Abstract

The protonation of a number of 4,6-dihydroxypyrimidine derivatives is studied, and the features of the electronic spectra of free bases and protonated forms are considered. It is shown that the alkyl substituents in position 2 increase the basicity of the compound, and the nitro group in position 5 leads to its decrease. In an acid medium (0.1–99.5% H2SO4), 4,6-dihydroxypyrimidine, 6-hydroxy-2-methylpyrimidine-4(3H)-one, and 6-hydroxy-2-ethylpyrimidine-4(3H)-one have two protonation stages, barbituric acid is protonated in three stages, and 6-hydroxy-2-methyl-5-nitropyrimidine-4(3H)-one and 6-hydroxy-2-ethyl-5-nitropyrimidine-4(3H)-one form a monocation.
Introduction
An important feature of pyrimidinediones is the presence of amphoteric properties and prototropic tautomerism. In neutral and alkaline environments, many of them form mono- and dianions. In a strong acidic medium, protonation occurs, which, due to the presence of several centers, proceeds with the formation of mono-, di-, and tricharged cations. It is obvious that tautomerism and acid–base properties have a strong influence on the properties and reactivity of compounds. These issues have repeatedly been the subject of research.1
In particular, protonation has a strong influence on the nitration of heterocyclic compounds. The main works in this direction are the cycle of research by Katritzky, which considers, among other things, the nitration of pyrimidinones.2 Using the same methods, we studied the effect of protonation on the nitration of 6-hydroxy-2-methylpyrimidine-4(3H)-one (1) and showed that the free base is involved in the process.3
Among the pyrimidine derivatives, the uracil analogue 4,6-dihydroxypyrimidine (2) can be distinguished. Its derivatives include the well-known barbituric acid (3), which is the parent compound for the same-name class of drugs. Recently, a number of 4,6-dihydroxypyrimidine derivatives have been found to have urease inhibition activity,4 and it is also used in the synthesis of compounds that are immune-activated NO production inhibitors.5 Nitration of 2 is proposed to obtain nitroform.6 Industrial production of the insensitive explosive 1,1-diamino-2,2-dinitroethylene is based on the nitration of 1.7
Several studies are devoted to the study of the structure and tautomerism of 2 and its 5-alkyl derivatives. Short and Thompson8 based on IR spectroscopy data suggested that in the solid state, it is in the lactam–lactim and dilactam forms (Figure 1, 2a, 2b, 2c, 2d). Based on nuclear magnetic resonance (NMR) and UV spectroscopy data, a number of Russian and Australian researchers also tended to prefer the existence of form 2a in solutions in organic solvents.9−11 Although this form dominates the solution, a small amount of dilactam (2c)12,13 is allowed. Khromov–Borisov9 based on NMR spectroscopy data and Katritzky14 based on basicity measurements suggested that zwitterionic forms (Figures 2 and 3, 2e, 4a–d) are important in aqueous solutions.
Figure 1.
Tautomeric forms of 4,6-dihydroxypyrimidine.
Figure 2.
Ionic forms of 4,6-dihydroxypyrimidine.
Figure 3.
Zwitterionic and dimeric forms of the derivatives of 4,6-dihydroxypyrimidine: 4a—R2 = R1 = H; 4b—R1 = CH3, R2 = H; 4c—R1 = C6H5, R2 = H; 4d—R1 = H, R2 = CH3; 5a—R1 = R2 = H; 5b—R1 = H, R2 = CH3; 5c—R1 = CH3, R2 = H; 5d—R1 = R2 = CH3.
Later, studies by Katrusiak and Katrusiak15 using X-ray structure analysis showed that 4,6-dihydroxypyrimidine exists in two crystalline polymorphic forms—molecular and ionic. In the first of them, the molecules are in the form (2a), while in the ionic polymorph, the molecules are differentiated into positive and negative ions (Figure 2, 2f and 2g).
An important argument for the existence of zwitterionic forms of 4,6-dihydroxypyrimidine derivatives is their asymmetric dimerization. Russian and Czech researchers16,17 have shown the formation of such compounds 5a–d in a series of N-methyl derivatives of 4,6-dihydroxypyrimidine (Figure 3). A crystal labile product of spontaneous dimerization of 4,6-dihydroxypyrimidine was obtained much later.18 Its structure was determined as 2-(4,6-dioxo-5-pyrimidinyl)-4,6-dioxo-1,2,3,5,5-pentahydropyrimidine (6). It was shown that after dissolution in dimethyl sulfoxide (DMSO), the reverse transformation to 2 occurs.
Even more attention is paid to tautomerism and acid–base properties of barbituric acid. It is known that the most stable neutral form in solutions is keto form 3a, which has been shown in numerous studies by both experimental and computational methods,19,20 but the existence of a crystalline enol polymorph has been proved only recently.21 The formation of mono- and dianions and their spectral properties are well studied.22 Their protonation is less studied, and Zuccarello23 considers the electronic spectra of barbituric acid and shows that in acidic media (up to 10 M HCl, Ho = 3.59), there is a cation that exists in two main equilibrium forms 3b and 3c with some percentage of 3d. Olah,24 in his work using the 1H NMR spectroscopy method, shows the formation of the triprotonated form of 3e in the medium trifluoromethanesulfonic acid (Ho = 14.1), but data on pKb are not given (Scheme 1).
Scheme 1. Protonation of Barbituric Acid.
A significant number of studies have been devoted to the peculiarities of protonation of thymine, uracil, 4,6-dihydroxypyrimidines. However, most of them focus on the issue of keto–enol tautomerism and forms of their existence in solutions and solid state, and not on the protonation site. For barbituric acid, there are no data of basicity constants for the second and third stages of protonation. Due to increased attention of researchers to 2-substituted 4,6-dihydroxypyrimidines as initial compounds for the synthesis of biologically active and energetic materials, major goals of this research were the investigation of the basicity of 6-hydroxy-2-methylpyrimidine-4(3H)-one (1), 4,6-dihydroxypyrimidine (2), barbituric acid (3), 6-hydroxy-2-ethylpyrimidine-4(3H)-one (7), 6-hydroxy-2-methyl-5-nitropyrimidine-4(3H)-one (8), and 6-hydroxy-2-ethyl-5-nitropyrimidine-4(3H)-one (9) (Figure 4) as well as the study of the influence of substituent in positions 2 and 5 on protonation direction and basicity constant determination.
Figure 4.
Comparison of the structures of 4,6-dihydroxypyrimidine derivatives with uracil and thymine.
It is well known that the chemical reactions of weak organic bases such as nitrosation, nitration, halogenation, etc., which take place in acidic media, could be strongly influenced by their acid–base properties. Basicity constants are a criterion for the quantitative assessment of acid–base properties and are used for the calculation of true rate constants of reactions. pKb values allow us to choose a correct acidity of the reaction medium. There are no experimentally determined pKb values for 2-substituted 4,6-dihydroxypyrimidines in the literature. Results of the present study could help us to obtain information about the structure and reactivity of 2-substituted 4,6-dihydroxypyrimidines and the mechanisms of reactions in acidic media. In addition, the basicity constants are reference values.
Results
Protonation of 4,6-dihydroxypyrimidine and its derivatives was studied in a 0.1–100% sulfuric acid medium. The main method of research was spectroscopy in the ultraviolet region (UV spectroscopy). Liquid chromatography–mass spectrometry (LC–MS) and 1H NMR were used to control possible chemical transformations.
There is no doubt that tautomeric transformations and dimerization of 2 and its derivatives can have a significant effect on protonation. Although according to IR, 1H NMR, and LC–MS data, the 2 obtained by us does not contain 6 and in the solid state is in the lactam–lactim form 2a, it was necessary to find the features of its behavior in solutions.
We studied its spontaneous dimerization in solutions in water, buffer solutions, and trifluoroacetic acid (TFA). In all cases, after dissolution 2, dimer 6 begins to form (by LC–MS). The process is slow: at room temperature, in an aqueous solution, the equilibrium is reached in 2–3 days with a 6 content of 4%; in acidic buffer solutions with a pH of 4.8–3.6 and TFA, the equilibrium concentration is reached in 1–2 days with a content of ∼1%, while there is no noticeable change in the UV spectrum. Thus, the acidic medium suppresses dimerization and it does not affect the pKb measurement. No dimeric forms were found in the study of 1, 3, and 7 solutions.
Protonation is a fast process, but if tautomeric transformations are slow and equilibrium is not achieved during the measurement, the result of pKb determination will be distorted. Therefore, our study monitored changes in the UV spectra over time. The study showed that the spectra of 1, 3, and 7 in solutions in water and sulfuric acid of all concentrations do not change, the substances are chemically stable, and if tautomeric transformations occur during dissolution, then equilibrium is achieved during the preparation of solutions (about 5–15 min).
A different behavior was found for aqueous solutions of 2: when registering the spectra 2–3 min after preparation, the value of extinction coefficient (ε) at the maximum at 254 nm has a value of ∼3000 L·mol–1·cm–1. Then, the optical density increases to the value of ε ∼ 8900 L·mol–1·cm–1. The change in optical density is well described by the first-order kinetic equation of the reaction and takes about an hour at 20 °C. The position of the maximum and the profile of the spectrum do not change. These observations can be interpreted as the transformation of the lactam–lactim form 2a in zwitterionic ion 2C.25 The ε value in a weak acid medium (0.5–1% H2SO4) is close to 9400 L·mol–1·cm–1 and is reached immediately after the dissolution of 2 (<5 min). Repeated registration of the spectra after 1–4 h shows no changes, and therefore, tautomerism does not interfere with the measurement of pKb.
In cases of 1, 2, and 7, there is a two-jump transition of change in the maxima position, which corresponds to two protonation stages. For 8 and 9, one protonation stage is observed, and for 3, three protonation stages are observed, Figures 5 and 9.
Figure 5.
Dependence of the absorption maximum position on the acidity: ○—6-hydroxy-2-methylpyrimidine-4(3H)-one (1), □—4,6-dihydroxypyrimidine (2), ●—6-hydroxy-2-ethylpyrimidine-4(3H)-one (7).
Figure 9.
UV spectra of free base and protonated forms of barbituric acid (3).
The spectra of 1, 2, and 7 are close to each other, which indicates their existence in the same tautomers. They are characterized by intense absorption with maxima at 200–204 nm (lg ε ≈4.3) and 252–254 nm (lg ε ≈ 4.0). The maximum at 252–254 nm is the most convenient for measuring pKb, since at these wavelengths, the influence of the solvent is minimal and the peak shift during the formation of cations does not lead to its going beyond the operating range of the spectrometer. Alkyl substituent at position 2 does not significantly affect the position of maxima, but there is a moderate increase in the extinction coefficients (Table 1).
Table 1. Absorption Maxima and Extinction Coefficients of Free Base and Cations of 4,6-Dihydroxypyrimidine and Its Derivatives.
| λmax, nm/lg (ε), L·mol–1·cm–1 |
||||||
|---|---|---|---|---|---|---|
| compounds | 1 | 2 | 3 | 7 | 8 | 9 |
| B | 252/4.07 | 252/3.97 | 254/3.00 | 253/4.08 | 324/3.72 | 325/3.74 |
| BH+ | 242/3.93 | 242/3.93 | 258/2.15 | 242/3.93 | 296/3.73 | 296/3.82 |
| BH22+ | 246/4.08 | 246/3.89 | 260/3.00 | 246/4.10 | ||
| BH33+ | 258/4.50 | |||||
The nitro group in 6-hydroxy-2-methyl-5-nitropyrimidin-4(3H)-one (8) and 6-hydroxy-2-ethyl-5-nitropyrimidin-4(3H)-one (9) does not significantly change a spectrum but causes a shift of maxima to the long-wavelength range.
During the transition from a weakly acidic to moderately acidic medium (H2SO4 with a concentration of 30–40%, Ho ∼ 1.7 to −2.5), changes in the 1, 2, and 7 spectra are the same, the main absorption peak shifts from 250–254 to 240–242 nm (Figures 6 and 7), and the extinction coefficient decreases.
Figure 6.
Changes in the UV spectra of 6-hydroxy-2-methylpyrimidin-4(3H)-one (1) upon its first protonation in solutions in 0.9–30.7% H2SO4.
Figure 7.
Changes in the UV spectra of 6-hydroxy-2-methylpyrimidin-4(3H)-one (1) upon its second protonation in solutions in 30–95.0% H2SO4.
An isobestic point is present on the group of spectra, which confirms the assumption that free and monoprotonated forms are present in this range of acidity (Figure 6). At Ho ∼ −2 to −3, a region with a constant spectrum is observed, which makes it possible to accept the spectra of compounds in this region for the spectra of monoprotonated forms.
With a further increase in Ho from −2 to −11, the absorption maximum shifts from 240 to 245 nm (Figure 7), accompanied by an increase in the extinction coefficient, and an isobestic point is also observed. In sulfuric acid with a concentration higher than 90% for 1 and 7, the spectrum changes stop and the diprotonated form is present in the solution. In the case of 2, the maximum shift continues up to 99.9% H2SO4.
In the case of nitro derivatives of 8 and 9, with an increase in acidity, the absorption maximum shifts to the short-wavelength region (Figure 8). One protonation stage is observed.
Figure 8.
UV spectra of 6-hydroxy-2-methyl-5-nitropyrimidine-4(3H)-one (8) in a solution of 6.7–95.9% H2SO4.
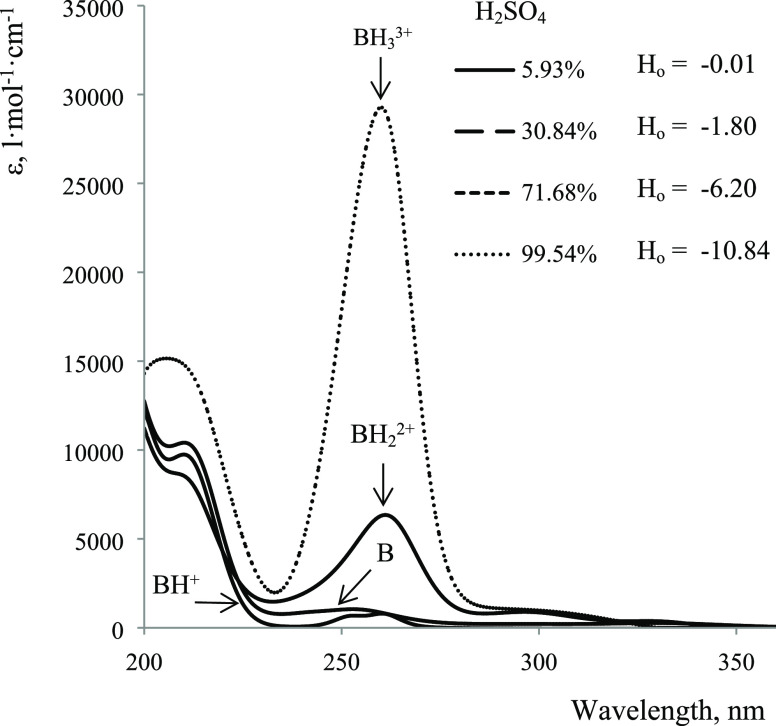
Changes in the spectrum of 3 with increasing acidity are more complex (Figure 9). Barbituric acid is an acid with pKa ≈ 4, so it is largely in the form of an anion in water, which has an intense absorption at 258 nm.22 When switching to solutions of sulfuric acid of 3–5%, ionization is suppressed and the neutral form prevails in the solution, which is characterized by an intense absorption below 220 nm.
With an increase in the concentration of sulfuric acid to 30%, a monotonous decrease in the optical density is observed at 210 nm and, especially, at 260 nm, which is replaced by an increase with a further increase in acidity. Thus, the optical density dependences at 210 and 260 nm (Figure 10) on Ho show three regions corresponding to three protonation stages: from 5 to 35%, monoprotonated form (BH+); from 35 to 70%, diprotonated form (BH22+); and from 70 to 100%, triprotonated form (BH33+) are generated. The spectra of 30, 70, and 99.5% H2SO4 were assumed to be mono-, di-, and triprotonated forms of 3, respectively, when calculating pKb.
Figure 10.
Change of extinction coefficient (at 262 nm) of barbituric acid (3) on acidity. Black circles—experiment, dotted line—calculation according to eq 4.
Discussion
pKb values were calculated using the Yates–McClelland method, which assumes that there is a proportional relationship between any values of the Hx acidity function26
| 1 |
| 2 |
Thus, the dependence of the logarithms of the ionization ratios (I) on the Hx acidity function is linear and the criterion for the applicability of Hx to the study of protonation of a weak base is the angular coefficient m = dHx/dHo close to 1. The function Hx indirectly indicates the type of protonation. The pKb value determined by the Ho acidity scale and equal to the acidity of the medium at the half-protonation point (log I = 0) was calculated by the equation
| 3 |
In this research, the acidity functions Ho,27−30Ha31,32 and HI,33Ho‴34 were used to analyze experimental data. The Hx acidity functions describe a type of protonation of very weak uncharged bases. When constructing them, the following reference reactions were used: Ho—protonation of nitroanilines; HI—protonation of primary amides; Ha—protonation of indoles; Ho‴—protonation of tertiary aromatic amines.
In the cases of 1, 2, 7, 8, and 9, the concentrations of free base (B), cation (BH+), and dication (BH22+) were calculated from UV spectroscopy data by Firordt’s method by two wavelengths corresponding to their maxima (Table 1). The mixture is analyzed using Firordt’s method by resolving the following system of equations
| 4 |
where A1 and A2 denote adsorption at λ1 and λ2, respectively; a1, a2, b1, and b2 are the molar absorption coefficients of X and Y at λ1 and λ2, respectively; CX and CY are the molar concentrations of X and Y in the mixture, respectively; and l is the thickness of the absorbing layer.35
For 3, the maximum shift is small, which complicates the use of this method, but there is a significant change in the optical density at ∼260 nm, so ionization ratio was calculated by the equation
| 5 |
The values of pKbn obtained by us are shown in Table 2. As can be seen for 1 and 2, they coincide with those given in ref (14). The introduction of a donor substituent in position 2 is expected to increase basicity. Replacing the methyl group with an ethyl group slightly reduces the basicity for the first protonation step, but increases it for the second.
Table 2. Basicity of 4,6-Dihydroxypyrimidine Derivatives.
| compound | pKb1 | pKb2 | pKb3 |
|---|---|---|---|
| 1 | 0.21;14 0.23;3 0.17 ± 0.01 | –6.10;3 −6.24 ± 0.48 | |
| 2 | –0.41 ± 0.03; −0.3014 | –7.06 ± 0.37 | |
| 3 | –0.90 ± 0.06 | –3.62 ± 0.02 | –9.27 ± 0.54 |
| 7 | –0.03 ± 0.04 | –5.81 ± 0.35 | |
| 8 | –6.04 ± 0.32 | ||
| 9 | –5.92 ± 0.80 | ||
| uracil36 | –2.38 | –7.3 (Ha) ∼ (−12.1 Ho)a | |
| thymine36 | –3.02 | –6.8 (Ha) ∼ (−10.9 Ho)a |
Acidity score at the half-protonation point.
The oxygen in the second position in barbituric acid (3) also acts in different directions. There is a strong decrease in basicity at the first stage of protonation, which correlates with the presence of pronounced acidic properties in 3 and is determined by its presence in the keto form. However, the second protonation of 3 is much easier than that of 1, 2, and 7, and the same seems to apply to the third stage of protonation.
The presence of a nitro group with a strong electron-acceptor effect is expected to significantly reduce the basicity of compounds; only the first protonation is observed in the studied range of acidity.
As indicated, the type of acidity function that best describes protonation is indirectly related to the direction of protonation. Table 3 shows the values of coefficient m. At the first protonation of 1 and 7, they are close to 1 on the Ho scale. The Ha scale is more suitable for 2, but using Ho also gives a satisfactory value for the m coefficient. Their difference from 1 is not high. This fact does not allow us to make a clear choice in favor of one of these acidity scales.
Table 3. pK*b and Coefficient m Values for 4,6-Dihydroxypyrimidine Derivatives.
| compound | pK*b1 | m | pK*b2 | m | pK*b3 | m |
|---|---|---|---|---|---|---|
| Ho | ||||||
| 1 | 0.18 ± 0.01 | 1.06 ± 0.04 | –2.84 ± 0.22 | 0.45 ± 0.04 | ||
| 2 | –0.32 ± 0.02 | 0.79 ± 0.02 | –2.91 ± 0.15 | 0.41 ± 0.03 | ||
| 3 | –2.09 ± 0.08 | 2.31 ± 0.07 | –4.14 ± 0.03 | 1.15 ± 0.01 | –5.99 ± 0.35 | 0.64 ± 0.04 |
| 7 | –0.026 ± 0.037 | 0.94 ± 0.05 | –2.81 ± 0.17 | 0.48 ± 0.03 | ||
| 8 | –2.76 ± 0.11 | 0.46 ± 0.02 | ||||
| 9 | –3.02 ± 0.41 | 0.51 ± 0.07 | ||||
| Ha | ||||||
| 1 | 0.00 ± 0.01 | 1.32 ± 0.05 | –3.68 ± 0.21 | 0.94 ± 0.06 | ||
| 2 | –0.47 ± 0.04 | 1.02 ± 0.03 | –3.67 ± 0.25 | 0.84 ± 0.07 | ||
| 3 | –2.49 ± 0.09 | 2.90 ± 0.09 | –5.32 ± 0.21 | 2.022 ± 0.08 | –6.10 ± 0.04 | 1.08 ± 0.08 |
| 7 | –0.19 ± 0.04 | 1.18 ± 0.06 | –3.75 ± 0.23 | 0.99 ± 0.06 | ||
| 8 | –3.72 ± 0.08 | 0.95 ± 0.02 | ||||
| 9 | –3.41 ± 0.37 | 0.87 ± 0.10 | ||||
| HI | ||||||
| 3 | –1.85 ± 0.09 | 1.58 ± 0.06 | –4.18 ± 0.10 | 0.84 ± 0.08 | –6.64 ± 0.39 | 0.57 ± 0.04 |
| Hr | ||||||
| 3 | –1.48 ± 0.11 | 1.16 ± 0.05 | –3.70 ± 0.08 | 0.52 ± 0.01 | –6.03 ± 0.61 | 0.33 ± 0.04 |
The unified character of the spectra of protonated forms of 1, 2, and 7 indicates their existence in similar tautomeric forms and a single protonation mechanism. When considering a possible proton attachment site for 4,6-dihydroxypyrimidine derivatives, their tautomerism must be taken into account. During the first protonation of 1, 2, and 7, the value of the coefficient m is close to 1 when using the acidity function Ho. It is different from the first protonation stage of thymine and uracil, which is described by the Ha function. The site of protonation is oxygen.36
This suggests the addition of a proton to the “pyridine” nitrogen atom of lactam–lactim form with the formation of cation 13a (Scheme 3). Although the protonation of the lactam–lactim form is possible, in our opinion, the presence of zwitterionic forms will be an important factor. In this case, it is preferable to attach a proton to the negative center of the zwitterionic form - the oxygen atom of carbonyl group. This process leads to the formation of a cation with localization of the charge on the "pyridine" nitrogen atom, the structure of which is the same as in the protonation of the lactam–lactim form. The final structure of the cation will be determined by the relative stability of its tautomeric forms. Protonation of the lactam–lactim form is possible at a low rate of its conversion to zwitterion and a low equilibrium concentration of the latter.
Scheme 3. Protonation of 4,6-Dihydroxypyrimidine (2), 6-Hydroxy-2-Methylpyrimidine-4(3H)-One (1), and 6-Hydroxy-2-Ethylpyrimidine-4(3H)-One (7).
The existence of ionic forms of 1 and 7 was previously assumed, but, unlike 2, there is no direct evidence for this. However, the presence of a certain amount of them in solutions cannot be excluded. Dimerization could indicate their presence, but it is not observed in aqueous solutions, probably due to the steric effect of the substituent in position 2. To test this assumption, we used LC–MS to study the interaction of 1 and 2 in an aqueous solution (molar ratio 1:1). Along with 6, the formation of a mixed dimer was achieved, presumably having the structure 12 (Scheme 2). Their equilibrium content, as in the case of 2, was ∼4%. The ratio of 6 and 12 is approximately 3:2, which indicates close interaction rates and equilibrium concentrations of zwitterion forms of 1 and 2.
Scheme 2. Formation of Dimer from 4,6-Dihydroxypyrimidine (1) and 6-Hydroxy-2-Methylpyrimidine-4(3H)-One (2).
The first protonation of 1, 2, and 7 is described by the Ho function constructed using anilines, so we can expect the addition of a proton to pyridine nitrogen atom (Scheme 3).
The comparison of the 1H NMR spectra of 2 in DMSO-d6 and acidic media shows that the base form of the cation can be the lactam–lactim forms, the ratio of proton integrals in 2 and 5 positions is 1:1. During monoprotonation in the TFA medium, a shift of proton signals at positions 2 and 5 to a weak field is observed (ΔδH = +0.98 and +0.81 ppm) and a larger shift at position 2 is in good agreement with the structure 13a (R = H) (Scheme 3), with a predominant localization of charge on nitrogen atoms. In addition, solutions contain up to 10% dilactam forms 13b (R = H). It is characterized by a methylene group signal: δH 2.24 ppm (d, 2H, J = 0.8 Hz). Its presence was also proved in ref (12) by the isotopic exchange of hydrogen at position 5 for deuterium in the D2SO4.
The protonation of substances 1 and 7 occurs in a similar way. When monocations are formed in TFA, the signals of protons in position 5 are shifted into a weak field: ΔδH = +0.92 and +0.83 ppm accordingly. The signal of the methyl group in monocation 13a (R = CH3) is also strongly shifted downfield: ΔδH = +0.49 ppm. The same effect is exerted by monoprotonation on the chemical shift of the protons of the ethyl group: ΔδH = +0.32 ppm for CH3 and ΔδH = +0.54 ppm for CH2. At the same time, no dilactam form is observed for 1. For 7, as well as for 2, up to 5% of the form of dilactam 13b (R = C2H5) is present.
The second protonation of 1, 2, and 7 is described by the Ha function constructed using amides, so we can expect the addition of a proton to oxygen (Scheme 3).
Other changes in the 1H NMR spectrum are observed during the formation of dication 2 in 95% sulfuric acid. Compared to the monocation, the signal of the proton in position 5 is even more biased into the weak field (ΔδH = +0.22 ppm). On the contrary, the signal of the proton in position 2 is shifted into a strong field (ΔδH = −0.32 ppm). This fact is in good agreement with structure 14a (R = H) (Scheme 3), and the second protonation occurs at carbonyl oxygen. The dilactam form 14b (R = H) is also present. It is characterized by the signal of the methylene group: δH = 2.15 ppm (s, 2H).
Comparison of the 1H NMR spectra of mono- and dications of 1 and 7 shows changes similar to those for compound 2. A shift of the signals of protons in position 5 in a weak field is observed: ΔδH = +0.24 and +0.23 ppm accordingly. This indicates that the position of the second protonation site is close to position 5. On the contrary, the signals of the alkyl group are shifted to strong field: ΔδH = −0.38 ppm for CH3 and ΔδH = −0.63 (CH2) and ΔδH = −0.46 (CH3) ppm for ethyl group. In the cases of 1, 7, and 6-hydroxy-2-isopropylpyrimidine-4(3H)-one (10), in contrast to 2, no forms of dilactam were found.
The 1H NMR spectra of the dication 14a in concentrated H2SO4, compared to the solution of the free base in DMSO-d6, show that in all cases, the proton signals in position 5 are strongly shifted to a weak field: R = CH3, ΔδH = +1.17 ppm; R = C2H5, ΔδH = +1.10 ppm; R = CH(CH3)2, ΔδH = +1.08 ppm. It can be seen that an increase in the donor effect of the alkyl group increases the electron density at the pyrimidine core and correlates with a decrease in pKb2.
The signal of the methyl group of dication 14a (R = CH3) is shifted to the weak field significantly less (ΔδH = +0.17 ppm) than in the case of thymine (ΔδH = +0.74 ppm), which is consistent with different protonation directions and may indicate a significant charge delocalization.
For dication 14a (R = C2H5), a shift of the signals of the methylene group to the weak field by 0.08 ppm is also observed, and the signals of the methyl group, on the contrary, are shifted to the strong field ΔδH = −0.34 ppm, which is typical of an ethyl group connected to electron-acceptor radical. In the case of 6-hydroxy-2-isopropylpyrimidine-4(3H)-one, all signals of the alkyl substituent are shifted to a strong field: CH ΔδH = −0.26 ppm and CH3 ΔδH = −0.31 ppm. In all cases, J does not change significantly.
All of this confirms the generality of the second protonation of 1, 2, and 7 and the location of the second protonation center on the oxygen atom of the carbonyl group.
For nitro derivatives, it is most likely to be found in the lactam–lactim and dilactim forms, but not in the dilactam form. 1H NMR spectra of 8 and 9 in DMSO-d6 and H2SO4 show the absence of a proton at position 5. The UV spectra of neutral and protonated forms of nitro derivatives do not differ, in principle, from the spectra of the initial compounds; a maximum shift is observed due to the presence of an acceptor substituent.
Protonation of 8 and 9 is described by the Ha function, which suggests the addition of a proton by oxygen of the carbonyl group. The observed shift of the substituent proton signals to a strong field (8: CH3, ΔδH = −0.14 ppm; 9: CH2, ΔδH = −0.08 ppm, CH3, ΔδH = −0.35 ppm; 12: CH, ΔδH = −0.26 ppm, CH3, ΔδH = −0.44 ppm) also indicates a different nature of protonation in contrast to the parent structures and is consistent with the addition of a proton to the carbonyl group.
The nitro group in position 5 reduces the basicity of nitrogen atoms and makes it difficult to delocalize the negative charge, preventing the formation of zwitterionic forms. Consequently, they do not make a significant contribution to protonation, which occurs on the oxygen of the carbonyl group (Scheme 4).
Scheme 4. Protonation of 6-Hydroxy-2-Methyl-5-Nitropyrimidine-4(3H)-One (8), 6-Hydroxy-2-Ethyl-5-Nitropyrimidine-4(3H)-One (9), and 6-Hydroxy-2-Isopropyl-5-Nitropyrimidine-4(3H)-One (11).

Analysis of the coefficients m during protonation of barbituric acid showed that for the first stage, it is satisfactorily described by the acidity function Hr; the second stage is satisfactorily described by the acidity functions Ho and HI; the third stage is described by the acidity function Ha. As already mentioned, the change in UV spectra during 3 protonation is fundamentally different from that observed for other studied compounds. A significant change in both the position and intensity of the absorption maxima is consistent with the available data on the structure of free and protonated forms of 3. Based on the structures of the monoprotonated and triprotonated forms, let us consider the possible structure of the diprotonated form. Note that the UV spectrum of BH22+ shows great similarity to the spectrum of BH33+, but is significantly different from BH+. This suggests a large contribution of the triketo form 3g in equilibrium with the diketo form 3f (Scheme 5).
Scheme 5. Protonation of Barbituric Acid (3).
Conclusions
4,6-Dihydroxypyrimidine and 6-hydroxy-2-methylpyrimidine-4(3H)-one are stronger bases compared to the isomeric thymine and uracil. The first protonation of 4,6-dihydroxypyrimidine, 6-hydroxy-2-methylpyrimidin-4(3H)-one, and 6-hydroxy-2-ethylpyrimidin-4(3H)-one easily occurs by the addition of a proton to the zwitterionic form. Thus, it is most likely that the primary protonation site is the pyridine nitrogen atom. The second protonation with the formation of a doubly charged cation occurs by the addition of a proton to the oxygen of the carbonyl group.
The presence of a nitro group at position 5 reduces the basicity of nitrogen atoms and prevents the formation of a zwitterion structure, which leads to a sharp decrease in basicity and protonation of 6-hydroxy-2-methyl-5-nitropyrimidine-4(3H)-one and 6-hydroxy-2-ethyl-5-nitropyrimidine-4(3H)-one by carbonyl groups.
Protonation of barbituric acid takes place in three stages, while its basicity at the first protonation step is lower than 4,6-dihydroxypyrimirine, which is due to its presence in the keto form. The formation of dication occurs at a lower acidity than 4,6-dihydroxypyrimidine, 6-hydroxy-2-methylpyrimidine-4(3H)-one, and 6-hydroxy-2-ethylpyrimidine-4(3H)-one.
Based on the experimental data obtained for all studied compounds, basicity constants and values of the m coefficient were calculated using various acidity functions.
Methods
The following substances for the study were synthesized using known methods: 4,6-dihydroxypyrimidine (2),37 barbituric acid (3),38 6-hydroxy-2-methylpyrimidine-4(3H)-one (1),39 6-hydroxy-2-ethylpyrimidine-4(3H)-one (7),40 6-hydroxy-2-isopropylpyrimidine-4(3H)-one (10),41 6-hydroxy-2-methyl-5-nitropyrimidine-4(3H)-one (8),42 6-hydroxy-2-ethyl-5-nitropyrimidine-4(3H)-one (9),43 and 6-hydroxy-2-isopropyl-5-nitropyrimidine-4(3H)-one (11).43 According to LC/MS and 1H NMR spectroscopy data, their purity is higher than 98%.
Solutions of sulfuric acid were prepared by mixing sulfuric acid (95%), oleum (105%), and distilled water. The concentration was determined titrometrically and by density with an error of no more than 0.1%.
Absorption spectra in the ultraviolet and visible regions were recorded in a temperature-controlled cell on a Specord M-40 spectrophotometer at 25 ± 0.1 °C. To control the stability of the solutions, the measurements were repeated up to three to five times for 3–6 h. Data were averaged. The deconvolution of spectra was carried out using the “OMNIC” program and mixed Gauss–Lorentz functions.
Liquid chromatography–mass spectrometry (LC–MS) experiments were performed using a Thermo Finnigan Surveyor MSQ with electrospray ionization (ESI, positive and negative modes).
1H NMR spectra were recorded on a Varian Mercury Plus instrument (300 MHz).
Infrared (IR) spectra were recorded on an Avatar 360 FTIR instrument in KBr.
Acknowledgments
This research was financially supported by the Mendeleev University of Chemical Technology (#T-2020-037).
Glossary
Abbreviations
- LC–MS
liquid chromatography–mass spectrometry
- IR
infrared
- UV
ultraviolet
- NMR
nuclear magnetic resonance
- DMSO
dimethyl sulfoxide
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00671.
Decomposition of UV spectra of the free base and protonated forms of 4,6-dihydroxypyrimidine derivatives; chemical shifts of protons in the free base and protonated form of 4,6-dihydroxypyrimidine derivatives; calculation of pKb values of 4,6-dihydroxypyrimidine derivatives; mass and UV spectra of dimer 6 and 12; and 1H NMR spectra (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Katritzky A. R.; Lagowski J. M. Prototropic tautomerism of heteroaromatic compounds. Adv. Heterocycl. Chem. 1963, 1, 311–437. 10.1016/S0065-2725(08)60529-2. [DOI] [PubMed] [Google Scholar]
- Johnson C. D.; Katritzky A. R.; Kingsland M.; Scriven E. F. V. Kinetics and mechanism of electrophilic substitution of heteroaromatic compounds. Part XXII. The nitration of pyrimidinones. J. Chem. Soc. B 1971, 1–4. 10.1039/J29710000001. [DOI] [Google Scholar]
- Kushtaev A. A.; D’yakonov A. V.; Yudin N. V.; Zbarskii V. L. Nitration Kinetics of 6-Hydroxy-2-methylpyrimidin-4(3H)-one and 2-Methoxy-2-methylimidazolidine-4,5-dione. Russ. J. Appl. Chem. 2009, 82, 1785. 10.1134/S1070427209100073. [DOI] [Google Scholar]
- Muhammad M. T.; Khan K. M.; Khan A.; Arshad F.; Fatima B.; Choudhary M. I.; Syed N.; Moin S. T.; et al. Syntheses of 4,6-dihydroxypyrimidine diones, their urease inhibition, in vitro, in silico, and kinetic studies. Bioorg. Chem. 2017, 75, 317–331. 10.1016/j.bioorg.2017.08.018. [DOI] [PubMed] [Google Scholar]
- Jansa P.; Holý A.; Dračínský M.; Kolman V.; Janeba Z.; Kostecká P.; Kmoníčková E.; Zídek Z. 5-Substituted 2-amino-4,6-dihydroxypyrimidines and 2-amino-4, 6-dichloropyrimidines: synthesis and inhibitory effects on immune-activated nitric oxide production. Med. Chem. Res. 2014, 23, 4482–4490. 10.1007/s00044-014-1018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu-Qiang B. I.; Wang B. Z.; Wang X. J. Study on the preparation technique of nitroform with high security. Chin. J. Explos. Propellants 2010, 33, 14–17. [Google Scholar]
- Bellamy A. J.FOX-7 (1,1-Diamino-2,2-dinitroethene). In High Energy Density Materials: Structure and Bonding; Klapötke T. M., Ed.; Springer-Verlag: Heidelberg, Germany, 2007; Vol. 125, pp 1–33. [Google Scholar]
- Short L. N.; Thompson H. W. 38. Infra-red spectra of derivatives of pyrimidine. J. Chem. Soc. 1952, 168–187. 10.1039/JR9520000168. [DOI] [Google Scholar]
- Kheifets G. M.; Khromov-Borisov N. V.; Koltsov A. I.; Volkenstein M. V. The proton magnetic resonance spectra and the structure of 4, 6-dihydroxy-pyrimidine and its derivatives. Tetrahedron 1967, 23, 1197–1209. 10.1016/0040-4020(67)85070-1. [DOI] [Google Scholar]
- Brown D. J.; Teitei T. Simple pyrimidines. VII. The fine structure of 4, 6-dihydroxypyrimidine. Aust. J. Chem. 1964, 17, 567–572. 10.1071/CH9640567. [DOI] [Google Scholar]
- Kheifets G. M.; Khromov-Borisov N. V. The structure of 4,6-dihydroxypyrimidine and its 5-Methyl Analog. Russ. J. Gen. Chem. 1964, 34, 3134–3135. [Google Scholar]
- Inoue Y.; Furutachi N.; Akanishi K. Tautomerism of 4-Hydroxy-and 4, 6-Dihydroxypyrimidine. J. Org. Chem. 1966, 31, 175–178. 10.1021/jo01339a037. [DOI] [Google Scholar]
- Berndt M.; Ingwer M.; Kwiatkowski J. S. Tautomerism and the spectroscopic properties of some 4,6-disubstituted pyrimedines. J. Mol. Struct. 1973, 19, 247–254. 10.1016/0022-2860(73)85268-8. [DOI] [Google Scholar]
- Katritzky A. R.; Popp F. D.; Waring A. J. Potentially tautomeric azines. Part VI. The fine structure of “4,6-dihydroxypyrimidines”. J. Chem. Soc. B 1966, 565–568. 10.1039/J29660000565. [DOI] [Google Scholar]
- Katrusiak A.; Katrusiak A. Ionic disparity of identical molecules in polymorphs. Org. Lett. 2003, 5, 1903–1905. 10.1021/ol034494r. [DOI] [PubMed] [Google Scholar]
- Kheifets G. M.; Khromov-Borisov N. V.; Kol’tov A. I. Dimers of N-Methyl Derivatives of 4,6-Pyrimidinediol. Russ. J. Org. Chem. 1966, 2, 1516–1523. [Google Scholar]
- Prystaš M. Nucleic acid components and their analogues. CII. N-Alkyl derivatives of 2-and 5-substituted 4-hydroxy-6 (1H)-pyrimidinones. Collect. Czech. Chem. Commun. 1967, 32, 4241–4259. 10.1135/cccc19674241. [DOI] [Google Scholar]
- Katrusiak A.; Katrusiak A. Crystal-stabilisation of an elusive 4,6-pyrimidinedione dimer. Tetrahedron Lett. 2007, 48, 1935–1938. 10.1016/j.tetlet.2007.01.096. [DOI] [Google Scholar]
- Delchev V. B. DFT ab initio study of the keto-enol tautomerism of barbituric acid. J. Struct. Chem. 2004, 45, 570–578. 10.1007/s10947-005-0031-8. [DOI] [Google Scholar]
- Valadbeigi Y.; Farrokhpour H.; Tabrizchi M. Theoretical study on the isomerization and tautomerism in barbituric acid. J. Struct. Chem. 2014, 25, 1805–1810. 10.1007/s11224-014-0452-0. [DOI] [Google Scholar]
- Schmidt M. U.; Brüning J.; Glinnemann J.; Hützler M. W.; Mörschel P.; Ivashevskaya S. N.; van de Streek J.; Braga D.; Maini L.; Chierotti M. R.; Gobetto R. The thermodynamically stable form of solid barbituric acid: the enol tautomer. Angew. Chem., Int. Ed. 2011, 50, 7924–7926. 10.1002/anie.201101040. [DOI] [PubMed] [Google Scholar]
- Fox J. J.; Shugar D. Absorption spectra and structure of barbituric acid derivatives as a function of pH. Bull. Soc. Chim. Belg. 1952, 61, 44–63. 10.1002/bscb.19520610105. [DOI] [Google Scholar]
- Zuccarello F.; Buemi G.; Gandolfo C.; Contino A. Barbituric and thiobarbituric acids: a conformational and spectroscopic study. Spectrochim. Acta, Part A 2003, 59, 139–151. 10.1016/S1386-1425(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Olah G. A.; Kiovsky T. E. Stable carbonium ions. LXX. Protonated nitroalkanes and nitroaromatic compounds. Cleavage of protonated nitroalkanes (cycloalkanes) to carbonium ions. J. Am. Chem. Soc. 1968, 90, 6461–6464. 10.1021/ja01025a040. [DOI] [Google Scholar]
- Tuan V. K.; Tkhan’ N. S.; Yudin N. V.; Kushtayev A. A. In Kinetics and Nitration Mechanism of 4,6-Dihydroxypyrimidine and Its Derivatives in the Presence of Nitrous Acid, Vserossiyskaya Konferentsiya. AKS-2019, Institut Organicheskoy Khimii Imeni N.D. Zelinskogo RAN: Moscow, Russia, 2019; pp 199–205.
- Yates K.; Wai H.; Welch G.; McClelland R. A. Medium dependence of acidity functions and activity coefficients in perchloric acid. J. Am. Chem. Soc. 1973, 95, 418–426. 10.1021/ja00783a018. [DOI] [Google Scholar]
- Paul M. A.; Long F. A. Ho and related indicator acidity function. Chem. Rev. 1957, 57, 1–45. 10.1021/cr50013a001. [DOI] [Google Scholar]
- Högfeldt E.; Bigeleisen J. Acidity Constants of Some Hammett Indicators in Heavy Water. The Hammett Acidity Function, Do, for DCl and D2SO4 Solutions. J. Am. Chem. Soc. 1960, 82, 15–20. 10.1021/ja01486a005. [DOI] [Google Scholar]
- Gillespie R. J.; Peel T. E.; Robinson E. A. Hammett acidity function for some super acid systems. I. Systems H2SO4-SO3, H2SO4-HSO3F, H2SO4-HSO3Cl, and H2SO4-HB(HSO4)4. J. Am. Chem. Soc. 1971, 93, 5083–5087. 10.1021/ja00749a021. [DOI] [Google Scholar]
- Jorgenson M. J.; Hartter D. R. A Critical Re-evaluation of the Hammett Acidity Function at Moderate and High Acid Concentrations of Sulfuric Acid. New Ho Values Based Solely on a Set of Primary Aniline Indicators. J. Am. Chem. Soc 1963, 85, 878–883. 10.1021/ja00890a009. [DOI] [Google Scholar]
- Yates K.; Riordan J. C. The HA acidity function and the mechanism of amide hydrolysis in hydrochloric acid. Can. J. Chem. 1965, 43, 2328–2335. 10.1139/v65-314. [DOI] [Google Scholar]
- Frederick G. D.; Poulter C. D. Extension of the HA acidity function into oleum mixtures. J. Am. Chem. Soc. 1975, 97, 1797–1801. 10.1021/ja00840a031. [DOI] [Google Scholar]
- Hinman R. L.; Lang J. The protonation of indoles. Basicity studies. The dependence of acidity functions on indicator structure. J. Am. Chem. Soc. 1964, 86, 3796–3806. 10.1021/ja01072a040. [DOI] [Google Scholar]
- Yates K.; Wai H.; Welch G.; McClelland R. A. Medium dependence of acidity functions and activity coefficients in perchloric acid. J. Am. Chem. Soc. 1973, 95, 418–426. 10.1021/ja00783a018. [DOI] [Google Scholar]
- Vlasova I. V.; Vershinin V. I. Determination of Binary Mixture Components by the Firordt Method with Errors Below the Specified Limit. J. Anal. Chem. 2009, 64, 553–558. 10.1134/S1061934809060033. [DOI] [Google Scholar]
- Benoit R. L.; Frechette M. 1H and 13C nuclear magnetic resonance and ultraviolet studies of the protonation of cytosine, uracil, thymine, and related compounds. Can. J. Chem. 1986, 64, 2348–2352. 10.1139/v86-387. [DOI] [Google Scholar]
- Hunds A.; Huels A. G.. Process for the Preparation of 4,6-Dihydroxypyrimidine. U.S. Patent US58471391998.
- Ahluwalia V. K.; Bhagat P.; Aggarwal R.; Chandra R.. Intermediates for Organic Synthesis; I. K. International Pvt. Ltd.: New Delhi, 2005; pp 280–281. [Google Scholar]
- Chylek Z.; Cudzilo S.; Diduszko R. R. Optimitization of 1,1-diamino-2,2-dinitroethane. Biul. WAT 2004, 54, 633–634. [Google Scholar]
- Wade J. J.Pyrimidines Substituted by Nitrogen-Containing Heterocyclic Rings as Intermediates. U.S. Patent US45365791985.
- Gershon H.; Braun R.; Scala A.; Rodin R. Pyrimidines. IV. 2-, 5-, and 2, 5-Substituted Chloropyrimidines. J. Med. Chem. 1964, 7, 808–811. 10.1021/jm00336a032. [DOI] [PubMed] [Google Scholar]
- Astrat’ev A. A.; Dashko D. V.; Mershin A. Y.; Stepanov A. I.; Urazgil’deev N. A. Some specific features of acid nitration of 2-substituted 4,6-dihydroxypyrimidines. Nucleophilic cleavage of the nitration products. Russ. J. Org. Chem. 2001, 37, 729–733. 10.1023/A:1012568305472. [DOI] [Google Scholar]
- Hymans W. E. 1,2,3-thiadiazolo[5,4-b]pyrimidin-4(5H)ones. J. Heterocycl. Chem. 1976, 13, 1141–1144. 10.1002/jhet.5570130548. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.