Abstract
BACKGROUND:
Characteristics of US blood donors with recent (RBI) or occult (OBI) hepatitis B virus (HBV) infection are not well defined.
METHODS:
Donors with RBI and OBI were identified by nucleic acid and serologic testing among 34.4 million donations during 2009–2015. Consenting donors were interviewed and their HBV S-gene sequenced.
RESULTS:
The overall rate of HBV-infected donors was 7.95 per 100,000; of these, 0.35 per 100,000 and 1.70 per 100,000 were RBI and OBI, respectively. RBI (n = 120) and OBI (n = 583) donors constituted 26% of all HBV-infected (n = 2735) donors. Detection of HBV DNA in 92% of OBI donors required individual donation nucleic acid testing. Donors with OBI compared to RBI were older (mean age, 48 vs 39 years; p < 0.0001) with lower median viral loads (9 vs. 529 IU/mL; p < 0.0001). A higher proportion of OBI than RBI donors were born or resided in an endemic country (39% vs. 5%; p= 0.0078). Seventy-seven percent of all RBI and OBI donors had multiple sex partners, an HBV-riskfactor. Of 40 RBI and 10 OBI donors whose S gene was sequenced, 33 (83%) and 6 (60%), respectively, carried HBV subgenotype A2; 18 (55%) and 2 (33%), respectively, shared an identical sequence. Infection with 1 or more putative HBV-immune-escape mutants was identified in 5 (50%) of OBI but no RBI donors.
CONCLUSION:
RBI and OBI continue to be identified at low rates, confirming the importance of comprehensive HBV DNA screening of US blood donations. HBV-infected donors require referral for care and evaluation and contact tracing; their HBV strains may provide important information on emergent genotypes.
In the United States, approximately 13.6 million units of whole blood and RBCs are donated annually1 from volunteer, nonremunerated donors.2 Donors are screened for high-risk behaviors before giving blood and are at low risk of infection by blood-borne pathogens. A small proportion of donors continue to be detected by nucleic acid testing (NAT) as having recent or established infection with human immunodeficiency virus (2.8 per 100,000 donations), hepatitis C virus (20.0 in 100,000 donations) and hepatitis B virus (HBV) (7.6 per 100,000 donations).3
Blood donation screening for HBV by NAT in the United States is performed in minipools (MPs) of 6–16 samples, depending on the manufacturer used, or individually. NAT detects HBV DNA after exposure within 19 to 27 days in MPs or within 10–18 days by ID NAT, which is less than the 30–38 days required for detection of HBV infection by serologic testing.4,5 The HBV residual transmission risk per blood unit transfused following the implementation of HBV NAT is approximately one per million, similar to human immunodeficiency virus and hepatitis C virus.4 Three large studies have provided insights into HBV NAT-positive blood donors in the United States. These studies investigated risk factors for infection,6 genetic diversity of infecting HBV strains between recent and established infections,7 and the prevalence of HBV among donations.3 None of these studies investigated a large population of recent HBV-infected (RBI) blood donors (i.e., HBV DNA yield or hepatitis B surface antigen [HBsAg] yield)8 or donors with occult HBV infection (OBI).
In this study, we report the rates of HBV DNA-positive US blood donors with RBI and OBI as the result of routine screening, and the characteristics of RBI and OBI donors whose frozen donated plasma units were available for study.
METHODS
Study subjects
We reviewed all American Red Cross (ARC) blood donations from allogeneic, English-speaking, nonmilitary blood donors aged 18 years or older collected from June 21, 2009, through April 28, 2015. Presenting donors had been informed that a portion of their blood donation, testing data, or demographics might be used for future research. Donors were eligible for follow-up in this study if identified as RBI or OBI and a frozen plasma unit was available.
RBI was defined as donors testing HBV DNA-reactive by NAT and nonreactive for HBsAg and HBV core antibody (HBcAb), as well as donors testing HBV DNA reactive and HBsAg confirmed positive but HBcAb nonreactive. The first group is referred to as HBV DNA yield and the second group is referred to as HBsAg yield. OBI was defined as HBV DNA reactive and HBcAb reactive but HBsAg nonreactive (with or without antibody to hepatitis B surface antigen [HBsAb]). Of note, blood donations in the United States are not screened for HBsAb. OBI was identified by finding HBV DNA in routine screening involving MPs of 16 unique donation samples or from additional testing using more sensitive methods.9 All HBV-infected donors (NAT and/or serology positive) were notified, counseled, and indefinitely deferred from donating blood. Donors who met the criteria for inclusion in this study were consented and invited to answer a questionnaire covering known risk factors for HBV transmission and to provide follow-up blood samples with financial compensation. Retrieved plasma units from the HBV-reactive donations and follow-up samples were stored frozen at −30 °C or below. The study was approved by the ARC Institutional Review Board.
HBV testing
All blood donations were screened for HBsAg and HBcAb (PRISM; Abbott Laboratories), including neutralization testing for confirmation of HBsAg reactivity, and for HBV DNA by NAT in MPs of 16 by transcription-mediated amplification (TMA) (Procleix Ultrio, Grifols, from 2009 until April 28, 2013, after which Ultrio Plus was used for the remaining 2-year period). Reactive MPs were resolved to ID followed by Ultrio or Ultrio Plus discriminatory assay to identify HBV DNA reactivity. The sensitivity for individual-sample HBV DNA (95% limit of detection) increased from 10.4 RJ/mL (95% confidence interval [Cl], 9.2–12.2) to 3.4 RJ/mL (95% CI, 3.0–4.1) with the conversion from Ultrio to Ultrio Plus.9 All HBcAb-reactive samples that were MP- or ID-TMA-nonreactive were retested individually by HBV polymerase chain reaction (PCR) (COBAS AmpliScreen, Multiprep method, Roche Molecular Diagnostics) having HBV DNA sensitivity of 4.41 RJ/mL (95% CI, 3.56–6.13).10 Only those HBcAb-reactive donations having NAT reactivity for HBV DNA by TMA or PCR were considered confirmed positive. Plasma units retrieved from HBV DNA-reactive donations, as well as follow-up blood samples, were tested for HBsAg and HBcAb, and for HBV DNA individually using TMA and HBsAb using an enzyme immunoassay (Monolisa, Bio-Rad Laboratories). Samples from HBV DNA-reactive and seronegative plasma units were retested in replicates of 10 by TMA for confirmation of HBV DNA; samples with reactivity in any replicate were considered confirmed Viral loads were determined for HBV DNA-confirmed-positive samples by HBV PCR, with a lower limit of quantitation of 20 IU/mL (SuperQuant, National Genetics Institute; Los Angeles, CA).11 Samples nonreactive for SuperQuant were reflexed to UltraQual HBV PCR (National Genetics Institute) (95% limit of detection of 0.9 IU/mL).12 Samples that were reactive by qualitative PCR but nonreactive by quantitative PCR were assigned the midpoint value of 9.4 IU/mL.4 Of note, throughout the study period HBV DNA yield samples were routinely submitted for viral load testing (if volume permitted) as part of the ARC routine confirmatory algorithm. Conversely, viral load testing of HBsAg yield of prevalent and OBI samples was not part of the ARC confirmatory algorithm throughout the study period; thus, the percentage of samples from each of these subsets that were submitted for viral load testing was less compared to HBV DNA yield samples. HBV DNA-confirmed-positive samples were also forwarded to the Division of Viral Hepatitis Laboratory, Centers for Disease Control and Prevention (CDC) for HBV DNA sequencing.
HBV DNA sequencing
DNA sequencing involved a 435-base-pair DNA segment amplified from the HBV S gene (between nucleotide positions 222 and 656), as previously described.13
Statistical analysis
Differences in proportions were determined by chi-square test or Fisher’s exact test when appropriate. For significant findings, the Bonferroni correction was applied for multiple comparisons. Differences between subgroups were analyzed using the one-way analysis of variance Welch test as appropriate for continuous variables followed by Tukey’s multiple comparison test Continuous variables with skewed distributions were tested by the Mann-Whitney U test or Kruskal-Wallis test followed by Dunn’s multiple comparison test when appropriate after applying Bonferroni corrections of the p values. Annual rates of HBV infection among blood donors with prevalent (i.e., HBV DNA reactive, HBsAg confirmed positive, and HBcAb reactive), RBI, or OBI during the study period were determined by dividing the number of confirmed-positive donations by the total number of donations tested. Poisson regression was used to investigate the linear trend of HBV prevalence in each subgroup. Two-tailed p values are reported.
RESULTS
Detection rates in RBI and OBI donors
A total of 34,390,972 allogeneic donations from 5,216,186 (26.1%) first-time and 14,791,009 (73.9%) repeat blood donors were collected from June 21, 2009, through April 28, 2015, in 42 states, the District of Columbia, and Puerto Rico. Testing of donations resulted in an overall RBI rate of 0.35 per 100,000 donations (n = 120; 95% CI, 0.29–0.42) and an OBI rate of 1.70 per 100,000 donations (n = 583; 95% CI, 1.56–1.84) with no trends in rates observed throughout the study period (Table 1). The 120 donors with RBI consisted of 48 donations that were HBV DNA yield and 72 donations that were HBsAg yield (Fig. 1). The overall HBV DNA rates, which included prevalent positive donors was 7.95 per 100,000 donations (n = 2735; 95% CI, 7.66–8.26). RBI and OBI constituted 26% of the total HBV DNA-confirmed-positive donors. Of these two groups, plasma units were available from 199 donors for HBV DNA sequencing (RBI 84 of 120 [70%]; OBI 115 of 583 [20%]) (Fig. 1). The remaining plasma units had been discarded prior to the study after release of repeatedly reactive screening result(s).
TABLE 1.
Frequency of detection of hepatitis B virus (HBV) infection markers among American Red Cross blood donors and donations, June 2009 to April 2015
| Year† | Total number of donations‡ | Number of HBV-infected donors* | HBV infection, rate per 100,000 blood donations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalent | RBI | OBI | Total HBV DNA positives§ | Prevalent§ | RBI§ | OBI§ | Total HBV DNA positives§ | ||
| 2009‖ | 3,386,143 | 104 | 6 | 55 | 165 | 3.07 (2.54–3.72) | 0.18 (0.08–0.39) | 1.62 (1.25–2.11) | 4.87 (4.18–5.68) |
| 2010 | 6,345,026 | 374 | 23 | 106 | 503 | 5.89 (5.33–6.52) | 0.36 (0.24–0.54) | 1.67 (1.38–2.02) | 7.93 (7.26–8.65) |
| 2011 | 6,185,839 | 433 | 24 | 116 | 573 | 7.00 (6.37–7.69) | 0.39 (0.26–0.58) | 1.88 (1.56–2.25) | 9.26 (8.54–10.05) |
| 2012 | 5,894,046 | 412 | 20 | 90 | 522 | 6.99 (6.35–7.70) | 0.34 (0.22–0.52) | 1.53 (1.24–1.88) | 8.86 (8.13–9.65) |
| 2013 | 5,516,557 | 333 | 22 | 86 | 441 | 6.04 (5.42–6.72) | 0.40 (0.26–0.60) | 1.56 (1.26–1.93) | 7.99 (7.28–8.78) |
| 2014 | 5,348,293 | 284 | 16 | 119 | 419 | 5.31 (4.73–5.96) | 0.30 (0.18–0.49) | 2.23 (1.86–2.66) | 7.83 (7.12–8.62) |
| 2015¶ | 1,715,068 | 92 | 9 | 11 | 112 | 5.36 (4.37–6.58) | 0.52 (0.28–1.00) | 0.64 (0.36–1.15) | 6.53 (5.43–7.86) |
| Total | 34,390,972 | 2032 | 120 | 583 | 2735 | 5.91 (5.66–6.17) | 0.35 (0.29–0.42) | 1.70 (1.56–1.84) | 7.95 (7.66–8.26) |
Prevalent infection = HBV DNA (+)/HBsAg (+)/HBcAb (+); RBI = HBV DNA (+)/HBsAg (−) or (+)/HBcAb (−); OBI = HBV DNA (+)/HBsAg (−)/HBcAb (+).
Abbreviations: RBI, recent hepatitis B infection; OBI, occult hepatitis B infection; HBsAg, hepatitis B surface antigen; HBcAb, hepatitis B core antibody.
HBV DNA positive by screening and/or additional confirmatory testing.
HBV DNA screened using the Procleix Ultrio assay from 2009 until April 28, 2013 (the first approx. 4 years) after which the Ultrio Plus assay (Grifols) was used for the remaining 2-year study period.
Includes only allogeneic blood donations.
No significance in linear trend was observed throughout the study period.
Includes donations collected from June 22, 2009, to December 31, 2009.
Includes donations collected from January 1,2015, to April 28, 2015.
Fig. 1.
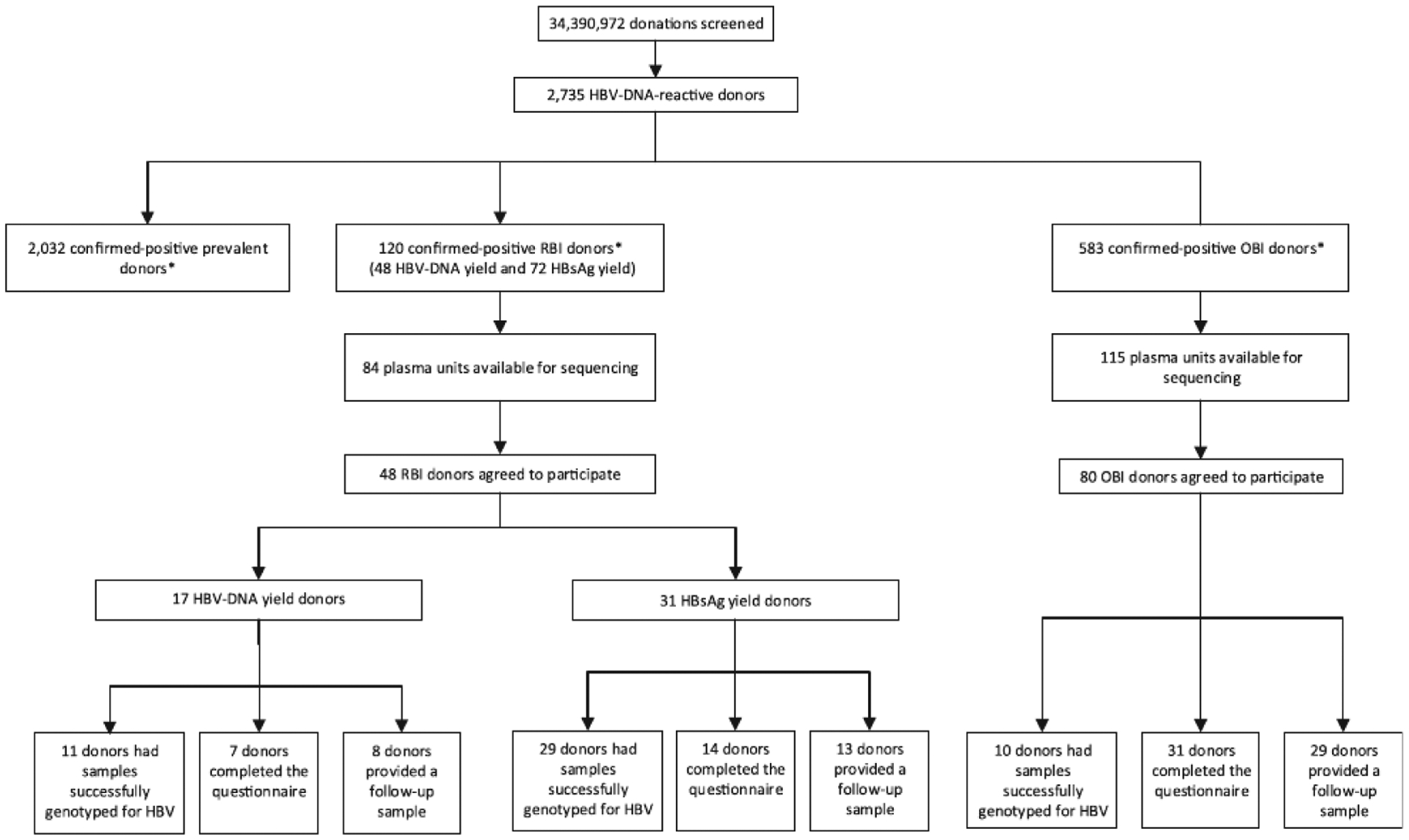
Flow chart showing the breakdown of American Red Cross HBV-infected blood donors and study participation, June 2009 to April 2015. * Viral load was obtained from 1248 (34%) prevalent, 43 (90%) HBV DNA yield, 51 (71%) HBsAg yield, and 201 (34%) OBI donors. HBV, hepatitis B virus; OBI, occult hepatitis B infection; RBI, recent hepatitis B infection.
In April 2013, the ARC converted from the Ultrio to the Ultrio Plus assay. Despite the conversion to a more sensitive assay, the proportion of RBI (chi square = 0.021; p = 0.885) and OBI (chi square = 1.203; p = 0.273) donations detected before and after Ultrio Plus was not different (Table 1). Of 583 donations ultimately shown to be OBI, 537 (92%) required additional ID NAT to detect HBV DNA. Of the OBI donors detected by MP NAT (n = 46), 6% (25 of 389) and 11% (21 of 194) were detected before and after conversion to Ultrio Plus, respectively, suggesting a positive impact of the more sensitive assay.
Demographic and virologic characteristics of RBI and OBI donors
The mean age of RBI donors was significantly younger than OBI donors (39 vs. 48 years, respectively; p < 0.0001) (Table 2). There were more male (64% and 65%) than female (36% and 35%) donors among RBI and OBI, respectively, with nearly identical proportions in the two groups (Table 2). The difference in racial/ethnic distribution between RBI and OBI donors was significant More RBI were detected among whites versus blacks (odds ratio [OR], 2.2; 95% CI, 1.3–3.7; p = 0.004) and whites versus Asians (OR, 25.2; 95% CI, 6.1–104.6; p < 0.0001), followed by more blacks versus Asians (OR, 11.7; 95% CI, 2.7–50.1; p < 0.0001), and donors who self-identified as “other” versus Asian (OR, 11.1; 95% CI, 2.1–57.4; p = 0.002). Conversely, more OBI were detected among Asian versus all other racial/ethnic groups (OR, 18.8; 95% CI, 4.5–75.6; p < 0.0001).
TABLE 2.
Characteristics of American Red Cross blood donors with prevalent, recent, and occult HBV infection (n = 2735), June 2009 to April 2015
| Characteristic | Prevalent infection (n = 2032) | RBI (n = 120) | OBI (n = 583) | p value* |
|---|---|---|---|---|
| Age (y), mean (95% CI) | 35 (34.0–35.3) | 39 (36.2–41.4) | 48 (47.3–49.6) | <0.0001† |
| Range | 15–84 | 16–72 | 16–86 | |
| Sex, number (%) | ||||
| Male | 1433 (71)‡ | 77 (64) | 380 (65) | 0.024 |
| Female | 599 (29)‡ | 43 (36) | 203 (35) | |
| Race/ethnicity, number (%) | ||||
| White | 238 (12)‡ | 73 (60)‡ | 171 (29)‡ | <0.0001§ |
| Black | 390 (19) | 22 (18) | 111 (19) | |
| Asian | 694 (34)‡ | 2 (2)‡ | 118 (20)‡ | |
| Hispanic | 47 (2) | 3 (3) | 20 (3) | |
| Other | 131 (6) | 6 (5) | 32 (5) | |
| Not known | 532 (26) | 14 (12)* | 131 (22) | |
| Donor status, number (%) | ||||
| First-time | 1896 (93)‡ | 36 (30)‡ | 417 (72)‡ | <0.0001 |
| Repeat | 136 (7)‡ | 84 (70)‡ | 166 (28)‡ | |
| Viral coinfection, number (%) | ||||
| HCV | 41 (2) | 12 (10) | 49 (8) | 0.608 |
| HIV-1 | 7 (<1) | 4 (3) | 12 (2) | |
| Location of residence by US region‖, number (%) | ||||
| Northeast | 413 (20) | 14 (12) | 106 (18) | <0.0001 |
| South | 694 (34) | 54 (45) | 212 (36) | |
| Midwest | 371 (18) | 35 (29) | 115 (20) | |
| West | 556 (27)‡ | 12 (10)‡ | 135 (23) | |
| Other | 17 (1)‡ | 5 (4) | 15 (3) | |
| HBsAb concentration in reactive donation, number (%) | ||||
| ≥ 10 mIU/mL | N/A | 3 (6) | 31 (39) | <0.0001 |
| < 10 mIU/mL | N/A | 45 (94) | 49 (61) | |
Prevalent infection = HBV DNA (+)/HBsAg (+)/HBcAb (+); recent infection = HBV DNA (+)/HBsAg (−) or (+)/HBcAb (−); OBI = HBV DNA (+)/HBsAg (−)/HBcAb (+).
Abbreviations: CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV-1, human immunodeficiency virus type 1; HBsAb, antibody to hepatitis B surface antigen; NA, not applicable; OBI, oocult hepatitis B infection; OR, odds ratio; RBI, recent hepatitis B infection.
P value based on χ2 test among the three categories of infection (prevalent, recent, or occult) unless otherwise specified.
P value based on one-way analysis of variance. The difference in means between each group is significant (Tukey’s multiple comparison).
Significant (adjusted residual after adjusting the p value for multiple comparisons).
Donors that did not self-identity race/ethnicity were excluded from analysis.
Regions defined by the US Census Bureau.25
RBI donors were nearly six times more likely to be repeat donors (OR, 5.9; 95% CI, 3.8–9.0; p < 0.0001) compared to OBI donors. In contrast, OBI donors were six times more likely to be first-time donors. Donations by repeat donors exceeded first-time donors by 3 to 1; 86% of repeat donors were white. Prior to the reactive donation, repeat RBI donors provided significantly more donations per donor than repeat OBI donors (mean, 13.2; 95% CI, 9.9–16.7 vs. 5.2; 95% CI, 3.2–7.1; p < 0.0001). Significantly more RBI than OBI donors were residents of the South versus West (OR, 2.9; 95% CI, 1.5–5.6; p = 0.001) or were residents of the Midwest versus the West (OR, 3.4; 95% CI, 1.7–6.9; p < 0.0001). Nearly 40% (212 of 568) of OBI donors were residents of the South versus other regions (Table 2).
Among confirmed-positive donations, median viral loads beginning with the earliest stage of infection included RBI donors (94 of 120, or 78% with quantifiable results) having 529 IU/mL (compromised of 43 HBV DNA yield donors having a median of 40 IU/mL and 51 HBsAg yield donors with a median of 12,400 IU/mL). Prevalent donors (1248 of 2032 [61%]) had median viral loads of 650 IU/mL, and OBI donors (201 of 583 [34%]) had median viral loads of 9 IU/mL (Fig. 2.) There was no association between hepatitis C virus or human immunodeficiency virus coinfection among donors with RBI or OBI. The proportion of donors with HBsAb concentrations of 10 mIU/mL or higher (the protective level) in donation plasma was significantly lower in RBI than in OBI donors (6% vs. 39%; p < 0.0001).
Fig. 2.
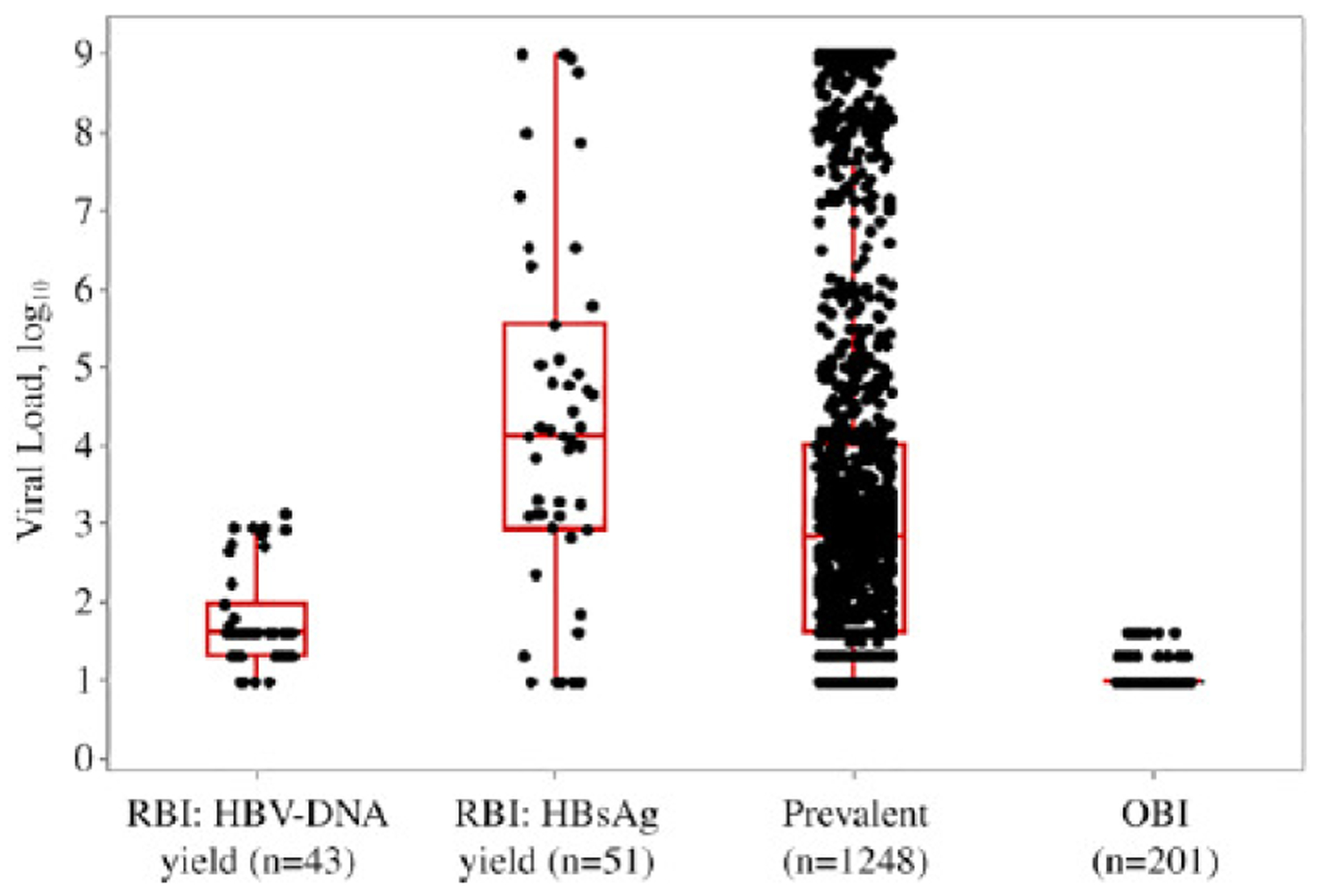
Viral load (VL; IU/mL) distribution of four classes of HBV-confirmed-positive donations among American Red Cross blood donors, June 2009 to April 2015. The median VL for each class is as follows; RBI: HBV-DNA yield = 40 IU/mL (1.60 log10 IU/mL), RBI: HBsAg yield = 12,400 IU/mL (4.09 log10 IU/mL), prevalent = 650 IU/mL (2.81 log10 IU/mL) and OBI = 9 IU/mL (0.97 log10 IU/mL). A significant difference in VL was observed between the following classes; RBI: HBV-DNA yield vs. all (p < 0.0001), RBI: HBsAg yield vs. OBI (p < 0.0001) and prevalent vs. OBI (p < 0.0001). RBI: HBV-DNA yield = HBV-DNA-confirmed-positive only; RBI: HBsAg yield = HBV-DNA-confirmed-positive and HBsAg reactive only; prevalent = HBV-DNA-confirmed-positive, HBsAg reactive, and HBcAb reactive; OBI = HBV-DNA-confirmed-positive, HBsAg non-reactive, and HBcAb reactive. OBI, occult hepatitis B infection; RBI, recent hepatitis B infection.
HBV genotypic characteristics among RBI and OBI donors
Of 84 RBI donors and 115 OBI donors with available plasma for study, 48 (57%) RBI and 80 (70%) OBI agreed to participate by providing additional information and/or samples (Fig. 1). The HBV S gene was successfully amplified and sequenced from 83% (40 of 48) of RBI donors and 13% (10 of 80) of OBI donors (p < 0.0001). Lower rates of sequencing of the S gene among OBI donors reflected their low viral loads (Table 2). HBV subgenotype A2 was predominant among RBI (83% [33 of 40]) and OBI (60% [6 of 10]), donors. HBV genotype D was carried by 10% (4 of 40) of RBI donors and by 30% (3 of 10) of OBI donors. One RBI donor and one OBI donor carried HBV genotype C, one RBI donor carried HBV genotype B, and another carried genotype H. The genotypic sequence diversity of HBV strains from RBI and OBI donors is presented in Fig. 3 along with HBV strains from cases reported to two CDC surveillance systems and from US HBV outbreaks.14,15 The differences in distribution of HBV A2 and non-A2 genotypes between RBI and OBI donors were not significant (p = 0.197). Among 39 A2 sequences, 20 (51%) were identical; 45% (18 of 40) were from RBI, and 20% (2 of 10) were from OBI donors. Of the A2 sequences, 55% (18 of 33) were from RBI and 33% (2 of 6) were from OBI donors.
Fig. 3.
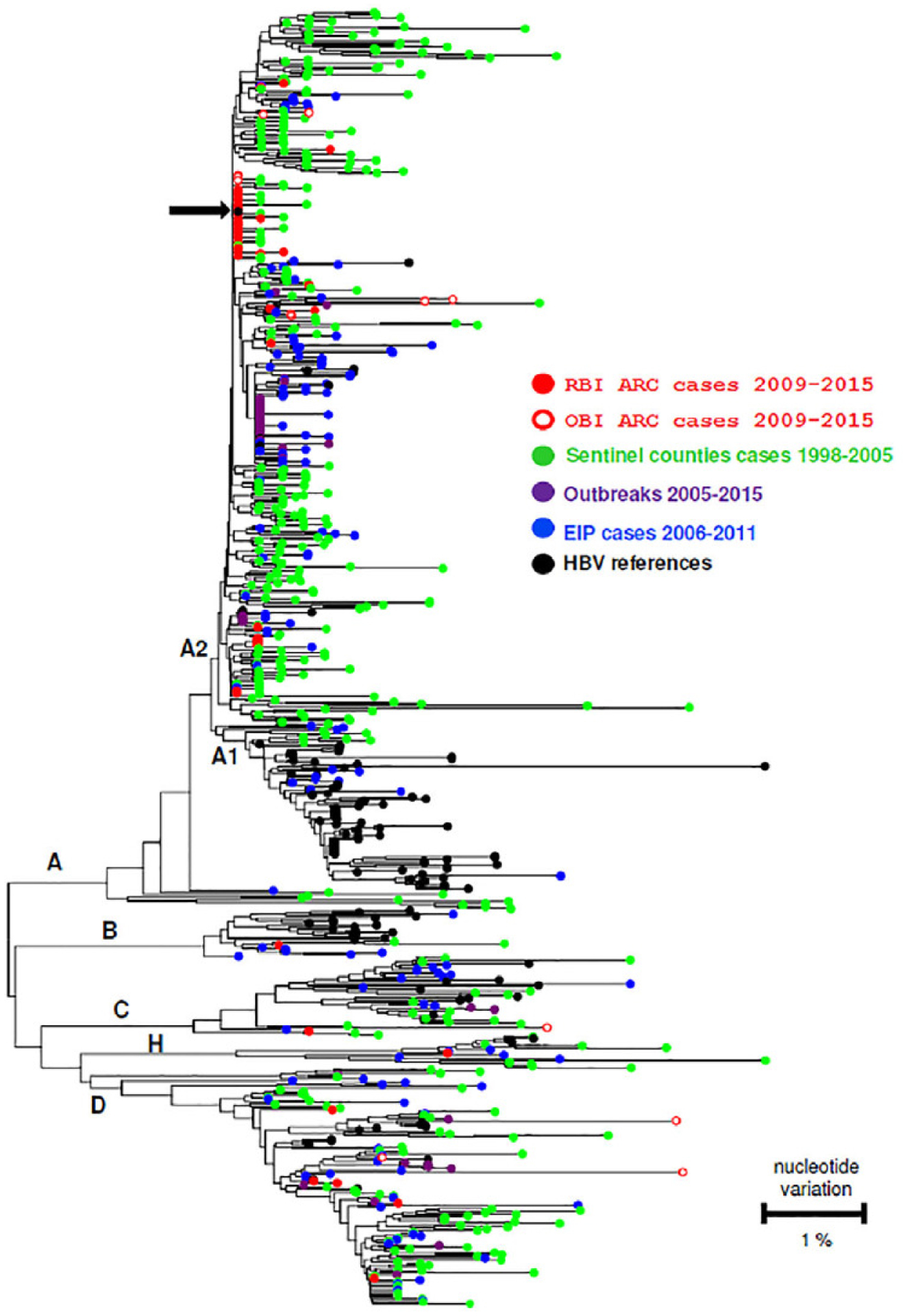
Phylogenetic tree constructed from a 442-bp DNA segment amplified from the hepatitis B virus (HBV) S gene in recent (RBI) and occult (OBI) American Red Cross (ARC) infected donors for whom HBV S gene was successfully amplified and sequenced (n = 50), and from representative cases from the CDC’s Sentinel Counties14 and Emerging Infections Program (EIP)15 surveillance studies, and outbreak cases. HBV references refers to one or more strain sequences for genotypes A, B, C, D and H obtained from GeneBank. The black arrow indicates the S gene sequence shared among 51% of the A2-infected ARC RBI and OBI donors.
Infection by HBV S-immune escape mutants
Nucleotide changes in the a determinant of the HBV S gene, which effectuate changes in amino acids in HBsAg that can confer immune-escape properties,16–18 were identified in 5 of 10 donors with OBI (Fig 4); all 5 had low viral loads (≤40 IU/mL) and only 1 had an HBsAb concentration of 10 mIU/mL or higher. Nine HBV variants with defects in HBsAg secretion also were observed among OBI donors, including one variant each of P120K; P120T, C121G, M133I, D144G, G145R, and C147Y, and two with D144E.
Fig. 4.
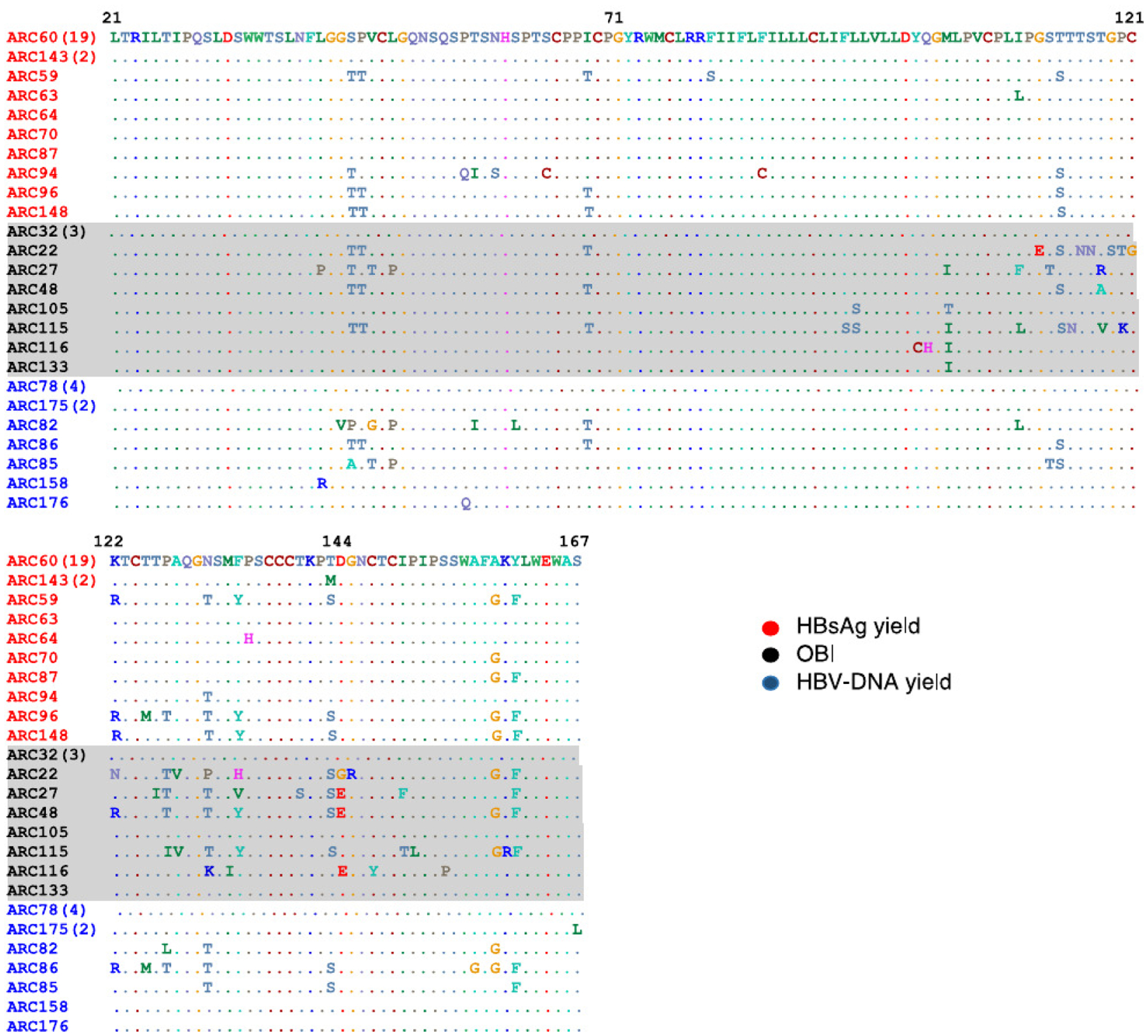
Alignment of amino acid sequences in a determinant of HBV S protein from infected donors for whom the HBV S gene was successfully sequenced (n = 50). Bach ARC number represents a unique donor, color coded based on case status - HBsAg yield (HBV-DNA-confirmed-positive and HBsAg reactive only [red]), HBV-DNA yield (HBV-DNA-confirmed positive only [blue]) and OBI (HBV-DNA-confirmed-positive/HBsAg non-reactive/HBcAb reactive [black, shaded gray]) donors. ARC22 carried the C121G, D144G and G145R mutations; ARC27 and ARC48 carried the D144B mutation; ARC115 carried theP120T mutation; ARC116 carried the P120K, M133I, D144E, and C147Y mutations. Abbreviations: ARC, American Red Cross; HBV, hepatitis B virus; OBI, occult hepatitis B infection.
Virologic course
Of the 128 participating donors, 50 (21 RBI and 29 OBI; Fig. 1) provided one or more follow-up blood samples (range, 1–6 follow-up samples per donor) from 33 to 2116 days following the reactive donation; no limit was provided on the number of samples or duration of donor follow-up. Among eight RBI donors classified as HBV DNA yield and who were followed (Fig. 1), all converted HBsAg and HBcAb, suggesting that primary OBI is uncommon (i.e., sustained HBV DNA reactivity without HBsAb and HBsAg nonreactivity).19 Among the 13 HBsAg yield donors who were followed (Fig. 1), 9 converted to HBcAb and HBsAb reactive, 3 converted HBcAb only, and 1 remained nonreactive for HBcAb and HBsAb (69 days after reactive donation). Both groups of RBI donors cleared or were in the process of clearing HBV DNA when followed; one donor classified as HBsAg yield (4.8% [1 of 21]) progressed to chronicity (ongoing HBV DNA, HBsAg and HBcAb reactivity, but HBsAb nonreactive 581 days after reactive donation). Among OBI donors followed, 45% (13 of 29) remained HBV DNA positive with low viral loads (<40 IU/mL) at a median of 388 (77–2116) days.
HBV risk factors among RBI and OBI donors
The proportion of RBI (44% [21 of 48]) and OBI (39% [31 of 80]) donors responding to the study questionnaire covering acknowledged risk factors for HBV transmission were similar (Table 3). Many of the 52 respondents reported one or more potential risk factors for HBV, but no significant difference was found between RBI and OBI groups except for a greater proportion of persons born or having resided in an HBV-endemic country among OBI donors than among RBI donors (39% vs. 5%; p = 0.0078). Notably, 77% (40 of 52) of the combined RBI and OBI groups reported multiple sex partners, including men who had sex with men, sex with an injection drug user, and/or sex with a person known to have hepatitis B.
TABLE 3.
Risk Factors of American Red Cross blood donors with recent and occult HBV infection who completed the study questionnaire (n = 52), June 2009 to April 2015
| RBI | OBI | |||
|---|---|---|---|---|
| Risk‡ | n = 21 | n = 31 | OR (95% CI) | p value§ |
| No, of sexual partners in lifetime (%) | ||||
| ≤ 1 | 6 (29) | 11 (35) | Reference | NS |
| > 1 | 15 (71) | 20 (65) | 1.4 (0.4–4.6) | |
| Men who had sex with men, number (%)‖ | 3 (27) | 1 (4) | 9.7 (0.9–107.2) | NS |
| Had sex with someone known to have STD or hepatitis B*, number (%) | 3 (14) | 2 (6) | 2.4 (0.4–16.0) | NS |
| Lived with someone known to have viral hepatitis, number (%) | 5 (24) | 6 (19) | 1.3 (0.3–5.0) | NS |
| Injecting drug use, number (%) | 1 (5) | 2 (6) | 0.7 (0.1–8.5) | NS |
| Incarceration, number (%) | 5(24) | 6 (19) | 1.3 (0.3–5.0) | NS |
| Tattoo, number (%) | 8 (38) | 9 (29) | 1.5 (0.5–4.9) | NS |
| Piercings, number (%) | ||||
| Ear | 9 (43) | 10 (32) | Reference | NS |
| Ear and body | 4 (19) | 0 (0) | 10.0 (0.5–210.2)† | |
| Body | 1 (5) | 0(0) | 3.3 (0.1–91.6)† | |
| Acupuncture, number (%) | 2 (10) | 4 (13) | 0.7 (0.1–4.3) | NS |
| Occupational exposure to blood, number (%) | 4 (19) | 7 (23) | 0.8 (0.2–3.2) | NS |
| Blood transfusion, number (%) | 4 (19) | 3 (10) | 2.2 (0.4–11.0) | NS |
| Hemodialysis, number (%) | 1 (5) | 0 (0) | 4.6 (0.2–118.7)† | NS |
| Hospitalized 2 yr before reactive donation, number (%) | 4 (19) | 7 (21) | 0.8 (0.2–3.2) | NS |
| Dental visit 2 yr before reactive donation, number (%) | ||||
| Cleaning only, without dental injection | 6 (29) | 7(23) | Reference | NS |
| Dental procedures otherthan cleaning | 9 (43) | 16 (52) | 0.7 (0.2–2.6) | |
| Born/resided in an HBV-endemic country, number (%) | 1 (5) | 12 (39) | 0.1 (0–0.7) | 0.0078 |
RBI = HBV DNA (+)/HBsAg (−) or (+)/HBcAb (−); OBI = HBV DNA (+)/HBsAg (−)/HBcAb (+).
Abbreviations: CI, confidence interval; HBcAb = hepatitis B core antibody; HBsAg = hepatitis B surface antigen; HBV, hepatitis B virus; OBI, occult hepatitis B infection; OR, odds ratio; RBI, recent hepatitis B infection; NS, not significant; STD, sexually transmitted disease.
Statistically adjusted to incorporate men only.
A fixed 0.5 correction was added to all cells due to zero counts.
Some cases had more than one risk.
p value calculated using Fisher’s exact test.
DISCUSSION
We used NAT to define HBV-infected blood donors with RBI (i.e., HBV-DNA yield or HBsAg yield)8 and OBI, and to further investigate their characteristics. All RBI donors who were followed seroconverted; therefore, primary OBI was not identified in this study. Also, we were unable to detect the presence of HBsAb-only OBI,20 as none of our donors were HBsAb positive as the only marker in the presence of HBV DNA During the study period, the overall rate of HBV infection (defined as HBV DNA confirmed positive with or without other serologic markers) was 7.95 per 100,000 donations, a rate similar to 7.6 per 100,000 donations reported for 2011–2012.3 Data from the ARC demonstrated decreases in overall HBV prevalence and incidence rates when four successive two-year periods from 2008–2015 were analyzed.21 The largest group of HBV-infected donors, that is, those who had all three HBV markers detected (ie., HBV DNA HBsAg, and HBcAb), were not examined in detail in this study. Our focus was investigating RBI and OBI donors. The rates of RBI and OBI donors were low, 0.35 and 1.70 per 100,000 donations, respectively, but not inconsequential. We found no trend in the rates of RBI and OBI during the study period in contrast to the observed decrease in the overall HBV prevalence and incidence in the ARC donor population.21 Some annual variability in the rates of RBI and OBI occurred and would be expected. This variation could have been partly the result of the relatively small annual number of RBI and OBI donors versus that of the total HBV-infected donor population. The change to a more sensitive HBV DNA detection assay (Ultrio Plus) could have contributed to variation in RBI and OBI rates, but did not appear to explain the absence of an observed decrease.
The use of sensitive HBV NAT has improved detection of HBsAg-nonreactive donations from HBV-infected donors, further reducing the risk of HBV transfusion transmission.4,22 It is noteworthy that HBV MP NAT failed to detect approximately 92% (46 of 593) of OBI donors; ID NAT is required for detection of OBI donors with low viral loads. Low viral loads and rates of MP NAT detection of OBI donors are consistent with previously published reports.4,11 In the United States (an HBV nonendemic country), addition of routine testing of blood donors for HBcAb has improved suspicion of OBI and prompted use of ID NAT to detect low levels of HBV DNA thus identifying OBI donors at risk for progression or transmitting HBV infection.23,24
Nearly one half of all RBI donors and more than one third of OBI donors resided in the southern region of the United States, as defined by the US Census Bureau (i.e., the District of Columbia, Alabama, Arkansas, Delaware, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, and West Virginia).25 This observation is consistent with previous reports3,14 that found the southern region was among regions with the highest rates of HBV-infected persons. Based on the CDC’s National Viral Hepatitis Surveillance Report for 2015, the southern states accounted for 83% of reported cases of acute hepatitis B, with rates above the national rate of 1.1 per 100,000 population.26
In this study, RBI was more frequently detected in repeat donors as well as among white donors. In contrast, the majority (93%) of all HBV DNA-positive blood donors identified were Asian first-time donors, consistent with a prior study.7 Most Asian donors had prevalent infection, but 2% had RBI, and 20% had OBI (Table 2). This observation is likely due to the migration of persons from eastern Asia (an HBV-endemie region) with chronic asymptomatic infection from mother-to-child transmission.27–30
Risks of HBV infection other than having resided or been born in an HBV-endemic country were not significantly different between RBI and OBI groups. More than three fourths (40 of 52) of the combined RBI and OBI donor groups in this study self-reported having a variety of sexual contacts associated with increased risk of HBV transmission. Unlike our earlier study, we used a questionnaire that did not establish the HBV infection status of the donor’s most recent sexual partner(s) and therefore could not confirm HBV exposure.22 Identifying the source of HBV is not simple because some HBV-infected donors had multiple potential sources for HBV and others had none. In the CDC’s National Notifiable Diseases Surveillance System for acute hepatitis B cases in 2015, one or more potential exposures/behaviors associated with HBV transmission were identified in 48% of cases.26 Since blood donors are prescreened for high-risk exposures/behaviors, identification of RBI among volunteer blood donors suggests that most of these donors were unaware of their HBV infection. Our findings suggest that RBI as well as OBI donors might benefit from counseling, including referral for evaluation of HBV infection and contact tracing.
HBV genotyping was more successful in samples from RBI than OBI donors reflecting the wide disparity between HBV DNA concentrations in these groups. Among 48 donors with RBI, 83% carried HBV subgenotype A2, a frequency consistent with a prior report of 67% of incident donors infected by genotype A.7 This frequency is similar to frequencies obtained from surveillance studies of the US general population conducted from 1999 to 2005 and 2006 to 2011,14,15 which found A2 rates among acute hepatitis B cases of 75% and 82%, respectively. Patients with primary infection by an A2 strain tend to progress to chronieity,31,32 adding to the reservoir of HBV carriers. Genotype A accounted for 35% of the US cases of chronic hepatitis B in 2001.30 A rapid population expansion in A2 strains appeared to occur between 1995 and 2002 in the United States, with A2 representing the overwhelming majority of genotype A strains circulating in the United States now.13,14
A substantia] proportion (51% [20 of 39]) of our sequenced A2-infected RBI donors carried HBV strains that shared an identical S gene nucleotide sequence. In molecular characterization of 450 acute hepatitis B cases infected with A2, 150 (33%) carried strains that had this shared S gene sequence.14 Detection of only 15 unique S gene sequences among 33 RBI blood donors infected with subgenolype A2 strains in our study, and identification of short terminal branches in the phylogenetic tree for these sequences indicated reduced genetic heterogeneity, consistent with prior studies of acute hepatitis B cases in the United States13–15
Of 80 participating OBI donors, 5 of 10 with available HBV DNA had HBV variants with amino acid substitutions in the a determinant of the S gene. It is likely that the number of HBV-infected donors in this study having mutations in the S gene was underestimated, considering that only 13% (10 of 80) of OBI donors were successfully genotyped, reflecting their low HBV DNA concentrations.11 Donors who were HBsAg negative may have been infected by HBV strains with impaired production or secretion of HBsAg.33–35 In a prior study including 33 OBI donors, 67% were identified with mutations that may have resulted in the absence of HBsAg production.11 Another explanation for the HBsAg-negative status in the OBI donors is that HBsAg is present but at levels below the detection limit of assays used. The HBV DNA concentration in all OBI donors was 40 IU/mL or lower. Higher HBsAg assay sensitivity would be required to detect possible HBsAg in infected individuals having such low viral loads (e.g., 0.02 ng/mL vs. 0.08–0.10 ng/mL of current assays).36 OBI donors who are HBV DNA positive provide ongoing evidence that they may be “currently” infected and therefore potentially infectious. Blood and blood products from donors with OBI have transmitted HBV, especially in the absence of neutralizing antibody.37,38 As such, it is noteworthy that of the OBI plasma units retrieved (n = 80), 61% had HBsAb levels of less than 10 mIU/mL (Table 2).
The major limitation of this study was the low percentage of retrieved OBI plasma units. The proportion of retrieved donations from RBI donors was higher than those from OBI donors (70% [84 of 120] vs. 20% [115 of 583]). Many of the plasma units from OBI donors were discarded after screening. It is likely that additional HBV mutants were present in OBI donors but not detected considering the small proportion of plasma units available for sequencing and that only a fragment of the S gene was sequenced. For both RBI and OBI, without whole genome and/or next generation sequencing, confirming genetic and epidemiologic relatedness is not possible.13 Finally, the questionnaire was based on self-reported history of risk behaviors/factors, obtained by telephone interview at variable periods after detection of infection, and possibly subject to recall bias.
In summary, this study showed that donations from US blood donors continue to have low rates of RBI and OBI. Risks for RBI or OBI among volunteer donors remain difficult to ascertain, although our data suggest that multiple sexual partners was a common exposure.6 A substantial proportion of the sequenced RBI were caused by HBV A2, likely indicating more frequent transmission of this genotype from chronically or acutely infected persons.13 Infection by HBV S gene escape mutants was exclusive to sequenced donors with OBI. Although the rates of RBI and OBI were low, our findings confirm the continuing importance of careful screening of all donations for evidence of HBV infection. All HBV-infeded donors, including those found to have RBI or OBI should receive counseling, including referral for care and contact tracing. To exclude the possibility of ongoing HBV infection, donors testing HBcAb reactive as their only HBV marker, should have additional sensitive ID NAT (<40 IU/mL) performed either by the blood collection agency or by their health care provider. Additional studies evaluating HBV DNA sequences from US donors will provide information on emerging genotypes and strains.
ACKNOWLEDGMENTS
The authors acknowledge and thank Elizabeth H. Hewitt and Anne M. Kaldun of the American Red Cross, for conducting all the donor interviews; Sakina Smith of the American Red Cross, who facilitated the preparation and shipment of samples for testing; and Daniel McGovern from the CDC Reference Laboratory for his help with the preanalytics of this study.
FINANCIAL DISCLOSURE
This work was supported by the Centers for Disease Control and Prevention [grant number 200-2013-56600].
ABBREVIATIONS:
- ARC
American Red Cross
- CI
confidence interval
- HBcAb
hepatitis B core antibody
- HBsAb
antibody to hepatitis B surface antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- ID
individual
- MPs
minipools
- NAT
nucleic add testing
- OBI
occult HBV infection
- OR
odds ratio
- PCR
polymerase chain reaction
- RBI
recent HBV-infected
- TMA
transcription-mediated amplification
Footnotes
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest
REFERENCES
- 1.Whitaker B, Rajbhandary S, Weinman S, et al. Trends in United States blood collection and transfusion: results from the 2013 AABB blood collection, utilization, and patient blood management survey. Transfusion 2016;56:2173–83. [DOI] [PubMed] [Google Scholar]
- 2.Dorsey KA, Moritz ED, Steele WR, et al. A comparison of human immunodeficiency virus, hepatitis C virus, hepatitis B virus, and human T-lymphotropic virus marker rates for directed versus volunteer blood donations to the American Red Cross during 2005 to 2010. Transfusion 2013;53:1250–6. [DOI] [PubMed] [Google Scholar]
- 3.Dodd RY, Notari EP, Nelson D, et al. Development of a multisystem surveillance database for transfusion-transmitted infections among blood donors in the United States. Transfusion 2016;56:2731–9. [DOI] [PubMed] [Google Scholar]
- 4.Stramer SL, Notari EP, Krysztof DE, et al. Hepatitis B virus testing by minipool nucleic acid testing does it improve blood safety? Transfusion 2013;53:2449–58. [DOI] [PubMed] [Google Scholar]
- 5.Zou S, Stramer SL, Notari EP, et al. Current incidence and residual risk of hepatitis B infection among blood donors in the United States. Transfusion 2009;49:1609–20. [DOI] [PubMed] [Google Scholar]
- 6.Custer B, Kfessler D, Vahidnia F, et al. Risk factors for retrovirus and hepatitis virus infections in accepted blood donors. Transfusion 2015;55:1093–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delwart E, Slikas E, Stramer SL, et al. Genetic diversity of recently acquired and prevalent HiV, hepatitis B virus, and hepatitis C virus infections in US blood donors. J Infect Dis 2012;205:375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollinger FB. Hepatitis B virus infection and transfusion medicine: science and the occult. Transfusion 2003;43:1001–26. [DOI] [PubMed] [Google Scholar]
- 9.Stramer SL, Krysztof DE, Brodsky JP, et al. Comparative analysis of triplex nucleic acid test assays in United States blood donors. Transfusion 2013;53:2525–37. [DOI] [PubMed] [Google Scholar]
- 10.Ohhashi Y, Pai A, Halait H, et al. Analytical and clinical performance evaluation of the Cobas TaqScreen MPX Test for use on the Cobas S 201 system. J Virol Methods 2010;165:246–53. [DOI] [PubMed] [Google Scholar]
- 11.Enjalbert F, Krysztof DE, Candotti D, et al. Comparison of seven hepatitis B virus (HBV) nucleic add testing assays in selected samples with discrepant HBV marker results from United States blood donors. Transfusion 2014;54:2485–95. [DOI] [PubMed] [Google Scholar]
- 12.Yang MH, Li L, Hung YS, et al. The efficacy of individual-donation and minipool testing to detect low-level hepatitis B virus DNA in Taiwan. Transfusion 2010;50:65–74. [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran S, Purdy MA, G-L X, et al. Recent population expansions of hepatitis B virus in the United States. J Virol 2014;88:13971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teshale EH, Ramachandran S, G-L X, et al. Genotypic distribution of hepatitis B virus (HBV) among acute cases of HBV infection, selected United States counties, 1999–2005. Clin Infect Dis 2011;53:751–6. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal K, Elevens RM, Kainer MA, et al. Epidemiology of acute hepatitis B in the United States from population-based surveillance, 2006–2011. Clin Infect Dis 2015;61:584–92. [DOI] [PubMed] [Google Scholar]
- 16.Sloan RD, Ijaz S, Moore PL, et al. Antiviral resistance mutations potentiate hepatitis B virus immune evasion through disruption of its surface antigen a determinant. Antivir Ther 2008; 13:439. [PubMed] [Google Scholar]
- 17.Aragri M, Alteri C, Battisti A, et al. Multiple hepatitis B virus (HBV) quasispecies and immune-escape mutations are present in HBV surface antigen and reverse transcriptase of patients with acute hepatitis B. J Infect Dis 2016;213:1897–905. [DOI] [PubMed] [Google Scholar]
- 18.Hossain MG, Ueda K. Investigation of a novel hepatitis B virus surface antigen (HBsAg) escape mutant affecting immunogenidty. PLoS One 2017;12:e0l6787l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuen M-F. Clinical implication of occult hepatitis B infection. Ann Blood 2017;2:1–10. [Google Scholar]
- 20.Allain J-P. Global epidemiology of occult HBV infection. Ann Blood 2017;2:1–13. [Google Scholar]
- 21.Crowder LA, Steele WR, Notari EP, et al. Epidemiology of hepatitis B virus, hepatitis C virus and human immunodeficiency virus in United States blood donors. Transfusion 2017;57:47A–A. [DOI] [PubMed] [Google Scholar]
- 22.Stramer SL, Wend U, Candotti D, et al. Nucleic acid testing to detect HBV infection in blood donors. New Engl J Med 2011; 364:236–47. [DOI] [PubMed] [Google Scholar]
- 23.Seo DH, Whang DH, Song EY, et al. Occult hepatitis B virus infection and blood transfusion. World J Hepatol 2015;7:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esposito A, Sabia C, lannone C, et al. Occult hepatitis infection in transfusion medidne: screening policy and assessment of current use of anti-HBc testing. Transfus Med Hemother 2017; 44:263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States Census Bureau. Geographic terms and concepts - census divisions and census regions; 2015. [cited 2018 Jun 29]. Available from: https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf
- 26.Centers for Disease Control and Prevention. Surveillance for viral hepatitis—United States, 2015; 2017 (cited 2018 Jun 7], Available from: https://www.cdc.gov/hepatitis/statistics/2015survdllance/index.htm
- 27.Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in US households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology 2016;63:388–97. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Guo N, Yu Q, et al. Risk factors for hepatitis B and C infection among blood donors in five Chinese blood centers. Transfusion 2015;55:388–94. [DOI] [PubMed] [Google Scholar]
- 29.Ranger-Rogez S, F D. Hepatitis B mother-to-child transmission. Expert Rev of Anti Infect Ther 2004;2:133–45. [DOI] [PubMed] [Google Scholar]
- 30.Chu CJ, Keeffe EB, Han SH, et al. Hepatitis B virus genotypes in the United States: results of a nationwide study. Gastroenterology 2003;125:444–51. [DOI] [PubMed] [Google Scholar]
- 31.Kogame M, Ishii K, Shiratori M, et al. Clinical characteristics of patients with acute hepatitis B genotype A. J Gastroenterol Hepatol 2010;25:A90. [Google Scholar]
- 32.Yamada N, Yotsuyanagi H, Okuse C, et al. Duration of HBs anti-genemia in patients with acute hepatitis B. Kanzo 2010;51:534–5. [Google Scholar]
- 33.Candotti D, Lin CK, Belkhiri D, et al. Occult hepatitis B infection in blood donors from South East Asia: molecular characterisation and potential mechanisms of occurrence. Gut 2012; 61:1744–53. [DOI] [PubMed] [Google Scholar]
- 34.Biswas S, Candotti D, Allain JP. Specific amino add substitutions in the S protein prevent its excretion in vitro and may contribute to occult hepatitis B virus infection. J VirolMethods 2013;87:7882–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberger KM, Bauer T, Bohm S, et al. High genetic variability of the group-specific a-determinant of hepatitis B virus surface antigen (HBsAg) and the corresponding fragment of the viral polymerase in chronic virus carriers lacking detectable HBsAg in serum. J Gen Virol 2000;81:1165–74. [DOI] [PubMed] [Google Scholar]
- 36.Martin LA Stramer SL, Kuhns MC, et al. Correlation of improved hepatitis B surface antigen detection limits with hepatitis B virus DNA nucleic add test yield in blood donations. Transfusion 2012;52:2201–8. [DOI] [PubMed] [Google Scholar]
- 37.Satake M, Taira R, Yugi H, et al. Infectivity of blood components with low hepatitis B virus DNA levels identified in a look-back program. Transfusion 2007;47:1197–205. [DOI] [PubMed] [Google Scholar]
- 38.Allain JP, Mihaljevic I, Gonzalez-Fraile MI, et al. Infectivity of bloodproducts from donors with occult hepatitis B virus infection. Transfusion 2013;53:1405–15. [DOI] [PubMed] [Google Scholar]


