1. INTRODUCTION
Allergen immunotherapy (AIT), the gradually increasing repeated administration of high doses of allergens to allergic patients, offers the potential for immune tolerance against reactions to the natural exposures to specific allergens. AIT may lead to the long‐lasting remission of allergic symptoms and is the only disease‐modifying intervention in IgE‐mediated allergic respiratory diseases.
This Pocket Guide was developed by an ARIA and EAACI joint study group from a background paper of the ARIA‐MASK study group and from the EAACI guidelines on allergen immunotherapy.
Bousquet J, Pfaar O, Togias A, et al. (2019). ARIA Care pathways for allergen immunotherapy. Allergy 2019; 74: 2087–2102.
Agache, Lau S, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: house dust mite‐driven allergic asthma. Allergy, 2019;74:855‐73.
AIT is a proven therapeutic option for the treatment of allergic rhinitis, conjunctivitis, and/or asthma using sublingual (SLIT) or subcutaneous (SCIT) routes.
However, AIT is more expensive than symptomatic treatments for allergic diseases (excluding biologicals). It is justified (i) in patients with rhinitis otherwise uncontrolled by symptomatic treatment or (ii) as an add‐on to regular asthma treatment in controlled or partially‐controlled asthmatic patients sensitised to house dust mites aiming to decrease asthma exacerbations, rescue and controller medication, and to improve quality of life.
Care pathways are structured multi‐disciplinary care plans detailing the key steps of patient care. They promote the translation of guideline recommendations to their application in clinical practice.
Although many international and national AIT guidelines have been produced, this is the first care pathway for AIT.
This pocket guide applies to sublingual (SLIT) and sub‐cutaneous (SCIT) immunotherapy for allergic rhinitis.
It has been revised by members from 65 countries (Figure 1).
FIGURE 1.
Countries with Pocket Guide members
2. ALLERGENS TO BE ADMINISTERED
The decision to prescribe AIT should be based on relevant symptoms during allergen exposure, demonstration of sensitisation to the relevant allergens, and availability of good‐quality extracts with proven efficacy and safety.
Some allergen extracts are approved for marketing in the EU (list in annex) with some others also approved by national health agencies.
For certain products, efficacy and safety have been demonstrated in appropriate clinical studies on adults and children. The extrapolation to untested products, allergens or a different population from the one evaluated in the trial is not appropriate and not in line with current guidelines as there is no class‐effect in AIT.
Both monosensitised and polysensitised patients can be treated. However, in the latter case, the most clinically relevant allergen(s) should be used when symptoms are clearly present with allergen source exposure and when allergy tests confirm clinical findings.
3. STRATIFICATION OF ALLERGIC PATIENTS
Precision medicine aims at the customisation of healthcare, tailored to the characteristics of each individual patient. The stratification of patients into subpopulations is the basis of clinical decision making (Figure 2).
FIGURE 2.
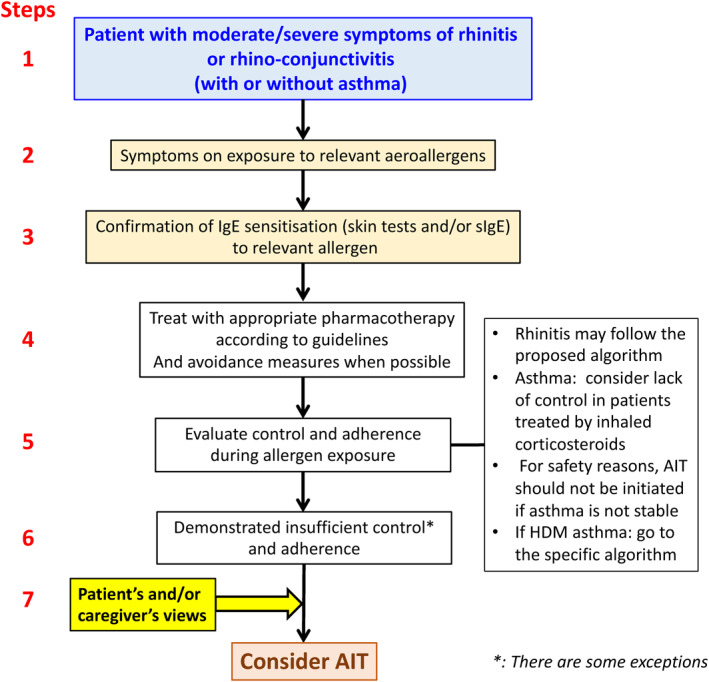
Proposed Flow of Precision Medicine approach in allergic diseases. *examples of exceptions: Thunderstorm‐induced asthma, patient with moderate rhinitis and severe asthma during pollen season
In allergic diseases, patient stratification is required to:
Propose the appropriate pharmacotherapy.
Identify the most suitable candidates for AIT.
Reduce the amount of time and resources needed to match the right patient to an optimal care management programme.
Optimise costs as expensive therapeutic interventions are not necessary or suitable for all patients.
Patient stratification may also help to improve the patient's engagement.
4.
4.1. Precision medicine in the indication of AIT
Precise diagnosis with history, skin prick tests and/or specific IgE and, if applicable, component‐resolved in vitro testing. In some cases, where the above‐mentioned diagnostic tools do not allow for precise diagnosis, allergen provocation testing (nasal, ocular and, in some cases, bronchial) may be needed.
Proven indications: Allergic rhinitis, conjunctivitis and/or asthma.
Symptoms predominantly induced by the relevant allergen exposure.
-
Patient stratification:
Poor control of nasal or ocular symptoms despite optimal medications according to guidelines with documented adherence to treatment.
Exceptions to requiring optimum symptomatic treatment prior to considering AIT include unacceptable side effects of the medications.
Allergic asthma fully controlled under background asthma medication (see EAACI HDM‐AIT GL)
However, for partially controlled asthma, HDM‐AIT may facilitate achieving asthma control (see EAACI HDM‐AIT GL)
Good clinical documentation of efficacy and safety for the AIT product with relevant trials.
The patient's (and caregiver's) views represent an essential component.
4.2. Biomarkers
There are currently no in vivo or in vitro biomarkers validated for monitoring the efficacy of AIT although several potential candidates are currently being investigated.
5. mHEALTH
Apps can be used:
To acquire real‐world evidence to confirm the efficacy of AIT in situations where randomised controlled trials are difficult to perform.
To assess air quality index including pollen exposure and air pollution.
By physicians and patients for stratification of patients and follow‐up.
6. RHINITIS (WITH OR WITHOUT CONJUNCTIVITIS) IN ADOLESCENTS AND ADULTS
The selection of pharmacotherapy and AIT for patients with AR and/or allergic conjunctivitis may be better supported by evidence algorithms to aid patients and healthcare professionals jointly determine the treatment and its step‐up or step‐down strategy depending on rhinitis control (shared decision‐making).
A simple algorithm is proposed as an aid for physicians to determine the treatment of their patients (Figure 3).
FIGURE 3.
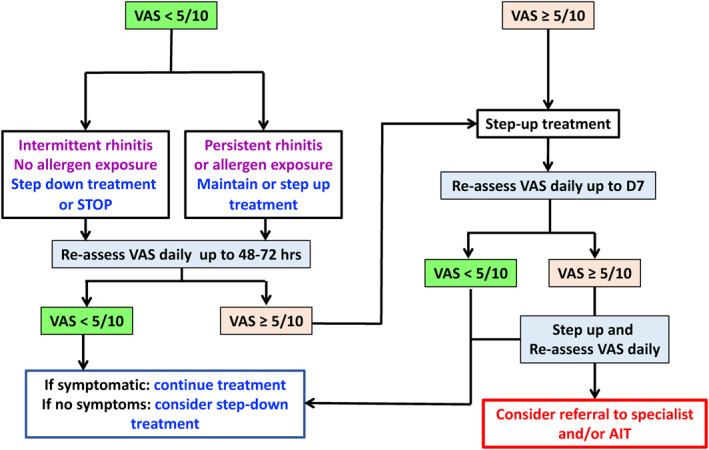
Treatment algorithm using visual analogue scale (VAS) for adolescents and adults AIT, allergen immunotherapy; VAS, visual analogue scale.
6.1. Treatment algorithm using visual analogue scale (VAS)
In the case of remaining ocular symptoms, add intra‐ocular treatment.
7. RHINITIS (WITH OR WITHOUT CONJUNCTIVITIS) IN CHILDREN
AIT is effective, has long‐term beneficial effects after cessation, and may delay or prevent the onset of asthma. AIT can be initiated in children with moderate/severe rhinitis that is not controlled by appropriate medications according to guidelines.
8. ASTHMA
An algorithm for HDM‐driven allergic asthma diagnosis and management is proposed by the EAACI guidelines.
For patients with concomitant allergic rhinitis and sensitised to house dust mite—with persisting asthma symptoms despite low‐moderate dose of inhaled corticosteroids—SLIT can be considered, provided FEV1 is >70% predicted.
House dust mite SLIT should initially be considered as an add‐on therapy to controller treatment, and reduction in asthma controllers should be performed gradually under the supervision of a physician.
Immunotherapy is not indicated for the treatment of acute exacerbations, and patients must be informed of the need to seek medical attention immediately if their asthma deteriorates suddenly (Figure 4).
FIGURE 4.
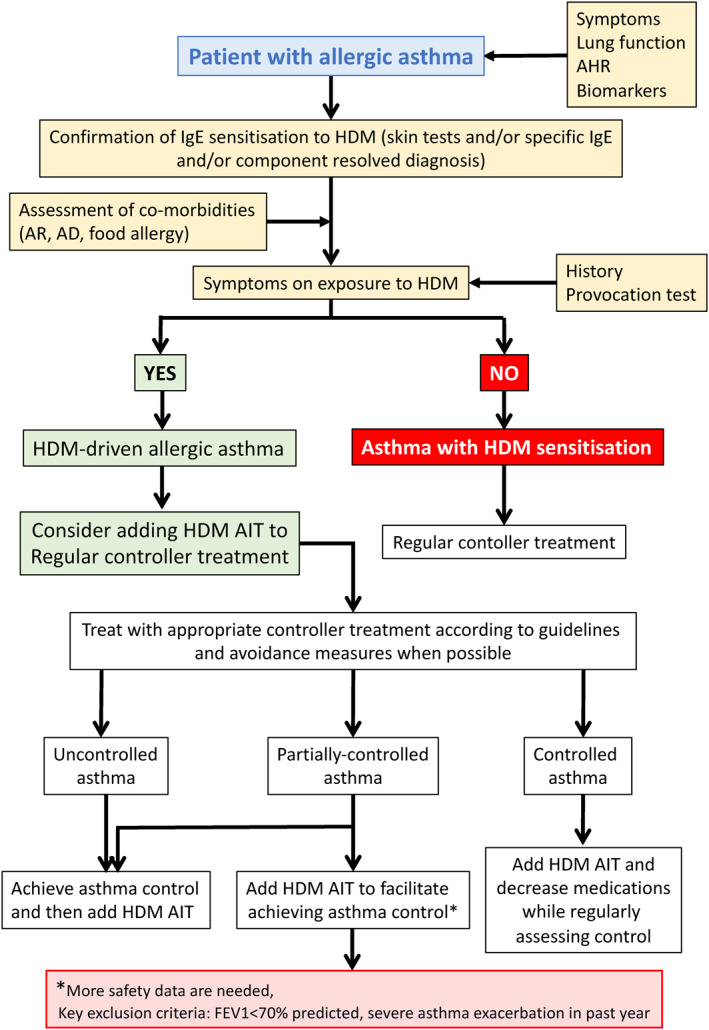
Algorithm for AIT in asthma
9. MULTIMORBIDITY
One strength of AIT is that it has the potential to control all allergic diseases related to a specific allergen, including rhinitis, conjunctivitis and asthma.
10. SAFETY
10.1. Subcutaneous immunotherapy (SCIT)
Local reactions: A typical reaction is redness and swelling at the injection site immediately or several hours after the injection. Sometimes, sneezing, nasal congestion or hives can occur.
Systemic reactions: Serious reactions to injections are very rare and require immediate medical attention. Symptoms of an anaphylactic reaction can include swelling in the throat, wheezing or tightness in the chest, nausea and dizziness. The most serious reactions develop within 30 min after the injection, and patients are advised to wait in their doctor's surgery for at least 30 min after an injection. Severe bronchospasm can also occur, especially in patients where asthma is not controlled.
10.2. Sublingual immunotherapy (SLIT)
Allergen drops or tablets have a more favourable safety profile than injections. The initial dose should be performed in the doctor's surgery, and patients are advised to remain in the surgery for at least 30 min after administration. Thereafter, SLIT can be administered at home once the first dose has been given under the supervision of a physician.
Allergic reactions: The majority of patients will experience mild local reactions of the oropharyngeal passage. This is usually controlled by predosing with an antihistamine 30 min before the administration of SLIT. Sometimes, sneezing, nasal congestion or hives can occur. Anaphylaxis is rarely described.
In some countries, SLIT tablets include a warning about possible severe allergic reactions, and adrenaline auto‐injectors are routinely recommended. This is not the case in Europe.
CONFLICT OF INTEREST
IAgache is an Associate Editor Allergy and CTA.
CA reports grants from Allergopharma, grants from Idorsia, Swiss National Science Foundation, Christine Kühne‐Center for Allergy Research and Education, European Commission's Horison's 2020 Framework Programme, Cure, Novartis Research Institutes, Astra Zeneca, scibase, advisory role in Sanofi/Regeneron, grants from Glakso Smith‐Kline, advisory role in scibase.
IA reports personal fees from Hikma, Roxall, Astra Zeneca, Menarini, UCB, Faes Farma, Sanofi, Mundipharma, Bial, Amgen, Stallergenes.
SBA reports grants from TEVA, personal fees from TEVA, AstraZeneca, Boehringer Ingelheim, GSK, Sanofi, Mylan.
VC reports personal fees from ALK, Allergy Therapeutics, LETI, Thermofisher, Merck, Astrazeneca, GSK.
TC reports grants and personal fees from Stallergenes.
PD reports personal fees from ALK‐Abello, Stallergenes‐Greer, Astra Zeneca, GlaxoSmithKline, Mylan, Sanofi.
SD reports personal fees and non‐financial support from ALK Abello, personal fees from Adiga, Biomay, Allergopharma, Anergis, Allergy Therapeutics.
TH reports personal fees from GSK, Mundipharma, Orion Pharma.
SH reports other from ALK‐Abelló, other from ALK‐Abelló.
EH reports personal fees from Sanofi, Novartis, GSK, AstraZeneca, Circassia, Nestlè Purina.
JCI reports personal fees from Faes Farma, Laboratorios Casasco Argentina, Abbott de Ecuador, EuroFarma Argentina.
MJ reports personal fees from ALK‐Abello, Allergopharma, Stallergenes, Anergis, Allergy Therapeutics, Circassia, Leti, Biomay, HAL, during the conduct of the study; personal fees from Astra‐Zeneka, GSK, Novartis, Teva, Vectura, UCB, Takeda, Roche, Janssen, Medimmune, Chiesi,.
LK reports grants and personal fees from Allergopharma, MEDA/Mylan, LETI Pharma, Sanofi, grants from Stallergenes, Quintiles, ASIT biotech, grants from ALK Abelló, Lofarma, AstraZeneca, GSK, Inmunotk, personal fees from Allergy Therapeut., HAL Allergie, Cassella med; and Membership: AeDA, DGHNO, Deutsche Akademie für Allergologie und klinische Immunologie, HNO‐BV, GPA, EAACI.
PK reports personal fees from Adamed, Berlin Chemie Menarini, Boehringer Ingelheim, AstraZeneca, Lekam, Novartis, Polpharma, GSK, Polpharma, Sanofi, teva.
VK reports other from GSK, non‐financial support from Mylan, AstraZeneca, Dimuna, Norameda.
SL reports personal fees from DBV, Sanofi Aventis, Allergopharma, ALK, Nutricia, Bencard.
EM reports personal fees from Sanofi, Novartis, AstraZeneca and Chiesi.
JM reports personal fees and other from SANOFI‐GENZYME & REGENERON, NOVARTIS, ALLAKOS, MITSUBISHI‐TANABE, MENARINI, UCB, ASTRAZENECA, GSK, MSD, grants and personal fees from MYLAN‐MEDA Pharma, URIACH Group.
MO reports personal fees from Hycor Diagnostics, Thermo Fisher Phadia.
YO reports personal fees from Torii Pharmaceutical Co., Ltd., Shionogi Pharmaceutical Co.,Ltd.
OP received research grants from Inmunotek S.L., Novartis and MINECO and has received fees for giving scientific lectures or participation in Advisory Boards from: Allergy Therapeutics, Amgen, AstraZeneca, Diater, GlaxoSmithKline, S.A, Inmunotek S.L, Novartis, Sanofi‐Genzyme and Stallergenes.
NGP reports personal fees from Novartis, Nutricia, HAL, MENARINI/FAES FARMA, SANOFI, MYLAN/MEDA, BIOMAY, AstraZeneca, GSK, MSD, ASIT BIOTECH, Boehringer Ingelheim, grants from Gerolymatos International SA, Capricare.
OP reports grants and personal fees from ALK‐Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, ASIT Biotech Tools S.A., Laboratorios LETI/LETI Pharma, Anergis S.A., Glaxo Smith Kline, grants from Biomay, Circassia, Pohl‐Boskamp, Inmunotek S.L., personal fees from MEDA Pharma/MYLAN, Mobile Chamber Experts (a GA2LEN Partner), Indoor Biotechnologies, Astellas Pharma Global, EUFOREA, ROXALL Medizin, Novartis, Sanofi‐Aventis and Sanofi‐Genzyme, Med Update Europe GmbH, streamedup! GmbH, John Wiley and Sons, AS.
DPreports grants and personal fees from GlaxoSmithKline, personal fees from Menarini, Pliva, Belupo, AbbVie, Novartis, MSD, Chiesi, Revenio, personal fees and non‐financial support from Boehringer Ingelheim, non‐financial support from Philips.
MR is on the Advisory board‐ A. Menarini ‐ Speaker ‐ Astra Zeneca, Novartis, Sanofi, Mylan.
FSRreports speaker and advisory fees from AstraZeneca, Novartis, Sanofi, GSK, Teva and Lusomedicamenta.
GR reports payment to his Institution from Allergo Pharma.
BSreports personal fees from Allergopharma, during the conduct of the study; grants from National Health Programm, grant, personal fees from Polpharma, ASTRA, personal fees from Mylan, Adamed, patient ombudsman, national Centre for Research and Development, Polish Allergology Society.
JS reports grants and personal fees from Sanofi, personal fees from GSK, Novartis, Astra Zeneca, Mundipharma, Faes Farma.
GS reports personal fees from ALK, and leds on the BSACI Rhinitis Guidelines and lead for EUFOREA on Allergic Rhinitis.
PSG reports personal fees from Allergopharma, ALK, grants from Bencard, grants and personal fees from Stallergenes.
JS reports personal fees from Mylan, F2F events.
ATB reports grants and personal fees from Teva, AstraZeneca, GSK Sanofi, Mundipharma, personal fees from Bial, Novartis.
MJTreports grants from European Commission, SEAIC, ISCIII, personal fees from Diater laboratory, Leti laboratory, Aimmune Therapeutics.
MW reports personal fees from ALK‐Abello, Allergopharma, AstraZeneca, Bencard, Genzyme, GlaxoSmithKline, HAL Allergy, LETI, Meda Pharma, Novartis, Sanofi, Stallergenes, Teva.
DW reports other from Optinose, ALK, Sanofi; past Co‐Chair of the Joint Task Force on Practice Parameters of the AAAAI and ACAAI. Second author of a recently published practice parameter on Rhinitis.
MW reports other from Aralez (Medexus), Pediapharm, Pfizer, Astra Zeneca, GSK, Alk.
MZ reports personal fees from Takeda.
TZ reports and Organizational affiliations: Commitee member: WHO‐Initiative “Allergic Rhinitis and Its Impact on Asthma” (ARIA). Member of the Board: German Society for Allergy and Clinical Immunology (DGAKI). Head: European Centre for Allergy Research Foundation (ECARF). Secretary General: Global Allergy and Asthma European Network (GA2LEN). Member: Committee on Allergy Diagnosis and Molecular Allergology, World Allergy Organization (WAO).


