Abstract
Child malnutrition (CM) is a global public health problem. It contributes to poor health in one in four children under five years worldwide and causes serious health problems in children including stunted, wasted, and overweight growth. These serious public health issues lead to a higher chance of living in poverty in adulthood and consequently reduced economic productivity and increases the serious national and international burden. Currently, there is no meaningful therapeutic intervention of CM, and the use of different therapeutic foods have shown exceedingly poor outcomes among supplemented malnourished children. The role of metabolites and lipids has been extensively recognized as early determinants of child health, but their contribution in CM and its pathobiology are poorly understood. This perspective provides a most recent update on these aspects. After briefly introducing the disciplines of metabolomics and lipidomics, we describe a mass spectrometry-based metabolic workflow for analysis of both metabolites and lipids and summarize several recent applications of metabolomics and lipidomics in CM. Finally, we discuss the future directions of the field towards the development of meaningful interventions for CM through metabolomics and lipidomics advances.
Graphical Abstract
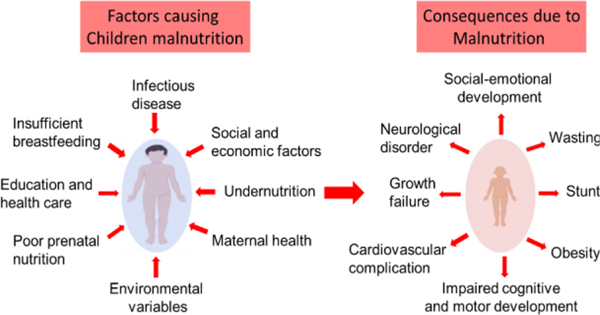
An overview of malnutrition
Malnutrition is a life threating condition for children in developing countries, which includes undernutrition (stunting and wasting) and overnutrition (obesity). According to a recent combined report by the United Nations Children’s Fund (UNICEF), the World Health Organization (WHO) and the World Bank Group, stunting affected approximately 22.2% (151 million) of children under 5 years of age, while wasting continued to threaten the lives of approximately 7.5% (501 million) of children under the age of 5. Furthermore, an estimated 5.6% (38 million) of children under the age of 5 globally were overweight in 2017 (UNICEF/WHO/World Bank Group, 2018)1. Moreover, Child malnutrition (CM) is directly or indirectly associated with 35% of deaths among children under five years worldwide2. Survivors with malnutrition suffer from numerous health problems including growth failures, cardiovascular complications, neurological disorders, impaired cognitive and motor development, social-emotional development, and increased risk of early morbidity and mortality3–5. Numerous factors are associated with CM including poor nutrition, maternal health, parental history, socio-economic status, microbiome and other pathogenic infections, education, healthcare, food insecurity, and other environmental variables (Figure 1)6–11. Low- and middle-income countries people who developed childhood malnutrition tending to become prone to many health related complexity including obesity and diabetics in adulthood (Ivana 2012 & Tzioumis 2014). Long-lasting impacts of malnutrition exacerbates the costs for the public health system and the poverty of the nation (Martins 2011).
Figure 1.
Some key factors associated with child malnutrition and its consequences in children health.
Recently, nutrient-based food supplementation, such as lipid-based nutrient supplements or Ready-To-Use Therapeutic Food (RUTFs), micronutrients such as vitamin A, zinc, iron individually or in combinations have been conducted on diverse CM studies9,12–15. Unfortunately, these interventions resulted in poor outcomes such as suboptimal growth (Choudhury 2016) among supplemented malnourished children15–18. Current knowledge regarding poor outcome by food supplementation in malnourished children is not quite understood (Uchiyama 2018 & Bhutta 2017). However, several studies recently emphasis that dysregulated epigenetics marks that were established at germ cells under malnourished condition could be transmitted and maintained in the subsequent offspring via the paternal lineage and impacts metabolism including lipids, thereby influencing health and disease risk (Uchiyama 2018 and Martinez 2014). Furthermore, failure of food supplementation in malnourished systems is expected because critical health problems due to malnutrition onset in most cases within the first two years of life and can persist even if nutrition becomes adequate later in life (Uchiyama 2018, Carlon SE 2009, Martinez 2014). Therefore, further research and analytical innovation is needed to improve our understanding of the pathobiology of severe malnutrition towards the development of meaningful therapeutic interventions for malnourished children, especially the molecular mechanisms underlying childhood health in the malnourished state.
Recently, discovery of metabolite or lipid-based biomarkers through metabolomics or lipidomics has increased our understanding of pathophysiology and improved therapeutic strategies for the development of precision medicine for many diseases with unmet challenges19–24. For example, significantly upregulated levels of corticosterone25 and sarcosine in prostate cancer20, 27-hydroxycholesterol levels in hypercholesterolemia and breast cancer26, and elevated levels of branched chain amino acids (BCAA) in pancreatic adenocarcinoma21 and myeloid leukemia27 are now linked with cancer progression and metastasis. Recently mass spectrometry-based metabolomics and lipidomics studies demonstrated that malnutrition results in serious dysfunction of the metabolome and lipidome profiles in malnourished children compared to healthy children and thus opens an emerging avenue to develop a new interventions for improved management of CM through identification of metabolic fingerprints7,23,28–30.
An overview of metabolomics, lipidomics and Mass Spectrometry (MS) techniques
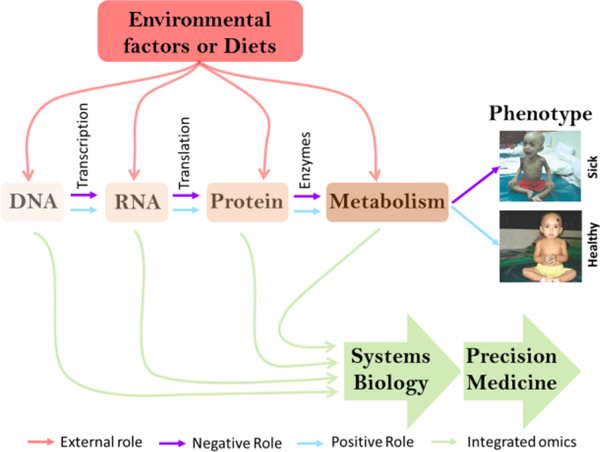
Metabolomics and lipidomics are newly emerging ‘Omics approaches in bioscience towards the development of precision therapeutic and clinical interventions (Figure 2). Metabolomics launched in 1999 and Lipidomics emerged in 2003 and both ‘Omics approaches have greatly advanced since that initial work, largely due to the development of mass spectrometry and its allied techniques19,31. Metabolomics involves the holistic characterization of small molecules, called metabolites, such as amino acids, organic acids, nucleotides, carbohydrates, and secondary metabolites in biological systems19,31. On the other hand, lipidomics elucidates the complete set of lipids such as free fatty acids (FFAs), saturated fatty acids (SFAs), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), glycerolipids, glycerophospholipids, sphingolipids, and sterols in a given biological system32.
Figure 2.
Model of precision medicine development through multi-omics approaches. Multi-omics includes genomics (study of gene), transcriptomics (study of gene expression), proteomics (study of protein), and metabolomics (study of small molecules metabolites or lipids). Systems biology followed by precision medicine incorporates comprehensive knowledge of multi-omics and external factors towards suitable therapy development.
Metabolites and lipids represent both the input and the output of cellular and physiological processes and their levels are therefore exquisitely sensitive to a wide range of perturbations linked to disease, genetic modification and environmental conditions19,33. Precision medicine or personalized medicine is an active component of today’s medical practice for protecting health and treating disease through understanding individual patient information. Metabolite or lipid levels represent both the downstream output of the genomics (study of the genome), epi-genomics (study of the chromatin occupancy), transcriptomics (study of the gene expression or transcript), and proteomics (study of protein expression), and the upstream input from the life style and/or environment, and therefore the metabolome and lipidome demonstrate an exciting inclusive tool for precision medicine initiatives (Figure 2). Recently, systems biology or integrated ‘Omics approaches have evolved as a scientific discipline to obtain a holistic understanding of global metabolism for the development of personalized medicine19,34.
Recent applications of Mass Spectrometry-based techniques such as direct MS analysis and chromatography coupled to MS analysis in metabolomics and lipidomics have made tremendous advances in biomedical research35–37. Numerous techniques and tools have been incorporated in modern mass spectrometry for metabolomics and lipidomics studies32,35,36,38. For example, direct MS technologies such as Time-of-Flight mass spectrometers (TOF-MS), orbitrap MS, Fourier transform ion cyclotron mass spectrometers (FT-ICR-MS), paper spray ionization mass spectrometry (PSI-MS) have broaden the metabolomics and lipidomics research by providing high spectral resolution and mass accuracy as well as simplified sample collection. Although direct MS techniques have been used for the analysis of large number of analytes, they have shown several limitations including susceptibility to ion suppression or enhancement, less efficient to distinguish unique metabolite ions from adduct and product ions as well as has inability to differentiate isomers39–42. On the other hand, chromatography coupled with MS provides high level of confidence, accuracy, and advantages such as reduction of matrix effects and ion suppression, isomer separation, retention time index for metabolite annotation, and accurate quantification of metabolite or lipid. For example, Gas Chromatography coupled to Mass Spectrometry (GC-MS) is highly advantageous for both volatile and nonvolatile complex mixture analyses following derivatization. GC interfaced to several mass analyzers including single quadrupole MS detectors, TOF-MS, or triple quadrupole MS and has been efficiently employed to study metabolome and lipidome of diverse biological studies32,35–38. Unfortunately, GC-MS is less efficient to detect the large number of polar metabolites expected. Therefore, efficient separation and detection of these compounds require liquid phase separation tools such as liquid chromatography (LC) coupled to MS.
Among all, Ultra High Performance Liquid Chromatography coupled with High Resolution Mass Spectrometry (UHPLC-HRMS) is a gold standard analytical tool for metabolomics or lipidomics studies due to its high-throughput, reproducibility, dynamic range of metabolome or lipidome coverage, and high mass accuracy24,43,44. UHPLC-HRMS-based metabolomics and lipidomics can be either untargeted, in which case global metabolites or lipid profiling is usually performed, or targeted, in which metabolites of interest are already known and directly analyzed45,46. The decision to perform an untargeted analysis versus a targeted analysis is typically decided based on whether there is a hypothesis. Untargeted metabolomics is often considered hypothesis generating because it aims to analyze as many metabolites as possible and then utilize bioinformatics to identify metabolites associated with the study population. This type of analysis requires defining the study population very well to ensure a well-represented sample set that then builds a hypothesis based on the differential metabolites. On the other hand, a targeted analysis typically begins with a hypothesis that suggests a key set of metabolites is altered and then uses the study population to investigate whether that smaller set of metabolites is different.
CM is a vast area of research and onset of CM is very complex. UHPLC-HRMS based metabolomics or lipidomics can be utilized to investigate this serious global public health problem towards the discovery of metabolic fingerprints for the early detection and management of CM. Recently, limited scale targeted metabolomics investigations into CM have been conducted using LC-MS and uncovered the remarkable potential of metabolites and lipids as a therapeutic interventions for malnourished children7,28,29,47. Future studies examining the untargeted global metabolome and lipidome of malnourished and healthy children or corresponding animal models will be warranted to address CM.
Metabolomics and lipidomics workflow
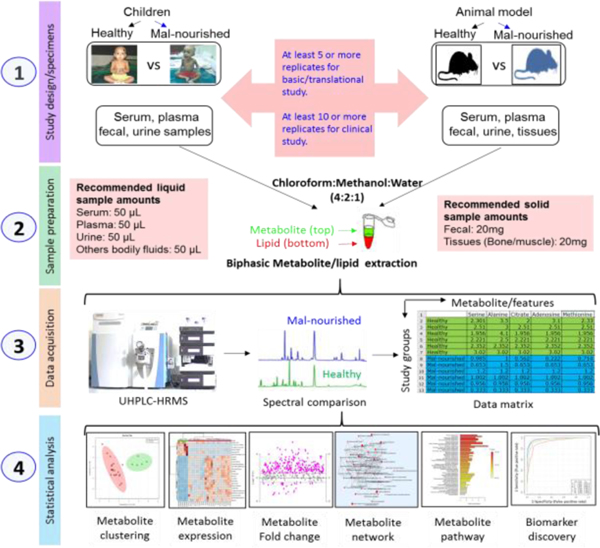
A typical workflow for metabolomics or lipidomics study includes four major steps such as study design (1), sample preparation (2), data acquisition (3), and statistical analysis (4) (Figure 3). Prior to any metabolomics or lipidomics study, proper study design is critically important. Metabolites and/or lipids change very rapidly in response to subtle changes in the environment. Therefore, it is essential to minimize any biological, technical or experimental variability while conducting metabolomics research towards the development of precision medicine. Consistency is one key to minimizing variation. To achieve reproducibility of metabolomics result, performing every single step the same as the first time is crucial. Prior to any metabolic profile analysis, understanding study types (basic or translational or clinical studies), study question, sample replication (at least 5 or more for basic translational study and at least 10 or more clinical study), sample randomization and stratification, sample categories (cell, microbes, parasites, tissue, organ, biofluids such as whole blood, plasma, serum, urine, saliva, seminal fluids, amniotic fluids, cerebrospinal fluid, synovial fluid, etc), sampling conditions (solid, suspension, adherent, trypsinizing, scratching, etc), sample preprocessing and storage conditions (temperature, biobanking, aliquoting, etc) are very important. Several extensive resources and reviews that have been published recently on study design of metabolomics and lipidomics19,48,49. In this section, we summarized key considerations during experimental design for global metabolomics and lipidomics using UHPLC-HRMS in biomedical research (Figure 3).
Figure 3.
High-throughput metabolomics and lipidomics workflow using UHPLC-HRMS. (Step 1) study design, which includes selection of study subjects for example human or animal, sample types and replications. (Step 2) sample preparation includes most commonly used biphasic (metabolite and lipid) extraction method following modified Folch methods (chloroform/methanol/water = 2:1:1). (Step 3) data collection and data matrix generation. (Step 4) Statistical analysis and data visualization includes clustering analysis by multivariate score plot and heatmap analysis, fold change analysis, metabolic pathway, and biomarker discovery.
The collected samples under proper experimental design are then prepared for metabolite/lipid extraction. Extraction methods for global metabolites and lipids have been extensively reviewed recently38. Most MS-based metabolomics or lipidomics techniques utilize biological extracts except imaging MS which utilize tissue slices50,51. In this workflow, we suggest most commonly used biphasic (metabolite and lipid) extraction method following modified Folch methods (chloroform/methanol/water = 2:1:1) (Figure 3) that our lab and others successfully utilized for diverse research in bioscience43,52,53. Quality control (QC) methods are critically needed for sample preparation that include proper internal standards (isotopically labeled) to account for extraction variations and measurement of total protein concentration (cells and tissue samples), total DNA/RNA concentration (cell and tissue), wet/dry tissue weight, or fluid volume are commonly utilize for pre/post-data normalization43.
The properly extracted metabolome or lipidome are then prepared for suitable MS analysis. During MS data acquisition, global LC-MS based metabolomics and lipidomics are commonly utilized for the analysis of metabolome or lipidome solutions36,38. On the other hand, tissue slices can be directly submitted to MS imaging45,51. The most popular MS ionization techniques for metabolomics or lipidomics are Electrospray ionization (ESI), Atmospheric Pressure Chemical Ionization (APCI), Atmospheric Pressure Photoionization (APPI), Matrix-assisted Laser Desorption/Ionization (MALDI), Desorption ESI (DESI), and Paper Spray Ionization Mass Spectrometry (PSI-MS). Of these approaches, ESI is by far the most common approach when coupled with LC for both metabolite and lipid analyses35,36,38. After MS data acquisition, spectral data are processed for deisotoping in order to generate a data matrix, followed by identification and quantification of metabolites or lipids species using data processing software or tools compatible with analytical techniques (eg. global, LC-MS, or imaging) (Mzmine, XCMS, Lipidsearch)54,55. The data matrix which contains both qualitative and quantitative information for metabolites or lipids are then ready for further statistical analysis to explore metabolite or lipid-based biomarker discovery and its underlying mechanism related to disease state (Figure 3).
Although, this section mainly focused UHPLC-HRMS technology, but, GC-MS or NMR (Nuclear Magnetic Resonance) technology-based metabolomics or lipidomics can be equally applied in children malnutrition following this workflow. Considering range of sensitivities and detection capacities of metabolomic technologies, LC-MS is highly sensitive and show higher detection capacity than GC-MS or NMR (Wishart 2011). However, elucidating some lipid isomers in complex mixtures by LC-MS is still problematic. Recently, LC-IMS-MS, integrated ion mobility spectrometry (IMS) between LC and MS separations, emerge as an efficient technique to identify isomeric changes of lipids occurring under complex mixtures (Kyle 2016 Analyst). Therefore, IMS could be a potentially important tools coupled with LC-MS to discern lipid analysis of malnutrition studies. On the other hand, although NMR is less sensitive but it is highly specific, reproducible and has ability to detect low concentrations of metabolites from a distinct specimens (Mahmud 2015, 2014 & Emmanuel 2018).
Applications in Children malnutrition
CM is a complex public health problem worldwide due to its severity and heterogeneity; however, MS-based metabolomics and lipidomics approaches recently offer an exciting opportunity to understand this complexity (Table 1). Moreau et al. recently reported an association of targeted metabolic signatures with growth and neurocognitive outcomes of nine-month-old children. Study identified that higher concentrations of hydroxy-sphingomyelin and essential amino acids and lower levels of acylcarnitines and bile acid conjugation are associated with improved health outcome of childen47. Semba et al. report a targeted metabolomics approach to measure 139 serum metabolites including amino acids, glycerophospholipids, sphingolipids, and other metabolites in 313 children based on Height-for-age Z-scores (HAZ) and weight-for-height Z-scores, aged 12–59 months, from rural Malawi, of whom 60% were stunted. Using LC-MS/MS, this study identified lower serum concentrations of all nine essential amino acids (tryptophan, isoleucine, leucine, valine, methionine, threonine, histidine, phenylalanine, lysine) and also lower serum concentrations of conditionally essential amino acids (arginine, glycine, glutamine), non-essential amino acids (asparagine, glutamate, serine), and six different sphingolipids compared with non-stunted children. This study also found an association of stunting with alterations in serum glycerophospholipid concentrations. Overall, Semba et al findings support the idea that children with a high risk of stunting may not be receiving an adequate dietary intake of essential amino acids and choline, an essential nutrient for the synthesis of sphingolipids and glycerophospholipids29. It was recognized that amino acids particularly leucine, tryptophan, and lysine are found very critical for childhood early developmental stages, where reduced levels of these amino acids was associated with stunted growth of children. Therefore, the metabolome findings from Semba et al could provide important insight towards the understanding of the management strategies for CM.
Table 1.
Metabolomic studies performed in children malnutrition studies.
| Study Design | Technique | Results and implications | Resources |
|---|---|---|---|
| Plasma samples were collected from children aged at 9 and 36 months (n=130)47 | LC-MS/MS, targeted | Revealed for the first time that metabolic signature is associated with neurodevelopment in an undernourished child cohort. Study identified 34 metabolites were associated with Height-for-Age z-score (HAZ), 37 with ΔHAZ (average HAZ), and 34 with full-scale IQ (e intelligence quotient). | 7,23,28–30,47,57 |
| Serum samples of children (stunted vs non-stunted) aged <5.0 years23,28,29 | LC-MS, targeted | Uncover the decreased concentrations of circulating amino acids including essential amino acids, conditionally essential amino acids, non-essential amino acids, phosphotidylcholine, and sphingolipids in children with stunted growth compared with nonstunted (Semba 2016). Unravel lower level of w3 and w6 long-chain PUFAs, carnitine, nerosteroids, and glutathione metabolism in stunted children (Semba 2017). | |
| Serum samples of children (Kwashiorkor vs marasmus vs community controls) aged <5.0 years56 | LC-MS, targeted | Clustering analysis identified children with SAM (kwashiorkor) were metabolically distinct from those with marasmus, and were more prone to severe metabolic disruptions. Several amino acids and biogenic amines, including those of the kynurenine-tryptophan pathways were highly reduced in SAM compared to other study groups. | |
| Serum samples of children (aged <5.0 years) with and without environmental enteric dysfunction (EED)30 | LC-MS, targeted | New insights into the relationship between gut permeability and alteration of serum metabolites (Semba 2016). | |
| Plasma samples of Children (aged <2.0 years)7 Low income countries vs high-income countries children 1. Gut microbes of malnourished vs well-nourished children Healthy vs stunted children |
LC-MS, targeted | For the first time, this study identified reduced metabolite production capabilities in children from low income countries compared with a high-income country. Consistent with Semba et al this study also identified that stunted children had reduced levels of essential amino acids as well as lower ratio of tryptophan to other neutral amino acids compared to the healthy group. Moreover, this study also identified that gut microbiota is associated with malnutrition and reduced amino acid levels. (kumar 2018) | |
| Fecal samples of children: malnourished with cholera, malnourished without cholera, and healthy57 | HPLC | Various short chain fatty acid metabolites (SCFA) such as acetate, propionate, valerate, and butyrate concentration were significantly lower in malnourished with cholera than that in the malnourished without cholera and healthy condition (Monira 2010) |
Semba et al. also evaluated 677 targeted serum metabolites in a cross-sectional study of 400 Malawian children aged 12–59 month, of whom 62% were stunted. Using LC-MS/MS following volcano plot based differential statistical analysis tools, this study revealed a potential association of stunted growth of children with lower serum concentrations of 1) ω−3 (n–3) and ω−6 (n–6) polyunsaturated fatty acids (PUFAs), 2) sulfated neurosteroids, which play a role in brain development, 3) carnitine, a conditionally essential nutrient with an important role in the carnitine shuttle for the metabolism of fatty acids and energy production, and 4) gamma-glutamyl amino acids, which represent an altered gamma-glutamyl cycle of glutathione metabolism. This study also identified significantly higher serum concentrations of five biomarkers such as cotinine, catechol sulfate, 4-vinylphenol sulfate; trigonelline (N’-methylnicotinate), and N-(2-furoyl) glycine associated to cigarette smoke exposure28. Remarkably, this metabolomics and lipidomics finding steered toward a clinical trial in order to explore efficacy of metabolic intervention towards the management of CM.
Furthermore, targeted LC-MS/MS was used by Giovanni et al in a study of 40 severe acute malnourished (SAM) Malawian children aged 9–59 month, of whom 21 with kwashiorkor (characterized by nutritional edema and metabolic disturbances, including hypoalbuminemia and hepatic steatosis) and 19 with marasmus (characterized by severe wasting) or living in the community (n = 157; 78 stunted and 79 non-stunted). This study identified significantly lower levels of several amino acids and biogenic amines, including those of the kynurenine-tryptophan metabolic pathway. This study summarized that children with kwashiorkor were metabolically distinct from those with marasmus, and were more prone to severe metabolic disruptions. Both SAM conditions (kwashiorkor and marasmus) showed distinct metabolic profiles from stunted and non-stunted controls, even after clinical stabilization, therefore, suggesting critical outcomes for future a SAM treatment plan56.
Moreover, Kumar et al. examined genome-scale metabolic modeling to understand the metabolic functions and interactions between individual species in the gut microbiota during healthy and malnutrition states of Bangladeshi children under the age of 5. Previously, it was reported persistent gut microbiota immaturity in Bangladeshi malnourished children8. Using targeted LC-MS/MS, Kumar et al. identified the gut microbial dysbiosis, which was associated with malnutrition and reduced plasma amino acid levels in Bangladeshi malnourished children. Therefore, this study provided a framework for future studies towards further characterization of gut microbial metabolic capabilities and their contribution to malnutrition7.
Conclusions and future perspectives
Nutrition has intense effects on human health throughout a person’s lifetime. Metabolome and lipidome at suitable physiological levels represent the early determinants of child health, and most importantly their levels are associated with cognitive and social development of children4,5. Metabolomics in a nutritional context has already been employed to investigate and characterize relevant features of the metabolic phenotype. As we discussed, LC-MS based targeted metabolic profiling studies of child malnutrition revealed some potential interventions for the reduction of child stunting and SAM7,28,47. Currently Ready-to-Use therapeutic food (RTUF), mostly based on metabolite or lipid supplementation, is a commonly used intervention for malnourished children12,58. Unexpectedly, RTUF led to exceedingly poor outcomes among supplemented malnourished children7,16–18,29. Although targeted metabolic studies based on LC-MS in child malnutrition provide some potential interventions for severe malnutrition, however, global profile of metabolome or lipidome in children with malnutrition is poorly studied and its expand application in CM could reveal more rigorous output on the pathophysiology and the management of SAM.
The metabolome and lipidome are the major molecular components of biological systems. According to The Human Metabolome Database (HMDB), there are over 40,000 metabolites and lipids that have already been identified or are likely to be found in the human body59. UHPLC-HRMS is an emerging analytical technique in biomedical sciences and has a unique strength to profile and identify the metabolic fingerprints in a wide variety of samples including biofluids and tissues19. In the recent years, untargeted metabolomics or lipidomics using UHPLC-HRMS technologies have enabled to detection over 3000 metabolic features, but have not yet been employed in CM based studies43,44. Notably, LC-MS based metabolomics or lipidomics technologies are often used to screen metabolite-based biomarker in a wide variety of bioscience disciplines such as inborn errors in metabolism60, genotyping61, pharmacometabolomics62, gut microbiome activity63, and nutrition-associated metabolic phenotyping64,65. Evidently, CM is a major health and wellness problem in developing countries where relatively rapid analytical technologies with little or no sample preparation and cost effective features are in high demand. Our group recently reported the use of paper spray ionization mass spectrometry (PSI-MS) for untargeted metabolomics research40. PSI-MS is a relatively new analytical technique allowing for rapid mass spectrometric analysis of biological samples with little or no sample preparation, incredibly faster data acquisition time (~1min), and much cheaper analysis40. There is a considerable interest in the clinic for the inexpensive storage and transportation of liquid biopsy samples such as blood, urine, saliva, and other bodily fluid as dried spots on paper. We believe PSI-MS could be considered the future bedside screening tool for high-throughput screening of global metabolome and lipidome of children with malnutrition.
In summary, emerging application of MS technologies-based metabolomics or lipidomics in CM could open exciting research avenue in global malnutrition research and may provide global public health researchers with novel platforms for investigating severe children malnutrition, uncovering new mechanistic insights through metabolic network that may lead to meaningful management strategy and cure.
Key messages.
Child malnutrition (CM) is a life threating global public health problem
Outcome from current management strategies for malnutrition are exceedingly poor
Emerging metabolomics and lipidomics approaches based on Mass Spectrometry techniques recently offer an exciting opportunity to understand the etiology and management of severe CM.
ACKNOWLEDGMENT
Research reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).WHO | Joint child malnutrition estimates - Levels and trends (2018. edition) http://www.who.int/nutgrowthdb/estimates2017/en/ (accessed May 9, 2019).
- (2).Martins VJB; Toledo Florêncio TMM; Grillo LP; Franco M. do C. P.; Martins PA; Clemente APG; Santos CDL; Vieira M. de F. A.; Sawaya AL Long-Lasting Effects of Undernutrition. Int J Environ Res Public Health 2011, 8 (6), 1817–1846. 10.3390/ijerph8061817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Grantham-McGregor S; Cheung YB; Cueto S; Glewwe P; Richter L; Strupp B. Developmental Potential in the First 5 Years for Children in Developing Countries. The Lancet 2007, 369 (9555), 60–70. 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Black RE; Allen LH; Bhutta ZA; Caulfield LE; de Onis M; Ezzati M; Mathers C; Rivera J. Maternal and Child Undernutrition: Global and Regional Exposures and Health Consequences. The Lancet 2008, 371 (9608), 243–260. 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- (5).Black RE; Victora CG; Walker SP; Bhutta ZA; Christian P; Onis M. de; Ezzati M; Grantham-McGregor S; Katz J; Martorell R; et al. Maternal and Child Undernutrition and Overweight in Low-Income and Middle-Income Countries. The Lancet 2013, 382 (9890), 427–451. 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- (6).Uchiyama R; Kupkova K; Shetty SJ; Linford AS; Pray-Grant MG; Wagar LE; Davis MM; Haque R; Gaultier A; Mayo MW; et al. Histone H3 Lysine 4 Methylation Signature Associated with Human Undernutrition. Proceedings of the National Academy of Sciences 2018, 115 (48), E11264–E11273. 10.1073/pnas.1722125115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Kumar M; Ji B; Babaei P; Das P; Lappa D; Ramakrishnan G; Fox TE; Haque R; Petri WA; Bäckhed F; et al. Gut Microbiota Dysbiosis Is Associated with Malnutrition and Reduced Plasma Amino Acid Levels: Lessons from Genome-Scale Metabolic Modeling. Metab. Eng 2018, 49, 128–142. 10.1016/j.ymben.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Subramanian S; Huq S; Yatsunenko T; Haque R; Mahfuz M; Alam MA; Benezra A; DeStefano J; Meier MF; Muegge BD; et al. Persistent Gut Microbiota Immaturity in Malnourished Bangladeshi Children. Nature 2014, 510 (7505), 417–421. 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Bhutta ZA; Berkley JA; Bandsma RHJ; Kerac M; Trehan I; Briend A. Severe Childhood Malnutrition. Nature Reviews Disease Primers 2017, 3, 17067. 10.1038/nrdp.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Morris SS; Cogill B; Uauy R. Effective International Action against Undernutrition: Why Has It Proven so Difficult and What Can Be Done to Accelerate Progress? 2008, 371, 14. [DOI] [PubMed] [Google Scholar]
- (11).Minak J; Kabir M; Mahmud I; Liu Y; Liu L; Haque R; Petri WA Evaluation of Rapid Antigen Point-of-Care Tests for Detection of Giardia and Cryptosporidium Species in Human Fecal Specimens. Journal of Clinical Microbiology 2012, 50 (1), 154–156. 10.1128/JCM.01194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Briend A; Akomo P; Bahwere P; de Pee S; Dibari F; Golden MH; Manary M; Ryan K. Developing Food Supplements for Moderately Malnourished Children: Lessons Learned from Ready-to-Use Therapeutic Foods. Food Nutr Bull 2015, 36 (1_suppl1), S53–S58. 10.1177/15648265150361S109. [DOI] [PubMed] [Google Scholar]
- (13).Ramakrishnan U; Nguyen P; Martorell R. Effects of Micronutrients on Growth of Children under 5 y of Age: Meta-Analyses of Single and Multiple Nutrient Interventions. Am. J. Clin. Nutr 2009, 89 (1), 191–203. 10.3945/ajcn.2008.26862. [DOI] [PubMed] [Google Scholar]
- (14).Ashorn P; Alho L; Ashorn U; Cheung YB; Dewey KG; Gondwe A; Harjunmaa U; Lartey A; Phiri N; Phiri TE; et al. Supplementation of Maternal Diets during Pregnancy and for 6 Months Postpartum and Infant Diets Thereafter with Small-Quantity Lipid-Based Nutrient Supplements Does Not Promote Child Growth by 18 Months of Age in Rural Malawi: A Randomized Controlled Trial. J Nutr 2015, 145 (6), 1345–1353. 10.3945/jn.114.207225. [DOI] [PubMed] [Google Scholar]
- (15).Mayo-Wilson E; Junior JA; Imdad A; Dean S; Chan XHS; Chan ES; Jaswal A; Bhutta ZA Zinc Supplementation for Preventing Mortality, Morbidity, and Growth Failure in Children Aged 6 Months to 12 Years of Age. Cochrane Database Syst Rev 2014, No. 5, CD009384. 10.1002/14651858.CD009384.pub2. [DOI] [PubMed] [Google Scholar]
- (16).Bhutta ZA; Das JK; Rizvi A; Gaffey MF; Walker N; Horton S; Webb P; Lartey A; Black RE Evidence-Based Interventions for Improvement of Maternal and Child Nutrition: What Can Be Done and at What Cost? The Lancet 2013, 382 (9890), 452–477. 10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
- (17).Adu-Afarwuah S; Lartey A; Okronipa H; Ashorn P; Peerson JM; Arimond M; Ashorn U; Zeilani M; Vosti S; Dewey KG Small-Quantity, Lipid-Based Nutrient Supplements Provided to Women during Pregnancy and 6 Mo Postpartum and to Their Infants from 6 Mo of Age Increase the Mean Attained Length of 18-Mo-Old Children in Semi-Urban Ghana: A Randomized Controlled Trial. Am. J. Clin. Nutr 2016, 104 (3), 797–808. 10.3945/ajcn.116.134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Christian P; Shaikh S; Shamim AA; Mehra S; Wu L; Mitra M; Ali H; Merrill RD; Choudhury N; Parveen M; et al. Effect of Fortified Complementary Food Supplementation on Child Growth in Rural Bangladesh: A Cluster-Randomized Trial. Int J Epidemiol 2015, 44 (6), 1862–1876. 10.1093/ije/dyv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wishart DS Emerging Applications of Metabolomics in Drug Discovery and Precision Medicine. Nat Rev Drug Discov 2016, 15 (7), 473–484. 10.1038/nrd.2016.32. [DOI] [PubMed] [Google Scholar]
- (20).Sreekumar A; Poisson LM; Rajendiran TM; Khan AP; Cao Q; Yu J; Laxman B; Mehra R; Lonigro RJ; Li Y; et al. Metabolomic Profiles Delineate Potential Role for Sarcosine in Prostate Cancer Progression. Nature 2009, 457 (7231), 910–914. 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- (21).Mayers JR; Wu C; Clish CB; Kraft P; Torrence ME; Fiske BP; Yuan C; Bao Y; Townsend MK; Tworoger SS; et al. Elevation of Circulating Branched-Chain Amino Acids Is an Early Event in Human Pancreatic Adenocarcinoma Development. Nat. Med 2014, 20 (10), 1193–1198. 10.1038/nm.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Nelson ER; Wardell SE; Jasper JS; Park S; Suchindran S; Howe MK; Carver NJ; Pillai RV; Sullivan PM; Sondhi V; et al. 27-Hydroxycholesterol Links Hypercholesterolemia and Breast Cancer Pathophysiology. Science 2013, 342 (6162), 1094–1098. 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Semba RD; Zhang P; Gonzalez-Freire M; Moaddel R; Trehan I; Maleta KM; Ordiz MI; Ferrucci L; Manary MJ The Association of Serum Choline with Linear Growth Failure in Young Children from Rural Malawi. Am. J. Clin. Nutr 2016, 104 (1), 191–197. 10.3945/ajcn.115.129684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lee B; Mahmud I; Marchica J; Dereziński P; Qi F; Wang F; Joshi P; Valerio F; Rivera I; Patel V; et al. Integrated RNA and Metabolite Profiling of Urine Liquid Biopsies for Prostate Cancer Biomarker Discovery. bioRxiv 2019, 599514. 10.1101/599514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Mahmud I; Lee B; Perera R; Garrett TJ Abstract 5273: Multi-Omics Approaches Reveal Potential Role for Corticosterone in Prostate Cancer. Cancer Res 2019, 79 (13 Supplement), 5273–5273. 10.1158/1538-7445.SABCS18-5273. [DOI] [Google Scholar]
- (26).Nelson ER; Wardell SE; Jasper JS; Park S; Suchindran S; Howe MK; Carver NJ; Pillai RV; Sullivan PM; Sondhi V; et al. 27-Hydroxycholesterol Links Hypercholesterolemia and Breast Cancer Pathophysiology. Science 2013, 342 (6162), 1094–1098. 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Hattori A; Tsunoda M; Konuma T; Kobayashi M; Nagy T; Glushka J; Tayyari F; McSkimming D; Kannan N; Tojo A; et al. Cancer Progression by Reprogrammed BCAA Metabolism in Myeloid Leukaemia. Nature 2017, 545 (7655), 500–504. 10.1038/nature22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Semba RD; Trehan I; Li X; Salem N; Moaddel R; Ordiz MI; Maleta KM; Kraemer K; Manary MJ Low Serum ω−3 and ω−6 Polyunsaturated Fatty Acids and Other Metabolites Are Associated with Poor Linear Growth in Young Children from Rural Malawi. Am J Clin Nutr 2017, 106 (6), 1490–1499. 10.3945/ajcn.117.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Semba RD; Shardell M; Sakr Ashour FA; Moaddel R; Trehan I; Maleta KM; Ordiz MI; Kraemer K; Khadeer MA; Ferrucci L; et al. Child Stunting Is Associated with Low Circulating Essential Amino Acids. EBioMedicine 2016, 6, 246–252. 10.1016/j.ebiom.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Semba RD; Shardell M; Trehan I; Moaddel R; Maleta KM; Ordiz MI; Kraemer K; Khadeer M; Ferrucci L; Manary MJ Metabolic Alterations in Children with Environmental Enteric Dysfunction. Sci Rep 2016, 6, 28009. 10.1038/srep28009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Nicholson JK; Lindon JC; Holmes E. “Metabonomics”: Understanding the Metabolic Responses of Living Systems to Pathophysiological Stimuli via Multivariate Statistical Analysis of Biological NMR Spectroscopic Data. Xenobiotica 1999, 29 (11), 1181–1189. 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- (32).Wenk MR Lipidomics: New Tools and Applications. Cell 2010, 143 (6), 888–895. 10.1016/j.cell.2010.11.033. [DOI] [PubMed] [Google Scholar]
- (33).Hao J; Liebeke M; Astle W; De Iorio M; Bundy JG; Ebbels TMD Bayesian Deconvolution and Quantification of Metabolites in Complex 1D NMR Spectra Using BATMAN. Nat Protoc 2014, 9 (6), 1416–1427. 10.1038/nprot.2014.090. [DOI] [PubMed] [Google Scholar]
- (34).Beebe K; Kennedy AD Sharpening Precision Medicine by a Thorough Interrogation of Metabolic Individuality. Computational and Structural Biotechnology Journal 2016, 14, 97. 10.1016/j.csbj.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Dettmer K; Aronov PA; Hammock BD MASS SPECTROMETRY-BASED METABOLOMICS. Mass Spectrom Rev 2007, 26 (1), 51–78. 10.1002/mas.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Lei Z; Huhman DV; Sumner LW Mass Spectrometry Strategies in Metabolomics. J. Biol. Chem 2011, 286 (29), 25435–25442. 10.1074/jbc.R111.238691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wood PL Mass Spectrometry Strategies for Clinical Metabolomics and Lipidomics in Psychiatry, Neurology, and Neuro-Oncology. Neuropsychopharmacology 2014, 39 (1), 24–33. 10.1038/npp.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Yang K; Han X. Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends Biochem. Sci 2016, 41 (11), 954–969. 10.1016/j.tibs.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Hughey CA; Rodgers RP; Marshall AG Resolution of 11,000 Compositionally Distinct Components in a Single Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrum of Crude Oil. Anal. Chem 2002, 74 (16), 4145–4149. [DOI] [PubMed] [Google Scholar]
- (40).Chamberlain CA; Rubio VY; Garrett TJ Strain-Level Differentiation of Bacteria by Paper Spray Ionization Mass Spectrometry. Anal. Chem 2019, 91 (8), 4964–4968. 10.1021/acs.analchem.9b00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Giavalisco P; Hummel J; Lisec J; Inostroza AC; Catchpole G; Willmitzer L. High-Resolution Direct Infusion-Based Mass Spectrometry in Combination with Whole 13C Metabolome Isotope Labeling Allows Unambiguous Assignment of Chemical Sum Formulas. Anal. Chem 2008, 80 (24), 9417–9425. 10.1021/ac8014627. [DOI] [PubMed] [Google Scholar]
- (42).Wang H; Liu J; Cooks RG; Ouyang Z. Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry. Angewandte Chemie International Edition 2010, 49 (5), 877–880. 10.1002/anie.200906314. [DOI] [PubMed] [Google Scholar]
- (43).Mahmud I; Sternberg S; Williams M; Garrett TJ Comparison of Global Metabolite Extraction Strategies for Soybeans Using UHPLC-HRMS. Anal Bioanal Chem 2017, 409 (26), 6173–6180. 10.1007/s00216-017-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Koelmel JP; Kroeger NM; Gill EL; Ulmer CZ; Bowden JA; Patterson RE; Yost RA; Garrett TJ Expanding Lipidome Coverage Using LC-MS/MS Data-Dependent Acquisition with Automated Exclusion List Generation. J. Am. Soc. Mass Spectrom 2017, 28 (5), 908–917. 10.1007/s13361-017-1608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Gill EL; Koelmel JP; Yost RA; Okun MS; Vedam-Mai V; Garrett TJ Mass Spectrometric Methodologies for Investigating the Metabolic Signatures of Parkinson’s Disease: Current Progress and Future Perspectives. Analytical Chemistry 2018, 90 (5), 2979–2986. 10.1021/acs.analchem.7b04084. [DOI] [PubMed] [Google Scholar]
- (46).Roberts LD; Souza AL; Gerszten RE; Clish CB Targeted Metabolomics. Curr Protoc Mol Biol 2012, CHAPTER, Unit30.2. 10.1002/0471142727.mb3002s98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Moreau GB; Ramakrishnan G; Cook HL; Fox TE; Nayak U; Ma JZ; Colgate ER; Kirkpatrick BD; Haque R; Petri WA Childhood Growth and Neurocognition Are Associated with Distinct Sets of Metabolites. EBioMedicine 2019. 10.1016/j.ebiom.2019.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Hayton S; Maker GL; Mullaney I; Trengove RD Experimental Design and Reporting Standards for Metabolomics Studies of Mammalian Cell Lines. Cell. Mol. Life Sci 2017, 74 (24), 4421–4441. 10.1007/s00018-017-2582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Vuckovic D. Improving Metabolome Coverage and Data Quality: Advancing Metabolomics and Lipidomics for Biomarker Discovery. Chem. Commun 2018, 54 (50), 6728–6749. 10.1039/C8CC02592D. [DOI] [PubMed] [Google Scholar]
- (50).Tsai Y-H; Menger RF; Drexler DM; Yost RA; Garrett TJ Ionization Sources and Mass Analyzers in MS Imaging. Bioanalysis 2015, 7 (20), 2629–2637. 10.4155/bio.15.187. [DOI] [PubMed] [Google Scholar]
- (51).Garrett TJ; Dawson WW Lipid Geographical Analysis of the Primate Macula by Imaging Mass Spectrometry. Methods Mol. Biol 2009, 579, 247–260. 10.1007/978-1-60761-322-0_12. [DOI] [PubMed] [Google Scholar]
- (52).Ulmer CZ; Jones CM; Yost RA; Garrett TJ; Bowden JA Optimization of Folch, Bligh-Dyer, and Matyash Sample-to-Extraction Solvent Ratios for Human Plasma-Based Lipidomics Studies. Analytica Chimica Acta 2018, 1037, 351–357. 10.1016/j.aca.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Ulmer CZ; Patterson RE; Koelmel JP; Garrett TJ; Yost RA A Robust Lipidomics Workflow for Mammalian Cells, Plasma, and Tissue Using Liquid-Chromatography High-Resolution Tandem Mass Spectrometry. In Lipidomics: Methods and Protocols; Bhattacharya SK, Ed.; Methods in Molecular Biology; Springer; New York: New York, NY, 2017; pp 91–106. 10.1007/978-1-4939-6996-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Koelmel JP; Kroeger NM; Ulmer CZ; Bowden JA; Patterson RE; Cochran JA; Beecher CWW; Garrett TJ; Yost RA LipidMatch: An Automated Workflow for Rule-Based Lipid Identification Using Untargeted High-Resolution Tandem Mass Spectrometry Data. BMC Bioinformatics 2017, 18 (1), 331. 10.1186/s12859-017-1744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Myers OD; Sumner SJ; Li S; Barnes S; Du X. Detailed Investigation and Comparison of the XCMS and MZmine 2 Chromatogram Construction and Chromatographic Peak Detection Methods for Preprocessing Mass Spectrometry Metabolomics Data. Anal. Chem 2017, 89 (17), 8689–8695. 10.1021/acs.analchem.7b01069. [DOI] [PubMed] [Google Scholar]
- (56).Di Giovanni V; Bourdon C; Wang DX; Seshadri S; Senga E; Versloot CJ; Voskuijl W; Semba RD; Trehan I; Moaddel R; et al. Metabolomic Changes in Serum of Children with Different Clinical Diagnoses of Malnutrition. J. Nutr 2016, 146 (12), 2436–2444. 10.3945/jn.116.239145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Monira S; Nakamura S; Gotoh K; Izutsu K; Watanabe H; Alam NH; Endtz H. Ph; Cravioto A; Ali Sk. I.; Nakaya T; et al. Gut Microbiota of Healthy and Malnourished Children in Bangladesh. Front Microbiol 2011, 2. 10.3389/fmicb.2011.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Schoonees A; Lombard M; Musekiwa A; Nel E; Volmink J. Ready-to-Use Therapeutic Food for Home-Based Treatment of Severe Acute Malnutrition in Children from Six Months to Five Years of Age. Cochrane Database Syst Rev 2013, No. 6, CD009000. 10.1002/14651858.CD009000.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Wishart DS; Feunang YD; Marcu A; Guo AC; Liang K; Vázquez-Fresno R; Sajed T; Johnson D; Li C; Karu N; et al. HMDB 4.0: The Human Metabolome Database for 2018. Nucleic Acids Res 2018, 46 (D1), D608–D617. 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Schulze A; Lindner M; Kohlmüller D; Olgemöller K; Mayatepek E; Hoffmann GF Expanded Newborn Screening for Inborn Errors of Metabolism by Electrospray Ionization-Tandem Mass Spectrometry: Results, Outcome, and Implications. Pediatrics 2003, 111 (6 Pt 1), 1399–1406. 10.1542/peds.111.6.1399. [DOI] [PubMed] [Google Scholar]
- (61).Kastenmüller G; Raffler J; Gieger C; Suhre K. Genetics of Human Metabolism: An Update. Hum Mol Genet 2015, 24 (R1), R93–R101. 10.1093/hmg/ddv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Clayton TA; Lindon JC; Cloarec O; Antti H; Charuel C; Hanton G; Provost J-P; Le Net J-L; Baker D; Walley RJ; et al. Pharmaco-Metabonomic Phenotyping and Personalized Drug Treatment. Nature 2006, 440 (7087), 1073–1077. 10.1038/nature04648. [DOI] [PubMed] [Google Scholar]
- (63).Nicholson JK; Holmes E; Wilson ID Gut Microorganisms, Mammalian Metabolism and Personalized Health Care. Nat. Rev. Microbiol 2005, 3 (5), 431–438. 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- (64).Bictash M; Ebbels TM; Chan Q; Loo RL; Yap IKS; Brown IJ; de Iorio M; Daviglus ML; Holmes E; Stamler J; et al. Opening up the “Black Box”: Metabolic Phenotyping and Metabolome-Wide Association Studies in Epidemiology. J Clin Epidemiol 2010, 63 (9), 970–979. 10.1016/j.jclinepi.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Gibbons C; Finlayson G; Dalton M; Caudwell P; Blundell JE METABOLIC PHENOTYPING GUIDELINES: Studying Eating Behaviour in Humans. Journal of Endocrinology 2014, 222 (2), G1–G12. 10.1530/JOE-14-0020. [DOI] [PubMed] [Google Scholar]