Abstract
Background
Initially, three-dose schedules were recommended for vaccines against human papillomavirus (HPV); subsequently recommendations have been updated to a schedule of two doses delivered at least six (minimum five) months apart for those aged <15 years at dose 1. We aimed to re-estimate effective HPV vaccination coverage in Australia, considering reduced-dose schedules and possible one-dose effectiveness. We also aimed to identify which of the three school visits was most commonly missed amongst two-dose only recipients, to inform optimal timing of visits.
Methods
National vaccination register data were used to estimate: i) vaccination coverage at December 2017, either with a complete course (three or two sufficiently-spaced doses (>151 days apart)), or at least one dose; ii) for each birth cohort offered vaccination, the percentage of the initially targeted cohort with a complete course, or at least one dose (reflecting uptake at the time the vaccine was offered); and iii) among two-dose only recipients, the percentage who missed each of three school visits.
Results
Including those with two sufficiently-spaced doses increased end-2017 coverage by 1.3–2.8% points in those vaccinated at school. Including those with at least one dose increased coverage further, by 6.5–9.5% points, mostly due to including those receiving multiple too-closely-spaced doses. One-dose coverage reached 90.9% and 86.9% in females and males respectively born in 2002.
Among those vaccinated at school who received only two doses, it was much more common to miss the first (31.0% females; 32.5% males) or the third visit in the school year (54.6% females; 48.6% males) than the second (14.1% females; 18.8% males).
Conclusions
Including those with two sufficiently-spaced doses has a very modest impact on HPV vaccine coverage in Australia. If receiving at least one dose offers substantial protection, these data suggest that the school-based program is now achieving close to 90% coverage on this measure.
Keywords: HPV, Vaccination, Immunization, Papillomavirus, Coverage, Immunization Schedule
Highlights
-
•
HPV coverage data in Australia does not take into account valid 2-dose courses.
-
•
Including two sufficiently-spaced HPV doses had only a modest impact on coverage.
-
•
Over 60% of 2-dose recipients had doses too closely spaced for a 2-dose schedule.
-
•
One-dose coverage reached 90.9%/86.9% in females/males born in 2002.
-
•
Two-dose only recipients most commonly missed the third or first school visit.
1. Introduction
Vaccines against human papillomavirus (HPV) were originally licensed with three-dose schedules (scheduled at 0,1–2,6 months spacing); however subsequent data suggested that two doses in younger adolescents were similarly immunogenic as three doses in young women, provided the doses were sufficiently spaced [1,2]. Consequently, a vaccine schedule of two doses delivered at least six (and minimum of five) months apart is now recommended for those who receive dose one when aged <15 years [1,2]. Data from an Indian cohort study also suggest that two doses spaced at least 6 months apart in 15–18 year olds are sufficiently immunogenic and efficacious against targeted type HPV infections [3]. As in many countries, Australia commenced its National HPV Vaccination Program (NHVP) based on a three-dose schedule, but has since transitioned to a two-dose schedule (commencing in February 2018; start of school year, concurrent with a switch from quadrivalent to nonavalent HPV vaccine). Dose-specific coverage was routinely reported prior to 2018, but two-dose coverage prior to 2018 does not reflect whether the doses were delivered far enough apart for them to meet the current criteria for acceptable two-dose spacing.
The aim of this study was to produce updated estimates of completed vaccine courses (either three doses, or two sufficiently-spaced doses), and to characterise the timing between doses among those who received only two doses in Australia when the three-dose schedule was in place (“two-dose recipients”). This information provides estimates of the proportion of the population who could retrospectively be considered adequately protected against HPV6/11/16/18, and insight into which doses tended to be missed in the school-based program when the three-dose schedule was used. We also report coverage with at least one dose over time, given accumulating evidence that one dose may provide significant protection [[4], [5], [6], [7]].
2. Methods
Data were extracted from the National HPV Vaccination Program Register (NHVPR; doses administered by December 31, 2017 and recorded on the NHVPR by August 8, 2018). The NHVPR systematically collected notifications of HPV vaccine doses delivered in school programs from all eight jurisdictions across Australia, and voluntary notifications of doses given through primary care. Completeness of recording of doses delivered through schools was extremely high given this was routinely reported to the register [8]. These comprise the majority of doses, as the routine program (for girls aged 12–13 years since 2007, and for same-age boys since 2013) is delivered through schools, as was a large component of the catch-up program for girls (aged up to 18, over 2007–2008) and boys (aged up to 15, over 2013–2014). Doses delivered through primary care (during the catch-up program for women aged up to 26, over 2007–2009, or for doses missed at school) are known to be under-reported to the NHVPR. Under-reporting of the third dose for women aged 18–26 years during the initial catch up program has been estimated at approximately 14% [9].
Coverage of completed courses included individuals receiving either three doses at accepted minimum intervals in compliance with guidelines [10], or two doses spaced at least 152 days (five months) apart. Partially vaccinated individuals were separated into those who received one dose only, or multiple doses that were too close. Coverage was calculated in two ways – firstly as a percent of the initially targeted cohort to reflect uptake at the time the vaccine was offered, and therefore the effectiveness of program delivery. The initially targeted cohort was defined as the estimated resident population (ERP) in the year the cohort turned 12, or the ERP in the year they first became eligible for vaccination (2007 for females; 2013 for males), for those who were already older than 12 when this occurred. Secondly, coverage was calculated as a proportion of the ERP in 2017 providing a snapshot of population coverage at the end of 2017, noting annual net immigration in the targeted cohorts. Data are provided only for cohorts who had turned 15 by the end of 2017, in accordance with the WHO standard method that reports coverage achieved by age 15. This takes into account variations in the age of individuals in the school grade that vaccination is offered, and allows time for catch-up doses outside of school to be captured [11].
To explore whether particular dose visits were more likely to be missed, we examined the month when each dose was received by two-dose recipients who were aged <15 at dose 1, in relation to when each dose was typically delivered through the three-dose school-based program (February–March [dose 1 visit]; May [dose 2 visit]; September/October [dose 3 visit]), based on required spacing, and timing of term breaks (Supplementary Figure 1). “Dose periods” were defined as the period starting from the visit when a particular dose was delivered in school until just before the next school visit for the subsequent dose (and therefore includes doses that were missed at school, but where the individual received a catch-up dose in the community prior to the next school visit). Those who received their first dose any time outside of the dose 1 period (February–April) were assumed to have missed the dose offered at the first school visit. Those who received their second and final dose in the dose 2 period (May–August) were assumed to have subsequently missed the dose offered at the third school visit. All others were assumed to have missed the dose 2 visit. The assumed timing of school visits for each dose was varied to see how this affected the findings. Females born in 1992 were excluded from this analysis as an accelerated schedule (0,1,4 months, commencing in April) was used in 2007 [12].
3. Results
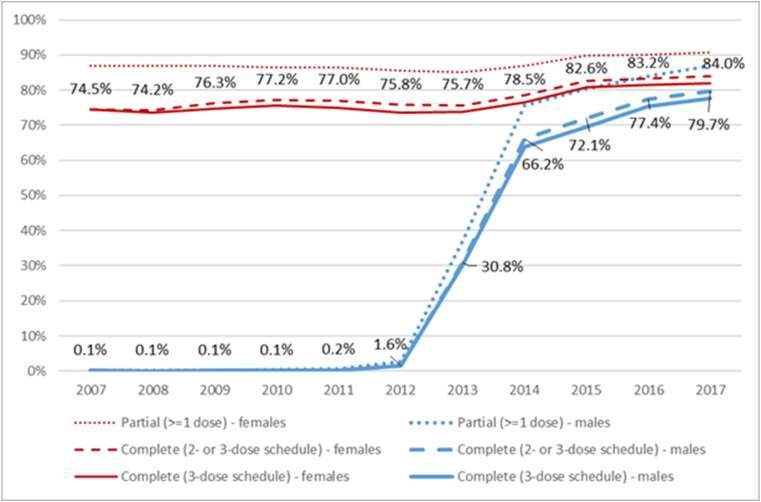
In cohorts offered HPV vaccination before age 15, including those who completed a valid two-dose course increased estimated coverage by 0.7–2.3% points in females and 1.9–2.7% points in males (Fig. 1, Supplementary Table 1). Including those who received either only one dose or multiple too-closely-spaced doses had a greater incremental effect on coverage, increasing coverage by a further 6.8–12.6% and 6.7–9.4% points in females and males respectively, reaching 90.9% and 86.9% in females and males respectively born in 2002.
Fig. 1.
Cumulative estimated HPV vaccine uptake among those turning 15 in that year, as a proportion of the population eligible at the target age (12 years)*, by sex and completion status, Australia (National HPV Vaccination Program Register data, as held August 8, 2018)
Cumulative uptake is presented as a proportion of those who were in Australia when aged 12 (and therefore eligible at the target age), in order to reflect effectiveness of program delivery. Labels are estimates of cumulative uptake of completed vaccine courses (valid 2- or 3-dose schedule). See also Supplementary Table 1.
* For those who were already aged >12 when the vaccine was first available through the National HPV Vaccination Program, uptake is as a proportion of the estimated resident population in the year the vaccine was first offered (2007 for females; 2013 for males).
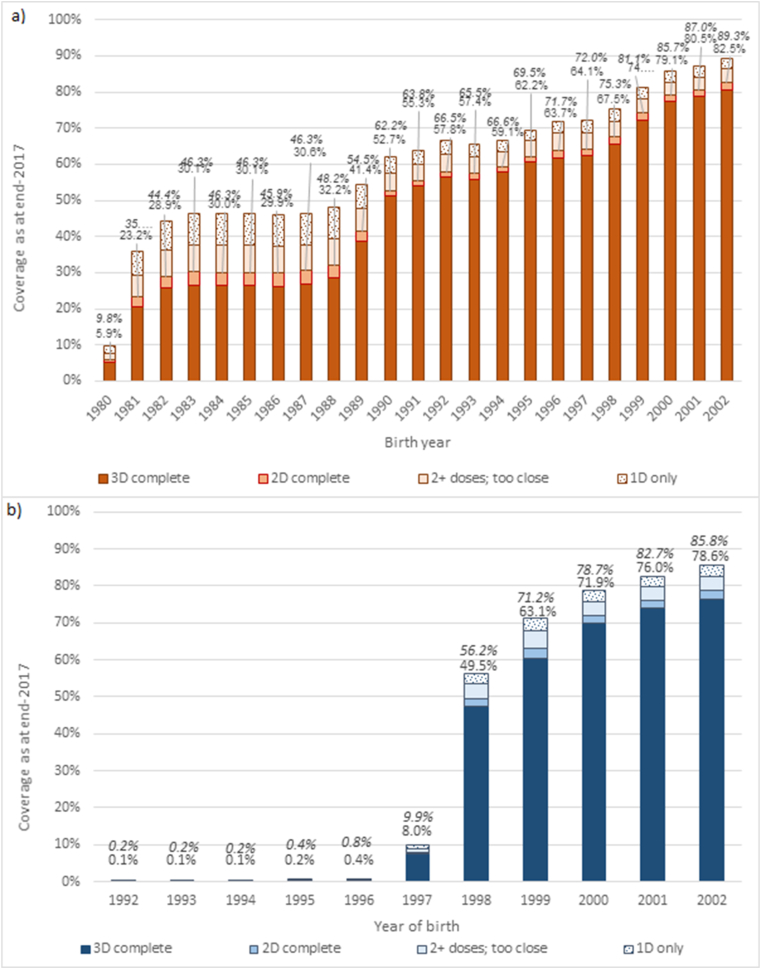
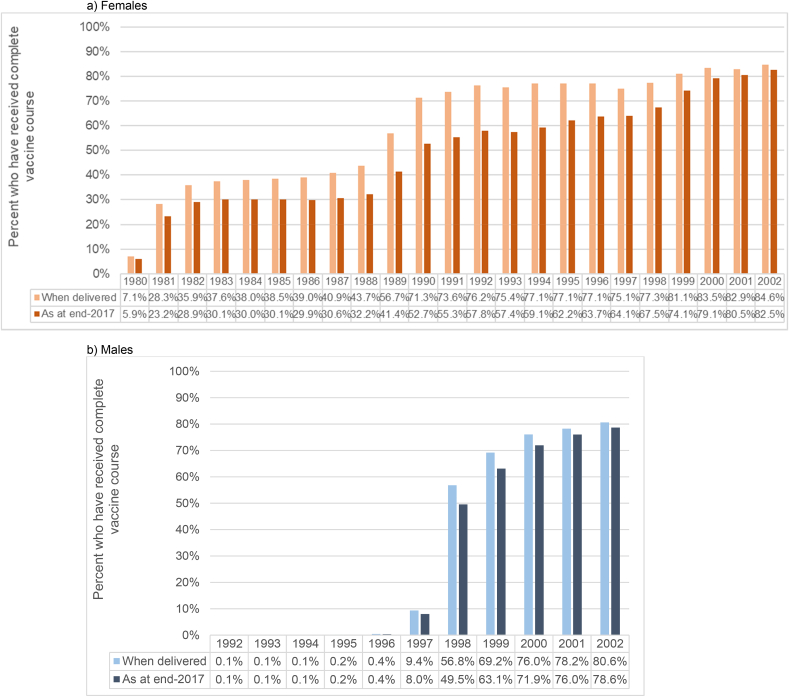
Fig. 2 shows estimated coverage (as at end-2017) by birth cohort, including completed three-dose courses and those with two sufficiently spaced doses (regardless of age at dose 1). Coverage for each birth cohort is lower than that shown in Fig. 1, due to net migration into Australia increasing the size of the ERP. Considering this wider number of birth cohorts, and how coverage is diluted over time due to net migration, including those who completed a sufficiently-spaced two-dose course increased estimated coverage by approximately 1.3–2.8% points for females and males offered vaccination at school, and 0.6–3.9% points in females aged 18 or older in 2007 predominantly offered vaccination through primary care (Supplementary Table 2). Taking into account coverage with any number of doses, coverage estimates rose by a further 6.5–9.5% points for females and males offered vaccination at school and 3.9–16.3% points in females aged 18 or older in 2007, compared to completion with either a sufficiently-spaced two- or three-dose course. Around half of this increase was due to including those who received one dose only, and half due to including those with multiple doses that were too closely spaced to meet the completion criteria for two- or three-dose schedules, but this varied for females and males offered vaccination at school (52–61% of the increase from including those with two too-close doses) and females predominantly offered vaccination through primary care (52–55% increase from one dose only) (Supplementary Table 3). A direct comparison between uptake when delivered and coverage as at end-2017, to show how coverage is diluted over time due to net migration, is shown in Fig. 3 (completed courses) and Supplementary Figure 2 (at least one dose).
Fig. 2.
Estimated HPV Vaccination Coverage as at end 2017, by sex and birth cohort.
Coverage is presented as a proportion of the estimated resident population at end-2017. Displayed values are coverage with completed vaccine courses (2- or 3-dose schedule spacing) in plain text, and coverage with at least one dose in italics. See also Supplementary Table 2.
Fig. 3.
Comparison of vaccine uptake (completed courses) when delivered and coverage as at end-2017, by sex and birth cohort.
The median timing between dose 1 and 2 among two-dose recipients was 112–121 days among females aged 18 or older in 2007 (predominantly vaccinated in primary care); and 59–111 days among females aged 17 or younger in 2007 (offered vaccination at school); but somewhat longer in males (predominantly offered vaccination at school; 114 days)(Supplementary Figure 3). Approximately one third of two-dose recipients had doses that were adequately spaced for a two-dose delivery schedule. When stratifying by sex and whether the group was predominantly offered vaccination at school versus in primary care, the percentage of two-dose recipients with doses at least 152 days apart was 33.3% among females aged 18 or older in 2007 (predominantly vaccinated in primary care); 28.1% among females aged 17 or younger in 2007 (offered vaccination at school); and 35.4% in males (predominantly offered vaccination at school).
Among two-dose recipients, 69.0% of females and 67.5% of males received their first dose at the expected time (February–April). The remainder (31.0% females; 32.5% males) who received their first dose in a later month potentially missed the first school visit (Table 1). Dose 2 was received at the expected time (May–August) by 54.6% and 48.6% of female and male two-dose recipients respectively, suggesting this group missed the third-dose visit. Sensitivity analyses exploring different assumed months for visits 1, 2 and 3 tended to increase the estimated proportion who missed the first school visit (up to 45.0%) and/or third visit (up to 57.4%), while the proportion who missed the second visit was consistently smaller than for either of the other two visits.
Table 1.
Estimated percent who missed each school visit, for varying assumptions of school visit timing, among two-dose recipients offered vaccine at school at the target age (females born 1993–2002; males born 1998–2002).
| Assumed dose periods |
Missed visit 1 |
Missed visit 2 |
Missed visit 3 |
|||
|---|---|---|---|---|---|---|
| D1; D2; D3 | Females | Males | Females | Males | Females | Males |
| Feb–Apr; May–Aug; Sep or later | 31.0% | 32.5% | 14.4% | 18.8% | 54.6% | 48.6% |
| Alternative assumptions | ||||||
| Feb–Apr; May–Sep; Oct or later | 31.0% | 32.5% | 5.5% | 10.1% | 63.5% | 57.4% |
| Feb–Apr; May–Jul; Aug or later | 31.0% | 32.5% | 20.7% | 24.1% | 48.3% | 43.3% |
| Jan–Apr; May–Aug; Sep or later | 30.1% | 31.8% | 15.3% | 19.6% | 54.6% | 48.6% |
| Feb–Apr; May-0.5 Sep; 0.5 Sep or later | 31.0% | 32.5% | 10.0% | 14.5% | 59.0% | 53.0% |
| Feb–Mar; Apr–Jul; Aug or later | 43.5% | 45.0% | 2.4% | 8.1% | 54.1% | 46.9% |
| Mar–Apr; May–Aug; Sep or later | 47.2% | 48.0% | −1.8% | 3.4% | 54.6% | 48.6% |
| Feb–Mar; Apr–Aug; Sep or later | 43.5% | 45.0% | −3.8% | 2.8% | 60.3% | 52.2% |
| Feb–Mar; Apr–Sep; Oct or later | 43.5% | 45.0% | −12.8% | −5.9% | 69.3% | 60.9% |
“Dose periods” were defined as the period starting from the visit when a particular dose was delivered in school until just before the next school visit for the subsequent dose (and therefore includes doses that were missed at school, but where the individual received a catch-up dose in the community prior to the next school visit). Those who received their first dose any time outside of the dose 1 period were assumed to have missed visit 1. Those who received their second and final dose in the dose 2 period were assumed to have subsequently missed visit 3. Percentage who missed visit 2 is the remainder after accounting for those estimated to have missed visits 1 or 3. Assumed visit timings where this percentage is < 0 are likely implausible.
4. Discussion
Including valid two-dose courses increases the estimated effective vaccine coverage in Australia by approximately two percentage points in cohorts offered vaccine at the target age. If two sufficiently-spaced doses at older ages also provide protection (as suggested by data from an Indian cohort) [3], effective coverage would be increased by up to almost four percentage points among females in birth cohorts aged 18 or older in 2007. This relatively modest increase in Australia, especially in those vaccinated at the target age, is because most two-dose recipients (approximately 69% females and 65% males) received their two doses closer than five months apart. If, as suggested by emerging evidence, one dose is sufficient to provide substantial protection, this would have a much greater effect on coverage (increasing it by up to 12.6% points compared to coverage with either a valid two- or three-dose course). In those offered vaccination at school, just over half of this increase would be a result of including those with too-closely-spaced doses (as opposed to only one dose). The relatively modest increase in coverage from two sufficiently-spaced doses, and the more substantial increase from including those who received multiple too-closely-spaced doses is understandable, as school doses were delivered approximately in line with a three-dose schedule of 0, 2, and 6 months. Therefore, among those who received both doses at school in the same calendar year, generally only those who missed the second school visit would have their doses sufficiently spaced to meet the two-dose completion criteria. This appeared to be the visit that was least likely to be missed in Australia, based on the month that doses were received in two-dose recipients.
A previous US study also reported a fairly modest effect on coverage from including valid two-dose courses, although there the effect increased over time and was larger in the period after the WHO recommended a two-dose schedule [13]. The US study reported that most (61.4%) of those who received only two vaccine doses received them at least five months apart, whereas the reverse was true in Australia. This difference likely reflects the very different delivery models in Australia (predominantly through schools) and the US (through primary care), as the timing of dose delivery in Australia will be clustered around the timing of school visits, which were usually less than five months apart prior to 2018.
The strengths of the current analysis include that it is based on a comprehensive national routine data collection, and includes several years of routine school-based vaccination prior to the introduction of the two-dose schedule. Completeness of recording of doses delivered through schools is very high [8], although a weakness is that completeness is known to be poorer for doses delivered through primary care, especially prior to mid-2008 when the NHVPR was established [9]. Incomplete recording on the NHVPR could have led to underestimates of the number of people with two sufficiently-spaced doses, but also could have led to underestimates of people who received three doses. These effects would work in opposite directions in terms of the incremental coverage from including those with valid two-dose courses, and so the overall effect is uncertain, but less likely to affect findings for the routinely-vaccinated cohorts who are mostly vaccinated at school.
Our findings also indicate that school-based delivery is highly effective, reaching close to 90% of the initially targeted cohort by age 15 with at least one dose, but also that coverage is diluted fairly substantially over time due to net migration into Australia. This is broadly consistent with earlier findings [9], but the extent of the difference has widened over time (for example by up to 18.1% points in females born in 1990, reducing apparent three-dose coverage from 69.2% to 51.1%). This potentially has implications for herd effects from vaccination if migrants are largely unvaccinated. From the perspective of cancer prevention, the effects are likely to be most important before around age 19–23, as mathematical models, which can give insight into otherwise unobservable processes, suggest that most women who eventually develop cervical cancer acquire the causal infection before that age [14]. The expansion to the National Immunisation Program in July 2017 to fund catch-up HPV vaccination up to age 19 for those who missed it at school may reduce this effect in future, as would increasing uptake of HPV vaccine in migrants' countries of origin, which could be expected as a consequence of the WHO's call to global action to eliminate cervical cancer as a public health problem [15].
Our exploration of the months in which doses were received by those who only received two doses suggest that it was much more common to miss the first (~32%) or the third school visit (~52%) than the second (~17%). This finding – that both the first and third school visits were commonly missed by two-dose recipients-may provide insights that remain relevant even now that Australia transitioned to a two-dose schedule from the 2018 school year – for example, that the first school visit should preferably not occur too early in the year. Potential reasons for not receiving the first vaccine dose at the first school visit could be that consent forms had not yet been returned. If the tendency to miss the third school visit was due to increasing absenteeism towards the end of the school year, rather than reluctance to receive a third injection, it is plausible that uptake of the now later scheduled second dose in the current two-dose schedule may be similarly affected. Data reporting the uptake of the two-dose nonavalent HPV vaccine course are awaited from the Australian Immunisation Register, which now records HPV vaccination data. In the interim, our estimates may provide insight into the likely coverage in the two-dose program.
In conclusion, including those with two sufficiently-spaced doses has a very modest impact on effective HPV vaccine coverage in Australia under the three-dose schedule, as around two thirds of two-dose recipients received their doses within five months. Increases in coverage would be more substantial if receiving at least one dose offers substantial protection. These data suggest that the school-based program is now achieving close to 90% coverage in the target cohort on this measure.
Declaration of competing interest
MAS reports grants from the National Health and Medical Research Council (Australia) and the Cancer Institute NSW, during the conduct of the study. KC reports grants from the National Health and Medical Research Council (Australia) and is co-principal investigator of an unrelated investigator-initiated trial of cervical screening in Australia (Compass; ACTRN12613001207707 and NCT02328872), which is conducted and funded by the VCS Foundation, a government-funded health promotion charity; the VCS Foundation received equipment and a funding contribution from Roche Molecular Systems and Ventana USA, but neither KC or her institution on her behalf have received direct funding from industry for this trial or any other project. JMLB was an investigator on investigator-initiated research grants that provided funding for laboratory testing for a study of cervical cancers (Seqirus) and recurrent respiratory papillomatosis (MSD) more than three years ago, but has never received personal financial benefits.
KW has no relevant interests to declare.
Acknowledgments
The National HPV Vaccination Program Register was owned and funded by the Australian Department of Health and operated by the VCS Foundation. The Department of Health permitted publication of the data in this article but played no role in its preparation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tvr.2021.200216.
Funding
Megan Smith receives salary support from the National Health and Medical Research Council (APP1159491) and Cancer Institute NSW (ECF181561).
Credit author statement
Megan Smith: Conceptualization, Methodology, Formal analysis, Writing – original draft, Funding acquisition. Karen Winch: Formal analysis, Data curation, Writing – review & editing, Karen Canfell: Writing – review & editing, Julia Brotherton: Conceptualization, Methodology, Writing – review & editing
Ethics
The Australian Department of Health approved the release of aggregated data from the National HPV Vaccination Program Register used in this analysis. As only aggregated data were used, ethics approval was not required.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization Human papillomavirus vaccines: WHO position paper, May 2017. Wkly. Epidemiol. Rec. 2017;92:241–268. [Google Scholar]
- 2.World Health Organization Meeting of the strategic advisory group of experts on immunization, April 2014 – conclusions and recommendations. Wkly. Epidemiol. Rec. 2014;89:221–236. [PubMed] [Google Scholar]
- 3.Basu P., Muwonge R., Bhatla N. Two-dose recommendation for Human Papillomavirus vaccine can be extended up to 18 years - updated evidence from Indian follow-up cohort study. Papillomavirus Res. 2019;7:75–81. doi: 10.1016/j.pvr.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreimer A.R., Rodriguez A.C., Hildesheim A. Proof-of-Principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J. Natl. Cancer Inst. 2011;103:1444–1451. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brotherton J.M., Budd A., Rompotis C. Is one dose of human papillomavirus vaccine as effective as three?: a national cohort analysis. Papillomavirus Res. 2019;8:100177. doi: 10.1016/j.pvr.2019.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markowitz L.E., Drolet M., Perez N. Human papillomavirus vaccine effectiveness by number of doses: systematic review of data from national immunization programs. Vaccine. 2018;36:4806–4815. doi: 10.1016/j.vaccine.2018.01.057. [DOI] [PubMed] [Google Scholar]
- 7.Sankaranarayanan R., Joshi S., Muwonge R. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. 2018;36:4783–4791. doi: 10.1016/j.vaccine.2018.02.087. [DOI] [PubMed] [Google Scholar]
- 8.Brotherton J.M., Murray S.L., Hall M.A. Human papillomavirus vaccine coverage among female Australian adolescents: success of the school-based approach. Med. J. Aust. 2013;199:614–617. doi: 10.5694/mja13.10272. [DOI] [PubMed] [Google Scholar]
- 9.Brotherton J.M.L., Liu B., Donovan B. Human papillomavirus (HPV) vaccination coverage in young Australian women is higher than previously estimated: independent estimates from a nationally representative mobile phone survey. Vaccine. 2014;32:592–597. doi: 10.1016/j.vaccine.2013.11.075. [DOI] [PubMed] [Google Scholar]
- 10.Chief Medical Officer . Australian Government Department of Health and Ageing; Canberra: 2009. Guidance on Revaccination where HPV Vaccine Doses Have Been Given at Less than Recommended Minimum Intervals.http://www1.health.gov.au/internet/immunise/publishing.nsf/Content/D5F6C9E0EEAE74FFCA25770300807AA3/$File/CMO-full-advice-hpv.pdf [Google Scholar]
- 11.Brotherton J.M.L., Bloem P.N. Population-based HPV vaccination programmes are safe and effective: 2017 update and the impetus for achieving better global coverage. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;47:42–58. doi: 10.1016/j.bpobgyn.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Brotherton J., Deeks S., Campbell-Lloyd S. Interim estimates of human papillomavirus vaccination coverage in the school-based program in Australia. Commun Dis Intell Q Rep. 2008;32:457–461. [PubMed] [Google Scholar]
- 13.Lin X., Rodgers L., Zhu L. Human papillomavirus vaccination coverage using two-dose or three-dose schedule criteria. Vaccine. 2017;35:5759–5761. doi: 10.1016/j.vaccine.2017.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger E., de Kok I., Groene E. Estimating the natural history of cervical carcinogenesis using simulation models: a CISNET comparative analysis. J. Natl. Cancer Inst. 2020;112(9):955–963. doi: 10.1093/jnci/djz227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . WHO; Geneva: 2020. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem.https://www.who.int/publications/i/item/9789240014107 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.