Abstract

Selective and sensitive moisture sensors have attracted immense attention due to their ability to monitor the humidity content in industrial solvents, food products, etc., for regulating industrial safety management. Herein, a hydroxy naphthaldehyde-based piezochromic luminogen, namely, 1-{[(2-hydroxyphenyl)imino]methyl}naphthalen-2-ol (NAP-1), has been synthesized and its photophysical and molecular sensing properties have been investigated by means of various spectroscopic tools. Owing to the synergistic effect of both aggregation-induced emission (AIE) and excited-state intramolecular proton transfer (ESIPT) along with the restriction of C=N isomerization, the probe shows bright yellowish-green-colored keto emission with high quantum yield after the interaction with a trace amount of water. This makes NAP-1 a potential sensor for monitoring water content in the industrial solvents with very low detection limits of 0.033, 0.032, 0.034, and 0.033% (v/v) from tetrahydrofuran (THF), acetone, dimethyl sulfoxide (DMSO), and methanol, respectively. The probe could be used in the food industry to detect trace moisture in the raw food samples. The reversible switching behavior of NAP-1 makes it suitable for designing an INHIBIT logic gate with an additional application in inkless writing. In addition, an Internet of Things-(IoT) based prototype device has been proposed for on-site monitoring of the moisture content by a smartphone via Bluetooth or Wi-Fi. The aggregated probe also has the ability to recognize Cu2+ from a purely aqueous medium via the chelation-enhanced quenching (CHEQ) mechanism, leading to ∼84% fluorescence quenching with a Stern–Volmer quenching constant of 1.46 × 104 M–1 and with an appreciably low detection threshold of 57.2 ppb, far below than recommended by the World Health Organization (WHO) and the United States Environmental Protection Agency (U.S. EPA). The spectroscopic and theoretical calculations (density functional theory (DFT), time-dependent DFT (TD-DFT), and natural bond orbital (NBO) analysis) further empower the understanding of the mechanistic course of the interaction of the host–guest recognition event.
Introduction
Water is an essential substance not only in human life but also in industrial chemical applications. However, it is a detrimental impurity in the organic solvents, thus preventing the smooth progress of organic synthesis in chemical industries.1 The presence of moisture can reduce the effectiveness as well as production yields of the organometallic compounds.2 Owing to the presence of moisture, column chromatography instruments may be damaged.3 For instance, the presence of a small quantity of moisture in the hydrocarbon solvents, used for storing active metals like sodium, potassium, etc., may not only reduce the quality of the metal but may also lead to severe accidents by explosions, owing to the formation of hydrogen gas.4 Along with this, it is also a fatal impurity in pharmaceutical products,5 processed food items,6 industrial oils, or fuel materials, leading to corrosion or severe mechanical damages. For the secure functioning of the aircraft, jet fuels also must be free of water.7 As an artifact, it is of great importance to quantify the moisture content in the organic solvents as well as other industrial items. There are various conventional methods to quantify the water content, such as Karl Fisher titration,8 chromatographic-mass spectrometry,9 distillation methods,10etc., but owing to their time-consuming, complicated techniques and limited on-site applicability, optical chemosensors based on colorimetric and fluorometric methods for trace-level moisture detection are immensely attractive for being a simple, low-cost, convenient, sensitive, and field-employable methodology.11,12 Moreover, a fluorometric methodology is highly advantageous due to its sensitivity and selectivity. Among different water detection fluorescence methodologies, photoinduced electron transfer (PET), intramolecular charge transfer (ICT), excited-state intramolecular proton transfer (ESIPT), aggregation-induced emission (AIE)/aggregation-induced enhanced emission (AIEE), aggregation-caused quenching (ACQ), and chemodosimetric approaches are well accepted. Among all of these fluorophores, AIE-active probes have drawn much attention over conventional ACQ-type fluorophores due to their unique feature of “turn-on” fluorescence behavior on aggregation,13 emerging as sensitive chemo- or biosensors.14 In principle, AIE-active molecules are poorly emissive in the organic solvents while strongly emissive in the presence of water/buffer due to the molecular aggregation,15,16 which is mainly attributed to the restricted intramolecular rotation (RIR), restricted intramolecular vibration (RIV), and/or restricted intramolecular motion (RIM).16 Along with this, ESIPT-active fluorophores, having intramolecular hydrogen bonding between a proton donor amino or hydroxyl group and a proton acceptor carbonyl oxygen or imine nitrogen in the ground state, are also very much attractive, owing to a large Stokes shift, dual emission behavior, and environment-dependent optical property variation for the structural alteration in the excited state.17,18 In principle, ESIPT is a four-step photochemical process, where proton relocation takes place from a proton donor to a proton acceptor unit in the excited state. In an ultrafast time span under photoexcitation, the enol (E) form is at first transformed into the excited enol, E*, followed by intramolecular proton transfer to the excited keto form, K*, and ultimately decay radiatively into the ground-state keto form (K). For AIEgens containing intramolecular hydrogen bonding, keto emission is mainly observed in the aggregated state, where intramolecular hydrogen bonding is invincible by solvent molecules. From this viewpoint, AIE fluorescence, owing to the aggregation of the ESIPT luminogen, is mainly the keto fluorescence.19,20 In general, the ESIPT probes are observed to acquire high quantum yield at higher concentrations or in the aggregated state, owing to their large Stokes shift, greatly reducing the chance of self-quenching of the fluorescence.21 Thus, the luminogen having both ESIPT and AIE characteristics may be an added advantage toward sensing applications.
Moreover, among various metal pollutants, the detection of copper is vital as it can lead to various health disorders, like cellular damage, Alzheimer’s disease, Wilson’s disease, etc.22 Accordingly, the United States Environmental Protection Agency (U.S. EPA) and the World Health Organization (WHO) have set the maximum permissible limit of Cu2+ within 15–25 μM.23 Therefore, the development of a simple chemosensor for the detection of this metal pollutant is quite in demand. For instance, hydroxy naphthaldehyde-based Schiff base molecules having electron donor azomethine “N” and phenolic “O” donor sites may be an ideal platform for binding such fatal metal ions. Upon binding with such metal ions, the chemosensors may exhibit either a turn-on fluorescence signal by the chelation-enhanced fluorescence mechanism (CHEF) or a “turn-off” fluorescence response via the chelation-enhanced quenching (CHEQ) mechanism. CHEF may originate from the blockage of the photoinduced electron transfer from ligand loan pairs, while CHEQ may originate via electron or energy transfer from the ligand to the metal center (ligand-to-metal charge transfer (LMCT)). Again, the concept of sensor development is ubiquitous. However, the developed sensor would be useful for society only if it could be interfaced with molecular electronics to make a portable prototype device, thereby moving the analytical achievement from the lab to the real field in a user-friendly way.
In this regard, as illustrated in Scheme 1, herein, we have investigated the optical sensing behavior of a hydroxyl naphthaldehyde-based ESIPT AIEgen, 1-{[(2-hydroxyphenyl)imino]methyl}naphthalen-2-ol (NAP-1). The luminogen exhibits AIE properties in organic solvents containing different water fractions. Unlike other Schiff bases, NAP-1 does not show fluorescence enhancement by hydrolytic cleavage of the azomethine linkage;24 it rather shows the OFF–ON AIE property by the formation of molecular aggregates. Thus, the probe can be effectively envisaged for trace-level moisture detection in various organic solvents including tetrahydrofuran (THF), acetone, dimethyl sulfoxide (DMSO), and MeOH with very low detection limits of 0.033, 0.032, 0.034, and 0.033% (v/v), respectively. The strong AIE emission with “naked-eye” yellowish-green fluorescence is observed by the combination of molecular planarization with restricted intramolecular rotation (RIR), which was validated by emission enhancement either through viscosity enhancement or by dropping off the temperature. The water detection phenomenon of the probe is reversible in the presence of activated molecular sieves (AMSs). Thus, it can be reused several times, and by means of this reversible behavior, it was possible to exploit it in inkless writing and the formulation of an INHIBIT logic gate. One step ahead, in the era of Industry 4.0, an Internet of Things (IoT)-based prototype has been proposed for interfacing the chemical data into a digital one for visualizing in a smartphone by Bluetooth or Wi-Fi. Moreover, the aggregated probe, NAP-1, due to the possession of N, O-binding sites, can selectively recognize Cu2+ through a visual OFF–ON–OFF fluorescence response via the CHEQ mechanism from a purely aqueous medium even in the background of other interfering metal ions with a binding constant of 2.34 × 108 M–1 and a limit of detection (LOD) of 57.2 ppb, which is far below compared to that of the permissible limit set by the WHO or U.S. EPA. The recognition properties have been thoroughly verified by UV–vis, fluorescence, Fourier transform infrared (FT-IR), 1H NMR spectrometric analyses, etc. In addition, all of the experimental findings were well corroborated with the DFT theoretical outcomes.
Scheme 1. Schematic Representation of the Proposed AIE and ESIPT Mechanism of NAP-1 and Its Application toward Trace-Level Moisture and Cu2+ Detection.
Results and Discussion
Single-Crystal X-ray Crystallographic Diffraction
Single-crystal X-ray diffraction can give better insight into the ground-state molecular conformation. X-ray-quality crystals of NAP-1 were obtained from the slow diffusion of tetrahydrofuran into hexane (1:1, v/v). NAP-1 crystallized in the orthorhombic Pbca space group. The crystallographic data are tabulated in Table S1, Supporting Information (SI). The molecular packing diagram is shown in Figure 1B, which shows that the molecule adopts a typical head-to-head or head-to-tail packing arrangement with a slightly twisted geometry. The compound bears one phenyl and one naphthyl fragment, bridged by a −CH=N unit, and the phenyl and naphthyl planes are twisted to 12.06° (Figure S5, SI), speculating a nonplanar geometry. As shown in Figure 1A, the hydroxyl oxygen, O(1), of the naphthalene unit is directed toward the −CH=N bond and the distance between that oxygen and the nitrogen, N(1), of the −CH=N unit is 1.95 Å (θ = 133.03°), revealing a strong intramolecular H-bonding interaction (OH···N interaction) susceptible to keto–enol tautomerism. This intramolecular hydrogen bonding accelerates the ESIPT process in the excited state. The torsion angle of the −CH=N unit (C4–C8–N3–C5) is 175.59°. The C–N (C8–N3) bond distance of the azomethine bond is found to be 1.32 Å. Meanwhile, the centroid-to-centroid distances between the amino phenyl units and hydroxy naphthalene units of the NAP-1 crystal are 4.310 and 4.413 Å, respectively (Figure 1C). The O–H bond distances are observed to be 0.84 (O2–H2) and 0.84 (O1–H1) Å and C–C bond distances range from 1.346 to 1.461 Å, which implies the presence of resonance in the system.
Figure 1.
(A) Oak Ridge thermal ellipsoid plot (ORTEP) diagram of NAP-1 with a 50% thermal ellipsoid, (B) unit cell packing diagram, (C) unit cell showing a π–π interaction (hydrogen atoms are omitted for clarity), and (D) one-dimensional polymeric chain.
Photophysical Characterization and Solvatochromic Properties
To check the photophysical properties of the developed chemosensor, NAP-1, the absorption and emission spectra were studied in eight different solvents with variable polarities, like dichloromethane (DCM), tetrahydrofuran (THF), 1,4-dioxane, methanol (MeOH), acetonitrile (MeCN), acetone, N,N-dimethylformamide (DMF), and dimethyl sulfoxide (DMSO). The chemosensor, NAP-1, shows different absorption and emission maxima with different absorbances and intensities, respectively, in different solvents (Figure 2A,B), which are summarized in Table S2, SI. The UV–vis spectrum of NAP-1 (10–5 M) in THF shows three major absorption peaks at 325, 385, and 460 nm. The lowest energy band at 460 nm may be due to the n → π* transition from imine25 along with the intramolecular charge transfer transition and the speculated presence of a keto tautomer. Other absorption peaks at 325 and 385 nm may typically arise due to the π → π* transition throughout the benzene and naphthalene skeleton, respectively, indicating the presence of an enol tautomer.26,27 Thus, from UV–vis absorption spectra, it is clear that NAP-1 exists in two tautomeric forms in the solution state and the corresponding ICT bands for keto and enol tautomers are obtained at 460 and 385 nm, respectively. With the increasing polarity of the solvents, the keto emission band of NAP-1 is slightly bathochromically shifted from 460 to 473 nm. Therefore, the sensitivity of the position of the absorption maxima of NAP-1 with the variation of solvent polarity is almost negligible,28 indicating a negligible influence of the inter- and intramolecular hydrogen bonding on the absorption spectra or the absence of the proton transfer process in the ground state.29
Figure 2.
(A) Absorption and (B) emission spectra of NAP-1 in different solvents, (C) Commission Internationale de l’Eclairage (CIE) chromaticity coordinates calculated from the emission spectra of NAP-1 in different solvents, and (D) emission spectral change of NAP-1 in MeOH upon the addition of an incremental amount of glycerol at room temperature (concentration: 10–5 M, excitation wavelength: 360 nm).
However, NAP-1 exhibited a profound effect on the fluorescence emission spectral properties with the variation of solvent polarity. Upon excitation at 360 nm, NAP-1 shows dual emission behavior (435–515 nm) in various solvents with different polarities (e.g., acetonitrile, acetone, 1,4-dioxane, etc.), which is a prominent characteristic of the ESIPT phenomenon in the excited state. The high-energy emission band is due to normal enol emission, while the low-energy emission band at a higher wavelength with a large Stokes shift is due to the keto emission by the ESIPT process. In the least polar solvent, dichloromethane, the compound exhibited only a single broad emission at 435 nm. In aprotic solvents, like 1,4-dioxane, acetone, acetonitrile, DMF, and DMSO, NAP-1 exhibits dual emission. With the increasing polarity of polar aprotic solvents (e.g., acetone, DMF, DMSO), the spectral position of the keto emission band of NAP-1 in the lower-energy region (493–516 nm) is bathochromically shifted. However, in polar protic solvents, like methanol, NAP-1 exhibits a strong emission in a longer-wavelength (516 nm) region along with a weaker emission in a shorter-wavelength (468 nm) region. Usually, the ESIPT process is inhibited in the polar protic solvent, like methanol, via intermolecular hydrogen bonding with the molecule. However, the appearance of an intense ESIPT peak in a longer-wavelength region (at 516 nm) in methanol might be ascribed to the formation of a methanol-induced encounter complex involving eight-membered rings between the intermolecular hydrogen-bonded methanol and the molecule, where proton transfer can still occur via a hydrogen-bonded solvent bridge (Scheme S1, SI).30 This facilitates the solvent-mediated proton transfer to exhibit the dominant keto emission. From Löwdin spin population analysis by a theoretical DFT study (Tables S3–S5, SI), it has also been observed that the charge density on the aldimine nitrogen atom is increased (from −0.05272 to −0.13198), while the charge density on the naphthyl oxygen atom is decreased (from −0.35539 to −0.31662) upon a methanol-mediated proton transfer process via an eight-membered-ring H-bonding configuration.30 Therefore, the theoretical study also supports that in the polar protic media like MeOH, proton transfer is possible via a hydrogen-bonded solvent bridge, facilitating the emission in the longer-wavelength region. However, in the presence of water, the emission peak is more red-shifted with enhanced signal intensity and naked-eye bright yellowish-green fluorescence, leading to the possibility of water-driven aggregation.31a Owing to the presence of an ESIPT group, the excited-state intramolecular proton transfer takes place from the proton donor (D) −OH site of naphthalene to the −CH=N– proton acceptor site (A) through a six-membered-ring hydrogen-bonding configuration due to the presence of the suitable bond distance (1.8 Å) required for the occurrence of ESIPT. This means that in the excited state phototautomerization takes place where the enol form is converted into the keto form through the ESIPT process, accelerated by the ground-state keto–enol tautomerism. Hence, the lower-energy emission band stands for the excited keto (k*) tautomer, produced by intramolecular proton transfer, while the higher-energy emission band stands for the excited enol (E*) tautomer.31b To further elucidate the emission spectral change in the presence of solvents with different polarities, the Commission Internationale de l’Eclairage (CIE) coordinates of the emission spectra in different solvents were also measured (Figure 2C).
To further validate the occurrence of the ESIPT process, a density functional theory (DFT) study has been performed using the B3LYP level of theory. The energy-minimized structures of the keto and enol tautomers are shown in Figure 3. The geometry-optimized structures of the enol form exhibit the optimization of the respective form with a shorter O–H distance (1.02 Å) compared to that of the N···H distance (1.63 Å), while the shortening of the N–H bond (1.04 Å) compared to that of the N···H distance of the enol form inferred the optimization of the keto form.32 From the theoretical calculations, it was inferred that the keto form is much more energetically stable than the enol form, divulging the inclination of the conversion of the enol form into the keto form through keto–enol tautomerization in the ground state and phototautomerization via the ESIPT process in the excited state, facilitated by intramolecular hydrogen bonding. Highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) plots with the corresponding energy values are shown in Scheme 2. Interestingly, from the geometry-optimized structure of the enol form (Figure 3C), it was observed that the enol tautomer has a twisted molecular conformation with an angle of 37.2° between the two planes, which is favorable for facile intramolecular rotation. Thus, the absorbed excitation energy is dissipated in the form of nonradiative transition, leading the enol tautomer to be almost nonemissive in nature, while in the keto form (Figure 2D) having almost a planar rigid structure, intramolecular rotation is prohibited, which strongly nullifies nonradiative relaxation and thus makes the keto tautomer highly emissive.33 Moreover, the planar conformation leads to more conjugation, which also well agrees with the red-shifted enhanced emission.34 Computed structural properties are summarized in Table S6, SI. The theoretical absorption peaks for enol and keto tautomers, calculated from time-dependent DFT (TD-DFT), are obtained at 400 and 423 nm, respectively, which are comparable to those obtained experimentally (Tables S7 and S8, SI). Natural bond orbital (NBO) population analysis (Tables S9 and S10, SI) revealed that the charge density over the proton acceptor, imine nitrogen, is decreased in the keto tautomer (−0.554) compared to that in the enol tautomer (−0.603). This gives a clear indication of the proton binding mechanism to the proton acceptor imine moiety from the proton donor hydroxyl moiety.35 Again, the calculated distance between hydroxyl H and imine N in the enol tautomer is 1.63 Å, which also shows strong intramolecular hydrogen bonding, facilitating the ESIPT process. The dipole moment of the keto tautomer (4.58 D) was observed to be greater than that of the enol tautomer (3.88 D), which possibly facilitates the proton transfer mechanism.36
Figure 3.
Geometry-optimized structures of the (A) enol and (B) keto tautomers; side view of (C) enol form (twisted structure) and (D) keto form (almost planar structure) of NAP-1.
Scheme 2. Schematic Representation of the Four-Step ESIPT Process.
pH Dependence of NAP-1
To get better insight into the photophysical characteristics, absorption and emission spectra were recorded at different pH values in the THF solvent (Figure S6, SI). NAP-1 exhibited a strong low-energy absorption band at 468 nm due to extensive intramolecular charge transfer (ICT) transition of the keto form. In neutral to acidic conditions, the enol tautomer predominantly exists over the keto tautomer as intramolecular proton transfer is prohibited at low to neutral pH conditions. However, in strongly acidic conditions (i.e., at pH < 4), the progressive quenching and disappearance of the red absorption band (n → π* band at 468 nm) might be due to the rupture of intramolecular hydrogen bonding between the proton donor and proton acceptor functionalities of NAP-1, followed by protonation at the hydroxy (−OH) functionalities.46a,46b Importantly, in strong basic conditions (i.e., at pH > 10), the −OH proton undergoes deprotonation with the concomitant generation of a new broad absorption peak at 500 nm. This may result from the fact that the hydroxyl group (−OH) in the NAP-1 molecule would gradually turn into a phenoxide ion via deprotonation, which will result in the enlarged conjugated system to induce chromogenic alteration from yellow to red.46c,46d Thus, NAP-1 shows pH-swiping characteristics, owing to the protonation and deprotonation of the hydroxy (−OH) functionalities.
Piezochromic Behavior
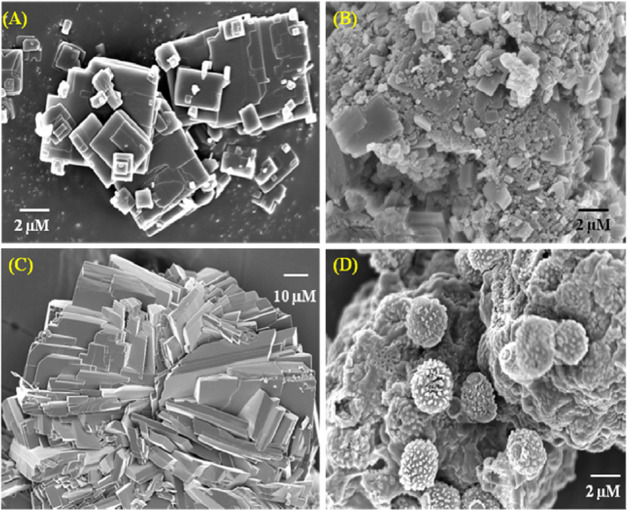
Piezochromic luminogens,37ai.e., the luminogens that can produce color alteration upon mechanical grinding or external pressure, are interesting in fabricating devices for pressure sensors, damage detectors, etc. Hence, we have investigated the solid-state optical properties of NAP-1. Figure 4 displays the emission spectra of NAP-1 in a pure solid state, resolving more prominent keto emission due to the restriction of intramolecular rotation as well as strong intramolecular hydrogen bonding. To check the piezochromic behavior of the compound, the as-synthesized material was ground thoroughly with a pestle and the emission spectral change was recorded. Interestingly, a bathochromic shift of the emission spectra was noted upon mechanical stimulation due to the destruction of weak intermolecular CH···O and CH···π interactions via planarization of the molecular conformation in the amorphized state.37b At the same time, the color of the compound in the room light changed from bright yellow to orangish-yellow (Figure S7, SI). To further support the morphological change of the compound upon grinding, field emission-scanning electron microscopy (FE-SEM) images (Figure 6) were collected, which disclosed the change of the crystalline nature of the as-prepared compound to the amorphous phase upon mechanical stimulation. Extra support for the piezochromic behavior of NAP-1 was acquired from powder X-ray diffraction (PXRD) analysis in the crystalline state and ground state. The sharp and intense peaks of NAP-1 in the crystalline state are weakened by the applied shear force, which is due to the demolition of the ordered structure or weak interactions in the pristine sample upon grinding (Figure S8, SI). Upon grinding, NAP-1 exhibited more diffused diffraction peaks compared to the pristine samples. This indicates that the crystalline-to-amorphous phase transition is responsible for the observed difference in the spectral features of ground and pristine samples of NAP-1.37c Therefore, both PXRD and FE-SEM analyses confirm the piezochromic behavior of NAP-1.
Figure 4.

Fluorescence emission spectral changes of NAP-1 in the solid state upon mechanical grinding.
Figure 6.
FE-SEM images of (A) as-synthesized, (B) ground, (C) crystalline, and (D) aggregated forms of NAP-1.
Aggregation-Induced Emission (AIE) Behavior with Trace-Level Moisture Detection
Encouraged by the unique photophysical properties, we investigated the analyte-dependent spectral response of NAP-1. Usually, the molecules having easily isomerizable units, like −CH=N, can quickly proceed through nonradiative decay of the excited state.16,38a Hence, inhibition of such a nonradiative decay process by the prohibition of the intramolecular rotation through the formation of aggregates may lead to aggregation-induced enhanced emission (AIE). To check the influence of aggregate formation on the photophysical properties, UV–vis and emission spectral studies were carried out in the presence of varying water contents (v/v), which may be helpful to detect trace levels of water in the organic solvents. For being one of the most commonly used polar aprotic solvents, tetrahydrofuran (THF) was chosen for water level detection, where THF acted as a good solvent and water as a poor solvent. Figure 5 clearly indicates that in pure THF, NAP-1 is almost nonemissive in nature, as in pure organic solvents, the compound easily undergoes intramolecular rotation along with the C=N isomerization, which leads to the destruction of the excited state via nonradiative deactivation. However, as water is a poor solvent for NAP-1, with the increasing water fraction, the fluorescence intensity of the keto emission increases due to the subtle change in the solvating power of the solvent mixture.
Figure 5.
(A) Emission spectral change upon the addition of an incremental water fraction (%, v/v) in THF, (B) corresponding intensity of the AIE emission at different water fractions (%, v/v), and (C) NAP-1 (in THF) containing different volume fractions of water (concentration: 10 μM, excitation wavelength: 360 nm).
After the water fraction increased to 60%, the fluorescence intensity is highly increased, indicating that the molecule prefers to stay in the aggregated form38b because of its insolubility in water, where intramolecular hydrogen bonding between hydroxyl and −CH=N groups becomes stabilized, which was beneficial for the ESIPT process. Thus, the probe molecule mainly exists in the keto form, owing to the protection of the hydrogen bond in the hydrophobic environment of aggregates.32 This facilitates the radiative decay via molecular planarity by restricting the intramolecular rotation, giving an enhanced bright yellowish-green-colored fluorescence, which is also visualized by the naked eye under a UV lamp. Interestingly, the fluorescence intensity of the enol emission of NAP-1 gradually decreased and the keto emission sharply increased up to ∼80% (v/v) of the water content with ∼14-fold emission enhancement, while at higher water fractions, the intensity decreased (keeping the ultimate concentration of the mixture solution constant at 10 μM). With the increasing water content, the intensity of the keto emission spectrum is highly increased with slight red shifting, which is a clear indication of the facile ESIPT process. This suggests that with the increasing water fraction the population of the aggregated species is also increased.27 Nonetheless, aggregation forced the molecule, NAP-1, to reside in a more apolar environment, partially restricting the ICT process,39,40 which is also reflected from bathochromic shifting. Hence, the enhancement of apparent emission intensity is induced by water-dependent aggregation.41,42 To get further insight into the AIE mechanism, UV–vis absorption spectral studies were also carried out in the presence of different water fractions (vol %) (Figure S9, SI). Interestingly, it was found that upon gradual addition of water the lower-energy absorption band is increased and the solutions containing 70–90% water fractions showed level-off tails of long wavelength in the UV–vis spectra, which typically suggested the formation of aggregates,33 leading to Mie scattering in the presence of water.40,43 Additionally, the sensing kinetics of the fluorescence enhancement was very fast. The AIE factor (αAIE) can be measured by comparing the quantum yields of the respective states, which can provide a quantitative idea about the fluorescence enhancement by the formation of aggregates. In THF, MeOH, and DMSO, the quantum yields of NAP-1 in solution were calculated to be 0.09, 0.14, and 0.07, respectively, whereas in the aggregated states, those were 0.94, 0.64, and 0.77, respectively, providing the corresponding AIE factors, αAIE values, to be 10.4, 4.6, and 11, respectively. The formation of aggregates was further verified by dynamic light scattering (DLS) (Figure S10, SI) and field emission-scanning electron microscopy (FE-SEM) analysis (Figure 6). From the DLS study, it was observed that in the presence of water, the size of NAP-1 (10 μM, THF) increased from 406 nm (0% water fraction) to 989 nm (80% water fraction), supporting the water-assisted aggregate formation. The aggregated form is stable almost up to 24 h, which was verified by DLS and fluorimetric studies (Figure S11, SI). Time-dependent responses of NAP-1 in THF and MeOH were recorded with the maximum water fraction (v/v) at 10 μM concentration. From the experimental results (Figure S12, SI), it was observed that only ∼5 s were required for fluorescence enhancement. This fast response made NAP-1 a potential sensor for trace-level water detection from commercial organic solvents.
Generally, in the system with both AIE and ESIPT processes, keto emission is the preferred one in the aggregated state, owing to strong intramolecular proton transfer, restricted intramolecular rotation, hydrophobic environment, etc. Thus, the enhancement of the keto emission band in the aggregated state well correlates with the fact. For this reason, in the aggregated state, NAP-1 relies on the synergistic effect of both AIE and ESIPT processes, which helped to exhibit strong enhanced keto emission with a large Stokes shift.44 Additionally, the enhancement of the keto emission intensity without a subtle spectral shift in the ice-cold solvents (acetone, THF, DCM, MeOH, 1,4-dioxane) compared to that in the solvents at room temperature also divulges the possibility of a restricted intramolecular rotation (RIR) mechanism as well as the restriction of solvent-coupled vibrational modes to enhance the AIE-induced emission intensity (Figure S13, SI).45,46 Again, at room temperature, the fluorescence spectral response in the methanolic solution of different viscosities using glycerol was analyzed. From Figure 2D, it can be inferred that with the increasing glycerol fraction, more specifically with the increasing viscosity, there occurs fluorescence enhancement, owing to the suppression of the intramolecular rotation, followed by facile ESIPT, making the molecule highly emissive. Thus, the analyses further validate the AIE-induced fluorescence enhancement by the RIR mechanism.47,48 Interestingly, in Figure S14, SI, it can be observed that with the increasing concentration of NAP-1 at room temperature from 0.1 to 100 μM, the intensity of the keto emission band is increased with a gradual decrease of the enol emission band. This speculates that in the excited state a facile proton transfer process takes place with the increasing concentration or aggregation of the compound, thereby facilitating the AIE-induced emission through RIR.49a Moreover, the AIE behavior of NAP-1 in THF/water suggests that the molecule would also exhibit fluorescence in the solid state. The restriction of intramolecular rotation inhibits the nonradiative relaxation pathway, which is also responsible for strong solid-state emission49b (Figure 4).
To explore the broad-range applicability of NAP-1 as a water sensor, we have also extended the experimentations with several other solvents (e.g., acetone, dimethyl sulfoxide, methanol, etc.) that are extensively used in the chemical industries (Figure S15, SI). NAP-1 could detect water content with very low LOD of 0.033, 0.032, 0.034, and 0.033% (v/v) and limit of quantitation (LOQ) of 0.110, 0.108, 0.114, and 0.111% (v/v) from THF, acetone, DMSO, and methanol, respectively (Figure S16, SI). Thus, the results obtained from Figure S17, SI indicate that NAP-1 can be successfully utilized as a potential water sensor for trace-level water detection from different industrial solvents.
To shed light on the sensing mechanism, 1H NMR analysis of NAP-1 in DMSO-d6 was carried out in the presence of an incremental addition of D2O (Figure S18, SI). It was observed that the extremely down-field-shifted hydroxyl proton signal at 10.38 ppm attributed to the ESIPT process is totally diminished, owing to the increase of the extent of intramolecular proton transfer upon a gradual increase of the water fraction, while the other hydroxyl proton at 15.7 ppm, the −CH=N proton signal at 9.5 ppm, and rest aromatic protons experience upfield shifting in the aggregated state.17
Food Sample Analysis for Trace-Level Moisture Detection: A Useful Tool for the Food Industry
As the moisture content affects the quality of the raw food products, it is highly desirable to monitor the moisture contents in the food industry by simple methodology. To check the applicability of the chemosensor toward monitoring the water content in food products, salt, sugar, and honey were chosen. The effectiveness of NAP-1 toward moisture detection was evaluated with an oven-dried sample as well as a sample kept in an open environment at room temperature for one month. When the food samples were added in NAP-1 (10 μM) in dry THF, the emission intensity at 515 nm was enhanced (Figure 7). Based on the relationship between the moisture content and emission intensity, it was found that honey contains the maximum moisture content, while sugar had the least. To verify the moisture content in each sample, a known amount of water was added to trace the emission intensity. From the results obtained, it can be inferred that NAP-1 has the excellent potential to monitor the moisture content in commercial food materials.
Figure 7.

Changes of emission intensities of NAP-1 (10 μM) in dry THF (λex = 360 nm) in the presence of commercial food products.
Application in Inkless Writing: A Step in Favor of Anti-counterfeiting Activities
Nowadays, a huge amount of paper is wasted just because the information written on the paper is not erasable. Additionally, the inks that are used for writing are also toxic to human health. In this regard, a rewritable fluorescent platform has drawn immense attention as a revolutionary greener solution using water as ink,50 thereby having great applications in the steganography and anti-counterfeiting activities too.
Owing to the reversible switching of NAP-1 by inclusion and exclusion of water, it could be exploited for potential applications in inkless writing using a fluorescence method. NAP-1-coated paper was fabricated by immersing the paper in a 1 mM THF solution of NAP-1, followed by vacuum drying. Figure 8 shows the representative images of the experimentation. “N” followed by “A” and “P” was written onto the NAP-1-impregnated paper using water as ink. The written letters could be clearly visualized by the naked eye under a UV lamp exhibiting a strong yellowish-green-colored fluorescence. Interestingly, the color was completely erased upon heating by the evaporation of water, and this could be repeated several times. Thus, NAP-1 could be successfully applied in inkless writing using a water-jet printer or a fountain pen with the “write–erase–write” facility, which would exhibit a substantial footprint in the domain of anti-counterfeiting activities.
Figure 8.
Photographic images demonstrating the write–erase–write property of an NAP-1-coated paper substrate.
Reversibility and Proposition of an INHIBIT Logic Gate
Any ideal chemosensor would be superior and highly acceptable compared to the other developed chemosensors if it can be reused several times. To evaluate the reversible nature of NAP-1, a variety of drying agents, like sodium sulfate, magnesium sulfate, and activated molecular sieves (AMSs), were checked to absorb the externally added water molecules present in the organic solvents. Interestingly, it was observed that AMS showed good reversibility of the chemosensor by absorbing the externally added water molecules from the organic solvents and the solution again became almost nonluminescent. This could be repeated several times without a significant loss of the potential of the chemosensor as an impurity detector. Additionally, AMS does not induce any change of the inherent property of the chemosensor as it is chemically inert in nature, leading to its successful utilization toward the reusability of the developed chemosensor. In Figure 9A, it is clear that initially NAP-1 was almost nonemissive in the organic solvents and became highly fluorescent in the presence of water. By the introduction of AMS to that solution, it again became almost nonfluorescent within few minutes, while the external addition of water again restored the initially obtained bright fluorescence. This phenomenon is repeatable several times, making NAP-1 a more efficient moisture sensor in commercial organic solvents.
Figure 9.

(A) Change of the fluorescence intensity of NAP-1 (in DMSO, 10 μM) at 515 nm upon alternate addition of water and activated molecular sieves (AMSs) and (B) truth table and corresponding INHIBIT logic gate based on water and AMS as chemical inputs.
Encouraged by the fascinating reversible behavior of NAP-1 upon the alternating presence of water and AMS, it is possible to interface the chemical output into molecular electronics with the aid of Boolean-type INHIBIT logic gates, which is attracting great interest in promising molecular-level smart optoelectronic device fabrication because of its noncommutative behavior.51a,51b For mimicking a two-input binary logic gate, herein, water (IN1) and AMS (IN2) have been exploited as the input signals, while the fluorescence emission intensity at 515 nm has been exploited as the output signal, OUTY1. The presence and absence of the input signals are designated as “0” and “1”, respectively, while the turn-on and turn-off fluorescence signals at 515 nm are designated as 1 and 0, respectively. As shown in the truth table (Figure 9B), four combinations of inputs are possible, and these are (0, 0), (0, 1), (1, 0), and (1, 1). In the absence of any inputs, the (0, 0) fluorescence intensity at 515 nm was very low; thus, the output signal, OUTY1, would remain in the OFF-state (0). Only in the presence of IN1 (water) and the absence of IN2 (AMS), i.e., (1, 0), NAP-1 would show a bright yellowish-green fluorescence, thereby leading to OUTY1 in the ON-state (1). If all of the inputs or only IN2 (i.e., (0, 1) or (1, 1)) is present, then again the fluorescence would remain in the OFF-state (0). Thus, the logic combinations led to the possible formulation of an INHIBIT molecular switch.
Proposition of IoT-Based Portable Prototype Device Development: Interfacing Chemistry with Modern Electronics
In the emerging Industry 4.0 and IoT era,51c,51d with the advancement of technologies, the data acquisition processes have become easier and more user-friendly. Thus, to verify the presence of moisture in any suspected sample, a portable device based on the developed chemosensor might be fabricated for on-site detection of the moisture content in the suspected sample, which might be enormously helpful in the industrial sector. The proposed device would be entirely composed of (a) a customized sample vial holder to place the sample vial, (b) a 360 nm UV light-emitting diode (LED) to excite the sample, (c) a light-dependent resistor (LDR) for measuring the light intensity, (d) ESP-12E for data acquisition and Bluetooth/Wi-Fi connectivity, and (e) an analog-to-digital converter (ADC) for data conversion.
The power of the device might be supplied by simply using a power bank so that the portable device could also be useful in remote areas. The working principle of the prototype is as follows:
-
(1)
At first, the vial containing our developed chemosensor solution with the suspected sample with air-tight capping would be placed in the vial holder of the device.
-
(2)
The UV LED would be used to excite the sample.
-
(3)
Consequently, the microcontroller platform (LDR) would measure the light intensity.
The LDR is connected with a series of resistors, and the output voltage across the LDR for the variation of light intensity would be measured in terms of the resistor divider rule between two resistors. This output circuit is connected to the analog-to-digital converter (ADC) of the ESP-12E for converting the voltage data into digitally coded data by translating the voltage data into the corresponding moisture content of the supplied sample. If the LDR is connected to a series of 10 kΩ resistors, the output voltage will vary in the range of 0–3.3 V. If the ADC is of 10 bit, then the sensitivity of the output voltage would be of the order of 3.3 V/210 = 0.0032 V. The digitalized data corresponding to the respective moisture content could be displayed on the liquid-crystal display (LCD) screen attached to the device. The translated data could be directly uploaded via a Bluetooth or Wi-Fi connection to a cloud-based server, where the data could be plotted for the quantification of the moisture content. This information could be directly transferred to the laptop/desktop or even a smartphone through self-developed android-based applications. Moreover, for easier communication and to fit the device as a “laymen’s prototype”, three buzzers (red, yellow, and green) would also be connected to it. When the moisture content in any packaged/suspected sample is within 5 and 10–50 and >50%, green, yellow, and red buzzers would buzz, respectively, based on the pretrained coding (Figure S19, SI). The workflow and schematic diagram of the prototype have been summarized in Scheme 3. The initial LDR response might be increased in the presence of an increasing amount of moisture content, which might be attributed to the consequent increase of fluorescence intensity. From the plot of LDR voltage and the moisture content, the sensitivity of the device could be measured. This IoT-based platform is particularly highly interesting because even any notification regarding an alarming level of moisture content could be dropped or live data could be streamed in the smartphone from a remote area without being present in the experimentation spot. Thus, the proposed IoT-based portable prototype device integrated with artificial intelligence would be a value-added addition to modern chemical technology for rapid and real-time monitoring of the moisture content.
Scheme 3. (a) Work Flow of the Function of the IoT-Based Device and (b) Schematic Diagram of the Proposed IoT-Based Prototype.
Investigation of Cu2+ Sensing via Chelation-Caused Quenching (CHEQ)
Owing to the availability of the hydroxyl groups and N-atom of the aldimine moiety, the response of NAP-1 in MeOH/H2O (60% v/v) at pH 7.4 and 10 μM concentration was investigated by the introduction of various metal ions in a purely aqueous medium. The compound showed strong yellowish-green fluorescence in the requisite condition. As demonstrated in Figure 10, it was observed that only Cu2+ induced a significant ∼84% fluorescence quenching, while the other metal ions (Mg2+, Na+, Hg2+, Cd2+, Zn2+, As3+) could not induce any subtle change in the fluorescence property of NAP-1. Moreover, with the increasing concentration of Cu2+, the fluorescence intensity was annihilated gradually with hypsochromic shifting, speculating the regeneration of the normal enol emission disrupting the ESIPT process. Thus, the result exhibited the immense selective affinity of NAP-1 toward Cu2+ in a purely aqueous medium. Moreover, the competitive experiments for the selectivity of Cu2+ in the background of other interfering metal ions also proved the best compatibility of NAP-1 with Cu2+ (Figures S20 and S21, SI), indicating its utility in various real-field analytical applications due to the possession of anti-interference characteristics toward Cu2+ detection. Furthermore, a good linear relationship of the fluorescence intensity and the concentration of Cu2+ was obtained with correlation coefficient, R2, = 0.96289 (Figure S22, SI). This gives a detection limit52a of Cu2+ as low as 57.2 ppb from a purely aqueous medium, which is far below the safe limit (1.3 ppm) set by the U.S. EPA.52b To investigate the proportional relationship between NAP-1 and Cu2+, Job’s plot was analyzed. In Figure S23, SI, it can be observed that when the mole fraction of the analyte was 0.5, the response was maximum. Thus, it could be predicted that NAP-1 maintains 1:1 binding stoichiometry with Cu2+. The corresponding association constant for 1:1 binding stoichiometry was obtained to be 2.34 × 108 M–1 from the Benesi–Hildebrand equation53 (Figure S24, SI). To investigate the response rate of NAP-1 toward Cu2+, a time-dependent study has been carried out using Cu2+ of different concentrations. In Figure S25, SI, it is noteworthy to infer that the reaction completed within 40–50 s and remained almost the same for the time period of ∼2 min. This short and faster response time due to strong complexation with Cu2+ suggests its real-time applicability. To determine the quenching constant, kSV, of NAP-1 toward Cu2+, the fluorescence intensity ratio, I0/I, was plotted against the concentration of Cu2+. It was observed that the Stern–Volmer plot (Figure 11) of Cu2+ bends downward instead of a linear relationship. The plausible reason behind this nonlinear Stern–Volmer plot may be that the fluorescence annihilation is caused by mixing of both static and dynamic quenching.54 Meanwhile, the downward curvature of the Stern–Volmer plot indicates the presence of two or more emitting species, suggesting the presence of the aggregate. Thus, we have applied an exponential quenching equation, I0/I = A ek[Q] + B, where A, B, and k are the constants.55 From the nonlinear curve fitting, I0/I = −10.19 e–14.29[Cu2+] + 11.1 (R2 = 0.98269), the Stern–Volmer quenching constant was calculated to be 1.46 × 104 M–1 by multiplying the constants A and k.
Figure 10.

(A) Emission spectral change of NAP-1 in MeOH with 60% water fraction (vol %) in the presence of Cu2+ (concentration: 10 μM, excitation wavelength: 360 nm). (B) Fluorescence images of NAP-1 in MeOH/H2O (60% v/v) at pH 7.4 in the presence of various metal ions under UV light (concentration of the metal ions: 10 μM).
Figure 11.
(A) Nonlinear Stern–Volmer plot for Cu2+ by the exponential quenching equation. (B) Stern–Volmer plot of NAP-1 in the presence of Cu2+ and other metal ions. Concentration of NAP-1 in methanol: 10 μM, excitation wavelength: 360 nm.
Plausible Cu2+-Sensing Mechanism
As Cu2+ is a paramagnetic ion possessing an empty d-shell, it has every possibility to quench the fluorophore emission via an electron or energy transfer mechanism. Additionally, among the relevant paramagnetic metal ions, Cu2+ has a comparatively high thermodynamic affinity toward NAP-1, owing to the possession of N, O-binding sites, leading to a fast kinetic process.56
To shed light on the plausible sensing mechanism, 1H NMR titration of NAP-1 in DMSO-d6 was carried out in the presence of an incremental addition of Cu2+ in DMSO-d6. As observed in Figure 12, upon gradual addition of Cu2+, the hydroxyl proton signals totally disappear, signifying direct binding of Cu2+ with NAP-1 through hydroxyl O-binding sites. Thus, the ESIPT process was disrupted, which was also verified from the blue-shifting of the emission spectrum of NAP-1 upon titration with Cu2+. Along with this, the −CH=N proton signal was largely down-field-shifted from 9.5 to 10.55 ppm, owing to the excitation energy or charge transfer from NAP-1 to the vacant d-orbital of Cu2+, leading to fast nonradiative relaxation of the excited state. This suggests the involvement of the imine nitrogen in the complexation. This is also well corroborated with the hard and soft acid base (HSAB) principle, where N being a borderline base would have affinity with borderline acid, Cu2+. Moreover, other aromatic protons also experience significant down-field shifting due to a decrease of electron density in the whole aromatic framework after complexation,57 further confirming the facilitation of the LMCT transition. Owing to the paramagnetic nature of Cu2+, the proton signals became broader upon further introduction of Cu2+,5858 indicating a possible interaction of Cu2+ with the host entity.
Figure 12.
1H NMR spectral response of NAP-1 (1 mM) in DMSO-d6 upon an incremental addition of Cu2+ (1 mM) in DMSO-d6, where addition of Cu2+ is (a) 0 μL, (b) 25 μL, (c) 60 μL, and (d) 100 μL.
Moreover, the interaction mechanism was investigated by cyclic voltammetric studies with tetrabutylammonium hexafluorophosphate as a supporting electrolyte using a glassy carbon electrode as a working electrode, a platinum wire as a counter electrode, and a saturated Ag/AgCl electrode as a reference electrode at the scan rate of 0.05 V/s, exploiting ferrocene as an internal standard. Upon addition of Cu2+, the initial oxidation peak of NAP-1 at 0.29 V moved to 0.41 V (Figure S26, SI). This indicates that in the presence of Cu2+ the oxidation of the chemosensor, NAP-1, became difficult. As in the presence of Cu2+, LMCT was facilitated, leading to oxation of NAP-1. Thus, after metal binding, NAP-1 became prone to be reduced, thereby impeding the oxidation process, which is also reflected from the electrochemical studies.
In addition to this, ESI-MS data was collected to further strengthen the binding of NAP-1 with Cu2+. It was observed that after the interaction with Cu2+, a new characteristic peak at 391 amu was obtained, suggesting the formation of the [NAP-1 + Cu2+] adduct, which also validated the formation of a 1:1 complex (Figure S27, SI). FT-IR studies revealed that the initial O–H and CH=N stretching frequencies of NAP-1 at 3422 and 1633 cm–1 reduced to 3409 and 1604 cm–1, respectively, validating the likely interaction of NAP-1 with Cu2+. Additionally, a new peak appeared at 1386 cm–1 due to the −NO3 stretching frequency of the ensuing [NAP-1–Cu2+] adduct (Figure S28, SI).
FE-SEM analysis was also performed to take a look at the change of morphology after Cu2+ addition. The addition of Cu2+ did not destroy the aggregated form as documented from FE-SEM analysis (Figure S29, SI) and DLS studies (Figure S30, SI) (revealing the average particle size of the adduct as 1053 nm); rather, a two-multimembered chelate ring was formed, maintaining the rigidity of the aggregate, though the intramolecular H-bonding was disrupted, which caused the reduction of fluorescence intensity.
To gain in-depth insight into the sensing mechanism toward detecting Cu2+, DFT studies have been carried out using the Turbomole (v7.0) software. The geometry-optimized structures of NAP-1 and the corresponding Cu2+ adduct have been shown in Figure 13, which exhibits that after the interaction with Cu2+, LUMO was mainly centralized on the metal center. Thus, theoretically, it was also verified that in the presence of Cu2+, electron transfer occurred from NAP-1 to the vacant metal orbital, as evidenced from 1H NMR spectral studies. This LMCT phenomenon led to fluorescence quenching of the aggregated compound by facilitating the nonradiative decay pathway in the excited state. Moreover, it was also seen that the interaction with the metal ion led to the reduction of the HOMO–LUMO energy band gap (2.73 eV) compared to that of the free sensor (3.29 eV) along with overall stabilization of the host/guest adduct by ∼1716 kJ/mol.
Figure 13.
Geometry-optimized structures of NAP-1 and the NAP-1–Cu2+ adduct with the corresponding HOMO–LUMO energy gaps.
A comparative literature survey has been summarized in Tables 1 and 2, which clearly exhibit the superiority of the present chemosensor, NAP-1, in terms of efficiency or practicability, making it unique in its congener.
Table 1. Comparative Literature Study of NAP-1 with Other Reported Water Chemosensors.
| entry | detection medium | LOD | response time | application in the food industry | application in inkless writing | ref |
|---|---|---|---|---|---|---|
| 1. | acetone, acetonitrile, ethanol, and isopropanol | 0.20, 0.38, 0.24, and 0.09% in acetone, acetonitrile, ethanol, and isopropanol, respectively | 10 min | no | no | (59) |
| 2. | acetone, acetonitrile, THF, DMSO, and 1,4-dioxane | 0.003% in CH3CN and THF, 0.006% in acetone, 0.007% in DMSO, and 0.008% in 1,4-dioxane | 10 s | yes | no | (60) |
| 3. | acetone and THF | 0.0042 and 0.0058 wt % in acetone and THF, respectively | fast | no | yes | (61) |
| 4. | THF and dioxane | 0.010% (113 ppm) and 0.019% (212 ppm) in THF and dioxane, respectively | not mentioned | no | no | (62) |
| 5. | THF and dioxane | 0.0063 and 0.21% (v/v) in THF and dioxane, respectively | not mentioned | no | no | (63) |
| 6. | DMSO | 0.18% v/v water in DMSO | 1 min | no | no | (64) |
| 7. | dioxane and acetonitrile | 0.22 and 0.15 wt % in dioxane and acetonitrile, respectively | not mentioned | no | no | (65) |
| 8. | acetonitrile | 0.25 wt % in acetonitrile | 1 day | no | no | (66) |
| 9. | acetonitrile | 0.003 wt % in acetonitrile | not mentioned | no | no | (67) |
| 10. | THF, acetone, DMSO, and methanol | 0.033, 0.032, 0.034, and 0.033% (v/v) in THF, acetone, DMSO, and methanol, respectively | 5 s | yes | yes | this work |
Table 2. Comparative Literature Study of NAP-1 with Other Reported Cu2+ Chemosensors.
| entry | detection medium | LOD | ref |
|---|---|---|---|
| 1. | acetonitrile | 270 ppb | (68) |
| 2. | acetonitrile | 81.28 ppb | (69) |
| 3. | CH3OH/aqueous HEPES buffer (10 mM, pH 7.4; 8:2, v/v) | 831.9 ppb | (70) |
| 4. | acetonitrile–water (2:1, v/v) | 127 ppb | (71) |
| 5. | DMSO | 198.12 ppb | (72) |
| 6. | Water | 4 ppm | (73) |
| 7. | MeOH | 7.9 ppb | (74) |
| 8. | MeOH | 15.3 ppb | (75) |
| 9. | acetonitrile | 158.75 ppb | (76) |
| 10. | purely aqueous medium | 57.2 ppb | this work |
Conclusions
In summary, an ESIPT-active piezochromic AIEgen, 1-{[(2-hydroxyphenyl)imino]methyl}naphthalen-2-ol (NAP-1), has been synthesized and its optical sensing properties have been thoroughly investigated. In the presence of a trace amount of water, bright yellowish-green-colored keto emission is facilitated by water-induced aggregation, as evidenced from FE-SEM, DLS, and theoretical DFT studies. In the presence of water, the probe exhibits high quantum yields of 0.94, 0.64, and 0.77, respectively, with the corresponding AIE factors (αAIE values) of 10.4, 4.6, and 11 in THF, MeOH, and DCM, respectively. Thus, NAP-1 can be improvised for trace-level moisture detection from commercial organic solvents. The probe could also be used in the food industry to detect trace moisture in the raw food samples, indicating its practical applicability. Regeneration of the sensory receptor could be exploited toward INHIBIT logic gate formulation as well as inkless writing as a revolutionary greener step using water as ink. One step ahead, an IoT-based prototype could be fabricated for on-spot detection of moisture content in the given sample by a smartphone via a wireless connection. The aggregated probe can selectively recognize a Cu2+-like toxic water pollutant (LOD = 57.2 ppb) from a purely aqueous medium via the CHEQ mechanism, which has also been theoretically verified. Thus, this work inspires us to work with more new probes, possessing both AIE and ESIPT characteristics, which will be utilized efficiently in various analytical applications for optical device fabrication in the near future.
Experimental Section
Materials
The preparatory chemicals (reagents and solvents) were of analytical grade and used as received without further purification. Salts of cations were purchased from Sigma-Aldrich. The solvents (e.g., DMSO, methanol, acetonitrile, tetrahydrofuran, 1,4-dioxane, DMF, acetone, dichloromethane) used for experimental purposes were of spectroscopic grade and purchased from Merck (India) Pvt. Ltd. and used after distillation to obtain dry solvents. Double-distilled water was utilized throughout the experimentation.
Instrumentation
1H and 13C NMR spectra were recorded using a Bruker 500 MHz NMR spectrometer. ESI-MS mass spectra were recorded on an Advion compact mass spectrometer (serial no. 3013-0140). Infrared spectra were recorded on a PerkinElmer FT-IR Spectrum 100 spectrophotometer. UV–vis spectra were recorded on a CARY 60 spectrophotometer. Solid- and solution-state fluorescence spectra were recorded in a Varian Cary Eclipse fluorescence spectrophotometer and a PerkinElmer LS 45 instrument. The X-ray crystallographic data were taken from Bruker D8 Venture (APEX-III) with a photon detector. Powder X-ray diffraction (PXRD) analysis was carried out by Bruker AXS (Germany) with a model no. of D8 FOCUS. FE-SEM analysis was carried out using Sigma HD, Zeiss, Oxford Instruments, Germany. The change of particle size before and after the interaction with water and Cu2+ was monitored by DLS studies (Zetasizer Nano ZS90). Cyclic voltammetry was performed in a CH instrument.
Synthesis and Characterization of NAP-1
The fluorescent chemosensor 1-{[(2-hydroxyphenyl)imino]methyl}naphthalen-2-ol (NAP-1) was synthesized in a one-pot synthetic pathway according to the conventional process with a slight modification.25 Briefly, 2-hydroxy-1-naphthaldehyde (172 mg, 1 mmol) in 10 mL of methanol was added dropwise in a 10 mL methanol solution of 2-aminophenol (109 mg, 1 mmol) under stirring conditions at room temperature (Scheme 4). After overnight stirring, a yellowish-orange-colored precipitate was obtained, and it was then filtered off and dried. Orange-colored crystals were obtained from the slow diffusion of the THF solution of NAP-1 into hexane. ESI-MS (m/z): [C17H13NO2] + H+: calcd, 264.29; found, 264.3 (Figure S1, SI); IR (KBr pellet, cm–1): 3422 (O–H), 1633 (CH=N) (Figure S2, SI); 1H NMR (500 MHz, DMSO-d6) (ppm) δ 15.7 (s, 1H, −OH), 10.4 (s, 1H, −OH), 9.5 (s, 1H, −CH=N), 8.39–8.38 (d, 1H), 7.94–7.92 (t, 1H), 7.82–7.80 (d, 1H), 7.69–7.68 (d, 1H), 7.51 (d, 1H), 7.49–7.48 (t, 1H), 7.29–7.26 (t, 1H), 7.13–6.94 (m, 2H), 6.80–6.78 (d, 1H) (Figure S3, SI); 13C NMR (DMSO-d6) (ppm) δ 178.35, 149.82, 148.90, 138.52, 134.46, 129.52, 128.96, 128.64, 127.26, 126.33, 125.70, 123.56, 120.34, 120.25, 118.04, 116.43, 108.17 (Figure S4, SI).
Scheme 4. Synthesis of NAP-1.

Preparation of Aggregates
A stock solution of NAP-1 was primarily prepared at 1 mM concentration in different organic solvents (like THF, DMSO, MeOH). This solution (5 mL) was transferred into an Erlenmeyer flask, and then water was added dropwise to the solution at stirring conditions, maintaining the final concentration at 10 μM, to prepare the aggregates suitable for FE-SEM analysis.
Determination of Water Content in Food Products
The food products (salt, sugar, honey) were obtained from a local market. Each of the samples was oven-dried, and then 10 mg of each was placed in 1 mL of dry THF separately and sonicated for 10 min for preparing a homogeneous mixture. Finally, this was mixed with NAP-1 in dry THF with a final concentration of 10 μM. After mixing, the fluorescence emission spectra were immediately recorded at 515 nm upon excitation at 360 nm.
Acknowledgments
RD gratefully acknowledges DST INSPIRE for her fellowship (INSPIRE Roll no.: [IF180252]). The authors acknowledge DSTBT sponsored project GAP-225612 (vide. sanction order no. 78(Sanc.)/ST/P/S&T/6G-1/2018 dated 31/01/2019) for support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00565.
The authors declare no competing financial interest.
Supplementary Material
References
- Šedivec V.; Flek J.. Handbook of Analysis of Organic Solvents; John Wiley, Inc.: New York, 1976. [Google Scholar]
- Jung H. S.; Verwilst P.; Kim W. Y.; Kim Fluorescent and colorimetric sensors for the detection of humidity or water content. Chem. Soc. Rev. 2016, 45, 1242–1256. 10.1039/C5CS00494B. [DOI] [PubMed] [Google Scholar]
- accessed March
- Williams D. B. G.; Lawton M. Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants. J. Org. Chem. 2010, 75, 8351. 10.1021/jo101589h. [DOI] [PubMed] [Google Scholar]
- Padivitage N. L.; Smuts J. P.; Armstrong D. W.. Specification of Drug Substances and Products: Development and Validation of Analytical Methods; Elsevier: Amsterdam, 2013. [Google Scholar]
- Rhim J. W. Increase in water vapor barrier property of biopolymer-based edible films and coatings by compositing with lipid materials. Food Sci. Biotechnol. 2004, 13, 528–535. [Google Scholar]
- Bitten J. New method for determining free-water content in fuel. Anal. Chem. 1968, 40, 960–962. 10.1021/ac60262a001. [DOI] [Google Scholar]
- Richter D. Neues Verfahren zur maßanalytischen Bestimmung des Wassergehaltes von Flüssigkeiten und festen Körpern. Beitrag zur Abhandlung von Dr. K. Fischer. Angew. Chem. 1935, 48, 776. 10.1002/ange.19350485003. [DOI] [Google Scholar]
- Inagaki S.; Morii N.; Numata M. Development of a reliable method to determine water content by headspace gas chromatography/mass spectrometry with the standard addition technique. Anal. Methods 2015, 7, 4816–4820. 10.1039/C5AY00832H. [DOI] [Google Scholar]
- Veillet S.; Tomao V.; Visinoni F.; Chemat F. New and rapid analytical procedure for water content determination: microwave accelerated Dean-Stark. Anal. Chim. Acta 2009, 632, 203–207. 10.1016/j.aca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Mishra H.; Misra V.; Mehata M. S.; Pant T. C.; Tripathi H. B. Fluorescence Studies of Salicylic Acid Doped Poly(vinyl alcohol) Film as a Water/Humidity Sensor. J. Phys. Chem. A 2004, 108, 2346–2352. 10.1021/jp0309365. [DOI] [Google Scholar]
- Ooyama Y.; Matsugasako A.; Oka K.; Nagano T.; Sumomogi M.; Komaguchi K.; Imae I.; Harima Y. Fluorescence PET (photo-induced electron transfer) sensors for water based on anthracene–boronic acid ester. Chem. Commun. 2011, 47, 4448–4450. 10.1039/c1cc10470e. [DOI] [PubMed] [Google Scholar]
- Mao L.; Liu Y.; Yang S.; Li Y.; Zhang X.; Wei Y. Recent advances and progress of fluorescent bio-/chemosensors based on aggregation-induced emission molecules. Dyes Pigm. 2019, 162, 611–623. 10.1016/j.dyepig.2018.10.045. [DOI] [Google Scholar]
- La D. D.; Bhosale S. V.; Jones L. A.; Bhosale S. V. Tetraphenylethylene-Based AIE-Active Probes for Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 12189–12216. 10.1021/acsami.7b12320. [DOI] [PubMed] [Google Scholar]
- Hong Y.; Lama J. W. Y.; Tang B. Z. Aggregation-induced emission: phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. 10.1039/b904665h. [DOI] [PubMed] [Google Scholar]
- Hong Y.; Lam J. W. Y.; Tang B. Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. 10.1039/c1cs15113d. [DOI] [PubMed] [Google Scholar]
- Sedgwick A. C.; Wu L.; Han H.-H.; Bull S. D.; He X.-P.; James T. D.; Sessler J. L.; Tang B. Z.; Tian H.; Yoon J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. 10.1039/C8CS00185E. [DOI] [PubMed] [Google Scholar]
- Li K.; Feng Q.; Niu G.; Zhang W.; Li Y.; Kang M.; Xu K.; He J.; Hou H.; Tang B. Z. Benzothiazole-Based AIEgen with Tunable Excited-State Intramolecular Proton Transfer and Restricted Intramolecular Rotation Processes for Highly Sensitive Physiological pH Sensing. ACS Sens. 2018, 3, 920–928. 10.1021/acssensors.7b00820. [DOI] [PubMed] [Google Scholar]
- Mei J.; Hong Y.; Lam J. W.; Qin A.; Tang Y.; Tang B. Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. 10.1002/adma.201401356. [DOI] [PubMed] [Google Scholar]
- Song Z.; Kwok R. T.; Zhao E.; He Z.; Hong Y.; Lam J. W.; Liu B.; Tang B. Z. Title: A Ratiometric Fluorescent Probe Based on ESIPT and AIE Processes for Alkaline Phosphatase Activity Assay and Visualization in Living Cells. ACS Appl. Mater. Interfaces 2014, 6, 17245–17254. 10.1021/am505150d. [DOI] [PubMed] [Google Scholar]
- Kwon J. E.; Park S. Y. Advanced Organic Optoelectronic Materials: Harnessing Excited-State Intramolecular Proton Transfer (ESIPT) Process. Adv. Mater. 2011, 23, 3615–3642. 10.1002/adma.201102046. [DOI] [PubMed] [Google Scholar]
- Lutsenko S.; Gupta A.; Burkhead J. L.; Zuzel V. Cellular multitasking: the dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch. Biochem. Biophys. 2008, 476, 22–32. 10.1016/j.abb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M.; Puri A. A review of permissible limits of drinking water. Indian J. Occup. Environ. Med. 2012, 16, 40–44. 10.4103/0019-5278.99696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik B.; Das D. Aggregation-Induced Emission or Hydrolysis by Water? The Case of Schiff Bases in Aqueous Organic Solvents. J. Phys. Chem. C 2018, 122, 3655–3661. 10.1021/acs.jpcc.7b12430. [DOI] [Google Scholar]
- a Guo A.; Zhu R.; Ren Y.; Dong J.; Feng L. A “turn-on” fluorescent chemosensor for aluminum ion and cell imaging application. Spectrochim. Acta, Part A 2016, 153, 530–534. 10.1016/j.saa.2015.09.009. [DOI] [PubMed] [Google Scholar]; b Jang Y. K.; Nam U. C.; Kwon H. L.; Hwang I. H.; Kim C. A selective colorimetric and fluorescent chemosensor based-on naphthol for detection of Al3+ and Cu2+. Dyes Pigm. 2013, 6–13. 10.1016/j.dyepig.2013.04.002. [DOI] [Google Scholar]
- Guo Z.-H.; Lei T.; Jin Z.-X.; Wang J.-Y.; Pei T-Shaped Donor–Acceptor Molecules for Low-Loss Red-Emission Optical Waveguide. Org. Lett. 2013, 15, 3530. 10.1021/ol4012025. [DOI] [PubMed] [Google Scholar]
- Panja S. K. J-type aggregation and thermochromic behavior of a schiff base in solution: Role of keto-enol tautomerization. Spectrochim. Acta, Part A 2020, 229, 117860 10.1016/j.saa.2019.117860. [DOI] [PubMed] [Google Scholar]
- a Singh R. B.; Mahanta S.; Kar S.; Guchhait N. Photo-Physical Properties of 1-Hydroxy-2-Naphthaldehyde: A Combined Fluorescence Spectroscopy and Quantum Chemical Calculations. Chem. Phys. 2007, 331, 373–384. 10.1016/j.chemphys.2006.11.007. [DOI] [Google Scholar]; b Chowdhury P.; Panja S.; Chakravorti S. Excited State Prototropic Activities in 2-Hydroxy 1- aphthaldehyde. J. Phys. Chem. A 2003, 107, 83–90. 10.1021/jp026404q. [DOI] [Google Scholar]; c Wu K.-C.; Cheng Y.-M.; Lin Y.-S.; Yeh Y.-S.; Pu S.-C.; Hu Y.-H.; Yu J.-K.; Chou P.-T. Competitive Intramolecular Hydrogen Bonding formation and Excited-State Proton Transfer Reaction in 1-[(Diethylamino)-methyl]-2-hydroxy-3-naphthaldehyde. Chem. Phys. Lett. 2004, 384, 203–209. 10.1016/j.cplett.2003.11.058. [DOI] [Google Scholar]
- a Morales A. R.; Schafer-Hales K. J.; Yanez C. O.; Bondar M. V.; Przhonska O. V.; Marcus A. I.; Belfield K. D. Excited State Intramolecular Proton Transfer and Photophysics of a New Fluorenyl Two-Photon Fluorescent Probe. ChemPhysChem 2009, 10, 2073–2081. 10.1002/cphc.200900032. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cohen M. D.; Flavian S. Topochemistry. Part XXV. The Absorption Spectra of Some N-Salicylideneanilines and Related Anils in Solution. J. Chem. Soc. B 1967, 321–328. 10.1039/j29670000321. [DOI] [Google Scholar]; c Itoh M.; Fujiwara Y. Transient Absorption and Two-Step Laser Excitation Fluorescence Studies of Photoisomerization in 2-(2- Hydroxyphenyl)Benzoxazole and 2-(2-Hydroxyphenyl) Benzothiazole. J. Am. Chem. Soc. 1985, 107, 1561–1565. 10.1021/ja00292a018. [DOI] [Google Scholar]
- a Pallavi P.; Kumar V.; Hussain M. D. W.; Patra A. Excited- State Intramolecular Proton Transfer-Based Multifunctional Solid- State Emitter: A Fluorescent Platform with “Write-Erase-Write” Function. ACS Appl. Mater. Interfaces 2018, 10, 44696–44705. 10.1021/acsami.8b14215. [DOI] [PubMed] [Google Scholar]; b Ghiggino K. P.; Scully A. D.; Leaver I. H. Effect of Solvent on Excited-State Intramolecular Proton Transfer in Benzotriazole Photostabilizers. J. Phys. Chem. A 1986, 90, 5089–5093. 10.1021/j100412a042. [DOI] [Google Scholar]
- a An B. K.; Lee D. S.; Lee J. S.; Park Y. S.; Song H. S.; Park S. Y. Strongly Fluorescent Organogel System Comprising Fibrillar Self-Assembly of a Trifluoromethyl-Based Cyanostilbene Derivative. J. Am. Chem. Soc. 2004, 126, 10232–10233. 10.1021/ja046215g. [DOI] [PubMed] [Google Scholar]; b Jana S.; Dalapati S.; Guchhait N. Proton Transfer Assisted Charge Transfer Phenomena in Photochromic Schiff Bases and Effect of −NEt2 Groups to the Anil Schiff Bases. J. Phys. Chem. A 2012, 116, 10948–10958. 10.1021/jp3079698. [DOI] [PubMed] [Google Scholar]
- Singh P.; Singh H.; Sharma R.; Bhargava G.; Kumar S. Diphenylpyrimidinone–salicylideneamine – new ESIPT based AIEgens with applications in latent fingerprinting. J. Mater. Chem. C 2016, 4, 11180–11189. 10.1039/C6TC03701A. [DOI] [Google Scholar]
- Xu L.; Li Y.; Li S.; Hu R.; Qin A.; Tang B. Z.; Su B. Enhancing the visualization of latent fingerprints by aggregation induced emission of siloles. Analyst 2014, 139, 2332–2335. 10.1039/C3AN02367B. [DOI] [PubMed] [Google Scholar]
- Barbon S. M.; Staroverov V. N.; Gilroy J. B. Effect of Extended π Conjugation on the Spectroscopic and Electrochemical Properties of Boron Difluoride Formazanate Complexes. J. Org. Chem. 2015, 80, 5226–5235. 10.1021/acs.joc.5b00620. [DOI] [PubMed] [Google Scholar]
- Sun C.; Su X.; Zhou Q.; Shi Y. Regular tuning of the ESIPT reaction of 3-hydroxychromone-based derivatives by substitution of functional groups. Org. Chem. Front. 2019, 6, 3093–3100. 10.1039/C9QO00722A. [DOI] [Google Scholar]
- Meisner Q. J.; Younes A. H.; Yuan Z.; Sreenath K.; Hurley J. J. M.; Zhu L. Excitation-Dependent Multiple Fluorescence of a Substituted 2-(2′-Hydroxyphenyl)benzoxazole. J. Phys. Chem. A 2018, 122, 9209–9223. 10.1021/acs.jpca.8b07988. [DOI] [PubMed] [Google Scholar]
- a Fu Y.; Qiu F.; Zhang F.; Mai Y.; et al. A dual-boron-cored luminogen capable of sensing and imaging. Chem. Commun. 2015, 51, 5298–5301. 10.1039/C4CC08551E. [DOI] [PubMed] [Google Scholar]; b Tharmalingam B.; Mathivanan M.; Dhamodiran G.; Mani K. S.; Paranjothy M.; Murugesapandian B. Star-Shaped ESIPT-Active Mechanoresponsive Luminescent AIEgen and Its On–Off–On Emissive Response to Cu2+/S2–. ACS Omega 2019, 4, 12459–12469. 10.1021/acsomega.9b00845. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Nagai S.; Yamashita M.; Tachikawa T.; Ubukata T.; Asami M.; Ito S. Efficient and versatile mechanochromic luminescence of phenanthroimidazolylbenzothiadiazoles: tricolor switching and directional control over the chromism. J. Mater. Chem. C 2019, 7, 4988–4998. 10.1039/C9TC00157C. [DOI] [Google Scholar]
- a Sturala J.; Etherington M. K.; Bismillah A. N.; Higginbotham H. F.; Trewby W.; Aguilar J. A.; Bromley E. H. C.; Avestro A.-J.; Monkman A. P.; McGonigal P. R. Excited-State Aromatic Interactions in the Aggregation-Induced Emission of Molecular Rotors. J. Am. Chem. Soc. 2017, 139, 17882–17889. 10.1021/jacs.7b08570. [DOI] [PubMed] [Google Scholar]; b Li K.; Feng Q.; Niu G.; Zhang W.; Li Y.; Kang M.; Xu K.; He J.; Hou H.; Tang B. Z. Benzothiazole-Based AIEgen with Tunable Excited- State Intramolecular Proton Transfer and Restricted Intramolecular Rotation Processes for Highly Sensitive Physiological pH Sensing. ACS Sens. 2018, 3, 920–928. 10.1021/acssensors.7b00820. [DOI] [PubMed] [Google Scholar]
- Chen W.; Zhang Z.; Li X.; Ågren H.; Su J. Highly sensitive detection of low-level water content in organic solvents and cyanide in aqueous media using novel solvatochromic AIEE fluorophores. RSC Adv. 2015, 5, 12191–12201. 10.1039/C4RA15199B. [DOI] [Google Scholar]
- Zhang Y.; Li D.; Li Y.; Yu J. Solvatochromic AIE luminogens as supersensitive water detectors in organic solvents and highly efficient cyanide chemosensors in water. Chem. Sci. 2014, 5, 2710–2716. 10.1039/c4sc00721b. [DOI] [Google Scholar]
- Hong Y.; Lam J. W. Y.; Tang B. Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. 10.1039/c1cs15113d. [DOI] [PubMed] [Google Scholar]
- Qian Y.; Cai M. M.; Zhou X.; Gao Z. Q.; Wang X. P.; Zhao Y.; Yan X.; et al. More than Restriction of Twisted Intramolecular Charge Transfer: Three-Dimensional Expanded #-Shaped Cross-Molecular Packing for Emission Enhancement in Aggregates. J. Phys. Chem. C 2012, 116, 12187–12195. 10.1021/jp212257f. [DOI] [Google Scholar]
- Zhu X.; Liu R.; Li Y.; Huang H.; Wang Q.; Wang D.; Zhu X.; Liu S.; Zhu H. An AIE-active boron-difluoride complex: multi-stimuli-responsive fluorescence and application in data security protection. Chem. Commun. 2014, 50, 12951–12954. 10.1039/C4CC05913A. [DOI] [PubMed] [Google Scholar]
- Singh P.; Singh H.; Sharma R.; Bhargava G.; Kumar S. Diphenylpyrimidinone–salicylideneamine – new ESIPT based AIEgens with applications in latent fingerprinting. J. Mater. Chem. C 2016, 4, 11180–11189. 10.1039/C6TC03701A. [DOI] [Google Scholar]
- Sedgwick A. C.; Wu L.; Han H.-H.; Bull S. D.; He X.-P.; James T. D.; Sessler J. L.; Tang B. Z.; Tian H.; Yoon J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. 10.1039/C8CS00185E. [DOI] [PubMed] [Google Scholar]
- a Ali R.; Gupta R. C.; Dwivedi S. K.; Misra A. Excited state proton transfer (ESIPT) based molecular probe to sense F– and CN– anions through a fluorescence “turn-on” response. New J. Chem. 2018, 42, 11746. 10.1039/C8NJ01435C. [DOI] [Google Scholar]; b Sarma S.; Bhowmik A.; Sarma M. J.; Banu S.; Phukan P.; Das D. K. Condensation product of 2-hydroxy-1-napthaldehyde and 2-aminophenol: Selective fluorescent sensor for Al3+ ion and fabrication of paper strip sensor for Al3+ ion. Inorg. Chim. Acta 2018, 469, 202–208. 10.1016/j.ica.2017.09.025. [DOI] [Google Scholar]; c Xing L.; Zheng X.; Sun W.; Yuan H.; Hu L.; Yan Z. UV–vis spectral property of a multi-hydroxyl Schiff-base derivative and its colorimetric response to some special metal ions. Spectrochim. Acta, Part A 2018, 203, 455–460. 10.1016/j.saa.2018.06.015. [DOI] [PubMed] [Google Scholar]; d Ulrich G.; Nastasi F.; Retailleau P.; Puntoriero F.; Ziessel R.; Campagna S. Luminescent Excited-State Intramolecular Proton-Transfer (ESIPT) Dyes Based on 4-Alkyne-Functionalized [2,2′-Bipyridine]-3,3′-diol Dyes. Chem. – Eur. J. 2008, 14, 4381–4392. 10.1002/chem.200701803. [DOI] [PubMed] [Google Scholar]
- Divya T. T.; Ramshad K.; Saheer V. C.; Chakkumkumarath L. Self-reversible mechanochromism and aggregation induced emission in neutral triarylmethanes and their application in water sensing. New J. Chem. 2018, 42, 20227–20238. 10.1039/C8NJ04479A. [DOI] [Google Scholar]
- Tharmalingam B.; Mathivanan M.; Dhamodiran G.; Mani K. S.; Paranjothy M.; Murugesapandian B. Star-Shaped ESIPT-Active Mechanoresponsive Luminescent AIEgen and Its On–Off–On Emissive Response to Cu2+ /S2–. ACS Omega 2019, 4, 12459–12469. 10.1021/acsomega.9b00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sun S.-S.; Lee A. J. Photophysics and evidence of excimer formation, linear bipyridines in solution and solid films. J. Photochem. Photobiol., A 2001, 140, 157–161. 10.1016/S1010-6030(01)00410-5. [DOI] [Google Scholar]; b Qi F.; Lin J.; Wang X.; Cui P.; Yan H.; Gong S.; Ma C.; Liu Z.; Huang W. New AIE-active pyrimidine-based boronfluoride complexes with high solid-state emission and reversible mechanochromism luminescence behavior. Dalton Trans. 2016, 45, 7278–7284. 10.1039/C6DT00292G. [DOI] [PubMed] [Google Scholar]
- Panda T.; Maiti D. K.; Panda M. K. Inkless Writing and Self-Erasing Security Feature of (Z)-1,2-Diarylacrylonitrile-Based Materials: A Confidential Data Communication. ACS Appl. Mater. Interfaces 2018, 10, 29100–29106. 10.1021/acsami.8b08279. [DOI] [PubMed] [Google Scholar]
- a Li G.; Bai L.; Tao F.; Deng A.; Wang L. A dual chemosensor for Cu2+ and Hg2+ based on rhodamine-terminated water-soluble polymer in 100% aqueous solution. Analyst 2018, 143, 5395–5403. 10.1039/C8AN01130C. [DOI] [PubMed] [Google Scholar]; b Singh A.; Verma P.; Laha S.; Samanta D.; Roy S.; Maji T. K. Photochromic Conjugated Microporous Polymer Manifesting BioInspired pcFRET and Logic Gate Functioning. ACS Appl. Mater. Interfaces 2020, 12, 20991–20997. 10.1021/acsami.0c05182. [DOI] [PubMed] [Google Scholar]; c Stankovic J. A. Research Directions for the Internet of Things. IEEE Internet Things J. 2014, 1, 3–9. 10.1109/JIOT.2014.2312291. [DOI] [Google Scholar]; d Gubbi J.; Buyya R.; Marusic S.; Palaniswami M. Internet of Things (IoT): A vision, architectural elements, and future directions. Future Gener. Comput. Syst. 2013, 29, 1645–1660. 10.1016/j.future.2013.01.010. [DOI] [Google Scholar]
- a Bej S.; Das R.; Hirani H.; Ghosh S.; Banerjee P. “Naked-eye” detection of CN– from aqueous phase and other extracellular matrices: an experimental and theoretical approach mimicking the logic gate concept. New J. Chem. 2019, 43, 18098–18109. 10.1039/C9NJ04528G. [DOI] [Google Scholar]; b You Y.; Han Y.; Lee Y.-M.; Park S. Y.; Nam W.; Lippard S. J. Phosphorescent Sensor for Robust Quantification of Copper(II) Ion. J. Am. Chem. Soc. 2011, 133, 11488–11491. 10.1021/ja204997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bej S.; Das R.; Murmu N. C.; Banerjee P. Selective Identification and Encapsulation of Biohazardous m-Xylene among a Pool of Its Other Constitutional C8 Alkyl Isomers by Luminescent d10 MOFs: A Combined Theoretical and Experimental Study. Inorg. Chem. 2020, 59, 4366–4376. 10.1021/acs.inorgchem.9b03306. [DOI] [PubMed] [Google Scholar]; b Bej S.; Hazra A.; Das R.; Saha S. K.; Corbella M.; Banerjee P. Exploratory studies of a multidimensionally talented simple MnII-based porous network: selective “turn-on” recognition @ cysteine over homocysteine with an indication of cystinuria and renal dysfunction. New J. Chem. 2020, 44, 14712–14722. 10.1039/D0NJ02265A. [DOI] [Google Scholar]; c Pandit N. R.; Bej S.; Mondal A.; Ghosh M.; Kostakis G. E.; Powell A. K.; Banerjee P.; Biswas B. Exploratory studies on azido-bridged complexes (Ni2+ and Mn2+) as dual colourimetric chemosensors for S2– and Ag+: combined experimental and theoretical outcomes with real field applications. Dalton Trans. 2020, 49, 13090–13099. 10.1039/D0DT02846K. [DOI] [PubMed] [Google Scholar]; d Paul S.; Das R.; Seth M.; Hirani H.; Murmu N. C.; Banerjee P. A Urea-Functionalized Chemoreceptor for Expeditious Chromogenic Recognition of Toxic Industrial Pollutants Cu2+ and CN– from Real Water Sources and Biofluids: Diagnosis of Wilson’s disease from Human Urine. Ind. Eng. Chem. Res. 2020, 59, 19077–19092. 10.1021/acs.iecr.0c02695. [DOI] [Google Scholar]
- Lakowicz J. R.Principles of Fluorescence Spectroscopy; Plenum Press: New York, 1986. [Google Scholar]
- Wei W.; Lu R.; Tang S.; Liu X. Highly cross-linked fluorescent poly(cyclotriphosphazene-co-curcumin) microspheres for the selective detection of picric acid in solution phase. J. Mater. Chem. A 2015, 3, 4604–4611. 10.1039/C4TA06828A. [DOI] [Google Scholar]
- Kramer R. Fluorescent Chemosensors for Cu2+ Ions: Fast, Selective, and Highly Sensitive. Angew. Chem., Int. Ed. 1998, 37, 772–773. . [DOI] [PubMed] [Google Scholar]
- Tan J.; Wang X.; Zhang Q.; Zhou H.; Yang J.; Wu J.; Tian Y.; Zhang X. Chalcone based ion-pair recognition towards nitrates and the application for the colorimetric and fluorescence turn-on determination of water content in organic solvents. Sens. Actuator, B 2018, 260, 727–735. 10.1016/j.snb.2017.12.186. [DOI] [Google Scholar]
- Selvan G. T.; Varadarju C.; Selvan R. T.; Enoch I. V. M. V.; Selvakumar P. M. On/Off Fluorescent Chemosensor for Selective Detection of Divalent Iron and Copper Ions: Molecular Logic Operation and Protein Binding. ACS Omega 2018, 3, 7985–7992. 10.1021/acsomega.8b00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J.; Wang X.; Zhang Q.; Zhou H.; Yang J.; Wu J.; Tian Y.; Zhang X. Chalcone based ion-pair recognition towards nitrates and the application for the colorimetric and fluorescence turn-on determination of water content in organic solvents. Sens. Actuator, B 2018, 260, 727–735. 10.1016/j.snb.2017.12.186. [DOI] [Google Scholar]
- Kim T.; Kim Y. A Water Indicator Strip: Instantaneous Fluorogenic Detection of Water in Organic Solvents, Drugs, and Foodstuffs. Anal. Chem. 2017, 89, 3768–3772. 10.1021/acs.analchem.7b00270. [DOI] [PubMed] [Google Scholar]
- Kumar P.; Sakla R.; Ghosh A.; Jose D. A. Reversible Colorimetric Sensor for Moisture Detection in Organic Solvents and Application in Inkless Writing. ACS Appl. Mater. Interfaces 2017, 9, 25600–25605. 10.1021/acsami.7b05335. [DOI] [PubMed] [Google Scholar]
- Chen W.; Zhang Z.; Li X.; Agren H.; Su J. Highly sensitive detection of low-level water content in organic solvents and cyanide in aqueous media using novel solvatochromic AIEE fluorophores. RSC Adv. 2015, 5, 12191–12201. 10.1039/C4RA15199B. [DOI] [Google Scholar]
- Zhang Y.; Liang C.; Jiang S. A solvatochromic cyanostilbene derivative as an intensity and wavelength-based fluorescent sensor for water in organic solvents. New J. Chem. 2017, 41, 8644–8649. 10.1039/C7NJ01361B. [DOI] [Google Scholar]
- Kim W. Y.; Shi H.; Jung H. S.; Cho D.; Verwilst P.; Lee J. Y.; Kim J. S. Coumarin-decorated Schiff base hydrolysis as an efficient driving force for the fluorescence detection of water in organic solvents. Chem. Commun. 2016, 52, 8675–8678. 10.1039/C6CC04285F. [DOI] [PubMed] [Google Scholar]
- Divya T. T.; Ramshad K.; Saheer V. C.; Chakkumkumarath L. Self-reversible mechanochromism and aggregation induced emission in neutral triarylmethanes and their application in water sensing. New J. Chem. 2018, 42, 20227–20238. 10.1039/C8NJ04479A. [DOI] [Google Scholar]
- Enoki T.; Ooyama Y. Colorimetric and ratiometric fluorescence sensing of water based on 9-methyl pyrido[3,4-b]indole-boron trifluoride complex. Dalton Trans. 2019, 48, 2086–2092. 10.1039/C8DT04527E. [DOI] [PubMed] [Google Scholar]
- Song Li.; Wu Y. W.; Chai W. X.; Tao Y. S.; Jiang C.; Wang Q. H. Fluorescence Quenching of a Europium Coordination Compound for the Detection of Trace Amounts of Water: Uncovering the Response Mechanism by Structural Confirmation. Eur. J. Inorg. Chem. 2015, 2264–2271. 10.1002/ejic.201500062. [DOI] [Google Scholar]
- Choi S. H.; Pang K.; Kim K.; Churchill D. G. Cu2+ Colorimetric Sensing and Fluorescence Enhancement and Hg2+ Fluorescence Diminution in “Scorpionate”-like Tetrathienyl-Substituted Boron-Dipyrrins. Inorg. Chem. 2007, 46, 10564–10577. 10.1021/ic701101j. [DOI] [PubMed] [Google Scholar]
- Baslak C.; Kursunlu A. N. Naked-eye fluorescent sensor for copper (II) ion based on naphthalene conjugate BODIPY dye, nlu. Photochem. Photobiol. Sci. 2018, 17, 1091–1097. 10.1039/C8PP00137E. [DOI] [PubMed] [Google Scholar]
- Roy S. B.; Rajak K. K. A quinoline appended naphthalene derivative based AIE active “turn– on” fluorescent probe for the selective recognition of Al3+ and colourimetric sensor for Cu2+: Experimental and computational studies. J. Photochem. Photobiol., A 2017, 332, 505–514. 10.1016/j.jphotochem.2016.09.015. [DOI] [Google Scholar]
- Harsha K. G.; Appalanaidu E.; Rao B. A.; Baggi T. R.; Rao V. J. ON–OFF Fluorescent Imidazole Derivative for Sensitive and Selective Detection of Copper(II) Ions, Russ. J. Org. Chem. 2020, 56, 158–168. 10.1134/S1070428020010248. [DOI] [Google Scholar]
- Bhalla V.; Singh H.; Kumar M. Triphenylene based copper ensemble for the detection of cyanide ions. Dalton Trans. 2012, 41, 11413–11418. 10.1039/c2dt31244a. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar V.; Das S.; Yadav R.; Hossain S.; Parihar R.; Subramaniam G.; Sen P. Novel Chemosensor for the Visual Detection of Copper(II) in Aqueous Solution at the ppm Level. Inorg. Chem. 2012, 51, 8664–8666. 10.1021/ic301399a. [DOI] [PubMed] [Google Scholar]
- Sadollahkhani A.; Hatamie A.; Nur O.; Willander M.; Zargar B.; Kazeminezhad I. Colorimetric Disposable Paper Coated with ZnO@ZnS Core–Shell Nanoparticles for Detection of Copper Ions in Aqueous Solutions. ACS Appl. Mater. Interfaces 2014, 6, 17694–17701. 10.1021/am505480y. [DOI] [PubMed] [Google Scholar]
- Mohan V.; Das N.; Jain V. K.; Khan T.; Pandey S. K.; Faizi M. S. H.; Daniel J.; Sen P. Highly Selective and Sensitive (PPB Level) Quinolin-Based Colorimetric Chemosensor for Cu(II). ChemistrySelect 2020, 5, 9435–9442. 10.1002/slct.202001814. [DOI] [Google Scholar]
- Chakraborty N.; Chakraborty A.; Das S. A pyrene based fluorescent turn on chemosensor for detection of Cu2+ ions with antioxidant nature. J. Lumin. 2018, 199, 302–309. 10.1016/j.jlumin.2018.03.042. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.