Key Points
Question
Is an unhealthy diet associated with clonal hematopoiesis of indeterminate potential, and are they together associated with an increased risk of adverse cardiovascular events?
Findings
In this cohort study of 44 111 adult participants in the UK Biobank, an unhealthy diet was independently associated with a 25% increased likelihood of the presence of clonal hematopoiesis of indeterminate potential and increases in adverse cardiovascular events and death.
Meaning
The results of this study suggest that unhealthy eating habits may be associated with the development of clonal hematopoiesis of indeterminate potential as well as substantially increased risk of adverse cardiovascular events and death.
Abstract
Importance
Clonal hematopoiesis of indeterminate potential (CHIP), the expansion of somatic leukemogenic variations in hematopoietic stem cells, has been associated with atherosclerotic cardiovascular disease. Because the inherited risk of developing CHIP is low, lifestyle elements such as dietary factors may be associated with the development and outcomes of CHIP.
Objective
To examine whether there is an association between diet quality and the prevalence of CHIP.
Design, Setting, and Participants
This retrospective cohort study used data from participants in the UK Biobank, an ongoing population-based study in the United Kingdom that examines whole-exome sequencing data and survey-based information on health-associated behaviors. Individuals from the UK Biobank were recruited between 2006 and 2010 and followed up prospectively with linkage to health data records through May 2020. The present study included 44 111 participants in the UK Biobank who were age 40 to 70 years, had data available from whole-exome sequencing of blood DNA, and were free of coronary artery disease (CAD) or hematologic cancer at baseline.
Exposures
Diet quality was categorized as unhealthy if the intake of healthy elements (fruits and vegetables) was lower than the median of all survey responses, and the intake of unhealthy elements (red meat, processed food, and added salt) was higher than the median. Diets were classified as healthy if the intake of healthy elements was higher than the median, and the intake of unhealthy elements was lower than the median. The presence of CHIP was detected by data from whole-exome sequencing of blood DNA.
Main Outcomes and Measures
The primary outcome was CHIP prevalence. Multivariable logistic regression analysis was used to examine the association between diet quality and the presence of CHIP. Multivariable Cox proportional hazards models were used to assess the association of incident events (acute coronary syndromes, coronary revascularization, or death) in each diet quality category stratified by the presence of CHIP.
Results
Among 44 111 participants (mean [SD] age at time of blood sample collection, 56.3 [8.0] years; 24 507 women [55.6%]), 2271 individuals (5.1%) had an unhealthy diet, 38 552 individuals (87.4%) had an intermediate diet, and 3288 individuals (7.5%) had a healthy diet. A total of 2507 individuals (5.7%) had CHIP, and the prevalence of CHIP decreased as diet quality improved from unhealthy (162 of 2271 participants [7.1%]) to intermediate (2177 of 38 552 participants [5.7%]) to healthy (168 of 3288 participants [5.1%]; P = .003 for trend). Compared with individuals without CHIP who had an intermediate diet, the rates of incident cardiovascular events progressively decreased among those with CHIP who had an unhealthy diet (hazard ratio [HR], 1.52; 95% CI, 1.04-2.22) and those with CHIP who had a healthy diet (HR, 0.99; 95% CI, 0.62-1.58) over a median of 10.0 years (interquartile range, 9.6-10.4 years) of follow-up.
Conclusions and Relevance
This cohort study suggests that an unhealthy diet quality may be associated with a higher prevalence of CHIP and higher rates of adverse cardiovascular events and death independent of CHIP status.
This cohort study uses data from adult participants in the UK Biobank to examine whether diet quality is associated with the risk of clonal hematopoiesis of indeterminate potential and whether they are together associated with an increased risk of incident cardiovascular events and death.
Introduction
Despite scientific advances in our understanding of coronary artery disease (CAD), lipid metabolism, sex-specific risk factors, germline genetic risk, and behavioral risk, age continues to be the dominant risk factor associated with CAD.1,2,3 The aging hematopoietic system has substantial implications for vascular homeostasis and age-associated dysfunction.4,5 However, the specific mechanisms through which the aging immune system is associated with atherosclerotic cardiovascular disease (CVD) are incompletely understood.
Clonal hematopoiesis of indeterminate potential (CHIP) is an age-associated clonal expansion in leukemogenic genes without any overt diagnosis of cancer and is detectable from next-generation sequencing of blood DNA.6,7,8 Clonal hematopoiesis of indeterminate potential is a common age-associated phenomenon found in up to 10% of individuals older than 70 years.9 While CHIP is a substantial risk factor for the development of hematologic cancer, it is associated with a larger absolute risk increase for CAD.10,11,12,13,14,15 Furthermore, a greater clonal fraction of CHIP variations in the blood is associated with a greater burden of coronary atherosclerosis and CAD risk, partly through inflammatory mediators.11,14 A whole-genome sequencing analysis of CHIP recently estimated its heritability at only approximately 3.6%.12 This observation raises questions regarding which exposures and behavioral factors may be associated with the development of CHIP. Smoking, a history of chemotherapy receipt or premature menopause, type 2 diabetes, and HIV infection have been associated with the presence of CHIP.6,16,17,18,19,20,21,22
Healthy diet is an important factor in the maintenance of optimal cardiovascular (CV) health.23 A health-promoting diet has long been associated with improvements in CV risk.24,25,26,27,28 Diets high in fruits and vegetables have been associated with reduced inflammatory burden and lower CV events, but the mechanisms that mediate these associations are incompletely understood.29,30 Some studies have suggested that diets high in fruits and vegetables and low in animal protein are associated with reductions in incident cancer diagnoses and recurrences among patients receiving cancer treatment.31,32,33,34,35,36 Diet, inflammation, and precancerous states such as CHIP are further associated through myeloid lineage propagation and clonal phenomena.37 For instance, in tet methylcytosine dioxygenase 2 (Tet2) knockout mice, bacterial translocation from the intestine and resultant activation of cytokine toll-like receptor 2 and interleukin 6 signaling were associated with clonal expansion and preleukemic myeloproliferation.38 We hypothesized that healthy dietary patterns are associated with a lower prevalence of CHIP and a reduction in incident CV events regardless of CHIP status. We also assessed whether a healthy dietary pattern was associated with incident CV events differentially by CHIP status.
We used data from the UK Biobank, an ongoing population-based cohort study in the United Kingdom, to investigate whether diet quality is associated with the presence of CHIP and whether a healthy diet is associated with mitigated risk of incident CV events and death in the context of CHIP.
Methods
The study was approved by the institutional review board of Massachusetts General Hospital. Participants provided informed consent for the use of their data at the time of enrollment in the UK Biobank. The institutional review board therefore determined that no additional informed consent was required for secondary use of data because the present study posed minimal risk to participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Study Population
Data were from the UK Biobank, a population-based cohort of more than 500 000 adults in the UK between ages 40 to 70 years at recruitment who were followed up prospectively.39 Individuals from the UK Biobank were recruited between 2006 and 2010 and followed up prospectively with linkage to health data records through May 2020. At the time of the baseline study visit, participants used a touch screen to provide detailed information about medical history, medication receipt, smoking history, and dietary habits among other lifestyle factors. Whole-exome sequencing of blood DNA was performed among a subset of individuals, and the first 50 000 of these sequences had been released at the time of the present study.40 Among those 50 000 individuals with available whole-exome sequencing data, approximately 25% were recruited at a study visit to receive magnetic resonance imaging, which introduced the potential for immortal time bias that is addressed in the analysis.
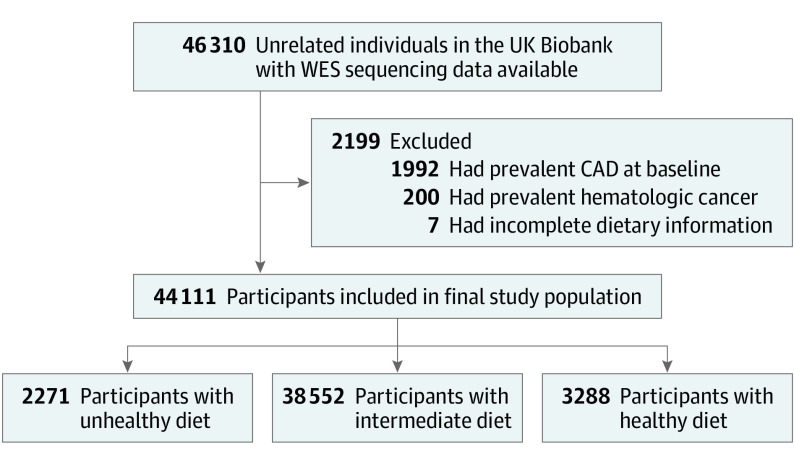
A total of 46 310 individuals in the UK Biobank had whole-exome sequencing data available. Of those, 1992 individuals were excluded because they had CAD, and 200 individuals were excluded because they had hematologic cancer at the time of the blood sample collection. Seven additional individuals were excluded based on incomplete responses on the food frequency survey, leaving a final cohort of 44 111 participants (Figure 1). All data were collected from study recruitment until August 2016.
Figure 1. Study Flow Diagram.
CAD indicates coronary artery disease; WES, whole-exome sequencing.
Exposures
The primary exposure was diet quality, which was rated using an ordinal categorical scale comprising unhealthy, intermediate, or healthy diet. To define the quality of a participant’s diet, we compared available questionnaire response fields with the 2015 Healthy Eating Index from the US Department of Health and Human Services (eMethods in the Supplement). Alternate validated standardized diet scores were considered, but missing elements precluded their use. The Healthy Eating Index recommends that a healthy diet be enhanced with healthy foods, including a variety of vegetables, whole fruits, whole grains, and lean meats or vegetarian protein.41
At enrollment, participants in the UK Biobank completed surveys regarding their frequency of intake of a range of common food and drink items using the Oxford WebQ survey.42 This self-administered online survey has been validated using biomarkers for protein, potassium, and sugar intake. It performs comparably well with longer and more administratively burdensome interviewer-based recall surveys.43 Diet quality was assessed based on food frequency questionnaire responses. Diet was categorized as unhealthy if the intake of healthy elements (fruits and vegetables) was lower than the median of all survey responses, and the intake of unhealthy elements (red meat, processed food, and added salt) was higher than the median. Diets were classified as healthy if the intake of healthy elements was higher than the median, and the intake of unhealthy elements was lower than the median. The remainder of individuals were categorized as having intermediate diet quality.
A dietary score adapted from the Diet Quality Score44 was also calculated and used to replicate the primary analysis (eMethods in the Supplement). To generate this score, diet quality was treated as a continuous variable by assigning point values to each of 3 dietary elements: fruits and vegetables, red and processed meats, and added salt. Individual intake was ranked as a percentile for the fruits and vegetables category (with highest quantile of intake assigned 3 points, lowest quantile assigned 1 point, and intermediate quantiles assigned 2 points) and the red and processed meat category (with lowest quantile of intake assigned 3 points, highest quantile assigned 1 point, and intermediate quantiles assigned 2 points). For the added salt category, usually or always adding salt to food was assigned 1 point, sometimes adding salt was assigned 2 points, and never adding salt was assigned 3 points. The total dietary quality score ranged from 3 to 9 for each individual.
Sequencing and CHIP Detection
Exomes from participants in the UK Biobank were sequenced from blood-derived DNA at the Regeneron Sequencing Center (Tarrytown, New York), as previously described.40 Genome Analysis Toolkit MuTect2 software, version 4 (Broad Institute), was used to analyze exomes for the detection of somatic variations according to a predefined list of curated leukemogenic CHIP-associated variations in the UK Biobank, as previously described.11,12 While a previous analysis of data from the UK Biobank was restricted to DNA methyltransferase 3A (DNMT3A; OMIM 602769) and tet methylcytosine dioxygenase 2 (TET2; OMIM 612839) gene variations,11 the present study, which used realigned UK Biobank exomes, was not restricted to DNMT3A and TET2 variations but used the similarly described protocol.11
Outcomes
The primary outcome was the presence of all CHIP, which was defined as the presence of somatic variations in leukemogenic CHIP drive genes with a variant allele fraction of more than 2%. A secondary end point was the presence of CHIP with large clones (variant allele fraction >10%), as CHIP clones higher than this threshold have been previously associated with adverse clinical outcomes.7,11,12,14
Additional models were used to test for an association between CHIP and incident adverse CV events (CAD or death, as previously described11) and to evaluate whether this association varied by diet quality category. Cardiovascular disease was defined as a history of myocardial infarction or revascularization with coronary artery bypass grafting or coronary angioplasty, with or without stenting. Incident events were ascertained through electronic health records linked to the UK Biobank (eMethods in the Supplement).
Statistical Analysis
Participant characteristics were compared between diet quality categories using an analysis of variance or the Kruskal-Wallis test, as appropriate, and a Pearson χ2 or Fisher exact test was used for categorical variables. The primary analysis evaluated the association of diet quality with CHIP using multivariable logistic regression models adjusted for age, sex, current tobacco smoking, the presence of type 2 diabetes, Townsend deprivation index score (with positive values indicating areas with high material deprivation, negative values indicating areas with low material deprivation, and 0 indicating areas with overall mean values), and the first 10 principal components of genetic ancestry. The first 10 principal components were derived through an analysis of associated genome-wide common genotypes.
To reduce the risk of confounding across diet quality categories owing to differing health status, we performed sensitivity analyses that were also adjusted for prevalent hypertension or hyperlipidemia and systolic blood pressure, total cholesterol, triglyceride, and blood glucose levels. Additional sensitivity analyses also included models adjusted for body mass index (calculated as weight in kilograms divided by height in meters squared), alcohol use, interaction of sex and diet, interaction of type 2 diabetes and diet, and interaction of age and diet as well as models excluding individuals with stroke or heart failure. In additional exploratory analyses, we tested the association of individual dietary elements with CHIP using multivariable logistic regression analysis, and we examined the association between diet quality and individual CHIP genes using χ2 tests.
We next assessed the association of diet quality categories with incident CAD or death according to CHIP status using Cox proportional hazards models adjusted for age, sex, current tobacco smoking, and the presence of type 2 diabetes. The Cox proportional hazards assumption was tested with Schoenfeld residuals and was met. Follow-up began at blood sample collection; those who had CAD before the blood sample collection were excluded from the analysis. To assess for potential immortal time bias, we performed an additional sensitivity analysis excluding the subset of individuals selected for exome sequencing because of the availability of cardiac magnetic resonance imaging data. We examined whether observed CAD consequences differed by CHIP status using interaction testing.
Given the possibility of a type I error owing to multiple comparisons, findings from the secondary analyses were considered exploratory. Analyses were conducted using R software, version 4.0.2 (R Foundation for Statistical Computing), with 2-sided P < .05 considered statistically significant.
Results
Study Cohort
Among 44 111 total participants, the mean (SD) age at the time of blood sample collection was 56.3 (8.0) years, and 24 507 participants (55.6%) were female (Table 1). A total of 24 731 participants (56.1%) never smoked tobacco, 3970 participants (9.0%) currently smoked tobacco, and 15 277 participants (34.6%) formerly smoked tobacco. The mean (SD) body mass index was 27.3 (4.8). Type 2 diabetes was present in 1013 participants (2.3%), heart failure in 95 participants (0.2%), and chronic kidney disease in 136 participants (0.3%).
Table 1. Participant Characteristics.
| Characteristic | No. (%) | P value | |||
|---|---|---|---|---|---|
| Total cohort | Diet qualitya | ||||
| Unhealthy | Intermediate | Healthy | |||
| Participants, No. | 44 111 | 2271 | 38 552 | 3288 | NA |
| Age, mean (SD), y | 56.3 (8.0) | 56.3 (8.1) | 56.4 (8.0) | 56.0 (7.8) | .02 |
| Sex | <.001 | ||||
| Female | 24 507 (55.6) | 849 (37.4) | 21 285 (55.2) | 2373 (72.2) | |
| Male | 19 604 (44.4) | 1422 (62.6) | 17 267 (44.8) | 915 (27.8) | |
| Smoking status | <.001 | ||||
| Never | 24 731 (56.1) | 946 (41.7) | 21 810 (56.6) | 1975 (60.1) | |
| Previous | 15 277 (34.6) | 850 (37.4) | 13 310 (34.5) | 1117 (34.0) | |
| Current | 3970 (9.0) | 467 (20.6) | 3322 (8.6) | 181 (5.5) | |
| BMI, mean (SD) | 27.3 (4.8) | 28.1 (4.8) | 27.4 (4.8) | 26.0 (4.6) | <.001 |
| Prevalent morbidities | |||||
| Heart failure | 95 (0.2) | 10 (0.4) | 78 (0.2) | 7 (0.2) | .07 |
| Type 2 diabetes | 1013 (2.3) | 58 (2.6) | 884 (2.3) | 71 (2.2) | .62 |
| Chronic kidney disease | 136 (0.3) | 3 (0.1) | 128 (0.3) | 5 (0.2) | .07 |
| Townsend deprivation index score, mean (SD)b | −1.58 (2.80) | −1.27 (2.93) | 1.63 (2.77) | 1.17 (2.93) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
Participant characteristics were compared between diet quality categories using analysis of variance or a Kruskal-Wallis test, as appropriate; a Pearson χ2 or Fisher exact test was used for categorical variables.
A score of 0 represents an area with overall mean values. Positive values indicate areas with high material deprivation, and negative values indicate areas with low material deprivation.
Dietary Elements and Quality Categories
Healthy dietary elements, including cooked and raw fruits and vegetables, had median daily values of approximately 2 tbsp (IQR, 0-4 tbsp; range, 0-50 tbsp) of raw vegetables, 2 tbsp (IQR, 1-3 tbsp; range, 0-50 tbsp) of cooked vegetables, 2 pieces (IQR, 0-4 pieces; range, 0-50 pieces) of fresh fruit, and 0 pieces (IQR, 0-1 piece; range, 0-100 pieces) of dried fruit. In the UK Biobank intake surveys, meat and unhealthy dietary elements were scored as weekly intake frequency, in which the median scores for unhealthy dietary elements were 2 points (IQR, 0-4 points; range, 0-5 points) for processed meat, 1 point (IQR, 0-2 points; range, 0-5 points) for beef, 1 point (IQR, 0-2 points; range, 0-5 points) for pork, and 1 point (IQR, 1-1 point; range, 0-5 points) for lamb (eMethods in the Supplement).
Among all participants, 3288 individuals (7.5%) had a healthy diet, 38 552 (87.4%) had an intermediate diet, and 2271 (5.1%) had an unhealthy diet. Compared with participants with healthy and intermediate diets, those with unhealthy diets had lower scores for healthy dietary elements (eg, raw salad: mean [SD] score, 3.71 [3.23] in the healthy group vs 2.26 [2.16] in intermediate group vs 1.30 [1.14] in the unhealthy group) and higher scores for unhealthy dietary elements (eg, added salt: mean [SD] score, 1.25 [0.43] in the healthy group vs 1.55 [0.77] in the intermediate group vs 3.30 [0.45] in the unhealthy group) based on the study definitions (eTable 1 and eFigure 1 in the Supplement).
Healthy dietary elements were correlated with each other (r = 0.12-0.35), as were unhealthy dietary elements (r = 0.01-0.44). Healthy and unhealthy dietary elements were generally inversely correlated (r = −0.15 to 0). Few of these correlations met the criteria for statistical significance (eFigure 2 in the Supplement).
Prevalence of CHIP
Among participants in the study cohort, 2507 individuals (5.7%) had CHIP, and large clones were present in 1035 individuals (2.3%). Age was positively associated with the prevalence of CHIP (eFigure 3 in the Supplement). Overall, 49 different CHIP genes were identified, and the most commonly altered CHIP-associated genes were DNMT3A in 1600 participants (63.8%), TET2 in 391 participants (15.6%), ASXL transcriptional regulator 1 (ASXL1; OMIM 612990) in 156 participants (6.2%), tumor protein p53 (TP53; OMIM 191170) in 41 participants (1.6%), and protein phosphatase magnesium/manganese-dependent 1D (PPM1D; OMIM 605100) in 39 participants (1.6%), which was consistent with previous studies (eTable 2 in the Supplement). Most individuals with CHIP had only 1 gene variation (eFigure 4 in the Supplement).
Diet Quality and CHIP
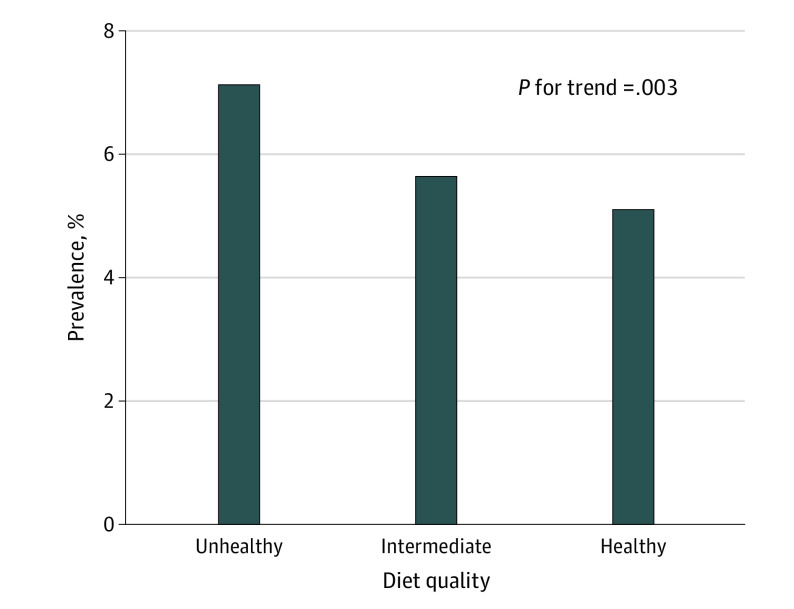
Clonal hematopoiesis of indeterminate potential was present in 162 of 2271 participants (7.1%) with an unhealthy diet, 2177 of 38 552 participants (5.7%) with an intermediate diet, and 168 of 3288 participants (5.1%) with a healthy diet. The unadjusted CHIP prevalence progressively decreased with healthier diet quality (7.1% among those with an unhealthy diet, 5.7% among those with an intermediate diet, and 5.1% among those with an unhealthy diet; P = .003 for trend) (Figure 2). This trend was similar for participants with large CHIP clones (77 of 2194 participants [3.4%] with an unhealthy diet, 897 of 37 655 participants [2.3%] with an intermediate diet, and 61 of 3227 participants [1.9%] with a healthy diet; P < .001 for trend) (eTable 3 and eFigure 5 in the Supplement).
Figure 2. Association Between Diet Quality and Prevalence of CHIP.
CHIP indicates clonal hematopoiesis of indeterminate potential.
Multivariable analysis adjusted for age, sex, the presence of type 2 diabetes, smoking status, Townsend deprivation index score, and the first 10 principal components of genetic ancestry indicated an association between unhealthy diet quality and CHIP prevalence, with an odds ratio (OR) of 1.25 (95% CI, 1.03-1.50; P = .02) compared with intermediate diet quality (eFigure 6 in the Supplement). Healthy diet quality compared with intermediate diet quality was associated with a lower CHIP prevalence (95% CI, 0.72-1.03; P = .16), but this difference was not statistically significant. These results were robust to a sensitivity analysis in which the model was adjusted for prevalent hypertension and hyperlipidemia as well as systolic blood pressure, total cholesterol, triglyceride, and blood glucose levels and to a separate sensitivity analysis in which individuals with ischemic stroke and heart failure were excluded (eTable 4 and eTable 5 in the Supplement). The findings were replicated using alternate coding of diet quality as a continuous variable (eTable 6 and eFigure 7 in the Supplement).
We next assessed whether diet quality categories were associated with specific CHIP-associated genes. We assessed DNMT3A, TET2, and ASXL1, the 3 genes with the most commonly observed CHIP-associated variations. Among 162 participants with CHIP who had an unhealthy diet, 95 individuals (58.6%) had DNMT3A variations, 26 individuals (16.0%) had TET2 variations, and 10 individuals (6.2%) had ASXL1 variations. No statistically significant differences were observed between the distribution of CHIP-associated genes across dietary classes using χ2 testing (eTable 7 and eFigure 8 in the Supplement).
Diet Quality and Adverse Cardiovascular Outcomes
A total of 3855 participants (8.7%) experienced incident CV events that occurred over a median of 10.0 years (IQR, 9.6-10.4 years). Of those, 276 of 2271 individuals (12.2%) had an unhealthy diet, 3382 of 38 552 individuals (8.8%) had an intermediate diet, and 197 of 3288 individuals (6.0%) had a healthy diet (eTable 8 in the Supplement). Improvement in diet quality category was associated with fewer incident events, with events occurring in 276 of 2271 participants (12.2%) with unhealthy diets, 3382 of 38 552 participants (8.8%) with intermediate diets, and 197 of 3288 participants (6.0%) with healthy diets (P < .001 for trend). This association remained after multivariable adjustment using a Cox proportional hazards model. Compared with an intermediate diet, an unhealthy diet was associated with a higher risk of CV events (hazard ratio [HR], 1.29; 95% CI, 1.14-1.46; P < .001), and a healthy diet was associated with a lower risk of CV events (HR, 0.75; 95% CI, 0.65-0.87; P < .001) (eFigure 9 in the Supplement).
Adverse Cardiovascular Outcomes by Diet Quality
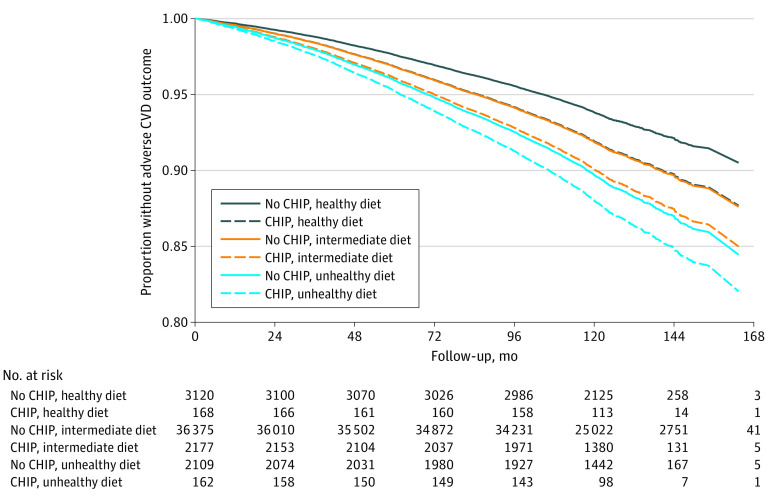
After multivariable adjustment, the presence of CHIP was independently associated with an increased risk of incident CV events (HR, 1.24; 95% CI, 1.11-1.39; P < .001) (Table 2). In a combined model, both CHIP and diet quality were independently associated with incident CV events. Compared with participants without CHIP who had an intermediate diet, those with CHIP who had an unhealthy diet had a higher risk of experiencing a CV event (HR, 1.52; 95% CI, 1.04-2.22). Those with CHIP who had an intermediate diet had a CV event risk (HR, 1.24; 95% CI, 1.10-1.40) similar to those without CHIP who had an unhealthy diet (HR, 1.29; 95% CI, 1.13-1.47). Individuals with CHIP who had a healthy diet also had a CV event risk (HR, 0.99; 95% CI, 0.62-1.58) that was similar to those without CHIP who had an intermediate diet (reference group). Those without CHIP who had a healthy diet had the lowest risk of a CV event (HR, 0.75; 95% CI, 0.64-0.87) (Figure 3; eFigure 10 in the Supplement). We observed similar relative diet quality–based stratification, albeit at systematically lower absolute event rates, among individuals with vs without CHIP. After excluding individuals with cardiac magnetic resonance imaging data, we found similar adverse CV event stratification by presence of CHIP and diet quality, suggesting the results were not observed because of immortal time bias (eTable 9 in the Supplement).
Table 2. Incident Cardiovascular Events and Deatha.
| Variable | HR (95% CI) | P value |
|---|---|---|
| Presence of CHIP | 1.24 (1.10-1.39) | <.001 |
| Diet | ||
| Unhealthy | 1.28 (1.13-1.24) | <.001 |
| Intermediate | 1 [Reference] | NA |
| Healthy | 0.75 (0.65-0.87) | <.001 |
| Age | 1.08 (1.08-1.14) | <.001 |
| Current smoking | 1.07 (1.0-1.14) | .04 |
| Type 2 diabetes | 2.08 (1.82-2.39) | <.001 |
| Female sex | 0.58 (0.54-0.62) | <.001 |
Abbreviations: CAD, coronary artery disease; CHIP, clonal hematopoiesis of indeterminate potential; HR, hazard ratio; NA, not applicable.
Multivariable-adjusted Cox proportional hazards model adjusted for age, sex, smoking status, diabetes status, and the first 10 principal components of genetic ancestry.
Figure 3. Survival Plot of Incident Cardiovascular Events and Deaths by CHIP Status and Diet Quality.
Time-to-event analysis of adverse cardiovascular events or death using a multivariate Cox proportional hazard model adjusted for presence of CHIP, diet quality, age, sex, smoking status, diabetes status, and first 10 principal components of genetic ancestry. CHIP indicates clonal hematopoiesis of indeterminate potential; CVD, cardiovascular disease.
Discussion
In this cohort study, an unhealthy diet that was low in fruits and vegetables and high in red meat was associated with an increased likelihood of the presence of CHIP, which is a known risk factor for both hematologic cancer and CVD.7,14 Among individuals with CHIP, those with healthier diets had the fewest incident CV events; however, this finding was not statistically significant. These results have several implications for understanding the associations between CHIP, health-associated behaviors, and CVD.
First, dietary patterns and content were found to be associated with the prevalence of CHIP, which is a novel CV risk factor of emerging importance. Deep targeted sequencing has indicated that most middle-aged adults have quiescent blood cell clones with cancer-associated variations; however, the additional factors and exposures that alter fitness and produce further clonal expansion to meet the criteria for CHIP are not yet understood.45,46 Putative mechanisms for diet include regulation of the bone marrow microenvironment, hematopoietic stem cell pool maintenance, and dietary associations with inflammation.47,48 Nutrients (such as vitamins B12, A, D, C, and folate) participate substantially in hematopoiesis.49,50 Vitamin C is a cofactor in the enzymatic activity of the tet family of DNA hydroxylases, of which TET2 is a negative regulator of hematopoietic stem cell self-renewal.51 In addition to consequences for hematopoietic stem cell regeneration and differentiation, a healthy diet high in fruits, vegetables, fiber, protein, and health-promoting fats is associated with lower inflammatory markers and healthy hematopoietic stem cells.47,52,53,54
Second, a diet low in fruits and vegetables and high in red and processed meats has been previously associated with increased CVD and hematologic cancer rates and, based on the present findings, has now been associated with increased CHIP prevalence, which is a shared risk factor for CVD and hematologic cancer.4,29,55,56,57,58,59,60,61 In vitro and animal studies have indicated that common inflammatory pathways can promote myeloid differentiation and clonal expansion of TET2 variant cell lines.38,62 Blood levels of interleukin 6, tumor necrosis factor α, leptin, and C-reactive protein are all increased in the sera of obese individuals.58,63 Increases in inflammatory cytokines such as tumor necrosis factor α have been reported to favor TET2-variant clonal hematopoiesis.62 In contrast, healthy diets high in fruits and vegetables have been associated with reduced cancer rates and lower systemic markers of inflammation.24,29,64,65,66
Third, the results of the current study support the notion that dietary pattern may mitigate the risk of excess CVD among individuals with CHIP. With the increasingly widespread use of next-generation sequencing in research studies and clinical settings, CHIP is increasingly being identified in asymptomatic individuals.12 Although there are several hypotheses regarding anti-inflammatory therapies, approved dedicated therapies for the reduction of CHIP-associated CVD are currently absent.10,11,13,14,67 Notably, this study found a graded additive association between improved diet quality and reduced incident CV events among individuals with CHIP. In the current analysis, a healthy diet was associated with a substantially lower CV event risk at the general population level. Given the increased CVD risk conferred by CHIP, the absolute risk reduction associated with diet modification may be greater among those with CHIP. Disclosure of an accumulation of CVD risk factors has been reported to promote greater adherence to dietary interventions.68 In a clinical context, when the absolute risk reduction of CVD is expected to be greater, individuals may be more willing to adopt new therapies and recommendations.68 Although prospective clinical trials will be necessary to establish efficacy, dietary interventions may provide a low-risk therapeutic intervention for individuals with CHIP.
Fourth, a dietary history survey is an inexpensive and widely available screening tool that may be used to optimize the diagnostic yield of CHIP testing. Conventional laboratory testing, including assessments of complete blood cell count and high-sensitivity C-reactive protein, does not accurately identify individuals with CHIP.7,11,12 However, the data from the present study suggest that dietary quality may be added to the constellation of risk factors for CHIP that may be used to enhance diagnostic yield.6,7,8,11,18,19,69 Requiring further study is male sex as a risk factor. In the primary analysis, consistent with previous studies, male sex was associated with an increased risk of CHIP; however, in sensitivity analyses that adjusted for systolic blood pressure, cholesterol, triglyceride, and blood glucose levels, sex was no longer a statistically significant covariate, although diet quality remained significant.7 In addition to smoking, dietary history represents an independent modifiable lifestyle factor that may help to prioritize individuals for CHIP testing.
Limitations
This study has limitations. First, the observational cross-sectional analysis cannot determine whether the association between diet quality and CHIP prevalence is causal. Longitudinal assessments of the evolution of CHIP are necessary to confirm the proposed temporal association between dietary patterns and the development of CHIP. Second, despite extensive covariate adjustment, the possibility of residual confounding from unmeasured variables cannot be ruled out. Third, this study was underpowered to test the consequences of specific dietary components as well as gene-specific associations. Fourth, although these observational analyses suggest that diet can be used to stratify CHIP-associated CV events, prospective randomized clinical trials are necessary to establish that improvements in diet quality reduce the CHIP-associated risk of CV events.
Conclusions
Clonal hematopoiesis of indeterminate potential is a newly recognized risk factor for CVD that is higher among individuals with unhealthy diets. Among individuals with CHIP, diet quality remains an independent risk factor that can be used to stratify CVD risk.
eMethods. Diet Quality Categorization, Diet Quality Treated as a Continuous Variable, Dietary Survey Response Scores, and Definition of Cardiovascular Events
eTable 1. Distribution of Dietary Elements
eTable 2. Frequency of CHIP Driver Genes
eTable 3. Association Between CHIP Prevalence and Diet Quality
eTable 4. Sensitivity Analysis Including Additional Covariates to Account for Potential Confounding in the Association Between CHIP Prevalence and Diet Quality
eTable 5. Sensitivity Analysis Showing Odds Ratios for the Presence of CHIP Predicted by Diet Quality After Excluding Patients With Prevalent Ischemic Stroke and Heart Failure From the Study Cohort
eTable 6. Odds Ratios for Presence of CHIP Predicted by a Continuous Dietary Score and Adjusted for Key Covariates
eTable 7. Frequency of CHIP Driver Gene Variations by Diet Quality Category
eTable 8. Incidence of Adverse Events by Diet Quality Category
eTable 9. Sensitivity Analysis of Incidence of Adverse Events by CHIP Presence and Diet Quality Category After Exclusion of Individuals With Cardiac MRI Data Available
eFigure 1. Distribution of Dietary Elements by Diet Quality Category and Overall
eFigure 2. Correlation Matrix of Dietary Element Intake Values
eFigure 3. CHIP Prevalence by Age Group
eFigure 4. Frequency of Individuals Harboring 1 or More CHIP Variation
eFigure 5. Prevalence of CHIP (VAF>0.02) and Large CHIP Clones (VAF>0.10) With Improved Diet Quality
eFigure 6. Odds Ratios for CHIP Presence Across Diet Quality Categories
eFigure 7. CHIP Prevalence by Diet Quality Treated as a Continuous Variable and Trichotomized
eFigure 8. CHIP Driver Variations in Diet Quality Categories
eFigure 9. Diet Quality Categories and Risk of Adverse Events With Simple Survival Analysis
eFigure 10. Forest Plot of Hazard Ratio of Incident Cardiovascular Events and Death Predicted by CHIP Status and Diet Quality Category
References
- 1.Hindy G, Aragam KG, Ng K, et al. Genome-wide polygenic score, clinical risk factors, and long-term trajectories of coronary artery disease. Arterioscler Thromb Vasc Biol. 2020;40(11):2738-2746. doi: 10.1161/ATVBAHA.120.314856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204-1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135-1143. doi: 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- 5.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110(8):1097-1108. doi: 10.1161/CIRCRESAHA.111.246876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477-2487. doi: 10.1056/NEJMoa1409405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488-2498. doi: 10.1056/NEJMoa1408617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472-1478. doi: 10.1038/nm.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khetarpal SA, Qamar A, Bick AG, et al. Clonal hematopoiesis of indeterminate potential reshapes age-related CVD: JACC review topic of the week. J Am Coll Cardiol. 2019;74(4):578-586. doi: 10.1016/j.jacc.2019.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abplanalp WT, Mas-Peiro S, Cremer S, John D, Dimmeler S, Zeiher AM. Association of clonal hematopoiesis of indeterminate potential with inflammatory gene expression in patients with severe degenerative aortic valve stenosis or chronic postischemic heart failure. JAMA Cardiol. 2020;5(10):1-6. doi: 10.1001/jamacardio.2020.2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bick AG, Pirruccello JP, Griffin GK, et al. Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation. 2020;141(2):124-131. doi: 10.1161/CIRCULATIONAHA.119.044362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bick AG, Weinstock JS, Nandakumar SK, et al. ; NHLBI Trans-Omics for Precision Medicine Consortium . Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature. 2020;586(7831):763-768. doi: 10.1038/s41586-020-2819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355(6327):842-847. doi: 10.1126/science.aag1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111-121. doi: 10.1056/NEJMoa1701719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natarajan P, Jaiswal S, Kathiresan S. Clonal hematopoiesis: somatic mutations in blood cells and atherosclerosis. Circ Genom Precis Med. 2018;11(7):e001926. doi: 10.1161/CIRCGEN.118.001926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King KY, Huang Y, Nakada D, Goodell MA. Environmental influences on clonal hematopoiesis. Exp Hematol. 2020;83:66-73. doi: 10.1016/j.exphem.2019.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019;366(6465):eaan4673. doi: 10.1126/science.aan4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honigberg MC, Zekavat SM, Niroula A, et al. Premature menopause, clonal hematopoiesis, and coronary artery disease in postmenopausal women. Circulation. 2021;143(5):410-423. doi: 10.1161/CIRCULATIONAHA.120.051775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bick AG, Popadin K, Thorball CW, et al. Increased CHIP prevalence amongst people living with HIV. medRxiv. Preprint posted online November 7, 2020. doi: 10.1101/2020.11.06.20225607 [DOI]
- 20.Bolton KL, Ptashkin RN, Gao T, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet. 2020;52(11):1219-1226. doi: 10.1038/s41588-020-00710-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coombs CC, Zehir A, Devlin SM, et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21(3):374-382. doi: 10.1016/j.stem.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zink F, Stacey SN, Norddahl GL, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130(6):742-752. doi: 10.1182/blood-2017-02-769869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; ARIC Study Investigators . Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690-1696. doi: 10.1016/j.jacc.2010.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseini B, Berthon BS, Saedisomeolia A, et al. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: a systematic literature review and meta-analysis. Am J Clin Nutr. 2018;108(1):136-155. doi: 10.1093/ajcn/nqy082 [DOI] [PubMed] [Google Scholar]
- 25.Sotos-Prieto M, Bhupathiraju SN, Mattei J, et al. Association of changes in diet quality with total and cause-specific mortality. N Engl J Med. 2017;377(2):143-153. doi: 10.1056/NEJMoa1613502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khera AV, Emdin CA, Drake I, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375(24):2349-2358. doi: 10.1056/NEJMoa1605086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dias JA, Wirfalt E, Drake I, et al. A high quality diet is associated with reduced systemic inflammation in middle-aged individuals. Atherosclerosis. 2015;238(1):38-44. doi: 10.1016/j.atherosclerosis.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 28.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48(4):677-685. doi: 10.1016/j.jacc.2006.03.052 [DOI] [PubMed] [Google Scholar]
- 29.Li J, Lee DH, Hu J, et al. Dietary inflammatory potential and risk of cardiovascular disease among men and women in the U.S. J Am Coll Cardiol. 2020;76(19):2181-2193. doi: 10.1016/j.jacc.2020.09.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reedy J, Krebs-Smith SM, Miller PE, et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr. 2014;144(6):881-889. doi: 10.3945/jn.113.189407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penuelas J, Krisztin T, Obersteiner M, et al. Country-level relationships of the human intake of N and P, animal and vegetable food, and alcoholic beverages with cancer and life expectancy. Int J Environ Res Public Health. 2020;17(19):7240. doi: 10.3390/ijerph17197240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keaver L, Ruan M, Chen F, et al. Plant- and animal-based diet quality and mortality among US adults: a cohort study. Br J Nutr. 2020;1-11. doi: 10.1017/S0007114520003670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diallo A, Deschasaux M, Latino-Martel P, et al. Red and processed meat intake and cancer risk: results from the prospective NutriNet-Santé cohort study. Int J Cancer. 2018;142(2):230-237. doi: 10.1002/ijc.31046 [DOI] [PubMed] [Google Scholar]
- 34.Fung TT, Hu FB, McCullough ML, Newby PK, Willett WC, Holmes MD. Diet quality is associated with the risk of estrogen receptor–negative breast cancer in postmenopausal women. J Nutr. 2006;136(2):466-472. doi: 10.1093/jn/136.2.466 [DOI] [PubMed] [Google Scholar]
- 35.Norat T, Lukanova A, Ferrari P, Riboli E. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer. 2002;98(2):241-256. doi: 10.1002/ijc.10126 [DOI] [PubMed] [Google Scholar]
- 36.Tavani A, La Vecchia C, Gallus S, et al. Red meat intake and cancer risk: a study in Italy. Int J Cancer. 2000;86(3):425-428. doi: [DOI] [PubMed] [Google Scholar]
- 37.Herault A, Binnewies M, Leong S, et al. Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. Nature. 2017;544(7648):53-58. doi: 10.1038/nature21693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meisel M, Hinterleitner R, Pacis A, et al. Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature. 2018;557(7706):580-584. doi: 10.1038/s41586-018-0125-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203-209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Hout CV, Tachmazidou I, Backman JD, et al. ; Geisinger-Regeneron DiscovEHR Collaboration; Regeneron Genetics Center . Exome sequencing and characterization of 49,960 individuals in the UK Biobank. Nature. 2020;586(7831):749-756. doi: 10.1038/s41586-020-2853-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.US Department of Health and Human Services; US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th ed. US Department of Health and Human Services and US Department of Agriculture; December 2015. Accessed March 15, 2020. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
- 42.Liu B, Young H, Crowe FL, et al. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011;14(11):1998-2005. doi: 10.1017/S1368980011000942 [DOI] [PubMed] [Google Scholar]
- 43.Greenwood DC, Hardie LJ, Frost GS, et al. Validation of the Oxford WebQ online 24-hour dietary questionnaire using biomarkers. Am J Epidemiol. 2019;188(10):1858-1867. doi: 10.1093/aje/kwz165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toft U, Kristoffersen LH, Lau C, Borch-Johnsen K, Jorgensen T. The Dietary Quality Score: validation and association with cardiovascular risk factors: the Inter99 study. Eur J Clin Nutr. 2007;61(2):270-278. doi: 10.1038/sj.ejcn.1602503 [DOI] [PubMed] [Google Scholar]
- 45.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. 2016;7:12484. doi: 10.1038/ncomms12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson CJ, Papula AL, Poon GYP, et al. The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science. 2020;367(6485):1449-1454. doi: 10.1126/science.aay9333 [DOI] [PubMed] [Google Scholar]
- 47.Bujko K, Cymer M, Adamiak M, Ratajczak MZ. An overview of novel unconventional mechanisms of hematopoietic development and regulators of hematopoiesis—a roadmap for future investigations. Stem Cell Rev Rep. 2019;15(6):785-794. doi: 10.1007/s12015-019-09920-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nahrendorf M, Swirski FK. Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res. 2015;116(5):884-894. doi: 10.1161/CIRCRESAHA.116.303550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cabezas-Wallscheid N, Buettner F, Sommerkamp P, et al. Vitamin A–retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell. 2017;169(5):807-823. doi: 10.1016/j.cell.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 50.Cortes M, Chen MJ, Stachura DL, et al. Developmental vitamin D availability impacts hematopoietic stem cell production. Cell Rep. 2016;17(2):458-468. doi: 10.1016/j.celrep.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cimmino L, Dolgalev I, Wang Y, et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell. 2017;170(6):1079-1095. doi: 10.1016/j.cell.2017.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burns SS, Kapur R. Putative mechanisms underlying cardiovascular disease associated with clonal hematopoiesis of indeterminate potential. Stem Cell Reports. 2020;15(2):292-306. doi: 10.1016/j.stemcr.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pietras EM, Mirantes-Barbeito C, Fong S, et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol. 2016;18(6):607-618. doi: 10.1038/ncb3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh RK, Chang HW, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73. doi: 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baena Ruiz R, Salinas Hernandez P. Diet and cancer: risk factors and epidemiological evidence. Maturitas. 2014;77(3):202-208. doi: 10.1016/j.maturitas.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 56.English DR, MacInnis RJ, Hodge AM, Hopper JL, Haydon AM, Giles GG. Red meat, chicken, and fish consumption and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1509-1514. [PubMed] [Google Scholar]
- 57.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288(20):2569-2578. doi: 10.1001/jama.288.20.2569 [DOI] [PubMed] [Google Scholar]
- 58.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104-1114. doi: 10.1161/CIRCULATIONAHA.115.020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83(2):456S-460S. doi: 10.1093/ajcn/83.2.456S [DOI] [PubMed] [Google Scholar]
- 60.Swerdlow DI, Holmes MV, Kuchenbaecker KB, et al. ; Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium . The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379(9822):1214-1224. doi: 10.1016/S0140-6736(12)60110-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wood AD, Strachan AA, Thies F, et al. Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Br J Nutr. 2014;112(8):1341-1352. doi: 10.1017/S0007114514001962 [DOI] [PubMed] [Google Scholar]
- 62.Abegunde SO, Buckstein R, Wells RA, Rauh MJ. An inflammatory environment containing TNFα favors Tet2-mutant clonal hematopoiesis. Exp Hematol. 2018;59:60-65. doi: 10.1016/j.exphem.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 63.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505-522. doi: 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haring B, Reiner AP, Liu J, et al. Healthy lifestyle and clonal hematopoiesis of indeterminate potential: results from the Women’s Health Initiative. J Am Heart Assoc. 2021;10(5):e018789. doi: 10.1161/JAHA.120.018789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hematdar Z, Ghasemifard N, Phishdad G, Faghih S. Substitution of red meat with soybean but not non-soy legumes improves inflammation in patients with type 2 diabetes; a randomized clinical trial. J Diabetes Metab Disord. 2018;17(2):111-116. doi: 10.1007/s40200-018-0346-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shah B, Newman JD, Woolf K, et al. Anti-inflammatory effects of a vegan diet versus the American Heart Association–recommended diet in coronary artery disease trial. J Am Heart Assoc. 2018;7(23):e011367. doi: 10.1161/JAHA.118.011367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sidlow R, Lin AE, Gupta D, et al. The clinical challenge of clonal hematopoiesis, a newly recognized cardiovascular risk factor. JAMA Cardiol. 2020;5(8):958-961. doi: 10.1001/jamacardio.2020.1271 [DOI] [PubMed] [Google Scholar]
- 68.Navar AM, Wang TY, Mi X, et al. Influence of cardiovascular risk communication tools and presentation formats on patient perceptions and preferences. JAMA Cardiol. 2018;3(12):1192-1199. doi: 10.1001/jamacardio.2018.3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dharan NJ, Yeh P, Bloch M, et al. ; ARCHIVE Study Group. Age-related clonal haematopoiesis is more prevalent in older adults with HIV: the ARCHIVE study. medRxiv. Preprint posted online November 22, 2020. doi: 10.1101/2020.11.19.20235069 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Diet Quality Categorization, Diet Quality Treated as a Continuous Variable, Dietary Survey Response Scores, and Definition of Cardiovascular Events
eTable 1. Distribution of Dietary Elements
eTable 2. Frequency of CHIP Driver Genes
eTable 3. Association Between CHIP Prevalence and Diet Quality
eTable 4. Sensitivity Analysis Including Additional Covariates to Account for Potential Confounding in the Association Between CHIP Prevalence and Diet Quality
eTable 5. Sensitivity Analysis Showing Odds Ratios for the Presence of CHIP Predicted by Diet Quality After Excluding Patients With Prevalent Ischemic Stroke and Heart Failure From the Study Cohort
eTable 6. Odds Ratios for Presence of CHIP Predicted by a Continuous Dietary Score and Adjusted for Key Covariates
eTable 7. Frequency of CHIP Driver Gene Variations by Diet Quality Category
eTable 8. Incidence of Adverse Events by Diet Quality Category
eTable 9. Sensitivity Analysis of Incidence of Adverse Events by CHIP Presence and Diet Quality Category After Exclusion of Individuals With Cardiac MRI Data Available
eFigure 1. Distribution of Dietary Elements by Diet Quality Category and Overall
eFigure 2. Correlation Matrix of Dietary Element Intake Values
eFigure 3. CHIP Prevalence by Age Group
eFigure 4. Frequency of Individuals Harboring 1 or More CHIP Variation
eFigure 5. Prevalence of CHIP (VAF>0.02) and Large CHIP Clones (VAF>0.10) With Improved Diet Quality
eFigure 6. Odds Ratios for CHIP Presence Across Diet Quality Categories
eFigure 7. CHIP Prevalence by Diet Quality Treated as a Continuous Variable and Trichotomized
eFigure 8. CHIP Driver Variations in Diet Quality Categories
eFigure 9. Diet Quality Categories and Risk of Adverse Events With Simple Survival Analysis
eFigure 10. Forest Plot of Hazard Ratio of Incident Cardiovascular Events and Death Predicted by CHIP Status and Diet Quality Category