Abstract
Native myocardium has limited regenerative potential post injury. Advances in lineage reprogramming have provided promising cellular sources for regenerative medicine in addition to research applications. Recently we have shown that adult mouse fibroblasts can be reprogrammed to expandable, multipotent, induced cardiac progenitor cells (iCPCs) by employing forced expression of five cardiac factors along with activation of canonical Wnt and JAK/STAT signaling. Here we aim to further characterize iCPCs by highlighting their safety, ease of attainability, and functionality within a three-dimensional cardiac extracellular matrix scaffold. Specifically, iCPCs did not form teratomas in contrast to embryonic stem cells when injected into immunodeficient mice. iCPC reprogramming was achieved in wild type mouse fibroblasts without requiring a cardiac-specific reporter, solely utilizing morphological changes to identify, clonally isolate, and expand iCPCs, thus increasing the versatility of this technology. iCPCs also show the ability to repopulate decellularized native heart scaffolds and differentiated into organized structures containing cardiomyocytes, smooth muscle, and endothelial cells. Optical mapping of recellularized scaffolds shows field-stimulated calcium transients that propagate across islands of reconstituted tissue and bipolar local stimulation demonstrates cell-cell coupling within scaffolds. Overall, iCPCs provide a readily attainable, scalable, safe, and functional cell source for a variety of application including drug discovery, disease modeling, and regenerative therapy.
Keywords: heart, reprogramming, cardiac progenitor cells, extracellular matrix, calcium transients
1. Introduction
Transdifferentiation or lineage reprogramming provides a powerful new avenue to generate desired cell types for research applications as well as for regenerative medicine applications. For the cardiovascular system, induced cardiomyocytes (iCMs) can be generated from starting mouse and human fibroblast populations using a combination of reprogramming transcription factors, Gata4, Mef2c, and Tbx5 [1–3]. Alternatively, the addition of Hand2 to Gata4, Mef2c and Tbx5 was found to improve the efficiency of generating iCMs [4]. Other approaches to produce iCMs from fibroblasts have also emerged including forced expression of key miRNAs or completely small molecule based reprogramming [5, 6]. Studies attempting to reprogram human fibroblasts to cardiac progenitor cells (CPCs) have identified a transient progenitor state but have not yet produce a stable, proliferative CPCs [7, 8]. More recently, we and others have demonstrated the ability to reprogram mouse fibroblasts to induced cardiac progenitor cells (iCPCs). However, the methods to generate iCPCs have varied. In our prior study, forced expression of a combination of 5 cardiac factors (Mesp1, Tbx5, Gata4, Nkx2.5 and Baf60c; referred to as MTGNB) was sufficient to reprogram mouse fibroblasts from a variety of tissue sources to iCPCs in the presence of activators or Wnt and Jak/STAT signaling [9]. An alternative approach used transient expression of Yamanaka factors in mouse secondary fibroblasts to create epigenetically unstable cells that can be directed by an appropriate combination of signaling molecules to become induced and expandable cardiac progenitor cells [10]. The iCPCs derived from both of these methods share key properties in that they are highly expandable and are lineage restricted in their potency to give rise to key cardiac lineages including cardiomyocytes, smooth muscle cells and endothelial cells. Furthermore, in a mouse myocardial infarction model, iCPCs improved outcomes [9, 10]. Thus, iCPCs are a promising cell source for cardioregenerative medicine applications providing a proliferative cell that may be able to accomplish more complete cardiac repair than delivery of terminally differentiated cardiac lineages. In addition, the iCPCs can provide another tool for cardiovascular basic research, drug development, and disease modeling.
The initial descriptions of iCPCs also raised important questions for future studies. In analogy to the generation of induced pluripotent stem cells (iPSCs), can clonally derived cell lines be formed rather than generating polyclonal populations of iCPCs? Such clonally derived lines may reduce heterogeneity and better define the potency of the iCPCs as both bipotent and tripotent iCPCs were identified by colony forming assays [9]. Lineage-restricted iCPCs are predicted to exhibit less tumorigenicity than pluripotent stem cells (PSCs) which exhibit a clear ability to generate teratomas, but this prediction has not been formally tested. Initial generation of iCPCs required the use of a cardiac reporter mouse line to identify reprogrammed cells, but to make this technology more broadly applicable, reprogramming of wild type fibroblast populations is desirable. Although the iCPCs are capable of in vitro differentiation, the process is inefficient requiring weeks of culture. The differentiated cardiomyocytes are immature and do not spontaneously contract, unless co-cultured with other contracting cardiomyocytes. Thus, approaches to improve the differentiation of iCPCs are needed.
Approaches to differentiate iCPCs as well as PSCs have focused primarily on soluble small molecules including key growth factors integral to cardiac development; however, less attention has focused on the role of extracellular matrix (ECM) in promoting efficient differentiation of cardiac lineages. During embryonic cardiac development, the ECM plays a variety of roles including providing direct signaling to the associated cells, contributing to structural organization, imposing relevant mechanical properties, and providing a reservoir of key signaling molecules [11]. Additional evidence for the ability of the native ECM to promote differentiation during reprogramming is evident from the more efficient reprogramming of pancreatic acinar cells to islet-like cells in vivo [12] or the more efficient generation of iCMs from cardiac fibroblasts in vivo [4, 13]. The cardiac ECM is complex and is composed of multiple proteins including collagens, fibronectins, lamins, elastin, thrombospondin and others as well as other key constituents including a variety of glycosaminoglycans such as hyaluronic acid. It is estimated that more than 100 distinct protein isoforms are present in the native cardiac ECM; therefore, it is difficult to reconstitute this complex microenvironment during monolayer or by aggregate-based in vitro differentiation methods. Most success in generating a robust preparation of cardiac ECM has come from efforts at decellularization of native cardiac tissue [14]. Perfusion-based decellularization of intact hearts has provided a suitable scaffold for repopulation with relevant cardiac lineage cells to recreate functional cardiac tissue. Most efforts at repopulating cardiac scaffolds have focused on delivering terminally differentiated cells such as cardiomyocytes that can partially repopulate the scaffold. However, a prior study implanted human PSC-derived cardiac progenitors to mouse heart ECM scaffolds and demonstrated the survival and differentiation of these cells to multiple cardiac lineages including functional cardiomyocytes [15]. Therefore, decellularized cardiac ECM is an appealing substrate to optimize the differentiation of iCPCs.
In the present study, we sought to advance the iCPC technology by developing clonal iCPCs, testing the tumorigenicity of iCPCs, reprogramming iCPCs in the absence of a reporter transgene, and generating functional cardiac tissue from iCPCs in heart ECM scaffolds.
2. Materials and Methods
2.1. Fibroblast isolation
Adult fibroblasts were isolated from mouse tissues from the indicated mouse lines including the double transgenic Nkx2.5eYFP/rtTA in C57BL/6J [9, 16], B6.Cg-Gt(ROSA)26Sor<tm1(rtTA*M2) (Jackson Laboratory 006965), and wild type C57BL/6J mice using methods as previously described [9, 16]. All experimental procedures and protocols involving mice were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison and conformed to US Animal Welfare Act and institutional guidelines. In brief, mouse heart and lung explants were obtained from 6–8 week-old mice, washed with phosphate buffered saline (PBS), minced to pieces around 1mm3 in size, trypsinized (0.25% trypsin-EDTA) for 10 min and plated on 0.1% gelatin coated dishes. The explants were grown in fibroblast medium (DMEM, 10% fetal bovine serum (FBS) with 1% NEAAs/L-glutamine/penicillin/streptomycin) for 10–12 days. Fibroblasts migrating from the explants were harvested (0.25% trypsin-EDTA) and then were filtered through 40 micron cell strainer (BD Biosciences, #352340), passaged twice in 10 cm petri dishes to be 90% confluent, and used for reprogramming experiments within two passages.
2.2. Reprogramming of fibroblasts to iCPCs
Primary fibroblasts were reprogrammed as previously described with minor modifications [16]. After two passages, primary fibroblasts were seeded in a gelatinized 12-well plate at density of 50,000 cells/well and cultured for two days in fibroblast medium. In the case of C57/BL6J wild type mice lung fibroblasts, cells were first infected for 48 hours with LentiV rtTA supernatant with 8μg/ml Polybrene (Sigma). The virus supernatant was then removed, and fibroblasts were maintained in culture for an additional 72 hours. Cardiac fibroblasts from transgenic mice expressing rtTA were plated as above for 48 hours, but not infected with the LentiV-rtTA. Next, on day 0 of the reprogramming protocol (Fig. 2A), fibroblasts were treated with LentiV-5F supernatant (pSAM2-huMesp1, pSAM2-mTbx5, pSAM2-mNkx2.5, pSAM2-GATA4, pSAM2-huBaf60c) for the next 48 hours with 8μg/ml Polybrene. The LentiV-5F medium was replaced with fibroblast media containing 4μg/ml doxycycline at day 2 and iCPC induction medium (DMEM, 10%FBS, 1%NEAA, 1%L-glutamine, 1% penicillin/streptomycin, 4μg/ml doxycycline (Sigma), 2.5uM BIO (Cayman Chemical), 103units /ml LIF (Millipore)) at day 4. Cells undergoing reprograming appeared morphologically different losing parental fibroblast morphology, progressing to high nuclear-cytoplasmic ratio and could be identified as small proliferative clusters between 2–4weeks. Medium was changed with iCPC induction medium every 5 days. The iCPC colonies were picked using clonal disks (Scienceware® cloning discs diam. 3.2 mm (1/8 in.), Sigma Aldrich) for single cell clonal passaging or iCPC bulk culture was used to establish stable cell lines. After reprogramming was achieved, the iCPCs were maintained in iCPC maintenance medium (iCPC induction medium without doxycycline). The iCPCs were split 1:5 in 10 cm dish at a density of 50–70,000 cells/mL, yielding 2–2.5 million cells per 10 cm plate.
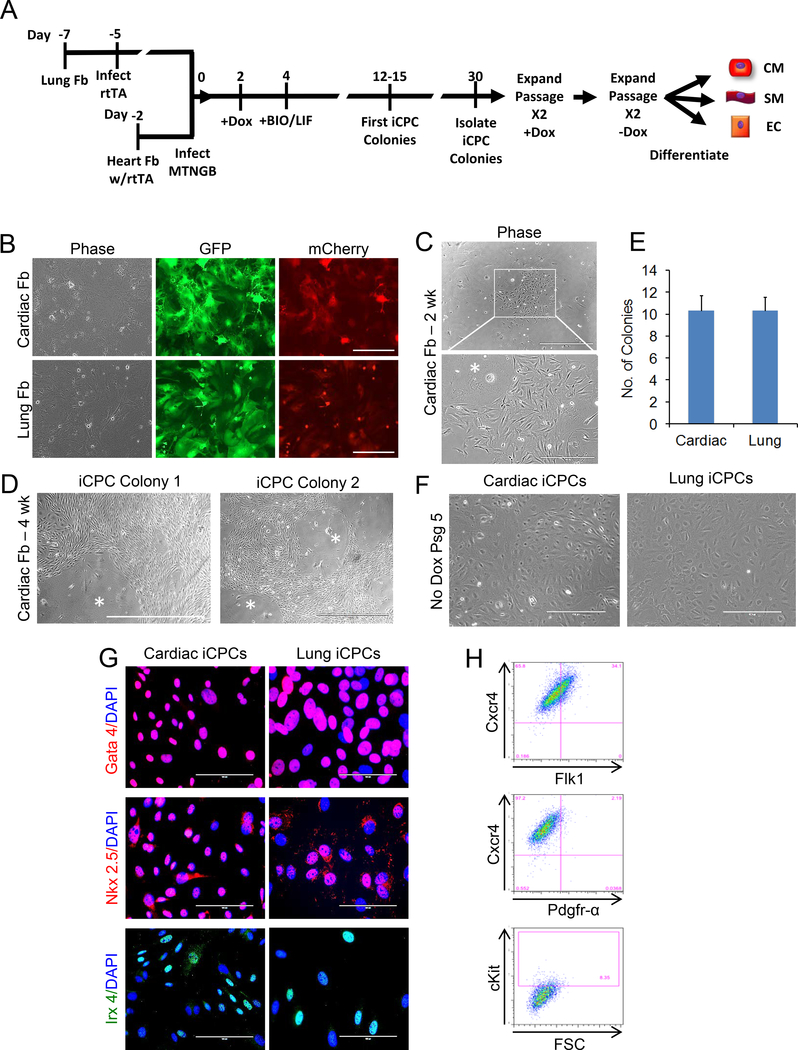
Fig. 2. iCPCs generated from reporterless adult mouse cardiac and lung fibroblasts and isolated based on morphological criteria.
(A) Schematic timeline of experimental design for reprogramming of adult wild type lung fibroblasts (derived from C57BL/6J mouse) and cardiac fibroblasts homozygous for the rtTA transgene (derived from R26-M2rtTA knock-in mouse). (B) Adult mouse lung and rtTA-cardiac fibroblasts were infected with a doxycycline inducible GFP expressing lentivirus that also contained an IRES-mCherry cassette, and greater than 90% of infected fibroblasts showed GFP/mCherry expression after doxycycline induction indicating a robustly functional expression system. (C) rtTA-cardiac fibroblasts were infected with 5 cardiac factors (MTGNB) and cultured in iCPC induction medium containing doxycycline, LIF and BIO. Cells undergoing reprogramming appeared morphologically different from fibroblasts and could be identified as small proliferative clusters after 2 weeks. (D) 4 weeks after induction of reprogramming of rtTA-cardiac fibroblasts, proliferative reporterless iCPC colonies were observed. (E) Number of reporterless iCPC colonies, derived from lung fibroblasts or rtTA-cardiac fibroblasts, observed 4 weeks after infection with 5 cardiac factors (50,000 starting fibroblasts) (n=3, SEM). (F) Reporterless iCPCs, derived from wild type lung fibroblasts and rtTA-cardiac fibroblasts, could be stably expanded without exogenous expression of cardiac factors. (G) Reporterless iCPCs expressed transcription factors Gata4, Nkx 2.5, and Irx4 which are associated with cardiac progenitor cells. (H) Flow cytometry revealed reporterless iCPC generated from rtTA-cardiac fibroblasts expressed Cxcr4, showed dim expression for Flk1, but only a small fraction was ckit positive.
2.3. Differentiation of iCPCs
The iCPCs were differentiated by cell aggregation using 24-well ultra-low attachment multiwell plates (Corning Life Sciences, CLS3473–24EA) for 4 days in cardiac differentiation medium (fibroblast medium, 5 μM IWP4 (Stemgent), 50 ng/ml Bmp4 (RD Systems), 10 ng/ml VEGF (RD Systems), and 30 ng/ml bFGF (RD Systems). Aggregates were then plated on 0.1% gelatin coated plates and cultured in fibroblast medium containing 1% serum for 35–52 days.
2.4. Immunocytochemistry and flow cytometry
For immunocytochemistry and flow cytometry, a previously described protocol with minor modifications was used [9, 16]. Cells were fixed with 4% paraformaldehyde at room temperature in PBS for 12min, permeabilized for intracellular staining with 0.1% Triton-X-100 in PBS (PBS-T) for 6 mins at room temperature, blocked with 2% goat or donkey serum + 5% BSA in PBS-T for 1 hour at room temperature, and stained overnight with specific primary antibodies in blocking buffer at 4°C. Primary antibodies to key cardiac transcription factors were used to characterize iCPCs, including Nkx2.5 (RD Systems – 1:100), Gata4 (Santa Cruz – 1:200), and Irx4 (Abgent- undiluted supernatant). The iCPC-differentiated cells were labeled with antibodies to alpha-actinin (Sigma - 1:250) and cardiac actin (Sigma - 1:400)to identify cardiomyocytes; SM-MHC (Biomedical Technologies – 1:250) to identify smooth muscle cells and CD31 (BD Pharmingen – 1:400) to identify endothelial cells. The cells were labeled with secondary antibodies Alexa Fuor 568 (ab175473)/ Alexa Fluor 594 (ab150116, ab150080)/Alexa Fluor-488(ab 150113) at 1:500 dilution for 1 hour after PBS-T wash steps and final nuclear staining (DAPI, ThermoFisher Scientific −1:1000) for 5 min before imaging with EVOS FL Auto imaging station. Flow cytometry analysis was done using cell surface markers, including Cxcr4(BioLegend 647/APC conjugate – 1:50), Flk1 (BD Pharmingen PE conjugate – 1:20), cKit (BD Pharmingen PE/Cy7 conjugate – 1:50), and PDGFRα (Santacruz PEconjugate – 1:20).
2.5. Teratoma assay
106 E14 mouse embryonic stem cells (mESCs) or lentiviral CMV-eGFP-labelled iCPCs (cardiac fibroblast-derived clonal, tri-potent iCPC line) suspended in 100μL Matrigel were injected subcutaneously in 12 NOD SCID mice (Charles River, NOD.CB17-Prkdcscid/NCrCrƖ). Mice were housed for 5–6 weeks until large visible tumors were observed for mESCs. Mice were anesthetized and the injection site was dissected to retrieve ectopic donor tissue if present. Tissue was fixed with 4% paraformaldehyde, embedded in paraffin and histology and immunofluorescence was performed to determine cell fate.
2.6. Decellularization of whole mouse heart
A previously described decellularization protocol with minor modifications was used [14]. C57BL6/J mice (6–8 weeks old) were sacrificed and the ascending aorta was cannulated with an 18-gauge blunted needle that was sutured to allow retrograde coronary perfusion (Langendorff). The hearts were perfused with heparinized PBS (HyClone) containing 10 μM adenosine at coronary artery perfusion pressure of 77.4 mm Hg for 10 min, transferred to a closed decellularization set-up, and perfused with 1% SDS (ThermoFisher Scientific) in deionized water for 12 hrs (Fig. 3A). This was followed by a 15 min wash with deionized water and then treatment with 1% Triton-X-100 (Sigma) in deioinized water for the next 30 min at a flow rate of 6 ml/min. During this time, the heart transitioned from pale brown color to translucent white. The heart was then perfused for the next 124 hrs with PBS wash buffer (without calcium and magnesium ions #70011044, Gibco, ThermoFisherScientific), antibiotics (Penicillin-Streptomycin, 10,000U/ml, Cat#15140122, ThermoFisher Scientific); 0.1% amphotericin B solution (Caisson Labs; ABL01–100ML), and protease and phosphate inhibitor cocktail (Cat# 5872S, Cell Signaling Technology), to clear the heart of surfactant remnants. After complete decellularization of the heart, the decellularized ECM (DC-ECM) scaffold was stored in fresh PBS wash buffer, pH 7.4, with protease and phosphatase inhibitors and antibiotics at 4°C for up to 2 weeks, before recellularization or other applications.
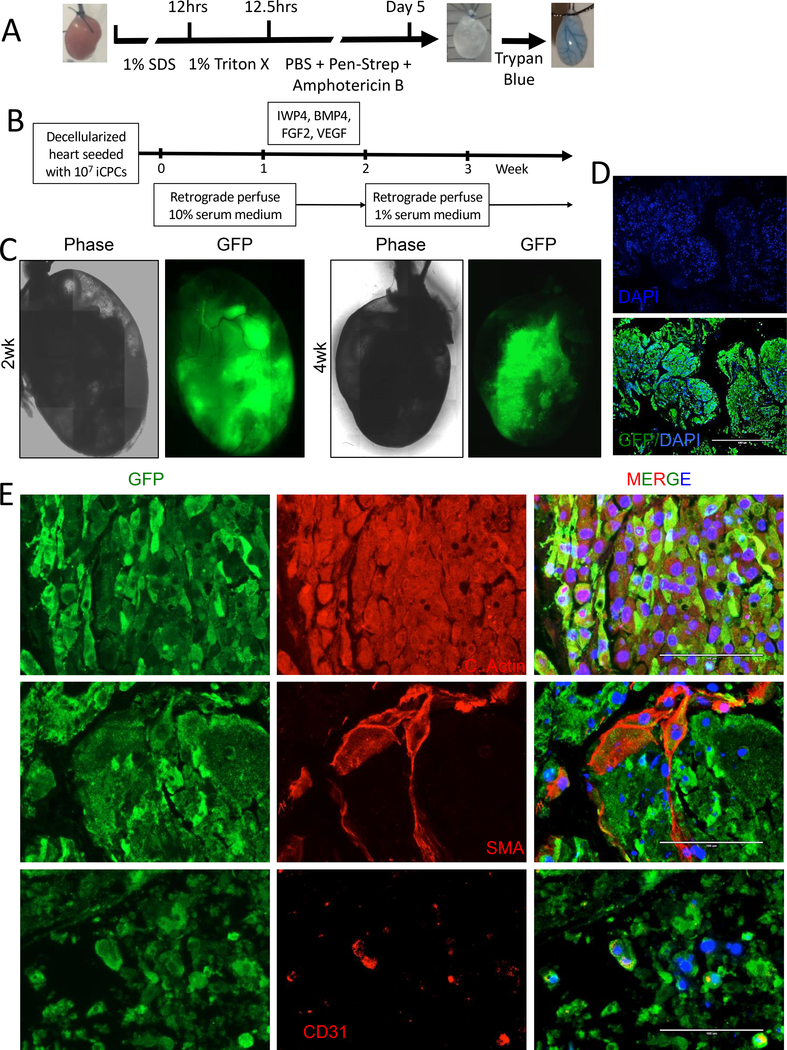
Fig. 3. iCPCs repopulate decellularized heart scaffold and differentiate into cardiac lineage cells.
(A) Schematic protocol for decellularization with images of aorta cannulated mouse heart before, after decellularization, and after decellularization with trypan blue perfusion. (B) Schematic of iCPC repopulation with 10 million GFP labelled iCPCs injected retrograde via an aortic cannula into decellularized whole heart scaffold. Scaffolds were placed in the incubator for 1–3 hr to allow cell attachment and then perfused for 4–5 weeks with media and growth factors/small molecules as indicated. (C) Live imaging was performed at various time points and showed that iCPCs attach and repopulate the decellularized scaffolds with phase contrast and epifluorescence for GFP. (D) GFP staining 3 weeks after recellularization revealed that iCPCs migrated and colonized the left ventricular myocardium region of the decellularized heart scaffold. (E) Epifluorescence images of sections from 4 week recellularized scaffolds for GFP marking iCPC progeny, DAPI to label nuclear DNA, cardiac actin for cardiomyocytes, smooth muscle actin from smooth muscle, and CD31 for endothelial cells. Scale bar = 1000uM for D and 100uM for E.
2.7. Recellularization of whole mouse heart scaffold
Scaffolds were placed in a modified version of previously described closed bioreactor system [14] in a 37° C, 5% CO2 incubator. Ten million iCPCs reprogrammed from either lung or cardiac fibroblasts and labeled with CMV-GFP expressing lentivirus were injected retrograde via an aortic cannula into decellularized whole mouse heart scaffolds. The injected scaffolds were maintained in DMEM 10% serum medium with antibiotics (1% Penicillin-Streptomycin, 0.1 % amphotericin B, and 0.1 % ciprofloxacin solution) without perfusion for 3 hours in a petri dish in a 37° C, 5% CO2 incubator to allow cell attachment. The recellularized scaffolds were then suspended in mid-air in a sterile 50mL syringe attached to a rubber stopper from above via an18-gauge blunted needle that was previously sutured to the ascending aorta to allow retrograde coronary perfusion, which was continued with the same buffer at a rate of 6 ml/min. Perfusate from the recellularized scaffold was then collected via gravity at the bottom of the 50mL syringe which was then returned via sterile silicone tubing back into a 100mL Erlenmeyer flask with a side arm from which the continuous pump collected media for retrograde perfusion. The media was changed via Erlenmeyer flask side arm every 3–4 days. After one week, we switched the perfusate to a differentiation medium (fibroblast medium with 5 mM IWP4 [Stemgent], 50 ng/ml Bmp4 [RD Systems], 10 ng/ml Vegf [RD Systems], and 30 ng/ml bFgf [RD Systems]) for an additional week. Thereafter, for weeks 3–5, the recellularized heart scaffolds were perfused with differentiation maintenance medium (DMEM, 1%FBS, 1% NEAA, 1% L-glutamine, 1% Pen/Strep). After 4 to 5 weeks, the recellularized heart scaffolds were subjected to analysis with histology, immunofluorescence labeling with confocal imaging or optical mapping of intracellular Ca2+ transients as subsequently described.
2.8. Immunofluorescence of mouse heart and receullarized heart scaffolds
Paraffin embedded mouse heart tissue was sectioned at 5 μm intervals, mounted on charged slides, and then incubated in an 80̊°C oven for 20 minutes to melt the paraffin. The sections were deparaffinized in 3 changes of xylene, 5 minutes each. Sections were hydrated through graded ethanol to deionized water exchanges and rinsed for 5 min. in dH2O. Antigen retrieval was performed in citrate buffer, pH 6.0 (10 mM citric acid, 0.05% tween 20), for 3 minutes in a Biocare decloaker (Biocare Medical, Concord, CA). The slides were cooled for 30 min and rinsed in PBS. 10% goat serum in PBS was used to serum block for one hour at room temperature. Primary antibodies in PBS with 1% goat serum was used to immunolabel cardiomyocytes overnight at 4°C and then rinsed for 5 min each for 3 washes of PBS. Secondary antibodies at 1:500 dilution (alexa 568/633/647) was added for 30 minutes at room temperature, in the dark, rinsed for 5 min in 3 washes of PBS, and rinsed in dH2O for 5 min. Coverslips were applied with Prolong Gold antifade reagent with DAPI (Invitrogen). Primary antibodies used and their respective dilutions were – Cardiac actin (Sigma – 1:50), CD31 (Abcam - 1:50), SMA (Sigma – 1:50), GFP (Abcam – 1:50).
2.9. Optical mapping of recellularized heart scaffolds
Recellularized mouse heart scaffolds were stained by cycling perfusion with calcium indicator Rhod-2 AM (10 μmol/L, Invitrogen, Carlsbad, CA) at 37°C for 30 minutes. After 20 minute washout and stabilization, the recellularized scaffolds were mapped with a MiCAM Ultima-L CMOS camera (SciMeda) at high spatial (100 × 100 pixels, 230 ± 20 μm/pixel) and temporal (333 – 1,000 frames/second) resolution. Excitation light was generated by HL-151 Halogen light system with a band-pass filter (520 ± 45 nm). Emission fluorescent signals were collected through a band-pass filter (590 ± 15 nm, Thorlabs, Newton, NJ). The acquired fluorescent signal was digitized, amplified, and visualized by custom program using Matlab software [17]. Field and point constant voltage electrical stimulation (1 – 5 Hz) of 1.5 times threshold were applied during mapping respectively.
2.10. Statistical Analysis
For comparisons of two samples assuming unequal variances, two tail t-test was done, and p<0.05 was regarded as the level of significance.
3. Results
3.1. Clonal iCPC cell lines are multipotent and do not form teratomas
Our previously reported iCPC lines were derived by combining several iCPC colonies and passaging to generate polyclonal cell lines [9]. However, to reduce heterogeneity as well as to evaluate the potency of single iCPC colonies, we generated clonal iCPC lines (using cardiac fibroblasts from Nkx-2.5eYFP/rtTA mouse as starting cell source) according to protocol published previously [16]. Twenty-four putative iCPCs colonies were identified using previously described morphological changes (eYFP fluorescence was not utilized). The colonies were selectively isolated and expanded. Only 50% of the colonies were able to generate stably reprogrammed clonal cell lines (at least 2–3 passages without dox induction) (Fig. 1A). To evaluate the cardiac potency of these clonal iCPC lines we randomly selected 8 of 12 stably reprogrammed clonal cell lines, differentiated them individually, and performed immunofluorescence for cardiomyocyte, endothelial and smooth muscle markers. Immunofluorescence revealed 6/8 clones were tripotent (CM, SM, EC) whereas 2 of 8 clones were bipotent (CM, EC) (Fig. 1B,C).
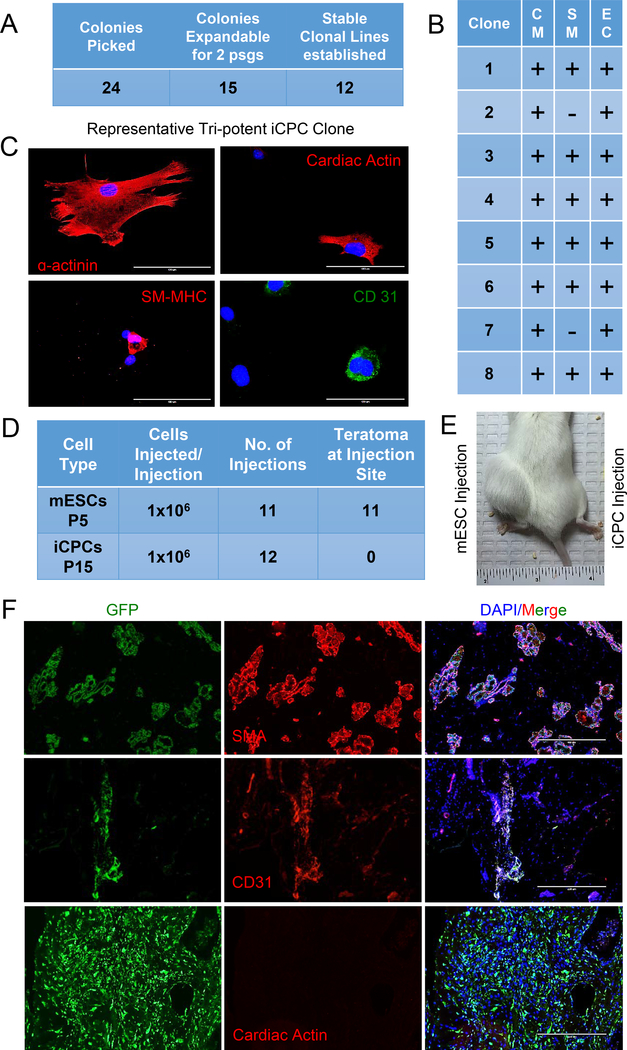
Fig. 1. Multipotent iCPC clonal cell lines can be established from single cell reprogramming events and clonal iCPCs do not form teratomas.
(A) Using cardiac fibroblasts and morphological changes to identify reprogramming, iCPC colonies were individually isolated and expanded to generate clonal cell lines. Only 50% of colonies produced stably expandable iCPC lines without exogenous doxycycline induction. (B) 8 Clonal iCPC cell lines were differentiated in vitro and immunofluorescence was performed to assess tri-lineage differentiation. The majority of iCPC clones were tripotent and could differentiate into cardiomyoctyes (CM), smooth muscle (SM) and endothelial cells (EC). (C) Representative immunofluorescence images showing a tripotent iCPC clone based on positive staining for α-actinin, SM-MHC and CD 31. (D) A single colony derived, tri-potent iCPC cell line (TrCl8) derived from cardiac fibroblasts was used for teratoma assay. Summary table for teratoma assays using 1 million mESCs injected subcutaneously into the left hind limb and 1 million CMV-GFP labeled iCPCs into right hind limb of NOD-SCID mice. (E) Representative mouse 6 weeks after injection of mESCs (left) leading to a large tumor (teratoma confirmed by histology, not shown) in contrast to iCPC injection (right) without visible tumor. (F) Immunolabeling of iCPCs injected in right hind limb after one month showed iCPCs differentiated into smooth muscle cells (SMA) and endothelial cells (CD31), but did not differentiate into cardiomyocytes (absence of cardiac actin staining).
One potential advantage of iCPCs, over PSC derivatives, is a reduction in the risk of tumor formation, as iCPC reprogramming does not transition through an intermediate pluripotent cell state. Even though our previous in vivo work in immunocompetent mice did not find any cardiac or off-target tumors, a teratoma assay performed using immunocompromised mice is a stringent method to evaluate tumorigenicity [18]. Therefore, we performed a teratoma assay comparing iCPCs and mESCs injected subcutaneously into the hind limb of immunodeficient (NOD/SCID) mice. One million GFP labeled iCPCs (suspended in Matrigel) were subcutaneously injected above right hindlimb, and one million E14 mESCs (suspended in Matrigel) were subcutaneously injected above left hind limb. In 11/11 mice injected with mESCs, large tumors were easily observed after 5–6 weeks. In contrast, injection of iCPCs did not result in gross tumor formation in 12 injected mice (Fig. 1D,E). However, upon dissecting the site of iCPC injection we noticed small tissue plugs in 3/12 animals. Histology analysis of iCPC tissue plugs did not reveal teratoma formation. Immunofluorescence using cardiac lineage markers revealed that iCPCs within the small tissue plugs had differentiated into vascular cells (endothelial and smooth muscle) based on expression of SMA and CD31. However, we could not detect expression for cardiac actin, suggesting cardiac differentiation may not be favored in this microenvironment (Fig. 1F). In the 9/12 animals that we could not detect gross tissue plugs, we did not perform further histology given the clear absence of teratoma formation. Overall, these data demonstrate that in contrast to ESCs, the iCPCs did not form teratomas under comparable conditions in this immunodeficient mouse model. Furthermore, the surviving iCPCs that were detected in tissue plugs preferentially differentiated into vascular cells and not cardiomyocytes perhaps reflective of the non-cardiac microenvironment.
3.2. Morphological criteria alone are sufficient to identify iCPC colonies and generate stably reprogrammed iCPC cell lines (Reporterless Reprogramming)
Cells undergoing iCPC reprogramming undergo a dramatic morphological change from parental fibroblasts and develop into highly proliferative iCPC colonies. Here we tested if morphology alone can be utilized to identify cells undergoing iCPC reprogramming. We utilized adult cardiac fibroblasts isolated from transgenic reverse tetracycline transactivator (rtTA) mice and adult lung fibroblasts from wild type mice. First, we infected cardiac and lung fibroblasts with a doxycycline inducible GFP-IRES-mcherry lentivirus (same construct also used for cardiac factor infection) and confirmed that the inducible expression system is efficient and functional (Fig 2B - >90% infection efficiency observed in both cell sources). Since our cardiac factors are doxycycline inducible, we had to infect the wild type lung fibroblasts with a lentivirus encoding reverse tetracycline transactivator (rtTA) (Fig. 2A). Next, we infected cardiac and lung fibroblasts with lentiviruses encoding 5 cardiac factors (Mesp1, Tbx5, Gata4, Nkx2.5, Baf60c) and cultured in iCPC induction medium containing doxycycline (Dox), leukemia inhibitory factor (LIF) and the GSK3β inhibitor BIO as previously reported (Fig. 2A) [9, 16]. Clusters of morphologically distinct, proliferative cells started to emerge at day 15 (Fig. 2C) and compact iCPC colonies were counted on day 30 (Fig. 2D, E). The cells were cultured in the presence of Dox for 2 passages (polyclonal passaging as reported previously [16]) followed by passaging in the absence of Dox. Both cardiac- and lung-derived reporterless iCPC lines were able to proliferate in iCPC maintenance medium (LIF+BIO without Dox) indicating stable reprograming to iCPC cell state was achieved. Reporterless-iCPCs expressed cardiac progenitor transcription factors including Nkx2.5, Tbx5, and Irx4 (Fig. 2G). Flow cytometry was used to assess for cell surface markers associated with CPCs [19–21]. Reporterless-iCPCs generated from cardiac fibroblasts were > 95% positive of Cxcr4, showed dim expression for Flk1 and had only subset of cells positive for cKit or Pdgfr-α (Fig. 2H). Together these results suggest that morphological criteria alone are sufficient to identify reprogramming events and generate stable iCPC cell lines from both cardiac and lung fibroblasts without using a cardiac progenitor-specific reporter.
3.3. Decellularization of whole mouse heart to generate cardiac ECM scaffold
In order to test the potency of iCPCs to differentiate and repopulate native cardiac ECM, we decellularized mouse hearts using a protocol developed by Ott et al. [14]. We used a modified Langendorff apparatus and performed detergent based retrograde coronary perfusion for decellularization followed by a prolonged antibiotic and antifungal rinse. At the end of the decellularization procedure, hearts decreased in size and obtained a blanched white coloration, commonly observed for perfusion-decellularized organs (Fig. 3A). The decellularized hearts maintained major vasculature conduits with the intact coronary vasculature visualized by perfusion with trypan blue (Fig 3A).
3.4. iCPCs repopulate de-cellularized heart scaffold and differentiate into cardiac lineage cells including cardiomyocytes, smooth muscle cells and endothelial cells
We tested if iCPCs delivered to the decellularized cardiac scaffold could repopulate the heart and differentiate into cardiac lineage cells. Ten million iCPCs labeled with CMV-GFP expressing lentivirus were injected retrograde via an aortic cannula into decellularized whole mouse heart scaffolds. The scaffolds were maintained in DMEM 10% serum medium without perfusion for 3 hours to allow cell attachment to scaffold, followed by continuous retrograde perfusion of medium in a closed bioreactor system for 4–5 weeks. Cell seeding was evident by the live imaging of GFP epifluorescence in the scaffolds (Fig. 3C). Recellularization was evident on gross inspection of the recellularized scaffolds by the opacification of the scaffolds and change in color. Cross sectioning of recellularized scaffold followed by GFP staining revealed that iCPCs had repopulated the decellularized scaffold (Fig. 3D). Immunolabeling followed by confocal imaging demonstrated the presence of cardiac actin positive cardiomyocytes that co-labeled with GFP in recellularized scaffolds. Likewise, some vascular structures were repopulated with SMA positive smooth muscle cells. In addition, CD-31 positive endothelial cells were detected (Fig. 3E). These data suggest that iCPCs differentiated into cardiac lineage cells within scaffolds. A total of 5 heart scaffolds (3 seeded with cardiac fibroblast-derived clonal iCPCs and 2 with lung fibroblast derived clonal iCPCs) were successfully recellularized and demonstrated repopulation of the heart scaffolds and differentiation of iCPCs into cardiac lineage cells.
3.5. Electrically stimulated Ca2 transients in iCPC recellularized cardiac scaffolds
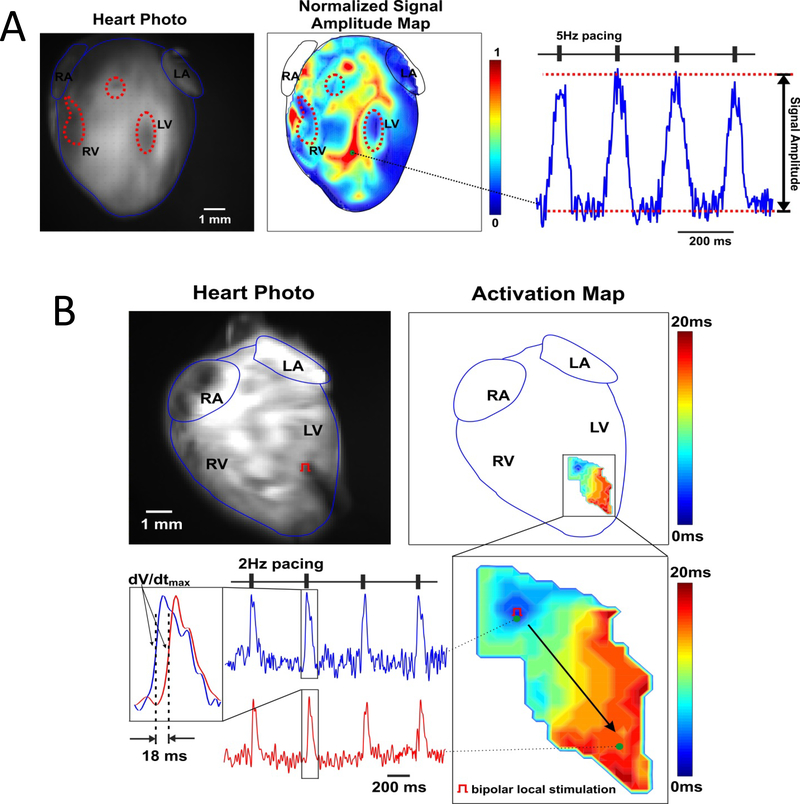
Although the iCPC-derived cardiomyocytes were able to effectively repopulate the decellularized hearts and differentiate into cardiac linage cells, we could not detect obvious contractions in the recellularized scaffolds. This lack of evidence of electro-mechanical coupling may reflect the limited degree of recellularization relative to the overall matrix scaffold and associated mechanical properties. In order to provide a more sensitive assay of iCPC-derived cardiomyocytes function, we tested their ability to generate and propagate calcium transients when electrically stimulated. Recellularized heart scaffolds were loaded with Ca2+-sensitive fluorescent dye Rhod-2 AM and subjected to optical mapping at 37oC. Fluorescent signal was detected from recellularized scaffolds in response to pacing (Fig. 4). Under field stimulation, multiple areas of the recellularized construct demonstrated appropriate electrically induced Ca2+ transients suggesting that electrically functional cardiomyocyte clusters were present in the scaffold (Fig. 4A). Bipolar local stimulation revealed that the Ca2+ signal propagated within cell clusters over several millimeters, indicating electrical coupling between the cardiomyocytes in the scaffold (Fig. 4B). Although the cell-cell coupling was not as rapid as in a native heart, with conduction velocity of 9.72 cm/s, the results demonstrated successful establishment of intercellular communication following differentiation in the recellularized scaffold. An additional 3 recellularized heart scaffolds underwent optical mapping and demonstrated functional Ca2+ transients upon electrical stimulation. Overall, these results suggest that the iCPC differentiated cardiomyocytes form 3-dimensional electrically coupled cardiac tissue within the cardiac ECM scaffolds.
Fig. 4. iCPC-derived cardiomyocytes mature in heart scaffold and show Ca2+ transients and cell-cell coupling.
High resolution fluorescent optical mapping of Ca2+ transients (CaTs) from recellularized cardiac scaffolds during field (A) and point (B) stimulation. (A) Fluorescent photo of Ca2+-sensitive dye Rhod-2-stained recellularized mouse heart (right panel). Highlighted by dotted red lines areas indicate regions of low fluorescence, presumably, due to a low cell density; these areas corresponded with areas with no CaT signals (dark blue) in normalized optical single amplitude distribution map, whereas other areas showed prominent Ca2+ signals during 5Hz field pacing stimulation as indicated by dark red color (middle panel – calcium activity correlated with GFP signal intensity during live imaging – Figure 4A, 4 wk scaffold). Such areas suggested live and functioning cardiomyocyte clusters with appropriate Ca2+ cycling distributed within the recellularized heart scaffold. On the right panel, a representative CaT recording from such clusters is shown. RA and LA – right and left atria, RV and LV – right and left ventricles. (B) Top panels: fluorescent photo of stained recellularized mouse heart (left) with pacing electrode positioned in the middle of the LV (red pacing symbol), and corresponding CaT activation map reconstructed during constant (2 Hz) pacing. Near the activation map, a color time scale shows CaT activation time. Below, an enlarged area of continuous propagation of CaT throughout the LV induced by point stimulation is shown together with representative CaT recordings collected from the beginning and the end of activated area. Activation time was calculated as a time at (dF/dt)max and used to calculate conduction velocity. Bipolar local stimulation revealed that the calcium signal propagated between the two sites shown, indicating cardiomyocyte cell-cell coupling within the scaffold.
4. Discussion
Building from our prior study describing the generation of mouse iCPCs using forced expression of defined factors (MTNGB) [9], here we demonstrate more broadly applicable methods to generate iCPCs and powerful new applications using iCPCs that enable the generation of functional cardiac tissue from them. The data demonstrate that iCPCs can be clonally derived and expanded into distinct cell lines. Furthermore, we demonstrate the generation and isolation of iCPCs from wild type mouse fibroblasts based on morphology criteria without the need for a genetically encoded reporter to identify iCPCs. Importantly, the obtained iCPCs do not exhibit the tumorigenicity that ESCs demonstrate in a teratoma assay. Seeding iCPCs in decellularized mouse heart scaffolds generates functional cardiac tissue evidenced by the presence of differentiated cardiomyocytes, endothelial and smooth muscle cells with propagation of intracellular Ca2+ transients within recellularized scaffolds.
The development of techniques for lineage reprogramming has typically employed transgenic cells that express a reporter protein or antibiotic resistance gene to identify the desired cell lineage. This approach was employed in the pioneering study first generating mouse iPSCs with the βgeo construct knocked into the Fbx15 gene [22]. In the case of human iPSCs, the knock-in of neomycin phosphotransferase in the OCT4 locus allowed isolation of iPSCs [23]. However, it was quickly appreciated that cell and colony morphology was adequate to allow identification and isolation of reprogrammed iPSCs without genetic markers [23, 24]. Similarly, we can identify morphological iCPC colonies composed of cells with a high nuclear to cytoplasmic ratio that are proliferative and generate stable iCPC lines without use of the Nkx2.5-EYFP reporter that was originally used. However, like iPSC reprogramming, not all colonies generate stable cells lines, likely due to incomplete reprogramming. In addition, each clonal cell line requires characterization for potency because both bipotent and tripotent lines are possible with MTNGB reprogramming. The ability to reprogram fibroblasts to iCPCs from potentially any mouse line expands this technology making it adaptable for a range of applications.
Because iCPCs hold promise as a therapeutic cell source for cardiac regeneration, the risk of tumorigenesis from delivering a live cell product requires assessment. PSCs can give rise to teratomas following transplantation, and clinical applications using terminal lineages derived from human PSCs have required detailed tumorigenesis safety testing [25]. The data suggest that iCPCs are committed to form cardiovascular lineages and thus theoretically do not exhibit the risk of teratoma formation that PSCs do. Our results confirm that iCPCs do not form teratomas when transplanted into immunocompromised mice in which mESCs readily form teratomas. Although some of the transplanted iCPCs survived and differentiated to smooth muscle and endothelial cells, there was no tumor formation identified after one month. This initial analysis of tumorigenesis demonstrates a clear reduction in the risk of tumorigenesis between mESCs and iCPCs, but further analysis with longer follow-up will be necessary to comprehensively evaluate the long-term risk of tumorigenesis related to iCPC transplantation.
Perfusion decellularized heart scaffolds provide a rich substrate for generation of functional cardiac tissue and ultimately a bioartificial heart [14]. The iCPCs readily attached to the decellularized scaffold and differentiated to relevant cardiac lineages including cardiomyocytes, endothelial and smooth muscle cells. The differentiated cardiomyocytes in the heart scaffolds showed an increase in cell size and sarcomere organization compared to in vitro differentiated iCPCs over a similar period of time. This finding suggests that in addition to the growth factors and small molecules that were perfused to promote differentiation of iCPCs, the appropriate cardiac ECM facilitated differentiation. The 3D scaffold also promoted the formation of areas of coupled and electrically functional cardiac tissue based on the results of optical mapping experiments demonstrating electrically stimulated Ca2+ transient that propagated several millimeters. Although the partially recellularized 3D heart scaffold was far from a completely functional heart, the present results provide evidence that iCPCs are a promising cell source to repopulate such scaffolds showing similarities to a previous study using human iPSC-derived cardiovascular progenitors to repopulate a mouse heart scaffold [15].
5. Conclusions
Our results advance iCPC technology demonstrating more broadly applicable methods for reprogramming and the ability to form electrically coupled cardiac tissue using iCPCs to recellularize heart scaffolds. The recellularized scaffolds suggest the possibility of therapeutic applications to replace damaged or dysfunctional heart tissue using iCPCs. Basic cardiovascular research, drug discovery, toxicity testing, and other in vitro applications are enabled by iCPC generated cardiac tissue.
6. Limitations and Future Directions
There remain a number of limitations and questions regarding this study of iCPCs. Clonal iCPC lines exhibit heterogeneity regarding cell surface markers and potency. These differences may be related to the heterogeneity of CPCs in normal development, but future studies are needed to understand and exploit this heterogeneity to obtain the optimal cell preparation for a given application. In addition, the starting source of fibroblasts for reprogramming may affect the results potentially impacting the differentiation capacity of the cells, but this has not been systematically evaluated. Although the iCPCs seeded and differentiated in the decellularized scaffolds, the process of seeding and differentiation requires further optimization to obtain more complete recellularization and promote formation of mature and functional cardiac tissue. The current studies have reprogrammed mouse primary fibroblasts, but this approach has not yet succeeded using human fibroblasts to generate proliferative iCPC lines. Furthermore, efforts to reprogram without integration of the transgenes for footprint free reprogramming in analogy to approaches at factor delivery now optimized for iPSC reprogramming will be important future improvements. The efficiency of reprogramming remains relatively low, and future efforts to further optimize this process potentially with improved culture conditions and small molecules are important.
Acknowledgements
We would like to thank the UWCCC Experimental Pathology Core (especially Joe Hardin) for help with immunohistochemistry experiments.
Funding
The following funding sources supported this work: NIH R01 HL129798 (TJK), U01 HL134764 (TJK), U01HL099773 (TJK), T32HL007936-17 (RAA), S10RR025644 (TJK), and AHA predoctoral fellowship 12PRE9520035 (PAL).
Footnotes
Transparency document
Conflict of Interest
TJK is a consultant for FujiFilm Cellular Dynamics Inc. RAA is a consultant for Cell Reprogramming and Therapeutics LLC. Pending patent applications are related to iCPC generation (TJK, PAL). EGS and ANR have ownership in Cellular Logistics Inc.
References
- [1].Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D, Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors, Cell, 142 (2010) 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D, Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state, Stem cell reports, 1 (2013) 235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, Tohyama S, Yuasa S, Kokaji K, Aeba R, Yozu R, Yamagishi H, Kitamura T, Fukuda K, Ieda M, Induction of human cardiomyocyte-like cells from fibroblasts by defined factors, Proc Natl Acad Sci U S A, 110 (2013) 12667–12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN, Heart repair by reprogramming non-myocytes with cardiac transcription factors, Nature, 485 (2012) 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ, MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes, Circ Res, 110 (2012) 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cao N, Huang Y, Zheng J, Spencer CI, Zhang Y, Fu JD, Nie B, Xie M, Zhang M, Wang H, Ma T, Xu T, Shi G, Srivastava D, Ding S, Conversion of human fibroblasts into functional cardiomyocytes by small molecules, Science, 352 (2016) 1216–1220. [DOI] [PubMed] [Google Scholar]
- [7].Islas JF, Liu Y, Weng KC, Robertson MJ, Zhang S, Prejusa A, Harger J, Tikhomirova D, Chopra M, Iyer D, Mercola M, Oshima RG, Willerson JT, Potaman VN, Schwartz RJ, Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors, Proc Natl Acad Sci U S A, 109 (2012) 13016–13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li XH, Li Q, Jiang L, Deng C, Liu Z, Fu Y, Zhang M, Tan H, Feng Y, Shan Z, Wang J, Yu XY, Generation of Functional Human Cardiac Progenitor Cells by High-Efficiency Protein Transduction, Stem Cells Transl Med, 4 (2015) 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lalit PA, Salick MR, Nelson DO, Squirrell JM, Shafer CM, Patel NG, Saeed I, Schmuck EG, Markandeya YS, Wong R, Lea MR, Eliceiri KW, Hacker TA, Crone WC, Kyba M, Garry DJ, Stewart R, Thomson JA, Downs KM, Lyons GE, Kamp TJ, Lineage Reprogramming of Fibroblasts into Proliferative Induced Cardiac Progenitor Cells by Defined Factors, Cell Stem Cell, 18 (2016) 354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang Y, Cao N, Huang Y, Spencer CI, Fu JD, Yu C, Liu K, Nie B, Xu T, Li K, Xu S, Bruneau BG, Srivastava D, Ding S, Expandable Cardiovascular Progenitor Cells Reprogrammed from Fibroblasts, Cell Stem Cell, 18 (2016) 368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rozario T, DeSimone DW, The extracellular matrix in development and morphogenesis: a dynamic view, Dev Biol, 341 (2010) 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA, In vivo reprogramming of adult pancreatic exocrine cells to beta-cells, Nature, 455 (2008) 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D, In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes, Nature, 485 (2012) 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA, Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart, Nat Med, 14 (2008) 213–221. [DOI] [PubMed] [Google Scholar]
- [15].Lu TY, Lin B, Kim J, Sullivan M, Tobita K, Salama G, Yang L, Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells, Nature communications, 4 (2013) 2307. [DOI] [PubMed] [Google Scholar]
- [16].Lalit PA, Rodriguez AM, Downs KM, Kamp TJ, Generation of multipotent induced cardiac progenitor cells from mouse fibroblasts and potency testing in ex vivo mouse embryos, Nat Protoc, 12 (2017) 1029–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lang D, Sulkin M, Lou Q, Efimov IR, Optical mapping of action potentials and calcium transients in the mouse heart, Journal of visualized experiments : JoVE, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].I. International Stem Cell, Assessment of established techniques to determine developmental and malignant potential of human pluripotent stem cells, Nature communications, 9 (2018) 1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G, Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines, Cell Stem Cell, 8 (2011) 228–240. [DOI] [PubMed] [Google Scholar]
- [20].Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, Gramolini A, Keller G, SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells, Nat Biotechnol, 29 (2011) 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nelson TJ, Faustino RS, Chiriac A, Crespo-Diaz R, Behfar A, Terzic A, CXCR4+/FLK-1+ biomarkers select a cardiopoietic lineage from embryonic stem cells, Stem Cells, 26 (2008) 1464–1473. [DOI] [PubMed] [Google Scholar]
- [22].Takahashi K, Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell, 126 (2006) 663–676. [DOI] [PubMed] [Google Scholar]
- [23].Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA, Induced pluripotent stem cell lines derived from human somatic cells, Science, 318 (2007) 1917–1920. [DOI] [PubMed] [Google Scholar]
- [24].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S, Induction of pluripotent stem cells from adult human fibroblasts by defined factors, Cell, 131 (2007) 861–872. [DOI] [PubMed] [Google Scholar]
- [25].Carpenter MK, Frey-Vasconcells J, Rao MS, Developing safe therapies from human pluripotent stem cells, Nat Biotechnol, 27 (2009) 606–613. [DOI] [PubMed] [Google Scholar]