Abstract
Decidual macrophages are in close contact with trophoblast cells during placenta development, and an appropriate crosstalk between these cellular compartments is crucial for the establishment and maintenance of a healthy pregnancy. During different phases of gestation, macrophages undergo dynamic changes to adjust to the different stages of fetal development. Trophoblast-secreted factors are considered the main modulators responsible for macrophage differentiation and function. However, the phenotype of these macrophages induced by trophoblast-secreted factors and the factors responsible for their polarization has not been elucidated. In this study, we characterized the phenotype and function of human trophoblast-induced macrophages. Using in vitro models, we found that human trophoblast educated macrophages (TEMs) were CD14+CD206+CD86− and presented an unusual transcriptional profile in response to TLR4/LPS activation characterized by the expression of type 1 Interferon beta (IFN-β) expression. IFN-β further enhance the constitutive production of soluble programmed cell death ligand 1 (PD-L1) from trophoblast cells. PD-1 blockage inhibited trophoblast induced macrophage differentiation. Soluble PD-L1 was detected in the blood of pregnant women and increased throughout the gestation. Collectively, our data suggest the existence of a regulatory circuit at the maternal fetal interface wherein IFN-β promotes sPD-L1 expression/secretion by trophoblast cells, which can then initiate a PD-L1/PD-1 mediated macrophage polarization towards an M2 phenotype, consequently decreasing inflammation. Macrophages then maintain the expression of sPD-L1 by the trophoblasts through IFN-β production induced through TLR4 ligation.
Circulating PD-L1 might also function as a marker for normal trophoblast immune modulation during early pregnancy.
Keywords: Trophoblast, macrophage, LPS, IFN-β, soluble PD-L1, PD1
Graphical Abstract
1. Introduction
Pregnancy exemplifies a unique immunological condition in which cells at the maternal-fetal interface create a delicate balance between the support of fetal growth and development, tolerance to paternal antigens, and appropriate responses to infection if it occurs[1]. This microenvironment is dynamic and continuously adjusts to the different stages of fetal development. As such there is an active process of adaptation and modulation from both the maternal and fetal sides [2]. The success of pregnancy depends on the appropriate communication between the fetal trophoblast and the immune cells present at the maternal-fetal interface including macrophages (reviewed in [3, 4]).
Macrophages comprise 20–25% of the total leukocyte population in early pregnancy, and their presence is maintained throughout pregnancy. Macrophages are classically categorized into M1 and M2 subtypes [5] with M1 macrophages geared towards clearing infections (pro-inflammatory) and M2 macrophages better poised for tissue remodeling and repair (anti-inflammatory). This classification follows the TH1 and TH2 nomenclature used to identify pro- and anti-inflammatory T cells [6] [7]. Despite the nomenclature similarities, no master regulator that directs macrophage differentiation has been described, emphasizing individual cell plasticity in the myeloid lineage rather than discrete cell types [8]. Decidual macrophages are differentiated from monocytes derived from the bone marrow [3, 9–12], which migrate from the bloodstream to the uterus during the pre-implantation period and differentiate into decidua-specific macrophages upon exposure to this local microenvironment [13–16]. Decidual macrophages have a high degree of plasticity that allows them to change their phenotypes based on the signals present at the implantation site. During the pre-implantation period, macrophages are mainly the M1 phenotype [17], they change to M2 phenotype following trophoblast attachment and invasion and then revert to M1 phenotype at the time of delivery [3, 18]. Inappropriate macrophage polarization during a specific developmental stage can have a detrimental effect on fetal development and pregnancy outcome [3]. Our current knowledge on the factors regulating macrophage polarization at the implantation site is however still limited.
The trophoblasts represent the first point of contact between the blastocyst and maternal decidua and play an active role in shaping the immunological milieu at the implantation site [1]. They have the ability to sense and respond to their microenvironment through the expression of pattern recognition receptors such as toll like receptors (TLRs), which can recognize specific molecular patterns released from the local cells including decidual stromal cells and immune cells, known as damage associated molecular patterns (DAMS) or molecules released by commensal or pathogenic bacteria (pathogen associated molecular patterns, PAMS) [19–22]. Trophoblast-secreted factors are able to recruit and modulate the differentiation and function of immune cells at the maternal-fetal interface throughout pregnancy[4]. We and others have shown that trophoblast-secreted factors present in the trophoblast conditioned media (CM) promote T cell differentiation into regulatory T cells (Tregs) and CD14+ monocytes into decidual-macrophages [13, 15, 23]. Indeed, we demonstrated that trophoblast CM could induce monocyte differentiation into macrophages characterized by gain of CD14 and CD16 surface expression and enhanced production of chemokines such as Interleukin-10 (IL-10) and CXCL10 as well as increased capacity for phagocytosis[15]. We designated these macrophages as trophoblast-educated macrophages (TEM)s. The functional properties of these TEMs and the specific factors in the trophoblast CM that are required for their differentiation have however, not been elucidated.
Thus, the objectives of this study were to further characterize the role of trophoblast- secreted factors in decidual macrophage differentiation by performing a phenotypic characterization of TEMs and transcriptional assessment of their response to TLR4 stimulation. In addition, we evaluated the potential factor(s) produced by the trophoblast cells responsible for macrophage polarization. Our findings show that TEMs are CD14+/CD206+/CD86− and present a unique transcriptional profile in response to TLR4/LPS stimulation dominated by type I Interferon (IFN)-responsive pathways. Moreover, we demonstrate that soluble PD-L1 (sPD-L1) secreted by trophoblast cells and present in the blood of pregnant women, is responsible for TEM polarization. These findings provide a better understanding of the unique immune regulatory network that is present at the implantation site and necessary for the success of pregnancy.
2. Material and Methods
2.1. Ethic statement
Studies with human blood monocytes were approved by the institutional Review Board (IRB) committee of Yale University with no written consent requirement (#2000021607). Monocytes were obtained from healthy individuals eligible for single donor platelet apheresis in a blood bank setting. Investigators had no access to any personal information. All the samples evaluated had no identifiers.
2.2. Reagents, antibodies and cell lines
The cell lines used in the experiments were the first trimester trophoblast cell line Swan 71 established in our laboratory (21) and 3A cells obtained from ATCC [24, 25]. Cells were cultured in Roswell Park Memorial Institute (RPMI) media supplemented with 10% fetal bovine serum (FBS), 1000 U/ml penicillin, 100 ug/ml streptomycin, 10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 100 nM non-essential amino acids, and 1mM sodium pyruvate, and maintained at 37°C with 5% CO2.
Lipopolysaccharides (LPS) isolated from Escherichia coli (0111:B4) was purchased from Sigma–Aldrich (St Louis, MO, USA). The magnetic multiplex beads were obtained from Bio-Rad Laboratories (Hercules CA, USA), and they were precoated with the antibodies recognizing Interleukins (IL) IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12 (p70), IL-17, Granulocyte Colony Stimulating Factor (G-CSF), Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF), Interferon-Gamma (IFN-γ), Monocyte chemoattractant protein-1 (MCP-1), Macrophage Inflammatory Proteins (MIP-1α/β), Tumor Necrosis Factor-α, (TNFα), C-C Motif Chemokine Ligand 5 (CCL5 or RANTES), Vascular endothelial growth factor (VEGF), C-X-C Motif Chemokine Ligand 10 (CXCL10), and growth-regulated oncogene-alpha (GRO-α).
2.3. Patient recruitment and serum sample collection
Study population
The description of the patients population, recruitment and characteristics has been previously described [26] as following:
This study included 506 serum samples collected between 3 to 13 weeks (25 to 96 days) of gestation. In order to include enough samples from the possible earliest timepoints in pregnancy, samples were collected from two prospectively followed pregnancy cohorts - women undergoing in vitro fertilization (IVF) treatment (n=40) and women with natural conceptions (n=102). The samples from the IVF cohort included 83 data points for normal pregnancies (2–6 data points per individual sample). The natural conception cohort included 270 data points for normal pregnancies (3–4 data points per individual sample).
A limitation of this cohort was the lack of racial diversity. The majority of the patients were Caucasian because of the country of enrollment (Denmark) [27]. Details for each cohort are described below.
IVF Cohort.
Recruitment of IVF patients and storage of samples were approved by the Yale institutional IRB with no written consent requirement (#2000021607). The study was deemed to have minimal harm to patients thus only verbal consent was requested. The investigators had no access to any personal information. The coded serum samples were provided to the investigators without any patient information. The patients eligible for participation in this study included those who were between 18–44 years old and underwent fresh or frozen day 3 or day 5 (blastocyst) embryo transfer during the period of October 2017 to July 2018. Exclusion criteria were patients with chronic autoimmune disease (such as lupus, thyroid antibodies, ulcerative colitis, or Crohn’s disease), diabetes and hypertension requiring medication treatment, endometriosis confirmed by laparoscopy, or current illness with inflammatory processes. Patients were also excluded if they had prior pregnancy losses, unless the tissue from the loss had undergone genetic testing and was determined to be chromosomally abnormal. Patients were asked to participate at the time of embryo transfer. Blood was collected by venipuncture into 10mL vacutainer tubes at the time showing the first positive β-hcg, then 8–12 days after embryo transfer, and then every 48 hours until an intrauterine pregnancy was confirmed using transvaginal ultrasound. Samples were left at room temperature for 60 minutes to allow for clotting and then centrifuged (Thermo Scientific Sorvall ST 16, Waltham, MA) at 3,000 RPM for ten minutes at room temperature. Serum was aliquoted into 1.5mL polypropylene RNase- and DNase-free microcentrifuge tubes and stored in -80°C freezers until ready for testing.
Naturally Conceived Cohort.
The natural conception cohort was part of a larger Danish prospective early pregnancy cohort (the PEP cohort) including women recruited through online advertisement in the period of 2016 – 2017. The characteristics of the patient population recruited in the study is described in a previous publication [27]. In short, healthy women at the age of 18 years or older with a singleton pregnancy and able to understand and sign written consent were eligible for participation. Exclusion criteria included history of recurrent pregnancy losses (≥3 losses, including biochemical pregnancies), any type of assisted reproductive techniques, uterine or tubal abnormalities assessed at the first visit, and ongoing substance abuse. The patient follow-up with serial blood draws and transvaginal ultrasound started as soon as the women expressed interest after a positive urine pregnancy test and continued every two weeks until completion of the first trimester (11–14 weeks of gestation). Blood was collected by venipuncture into vacutainer separator tubes (BD Diagnostics, Franklin Lakes, NJ, USA), and allowed to clot for 15 minutes at room temperature and then centrifuged (Hettich Rotina 380 R, Andreas Hettich GmbH, Tuttlingen, Germany) at 3500rpm at 5°C for 10 min. Serum was aliquoted into the same plastic vials as those from the IVF cohort and stored in -80°C freezers until ready for testing. All specimens from both cohorts were transported in liquid nitrogen to Yale University.
2.4. Human primary trophoblast isolation and culture
Human primary trophoblast cells were isolated from first trimester elective terminations as previously described [24]. A signed written consent form was obtained from the patients. The use of placental tissues, specimens and consent forms was approved by the Yale University Human Investigation Committee (#2000021607). The tissue specimen was collected in cold, sterile phosphate-buffered saline (PBS) and immediately transported to the laboratory for cell culture preparation. Briefly, first trimester placental villous tissues were cut and digested in PBS supplemented with 0.25% Trypsin (Gibco, Grand Island, NY, USA) for 10 min at 37℃ with gentle agitation. An equal volume of 10% FBS (Gibco, Grand Island, NY, USA) and Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Grand Island, NY, USA) was added to inactivate the trypsin. The supernatant was collected and centrifuged at 1500 rpm at room temperature for 10 min. The pellet was resuspended in 5 ml DMEM media supplemented with 10% FBS. This suspension was laid over lymphocyte separation media (ICN Biomedicals, Inc., Aurora, OH, USA) and centrifuged at 2000 rpm for 20 min. The interface containing the trophoblast cells was collected and centrifuged at 1500 rpm for 10 min. Cells were resuspended in DMEM with 10% FBS and then plated on a 6-well plate to grow.
2.5. Preparation of trophoblast conditioned media (CM)
First trimester trophoblast Swan 71 cells [28] or first trimester trophoblast primary cell cultures were plated at 5×10^5 cells/100 mm dish with DMEM-F12 media containing 10% FBS and allowed to attach overnight. Media were then changed to DMEM-F12 media containing 1% FBS and incubated for additional 48 hrs. Cell supernatant was collected, clarified, aliquoted, and stored at -80℃ until use.
2.6. Monocyte isolation
CD14+ monocytes were isolated from concentrated leukocytes from non-pregnant females between the ages of 25–35 (see Ethic Statement). CD14+ monocyte isolation was performed as previously described [15, 29]. Briefly, leukocytes were diluted 1:1 in PBS, laid onto lymphocyte separation medium and centrifuged without brake at 2000 rpm at room temperature for 20 min. The mononuclear layer was then resuspended in PBS and centrifuged at 800g at room temperature for 10 min. The pellet was then resuspended in Easy Sep buffer (0.5% bovine serum albumin, 2 mm ethylenediaminetetraacetic acid, pH 7.2), and the human CD14+ monocytes were purified by positive selection using the monocyte isolation kit II (EasySep, Vancouver, BC CA) according to the manufacture’s protocol. The purity of the CD14+ cells was >95% as determined by flow cytometry.
2.7. Macrophage differentiation
Freshly isolated CD14+ cells were plated at 2 × 106 cell/well in DMEM/F-12 media containing 1% FBS. Cells were then treated with or without 50% conditioned media from the first trimester trophoblast cell line Sw.71, or 25 ng/mL GM-CSF, or 50 ng/mL M-CSF at 37°C in a humidified atmosphere (5% CO2) for a total of 7 days; media were refreshed once every 2 days as previously described[15]. The macrophages treated with GM-CSF and M-CSF were designated as M1-like and M2-like respectively, and macrophages differentiated with trophoblast conditioned media were designated as Trophoblast-Educated Macrophages (TEMs). For the LPS studies, the macrophages differentiated for 6 days were treated with 10 ng/ml LPS for 24 hrs, and the cells and supernatants were collected. For the PD-1 blocking studies, the purified CD14+ cells were treated with an anti-PD-1 mAb for 7 days in the presence of trophoblast CM or GM-CSF or M-CSF. The anti-PD-1 mAb treatment was refreshed once every 2 days. At day 7 of the study, cells and supernatants were collected and assessed for markers of differentiation by flow cytometry and for cytokines/chemokines by SimplePlex (ELLA) and Luminex.
2.8. Flow cytometry
The cells collected from differentiation cultures were analyzed by flow cytometry (FACSCalibur, Beckton Dickinson, Franklin Lakes, NJ, USA). PE-conjugated anti human-CD14 and FITC-conjugated anti human-CD16 Abs were purchased from eBioscience (San Diego, California) and used at a 1:50 dilution. The fluorescein isothiocyanate (FITC)-conjugated anti-human CD206 antibody (Biolegend, USA), phycoerythrin (PE)-conjugated anti-human CD14 antibody (Biolegend, USA) and PE-Cy5-conjugated anti-human CD86 antibody (Biolegend, USA) were used following the instructions from the manufacturers. FACS data were analyzed by FlowJo (Treestar, Ashland, OR, USA).
2.9. Cytokine and soluble PD-L1 analysis evaluation by ELLA
The cytokine profile of supernatants from the differentiated macrophages with or without LPS stimulation was determined using the Simple Plex immunoassay system (ELLA, Protein Simple, San Jose, CA) as previously described [30]. Briefly, 50 μl of sample was added to sample inlet ports on a cartridge, and each sample from a single sample inlet port was split into multiple parallel channels. Each channel was specific for one particular analyte and subjected to a typical sandwich immunoassay protocol. The entire immunoassay procedure was automated, and the analyzed results were obtained using the manufacture-encoded calibration curves.
2.10. Quantitative RT-PCR
RNA was extracted using the RNeasy Kit (Qiagen, Valencia, CA) and an equal amount of RNA (1 μg) was used for cDNA synthesis using iScript Reverse Transcription Supermix (Bio-Rad Laboratories, Hercules, CA, USA). The cDNA was diluted to 1:5 in nuclease-free water, and 5 μl were used in the polymerase chain reaction (PCR). The gene-specific primers and iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories) were added to the cDNA and run on the CFX96 C1000 system Quantitative PCR machine (Bio-Rad Laboratories). The primer sequences were previously described [31]. The amount of target relative to a calibrator was computed by 2−ΔΔCT, and the housekeeping gene β-actin (ACTB) or GAPDH was used for normalization.
2.11. PCR array
RNA was extracted using the RNeasy kit. RNA concentration and purity were assessed using spectrophotometric analyses of 260/280 ratios, and only samples with values of 1.8 or above were used for analysis. For quantitative analysis of messenger RNA (mRNA), 1 μg of RNA from each sample was reverse-transcribed using the RT2 First Strand Kit (Qiagen, Valencia, CA) as per manufacture’s protocol and used for the RT2 Profiler PCR Human Toll-Like Receptor Signaling Pathway Array (cat# PAHS-108Z) as directed to run on a Bio-Rad CFX96 cycler. Fold change analysis was performed using QIAGEN’S GeneGlobe Data Analysis Center (https://dataanalysis.qiagen.com/pcr/arrayanalysis).
2.12. Western Blot
Protein extraction was performed using Cell Lysis Buffer (Cell Signaling, Danvers, Ma, USA), and the total protein concentration was determined using the Pierce BCA Assay Kit (Thermo Fisher, Rockford, IL, USA). Equivalent amount of total protein was boiled for 5 min, separated on 10% Sodium Dodecyl Sulfate PolyAcrylamide Gel Electrophoresis (SDS-PAGE) and transferred to a Polyvinylidene fluoride (PVDF) membrane (Perkin Elmer, Shelton, CT, USA). Membranes were blocked with 5% nonfat milk for 1 h at room temperature and incubated with primary antibodies at 4℃ overnight. The membranes were washed and incubated with corresponding HRP-labeled secondary antibodies at room temperature for 2 h. Blots were imaged with the Kodak Image Station 400 (Eastman Kodak, Rochester, USA).
2.13. Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Science (SPSS, IBM, New York, New York) for windows and Prism software, version 5 (GraphPad, San Diego, CA). Differences between two groups were analyzed using Student’s t-test. The differences among multiple groups were analyzed by one-way ANOVA and Chi-square test. P-values less than 0.05 were considered significant. All the experiments were done in triplicate and a minimum of three independent experiments.
3. Results
3.1. Induction of a unique population of macrophages by trophoblast CM
Previously we reported that exposure of CD14+ peripheral blood monocytes to trophoblast CM induced their differentiation into decidual like-macrophages. As stated above, we designated them as Trophoblast-Educated Macrophages (TEMs)[15]. Our first objective was to further characterize the phenotype of these macrophages differentiated by trophoblast-secreted factors in terms of their heterogeneity. Figure 1 shows flow cytometry strategy for the characterization of monocytes isolated from peripheral blood. As previously shown, monocytes are a heterogeneous population [32], where classical monocytes have high CD14 (CD14high) and nonclassical monocytes have low CD14 (CD14dim),[33, 34]; and they are mainly the M1 (inflammatory) type[35] characterized by being CD86+/CD206− (Fig. 1A). The CD14dim population is double negative (CD86−/CD206−) (Fig. 1A). We isolated the CD14+ monocytes from PBMCs by magnetic beads and exposed them to trophoblast conditioned media (CM) for 7 days and evaluated their phenotype by flow cytometry. Exposure of these CD14+ monocytes to trophoblast CM promoted their differentiation into macrophages that maintained their CD14 expression and further gained CD16 expression (CD14+/CD16+) (Fig. 1B) [15]. Furthermore, they gained CD206 and lost CD86 expression, suggesting a differentiation towards a M2 phenotype that resembled decidual macrophages (CD14+/CD206+/CD86−) [9, 12, 36–38](Fig. 1B).Isotypes for the different markers are shown in Sup. Fig. 1. Accordingly, these in vitro results suggest that the placenta, more specifically trophoblast-secreted factors play an important role in the process of differentiation of local macrophages.
Figure 1. Phenotype of peripheral blood monocytes before and after differentiation stimulated by Trophoblast CM.
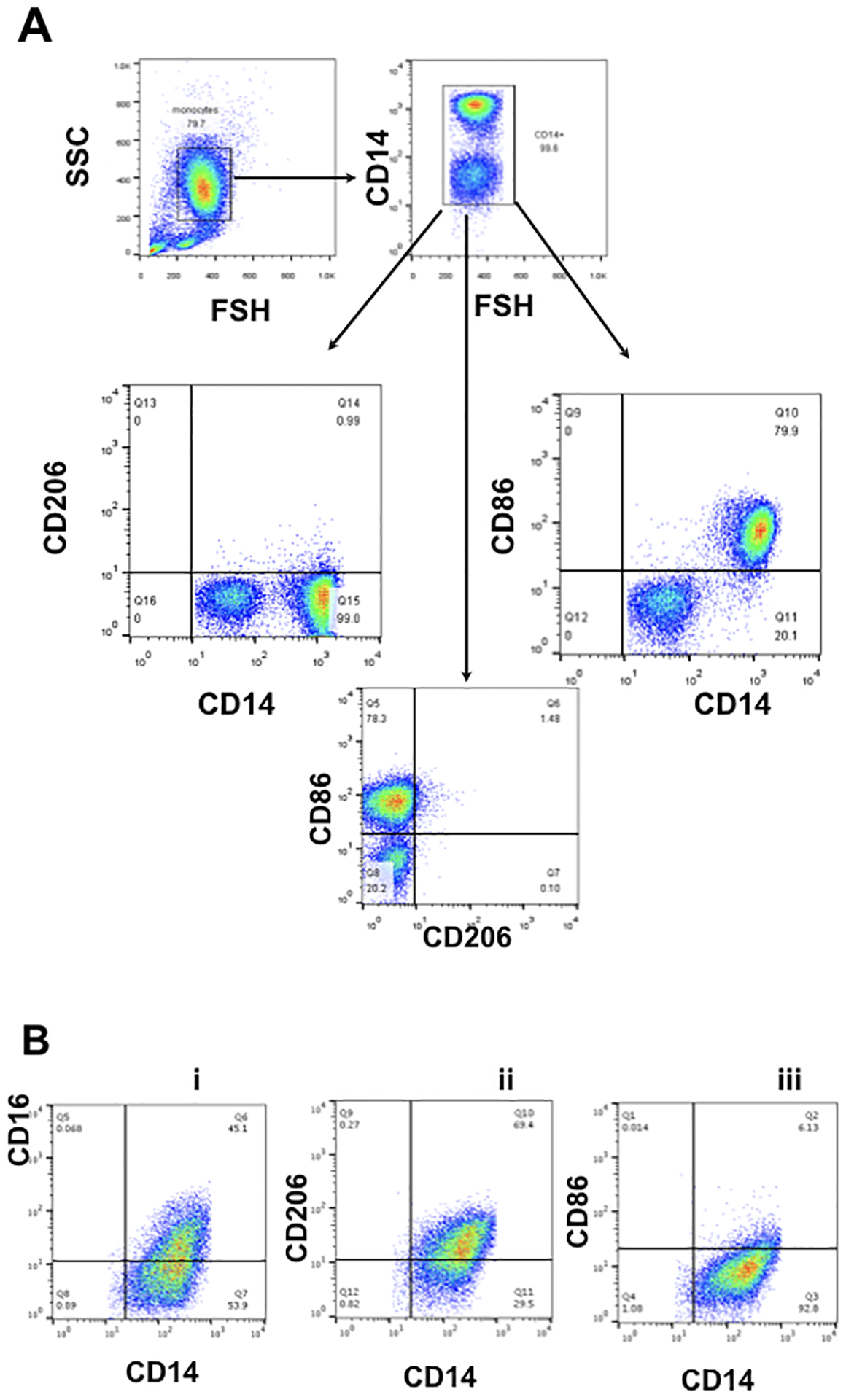
A. Gating strategy was set up for characterization of circulating monocytes. Isolated peripheral blood CD14+ monocytes express CD14 and CD86 but not CD206 (CD14+/CD206−/CD86+)
B. Freshly isolated CD14+ monocytes were exposed to first trimester trophoblast CM for 6 days and then analyzed by flow cytometry for CD14, CD86, CD206 and CD16. Following treatment with trophoblast CM, monocytes/macrophages maintained CD14 expression, and gained CD16 and CD206, but lost CD86 (CD14+/CD16+/CD206+ /CD86−). Representative figure of 6 independent experiments with each done in triplicate.
3.2. Differential response of TEMs to LPS
The basal cytokine profile as well as the cytokine profile in response to stimulus reflect the functional differentiation status of monocytes/macrophages [39–41]. Thus, to determine the functional consequence of differentiation stimulated by trophoblast CM, we first analyzed the basal cytokine secretion profile of TEMs and compared them to the M-CSF differentiated macrophages (MDMs), which had a classical M2-like phenotype. Thus, CD14+ monocytes were differentiated in the presence of trophoblast CM or M-CSF for five days. To determine the factors secreted by the differentiated cells and not the CM, the cultures were replaced with fresh media for additional 24h and collected for cytokine characterization. Interestingly, TEMs presented a distinct secretory cytokine profile characterized by a lower level of TNF-α and higher levels of TGF-β and IL-10 than that in MDMs (Fig.2).
Figure 2. Differential cytokine profile between M-CSF polarized macrophages (MDMs) and trophoblast- polarized macrophages (TEMs).
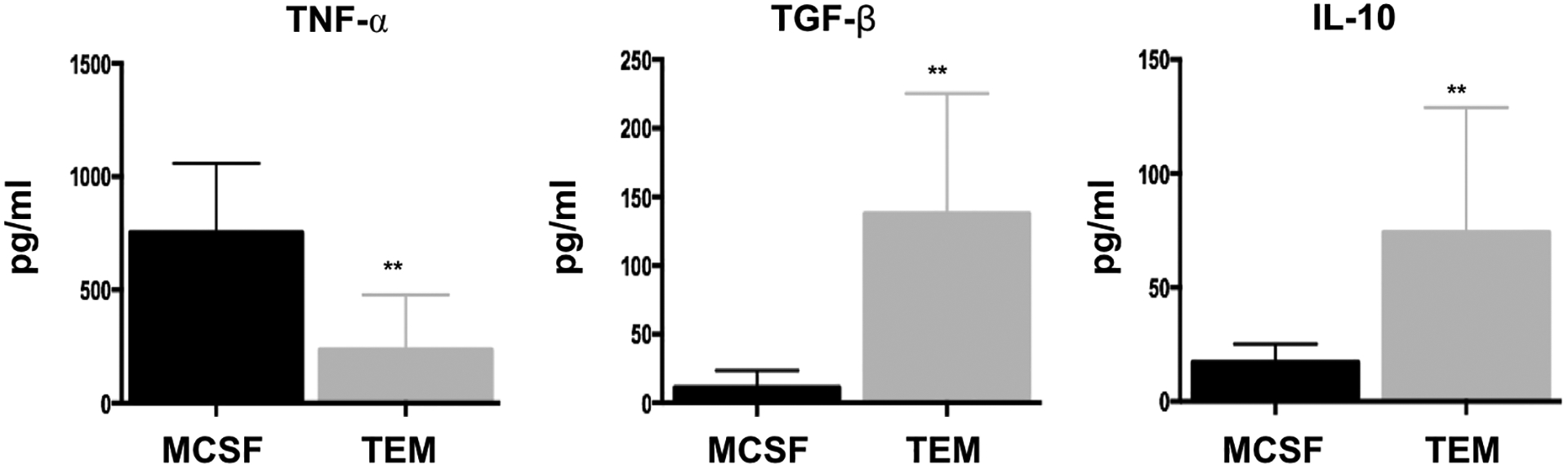
Freshly isolated CD14+ monocytes were differentiated with trophoblast CM (TEM) or M-CSF (MDM) for 6 days. Following differentiation their basal cytokine profile was quantified. TEMs presented a distinct cytokine profile compared to MDMs characterized by lower secretion of TNF-α, but higher secretion of TGF-β and IL-10. Data presented as mean + SD and are shown for 6 independent experiments with each done in triplicate. ** p=0.01
Next, we characterized the functional response of TEMs and MDMs to TLR stimulation with a focus on TLR4 signaling using LPS. Thus, upon 7 days of differentiation by trophoblast CM (for TEMs) or M-CSF (for MDMs) treatment, the cells were exposed to LPS (10 ng/ml) for 8 hours and their response was first characterized by determining the gene profile of cytokines and chemokines using a cytokine/chemokine PCR array. We observed distinct responses from the two types of macrophages. Whereas the MDM’s response to LPS was associated with increased mRNA levels of IL-1β, PTGS2, IL-6, IL-1α, and TNF-⍺ (Fig. 3A), the TEM’s response revealed a predominantly non-classical profile characterized by an increase in type-1 IFNs (Fig. 3B). In order to confirm the results from the PCR array, we performed RT-qPCR using mRNA samples from TEMs and MDMs treated with LPS (10ng/ml) for 8 hours. When we compared the magnitude of the response between the two cell types, TEMs presented a significant lower expression of TNFα, IL-6 but a much higher expression of the chemokine CCL5 than MDMs (Fig.4) This profile is consistent with the results from the PCR array (Fig. 3).
Figure 3. Differential response to TLR4 ligation by LPS.
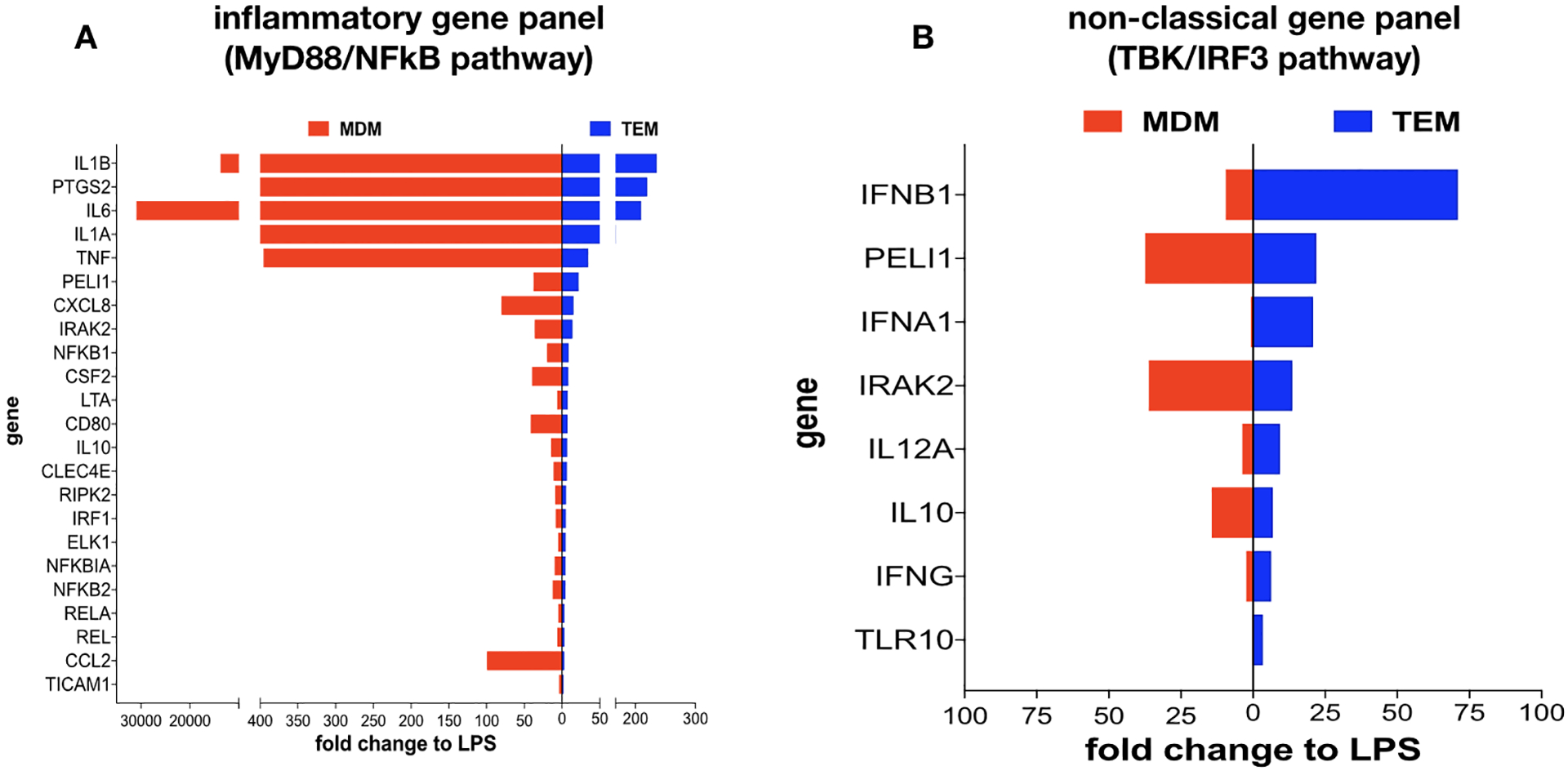
Freshly isolated CD14+ monocytes were differentiated with trophoblast CM (TEMs) or M-CSF (MDMs) for 6 days. Following differentiation cells were exposed to LPS for 8 hrs and their response to LPS was characterized by cytokine PCR array.
A. LPS treatment induced a higher mRNA expression of pro-inflammatory cytokines in MDMs than in TEMs. Fold changes in mRNA expression were calculated for each group treated with LPS in relation to their respective control (treated with vehicle)
B. Type I IFN-β and IFN-α and associated genes were highly induced by LPS treatment in TEMs compared to MDMs. Fold changes in mRNA expression were calculated for each group treated with LPS in relation to their respective control (treated with vehicle).
Figure 4. Validation of the differential cytokine profile in response to TLR4 ligation by LPS.
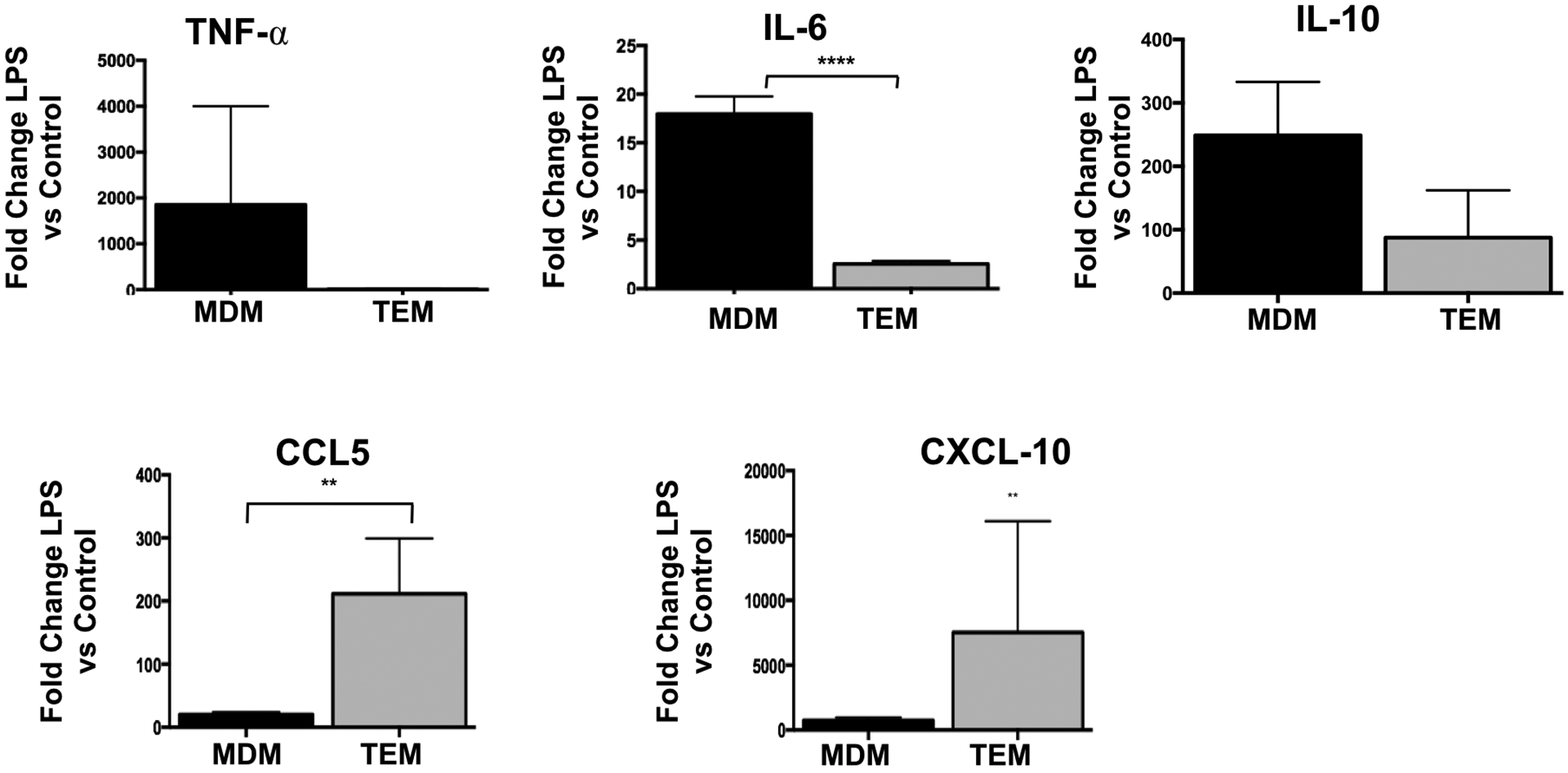
Freshly isolated CD14+ monocytes were differentiated with trophoblast CM (TEM) or M-CSF (MDM) for 6 days. Following differentiation cells were exposed to LPS for 24 hrs and mRNA of each cytokine was quantified by qPCR using specific primers. Data was presented for each cytokine as fold changes in mRNA expression calculated for each group treated with LPS in relation to their respective control (treated with vehicle). Data presented as mean + SD and are shown for 6 independent experiments with each done in triplicate. ** p=0.01; **** p=0.001
3.3. Activation of TLR4/TBK/IRF3 pathway in TEMs
We then sought to characterize the differential pathways activated in TEMs and MDMs (classical M2 macrophages) in response to TLR4 ligation by LPS. TLR4 ligation by LPS has been shown to induce two distinct pathways: the classical MyD88-NFkB signaling cascade leading to the production of pro-inflammatory cytokines [42] and the TBK/IRF3 pathway leading to the production of type-1 interferons [43, 44]. We hypothesized that in the TEMs, TLR4 ligation by LPS preferentially would signal through the non-classical TBK/IRF3 pathway since we did not observe a favored increase in NF-κB-regulated pro-inflammatory cytokines. To test this hypothesis, TEMs and MDMs were treated with LPS (10 ng/ml) and samples were collected at 15, 30, 60, 240, and 480 mins post treatment. We first determined the effect of LPS on the MyD88/NFκB pathway by evaluating IκBa, an inhibitor of p65 nuclear translocation [45]. A decrease in IκBa would suggest its degradation and consequently p65 nuclear translocation and the induction of p65-induced gene transcription. As we hypothesized, we did not observe IkBa degradation in the TEMs upon TLR4 ligation with LPS (Fig. 5B). In contrast, we observed low levels of IkBa in MDMs, suggestive of its degradation (Fig. 5A). These findings suggest that in TEMs, LPS may promote the activation of the non-classical pathway TBK/IRF3 pathway. To confirm it, we evaluated the activation status of the TBK/IRF3 pathway in TEMs by determining the phosphorylation status of TBK after LPS stimulation. We observed an increase in the levels of phosphorylated TBK (pTBK) within 15 min of exposure to LPS. In addition, we also observed an increase in the levels of phosphorylated IRF3 (pIRF3), a transcription factor downstream of TBK and a major regulator of type I IFN expression [44, 46, 47]. Total TBK and IRF3 expression was not affected by LPS treatment (Fig. 5C). The observed changes on pTBK and pIRF3 was transient with their levels resuming to baseline by 240 mins (Fig.5C). On the other hand, no changes of pIRF3 or pTBK were observed in MDMs (data not shown). Then we evaluated the mRNA expression of IFN-α and IFN-β in TEMs following LPS treatment. In accordance, TLR4 ligation by LPS in TEMs resulted in an increase in mRNA levels of IFN-α and IFN-β (Fig. 6A) as well as an increase in the antimicrobial factors beta-defensin-1, -2, and SLP1 (Fig. 6B). These findings demonstrate that in TEMs, LPS promotes the expression of Type I Interferon through the TLR4/ TBK/ IRF3 pathway.
Figure 5. LPS-TLR4 ligation in TEMs is associated with the activation of the MyD88 independent pathway TBK/IRF3.
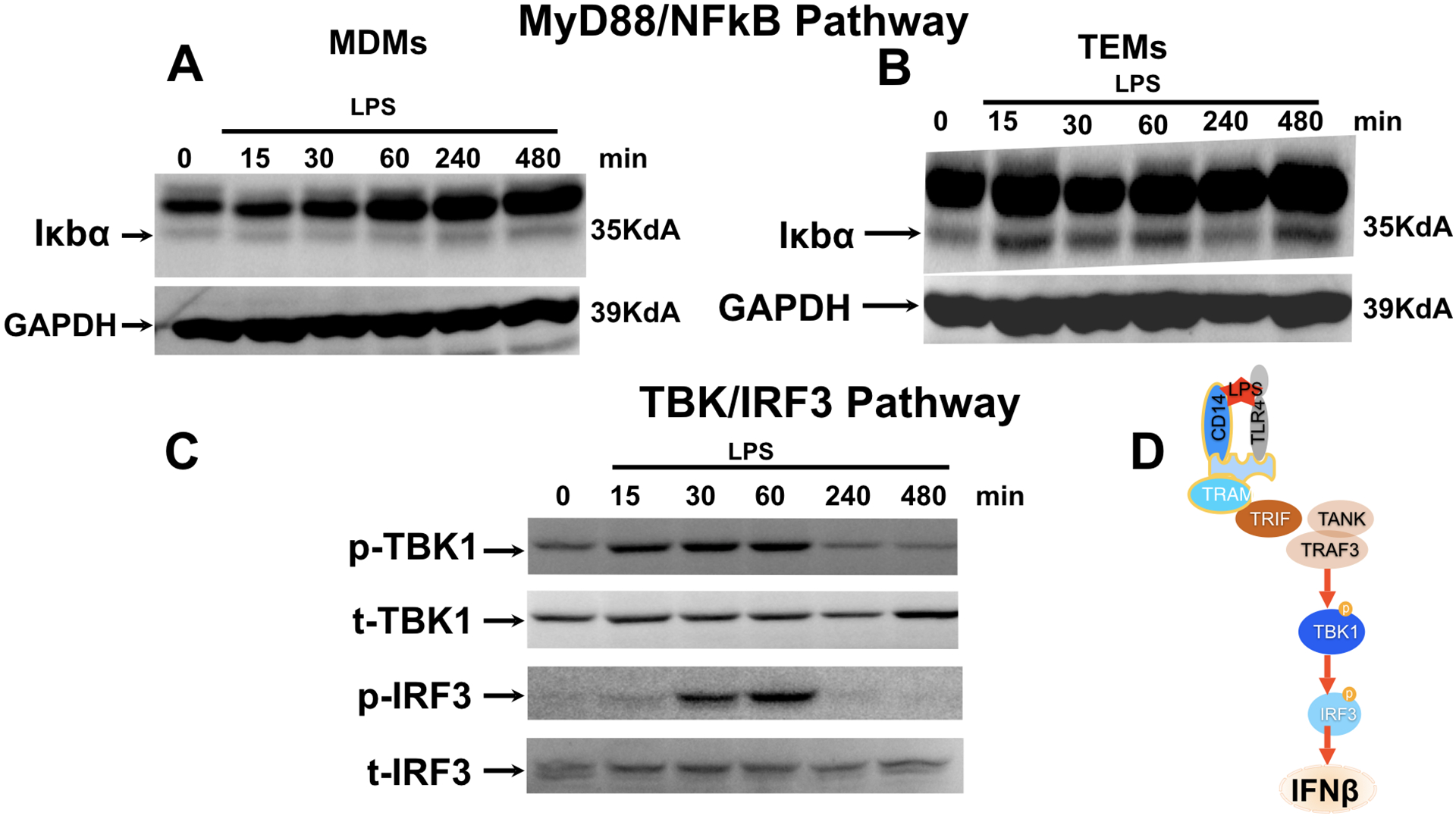
Freshly isolated CD14+ monocytes were differentiated with trophoblast CM (TEM) or M-CSF (MDM) for 6 days followed by treatment with LPS (10 ng/ml) for 15, 30, 60, 240, and 480 mins.
A. Effect of LPS treatment on MyD88/NFκB pathway was determined in M-CSF polarized macrophages (MDMs) by analyzing levels of IκBα by Western blot analysis. Note the low levels of IκBα expression in MDM.
B. Effect of LPS treatment on MyD88/NFκB pathway was determined in trophoblast educated macrophages (TEMs) by analyzing levels of IκBα by Western blot analysis. Note the continue high expression of IκBα in the presence of LPS treatment.
C. Effect of LPS treatment on TBK/IRF3 pathway was determined by analyzing phosphorylation status of TBK and IRF3 by western blot analysis. pTBK1=phosphorylated TBK; tTBK1=total TBK1. pIRF3= phosphorylated IRF3; t-IRF3=total IRF3. Representative figure of 3 independent experiments.
Figure 6. LPS-TLR4 ligation promotes type I IFN and beta defensins in TEMs.
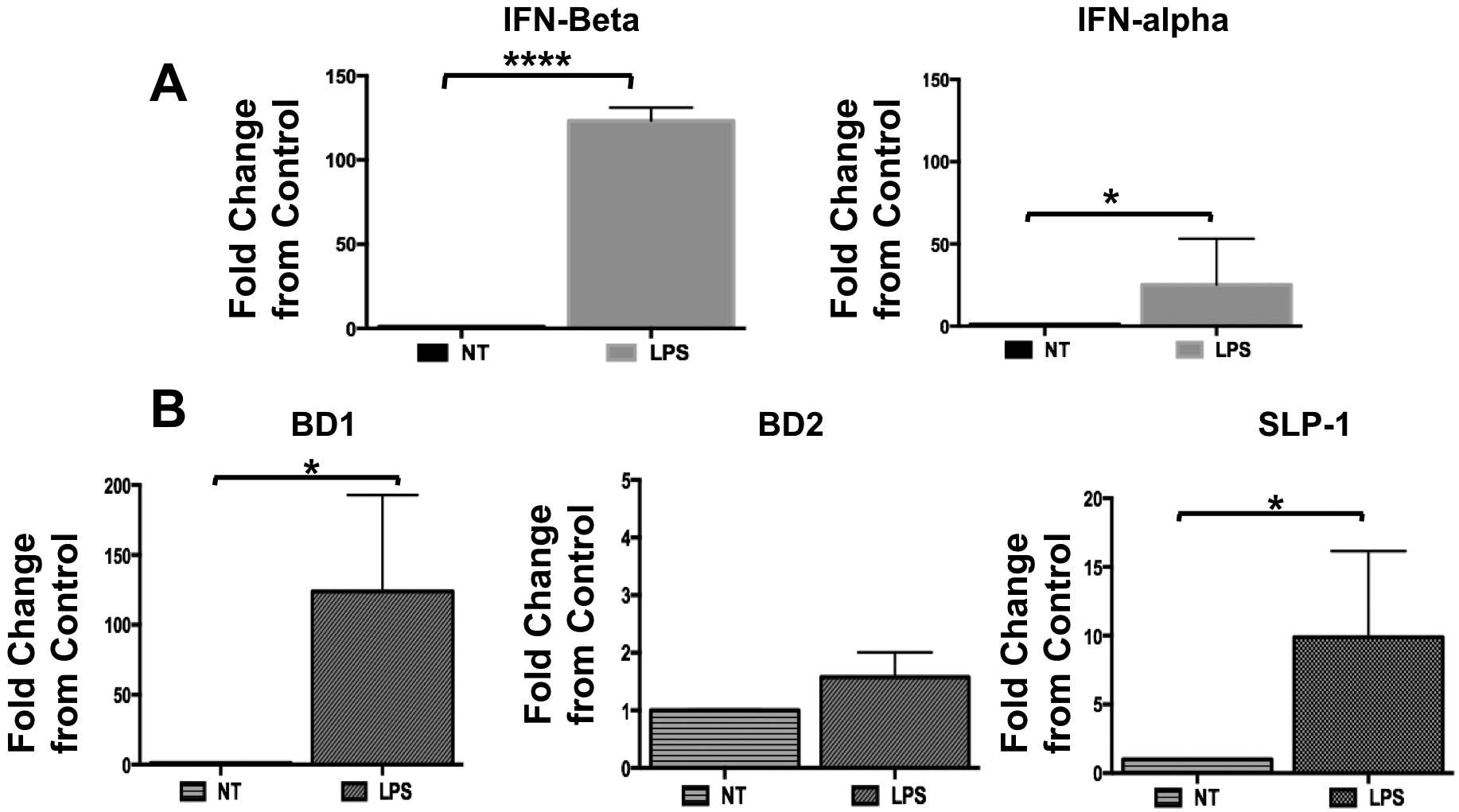
Freshly isolated CD14+ monocytes were differentiated with trophoblast CM (TEM) for 6 days. Following differentiation cells were treated with LPS (10 ng/ml) for 8 hours. mRNA expression of type I IFNs and the ISGs BD1, BD2 and SLP-1 was determined by qPCR. Data presented as mean + SD and are shown for 3 independent experiments with each done in triplicate
A. Type I IFN-α and INF-β mRNA expression in TEMs treated with LPS. Fold changes in mRNA expression were calculated for each group treated with LPS in relation to their respective control (treated with vehicle).
B. Expression of anti-microbial factors, beta defensins 1 and 2 (BD1 and BD2) and SLP-1 in TEMs treated with LPS. Fold changes in mRNA expression were calculated for each group treated with LPS in relation to their respective control (treated with vehicle). N= 6 independent experiments and each one done in triplicates. Data presented as mean + SD and are shown for 6 independent experiments with each done in triplicate. * p=0.05; **** p=0.001
To further confirm the involvement of TBK and IRF3 in TLR4-induced type 1 IFN response in TEMs, we exposed TEMs to LPS (10 ng/ml) in the presence or absence of BX795 (10 nM) [48]. BX795 is a potent and specific inhibitor of TBK1 and blocks the phosphorylation, nuclear translocation, and transcriptional activity of IRF3, and hence, the production of IFN-β [49]. We found that in the presence of BX795, LPS-induced phosphorylation of IRF3 was inhibited (Fig. 7A). More importantly, we observed a significant suppression of LPS-induced IFN-α and IFN-β expression in TEMs (Fig 7B). In addition, LPS-induced increase in CCL5, CXCL10 and the anti-microbial factors BD1, BD2, and SLP-1 were also curtailed in the presence of BX795 (Fig. 8). Collectively, these data demonstrate that the dominant signaling in TEMs in response to LPS is the non-classical TLR4/TBK1/IRF3/IFN pathway.
Figure 7. TLR4 ligation by LPS promotes IFN-β expression through the TBK-IRF3 pathway.
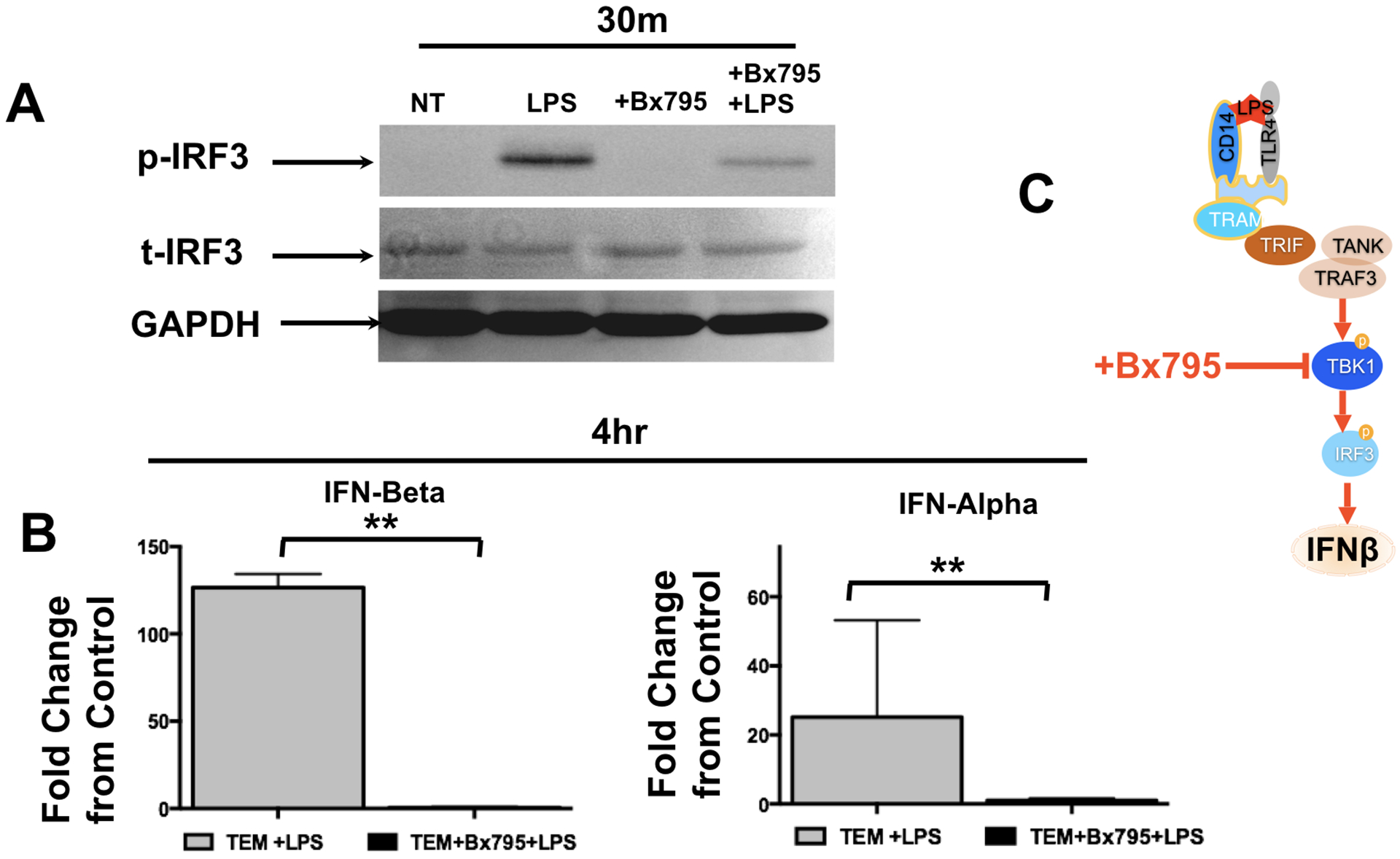
Freshly isolated CD14+ monocytes were differentiated with trophoblast CM (TEM) for 6 days. Following differentiation cells were treated with LPS (10 ng/ml) in the presence or absence of the TBK inhibitor BX795, after which protein was extracted.
A. Phosphorylation of IRF3 was determined western blot analysis in TEMs treated with LPS in the presence or absence of BX795. LPS enhanced pTBK and this effect was blocked by BX795. Representative figure of 3 independent experiments.
B. LPS treatment increases IFN-α and IFN-β mRNA expression in TEMs, which was inhibited in the presence of BX795. Data was presented as fold change of LPS (treated) vs. Vehicle (control). n=6. **P<0.01; data is presented as Mean±SEM.
Figure 8. LPS-TLR4 ligation in TEMs induce IFN-β-related genes via TBK/IRF3 signaling.
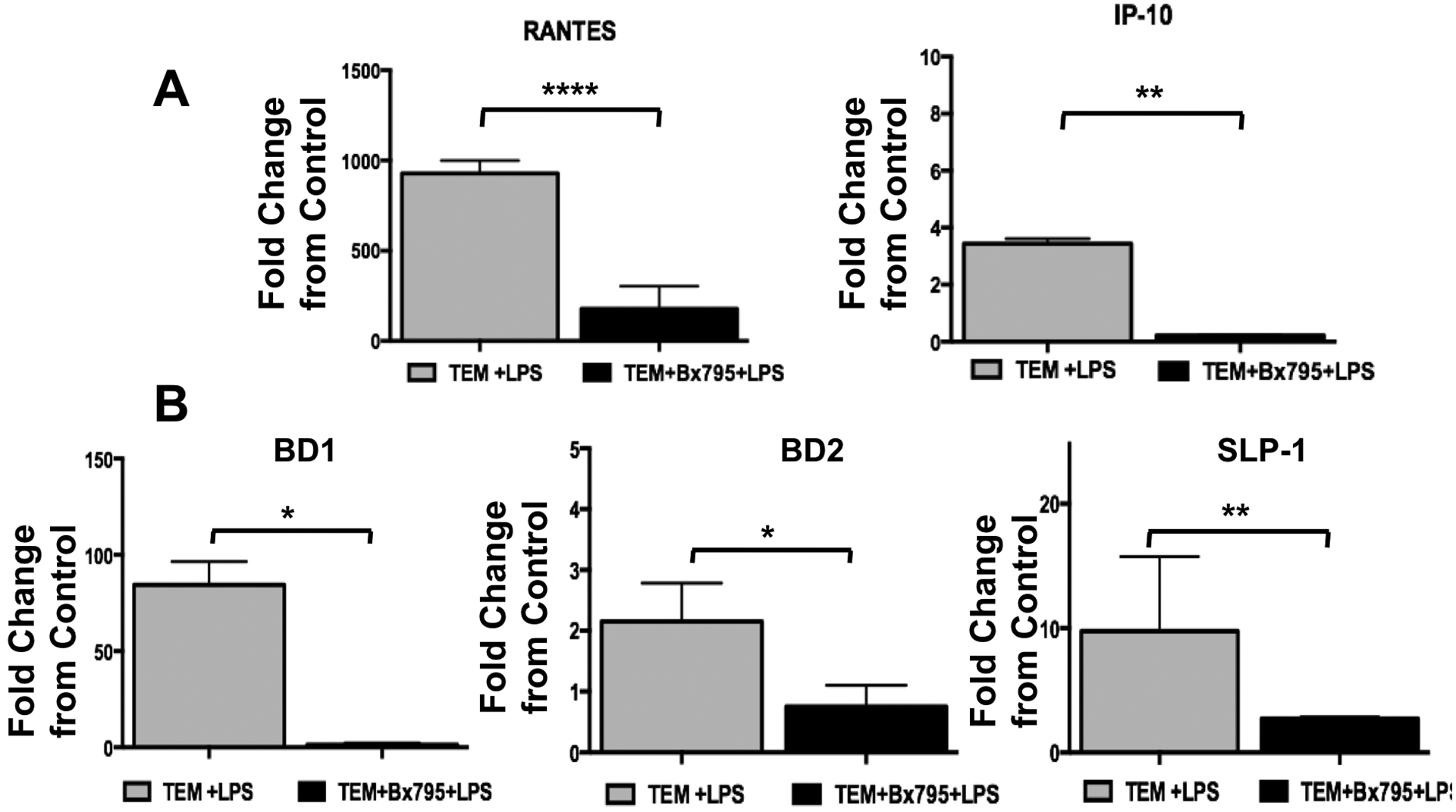
TEMs were exposed to LPS for 4 hrs in the presence or absence of the TBK inhibitor BX795, after which RNA was extracted and mRNA expression was determined by qPCR
A. LPS treatment increased CCL5 (RANTES) and CXCL10 (IP-10) mRNA expression in TEMs, which was inhibited in the presence of BX795. Data was presented as fold change of LPS (treated) vs. Vehicle (control).
B. LPS treatment increases BD1, BD2 and SLP-1 mRNA expression in TEMs, which is inhibited in the presence of BX795. Data was presented as fold change of LPS (treated) vs. Vehicle (control). *P<0.05; **P<0.01; ****P<0.001; data is presented as Mean±SEM. n=6
3.4. The role of PD-1/PD-L1 pathway in trophoblast-induced macrophage polarization
Our next objective was to elucidate the potential factors produced by trophoblast cells responsible for the phenotype of TEMs. Programmed cell death-1 (PD-1) and its ligand (PD-L1) are key modulators of immune cell function and differentiation and have been suggested to play an important role in macrophage polarization and function [50]. During early pregnancy, PD-L1 expression is observed in syncytiotrophoblasts, cytotrophoblasts and extravillous trophoblast cells [51] indicating the involvement of trophoblast cells in modulating immune cells. Since we observed the expression of PD-1 in decidual macrophages [51], we hypothesized that trophoblast cells would express and secrete PD-L1, which could be the key mediator of macrophage differentiation leading to the TEM phenotype. To test this hypothesis, CD14+ monocytes were incubated with trophoblast CM in the presence or absence of a specific PD-1-blocking antibody, which would prevent the engagement of PD-L1 (secreted by trophoblast) on PD-1 expressed by CD14+ monocytes and consequently block the activation of the PD-L1/PD-1 pathway. Our data showed that CD14+ monocytes exposed to trophoblast CM with an isotype control IgG differentiated into CD14+/CD206+/CD86−; however, when the same trophoblast CM was added in the presence of PD-1 blocking antibody we observed a decrease in the CD206+ cells and an increase in the CD86+ macrophages (Fig. 9B) that resembled the percentages of the CD86+ and CD206+ macrophages observed in the GM-CSF differentiated group, which were the classical M1 phenotype (Fig. 9C). These data suggest that by blocking PD-1, trophoblast CM fails to induce a M2 macrophage differentiation.
Figure 9. Trophoblast derived soluble PD-L1 promotes M2 like macrophage polarization.
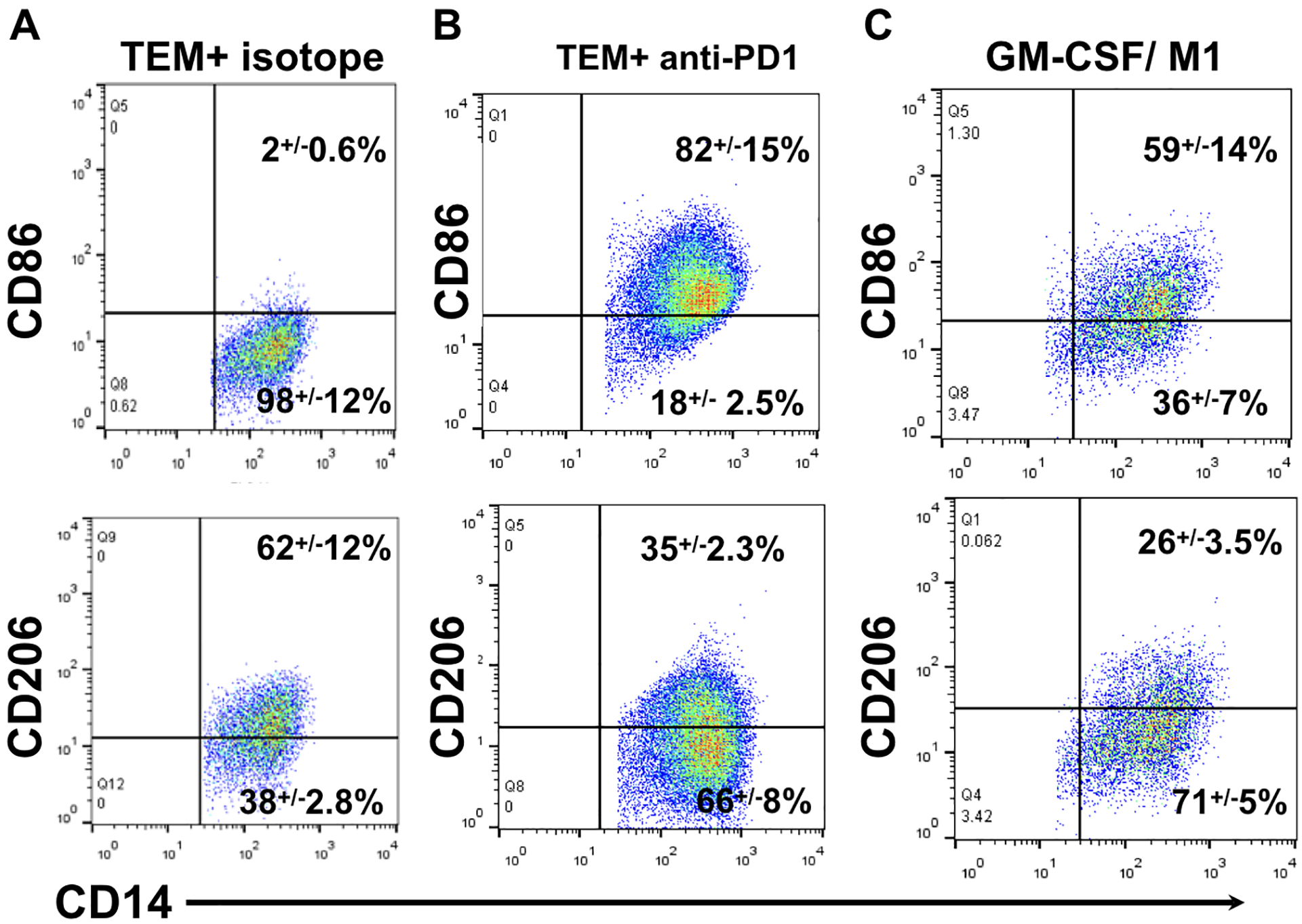
Freshly isolated CD14+ monocytes undergo differentiation in the presence of GM-CSF (M1 like) or trophoblast CM in the presence or absence of anti-PD-1 mAb or control isotype for 7 days, after which cells were evaluated by flow cytometry.
A. Flow cytometry of freshly isolated CD14+ monocytes differentiated in the presence of trophoblast CM and isotype control. TEMs are CD14+/CD206+/CD86−.
B. Flow cytometry of freshly isolated CD14+ monocytes differentiated in the presence of trophoblast CM and PD-1 blocking monoclonal antibody. The presence of the PD-1 blocking mAb prevented trophoblast induced M2-like polarization, leading to macrophages polarization characterized as increased CD86+ and decreased D206+ cells, similar to the polarization seen with GM-CSF treatment (M1 like).
C. Flow cytometry of freshly isolated CD14+ monocytes differentiated in the presence of GM-CSF. GM-CSF induced the polarization of CD14+ with a relatively high fraction of CD86+ and a low fraction of CD206+ macrophages. Representative figure of 3 independent experiments in triplicates.
Furthermore, the response to LPS in the macrophages differentiated with trophoblast CM in the presence of PD-1 blocking antibody was reversed from the type I IFN expression status towards a pro-inflammatory cytokine profile with significantly higher levels of TNF-α and IL-6 expression (Fig 10). This phenotype resembles M1-like macrophages, as demonstrated by the type of response observed with GM-CSF-differentiated macrophages (Fig 10). To further prove the role of PD-1/PD-L1 pathway in macrophage polarization, we treated GM-CSF-differentiated macrophages (M1-like) with recombinant PD-L1 (PDL-1FC) and showed that PD-L1 was able to promote their polarization towards an M2 phenotype (CD14+CD206+) (Sup Fig. 1)[51].
Figure 10. Inhibition of PD-1 changes the cytokine profile of trophoblast induced macrophage polarization.
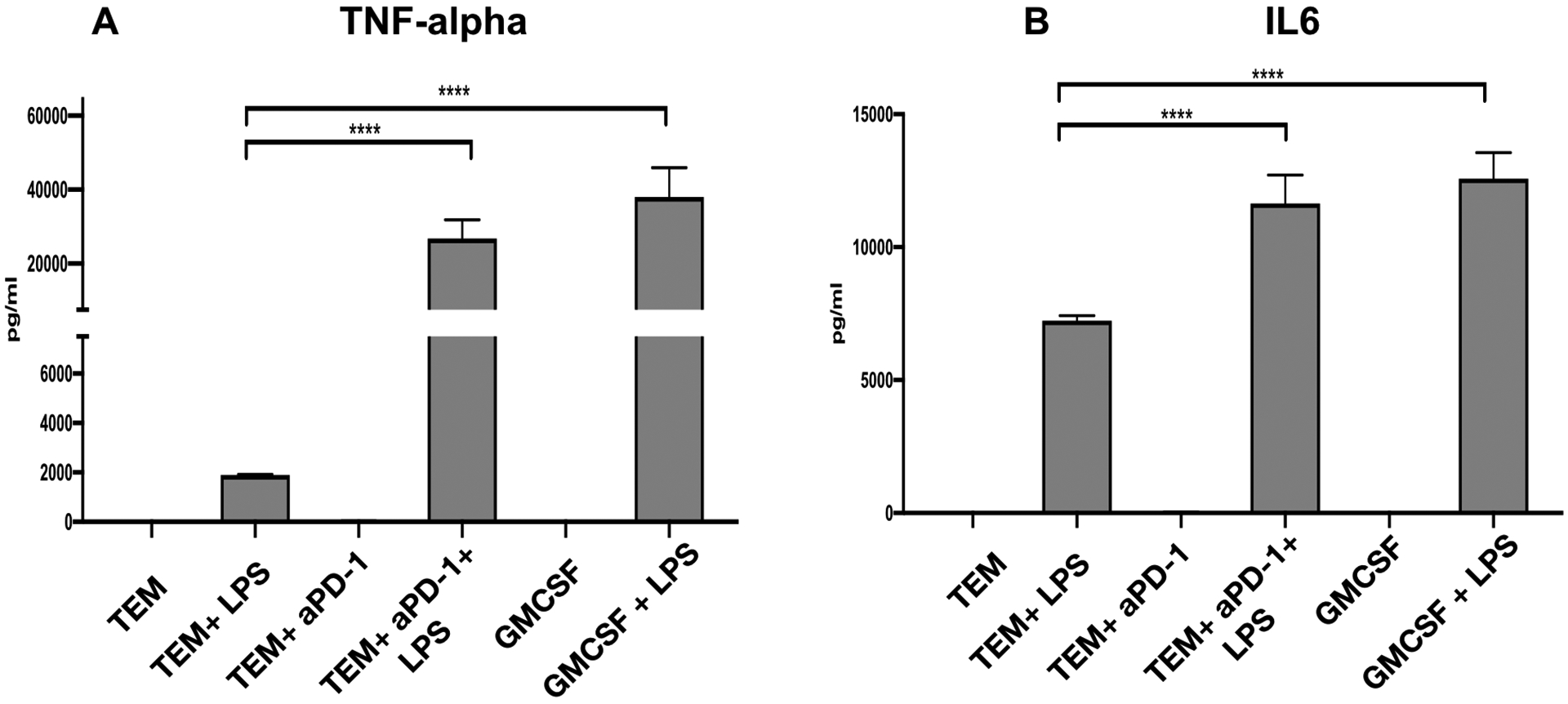
Freshly isolated CD14+ monocytes were exposed to TCM in the presence or absence of anti-PD-1 mAb for 7 days, after which cell were exposed to LPS (10 ng/ml) for 24 and TNF-β and IL6 cytokine responses were determined by ELLA assay. Note the differential response of trophoblast educated macrophages (TEMs) when PD1 was blocked during differentiation with a blocking anti-PD-1 mAB.
A. TEMs are characterized by low expression of TNF-α in response to LPS. However, the presence of anti-PD-1 mAB during differentiation leads to high levels of TNFα expression in response to LPS;
B. TEMs are characterized by low expression of IL-6 in response to LPS. However, the presence of anti-PD1 mAB during differentiation leads to high levels of IL-6 expression in response to LPS. TEM=trophoblast educated macrophages. TEM+LPS= trophoblast educated macrophages treated with LPS for 24 hours TEMαPD-1= trophoblast educated macrophages differentiated in the presence of α-PD1 monoclonal antibody. TEMαPD-1+LPS= trophoblast educated macrophages differentiated in the presence of αPD-1 monoclonal antibody treated with LPS for 24 hours. GMCSF= macrophages differentiated in the presence of GM-CSF. GMCSF+LPS= GM-CSF differentiated macrophages treated with LPS for 24 hours. Data presented as mean + SD and are shown for 3 independent experiments with each done in triplicate. ****P<0.001;
Taken together, these results demonstrate that by blocking PD-1 in the monocytes, we can prevent trophoblast induced differentiation of CD14+ monocytes into M2 macrophages, suggesting that the PD-1/PD-L1 signaling pathway is a major effector of trophoblast-induced macrophage polarization.
3.5. Expression and secretion of soluble PD-L1 (sPD-L1) by trophoblast cells
The findings described above using trophoblast CM suggest that the effect on macrophage polarization is due to the secreted soluble form of PD-L1 but not its membranal form. Therefore, our next objective was to characterize the expression of PD-L1 in trophoblast cells using first trimester trophoblast cell lines Swan 71 and 3A as well as the primary cultures of trophoblast cells isolated from first trimester placentas (obtained from elective terminations). Firstly, we confirmed that trophoblast cells constitutively express PD-L1 by western blot analysis. As shown in supplementary figure 2, positive PD-L1 protein expression was observed in cell lysates from first trimester trophoblast Swan 71 cell line collected at multiple times (Sup Fig.2). Similar results were observed with 3A and the primary cultures of trophoblast cells (Sup Fig. 3A). Next, we evaluated if PD-L1 was secreted by collecting culture supernatants from the trophoblast cells at different time points, and sPD-L1 was quantified using the ELLA platform. As shown in Figure 11A, sPD-L1 was detected in the supernatants of trophoblast Swan 71 cells and its concentration increased in a time dependent manner suggesting constitutive production and secretion of sPD-L1 by trophoblast cells. Similar time dependent increases of sPD-L1 concentrations were also observed in the supernatants of trophoblast primary cultures (Sup. Fig. 3A).
Figure 11. Trophoblast constitutively secrete soluble PD-L1, and it is regulated by IFN-β.
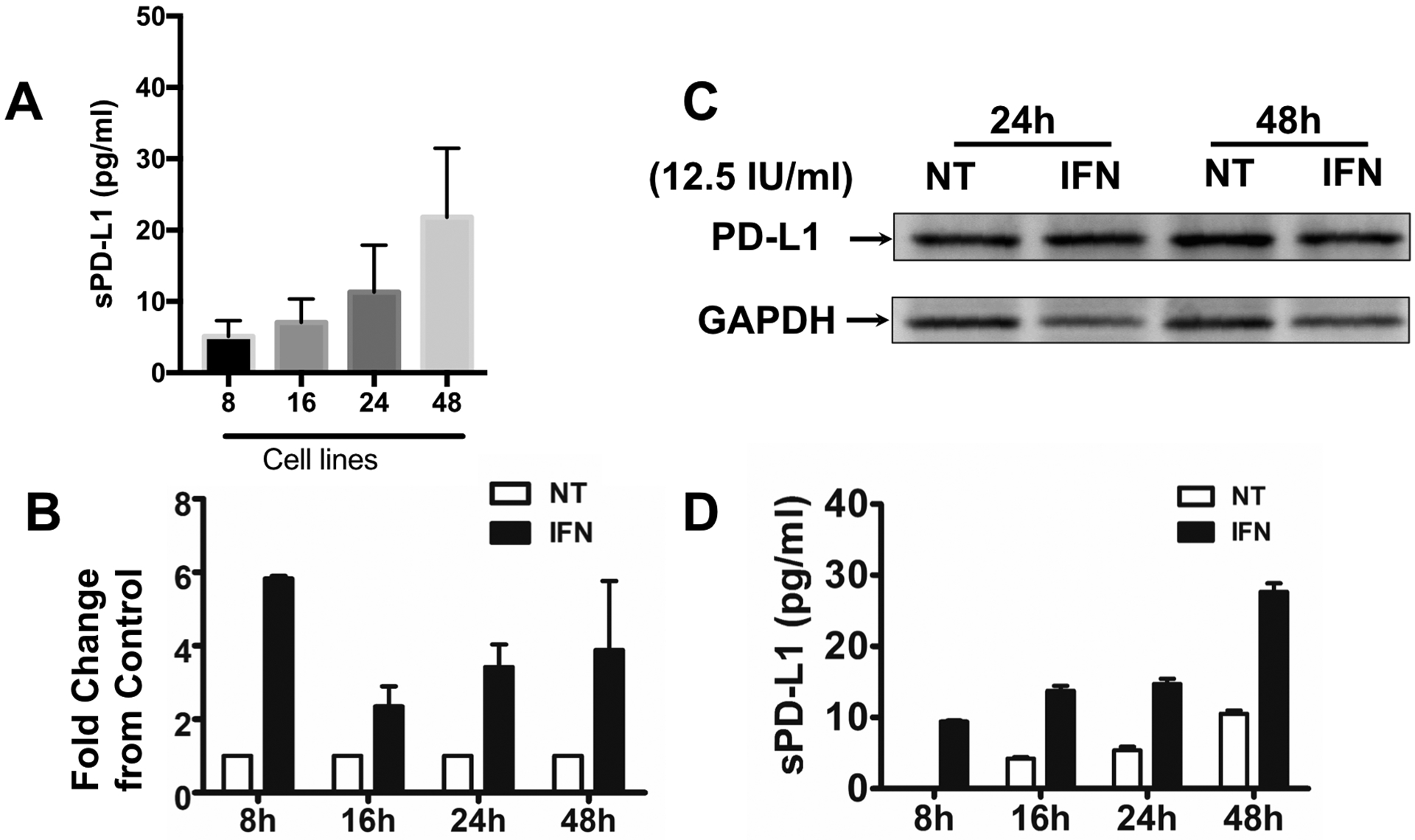
A. Expression levels of secreted PD-L1 detected in trophoblast supernatants by ELLA assay. Supernatants were collected from 100% confluent Swan 71 trophoblast cells at different times and PD-L1 expression was quantified by ELLA assay. Note the increase of sPD-L1 in the supernatant in a time dependent manner.
B. Trophoblast Swan 71 cells were treated with or without IFN-β (12.5 IU/ml) for 8, 16, 24 and 48h, after which PD-L1 mRNA expression was determined by qPCR. IFN-β induced a 6-fold increase in mRNA expression within 8h of treatment and remained higher than the control for an additional 48h. Data is presented as fold changes between the IFN-β treated group compared to the non-treated control group.
C. Trophoblast Swan 71 cells were treated with or without IFN-β (12.5 IU/ml) for 24 and 48h, after which PD-L1 protein expression was determined in total cell lysate by western blot analysis. Trophoblast cells constitutively express intracellular PDL-1 protein, but it is not affected by IFN-β treatment.
D. Trophoblast Swan 71 cells were treated with or without IFN-β (12. 5 IU/ml) for 24 and 48 hr, after which supernatants were collected and sPD-L1 protein expression was determined by ELLA assay. IFN-β treatment of trophoblast cells enhances the concentration of soluble PD-L1 detected in the supernatants in a time dependent manner. n=6 individual experiments.
3.6. Stimulation of sPD-L1 secretion by INF-β in trophoblast cells.
Our next objective was to characterize the regulation of PD-L1 expression and secretion in trophoblast cells by identifying the potential factors that could promote sPD-L1 expression. Since PD-L1 is an interferon-stimulated gene (ISG) and is induced by IFN-β in the trophoblast [52], we tested the hypothesis that a regulatory network would exist wherein IFN-β expressed by macrophages could enhance PD-L1 expression in trophoblast cells. To test this hypothesis, we treated trophoblast cells with IFN-β (12.5 IU/ml) for 8,16, 24 and 48 hours and evaluated PD-L1 mRNA and protein expression. Interestingly, treatment of trophoblast Swan 71 cells with IFN- β induced a time dependent increase in PD-L1 mRNA (Fig. 11B) but this was not associated with a corresponding increase in the intra-cellular PD-L1 protein levels (cell lysate) (Fig. 11C). The lack of correlation between the mRNA and intracellular protein levels could be attributed to either post-transcriptional regulation or continuous secretion of the protein. To test the latter possibility, we collected supernatants from trophoblast cell line Swan 71 following treatment with IFN-β (12.5 IU/ml) and quantified the protein concentration of secreted PD-L1. As shown in Figure 11D, protein levels detected in the supernatants of trophoblast Swan 71 cells increased in a time dependent manner and were further enhanced when trophoblast cells were treated with IFN-β. Similar results were found with 3A trophoblast cells (Sup Fig. 3B). The regulation of PD-L1 by IFN-β is specific as shown by a dose dependent increase on PD-L1 mRNA expression observed in Swan 71 cell line as well as in primary cultures of first trimester trophoblast (Sup Fig. 3C and D). These findings show that the trophoblasts constitutively secrete sPD-L1, and its expression and secretion are enhanced by IFN-β.
3.7. sPD-L1 expression in serum from pregnant women through early gestation.
Finally, we evaluated whether the in vitro observation could be correlated with the in vivo condition during human pregnancy. Thus, we sought to evaluate whether we could detect the presence of circulating sPD-L1 in the serum of pregnant women and the earliest stage of the pregnancy that could be measured. To achieve this objective, we determined the levels of sPD-L1 in serum samples obtained during the first trimester of pregnancy using ELLA assay. Serum samples were categorized into the following groups: peri-implantation phase (4–5 weeks), histotrophic phase (6–8 weeks), and perfusion phase (9–13 weeks), based on gestational age at the time of blood draw. These phases are histologically accepted and correlates with the different stages of placentation. As shown in Fig. 12, we observed that sPD-L1 could be detected and measured in the serum of pregnant women as early as 4–5 weeks. Moreover, through the entire first trimester period, the sPD-L1 circulating concentrations increased as the pregnancy progressed, reaching the highest level at the perfusion phase, the time of establishment of the uteroplacental circulation (Fig. 12).
Figure 12. Determination of circulating soluble PD-L1 in the serum of pregnant women during early gestation.
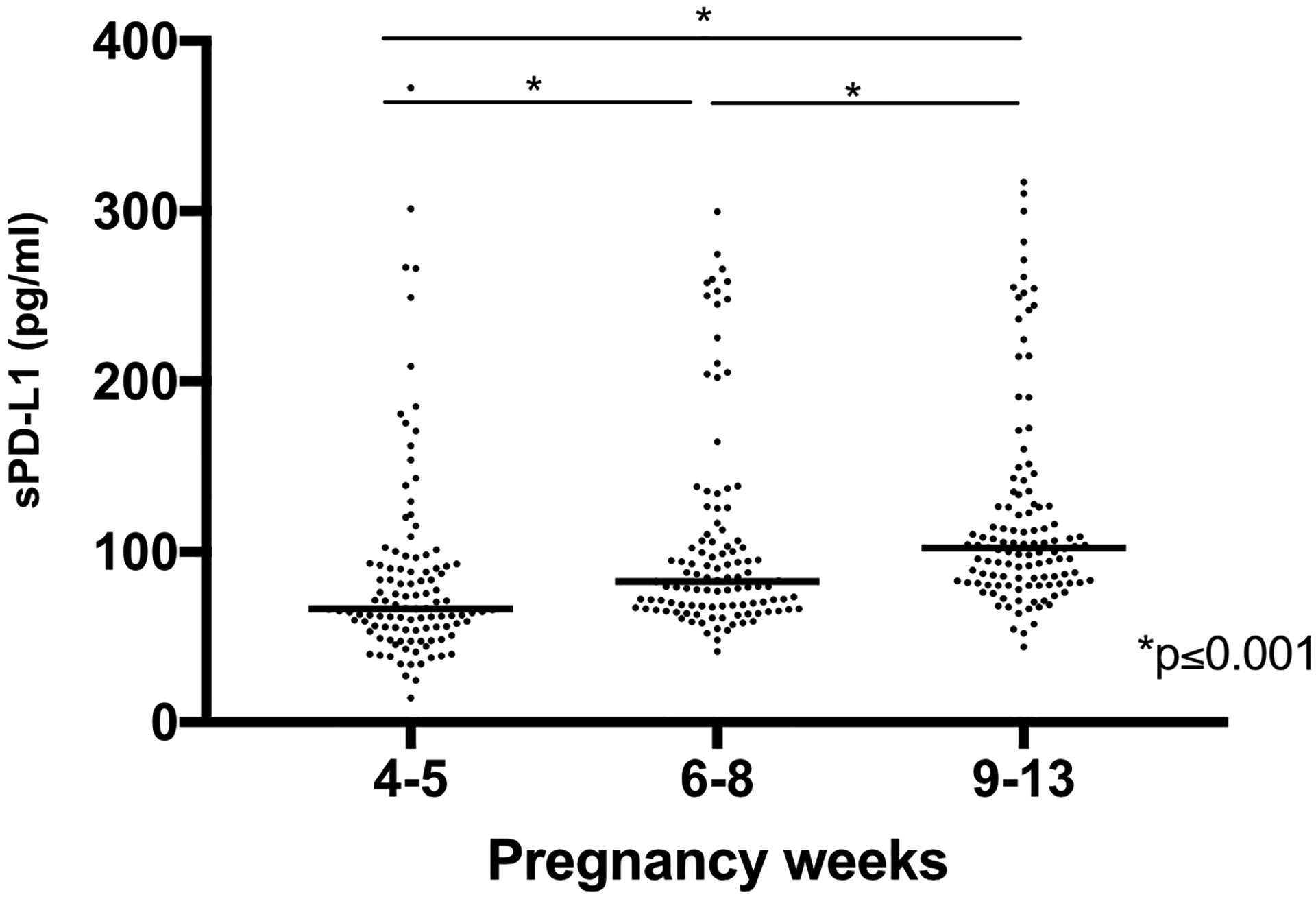
Serum samples were collected from pregnant women at gestational weeks 4–13 and soluble PD-L1 was analyzed by ELLA system. *p<0.001.
4. Discussion
Macrophage polarization in response to the microenvironment is an essential step for maintaining tissue homeostasis, and during pregnancy is essential for its success [3]. In this study we demonstrate that trophoblasts play a critical role in the process of macrophage polarization, which is mediated, in part, by the expression of soluble PD-L1. The present study describes, for the first time, the characterization of the trophoblast educated macrophages (TEMs) that are differentiated by trophoblast-secreted factors, and demonstrates that the trophoblasts have the capacity to induce, in vitro, the polarization of macrophages into a phenotype that resembles decidual macrophages positive for CD206 and negative for CD86. And in stark contrast to circulating monocytes or M-CSF-differentiated macrophages, TEMs stimulated by TLR4 are characterized by a type I interferon signature.
Macrophages are one of the main leukocyte populations present throughout gestation[53], characterized by a high degree of plasticity and heterogeneity [54]. As macrophage differentiation is specifically regulated and heavily influenced by their environment, we proposed that the embryo, more specifically the trophoblast cells, might play a role in their differentiation. In order to prove our hypothesis, we developed an in vitro system that could mimic the interaction between these two cell types and showed that trophoblast-secreted factors promoted macrophage differentiation into a unique phenotype that resembled decidual macrophages (CD14+/CD16+/CD206+ /CD86−)[9, 51].
When we evaluated the gene expression profile of these trophoblast educated macrophages (TEMs) and compared them with peripheral blood monocytes or M-CSF (M2-like) differentiated macrophages, we observed that TEMs had transcripts such as IFN-α and IFN-β indicative of immune regulation and tissue repair. Interestingly, TEM’s response to TLR4 stimulation with LPS was associated with the upregulation of the expression of TRIF/IRF3-dependent genes such as IFN-β, CCL5 and CXCL10 as well as the increased expression of CD16. Monocytes/macrophages respond to Gram-negative bacteria through the TLR4 signaling [55, 56] and it goes through two distinct pathways depending on the involvement of the adaptor molecules MyD88 or TRIF [43, 55–57]. Either pathway leads to distinct outcomes: the MyD88-dependent pathway is characterized by a dominant pro-inflammatory response (IL-6, TNF-α, CXCL1) through the activation of NF-kB [58, 59]; whereas the TRIF-dependent pathway leads to the phosphorylation of TBK and IRF3 and type I IFN expression [43]. In this study we show that the response of TLR4 to LPS in macrophages differentiated in the presence of trophoblast-derived factors are mainly through the TRIF/TBK/IRF3-dependent pathway leading to the expression of type I IFN-β. Our findings are consistent with the report of decreased expression of inflammatory cytokines in the monocytes/macrophages isolated from pregnant women and challenged by LPS compared to the LPS treated macrophages from non-pregnant women [53]. This differential response was suggested as a mechanism of tolerance to LPS [53]. However, our findings of the activation of the TLR4/TBK/IRF3 pathway and expression of type I IFN suggest that the polarization of decidual macrophages is associated not only with LPS tolerance but also with immune regulation and protection against viral infections, characteristics of M2b macrophages [60].
Polarized macrophages can be generally classified in two main groups: M1 or classically activated pro-inflammatory macrophages and M2 or alternatively activated macrophages which drive immune regulation and tissue remodeling [61]. However, this classification has been shown to be an oversimplification [62]. M2 macrophages can be further subdivided into M2a, M2b, M2c and M2d based upon the applied stimuli and the resultant transcriptional changes [63, 64]. When we compared TEMs with the classical M-CSF differentiated M2 macrophages we observed some similarities but also some major differences between the two types, suggesting that TEMs are potentially a unique subtype of M2b macrophages.
M2b macrophages are known as regulatory macrophages, they can control the depth of the immune response and the inflammatory reaction [63] and promote tissue repair, such as in case of spinal cord injury and myocardial ischemia/reperfusion injury where they have shown to contribute to recovery of these injuries [65, 66]. Likewise, decidual macrophages are essential for the continuous tissue renewal associated with trophoblast invasion and placental growth [67]. Comparably, we showed that TEMs had a higher capacity of promoting wound repair than monocytes or M-CSF differentiated macrophages [15]. The induction of IRF3 by TLR4 stimulation further suggests that TEMs are more resembling a M2b phenotype. IRF3 has been shown to play a core role in M2b activation [68] and promote the expression of type I IFN-β, another characteristic of M2 macrophages [68].
An additional characteristic of decidual macrophages and TEMs is CD16 expression (CD14+/CD16+), which may be a potential mechanism by which trophoblast cells modulate TLR4 response to LPS in macrophages. Indeed, Shalova et al. [69] reported that CD16 expression in monocytes contributed to the enhanced expression of the TLR4/TRIF-dependent genes.
What is the functional consequence of trophoblast-induced macrophage polarization and the preferential IFN-β response upon TLR stimulation? IFN-β is a major mediator of antiviral response [70, 71]. In addition to its antiviral role, IFN-β has been reported to function as an immune modulator by limiting immune responses [72] and regulating the recruitment of immune cells [31, 73]. The immune regulatory functions of IFN-β are meditated by the expression of Interferon-Stimulated Genes (ISGs), which can promote apoptosis [74], inhibit cell recruitment [75] and induce cell differentiation[76]. PD-L1 belongs to the ISG family and is an important immune modulatory factor [77, 78].
Recent studies have shown that the PD-1/PD-L1 pathway is critical to the regulation of immune cell homeostasis, specially T cell activation in relation to peripheral tolerance in several malignancies such as cancer as well as during pregnancy to paternal antigens [79, 80]. During pregnancy, PD-L1 was shown to be expressed in the placenta of normal pregnancies, primarily in the trophoblasts, and its expression was significantly decreased in pregnancy complications such as recurrent miscarriages [18, 51, 80, 81]. These findings suggest that the placenta can modulate decidual immune cells and that alterations on PD1/PD-L1 axis may affect the crosstalk between the placenta and the maternal immune system [82].
More recently, we and others have shown a role of the PD-1/PD-L1 axis in macrophage differentiation [50] [3, 51, 81]; and since pregnancy is a dynamic and highly regulated immunologic process, the success of the pregnancy requires that the macrophage activation status remains appropriately regulated throughout pregnancy. Here, we demonstrate that trophoblast secreted sPD-L1 may be one of the main mediators of macrophage polarization during pregnancy. Indeed, when we used trophoblast condition media to co-culture with monocytes, we showed that macrophages differentiated into CD14+/CD206+/CD86−; however, if we blocked the PD-1 pathway in the monocytes, we were able to inhibit the effect of trophoblast condition media and to shift macrophage differentiation towards CD14+/CD206−/CD86+. Notably, PD-1 blockage enhanced the production of pro-inflammatory cytokines further confirming the modulatory effects of trophoblast-secreted sPD-L1 on macrophage differentiation.
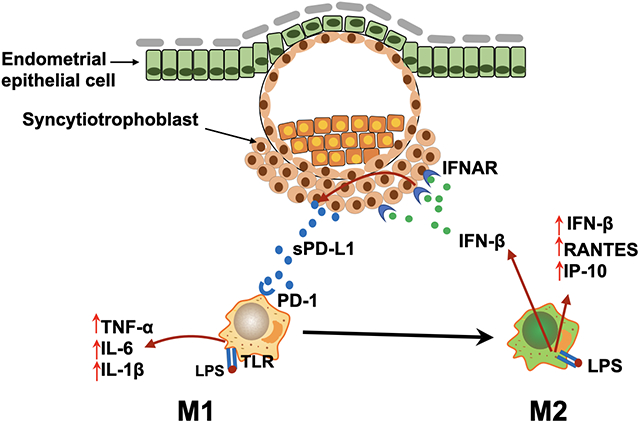
During early pregnancy, macrophages present at the implantation site possess characteristics associated with M1 phenotype[3]; however, as pregnancy progresses and the developing placenta invades the endometrium and establishes a close contact with decidual cells, the trophoblasts can change the microenvironment and induce the M2 polarization characteristic of decidual macrophages[10] (Fig. 13). Using the in vitro model described in this study we are able to recapitulate the trophoblast’s function of regulating macrophage polarization. Consequently, we propose that the signals originated from trophoblast cells, by regulating macrophage polarization, are responsible for the shift of the inflammatory milieu at the implantation site (Fig 13).
Figure 13. Regulatory circuit at the maternal fetal interface promoting M2 macrophage polarization.
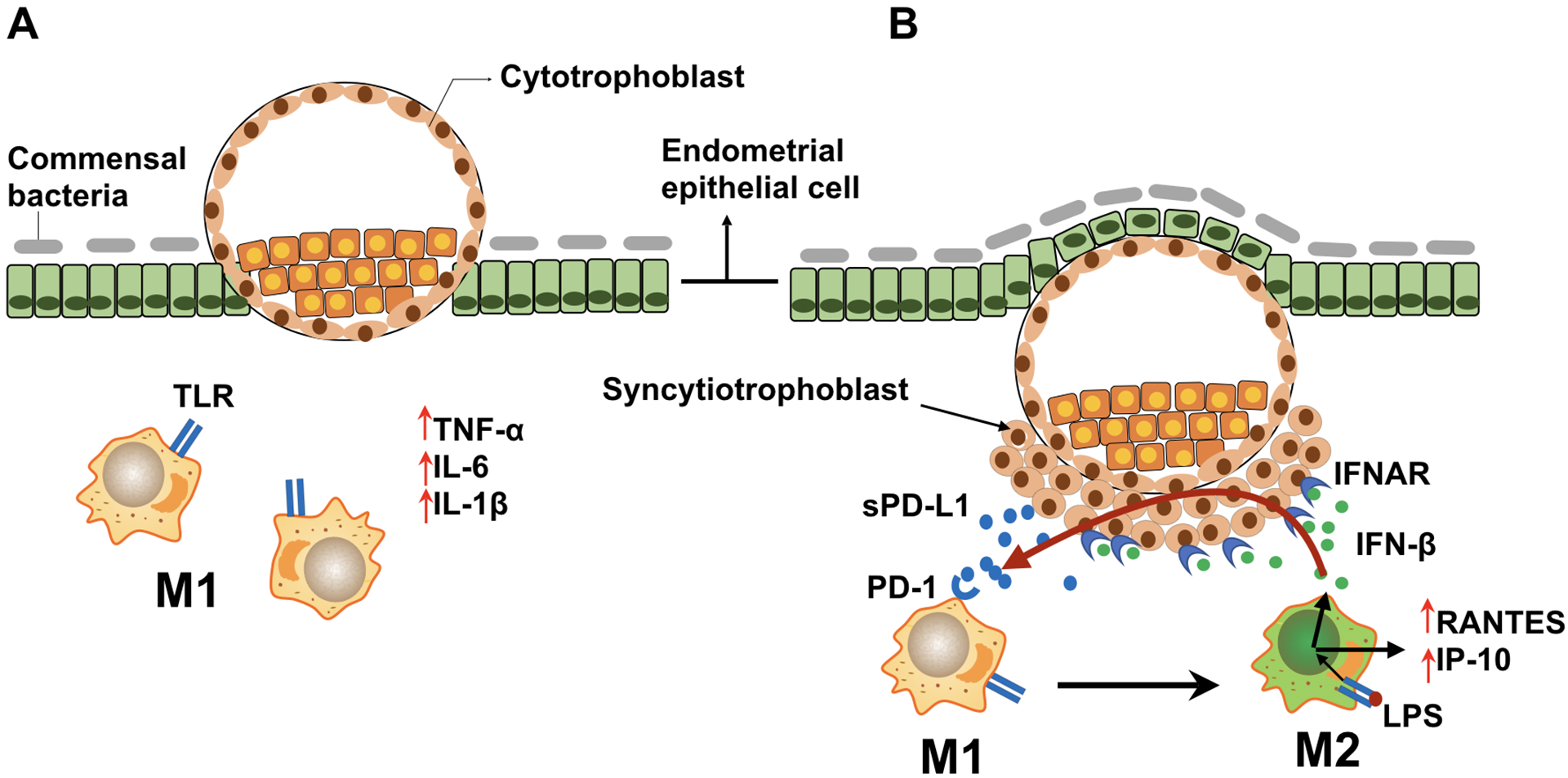
During gestation, macrophages undergo dynamic changes, predominantly displaying the M1 or M2 phenotype.
A. During the peri-implantation period, activated endometrial/decidua macrophages (M1 phenotype) produce inflammatory cytokines and chemokines (e.g. TNF-α, IL-6, IL-1β), inducing pro-inflammatory responses necessary for embryo implantation and trophoblast invasion
B. As the trophoblasts invade deeply into the uterine stroma, trophoblast–secreted factors (such as sPD-L1) promote the shift of decidual macrophages into M2 phenotype. LPS/TLR4 ligation induces the expression of IFN-β, which further promotes sPD-L1 expression/secretion by trophoblast cells. The upregulated sPD-L1 enhances macrophage polarization towards an M2b phenotype that consequently decreases inflammation and promotes tissue repair and tolerance.
In line with previous studies [80, 83], we found that both membrane and soluble PD-L1 constitutively expressed in trophoblast. However, compared with membrane PD-L1 protein, we observed high levels of sPD-L1 in the supernatant of trophoblast cells which was further enhanced by exposure to IFN-β. The presence of a secreted form of PD-L1 observed from the trophoblast could explain the source of the detected circulating sPD-L1 identified in the serum of pregnant women. A previous report showed that circulating PD-L1 protein expression increased from week 14 of gestation until the end of pregnancy [83, 84] However, it is unknow how early PD-L1 is produced and detectable in the maternal circulation. In this study we were able to detect sPD-L1 in the serum of pregnant women as early as 4 weeks of gestation and its rise throughout the first trimester. To our knowledge, this is the first study to report the presence of circulating sPD-L1 in the serum of normal pregnancies during the first trimester. Therefore, based on these clinical observations and our new findings reported in this study, we postulate that the increase in sPD-L1 in the maternal blood likely indicates the status of placental growth and the immune modulatory function to promote and maintain immune tolerance during gestation[80]. An important component of this immune modulatory function is associated with macrophage polarization (Fig. 13).
5. Conclusion
In conclusion, our findings suggest the existence of a regulatory circuit at the maternal-fetal interface wherein IFN-β promotes sPD-L1 expression/secretion by trophoblast cells, which then initiates a PD-L1/PD-1 mediated macrophage polarization towards an M2b phenotype, leading to inhibition of inflammation and promotion of tissue repair and tolerance. Our findings have important implications for better understanding of the physiological mechanisms that control macrophage differentiation, promote maternal-fetal tolerance and identify potential biomarkers to monitor placental growth and immune modulation throughout gestation.
Supplementary Material
Acknowledge
This work was supported in part by NIH grant NIAID 1R01AI145829-01. We thank Prof. Ayesha Alvero and Dr. Zhong Dong for the constructive criticism of the manuscript.
Footnotes
STUDY FUNDING/COMPETING INTEREST(S)
The authors received no specific funding for this work and disclose no conflicts of interest
References
- 1.Mor G, Aldo P, Alvero AB (2017) The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 17, 469–482. [DOI] [PubMed] [Google Scholar]
- 2.Mor G, Cardenas I, Abrahams V, Guller S (2011) Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 1221, 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang YH, He M, Wang Y, Liao AH (2017) Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Frontiers in immunology 8, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mor G and Cardenas I (2010) The immune system in pregnancy: A unique complexity. Am J Reprod Immunol 63, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez FO, Gordon S, Locati M, Mantovani A (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177, 7303–11. [DOI] [PubMed] [Google Scholar]
- 6.Athanassakis I, Papadimitriou L, Koumantakis E, Vassiliadis S (2000) Th1- and Th2-type lymphokine-assisted induction and release of chemokine receptors from primary human trophoblast cells. Hum Immunol 61, 651–7. [DOI] [PubMed] [Google Scholar]
- 7.Saito S (2000) Cytokine network at the feto-maternal interface. J Reprod Immunol 47, 87–103. [DOI] [PubMed] [Google Scholar]
- 8.Houser BL, Tilburgs T, Hill J, Nicotra ML, Strominger JL (2011) Two unique human decidual macrophage populations. J Immunol 186, 2633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gustafsson C, Mjosberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, Ernerudh J (2008) Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One 3, e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houser BL, Tilburgs T, Hill J, Nicotra ML, Strominger JL (2011) Two unique human decidual macrophage populations. J Immunol 186, 2633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntire RH, Ganacias KG, Hunt JS (2008) Programming of human monocytes by the uteroplacental environment. Reprod Sci 15, 437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ning F, Liu H, Lash GE (2016) The Role of Decidual Macrophages During Normal and Pathological Pregnancy. Am J Reprod Immunol 75, 298–309. [DOI] [PubMed] [Google Scholar]
- 13.Svensson-Arvelund J, Mehta RB, Lindau R, Mirrasekhian E, Rodriguez-Martinez H, Berg G, Lash GE, Jenmalm MC, Ernerudh J (2015) The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol 194, 1534–44. [DOI] [PubMed] [Google Scholar]
- 14.Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J (2011) Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol 187, 3671–82. [DOI] [PubMed] [Google Scholar]
- 15.Aldo PB, Racicot K, Craviero V, Guller S, Romero R, Mor G (2014) Trophoblast Induces Monocyte Differentiation Into CD14+/CD16+ Macrophages. Am J Reprod Immunol 72, 270–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fest S, Aldo PB, Abrahams VM, Visintin I, Alvero A, Chen R, Chavez SL, Romero R, Mor G (2007) Trophoblast-macrophage interactions: a regulatory network for the protection of pregnancy. Am J Reprod Immunol 57, 55–66. [DOI] [PubMed] [Google Scholar]
- 17.Jaiswal MK, Mallers TM, Larsen B, Kwak-Kim J, Chaouat G, Gilman-Sachs A, Beaman KD (2012) V-ATPase upregulation during early pregnancy: a possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction 143, 713–25. [DOI] [PubMed] [Google Scholar]
- 18.Yao Y, Xu XH, Jin L (2019) Macrophage Polarization in Physiological and Pathological Pregnancy. Frontiers in immunology 10, 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrahams VM (2008) Pattern recognition at the maternal-fetal interface. Immunol Invest 37, 427–47. [DOI] [PubMed] [Google Scholar]
- 20.Koga K and Mor G (2007) Toll-like receptors and pregnancy. Reprod Sci 14, 297–9. [DOI] [PubMed] [Google Scholar]
- 21.Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G (2005) A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol 175, 8096–104. [DOI] [PubMed] [Google Scholar]
- 22.Koga K and Mor G (2010) Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol 63, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramhorst R, Fraccaroli L, Aldo P, Alvero AB, Cardenas I, Leiros CP, Mor G (2012) Modulation and recruitment of inducible regulatory T cells by first trimester trophoblast cells. Am J Reprod Immunol 67, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Straszewski-Chavez SL, Abrahams VM, Alvero AB, Aldo PB, Ma Y, Guller S, Romero R, Mor G (2009) The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta 30, 939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiter JL, Drendel HM, Chakraborty S, Schellinger MM, Lee MJ, Mor G (2017) Cytogenetic features of human trophoblast cell lines SWAN-71 and 3A-subE. Placenta 52, 17–20. [DOI] [PubMed] [Google Scholar]
- 26.Kaislasuo J, Simpson S, Petersen JF, Peng G, Aldo P, Lokkegaard E, Paidas M, Pal L, Guller S, Mor G (2019) IL-10 to TNFalpha ratios throughout early first trimester can discriminate healthy pregnancies from pregnancy losses. Am J Reprod Immunol, e13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friis Petersen J, Friis-Hansen LJ, Jensen AK, Nyboe Andersen A, Lokkegaard ECL (2019) Early pregnancy reference intervals; 29 serum analytes from 4 to 12 weeks’ gestation in naturally conceived and uncomplicated pregnancies resulting in live births. Clinical chemistry and laboratory medicine : CCLM / FESCC. [DOI] [PubMed] [Google Scholar]
- 28.Straszewski-Chavez SL, Abrahams VM, Aldo PB, Mor G (2005) Isolation and characterization of a novel telomerase-immortalized human first trimester trophoblast cell line. . Placenta 26, A.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldo PB, Craveiro V, Guller S, Mor G (2013) Effect of culture conditions on the phenotype of THP-1 monocyte cell line. Am J Reprod Immunol 70, 80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aldo P, Marusov G, Svancara D, David J, Mor G (2016) Simple Plex() : A Novel Multi-Analyte, Automated Microfluidic Immunoassay Platform for the Detection of Human and Mouse Cytokines and Chemokines. Am J Reprod Immunol 75, 678–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon JY, Aldo P, You Y, Ding J, Racicot K, Dong X, Murphy J, Glukshtad G, Silasi M, Peng J, Wen L, Abrahams VM, Romero R, Mor G (2018) Relevance of placental type I interferon beta regulation for pregnancy success. Cell Mol Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geissmann F, Jung S, Littman DR (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82. [DOI] [PubMed] [Google Scholar]
- 33.Sadeghi HM, Schnelle JF, Thoma JK, Nishanian P, Fahey JL (1999) Phenotypic and functional characteristics of circulating monocytes of elderly persons. Exp Gerontol 34, 959–70. [DOI] [PubMed] [Google Scholar]
- 34.Metcalf TU, Wilkinson PA, Cameron MJ, Ghneim K, Chiang C, Wertheimer AM, Hiscott JB, Nikolich-Zugich J, Haddad EK (2017) Human Monocyte Subsets Are Transcriptionally and Functionally Altered in Aging in Response to Pattern Recognition Receptor Agonists. J Immunol 199, 1405–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almeida J, Bueno C, Alguero MC, Sanchez ML, de Santiago M, Escribano L, Diaz-Agustin B, Vaquero JM, Laso FJ, San Miguel JF, Orfao A (2001) Comparative analysis of the morphological, cytochemical, immunophenotypical, and functional characteristics of normal human peripheral blood lineage(-)/CD16(+)/HLA-DR(+)/CD14(-/lo) cells, CD14(+) monocytes, and CD16(-) dendritic cells. Clin Immunol 100, 325–38. [DOI] [PubMed] [Google Scholar]
- 36.Heikkinen J, Mottonen M, Komi J, Alanen A, Lassila O (2003) Phenotypic characterization of human decidual macrophages. Clin Exp Immunol 131, 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slukvin II, Watkins DI, Golos TG (2001) Phenotypic and functional characterization of rhesus monkey decidual lymphocytes: rhesus decidual large granular lymphocytes express CD56 and have cytolytic activity. J Reprod Immunol 50, 57–79. [DOI] [PubMed] [Google Scholar]
- 38.Bulmer JN, Pace D, Ritson A (1988) Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Dev 28, 1599–613. [DOI] [PubMed] [Google Scholar]
- 39.Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, Glass CK (2014) Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bujko A, Atlasy N, Landsverk OJB, Richter L, Yaqub S, Horneland R, Oyen O, Aandahl EM, Aabakken L, Stunnenberg HG, Baekkevold ES, Jahnsen FL (2018) Transcriptional and functional profiling defines human small intestinal macrophage subsets. J Exp Med 215, 441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar S and Jack R (2006) Origin of monocytes and their differentiation to macrophages and dendritic cells. J Endotoxin Res 12, 278–84. [DOI] [PubMed] [Google Scholar]
- 42.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA Jr. (1998) MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 2, 253–8. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–3. [DOI] [PubMed] [Google Scholar]
- 44.Charrel-Dennis M, Latz E, Halmen KA, Trieu-Cuot P, Fitzgerald KA, Kasper DL, Golenbock DT (2008) TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell host & microbe 4, 543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Picard C, Casanova JL, Puel A (2011) Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clin Microbiol Rev 24, 490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolb JP, Casella CR, SenGupta S, Chilton PM, Mitchell TC (2014) Type I interferon signaling contributes to the bias that Toll-like receptor 4 exhibits for signaling mediated by the adaptor protein TRIF. Sci Signal 7, ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doyle S, Vaidya S, O’Connell R, Dadgostar H, Dempsey P, Wu T, Rao G, Sun R, Haberland M, Modlin R, Cheng G (2002) IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17, 251–63. [DOI] [PubMed] [Google Scholar]
- 48.Bai LY, Chiu CF, Kapuriya NP, Shieh TM, Tsai YC, Wu CY, Sargeant AM, Weng JR (2015) BX795, a TBK1 inhibitor, exhibits antitumor activity in human oral squamous cell carcinoma through apoptosis induction and mitotic phase arrest. European journal of pharmacology 769, 287–96. [DOI] [PubMed] [Google Scholar]
- 49.Clark K, Plater L, Peggie M, Cohen P (2009) Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: a distinct upstream kinase mediates Ser-172 phosphorylation and activation. J Biol Chem 284, 14136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho HY, Choi EK, Lee SW, Jung KO, Seo SK, Choi IW, Park SG, Choi I, Lee SW (2009) Programmed death-1 receptor negatively regulates LPS-mediated IL-12 production and differentiation of murine macrophage RAW264.7 cells. Immunol Lett 127, 39–47. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Ma L, Hu X, Ji J, Mor G, Liao A (2019) The role of the PD-1/PD-L1 axis in macrophage differentiation and function during pregnancy. Hum Reprod 34, 25–36. [DOI] [PubMed] [Google Scholar]
- 52.Ivashkiv LB and Donlin LT (2014) Regulation of type I interferon responses. Nat Rev Immunol 14, 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faas MM and de Vos P (2017) Uterine NK cells and macrophages in pregnancy. Placenta 56, 44–52. [DOI] [PubMed] [Google Scholar]
- 54.Genard G, Lucas S, Michiels C (2017) Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies. Front Immunol 8, 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S (1999) Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11, 443–51. [DOI] [PubMed] [Google Scholar]
- 56.Akira S and Sato S (2003) Toll-like receptors and their signaling mechanisms. Scand J Infect Dis 35, 555–62. [DOI] [PubMed] [Google Scholar]
- 57.Dobrovolskaia MA and Vogel SN (2002) Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect 4, 903–14. [DOI] [PubMed] [Google Scholar]
- 58.Kawai T and Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11, 373–84. [DOI] [PubMed] [Google Scholar]
- 59.Kondo T, Kawai T, Akira S (2012) Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol 33, 449–58. [DOI] [PubMed] [Google Scholar]
- 60.Wang LX, Zhang SX, Wu HJ, Rong XL, Guo J (2019) M2b macrophage polarization and its roles in diseases. J Leukoc Biol 106, 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinez FO, Sica A, Mantovani A, Locati M (2008) Macrophage activation and polarization. Front Biosci 13, 453–61. [DOI] [PubMed] [Google Scholar]
- 62.Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, Roy S (2015) Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol 185, 2596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang N, Liang H, Zen K (2014) Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Frontiers in immunology 5, 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oishi S, Takano R, Tamura S, Tani S, Iwaizumi M, Hamaya Y, Takagaki K, Nagata T, Seto S, Horii T, Osawa S, Furuta T, Miyajima H, Sugimoto K (2016) M2 polarization of murine peritoneal macrophages induces regulatory cytokine production and suppresses T-cell proliferation. Immunol 149, 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gensel JC, Wang Y, Guan Z, Beckwith KA, Braun KJ, Wei P, McTigue DM, Popovich PG (2015) Toll-Like Receptors and Dectin-1, a C-Type Lectin Receptor, Trigger Divergent Functions in CNS Macrophages. J Neurosci 35, 9966–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gensel JC and Zhang B (2015) Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res 1619, 1–11. [DOI] [PubMed] [Google Scholar]
- 67.Mor G and Abrahams VM (2003) Potential role of macrophages as immunoregulators of pregnancy. Reprod Biol Endocrinol 1, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chistiakov DA, Myasoedova VA, Revin VV, Orekhov AN, Bobryshev YV (2018) The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 223, 101–111. [DOI] [PubMed] [Google Scholar]
- 69.Shalova IN, Kajiji T, Lim JY, Gomez-Pina V, Fernandez-Ruiz I, Arnalich F, Iau PT, Lopez-Collazo E, Wong SC, Biswas SK (2012) CD16 regulates TRIF-dependent TLR4 response in human monocytes and their subsets. J Immunol 188, 3584–93. [DOI] [PubMed] [Google Scholar]
- 70.Wang WB, Levy DE, Lee CK (2011) STAT3 negatively regulates type I IFN-mediated antiviral response. J Immunol 187, 2578–85. [DOI] [PubMed] [Google Scholar]
- 71.Trinchieri G (2010) Type I interferon: friend or foe? J Exp Med 207, 2053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J (2011) Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–23. [DOI] [PubMed] [Google Scholar]
- 73.Racicot K, Aldo P, El-Guindy A, Kwon JY, Romero R, Mor G (2017) Cutting Edge: Fetal/Placental Type I IFN Can Affect Maternal Survival and Fetal Viral Load during Viral Infection. J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karaghiosoff M, Steinborn R, Kovarik P, Kriegshauser G, Baccarini M, Donabauer B, Reichart U, Kolbe T, Bogdan C, Leanderson T, Levy D, Decker T, Muller M (2003) Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat Immunol 4, 471–7. [DOI] [PubMed] [Google Scholar]
- 75.Jablonska J, Wu CF, Andzinski L, Leschner S, Weiss S (2014) CXCR2-mediated tumor-associated neutrophil recruitment is regulated by IFN-beta. Int J Cancer 134, 1346–58. [DOI] [PubMed] [Google Scholar]
- 76.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I (2014) Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, Parisi G, Saus CP, Torrejon DY, Graeber TG, Comin-Anduix B, Hu-Lieskovan S, Damoiseaux R, Lo RS, Ribas A (2017) Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep 19, 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Staples KJ, Nicholas B, McKendry RT, Spalluto CM, Wallington JC, Bragg CW, Robinson EC, Martin K, Djukanovic R, Wilkinson TM (2015) Viral infection of human lung macrophages increases PDL1 expression via IFNbeta. PLoS One 10, e0121527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salvany-Celades M, van der Zwan A, Benner M, Setrajcic-Dragos V, Bougleux Gomes HA, Iyer V, Norwitz ER, Strominger JL, Tilburgs T (2019) Three Types of Functional Regulatory T Cells Control T Cell Responses at the Human Maternal-Fetal Interface. Cell Rep 27, 2537–2547.e5. [DOI] [PubMed] [Google Scholar]
- 80.Enninga EAL, Harrington SM, Creedon DJ, Ruano R, Markovic SN, Dong H, Dronca RS (2018) Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am J Reprod Immunol 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang YH, Tian M, Tang MX, Liu ZZ, Liao AH (2015) Recent Insight into the Role of the PD-1/PD-L1 Pathway in Feto-Maternal Tolerance and Pregnancy. Am J Reprod Immunol 74, 201–8. [DOI] [PubMed] [Google Scholar]
- 82.Meggyes M, Miko E, Szigeti B, Farkas N, Szereday L (2019) The importance of the PD-1/PD-L1 pathway at the maternal-fetal interface. BMC Pregnancy Childbirth 19, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS (2003) B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod 68, 1496–504. [DOI] [PubMed] [Google Scholar]
- 84.Kshirsagar SK, Alam SM, Jasti S, Hodes H, Nauser T, Gilliam M, Billstrand C, Hunt JS, Petroff MG (2012) Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 33, 982–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


