Abstract
Background:
Very few studies have explored the utility of subjective cognitive complaints (SCCs) in primary care settings.
Objective:
We aim to investigate associations between SCCs (item-level), objective cognitive function (across domains and global), and mood in a diverse primary care population, including subjects with mild cognitive impairment.
Methods:
We studied 199 (75.9% females; 57.8% Hispanics; 42.2% African Americans) older adults (mean age 72.5 years) with memory concerns at a primary care clinic. A five-item SCC questionnaire, and objective cognitive assessments, including the Montreal Cognitive Assessment (MoCA) and the Geriatric Depression Scale, were administered.
Results:
Logistic regression analyses showed associations between SCC score and depressive symptoms. A memory-specific (“memory worsening”) SCC predicted scores on the MoCA (p = 0.005) in Hispanics.
Conclusion:
SCCs are strongly linked to depressive symptoms in African Americans and Hispanics in a primary care setting; a specific type of SCC is related to global cognitive function in Hispanics.
Keywords: Cognitive function, cross-sectional, depressive symptoms, primary care, subjective health complaint, underserved populations
INTRODUCTION
Subjective cognitive complaints (SCCs) are self-reported changes in cognitive function or concerns about memory that are not detected objectively through neuropsychological testing [1]. Longitudinal studies suggest that SCCs manifest before the onset of clinical impairment and may serve as early warning signs of cognitive decline [2] and dementia [3, 4]. In contrast to these studies, a recent meta-analysis of cross-sectional studies argued that comprehensive measures of SCCs are not strongly related to objective cognitive function. Instead, SCCs are more associated with depressive symptoms [5]. It is worth noting that these studies were conducted in mostly White older adult populations. This is a key limitation in the field. While depression is historically known as a component of a differential diagnosis for dementia, growing evidence suggests that new onset psychiatric symptoms may represent early stages of cognitive decline and dementia [6–8]. Thus, the onset and development of SCCs and depressive symptoms may differ. This relationship becomes more important among Hispanics and African Americans (AAs) who report higher levels of depressive symptoms [9] and are much more likely to develop Alzheimer’s disease (AD) and other dementias compared to Whites [10]. Also, Hispanics are more likely to report higher depressive symptoms in comparison with AAs [11], while AAs are more likely to develop AD or other dementias compared to Hispanics [10].
In AAs, associations between SCCs and objective cognitive function have been mixed in the literature, with some clinic-based studies reporting relationships between SCCs and current cognitive functioning and progression to dementia [12, 13]. Other community-based studies reported no cross-sectional relationships [14–16] but have reported that SCCs merely reflect depressive symptoms in a primary care setting [17]. Furthermore, in a study looking at cultural differences in SCCs, AAs endorsed fewer SCCs than Whites [14]; however, these results have been inconsistent.
For example, a study reported that Hispanic older populations report more SCCs than non-Hispanic Whites despite similar levels of cognitive impairment [18]. Furthermore, SCC endorsements in Hispanics were more correlated with depressive symptoms, but this relationship did not exist in non-Hispanic Whites [18]. Other cross-sectional studies have found stronger associations between depressive symptoms than objective cognitive function in a community-based Hispanic population [19] and in a population recruited in a primary care setting [20].
Neuropsychological tests used for assessing cognitive function may not be sensitive to detect subtle levels of cognitive impairment, while SCCs are shown to precede impairments in cognitive function identified through neurophysiological testing. This may explain why cross-sectional relationships are not observed between SCCs and cognitive function. However, the association between multiple types of item-level SCCs and domains of cognitive function has not been addressed nor compared in AAs versus Hispanics from the same catchment area in a primary care setting. Considering the relatively few studies of SCCs in AAs and Hispanic primary care populations as well as the high rates of depressive symptoms and dementia in these groups, we endeavored to examine cross-cultural comparisons of multiple validated SCCs at the item-level in AAs and Hispanics from an urban community-based primary care population. Our objectives were to: 1) investigate SCC endorsement in AAs versus Hispanics in a primary care clinic, and 2) investigate associations between SCCs (item-level), objective cognitive function (global and across domains), and depressive symptoms. On the basis of previous studies, we hypothesized that SCCs would be more strongly associated with depressive symptoms than objective cognitive function in AAs and Hispanics. The purpose of this study is to improve the understanding of and the utility of SCCs in clinical assessments of AA and Hispanic primary care populations.
METHODS
Participants
Participants were enrolled in a study based in a primary care clinic in Bronx, NY. The main aim of the study was to develop and validate a 5-min cognitive screening tool to improve dementia care in primary care patient populations when cognitive concerns are present. Participants included community-dwelling older adults with cognitive concerns (aged 65 and older) who were recruited at their primary care clinic appointment and received all study-related assessments on the same day. Recruitment was offered in the waiting area by either the research assistant, participant family member, or clinic personnel. Potential participants completed a “yes/no” 2-item questionnaire, with respect to concerns about memory at their primary care appointment and subjects were assessed on the same day. Participants were evaluated in a quiet, well-lit room by a bilingual research assistant supervised by a licensed neuropsychologist. All assessments were administered in the participant’s preferred language (Spanish or English), classified as the “assessment language.” Participants with a previous cognitive diagnosis (i.e., dementia or MCI) or who were taking prescribed anti-dementia medications reported in the electronic medical record (EMR) were excluded. For this analysis, only participants who self-identified as AA or Hispanic were included. All participants provided written, informed consent to participate. The study was approved by the institutional review board at the Albert Einstein College of Medicine, Bronx, NY.
Objective cognitive function assessment
Objective cognitive function was assessed with neuropsychological instruments that measure cognitive domains of episodic memory (Hopkins Verbal Learning Test-Revised [HVLT-total and HVLT-delayed]) [21], attention/processing speed (Symbol Digit Modalities Test [SDMT]) [22], executive function (free and copy drawings of the Clock Drawing Test [CDT free and CDT copy]) [23, 24], language (a semantic fluency “animals” test [25, 26], a phonemic fluency “FAS” test [27], the 15-item Boston Naming Test Second Version [BNT-2] [28]), and a global cognition assessment (the Montreal Cognitive Assessment [MoCA]) [29]. To create an objective cognitive composite, we utilized a well-established statistical approach to combine cognitive tests [30]. Specifically, standard scores were calculated for each objective cognitive test controlling for age, education years, and assessment language. Standard scores on individual objective cognitive tests were then summed together to create a composite score classified as “overall cognition.” Similar cognitive composites have been associated with AD-related pathology [31, 32]. Objective cognitive function tests were analyzed separately and as a composite score.
Depressive symptoms assessment
Depressive symptoms were assessed with the 30-item Geriatric Depression Scale (GDS) [33]. The GDS ranges from 0–30, with high scores signifying higher depressive symptoms.
Subjective cognitive complaint assessment
SCCs were assessed with five questions that have previously demonstrated high internal validity and associations with objective cognitive function and AD [34–36]. SCCs are numbered and listed in Figs. 1 and 2 and Table 2. SCCs 1, 2, and 4 originated from the Albert Einstein Health Self-Assessment Form [34]. SCC 3 was selected from the Cognitive Change Index (CCI) [35]. Finally, SCC 5 is a memory item selected from GDS (GDS item 14). All SCCs (except for SCC 5) were completed before objective cognitive function tests and GDS. All SCCs were analyzed separately and not as a composite. Further SCCs were classified as types: SCC 1 = age-related memory status, SCC 2 = current memory problem, SCC 3 = worsening memory, SCC 4 = non-memory cognitive concern, SCC 5 = memory worse than most people. SCCs 1, 2, 3, and 5 are memory-specific, whereas SCC 4 is a non-memory SCC.
Fig. 1.
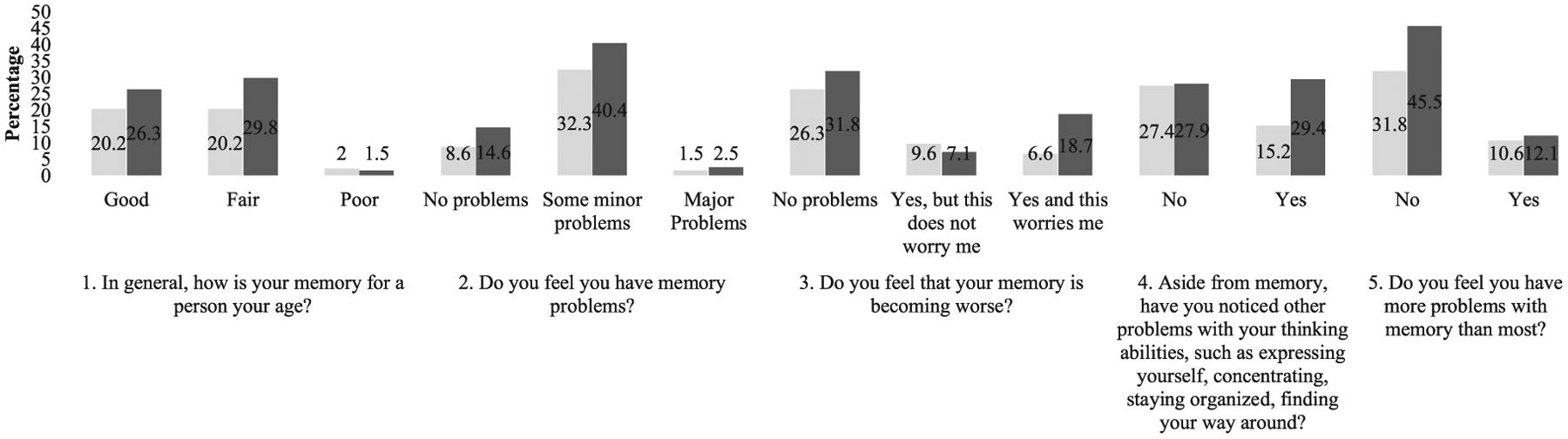
Data are presented as percentages (%) of the total (n = 199) subjective cognitive complaint responses.  African American;
African American;  Hispanic.
Hispanic.
Fig. 2.
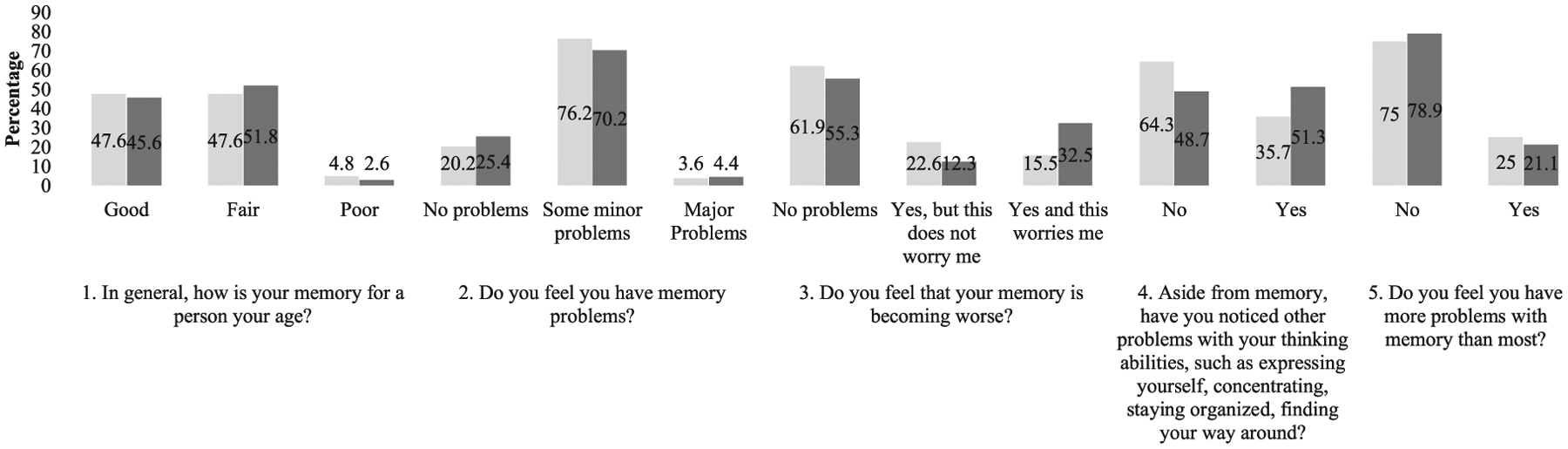
Data are presented as percentages (%) of subjective cognitive complaint responses within groups.  African American;
African American;  Hispanic.
Hispanic.
Table 2.
Odds ratios (OR), confidence intervals (95% CI), and p-values (p) for the associations between objective cognitive function, subjective cognitive complaints and depressive symptoms by race/ethnicity
| Assessment | Race/Ethnicity | OR (95% CI) | p |
|---|---|---|---|
| SCC 1. “In general, how is your memory for a person your age?” | |||
| GDS | AA | 1.148 (1.043–1.264) | 0.005* |
| Hispanic | 1.115 (1.040–1.194) | 0.002* | |
| MoCA | AA | 1.030 (0.914–1.161) | 0.626 |
| Hispanic | 0.905 (0.807–1.016) | 0.092 | |
| HVLT-total | AA | 1.031 (0.920–1.156) | 0.601 |
| Hispanic | 1.031 (0.935–1.136) | 0.540 | |
| HVLT-delayed | AA | 1.085 (0.884–1.332) | 0.433 |
| Hispanic | 0.980 (0.836–1.150) | 0.806 | |
| SDMT | AA | 1.038 (0.990–1.088) | 0.120 |
| Hispanic | 0.968 (0.921–1.017) | 0.190 | |
| Semantic fluency | AA | 0.982 (0.868–1.111) | 0.775 |
| Hispanic | 0.978 (0.887–1.079) | 0.659 | |
| Phonemic fluency | AA | 1.003 (0.961–1.046) | 0.896 |
| Hispanic | 0.969 (0.928–1.012) | 0.154 | |
| CDT free | AA | 1.063 (0.707–1.598) | 0.770 |
| Hispanic | 0.719 (0.511–1.011) | 0.058 | |
| CDT copy | AA | 0.812 (0.485–1.358) | 0.427 |
| Hispanic | 0.850 (0.570–1.268) | 0.425 | |
| Overall cognition | AA | 1.059 (0.946–1.185) | 0.323 |
| Hispanic | 0.972 (0.880–1.074) | 0.575 | |
| SCC 2. “Do you feel you have memory problems?” | |||
| GDS | AA | 1.102 (1.045–1.383) | 0.010 |
| Hispanic | 1.109 (1.021–1.205) | 0.014 | |
| MoCA | AA | 1.079 (0.921–1.264) | 0.348 |
| Hispanic | 0.997 (0.876–1.133) | 0.958 | |
| HVLT-total | AA | 1.042 (0.899–1.207) | 0.914 |
| Hispanic | 1.006 (0.901–1.124) | 0.983 | |
| HVLT-delayed | AA | 0.997 (0.772–1.287) | 0.983 |
| Hispanic | 0.934 (0.778–1.121) | 0.462 | |
| SDMT | AA | 1.010 (0.952–1.073) | 0.734 |
| Hispanic | 0.996 (0.943–1.053) | 0.895 | |
| Semantic fluency | AA | 1.005 (0.854–1.182) | 0.954 |
| Hispanic | 1.011 (0.906–1.128) | 0.850 | |
| Phonemic fluency | AA | 1.019 (0.964–1.077) | 0.501 |
| Hispanic | 1.028 (0.978–1.081) | 0.271 | |
| CDT free | AA | 1.044 (0.602–1.811) | 0.878 |
| Hispanic | 1.044 (0.723–1.506) | 0.820 | |
| CDT copy | AA | 0.630 (0.317–1.254) | 0.189 |
| Hispanic | 0.880 (0.557–1.391) | 0.585 | |
| Overall cognition | AA | 1.046 (0.911–1.202) | 0.520 |
| Hispanic | 1.003 (0.895–1.125) | 0.955 | |
| SCC 3. “Do you feel that your memory is becoming worse?” | |||
| GDS | AA | 1.148 (1.039–1.269) | 0.007* |
| Hispanic | 1.125 (1.053–1.202) | 0.001* | |
| MoCA | AA | 1.051 (0.928–1.191) | 0.434 |
| Hispanic | 0.836 (0.738–0.947) | 0.005* | |
| HVLT-total | AA | 1.026 (0.913–1.153) | 0.667 |
| Hispanic | 0.976 (0.889–1.072) | 0.618 | |
| HVLT-delayed | AA | 1.080 (0.874–1.334) | 0.478 |
| Hispanic | 0.902 (0.770–1.056) | 0.199 | |
| SDMT | AA | 1.032 (0.985–1.082) | 0.186 |
| Hispanic | 0.965 (0.919–1.013) | 0.151 | |
| Semantic fluency | AA | 1.079 (0.949–1.227) | 0.245 |
| Hispanic | 0.958 (0.869–1.055) | 0.382 | |
| Phonemic fluency | AA | 1.029 (0.985–1.075) | 0.197 |
| Hispanic | 0.941 (0.899–0.986) | 0.010 | |
| CDT free | AA | 1.304 (0.852–1.995) | 0.222 |
| Hispanic | 0.661 (0.472–0.927) | 0.016 | |
| CDT copy | AA | 0.954 (0.565–1.613) | 0.861 |
| Hispanic | 0.817 (0.553–1.205) | 0.307 | |
| Overall cognition | AA | 1.086 (0.967–1.220) | 0.163 |
| Hispanic | 0.906 (0.817–1.005) | 0.063 | |
| SCC 4 “Aside from memory, have you noticed other problems with your thinking abilities, such as expressing yourself, concentrating, staying organized, finding your way around?” | |||
| GDS | AA | 1.096 (1.001–1.200) | 0.047 |
| Hispanic | 1.265 (1.147–1.395) | <0.001* | |
| MoCA | AA | 0.875 (0.767–0.997) | 0.045 |
| Hispanic | 0.976 (0.872–1.093) | 0.983 | |
| HVLT-total | AA | 0.999 (0.889–1.122) | 0.983 |
| Hispanic | 1.025 (0.929–1.131) | 0.620 | |
| HVLT-delayed | AA | 0.979 (0.796–1.204) | 0.842 |
| Hispanic | 0.996 (0.848–1.171) | 0.965 | |
| SDMT | AA | 1.033 (0.985–1.084) | 1.000 |
| Hispanic | 1.000 (0.951–1.051) | 0.704 | |
| Semantic fluency | AA | 1.025 (0.904–1.161) | 0.704 |
| Hispanic | 0.953 (0.861–1.055) | 0.355 | |
| Phonemic fluency | AA | 1.000 (0.958–1.044) | 0.993 |
| Hispanic | 0.969 (0.927–1.013) | 0.169 | |
| CDT free | AA | 1.148 (0.757–1.741) | 0.517 |
| Hispanic | 0.757 (0.538–1.065) | 0.110 | |
| CDT copy | AA | 0.796 (0.473–1.339) | 0.390 |
| Hispanic | 0.863 (0.576–1.291) | 0.472 | |
| Overall cognition | AA | 1.019 (0.911–1.139) | 0.746 |
| Hispanic | 1.000 (0.906–1.104) | 1.000 | |
| SCC 5 “Do you feel you have more problems with memory than most?” | |||
| GDS | AA | 1.208 (1.074–1.358) | 0.002* |
| Hispanic | 1.280 (1.137–1.442) | <0.001* | |
| MoCA | AA | 1.002 (0.873–1.151) | 0.973 |
| Hispanic | 0.924 (0.796–1.071) | 0.294 | |
| HVLT-total | AA | 0.977 (0.856–1.115) | 0.733 |
| Hispanic | 0.938 (0.826–1.065) | 0.324 | |
| HVLT-delayed | AA | 1.007 (0.800–1.267) | 0.954 |
| Hispanic | 0.942 (0.766–1.158) | 0.570 | |
| SDMT | AA | 1.030 (0.976–1.085) | 0.281 |
| Hispanic | 0.999 (0.938–1.064) | 0.978 | |
| Semantic fluency | AA | 0.918 (0.808–1.043) | 0.190 |
| Hispanic | 0.975 (0.925–1.027) | 0.340 | |
| Phonemic fluency | AA | 0.975 (0.925–1.027) | 0.340 |
| Hispanic | 0.968 (0.915–1.023) | 0.251 | |
| CDT free | AA | 0.844 (0.532–1.340) | 0.473 |
| Hispanic | 0.868 (0.590–1.276) | 0.471 | |
| CDT copy | AA | 1.842 (0.961–3.531) | 0.066 |
| Hispanic | 0.874 (0.535–1.429) | 0.591 | |
| Overall cognition | AA | 0.966 (0.853–1.095) | 0.590 |
| Hispanic | 0.960 (0.843–1.094) | 0.541 | |
Logistic regressions were adjusted for age, sex, education, and assessment language,
p < 0.01. AA, African American; GDS, Geriatric Depression Scale; MoCA, Montreal Cognitive Assessment; HVLT, Hopkins Verbal Learning Test; SDMT, Symbol Digit Modalities Test; CDT, Clock Drawing Test.
Data analysis
Participants’ demographic characteristics, objective cognitive function scores, and SCCs were compared between AAs and Hispanics using independent samples t-test for continuous variables, and Pearson’s chi-square tests for categorical variables. Independent samples t-tests were conducted after using Levene’s test for equality of variances. Where necessary, unequal-variance t-tests were used to alleviate heteroscedasticity. Some participants did not complete all assessments; therefore, to account for missing data, we included the number of participants who completed each test in Table 1. To investigate mean differences in depressive symptoms and objective cognitive function by SCCs, we ran ANOVAs (stratified by race/ethnicity) with GDS and objective cognitive function tests as dependent variables. To examine associations as a function of race/ethnicity, we performed stratified (by race/ethnicity) logistic regression analyses between SCCs, GDS, and objective cognitive function. Logistic models are presented in Table 2 with 95% confidence intervals (CI), odds ratio (OR), and p-value. Each regression model included age, sex, education years, and assessment language as covariates. Since AAs were all English speakers, assessment language was not included as a covariate in AAs. All models examined the associations of objective cognitive function and depressive symptoms for each SCC. To run logistic regression analyses, SCCs 1, 2, and 3 were converted to dichotomous variables: SCC 1, “good” = 0, “fair” and “poor” = 1; SCC 2, “no problems” = 0, “minor problems” and “major problems” = 1; SCC 3, “no problems” = 0, “yes and this worries me” and “yes, but, this does not worry me” = 1. In SCCs 4 and 5, “no” remained coded as 0, and “yes” as 1. Since SCC 5 is number 14 from GDS, we created a new GDS variable (GDS minus item 14) to use only for SCC 5 regressions. ANOVAs, chi-squares, and t-tests were performed on non-dichotomized SCCs.
Table 1.
Demographic characteristics, objective cognitive function scores, and depressive symptoms (n = 199)
| Variables | African American (n = 84) | Hispanic (n = 115) | ||
|---|---|---|---|---|
| Female, n (%) | 63 | (31.7%) | 88 | (44.2%) |
| Age* | 73.94 | (6.22) | 71.47 | (5.36) |
| Education* | 12.65 | (2.86) | 10.39 | (4.07) |
| Geriatric Depression Scale (n = 195) | 6.69 | (5.22) | 8.72 | (6.71) |
| Montreal Cognitive Assessment (n = 186)* | 18.06 | (4.10) | 16.32 | (3.95) |
| Hopkins Verbal Learning Test-Total* | 15.25 | (4.37) | 16.90 | (4.34) |
| Hopkins Verbal Learning Test- | 3.96 | (2.45) | 4.32 | (2.53) |
| Delayed Recall (n = 198) | ||||
| Symbol Digit Modalities Test (n = 198) | 23.75 | (10.72) | 20.30 | (10.48) |
| Clock Drawing Test free (n = 198) | 3.19 | (1.15) | 3.23 | (1.22) |
| Clock Drawing Test copy- (n = 198) | 3.89 | (0.96) | 3.58 | (1.00) |
| Semantic fluency (n = 197) | 14.41 | (3.86) | 13.27 | (3.92) |
| Phonemic fluency (n = 196) | 24.70 | (10.83) | 21.65 | (9.46) |
| Overall cognition (n = 193) | z = 0.03 | (1.03) | z = −0.03 | (0.98) |
Data are presented as mean ± (SDs), z-score ± (SDs) or number (proportion %).
p < 0.01 between African Americans and Hispanics. Total n shown for variables with missing data.
We conducted sensitivity analyses to account for potentially undiagnosed dementia cases. Dementia was defined as a score of two standard deviations (SD) or more below the age and education years normed mean scores on two or more objective cognitive tests.
To account for multiple comparisons within AA and Hispanic groups, we applied Bonferroni correction, and set an alpha level of 0.01. All analyses were conducted using IBM SPSS version 25.2.
RESULTS
A total of 199 participants (age: 72.5 ± 5.9, education years: 11.3 ± 3.8, 75.9% females, 57.8% Hispanics, 42.2% AAs) were evaluated. The overall distribution of objective cognitive performance and depressive symptoms are shown in Supplementary Table 1. Demographic data as a function of race/ethnicity are demonstrated in Table 1. Relative to AAs (n = 84), Hispanics (n = 115) were significantly younger (p = 0.003) and less educated (p < 0.001). Hispanics performed significantly worse on MoCA (p = 0.004) but, performed significantly better on HVLT-total (p = 0.009). However, there were no significant group differences in objective cognitive function after adjusting for covariates: age, sex, assessment language, and education years. In the Hispanic sample, participants interviewed in English (n = 19) performed significantly better on SDMT than participants interviewed in Spanish (n = 96), (p ≤ 0.001); however, this difference did not hold after adjusting for age, sex, and education years.
Figure 1 illustrates the distribution of SCC responses (numbered 1–5) of the total sample, whereas Fig. 2 illustrates the distribution of SCC responses within each subject group (AAs and Hispanics). Chi-square results showed that endorsements of worsening memory (SCC 3) and non-memory cognitive concerns (SCC 4) significantly differed between AAs and Hispanics. Hispanics endorsed “Yes, and this worries me,” while AAs endorsed “Yes, but this does not worry me” (p = 0.004). Endorsements also significantly differed by assessment language in the Hispanic group. Hispanics assessed in Spanish endorsed non-memory cognitive concerns (SCC 4): “yes” (26.4%), while those assessed in English endorsed “no” (34%) (p = 0.004).
ANOVA results for SCCs and depressive symptoms suggested that Hispanics who endorsed SCC items 1–5 had significantly higher mean scores for depressive symptoms. AAs who endorsed 2, 3, and 5 had significantly higher depressive symptoms (SCC 1, AAs: p = 0.014; Hispanics: p = 0.001), (SCC 2, AAs: p = 0.004; Hispanics: p < 0.001), (SCC 3, AAs: p = 0.003; Hispanics: p = 0.001), (SCC 4, AAs: p = 0.038; Hispanics: p < 0.001) (SCC 5, AAs: p < 0.001; Hispanics: p < 0.001).
Table 2 demonstrates the logistic regression models of the five SCCs, depressive symptoms (GDS), and objective cognitive function in AAs and Hispanics adjusted for covariates. SCCs were treated as dependent variables for all logistic regressions. To examine objective cognitive function and SCCs at an item-level, we performed multiple logistic regressions. As such, ten logistic regressions (one for each objective cognitive function assessment, overall cognition, and depressive symptoms) stratified by race/ethnicity were conducted for each SCC. In both AAs and Hispanics, endorsements of current memory difficulties (SCC1), worsening memory (SCC 3), and memory worse than most people (SCC 5) were strongly associated with depressive symptoms. In the Hispanic group, non-memory cognitive concern was associated with depressive symptoms. Additionally, lower (worse) scores on the MoCA were associated with increased odds of reporting worsening memory (SCC 3).
We examined associations between assessment language and SCCs in the Hispanic group, adjusted for the same covariates as above. For the former, SCCs were dependent variables for each logistic regression model. However, there were no significant findings.
Sensitivity analyses
To examine whether depressive symptoms confounded relationships between SCCs and objective cognitive function, we reran all logistic models adding GDS (excluding GDS item 14 for SCCs 1–5) as an additional covariate. There were no changes in results. As for assessment language models, Hispanics assessed in Spanish were more likely to endorse memory worse than most people (SCC 5) (OR = 0.04, 95% CI 0.01 – 0.32, p = 0.002). However, there were no significant interaction effects.
We conducted sensitivity analyses to account for potentially undiagnosed cases of dementia and MCI, which may have influenced the results. We only excluded four participants with possible dementia (2 AAs and 2 Hispanics) defined as a score of two SDs or more below the age and education adjusted means on two or more objective cognitive tests. After excluding these four participants, results from the primary model remained the same. Overall, our results were not impacted by patients with undiagnosed dementia or depressive symptoms. In addition, seventeen participants (7 (3.6%) AAs and 10 (5.1%) Hispanics, p = 0.015) scored 1.5 SDs or more below the age and education adjusted means on two or more objective cognitive tests corresponding to a diagnosis of MCI using Petersen criteria [37]. Results from our analysis excluding these individuals eliminated prior GDS associations with age-related memory status (SCC 1) and worsening memory (SCC 3) in AAs. Furthermore, a significant association was observed between worsening memory (SCC 3) and phonemic fluency in Hispanics (OR = 0.93, CI = 0.88–0.98, p = 0.007).
DISCUSSION
The present findings indicate that although most SCCs have a stronger association with depressive symptoms in both groups, a specific SCC about worsening memory was predictive of global objective cognitive function in Hispanics. The strong links found between SCCs and depressive symptoms are consistent with prior reports in Burmester et al.’s (2016) meta-analysis [5] and findings from previous community and clinic-based studies, including primary care settings [14, 17, 18, 20].
In contrast to the minimal literature in Hispanics, we found that complaints about worsening memory were predictive of global cognitive function in Hispanics, raising the possibility that this particular SCC could be a valuable screening tool in primary care clinics when assessing cognitive impairment. However, further investigation is necessary to justify the utility of this particular SCC in a larger population of Hispanics. As such, these results for the MoCA are novel and diverge from research that did not find any links between SCCs and objective cognitive function in Hispanics from a primary care setting [20].
Our results indicate that SCCs are not related to objective cognitive function in AAs, specifically in primary care settings. These results are consistent with community-based studies in AAs that did not find any cross-sectional relationships [15–17] but, differs from a clinic-based study that found memory-specific SCCs related to objective cognitive function [12].
We also observed SCC variations by race/ethnicity, assessment language, and severity of depressive symptoms. In general, Hispanics reported more SCCs compared to AAs. Hispanics, as a group, and participants assessed in Spanish more so than Hispanics assessed in English, reported more “worry” regarding their worsening memory.
Additional analyses revealed that assessment language predicted endorsements of the GDS memory item, “Do you feel you have more problems with memory than most?” in English and Spanish speaking Hispanic groups. These findings may be driven by cultural relevance, appropriateness of expression, or possible misinterpretation of meaning in terms of perceived health [38]. Furthermore, these findings present a significant cultural factor that should be explored in future studies. In terms of the magnitude of depressive symptoms, Hispanics and AAs with mild depressive symptoms reported more “worry” about their worsening memory and “poor” age-related memory status. Hispanics with severe levels of depressive symptoms endorsed “major” current memory problems.
A clear explanation of why we observed racial and ethnic differences in SCC endorsements including, an association with cognitive function in Hispanics but, not in AAs remains elusive. We speculate that differences in cultural, lifestyle, and prevalence of other non-dementia medical factors amongst groups could influence such differences. However, the AA and Hispanic patients were recruited from the same primary care population and resided in the same catchment area. Studies have found that Hispanics with increased acculturation [39] and Major Depressive Disorder [40] are more likely to report higher somatic symptoms to their primary care physician than other racial and ethnic groups. Another study found that Hispanics who presented with cognitive concerns had higher inflammatory markers and endothelial dysfunction than those without cognitive concerns [19]. Other studies imply that cardiovascular risk factors and Parkinson’s disease are associated with increased cognitive complaints [17, 41]. Future studies should account for the effects of the factors as described above.
Collectively, our study offers methodological strengths. As recommended by the Subjective Cognitive Decline Initiative (SCD-I) working group [3], we investigated SCCs and objective cognitive assessments at an item level, which allowed us to determine differential patterns of SCC endorsement across racial and ethnic groups. To our knowledge, no study has directly compared SCCs in AAs to Hispanics. As such, we view these current findings as a first attempt to compare diverse and understudied groups. We also examined a range of comprehensive measures for assessing SCCs, including scaled and dichotomous SCCs derived from several validated measures. In one of our SCC queries, we utilized “age” as a reference point to compare memory changes amongst peers, which reduced the likelihood of over-endorsement. When older adults are not given a reference point, they are more likely to report their baseline cognitive function but not report cognitive changes over time [42]. Another recommendation we applied from the SCD-I working group [3] was our recruitment method. We inquired about a cognitive “concern” in a primary care community-based setting instead of a memory clinic. Patients visiting memory clinics are more likely to have preclinical AD [3]. Therefore, we maximized the likelihood of identifying a subgroup of patients with non-normative cognitive changes. We performed sensitivity analyses to address the possible effects of depressive symptoms and possible dementia on our primary results. Finally, evidence suggests that native English speakers outperform native Spanish speakers on neuropsychological tests [43], especially on language-mediated tests [44]. In contrast to a previous study [20], we provided evidence of the findings’ strength by controlling for assessment language effects as well as examining its influences on SCC endorsements.
It is important to note that the current study had several limitations. Overall, our sample size was based on a convenient clinic-based sample of participants with cognitive concerns. The study did not include a comparable sample of participants without cognitive concerns, limiting the generalizability of the results. Furthermore, our AA sample was relatively smaller than our Hispanic sample, therefore, future studies that may include a demographically matched sample with more AAs will be important to indicate the generalizability of the results. As AAs and Hispanics may have different norms to determine cognitive impairment, our study design did not permit us to make a formal cognitive diagnosis of MCI or dementia. Although cognitive norms were not adjusted for race, testing was administered in the participant’s preferred language. Future studies could explore differences in SCCs in relation to cognitive diagnoses and racial and ethnic groups. Another limitation was that we did not investigate possible variations of SCCs among Hispanics of different origins. The prevalence of dementia differs in diverse ethnic groups [45]. Therefore, future research should investigate SCCs in disparate Hispanic ethnic groups (e.g., Caribbean, South America, etc.). Although our SCCs were validated in Spanish-speaking populations, these SCCs were not explicitly developed for older native Spanish speakers. As observed in our results, SCC endorsement differed and was predicted by assessment language even within similar cultural groups. Thus, further studies would be strengthened by assessing more Spanish-speaking populations to validate SCCs accurately for this community. We also did not collect informant reports of SCCs. Informant reports of SCCs have consistently confirmed validity and relevance to the early manifestation of AD and other dementias, especially when combined with self-reported SCCs [46, 47].
Furthermore, we cannot conclude whether SCCs hold a predictive value of future cognitive decline in AAs and Hispanics due to our cross-sectional approach. Recent longitudinal studies have demonstrated associations between SCCs and cognitive decline in AAs [13, 48] and Hispanics [49]. More longitudinal research is necessary to examine the predictive utility of SCCs and future objective cognitive decline in diverse populations.
The current findings suggest that health providers should assess depressive symptoms as well as cognitive impairment when specific types of SCCs are reported in AAs and Hispanics, particularly in primary care settings. Other studies have found associations between SCCs and other psychological measures such as anxiety and neuroticism [50, 51]. Future studies should investigate the effects of these psychiatric symptoms in diverse populations.
In conclusion, the current study replicates and supplements cross-sectional findings in Hispanics and AAs showing that depressive symptoms were strongly associated with SCCs in both groups. Moreover, endorsements of specific types of SCCs may indicate underlying cognitive impairment in Hispanics but not in AAs in primary care. More research is required about the correlates and predictive value of specific SCCs and cognitive decline in similar groups. Ultimately, a comprehensive understanding of the utility of SCCs in diverse, underrepresented groups will improve current SCC instruments in primary care clinics, facilitating the early detection of dementia.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number UG3NS105565. The authors would like to express their gratitude to the participants and study team involved in data collection and management in the 5-Cog Battery for Detecting Cognitive Impairment and Dementia project.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-1399r3).
Footnotes
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-201399.
REFERENCES
- [1].Studart AN, Nitrini R (2016) Subjective cognitive decline: The first clinical manifestation of Alzheimer’s disease? Dement Neuropsychol 10, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mendonça MD, Alves L, Bugalho P (2016) From subjective cognitive complaints to dementia: Who is at risk?: A systematic review. Am J Alzheimers Dis Other Demen 31, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Molinuevo JL, Rabin LA, Amariglio R, Buckley R, Dubois B, Ellis KA, Ewers M, Hampel H, Klöppel S, Rami L (2017) Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement 13, 296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10, 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burmester B, Leathem J, Merrick P (2016) Subjective cognitive complaints and objective cognitive function in aging: A systematic review and meta-analysis of recent cross-sectional findings. Neuropsychol Rev 26, 376–393. [DOI] [PubMed] [Google Scholar]
- [6].Chong TWH, Lautenschlager NT, Anstey KJ, Bryant C (2020) Anxiety disorders in late life—why are we not more worried? Int J Geriatr Psychiatry 35, 955–961. [DOI] [PubMed] [Google Scholar]
- [7].Donovan NJ, Amariglio RE, Zoller AS, Rudel RK, Gomez-Isla T, Blacker D, Hyman BT, Locascio JJ, Johnson KA, Sperling RA (2014) Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer’s disease. Am J Geriatr Psychiatry 22, 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S (2002) Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA 288, 1475–1483. [DOI] [PubMed] [Google Scholar]
- [9].Jang Y, Chiriboga DA, Kim G, Phillips K (2008) Depressive symptoms in four racial and ethnic groups: The survey of older Floridians (SOF). Res Aging 30, 488–502. [Google Scholar]
- [10].(2020) 2020 Alzheimer’s disease facts and figures. Alzheimers Dement 16, 391–460. [DOI] [PubMed] [Google Scholar]
- [11].Minsky S, Vega W, Miskimen T, Gara M, Escobar J (2003) Diagnostic patterns in Latino, African American, and European American psychiatric patients. Arch Gen Psychiatry 60, 637–644. [DOI] [PubMed] [Google Scholar]
- [12].Boggess MB, Barber JM, Jicha GA, Caban-Holt A (2020) Subjective memory complaints are an important surrogate for objective cognitive performance in African Americans. Alzheimer Dis Assoc Disord 34, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].John SE, Evans SA, Hanfelt J, Loring DW, Goldstein FC (2020) Subjective memory complaints in White and African American participants. J Geriatr Psychiatry Neurol 33, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Blazer DG, Hays JC, Fillenbaum GG, Gold DT (1997) Memory complaint as a predictor of cognitive decline: A comparison of African American and White elders. J Aging Health 9, 171–184. [DOI] [PubMed] [Google Scholar]
- [15].Jackson JD, Rentz DM, Aghjayan SL, Buckley RF, Meneide TF, Sperling RA, Amariglio RE (2017) Subjective cognitive concerns are associated with objective memory performance in Caucasian but not African-American persons. Age Ageing 46, 988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sims RC, Whitfield KE, Ayotte BJ, Gamaldo AA, Edwards CL, Allaire JC (2011) Subjective memory in older African Americans. Exp Aging Res 37, 220–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sperling SA, Tsang S, Williams IC, Park MH, Helenius IM, Manning CA (2017) Subjective memory change, mood, and cerebrovascular risk factors in older African Americans. J Geriatr Psychiatry Neurol 30, 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Harwood D, Barker W, Ownby R, Duara R (1998) Memory complaints in the elderly: A comparative analysis of informant and subject reports among Hispanics and White non-Hispanics. Clin Gerontol 18, 56–60. [Google Scholar]
- [19].Hall JR, Wiechmann A, Johnson LA, Edwards M, O’Bryant SE (2018) Characteristics of cognitively normal Mexican-Americans with cognitive complaints. J Alzheimers Dis 61, 1485–1492. [DOI] [PubMed] [Google Scholar]
- [20].Zlatar ZZ, Muniz MC, Espinoza SG, Gratianne R, Gollan TH, Galasko D, Salmon DP (2018) Subjective cognitive decline, objective cognition, and depression in older Hispanics screened for memory impairment. J Alzheimers Dis 63, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Benedict RHB, Schretlen D, Groninger L, Brandt J (1998) Hopkins verbal learning test – revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 12, 43–55. [Google Scholar]
- [22].Smith A (1973) Symbol digit modalities test, Western Psychological Services, Los Angeles. [Google Scholar]
- [23].Mendez MF, Ala T, Underwood KL (1992) Development of scoring criteria for the clock drawing task in Alzheimer’s disease. J Am Geriatr Soc 40, 1095–1099. [DOI] [PubMed] [Google Scholar]
- [24].Shulman KI (2000) Clock-drawing: Is it the ideal cognitive screening test? Int J Geriatr Psychiatry 15, 548–561. [DOI] [PubMed] [Google Scholar]
- [25].Goodglass H, Kaplan E (1972) The assessment of aphasia and related disorders, Lea & Febiger, Philadelphia. [Google Scholar]
- [26].Rosen WG (1980) Verbal fluency in aging and dementia. J Clin Exp Neuropsychol 2, 135–146. [Google Scholar]
- [27].Spreen O, Benton AL (1977) Neurosensory center comprehensive examination for aphasia (NCCEA), revised edition: Manual of instructions, Neuropsychology Laboratory, University of Victoria. [Google Scholar]
- [28].Kaplan E, Goodglass H, Weintraub S (1983) The Boston Naming Test (2nd ed.), Lea & Febiger, Philadelphia. [Google Scholar]
- [29].Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [30].Riordan H (2017) Constructing composites to optimise cognitive outcomes. J Clin Stud 9, 40–45. [Google Scholar]
- [31].Jonaitis EM, Koscik RL, Clark LR, Ma Y, Betthauser TJ, Berman SE, Allison SL, Mueller KD, Hermann BP, Van Hulle CA (2019) Measuring longitudinal cognition: Individual tests versus composites. Alzheimers Dement (Amst) 11, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Malek-Ahmadi M, Chen K, Perez SE, He A, Mufson EJ (2018) Cognitive composite score association with Alzheimer’s disease plaque and tangle pathology. Alzheimers Res Ther 10, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17, 37–49. [DOI] [PubMed] [Google Scholar]
- [34].Rabin LA, Wang C, Katz MJ, Derby CA, Buschke H, Lipton RB (2012) Predicting Alzheimer’s Disease: Neuropsychological tests, self-reports, and informant reports of cognitive difficulties. J Am Geriatr Soc 60, 1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rattanabannakit C, Risacher SL, Gao S, Lane KA, Brown SA, McDonald BC, Unverzagt FW, Apostolova LG, Saykin AJ, Farlow MR (2016) The cognitive change index as a measure of self and informant perception of cognitive decline: Relation to neuropsychological tests. J Alzheimers Dis 51, 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kaup AR, Nettiksimmons J, LeBlanc ES, Yaffe K (2015) Memory complaints and risk of cognitive impairment after nearly 2 decades among older women. Neurology 85, 1852–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256, 183–194. [DOI] [PubMed] [Google Scholar]
- [38].Hunt SM, Bhopal R (2004) Self report in clinical and epidemiological studies with non-English speakers: The challenge of language and culture. J Epidemiol Community Health 58, 618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bauer AM, Chen C-N, Alegria M (2012) Prevalence of physical symptoms and their association with race/ethnicity and acculturation in the United States. Gen Hosp Psychiatry 34, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dunlop BW, Still S, LoParo D, Aponte-Rivera V, Johnson BN, Schneider RL, Nemeroff CB, Mayberg HS, Craighead WE (2020) Somatic symptoms in treatment-naïve Hispanic and non-Hispanic patients with major depression. Depress Anxiety 37, 156–165. [DOI] [PubMed] [Google Scholar]
- [41].Erro R, Santangelo G, Barone P, Picillo M, Amboni M, Longo K, Giordano F, Moccia M, Allocca R, Pellecchia MT (2014) Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J Geriatr Psychiatry Neurol 27, 276–281. [DOI] [PubMed] [Google Scholar]
- [42].Tandetnik C, Farrell MT, Cary MS, Cines S, Emrani S, Karlawish J, Cosentino S (2015) Ascertaining subjective cognitive decline: A comparison of approaches and evidence for using an age-anchored reference group. J Alzheimers Dis 48, S43–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Loewenstein DA, Arguüelles T, Barker WW, Duara R (1993) A comparative analysis of neuropsychological test performance of Spanish-speaking and English-speaking patients with Alzheimer’s disease. J Gerontol 48, P142–P149. [DOI] [PubMed] [Google Scholar]
- [44].Kisser JE, Wendell CR, Spencer RJ, Waldstein SR (2012) Neuropsychological performance of native versus non-native English speakers. Arch Clin Neuropsychol 27, 749–755. [DOI] [PubMed] [Google Scholar]
- [45].González HM, Tarraf W, Schneiderman N, Fornage M, Vásquez PM, Zeng D, Youngblood M, Gallo LC, Daviglus ML, Lipton RB (2019) Prevalence and correlates of mild cognitive impairment among diverse Hispanics/Latinos: Study of Latinos-investigation of neurocognitive aging results. Alzheimers Dement 15, 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Buckley R, Saling M, Ellis K, Rowe C, Maruff P, Macaulay LS, Martins R, Masters C, Savage G, Rainey-Smith S (2015) Self and informant memory concerns align in healthy memory complainers and in early stages of mild cognitive impairment but separate with increasing cognitive impairment. Age Ageing 44, 1012–1019. [DOI] [PubMed] [Google Scholar]
- [47].Gifford KA, Liu D, Carmona H, Lu Z, Romano R, Tripodis Y, Martin B, Kowall N, Jefferson AL (2015) Inclusion of an informant yields strong associations between cognitive complaint and longitudinal cognitive outcomes in non-demented elders. J Alzheimers Dis 43, 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Arvanitakis Z, Leurgans SE, Fleischman DA, Schneider JA, Rajan KB, Pruzin JJ, Shah RC, Evans DA, Barnes LL, Bennett DA (2018) Memory complaints, dementia, and neuropathology in older blacks and whites. Ann Neurol 83, 718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fernandez-Blazquez MA, Ávila-Villanueva M, Maestú F, Medina M (2016) Specific features of subjective cognitive decline predict faster conversion to mild cognitive impairment. J Alzheimers Dis 52, 271–281. [DOI] [PubMed] [Google Scholar]
- [50].Balash Y, Mordechovich M, Shabtai H, Giladi N, Gurevich T, Korczyn AD (2013) Subjective memory complaints in elders: Depression, anxiety, or cognitive decline? Acta Neurol Scand 127, 344–350. [DOI] [PubMed] [Google Scholar]
- [51].Pearman A, Hertzog C, Gerstorf D (2014) Little evidence for links between memory complaints and memory performance in very old age: Longitudinal analyses from the Berlin aging study. Psychol Aging 29, 828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


