized in general and geriatric psychiatry.
Still, as diagnoses were clinical, there might have been individuals with mixed or other pathology among the cases. Another limitation of this study is that prescriptions written in the early 1990s, outside of the region of Västerbotten, or by private practitioners, were not registered. Although this might have led to an underrepresentation of treatments in the study, it should not introduce any systematic bias between cases and controls. The number of persons prescribed herpes antiviral treatments was small, which should be considered when interpreting the results.
The frequency and duration of antiviral drug treatment may be of great importance for the association of antiviral treatment with risk of later AD development. The lack of such data in the present study is a limitation; however, considering the low number of treatments, further differentiation would have been difficult even if data were available.
To avoid losing cases, we used matching with replacement, that is, a procedure in which some controls were matched more than once. Importantly, the proportions of antiviral treatment were similar among both unique controls and controls as matched, indicating that the results were not driven by the matching with replacement.
An association between a longer follow‐up time and the probability of having received antiviral treatment (P = .001) was seen and this potential confounder was handled by including the follow‐up time variable in the regression analyses. Reverse causality, that is, that individuals with AD were not prescribed antiviral medication prior to their diagnosis because of something related to the development of their disease, was also considered. One such potential factor could be incapability to seek medical care due to cognitive impairment. However, those who developed AD did not have fewer prescriptions as they approached their time of diagnosis and did not have a longer time between prescription and their end‐of‐follow‐up compared to the controls. Also, we found several prescriptions for individuals with AD even after diagnosis. Together, these observations indicate that reverse causality could not explain the findings.
In summary, this and previous registry‐based observational studies have indicated an association of antiviral treatment with lower risk of major neurocognitive disorder. 26 , 27 , 28 , 29 Ideally, this possible effect should be investigated in randomized studies; however, this might be complicated due to the long follow‐up time and large study population needed. An important question is when the antiviral treatment should be administrated to have its greatest preventive effect, and what dosage and duration of treatment are needed for prevention. Previous epidemiological studies suggest that HSV reactivation could precede the development of AD by approximately 8 to 10 years. 6 , 8 One of the most common symptoms of reactivation is recurrent oral ulcers, a condition also linked to APOE ε4 carriage. 12 , 35 Hence middle‐aged individuals or older with HSV1 reactivation symptoms, such as oral ulcers, could possibly benefit from oral antiviral treatment to prevent later AD development; however, this warrants further study.
5. CONCLUSION
In conclusion, this population‐based nested case‐control study indicated a 70% lower risk for developing AD with the use of antiviral drugs, among HSV1 carriers. This finding suggests that antiviral drug use might prevent later development of HSV1‐associated AD, which could possibly be an incentive for clinicians to more liberally consider oral antiviral treatment to individuals presenting HSV reactivation symptoms.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
The authors wish to express their gratitude to Professor Emeritus Lars‐Göran Nilsson, Department of Psychology, Stockholm University, the former Director and Principal Investigator of Betula cohort study, and to Research Coordinator Annelie Nordin Adolfsson, Department of Clinical Sciences, Umeå University, for their invaluable contribution to the data collection. This study was supported financially by grants from Region Västerbotten, Wallenberg Centre for Molecular Medicine (WCMM) at Umeå University, the Swedish Dementia Association, the Swedish Alzheimer Fund, and the Umeå University Foundation for Medical Research to Hugo Lövheim. Ongoing work in the Betula project is supported by scholar grants from the Knut and Alice Wallenberg (KAW‐scholar) Foundation to Lars Nyberg. This work was further funded by a grant from the Swedish Research Council for Health, Working Life and Welfare (Dnr 2013‐2056) to Maria Josefsson.
Hemmingsson E‐S, Hjelmare E, Weidung B, et al. Antiviral treatment associated with reduced risk of clinical Alzheimer's disease—A nested case‐control study. Alzheimer's Dement. 2021;7:e12187. 10.1002/trc2.12187
REFERENCES
- 1. Wortmann M. Dementia: a global health priority – highlights from an ADI and World Health Organization report. Alzheimer Res Ther. 2012;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Itzhaki RF, Lathe R, Balin BJ et al. Microbes and Alzheimer's disease. J Alzheimer Dis. 2016;51:979‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mangold CA, Szpara ML. Persistent Infection with Herpes Simplex Virus 1 and Alzheimer’s Disease—A Call to Study How Variability in Both Virus and Host may Impact Disease. Viruses. 2019;11(10):966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olsson J, Kok E, Adolfsson R, Lovheim H, Elgh F. Herpes virus seroepidemiology in the adult Swedish population. Immunity Ageing. 2017;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Looker KJ, Magaret AS, May MT, et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One. 2015;10:e0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lovheim H, Gilthorpe J, Adolfsson R, Nilsson LG, Elgh F. Reactivated herpes simplex infection increases the risk of Alzheimer's disease. Alzheimer Dement. 2015;11:593‐599. [DOI] [PubMed] [Google Scholar]
- 7. Letenneur L, Peres K, Fleury H, et al. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer's disease: a population‐based cohort study. PLoS One. 2008;3:e3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lovheim H, Gilthorpe J, Johansson A, Eriksson S, Hallmans G, Elgh F. Herpes simplex infection and the risk of Alzheimer's disease: a nested case‐control study. Alzheimer Dement. 2015;11:587‐592. [DOI] [PubMed] [Google Scholar]
- 9. Lovheim H, Norman T, Weidung B, et al. Herpes simplex virus, APOEvarepsilon4, and cognitive decline in old age: results from the Betula Cohort Study. J Alzheimer Dis. 2019;67:211‐220. [DOI] [PubMed] [Google Scholar]
- 10. Lopatko Lindman K, Weidung B, Olsson J, et al. A genetic signature including apolipoprotein Eepsilon4 potentiates the risk of herpes simplex‐associated Alzheimer's disease. Alzheimer Dement. 2019;5:697‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linard M, Letenneur L, Garrigue I, Doize A, Dartigues JF, Helmer C. Interaction between APOE4 and herpes simplex virus type 1 in Alzheimer's disease. Alzheimer Dement. 2020;16:200‐208. [DOI] [PubMed] [Google Scholar]
- 12. Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet. 1997;349:241‐244. [DOI] [PubMed] [Google Scholar]
- 13. Burgos JS, Ramirez C, Sastre I, Bullido MJ, Valdivieso F. ApoE4 is more efficient than E3 in brain access by herpes simplex virus type 1. Neuroreport. 2003;14:1825‐1827. [DOI] [PubMed] [Google Scholar]
- 14. Burgos JS, Ramirez C, Sastre I, Valdivieso F. Effect of apolipoprotein E on the cerebral load of latent herpes simplex virus type 1 DNA. J Virol. 2006;80:5383‐5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pandey JP, Olsson J, Weidung B, Kothera RT, Johansson A, Eriksson S, Hallmans G, Elgh F, Lövheim H. An Ig γ Marker Genotype Is a Strong Risk Factor for Alzheimer Disease, Independent of Apolipoprotein E ε4 Genotype. The Journal of Immunology. 2020;205(5):1318–1322. 10.4049/jimmunol.2000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella‐zoster. Lancet Neurol. 2007;6:1015‐1028. [DOI] [PubMed] [Google Scholar]
- 17. Wozniak MA, Frost AL, Itzhaki RF. Alzheimer's disease‐specific tau phosphorylation is induced by herpes simplex virus type 1. J Alzheimer Dis. 2009;16:341‐350. [DOI] [PubMed] [Google Scholar]
- 18. Readhead B, Haure‐Mirande JV, Funk CC, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99:64‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bearer EL. HSV, axonal transport and Alzheimer's disease: in vitro and in vivo evidence for causal relationships. Future Virol. 2012;7:885‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olsson J, Lovheim H, Honkala E, Karhunen PJ, Elgh F, Kok EH. HSV presence in brains of individuals without dementia: the TASTY brain series. Dis Models Mechan. 2016;9:1349‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, et al. Alzheimer's disease‐associated beta‐amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron. 2018;99:56‐63.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Chiara G, Piacentini R, Fabiani M, et al. Recurrent herpes simplex virus‐1 infection induces hallmarks of neurodegeneration and cognitive deficits in mice. PLoS Pathog. 2019;15:e1007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cairns DM, Rouleau N, Parker RN, Walsh KG, Gehrke L, Kaplan DL. A 3D human brain‐like tissue model of herpes‐induced Alzheimer's disease. Sci Adv. 2020;6:eaay8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soscia SJ, Kirby JE, Washicosky KJ, et al. The Alzheimer's disease‐associated amyloid beta‐protein is an antimicrobial peptide. PLoS One. 2010;5:e9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar DK, Choi SH, Washicosky KJ, et al. Amyloid‐beta peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016;8:340ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopatko Lindman K, Hemmingsson ES, Weidung B, et al. Herpesvirus infections, antiviral treatment, and the risk of dementia—a registry‐based cohort study in Sweden. Alzheimer Dement. 2021;7:e12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tzeng NS, Chung CH, Lin FH, et al. Anti‐herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections‐a nationwide, population‐based cohort study in Taiwan. Neurotherapeutics. 2018;15:417‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen VC, Wu SI, Huang KY, et al. Herpes zoster and dementia: a nationwide population‐based cohort study. J Clin Psychiatry. 2018;79. [DOI] [PubMed] [Google Scholar]
- 29. Bae S, Yun S, Kim M, Yoon W, Lim JS, Lee S, Choi S‐H, Kim YS, Woo JH, Kim SY, Kim S‐H. Association of herpes zoster with dementia and effect of antiviral therapy on dementia: a population‐based cohort study. European Archives of Psychiatry and Clinical Neuroscience. 2020. 10.1007/s00406-020-01157-4. [DOI] [PubMed] [Google Scholar]
- 30. Nilsson L‐G, Backkman L, Erngrund K, et al. The betula prospective cohort study: memory, health, and aging. Aging Neuropsychol Cogn. 1997;4:1‐32. [Google Scholar]
- 31. Nilsson L‐G, Adolfsson R, Bockman L, de Frias CM, Molander B, Nyberg L. Betula: a prospective cohort study on memory, health and aging. Aging, Neuropsychol Cogn. 2004;11:134‐148. [Google Scholar]
- 32. Nyberg L, Boraxbekk CJ, Sörman DE, et al. Biological and environmental predictors of heterogeneity in neurocognitive ageing: evidence from Betula and other longitudinal studies. Ageing Res Rev. 2020;64:101184. [DOI] [PubMed] [Google Scholar]
- 33. Sundstrom A, Marklund P, Nilsson LG, et al. APOE influences on neuropsychological function after mild head injury: within‐person comparisons. Neurology. 2004;62:1963‐1966. [DOI] [PubMed] [Google Scholar]
- 34. Juto P, Settergren B. Specific serum IgA, IgG and IgM antibody determination by a modified indirect ELISA‐technique in primary and recurrent herpes simplex virus infection. J Virol Methods. 1988;20:45‐55. [DOI] [PubMed] [Google Scholar]
- 35. Koelle DM, Magaret A, Warren T, Schellenberg GD, Wald A. APOE genotype is associated with oral herpetic lesions but not genital or oral herpes simplex virus shedding. Sexually Transmit Infect. 2010;86:202‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]


