Abstract
Purpose:
Investigate the ability of Fluorothymidine F-18 (FLT) positron emission tomography (PET) combined with Computed Tomography (CT) at 6 weeks to predict treatment response at 12 weeks after treatment with pembrolizumab.
Methods:
Five patients with unresectable stage IV melanoma were included in this single-institution pilot study. Patients underwent FLT-PET/CT (baseline and 6 weeks) and CT (baseline and 12 weeks). FLT-PET/CT response and CT response were assessed using PET Response Criteria in Solid Tumors (PERCIST) and immune Response Evaluation Criteria in Solid Tumors (iRECIST), respectively. Patients were categorized as responders (complete response (CR), partial response (PR)) and non-responders (stable disease (SD), progressive disease (PD)). Agreement between 6-week FLT-PET/CT and 12-week CT was calculated using Kappa’s Cohen agreement. Eight baseline FLT-PET/CT parameters were extracted: standardized uptake value (SUV) max, SUVpeak, SUVSD, SUVmean, Proliferative Tumor Volume (PTV), Total Lesion Proliferation (TLP), BLR (Bone marrow-to-Liver SUVmax ratio), and SLR (Spleen-to-Liver SUVmax ratio). Eight delta-parameters were extracted at 6 weeks by calculating variation in FLT uptake as percentage change from baseline.
Results:
Agreement between 6-week FLT-PET/CT and 12-week CT was Kappa = 0.615, p = 0.025. Three of five patients were categorized as responders on CT by iRECIST. At baseline, responders had a lower mean PTV and a higher BLR. At 6-weeks, responders demonstrated a decrease in tumor volume and tumor proliferation.
Conclusions:
Our study illustrates the potential for FLT-PET/CT as an early predictor of response for patients with metastatic melanoma on anti-PD1 immunotherapy. Larger studies are indicated to confirm these findings.
Keywords: Melanoma, FLT-PET, Pembrolizumab
INTRODUCTION
Metastatic melanoma remains a lethal disease despite recent advances in surveillance and treatment. Immunotherapy has nearly completely superceded traditional chemotherapy in the management of advanced melanoma patients who traditionally survived less than one year (1). In the KEYNOTE-006 trial, the anti-PD-1 antibody pembrolizumab was more effective at prolonging progression free and overall survival than the previous standard of care treatment, ipilimumab. The latest results suggest that the response to treatment persists even after concluding immunotherapy (2). While combination therapy including both ipilimumab and nivolumab may be superior to single agent nivolumab, the added toxicity of ipilimumab remains a significant barrier to implementation. It is increasingly important to identify patients who progress early in the treatment course so that combination strategies can be administered. Identifying patients who are progressing on single agent immunotherapy is complicated by the phenomenon of pseudoprogression. Pseudoprogression describes an initial increase in size of the tumor lesion due to inflammatory infiltrate followed by a subsequent decrease in tumor burden, that has been observed in patients undergoing immunotherapy (3). Pseudoprogression may lead to the premature termination of anti-PD1 therapy in patients who may have in fact received benefits had they continued with treatment. Hyperprogression, in contrast, is the occurrence of rapid disease progression soon after starting treatment with immune checkpoint blockades. Hyperprogression occurs in 9% of patients treated with PD-1 inhibitors and is associated with inferior clinical outcome (4). Nonetheless, due to the known existence of pseudoprogression patients with hyperprogression are often continued on treatment for a full 12 week course due to the absence of available biomarkers to identify them (5). CT and FDG-PET/CT are the current methods for evaluating immunotherapy response, but neither is sufficient for predicting atypical immune-related responses (6).
[18F]-fluoro-thymidine (FLT) PET is an imaging biomarker for cellular proliferation that can identify changes in proliferation in successfully treated patients (7). FLT is a substrate for thymidine kinase that is transported into the cell during DNA synthesis and subsequently trapped, but not incorporated into the DNA itself, and thus FLT uptake serves as a suitable marker of active DNA synthesis in vivo (8). FLT-PET has already been found to be a suitable predictor of early response in lymphoma patients treated with targeted therapy, and, in fact, was shown to be superior to FDG-PET (9). FLT-PET has also been found to be useful in monitoring patients with melanoma brain metastasis who undergo targeted therapy or immunotherapy. In a case series, FLT-PET was used to guide treatment decisions in two patients with brain metastases (10). A phase II open label study has recently opened to use FLT-PET to predict progression-free survival in ninety patients with BRAFV600E/K mutated unresectable stage IIIC/IV melanoma. Outcomes will be correlated with PET/CT imaging response (11). FLT-PET is a suitable strategy to assess immunotherapy treatments because it is uniquely positioned to measure a decline in tumor cell proliferation, one of the earliest changes following response to immunotherapy with a checkpoint inhibitor (12).
We hypothesize that we will detect a decreased proliferative signal following successful immunotherapy since most malignant cells have proliferation rates (and indices) that are greater than tumor infiltrating immune cells. FLT-PET offers a chance to better understand patients’ response to therapy and distinguish true progression from pseudoprogression. This insight is not only useful for diagnostic purposes, but also for the purpose of making clinical decisions about treatment course.
There were four main aims of this pilot study: 1) To determine the agreement between four categories measured on CT and FLT-PET (response: complete (metabolic) response (CR) or partial (metabolic) response (PR), non-response: stable (metabolic) disease (SD) or progressive (metabolic) disease (PD)); 2) To determine the baseline biomarkers in objective responders compared to non-objective responders; 3) To determine delta (baseline compared to 6-week) biomarkers in objective responders versus non-objective responders; 4) To determine the association of these FLT-PET metrics (iRECIST, irRC, and volumetric criteria) with overall survival data.
METHODS
Study Population
In this pilot study, adult patients diagnosed with unresectable stage IV melanoma selected for immunotherapy with pembrolizumab were eligible for enrollment. Both newly diagnosed and relapsed patients with radiological or clinical evidence of disease were eligible. Exclusion criteria included prior treatment with anti-PD1 immunotherapy, indications for other anti-neoplastic or immunosuppressive therapy, known additional malignancy, and uncontrolled intercurrent illness. Five patients were recruited for the study, Table 1. This prospective clinical trial was approved by the Columbia University Medical Center Institutional Review Board (Protocol AAAO2558). All participants signed written informed consent to participate in the study.
TABLE 1.
Demographics and Clinical Characteristics of Patients Included in the Study
| Patient ID | 1 | 2 | 3 | 4 | 5 | Median (range) |
|---|---|---|---|---|---|---|
| Clinical characteristics | ||||||
| Age (years) | 44 | 62 | 43 | 53 | 31 | 44 (31:62) |
| Gender | F | M | M | M | M | |
| Ethnicity | White | White | White | White | White | |
| Overall survival (months) | 25 | 9 | 7 | 10 | 4 | 9 (4:25) |
| Status | Alive | Alive | Alive | Dead | Dead | |
| Stage | ||||||
| AJCC classification | M1c | M1a | M1c | M1c | M1c | |
| Sites of Metastatic Disease | Bone, lung, nodal, soft tissue | Lymph nodes | Soft tissue, liver lesion | Lung, stomach, soft tissue | Pleura | |
| LDH UI/L | 141 | 169 | 233 | 442 | 365 | 233 (141:442) |
| Treatment | ||||||
| Drug | Pembrolizumab | Pembrolizumab | Pembrolizumab | Pembrolizumab | Pembrolizumab | |
| Response 6 weeks (FLT-PET, PERCIST) | PR | PR | SD | SD | PD | |
| Response – 12 weeks (CT, RECIST) | CR | PR | PR | SD | PD | |
| Baseline FLT-PET | ||||||
| Uptake | ||||||
| SUV max | 12.1 | 5.9 | 5,5 | 8.4 | 5.2 | 5.9 (5.2:12.1) |
| SUV peak | 9.7 | 4.8 | 3,7 | 7.4 | 3.9 | 4.8 (3.7:9.7) |
| SUV mean | 3.7 | 3.8 | 3,1 | 5.2 | 2.8 | 3.7 (2.8:5.2) |
| SUV SD | 1 | 0.7 | 0,8 | 1.1 | 0.5 | 0.8 (0.5:1.1) |
| Tumor Burden | ||||||
| TLP | 226 | 106 | 14 | 349 | 353 | 225 (14:353) |
| PTV | 60 | 28 | 5 | 67 | 128 | 60 (5:128) |
| Healthy Hematopoietic Tissue Metabolism | ||||||
| SLR | 34% | 41% | 33% | 60% | 61% | 41% (33%:61%) |
| BLR | 137% | 125% | 129% | 98% | 79% | 125% (79%:137%) |
| 6-week (Delta) FLT-PET | ||||||
| Uptake | ||||||
| Delta-SUV max | −75% | −40% | −22% | −14% | 233% | −22% (−75%:233%) |
| Delta-SUV peak | −74% | −53% | −15% | −15% | 295% | −15% (−74%:295%) |
| Delta-SUV mean | −54% | −52% | −17% | −17% | 203% | −17% (−54%:203%) |
| Delta-SD | −89% | −63% | −17% | −31% | 5% | −31% (−89%:5%) |
| Tumor Burden | ||||||
| Delta- TLP | −91% | −85% | −32% | 63% | 8773% | −32% (−91%:8773%) |
| Delta- PTV | −79% | −69% | −18% | 96% | 2830% | −18% (−79%:2830%) |
| Healthy Hematopoietic Tissue Metabolism | ||||||
| Delta-SLR | 47% | 0% | −9% | −27% | 4% | 0% (−27%:47%) |
| Delta-BLR | −4% | 12% | 21% | 38% | 9% | 12% (−4%:38%) |
Treatment Protocol
Beginning at week 0 of the study, study participants received a 200 mg dose of pembrolizumab administered as a 30-minute intravenous (IV) infusion every 3 weeks for up to 2 years (Figure 1). Concomitant treatment medications were administered at the discretion of the treating physician in keeping with the standards of medical care, excluding live vaccines and other systemic antineoplastic therapies.
Figure 1:
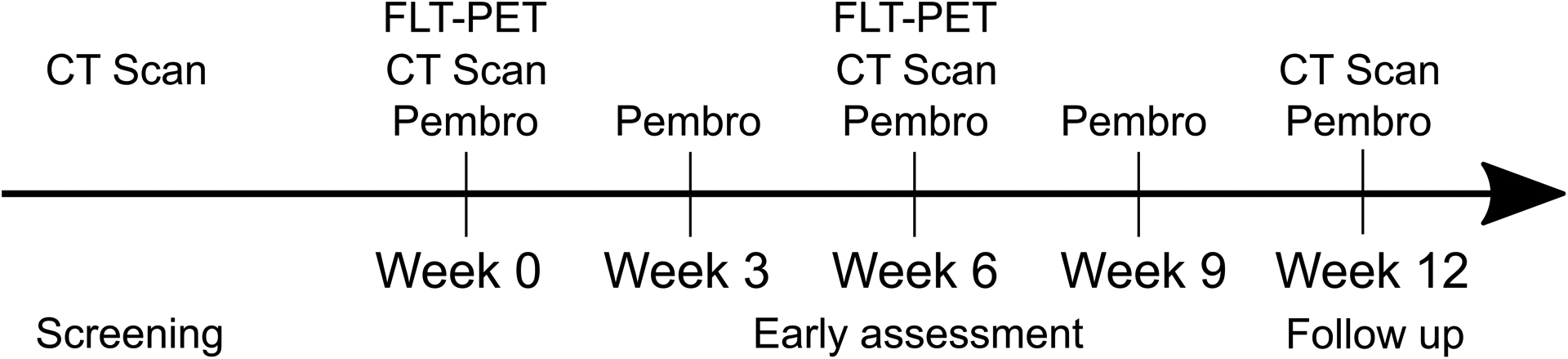
Timeline of treatment and imaging procedures.
Imaging Protocol
All subjects underwent baseline radiological tumor assessment by conventional CT imaging of any region of known or suspected disease and [18F]-FLT-PET imaging occurred after the first dose of pembrolizumab, within 28 days of administration. [18F]-FLT was produced in the PET/radiochemistry facility at CUMC under PET cGMP conditions as approved by the Food and Drug Administration through investigational new drug number 122435. Synthesis of this radiotracer was carried out using a novel synthesis module obtained from Neptis, SA (Belgium).
Study participants were administered approximately 185 MBq (5 mCi) of 18F-FLT intravenously. A PET/CT scan was performed after an approximately 30-minute wait time (+/− 60 minutes). PET/CT scans were performed on Siemens Biograph 40 (Siemens - Erlangen, Germany) with a 40-detector helical CT. PET/CT image acquisition followed standard procedure in our PET Center, and the entire body from vertex to toes was imaged. PET acquisition time was based on patient weight.
Repeat whole body [18F]-FLT-PET imaging was performed at week 6 using the same instrument and image acquisition protocol as baseline. Repeat CT imaging of all areas of known or suspected disease was performed at week 12 also utilizing the same instrument and image acquisition protocols as baseline. Response assessment of CT imaging was performed using iRECIST.
Image Analysis
PET/CT images were reviewed and analyzed on a Siemens SYNGO.VIA workstation, Version VB20A (Siemens - Erlangen, Germany) by a fellowship-trained nuclear radiologist (R.Y., 4 years of experience) with dual board certification in diagnostic radiology and nuclear medicine.
All measurable tumor lesions were segmented on every slice of the PET scan where the lesion is visible. Baseline and post-therapy FLT-PET/CT scans were analyzed quantitatively by measuring maximum, peak, mean, and standard deviation of the standardized uptake values (SUV) of all tumor lesions, healthy hematopoietic tissue (spleen and bone marrow), and liver as background. Additionally, the proliferative tumor volume (PTV) was measured using three-dimensional regions of interest around tumor lesions, using a threshold of 40% of SUVmax. Total lesion proliferation (TLP) was calculated using the formula: TLP = PTV × SUVmean. Methodology for FLT quantification was similar to a prior study using FLT-PET/CT (13,14). We extracted 8 biomarkers from baseline FLT-PET characterizing FLT uptake (SUVmax, SUVpeak, SUVmean, SUVSD, TLP, PTV, SLR, BLR) and 8 biomarkers characterizing the changes between baseline and 6 weeks (delta-SUVmax, delta-SUVpeak, delta-SUVmean, delta-SUVSD, delta-TLP, delta-PTV, delta-SLR, delta-BLR). Variations in quantitative FLT uptake were calculated as percentage change between baseline and post-therapy PET/CT scans. For patients with multiple tumor lesions, the “hottest” lesion with the highest SUVmax was selected for SUVmax and SUVpeak. For PTV and TLP, the sum of these measures for all tumor lesions was used. Proliferation of healthy hematopoietic tissue was determined using liver uptake as background and a ratio of SUVmax of spleen or marrow to SUVmax of liver. For bone marrow FLT uptake, the L3 vertebral body was measured.
Responses Assessment
Early response assessment on FLT-PET/CT at 6 weeks was compared with objective response on CT at 12 weeks to assess the utility of FLT-PET/CT as an early predictor of response. Response assessment on FLT-PET/CT imaging at 6 weeks was performed using immune PET Response Criteria in Solid Tumors (iPERCIST) (15).
Statistical Analysis
The primary outcome was to explore the predictive value of FLT-PET at 6 weeks to predict the best-overall response on CT-scan at 12 weeks using iRECIST. Patients were divided into two groups according to the best overall response among all patients included in this study. For FLT-PET, patients were classified as responders: complete metabolic response (CMR), partial metabolic response (PMR), or non-responders: stable metabolic disease (SMD), progressive metabolic disease (PMD). Tumor response and progression on CT were evaluated using iRECIST following CT imaging at week 12 of the study, responders: complete response (CR), partial response (PR), or non-responders: stable disease (SD), progressive disease (PD). Agreements between different FLT-PETs and CTs were evaluated using Cohen’s kappa coefficient. Kappa-values are reported using the benchmarks of Landis and Koch: almost perfect (0.81–1.00), substantial (0.61–0.81), moderate (0.41–0.60), fair (0.21–0.40), slight (0.01–0.20), and poor (< 0.01). Since the response assessment is ordinal (CR, PR, SD, PD), we used a linearly weighted kappa.
Imaging biomarkers (SUVmax, SUVmean, SUVpeak, SUVSD, TLP, PTV, SLR, BLR) were compared at baseline between responders and non-responders. Additionally, the change in biomarkers (delta-SUVmax, delta-SUVpeak, delta-SUVmean, delta-SUVSD, delta-TLP, delta-PTV, delta-SLR, delta-BLR) was calculated at 6 weeks compared to baseline in responders versus non-responders.
The secondary outcome was to compare overall survival (OS) determined by iRECIST assessments at 12 weeks using median survival time and log-rank testing (Kaplan-Meier analysis). The difference in OS between those groups was assessed using log-rank testing (Kaplan-Meier analysis), and a p-value less than 0.05 was considered significant.
The tertiary outcome was to evaluate the frequency of pseudoprogression and immune related adverse events on FLT-PET.
Due to the small sample size, descriptive statistics were used to describe the population and compare patients with objective response and without objective response. Analysis was conducted using Microsoft Excel (v2019, Microsoft, USA, 2019), SPSS (v25.0, IBM, USA, 2017), and R (Version 1.2.1335).
RESULTS
Patient Characteristics
All five patients underwent FLT-PET/CT at baseline (pre-therapy) and 6 weeks post therapy (early therapy FLT-PET). Four patients underwent staging CT at baseline and 12 weeks post therapy. One patient (patient #5) underwent staging CT at baseline, but did not undergo post-therapy CT at 12 weeks, as the patient was taken off study due to symptomatic clinical progression from impending cardiac tamponade from malignant pleural disease and radiological progression on early therapy FLT-PET. For this patient, the CT from the FLT-PET/CT was used for iRECIST response assessment.
Table 1 summarizes the patient demographics, clinical characteristics including melanoma stage, response assessment, and variations in FLT uptake between pre-therapy and early therapy FLT-PET/CT scans. The median age was 44 years (range 31–62 years). All five patients had stage IV melanoma (4 stage M1c, 1 stage M1a). The median lactate dehydrogenase (LDH) was 233 UI/L, (range 141–442). Table 2 summarizes the patient characteristics for those who achieved objective response and no objective response. Table 3 shows the per-lesion response to treatment.
TABLE 2.
Patient Characteristics Separated by Those Patients Who Achieved Objective Response and No Objective Response on Treatment With Pembrolizumab
| Patient ID | No Objective Response | Objective Response | ||
|---|---|---|---|---|
| Mean | Mean | |||
| Clinical characteristics | ||||
| Age (years) | 42 | 50 | ||
| Gender F/M | ||||
| Ethnicity | ||||
| Overall survival (months) | 7 | 14 | ||
| Status Alive/Dead | ||||
| Stage | ||||
| AJCC classification | M1c: 2 | M1a: 1, M1c: 2 | ||
| LDH UI/L | 404 | 181 | ||
| Treatment | ||||
| Drug | Pembrolizumab: 2 | Pembrolizumab: 3 | ||
| Baseline FLT-PET | ||||
| Uptake | ||||
| SUV max | 6.8 | 7.8 | ||
| SUV peak | 5.7 | 6.1 | ||
| SUV mean | 4 | 3.6 | ||
| SUV SD | 0.8 | 0.8 | ||
| Tumor Burden | ||||
| TLP | 351.1 | 115.1 | ||
| PTV | 97.4 | 30.8 | ||
| SLR | 0.6 | 0.4 | ||
| BLR | 0.9 | 1.3 | ||
| FLT-PET at 6 weeks | ||||
| Uptake | ||||
| Delta-SUV max | 1.1 | −0.5 | ||
| Delta-SUV peak | 1.4 | −0.5 | ||
| Delta-SUV mean | 0.9 | −0.4 | ||
| Delta-SD | −0.1 | −0.6 | ||
| Tumor Burden | ||||
| Delta-TLP | 44.2 | −0.7 | ||
| Delta-PTV | 14.6 | −0.6 | ||
| Delta-SLR | −0.1 | 0.1 | ||
| Delta-BLR | 0.2 | 0.1 | ||
TABLE 3.
Per-Lesion Response to Treatment Showing Change in Outcome Measures from Baseline to 6 Week Follow Up
| Lesion | 1.1 | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 | 2.1 | 3.1 | 3.2 | 3.3 | 4.1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Lung | Adrenal | LN | Peritoneum | Bone | Peritoneum | Pleural | Skin | Skin | Skin | Lung |
| Response to treatment | |||||||||||
| 6-week FLT-PET | PR | PR | PR | PR | PR | PR | PD | SD | SD | SD | PR |
| Best overall response | CR | CR | CR | CR | CR | CR | PD | SD | SD | SD | PR |
| Baseline FLT-PET | |||||||||||
| Uptake | |||||||||||
| SUV max | 4.62 | 6.81 | 6.14 | 4.61 | 12.1 | 5.44 | 5.23 | 8.39 | 6.43 | 7.55 | 5.94 |
| SUV peak | 3.69 | 5.39 | 4.99 | 2.86 | 9.71 | 3.66 | 3.92 | 7.41 | 5.26 | 4.97 | 4.84 |
| SUV mean | 2.55 | 3.89 | 3.93 | 2.66 | 7.33 | 3.26 | 2.76 | 5.62 | 3.77 | 4.69 | 3.88 |
| SUV SD | 0.56 | 1.05 | 0.88 | 0.74 | 1.79 | 0.88 | 0.52 | 1.24 | 0.93 | 1.2 | 0.78 |
| Tumor Burden | |||||||||||
| TLP | 64.9 | 26.1 | 61.7 | 4.95 | 60.3 | 7.58 | 353.27 | 288.7 | 50.51 | 9.62 | 102.31 |
| PTV | 25.5 | 6.7 | 15.7 | 1.86 | 8.2 | 2.32 | 128.11 | 51.34 | 13.38 | 2.05 | 26.36 |
| 6-week FLT-PET | |||||||||||
| Uptake | |||||||||||
| Delta-SUV max | −35% | −100% | −67% | −100% | −100% | −100% | 233% | −14% | −48% | −30% | −40% |
| Delta-SUV peak | −32% | −100% | −64% | −100% | −100% | −100% | 295% | −15% | −53% | −15% | −53% |
| Delta-SUV mean | −31% | −100% | −64% | −100% | −100% | −100% | 207% | −19% | −52% | −27% | −51% |
| Delta-SD | −25% | −100% | −74% | −100% | −100% | −100% | 340% | −23% | −52% | −23% | −58% |
| Tumor Burden | |||||||||||
| Delta-TLP | −71% | −100% | −95% | −100% | −100% | −100% | 8690% | 87% | −71% | 27% | −85% |
| Delta-PTV | −59% | −100% | −87% | −100% | −100% | −100% | 2757% | 133% | −40% | 74% | −70% |
Tumor Response Assessment Categorization on FLT-PET (6 week) compared to CT (12 week)
Agreement between CT and FLT-PET was substantial (Kappa = 0.615, p = 0.025), Table 4. Using iRECIST, one patient had CR, two patients had PR, one patient had SD, and one patient had PD. While no patients had CMR, two patients had PMR, two patients had SMD, and one patient had PMD on early FLT-PET/CT using iPERCIST. Two discordant cases were patient #1, who had PMR on FLT-PET/CT and CR on CT, and patient #3, who had SMD on FLT-PET/CT and PR on CT (Figure 2). Patient #1 had multi-organ metastases including bone, lung, nodes, and peritoneum. Her FLT-PET-based response was categorized as PR since all of her lesions demonstrated resolution of FLT uptake, except two lesions with residual FLT uptake above background: right upper lung mass (SUVpeak decreased from 3.7 to 2.5) and peripancreatic lymph node (SUVpeak 5.0 to 1.8). All of her lesions resolved on post therapy CT at 12 weeks. Patient #3 had a solitary FLT-avid left inguinal lymph node metastasis and non-FLT-avid sub-centimeter pulmonary metastasis. His FLT-PET-based response was categorized as SD since the inguinal lymph node (target lesion) decreased by 13.5% (SUVpeak 3.7 to 3.2) and did not meet 30% decrease threshold for PR, while on CT, the left inguinal node (target lesion) decreased by 31.6% (19 mm to 13 mm) to meet the 30% decrease threshold for PR.
TABLE 4.
Tumor Response Assessment With FLT-PET at 6 Weeks Compared to CT at 12 Weeks Showed Substantial Agreement Between These Imaging Tests
| 12-Week CT | ||||||
|---|---|---|---|---|---|---|
| CR | PR | SD | PD | Total | ||
| 6-Week FLT-PET | CR | 0 | 0 | 0 | 0 | 0 |
| PR | 1 | 1 | 0 | 0 | 2 | |
| SD | 0 | 1 | 1 | 0 | 2 | |
| PD | 0 | 0 | 0 | 1 | 1 | |
| Total | 1 | 2 | 1 | 1 | 5 | |
Figure 2:
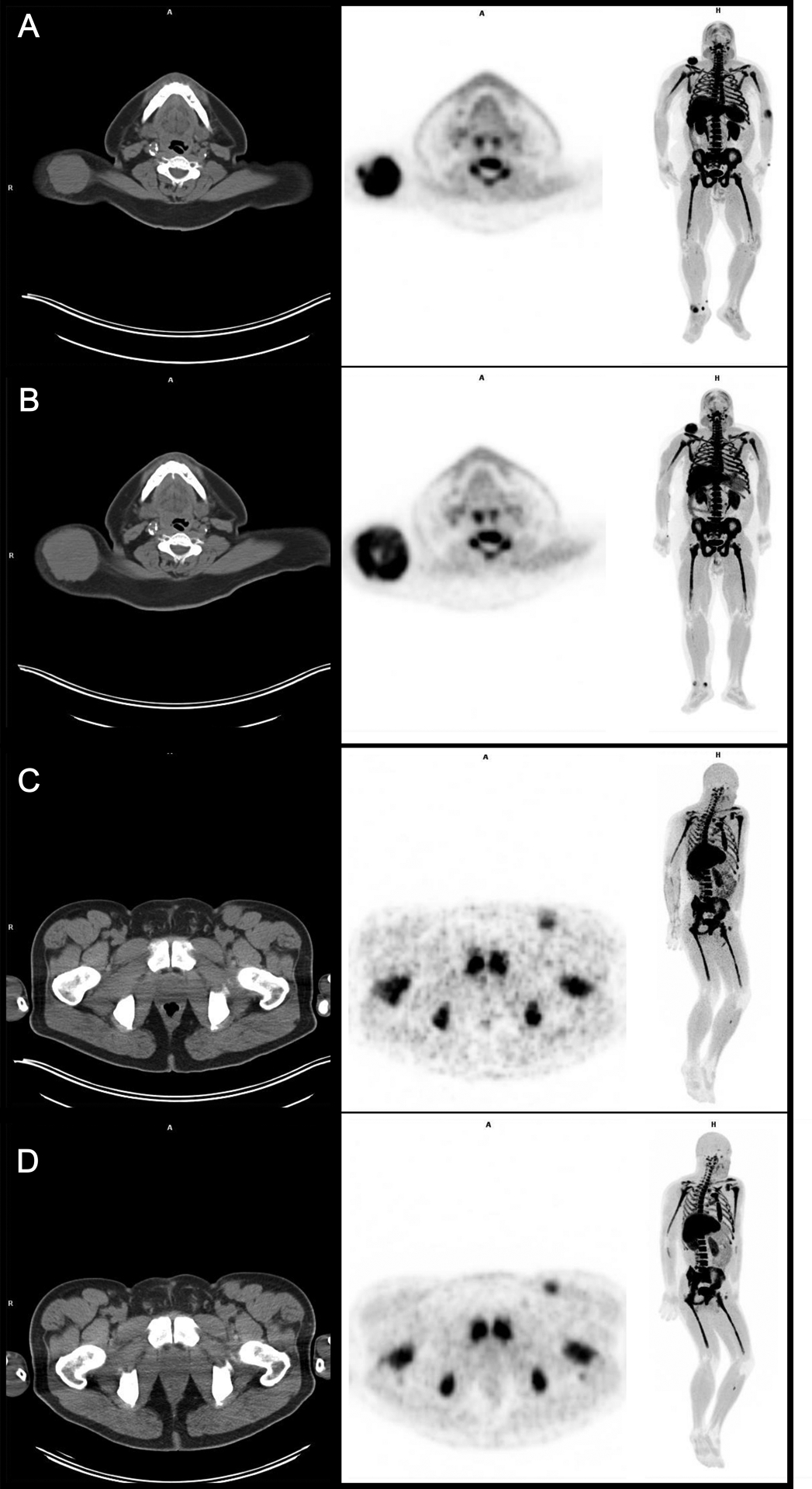
Left to right images: CT, FLT-PET, and FLT-PET whole body maximum intensity projection (MIP). A and B) A shows the baseline scans and B shows the follow-up scans. Patient 4’s dominant lesion in the right subcutaneous back increased in size on CT during the follow up FLT-PET scan from 52 mm to 72 mm, however the SUV of the lesion decreased by −14.4%. Thus, there was a discordance between CT size and FLT-PET SUV on that scan. Ultimately, the patient had stable disease on follow up CT 12 weeks later which suggests that the FLT was a better and earlier predictor of the ultimate outcome.
C and D) C shows the baseline scans and D shows the follow-up scans. The patient’s left inguinal node did not change on CT during the follow up FLT-PET and remained at 19 mm short axis but FLT SUV decreased 21.9%. Ultimately, the patient had partial response on follow up CT 12 weeks later which again demonstrates the advantage of FLT over CT as an early predictor of response.
Association of baseline FLT-PET biomarkers with Best Overall Response
Baseline biomarkers of FLT uptake and CT-based iRECIST objective response outcomes are demonstrated in Table 1. Best overall response was defined as response (CR, PR) or non-response (SD, PD). Figure 3 shows the relationship between SUVmax, PTV, and BLR on baseline FLT-PET and CT-based RECIST objective response when patients are dichotomized between responders and non-responders. Responders had a trend toward higher BLR and lower PTV at baseline compared to non-responders (Figure 3B). However, SUVmax on baseline FLT-PET did not demonstrate a trend between responders and non-responders.
Figure 3:
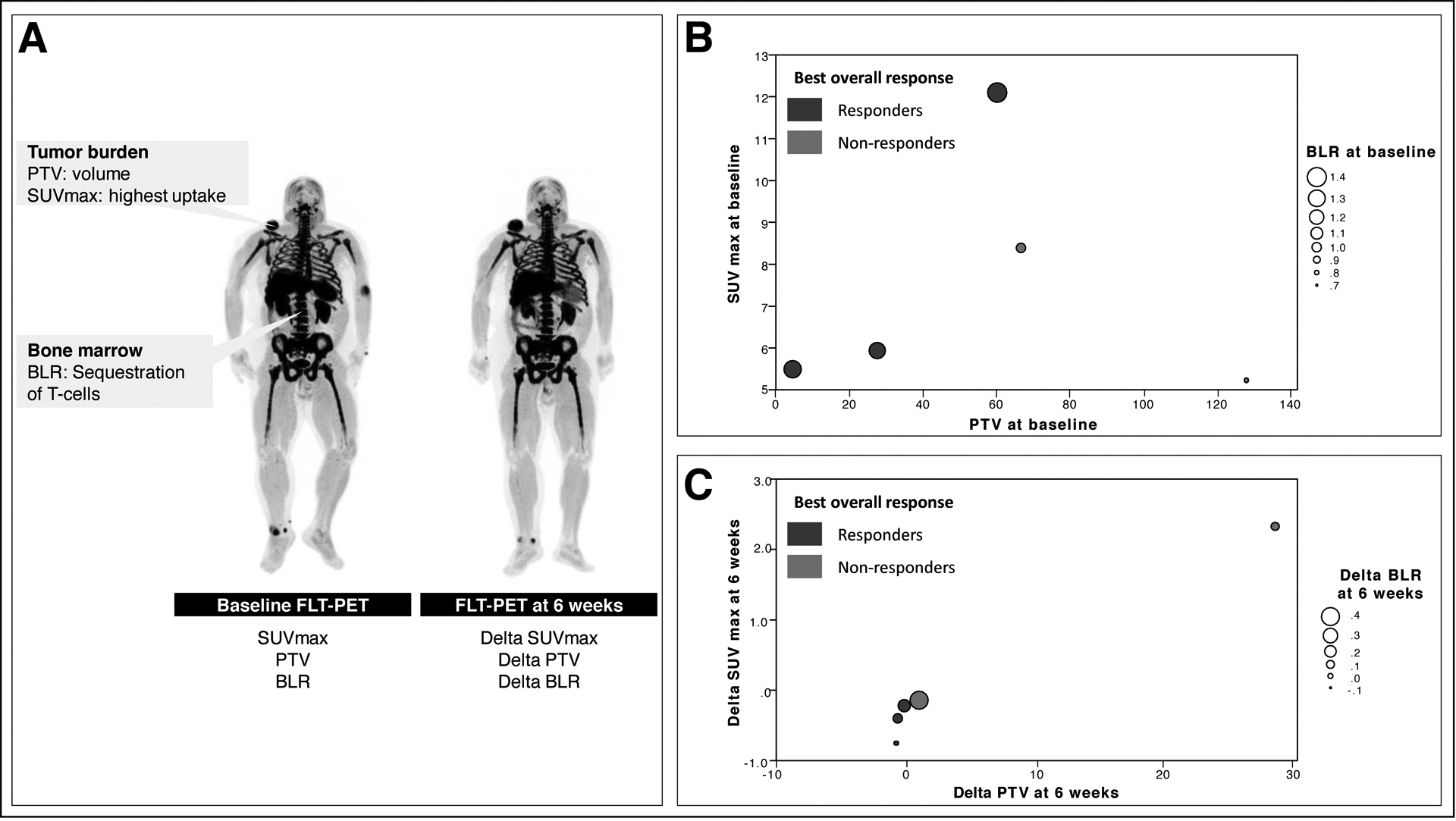
Association between pre-therapy FLT-PET vs. CT objective response at 12 weeks. A) Baseline markers in FLT-PET include PTV, BLR, and SUV. Delta-biomarkers are calculated as the change from baseline to 6 weeks. B) Responders have a trend towards greater BLR at baseline and lower PTV at baseline. C) Responders have a trend toward lower delta-PTV compared to non-responders.
Baseline and early therapy FLT-PET splenic uptake (SLR) did not demonstrated a trend between responders and non-responders.
Association of 6-week FLT-PET biomarkers with Best Overall Response
Delta-SUVmax, delta-SUVpeak, delta-SUVmean, delta-SUVSD, delta-TLP, delta-PTV, delta-SLR, delta-BLR were calculated to compare the change in response from baseline to 6-weeks on FLT-PET. These biomarkers were then correlated with best overall response as measured by CT-based iRECIST objective response, Figure 4.
Figure 4:
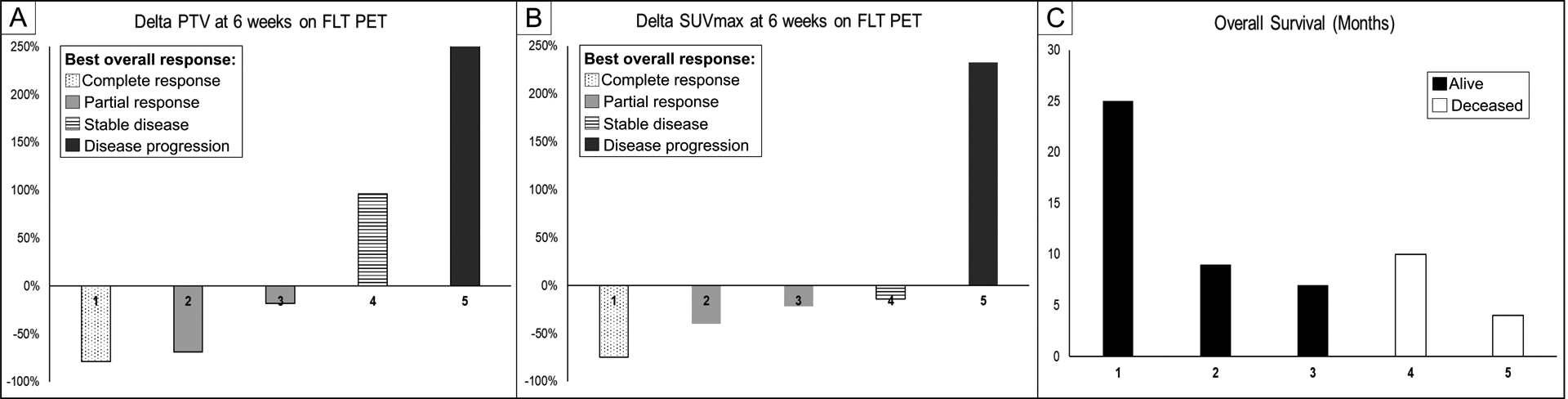
A) Percent change in FLT SUVmax from baseline to early FLT-PET/CT at 6 weeks. Upper limit of the y-axis is 250%, but the value of patient 5 was +2800%. B) Percent change in FLT PTV from baseline to early FLT-PET/CT at 6 weeks. C) Overall survival of patients in months.
Delta-SUVmax, SUVmean, and SUVpeak of tumor FLT uptake between pre-therapy and early therapy FLT-PET scans showed a trend towards correlation with CT-based response assessment (Table 1, Figure 4A, Figure 5). For example, the one patient (patient 5) with PMD had the highest percentage increase in delta-SUVmax of +233%, while the one patient (patient 1) with CMR had the percentage decrease in SUVmax of −75%. Delta-SUVmax allowed for discrimination between non-response in patient 4 (−14%) and response in patient 3 (−22%), whereas these patients had the same delta-SUVmean (−17%) and delta-SUVpeak (−15%), suggesting that delta-SUVmax may be a more sensitive metric.
Figure 5:
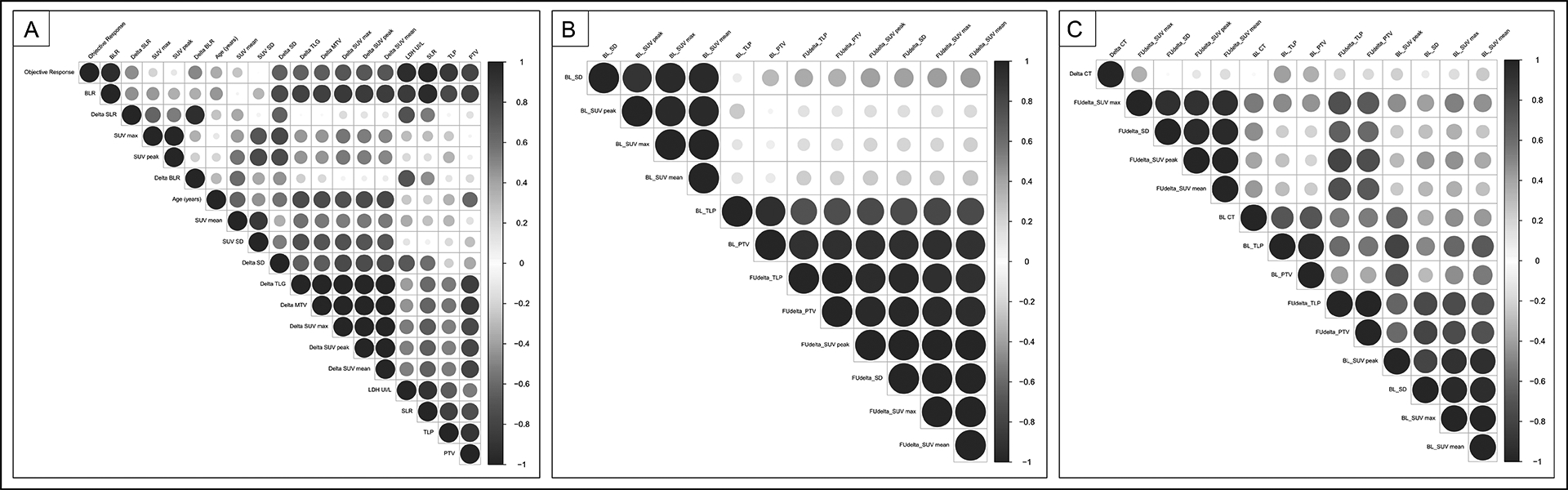
Correlogram between baseline and delta imaging biomarkers.
Correlation between pretreatment imaging biomarkers: FDG uptake of tumor, bone marrow, spleen (SUV), metabolic tumor burden (PTV, TLP), HISUV and metabolic proliferative biomarkers in hematopoietic tissues (BLR, SLR). Spearman’s correlation coefficients were used. The strength of each statistically significant correlation is displayed using a color code and the size of the circle. Clusters are performed using an unsupervised approach.
A. Per patient analysis (n=5 pts)
B. Per lesion analysis including PET at 6 weeks (n=13 lesions)
C. Per lesion analysis including PET at 6 weeks and CT at 12 weeks (n=8 lesions)
Delta-PTV and delta-TLP also demonstrated a similar trend (Figure 4B), with both parameters allowing for clearer discrimination between non-response and response than SUVmax, for example, PTV increased by +96% in patient #4 with non-response and decreased by −18% in patient #3 with response, compared to −14% and −22% for SUVmax, respectively.
On early therapy FLT-PET, responders had a trend towards greater decrease in delta-PTV compared to non-responders (Figure 3C). No trend was observed for delta-SUVmax and delta-BLR.
Association of Response Classification with Overall Survival
The relationship between objective response on early therapy FLT-PET and OS is shown in Figure 4C. There was a trend towards decreased OS in 2 patients who were non-responders as compared with OS in 3 patients who were responders. Patient 1, 2, and 3 were alive 25, 10, and 9 months, respectively and these patients demonstrated complete response (patient 1) and partial response (patients 2 and 3). Patients 4 and 5 who demonstrated progressive disease and stable disease, respectively, died within 3 to 7 months. Figure 6 shows the disease specific survival curve for patients divided into responders (CR and PR) and non-responders (SD and PD).
Figure 6:
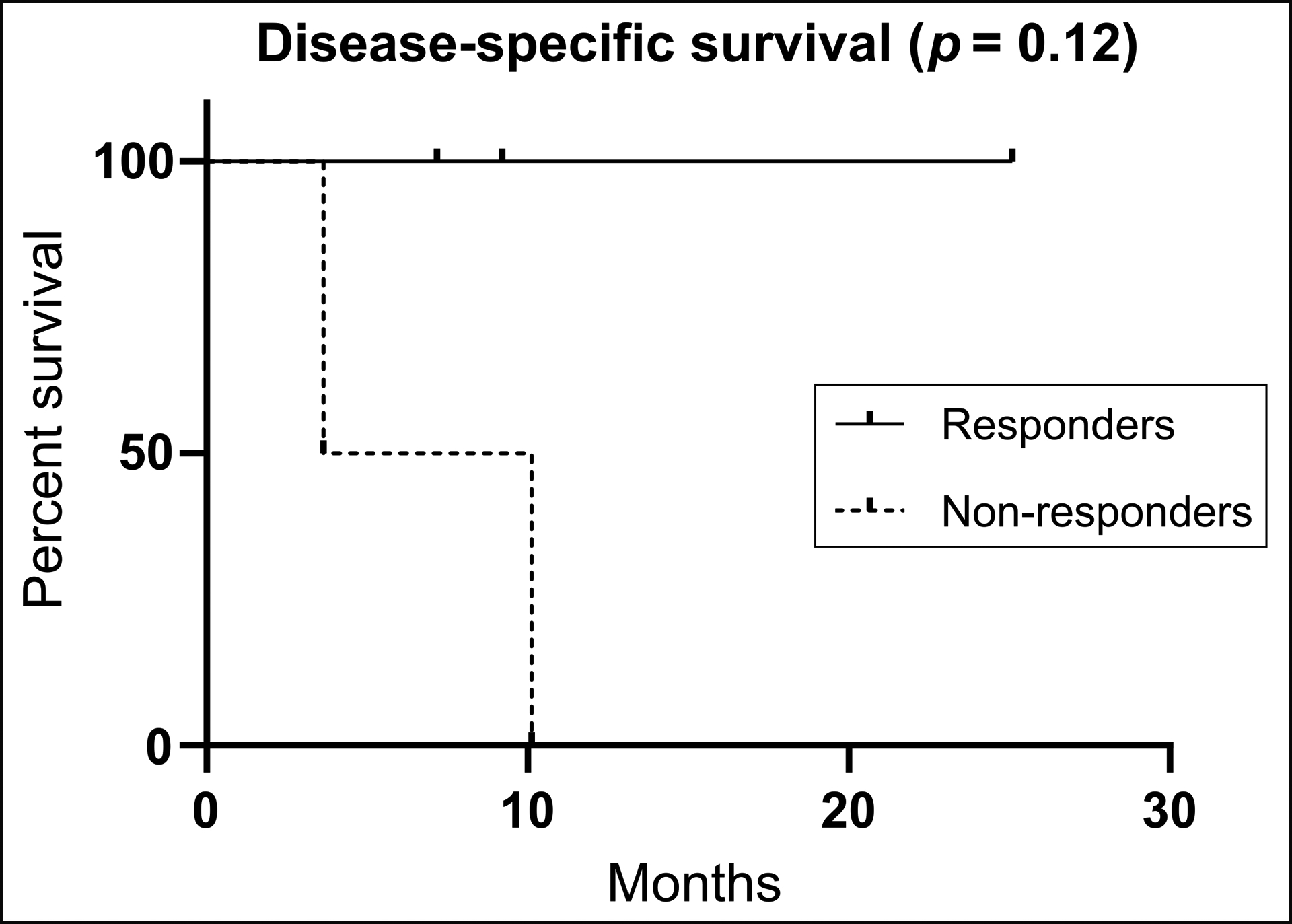
Disease-specific survival curve showing percent survival for responders (complete response and partial response) and non-responders (stable disease and disease progression)
Pseudoprogression and Immune-related Adverse Events on FLT-PET
No pseudoprogression was observed on FLT-PET in 5 patients and 13 lesions. No immune-related adverse events were observed in 5 patients.
DISCUSSION
This pilot study evaluated the feasibility of FLT-PET as an early predictive biomarker for response in patients with metastatic melanoma treated with immunotherapy. FLT-PET is a promising modality for evaluating disease progression in patients treated with immunotherapy. We predict that FLT-PET will be more sensitive than FDG-PET and CT-scan in distinguishing between true progression and pseudoprogression. Because CT-scans evaluate tissue density, it is unable to distinguish tumor infiltrating lymphocytes from tumor cells as the density of the tumor cells, as these cells have the same density. Thus, pseudoprogression and true progression could not be distinguished with a density-based metric. Similarly, FDG-PET uses glucose uptake to measure tumor cell proliferation. Because immune cells and tumor cells both have high glucose consumption, it is difficult to distinguish between immune cell infiltrate compared to increased density of tumor cells on FDG-PET. FLT-PET measures a radiolabeled DNA nucleoside, thymidine, which is present in higher levels in tumor cells rather than immune cells (16,17). Our study is the first study to evaluate FLT-PET in patients with metastatic melanoma on anti-PD1 immunotherapy and compare early response classification on FLT-PET with RECIST-based CT objective response and investigate novel FLT biomarkers. Significantly, in this population of 5 patients, only one patient demonstrated increased SUV on FLT-PET and this patient was, in fact, a hyper-progresser.
FLT-PET has shown promise in detecting early treatment response in various malignancies including lung, brain, head and neck, and breast cancers (18). However, only 3 FLT-PET studies have been performed in patients with melanoma. One study examined FLT-PET/MRI in 5 patients with melanoma brain metastases on targeted therapy or immunotherapy. Two patients of 5 completed pre- and post-therapy FLT-PET/MRI, one demonstrating concordant delta-FLT uptake and size, while another showed a mixed response pattern (10).
Our results demonstrated a trend in 5 patients that metabolic changes on early therapy FLT-PET at 6 weeks could predict iRECIST-based objective response on CT at 12 weeks, as there was substantial agreement between FLT-PET and CT as measured by Cohens Kappa coefficient.
In this study, we evaluated the prognostic value of baseline FLT biomarkers. This could be used to guide the decision to start treatment. At baseline, a lower PTV, SUVmax, and a higher BLR was associated with response. These baseline biomarkers could have significant clinical implications. For example, if we are able to differentiate responders from non-responders at baseline, before therapy is initiated, clinicians can avoid unnecessary treatment of patients who will not receive benefit. This will both reduce morbidity from unnecessary treatment and save heathcare dollars. BLR could be a useful metric to differentiate between responders and non-responders early in the treatment course. Strikingly, increased tumor volume and increased BLR on baseline FDG-PET has been found to be associated with decreased OS and progression free survival (PFS) (14) as well as with transcriptomic profiles including regulatory T-cell markers.
We demonstrated that 6-week FLT biomarkers can be used to predict treatment efficacy. Several studies have evaluated changes in imaging biomarkers in predicting response to therapy. One study evaluated FLT-PET in melanoma patients with regional lymph node metastases receiving dendritic cell vaccine therapy (19). FLT uptake was significantly higher in vaccinated lymph nodes as compared with control nodes injected with placebo. Correlation with immunohistochemistry confirmed activation of CD4+ and CD8+ T-cell and the authors suggested that delta-FLT-PET can be used to differentiate early responders from non-responders through visualization of immune cell activation (19). A study of FDG and FLT-PET scans on patients with advanced melanoma before and after therapy with CTLA4-blocking antibody, tremelimumab showed no significant delta-FDG or delta-FLT of metastatic lesions after 2 months of tremelimumab. However, there was a significant increase in FLT splenic suggesting release of CTLA4 checkpoint and subsequent T-cell proliferation in the spleen. Increased splenic uptake was not observed on FDG-PET (20).
Our study did not observe a trend in delta-SLR between responders and non-responders and all patients except patient #1 with CR demonstrated an increased in splenic uptake on early FLT-PET.
Delta-FLT biomarkers were associated with objective response as measured by CT-based iRECIST criteria. Patients with a greater decrease in FLT uptake, as measured by delta-SUV max, delta-PTV, and delta-TLP, had better objective response, and patients with lesser decrease or even an increase in FLT uptake had worse objective response. Similarly, this information at the 6-week time point is useful in guiding decisions-making regarding whether to continue treatment. A trend was observed for OS, as FLT-PET responders had higher OS than non-responders.
No pseudoprogression was observed on FLT-PET in 5 patients and 13 lesions, and no immune-related adverse events were observed. Notably however, one patient with increased PTV but decreased SUV at 6 weeks did display stable disease at 12 weeks suggesting that increase in tumor volume occurred early in treatment but was not associated with increased metabolism in contrast the hyperprogressing patient who had increases in both PTV and SUV. One potential application of FLT-PET is to identify non-responders with significant progression of disease (hyperprogression), such as patient #5 in our study, at an earlier time-point to facilitate earlier transition to alternative therapy or supportive care. Hyperprogression is reported to occur in 9% of patients on anti-PD1/PDL-1 therapy and can be diagnosed using tumor growth rate of serial CT scans (4). It is plausible that FLT-PET may be able to detect hyperprogression earlier through rapid and substantial increased in FLT biomarkers (i.e. delta-SUVmax, PTV) and be advantageous over CT scans (21,22). Recently, a study using FDG-PET in patients with metastatic melanoma on anti-PD1 immunotherapy showed that higher total metabolic tumor volume (TMTV) and BLR on baseline FDG-PET correlated with shorter PFS and OS (14). In our study, non-responders similarly had higher PTV, but conversely have lower BLR compared to non-responders. This discordance may be explained from our limited sample size or differences in glucose metabolism and cell proliferation in bone marrow.
These results support our hypothesis that FLT-PET could potentially detect early response to anti-PD1 immunotherapy through in vivo imaging of DNA synthesis and cell proliferation. While our study only had 5 patients, these results would provide justification for a larger prospective study to test the utility of FLT-PET as an early predictive biomarker to guide management.
The main limitation of our study is the small sample size of 5 patients, which precluded adequate power and evaluation of statistical significance. Recruitment into the study may have been limited by the advanced disease and aggressive nature of metastatic melanoma. Our hypothesis that FLT-PET may have utility in differentiating true progression from pseudoprogression could not be tested since no patients had pseudoprogression in our study. Another limitation is our approach in using liver background uptake to normalize spleen and bone marrow uptake (SLR and BLR). While this approach has been used in FDG-PET, the high physiological liver uptake in FLT-PET may have precluded detectable trends in our cohort. Similarly, we used PERCIST 1.0 for our FLT response assessment, which was designed and validated in FDG-PET, but has not be proved in FLT-PET.
CONCLUSION:
This study demonstrates the feasibility of using FLT-PET as an early predictor of response for patients with metastatic melanoma treated with anti-PD1 immunotherapy. Assessing treatment response in this setting is challenging using available conventional imaging due to well established phenomena of delayed response to immunotherapy. Identification of changes in metabolic activity at 6 weeks has the potential to enable clinicians to determine whether patients are responding to therapy earlier in the treatment course, which would allow for patients to change treatments earlier if single agent immunotherapy is not effective. Further studies with larger cohorts of patients are indicated to validate and demonstrate the significance of the results of this pilot study.
Acknowledgment:
We would like to acknowledge Merck Pharmaceuticals for funding this study.
Footnotes
Conflict of Interest Disclosures: None
REFERENCES
- 1.Maverakis E, Cornelius LA, Bowen GM, et al. : Metastatic melanoma - a review of current and future treatment options. Acta Derm Venereol 2015; 95: 516–24. [DOI] [PubMed] [Google Scholar]
- 2.Lugowska I, Teterycz P, Rutkowski P: Immunotherapy of melanoma. Contemp Oncol (Pozn) 2018; 22:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiou VL, Burotto M: Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol 2015; 33: 3541–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champiat S, Dercle L, Ammari S, et al. : Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017; 23:1920–1928 [DOI] [PubMed] [Google Scholar]
- 5.Saenger YM, Wolchok JD: The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun 2008. 8:1. [PMC free article] [PubMed] [Google Scholar]
- 6.Kwak JJ, Tirumani SH, Abbeele ADVd, et al. : Cancer Immunotherapy: Imaging Assessment of Novel Treatment Response Patterns and Immune-related Adverse Events. RadioGraphics 2015; 35: 424–437 [DOI] [PubMed] [Google Scholar]
- 7.Shinomiya A, Kawai N, Okada M, et al. : Evaluation of 3’-deoxy-3’-[18F]-fluorothymidine (18F-FLT) kinetics correlated with thymidine kinase-1 expression and cell proliferation in newly diagnosed gliomas. Eur J Nucl Med Mol Imaging 2013; 40: 175–85 [DOI] [PubMed] [Google Scholar]
- 8.Crandall JP, Tahari AK, Juergens RA, et al. : A comparison of FLT to FDG PET/CT in the early assessment of chemotherapy response in stages IB–IIIA resectable NSCLC. 2017; EJNMMI Res 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Graf N, Herrmann K, et al. : FLT-PET is superior to FDG-PET for very early response prediction in NPM-ALK-positive lymphoma treated with targeted therapy. Cancer Res 2012; 72: 5014–24 [DOI] [PubMed] [Google Scholar]
- 10.Nguyen NC, Yee MK, Tuchayi AM, et al. : Targeted Therapy and Immunotherapy Response Assessment with F-18 Fluorothymidine Positron-Emission Tomography/Magnetic Resonance Imaging in Melanoma Brain Metastasis: A Pilot Study. Front Oncol 2018; 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Hiel B, Haanen J, Stokkel MPM, et al. : Vemurafenib plus cobimetinib in unresectable stage IIIc or stage IV melanoma: response monitoring and resistance prediction with positron emission tomography and tumor characteristics (REPOSIT): study protocol of a phase II, open-label, multicenter study. BMC Cancer 2017; 17: 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riaz N, Havel JJ, Makarov V, et al. : Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 2017; 171:934–949.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi S, Zhongyi Y, Yingjian Z, et al. : (18)F-FLT and (18)F-FDG PET/CT in Predicting Response to Chemoradiotherapy in Nasopharyngeal Carcinoma: Preliminary Results. Sci Rep 2017; 7:40552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seban RD, Nemer JS, Marabelle A, et al. : Prognostic and theranostic 18F-FDG PET biomarkers for anti-PD1 immunotherapy in metastatic melanoma: association with outcome and transcriptomics. Eur J Nucl Med Mol Imaging, 2019; 46: 2298–2310 [DOI] [PubMed] [Google Scholar]
- 15.Wahl RL, Jacene H, Kasamon Y, et al. : From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 50 Suppl 2009; 1:122s–50s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taheri MR, Wickremasinghe RG, Hoffbrand AV: Alternative metabolic fates of thymine nucleotides in human cells. The Biochemical Journal 1981; 194:451–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocklin RE: Modulation of cellular-immune responses in vivo and in vitro by histamine receptor-bearing lymphocytes. The Journal of Clinical Investigation 1976; 57:1051–1058, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanghera B, Wong WL, Sonoda LI, et al. : FLT PET-CT in evaluation of treatment response. Indian J Nucl Med 2014; 29:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aarntzen EH, Srinivas M, De Wilt JH, et al. : Early identification of antigen-specific immune responses in vivo by [18F]-labeled 3’-fluoro-3’-deoxy-thymidine ([18F]FLT) PET imaging. Proc Natl Acad Sci U S A 2011; 108:18396–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribas A, Benz MR, Allen-Auerbach MS, et al. : Imaging of CTLA4 blockade-induced cell replication with (18)F-FLT PET in patients with advanced melanoma treated with tremelimumab. J Nucl Med 2010; 51:340–6 [DOI] [PubMed] [Google Scholar]
- 21.Dercle L, Seban RD, Lazarovici J, et al. : (18)F-FDG PET and CT Scans Detect New Imaging Patterns of Response and Progression in Patients with Hodgkin Lymphoma Treated by Anti-Programmed Death 1 Immune Checkpoint Inhibitor. J Nucl Med 2018; 59:15–24 [DOI] [PubMed] [Google Scholar]
- 22.Dercle L, Ammari S, Seban RD, et al. : Kinetics and nadir of responses to immune checkpoint blockade by anti-PD1 in patients with classical Hodgkin lymphoma. Eur J Cancer 2018; 91:136–144 [DOI] [PubMed] [Google Scholar]


