Abstract
Background
Tuberculosis (TB) may facilitate carcinogenesis. We performed a case-control study of the association between TB and cancer in Xinjiang, a high TB endemic area of China.
Methods
From January 2016 to December 2018, a total of 45,455 patients hospitalized in Xinjiang Cancer Hospital were consecutively enrolled and divided into a malignant tumor group (n = 32,539) and a benign tumor group (n = 12,916). Patients with active and previous TB before the diagnosis of cancer were retrospectively identified in the two groups.
Results
A significantly higher proportion of TB was found in the malignant tumor group (n = 1776, 5.46%) than in the control (benign tumor) group (n = 175, 1.35%) (p < 0.0001). The highest and lowest proportions of TB in the malignant group were in patients with non-Hodgkin’s lymphoma (16.74%) and thyroid cancer (0.77%), respectively. In multivariate analysis adjusting for age, sex, and ethnicity, TB remained an independent risk factor for all cancers (odds ratio (OR) 1.68; 95% confidence interval (CI) 1.43–1.97). Furthermore, TB was associated with a significantly higher risk of non-Hodgkin’s lymphoma, cervical cancer, esophageal cancer, “other” cancers, ovarian cancer, and breast cancer. Moreover, females with TB were more likely to develop cancer than males (p < 0.0001), except for esophageal cancer and lymphoma.
Conclusion
TB patients have an elevated cancer risk. A screening strategy for TB should be taken into consideration before treatment in patients with some cancer types that are associated with a high proportion of TB.
Keywords: Tuberculosis, Malignant Cancer, Case-control study
Background
Tuberculosis (TB) is an old disease that has affected humans more than 8000 years ago [1, 2]. To date, most TB is completely curable with a timely diagnosis and correct drug treatment [2, 3]. Globally, in 2015, the age-standardized tuberculosis incidence rates (per 100 000 people) among men and women were 154.4 (140.0–172.2) and 86.3 (78.0–97.4), respectively [4]. The age-standardized tuberculosis mortality rates (per 100 000 people) among men and women were 21.9 (16.5–;29.5) and 10.8 (8.5–13.1), respectively [4]. With declining trends in mortality, incidence, and prevalence of TB in human immunodeficiency virus (HIV)-negative individuals, challenges remain in most Asia [4, 5].
Western China is a high endemic area for TB [6, 7]. The patient diagnosis rate of pulmonary TB was 0.34 (95% confidence interval (CI) 0.25–0.44) in the 2010–2011 Xinjiang survey [6]. In addition, the burden of multidrug-resistant and extensively drug-resistant TB cases was also found to be substantial in the Xinjiang area [7]. TB may induce a chronic inflammatory state and impair T-cell-mediated immunity, contributing to the development of cancer [8–10]. Indeed, a number of epidemiologic studies have shown that the risk of cancer is the greatest in the first 2 years after the diagnosis of TB [9–13] but remains elevated for long periods [12, 14-18]. Understanding the risk of the development of cancer after TB in a highly endemic area has some practical clinical significance, such as improving the prevention and diagnosis of cancer.
However, limited data are available from Western China to support the link between cancer and TB. Data from such areas might add valuable insights into the relationship between TB and malignant cancer. Therefore, we performed a retrospective case-control study to compare the proportion of TB in malignant tumor patients and benign tumor patients.
Methods
Patient selection method
From January 2016 to December 2018, a total of 46,371 inpatients were treated at Xinjiang Cancer Hospital, a tertiary cancer center in Xinjiang. This cohort was then divided into two groups, namely, the malignant tumor group (n = 32,539) and the control (benign tumor) group (n = 12,916) (Table 1), based on the definitive pathological diagnosis confirmed by at least 2 experienced pathologists. Patients who were diagnosed with TB after being diagnosed with cancer (n = 272) and those lacking definitive pathological reports (n = 644) were excluded from this study. This study was performed in accordance with the principles of the Declaration of Helsinki.
Table 1.
Cancer risk in patients with tuberculosis (TB) stratified by patient characteristics
| Characteristics | Malignant tumor group (N = 32,539) |
Benign tumor group (N = 12,916) |
P | ||
|---|---|---|---|---|---|
| No | % | No | % | ||
| Sex | < 0.0001 | ||||
| Male | 12,420 | 38.17 | 791 | 6.12 | |
| Female | 20,119 | 61.83 | 12,125 | 93.88 | |
| Age, years | < 0.0001 | ||||
| Median | 55.0 | – | 41.4 | – | |
| Range | 2–99 | – | 5–91 | – | |
| Ethnicity | 0.2155 | ||||
| Han | 21,845 | 67.13 | 8734 | 67.62 | |
| Non-Han | 10,694 | 32.87 | 4182 | 32.38 | |
| Tuberculosis | < 0.0001 | ||||
| TB | 1776 | 5.46 | 175 | 1.35 | |
| Non-TB | 30,763 | 94.54 | 12,741 | 98.65 | |
Identification of tuberculosis patients
A retrospective TB survey was performed in the two groups. Patients with active and previous TB before the diagnosis of cancer (benign or malignant) were obtained from the Department of TB Registry in Xinjiang Cancer Hospital. Among the TB patients, 1673 had pulmonary TB, 103 had extrapulmonary TB in the malignant tumor group, and 153 and 22 patients had pulmonary and extrapulmonary TB in the control group, respectively. In the malignant tumor group, most patients with TB disease had previous pulmonary TB, according to clinical and radiological indicators such as old pulmonary TB (n = 1690). Active pulmonary TB was found in 78 patients in the malignant tumor group who were positive for smear or culture results (n = 12) or had radiographic abnormalities consistent with active pulmonary TB (n = 66). In the control group, 167 patients had previous pulmonary TB, and only 2 patients had radiographic abnormalities consistent with active pulmonary TB. Only 8 and 6 patients in the malignant tumor and control groups, respectively, had a past medical history of pulmonary TB diagnosed by their doctors.
In both groups, when patients were hospitalized multiple times, only the first hospitalization was included in this study. “Other cancer” refers to any cancer defined by type or site accounting for less than 5%, including cancer originating in the testis, vulva, penis, vagina, labia majora, labia minora, perineum, scrotum, hydatidiform mole, chorion, notochord, adrenal cortex, neuroendocrine system, mediastinum, sweat glands, mesothelium, thymus, or umbilical tube.
Statistical analysis
Data were analyzed using SPSS (version 25.0; SPSS, Chicago, IL). To identify the risk of cancer associated with TB, age, sex, and ethnicity were adjusted for in the statistical analysis. A binary logistic regression model was used to estimate the adjusted odds ratio (OR) and 95% confidence interval (CI). The variables related to the event of interest (cancer) were introduced into the model in an unconditional way. A 2-tailed P value < 0.05 was considered statistically significant.
Results
TB occurred more frequently in malignant cancer than in benign tumor patients
To assess the relationship between TB and tumors, we assigned the inpatients to two groups: the control group (benign tumor group, n = 12,916) and the study group (malignant tumor group, n = 32,539). The identified proportions of TB in the control and malignant groups are presented in Table 1. In total, 175 patients (1.35%) in the control group and 1776 patients (5.46%) in the study group had TB prior to the diagnosis of cancer (p < 0.0001). In 2010, the prevalence rate of pulmonary TB in the general population was 0.92-2.65% [19], which is comparable to the proportion of TB in the control group. In univariate analysis, TB was a risk factor for malignant cancer, in addition to traditional risk factors, such as age and sex (p < 0.0001). However, no significant difference in the proportion of TB was found between patients of Han and non-Han ethnicities.
TB may be associated with an increased risk of specific cancer types
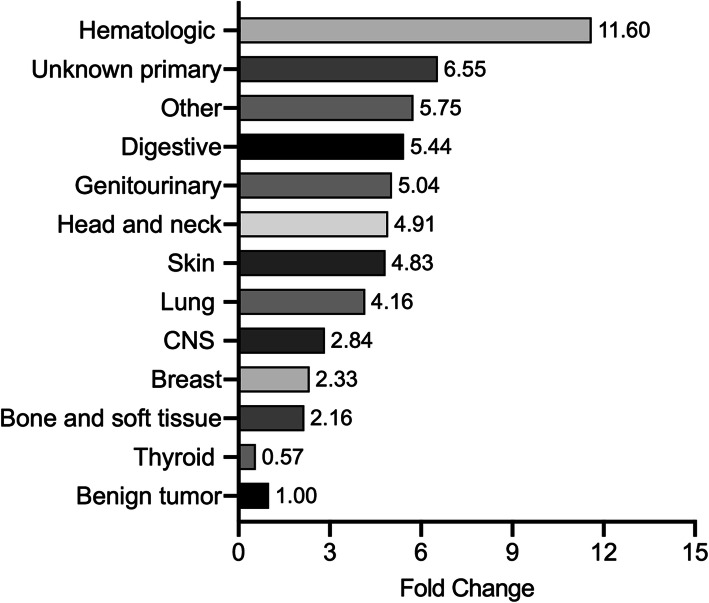
The proportion of TB may vary among patients with different cancer sites or types. The proportion of TB in patients with different cancer sites/types and the multivariate logistic regression analysis results stratified by cancer site/type and sex are shown in Table 2. The highest proportion of TB was found in patients with hematologic malignancies (15.66%), followed by those with unknown primary cancer (8.84%), “other” cancers (7.76%), digestive tract cancer (7.35%), genitourinary cancer (6.81%), head and neck cancer (6.63%), skin cancer (6.52%), central nervous system (CNS) cancer (3.83%), breast cancer (3.14%), and bone and soft tissue sarcoma (2.92%). In contrast, the lowest proportion of TB was found in patients with thyroid cancer (0.77%). The fold change in the proportion of TB for different cancer sites in the study group compared with the control (benign tumor) group is also shown in Fig. 1.
Table 2.
Proportion of tuberculosis (TB) and cancer risk in patients stratified by tumor site
| Cancer type (N.) | Total | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N. of TB | % | Adjusted OR* (95% CI) |
N. of TB | % | Adjusted ORa (95% CI) |
N. of TB | % | Adjusted ORa (95% CI) |
|
| Benign tumor (n = 12,916) | 175 | 1.35 | Reference | 26 | 3.27 | Reference | 149 | 1.23 | Reference |
| Malignant cancers (n = 32,539) | 1776 | 5.46 | 1.68 (1.43–1.97) | 861 | 6.93 | 1.12 (0.79–1.60) | 915 | 4.55 | 1.83 (1.53–2.19) |
| Lung cancer (n = 3968) | 223 | 5.62 | 1.44 (1.06–1.95) | 165 | 6.48 | 1.27 (0.83–1.94) | 58 | 4.08 | 1.57 (1.04–2.37) |
| Adenocarcinoma (n = 2046) | 91 | 4.45 | 2.05 (1.43–2.95) | 59 | 5.58 | 1.72 (1.06–2.81) | 32 | 3.34 | 2.09 (1.28–3.41) |
| Squamous cell carcinoma (n = 763) | 57 | 7.47 | 1.35 (0.82–2.22) | 50 | 7.37 | 1.53 (0.90–2.62) | 7 | 8.24 | NA |
| Small cell (n = 497) | 25 | 5.03 | NA | 22 | 5.66 | NA | 3 | 2.78 | NA |
| Other (n = 662) | 50 | 7.55 | 1.38 (0.87–2.18) | 34 | 8.04 | 1.44 (0.81–2.57) | 16 | 6.69 | NA |
| Extrapulmonary tumor (n = 28,571) | 1553 | 5.44 | 1.72 (1.46–2.02) | 696 | 7.05 | 1.17 (0.82–1.68) | 857 | 4.59 | 1.88 (1.57–2.24) |
| CNS (n = 287) | 11 | 3.83 | NA | 6 | 3.33 | NA | 5 | 4.67 | NA |
| Thyroid (n = 4784) | 37 | 0.77 | 0.30 (.021–0.44) | 6 | 0.57 | NA | 31 | 0.83 | 0.42 (0.28–0.62) |
| Head and neck (n = 799) | 53 | 6.63 | 1.52 (1.01–2.30) | 35 | 6.52 | 1.09 (0.66–1.81) | 18 | 6.87 | NA |
| Digestive (n = 8722) | 641 | 7.35 | 1.33 (1.05–1.68) | 476 | 8.10 | 1.17 (0.80–1.70) | 165 | 5.80 | 1.41 (1.05–1.88) |
| Esophagus (n = 1611) | 241 | 14.96 | 2.23 (1.65–3.02) | 185 | 16.53 | 2.49 (1.64–3.79) | 56 | 11.38 | 1.91 (1.24–2.95) |
| Gastric-esophageal junction (n = 522) | 46 | 8.81 | 0.80 (0.48–1.33) | 38 | 8.70 | 0.69 (0.39–1.22) | 8 | 9.41 | NA |
| Stomach (n = 2055) | 141 | 6.86 | 1.12 (0.80–1.56) | 111 | 7.58 | 1.04 (0.68–1.60) | 30 | 5.08 | 1.20 (0.76–1.90) |
| Colon, rectum and anus (n = 2424) | 124 | 5.12 | 0.74 (0.53–1.03) | 83 | 5.79 | 0.69 (0.44–1.08) | 41 | 4.14 | 0.85 (0.55–1.29) |
| Liver (n = 1316) | 53 | 4.03 | 0.50 (0.32–0.79) | 41 | 4.05 | 0.53 (0.32–0.87) | 12 | 3.96 | NA |
| Biliary tract (n = 369) | 19 | 5.15 | NA | 8 | 5.44 | NA | 11 | 4.95 | NA |
| Pancreas (n = 425) | 17 | 4.00 | NA | 10 | 3.82 | NA | 7 | 4.29 | NA |
| Hematologic malignancies (n = 894) | 140 | 15.66 | 4.88 (3.66–6.51) | 88 | 18.14 | 4.07 (2.66–6.21) | 52 | 12.71 | 4.87 (3.39–7.01) |
| Non-Hodgkin's lymphoma (n = 675) | 113 | 16.74 | 4.57 (3.35–6.24) | 71 | 19.72 | 4.27 (2.74–6.66) | 42 | 13.33 | 4.21 (2.80–6.33) |
| Hodgkin's lymphoma (n = 122) | 20 | 16.39 | NA | 12 | 18.46 | NA | 8 | 14.04 | NA |
| Multiple myeloma (n = 87) | 6 | 6.90 | NA | 4 | 7.41 | NA | 2 | 6.06 | NA |
| Leukemia (n = 10) | 1 | 10.00 | NA | 1 | 16.67 | NA | 0 | 0.00 | NA |
| Genitourinary (n = 5711) | 389 | 6.81 | 2.22 (1.82–2.70) | NA | NA | NA | NA | NA | NA |
| Cervix (n = 2839) | 261 | 9.19 | 3.49 (2.79–4.38) | NA | NA | NA | NA | NA | NA |
| Uterus (n = 780) | 22 | 2.82 | NA | NA | NA | NA | NA | NA | NA |
| Ovary (n = 1045) | 56 | 5.36 | 1.88 (1.33–2.64) | NA | NA | NA | NA | NA | NA |
| Prostate (n = 277) | 16 | 5.78 | NA | NA | NA | NA | NA | NA | NA |
| Bladder (n = 314) | 17 | 5.41 | NA | 14 | 6.06 | NA | 3 | 3.61 | NA |
| Kidney (n = 456) | 17 | 3.73 | NA | 13 | 4.59 | NA | 4 | 2.31 | NA |
| Skin cancer (n = 491) | 32 | 6.52 | 0.84 (0.51–1.37) | 15 | 6.05 | NA | 17 | 7.00 | NA |
| Melanoma (n = 174) | 12 | 6.90 | NA | 6 | 7.06 | NA | 6 | 6.74 | NA |
| Nonmelanoma (n = 317) | 20 | 6.31 | NA | 9 | 5.52 | NA | 11 | 7.14 | NA |
| Bone and soft tissue (n = 718) | 21 | 2.92 | NA | 9 | 2.59 | NA | 13 | 3.50 | NA |
| Breast (n = 5443) | 171 | 3.14 | 1.29 (1.02–1.62) | 0 | 0.00 | NA | 171 | 3.16 | 1.36 (1.08–1.73) |
| Unknown primary (n = 181) | 16 | 8.84 | NA | 8 | 8.16 | NA | 8 | 9.64 | NA |
| Other* (n = 541) | 42 | 7.76 | 1.93 (1.28–2.90) | 10 | 4.46 | NA | 32 | 10.09 | 2.41 (1.53–3.80) |
Notes: NA not applicable, N number
*, this category included cancers originating from the testis, vulva, penis, vagina, labia majora, labia minora, perineum, scrotum, hydatidiform mole, chorion, notochord, adrenal cortex, neuroendocrine system, mediastinum, sweat glands, mesothelium, thymus, umbilical tube and so on
OR, odds ratio; 95% CI, 95% confidence interval; CNS, central nervous system;
*The OR was adjusted by sex and age (as a continuous variable)
a The OR was adjusted by age (as a continuous variable)
Fig. 1.
The fold change in the proportion of tuberculosis in patients with different cancer sites in the malignant group compared with the benign tumor group. Note, CNS, central nervous system
In the multivariate logistic regression analysis, TB remained an independent risk factor for all cancers (OR 1.68, 95% CI 1.43-1.97), including lung cancer (OR 1.44, 95% CI 1.0-1.95) and extrapulmonary cancer (OR 1.72, 95% CI 1.46-2.02), after adjusting for sex and age. Analysis stratified by specific types of cancer revealed high risks of non-Hodgkin’s lymphoma, cervical cancer, esophageal cancer, “other” cancer, ovarian cancer, head and neck cancer, and breast cancer and reduced risks of thyroid and liver cancer after TB. No statistically significant elevations in the risk of cancers originating from the gastric-esophageal junction, stomach, colon, rectum, anus, or skin were observed. With the exception of esophageal cancer and non-Hodgkin’s lymphoma, the relative cancer risk after TB was significantly elevated in females but not in males in the study group.
Discussion
The current case-control study was performed in Xinjiang Province in China, where the prevalence of pulmonary TB is high [6, 20]. In this study, we found a greater proportion of patients with malignant cancers than of those with benign tumors who had a previous TB. Prior TB might increase the risks of lung cancer and some extrapulmonary cancers, including hematologic malignancies, esophageal cancer, genitourinary cancers, head and neck cancer, breast cancer, and “other” cancers. In accord with several prior studies [9, 11, 13, 15, 21], our results indicate an association between TB and certain cancer types (Table 3).
Table 3.
A summary of studies of the risk of cancer in tuberculosis (TB) patients compared with the general population
| Type of TB infection | Type of study | Sample Size | Incidence of cancer in TB patients | Hazard ratio or relative risk |
Extrapulmonary cancer type |
PMID | |
|---|---|---|---|---|---|---|---|
|
Kuo et al. 2013 |
Latent | Retrospective population-based study | 530 | 7.91% |
All cancers: 2.07, 95% CI, 1.90-2.26 Nonpulmonary cancer: 1.71, 95% CI: 1.54-1.90 |
Head and neck, Digestive tract, Skin, Bladder, Hematological malignancies | 23,652,313 |
| Simonsen et al. 2014 | Active | Cohort study | 15,024 | 11.6% |
All cancers: 1.52, 95% CI 1.45-1.59; Extrapulmonary cancer: 1.29, 95% CI: 1.22-1.36 |
Malignant pleural mesothelioma, Hodgkin’s lymphoma, ovarian, non-Hodgkin’s malignant lymphoma | 25,216,835 |
| Su et al. 2016 | Latent |
Retrospective population-based study |
11,522 | 8.46% |
All cancers: 2.29, 95% CI: 1.26-4.17 Nonpulmonary cancer: NA |
Multiple myeloma, kidney, bladder, hepatobiliary, gastrointestinal | 26,825,880 |
| Everatt et al. 2017 | Active | Retrospective cohort study | 21,986 | 7.2% |
All cancers: 1.76, 95% CI: 1.67-1.85; Nonpulmonary cancer: 1.41, 95% CI: 1.33-1.50 |
Mouth and pharynx, esophagus, stomach, larynx, cervix uteri, leukemia, hematological cancers, “other” cancers | 28,572,839 |
|
Chen et al., 2020 |
Mixed | Case-control study | 32,539 cases and 12,916 controls | NA |
All cancers: 1.68, 95% CI: 1.43-1.97; Nonpulmonary cancer: 1.72, 95% CI: 1.46-2.02 |
Non-Hodgkin’s lymphoma, cervical cancer, esophageal cancer, “other” cancer, ovarian cancer, head and neck cancer, breast cancer | This study |
NA not available
Chronic inflammation during TB may lead to cancer development [22-25]. However, the etiopathogenesis of this association is still imperfectly known. In lung cancer patients, TB-associated chronic inflammation in the lungs could cause clastogenic activity in the DNA of bronchial epithelium [22]. Furthermore, intracellular Mycobacterium TB DNA may induce neoplastic transformation of bronchial epithelial cells via lateral gene transfer [22]. Moreover, TB [26] and other chronic inflammatory disorders [27] might exert a huge influence on the secretory inflammation profiles of T helper cells and decrease antigen-specific T-cell responses in the pulmonary and lymphoid compartments [23].
Although far more males than females have TB globally [28, 29], TB-infected females than males often have more precarious social and economic positions [29, 30]. In this study, females seemed to have a higher risk of developing cancer than males after TB, although esophageal cancer and non-Hodgkin’s lymphoma were exceptions, suggesting that TB may contribute to the sex-specific development of malignant cancer. Indeed, estrogen signaling in females may contribute to a complex and sex-specific link between TB and cancer [31], such as TB could reprogram macrophages to a nonbactericidal M2 phenotype [28, 32-35]. In addition, some unadjusted confounding factors, such as autoimmune disorders, preferably occurred in females and were linked to lung and extrapulmonary cancer risk [36-38]. However, more future prospective studies on female sex and cancer risk are warranted.
Indeed, prior TB has been consistently found to be associated with a subsequent high risk of hematological malignancies, including non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, multiple myeloma, and leukemia. In addition, more than one published study has shown that patients with TB are relatively more susceptible to cancer in the digestive tract, genitourinary tract, and head and neck. Consistent with these findings, we showed that TB patients have elevated risks of esophageal cancer, cervical cancer, head and neck cancer, and ovarian cancer. Furthermore, we showed that TB patients had an elevated risk of breast cancer. However, our data did not show that there was an elevated risk of skin cancer after TB. In contrast to the increased risk of hepatobiliary cancers shown by Su et al. [15], we found that TB has a protective effect against liver cancers (OR 0.50, 95% CI 0.32-0.79). However, the effect of TB on liver cancer is still controversial [39]. The differences in the risk of specific types of cancer risk in TB patients might be due to both differences in the study populations and differences in the research design.
This study was performed in a highly endemic area for TB with a large sample size. However, the current study was limited by estimating the real incidence of cancer after TB. In our study, we adjusted for age, sex, and ethnicity but lacked data to examine the role of tobacco smoking and other confounding factors associated with both TB and cancer. Furthermore, the misclassification of TB was also possible, although we included both active and inactive TB cases.
In clinical practice, clinicians should be alert to the association between cancer and TB. Some cohort studies have shown that the incidence of cancers in TB patients ranged from 7.2 to 11.6% (Table 3). The present study indicated that approximately 5% of cancer patients had prior TB in a highly endemic area in China. Indeed, some scientists have reported that the reactivation of latent TB infection has detrimental effects on outcomes in cancer patients during treatment with immune checkpoint inhibitors [40] and intensive chemotherapy [40-43]. Thus, we recommend screening for latent or active TB before initiating immunotherapy and intensive chemotherapy in most cancer patients. However, we were unable to calculate the benefits of this screening strategy in cancer patients. A future prospective study assessing the relationship of TB with prognosis in cancer patients is warranted.
Conclusions
Our data showed that a greater proportion of cancer patients than benign tumor patients with a history of TB, indicating a high risk of cancer in TB patients. Prior TB may increase the risk of cancer, including hematologic malignancies, esophageal cancer, genitourinary cancers, head and neck cancer, breast cancer, and “other” cancers.
Acknowledgments
We appreciate the valuable comments on our study by Dr. Yong Zhou and Dr. Gui-Qin Yao.
Abbreviations
- TB
Tuberculosis
- OR
Odds ratio
- CI
Confidence interval
- CNS
Central nervous system
Authors’ contributions
S. Y. and D.M. J. contributed equally to the initial conception, design and coordination of this study. All authors provided input and guidance in the revision and development of the study. Statistical support in preparing the study was provided by G.L. C and L. G. The manuscript was drafted by G.L. C. and D.M. J. Correspondence to Shun’e Yang or Dong-Mei Ji. All authors have read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81802362). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to privacy/ethical restrictions but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Xinjiang Medical University Institutional Review Board (number K-2021004). Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors have declared that no competing interests exist.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guang-Liang Chen and Li Guo contributed equally to this work.
References
- 1.Hershkovitz I, Donoghue HD, Minnikin DE, May H, Lee OY, Feldman M, et al. Tuberculosis origin: the Neolithic scenario. Tuberculosis (Edinb). 2015;95(Suppl 1):S122-6. 10.1016/j.tube.2015.02.021. [DOI] [PubMed]
- 2.Global tuberculosis report 2018. Geneva: World Health Organization; 2018. Licence: CCBY-NCSA3.0IGO.
- 3.Padayatchi N, Daftary A, Naidu N, Naidoo K, Pai M. Tuberculosis: treatment failure, or failure to treat? Lessons from India and South Africa. BMJ Glob Health. 2019;4(1):e001097. 10.1136/bmjgh-2018-001097. [DOI] [PMC free article] [PubMed]
- 4.Kyu HH, Maddison ER, Henry NJ, Mumford JE, Barber R, Shields C, et al. The global burden of tuberculosis: results from the global burden of disease study 2015. Lancet Infect Dis. 2018;18(3):261-84. 10.1016/S1473-3099(17)30703-X. [DOI] [PMC free article] [PubMed]
- 5.Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med. 2016;13(10):e1002152. 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed]
- 6.Mijiti P, Yuehua L, Feng X, Milligan PJ, Merle C, Gang W, et al. Prevalence of pulmonary tuberculosis in western China in 2010-11: a population-based, cross-sectional survey. Lancet Glob Health. 2016;4(7):e485-94. 10.1016/S2214-109X(16)30074-2. [DOI] [PubMed]
- 7.Qi YC, Ma MJ, Li DJ, Chen MJ, Lu QB, Li XJ, et al. Multidrug-resistant and extensively drug-resistant tuberculosis in multiethnic region, Xinjiang Uygur autonomous region, China. PLoS One. 2012;7(2):e32103. 10.1371/journal.pone.0032103. [DOI] [PMC free article] [PubMed]
- 8.Cao S, Li J, Lu J, Zhong R, Zhong H. Mycobacterium tuberculosis antigens repress Th1 immune response suppression and promotes lung cancer metastasis through PD-1/PDl-1 signaling pathway. Cell Death Dis. 2019;10(2):44. doi: 10.1038/s41419-018-1237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonsen DF, Farkas DK, Sogaard M, Horsburgh CR, Sorensen HT, Thomsen RW. Tuberculosis and risk of cancer: a Danish nationwide cohort study. Int J Tuberc Lung Dis. 2014;18(10):1211–1219. doi: 10.5588/ijtld.14.0161. [DOI] [PubMed] [Google Scholar]
- 10.Shu CC, Liao KM, Chen YC, Wang JJ, Ho CH. The burdens of tuberculosis on patients with malignancy: incidence, mortality and relapse. Sci Rep. 2019;9(1):11901. doi: 10.1038/s41598-019-48395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo SC, Hu YW, Liu CJ, Lee YT, Chen YT, Chen TL, et al. Association between tuberculosis infections and nonpulmonary malignancies: a nationwide population-based study. Br J Cancer. 2013;109(1):229-34. 10.1038/bjc.2013.220. [DOI] [PMC free article] [PubMed]
- 12.Yu YH, Liao CC, Hsu WH, Chen HJ, Liao WC, Muo CH, et al. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol. 2011;6(1):32-7. 10.1097/JTO.0b013e3181fb4fcc. [DOI] [PubMed]
- 13.Everatt R, Kuzmickiene I, Davidaviciene E, Cicenas S. Nonpulmonary cancer risk following tuberculosis: a nationwide retrospective cohort study in Lithuania. Infect Agent Cancer. 2017;12(1):33. doi: 10.1186/s13027-017-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everatt R, Kuzmickiene I, Davidaviciene E, Cicenas S. Incidence of lung cancer among patients with tuberculosis: a nationwide cohort study in Lithuania. Int J Tuberc Lung Dis. 2016;20(6):757–763. doi: 10.5588/ijtld.15.0783. [DOI] [PubMed] [Google Scholar]
- 15.Su VY, Yen YF, Pan SW, Chuang PH, Feng JY, Chou KT, et al. Latent tuberculosis infection and the risk of subsequent Cancer. Medicine (Baltimore). 2016;95(4):e2352. 10.1097/MD.0000000000002352. [DOI] [PMC free article] [PubMed]
- 16.Kamboj M, Sepkowitz KA. The risk of tuberculosis in patients with cancer. Clin Infect Dis. 2006;42(11):1592–1595. doi: 10.1086/503917. [DOI] [PubMed] [Google Scholar]
- 17.Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One. 2011;6(3):e17479. doi: 10.1371/journal.pone.0017479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia Z, Cheng S, Ma Y, Zhang T, Bai L, Xu W, et al. Tuberculosis burden in China: a high prevalence of pulmonary tuberculosis in household contacts with and without symptoms. BMC Infect Dis. 2014;14(1):64. 10.1186/1471-2334-14-64. [DOI] [PMC free article] [PubMed]
- 19.Li XX, Zhang H, Jiang SW, Liu XQ, Fang Q, Li J, et al. Geographical distribution regarding the prevalence rates of pulmonary tuberculosis in China in 2010. Zhonghua Liu Xing Bing Xue Za Zhi. 2013;34(10):980-4. [PubMed]
- 20.Xia Y, Xin D, Chen W, Zhang H, Liu X, Xue L, et al. Pulmonary tuberculosis prevalence among different regions in China in. Chinese Journal of Antituberculosis. 2010;2012:803-7.
- 21.Omoti CE, Olu-Eddo AN, Nwannadi AI. Coexistence of TB and adult hematological cancers in Benin City, Nigeria. Trop Doct. 2009;39(4):205–207. doi: 10.1258/td.2009.080348. [DOI] [PubMed] [Google Scholar]
- 22.Molina-Romero C, Arrieta O, Hernandez-Pando R. Tuberculosis and lung cancer. Salud Publica Mex. 2019;61(3):286–291. doi: 10.21149/10090. [DOI] [PubMed] [Google Scholar]
- 23.Chen GL, Xia ZG, Jin J, Yu BH, Cao J. Characterization of artificial pneumothorax-unrelated Pyothorax-associated lymphoma. J Oncol. 2021;2021:3869438. doi: 10.1155/2021/3869438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12(8):715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 25.Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park) 2011;25(5):400–410. [PubMed] [Google Scholar]
- 26.Qiu L, Huang D, Chen CY, Wang R, Shen L, Shen Y, et al. Severe tuberculosis induces unbalanced upregulation of gene networks and overexpression of IL-22, MIP-1alpha, CCL27, IP-10, CCR4, CCR5, CXCR3, PD1, PDL2, IL-3, IFN-beta, TIM1, and TLR2 but low antigen-specific cellular responses. J Infect Dis. 2008;198(10):1514-9. 10.1086/592448. [DOI] [PMC free article] [PubMed]
- 27.Cope AP. Studies of T-cell activation in chronic inflammation. Arthritis Res. 2002;4(Suppl 3):S197–S211. doi: 10.1186/ar557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nhamoyebonde S, Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis. 2014;209(Suppl 3):S100–S106. doi: 10.1093/infdis/jiu147. [DOI] [PubMed] [Google Scholar]
- 29.Connolly M, Nunn P. Women and tuberculosis. World Health Stat Q. 1996;49(2):115–119. [PubMed] [Google Scholar]
- 30.Srivastava K, Kant S, Narain A, Bajpai J. Tuberculosis in women: A reflection of gender inequity. 2018:PA531.
- 31.Keselman A, Fang X, White PB, Heller NM. Estrogen signaling contributes to sex differences in macrophage polarization during asthma. J Immunol. 2017;199(5):1573–1583. doi: 10.4049/jimmunol.1601975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Routley CE, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound Repair Regen. 2009;17(1):42–50. doi: 10.1111/j.1524-475X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 33.Menzies FM, Henriquez FL, Alexander J, Roberts CW. Selective inhibition and augmentation of alternative macrophage activation by progesterone. Immunology. 2011;134(3):281–291. doi: 10.1111/j.1365-2567.2011.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An D, Kasper DL. Testosterone: more than having the guts to win the tour de France. Immunity. 2013;39(2):208–210. doi: 10.1016/j.immuni.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Najafi M, Hashemi Goradel N, Farhood B, Salehi E, Nashtaei MS, Khanlarkhani N, et al. Macrophage polarity in cancer: a review. J Cell Biochem. 2019;120(3):2756-65. 10.1002/jcb.27646. [DOI] [PubMed]
- 36.Bordignon V, Bultrini S, Prignano G, Sperduti I, Piperno G, Bonifati C, et al. High prevalence of latent tuberculosis infection in autoimmune disorders such as psoriasis and in chronic respiratory diseases, including lung cancer. J Biol Regul Homeost Agents. 2011;25(2):213-20. [PubMed]
- 37.Hemminki K, Liu X, Ji J, Sundquist J, Sundquist K. Effect of autoimmune diseases on risk and survival in histology-specific lung cancer. Eur Respir J. 2012;40(6):1489–1495. doi: 10.1183/09031936.00222911. [DOI] [PubMed] [Google Scholar]
- 38.Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev. 2017;16(10):1049–1057. doi: 10.1016/j.autrev.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Dobler CC, Cheung K, Nguyen J, Martin A. Risk of tuberculosis in patients with solid cancers and hematological malignancies: a systematic review and meta-analysis. Eur Respir J. 2017;50(2). [DOI] [PubMed]
- 40.Anastasopoulou A, Ziogas DC, Samarkos M, Kirkwood JM, Gogas H. Reactivation of tuberculosis in cancer patients following administration of immune checkpoint inhibitors: current evidence and clinical practice recommendations. J Immunother Cancer. 2019;7(1):239-51. 10.1186/s40425-019-0717-7. [DOI] [PMC free article] [PubMed]
- 41.World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva; 2018. https://pubmed.ncbi.nlm.nih.gov/30277688/. [PubMed]
- 42.Force USPST, Bibbins-Domingo K, Grossman DC, Curry SJ, Bauman L, Davidson KW, et al. Screening for latent tuberculosis infection in adults: US preventive services task Force recommendation statement. JAMA. 2016;316(9):962-9. [DOI] [PubMed]
- 43.Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000, 161(4 Pt 2):S221-S247. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy/ethical restrictions but are available from the corresponding author on reasonable request.