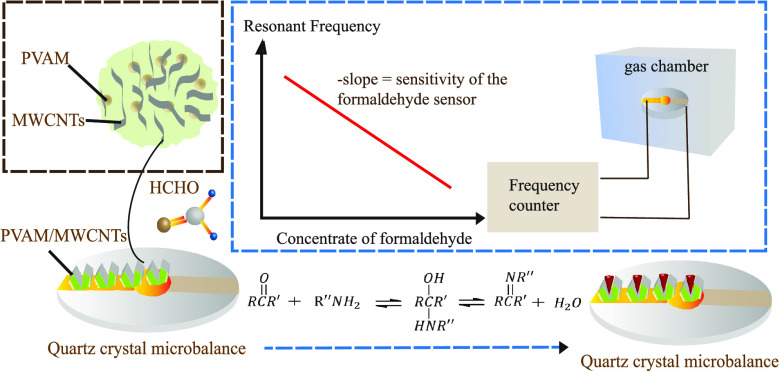
Abstract
Two formaldehyde detection methods are proposed by applying composite film quartz crystal microbalance (QCM) sensors. QCM sensor coated with PAAm/MWCNTs and PVAm/MWCNTs shows excellent characteristics of lower limit and high sensitivity. The lower limit of PVAm/MWCNTs is 0.5 ppm, and its detection sensitivity is 0.74 ppm/Hz. Upon working at different concentrations of formaldehyde and fabricating in different proportions, the reuse performance, gas selectivity, and response at room temperature show contrasting results. The main advantages of the two sensors presented are fast reaction, low cost, and easy manufacture. Compared to other formaldehyde sensors based on QCM, the PAAm/MWCNT- and PVAm/MWCNT-coated QCM sensors are able to concurrently show excellent selectivity, reuse performance, and high sensitivity, which is of great significance to detect the environmental quality.
Introduction
Formaldehyde (HCHO) is widely acknowledged as one of the most important chemical raw materials, which is characterized by active chemical properties, high purity, and low cost.1 The world produces more than 10 million tons of formaldehyde every year.2 Formaldehyde is in high demand in the manufacturing of building plates, plywood, and lacquer materials. Thus, HCHO is released to the environment as a volatile compound in large quantities.2−6 HCHO can cause serious health problems on long-term exposure even at a low concentration. HCHO has been identified as a mutation-inducing substance in organisms and a human carcinogen that can increase irritation symptoms and cause respiratory distress and immune system disorders, which has been confirmed by microbial and mouse experiments.2,7−9 For pregnant women, long-term exposure to HCHO at low concentrations will cause fetal deformities and necrosis due to allergic reactions.10 Scientists have found that formaldehyde is also one of the chemical mediators of apoptosis.11 Researchers have made a lot of efforts on formaldehyde sensors and invented a variety of products and analytical detection methods. At present, several methods are being commonly used, among which spectrophotometry has the advantages of low cost and easy handling, but it shows low sensitivity and the material is susceptible to deterioration.12−14 Chromatography has become very common for detecting formaldehyde, which is not easily influenced by environmental factors and shows outstanding gas separation performance with high sensitivity and precision. Other methods such as electrochemical detection, high-performance liquid chromatography, and photometric and fluorometric determination are suited for real-time monitoring, and they often require large, expensive laboratory equipment, harsh experimental conditions, and has complex sampling techniques.15,16 At present, the fastest detector for testing formaldehyde is the PPM-400 portable gas detection instrument from United Kingdom, which can determine formaldehyde at the level of 0.01 ppm. However, it is vulnerable to interference by other volatile organic gases and has a short life. Among the above methods, QCM is widely used for its high sensitivity, fast response, simplicity, and low experimental cost, and it is confirmed to be the most attractive and widespread technique for measuring HCHO at low concentrations. QCM can be functionalized by several coating methods, including drop-casting, spin coating, electrospinning, chemical vapor deposition, and molecular imprinting.17 QCM can detect mass changes at the nanogram level.3,10 AT-cut quartz wafer is coated with a selective film, which can absorb gas, and the surface mass of quartz crystal increases. The mechanical resonance frequency of crystal changes due to the inverse piezoelectric effect of quartz crystal so that the concentration of target gas can be detected.18 The coated QCM sensor is put into the gas chamber. After the reaction between the gas and the sensitive film, the film mass increases and the frequency of the QCM sensor decreases. Finally, the concentration of the gas to be measured is calculated using the frequency shift. The performance of the existing QCM for measuring formaldehyde is largely determined by the surface coating material.16,19−21 Repeatability, selectivity, and sensitivity are the key performance metrics to test formaldehyde detection for QCM. Polymer nanofibers are characterized by large surface–volume ratio, superior mechanical properties, and high porosity.10,22−24 The experimental results showed that the detection limit of the PAAm/MWCNT sensor reached 1 ppm, and the PVAm/MWCNT sensor could be used to detect formaldehyde at a lower concentration, with various parameters superior to PAAm/MWCNTs.
Table 1 lists the results of using QCM as a formaldehyde sensor in recent years. It can be seen that scientists have discovered a variety of polymers and oxides and made them into composite membranes to produce sensitive membranes and coated them on QCM to test formaldehyde. Molecularly imprinted polymers (MIPs) were used to make composite membranes selective to formaldehyde,25 but it needs a relatively complex manufacturing process. Using the electrospinning technology, materials can be fabricated with cross-linked network structures, large specific surface areas, high porosity, and large packing density, which means the sensor can achieve higher sensitivity. But the above two methods need a relatively complex manufacturing process. To prepare fibers by electrospinning, the polymer must reach a certain relative molecular mass. In the literature, the test ranges for the concentration of formaldehyde are mostly 1–100 or 1–30 ppm.26−31 This article mainly discusses the detection of formaldehyde in the low concentration (0.5-6 ppm) range, which is more suitable for most real-life scenarios. For the detection of low-concentration formaldehyde, we prefer to choose low-cost materials that do not contain metal ions, to avoid environmental pollution. The sensitivity and response time in the low-concentration formaldehyde test in this paper are comparable to previous results. The materials used have the advantages of not requiring complex preparation processes, low cost, and no environmental pollution.
Table 1. Results of Previous Work.
| refs | materials | sensitivity | response/recovery time | limit | year |
|---|---|---|---|---|---|
| (32) | amine-functionalized SBA-15 | not given | 11/15 s | not given | 2007 |
| (26) | PEI/PVA | 0.5 Hz/ppm | not given | not given | 2010 |
| (33) | PEI/PS | 1.7 Hz/ppm | not given | 3 ppm | 2011 |
| (27) | PEI/BC | 3.15 Hz/ppm | not given | 10 ppm | 2011 |
| (28) | PEI-functionalized TiO2 fibers | 0.014 Hz/ppm | <120 s | 1.2 ppm | 2012 |
| (34) | PAN/PVA | not given | not given | 0.5 ppm | 2013 |
| (29) | chitosan + PEI | 0.172 Hz/ppm | not given | not given | 2014 |
| (35) | PEI/MWCNTs | 0.82 Hz/ppm | 114/127 s | 0.6 ppm | 2015 |
| (36) | graphene oxide | 22.9 Hz/ppm | 60 s | 0.06 ppm | 2016 |
| (30) | Cu(DDS)2Cl2(MeOH2) | not given | 50 s | 50 ppb | 2017 |
| (37) | diamino diphenyl sulfone (DDS) | 3.68–5.94 Hz/ppm | 60 s (1.7 ppm) | 1 ppm | 2017 |
| (38) | PDA/PODS | 10 Hz/ppm | not given | not given | 2018 |
| (39) | copper manganese composite oxide | 167 Hz/ppm | 60 s | 0.3 ppm | 2018 |
| (40) | PDA/HMSSs | 21.4–51.8 Hz/ppm | 5–9/3–15 s | 100 ppb | 2018 |
| (31) | CuO/ZnO nanofiber | 0.743 Hz/ppm | not given | 41 ppb | 2019 |
| (3) | calix[4]arene cage (SCC) | 18.324 Hz/ppm | not given | 0.67 ppb | 2020 |
Materials and Method
Principle and Numerical Calculation of QCM
When generating standing sound waves in an AY-cut quartz crystal, the intrinsic positive frequency of quartz crystal f0 is
| 1 |
where Aq is the effective area of a quartz crystal, μ is the shear modulus of the crystal, ρq is the density, and M represents the mass. On evenly coating the QCM with the electrode, the frequency change Δf caused by mass change ΔM is18
| 2 |
where Ae is the electrode area. For AT-cut quartz crystals with density ρq = 2.648 g/cm2 and shear modulus μq = 2.947 × 1011/(cm·s2), the formula for QCM frequency response is
| 3 |
where Δt is the thickness of the composite membrane. This formula proves that frequency variation of QCM is inversely proportional to the film mass on the electrode surface, that is, as the film mass increases, the frequency of QCM decreases.
For selecting a coating material, we mainly consider the following four aspects: the material should react reversibly with formaldehyde; it should have excellent adsorption capacity; the reactive surface area of the material should be maximum; and the difficulty of coating material preparation and the environmental contamination of the raw materials required for preparation should be concerned. According to the element structure of the main chain of the macromolecule, polymers can be divided into carbon chain polymers, heterochain polymers, and elemental organic polymers. For this work, we choose polymers rich in amine groups among carbon chain polymers.
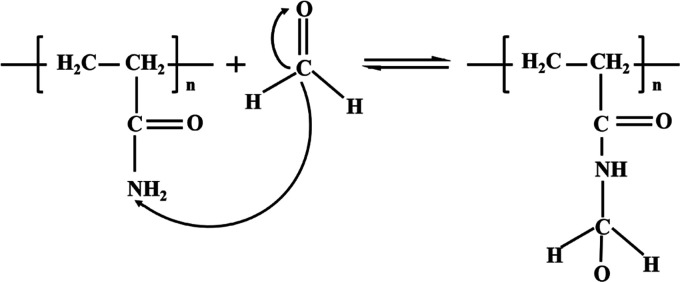
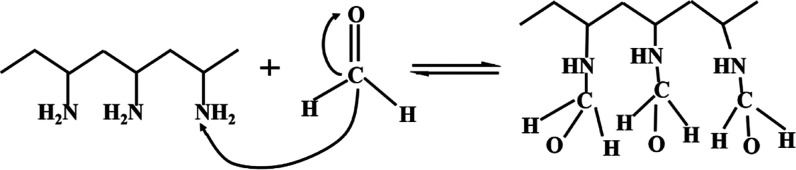
Polyacrylamide (PAAm) is a linear polymer with the chemical formula (C3H5NO)n. It is a hard glassy solid at room temperature. The products include glue, latex and white powder, translucent beads, and flakes. Polyvinylamine (PVAm) is a kind of polymer with rich activated primary amine group, high charge density, and controllable molecular chain length, which are very suitable for formaldehyde detection.34,41 The porous structure of the coating may accelerate the adsorption and desorption processes, thereby increasing the response rate.34 The reaction between formaldehyde and PAAm and PVAm is shown in Figures 1 and 2, respectively. The sensor’s sensing principle is based on the adsorption theory, which mainly involves the reversible nucleophilic addition reaction between formaldehyde molecules and primary amine groups in PAAm and PVAm. The positive reaction is the process of adsorbing formaldehyde, and the reverse reaction is the desorption process of formaldehyde, as shown in Figure 1. The carbon–oxygen double bond of the formaldehyde molecule has a σ bond and a π bond, and the carbon–oxygen double bond in the molecule exhibits polarity because of the electronegativity difference between carbon and oxygen atoms. The oxygen atom has a stronger electron adsorption force, which causes the common electron pair in the carbon–oxygen double bond to move to the oxygen atom. Therefore, the oxygen atom is negatively charged and the carbon atom is positively charged, which eventually causes the nucleophile to move to the carbon atom first and combine with it. At the same time, there is a lone pair of electrons in the amine group, which can easily become a nucleophile (negatively charged) to attack the electrophile (positively charged). After the reaction is complete, the carbon–oxygen double bond in the formaldehyde molecule is broken, forming two new covalent bonds via a chemical adsorption reaction, which can improve the adsorption of the composite sensitive membrane with formaldehyde, and the reversibility of the reaction ensures that the QCM sensor has excellent repeatability. The primary amine group in the PVAm molecule is higher than that of PAAm. MWCNT is considered a promising nanofiller in polymer nanocomposites due to its excellent properties, such as high mechanical strength and toughness, as well as high surface energy and small size. It is possible to enhance the sensitivity and selectivity by surface treatments and organic and inorganic functionalization.42,43 Therefore, the PVAm/MWCNT composite film QCM sensor has superior sensitivity and a detection limit that can reach 0.5 ppm.
Figure 1.
Principle of nucleophilic addition reaction between PAAm and formaldehyde.
Figure 2.
Principle of nucleophilic addition reaction between PVAm and formaldehyde.
QCM Sensor Detection System
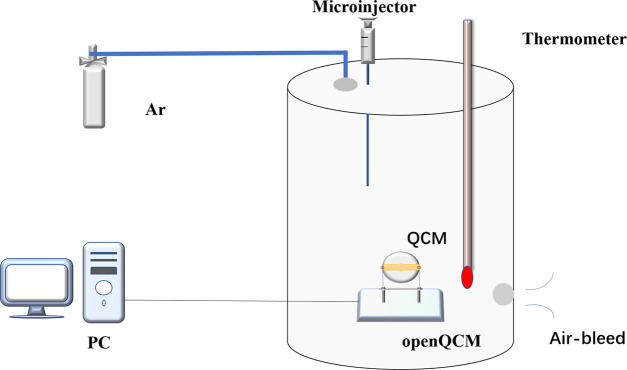
In this paper, a set of OpenQCM detection system for measuring gas concentration is designed, as shown in Figure 3. The system includes an OpenQCM tester, a computer, a high-purity argon bottle, a temperature-controlled gas chamber, a microsyringe, a thermometer, and a stopwatch. The volume of the gas chamber is 5.47 L, and the upper end of the gas chamber is the main inlet of argon, the mouth of the microsyringe, and the mouth of the thermometer. An exhaust hole is set in the lower right corner of the gas chamber to expel gas from the gas chamber. The purpose of the microinjector is to inject a certain concentration of formaldehyde into the gas chamber to create an environment with different concentrations of formaldehyde. The density of argon is higher than that of air and formaldehyde. Argon is helpful to exhaust air and water vapor in the air chamber or formaldehyde residual gas and vent hole, which can be used to remove harmful gas from the air chamber for pollution-free treatment. OpenQCM software and OpenQCM hardware are installed in the computer to measure, read, and save data. The rated frequency is 1–25 MHz, the supply voltage is +3.3 V, the power is 66 mV, and the temperature sensor is a 10 K thermosensitive resistor. Figure 4 is the schematic diagram of HCHO adsorption of QCM sensor. In early gas environment under test, the gas adsorption on the composite film cause mass increases, the frequency by the steady state suddenly drop, when frequency stability is not reduced. It means that the composite film is saturated, then the concentration of the gas under test can be calculated by the frequency response value.
Figure 3.
OpenQCM testing system.
Figure 4.
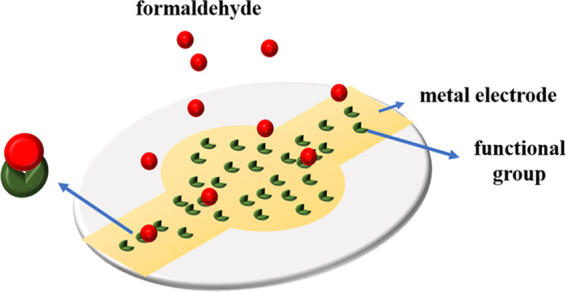
Diagram of QCM adsorption formaldehyde.
Preparation of Composite Film on QCM
Raw materials required for the preparation and coating of the composite membrane include polyacrylamide (PAAm), polyvinylamine (PVAm), polyvinylpiroketone (PVP), anhydrous ethanol, acetone, and deionized water. The temperature of the preparation environment is 25 °C. MWCNTs (300 mg) and PVP dispersing agent (60 mg) weighed using a microbalance were poured into a beaker, 99.64 mL of deionized water was added, the beaker mouth was sealed with plastic wrap (to prevent water evaporation in the solution), then twice sealed with tin foil, and put into an ultrasonic cleaner for ultrasonic stirring for 30 min (i.e., 5 min × 6 times) to ensure the uniform dispersion of MWCNTs. After ultrasound treatment, the dispersion was centrifuged and settled by centrifugation at a rate of 2000 r/min. The centrifugation time was set at 30 min. The upper liquid was 0.3 wt % MWCNT water dispersion. PAAm solid particles (200 mg) were weighed with a microbalance, carefully poured into a clean beaker, 39.8 mL of high-purity deionized water was added to the beaker, stirred thoroughly with a glass rod to obtain a concentration of 5 mg/mL PAAm aqueous solution, the beaker was sealed with plastic wrap and tin foil, and finally the beaker solution was put in an ultrasonic cleaner and ultrasonically stirred for 30 min to obtain a 5 mg/mL PAAm aqueous dispersion. PVAm particles (262.5 mg) were taken in a beaker, 35 mL of deionized water was poured into it and sealed with plastic wrap and tin foil, and finally the beaker solution was put in a KQ218 ultrasonic cleaner for ultrasonic shaking for half an hour to completely disperse PVAm in deionized water. Then, 7.5 mg/mL PVAm aqueous dispersion was obtained. A disposable dropper was used to take 0.3 wt % MWCNTs and 5 mL of PAAm, PVAm aqueous dispersion, slowly dripped into the beaker, gently shaken to obtain a full mixture of PAAm and MWCNTs, stirred with a glass rod for 2 min, and used plastic wrap to seal the beaker. To ensure that the MWCNTs and PAAm aqueous dispersion are fully mixed, we use tin foil to seal the beaker, which was then placed in an ultrasonic cleaner with ultrasonic stirring for 30 min, and finally prepare the required PAAm/MWCNT composite membrane.
To reduce the influence of external factors on the composite film, the beakers, two buckets of drying dishes, and glass rods used in the experiment were thoroughly cleaned with acetone, anhydrous ethanol, and deionized water before the composite film was made and then dried in a drying oven at a high temperature. The PAAm dispersion solution and 0.3 wt % MWCNTs were mixed to prepare the required PAAm/MWCNT composite membrane solution. The coating platform is shown in Figure 5. The composite film was prepared by the gas atomization method, and the principle of mist film formation was adopted using the carrier gas pressure to compress the dispersion liquid. The film was uniformly sprayed on the QCM gold electrode, and with the increase of spraying time, the film would gradually form. This process has the characteristics of low cost, easy operation, and rapid coating, which can be used for materials with low water-soluble viscosity. Before making the composite film, the QCM chip should be carefully cleaned, and the composite film solution should be loaded into the spray pen after cleaning for spraying.
Figure 5.
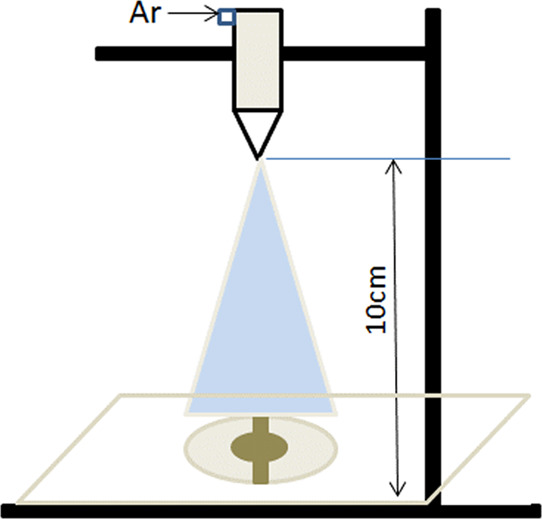
Composite film QCM coating platform.
Study on the Characteristics of Formaldehyde Sensor Based on the PAAm/MWCNT Composite Membrane
The QCM sensors were tested before injecting formaldehyde to test the stability of the sensors; the jitter diagram of the sensor over 787 s is shown in Figure 6, where the room temperature was 26 °C. It can be seen that the steady-state frequency value of the QCM sensor lies below 0.4 Hz without injecting formaldehyde; therefore, the subsequent test results will not be affected by frequency fluctuation errors. To study the sensitivity of the composite sensitive film sensor to formaldehyde, MWCNTs, PAAm, and PAAm/MWCNTs were coated on the electrode to obtain three sensors. The three sensors were put into the gas chamber one by one; argon was pumped to remove the water vapor and other interfering gases in the gas chamber; and the influence of external factors on the experiment was determined. With OpenQCM detection system frequency of testing sensor, 4 ppm of formaldehyde was injected into the gas chamber when the QCM sensor reaches a certain value of frequency, to test the changes in sensor frequency. When the sensor frequency reaches a stable value, argon is injected again to drain the gas chamber, gas indoor formaldehyde content is reduced, adverse reaction is triggered, and formaldehyde molecules in the composite membrane peel from the composite film. Finally, the adsorption is completely ceased. The composite film thickness has an influence on sensitivity. According to the Sauerbrey equation, the frequency of the QCM sensor is inversely proportional to the mass of the sensitive film coated on the electrode, so the thickness of the sensitive film can be determined according to the change of the QCM frequency. The more massive the sensitive membrane, the more formaldehyde can be adsorbed molecularly by the membrane surface, so increasing the film thickness can improve the sensitivity of the sensor. However, experiments show that the response time and recovery time are longer with the thicker film on the QCM electrode, and when the film thickness is too large, the effective adsorption area of the composite film will reach a fixed value and the QCM chip will stop oscillating. Therefore, to maximize the sensitivity, the optimal film thickness should be determined experimentally. Different film thicknesses can be obtained through different spraying times, and the response characteristics at 3 ppm were detected. To study the influence of different proportions of PAAm/MWCNTs membrane on the characteristics of sensors, four thin films with a 0.5:1/1:1/1:2/2:1 volume ratio of PAAm/MWCNTs were prepared, with a thickness of 0.25 μm, and the frequency difference before and after coating is 5593.0 Hz at room temperature. The four sensors were detected in the environment of 4 ppm formaldehyde after 24 h after drying. To study the response characteristics of the sensor to different concentrations of formaldehyde, a sensor with a 1:1 composite membrane ratio of PAAm/MWCNTs and a frequency difference of 5593.0 Hz before and after coating was prepared and placed in an air chamber to measure different concentrations of formaldehyde at room temperature. Repeatability refers to the degree of consistency of each measurement when the sensor detects the same physical quantity, which is an important index to describe the performance of the sensor. The response characteristics were tested in a 3 ppm environment, and the desorption process of formaldehyde was completed by injecting high-purity argon into the detection chamber, and the recovery performance of the QCM sensor to formaldehyde was tested. The same operation was repeated three times. The QCM sensor is vulnerable to interference by other volatile organic gases during gas detection. To test the selectivity of the sensor, four kinds of VOCs were used to study the selectivity of PAAm/MWCNTs-QCM. Under the same experimental conditions, 4 ppm formaldehyde, ethanol, chloroform, and propylene oxide were injected into the gas chamber for testing. The QCM sensor was connected to the OpenQCM detection system and put into the temperature control test box. Formaldehyde (3 ppm) was injected into the test box, and the temperature in the thermostatic test box was adjusted. The influence of temperature on the gas-sensitive characteristics of the sensor was tested from 25 to 85 °C at intervals of 5 °C.
Figure 6.
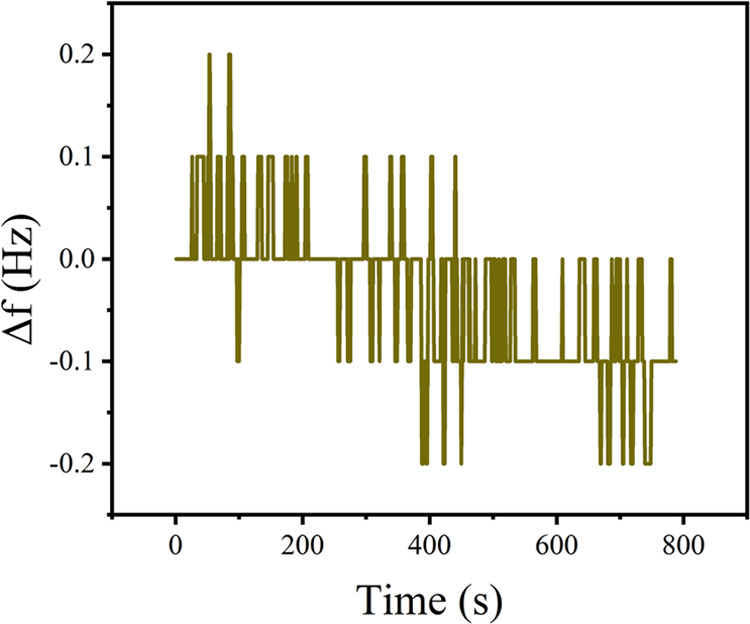
Stability of QCM sensor over 787 s.
Formaldehyde Sensor Characteristics Based on the PVAm/MWCNT Composite Membrane
PVAm particles were added to deionized water, and ultrasonic oscillation was performed in the ultrasonic cleaner for 30 min, which was the same as the preparation method of PAAm/MWCNTs. MWCNTs and PVP dispersants were added to deionized water to prepare dispersants. The dispersion of MWCNTs and PVP was mixed at 1:1 and then oscillated to obtain the composite membrane solution. The solution was atomized and coated to obtain PVAm-QCM and PVAm/MWCNTs-QCM sensors. PVAm-QCM and PVAm/MWCNTs-QCM sensors were compared, and they were put into a 4 ppm formaldehyde environment for comparison. To test the measuring range of the sensors, 0.5, 1, 2, 3, 4, and 6 ppm formaldehyde were injected into the gas chamber for testing. The gas chamber was injected with 3 ppm formaldehyde. When the signal was in steady state, argon was injected for desorption, and the operation was repeated three times. The selectivity and temperature characteristic test was performed in the same manner as the PAAm/MWCNT test.
Results and Discussion
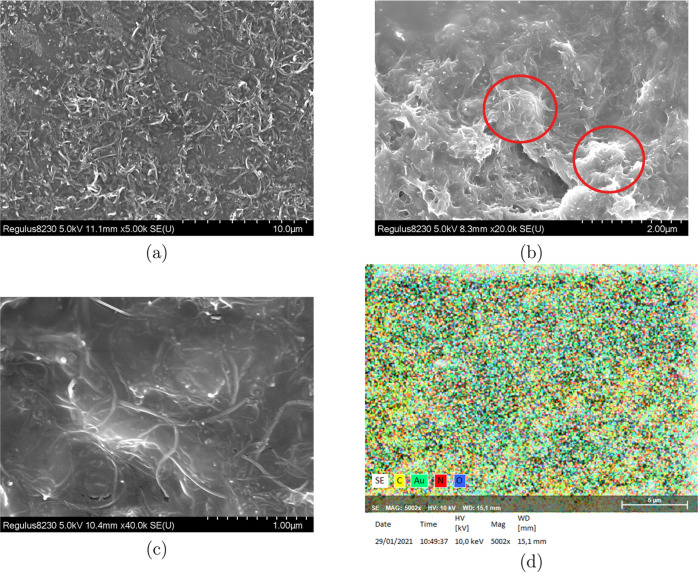
Figure 7 shows the electron microscopy images of PAAm/MWCNT and PVAm/MWCNT membrane samples. It can be seen that there are uniformly distributed multiwalled carbon nanotubes on the surface of the QCM. The dense network distribution can indicate that the surface area of the material is greatly increased. Ultrasonic treatment is 30 min, and Figure 7b shows the result of PAAm/MWCNTs with ultrasonic treatment for 20 min. It can be seen that a suitable ultrasonic time can effectively reduce the densification of MWCNTs, thereby achieving uniform dispersion of MWCNTs in the polymer. Elemental analysis shows that carbon, nitrogen, and oxygen are evenly distributed on the gold electrode. Figure 8 shows the Fourier transform infrared (FT-IR) spectra of PAAm/MWCNTs and PVAm/MWCNTs. The FT-IR graph shows that PAAm/MWCNTs and PVAm/MWCNTs have characteristic peaks of free −NH2 at 3154 and 3179 cm–1, respectively. N–H bending vibration absorption peaks appear at 1647 and 1650 cm–1. The graph indicates the existence of primary amine groups of the composite membrane.
Figure 7.
(a) SEM image of evenly mixed PAAm/MWCNTs, and (b) SEM image of not evenly mixed PAAM/MWCNTs, (c) SEM image of mixed PVAm/MWCNTs, and (d) result of elemental analysis.
Figure 8.
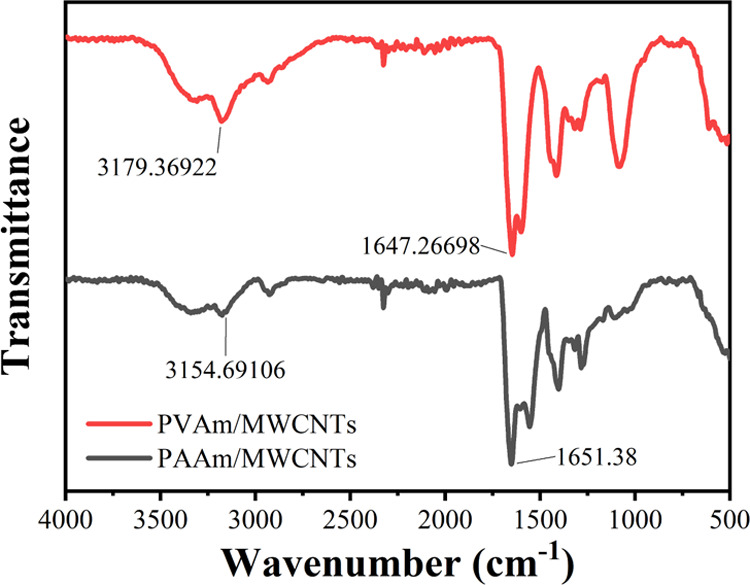
FT-IR graph of PAAm/MWCNTs and PVAm/MWCNTs.
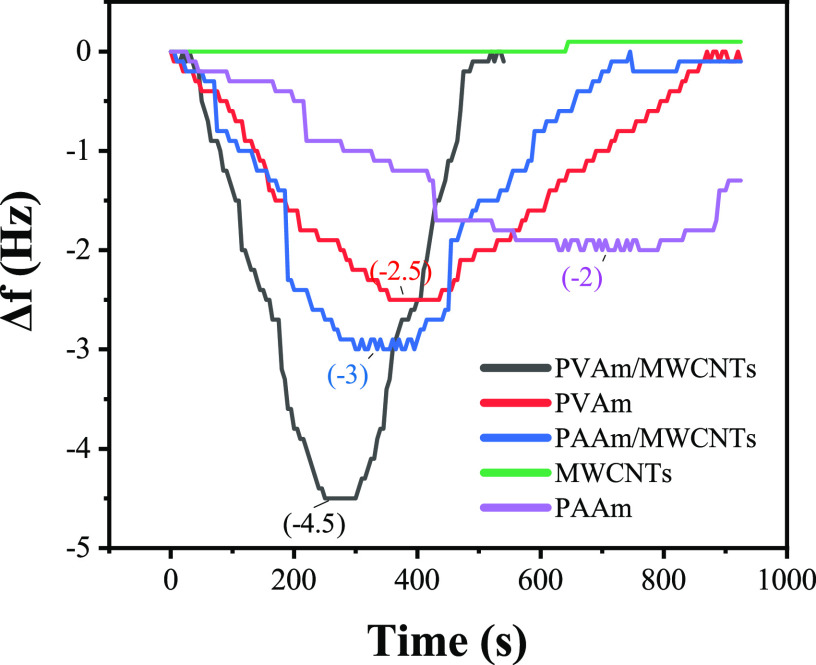
The frequency response of PAAm/MWCNTs-QCM and PVAm/MWCNTs-QCM at 4 ppm is shown in Figure 9.
Figure 9.
Comparison of the gas-sensitive characteristics of PAAm, PAAm/MWCNTs, PVAm, and PVAm/MWCNTs membrane to 4 ppm formaldehyde.
The frequency response and response time are shown in Table 2. The response time is defined as the time from the beginning of formaldehyde injection to the test frequency value that fluctuates and stabilizes within 0.4 Hz
Table 2. Frequency and Time Response of the Four Kinds of Sensors at 4 ppm.
| coated material | frequency response (Hz) | response time (s) | recovery time (s) |
|---|---|---|---|
| PAAm | 2 | 140 | 160 |
| PAAm/MWCNTs | 3 | 80 | 100 |
| PVAm | 2.5 | 365 | 200 |
| PVAm/MWCNTs | 4.5 | 485 | 195 |
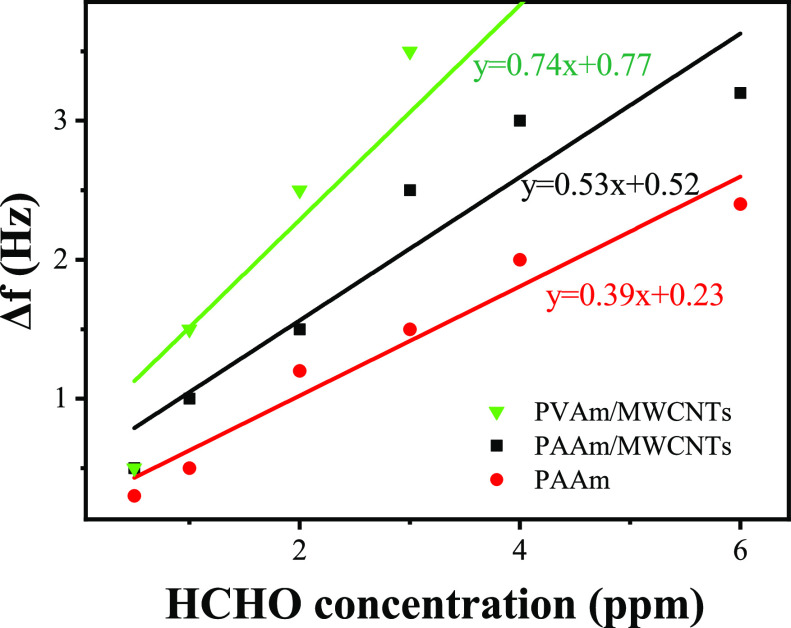
From Figure 9 and Table 2, all of the five kinds of sensors can return to the initial value. The response value of single-film QCM sensors was smaller than those of composite membrane QCM sensors. As for the response time, sensors coated with PAAm and PAAm/MWCNTs respond quicker than the sensors coated with PVAm and PVAm/MWCNTs, and composite film sensors respond quicker than the single-film sensors. A single MWCNT membrane sensor showed little response to formaldehyde. It was proved that the frequency change of the composite membrane sensors was caused by the nucleophilic addition reaction between the primary amine group in the PAAm or PVAm molecule and the formaldehyde molecule, and the mass increase was caused by the adsorption of the formaldehyde molecule on the QCM electrode. The frequency response of the composite film is more apparent than that of the single-film sensor, which is due to the specific surface area of the MWCNTs nanofiber film, which is conducive to the diffusion of formaldehyde gas and has more formaldehyde adsorption points per unit area. The linear fitting curve of the relationship between QCM frequency and formaldehyde concentration is shown in Figure 10. The sensitivities of PAAm/MWCNTs-QCM and PAAm-QCM were 0.52 and 0.39 Hz/ppm, respectively, and that of PVAm/MWCNT-QCM was 0.74 Hz/ppm.
Figure 10.
Relationship between frequency variation and concentration of PAAm, PAAm/MWCNTs, and PVAm/MWCNTs-QCM.
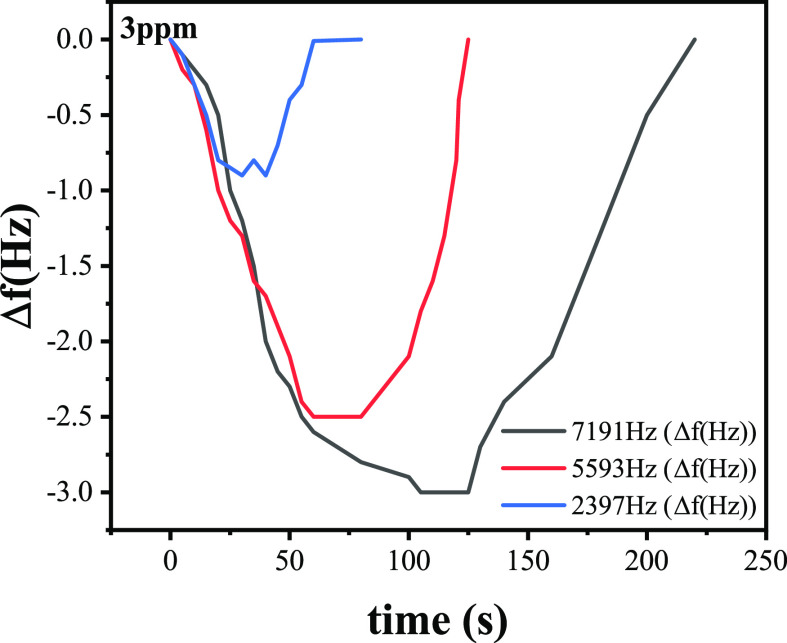
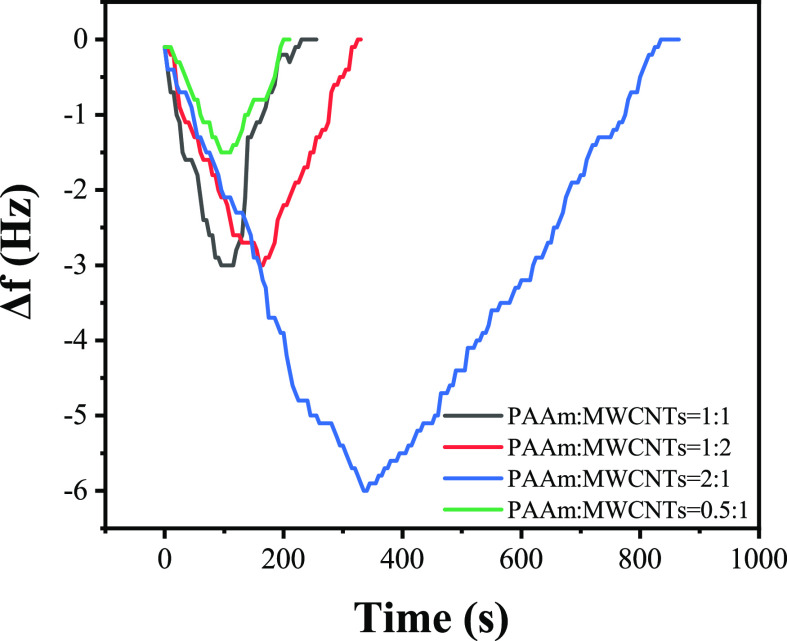
Gas sensing characteristics of PAAm/MWCNTs-QCM with different film thicknesses at 3 ppm formaldehyde are shown in Figure 11. The test results of different PAAm/MWCNTs-QCM at 3 ppm formaldehyde are shown in Table 3. The frequency difference before and after coating is used to represent the film load. According to the experimental results, within a certain range, the response value of the PAAm/MWCNT composite film sensor is proportional to the thickness of the film, and it can be seen from the experimental results that its response and recovery time increase with the increases of film thickness. It can be seen that the composite film thickness has a significant influence on the QCM sensor. When the frequency difference caused by coating thickness is 2397.0 Hz, the response value is 0.9 Hz, because its response value is low and it is not easy to detect in the complex test environment. The frequency difference caused by coating thickness is 7191.0 Hz, and the sensor response value is 3.0 Hz, compared with the value of 5593.0 frequency difference, which is not significantly different, but its response and recovery time are significantly extended, thereby affecting the test efficiency. According to the analysis of this phenomenon, it is believed that the increase of loading amount of the sensitive membrane leads to the delay of the gas transfer rate of the sensor in the fiber material. Theoretically, the larger load of sensitive film, the more gas the film can absorb. However, due to the limitation of actual materials, excessive film thickness will reduce the ratio of surface area to thickness and the porosity of the composite film, thus reducing the overall performance of the QCM sensor. In the experiment, the resonant frequency of the QCM is 10 MHz and the electrode radius is 3 mm. Therefore, it can be calculated that the change in frequency of 1 Hz is caused by a mass change of 1.25 ng for practical application consideration; it requires a trade-off between response time and response value, frequency difference caused by coating thickness was selected as 5593.0 Hz, and the optimal thickness of the sensitive film was calculated as 0.25 μm. Gas sensing characteristics of PAAm/MWCNTs with different ingredient proportions of composite film detected at 4 ppm formaldehyde are shown in Figure 12. The test results of QCM for 4 ppm formaldehyde are shown in Table 4,
Figure 11.
Gas sensing characteristics of different PAAm/MWCNTs-QCM at 3 ppm formaldehyde.
Table 3. Test Results of PAAm/MWCNTs-QCM at 3 ppm Formaldehyde with Different Film Thicknesses.
| frequency difference before and after film coating (Hz) | frequency response value (Hz) | the response time (s) | recovery time (s) |
|---|---|---|---|
| 2397.0 | 0.9 | 31 | 35 |
| 5593.0 | 2.5 | 60 | 65 |
| 7191.0 | 3.0 | 120 | 130 |
Figure 12.
Gas sensing characteristics of PAAm/MWCNTs-QCM with different ingredient proportions at 4 ppm formaldehyde.
Table 4. Test Results of PAAm/MWCNTs-QCM at Different Proportions to 4 ppm Formaldehyde.
| PAAm/MWCNTs proportion | response time (s) | recovery time (s) | frequency response value (Hz) |
|---|---|---|---|
| 0.5:1 | 95 | 100 | 1.4 |
| 1:1 | 100 | 93 | 2.5 |
| 1:2 | 175 | 163 | 2.5 |
| 2:1 | 345 | 483 | 6.1 |
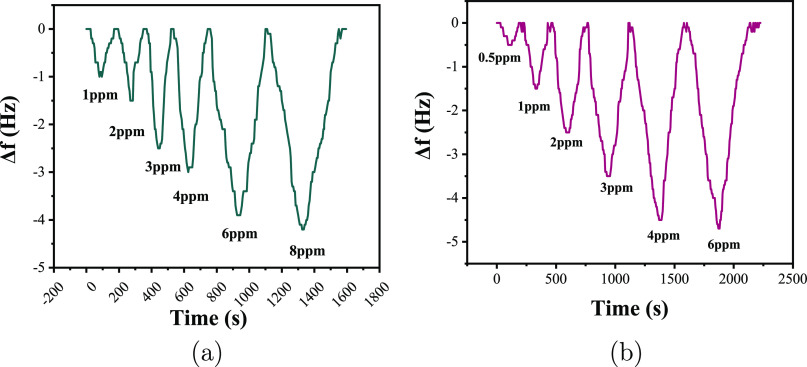
By analyzing the experimental data, it can be concluded that the sensor with a ratio of 2:1 has the highest response value because more formaldehyde molecules have nucleophilic addition reaction with PAAm, but its response and recovery time are also the longest. Compared with the other three ratio sensors, the response value of the 1:1 ratio sensor matched with that of 1:2, about 1.5 Hz higher than that of the sensor matched with 0.5:1, and its response time and recovery time were shortened by 70 s compared with that of the sensor matched with 1:2. When the ratio is 1:1, the formaldehyde molecule and PAAm have an appropriate effective area and the carbon nanotube fully disperses PAAm to maximize the contact with the formaldehyde molecule, and the nucleophilic addition reaction occurs. Similar to response value, response time and recovery time are also the main performance indicators for the evaluation of the sensor; in summary, the optimal ratio of PAAm and MWCNTs is 1:1, which is adopted in subsequent experiments. Formaldehyde gas of 0.5, 1, 2, 3, 4, 6, and 8 ppm was successively injected into the gas chamber and tested separately. The gas sensing characteristics of PAAm/MWCNTs-QCM and PAAm/MWCNTs-QCM to formaldehyde at different concentrations are shown in Figure 13.
Figure 13.
(a) Gas sensing characteristics of PAAm/MWCNTs-QCM to different concentrations of formaldehyde and (b) sensitive characteristics of PVAm/MWCNTs-QCM at different concentrations of formaldehyde.
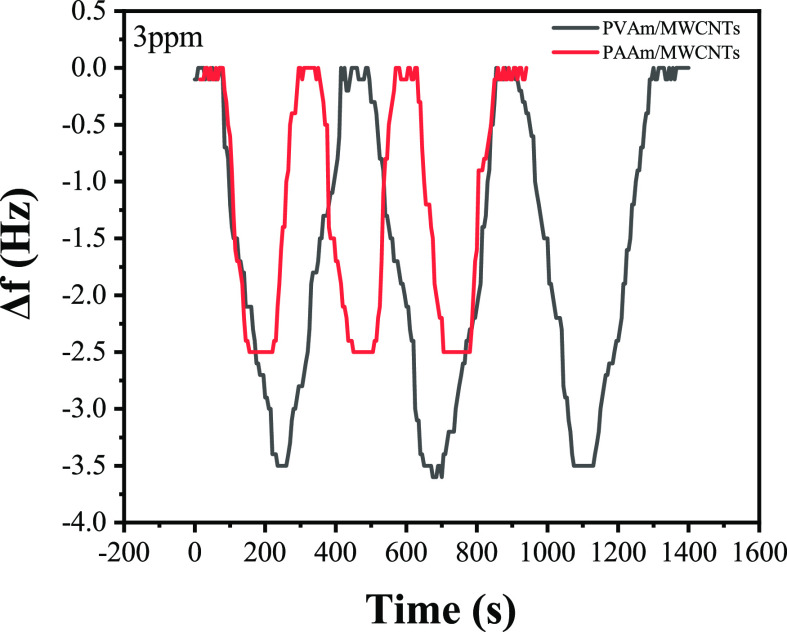
It can be seen from Figure 13 that the reaction between composite membrane and formaldehyde molecules leads to an increase in the mass of the composite film, which leads to a decrease in the frequency of the QCM sensor. When the frequency drops to a dynamic equilibrium, the sensor frequency continues to decrease if the concentration is increased. To test the recovery characteristics of the sensor, argon gas is slowly injected into the gas chamber and the frequency of the QCM sensor will be out of the dynamic equilibrium state. With the decrease of formaldehyde concentration, the frequency will gradually increase and finally returns to the initial state. To complete the desorption process, argon can be injected into the gas chamber for a period of time to reduce the formaldehyde concentration in the gas chamber to zero. According to the analysis data, the two composite film QCM sensors had a good response to formaldehyde, that is, the recovery was fast and relatively complete and the baseline did not drift, and the low limit of the PAAm/MWCNTs sensor detection was 1 ppm and that of PVAm/MWCNTs is 0.5 ppm. When the formaldehyde concentration exceeded 8 ppm (6 ppm for PVAm/MWCNTs), the response value of the QCM sensor did not increase significantly any longer, indicating that the composite film did not absorb formaldehyde molecules. It is inferred that the reason for this phenomenon is that the reaction between formaldehyde molecules and composite film reaches a dynamic balance, and the composite film no longer absorbs formaldehyde. Excessive formaldehyde will cause the composite membrane to produce “toxic phenomenon”. The PAAm/MWCNTs-QCM sensor and PVAm/MWCNTs-QCM sensor were tested in the 3 ppm environment alternated to desorption environment three times. The results are presented in Figure 14.
Figure 14.
Repetitive detection of PAAm/MWCNTs and PVAm/MWCNTs-QCM in the 3 ppm formaldehyde environment.
It can be seen from the figure that the response value of the sensor to the 3 ppm formaldehyde environment is 2.5 Hz and that of PAAm/MWCNTs-QCM is 3.5 Hz, and the results of the three times experiment are essentially the same. After argon was injected, the curve returned to the initial position, indicating that the sensors have good repeatability and good reversibility and that it can be reused many times, thereby reducing the cost.
For the selectivity test of the sensor, the test result after injecting the interfering gas is shown in Figure 15.
Figure 15.
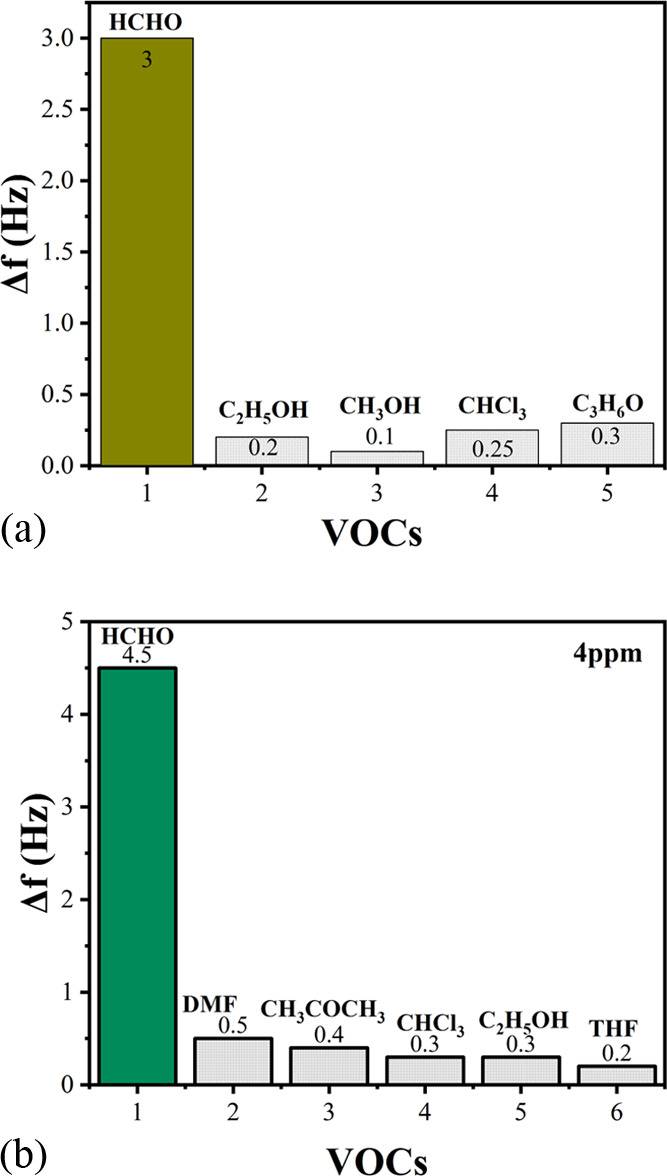
(a) Selectivity detection of PAAm/MWCNTs-QCM and (b) selectivity detection of PVAm/MWCNTs-QCM.
It can be observed from the figure that the response value of the sensor to formaldehyde is far greater than those to other gas; for PAAm/MWCNTs-QCM sensor, the frequency response of formaldehyde is 3.0 Hz and that of PAAm/MWCNTs is 4.5 Hz. For other organic gases, the response degree of the sensor to them from high to low is C3H6O, CHC13, C2H5OH, CH3OH, the response of the sensor for the gas is less than 0.5 Hz, it can be inferred that PAAm/MWCNTs-QCM has superior selectivity to the formaldehyde gas and is almost unaffected by other gases.
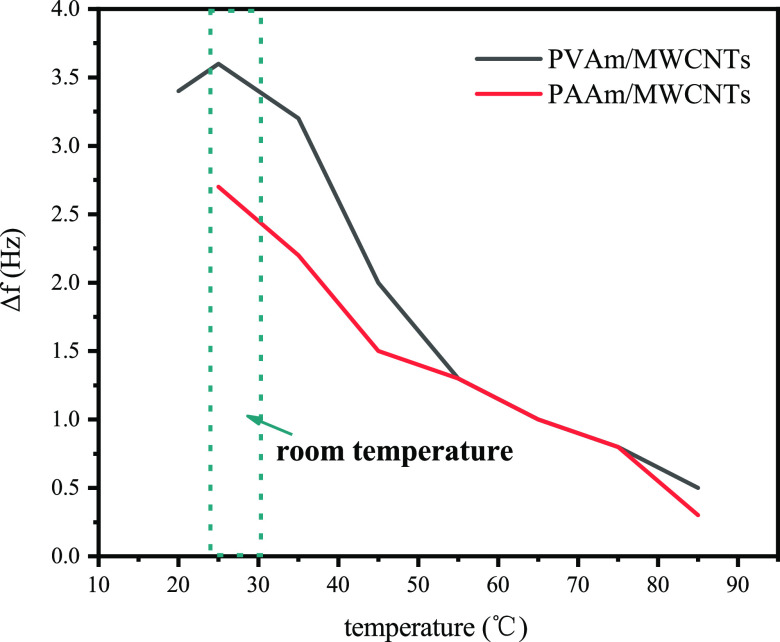
The response characteristics of the two composite membrane sensors at different temperatures are shown in Figure 16.
Figure 16.
Influence of temperature on the gas sensitivity characteristics of PAAm/MWCNTs-QCM and PVAm/MWCNTs-QCM.
The curve in the above figure shows that the sensor’s response is inversely proportional to temperature. In the high-temperature environment, there is a rapid desorption of formaldehyde during the adsorption of formaldehyde by a composite membrane. Therefore, the adsorption of formaldehyde by the composite film of QCM sensor is less, resulting in a relatively low response value. The data also showed that it worked well at room temperature and had a high response value, which could be used for formaldehyde detection in daily life.
Conclusions
In this paper, PAAm/MWCNTs and PVAm/MWCNTs composite membranes were prepared by the air-spray method. The gas-sensitive characteristics to the formaldehyde environment at room temperature were explored. At room temperature, the influence of the composite film thickness of PAAm/MWCNTs and the proportion of the configuration of PAAm/MWCNTs on the QCM sensor was studied. The optimal process parameters were determined by multiple tests: frequency difference before and after the formation of the film, 5593.0 Hz; film thickness, 0.25 μm; and proportion of the configuration of the composite film, 1:1. The gas sensitivity of the sensor at different concentrations of formaldehyde was analyzed. The experimental results show that the sensor has a lower limit of 1 ppm for formaldehyde, a detection range of 1–8 ppm, and a sensitivity of 0.53 Hz/ppm. The sensor also has good reproducibility, selectivity, and high sensitivity at room temperature. The mechanism of the interaction between PAAm/MWCNT composite film and formaldehyde is mainly due to the reversible nucleophilic addition reaction between PAAm primary amine groups and formaldehyde molecules at room temperature. To further reduce the monitoring lower limit of the sensor, sensitive materials with more primary amine groups were selected to produce the optimal PVAm/MWCNT composite membrane QCM sensor, and the gas sensing characteristics of the sensor to the environment with different concentrations of formaldehyde were analyzed. The experimental results show that the sensor has a minimum detection limit of 0.5 ppm, a detection range of 0.5–6 ppm, and a sensitivity of 0.74 Hz/ppm. The sensor also has good reproducibility, selectivity, and high response at room temperature. The two kinds of sensors designed in this paper are characterized by fast response, low cost, and easy preparation. They can be used to detect a low concentration of formaldehyde, which is of great significance for the detection of environmental quality.
Acknowledgments
This work was supported by the Natural Science Foundation of China (no. 61675025).
The authors declare no competing financial interest.
Author Status
† Author Qi Li studied as a postgraduate in Beijing Institute of Technology, and graduated at 2016, he is now an employee of Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
References
- Cleaves H. J. II The prebiotic geochemistry of formaldehyde. Precambrian Res. 2008, 164, 111–118. 10.1016/j.precamres.2008.04.002. [DOI] [Google Scholar]
- Korpan Y. I.; Gonchar M. V.; Sibirny A. A.; Martelet C.; El’skaya A. V.; Gibson T. D.; Soldatkin A. P. Development of highly selective and stable potentiometric sensors for formaldehyde determination. Biosens. Bioelectron. 2000, 15, 77–83. 10.1016/S0956-5663(00)00054-3. [DOI] [PubMed] [Google Scholar]
- Temel F. One novel calix[4]arene based QCM sensor for sensitive, selective and high performance-sensing of formaldehyde at room temperature. Talanta 2020, 211, 120725 10.1016/j.talanta.2020.120725. [DOI] [PubMed] [Google Scholar]
- Flyvholm M.-A.; Andersen P. Identification of formaldehyde releasers and occurrence of formaldehyde and formaldehyde releasers in registered chemical products. Am. J. Ind. Med. 1993, 24, 533–552. 10.1002/ajim.4700240505. [DOI] [PubMed] [Google Scholar]
- Kawamura K.; Kerman K.; Fujihara M.; Nagatani N.; Hashiba T.; Tamiya E. Development of a novel hand-held formaldehyde gas sensor for the rapid detection of sick building syndrome. Sens. Actuators, B 2005, 105, 495–501. 10.1016/j.snb.2004.07.010. [DOI] [Google Scholar]
- Que Z.; Furuno T.; Katoh S.; Nishino Y. Evaluation of three test methods in determination of formaldehyde emission from particleboard bonded with different mole ratio in the urea-formaldehyde resin. Build. Sci. 2007, 42, 1242–1249. 10.1016/j.buildenv.2005.11.026. [DOI] [Google Scholar]
- Kriebel D.; Sama S. R.; Cocanour B. Reversible pulmonary responses to formaldehyde. A study of clinical anatomy students. Am. Rev. Respir. Dis. 1993, 148, 1509–1515. 10.1164/ajrccm/148.6_Pt_1.1509. [DOI] [PubMed] [Google Scholar]
- Bosetti C.; McLaughlin J.; Tarone R.; Pira E.; La Vecchia C. Formaldehyde and cancer risk: a quantitative review of cohort studies through 2006. Ann. Oncol. 2008, 19, 29–43. 10.1093/annonc/mdm202. [DOI] [PubMed] [Google Scholar]
- Monticello T. M.; Swenberg J. A.; Gross E. A.; Leininger J. R.; Kimbell J. S.; Seilkop S.; Starr T. B.; Gibson J. E.; Morgan K. T. Correlation of Regional and Nonlinear Formaldehyde-induced Nasal Cancer with Proliferating Populations of Cells. Cancer Res. 1996, 56, 1012–1022. [PubMed] [Google Scholar]
- Wang L.; Gao J.; Xu J. QCM formaldehyde sensing materials: Design and sensing mechanism. Sens. Actuators, B 2019, 293, 71–82. 10.1016/j.snb.2019.04.050. [DOI] [Google Scholar]
- Squire R. A.; Cameron L. L. An analysis of potential carcinogenic risk from formaldehyde. Regul. Toxicol. Pharmacol. 1984, 4, 107–129. 10.1016/0273-2300(84)90034-5. [DOI] [PubMed] [Google Scholar]
- Möhlmann G. R. Formaldehyde Detection in Air by Laser-Induced Fluorescence. Appl. Spectrosc. 1985, 39, 98–101. 10.1366/0003702854249088. [DOI] [Google Scholar]
- Bricker C. E.; Johnson H. R. Spectrophotometric Method for Determining Formaldehyde. Ind. Eng. Chem., Anal. Ed. 1945, 17, 400–402. 10.1021/i560142a021. [DOI] [Google Scholar]
- West P. W.; Sen B. Spectrophotometric determination of traces of formaldehyde. Fresenius’ Z. Anal. Chem. 1956, 153, 177–183. 10.1007/BF00460217. [DOI] [Google Scholar]
- Shimomura T.; Itoh T.; Sumiya T.; Mizukami F.; Ono M. Electrochemical biosensor for the detection of formaldehyde based on enzyme immobilization in mesoporous silica materials. Sens. Actuators, B 2008, 135, 268–275. 10.1016/j.snb.2008.08.025. [DOI] [Google Scholar]
- Flueckiger J.; Ko F.; Cheung K. Microfabricated Formaldehyde Gas Sensors. Sensors 2009, 9, 9196–9215. 10.3390/s91109196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian T.; Hidayat S. N.; Rianjanu A.; Dharmawan A. B.; Wasisto H. S.; Triyana K. Intelligent Mobile Electronic Nose System Comprising a Hybrid Polymer-Functionalized Quartz Crystal Microbalance Sensor Array. ACS Omega 2020, 5, 29492–29503. 10.1021/acsomega.0c04433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbrey G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. 10.1007/BF01337937. [DOI] [Google Scholar]
- Kumar A.; Brunet J.; Varenne C.; Ndiaye A.; Pauly A.; Penza M.; Alvisi M. Tetra-tert-butyl copper phthalocyanine-based QCM sensor for toluene detection in air at room temperature. Sens. Actuators, B 2015, 210, 398–407. 10.1016/j.snb.2015.01.010. [DOI] [Google Scholar]
- Öztürk S.; Kösemen A.; Kösemen Z. A.; Kílínç N.; Öztürk Z. Z.; Penza M. Electrochemically growth of Pd doped ZnO nanorods on QCM for room temperature VOC sensors. Sens. Actuators, B 2016, 222, 280–289. 10.1016/j.snb.2015.08.083. [DOI] [Google Scholar]
- Chen W.; Deng F.; Xu M.; Wang J.; Wei Z.; Wang Y. GO/Cu2O nanocomposite based QCM gas sensor for trimethylamine detection under low concentrations. Sens. Actuators, B 2018, 273, 498–504. 10.1016/j.snb.2018.06.062. [DOI] [Google Scholar]
- Amini N.; Kalaee M.; Mazinani S.; Pilevar S.; Ranaei-Siadat S.-O. Morphological optimization of electrospun polyacrylamide/MWCNTs nanocomposite nanofibers using Taguchi’s experimental design. Int. J. Adv. Des. Manuf. Technol. 2013, 69, 139–146. 10.1007/s00170-013-5006-x. [DOI] [Google Scholar]
- Sill T. J.; von Recum H. A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Evingour G. A.; Pekcan N.. Optical, Mechanical, and Electrical Properties of Polymer Composites Doped by Multiwalled Carbon Nanotubes. In Carbon Nanotubes - Current Progress of their Polymer Composites, 2016. [Google Scholar]
- Hussain M.; Kotova K.; Lieberzeit P. A. Molecularly Imprinted Polymer Nanoparticles for Formaldehyde Sensing with QCM. Sensors 2016, 16, 1011 10.3390/s16071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Ding B.; Sun M.; Yu J.; Sun G. Nanofibrous polyethyleneimine membranes as sensitive coatings for quartz crystal microbalance-based formaldehyde sensors. Sens. Actuators, B 2010, 144, 11–17. 10.1016/j.snb.2009.08.023. [DOI] [Google Scholar]
- Hu W.; Chen S.; Liu L.; Ding B.; Wang H. Formaldehyde sensors based on nanofibrous polyethyleneimine/bacterial cellulose membranes coated quartz crystal microbalance. Sens. Actuators, B 2011, 157, 554–559. 10.1016/j.snb.2011.05.021. [DOI] [Google Scholar]
- Wang X.; Cui F.; Lin J.; Ding B.; Yu J.; Al-Deyab S. S. Functionalized nanoporous TiO2 fibers on quartz crystal microbalance platform for formaldehyde sensor. Sens. Actuators, B 2012, 171-172, 658–665. 10.1016/j.snb.2012.05.050. [DOI] [Google Scholar]
- Wang N.; Wang X.; Jia Y.; Li X.; Yu J.; Ding B. Electrospun nanofibrous chitosan membranes modified with polyethyleneimine for formaldehyde detection. Carbohydr. Polym. 2014, 108, 192–199. 10.1016/j.carbpol.2014.02.088. [DOI] [PubMed] [Google Scholar]
- Wang L.; Wang Z.; Xiang Q.; Chen Y.; Duan Z.; Xu J. High performance formaldehyde detection based on a novel copper (II) complex functionalized QCM gas sensor. Sens. Actuators, B 2017, 248, 820–828. 10.1016/j.snb.2016.12.015. [DOI] [Google Scholar]
- Diltemiz S. E.; Ecevit K. High-performance formaldehyde adsorption on CuO/ZnO composite nanofiber coated QCM sensors. J. Alloys Compd. 2019, 783, 608–616. 10.1016/j.jallcom.2018.12.237. [DOI] [Google Scholar]
- Zhu Y.; Li H.; Zheng Q.; Xu J.; Li X. Amine-Functionalized SBA-15 with Uniform Morphology and Well-Defined Mesostructure for Highly Sensitive Chemosensors To Detect Formaldehyde Vapor. Langmuir 2012, 28, 7843–7850. 10.1021/la300560j. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Wang X.; Lin J.; Ding B.; Yu J.; Pan N. Nanoporous polystyrene fibers functionalized by polyethyleneimine for enhanced formaldehyde sensing. Sens. Actuators, B 2011, 152, 316–323. 10.1016/j.snb.2010.12.028. [DOI] [Google Scholar]
- Huang W.; Wang X.; Jia Y.; Li X.; Zhu Z.; Li Y.; Si Y.; Ding B.; Wang X.; Yu J. Highly sensitive formaldehyde sensors based on polyvinylamine modified polyacrylonitrile nanofibers. RSC Adv. 2013, 3, 22994 10.1039/c3ra44671a. [DOI] [Google Scholar]
- Tai H.; Bao X.; He Y.; Du X.; Xie G.; Jiang Y. Enhanced Formaldehyde-Sensing Performances of Mixed Polyethyleneimine-Multiwalled Carbon Nanotubes Composite Films on Quartz Crystal Microbalance. IEEE Sens. J. 2015, 15, 6904–6911. 10.1109/JSEN.2015.2468073. [DOI] [Google Scholar]
- Yang M.; He J. Graphene oxide as quartz crystal microbalance sensing layers for detection of formaldehyde. Sens. Actuators, B 2016, 228, 486–490. 10.1016/j.snb.2016.01.046. [DOI] [Google Scholar]
- Wang L.; Zhu Y.; Xiang Q.; Cheng Z.; Chen Y.; Xu J. One novel humidity-resistance formaldehyde molecular probe based hydrophobic diphenyl sulfone urea dry-gel: Synthesis, sensing performance and mechanism. Sens. Actuators, B 2017, 251, 590–600. 10.1016/j.snb.2017.05.074. [DOI] [Google Scholar]
- Wang L.; Yu Y.; Xiang Q.; Xu J.; Cheng Z.; Xu J. PODS-covered PDA film based formaldehyde sensor for avoiding humidity false response. Sens. Actuators, B 2018, 255, 2704–2712. 10.1016/j.snb.2017.09.082. [DOI] [Google Scholar]
- Yang M.; He J. A copper-manganese composite oxide as QCM sensing layers for detection of formaldehyde gas. RSC Adv. 2018, 8, 22–27. 10.1039/C7RA11427C. [DOI] [Google Scholar]
- Zong J.; Zhang Y. S.; Zhu Y.; Zhao Y.; Zhang W.; Zhu Y. Rapid and highly selective detection of formaldehyde in food using quartz crystal microbalance sensors based on biomimetic poly-dopamine functionalized hollow mesoporous silica spheres. Sens. Actuators, B 2018, 271, 311–320. 10.1016/j.snb.2018.05.089. [DOI] [Google Scholar]
- Gao B.; Lu J.; Zhuang R.; Zhang G. Preparation of poly(vinyl amine)-grafted crosslinked poly(vinyl alcohol) microspheres. J. Appl. Polym. Sci. 2009, 114, 3487–3494. 10.1002/app.30892. [DOI] [Google Scholar]
- Wu G.-H.; Liu S.-Q.; Wu X.-Y.; Ding X.-M. Influence of MWCNTs modified by silane coupling agent KH570 on the properties and structure of MWCNTs/PLA composite film. J. Polym. Res. 2016, 23, 155. 10.1007/s10965-016-1024-3. [DOI] [Google Scholar]
- Maity D.; Kumar R. T. R. Polyaniline Anchored MWCNTs on Fabric for High Performance Wearable Ammonia Sensor. ACS Sens. 2018, 3, 1822–1830. 10.1021/acssensors.8b00589. [DOI] [PubMed] [Google Scholar]