Abstract
Pseudomonas aeruginosa is one of the main microbial species colonizing the lungs of cystic fibrosis patients and is responsible for the decline in respiratory function. Despite the hostile pulmonary environment, P. aeruginosa is able to establish chronic infections thanks to its strong adaptive capacity. Various longitudinal studies have attempted to compare the strains of early infection with the adapted strains of chronic infection. Thanks to new ‘-omics’ techniques, convergent genetic mutations, as well as transcriptomic and proteomic dysregulations have been identified. As a consequence of this evolution, the adapted strains of P. aeruginosa have particular phenotypes that promote persistent infection.
Keywords: adaptation, cystic fibrosis, genomic, phenotype, Pseudomonas aeruginosa
Data Summary
Supporting data are available in Table S1, available with the online version of this article.
Impact Statement.
The chronic lung infections caused by Pseudomonas aeruginosa are associated with the deterioration of pulmonary functions and general health of cystic fibrosis (CF) patients. The difficulty of efficiently eradicating this pathogen comes from its ability to evolve towards high-persistence phenotypes through genetic adaptation. Understanding the basis and the determinants of this evolution is, thus, essential for the identification of new strategies to limit lung colonization by P. aeruginosa . The sequencing studies performed on CF isolates have highlighted numerous different evolutionary paths taken by the bacterium, leading to an intense intrapatient and interpatient diversification of P. aeruginosa populations. Fortunately, the identification of convergent patterns of adaptation is now possible thanks to the increasing number of research studies focused on CF isolates worldwide. Previous reviews on the topic often focused on particular aspects of P. aeruginosa adaptation, such as the genome dynamic, diversification processes or metabolism. In the present review, all the different aspects, as well as the latest publications on the topic, have been compiled to provide an updated and broader viewpoint of P. aeruginosa adaptation to the CF environment. This review also highlights convergent adaptation patterns involving intergenic regions, and transcriptomic and proteomic profiles of P. aeruginosa , not fully explored until now.
Introduction
The ability of Pseudomonas aeruginosa to establish a chronic infection in cystic fibrosis (CF) lungs despite a wide range of stress sources highlights its high adaptability. In fact, the high plasticity of the P. aeruginosa core and accessory genome allows the bacterium to colonize a wide variety of environments, such as soils, water or abiotic surfaces [1–4]. However, P. aeruginosa adaptive processes have been especially described in the context of pulmonary infections. Indeed, the chronicity of P. aeruginosa CF lung infections and the difficulty in treating them make it essential to understand the mechanisms of the persistence. Moreover, this chronic infectious disease offers a rare opportunity to study long-term microbial evolution within a human host. The creation of CF centres has facilitated the conservation of the different micro-organisms isolated from CF patient sputa, allowing the constitution of longitudinal isolate banks from numerous subjects. This also contributed to the identification of highly transmissible P. aeruginosa strains such as the lineages DK2, AUST-02, LES (Liverpool epidemic strain) and C that are epidemic in Denmark, Australia and the UK, respectively [5]. Thanks to the development of next-generation sequencing methods, many studies have focused on longitudinal genetic adaptation of P. aeruginosa to the CF lung environment (Table 1). In 2006, Smith and colleagues were the first to describe a genetic evolution of a clonal lineage of P. aeruginosa in vivo by sequencing two P. aeruginosa strain isolates collected 7.5 years apart from the same patient [6]. Following studies were performed on a broader range of isolates from unique patients [7–12] or on transmissible lineages such as DK2 or AUST-02 [13–15]. Finally, Marvig et al. and Klockgether et al. combined both approaches to study the genomics of, respectively, 474 and 262 isolates from more than thirty patients [16–18].
Table 1.
Genomic studies performed on longitudinal CF isolates of P. aeruginosa
The list of genes or intergenic regions identified in these studies was used to highlight the most mutated regions in Tables 2 and 3. The most representative genomic studies performed on P. aeruginosa CF sequential isolates whose isolations were spaced by at least 1 year were selected.
|
Sequencing type |
No. of patients |
No. of sequenced isolates |
Time span of isolate evolution (years) |
No. of studied lineages or clone types |
Identification of positively selected genes |
Reference |
|---|---|---|---|---|---|---|
|
Whole-genome |
1 |
2 |
7.5 |
1 |
No |
[6] |
|
Gene-targeted |
29 |
58 |
5–20 |
nd |
No |
|
|
Whole-genome |
1 |
45 |
20 |
1 (PA14) |
No |
[7] |
|
1 |
63 |
23 |
1 (C) |
No |
||
|
Whole-genome |
6* |
12* |
35 max.* |
1 (DK2)* |
No |
[13]* |
|
Whole-genome |
21* |
55* |
36* |
1 (DK2)* |
Yes |
[14]* |
|
Whole-genome |
1 |
18 |
32 |
1 (DK1) |
No |
[9] |
|
Whole-genome |
1 |
13 |
6 |
1 |
Yes |
[8] |
|
1 |
14 |
20 |
1 |
|||
|
Whole-genome |
34 |
474 |
1–8 |
53 (36 for PE) |
Yes |
[16] |
|
Whole-genome |
4 |
26 |
17–19 |
6 |
Yes |
[18] |
|
Whole-genome |
1 |
2 |
6.9 |
1 (OC4A) |
No |
[10] |
|
Whole-genome |
1 |
2 |
3 |
1 |
No |
[12] |
|
Whole-genome |
32 (12 for PE) |
262 |
<15–35 |
12 |
Yes |
[17] |
|
Whole-genome |
13 (6 for PE) |
63 |
3–4 |
1 (AUST-02) |
Yes |
[15] |
|
Whole-genome |
1 |
40 |
8 |
1 |
Yes |
[11] |
|
Reanalysis of whole- genome sequencing |
68 |
534 |
nd |
44 |
Yes |
[81] |
nd, Not determined in the study; PE, parallel evolution.
*Isolates sequenced by Yang et al. were also used in the study by Marvig et al., making the results of these two studies interconnected [13, 14].
By gathering the results of these different longitudinal studies, we aim to provide an updated description of the main genetic adaptations of P. aeruginosa to the CF lung environment. In this review, we will also discuss how these alterations affect transcriptomic and proteomic profiles of P. aeruginosa thanks to the latest studies performed on clinical CF isolates. Finally, common phenotypes of CF-adapted P. aeruginosa will be described.
Genomic adaptation of P. aeruginosa
P. aeruginosa genome accumulates mutations during establishment of chronic colonization
Types and frequency of mutational events
Longitudinal genomic studies highlighted that late isolates of P. aeruginosa present numerous genetic modifications in comparison to early isolates. Small mutational events such as SNPs or short insertions and deletions (indels) have been described as the major driver of these modifications. Indeed, the P. aeruginosa genome was shown to accumulate a median of 3 SNPs per year, varying between 0.5 and 14 SNPs per year [6–9, 12, 14–18]. Small indels have also been reported at rates ranging from 0.4 to 2.7 indels per year (0.1 to 0.28 indels per SNP) [12, 14, 17]. These modifications could be observed on both core and accessory genomes of clinical isolates, depending on the use of a reference strain for gene annotation. Indeed, while several studies focused on the annotated genes in PAO1 or PA14 [6, 7, 13, 16, 17], others were able to identify SNPs in clone-specific genes using a related ancestral isolate as a reference [8, 9, 12, 14, 17]. The presence of accessory elements such as genomic islands and prophages could also be predicted in silico [10, 11].
The role of the accessory genome is in fact increasingly considered for understanding P. aeruginosa adaptive processes, due to its plasticity and the richness of its encoded functions [4, 19]. Indeed, the P. aeruginosa pathogenic islands (PAPIs) and several LES prophages were shown to affect diversification processes and important pathoadaptive phenotypes of P. aeruginosa , including its ability to establish in vivo and its antibiotic resistance [20–25]. Such elements can be horizontally transferred between P. aeruginosa or even between different microbial species through mechanisms of phage infection or pilus-mediated conjugation of excised and circularized genomic islands [4, 26–30]. However, acquisition of novel DNA through horizontal gene transfer remains rare [31, 32] and the genome of P. aeruginosa rather tends to shrink during its adaptation in CF lungs. Rau et al. described that the P. aeruginosa DK2 lineage underwent a loss of a mean of 4.2 kbp per year [31]. Deletions of more than 1000 bp have been observed in other lineages (in 10 out of 12 lineages in the study of Klockgether and colleagues), with the size of deleted regions reaching 188 kb [6, 7, 11, 17]. Here again, these deletions were shown to affect both core and accessory genomes, as prophages and genomic islands were shown to be partially or totally lost during P. aeruginosa adaptation to the CF environment [11, 27, 28, 31, 33–35]. Notably, the genomic islands PAPI-1 and the P. aeruginosa genomic island-2 (PAGI-2) were found either excised or impacted by deletions in CF isolates [27, 28]. In contrast, other elements of the accessory genome seem less prone to deletions, as the toxin–antitoxin systems, the clustered regularly interspaced short palindromic repeats (CRISPR) spacers and the genomic island PAGI-1 are well conserved in CF isolates [36–38].
In addition to deletions, the P. aeruginosa genome can undergo important chromosomal rearrangements that often involve accessory mobile elements, such as transposons and integrons [4, 19]. The insertion sequence IS6100 was identified as the main perpetrator of the frequent chromosomal inversions observed in the CF strains from clone C [35, 39]. Besides disrupting the reading frame of neighbouring genes [39], such chromosomal rearrangements can have pleiotropic consequences through modifications of regulatory regions or DNA topology [40]. By assessing the phenotype–genotype relationship of 44 isolates from a single patient, Darch et al. highlighted that the phenotypic diversity observed between CF isolates was mainly due to homologous recombination mechanisms [41]. However, this result and the high recombination rate obtained were then shown to mainly arise from false-positive events. New bio-informatics analyses of the same sequencing data with correcting filters indeed indicated lower recombination rates [42, 43]. These discrepancies emphasize the importance of bio-informatics tools and settings for the identification of recombination events, and more broadly for all genomic comparisons. In that respect, the detection of genetic alterations can be improved by combining second- and third-generation sequencing methods: while second-generation sequencing such as Illumina provides short reads with low error rates, the longer reads generated by third-generation sequencing allow a better detection of recombination events and large chromosomal rearrangements.
Hypermutability
The rate of spontaneous mutations can be affected by the genetic background of the strain, and even enhanced by previous mutational events. For instance, the high rates of deletion observed by Rau et al. can be attributed to stochasticity or to the presence of missense mutations in the coding sequences of the exonucleases sbcB and sbcC implicated in recombination [31]. In the same way, the well-known hypermutable phenotype of P. aeruginosa arises from genetic alterations of DNA repair systems. Indeed, mutations in mutS/mutL and uvrD genes are commonly observed in CF isolates and induce a significant increase of the mutation rate [44]. Chromosomal inversions were also shown to disrupt the reading frame of mutS and induce hypermutability in clinical strains from the C lineage [39]. Hypermutable isolates, thus, accumulate a mean of 16-fold more mutations, with a median of 48 SNPs per year (range of 2 to more than 350 SNPs per year) [7, 8, 15, 17, 45].
Hypermutability increases the genetic diversity of the P. aeruginosa population in CF lungs, an advantageous feature for adaptability to stressful conditions [8, 14, 16, 17, 46, 47]. Indeed, it has been shown that antibiotic exposure promotes the emergence of hypermutability in P. aeruginosa , then favouring acquisition of antibiotic resistance [45, 48–51]. However, Mehta and colleagues also observed that some hypermutable lineages would spontaneously decline and disappear from the evolving population [49]. This phenomenon could be explained by an accumulation of neutral and/or slightly deleterious mutations whose probability is also increased by hypermutability. Moreover, the fitness benefit of hypermutators seems to be restricted to the conditions in which they evolved, as the accumulated hitchhiking mutations can constitute a burden in non-selective conditions [49, 50]. Hypermutability is, thus, a double-edged sword that does not ensure the success of P. aeruginosa adaptation. Indeed, hypermutators rarely dominate the colonizing population and coexist with normo-mutable isolates in CF lungs, potentially through colonization of specific niches [8, 9, 14]. Compensation of the hypermutator phenotype through secondary mutations has also been reported during adaptation to CF environment [8], suggesting an importance of the phenotype at certain stages of evolution. This hypothesis is supported by the high prevalence of hypermutators in CF cohorts. Since the first estimations by Oliver [44], several studies in European and American cohorts confirmed that a mean of 28 % of CF patients were infected by at least one hypermutable isolate of P. aeruginosa [44, 52–55]. Finally, despite a high prevalence and an increased ability to develop antibiotic resistance, the impacts of infection by hypermutable P. aeruginosa on clinical outcome are unclear. While an association between the presence of hypermutators and the deterioration of lung function was described in English and French cohorts [56, 57], such a result was not confirmed in an Israeli cohort [55]. Moreover, Klockgether and colleagues did not highlight a correlation between annual rate of sequence variation and the severity of the clinical course of German CF patients [17].
Accumulation of mutations relies on selection mechanisms
The accumulation of mutations in the P. aeruginosa genome could be the result of genetic drift or neutral selection, during which mutations are stochastically fixed regardless of their impact. However, due to the stressful conditions inherent to the CF lung environment, mutations are actually selected because of their beneficial effect on bacterial fitness. As non-synonymous mutations are more likely to affect protein function and eventually fitness, selective mechanisms can be quantified by the non-synonymous to synonymous mutations ratio (d N/d S). This ratio can be calculated over different scales – from all coding regions of the pangenome to specific coding regions. Three type of selective mechanisms, thus, can be observed: (i) a d N/d S value over one testifies to positive selection, (ii) a value under one indicates purifying or negative selection, and (iii) a close to one ratio depicts typical genetic drift.
These three selective mechanisms have been observed for the P. aeruginosa genome during adaptation to the CF environment. Several studies have highlighted positive selection mechanisms at the genome scale (d N/d S of 1.4 and 2) [6, 12], whereas negative selection was observed in others (d N/d S between 0.33 and 0.79) [7–9, 14]. In fact, selective mechanisms appear to vary according to the colonization time and clinical status of patients, affecting the accumulation of mutations and the composition of the accessory genome. Klockgether and colleagues observed that the P. aeruginosa genome presented d N/d S ratios ranging from 0.39 to 1.66 according to the colonization time, mutability of isolates and the severity of infection [17]. A fluctuation of positive, neutral and negative selections with time was depicted for hypermutable strains causing severe and mild infections, and for normo-mutable isolates from mildly affected patients. Interestingly, only genomes of normo-mutable isolates from patients with severe infection presented a signature of positive selection during almost all the course [17]. A relationship between the severity and the accessory genome was also observed as isolates causing severe and mild infections presented divergent repertories of accessory genes. Similar observations were previously made for persistent and eradicated CF isolates [17, 32]. In addition, Cramer et al. and Markussen et al. observed a rapid genetic diversification during the first clades followed by coexistence of more stable sublineages of PA14 and DK1, respectively [7, 9]. Similarly, the DK2 lineage was shown to have accumulated most mutations before 1979 in order to ensure its success in several hosts, after which negative selection was observed [13]. In both studies, late P. aeruginosa isolates tended to accumulate fewer mutations than early ones, suggesting modifications of selection mechanisms over the time [7, 9, 11, 13]. Mutations are indeed less likely to improve fitness and, thus, to be fixed once P. aeruginosa is adapted to the CF environment. Compensation of the hypermutable phenotype by secondary mutations observed by Feliziani and colleagues [8] supports this notion, as it can rebalance the mutation rate to a regular level after a stage of rapid diversification and adaptation. Finally, several recent research studies on non-CF infections reported that P. aeruginosa adaptive mechanisms occur at the very beginning of the colonization, emphasizing the underappreciated role of genetic adaptation in acute infections [51, 58, 59]. Altogether, these results indicate that different modes of selection arise with time, according to infection stage and severity. Thus, we suggest that positive selection first occurs during acute infections, which often severely affect patient clinical status. Thereafter, neutral or negative selection is promoted as P. aeruginosa adapts and the infection becomes chronic.
Although general trends of positive or negative selection can be observed for the global genome, it is important to note that selection can vary considerably according to the DNA segment. Thus, genes from the antibiotic resistome can appear positively selected despite negative selection at the genome scale [8, 45]. In contrast, negative selection is particularly depicted in the accessory genome of P. aeruginosa , where loss of DNA and accumulation of synonymous SNPs are promoted by mutational hotspots and genomic instability [6, 10, 31, 32, 60, 61]. However, the negative selection in accessory segments compared to the core genome can sometimes be offset by DNA acquisition through horizontal gene transfer, as described in the clones C and PA14 [10, 60]. Finally, the genetic background of P. aeruginosa can also influence selection and fixation of mutations in particular genes through epistatic mechanisms. Certain genetic alterations, thus, may be positively selected due to their compensatory effect on former polymorphisms or in a given genetic background, as depicted in several cases. Damkiaer and colleagues observed that a single rpoD mutation induced alginate overproduction only in a particular genetic background of the DK2 lineage and, thus, was positively selected [62]. Genic alterations of mexT were shown to compensate the effects of lasR inactivation, suggesting that positive selection of this mutation may be promoted in lasR-negative isolates [63–66]. In the same way, mutations reverting the mutator phenotype might be positively selected only after alteration of the genes from DNA repair systems [8].
Besides colonization time, infection severity and the genetic background of isolates, spatial isolation can affect the dynamics of selection mechanisms. Indeed, it is now well understood that micro-organisms can be subject to highly different selective pressures according to the environment. The heterogeneity of the CF lung ecosystem generates ecological microniches with variable physicochemical and biotic characteristics and, thus, variable selective forces. As a result, a phenomenon of adaptive radiation can be observed during P. aeruginosa adaptation to the CF environment. Divergent evolutionary patterns have indeed been depicted between clonally related isolates that have evolved in sinuses or in lungs [9], and even between clones isolated from different lung regions [67]. In both studies, isolates evolved independently within the different regions, as no phenomenon of convergent evolution could be observed. Instead, genotypic and phenotypic diversification was shown to be driven by the spatial isolation of strains [9, 67]. This diversification leads to the coexistence of numerous clonal lineages in the CF airways, as excellently reviewed by Winstanley and colleagues [46].
In addition to this intra-clonal diversification, the heterogeneity of P. aeruginosa populations is promoted by the coexistence of several lineages within the lungs of CF patients. Thus, from a single sputum sample, different P. aeruginosa lineages are frequently isolated that were independently acquired from the environment or from other CF patients, especially for LES-derived lineages [46, 68, 69]. Williams and colleagues observed that the prevalence of each lineage within a patient was highly dynamic during the course of infection, affecting considerably the diversification processes of P. aeruginosa [69]. On the one hand, the lung colonization by divergent lineages was shown to bring more genetic diversity than the in situ evolution of P. aeruginosa . On the other hand, competition between lineages appeared to select for particular genotypes and, thus, influence the diversification processes of P. aeruginosa . In a CF patient, the replacement of a LES lineage by another, thus, could be associated with an increased frequency of pathoadaptive mutations in the lasR gene [69]. The other way round, one would also expect that the presence of certain genotypes within lungs can either promote or limit superinfection by other P. aeruginosa lineages and, thus, interclonal diversification. This phenomenon can be extended to the colonization by other microbial species, as they have to cope with heterogeneous, adapted and niche-specialized populations of P. aeruginosa .
This genetic and phenotypic diversification of P. aeruginosa raises important issues concerning the sampling and the study of bacterial colonies from CF expectorations: a single colony is not representative of the infecting P. aeruginosa metapopulation [46]. In the case of longitudinal genomic studies, the sequencing of a single strain per time point is an important limitation and provides only a restricted fraction of the different evolutionary paths that the bacterium has taken. This issue obviously feeds through to all genotypic and phenotypic characterizations of CF P. aeruginosa strains, but is increasingly taken into account for sequencing studies and the determination of antibiotic-resistance profiles [34, 45, 68, 69].
CF-adapted P. aeruginosa present pathoadaptive mutations
Coding regions
Despite the diversification processes of P. aeruginosa , the high number of genomic studies (Table 1) performed on sequential isolates allowed the identification of convergent patterns of adaptation. In addition to the d N/d S calculation, genes under positive selection were brought out through different approaches: Marvig and colleagues determined genes that accumulated more mutations than what would be predicted if mutations were randomly distributed across the genome [14, 16, 18]. In other studies, thresholds were set to establish lists of genes that were hit by a minimum quantity of independent mutations and/or in a minimum number of lineages [8, 11, 17].
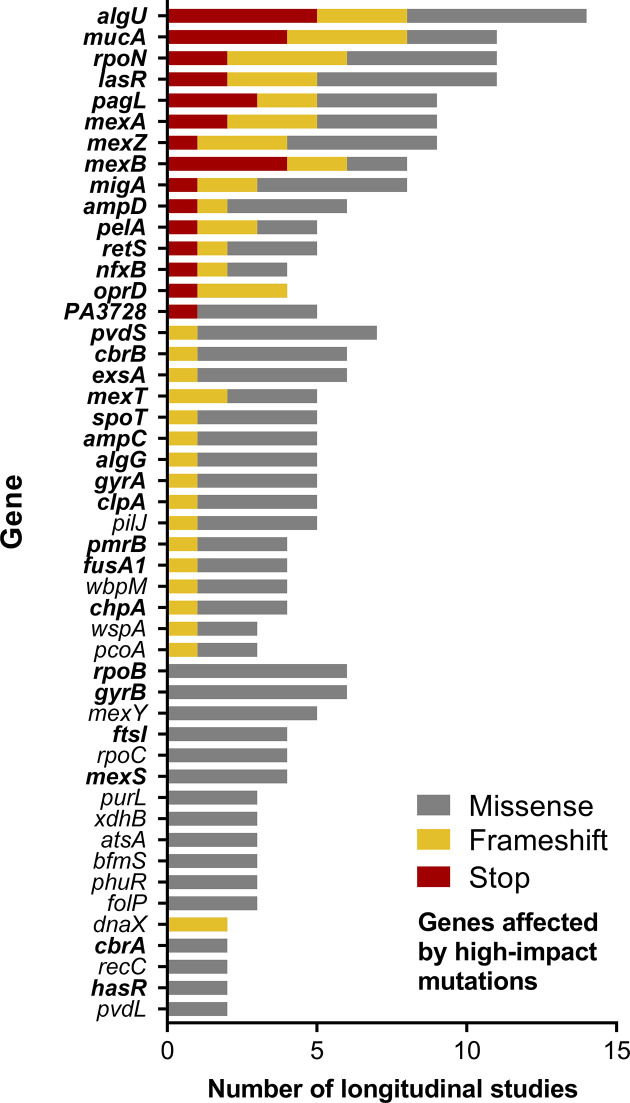
In order to have a global overview of the mutated genes during P. aeruginosa adaptation, the results of 13 longitudinal studies were examined (Table 1). Table 2 provides a list of 48 P . aeruginosa coding regions that have been identified as non-synonymously mutated in at least three of these studies. Different types of mutations, thus, were highlighted (missense, frameshift and stop), but their impacts also rely on their position in the gene. Despite the change of a single amino acid, missense mutations can indeed have drastic consequences on translation efficiency or protein function, especially when they affect important functional domains [6, 17, 63]. Missense mutations were notably predicted to drastically affect the protein function of RpoB and GyrB [17], or even induce total loss-of-function of MexS [6] (Fig. 1).
Table 2.
P. aeruginosa genes identified as non-synonymously mutated in at least three independent longitudinal studies
The characteristics of the 13 studies used for the intragenic regions are listed in Table 1.
|
Longitudinal studies |
|||||
|---|---|---|---|---|---|
|
Gene name |
PAO1 locus |
Product |
Positive selection |
No. |
Reference |
|
gyrB |
PA0004 |
DNA gyrase subunit B |
Yes |
8 |
|
|
pvdS |
PA2426 |
Sigma factor |
No |
8 |
|
|
mexA |
PA0425 |
RND multidrug efflux membrane fusion protein MexA precursor |
No |
6 |
|
|
mexY |
PA2018 |
Multidrug efflux protein |
No |
6 |
|
|
mexZ |
PA2020 |
Transcriptional regulator of multidrug efflux pump |
Yes |
6 |
|
|
gyrA |
PA3168 |
DNA gyrase subunit A |
No |
6 |
|
|
ftsI |
PA4418 |
Penicillin-binding protein 3 |
No |
6 |
|
|
mexB |
PA0426 |
RND multidrug efflux transporter |
No |
6 |
|
|
oprD |
PA0958 |
Basic amino acid, basic peptide and imipenem outer-membrane porin |
No |
6 |
|
|
migA |
PA0705 |
α-1,6-Rhamnosyltransferase |
No |
5 |
|
|
algU |
PA0762 |
RNA polymerase sigma factor |
Yes |
5 |
|
|
lasR |
PA1430 |
Transcriptional regulator of QS |
Yes |
5 |
|
|
pmrB |
PA4777 |
Two-component regulator system signal sensor kinase |
No |
5 |
|
|
mucA |
PA0763 |
Anti-sigma factor |
Yes |
5 |
|
|
algG |
PA3545 |
Alginate-C5-mannuronan-epimerase |
No |
5 |
|
|
mexS |
PA2491 |
Probable oxidoreductase |
Yes |
4 |
[6, 15–17] |
|
mexT |
PA2492 |
Transcriptional regulator of multidrug efflux pump |
No |
4 |
|
|
rpoB |
PA4270 |
DNA-directed RNA polymerase β chain |
No |
4 |
|
|
chpA |
PA0413 |
Component of chemotactic signal transduction system |
No |
4 |
|
|
wbpM |
PA3141 |
Nucleotide sugar epimerase/dehydratase |
No |
4 |
|
|
fusA1 |
PA4266 |
Elongation factor G |
Yes |
4 |
|
|
rpoN |
PA4462 |
RNA polymerase C-54 factor |
Yes |
4 |
|
|
pagL |
PA4661 |
Lipid A 3-O-deacylase |
Yes |
4 |
|
|
retS |
PA4856 |
Regulator of exopolysaccharide and type III secretion |
No |
4 |
|
|
rpoC |
PA4269 |
DNA-directed RNA polymerase subunit β |
No |
3 |
|
|
exsA |
PA1713 |
Transcriptional regulator of T3SS |
No |
3 |
[6, 7, 12] |
|
ampC |
PA4110 |
β-Lactamase/d-alanine carboxypeptidase |
Yes |
3 |
|
|
atsA |
PA0183 |
Arylsulfatase |
No |
3 |
|
|
pilJ |
PA0411 |
Twitching motility protein |
Yes |
3 |
|
|
xdhB |
PA1523 |
Xanthine dehydrogenase |
No |
3 |
[7, 8, 10] |
|
dnaX |
PA1532 |
DNA polymerase subunits γ and τ |
No |
3 |
|
|
pcoA |
PA2065 |
Copper resistance protein A precursor |
No |
3 |
|
|
pvdL |
PA2424 |
Non-ribosomal peptide synthase, pyoverdine biosynthesis |
No |
3 |
[9–11] |
|
clpA |
PA2620 |
ATP-binding protease component |
Yes |
3 |
|
|
pelA |
PA3064 |
Glycohydrolase involved in Pel biosynthesis |
No |
3 |
|
|
hasR |
PA3408 |
Haem uptake outer-membrane receptor precursor |
No |
3 |
|
|
wspA |
PA3708 |
Chemotaxis transducer |
No |
3 |
|
|
PA3728 |
PA3728 |
ATPase |
Yes |
3 |
|
|
purL |
PA3763 |
Phosphoribosylformylglycinamidine synthase |
Yes |
3 |
|
|
bfmS |
PA4102 |
Histidine kinase sensor |
No |
3 |
|
|
recC |
PA4285 |
Exodeoxyribonuclease V subunit γ |
No |
3 |
[7, 8, 10] |
|
ampD |
PA4522 |
N-Acetyl-anhydromuranmyl-l-alanine amidase |
No |
3 |
[6, 7, 17] |
|
nfxB |
PA4600 |
Transcriptional regulator |
Yes |
3 |
[16–18] |
|
phuR |
PA4710 |
Putative haem/haemoglobin uptake outer-membrane receptor |
No |
3 |
|
|
cbrA |
PA4725 |
Two-component sensor CbrA |
No |
3 |
|
|
cbrB |
PA4726 |
Two-component response regulator CbrB |
No |
3 |
[7, 9, 13] |
|
folP |
PA4750 |
Dihydropteroate synthase |
No |
3 |
|
|
spoT |
PA5338 |
Guanosine-3',5'-bis(diphosphate) 3'-pyrophosphohydrolase |
Yes |
3 |
|
Fig. 1.
Number of longitudinal studies identifying stop (red), frameshifts (yellow) or missense (grey) mutations in 48 genes. Non-synonymously mutated genes and corresponding types of mutations were recovered from the longitudinal studies listed in Table 1. Genes in bold were affected by mutations predicted to have a drastic impact on protein function [17] or induce a partial or total loss-of-function [6].
Nonsense mutations and frameshifts induced by insertions and deletions are predicted as high-impact mutations as they induce a disruption and/or an interruption of translation. Most of the genes described in Table 2 have been shown to accumulate high-impact mutations during P. aeruginosa adaptation during longitudinal studies (Fig. 1). It is especially the case for numerous global regulators, such as mucA, algU, rpoN and lasR, but also regulators related to antibiotic resistance (nfxB, mexZ) or type III secretion (retS, exsA).
The role of these genes in P. aeruginosa adaptation to the CF environment was confirmed in larger cohorts of clinical isolates, but through a wide variety of mutations. In that respect, 173 unique lasR variants have been detected by gene-targeted sequencing of 2583 CF isolates, with most of them inducing a loss of function [63]. Mutations in mucoidy related genes have also been researched in P. aeruginosa isolates from CF patients [70–73]. A recent study in a Brazilian cohort identified 30 new mutations in the algUmucABD operon and confirmed the high frequency of the mucA22 mutation, inducing a premature stop codon in the mucA gene [74]. However, it is noteworthy that high-impact mutations do not inevitably induce a complete loss of function. Feltner and colleagues indeed observed a retained LasR activity in 25 % of cases despite missense or even nonsense mutations in the lasR sequence [63]. Similarly, P. aeruginosa strains carrying the nonsense mucA22 mutation were recently shown to respond highly differently than ΔmucA mutants to acidified nitrite conditions [75]. These results highlight the complexity of fully evaluating the consequences of mutations on protein features, even for ones predicted to induce a drastic impact or a loss of protein function. It is particularly the case for global transcriptomic regulators as their alteration, however small, can affect the expression and function of numerous other genes.
Synonymous mutations can also have beneficial or detrimental impacts on fitness through alteration of protein folding, translation efficiency and rate [76, 77]. Adaptive synonymous mutations with an associated gain of fitness have been highlighted during experimental evolution of Pseudomonas fluorescens [78, 79]. Thus, it would not be surprising that synonymous mutations also contribute to P. aeruginosa adaptation in CF lungs, although their impact is still rarely considered.
Intergenic regions
None of the previous studies assessed whether positive selection also occurred in non-coding regions, although intergenic mutations were identified. Recently, an analogous ratio to d N/d S was described to assess selective mechanisms occurring in non-coding regions, where d N is replaced by the number of intergenic SNPs per intergenic site (d I) [80]. Even though this method has not been used on a P. aeruginosa genome yet, the signature of purifying selection was observed for intergenic sites of other species such as Escherichia coli or Staphylococcus aureus [80, 81]. However, Khademi and colleagues reanalysed the sequencing data of intergenic regions from several longitudinal P. aeruginosa genomic studies [6, 14, 16] and were able to establish a list of adaptive non-coding regions mutated in at least 3 of the 44 studied lineages [81] (Table 3).
Table 3.
Selection of P. aeruginosa intergenic regions under positive selection
Mutations in intergenic regions were identified as positively selected by Khademi et al. [81] and selected for this table according to their number, the number of affected lineages and the number of longitudinal studies highlighting mutations in the same intergenic region. The complete list is shown in Table S1.
|
Upstream/downstream genes |
Upstream/ downstream PAO1 locus |
Upstream/downstream products |
No. of intergenic mutations |
No. of lineages |
Reference |
||
|---|---|---|---|---|---|---|---|
|
phuS // phuR |
PA4709 // PA4710 |
PhuS/haem/haemoglobin uptake outer-membrane receptor |
40 |
4 |
|||
|
PA0428 // PA0429 |
PA0428 // PA0429 |
Probable ATP-dependent RNA helicase/hypothetical protein |
34 |
10 |
[81] |
||
|
PA4786 // PA4787 |
PA4786 // PA4787 |
Probable short-chain dehydrogenase/probable transcriptional regulator |
28 |
12 |
[81] |
||
|
PA4690.5 // PA4691 |
PA4690.5 // PA4691 |
16S ribosomal RNA/hypothetical protein |
54 |
6 |
[81] |
||
|
PA2535 // PA2536 |
PA2535 // PA2536 |
Probable oxidoreductase/probable phosphatidate cytidylyltransferase |
18 |
6 |
[7, 81] |
||
|
motY // pyrC |
PA3526 // PA3527 |
Probable outer-membrane protein precursor/dihydroorotase |
32 |
6 |
[81] |
||
|
PA3230 // PA3231 |
PA3230 // PA3231 |
Conserved hypothetical protein/conserved hypothetical protein |
24 |
7 |
[81] |
||
|
algL // algl |
PA3547 // PA3548 |
Poly(β-d-mannuronate) lyase precursor/alginate O-acetyltransferase |
14 |
6 |
[7, 81] |
||
|
PA0976.1 // PA0977 |
PA0976.1 // PA0977 |
tRNA-Lys/hypothetical protein |
26 |
6 |
[81] |
||
|
rplU // ispB |
PA4568 // PA4569 |
50S ribosomal protein L21/octaprenyldiphosphate synthase |
22 |
7 |
[81] |
||
|
phzM // phzA1 |
PA4209 // PA4210 |
Probable phenazine-specific methyltransferase |
12 |
6 |
[7, 81] |
||
|
oprO // PA3281 |
PA3280 // PA3281 |
Pyrophosphate-specific outer-membrane porin precursor/hypothetical protein |
10 |
5 |
[7, 81] |
||
|
ldh // PA3419 |
PA3418 // PA3419 |
Leucine dehydrogenase |
10 |
5 |
[7, 81] |
||
|
ampR // ampC |
PA4109 // PA4110 |
Transcriptional regulator/β-lactamase precursor |
12 |
4 |
[9, 81] |
||
|
PA5160.1 // rmlB |
PA5160.1 // PA5161 |
tRNA-Thr/dTDP-d-glucose 4,6- dehydratase |
16 |
6 |
[81] |
||
Genes of which the promoter is located in the impacted intergenic region are underlined.
Interestingly, some of the adaptive intergenic regions identified have been found to be mutated in other longitudinal studies of which the sequencing data were not used in the analysis by Khademi et al. [7, 9, 18], supporting their role in P. aeruginosa adaptation. Table 3 presents the 15 adaptive intergenic regions most frequently mutated, i.e. regions that accumulated the highest number of mutations, in the most elevated number of lineages and longitudinal studies. The complete table is shown in Table S1. Mutations in the phuS/phuR intergenic region were identified in the largest number of studies and at significant rates. Finally, we notice that mutations occurred in the intergenic region between ampR and ampC, a gene that was also identified as pathoadaptive (Table 2). Genetic modifications of intergenic regions, thus, appear to also play a role in P. aeruginosa adaptation to the CF environment, potentially through the transcriptomic dysregulation of surrounding genes [81].
It is noteworthy that the sequencing results of the 13 longitudinal studies analysed in this review could be connected thanks to the genomic annotations from the reference strains PAO1 or PA14. Thus, Tables 2 and 3 are not representative of the numerous mutations occurring within genes or intergenic regions specific to clinical isolates. Moreover, the accessory genome of P. aeruginosa presents very divergent profiles according to the isolates, with great variations in its composition and its organization [4, 19]. As a result, accessory elements present a higher sequence diversity [27, 32, 60, 82], limiting the establishment of convergent evolutionary patterns within the accessory genome. Nonetheless, it needs to be kept in mind that some accessory genes can have homologous functions other than those present in the core genome [4, 19] and, thus, sometimes compensate a mutation in a conserved gene.
Phenotypical signatures of CF-adapted P. aeruginosa
P. aeruginosa adapts its expression profiles to the CF environment
Gene expression
The comparison of transcriptomes or proteomes of sequential clinical isolates seems to be the most suitable for assessing impacts of P. aeruginosa adaptation on global expression profiles. Several longitudinal studies indeed performed transcriptional profiling and observed differences of global transcript abundance between early and late isolates [9, 13], but also on specific expressed genes [12, 13, 83–85]. Table 4(a) lists 41 P . aeruginosa genes differentially expressed between early and late CF isolates. Convergent patterns of expression could be identified in vitro in late isolates in comparison to related early isolates, for instance, a down-regulation of genes involved in secretion (Hcp secretion island I), the pseudomonas quinolone signal (PQS) and phenazine biosynthesis. Interestingly, more than a half of dysregulated genes presented in Table 4 have been shown to be part of RpoN, AlgU or LasR regulons, underscoring their significance in P. aeruginosa adaptive mechanisms [86–89].
Table 4.
P. aeruginosa transcriptomic alterations during adaptation to the CF lung environment
Square colour indicates gene expression: up-regulation (red), down-regulation (green), undetermined (light grey), divergent according to studies (dark grey). (a) Gene expression in late isolates in comparison to related early isolates of P. aeruginosa . The 41 genes with a convergent pattern identified in at least four isolates were selected [12, 13, 83–85]. (b) Gene expression in clinical CF isolates in vivo (CF sputum, explanted lungs or zebra fish infection) in comparison to growth in vitro [91–94]. (c) Gene expression in PAO1 in vivo (murine infection model of acute pneumonia) in comparison to growth in vitro [95].
|
Gene name |
PAO1 locus |
Product |
(a) Expression in late isolates |
(b) Expression in CF isolates in vivo |
(c) Expression in PAO1 in vivo |
|||
|---|---|---|---|---|---|---|---|---|
|
PA1323 |
PA1323f |
Hypothetical protein |
||||||
|
PA1324 |
PA1324f |
Hypothetical protein |
||||||
|
PA1471 |
PA1471 |
Hypothetical protein |
||||||
|
PA1559 |
PA1559 |
Hypothetical protein |
||||||
|
PA1592 |
PA1592 |
Hypothetical protein |
||||||
|
mexX |
PA2019 |
RND multidrug efflux membrane fusion protein |
Key |
|||||
|
PA2485 |
PA2485 |
Hypothetical protein |
Up-regulation |
|||||
|
PA3691 |
PA3691g |
Hypothetical protein |
Down-regulation |
|||||
|
lptF |
PA3692g |
Lipotoxon F |
Undetermined |
|||||
|
PA3819 |
PA3819 |
Conserved hypothetical protein |
Divergent |
|||||
|
osmE |
PA4876 |
Osmotically inducible lipoprotein |
||||||
|
PA4880 |
PA4880 |
Probable bacterioferritin |
||||||
|
PA5212 |
PA5212 |
Hypothetical protein |
||||||
|
PA0045 |
PA0045 |
Hypothetical protein |
||||||
|
PA0046 |
PA0046 |
Hypothetical protein |
||||||
|
PA0047 |
PA0047 |
Hypothetical protein |
||||||
|
tagQ1 |
PA0070a |
TagQ1 |
||||||
|
pppA |
PA0075a |
PppA |
||||||
|
tagF1 |
PA0076a |
Hcp secretion island I (HSI-I) T6SS |
||||||
|
icmF1 |
PA0077a |
Hcp secretion island I (HSI-I) T6SS |
||||||
|
tssL1 |
PA0078b |
Hcp secretion island I (HSI-I) T6SS |
||||||
|
tssK1 |
PA0079b |
Hcp secretion island I (HSI-I) T6SS |
||||||
|
tssJ1 |
PA0080b |
Hcp secretion island I (HSI-I) T6SS |
||||||
|
ttsA1 |
PA0082c |
Hcp secretion island I (HSI-I) T6SS |
||||||
|
ttsB1 |
PA0083c |
Hcp secretion island I (HSI-I) T6SS |
||||||
|
ttsC1 |
PA0084c |
Hcp secretion island I (HSI-I) T6SS |
||||||
|
hcp1 |
PA0085 |
Hcp secretion island I (HSI-I) T6SS |
||||||
|
tagJ1 |
PA0086d |
Hcp secretion island I (HSI-I) T6SS |
||||||
|
tssE1 |
PA0087d |
Hcp secretion island I (HSI-I) T6SS |
||||||
|
tssG1 |
PA0089d |
Hcp secretion island I (HSI-I) T6SS |
||||||
|
clpV1 |
PA0090d |
ClpV1 |
||||||
|
pqsC |
PA0998e |
β-Keto-acyl-acyl-carrier protein synthase |
||||||
|
pqsD |
PA0999e |
Acetyl CoA ACP transacetylase |
||||||
|
phnA |
PA1001 |
Phenazine biosynthesis protein |
||||||
|
HsiB2 |
PA1657 |
Conserved hypothetical protein |
||||||
|
hcnA |
PA2193 |
Hydrogen cyanide synthase |
||||||
|
tse5 |
PA2684 |
Cell wall/membrane/envelope biogenesis |
||||||
|
PA3021 |
PA3021 |
Hypothetical protein |
||||||
|
PA3729 |
PA3729 |
Conserved hypothetical protein |
||||||
|
cytN |
PA4133 |
Cytochrome c oxidase subunit |
||||||
|
PA4317 |
PA4317 |
Hypothetical protein |
Genes annotated with an identical letter belong to the same operon.
Genes in bold respond to the following criteria: (i) convergent expression in CF late isolates in comparison to early ones, (ii) convergent expression in vivo in comparison to in vitro growth,and (iii) specific dysregulations in vivo in comparison to PAO1.
It is important to remember that gene expression relies highly on growth conditions and that in vitro patterns are not necessarily representative of what happens in vivo. Thanks to the advance of transcriptomic methods, recent studies evaluated P. aeruginosa global gene expression ‘in vivo’, i.e. directly on clinical populations within sputum [90–92], ex-planted lungs from CF patients [93], or during non-human infection models [94]. Transcriptomic patterns induced by in vivo conditions are presented in Table 4(b). Comparable transcriptomic dysregulations to those observed for in vitro transcriptomic analyses were depicted for more than a half of the genes listed in Table 4(a, b), including the down-regulation of genes from the Hcp secretion island. Interestingly, these dysregulations seem to be specific to CF clinical isolates. The PAO1 reference strain, not adapted to the CF-environment, was shown to present a very divergent, if not opposite, transcriptomic pattern during in vivo infection. These results were nonetheless obtained using a murine model of acute pneumonia and should be confirmed in a chronic infection context [95] (Table 4c). Altogether, these transcriptomic studies underscored the role of several genes in P. aeruginosa adaptation to the CF environment due to: (i) convergent expression in CF-adapted isolates in comparison to non-adapted ones, (ii) convergent expression in vivo in comparison to in vitro growth, and (iii) specific dysregulations in vivo in comparison to PAO1. Genes meeting these three criteria are highlighted in Table 4.
Protein expression
P. aeruginosa protein expression during CF infections was mainly assessed by evaluating proteomic changes between clinical and reference strains or under certain conditions, as reviewed by Hare and Cordwell and by Kamath et al. [96, 97]. More recently, this approach was used to evaluate proteome responses of a set of clinical isolates cultivated under different conditions of nutrient and oxygen availability [98–100]. Clinical P. aeruginosa isolates presented a distinct proteome profile from PAO1, with convergent expression of many proteins despite a high genomic and phenotypic diversity between isolates. An over-expression of proteins involved in amino acid biosynthesis or drug resistance, with the example of MexY was specifically noted for clinical isolates [98, 99]. Several proteins involved in motility, chemotaxis and adhesion features were also down-regulated, including proteins from the Fli and Pil systems, confirming previous observations [96, 97].
To our knowledge, differences of the global proteome between early and CF-adapted clonal isolates of P. aeruginosa , however, have not been assessed yet, limiting the establishment of direct relationships between genetic adaptation to the CF environment and protein expression. Nonetheless, a recent study described the P. aeruginosa proteome directly from CF sputum. By comparing protein expression in the P. aeruginosa population from 35 samples, Wu and colleagues, thus, were able to identify a convergent pattern of protein expression in vivo [101] (Table 5a). Some of the proteins identified as more abundantly produced by clinical isolates than by PAO1 were found also to be highly produced in vitro, with the example of the chaperone Hfq and the phosphate transporter PtsS (Table 5b) [98, 99, 101–103]. Here again, protein expression pattern appears to largely rely on growth conditions (Table 5b).
Table 5.
P. aeruginosa proteomic expression in vivo in comparison to in vitro conditions
Square colour indicates protein expression: up-regulation (red), down-regulation (green), undetermined (light grey). (a) Protein expression in P. aeruginosa populations from CF sputa, in comparison to populations grown in vitro [101]. The 15 proteins identified with a convergent pattern within the most samples were selected. (b) Protein expression in P. aeruginosa CF isolates in comparison to PAO1 determined in vitro in minimal medium M9 [99], rich medium LB [98, 102, 103] or in sputum-like media SCFM [99] or ASMDM (artificial sputum medium with high molecular mass DNA and mucin) [103], for the 15 proteins identified as expressed in vivo. NA, Not available.
|
Protein name |
PAO1 locus |
Product |
(a) In vivo vs in vitro |
(b) In vitro vs PA01 |
||||
|---|---|---|---|---|---|---|---|---|
|
Expression in CF sputa |
No. of samples with convergent pattern |
No. of samples with detected protein |
Expression in minimal medium |
Expression in rich medium |
Expression in sputum-like media |
|||
|
OprD |
PA0958 |
Outer-membrane porin precursor |
20 |
25 |
||||
|
OprH |
PA1178 |
PhoP/Q and low Mg2+ inducible outer-membrane protein H1 precursor |
27 |
33 |
||||
|
PA1288 |
PA1288 |
Probable outer-membrane protein precursor |
26 |
33 |
||||
|
OprI |
PA2853 |
Outer-membrane lipoprotein OprI precursor |
26 |
35 |
||||
|
AlgE |
PA3544 |
Alginate production outer-membrane protein AlgE precursor |
20 |
21 |
||||
|
FumC1 |
PA4470 |
Fumarate hydratase |
24 |
30 |
||||
|
PhuR |
PA4710 |
Haem/haemoglobin uptake outer-membrane receptor precursor |
22 |
32 |
||||
|
PA4793 |
PA4793 |
Hypothetical protein |
23 |
31 |
||||
|
PA4837 |
PA4837 |
Probable outer-membrane protein precursor |
28 |
31 |
||||
|
Hfq |
PA4944 |
Hfq |
19 |
29 |
||||
|
PstS |
PA5369 |
Phosphate ABC transporter, periplasmic phosphate-binding protein |
25 |
26 |
||||
|
NA |
NA |
TonB-dependent receptor |
24 |
25 |
||||
|
Icd |
PA2623 |
Isocitrate dehydrogenase |
21 |
30 |
||||
|
RpsB |
PA3656 |
30S ribosomal protein S2 |
20 |
28 |
||||
|
RplS |
PA3742 |
50S ribosomal protein L19 |
23 |
26 |
||||
Convergent phenotypes are selected by P. aeruginosa adaptation
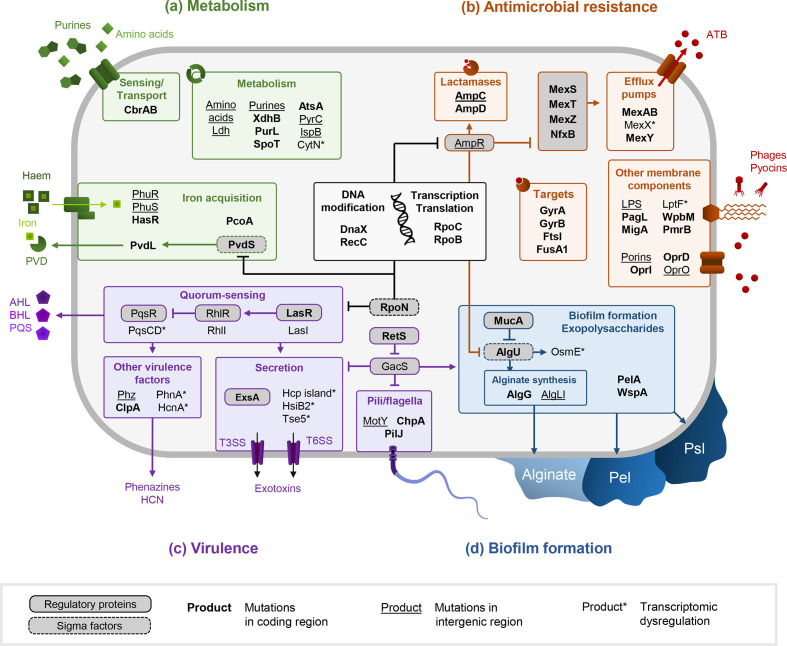
As a result of the diversification of genetic, transcriptomic and proteomic profiles, CF-adapted P. aeruginosa can present various phenotypic signatures (Fig. 2) [46, 47, 104]. Although these are often found to be patient dependent [17, 105], similar phenotypes are frequently observed in adapted P. aeruginosa isolates, including alterations of metabolism, antibiotic resistance, biofilm and virulence. These phenotypes are associated with chronic infections as they promote bacterial persistence within lungs and have been extensively described [46, 47, 104, 106–108]. Interestingly, an analogous phenotypic diversification could be recently reproduced in vitro by experimental evolution in CF-mimicking conditions. Schick and colleagues observed that the complexity and the viscosity of the synthetic cystic fibrosis sputum medium (SCFM) containing mucin was sufficient to induce several common phenotypes of CF strains, such as antibiotic resistance, biofilm formation, loss of motility and production of virulence factors [109].
Fig. 2.
Pathways related to metabolism (a), antimicrobial resistance (b), virulence (c) and biofilm formation (d) altered during P. aeruginosa adaptation to the CF environment. DNA sequences of products in bold have been shown to accumulate non-synonymous mutations. Intergenic regions surrounding products that are underscored are mutated. Late isolates present a convergent transcriptomic dysregulation of the products marked by asterisks in comparison to early isolates.
Metabolic alterations
The energetic metabolism of P. aeruginosa is largely affected by its adaptation to the CF environment. As a consequence of non-synonymous mutations in numerous metabolism-related genes, adapted P. aeruginosa strains present a differential and adjusted assimilation of the nutrients present in the CF lung (Fig. 2a) [17, 67, 105, 107, 110]. Auxotrophy or reduction of catabolic capacities are frequently observed and arise from either low or high molecule availability in the CF environment. Amino acid auxotrophy often arises in CF-adapted P. aeruginosa due to the high abundance of these molecules in CF sputum [107, 110–112]; in addition, purine auxotrophy can be established in DNA-rich sputa [113]. Development of new metabolic capacities can nonetheless arise through enrichment of the accessory genome in metabolic functions [17, 28, 60]. This adjusted metabolism increases P. aeruginosa fitness in the CF environment, but it often results in a slowed growth in laboratory conditions in comparison to non-adapted isolates [7, 8, 12, 13, 18, 107, 110, 114]. This modification of metabolic activities can limit effective detection and treatment of infecting P. aeruginosa , as illustrated by the emergence of highly resistant small colony variants (SCVs) and viable but non-culturable (VBNC) isolates [115–117].
Antimicrobial resistance and biofilm
Another feature limiting treatment of P. aeruginosa infection is the development of resistance mechanisms to antimicrobials. In comparison to early strains, late P. aeruginosa isolates present a greater antibiotic resistance acquired through different mechanisms: (i) alteration of antibiotic transport, (ii) increase of antibiotic degradation, and (iii) alteration of antibiotic targets [118]. The alteration of antibiotic transport is characterized by a decrease of antibiotic input through reduction of porin activities, and in an increase of drug output through modification of the efflux pumps activity. Particularly, oprD repression and mexAB overexpression, induced by mutations in their own coding sequences or in their regulators, are frequently responsible for β-lactam resistance in CF P. aeruginosa (Fig. 2b) [10, 118, 119]. Such resistance can also be promoted by the genome enrichment of accessory genes involved in multidrug secretion. The many transporters constituting the accessory genome of the LES epidemic strain, thus, contribute to its high antibiotic resistance and its epidemiological success [23]. The increase in antibiotic degradation is mainly perpetrated by an overproduction of the cephalosporinase AmpC, induced by mutations in the ampCD genes but also in the coding sequencing of their regulator AmpR (Fig. 2b) [118]. Finally, the increase of P. aeruginosa multidrug resistance can also involve the alteration of several antibiotic targets, such as the DNA gyrase GyrAB, the penicillin-binding protein FtsI or the lipopolysaccharide (LPS) of the bacterial outer membrane [11, 118, 120, 121] (Fig. 2b). The latter undergoes important alterations of its three components during P. aeruginosa adaptation to the CF environment. Mutations in pmrB, migA and pagL are associated with structural modifications of the lipid A part of the LPS, inducing resistance to polymyxins [10–12, 120, 122]. The alteration of MigA and LptF can also affect the synthesis of the core oligosaccharide and the transport of the mature LPS, although their impact on antibiotic resistance remains poorly understood [121, 123, 124]. Finally, CF isolates often lack the O-antigen polysaccharide of the LPS due to mutations in wbp genes, resulting in lower virulence and increased tolerance to gentamicin [121, 125].
Besides antibiotics, LPS modifications also affect P. aeruginosa resistance to phages and bacteriocins [120]. In CF-adapted P. aeruginosa , mutations in LPS biosynthesis genes were shown to decrease phage susceptibility by hampering LPS-mediated recognition [120, 126]. In contrast, chronic CF isolates are often more susceptible to the P. aeruginosa -produced bacteriocins, pyocins, due to an improved access to the cell envelope following the structural alterations of the O-antigen [120, 127, 128]. However, pyocin production is also frequently reduced in chronic CF P. aeruginosa [126, 127].
Resistance to antimicrobials is also associated with an increased formation of biofilm. The exopolysaccharide matrix, constituted of varying proportions of Pel, Psl or alginate molecules according to the strain, indeed allows the constitution of a physical and chemical barrier against antimicrobials (Fig. 2d) [129–132]. CF-adapted strains often present an up-regulation of Pel, Psl and/or alginate exopolysaccharides production; hence, increasing biofilm formation, modifying the composition of its matrix and favouring antimicrobial resistance [130]. Pel and Psl overproduction is, thus, responsible for the persistence phenotype of rugose small colony variants (RSCVs) in CF P. aeruginosa [133, 134]. Mucoid isolates, mainly arising from mucA alterations inducing alginate overproduction, are also associated with poorer clinical outcome and greater inflammation [135–138]. Interestingly, mucoid and non-mucoid isolates are often co-isolated from CF patients, due to diversification or reversion of the phenotype through compensatory mutations, in algU for instance (Fig. 2d) [11, 18, 72]. Sessile lifestyle is also promoted by a loss of motility linked to inhibition of pili and flagella synthesis [10, 12, 18]. Alterations of these membrane components, as well as LPS modification and biofilm formation, reduce the induction of the host inflammasome and, thus, efficient bacterial elimination from the lungs [106, 108].
Virulence
In the same way, P. aeruginosa -adapted isolates have been shown to secrete fewer virulence factors, which are both immunogenic and costly to produce [10, 18, 108]. Iron plays a pivot role in bacterial virulence and its acquisition is affected during P. aeruginosa adaptation to the CF environment. Alteration of pyoverdine siderophore synthesis through mutations in the regulator pvdS and the pvd genes is often observed, inducing a loss of virulence [125, 139, 140]. In contrast, iron acquisition through haem is promoted in adapted isolates thanks to the up-regulation of Phu and Has systems (Fig. 2a) [139, 141]. Changes in the accessory genome composition also undoubtedly affect P. aeruginosa virulence, as chronic or eradicated CF isolates present a different repertory of accessory functions than virulent ones [17, 32]. Alteration of the genomic islands PAPI-1 and PAPI-2 and the LES phages can greatly lower P. aeruginosa virulence [21, 22, 24]. In connection with this, CF isolates from chronic infection strains often lacks the PAPI-2 encoded cytotoxin ExoU. They instead harbour the type III secretion system (T3SS) effector ExoS, which is chromosomally encoded and has less virulent properties than ExoU [142–145]. However, mutations in major virulence and quorum-sensing (QS) regulators, such as retS, exsA or lasR, are the main perpetrators of the low-virulence state of chronic P. aeruginosa (Fig. 2c).
QS rewiring and modification of microbial interactions
The alterations of QS systems suggest that P. aeruginosa adaptation goes along with a reduction of social behaviours. This hypothesis is supported by the high frequency of lasR mutations that are also acquired during in vitro evolution of P. aeruginosa [146, 147]. On the one hand, the emergence of lasR-mutant social cheaters within the bacterial population suggest a loss of intra-species cooperative behaviours as these mutants will benefit from extracellular factors produced by other members without paying the energy cost [148–150]. However, this also indicates that QS activities and social behaviours need to be considered at the whole population scale. On the other hand, several recent studies depicted that lasR mutants isolated from CF infections retained an active QS through a lasR-independent induction of the Rhl system. This phenomenon was often related to compensatory mutations in the pathoadaptive mexT gene [63–66], and not by alteration of rhl genes. The latter are indeed rarely mutated during P. aeruginosa evolution within CF lungs, underscoring the importance of maintaining a functional Rhl system during chronic infections. Instead of a loss of QS, P. aeruginosa adaptation to the CF lung rather induces a rewiring of QS networks for the benefit of a Rhl-mediated social behaviour within the bacterial population. Furthermore, the intra-species interactions of P. aeruginosa do not seem to involve pyocins anymore, since both pyocin resistance and production are frequently reduced in chronic isolates [120, 126, 127]. However, pyocins and many of the QS-regulated factors also play a critical role in interspecies interactions, such as the type VI secretion system (T6SS) and pyocyanin (Fig. 2c) [151–155]. And indeed, an increasing number of studies highlight an evolution of P. aeruginosa interactions with other co-colonizing micro-organisms in the CF environment [155–160].
Concluding remarks and future perspectives
The numerous sequencing studies performed on clinical isolates allowed the description of the main genetic mechanisms of P. aeruginosa adaptation to the CF environment. This adaptation mainly relies on the accumulation and the selection of small mutations in pathoadaptive genes. For the first time, this phenomenon was recently shown to occur within intergenic regions as well. As these non-coding elements were rarely taken into account in genomic studies, reanalyses of the vast amount of sequencing data already available should allow a better examination of their role in the P. aeruginosa adaptation process. At the same time, the ambiguous impact of recombination and large chromosomal rearrangements on pathoadaptation could be clarified by combining second- and third-generation sequencing methods to assemble complete genomes.
Alteration of pathoadaptive elements allows the establishment of persistence phenotypes in P. aeruginosa , such as high antibiotic resistance through an increased efficiency of antimicrobial efflux, an enhanced ability to form biofilm and a slowed metabolism. In addition, the low-virulence state of CF-adapted P. aeruginosa limits the proper functioning of the host immune responses. However, the precise relationship between these phenotypes and the P. aeruginosa genotype remains difficult to evaluate, especially due to the intense diversification occurring during adaptation and the pleiotropic effects of most mutations. The study of several isolates per time point throughout longitudinal studies would allow a better overview of the different evolutionary paths taken by the bacterium within CF lungs. Assessing the changes in gene and protein expressions during P. aeruginosa adaptation thanks to -omics methods can also address some of these issues, with particular attention to the expression conditions. Transcriptomic and proteomic studies in vivo or in CF-like conditions, thus, appears essential to gain more insight in the physiological adaptation of P. aeruginosa to the CF environment.
The description of P. aeruginosa adaptive process ensures a better understanding of the selection forces that drive its evolution within the CF lung. While some of them are already known, such as antibiotic and oxidative stresses, other selective pressures remain little explored. Due to the polymicrobial nature of CF infections, the role of other microbial communities in P. aeruginosa adaptive mechanisms deserves more consideration. The activities of native or co-colonizing micro-organisms can deeply affect the environment characteristics, such as the distribution and availability of nutrients, iron or antimicrobial molecules. Moreover, a range of microbial interactions can either limit or promote P. aeruginosa persistence and, thus, adaptation within CF lung infections [156–158, 160–162]. In line with this, the presence of Staphylococcus aureus has been shown to promote P. aeruginosa colonization [163], whereas the latter was negatively associated with infection by other pathogens such as Burkholderia cepacia and Stenotrophomonas maltophilia [164]. Besides pathogens, the role of the normal lung microbiota is increasingly considered since commensal anaerobes have been shown to impact the antibiotic resistance and virulence of P. aeruginosa [161, 162]. Thus, the presence of these micro-organisms may influence establishment and adaptation of P. aeruginosa in the CF environment. Ultimately, the comprehensive understanding of this adaptation appears pivotal to limit the establishment of chronic P. aeruginosa infections.
Supplementary Data
Funding information
This work was supported by grants from the Fondation pour la Recherche Médicale (grant number ECO20170637499 to L. C.), the Finovi foundation (to K. M.), and the associations ‘Vaincre la mucoviscidose’ and ‘Gregory Lemarchal’ to (K. M.).
Author contributions
K. M. and L. C. were primarily responsible for writing the original draft. K. M., L. C. and F. V. contributed to the review and editing of the final version.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: CF, cystic fibrosis; LPS, lipopolysaccharide; PAPI, Pseudomonas aeruginosa pathogenic island; QS, quorum sensing; T3SS, type III secretion system; T6SS, type VI secretion system.
All supporting data, code and protocols have been provided within the article or through supplementary data files. One supplementary table is available with the online version of this article.
References
- 1.Moradali MF, Ghods S, Rehm BHA. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elabed H, González-Tortuero E, Ibacache-Quiroga C, Bakhrouf A, Johnston P, et al. Seawater salt-trapped Pseudomonas aeruginosa survives for years and gets primed for salinity tolerance. BMC Microbiol. 2019;19:142. doi: 10.1186/s12866-019-1499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewenza S, Abboud J, Poon K, Kobryn M, Humplik I, et al. Pseudomonas aeruginosa displays a dormancy phenotype during long-term survival in water. PLoS One. 2018;13:e0198384. doi: 10.1371/journal.pone.0198384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kung VL, Ozer EA, Hauser AR. The accessory genome of Pseudomonas aeruginosa . Microbiol Mol Biol Rev. 2010;74:621–641. doi: 10.1128/MMBR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkins MD, Somayaji R, Waters VJ. Epidemiology, biology, and impact of clonal Pseudomonas aeruginosa infections in cystic fibrosis. Clin Microbiol Rev. 2018;31:e00019-18. doi: 10.1128/CMR.00019-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA. 2006;103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer N, Klockgether J, Wrasman K, Schmidt M, Davenport CF, et al. Microevolution of the major common Pseudomonas aeruginosa clones C and PA14 in cystic fibrosis lungs. Environ Microbiol. 2011;13:1690–1704. doi: 10.1111/j.1462-2920.2011.02483.x. [DOI] [PubMed] [Google Scholar]
- 8.Feliziani S, Marvig RL, Luján AM, Moyano AJ, Di Rienzo JA, et al. Coexistence and within-host evolution of diversified lineages of hypermutable Pseudomonas aeruginosa in long-term cystic fibrosis infections. PLoS Genet. 2014;10:e1004651. doi: 10.1371/journal.pgen.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markussen T, Marvig RL, Gómez-Lozano M, Aanæs K, Burleigh AE, et al. Environmental heterogeneity drives within-host diversification and evolution of Pseudomonas aeruginosa . mBio. 2014;5:e01592-14. doi: 10.1128/mBio.01592-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianconi I, Jeukens J, Freschi L, Alcalá-Franco B, Facchini M, et al. Comparative genomics and biological characterization of sequential Pseudomonas aeruginosa isolates from persistent airways infection. BMC Genomics. 2015;16:1105. doi: 10.1186/s12864-015-2276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianconi I, D’Arcangelo S, Esposito A, Benedet M, Piffer E, et al. Persistence and microevolution of Pseudomonas aeruginosa in the cystic fibrosis lung: a single-patient longitudinal genomic study. Front Microbiol. 2018;9:3242. doi: 10.3389/fmicb.2018.03242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Mansfeld R, de Been M, Paganelli F, Yang L, Bonten M, et al. Within-host evolution of the Dutch high-prevalent Pseudomonas aeruginosa clone ST406 during chronic colonization of a patient with cystic fibrosis. PLoS One. 2016;11:e0158106. doi: 10.1371/journal.pone.0158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Jelsbak L, Marvig RL, Damkiaer S, Workman CT, et al. Evolutionary dynamics of bacteria in a human host environment. Proc Natl Acad Sci USA. 2011;108:7481–7486. doi: 10.1073/pnas.1018249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marvig RL, Johansen HK, Molin S, Jelsbak L. Genome analysis of a transmissible lineage of Pseudomonas aeruginosa reveals pathoadaptive mutations and distinct evolutionary paths of hypermutators. PLoS Genet. 2013;9:e1003741. doi: 10.1371/journal.pgen.1003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wee BA, Tai AS, Sherrard LJ, Ben Zakour NL, Hanks KR, et al. Whole genome sequencing reveals the emergence of a Pseudomonas aeruginosa shared strain sub-lineage among patients treated within a single cystic fibrosis centre. BMC Genomics. 2018;19:644. doi: 10.1186/s12864-018-5018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marvig RL, Sommer LM, Molin S, Johansen HK. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat Genet. 2015;47:57–64. doi: 10.1038/ng.3148. [DOI] [PubMed] [Google Scholar]
- 17.Klockgether J, Cramer N, Fischer S, Wiehlmann L, Tümmler B. Long-term microevolution of Pseudomonas aeruginosa differs between mildly and severely affected cystic fibrosis lungs. Am J Respir Cell Mol Biol. 2018;59:246–256. doi: 10.1165/rcmb.2017-0356OC. [DOI] [PubMed] [Google Scholar]
- 18.Marvig RL, Dolce D, Sommer LM, Petersen B, Ciofu O, et al. Within-host microevolution of Pseudomonas aeruginosa in Italian cystic fibrosis patients. BMC Microbiol. 2015;15:218. doi: 10.1186/s12866-015-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu X, Kulasekara BR, Lory S. Role of horizontal gene transfer in the evolution of Pseudomonas aeruginosa virulence. Genome Dyn. 2009;6:126–139. doi: 10.1159/000235767. [DOI] [PubMed] [Google Scholar]
- 20.Brockhurst MA, Buckling A, Rainey PB. The effect of a bacteriophage on diversification of the opportunistic bacterial pathogen, Pseudomonas aeruginosa . Proc Biol Sci. 2005;272:1385–1391. doi: 10.1098/rspb.2005.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winstanley C, Langille MGI, Fothergill JL, Kukavica-Ibrulj I, Paradis-Bleau C, et al. Newly introduced genomic prophage islands are critical determinants of in vivo competitiveness in the Liverpool epidemic strain of Pseudomonas aeruginosa . Genome Res. 2009;19:12–23. doi: 10.1101/gr.086082.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison EM, Carter MEK, Luck S, Ou H-Y, He X, et al. Pathogenicity islands PAPI-1 and PAPI-2 contribute individually and synergistically to the virulence of Pseudomonas aeruginosa strain PA14. Infect Immun. 2010;78:1437–1446. doi: 10.1128/IAI.00621-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dettman JR, Rodrigue N, Aaron SD, Kassen R. Evolutionary genomics of epidemic and nonepidemic strains of Pseudomonas aeruginosa . Proc Natl Acad Sci USA. 2013;110:21065–21070. doi: 10.1073/pnas.1307862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemieux A-A, Jeukens J, Kukavica-Ibrulj I, Fothergill JL, Boyle B, et al. Genes required for free phage production are essential for Pseudomonas aeruginosa chronic lung infections. J Infect Dis. 2016;213:395–402. doi: 10.1093/infdis/jiv415. [DOI] [PubMed] [Google Scholar]
- 25.Subedi D, Vijay AK, Kohli GS, Rice SA, Willcox M. Comparative genomics of clinical strains of Pseudomonas aeruginosa strains isolated from different geographic sites. Sci Rep. 2018;8:15668. doi: 10.1038/s41598-018-34020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu X, Gurkar AU, Lory S. Interstrain transfer of the large pathogenicity island (PAPI-1) of Pseudomonas aeruginosa . Proc Natl Acad Sci USA. 2006;103:19830–19835. doi: 10.1073/pnas.0606810104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klockgether J, Würdemann D, Reva O, Wiehlmann L, Tümmler B. Diversity of the abundant pKLC102/PAGI-2 family of genomic islands in Pseudomonas aeruginosa . J Bacteriol. 2007;189:2443–2459. doi: 10.1128/JB.01688-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathee K, Narasimhan G, Valdes C, Qiu X, Matewish JM, et al. Dynamics of Pseudomonas aeruginosa genome evolution. Proc Natl Acad Sci USA. 2008;105:3100–3105. doi: 10.1073/pnas.0711982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter MQ, Chen J, Lory S. The Pseudomonas aeruginosa pathogenicity island PAPI-1 is transferred via a novel type IV pilus. J Bacteriol. 2010;192:3249–3258. doi: 10.1128/JB.00041-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James CE, Fothergill JL, Kalwij H, Hall AJ, Cottell J, et al. Differential infection properties of three inducible prophages from an epidemic strain of Pseudomonas aeruginosa . BMC Microbiol. 2012;12:216. doi: 10.1186/1471-2180-12-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rau MH, Marvig RL, Ehrlich GD, Molin S, Jelsbak L. Deletion and acquisition of genomic content during early stage adaptation of Pseudomonas aeruginosa to a human host environment. Environ Microbiol. 2012;14:2200–2211. doi: 10.1111/j.1462-2920.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 32.Bezuidt OKI, Klockgether J, Elsen S, Attree I, Davenport CF, et al. Intraclonal genome diversity of Pseudomonas aeruginosa clones CHA and TB. BMC Genomics. 2013;14:416. doi: 10.1186/1471-2164-14-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma P, Gupta SK, Rolain J-M. Whole genome sequencing of bacteria in cystic fibrosis as a model for bacterial genome adaptation and evolution. Expert Rev Anti Infect Ther. 2014;12:343–355. doi: 10.1586/14787210.2014.887441. [DOI] [PubMed] [Google Scholar]
- 34.Fothergill JL, Mowat E, Ledson MJ, Walshaw MJ, Winstanley C. Fluctuations in phenotypes and genotypes within populations of Pseudomonas aeruginosa in the cystic fibrosis lung during pulmonary exacerbations. J Med Microbiol. 2010;59:472–481. doi: 10.1099/jmm.0.015875-0. [DOI] [PubMed] [Google Scholar]
- 35.Römling U, Schmidt KD, Tümmler B. Large genome rearrangements discovered by the detailed analysis of 21 Pseudomonas aeruginosa clone C isolates found in environment and disease habitats. J Mol Biol. 1997;271:386–404. doi: 10.1006/jmbi.1997.1186. [DOI] [PubMed] [Google Scholar]
- 36.Harmer C, Alnassafi K, Hu H, Elkins M, Bye P, et al. Modulation of gene expression by Pseudomonas aeruginosa during chronic infection in the adult cystic fibrosis lung. Microbiology. 2013;159:2354–2363. doi: 10.1099/mic.0.066985-0. [DOI] [PubMed] [Google Scholar]
- 37.Andersen SB, Ghoul M, Griffin AS, Petersen B, Johansen HK, et al. Diversity, prevalence, and longitudinal occurrence of type II toxin-antitoxin systems of Pseudomonas aeruginosa infecting cystic fibrosis lungs. Front Microbiol. 2017;8:1180. doi: 10.3389/fmicb.2017.01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.England WE, Kim T, Whitaker RJ. Metapopulation structure of CRISPR-Cas immunity in Pseudomonas aeruginosa and its viruses. mSystems. 2018;3:e00075-18. doi: 10.1128/mSystems.00075-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kresse AU, Dinesh SD, Larbig K, Römling U. Impact of large chromosomal inversions on the adaptation and evolution of Pseudomonas aeruginosa chronically colonizing cystic fibrosis lungs. Mol Microbiol. 2003;47:145–158. doi: 10.1046/j.1365-2958.2003.03261.x. [DOI] [PubMed] [Google Scholar]
- 40.Dorman CJ, Bogue MM. The interplay between DNA topology and accessory factors in site-specific recombination in bacteria and their bacteriophages. Sci Prog. 2016;99:420–437. doi: 10.3184/003685016X14811202974921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darch SE, McNally A, Harrison F, Corander J, Barr HL, et al. Recombination is a key driver of genomic and phenotypic diversity in a Pseudomonas aeruginosa population during cystic fibrosis infection. Sci Rep. 2015;5:7649. doi: 10.1038/srep07649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams D, Paterson S, Brockhurst MA, Winstanley C. Refined analyses suggest that recombination is a minor source of genomic diversity in Pseudomonas aeruginosa chronic cystic fibrosis infections. Microb Genom. 2016;2:e000051. doi: 10.1099/mgen.0.000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darch SE, McNally A, Corander J, Diggle SP. Response to ‘Refined analyses suggest that recombination is a minor source of genomic diversity in Pseudomonas aeruginosa chronic cystic fibrosis infections’ by Williams et al. (2016) Microb Genom. 2016;2:e000054. doi: 10.1099/mgen.0.000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliver A. Mutators in cystic fibrosis chronic lung infection: prevalence, mechanisms, and consequences for antimicrobial therapy. Int J Med Microbiol. 2010;300:563–572. doi: 10.1016/j.ijmm.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Colque CA, Albarracín Orio AG, Feliziani S, Marvig RL, Tobares AR, et al. Hypermutator Pseudomonas aeruginosa exploits multiple genetic pathways to develop multidrug resistance during long-term infections in the airways of cystic fibrosis patients. Antimicrob Agents Chemother. 2020;64:e02142-19. doi: 10.1128/AAC.02142-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winstanley C, O’Brien S, Brockhurst MA. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 2016;24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark ST, Guttman DS, Hwang DM. Diversification of Pseudomonas aeruginosa within the cystic fibrosis lung and its effects on antibiotic resistance. FEMS Microbiol Lett. 2018;365:fny026. doi: 10.1093/femsle/fny026. [DOI] [PubMed] [Google Scholar]
- 48.Davies EV, James CE, Brockhurst MA, Winstanley C. Evolutionary diversification of Pseudomonas aeruginosa in an artificial sputum model. BMC Microbiol. 2017;17:3. doi: 10.1186/s12866-016-0916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta HH, Prater AG, Beabout K, Elworth RAL, Karavis M. The essential role of hypermutation in rapid adaptation to antibiotic stress. Antimicrob Agents Chemother. 2019;63:e00744-19. doi: 10.1128/AAC.00744-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cabot G, Zamorano L, Moyà B, Juan C, Navas A, et al. Evolution of Pseudomonas aeruginosa antimicrobial resistance and fitness under low and high mutation rates. Antimicrob Agents Chemother. 2016;60:1767–1778. doi: 10.1128/AAC.02676-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khil PP, Dulanto Chiang A, Ho J, Youn J-H, Lemon JK, et al. Dynamic emergence of mismatch repair deficiency facilitates rapid evolution of ceftazidime-avibactam resistance in Pseudomonas aeruginosa acute infection. mBio. 2019;10:e01822-19. doi: 10.1128/mBio.01822-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall LMC, Henderson-Begg SK. Hypermutable bacteria isolated from humans – a critical analysis. Microbiology. 2006;152:2505–2514. doi: 10.1099/mic.0.29079-0. [DOI] [PubMed] [Google Scholar]
- 53.Oliver A, Mena A. Bacterial hypermutation in cystic fibrosis, not only for antibiotic resistance. Clin Microbiol Infect. 2010;16:798–808. doi: 10.1111/j.1469-0691.2010.03250.x. [DOI] [PubMed] [Google Scholar]
- 54.Rees VE, Deveson Lucas DS, López-Causapé C, Huang Y, Kotsimbos T. Characterization of hypermutator Pseudomonas aeruginosa isolates from patients with cystic osis in Australia. Antimicrob Agents Chemother. 2019;63:e02538-18. doi: 10.1128/AAC.02538-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Auerbach A, Kerem E, Assous MV, Picard E, Bar-Meir M. Is infection with hypermutable Pseudomonas aeruginosa clinically significant? J Cyst Fibros. 2015;14:347–352. doi: 10.1016/j.jcf.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Waine DJ, Honeybourne D, Smith EG, Whitehouse JL, Dowson CG. Association between hypermutator phenotype, clinical variables, mucoid phenotype, and antimicrobial resistance in Pseudomonas aeruginosa . J Clin Microbiol. 2008;46:3491–3493. doi: 10.1128/JCM.00357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferroni A, Guillemot D, Moumile K, Bernede C, Le Bourgeois M, et al. Effect of mutator P. aeruginosa on antibiotic resistance acquisition and respiratory function in cystic fibrosis. Pediatr Pulmonol. 2009;44:820–825. doi: 10.1002/ppul.21076. [DOI] [PubMed] [Google Scholar]
- 58.Wang K, Chen Y-Q, Salido MM, Kohli GS, Kong J-L, et al. The rapid in vivo evolution of Pseudomonas aeruginosa in ventilator-associated pneumonia patients leads to attenuated virulence. Open Biol. 2017;7:170029. doi: 10.1098/rsob.170029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Persyn E, Sassi M, Aubry M, Broly M, Delanou S, et al. Rapid genetic and phenotypic changes in Pseudomonas aeruginosa clinical strains during ventilator-associated pneumonia. Sci Rep. 2019;9:4720. doi: 10.1038/s41598-019-41201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fischer S, Klockgether J, Morán Losada P, Chouvarine P, Cramer N, et al. Intraclonal genome diversity of the major Pseudomonas aeruginosa clones C and PA14. Environ Microbiol Rep. 2016;8:227–234. doi: 10.1111/1758-2229.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiehlmann L, Wagner G, Cramer N, Siebert B, Gudowius P, et al. Population structure of Pseudomonas aeruginosa . Proc Natl Acad Sci USA. 2007;104:8101–8106. doi: 10.1073/pnas.0609213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Damkiaer S, Yang L, Molin S, Jelsbak L. Evolutionary remodeling of global regulatory networks during long-term bacterial adaptation to human hosts. Proc Natl Acad Sci USA. 2013;110:7766–7771. doi: 10.1073/pnas.1221466110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feltner JB, Wolter DJ, Pope CE, Groleau M-C, Smalley NE, et al. LasR variant cystic fibrosis isolates reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa . mBio. 2016;7:e01513-16. doi: 10.1128/mBio.01513-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen R, Déziel E, Groleau M-C, Schaefer AL, Greenberg EP. Social cheating in a Pseudomonas aeruginosa quorum-sensing variant. Proc Natl Acad Sci USA. 2019;116:7021–7026. doi: 10.1073/pnas.1819801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kostylev M, Kim DY, Smalley NE, Salukhe I, Greenberg EP, et al. Evolution of the Pseudomonas aeruginosa quorum-sensing hierarchy. Proc Natl Acad Sci USA. 2019;116:7027–7032. doi: 10.1073/pnas.1819796116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cruz RL, Asfahl KL, Van den Bossche S, Coenye T, Crabbé A. RhlR-regulated acyl-homoserine lactone quorum sensing in a cystic fibrosis isolate of Pseudomonas aeruginosa . mMBio. 2020;11:e00532-20. doi: 10.1128/mBio.00532-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jorth P, Staudinger BJ, Wu X, Hisert KB, Hayden H, et al. Regional isolation drives bacterial diversification within cystic fibrosis lungs. Cell Host Microbe. 2015;18:307–319. doi: 10.1016/j.chom.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams D, Evans B, Haldenby S, Walshaw MJ, Brockhurst MA, et al. Divergent, coexisting Pseudomonas aeruginosa lineages in chronic cystic fibrosis lung infections. Am J Respir Crit Care Med. 2015;191:775–785. doi: 10.1164/rccm.201409-1646OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams D, Fothergill JL, Evans B, Caples J, Haldenby S, et al. Transmission and lineage displacement drive rapid population genomic flux in cystic fibrosis airway infections of a Pseudomonas aeruginosa epidemic strain. Microb Genom. 2018;4:000167. doi: 10.1099/mgen.0.000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anthony M, Rose B, Pegler MB, Elkins M, Service H, et al. Genetic analysis of Pseudomonas aeruginosa isolates from the sputa of Australian adult cystic fibrosis patients. J Clin Microbiol. 2002;40:2772–2778. doi: 10.1128/JCM.40.8.2772-2778.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bragonzi A, Wiehlmann L, Klockgether J, Cramer N, Worlitzsch D, et al. Sequence diversity of the mucABD locus in Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology. 2006;152:3261–3269. doi: 10.1099/mic.0.29175-0. [DOI] [PubMed] [Google Scholar]
- 72.Ciofu O, Lee B, Johannesson M, Hermansen NO, Meyer P, et al. Investigation of the algT operon sequence in mucoid and non-mucoid Pseudomonas aeruginosa isolates from 115 Scandinavian patients with cystic fibrosis and in 88 in vitro non-mucoid revertants. Microbiology. 2008;154:103–113. doi: 10.1099/mic.0.2007/010421-0. [DOI] [PubMed] [Google Scholar]
- 73.Pulcrano G, Iula DV, Raia V, Rossano F, Catania MR. Different mutations in mucA gene of Pseudomonas aeruginosa mucoid strains in cystic fibrosis patients and their effect on algU gene expression. New Microbiol. 2012;35:295–305. [PubMed] [Google Scholar]
- 74.Candido Caçador N, Paulino da Costa Capizzani C, Gomes Monteiro Marin Torres LA, Galetti R, Ciofu O, et al. Adaptation of Pseudomonas aeruginosa to the chronic phenotype by mutations in the algTmucABD operon in isolates from Brazilian cystic fibrosis patients. PLoS One. 2018;13:e0208013. doi: 10.1371/journal.pone.0208013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Panmanee W, Su S, Schurr MJ, Lau GW, Zhu X, et al. The anti-sigma factor MucA of Pseudomonas aeruginosa: dramatic differences of a mucA22 vs. a ΔmucA mutant in anaerobic acidified nitrite sensitivity of planktonic and biofilm bacteria in vitro and during chronic murine lung infection. PLoS One. 2019;14:e0216401. doi: 10.1371/journal.pone.0216401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brule CE, Grayhack EJ. Synonymous codons: choose wisely for expression. Trends Genet. 2017;33:283–297. doi: 10.1016/j.tig.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kristofich J, Morgenthaler AB, Kinney WR, Ebmeier CC, Snyder DJ, et al. Synonymous mutations make dramatic contributions to fitness when growth is limited by a weak-link enzyme. PLoS Genet. 2018;14:e1007615. doi: 10.1371/journal.pgen.1007615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bailey SF, Hinz A, Kassen R. Adaptive synonymous mutations in an experimentally evolved Pseudomonas fluorescens population. Nat Commun. 2014;5:4076. doi: 10.1038/ncomms5076. [DOI] [PubMed] [Google Scholar]
- 79.Lebeuf-Taylor E, McCloskey N, Bailey SF, Hinz A, Kassen R. The distribution of fitness effects among synonymous mutations in a gene under directional selection. Elife. 2019;8:e45952. doi: 10.7554/eLife.45952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thorpe HA, Bayliss SC, Hurst LD, Feil EJ. Comparative analyses of selection operating on nontranslated intergenic regions of diverse bacterial species. Genetics. 2017;206:363–376. doi: 10.1534/genetics.116.195784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khademi SMH, Sazinas P, Jelsbak L. Within-host adaptation mediated by intergenic evolution in Pseudomonas aeruginosa . Genome Biol Evol. 2019;11:1385–1397. doi: 10.1093/gbe/evz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ernst RK, D′Argenio DA, Ichikawa JK, Bangera MG, Selgrade S, et al. Genome mosaicism is conserved but not unique in Pseudomonas aeruginosa isolates from the airways of young children with cystic fibrosis. Environ Microbiol. 2003;5:1341–1349. doi: 10.1111/j.1462-2920.2003.00518.x. [DOI] [PubMed] [Google Scholar]
- 83.Huse HK, Kwon T, Zlosnik JEA, Speert DP, Marcotte EM, et al. Parallel evolution in Pseudomonas aeruginosa over 39,000 generations in vivo. mBio. 2010;1:e00199-10. doi: 10.1128/mBio.00199-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rau MH, Hansen SK, Johansen HK, Thomsen LE, Workman CT, et al. Early adaptive developments of Pseudomonas aeruginosa after the transition from life in the environment to persistent colonization in the airways of human cystic fibrosis hosts. Environ Microbiol. 2010;12:1643–1658. doi: 10.1111/j.1462-2920.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- 85.Lee B, Schjerling CK, Kirkby N, Hoffmann N, Borup R, et al. Mucoid Pseudomonas aeruginosa isolates maintain the biofilm formation capacity and the gene expression profiles during the chronic lung infection of CF patients. APMIS. 2011;119:263–274. doi: 10.1111/j.1600-0463.2011.02726.x. [DOI] [PubMed] [Google Scholar]
- 86.Schuster M, Lostroh CP, Ogi T, Greenberg EP. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol. 2003;185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol. 2003;185:2080–2095. doi: 10.1128/JB.185.7.2080-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Damron FH, Owings JP, Okkotsu Y, Varga JJ, Schurr JR, et al. Analysis of the Pseudomonas aeruginosa regulon controlled by the sensor kinase KinB and sigma factor RpoN. J Bacteriol. 2012;194:1317–1330. doi: 10.1128/JB.06105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schultz A, Stick S. Early pulmonary inflammation and lung damage in children with cystic fibrosis: early inflammation and lung damage in CF. Respirology. 2015;20:569–578. doi: 10.1111/resp.12521. [DOI] [PubMed] [Google Scholar]
- 90.Gifford AH, Willger SD, Dolben EL, Moulton LA, Dorman DB, et al. Use of a multiplex transcript method for analysis of Pseudomonas aeruginosa gene expression profiles in the cystic fibrosis lung. Infect Immun. 2016;84:2995–3006. doi: 10.1128/IAI.00437-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossi E, Falcone M, Molin S, Johansen HK. High-resolution in situ transcriptomics of Pseudomonas aeruginosa unveils genotype independent patho-phenotypes in cystic fibrosis lungs. Nat Commun. 2018;9:3459. doi: 10.1038/s41467-018-05944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cornforth DM, Dees JL, Ibberson CB, Huse HK, Mathiesen IH, et al. Pseudomonas aeruginosa transcriptome during human infection. Proc Natl Acad Sci USA. 2018;115:E5125–E5134. doi: 10.1073/pnas.1717525115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kordes A, Preusse M, Willger SD, Braubach P, Jonigk D, et al. Genetically diverse Pseudomonas aeruginosa populations display similar transcriptomic profiles in a cystic fibrosis explanted lung. Nat Commun. 2019;10:3397. doi: 10.1038/s41467-019-11414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumar SS, Tandberg JI, Penesyan A, Elbourne LDH, Suarez-Bosche N, et al. Dual transcriptomics of host-pathogen interaction of cystic fibrosis isolate Pseudomonas aeruginosa PASS1 with zebrafish. Front Cell Infect Microbiol. 2018;8:406. doi: 10.3389/fcimb.2018.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Damron FH, Oglesby-Sherrouse AG, Wilks A, Barbier M. Dual-seq transcriptomics reveals the battle for iron during Pseudomonas aeruginosa acute murine pneumonia. Sci Rep. 2016;16:39172. doi: 10.1038/srep39172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hare NJ, Cordwell SJ. Proteomics of bacterial pathogens: Pseudomonas aeruginosa infections in cystic fibrosis - a case study. Proteomics Clin Appl. 2010;4:228–248. doi: 10.1002/prca.200900144. [DOI] [PubMed] [Google Scholar]
- 97.Kamath KS, Kumar SS, Kaur J, Venkatakrishnan V, Paulsen IT, et al. Proteomics of hosts and pathogens in cystic fibrosis. Proteomics Clin Appl. 2015;9:134–146. doi: 10.1002/prca.201400122. [DOI] [PubMed] [Google Scholar]
- 98.Penesyan A, Kumar SS, Kamath K, Shathili AM, Venkatakrishnan V, et al. Genetically and phenotypically distinct Pseudomonas aeruginosa cystic fibrosis isolates share a core proteomic signature. PLoS One. 2015;10:e0138527. doi: 10.1371/journal.pone.0138527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kamath KS, Pascovici D, Penesyan A, Goel A, Venkatakrishnan V, et al. Pseudomonas aeruginosa cell membrane protein expression from phenotypically diverse cystic fibrosis isolates demonstrates host-specific adaptations. J Proteome Res. 2016;15:2152–2163. doi: 10.1021/acs.jproteome.6b00058. [DOI] [PubMed] [Google Scholar]
- 100.Kamath KS, Krisp C, Chick J, Pascovici D, Gygi SP, et al. Pseudomonas aeruginosa proteome under hypoxic stress conditions mimicking the cystic fibrosis lung. J Proteome Res. 2017;16:3917–3928. doi: 10.1021/acs.jproteome.7b00561. [DOI] [PubMed] [Google Scholar]
- 101.Wu X, Siehnel RJ, Garudathri J, Staudinger BJ, Hisert KB, et al. In vivo proteome of Pseudomonas aeruginosa in airways of cystic fibrosis patients. J Proteome Res. 2019;18:2601–2612. doi: 10.1021/acs.jproteome.9b00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hare NJ, Solis N, Harmer C, Marzook NB, Rose B, et al. Proteomic profiling of Pseudomonas aeruginosa AES-1R, PAO1 and PA14 reveals potential virulence determinants associated with a transmissible cystic fibrosis-associated strain. BMC Microbiol. 2012;12:16. doi: 10.1186/1471-2180-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hare NJ, Soe CZ, Rose B, Harbour C, Codd R, et al. Proteomics of Pseudomonas aeruginosa Australian epidemic strain 1 (AES-1) cultured under conditions mimicking the cystic fibrosis lung reveals increased iron acquisition via the siderophore pyochelin. J Proteome Res. 2012;11:776–795. doi: 10.1021/pr200659h. [DOI] [PubMed] [Google Scholar]
- 104.Sousa AM, Pereira MO. Pseudomonas aeruginosa diversification during infection development in cystic fibrosis lungs – a review. Pathogens. 2014;3:680–703. doi: 10.3390/pathogens3030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klockgether J, Miethke N, Kubesch P, Bohn Y-S, Brockhausen I, et al. Intraclonal diversity of the Pseudomonas aeruginosa cystic fibrosis airway isolates TBCF10839 and TBCF121838: distinct signatures of transcriptome, proteome, metabolome, adherence and pathogenicity despite an almost identical genome sequence. Environ Microbiol. 2013;15:191–210. doi: 10.1111/j.1462-2920.2012.02842.x. [DOI] [PubMed] [Google Scholar]
- 106.Faure E, Kwong K, Nguyen D. Pseudomonas aeruginosa in chronic lung infections: how to adapt within the host? Front Immunol. 2018;9:2416. doi: 10.3389/fimmu.2018.02416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.La Rosa R, Johansen HK, Molin S. Adapting to the airways: metabolic requirements of Pseudomonas aeruginosa during the infection of cystic fibrosis patients. Metabolites. 2019;9:234. doi: 10.3390/metabo9100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Riquelme SA, Wong Fok Lung T, Prince A. Pulmonary pathogens adapt to immune signaling metabolites in the airway. Front Immunol. 2020;11:385. doi: 10.3389/fimmu.2020.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schick A, Kassen R. Rapid diversification of Pseudomonas aeruginosa in cystic fibrosis lung-like conditions. Proc Natl Acad Sci USA. 2018;115:10714–10719. doi: 10.1073/pnas.1721270115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.La Rosa R, Johansen HK, Molin S. Convergent metabolic specialization through distinct evolutionary paths in Pseudomonas aeruginosa . mBio. 2018;9:e00269-18. doi: 10.1128/mBio.00269-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Palmer KL, Aye LM, Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol. 2007;189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barth AL, Pitt TL. The high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pseudomonas aeruginosa . J Med Microbiol. 1996;45:110–119. doi: 10.1099/00222615-45-2-110. [DOI] [PubMed] [Google Scholar]
- 113.Kumar SS, Penesyan A, Elbourne LDH, Gillings MR, Paulsen IT. Catabolism of nucleic acids by a cystic fibrosis Pseudomonas aeruginosa isolate: an adaptive pathway to cystic fibrosis sputum environment. Front Microbiol. 2019;10:1199. doi: 10.3389/fmicb.2019.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cramer N, Fischer S, Hedtfeld S, Dorda M, Tümmler B. Intraclonal competitive fitness of longitudinal cystic fibrosis Pseudomonas aeruginosa airway isolates in liquid cultures. Environ Microbiol. 2020;22:2536. doi: 10.1111/1462-2920.14924. [DOI] [PubMed] [Google Scholar]
- 115.Evans TJ. Small colony variants of Pseudomonas aeruginosa in chronic bacterial infection of the lung in cystic fibrosis. Future Microbiol. 2015;10:231–239. doi: 10.2217/fmb.14.107. [DOI] [PubMed] [Google Scholar]
- 116.Mangiaterra G, Amiri M, Di Cesare A, Pasquaroli S, Manso E, et al. Detection of viable but non-culturable Pseudomonas aeruginosa in cystic fibrosis by qPCR: a validation study. BMC Infect Dis. 2018;18:701. doi: 10.1186/s12879-018-3612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Al Ahmar R, Kirby BD, Yu HD. Culture of small colony variant of Pseudomonas aeruginosa and quantitation of its alginate. J Vis Exp. 2020;156:e60466. doi: 10.3791/60466. [DOI] [PubMed] [Google Scholar]
- 118.López-Causapé C, Cabot G, Del Barrio-Tofiño E, Oliver A. The versatile mutational resistome of Pseudomonas aeruginosa . Front Microbiol. 2018;9:685. doi: 10.3389/fmicb.2018.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suresh M, Nithya N, Jayasree PR, Vimal KP, Manish Kumar PR. Mutational analyses of regulatory genes, mexR, nalC, nalD and mexZ of mexAB-oprM and mexXY operons, in efflux pump hyperexpressing multidrug-resistant clinical isolates of Pseudomonas aeruginosa . World J Microbiol Biotechnol. 2018;34:83. doi: 10.1007/s11274-018-2465-0. [DOI] [PubMed] [Google Scholar]
- 120.Huszczynski SM, Lam JS, Khursigara CM. The role of Pseudomonas aeruginosa lipopolysaccharide in bacterial pathogenesis and physiology. Pathogens. 2019;9:6. doi: 10.3390/pathogens9010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maldonado RF, Sá-Correia I, Valvano MA. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol Rev. 2016;40:480–493. doi: 10.1093/femsre/fuw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bricio-Moreno L, Sheridan VH, Goodhead I, Armstrong S, Wong JKL, et al. Evolutionary trade-offs associated with loss of PmrB function in host-adapted Pseudomonas aeruginosa . Nat Commun. 2018;9:2635. doi: 10.1038/s41467-018-04996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Poon KKH, Westman EL, Vinogradov E, Jin S, Lam JS. Functional characterization of MigA and WapR: putative rhamnosyltransferases involved in outer core oligosaccharide biosynthesis of Pseudomonas aeruginosa . J Bacteriol. 2008;190:1857–1865. doi: 10.1128/JB.01546-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dößelmann B, Willmann M, Steglich M, Bunk B, Nübel U, et al. Rapid and consistent evolution of colistin resistance in extensively drug-resistant Pseudomonas aeruginosa during morbidostat culture. Antimicrob Agents Chemother. 2017;61:e00043-17. doi: 10.1128/AAC.00043-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cullen L, Weiser R, Olszak T, Maldonado RF, Moreira AS, et al. Phenotypic characterization of an international Pseudomonas aeruginosa reference panel: strains of cystic fibrosis (CF) origin show less in vivo virulence than non-CF strains. Microbiology. 2015;161:1961–1977. doi: 10.1099/mic.0.000155. [DOI] [PubMed] [Google Scholar]
- 126.Römling U, Fiedler B, Bosshammer J, Grothues D, Greipel J, et al. Epidemiology of chronic Pseudomonas aeruginosa infections in cystic fibrosis. J Infect Dis. 1994;170:1616–1621. doi: 10.1093/infdis/170.6.1616. [DOI] [PubMed] [Google Scholar]
- 127.Ghoul M, West SA, Johansen HK, Molin S, Harrison OB, et al. Bacteriocin-mediated competition in cystic fibrosis lung infections. Proc Biol Sci. 2015;282:20150972. doi: 10.1098/rspb.2015.0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Redero M, López-Causapé C, Aznar J, Oliver A, Blázquez J, et al. Susceptibility to R-pyocins of Pseudomonas aeruginosa clinical isolates from cystic fibrosis patients. J Antimicrob Chemother. 2018;73:2770–2776. doi: 10.1093/jac/dky261. [DOI] [PubMed] [Google Scholar]
- 129.Franklin MJ, Nivens DE, Weadge JT, Howell PL. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front Microbiol. 2011;2:167. doi: 10.3389/fmicb.2011.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Colvin KM, Irie Y, Tart CS, Urbano R, Whitney JC, et al. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ Microbiol. 2012;14:1913–1928. doi: 10.1111/j.1462-2920.2011.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, et al. The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa . PLoS Pathog. 2011;7:e1001264. doi: 10.1371/journal.ppat.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Billings N, Millan M, Caldara M, Rusconi R, Tarasova Y, et al. The extracellular matrix component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2013;9:e1003526. doi: 10.1371/journal.ppat.1003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harrison JJ, Almblad H, Irie Y, Wolter DJ, Eggleston HC, et al. Elevated exopolysaccharide levels in Pseudomonas aeruginosa flagellar mutants have implications for biofilm growth and chronic infections. PLoS Genet. 2020;16:e1008848. doi: 10.1371/journal.pgen.1008848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Starkey M, Hickman JH, Ma L, Zhang N, De Long S, et al. Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. J Bacteriol. 2009;191:3492–3503. doi: 10.1128/JB.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Henry RL, Mellis CM, Petrovic L. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr Pulmonol. 1992;12:158–161. doi: 10.1002/ppul.1950120306. [DOI] [PubMed] [Google Scholar]
- 136.Parad RB, Gerard CJ, Zurakowski D, Nichols DP, Pier GB. Pulmonary outcome in cystic fibrosis is influenced primarily by mucoid Pseudomonas aeruginosa infection and immune status and only modestly by genotype. Infect Immun. 1999;67:4744–4750. doi: 10.1128/IAI.67.9.4744-4750.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hengzhuang W, Wu H, Ciofu O, Song Z, Høiby N. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2011;55:4469–4474. doi: 10.1128/AAC.00126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Malhotra S, Hayes D, Wozniak DJ. Mucoid Pseudomonas aeruginosa and regional inflammation in the cystic fibrosis lung. J Cyst Fibros. 2019;18:796–803. doi: 10.1016/j.jcf.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cornelis P, Dingemans J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front Cell Infect Microbiol. 2013;3:75. doi: 10.3389/fcimb.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Minandri F, Imperi F, Frangipani E, Bonchi C, Visaggio D, et al. Role of iron uptake systems in Pseudomonas aeruginosa virulence and airway infection. Infect Immun. 2016;84:2324–2335. doi: 10.1128/IAI.00098-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marvig RL, Damkiær S, Khademi SMH, Markussen TM, Molin S, et al. Within-host evolution of Pseudomonas aeruginosa reveals adaptation toward iron acquisition from hemoglobin. mBio. 2014;5:e00966-14. doi: 10.1128/mBio.00966-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ballarini A, Scalet G, Kos M, Cramer N, Wiehlmann L, et al. Molecular typing and epidemiological investigation of clinical populations of Pseudomonas aeruginosa using an oligonucleotide-microarray. BMC Microbiol. 2012;12:152. doi: 10.1186/1471-2180-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shaver CM, Hauser AR. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect Immun. 2004;72:6969–6977. doi: 10.1128/IAI.72.12.6969-6977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sawa T, Shimizu M, Moriyama K, Wiener-Kronish JP. Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: a review. Crit Care. 2014;18:668. doi: 10.1186/s13054-014-0668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sarges EDSNF, Rodrigues YC, Furlaneto IP, de Melo MVH, Brabo GLDC, et al. Pseudomonas aeruginosa type III secretion system virulotypes and their association with clinical features of cystic fibrosis patients. Infect Drug Resist. 2020;13:3771–3781. doi: 10.2147/IDR.S273759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tognon M, Köhler T, Gdaniec BG, Hao Y, Lam JS, et al. Co-evolution with Staphylococcus aureus leads to lipopolysaccharide alterations in Pseudomonas aeruginosa . ISME J. 2017;11:2233–2243. doi: 10.1038/ismej.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao K, Du L, Lin J, Yuan Y, Wang X, et al. Pseudomonas aeruginosa quorum-sensing and type vi secretion system can direct interspecific coexistence during evolution. Front Microbiol. 2018;9:2287. doi: 10.3389/fmicb.2018.02287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:411–414. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 149.Sandoz KM, Mitzimberg SM, Schuster M. Social cheating in Pseudomonas aeruginosa quorum sensing. Proc Natl Acad Sci USA. 2007;104:15876–15881. doi: 10.1073/pnas.0705653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dandekar AA, Chugani S, Greenberg EP. Bacterial quorum sensing and metabolic incentives to cooperate. Science. 2012;338:264–266. doi: 10.1126/science.1227289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tashiro Y, Yawata Y, Toyofuku M, Uchiyama H, Nomura N. Interspecies interaction between Pseudomonas aeruginosa and other microorganisms. Microbes Environ. 2013;28:13–24. doi: 10.1264/jsme2.ME12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sana TG, Berni B, Bleves S. The T6SSs of Pseudomonas aeruginosa strain PAO1 and their effectors: beyond bacterial-cell targeting. Front Cell Infect Microbiol. 2016;6:61. doi: 10.3389/fcimb.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nguyen AT, Oglesby-Sherrouse AG. Interactions between Pseudomonas aeruginosa and Staphylococcus aureus during co-cultivations and polymicrobial infections. Appl Microbiol Biotechnol. 2016;100:6141–6148. doi: 10.1007/s00253-016-7596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hotterbeekx A, Kumar-Singh S, Goossens H, Malhotra-Kumar S. In vivo and In vitro interactions between Pseudomonas aeruginosa and Staphylococcus spp. Front Cell Infect Microbiol. 2017;7:106. doi: 10.3389/fcimb.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fourie R, Pohl CH. Beyond Antagonism: The Interaction Between Candida Species and Pseudomonas aeruginosa . J Fungi. 2019;5:34. doi: 10.3390/jof5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Baldan R, Cigana C, Testa F, Bianconi I, De Simone M, et al. Adaptation of Pseudomonas aeruginosa in cystic fibrosis airways influences virulence of Staphylococcus aureus in vitro and murine models of co-infection. PLoS One. 2014;9:e89614. doi: 10.1371/journal.pone.0089614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Michelsen CF, Christensen A-MJ, Bojer MS, Høiby N, Ingmer H, et al. Staphylococcus aureus alters growth activity, autolysis, and antibiotic tolerance in a human host-adapted Pseudomonas aeruginosa lineage. J Bacteriol. 2014;196:3903–3911. doi: 10.1128/JB.02006-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Frydenlund Michelsen C, Hossein Khademi SM, Krogh Johansen H, Ingmer H, Dorrestein PC, et al. Evolution of metabolic divergence in Pseudomonas aeruginosa during long-term infection facilitates a proto-cooperative interspecies interaction. ISME J. 2016;10:1323–1336. doi: 10.1038/ismej.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Briaud P, Camus L, Bastien S, Doléans-Jordheim A, Vandenesch F, et al. Coexistence with Pseudomonas aeruginosa alters Staphylococcus aureus transcriptome, antibiotic resistance and internalization into epithelial cells. Sci Rep. 2019;9:16564. doi: 10.1038/s41598-019-52975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Camus L, Briaud P, Bastien S, Elsen S, Doléans-Jordheim A, et al. Trophic cooperation promotes bacterial survival of Staphylococcus aureus and Pseudomonas aeruginosa . ISME J. 2020;14:3093–3105. doi: 10.1038/s41396-020-00741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Flynn JM, Cameron LC, Wiggen TD, Dunitz JM, Harcombe WR, et al. Disruption of cross-feeding inhibits pathogen growth in the sputa of patients with cystic fibrosis. mSphere. 2020;5:e00343-20. doi: 10.1128/mSphere.00343-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Scott JE, O’Toole GA. The yin and yang of Streptococcus lung infections in cystic fibrosis: a model for studying polymicrobial interactions. J Bacteriol. 2019;201:e00115-19. doi: 10.1128/JB.00115-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cigana C, Bianconi I, Baldan R, De Simone M, Riva C, et al. Staphylococcus aureus impacts Pseudomonas aeruginosa chronic respiratory disease in murine models. J Infect Dis. 2018;217:933–942. doi: 10.1093/infdis/jix621. [DOI] [PubMed] [Google Scholar]
- 164.Granchelli AM, Adler FR, Keogh RH, Kartsonaki C, Cox DR. Microbial interactions in the cystic fibrosis airway. J Clin Microbiol. 2018;56:e00354-18. doi: 10.1128/JCM.00354-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.