Abstract

Noncatalyzed, regio- and stereoselective hypochlorite oxidation of 3-aminothieno[2,3-b]pyridine-2-carboxamides is presented. Unexpectedly, the oxidation proceeded by different mechanistic pathways, and different products were formed, depending on the nature of solvents used. A possible mechanism, the structure of products, kinetics and dynamics of intramolecular processes, and biological activity of products are discussed.
Introduction
Thieno[2,3-b]pyridines belong to a privileged class of compounds that has for a long time attracted great interest for its beneficial effects in the treatment of many diseases.1−5 Recently, there have been a number of reports concerning the biological activity of thienopyridines. Recently, 3,6-diaminothieno[2,3-b]pyridines 1 were identified as selective inhibitors of the plasmodial glycogen synthase kinase-3 PfGSK-3,6−9 inhibitors of bacterial histidine kinase autophosphorylation,10 heat shock protein Hsp90 and serine/threonine kinase B-Raf inhibitors,11 TGF-βR1 modulators,12 and inhibitors of infectious prion isoform PrPSc replication13 (Figure 1). Easily available14 thieno[2,3-b]quinolines 2 have been reported as phosphoinositide specific-phospholipase C-γ (PLC-γ) enzyme inhibitors15−19 with significant antiproliferative activities against a range of human cancer cell lines20−25 and as potent antiplatelet agents.26 4-Aminothienopyridine-3-carbonitriles 3 or their derivatives showed good activity against Staphylococcus epidermidis,27Leishmania amazonensis,28 and Mayaro virus29 and were also recognized as protein kinase C θ (PKCθ) inhibitors30−32 for treatment of autoimmune and inflammatory diseases. Several thienopyridines have been developed as bone anabolic agents33 (4, Figure 1), inhibitors of 15-prostaglandin dehydrogenase 5 useful for tissue regeneration,34,35 alkaline phosphatase enhancers 6 for osteoporosis treatment,36 hepatitis C virus inhibitors 7,37 anti-HIV agents 8,38 highly selective 5-hydroxytryptamine (5-HT)4 receptor agonists and memory enhancers such as PRX-03140 (9),39,40 and negative allosteric modulators of metabotropic GluR5 receptors (10).41 A series of 3-amino-4-methylthieno[2,3-b]pyridine-2-carboxamides 11–14 were reported42−53 as selective muscarinic acetylcholine receptor 4 (M4) positive allosteric modulators that displayed in vivo efficacy in preclinical models of antispsychotic drug effects. Some thienopyridines are useful as insecticides,54,55 pesticides,56 herbicide antidotes with respect to 2,4-dichlorophenoxyacetic acid (2,4-D),57,58 and plant growth regulators.59 Easily acccessible60 3-aminothienopyridine-5-carboxylic acids 15 were identified and developed as a novel class of IKKβ inhibitors,61 ubiquitin C-terminal hydrolase-L1 (UCH-L1) inhibitors,62 and HIV-1 integrase inhibitors.63
Figure 1.

Biologically active thieno[2,3-b]pyridines.
A functionalization of substituents and a new ring addition to the thieno[2,3-b]pyridine core system may be used to enhance and broaden the application of compounds. Thus, biological activities exhibited by ring-fused thienopyridines include antimicrobial and antiprotozoal,64−67 antihistaminic,68 antiproliferative/anticancer,69−71 anti-Alzheimer,72 anticonvulsant, and neurotropic73−76 effects. Pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidines were reported as phosphodiesterase type 4 inhibitors,77 multitarget Ser/Thr kinase inhibitors,78 Cdc7 kinase inhibitors,79 and hepatic gluconeogenesis inhibitors.80
In continuation of our studies on the synthesis and biological properties of thienopyridines,81−90 we wish now to report the synthesis and structures of new polyheterocyclic ensembles prepared by noncatalyzed oxidative dimerization of 3-aminothieno[2,3-b]pyridine-2-carboxamides.
In general, selective oxidation of thienopyridines with a variety of reagents can be considered as an effective route to functionalization of the bicyclic core and has been used for preparation of the corresponding N-oxides 16 and 17,91−96 halo derivatives 18 and 19,94,97 S-oxides 20–22,91,95,98,99 sulfones 23 and 24,95,98−100 and functionalized molecules 25–29 (Scheme 1).
Scheme 1. Oxidation of Thieno[2,3-b]pyridines and the Reactions of Oxidation Products.
R = H, Et. (i) 30% H2O2, and AcOH, 55 °C; (ii) meta-chloroperbenzoic acid (MCPBA) in HCCl3 or CH2Cl2–EtOAc and at 0 °C; (iii) magnesium monoperoxyphthalate hexahydrate, AcOH, and 25 °C; (iv) Hal2, Ag2SO4, and conc. H2SO4; (v) Br2, dry Et2O or CH2Cl2, and 0 °C; (vi) Cl2, HCCl3–H2O, and reflux 3 h; (vii) Cl2, HCCl3–H2O, 0–10 °C, and 3 h; (viii) NaOCl, conc. H2SO4, and tetrahydrofuran (THF)–H2O; (ix) aqueous (aq.) NaOCl, HCl, and room temperature (r.t.); (x) Me2NC(O)Cl, Me3SiCN, and CH3CN; (xi) POCl3, CHCl3, 100 °C, and 3 h; (xii) Bu4N+ Br–, CH2Cl2, (CF3SO2)2O, 0 °C, and 16 h; (xiii) (1) HNO3, H2SO4, and 90–120 °C; (2) Fe, AcOH, and 100 °C; (xiv) (1) HNO3, AcOH, and 120 °C; (2) Sn, HCl, and 25 °C; (xv) KSCN, CH2Cl2–H2O, PhC(O)Cl, and 25 °C; (xvi) PhICl2 (IBDC) and MeCN–H2O; and (xvii) Cl2, CCl4–H2O, and 0 °C.
However, a survey of the literature has revealed the lack of studies on the oxidation of easily available and biologically active 3-aminothieno[2,3-b]pyridines. These studies can be helpful in consideration of possible metabolic in vivo oxidation pathways for thienopyridine drugs.
First, we considered the oxidation of 3-aminothieno[2,3-b]pyridine-2-carboxamides and 2-acyl-3-aminothieno[2,3-b]pyridines as the possible route toward polyheterocyclic ensembles having pyrazole or isoxazole fragments (Scheme 2).
Scheme 2. Expected Oxidation Pathways for 2-Substituted 3-Aminothieno[2,3-b]pyridines.
In fact, the preparation of benz[c]isoxazoles by oxidation of ortho-aminophenyl ketones with monopersulphate was reported101 by Bamberger and Elger as far back as in 1903. The more modern improved methods of the oxidation involve the use of oxone,102 [bis(acetoxy)iodo]benzene,103−105 NaOCl in ethanolic NaOH solution,106 MCPBA,107 and H2O2–AcOH108 as oxidizing agents. 3-Indazolones109,110 or their heteroanalogs110,111 can be prepared by hypervalent iodine oxidation of anthranilamides or related heterocyclic ortho-amino carboxamides (Scheme 3). The improved procedure for the synthesis of 3-indazolones was proposed by Dai et al. and based on the use of a copper–air catalytic system for intramolecular N–N bond formation.112 Recently, an approach to construct N,N′-diarylindazol-3-ones has been developed113 using the tandem sequence of Chan–Evans–Lam oxidative C–N cross-coupling of anthranilamides with aryl boronic acids, followed by dimethyl sulfoxide (DMSO)/air oxidative N–N coupling.
Scheme 3. Intramolecular N–N Oxidative Coupling of Anthranilamides and Related Substrates.
(i) PhI(OC(O)CF3)2 (phenyliodine bis(trifluoroacetate) (PIFA)), CH2Cl2, trifluoroacetic acid (TFA), 0 °C, and 45–81%; (ii) CuBr (20 mol %), DMSO, air, 120 °C, and 56–99%; and (iii) PIFA, dimethylformamide (DMF)–H2O, r.t., and 4 h.
With these points in mind, we decided to investigate the oxidation of 3-aminothieno[2,3-b]pyridine-2-carboxamides using a commercial bleach (aq. NaOCl) solution. To our surprise, preliminary results showed114 that the expected pyrazole ring formation did not take place and that the reaction proceeded in a more complex way to afford unusual oxidative dimerization products.
In this paper, we wish to report the detailed studies on the new reaction and its scope and limitations, the structure, stereochemistry of products in the solid state and in a solution, the kinetics and dynamics of intramolecular processes in a solution, and the results of biological in silico studies.
Results and Discussion
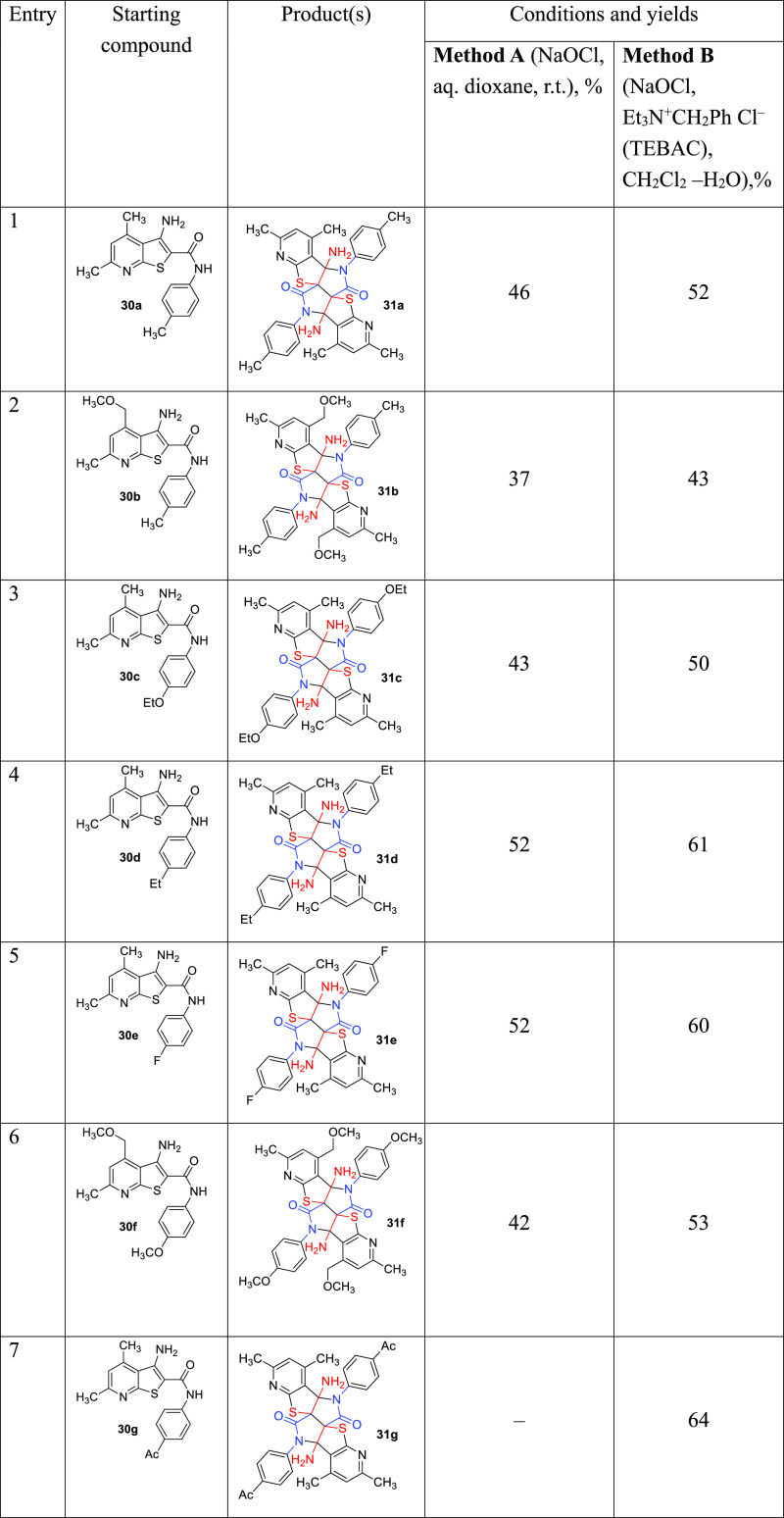
We found that thienopyridines 30 readily undergo oxidation upon treatment with a 10-fold excess of aq. NaOCl in aqueous dioxane to afford the new products of oxidative dimerization, pyrido[3′′′′,2′′′′:4‴,5‴]thieno[2‴,3‴:4″,5″]pyrrolo[3″,4″:3′,4′]pyrrolo-[2′,3′:4,5]thieno[2,3-b]pyridine-6,13-diones 31 in moderate (37–55%) yields (method A, Scheme 4 and Table 1). Somewhat better results (43–64%) were achieved when the oxidation proceeded under phase transfer catalyst (PTC) conditions in a CH2Cl2–water system (method B). The scope of the substrates is limited to those thienopyridine-2-carboxamides bearing mostly electron-donating groups in aryl substituents. Thus, we failed to obtain any oxidation products from thienopyridine 30l (R = Me, Ar = 4-NO2C6H4, Table 1, entry 12), though thienopyridines 30g and 30h bearing less strong withdrawing groups (Ar = 4-AcC6H4, 4-CF3C6H4, Table 1, entries 7 and 8) reacted well. Surprisingly, when the reaction was conducted in aq. EtOH, a mixture of polycycles 31 (28–29%) and oxidation/solvolysis products 32 (14–15%) was obtained (method C, Scheme 4 and Table 2).
Scheme 4. Oxidation of 3-Aminothieno[2,3-b]pyridine-2-carboxamides with Bleach Solution under Different Conditions.
Table 1. Reaction Scope, Yields, and Conditions.
Table 2. Results of Oxidation of Thienopyridines 30a and 30b with NaOCl in EtOHa.
Conditions: aq. NaOCl, EtOH, r.t., and 6–9 h.
To examine whether other oxidants are suitable for preparation of polycycles 31 from thienopyridines 30, we performed the reaction of compound 30d (R = Me, Ar = 4-EtC6H4) with MCPBA and magnesium monoperoxyphthalate (MMPP) (Scheme 5). In both cases, only simple S-oxidation products 33 and 34 were isolated.
Scheme 5. Reactions of 3-Aminothieno[2,3-b]pyridine-2-carboxamides with MCPBA and MMPP.
The structure of 2-ethoxy-4,6-dimethyl-N-(4-methylphenyl)-3-oxo-2,3-dihydrothieno[2,3-b]pyridine-2-carboxamide 32a was studied in detail using NMR spectroscopy, including two-dimensional (2D) NMR heteronuclear single-quantum correlation (HSQC) and heteronuclear multiple bond correlation (HMBC) techniques (Figure 2), high-resolution mass spectrometry (HRMS), and elemental analysis.
Figure 2.
Chemical shifts in the 1H and 13C NMR spectra of 32a (DMSO-d6, 25 °C).
As we can see from Figure 2, in the 1H NMR spectrum, diastereotopic protons of OCH2 appeared as two doublets of quartets with coupling constants 2J 14.2 Hz and 3J 6.9 Hz due to the presence of the neighboring chiral C-2 carbon atom. In the 1H NMR spectrum of the related thienopyridine 32b, two doublets of quartets of OCH2 (2J 14.5 Hz), as well as AB quartet of methylene protons CH2OMe (2J 12.8 Hz), were observed. The signals of keto carbons at δ 197.3–197.4 ppm were also observed in the 13C NMR spectra of 32a and 32b. In the IR spectra, bands at ν 1640–1650 and 1690–1695 cm–1 can be assigned to the stretches of keto and amide C=O groups, respectively.
Formation of compounds 32a and 32b can be rationalized by the following mechanistic sequence (Scheme 6). We suggest that electrophilic chlorination occurs at the C-2 position with formation of stabilized cation A, followed by deprotonation, nucleophilic substitution of a chlorine atom with an ethoxide ion, and hydrolysis.
Scheme 6. Plausible Mechanism for Formation of Thienopyridines 32.
The compounds 31 are colorless, high melting crystalline solids, sparingly soluble in most organic solvents, except for acetone, CH2Cl2, DMF, and DMSO. The IR spectra of polycycles 31 differ from the spectra of compounds 30 and 32. Thus, the latter spectra revealed the typical absorption bands of amide carbonyls ν C=O in the region of 1630–1645 cm–1 while the bands at 1730–1740 cm–1, which are due to five-membered lactam carbonyl stretches, were observed in the spectra of 31.
Another interesting issue is the stereochemical features of polycyclic oxidation products. Compounds 31 exhibit four chiral centers giving rise to eight possible pairs of diastereomers. However, only one set of signals corresponding to one of the possible stereoisomeric pairs was observed in the 1H and 13C NMR spectra at high temperatures (80–120 °C). In the spectra of compounds 31b, 31f, and 31j, which have prochiral methoxymethyl groups, the signals of diastereotopic hydrogens appeared as a pair of doublets with germinal coupling constant of 14.5–15.7 Hz.
The X-ray study of the crystal structure of compound 31c (Figure 3) showed that products exist as a pair of (R,R,R,R)/(S,S,S,S) enantiomers only. The molecule of 31c has a second-order symmetry axis passing through the center of the C–C bond common to both lactam rings. After recrystallization from DMSO, two solvent molecules, which are linked through hydrogen bonds to the amino groups, also filled the crystal unit cell (Figure 4). The geometry of DMSO-d6 molecules captured in the crystal lattice showed no difference, neither in bond lengths nor in angles, with the results reported for DMSO-d6 single crystal at 100 K.115
Figure 3.
General view of the molecule of compound 31c (as solvate with two molecules of DMSO-d6) with atom numbering (by X-ray data).
Figure 4.
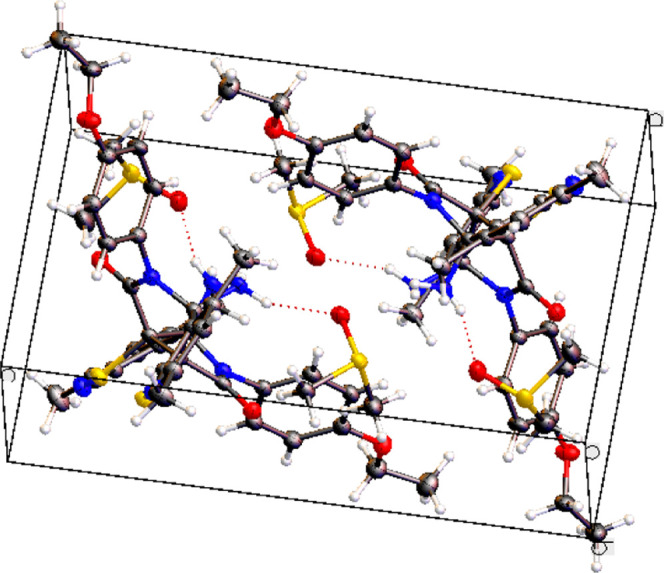
Crystal packing of the (R,R,R,R)/(S,S,S,S)-pair and four DMSO-d6 molecules in the unit cell of racemic compound 31c.
Intermolecular hydrogen bonds between 31c and DMSO-d6 molecules are somewhat different from each other. Thus, the intermolecular bond O(5)···H(6B) has an interatomic distance of 2.173 Å and bond angles S(3)–O(5)···H(6B) and N(6)–H(6BB)···O(5) equal to 132.4 and 157.5°, respectively. The bond O(6)···H(5A) has a length of 2.139 Å, and the angles S(4)–O(6)···H(5A) and N(5)–H(5A)···O(6) were found to be 157.6 and 125.9°, respectively.
The main stereochemical and structural features of molecule 31c are the following.
-
(1)
All four five-membered rings are almost planar (the average deviation of atoms from planes does not exceed 0.1 Å), and pyridine rings lie in the planes of thiophene fragments. Four five-membered cycles form a folded structure in which the atoms common for two [C(6) and C(14)] and three [C(8) and C(16)] cycles almost have no distortions of bond angles, and the interatomic distances are close to the standard Csp3–Csp3 bond lengths.
-
(2)
The angle between the central planes of lactam rings (Figure 5) C(16)–C(8)–N(1)–C(7)–C(8) (plane 1) and C(16)–C(8)–C(14)–N(2)–C(15) (plane 2) is equal to 125.8°, and the adjacent planes of thiophene rings S(1)–C(1)–C(5)–C(6)–C(16) (plane 3) and C(8)–C(14)–C(13)–C(9)–S(2) (plane 4) form angles close to 115° with lactam cycles (angles between planes 1–3 and 2–4 are equal to 114.7 and 115.2°, respectively). With regard to the folded system of five-membered rings, the amino groups are located on one side and both sulfur atoms on the other side in pseudoaxial positions, while carbonyl occupied pseudoequatorial positions.
-
(3)
The C–NH2 bonds are almost parallel to each other; the amino groups are linked by intramolecular hydrogen bonds with the interatomic distances H···N(6) and N(5)–N(6) equal to 2.620 and 3.008 Å and the angle N(5)–H···N(6), 150.0°. The arrangement of amino groups on one side of the rigid central tetracyclic structure allows one to assume the properties of proton sponges116,117 for compounds 31 and favors the preparation of more complex supramolecular structures.
-
(4)
In the crystal, the aryl substituents are rotated by 108.0 and 64.8° out of the plane of lactam rings [cycles C(17)···C(22) and C(23)···C(28)]. We believe that such preferred non-coplanar orientation is typical for all para-substituted aryls (compounds 31a–h). As a result, the ortho-hydrogen atoms of aryl substituents are in different chemical environments. Thus, hydrogen atoms H(15) and H(24), which are located on the same side of the central core with amino groups, are at an almost equal distance from nitrogens of amino groups and carbonyl oxygens: the interatomic distances H(15)···O(2), H(15)···N(6), H(24)···O(1), and H(24)···N(5) are equal to 2.931, 3.297, 3.276, and 3.297 Å, respectively.
Figure 5.

Central fragment of molecule 31c (by X-ray).
This atomic neighborhood would result in deformation of the electron shells of H(15) and H(24); therefore, their signals in the 1H NMR spectra are expected to be shifted to the weaker field. In contrast, the distances H(22)–pyridine ring N(4) and H(27)–pyridine ring N(3) were found to be ∼3.5 Å and due to the shielding effect of aromatic pyridine rings, the signals of ortho-hydrogens H(22) and H(27) should be shifted to the anomalously stronger fields. In fact, these shielding/deshielding effects of aryl ortho-hydrogen atoms were indeed observed in the 1H NMR spectra recorded at −40 °C (Figure 6), when the rotation around the N–C(Ar) bond was almost negligible.
Figure 6.

Evolution of 1H NMR signals (acetone-d6) of compounds 31f (left) and 31e (right) upon changing the temperature from −40 to +24 °C (the downfield region of spectra is presented; black lines, experimental; red lines, theoretical; k, rate constant for the degenerate rotation of aryl substituent around the exocyclic Ar–N bond; and A and B, different rotamers of compounds 31e and 31f, respectively).
As we can see from Figure 6, four signals from four nonequivalent hydrogens of para-substituted benzene ring are observed in the downfield region of low-temperature NMR spectra of polycycles 31e and 31f, and the sharp singlets at δ 6.7 and 7.0 ppm belong to pyridine protons. According to the correlated spectroscopy (COSY) experiment, the signals at δ 6.3–6.5 and 7.4–7.6 ppm should be assigned to aryl H-2′ and H-6′ protons, and the signals at δ 6.3–6.5 and 7.4–7.6 ppm should be attributed to H-3′ and H-5′ atoms, respectively.
Hence, one may conclude that at low temperatures (≤−40 °C), the intramolecular rotation of the aryl fragment along the N–Ar bond is slow and it may be assumed that the molecular geometry in cold solutions is essentially similar to that in crystals. Upon heating of solutions of compounds 31, the 1H NMR spectra revealed time evolution of signals, and final coalescence takes place near room temperature (Figure 6).
The kinetic and activation parameters of rotamerization were estimated by analysis of NMR line shapes of aryl H-2′,6′ and H-3′,5′ signals in the 1H NMR spectra of 31e and 31f recorded in deuteroacetone upon cooling.
It is noteworthy that the spectral lines in the 1H NMR spectrum of 31e tend to broaden, and the spectrum was poorly resolved due to the partial spontaneous crystallization at below −40 °C. Therefore, for the theoretical modeling of NMR spectra, we used the chemical shifts of the indicated protons at −40 °C as reference values. The multiplets of H-3′,5′ atoms, which appeared as doublets of doublets collapsed to triplets due to spin–spin coupling to fluorine atom and H-2′,6′ atoms, are well resolved, and the spectral picture at −40 °C is closer to the spectrum in the absence of exchange than to the state of intermediate exchange (Figure 6). The line width at half maximum of the reference signals was taken equal to 2.8 Hz similar to 31f, and spin–spin coupling constants were accepted as those reported for 4-fluoroaniline.118 Theoretical spectra (Figure 6, red lines) were simulated by variation of rate constants for the exchange of reference proton signals in the aryl rings, and these theoretical spectra were compared with temperature-dependent experimental spectra. The calculated activation energy values ΔG‡, ΔH, and ΔS for intramolecular degenerate rotation in the molecules of compounds 31e and 31f are given in Figure 7.
Figure 7.
Changes in Gibbs free energies (ΔG‡) at the temperature-dependent rotamerization of compounds 31f (up) and 31e (down).
The computer simulation of 1H NMR spectra and calculation of exchange rate constants were performed using gNMR 5.0.6.0 software package.119 First, a series of temperature-dependent experimental 1H NMR spectra were exported to Galactic (*.spc) files using JEOL Delta 5.3 software (https://nmrsupport.jeol.com/) for further conversion into gNMR compatible.spg files using the gCVT program (included in the gNMR package). Next, for the spectrum recorded in the absence of exchange, the line shapes of the reference proton signals were theoretically modeled. Then, by program-driven varying of the chemical shifts, the width at half maximum and the spin–spin coupling constant (if any) line shapes were optimized using the least-squares method with the line shapes experimentally observed in.spg files.
The spectra, calculated with a fixed difference between chemical shifts of the reference signals, fixed width at half maximum, and coupling constants, were correlated with a series of other experimental temperature-dependent spectra by varying the exchange rate constant. As a result, the rate constants were determined for each spectrum at the corresponding temperature (see Figure 7), and the changes in Gibbs free energies (ΔG‡) were calculated for each rate constant using the Arrhenius equation. The enthalpy (ΔH‡) and entropy (ΔS‡) of activation were calculated by treating the dependence of ΔG‡vs temperature using least-squares linearization with a correlation coefficient of at least 0.99 (see Figure 7). According to our estimates based on the known data,120 we assume that error of the exchange rate constant k determination does not exceed 5%. This gives an estimated error for ΔG‡, 0.04 kcal/mol (0.15 kJ/mol); ΔH‡, 0.19 kcal/mol (0.8 kJ/mol); and ΔS‡, 0.23 cal/(mol·K) (0.96 J/(mol·K)).
The very close values of the activation parameters determined for compounds 31e and 31f allowed us to suggest a small influence of a substituent in the para-position on the activation barrier. The rather high differences between the rate constants are supposedly associated with the weight of the substituent(s) in the aromatic ring, since more heavy molecular fragments are prone to slower rotation.
The conformational analysis of the X-ray-determined structure of 31c revealed that upon rotation along the N–Ar bond and when the bonds C(17)–C(18) and N(2)–C(15) are eclipsed, the interatomic distances H(18)···N(6) and H(18)···O(2) are reduced to 1.7 and 1.9 Å, respectively. In both cases, the distances are longer than the sum of van der Waals radii of the atoms; therefore, one may conclude that carbonyl and amino groups are nearly equal with regard to steric restrictions of free rotation of aryl substituents.
When the temperature of DMSO-d6 solutions of compounds 31 was further increased from room temperature to 120–140 °C, the signals of ortho- and meta-hydrogens in 1H NMR spectra collapsed to two doublets typical for para-disubstituted aromatics. These high-temperature spectra are given in the Supporting Information, and the full assignment of the signals using 2D HSQC and HMBC experiments was also performed at 120–140 °C.
In the case of ortho-substituted aryl derivatives 31i and 31j, free rotation along N–Ar is absent and the 1H NMR spectra revealed no significant changes (excluding the usual temperature-dependent small chemical shifts) at any temperature from −40 to +120 °C. Only a typical ABCD-pattern of ortho-substituted aromatics was observed in the 1H NMR spectra of 31i and 31j; in addition, two methyl groups of both ortho-tolyl substituents appeared as one singlet in the NMR spectrum of 316i. In other words, both N–Ar substituents in the molecules of 31i and 31j are located symmetrically with respect to the central heterocyclic core. Furthermore, nuclear Overhauser enhancement spectroscopy (NOESY) experiments showed no correlation peaks between methyl and amino hydrogens, allowing one to suggest that CH3 and NH2 groups in 31i are located in anti positions to each other. We believe that 2-bromophenyl and amino groups in 31j are arranged in the same way. Hindered rotation along the N–Ar bond was also observed in the NMR spectrum of 31k bearing 3,4-disubstituted aryls, and a variable temperature study showed the same evolution of signals as was observed for compounds 31a–h. The molecular ions of compounds 31 are rather unstable under electron ionization (EI) conditions. The mass spectra revealed the fragment ions [M – 2Ar–N=C=O]+ and [Ar–N=C=O]+, which are typical for all compounds 31.
Mechanism of Formation of Compounds 31
Evidently, the reaction of thienopyridines 30 with the bleach proceeds as a kind of oxidative dimerization, with a cleavage of N–H and C(2)=C(3) bonds and formation of three new σ-bonds (Figure 8). It is noteworthy that neither pyridine nitrogen nor sulfur atoms are involved in the oxidation.
Figure 8.
Cleavage and formation of bonds in the bleach-based oxidation reaction of thienopyridines 30. Breaking bonds are shown in red and forming bonds in blue bold dashed lines.
We suggest two possible mechanistic pathways for the oxidative dimerization of thienopyridines 30. The first plausible mechanism (#1) is shown in Scheme 7. We suppose that the specificity of the new unusual oxidative dimerization is determined by the presence of HOCl (or Cl+) that appeared due to the hydrolysis of NaOCl in aqueous solution. In the first step, Cl+ or free HOCl reacts as the electrophile with thienopyridine 30 to afford resonance-stabilized cation A. We also suggest that a parallel process of alkaline-promoted deprotonation of amide with the formation of anion B occurs. The reaction between A and B leads to formation of a new intramolecular C–N bond; next, the carbocation-initiated multistep cascade process occurs, affording polycycles 31.
Scheme 7. Plausible Mechanism #1 for Formation of Polycycles 31.
Another possible mechanism (#2) is depicted in Scheme 8. Bleach (or HOCl that appeared due to hydrolysis) might act as a single electron transfer (SET) oxidant to produce cation-radical species 35. Their dimerization leads to dications 36, which undergo double intramolecular heterocyclization to afford polycycles 31. However, both proposed mechanisms are disputable and require further diligent studies.
Scheme 8. Plausible Mechanism #2 for Formation of Polycycles 31.
In Silico Biological Studies
The prediction of targeted biological activity of new compounds 31 was performed using the unique QSAR package “Microcosm BioS”121 by the method of maximum similarity with the reference structures. As reference compounds, we used the set of compounds that were previously studied for various types of targeted biological activity. As target proteins, we selected acetylcholinesterase; proto-oncogene tyrosine-protein kinase (Src), disintegrin and metalloproteinase domain-containing protein 10 (ADAM10), ADAM17, FXN frataxin, and neurokinin 1 receptor. For compounds 31a, 31b, 31d, 31f, 31i, and 31k, the indices of the expected biological activity of the tested structures for targets such as acetylcholinesterase and proto-oncogene tyrosine-protein kinase (Src) were equal to 2. Therefore, these structures can be considered promising candidates for docking studies and biological tests in vitro and in vivo.
According to the Microcosm BioS prediction, compounds 31a, 31b, 31d–f, and 31i–k are of interest as possible inhibitors of acetylcholinesterase, i.e., by reducing the biological activity of AChE and increasing the level of acetylcholine in the brain, and can be used for the treatment of Alzheimer’s disease (AD). In addition, compounds 31b, 31f, and 31i are likely to have inhibitory effects against proto-oncogene tyrosine-protein kinase (Src). The results of the in silico studies are given in the Supporting Information.
Conclusions
In summary, we have developed new oxidative dimerization of 3-aminothieno[2,3-b]pyridine-2-carboxamides upon treatment with commercial bleach leading to the formation of the unusual polyheterocyclic ensembles. The reaction proceeds in a highly stereoselective manner to give only one (R,R,R,R/S,S,S,S) out of the eight possible enantiomeric pairs. The preliminary results of in silico experiments indicate that the new compounds are promising candidates for further studies to identify new inhibitors of acetylcholinesterase and proto-oncogene tyrosine-protein kinase (Src). The studies on the biological activity of compounds are currently underway. The unique stereochemistry and the cis-arrangement of two amino groups make the molecules suitable for use as a good platform for supramolecular architectures.
Experimental Section
IR spectra were obtained using a Fourier transform infrared (FTIR) PerkinElmer Spectrum Two instrument in attenuated total reflection (ATR) mode. 1H and 13C NMR spectra were recorded on an Agilent 400/54 spectrometer (400 and 100 MHz, respectively) in DMSO-d6 or CDCl3 using tetramethylsilane (TMS) or residual solvent peaks as internal standards. COSY, 1H–13C HSQC, and 1H–13C HMBC spectra were obtained using an Agilent 400/54 spectrometer. Low-temperature 1H NMR spectra were recorded on a JEOL JNM-ESA spectrometer (400 MHz) in acetone-d6 using TMS as an internal standard. Mass spectra were recorded on a Varian CH-6 mass spectrometer with direct sample injection at 50–180 °C, using the ionization method, EI. Elemental analysis was performed on a Hewlett Packard HP-185B CHN-analyzer. Melting points were determined on a Stuart SMO 30 apparatus.
Single crystals C40H36D12N6O6S4 of compound 31c (as solvate with DMSO-d6) were grown from DMSO-d6. A suitable crystal was selected and studied on a SuperNova, Dual, Cu at zero, AtlasS2 diffractometer. The crystal was kept at 100.00(10) K during data collection. Using Olex2,122 the structure was solved with the ShelXT structure solution program123 using Intrinsic Phasing and refined with the ShelXL refinement package124 using least-squares minimization. The X-ray crystal structure of 31c has been deposited at the Cambridge Crystallographic Data Centre (CCDC 1816549). High-resolution mass spectra were obtained using a Bruker Maxis spectrometer (electrospray ionization-time-of-flight (ESI-TOF), MeCN solution, using HCO2Na–HCO2H for calibration).
Crystal Data for C40H36D12N6O6S4 (M = 849.16 g/mol)
Triclinic, space group P1̅ (no. 2), a = 11.0531(3) Å, b = 12.6868(3) Å, c = 16.6049(5) Å, α = 75.757(2)°, β = 83.114(2)°, γ = 66.615(3)°, V = 2070.87(11) Å3, Z = 2, T = 100.00(10) K, μ(Cu Kα) = 2.547 mm–1, Dcalc = 1.362 g/cm3, 30 796 reflections measured (7.778 ≤ 2θ ≤ 148.988°), and 8458 unique (Rint = 0.0517, Rsigma = 0.0366), which were used in all calculations. The final R1 was 0.0502 (I > 2σ(I)), and wR2 was 0.1363 (all data).
General Procedure for the Synthesis of 3-Amino-N-arylthieno[2,3-b]pyridine-2-carboxamides 30a–l
To a mixture of 4,6-dimethyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile125,126 or 4-(methoxymethyl)-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile127 (20 mmol) and 10% aq. KOH solution (11.2 mL, 20 mmol) in DMF (20 mL), the corresponding N-aryl-2-chloroacetamide (20 mmol) was added. The resulting mixture was stirred for 30–40 min at r.t. (the formation of a white precipitate of the S-alkylation product may be observed). Then, another portion of 10% aq. KOH solution (11.2 mL, 20 mmol) was added, and the mixture was stirred for 0.5–1 h until a precipitate was formed. The yellow solid was filtered off, washed with cold aqueous ethanol, and dried to give 3-aminothieno[2,3-b]pyridine-2-carboxamides 30a–l in 67–84% yield. The products were sufficiently pure and were used in the next step without further purification. Full details of the preparation and the spectral data of 30a–l are given in the Supporting Information. Some representative examples are given below.
3-Amino-N-(4-fluorophenyl)-4,6-dimethylthieno[2,3-b]pyridine-2-carboxamide (30e)
 Compound 30e was prepared according to the
general procedure in 83% yield as yellow crystals, mp 208–209
°C. 1H NMR (400 MHz, DMSO-d6) δ, 9.45 (CONH), 7.59 (dd, JH–H = 9.5 Hz, JH–F = 5.2 Hz, 2H,
H-2 and H-6 Ar), 7.11 (dd, JH–H = 9.5 Hz, JH–F = 9.5 Hz, 2H,
H-3 and H-5 Ph), 7.01 (s, 1H, H-5), 6.88 (s, 2H, NH2),
2.69 (s, 3H, CH3-4), 2.47 (s, 3H, CH3-6); 13C NMR (100 MHz, DMSO-d6) δ
164.7 (C=O), 159.6 (C-7a), 159.3 (C-6), 159.0 (d, JC–F = 240.0 Hz, C-4 Ar), 149.6 (C-3), 145.3 (C-4),
135.3 (d, JC–F = 2.8 Hz, C-1 Ar),
123.9 (d, JC–F = 8.0 Hz, 2C, C-2
and C-6 Ar), 123.2 (C-3a), 122.5 (C-5), 115.5 (d, JC–F = 22.5 Hz, 2C, C-3 and C-5 Ar), 96.7 (C-2),
24.2 (CH3-6), 20.1 (CH3-4); MS, m/z (I, %): 315 (57, M), 257 (88),
256 (57), 177 (45), 111 (100), 95 (34), 41 (22). Found, C 61.05, H
4.38, N 13. C16H14FN3OS. M 315. Calcd:
C 60.94, H 4.47, N 13.32. HRMS (ESI) calcd for C16H15FN2OS (M + H)+: 316.0914; found: 316.0910.
Compound 30e was prepared according to the
general procedure in 83% yield as yellow crystals, mp 208–209
°C. 1H NMR (400 MHz, DMSO-d6) δ, 9.45 (CONH), 7.59 (dd, JH–H = 9.5 Hz, JH–F = 5.2 Hz, 2H,
H-2 and H-6 Ar), 7.11 (dd, JH–H = 9.5 Hz, JH–F = 9.5 Hz, 2H,
H-3 and H-5 Ph), 7.01 (s, 1H, H-5), 6.88 (s, 2H, NH2),
2.69 (s, 3H, CH3-4), 2.47 (s, 3H, CH3-6); 13C NMR (100 MHz, DMSO-d6) δ
164.7 (C=O), 159.6 (C-7a), 159.3 (C-6), 159.0 (d, JC–F = 240.0 Hz, C-4 Ar), 149.6 (C-3), 145.3 (C-4),
135.3 (d, JC–F = 2.8 Hz, C-1 Ar),
123.9 (d, JC–F = 8.0 Hz, 2C, C-2
and C-6 Ar), 123.2 (C-3a), 122.5 (C-5), 115.5 (d, JC–F = 22.5 Hz, 2C, C-3 and C-5 Ar), 96.7 (C-2),
24.2 (CH3-6), 20.1 (CH3-4); MS, m/z (I, %): 315 (57, M), 257 (88),
256 (57), 177 (45), 111 (100), 95 (34), 41 (22). Found, C 61.05, H
4.38, N 13. C16H14FN3OS. M 315. Calcd:
C 60.94, H 4.47, N 13.32. HRMS (ESI) calcd for C16H15FN2OS (M + H)+: 316.0914; found: 316.0910.
3-Amino-4-(methoxymethyl)-6-methyl-N-(4-methoxyphenyl)thieno[2,3-b]pyridine-2-carboxamide (30f)
 Compound 30f was prepared according to the
general procedure in 69% yield as pale yellow crystals, mp 169–171
°C. 1H NMR (400 MHz, DMSO-d6) δ, 9.37 (CONH), 7.54 (d, J = 8.9 Hz, 2H,
H-2 and H-6 Ar), 7.21 (s, 1H, H-5), 7.02 (s, 2H, NH2),
6.88 (d, J = 8.9 Hz, 2H, H-3 and H-5 Ar), 4.82 (s,
2H, CH2O), 3.72 (s, 3H, CH3OAr), 3.37 (s, 3H, CH3OCH2), 2.55 (s, 3H,
CH3-6); 13C NMR (100 MHz, DMSO-d6) δ 164.3 (C=O), 159.7 (C-6), 159.6 (C-7a),
156.0 (C-4 Ar), 148.3 (C-3), 143.5 (C-4), 132.2 (C-1 Ar), 123.6 (2C,
C-2 and C-6 Ar), 122.9 (C-3a), 120.6 (C-5), 114.0 (2C, C-3 and C-5
Ar), 98.1 (C-2), 71.9 (CH2O), 58.1 (CH3OCH2), 55.6 (CH3OAr), 24.5
(CH3-6); MS, m/z (I, %): 357 (42, M), 326 (31), 235 (45), 234 (33), 205 (12),
176 (32), 123 (100), 95 (41), 80 (22), 43 (13). Found, C 60.35, H
5.50, N 11.94. C18H19N3O3S. M 357.43. Calcd, C 60.49, H 5.36, N 11.76. HRMS (ESI) calcd for
C18H19N3NaO3S (M + Na)+: 380.1050; found: 380.1039.
Compound 30f was prepared according to the
general procedure in 69% yield as pale yellow crystals, mp 169–171
°C. 1H NMR (400 MHz, DMSO-d6) δ, 9.37 (CONH), 7.54 (d, J = 8.9 Hz, 2H,
H-2 and H-6 Ar), 7.21 (s, 1H, H-5), 7.02 (s, 2H, NH2),
6.88 (d, J = 8.9 Hz, 2H, H-3 and H-5 Ar), 4.82 (s,
2H, CH2O), 3.72 (s, 3H, CH3OAr), 3.37 (s, 3H, CH3OCH2), 2.55 (s, 3H,
CH3-6); 13C NMR (100 MHz, DMSO-d6) δ 164.3 (C=O), 159.7 (C-6), 159.6 (C-7a),
156.0 (C-4 Ar), 148.3 (C-3), 143.5 (C-4), 132.2 (C-1 Ar), 123.6 (2C,
C-2 and C-6 Ar), 122.9 (C-3a), 120.6 (C-5), 114.0 (2C, C-3 and C-5
Ar), 98.1 (C-2), 71.9 (CH2O), 58.1 (CH3OCH2), 55.6 (CH3OAr), 24.5
(CH3-6); MS, m/z (I, %): 357 (42, M), 326 (31), 235 (45), 234 (33), 205 (12),
176 (32), 123 (100), 95 (41), 80 (22), 43 (13). Found, C 60.35, H
5.50, N 11.94. C18H19N3O3S. M 357.43. Calcd, C 60.49, H 5.36, N 11.76. HRMS (ESI) calcd for
C18H19N3NaO3S (M + Na)+: 380.1050; found: 380.1039.
General Procedures for the Oxidation of Thienopyridines 30 with NaOCl
Method A
A solution of the corresponding thienopyridine 30a–f, 30i, and 30k (2.0 mmol) in 1,4-dioxane (20 mL) was treated with aq. 10% NaOCl (5 mL). The solution was stirred at r.t. for 3–8 h until no starting compound was detected using thin-layer chromatography (TLC). Then, the mixture was treated with cold water (100 mL) and stirred until the formation of precipitate stopped. The solid was filtered off, washed with cold water (2 × 10 mL), and air-dried. The crude product was purified by flash chromatography (silica gel, pethroleum ether–EtOAc 30–100%) to afford compounds 31a–f, 30i, and 30k as colorless crystals and white or beige powders in 37–55% yield.
Method B
A mixture of thienopyridine 30a–k (2 mmol), CH2Cl2 (40 mL), 10% aq. NaOCl (8 mL), and benzyltriethylammonium chloride (TEBAC) (30 mg) was stirred at r.t. for 4–10 h until full conversion of the thienopyridine (as monitored using TLC). The organic layer was separated; the aqueous layer was extracted with CH2Cl2 (2 × 10 mL). The combined organic phases were washed with water (2 × 10 mL), dried over Na2SO4, and concentrated under reduced pressure to 1/4 of the volume. The residue was treated with hexane and left to stand for crystallization to give the desired products 31a–k in 43–64% yields.
Method C
A mixture of thienopyridine 30a, 30b (2 mmol), EtOH (80 mL), and 10% aq. NaOCl (5 mL) was stirred at r.t. for 6–9 h until no starting compound was detected using TLC. The mixture was poured into cold water (100 mL) and stirred until formation of the precipitate stopped. The solid was filtered off, washed with cold water (2 × 10 mL), and air-dried. The crude product was separated by flash chromatography on a Biotage KP-Sil column (50 g) using a gradient of 10–30% EtOAc in CH2Cl2 as the mobile phase to give compounds 31a and 31b and 32a and 32b.
Full details of the preparation and the spectral data of 31a–k and 32a and 32b are given in the Supporting Information. Some representative examples are given below.
(5aR,7aR,12aR,14aR/5aS,7aS,12aS,14aS)-7a,14a-Diamino-1,8-bis(methoxymethyl)-7,14-bis(4-methoxyphenyl)-3,10-dimethyl-7,7a,14,14a-tetrahydro-6H,13H-pyrido[3′′′′,2′′′′:4‴,5‴]thieno[2‴,3‴:4″,5″]pyrrolo-[3″,4″:3′,4′]pyrrolo[2′,3′:4,5]thieno[2,3-b]pyridine-6,13-dione (31f)
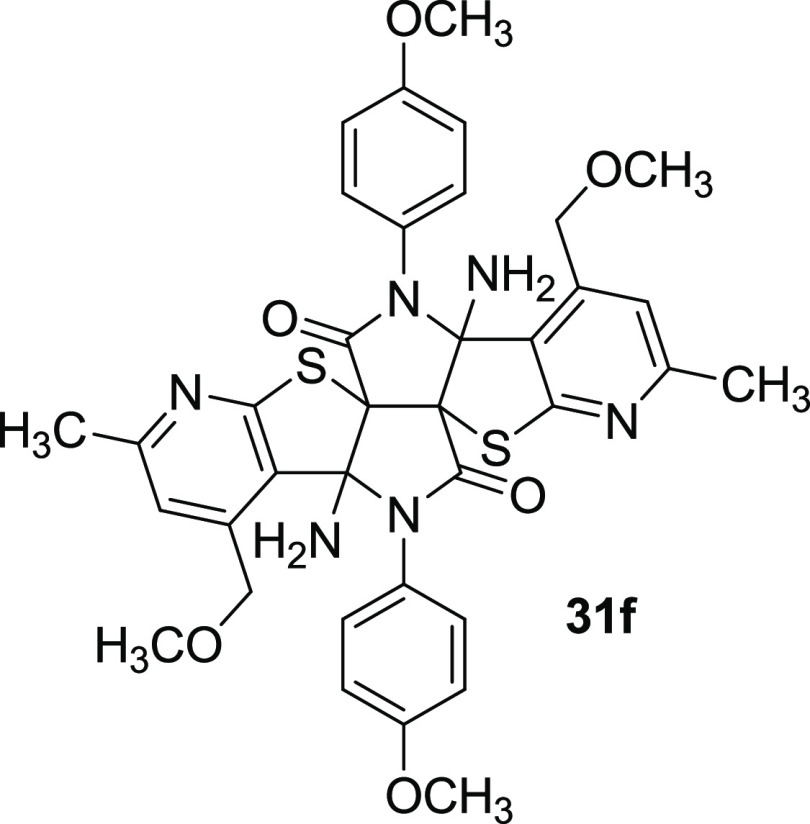 Compound 31f was prepared according to the
general procedure in 42% (method A) and 53% (method B) yields as a
beige solid, mp 203–205 °C (from EtOAc). 1H
NMR (400 MHz, DMSO-d6, 120 °C) δ
6.95 (d, 3J = 8.0 Hz, 4H, 2 × H-3
and 2 × H-5 Ar), 6.94 (s, 2H, H-2, H-10), 6.88 (d, 3J = 8.0 Hz, 4H, 2 × H-2 and 2 × H-5 Ar),
4.32 (d, 2J = 14.9 Hz, 2H, 2 × CH2O), 3.79 (s, 6H, 2 × CH3OAr), 3.43 (br s,
4H, 2 × NH2), 3.07 (d, 2J = 14.9 Hz, 2H, 2 × CH2O), 2.68 (s, 6H, 2 ×
CH2OCH3),
2.43 (s, 6H, CH3-3 and CH3-10).
Compound 31f was prepared according to the
general procedure in 42% (method A) and 53% (method B) yields as a
beige solid, mp 203–205 °C (from EtOAc). 1H
NMR (400 MHz, DMSO-d6, 120 °C) δ
6.95 (d, 3J = 8.0 Hz, 4H, 2 × H-3
and 2 × H-5 Ar), 6.94 (s, 2H, H-2, H-10), 6.88 (d, 3J = 8.0 Hz, 4H, 2 × H-2 and 2 × H-5 Ar),
4.32 (d, 2J = 14.9 Hz, 2H, 2 × CH2O), 3.79 (s, 6H, 2 × CH3OAr), 3.43 (br s,
4H, 2 × NH2), 3.07 (d, 2J = 14.9 Hz, 2H, 2 × CH2O), 2.68 (s, 6H, 2 ×
CH2OCH3),
2.43 (s, 6H, CH3-3 and CH3-10).
13C NMR (100 MHz, DMSO-d6, 120 °C) δ 168.9 (2C, C-6 and C-13), 161.9 (2C, C-4a and C-11a), 160.7 (2C, C-3 and C-10), 160.6 (2C, 2 × C-4 Ar), 148.8 (2C, C-1 and C-8), 132.0 (2C, 2 × C-1 Ar), 128.0 (4C, 2 × C-2 and 2 × C-6 Ar), 125.0 (2C, C-7b and C-14b), 118.1 (2C, C-2 and C-9), 115.2 (4C, 2 × C-3 and 2 × C-5 Ar), 88.0 (2C, C-5a and C-12a), 72.0 (2C, C-7a and C-14a), 69.5 (2C, 2 × CH2OCH3), 56.0 (2C, 2 × CH2OCH3), 24.3 (2C, CH3-3 and CH3-10).
HRMS (ESI) calcd for C36H36N6NaO6S2 (M + Na)+: 735.2041; found: 375.2035. MS, EI, 70 eV, m/z (I, %): 484 (13), 414 [M – 2ArN=C=O] (12), 365 (22), 149 [ArN=C=O] (70), 121 [ArN=C=O – 28] (100), 107 (21), 62 (27), 43 (47).
Found: C 60.81, H 4.94, N 11.67. C36H36N6O6S2. M 712.84. Calcd: C 60.66, H 5.09, N 11.79%.
(5aR,7aR,12aR,14aR/5aS,7aS,12aS,14aS)-7a,14a-Diamino-1,3,8,10-tetramethyl-7,14-bis[4-(trifluoromethyl)phenyl]-7,7a,14,14a-tetrahydro-6H,13H-pyrido[3′′′′,2′′′′:4‴,5‴]thieno[2‴,3‴:4″,5″]pyrrolo-[3″,4″:3′,4′]pyrrolo[2′,3′:4,5]thieno[2,3-b]pyridine-6,13-dione (31h)
 Compound 31h was prepared by method B in 62%
yield as a white powder, mp 213–215 °C (from CH2Cl2). 1H NMR (400 MHz, CDCl3) δ
7.66 (d, 3J = 8.3 Hz, 4H, 2 × H-3
and 2 × H-5 Ar), 7.13 (m, 4H, 2 × H-2 and 2 × H-6 Ar),
6.52 (s, 2H, H-2, H-9), 3.07 (s, 4H, 2 × NH2), 2.44
(s, 6H, CH3-3 and CH3-10), 1.43 (s, 6H, CH3-1 and CH3-8); 13C NMR (100 MHz, CDCl3) δ 169.2 (2C, C-6 and C-13),162.6 (2C, C-4 and C-11a),
146.5 (2C, C-1 and C-8), 161.0 (2C, C-3 and C-10), 137.9 (4C, q, 4JC–F = 1.4 Hz, C-2 and
C-6 Ar), 131.9 (2C, q, 2JC–F = 32.8, 2 × C-4 Ar), 131.1 (2C, 2 × C-1 Ar), 126.7 (4C,
q, 3JC–F = 3.8 Hz, 2
× C-3 and 2 × C-5 Ar), 124.6 (2C, C-7b and 14b), 123.6 (2C,
q, 1JC–F = 273.0 Hz,
2 × CF3), 122.9 (2C, C-2 and C-9), 88.5 (2C, C-7a
and 14a), 71.9 (2C, C-5a and 12a), 23.7 (2C, CH3-3 and
CH3-10), 17.7 (2C, CH3-1 and CH3-8);
HRMS (ESI) calcd for C34H27F6N6O2S2 (M + H)+: 729.1536;
found: 729.1533.
Compound 31h was prepared by method B in 62%
yield as a white powder, mp 213–215 °C (from CH2Cl2). 1H NMR (400 MHz, CDCl3) δ
7.66 (d, 3J = 8.3 Hz, 4H, 2 × H-3
and 2 × H-5 Ar), 7.13 (m, 4H, 2 × H-2 and 2 × H-6 Ar),
6.52 (s, 2H, H-2, H-9), 3.07 (s, 4H, 2 × NH2), 2.44
(s, 6H, CH3-3 and CH3-10), 1.43 (s, 6H, CH3-1 and CH3-8); 13C NMR (100 MHz, CDCl3) δ 169.2 (2C, C-6 and C-13),162.6 (2C, C-4 and C-11a),
146.5 (2C, C-1 and C-8), 161.0 (2C, C-3 and C-10), 137.9 (4C, q, 4JC–F = 1.4 Hz, C-2 and
C-6 Ar), 131.9 (2C, q, 2JC–F = 32.8, 2 × C-4 Ar), 131.1 (2C, 2 × C-1 Ar), 126.7 (4C,
q, 3JC–F = 3.8 Hz, 2
× C-3 and 2 × C-5 Ar), 124.6 (2C, C-7b and 14b), 123.6 (2C,
q, 1JC–F = 273.0 Hz,
2 × CF3), 122.9 (2C, C-2 and C-9), 88.5 (2C, C-7a
and 14a), 71.9 (2C, C-5a and 12a), 23.7 (2C, CH3-3 and
CH3-10), 17.7 (2C, CH3-1 and CH3-8);
HRMS (ESI) calcd for C34H27F6N6O2S2 (M + H)+: 729.1536;
found: 729.1533.
Found: C 56.14, H 3.51, N 11.45. C34H28F6N6O2S2. M 728.731. Calcd: C 56.04, H 3.60, N 11.5%.
(5aR,7aR,12aR,14aR/5aS,7aS,12aS,14aS)-7a,14a-Diamino-7,14-bis(3-chloro-4-methylphenyl)-1,3,8,10-tetramethyl-7,7a,14,14a-tetrahydro-6H,13H-pyrido[3′′′′,2′′′′:4‴,5‴]thieno[2‴,3‴:4″,5″]pyrrolo[3″,4″:3′,4′]pyrrolo[2′,3′:4,5]thieno[2,3-b]pyridine-6,13-dione (31k)
 Compound 31k was prepared according to the
general procedure in 55% (method A) and 63% (method B) yields as a
beige solid, mp 262–264 °C (from CH2Cl2). 1H NMR (400 MHz, DMSO-d6, 90 °C) δ 7.34 (d, 3J = 8.1 Hz, 2H, 2 × H-5 Ar), 7.16 (s, 2H, 2 × H-2 Ar), 6.71
(d, 3J = 8.1 Hz, 2H, 2 × H-6 Ar),
6.67 (s, 2H, H-2 and H-10), 3.60 (s, 4H, 2 × NH2),
2.37 (s, 6H, CH3-3 and CH3-10), 2.36 (s, 6H,
2 × CH3-Ph), 1.57 (s, 6H, CH3-1 and CH3-8).
Compound 31k was prepared according to the
general procedure in 55% (method A) and 63% (method B) yields as a
beige solid, mp 262–264 °C (from CH2Cl2). 1H NMR (400 MHz, DMSO-d6, 90 °C) δ 7.34 (d, 3J = 8.1 Hz, 2H, 2 × H-5 Ar), 7.16 (s, 2H, 2 × H-2 Ar), 6.71
(d, 3J = 8.1 Hz, 2H, 2 × H-6 Ar),
6.67 (s, 2H, H-2 and H-10), 3.60 (s, 4H, 2 × NH2),
2.37 (s, 6H, CH3-3 and CH3-10), 2.36 (s, 6H,
2 × CH3-Ph), 1.57 (s, 6H, CH3-1 and CH3-8).
13C NMR (100 MHz, DMSO-d6, 80 °C) δ 168.8 (2C, C-6 and C-13), 161.8 (2C, C-4a and C-11a), 160.1 (2C, C-3 and C-10), 148.2 (2C, C-1 and C-8), 137.2 (2C, 2 × C-4 Ar), 134.3 (2C, 2 × C-1 Ar), 133.8 (2C, 2 × C-3 Ar), 131.8 (2C, 2 × C-5 Ar), 131.4 (2C, 2 × C-2 Ar), 129.5 (2C, 2 × C-8 Ar), 126.1 (2C, C-7b and C-14b), 123.0 (2C, C-2 and C-9), 89.2 (2C, C-5a and C-12a), 72.6 (2C, C-7a and C-14a), 23.6 (2C, CH3-3 and CH3-10), 19.5 (2C, 2 × CH3-4 Ar), 17.9 (2C, CH3-1 and CH3-8); HRMS (ESI) calcd for C34H31Cl2N6O2S2 (M + H)+: 689.1322; found: 689.1333.
MS, EI, 70 eV, m/z (I, %): 398 (8), 396 (22), 354 [M – 2 ArN=C=O] (43), 326 (22), 169 (24), 167 (60), 141 (89), 139 (100), 91 (37), 43 (51).
Found: C, 59.18, H 4.49, N 12.27. C34H30Cl2N6O2S2. M 689.68. Calcd: C, 59.21, H 4.38, N 12.19%.
2-Ethoxy-4,6-dimethyl-N-(4-methylphenyl)-3-oxo-2,3-dihydrothieno[2,3-b]pyridine-2-carboxamide (32a)
 Compound 32a was prepared according to the
method C in 15% yield as a white powder, mp 98–99 °C (from
EtOAc). 1H NMR (400 MHz, DMSO-d6) δ 9.89 (s, 1H, CONH), 7.50 (d, 3J = 8.4 Hz, 2H, H-2 and H-6 Ar), 7.1 (d, 3J = 8.4 Hz, 2H, H-3 and H-5 Ar), 7.07 (s, 1H, H-5), 3.75 (dq, J = 6.9 Hz, J = 14.2 Hz, 1H, CH3CH2O), 3.39 (dq, J = 6.9, 14.2 Hz, 1H, CH3CH2O), 2.54 (s, 3H, CH3-4), 2.44 (s, 3H, CH3-6), 2.24 (s, 3H, CH3–Ar), 1.24 (t, 3J = 6.9 Hz, 3H, CH3CH2O); 13C NMR (100 MHz, DMSO-d6) δ 197.3 (C=O ketone), 172.5 (C-7a), 166.6
(C-6), 165.6 (C(O)NH), 150.9 (C-4), 135.4 (C-1 Ar), 134.9 (C-4 Ar),
129.5 (2C, C-3 and C-5 Ar), 123.3 (C-5), 121.1 (2C, C-2 and C-6 Ar),
119.7 (C-3a), 95.0 (C-2), 62.6 (CH3CH2O), 24.8 (CH3-6), 20.9 (CH3-Ar),
18.1 (CH3-4), 15.3 (CH3CH2O).
Compound 32a was prepared according to the
method C in 15% yield as a white powder, mp 98–99 °C (from
EtOAc). 1H NMR (400 MHz, DMSO-d6) δ 9.89 (s, 1H, CONH), 7.50 (d, 3J = 8.4 Hz, 2H, H-2 and H-6 Ar), 7.1 (d, 3J = 8.4 Hz, 2H, H-3 and H-5 Ar), 7.07 (s, 1H, H-5), 3.75 (dq, J = 6.9 Hz, J = 14.2 Hz, 1H, CH3CH2O), 3.39 (dq, J = 6.9, 14.2 Hz, 1H, CH3CH2O), 2.54 (s, 3H, CH3-4), 2.44 (s, 3H, CH3-6), 2.24 (s, 3H, CH3–Ar), 1.24 (t, 3J = 6.9 Hz, 3H, CH3CH2O); 13C NMR (100 MHz, DMSO-d6) δ 197.3 (C=O ketone), 172.5 (C-7a), 166.6
(C-6), 165.6 (C(O)NH), 150.9 (C-4), 135.4 (C-1 Ar), 134.9 (C-4 Ar),
129.5 (2C, C-3 and C-5 Ar), 123.3 (C-5), 121.1 (2C, C-2 and C-6 Ar),
119.7 (C-3a), 95.0 (C-2), 62.6 (CH3CH2O), 24.8 (CH3-6), 20.9 (CH3-Ar),
18.1 (CH3-4), 15.3 (CH3CH2O).
MS, EI, 70 eV, m/z (I, %): 356 [M+] (14), 223 [M – C2H4–C7H7N] (76), 194 [223 – CHO] (100), 166 [194 – C=O], 106 [C7H8N] (17), 91 [C7H7] (10).
Found: C, 64.02; H, 5.66; N, 7.86. C19H20N2O3S. M 356. Calcd: C, 64.13; H, 5.47; N, 7.71%.
2-Ethoxy-4-(methoxymethyl)-6-methyl-N-(4-methylphenyl)-3-oxo-2,3-dihydrothieno[2,3-b]pyridine-2-carboxamide (32b)
 Compound 32b was prepared according to the
method C in 14% yield as a white powder, mp 111–113 °C
(from EtOAc). 1H NMR (400 MHz, DMSO-d6) δ 9.91 (s, 1H, CONH), 7.52 (d, 3J = 8.4 Hz, 2H, H-2 and H-6 Ar), 7.11 (s, 1H, H-5), 7.09
(d, 3J = 8.4 Hz, 2H, H-3 and H-5 Ar),
4.29 (d, 2J = 12.8 Hz, 1H, CH3OCH2), 3.78 (dq, 3J = 7.0 Hz, 2J = 14.5 Hz, 1H,
CH3CH2O), 3.37 (dq, 3J = 7.0 Hz, 2J = 14.5 Hz, 1H, CH3CH2O), 3.05 (d, 2J = 12.8 Hz, 1H, CH3OCH2), 3.01 (s, 1H, CH3O), 2.46 (s, 3H, CH3-6), 2.22 (s, 3H, CH3–Ar), 1.24 (t, J = 7.0 Hz, 3H, CH3CH2O).
Compound 32b was prepared according to the
method C in 14% yield as a white powder, mp 111–113 °C
(from EtOAc). 1H NMR (400 MHz, DMSO-d6) δ 9.91 (s, 1H, CONH), 7.52 (d, 3J = 8.4 Hz, 2H, H-2 and H-6 Ar), 7.11 (s, 1H, H-5), 7.09
(d, 3J = 8.4 Hz, 2H, H-3 and H-5 Ar),
4.29 (d, 2J = 12.8 Hz, 1H, CH3OCH2), 3.78 (dq, 3J = 7.0 Hz, 2J = 14.5 Hz, 1H,
CH3CH2O), 3.37 (dq, 3J = 7.0 Hz, 2J = 14.5 Hz, 1H, CH3CH2O), 3.05 (d, 2J = 12.8 Hz, 1H, CH3OCH2), 3.01 (s, 1H, CH3O), 2.46 (s, 3H, CH3-6), 2.22 (s, 3H, CH3–Ar), 1.24 (t, J = 7.0 Hz, 3H, CH3CH2O).
13C NMR (100 MHz, DMSO-d6) δ 197.4 (C=O ketone), 172.7 (C-7a), 166.5 (C-6), 165.5 (C(O)NH), 151.1 (C-4), 135.4 (C-1 Ar), 133.9 (C-4 Ar), 129.7 (2C, C-3 and C-5 Ar), 123.3 (C-5), 121.2 (2C, C-2 and C-6 Ar), 119.8 (C-3a), 94.8 (C-2), 69.5 (CH2O), 62.6 (CH3CH2O), 58.2 (CH3O), 24.9 (CH3-6), 21.0 (CH3–Ar), 15.3 (CH3CH2O).
MS, EI, 70 eV, m/z (I, %): 386 [M+] (11), 358 [M – C2H4] (24), 355 [M – CH3O] (12), 280 [M – C7H7N] (100), 106 [C7H8N] (17), 91 [C7H7] (10).
Found: C, 62.05; H, 5.86; N, 7.28. C20H22N2O4S. M 386. Calcd: C, 62.16; H, 5.74; N, 7.25%.
Oxidation of Thienopyridine 30d with MCPBA
meta-Chloroperbenzoic acid (MCPBA) (740 mg, 3 mmol, 70%) in dry CH2C12 (10 mL) was added to a magnetically stirred solution of 3-amino-N-(4-ethylphenyl)-4,6-dimethylthieno[2,3-b]pyridine-2-carboxamide 30d (650 mg, 2 mmol) in dry CH2Cl2 (50 mL). The reaction was kept at r.t. and monitored occasionally using TLC. After the reaction was complete, aqueous 0.2 M NaOH was added to the reaction mixture, and the aqueous layer was extracted with CH2Cl2 (2 × 20 mL). The combined organic layer was washed with brine (3 × 40 mL), dried (Na2SO4), and concentrated. The residue was subjected to column chromatography on silica gel (hexane/acetone = 1:2) to afford sulfoxide 33 and sulfone 34 in 38 and 23% yields, respectively.
Oxidation of Thienopyridine 30d with MMPP
A mixture of magnesium monoperoxyphthalate (MMPP) (2.48 g, 4 mmol) and 3-amino-N-(4-ethylphenyl)-4,6-dimethylthieno[2,3-b]pyridine-2-carboxamide 30d (650 mg, 2 mmol) in dry CH3CN (40 mL) was refluxed for 3 h. After the reaction was complete (TLC), the mixture was poured into cold water (120 mL). The resulting precipitate was filtered off, air-dried, and purified by column chromatography (SiO2, CH2Cl2/acetone = 15:2) to give pure 3-amino-N-(4-ethylphenyl)-4,6-dimethylthieno[2,3-b]pyridine-2-carboxamide 1,1-dioxide 34 in 48% yield.
3-Amino-N-(4-ethylphenyl)-4,6-dimethylthieno[2,3-b]pyridine-2-carboxamide 1-Oxide (33)
 Compound 33 was prepared by oxidation of 30d with MCPBA in 38% yield as pale yellow crystals, mp 288–289
°C. 1H NMR (400 MHz, DMSO-d6, 80 °C) δ 9.06 (s, 1H, CONH), 8.25 (br s, 2H, NH2), 7.51 (d, 3J = 8.6 Hz, 2H, H-2
and H-5 Ar), 7.36 (s, 1H, H-5), 7.12 (d, 3J = 8.6, 2H, H-3 and H-5 Ar), 2.65 (s, 3H, CH3-4), 2.55
(s, 3H, CH3-6), 2.54 (q, 3J = 7.5 Hz, 2H, CH2CH3), 1.12 (t, 3J = 7.5 Hz, 3H, CH2CH3).
Compound 33 was prepared by oxidation of 30d with MCPBA in 38% yield as pale yellow crystals, mp 288–289
°C. 1H NMR (400 MHz, DMSO-d6, 80 °C) δ 9.06 (s, 1H, CONH), 8.25 (br s, 2H, NH2), 7.51 (d, 3J = 8.6 Hz, 2H, H-2
and H-5 Ar), 7.36 (s, 1H, H-5), 7.12 (d, 3J = 8.6, 2H, H-3 and H-5 Ar), 2.65 (s, 3H, CH3-4), 2.55
(s, 3H, CH3-6), 2.54 (q, 3J = 7.5 Hz, 2H, CH2CH3), 1.12 (t, 3J = 7.5 Hz, 3H, CH2CH3).
13C NMR (100 MHz, DMSO-d6, 80 °C) δ 165.4 (C-7a), 164.7 (C=O), 162.0 (C-6), 158.5 (C-3), 146.1 (C-4), 139.4 (C-4 Ar), 136.7 (C-1 Ar), 128.7 (C-5), 128.1 (2C, C-3 and C-5 Ar), 123.1 (C-3a), 121.5 (2C, C-2 and C-6 Ar), 101.9 (C-2), 28.1 (CH2CH3), 24.0 (CH3-6), 19.9 (CH3-4), 16.1 (CH2CH3).
MS, EI, 70 eV, m/z (I, %): 341 [M+] (34), 325 (16), 225 (100), 205 (11), 177 (34), 147 (68), 105 (71), 93 (18).
Found: C, 63,17; H, 5.73; N, 12.48. C18H19N3O2S. M 341.429. C, 63.32; H, 5.61; N, 12.31%.
3-Amino-N-(4-ethylphenyl)-4,6-dimethylthieno[2,3-b]pyridine-2-carboxamide 1,1-Dioxide (34)
 Compound 34 was prepared according to the abovementioned
procedures in 23 (by oxidation with MCPBA) and 48% yields (by oxidation
with MMPP) as pale yellow crystals; mp > 275 °C (sublimation). 1H NMR (400 MHz, DMSO-d6, 80 °C)
δ 8.31 (br s, 2H, NH2), 7.99 (s, 1H, CONH), 7.46
(s, 1H, H-5), 7.45 (d, 3J = 8.2 Hz, 2H,
H-2 and H-5 Ar), 7.17 (d, 3J = 8.2 Hz,
2H, H-3 and H-5 Ar), 2.71 (s, 3H, CH3-4), 2.59 (s, 3H,
CH3-6), 2.58 (q, 3J = 7.7 Hz,
2H, CH2CH3), 1.17 (t, 3J = 7.7 Hz, 3H, CH2CH3).
Compound 34 was prepared according to the abovementioned
procedures in 23 (by oxidation with MCPBA) and 48% yields (by oxidation
with MMPP) as pale yellow crystals; mp > 275 °C (sublimation). 1H NMR (400 MHz, DMSO-d6, 80 °C)
δ 8.31 (br s, 2H, NH2), 7.99 (s, 1H, CONH), 7.46
(s, 1H, H-5), 7.45 (d, 3J = 8.2 Hz, 2H,
H-2 and H-5 Ar), 7.17 (d, 3J = 8.2 Hz,
2H, H-3 and H-5 Ar), 2.71 (s, 3H, CH3-4), 2.59 (s, 3H,
CH3-6), 2.58 (q, 3J = 7.7 Hz,
2H, CH2CH3), 1.17 (t, 3J = 7.7 Hz, 3H, CH2CH3).
13C NMR (100 MHz, DMSO-d6, 80 °C) δ 163.6 (C-6), 161.4 (C-7a), 161.4 (C=O), 155.3 (C-3), 146.8 (C-4), 140.3 (C-4 Ar), 135.7 (C-1 Ar), 130.2 (C-5), 128.4 (2C, C-3 and C-5 Ar), 121.4 (2C, C-2 and C-6 Ar), 118.5 (C-3a), 96.4 (C-2), 28.0 (CH2CH3), 23.9 (CH3-6), 20.3 (CH3-4), 15.7 (CH2CH3).
MS, EI, 70 eV, m/z (I, %): 357 [M+] (11), 341 (22), 237 (45), 225 (60), 205 (100), 177 (14), 147 (48), 105 (77), 93 (23).
Found: C, 60.57; H, 5.23; N, 11.88. C18H19N3O3S. M 357.428. Calcd: C, 60.49; H, 5.36; N, 11.76%.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00341.
The study was carried out under the financial support of the Ministry of Science and High Education of the Russian Federation (project FZEZ-2020-0004).
The authors declare no competing financial interest.
Supplementary Material
References
- Paronikyan E. G.; Noravyan A. S.; Vartanyan S. A. Synthesis, transformations, and pharmacological properties of thienopyridines. Pharm. Chem. J. 1987, 21, 309. 10.1007/BF00757480. [DOI] [Google Scholar]
- Bakhite E. A.-G. Recent trends in the chemistry of thienopyridines. Phosphorus, Sulfur Silicon Relat. Elem. 2003, 178, 929. 10.1080/10426500307855. [DOI] [Google Scholar]
- Litvinov V. P.; Dotsenko V. V.; Krivokolysko S. G.. The Chemistry of Thienopyridines. In Advances in Heterocyclic Chemistry; Katritzky A. R., Ed.; Elsevier Science Ltd.: Oxford, 2007; Vol. 93, pp 117–178. [Google Scholar]
- Litvinov V. P.; Dotsenko V. V.; Krivokolysko S. G. Thienopyridines: synthesis, properties, and biological activity. Russ. Chem. Bull. 2005, 54, 864. 10.1007/s11172-005-0333-1. [DOI] [Google Scholar]
- Dotsenko V. V.; Buryi D. S.; Lukina D. Y.; Krivokolysko S. G. Recent advances in the chemistry of thieno[2,3-b]pyridines. 1. Methods of synthesis of thieno[2,3-b] pyridines. Russ. Chem. Bull. 2020, 69, 1829. 10.1007/s11172-020-2969-2. [DOI] [Google Scholar]
- Schweda S. I.; Alder A.; Gilberger T.; Kunick C. 4-Arylthieno[2,3-b]pyridine-2-carboxamides Are a New Class of Antiplasmodial Agents. Molecules 2020, 25, 3187 10.3390/molecules25143187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masch A.; Nasereddin A.; Alder A.; Bird M. J.; Schweda S. I.; Preu L.; Doerig C.; Dzikowski R.; Gilberger T. W.; Kunick C. Structure–activity relationships in a series of antiplasmodial thieno[2,3-b]pyridines. Malar. J. 2019, 18, 89 10.1186/s12936-019-2725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masch A.; Kunick C. Selective inhibitors of Plasmodium falciparum glycogen synthase-3 (PfGSK-3): New antimalarial agents?. Biochim. Biophys. Acta, Proteins Proteomics 2015, 1854, 1644. 10.1016/j.bbapap.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Fugel W.; Oberholzer A. E.; Gschloessl B.; Dzikowski R.; Pressburger N.; Preu L.; Pearl L. H.; Baratte B.; Ratin M.; Okun I.; Doerig C.; Kruggel S.; Lemcke T.; Meijer L.; Kunick C. 3,6-Diamino-4-(2-halophenyl)-2-benzoylthieno[2,3-b]pyridine-5-carbonitriles are selective inhibitors of Plasmodium falciparum Glycogen Synthase Kinase-3. J. Med. Chem. 2013, 56, 264. 10.1021/jm301575n. [DOI] [PubMed] [Google Scholar]
- Gilmour R.; Foster J. E.; Sheng Q.; McClain J. R.; Riley A.; Sun P.-M.; Ng W. L.; Yan D.; Nicas T. I.; Henry K.; Winkler M. E. New Class of Competitive Inhibitor of Bacterial Histidine Kinases. J. Bacteriol. 2005, 187, 8196. 10.1128/JB.187.23.8196-8200.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anighoro A.; Pinzi L.; Marverti G.; Bajorath J.; Rastelli G. Heat shock protein 90 and serine/threonine kinase B-Raf inhibitors have overlapping chemical space. RSC Adv. 2017, 7, 31069. 10.1039/C7RA05889F. [DOI] [Google Scholar]
- Bonafoux D.; Lee W.-C. Strategies for TGF-β modulation: a review of recent patents. Expert Opin. Ther. Pat. 2009, 19, 1759. 10.1517/13543770903397400. [DOI] [PubMed] [Google Scholar]
- May B. C. H.; Zorn J. A.; Witkop J.; Sherrill J.; Wallace A. C.; Legname G.; Prusiner S. B.; Cohen F. E. Structure-Activity Relationship Study of Prion Inhibition by 2-Aminopyridine-3,5-dicarbonitrile-Based Compounds: Parallel Synthesis, Bioactivity, and in Vitro Pharmacokinetics. J. Med. Chem. 2007, 50, 65. 10.1021/jm061045z. [DOI] [PubMed] [Google Scholar]
- Dotsenko V. V.; Krivokolysko S. G.; Chernega A. N.; Litvinov V. P. Anilinomethylidene derivatives of cyclic 1,3-dicarbonyl compounds in the synthesis of new sulfur-containing pyridines and quinolines. Russ. Chem. Bull. 2002, 51, 1556. 10.1023/A:1020939712830. [DOI] [Google Scholar]
- Reynisson J.; Court W.; O’Neill C.; Day J.; Patterson L.; McDonald E.; Workman P.; Katan M.; Eccles S. A. The identification of novel PLC-γ inhibitors using virtual high throughput screening. Bioorg. Med. Chem. 2009, 17, 3169. 10.1016/j.bmc.2009.02.049. [DOI] [PubMed] [Google Scholar]
- Hung J. M.; Arabshahi H. J.; Leung E.; Reynisson J.; Barker D. Synthesis and cytotoxicity of thieno[2,3-b]pyridine and furo[2,3-b]pyridine derivatives. Eur. J. Med. Chem. 2014, 86, 420. 10.1016/j.ejmech.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Leung E.; Hung J. M.; Barker D.; Reynisson J. The effect of a thieno[2,3-b]pyridine PLC-γ inhibitor on the proliferation, morphology, migration and cell cycle of breast cancer cells. Med. Chem. Commun. 2014, 5, 99. 10.1039/C3MD00290J. [DOI] [Google Scholar]
- Arabshahi H. J.; Leung E.; Barker D.; Reynisson J. The development of thieno[2,3-b]pyridine analogues as anticancer agents applying in silico methods. MedChemComm 2014, 5, 186. 10.1039/c3md00320e. [DOI] [Google Scholar]
- Reynisson J.; Jaiswal J. K.; Barker D.; D’mello S. A. N.; Denny W. A.; Baguley B. C.; Leung E. Y. Evidence that phospholipase C is involved in the antitumour action of NSC768313, a new thieno[2,3-b]pyridine derivative. Cancer Cell Int. 2016, 16, 18 10.1186/s12935-016-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rensburg M.; Leung E.; Haverkate N. A.; Eurtivong C.; Pilkington L. I.; Reynisson J.; Barker D. Synthesis and antiproliferative activity of 2-chlorophenyl carboxamide thienopyridines. Bioorg. Med. Chem. Lett. 2017, 27, 135. 10.1016/j.bmcl.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Eurtivong C.; Reynisdóttir I.; Kuczma S.; Furkert D. P.; Brimble M. A.; Reynisson J. Identification of anticancer agents based on the thieno[2,3-b]pyridine and 1H-pyrazole molecular scaffolds. Bioorg. Med. Chem. 2016, 24, 3521. 10.1016/j.bmc.2016.05.061. [DOI] [PubMed] [Google Scholar]
- Eurtivong C.; Semenov V.; Semenova M.; Konyushkin L.; Atamanenko O.; Reynisson J.; Kiselyov A. 3-Amino-thieno[2,3-b]pyridines as microtubule-destabilising agents: Molecular modelling and biological evaluation in the sea urchin embryo and human cancer cells. Bioorg. Med. Chem. 2017, 25, 658. 10.1016/j.bmc.2016.11.041. [DOI] [PubMed] [Google Scholar]
- Zafar A.; Sari S.; Leung E.; Pilkington L. I.; van Rensburg M.; Barker D.; Reynisson J. GPCR Modulation of Thieno[2,3-b]pyridine Anti-Proliferative Agents. Molecules 2017, 22, 2254 10.3390/molecules22122254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar A.; Pilkington L. I.; Haverkate N. A.; van Rensburg M.; Leung E.; Kumara S.; Denny W. A.; Barker D.; Alsuraifi A.; Hoskins C.; Reynisson J. Investigation into Improving the Aqueous Solubility of the Thieno[2,3-b]pyridine Anti-Proliferative Agents. Molecules 2018, 23, 145 10.3390/molecules23010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijan S.; Markotić A.; Mastelić A.; Režić-Mužinić N.; Pilkington L. I.; Reynisson J.; Čulić V. Č. Glycosphingolipid expression at breast cancer stem cells after novel thieno[2,3-b]pyridine anticancer compound treatment. Sci. Rep. 2020, 10, 11876 10.1038/s41598-020-68516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binsaleh N. K.; Wigley C. A.; Whitehead K. A.; van Rensburg M.; Reynisson J.; Pilkington L. I.; Barker D.; Jones S.; Dempsey-Hibbert N. C. Thieno[2,3-b]pyridine derivatives are potent anti-platelet drugs, inhibiting platelet activation, aggregation and showing synergy with aspirin. Eur. J. Med. Chem. 2018, 143, 1997. 10.1016/j.ejmech.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Pinheiro L. C. S.; Abreu P. A.; Afonso I. F.; Leal B.; Corrêa L. C. D.; Borges J. C.; Marques I. P.; Lourenço A. L.; Sathler P.; dos Santos A. L.; Medeiros C. A.; Cabral L. M.; Júnior M. L. O.; Romeiro G. A.; Ferreira V. F.; Rodrigues C. R.; Castro H. C.; Bernardino A. M. R. Identification of a potential lead structure for designing new antimicrobials to treat infections caused by Staphylococcus epidermidis-resistant strains. Curr. Microbiol. 2008, 57, 463. 10.1007/s00284-008-9234-5. [DOI] [PubMed] [Google Scholar]
- Pinheiro L. C. S.; Tonioni R.; Sathler P. C.; Castro H. C.; Bernardino A. M. R.; Magalhães U. O.; Cabral L.; Rodrigues C. R.; Borges J. C.; dos Santos M. S.; Ferreira V. F.; Braga S. N.; Bourguignon S. C.; Santos D. O. Searching for new antileishmanial lead drug candidates: Synthesis, biological and theoretical evaluations of promising thieno[2,3-b]pyridine derivatives. J. Microbiol. Antimicrob. 2012, 4, 32. 10.5897/JMA11.109. [DOI] [Google Scholar]
- Amorim R.; Ferreira de Meneses M. D.; Borges J. C.; Pinheiro L. C. S.; Caldas L. A.; Cirne-Santos C. C.; de Mello M. V. P.; de Souza A. M. T.; Castro H. C.; Paixão I. C. N. P.; Campos R. M.; Bergmann I. E.; Malirat V.; Bernardino A. M. R.; Rebello M. A.; Ferreira D. F. Thieno[2,3-b]pyridine derivatives: a new class of antiviral drugs against Mayaro virus. Arch. Virol. 2017, 162, 1577. 10.1007/s00705-017-3261-0. [DOI] [PubMed] [Google Scholar]
- Boschelli D. H.; Wu B.; Barrios Sosa A. C.; Chen J.; Asselin M.; Cole D. C.; Lee J.; Yang X.; Chaudhary D. Synthesis and PKCθ inhibitory activity of a series of 4-(indol-5-ylamino)thieno[2,3-b]pyridine-5-carbonitriles. Bioorg. Med. Chem. Lett. 2008, 18, 2850. 10.1016/j.bmcl.2008.03.077. [DOI] [PubMed] [Google Scholar]
- Tumey L. N.; Boschelli D. H.; Lee J.; Chaudhary D. 2-Alkenylthieno[2,3-b]pyridine-5-carbonitriles: Potent and selective inhibitors of PKCθ. Bioorg. Med. Chem. Lett. 2008, 18, 4420. 10.1016/j.bmcl.2008.06.040. [DOI] [PubMed] [Google Scholar]
- Wu B.; Boschelli D. H.; Lee J.; Yang X.; Chaudhary D. Second generation 4-(4-methyl-1H-indol-5-ylamino)-2-phenylthieno[2,3-b]pyridine-5-carbonitrile PKCθ inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 766. 10.1016/j.bmcl.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Saito K.; Nakao A.; Shinozuka T.; Shimada K.; Matsui S.; Oizumi K.; Yano K.; Ohata K.; Nakai D.; Nagai Y.; Naito S. Discovery and structure–activity relationship of thienopyridine derivatives as bone anabolic agents. Bioorg. Med. Chem. 2013, 21, 1628. 10.1016/j.bmc.2013.01.071. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Desai A.; Yang S. Y.; Bae K. B.; Antczak M. I.; Fink S. P.; Tiwari S.; Willis J. E.; Williams N. S.; Dawson D. M.; Wald D.; Chen W.-D.; Wang Z.; Kasturi L.; Larusch G. A.; He L.; Cominelli F.; Di Martino L.; Djuric Z.; Milne G. L.; Chance M.; Sanabria J.; Dealwis C.; Mikkola D.; Naidoo J.; Wei S.; Tai H.-H.; Gerson S. L.; Ready J. M.; Posner B.; Willson J. K. V.; Markowitz S. D. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 2015, 348, aaa2340 10.1126/science.aaa2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antczak M. I.; Zhang Y.; Wang C.; Doran J.; Naidoo J.; Voruganti S.; Williams N. S.; Markowitz S. D.; Ready J. M. Inhibitors of 15-Prostaglandin Dehydrogenase To Potentiate Tissue Repair. J. Med. Chem. 2017, 60, 3979. 10.1021/acs.jmedchem.7b00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K.; Shinozuka T.; Nakao A.; Kiho T.; Kunikata T.; Shiiki T.; Nagai Y.; Naito S. Synthesis and structure-activity relationship of 4-alkoxy-thieno[2,3-b]pyridine derivatives as potent alkaline phosphatase enhancers for osteoporosis treatment. Bioorg. Med. Chem. Lett. 2019, 29, 1769. 10.1016/j.bmcl.2019.05.014. [DOI] [PubMed] [Google Scholar]
- Wang N.-Y.; Zuo W.-Q.; Xu Y.; Gao C.; Zeng X.-X.; Zhang L.-D.; You X.-Y.; Peng C.-T.; Shen Y.; Yang S.-Y.; Wei Y.-Q.; Yu L.-T. Discovery and structure–activity relationships study of novel thieno[2,3-b]pyridine analogues as hepatitis C virus inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1581. 10.1016/j.bmcl.2014.01.075. [DOI] [PubMed] [Google Scholar]
- Sztuba-Solinska J.; Shenoy S. R.; Gareiss P.; Krumpe L. R. H.; Le Grice S. F. J.; O’Keefe B. R.; Schneekloth J. S. Jr. Identification of Biologically Active, HIV TAR RNA-Binding Small Molecules Using Small Molecule Microarrays. J. Am. Chem. Soc. 2014, 136, 8402. 10.1021/ja502754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler E. G.; Shacham S.; Noiman S.; Lezoualc’h F.; Robert S.; Gastineau M.; Rutkowski J.; Marantz Y.; Dumuis A.; Bockaert J.; Gold P. E.; Ragozzino M. E. VRX-03011, a novel 5-HT4 agonist, enhances memory and hippocampal acetylcholine efflux. Neuropharmacology 2007, 53, 563. 10.1016/j.neuropharm.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Johnson D. E.; Drummond E.; Grimwood S.; Sawant-Basak A.; Miller E.; Tseng E.; McDowell L. L.; Vanase-Frawley M. A.; Fisher K. E.; Rubitski D. M.; Stutzman-Engwall K. J.; Nelson R. T.; Horner W. E.; Gorczyca R. R.; Hajos M.; Siok C. J. The 5-Hydroxytryptamine4 Receptor Agonists Prucalopride and PRX-03140 Increase Acetylcholine and Histamine Levels in the Rat Prefrontal Cortex and the Power of Stimulated Hippocampal θ Oscillations. J. Pharmacol. Exp. Ther. 2012, 341, 681. 10.1124/jpet.112.192351. [DOI] [PubMed] [Google Scholar]
- Nógrádi K.; Wágner G.; Domány G.; Bobok A.; Magdó I.; Kolok S.; Mikó-Bakk M. L.; Vastag M.; Sághy K.; Gyertyán I.; Kóti J.; Gál K.; Farkas S.; Keserü G. M.; Greiner I.; Szombathelyi Z. Thieno[2,3-b]pyridines as negative allosteric modulators of metabotropic GluR5 receptors: Lead optimization. Bioorg. Med. Chem. Lett. 2015, 25, 1724. 10.1016/j.bmcl.2015.02.073. [DOI] [PubMed] [Google Scholar]
- Shirey J. K.; Xiang Z.; Orton D.; Brady A. E.; Johnson K. A.; Williams R.; Ayala J. E.; Rodriguez A. L.; Wess J.; Weaver D.; Niswender C. M.; Conn P. J. An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat. Chem. Biol. 2008, 4, 42. 10.1038/nchembio.2007.55. [DOI] [PubMed] [Google Scholar]
- Nawaratne V.; Leach K.; Suratman N.; Loiacono R. E.; Felder C. C.; Armbruster B. N.; Roth B. L.; Sexton P. M.; Christopoulos A. New Insights into the Function of M4 Muscarinic Acetylcholine Receptors Gained Using a Novel Allosteric Modulator and a DREADD (Designer Receptor Exclusively Activated by a Designer Drug). Mol. Pharmacol. 2008, 74, 1119. 10.1124/mol.108.049353. [DOI] [PubMed] [Google Scholar]
- Chan W. Y.; McKinzie D. L.; Bose S.; Mitchell S. N.; Witkin J. M.; Thompson R. C.; Christopoulos A.; Lazareno S.; Birdsall N. J. M.; Bymaster F. P.; Felder C. C. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 10978. 10.1073/pnas.0800567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaratne V.; Leach K.; Felder C. C.; Sexton P. M.; Christopoulos A. Structural Determinants of Allosteric Agonism and Modulation at the M4 Muscarinic Acetylcholine Receptor. Identification of ligand-specific and global activation mechanisms. J. Biol. Chem. 2010, 285, 19012. 10.1074/jbc.M110.125096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K.; Loiacono R. E.; Felder C. C.; McKinzie D. L.; Mogg A.; Shaw D. B.; Sexton P. M.; Christopoulos A. Molecular Mechanisms of Action and In Vivo Validation of an M4 Muscarinic Acetylcholine Receptor Allosteric Modulator with Potential Antipsychotic Properties. Neuropsychopharmacology 2010, 35, 855. 10.1038/npp.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. P.; Bridges T. M.; Gentry P. R.; Brogan J. T.; Kane A. S.; Jones C. K.; Brady A. E.; Shirey J. K.; Conn P. J.; Lindsley C. W. Synthesis and Structure–Activity Relationships of Allosteric Potentiators of the M4 Muscarinic Acetylcholine Receptor. ChemMedChem 2009, 4, 1600. 10.1002/cmdc.200900231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon R. L.; Millan M. J. LY2033298, a positive allosteric modulator at muscarinic M4 receptors, enhances inhibition by oxotremorine of light-induced phase shifts in hamster circadian activity rhythms. Psychopharmacology 2012, 224, 231. 10.1007/s00213-012-2743-8. [DOI] [PubMed] [Google Scholar]
- Le U.; Melancon B. J.; Bridges T. M.; Vinson P. N.; Utley T. J.; Lamsal A.; Rodriguez A. L.; Venable D.; Sheffler D. J.; Jones C. K.; Blobaum A. L.; Wood M. R.; Daniels J. S.; Conn P. J.; Niswender C. M.; Lindsley C. W.; Hopkins C. R. Discovery of a selective M4 positive allosteric modulator based on the 3-amino-thieno[2,3-b]pyridine-2-carboxamide scaffold: Development of ML253, a potent and brain penetrant compound that is active in a preclinical model of schizophrenia. Bioorg. Med. Chem. Lett. 2013, 23, 346. 10.1016/j.bmcl.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh T.; Valant C.; Crosby I. T.; Sexton P. M.; Christopoulos A.; Capuano B. Probing Structural Requirements of Positive Allosteric Modulators of the M4 Muscarinic Receptor. J. Med. Chem. 2013, 56, 8196. 10.1021/jm401032k. [DOI] [PubMed] [Google Scholar]
- Croy C. H.; Schober D. A.; Xiao H.; Quets A.; Christopoulos A.; Felder C. C. Characterization of the Novel Positive Allosteric Modulator, LY2119620, at the Muscarinic M2 and M4 Receptors. Mol. Pharmacol. 2014, 86, 106. 10.1124/mol.114.091751. [DOI] [PubMed] [Google Scholar]
- Schober D. A.; Croy C. H.; Xiao H.; Christopoulos A.; Felder C. C. Development of a Radioligand, [3H]LY2119620, to Probe the Human M2 and M4 Muscarinic Receptor Allosteric Binding Sites. Mol. Pharmacol. 2014, 86, 116. 10.1124/mol.114.091785. [DOI] [PubMed] [Google Scholar]
- Huynh T.; Valant C.; Crosby I. T.; Sexton P. M.; Christopoulos A.; Capuano B. Synthesis and Pharmacological Evaluation of M4 Muscarinic Receptor Positive Allosteric Modulators Derived from VU10004. ACS Chem. Neurosci. 2015, 6, 838. 10.1021/acschemneuro.5b00035. [DOI] [PubMed] [Google Scholar]
- Bakhite E. A.; Abd-Ella A. A.; El-Sayed M. E. A.; Abdel-Raheem S. A. A. Pyridine Derivatives as Insecticides. Part 1: Synthesis and Toxicity of Some Pyridine Derivatives Against Cowpea Aphid, Aphis craccivora Koch (Homoptera: Aphididae). J. Agric. Food Chem. 2014, 62, 9982. 10.1021/jf503992y. [DOI] [PubMed] [Google Scholar]
- El-Dean A. M. K.; Abd-Ella A. A.; Hassanien R.; El-Sayed M. E. A.; Abdel-Raheem S. A. A. Design, Synthesis, Characterization, and Insecticidal Bioefficacy Screening of Some New Pyridine Derivatives. ACS Omega 2019, 4, 8406. 10.1021/acsomega.9b00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akula N.; Trivedi P.; Han F. Q.; Wang N. Identification of small molecule inhibitors against SecA of Candidatus Liberibacter asiaticus by structure based design. Eur. J. Med. Chem. 2012, 54, 919. 10.1016/j.ejmech.2012.05.035. [DOI] [PubMed] [Google Scholar]
- Dotsenko V. V.; Buryi D. S.; Lukina D. Y.; Stolyarova A. N.; Aksenov N. A.; Aksenova I. V.; Strelkov V. D.; Dyadyuchenko L. V. Substituted N-(thieno[2,3-b]pyridine-3-yl)acetamides: synthesis, reactions, and biological activity. Monatsh. Chem. 2019, 150, 1973. 10.1007/s00706-019-02505-4. [DOI] [Google Scholar]
- Dotsenko V. V.; Muraviev V. S.; Lukina D. Y.; Strelkov V. D.; Aksenov N. A.; Aksenova I. V.; Krapivin G. D.; Dyadyuchenko L. V. Reaction of 3-Amino-4,6-diarylthieno[2,3-b]pyridine-2-carboxamides with Ninhydrin. Russ. J. Gen. Chem. 2020, 90, 948. 10.1134/S1070363220060043. [DOI] [Google Scholar]
- Buryi D. S.; Dotsenko V. V.; Aksenov N. A.; Aksenova I. V.; Krivokolysko S. G.; Dyadyuchenko L. V. Synthesis and properties of 4,6-dimethyl-5-pentyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile and 3-amino-4,6-dimethyl-5-pentylthieno[2,3-b]pyridines. Russ. J. Gen. Chem. 2019, 89, 1575. 10.1134/S1070363219080061. [DOI] [Google Scholar]
- Dotsenko V. V.; Krivokolysko S. G.; Chernega A. N.; Litvinov V. P. Anilinomethylidene derivatives of cyclic 1,3-dicarbonyl compounds in the synthesis of new sulfur-containing pyridines and quinolines. Russ. Chem. Bull. 2002, 51, 1556. 10.1023/A:1020939712830. [DOI] [Google Scholar]
- Nagarajan S.; Doddareddy M. R.; Choo H.; Cho Y. S.; Oh K.-S.; Lee B. H.; Pae A. N. IKKβ inhibitors identification part I: Homology model assisted structure based virtual screening. Bioorg. Med. Chem. 2009, 17, 2759. 10.1016/j.bmc.2009.02.041. [DOI] [PubMed] [Google Scholar]
- Mermerian A. H.; Case A.; Stein R. L.; Cuny G. D. Structure–activity relationship, kinetic mechanism, and selectivity for a new class of ubiquitin C-terminal hydrolase-L1 (UCH-L1) inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 3729. 10.1016/j.bmcl.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Dayam R.; Al-Mawsawi L. Q.; Zawahir Z.; Witvrouw M.; Debyser Z.; Neamati N. Quinolone 3-Carboxylic Acid Pharmacophore: Design of Second Generation HIV-1 Integrase Inhibitors. J. Med. Chem. 2008, 51, 1136. 10.1021/jm070609b. [DOI] [PubMed] [Google Scholar]
- Kamal A. M.; Radwan S. M.; Zaki R. M. Synthesis and biological activity of pyrazolothienotetrahydroisoquinoline and [1,2,4]triazolo[3,4-a]thienotetrahydro-isoquinoline derivatives. Eur. J. Med. Chem. 2011, 46, 567. 10.1016/j.ejmech.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Quintela J. M.; Peinador C.; González L.; Iglesias R.; Paramá A.; Álvarez F.; Sanmartín M. L.; Riguera R. Piperazine N-substituted naphthyridines, pyridothienopyrimidines and pyridothienotriazines: new antiprotozoals active against Philasterides dicentrarchi. Eur. J. Med. Chem. 2003, 38, 265. 10.1016/S0223-5234(03)00032-1. [DOI] [PubMed] [Google Scholar]
- El-Deen E. M. M.; Abd El-Meguid E. A.; Hasabelnaby S.; Karam E. A.; Nossier E. S. Synthesis, docking studies, and in vitro evaluation of some novel thienopyridines and fused thienopyridine–quinolines as antibacterial agents and DNA gyrase inhibitors. Molecules 2019, 24, 3650 10.3390/molecules24203650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki R. M.; Kamal El-Dean A. M.; Radwan S. M.; Ammar M. A. Synthesis, reactions, and antimicrobial activity of novel heterocyclic compounds containing cyclopenta[d]thieno[2,3-b]pyridine moiety and related fused heterocycles. Russ. J. Bioorg. Chem. 2020, 46, 85. 10.1134/S1068162020010148. [DOI] [Google Scholar]
- Quintela J. M.; Peinador C.; Veiga M. C.; Botana L. M.; Alfonso A.; Riguera R. Synthesis, antihistaminic and cytotoxic activity of pyridothieno- and pyridodithienotriazines. Eur. J. Med. Chem. 1998, 33, 887. 10.1016/S0223-5234(99)80013-0. [DOI] [Google Scholar]
- Hassan A. Y.; Sarg M. T.; El-Sebaey S. A. Synthesis and antitumor evaluation of some new derivatives and fused heterocyclic compounds derived from thieno[2,3-b]pyridine. J. Heterocycl. Chem. 2019, 56, 3102. 10.1002/jhet.3709. [DOI] [Google Scholar]
- Hassan A. Y.; Sarg M. T.; El-Sebaey S. A. Synthesis and antitumor evaluation of some new derivatives and fused heterocyclic compounds derived from thieno[2,3-b]pyridine: Part 2. J. Heterocycl. Chem. 2020, 57, 694. 10.1002/jhet.3810. [DOI] [Google Scholar]
- Al-Huniti M. H.; El-Abadelah M. M.; Zahra J. A.; Sabri S. S.; Ingendoh A. Facile synthesis of some novel pyrido[3’,2’:4,5]thieno[2,3-b][1,4]thiazine-8-carboxylic acids. Molecules 2007, 12, 497. 10.3390/12030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attaby F. A.; Abdel-Fattah A. M.; Shaif L. M.; Elsayed M. M. Reactions, Anti-Alzheimer and Anti COX-2 Activities of the Newly Synthesized 2-Substituted Thienopyridines. Curr. Org. Chem. 2009, 13, 1654. 10.2174/138527209789578135. [DOI] [Google Scholar]
- Dabaeva V. V.; Bagdasaryan M. R.; Noravyan A. S.; Dzhagatspanyan I. A.; Nazaryan I. M.; Akopyan A. G. Synthesis and neurotropic activity of new pyrimido[4′,5′:4,5]thieno[2,3-b]quinoline derivatives. Pharm. Chem. J. 2015, 49, 587. 10.1007/s11094-015-1334-5. [DOI] [Google Scholar]
- Paronikyan E. G.; Akopyan S. F.; Noravyan A. S.; Dzhagatspanyan I. A.; Nazaryan I. M.; Akopyan A. G. Synthesis and neurotropic activity of 6-thio-substituted pyrano[3,4-c]pyridine and 1-aminopyrano[4,3-d]thieno[2,3-b]pyridine derivatives and 9-substituted pyrido[2,3-b]thieno[3,2-d]pyrimidines. Pharm. Chem. J. 2010, 44, 183. 10.1007/s11094-010-0426-5. [DOI] [Google Scholar]
- Sirakanyan S. N.; Ovakimyan A. A.; Noravyan A. S.; Minasyan N. S.; Dzhagatspanyan I. A.; Nazaryan I. M.; Akopyan A. G. Synthesis and neurotropic activity of 8-amino derivatives of condensed thieno[3,2-d]- and furo[3,2-d]pyrimidines. Pharm. Chem. J. 2014, 47, 655. 10.1007/s11094-014-1026-6. [DOI] [Google Scholar]
- Paronikyan E. G.; Akopyan S. F.; Noravyan A. S.; Mamyan S. S.; Paronikyan R. G.; Dzhagatspanyan I. A. Synthesis and anticonvulsant activity of condensed thieno[2,3-e]pyrrolo[1,2-a]pyrimidin-8,12-diones. Pharm. Chem. J. 2013, 47, 92. 10.1007/s11094-013-0902-9. [DOI] [Google Scholar]
- Taltavull J.; Serrat J.; Gràcia J.; Gavaldà A.; Andrés M.; Córdoba M.; Miralpeix M.; Vilella D.; Beleta J.; Ryder H.; Pagès L. Synthesis and biological activity of pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidines as phosphodiesterase type 4 inhibitors. J. Med. Chem. 2010, 53, 6912. 10.1021/jm100524j. [DOI] [PubMed] [Google Scholar]
- Loidreau Y.; Deau E.; Marchand P.; Nourrisson M.-R.; Logé C.; Coadou G.; Loaëc N.; Meijer L.; Besson T. Synthesis and molecular modelling studies of 8-arylpyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-4-amines as multitarget Ser/Thr kinases inhibitors. Eur. J. Med. Chem. 2015, 92, 124. 10.1016/j.ejmech.2014.12.038. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Tovar C.; Yin X.; Xu Q.; Todorov I. T.; Vassilev L. T.; Chen L. Synthesis and evaluation of pyrido-thieno-pyrimidines as potent and selective Cdc7 kinase inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 319. 10.1016/j.bmcl.2008.11.093. [DOI] [PubMed] [Google Scholar]
- Ma F.; Liu J.; Zhou T.; Lei M.; Chen J.; Wang X.; Zhang Y.; Shen X.; Hu L. Discovery and structure-activity relationships study of thieno[2,3-b]pyridine analogues as hepatic gluconeogenesis inhibitors. Eur. J. Med. Chem. 2018, 152, 307. 10.1016/j.ejmech.2018.04.028. [DOI] [PubMed] [Google Scholar]
- Kosulina D. Y.; Vasilin V. K.; Stroganova T. A.; Kaklyugina T. Y.; Krapivin G. D. Furan ring recyclization in 2-furfurylthieno[2,3-b]pyridines: An intramolecular N-alkylation of pyrrole ring under acid conditions. J. Heterocycl. Chem. 2010, 47, 309. 10.1002/jhet.311. [DOI] [Google Scholar]
- Stroganova T. A.; Vasilin V. K.; Zelenskaya E. A.; Red’kin V. M.; Krapivin G. D. Some transformations of tertiary N-furfurylamides of aromatic and heteroaromatic carboxylic acids under acidic conditions. Synthesis 2008, 2008, 3088. 10.1055/s-2008-1067267. [DOI] [Google Scholar]
- Stroganova T. A.; Vasilin V. K.; Krapivin G. D. Transformations of 3-Amino-N-[2-(5-methyl-2-furyl)ethyl]thieno[2,3-b]pyridine-2-carboxamides in acidic media. Synlett 2016, 27, 1569. 10.1055/s-0035-1561400. [DOI] [Google Scholar]
- Lebedyeva I. O.; Dotsenko V. V.; Turovtsev V. V.; Krivokolysko S. G.; Povstyanoy V. M.; Povstyanoy M. V. The Thorpe–Ziegler-type reaction of 3-cyanopyridine-2(1H)-thiones with Biginelli 6-bromomethyl-3,4-dihydropyrimidin-2(1H)-ones: cascade assembling of tetra- and pentacyclic heterocyclic scaffolds. Tetrahedron 2012, 68, 9729. 10.1016/j.tet.2012.09.041. [DOI] [Google Scholar]
- Dotsenko V. V.; Krivokolysko S. G.; Chernega A. N.; Litvinov V. P. Fused sulfur-containing pyridine systems. 1. Synthesis and structures of tetrahydropyrido-thieno-pyridinone and tetrahydropyrido-thiopyrano-pyridinone derivatives. Russ. Chem. Bull. 2003, 52, 969. 10.1023/A:1024420930528. [DOI] [Google Scholar]
- Dotsenko V. V.; Krivokolysko S. G.; Litvinov V. P. A novel approach to the synthesis of partially hydrogenated dipyridothiophenes. Mendeleev Commun. 2004, 14, 30. 10.1070/MC2004v014n01ABEH001882. [DOI] [Google Scholar]
- Stroganova T. A.; Vasilin V. K.; Krapivin G. D.; Strelkov V. D.; Dyadyuchenko L. V. Synthesis of N-alkylated benzo- and pyridothienopyrrolo-[1,2a][1,4]diazepin-6-ones acting as antidotes against the herbicide 2,4-D. Chem. Heterocycl. Compd. 2016, 52, 45. 10.1007/s10593-016-1830-x. [DOI] [Google Scholar]
- Buryi D. S.; Dotsenko V. V.; Levashov A. S.; Lukina D. Y.; Strelkov V. D.; Aksenov N. A.; Aksenova I. V.; Netreba E. E. Synthesis of 4,6-disubstituted 2-thioxo-1,2-dihydropyridine-3-carbonitriles by the reaction of acetylenic ketones with cyanothioacetamide. Russ. J. Gen. Chem. 2019, 89, 886. 10.1134/S1070363219050050. [DOI] [Google Scholar]
- Chigorina E. A.; Bespalov A. V.; Dotsenko V. V. Synthesis and Cyclizations of N-(Thieno[2,3-b]pyridin-3-yl)cyanoacetamides. Russ. J. Gen. Chem. 2019, 89, 2018. 10.1134/S1070363219100062. [DOI] [Google Scholar]
- Dotsenko V. V.; Krivokolysko S. G.; Krivokolysko B. S.; Frolov K. A. A New approach to the synthesis of functional derivatives of 3-(4-pyridinyl)-1H-indole and 4-(1H-indol-3-yl)thieno[2,3-b]pyridine. Russ. J. Gen. Chem. 2018, 88, 682. 10.1134/S1070363218040114. [DOI] [Google Scholar]
- Klemm L. H.; Barnish I. T.; Zell H. Chemistry of thienopyridines. VIII. Substitution products derived from thieno[2,3-b]pyridine 7-oxide. J. Heterocycl. Chem. 1970, 7, 81. 10.1002/jhet.5570070112. [DOI] [Google Scholar]
- Lucas S. C. C.; Moore J. E.; Donald C. S.; Hawkins J. L. Synthesis of 4-arylthieno[2,3-b]pyridines and 4-aminothieno[2,3-b]pyridines via a regioselective bromination of thieno[2,3-b]pyridine. J. Org. Chem. 2015, 80, 12594. 10.1021/acs.joc.5b01735. [DOI] [PubMed] [Google Scholar]
- Chen H.-Y.; Nikolka M.; Wadsworth A.; Yue W.; Onwubiko A.; Xiao M.; White A. J. P.; Baran D.; Sirringhaus H.; McCulloch I. A thieno[2,3-b]pyridine-flanked diketopyrrolopyrrole polymer as an n-type polymer semiconductor for all-polymer solar cells and organic field-effect transistors. Macromolecules 2018, 51, 71. 10.1021/acs.macromol.7b00934. [DOI] [Google Scholar]
- Peixoto D.; Begouin A.; Queiroz M.-J. R. P. Synthesis of 2-(hetero)arylthieno[2,3-b] or [3,2-b]pyridines from 2,3-dihalopyridines, (hetero)arylalkynes, and Na2S. Further functionalizations. Tetrahedron 2012, 68, 7082. 10.1016/j.tet.2012.06.057. [DOI] [Google Scholar]
- Klemm L. H.; Mathur S. B.; Zell R.; Merrill R. E. Chemistry of thienopyridines. XII. Selective formation of N-oxides, sulfoxides, and sulfones in some tricyclic systems. J. Heterocycl. Chem. 1971, 8, 931. 10.1002/jhet.5570080608. [DOI] [Google Scholar]
- Klemm L. H.; Muchiri D. R.; Anderson M.; Salbador W.; Ford J. Chemistry of thienopyridines. XLII. Three novel compounds derived from thienopyridine N-oxides. J. Heterocycl. Chem. 1994, 31, 261. 10.1002/jhet.5570310146. [DOI] [Google Scholar]
- Klemm L. H.; Merrill R. E.; Lee F. H. W.; Klopfenstein C. E. Chemistry of thienopyridines. XVII. Direct halogenation of thieno[2,3-b]pyridine. J. Heterocycl. Chem. 1974, 11, 205. 10.1002/jhet.5570110218. [DOI] [Google Scholar]
- Klemm L. H.; Weakley T. J.; Yoon M. X-ray crystallographic and NMR structural studies of trans-2,3-dichloro-5-ethyl-2,3-dihydrothieno[2 3-b]pyridine syn-1-oxide reactions of thiophene rings with hypochlorite reagents. J. Heterocycl. Chem. 1999, 36, 1077. 10.1002/jhet.5570360440. [DOI] [Google Scholar]
- Klemm L. H.; Merrill R. E.; Lee F. H. W. Chemistry of thienopyridines. XIX. Further studies on chlorination and S-oxidation in the thieno[2,3-b]pyridine system. J. Heterocycl. Chem. 1974, 11, 535. 10.1002/jhet.5570110417. [DOI] [Google Scholar]
- Klemm L. H.; Merrill R. E. Chemistry of thienopyridines. XIII. Selective formation of sulfones in bi- and tricyclic systems. Thieno[2,3-b]pyridine 1,1-dioxide as a dienophile. J. Heterocycl. Chem. 1972, 9, 293. 10.1002/jhet.5570090217. [DOI] [Google Scholar]
- Bamberger E.; Elger F. Weitere Beiträge zur Kenntniss der Anthranile. Ber. Dtsch. Chem. Ges. 1903, 36, 3645. 10.1002/cber.190303603164. [DOI] [Google Scholar]
- Chiarini M.; Del Vecchio L.; Marinelli F.; Rossi L.; Arcadi A. Synthesis of 3-substituted 2,1-benzisoxazoles by the oxidative cyclization of 2-aminoacylbenzenes with Oxone. Synthesis 2016, 48, 3017. 10.1055/s-0035-1561471. [DOI] [Google Scholar]
- Velezheva V. S.; Marshakov V. Y.; Mel’man A. I.; Kurkovskaya L. N.; Suvorov N. N. Transformation of 2-arylmethylene-3-inolinones to 4-(2-acetylaminobenzoyl)-5-aryl-1,2,3-triazoles. J. Org. Chem. USSR 1988, 24, 1379. [Google Scholar]
- Prakash O.; Saini R. K.; Singh S. P.; Varma R. S. Hypervalent iodine oxidation of o-aminochalcones: A novel synthesis of 3-(β-styryl)-2,1-benzisoxazoles. Tetrahedron Lett. 1997, 38, 3147. 10.1016/S0040-4039(97)00564-9. [DOI] [Google Scholar]
- Dyall L. K.; Harvey J. J.; Jarman T. B. Oxidative Cyclizations. VIII. Mechanisms of Oxidation of ortho-Substituted Benzenamines and Improved Cyclizations by Bis(acetato-O)Phenyliodine. Aust. J. Chem. 1992, 45, 371. 10.1071/CH9920371. [DOI] [Google Scholar]
- Dyall L. K. Oxidative cyclizations. VII. Cyclization of 2-substituted anilines with alkaline hypohalite. Aust. J. Chem. 1984, 37, 2013. 10.1071/CH9842013. [DOI] [Google Scholar]
- Tian X.; Song L.; Farshadfar K.; Rudolph M.; Rominger F.; Oeser T.; Ariafard A.; Hashmi A. S. K. Acyl Migration versus Epoxidation in Gold Catalysis: Facile, Switchable, and Atom-Economic Synthesis of Acylindoles and Quinoline Derivatives. Angew. Chem., Int. Ed. 2020, 59, 471. 10.1002/anie.201912334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A.; Wayne Moran H.; Shamblee D. A.; Welstead W. J. Jr.; Nolan J. C.; Sancilio L. F.; Graff G. Antiinflammatory agents. 4. Syntheses and biological evaluation of potential pro-drugs of 2-amino-3-benzoylbenzeneacetic acid and 2-amino-3-(4-chlorobenzoyl)benzeneacetic acid. J. Med. Chem. 1990, 33, 2296. 10.1021/jm00170a039. [DOI] [PubMed] [Google Scholar]
- Correa A.; Tellitu I.; Domínguez E.; SanMartin R. Novel Alternative for the N–N Bond Formation through a PIFA-Mediated Oxidative Cyclization and Its Application to the Synthesis of Indazol-3-ones. J. Org. Chem. 2006, 71, 3501. 10.1021/jo060070+. [DOI] [PubMed] [Google Scholar]
- Correa A.; Tellitu I.; Domínguez E.; SanMartin R. An advantageous synthesis of new indazolone and pyrazolone derivatives. Tetrahedron 2006, 62, 11100. 10.1016/j.tet.2006.09.031. [DOI] [Google Scholar]
- Reddy A. C. S.; Narsaiah B.; Venkataratnam R. V. A Novel method for the synthesis of isoxazolo and pyrazolo pyridines using hypervalent iodine reagent. Synth. Commun. 1997, 27, 2217. 10.1080/00397919708003374. [DOI] [Google Scholar]
- Dai G.; Yang L.; Zhou W. Copper-catalyzed oxidative dehydrogenative N–N bond formation for the synthesis of N,N′-diarylindazol-3-ones. Org. Chem. Front. 2017, 4, 229. 10.1039/C6QO00573J. [DOI] [Google Scholar]
- Liu S.; Xu L.; Wei Y. One-Pot, Multistep Reactions for the Modular Synthesis of N,N′-Diarylindazol-3-ones. J. Org. Chem. 2019, 84, 1596. 10.1021/acs.joc.8b02548. [DOI] [PubMed] [Google Scholar]
- Stroganova T. A.; Vasilin V. K.; Dotsenko V. V.; Aksenov N. A.; Krapivin G. D. Reaction of thieno[2,3-b]pyridines with sodium hypochlorite: An unusual and stereoselective one-pot approach to dimeric pyrrolo[2′,3′:4,5]thieno[2,3-b]pyridines. Tetrahedron Lett. 2019, 60, 997. 10.1016/j.tetlet.2019.03.012. [DOI] [Google Scholar]
- Reuter H. Structural parameters of dimethyl sulfoxide, DMSO, at 100 K, based on a redetermination by use of high-quality single-crystal X-ray data. Acta Crystallogr., Sect. E: Crystallogr. Commun. 2017, 73, 1405. 10.1107/S2056989017012464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamas-Saiz A. L.; Foces-Foces C.; Elguero J. Proton sponges. J. Mol. Struct. 1994, 328, 297. 10.1016/0022-2860(94)08367-3. [DOI] [Google Scholar]
- Pozharskii A. F. Naphthalene ’proton sponges’. Russ. Chem. Rev. 1998, 67, 1. 10.1070/RC1998v067n01ABEH000377. [DOI] [Google Scholar]
- Risley J. M.; Kastanis J. P.; Young A. M. Determination of the second-order 1H NMR parameters for the aromatic protons in 4-fluoroaniline and application to the analysis of the 1H NMR spectra for the aromatic protons in N4-(4′-fluorophenyl)succinamic acid and in N4-(4′-fluorophenyl)-3,3-difluorosuccinamic acid. J. Fluorine Chem. 2011, 132, 269. 10.1016/j.jfluchem.2011.02.003. [DOI] [Google Scholar]
- Budzelaar P. H. M.gNMR for Windows (5.0.6.0). NMR Simulation Program; Cherwell Scientific, 2006.
- Aganov A. V.; Klochkov V. V.; Samitov Y. Y. New Aspects of the Application of Nuclear Magnetic Resonance to the Study of Chemical Exchange Processes. Russ. Chem. Rev. 1985, 54, 931. 10.1070/RC1985v054n10ABEH003123. [DOI] [Google Scholar]
- Vassiliev P. M.; Spasov A. A.; Kosolapov V. A.; Kucheryavenko A. F.; Gurova N. A.; Anisimova V. A.. Consensus Drug Design Using IT Microcosm. In Challenges and Advances in Computational Chemistry and Physics; Gorb L.; Kuz’min V.; Muratov E., Eds.; Springer Science + Business Media: Dordrecht, Netherlands, 2014; Chapter 12, Vol. 17, pp 369–431. [Google Scholar]
- Dolomanov O. V.; Bourhis L. J.; Gildea R. J.; Howard J. A. K.; Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339. 10.1107/S0021889808042726. [DOI] [Google Scholar]
- Sheldrick G. M. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3. 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narushyavichus É. V.; Garalene V. N.; Krauze A. A.; Dubur G. Y. Cardiotropic activity of pyridin-2(1H)-ones. Pharm. Chem. J. 1989, 23, 983. 10.1007/BF00764710. [DOI] [Google Scholar]
- Gallagher T.; Hirschhäuser C.. 1,2-Dihydro-4,6-dimethyl-2-thioxo-3-pyridinecarbonitrile. Encyclopedia of Reagents for Organic Synthesis; John Wiley and Sons, 2001. [Google Scholar]
- Kaigorodova E. A.; Konyushkin L. D.; Niyazymbetov M. E.; Kvak S. N.; Zaplishny V. N.; Litvinov V. P. Electrochemical synthesis and studies of substituted 2-thiopyridines. Russ. Chem. Bull. 1994, 43, 2095. 10.1007/BF00700178. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.