Abstract
Effects of different treatments on the bioavailability of lead (Pb) in soil from a smelter emission contaminated site in Joplin, Missouri, were evaluated in a mouse model. Similar estimates of relative bioavailability for Pb in untreated or treated soil were obtained in mice and in the well-established juvenile swine model. In the mouse model, treatments that used phosphate (phosphoric acid or triple superphosphate) combined with iron oxide or biosolids compost significantly reduced soil Pb bioavailability. Notably, effects of these remediation procedures were persistent, given that up to 16 years had elapsed between soil treatment and sample collection. Remediation of soils was associated with changes in Pb species present in soil. Differences in Pb species in ingested soil and in feces from treated mice indicated that changes in Pb speciation occurred during transit through the gastrointestinal tract. Use of the mouse model facilitates evaluation of remediation procedures and allows monitoring of the performance of procedures under laboratory and field conditions.
Graphical Abstract
INTRODUCTION
A coupled exposure-dose model has recently identified soil and dust as predominant sources of lead (Pb) exposure in 1 to 6-year old children.1 The significant contribution of soil Pb to childhood exposure to this toxic metal reflects persistent elevation of urban soil Pb levels over the past few decades2, a period during which Pb levels in other media (e.g., food, air) have declined.3–5 Because children are vulnerable to myriad adverse effects of Pb, reducing exposure to Pb from soil or dust ingestion is an important public health goal.6 A variety of interventions have been used to minimize exposure of children to Pb, although the effectiveness of some strategies has not been fully demonstrated.7 Historically, exposure of children to Pb has been reduced by removal of Pb-contaminated soil and replacement with uncontaminated soil.8 For example, removal and replacement of Pb-contaminated soil at the Bunker Hill Idaho Superfund site contributed to reduced exposure of children.9,10 Because soil removal and replacement can be expensive and difficult, in situ solidification and stabilization of metals in soil can be used to reduce risks associated with continued presence of contaminants.11 Successful remediation by metal stabilization results in formation of Pb compounds and sorption products in soil that are unlikely to migrate into groundwater or to be bioavailable if ingested.12 For Pb in soil, solidification and stabilization involves addition of phosphorus as phosphate (P) to promote formation of stable and relatively less soluble Pb-P species.13
Here, we examined effects of various treatments on soil Pb bioavailability using soil samples from long-term test plots at a Pb, Zn, and Cd-contaminated Superfund site in Joplin, Missouri. All treatments evaluated involved P application to soil; in some cases, P treatment was combined with addition of iron-rich byproduct or biosolids compost.14 Effects of these treatments on soil Pb bioavailability were evaluated in a mouse model.15 To our knowledge, the current study examines the effect of the longest interval between amendment and RBA evaluation thus far reported. All remediation strategies reduced tissue-specific Pb relative bioavailability (RBA) estimates for soils collected at 3 or 16 years after treatment. Changes in bioavailability were associated with changes in Pb species found in soil. Ultimately, understanding linkages between alterations in Pb speciation in treated soils and changes in Pb bioavailability will benefit public health by improving the effectiveness of soil remediation strategies.
METHODS AND MATERIALS
Origin of test soils –
In 1997, a field trial to evaluate several soil remediation procedures began at a Joplin, Missouri, site contaminated by Pb smelter emissions.14 Additional information on soil treatments, sample collection and processing, and elemental composition of soils is provided in Supplemental Information.
A <250 μm fraction prepared from untreated or treated soil was used to determine the Pb RBA. Soil treatments were 1% P as phosphoric acid (PA), 3.2% P as triple super phosphate (TSP), 2.5% Fe as iron-rich waste material and 1% P as TSP, and 10% biosolids compost and 1% P as TSP. The untreated soil was collected at the time of treatment and the 1% P as PA soil sample was collected about three years after treatment. Remaining soil samples were collected in 2013 about 16 years after treatment. Mouse assays to determine Pb RBA for untreated and treated soils were performed in 2016.
Mouse assay –
Young adult female C57BL/6 mice were exposed to Pb by consumption of powdered AIN-93G rodent diet which was amended with each soil described above or with Pb acetate, a soluble Pb compound.15 Test diets contained about 3 to 25 mg Pb per kg (parts per million, ppm). During the 9-day assay, mice were housed in groups of 3 in metabolic cages (Lab Products, Seaford, DE) with free access to amended diet and drinking water. At termination, mice were euthanized with CO2. A heparinized blood sample was collected by cardiac puncture and kidneys were removed. Following evisceration and skin removal, carcasses were defleshed in dermestid beetle cultures to provide nearly complete skeletons for bone Pb determination. Because the unit of analysis was the metabolic cage, samples from the 3 mice in each cage were pooled by tissue type for determination of Pb concentrations.
Tissue Pb determination –
Pooled kidneys and skeletons from each cage were homogenized using a model 6850 freezer mill (Spex, Metuchen, NJ). Aliquots of tissue homogenates were digested in a closed vessel microwave reaction system (CEM Microwave, Matthews, NC) in ultra-high purity nitric acid (SCP Science, Champlain, NY). Digested samples were diluted with deionized water to 5 to 10% nitric acid concentration before analysis. Samples of test diets were acid digested as described for tissue samples. Pb concentrations in tissue and diet samples were determined by inductively-coupled plasma-mass spectrometry using a X-Series II ICP/MS (SCP Science). Pb levels in pooled blood samples from each mouse in a metabolic cage were determined by anodic stripping voltammetry (LeadCare Ultra, Magellan Diagnostics, North Billerica, MA). Quality control samples run with each digestion batch included reagent blanks, blank spikes, matrix spikes, and NIST SRM 955c (caprine blood) (Table S1), 1577c (bovine liver), 1486 (bone meal), and 2710A (Montana I soil).
Pb speciation –
Speciation of Pb in soil and feces was identified from X-ray absorption spectra collected at the DuPont-Northwestern-Dow Collaborative Access Team Sector 5, beam line 5BM-D, at the Advanced Photon Source of the Argonne National Laboratory in Lemont, IL. Analytical details are provided in Supplemental Information.
Data analysis –
All statistical analyses were performed using SAS/STAT software, Version 9.3 of the SAS System for Windows SAS software. RBA was estimated as the ratio of linear regression slopes (m) for relationships between cumulative Pb dose (mg) and tissue Pb level (mg/kg or mg/L)
where mtest and mref are linear slopes for the test soil and reference groups, respectively.16 Effects of soil treatments on RBA were evaluated using two approaches that used different data to calculate mtest and mref. The first approach calculated mref using data for mice fed diets amended with Pb acetate (mPbAc). For the second approach, mref and mtest were calculated from data for mice fed diets amended with untreated (muntreated) or treated soil (mtreated), respectively. Rationales for the two approaches are presented in the Results and Discussion section. For either approach, regression slopes were estimated by simultaneously fitting test and reference group tissue data (blood, bone, kidney) to linear regression models sharing common intercepts using SAS PROC REG.17 Confidence intervals for RBAs and heteroscedastic-consistent covariances for parameter estimates were obtained as previously described.16,18 Studentized residuals (>3 or <−3) were used to identify statistical outliers, which were excluded from regression models.
RESULTS AND DISCUSSION
Comparison of RBA estimates from mouse and juvenile swine models –
The juvenile swine model has been widely used to estimate RBA of Pb in soil.16 Similar estimates of tissue-specific Pb RBA for NIST SRM 2710A in juvenile swine and mouse indicated that the mouse model was a useful alternative to the swine model.15 This conclusion was confirmed by an independent comparison of soil Pb RBA estimates from juvenile swine and mouse.19 In the work reported here, we compared tissue-specific RBA estimates from mouse and juvenile swine using an untreated and a treated soil sample. Tissue-specific RBA estimates for untreated soil were relatively high (> 60%) in both animal models (Supporting Information, Figure S1). A comparison of RBA estimates for PA-treated soil in juvenile swine and mouse were also similar; although, based on 90% confidence limits, mouse RBAs based on the bone metric tended to be higher (p<0.1) than corresponding RBA estimates from swine (Supporting Information, Figure S2).
Effect of remediation on soil Pb RBA –
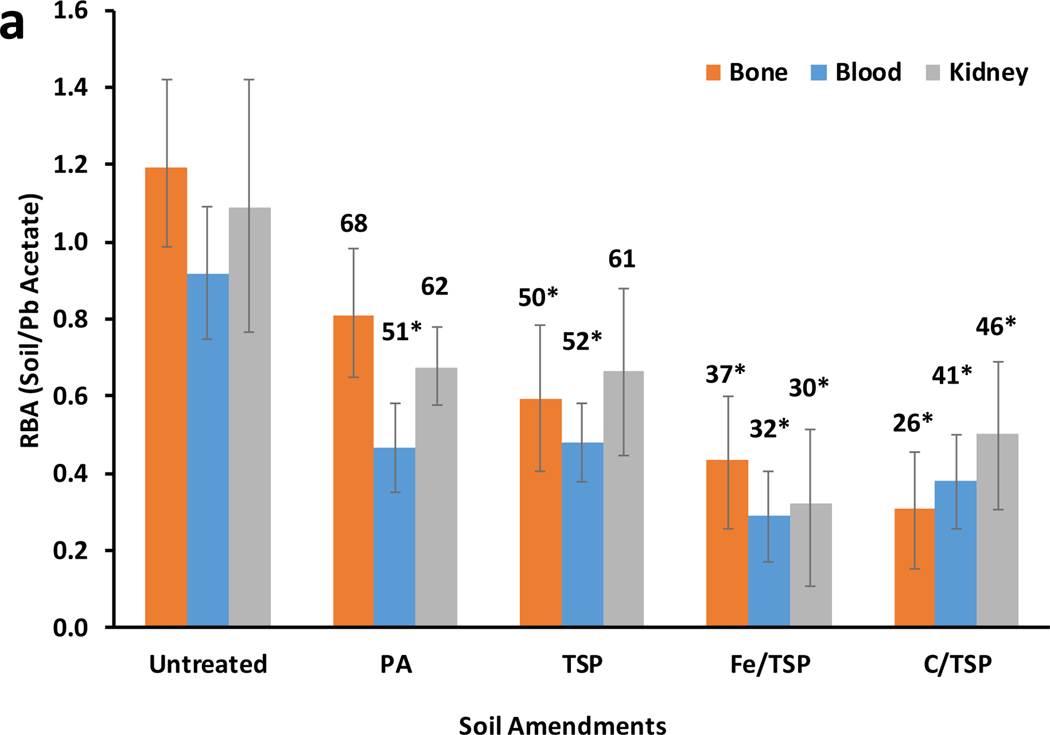
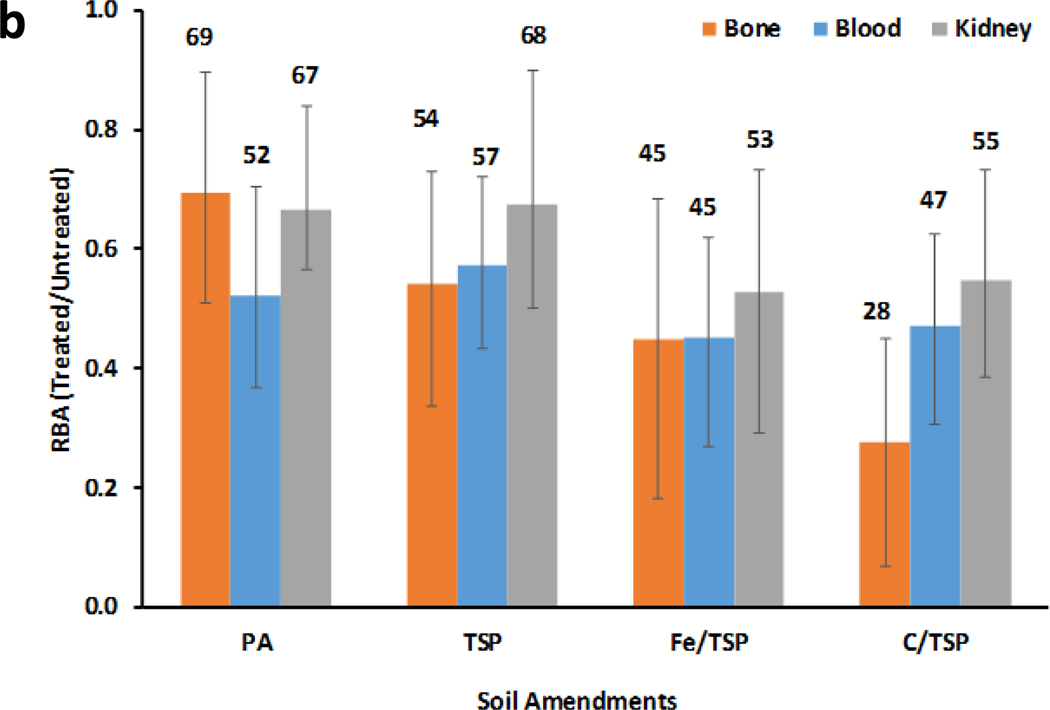
RBAs for Pb in untreated and treated soils shown in Figure 1 were calculated in two different ways. For results in Figure 1a, RBAs were calculated as the ratio of slopes of the dose-tissue regression models for soil and Pb acetate (RBA = msoil/mPbAc). This approach has been commonly used to calculate RBA.15,16 Figure 1a shows the treatment effect ratio (TER), the ratio of mean RBAs (100*RBAtreated/RBAuntreated) for each tissue.13 TERs for treated soils for which 95% confidence limits on the mean RBA did not overlap with the mean for untreated soil were considered significantly different (p<0.05) from control RBA. In Figure 1b, RBAs were estimated from dose-tissue regression slopes for treated and untreated soils (RBA = mtreated/muntreated). This approach directly compared regression slopes for treated and untreated soils and eliminated uncertainty in the estimate of the slope derived from tissue data from Pb acetate-treated mice. Mean tissue-specific RBAs for treated soils for which the 95% confidence limits excluded 1 were considered significantly different (p<0.05) from the corresponding tissue-specific RBA for untreated soil.
Figure 1.
Tissue-specific Pb RBAs for untreated or treated soils in the mouse. Treatments are phosphoric acid (PA), triple super phosphate (TSP) alone or combined with iron oxide (Fe) or biosolids compost (C). Error bar show 95% confidence limits for mean values. Each soil was assayed in 27 mice; Pb acetate was assayed in 135 mice.
a). RBAs for untreated or treated soils expressed relative to bioavailability of Pb acetate. (RBA= slopesoil/slopePb acetate). Number above bars are tissue-specific TERs. As defined, there is no TER for the untreated control. Asterisks denote TERs for treated soils for which 95% confidence limits on the mean RBA do not overlap with untreated soil.
b). RBAs for treated soils expressed relative to untreated soil (RBA= slopetreated/slopeuntreated). Number above bars are tissue-specific RBAs.
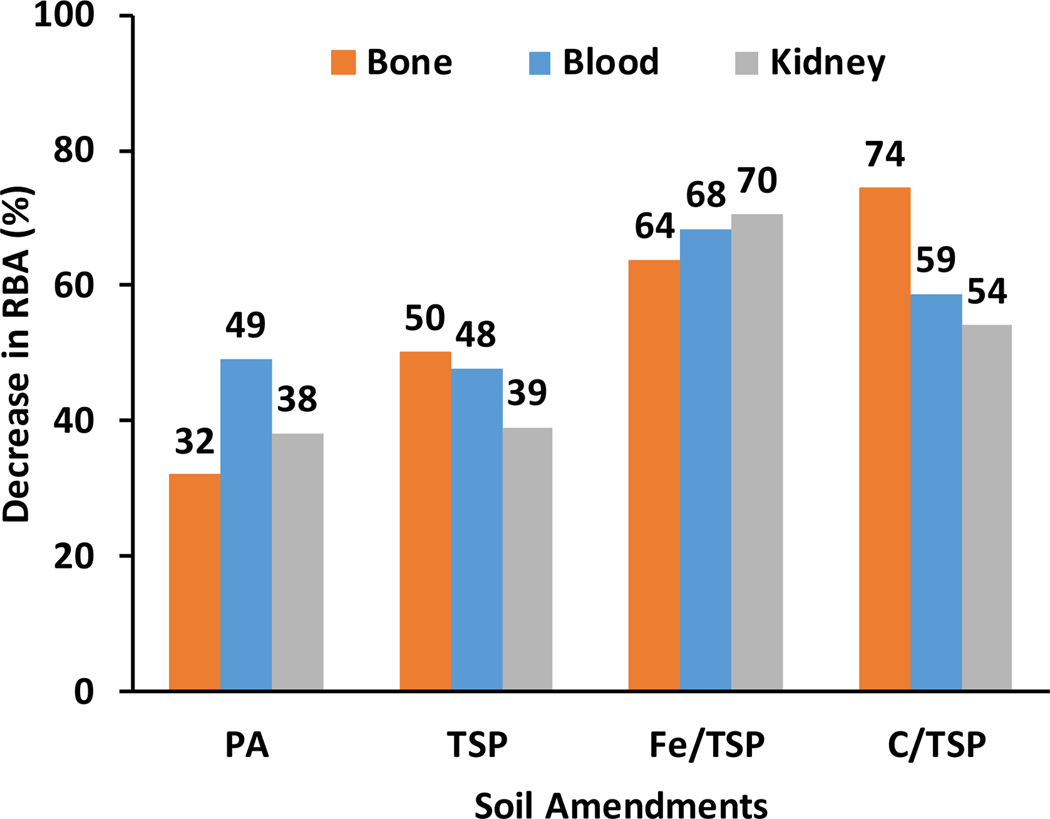
Based on the common approach (RBA = msoil/mPbAc), all soil treatments had tissue-specific effects on RBA estimates (Figure 1a). The decrease in RBA (1-TER) ranged from 32% to 74% for bone, 49% to 68% for blood, and 38% to 70% for kidney (Figure 2). For all treated soils, RBAs estimated from blood data were significantly lower than those estimated for untreated soil. RBAs estimated from bone data for TSP, Fe/TSP and C/TSP treatments, but not PA treatment, were significantly different from untreated soil. RBAs estimated from kidney data were not significantly different for untreated, PA-, or TSP-treated soil. However, both Fe/TSP and C/TSP treatments significantly reduced RBA estimates based on kidney data. TERs ranged from 0.26 to 0.69, with the lowest TERs observed for Fe/TSP- and C/TSP-treated soils. Adsorption of Pb to or its incorporation into Fe (hydr)oxides present in Fe/TSP-treated soil could account for the reduced Pb RBA.20,21 Reduced Pb RBA for Fe/TSP-treated soil could also reflect competition between soil-derived Fe and Pb for divalent metal transporter 1-mediated transfer across the gastrointestinal barrier.22–24 Reduced Pb RBA in C/TSP-treated soil was consistent with findings that soil amendment with biosolids compost reduced Pb bioavailability and bioaccessibility.25,26 Notably, mean RBAs of untreated soil based on bone tended to be greater than 1; however, based on the 95% confidence limits, the means were not significantly different from 1. Estimates of RBA >1 suggest that the soil contained for a Pb species that was more bioavailable than Pb+2+ produced from dissociation of lead acetate, the soluble reference Pb compound. RBAs>1 have been reported from swine studies of soils enriched with highly soluble lead carbonate41. However, as noted below, there was no evidence of lead carbonate or other highly soluble forms of Pb in the diets that were amended with soil and fed to mice.
Figure 2.
Decrease in Pb RBA (%) for treated soils evaluated in mouse model tissues. Numbers are % decrease (1-TER for RBA = msoil/mPbAc).
As shown in Figure 1b, direct comparison of dose-tissue slopes for treated and untreated soils (RBA = mtreated/muntreated) indicated that application of each soil treatment significantly reduced bioavailability relative to the control soil. The decrease in RBA (1-TER) ranged from 31% to 72% for bone, 43% to 55% for blood, and 32% to 47% for kidney.
The present study provides unique data on long-term effects of remediation on Pb RBA, showing that soil treatments produced changes in bioavailability that persisted for years after application. Earlier work found that PA treatment of this soil reduced Pb RBA at 78 months after application.14,27 Comparison of Pb RBA estimates for TSP-, C/TSP-, or Fe/TSP-treated soil with that for untreated soil showed that effects of each remediation procedure persisted for 16 years under field conditions. Because the mouse RBA estimates were obtained using soils collected approximately 16 years following treatment, these results provide no information about temporal changes in RBAs during the interval between treatment and our study. Based on overlapping 95% confidence limits of tissue-specific RBAs for all treatments, the magnitude of effect of different treatments on tissue-specific RBA estimates were indistinguishable. However, for some tissues, there appeared to be a treatment-related trend in TER. For example, for bone Pb, the rank order of TERs was PA > TSP > Fe/TSP > C/TSP. Although this study did not examine the value of the different tissue-specific RBA estimates in prediction of the efficacy of treatment, a toxicokinetic argument can be made for bone as a useful metric of cumulative Pb absorption. In the mouse model, skeletal Pb accounted for about 90% of the retained Pb dose which was less than 3% of the ingested Pb dose. Because higher Pb concentrations were attained in bone than in other tissues, determination of bone Pb levels provided a more precise estimate of Pb absorption. Furthermore, the role of bone as a site for long-term retention of Pb28–30 and as a target for chronic Pb toxicity31 underscored the importance of bone Pb as a metric of cumulative absorbed Pb dose.
Pb speciation and bioavailability.
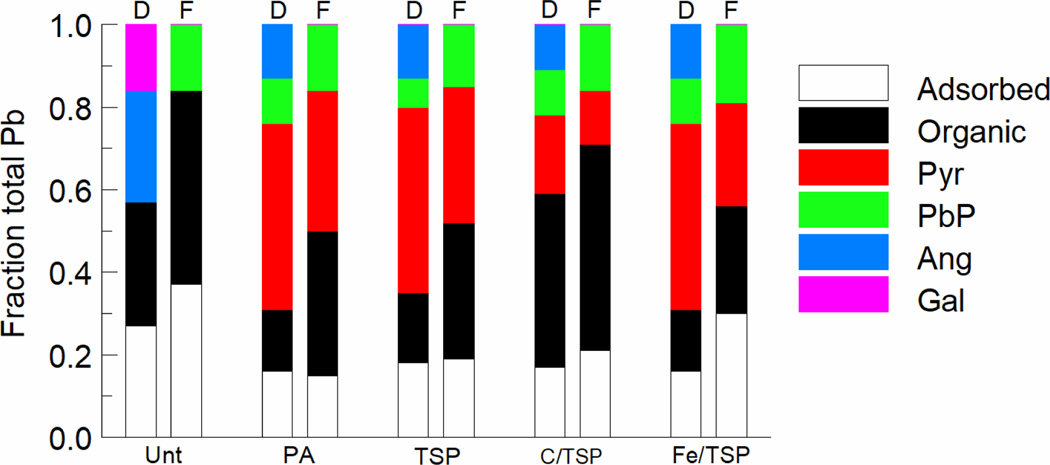
Linear combination of spectra from diets and feces provided information on Pb speciation (Figures S3 and S4). In addition, changes in the elemental composition of untreated and treated soils reflected persistent changes in soil P levels consistent with remediation strategies focused on formation of relatively insoluble Pb-P complexes (Supporting Information Figure S5). Pb speciation profiles in diets consumed by mice and in feces produced by mice during feeding of these diets were examined to provide insights into the relation between Pb speciation and gastrointestinal bioavailability. Figure 3 shows the fractional composition of Pb species consumed in diet and excreted in feces.
Figure 3.
Pb speciation in diet consumed and feces excreted by mice ingesting soil-amended diets. Relative amounts of each Pb species (fraction of total Pb) present in diet (D) and in feces (F) during the standard assay period were calculated using Pb speciation data derived from spectral analysis of diet and feces. Treatments as identified in Figure 1. PbP is trilead diphosphate (Pb3(PO4)2), Ang is anglesite (PbSO4), Gal is galena (PbS), Pyr is pyromorphite. Linear combination fitting spectra are shown in Fig. S3, and Pb standards utilized are shown in Fig. S4.
Determination of Pb species in diets containing untreated or treated soil provided information on effects of treatments and of incorporation of soil into diet on Pb speciation. Compared to diet prepared with untreated soil, diets prepared with treated soils were distinguished by the absence of galena (Gal), lower levels of anglesite (Ang) and adsorbed Pb, and the presence of pyromorphite (Pyr) and trilead diphosphate (PbP). The presence of Pyr and PbP and reduced levels of Gal and Ang in all diets prepared with treated soils suggested that extensive chemical transformation was associated with treatment of soil and incorporation of soil into diet. Earlier work found that treatment of soils with P in various chemical forms led to Pyr formation.13,32–35 The fraction of Pb bound to organic molecules was lower in diets prepared with PA-, TSP-, or Fe/TSP-treated soils and higher in diet prepared with C/TSP-treated soil than in diets prepared from untreated soil.
Comparison of Pb species in diets and feces provided information about transformation and fate of Pb species during gastrointestinal tract transit. Gal and Ang, which accounted for about 40% of Pb in diet prepared with untreated soil, were absent from feces. Ang, a component of all diets prepared from treated soils, was also absent from feces. Thus, in the mouse, Gal and Ang were highly bioavailable or were transformed to other Pb species in the gastrointestinal tract. By comparison, estimated Pb RBAs from juvenile swine for Gal-enriched soils (RBA = 1%) or Ang-rich soils (RBA=36%) were relatively low.16 Different estimates of Pb RBA for Gal- or Ang-enriched soils obtained in mouse and juvenile swine could reflect uncharacterized differences in the physical and chemical properties of soils used in different assays, different ratios of Pb species present in test soils, or species-specific differences in gastrointestinal transformation and uptake of these Pb compounds. Adsorbed Pb fractions in diet and feces were similar in mice that ingested diet with PA-, TSP, or C/TSP-treated soil. In mice ingesting diet amended with untreated or Fe/TSP-treated soil, the adsorbed Pb fractions in feces were higher than in diet. The fraction of organically bound Pb was higher in feces than in diet for mice that ingested any of the soil-amended diets. All diets prepared with treated soils contained Pyr and PbP. Notably, in feces from mice ingesting a diet prepared with treated soil, the fraction of Pyr was lower and the fraction of PbP was higher than in the corresponding diet. Different fractional Pyr levels in diet and feces could reflect abiotic or biotic transformation of Pyr in the gastrointestinal tract. Bacteria of genus Pseudomonas can dissolve Pyr to provide soluble P.36,37 Similar reactions meditated by gastrointestinal tract microbiota could change the chemical state of ingested Pb within the lumen of the gastrointestinal tract and affect its bioavailability. Other work has shown that interactions that occur between P and Pb in the gastrointestinal tract can directly alter the bioavailability of Pb.38. Notably, if the gastrointestinal microbiota play an important in the transformation of Pb species, it will be important to evaluate both similarities and differences in the structure and function of mouse and human gastrointestinal systems of mice and human as factors in transformation,39 Understanding transformation of Pb species that occur during gastrointestinal transit could provide useful information for development of new soil remediation strategies.
The current study shows that treatment of soils with P alone or in combination with other agents resulted in significant and long-lasting reductions in Pb RBAs. Because this study was focused on a few time points long removed from the time of soil treatment, it does not provide information on temporal changes in soil Pb speciation or in Pb bioavailability. Additional studies involving repeated sampling of remediated sites will be needed to characterize the time dependence of such changes. Differences in inorganic and organic components of soils that affect formation of insoluble Pb-P species and the efficacy of soil remediation procedures also require additional study.40,41 Determination of soil Pb RBA in the mouse gives a direct measure of the efficacy of treatment that can be coupled with determination of Pb speciation in treated soil to provide insights into the design of more effective soil-specific remediation processes.
Supplementary Material
ACKNOWLEDGEMENTS
RLC collected and prepared soils used in this study with funding provided by SERDP ER-1742 Project “Mechanisms and Permanence of Sequestered Lead and Arsenic in Soils: Impact on Human Bioavailability” Pb speciation analysis was performed at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) located at Sector 5 of the Advanced Photon Source (APS). DND-CAT is supported by Northwestern University, E.I. DuPont de Nemours & Co., and The Dow Chemical Company. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357. Portions of this work were funded by U.S. Environmental Protection Agency Office of Superfund Remediation and Technology Innovation (OSRTI) under contract EP-W-09–031. This document has been subjected to review by the National Exposure Research Laboratory (NERL) and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Abbreviations:
- RBA
relative bioavailability
- Pb
lead
- P
phosphate
- Ppm
parts per million
- Pyr
pyromorphite
- PbP
trilead diphosphate
- Gal
galena
- Ang
anglesite
Footnotes
SUPPORTING INFORMATION. Origin, collection and processing of soil samples, Lead Speciation, Comparison of soil Pb RBA estimates in mouse and juvenile swine, Figure S1: Pb RBA estimates for untreated soil in mouse and juvenile swine models, Figure S2: Pb RBA estimates for PA-treated soil in mouse and juvenile swine models
REFERENCES
- (1).Zartarian V; Xue J; Tornero-Velez R; Brown J. Children’s Lead Exposure: A multimedia modeling analysis to guide public health decision-making. Environ. Health Perspect 2017, 125 (9), 097009. DOI 10.1289/EHP1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Datko-Williams L; Wilkie A; Richmond-Bryant J. Analysis of U.S. soil lead (Pb) studies from 1970 to 2012. Sci. Total Environ 2014, 468–469, 854–863; DOI 10.1016/j.scitotenv.2013.08.089. [DOI] [PubMed] [Google Scholar]
- (3).Bolger PM; Yess NJ; Gunderson EL; Troxell TC; Carrington CD Identification and reduction of sources of dietary lead in the United States. Food. Addit. Contam 1996, 13 (1), 53–60. [DOI] [PubMed] [Google Scholar]
- (4).EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on lead in food. EFSA Journal 2010, 8 (4), 157; DOI: 10.2903/j.efsa.2010.1570. [DOI] [Google Scholar]
- (5).Richmond-Bryant J; Meng Q; Davis A; Cohen J; Lu SE; Svendsgaard D; Brown JS; Tuttle L; Hubbard H; Rice J; Kirrane E; Vinikoor-Imler LC; Kotchmar D; Hines EP; Ross M. The influence of declining air lead levels on blood lead-air lead slope factors in children. Environ. Health Perspect 2014, 122 (7), 754–760; DOI 10.1289/ehp.1307072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bellinger DC The protean toxicities of lead: new chapters in a familiar story. Int. J. Environ. Res. Public Health 2011, 8 (7), 2593–2628; DOI 10.3390/ijerph8072593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Nussbaumer-Streit B; Yeoh B; Griebler U; Pfadenhauer LM; Busert LK; Lhachimi SK; Lohner S; Gartlehner G. Household interventions for preventing domestic lead exposure in children. Cochrane Database Syst Rev. 2016, 10, CD006047; DOI 10.1002/14651858.CD006047.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kumpiene J; Lagerkvist A; Maurice C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments-a review. Waste Manag. 2008, 28 (1), 215–225; DOI 10.1016/j.wasman.2006.12.012. [DOI] [PubMed] [Google Scholar]
- (9).Sheldrake S; Stifelman M. A case study of lead contamination cleanup effectiveness at Bunker Hill. Sci. Total Environ 2003, 303 (1–2), 105–123. [DOI] [PubMed] [Google Scholar]
- (10).von Lindern I; Spalinger S; Petroysan V; von Braun M. Assessing remedial effectiveness through the blood lead:soil/dust lead relationship at the Bunker Hill Superfund Site in the Silver Valley of Idaho. Sci. Total Environ 2003, 303 (1–2), 139–170. [DOI] [PubMed] [Google Scholar]
- (11).National Research Council. Innovations in ground water and soil cleanup: From concept to commercialization. pp. 80–166, The National Academies Press: Washington, D.C, 1997. [Google Scholar]
- (12).Bolan N; Kunhikrishnan A; Thangarajan R; Kumpiene J; Park J; Makino T; Kirkham MB; Scheckel K. Remediation of heavy metal(loid)s contaminated soils--to mobilize or to immobilize? J. Hazard. Mater 2014, 266, 141–166; DOI 10.1016/j.jhazmat.2013.12.018. [DOI] [PubMed] [Google Scholar]
- (13).Scheckel KG; Diamond GL; Burgess MF; Klotzbach JM; Maddaloni M; Miller BW; Partridge CR; Serda SM Amending soils with phosphate as means to mitigate soil lead hazard: a critical review of the state of the science. J. Toxicol. Environ. Health B Crit. Rev 2013, 16 (6), 337–380; DOI 10.1080/10937404.2013.825216. [DOI] [PubMed] [Google Scholar]
- (14).Ryan JA; Scheckel KG; Berti WR; Brown SL; Casteel SW; Chaney RL; Hallfrisch J; Doolan M; Grevatt P; Maddaloni M; Mosby D. Reducing children’s risk from lead in soil. Environ. Sci. Technol 2004, 38 (1), 18A–24A. [DOI] [PubMed] [Google Scholar]
- (15).Bradham KD; Green W; Hayes H; Nelson C; Alava P; Misenheimer J; Diamond GL; Thayer WC; Thomas DJ Estimating relative bioavailability of soil lead in the mouse. J. Toxicol. Environ. Health. A 2016, 79 (24), 1179–1182; DOI: 10.1080/15287394.2016.1221789 [DOI] [PubMed] [Google Scholar]
- (16).Casteel SW; Weis CP; Henningsen GM; Brattin WJ Estimation of relative bioavailability of lead in soil and soil-like materials using young swine. Environ. Health Perspect 2006, 114 (8), 1162–1171; DOI 10.1289/ehp.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Finney DJ Statistical methods in biological assay 3rd ed.; Charles Griffin & Co.: London, U.K., 1978. [Google Scholar]
- (18).Long JS; Ervin LH Using heteroscedasticity consistent standard errors in the linear regression model. Am. Stat 2000, 54 (3), 217–224; DOI 10.1080/00031305.2000.10474549 [DOI] [Google Scholar]
- (19).Li SW; Sun HJ; Wang G; Cui XY; Juhasz AL; Li HB; Ma LQ Lead relative bioavailability in soils based on different endpoints of a mouse model. J. Hazard. Mater 2017, 326, 94–100; DOI 10.1016/j.jhazmat.2016.12.023. [DOI] [PubMed] [Google Scholar]
- (20).Shi Z; Allen HE; Di Toro DM; Lee S-Z; Harsh JB Predicting PbII adsorption on soils: the roles of soil organic matter, cation competition and iron (hydr)oxides. Environ. Chem 2013, 10 (6), 465–474; DOI 10.1071/EN13153 [DOI] [Google Scholar]
- (21).Zeng G; Wan J; Huang D; Hu L; Huang C; Cheng M; Xue W; Gong X; Wang R; Jiang D. Precipitation, adsorption and rhizosphere effect: The mechanisms for phosphate-induced Pb immobilization in soils-A review. J. Hazard. Mater 2017, 339, 354–367; DOI 10.1016/j.jhazmat.2017.05.038. [DOI] [PubMed] [Google Scholar]
- (22).Bannon DI; Abounader R; Lees PS; Bressler JP Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am. J. Physiol. Cell. Physiol 2003, 284 (1), C44–50; DOI 10.1152/ajpcell.00184.2002 [DOI] [PubMed] [Google Scholar]
- (23).Bressler JP; Olivi L; Cheong JH; Kim Y; Bannon D. Divalent metal transporter 1 in lead and cadmium transport. Ann. N.Y. Acad. Sci 2004, 1012, 142–152. [DOI] [PubMed] [Google Scholar]
- (24).Elsenhans B; Janser H; Windisch W; Schümann K. Does lead use the intestinal absorptive pathways of iron? Impact of iron status on murine 21°Pb and 59Fe absorption in duodenum and ileum in vivo. Toxicology. 2011, 284 (1–3), 7–11; DOI 10.1016/j.tox.2011.03.005. [DOI] [PubMed] [Google Scholar]
- (25).Brown S; Chaney RL; Hallfrisch JG; Xue Q. Effect of biosolids processing on lead bioavailability in an urban soil. J. Environ. Qual 2003, 32 (1),100–108. [DOI] [PubMed] [Google Scholar]
- (26).Farfel MR; Orlova AO; Chaney RL; Lees PS; Rohde C; Ashley PJ Biosolids compost amendment for reducing soil lead hazards: a pilot study of Orgro amendment and grass seeding in urban yards. Sci. Total Environ 2005, 340 (1–3), 40:81–95; DOI 10.1016/j.scitotenv.2004.08.018. [DOI] [PubMed] [Google Scholar]
- (27).U.S. Environmental Protection Agency. 2004. Mine waste technology program, phosphate stabilization of heavy metals: Contaminated mine waste yard soils, Joplin, Missouri, NPL site. Cincinnati, OH: National Risk Management Research Laboratory, U.S. Environmental Protection Agency. EPA/600/R-04/090 (https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryId=87290). [Google Scholar]
- (28).Rabinowitz MB Toxicokinetics of bone lead. Environ. Health Perspect 1991, 91, 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Smith DR; Osterloh JD; Niemeyer S; Flegal AR Stable isotope labeling of lead compartments in rats with ultralow lead concentrations. Environ. Res 1992, 57 (2), 190–207. [DOI] [PubMed] [Google Scholar]
- (30).Lever SZ; Scheffel U. Regional distribution of 203PbCl2 in the mouse after intravenous injection. Neurotoxicology. 1998, 19 (2), 197–207. [PubMed] [Google Scholar]
- (31).Beier EE; Holz JD; Sheu TJ; Puzas JE Elevated lifetime lead exposure impedes osteoclast activity and produces an increase in bone mass in adolescent mice. Toxicol. Sci 2016, 49 (2), 277–288; DOI 10.1093/toxsci/kfv234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hettiarachchi GM; Pierzynski GM; Ransom MD In situ stabilization of soil lead using phosphorus. J. Environ. Qual 2001, 30 (4), 1214–21. [DOI] [PubMed] [Google Scholar]
- (33).Yang J; Mosby DE; Casteel SW; Blanchar RW Lead immobilization using phosphoric acid in a smelter-contaminated urban soil. Environ Sci Technol. 2001, 35 (17). 3553–3559. [DOI] [PubMed] [Google Scholar]
- (34).Eusden JD Jr; Gallagher L; Eighmy TT; Crannell BS; Krzanowski JR; Butler LG; Cartledge FK; Emery EF; Shaw EL; Francis CA Petrographic and spectroscopic characterization of phosphate-stabilized mine tailings from Leadville, Colorado. Waste Manag. 2002, 22 (2), 117–135. [DOI] [PubMed] [Google Scholar]
- (35).Baker LR; Pierzynski GM; Hettiarachchi GM; Scheckel KG; Newville M. Micro-x-ray fluorescence, micro-x-ray absorption spectroscopy, and micro-x-ray diffraction investigation of lead speciation after the addition of different phosphorus amendments to a smelter-contaminated soil. J. Environ. Qual 2014, 43 (2), 488–97. DOI 10.2134/jeq2013.07.0281. [DOI] [PubMed] [Google Scholar]
- (36).Topolska J; Latowski D; Kaschabek S; Manecki M; Merkel BJ; Rakovan J. Pb remobilization by bacterially mediated dissolution of pyromorphite Pb5(PO4)3Cl in presence of phosphate-solubilizing Pseudomonas putida. Environ. Sci. Pollut Res. Int 2014, 21 (2), 1079–1089; DOI 10.1007/s11356-013-1968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Drewniak L; Skłodowska A; Manecki M; Bajda T. Solubilization of Pb-bearing apatite by bacteria isolated from polluted environment. Chemosphere. 2017, 171, 302–307; DOI 10.1016/j.chemosphere.2016.12.056. [DOI] [PubMed] [Google Scholar]
- (38).Juhasz AL; Gancarz D; Herde C; McClure S; Scheckel KG; Smith E. In situ formation of pyromorphite is not required for the reduction of in vivo Pb relative bioavailability in contaminated soils. Environ. Sci. Technol 2014, 48(12), 7002–7009; DOI 10.1021/es500994u. [DOI] [PubMed] [Google Scholar]
- (39).Nguyen TL; Vieira-Silva S; Liston A; Raes J. How informative is the mouse for human gut microbiota research? Dis. Model Mech 2015, 8, 1–16. DOI 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Karna RR; Noerpel MR; Luxton TP; Scheckel KG Point of zero charge: Role of pyromorphite formation and bioaccessibility of lead and arsenic in phosphate-amended soils. Soil Syst. 2018, 2, 22; DOI 10.3390/2020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Landrot G, Khaokaew S. Lead speciation and association with organic matter in various particle-size fractions of contaminated soils. Environ. Sci. Technol 2018, 52(12),6780–6788. DOI 10.1021/acs.est.8b00004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.