PURPOSE
In SOLO1, maintenance olaparib (300 mg twice daily) significantly improved progression-free survival (PFS) for patients with newly diagnosed BRCA1- and/or BRCA2-mutated advanced ovarian cancer compared with placebo (hazard ratio [HR], 0.30; 95% CI, 0.23 to 0.41; median not reached v 13.8 months). We investigated PFS in SOLO1 for subgroups of patients based on preselected baseline factors.
PATIENTS AND METHODS
Investigator-assessed PFS subgroup analyses of SOLO1 included clinical response after platinum-based chemotherapy (complete [CR] or partial response [PR]), surgery type (upfront or interval surgery), disease status after surgery (residual or no gross residual disease), and BRCA mutation status (BRCA1 or BRCA2). Additionally, we evaluated PFS in patients with stage III disease who underwent upfront surgery and had no gross residual disease. We also report objective response rate.
RESULTS
The risk of disease progression or death was reduced with olaparib compared with placebo by 69% (HR, 0.31; 95% CI, 0.21 to 0.46) and 63% (HR, 0.37; 95% CI, 0.24 to 0.58) in patients undergoing upfront or interval surgery; 56% (HR, 0.44; 95% CI, 0.25 to 0.77) and 67% (HR, 0.33; 95% CI, 0.23 to 0.46) in patients with residual or no residual disease after surgery; 66% (HR, 0.34; 95% CI, 0.24 to 0.47) and 69% in women with clinical CR or PR at baseline (HR, 0.31; 95% CI, 0.18 to 0.52); and 59% (HR, 0.41; 95% CI, 0.30 to 0.56) and 80% (HR 0.20; 95% CI, 0.10 to 0.37) in patients with a BRCA1 or BRCA2 mutation, respectively.
CONCLUSION
Patients with newly diagnosed advanced ovarian cancer achieve substantial benefit from maintenance olaparib treatment regardless of baseline surgery outcome, response to chemotherapy, or BRCA mutation type.
INTRODUCTION
For patients with newly diagnosed advanced ovarian cancer (OC), the standard of care is cytoreductive surgery and platinum-based chemotherapy.1,2 Most patients have no evidence of disease (NED) after treatment, but approximately 70% will relapse within 3 years of diagnosis.2 After recurrence, most patients receive multiple additional lines of treatment and will eventually die as a result of the disease.
CONTEXT
Key Objective
To explore whether all patients receiving first-line olaparib maintenance (compared with surveillance alone) will benefit from treatment regardless of baseline characteristics (preselected covariates), including those with favorable prognostic features (eg, patients with complete cytoreduction, those with complete response after platinum-based chemotherapy, or those with stage III disease who underwent upfront surgery and had no gross residual disease), or BRCA mutation status and report objective response rate to better understand the olaparib treatment effect in patients with newly diagnosed BRCA-mutated advanced ovarian cancer.
Knowledge Generated
SOLO1 subgroup analyses of PFS reported here were consistent with those previously reported in the overall study population, demonstrating that olaparib maintenance therapy was of substantial benefit in all reported patient subgroups.
Relevance
Regardless of patient baseline outcomes from surgery and chemotherapy or BRCA mutation type, patients with newly diagnosed advanced ovarian cancer are at high risk of disease progression and benefit from maintenance olaparib treatment.
Olaparib, a poly(ADP-ribose) polymerase (PARP) inhibitor, has demonstrated efficacy in several tumor types, including advanced OC, breast, prostate, and pancreatic cancers.3-7 Olaparib is approved in the United States, the European Union, and other countries as maintenance treatment for women with germline or somatic BRCA-mutated advanced OC who are in response to first-line platinum-based chemotherapy based on the phase III SOLO1 study (ClinicalTrials.gov identifier: NCT01844986).8,9 SOLO110 reported a substantial improvement in progression-free survival (PFS) after maintenance olaparib (tablets) versus placebo in patients with newly diagnosed advanced OC and a BRCA1 or BRCA2 mutation (Kaplan-Meier estimate of rate of freedom from disease progression or death at 3 years, 60% v 27%, respectively; hazard ratio [HR], 0.30; 95% CI, 0.23 to 0.41). In contrast to some contemporary trials in this setting (GOG-0218,11 ENGOT-OV26/GOG-3012/PRIMA,12 IMagyn050,13 AGO-OVAR1614), SOLO1 recruited patients regardless of prior surgical status; patients could have undergone upfront or interval cytoreductive surgery and have residual or no gross residual disease; however, in SOLO1, patients were required to have a BRCA-mutated tumor.
Baseline factors that may affect outcomes of patients with newly diagnosed advanced OC include tumor response (complete response [CR] v partial response [PR]) after platinum-based chemotherapy, BRCA mutation status, and timing of cytoreductive surgery (interval v upfront), as well as outcomes after surgery (residual v no gross residual disease).15,16 Surgical outcome has been reported as 1 of the most important independent prognostic factors for survival,16 with a significant survival advantage observed in patients with no gross residual disease compared with those with residual tumor burden of 1 to 10 mm or > 10 mm in diameter.17 We wished to explore whether all patients receiving first-line olaparib maintenance (compared with surveillance alone) benefit from treatment regardless of baseline characteristics, including those with favorable prognostic features (eg, complete cytoreduction or CR after platinum-based chemotherapy). In patients with no evidence of gross residual disease after surgery, it is likely that micrometastatic disease remains in almost all cases, and the risk of recurrence remains high18; despite being associated with a better prognosis, most of these patients will experience relapse later. It is possible that these patients may obtain even greater benefit from maintenance olaparib than those who have evidence of disease at baseline, because patients who initiate treatment with NED have longer PFS versus those with evidence of disease at baseline.19-21
We report the efficacy of olaparib in SOLO1 in terms of PFS using preselected baseline characteristics of surgical status and response after completion of platinum-based chemotherapy, patients with stage III disease who underwent upfront surgery and had no gross residual disease, and BRCA mutation status. We also report objective response rate (ORR) evaluated in women with radiologic evidence of disease at baseline to better understand the olaparib treatment effect in patients with newly diagnosed BRCA-mutated advanced OC.
PATIENTS AND METHODS
Study Design
SOLO1 was a phase III, multicenter, randomized, double-blind study.10 Patients had newly diagnosed confirmed advanced (International Federation of Gynecology and Obstetrics [FIGO] stage III or IV) high-grade serous or endometrioid OC, primary peritoneal cancer, and/or fallopian tube cancer, were in clinical CR (defined as no radiologic evidence of disease and normal cancer antigen–125 [CA-125] level) or PR (≥ 30% decrease in sum of diameters of target lesions or no radiologic evidence of disease after chemotherapy but abnormal CA-125 level) after platinum-based chemotherapy, and had deleterious or suspected deleterious germline or somatic BRCA mutation (Data Supplement provides testing details). Patients with stage III disease had undergone cytoreductive surgery before chemotherapy (upfront) or after initiation but before completion of chemotherapy (interval), and those with stage IV disease had undergone biopsy and/or upfront or interval cytoreductive surgery. Full inclusion/exclusion criteria have been published previously.10
Study Treatments
After completion of first-line platinum-based chemotherapy, patients were randomly assigned 2:1 to maintenance olaparib tablets (300 mg twice daily) or placebo (Fig 1). Random assignment was stratified according to clinical response after platinum-based chemotherapy (CR or PR). Treatment was continued until investigator-assessed objective disease progression (modified Response Evaluation Criteria In Solid Tumors [RECIST] version 1.1). After 2 years of treatment, patients with CR or NED discontinued treatment; those with evidence of disease could continue treatment.10
FIG 1.
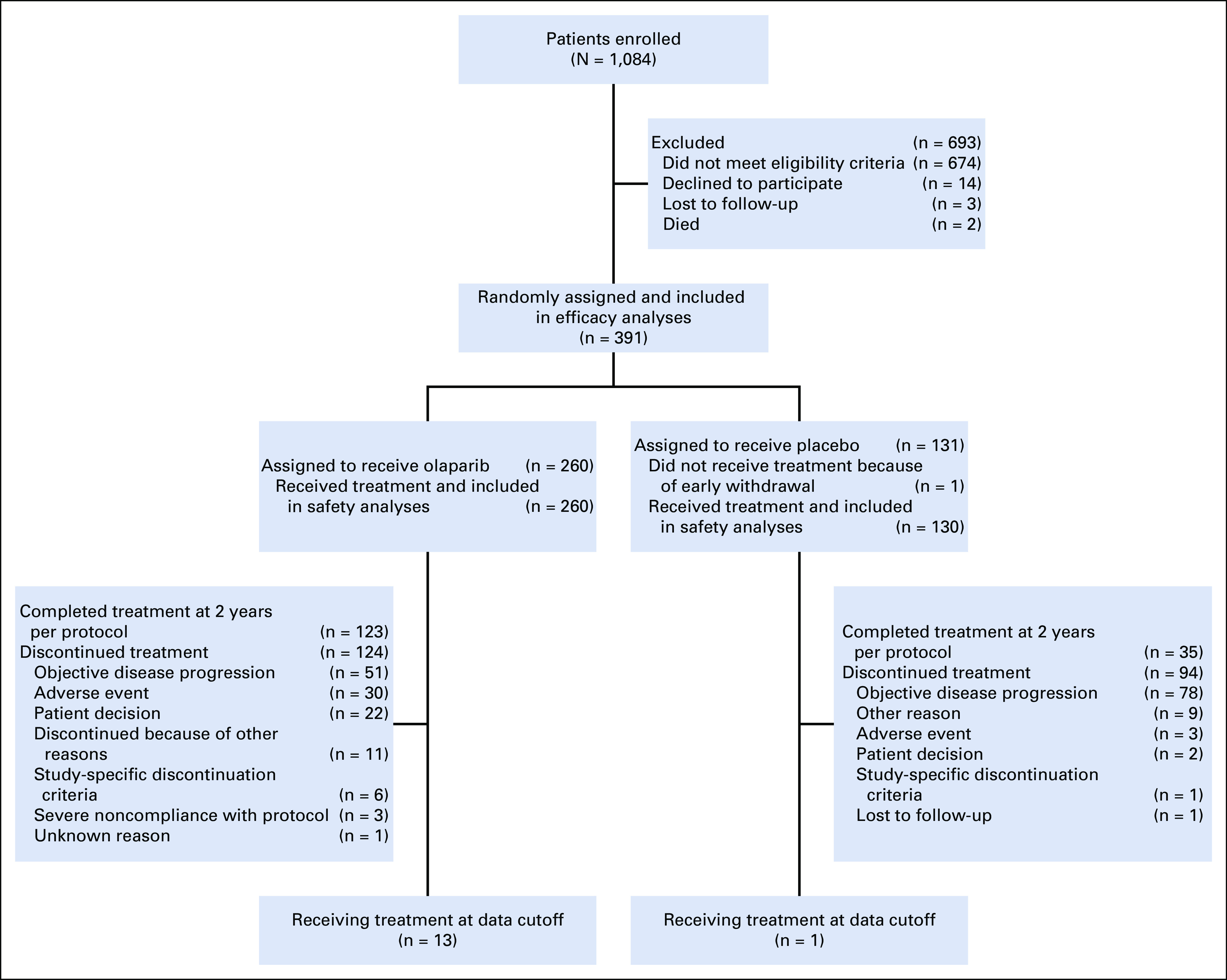
CONSORT diagram.
Study Outcome Measures
The primary efficacy analysis data cutoff (DCO) was May 17, 2018.10 Subgroup analyses reported here evaluated investigator-assessed PFS by modified RECIST (version 1.1) at the primary DCO. We used preselected covariates defined as clinically relevant for study patients. Prespecified subgroup analyses included clinical response after platinum-based chemotherapy (CR or PR) and BRCA mutation status (BRCA1/BRCA2). Subgroup efficacy analyses were also performed based on timing of surgery (upfront/interval; exploratory) and surgery outcome (macroscopic residual or no gross residual disease; prespecified) reported using data collected by electronic case report form (eCRF). PFS was also evaluated in patients with stage III disease with no gross residual disease after upfront surgery to determine the value of maintenance olaparib in patients with favorable prognostic features.
ORR (modified RECIST) was a secondary end point evaluated in women with radiologic evidence of disease at baseline. ORR was calculated based on overall visit responses from each postbaseline RECIST assessment (investigator assessed) before detection of progression or initiation of subsequent anticancer therapy.
Statistical Analysis
For subgroup analyses of PFS, the HRs (olaparib:placebo) and associated 95% CIs were calculated from a Cox proportional hazards model that contained the treatment term, factor (subgroup), and treatment-by-factor interaction term. CIs were calculated using a profile likelihood approach.22 An HR < 1 favored olaparib. Subgroup analyses of PFS were not powered to detect a statistically significant difference between subgroups evaluated. ORR was summarized by the number and percentage of patients with measurable disease at baseline. Statistical analyses were performed with SAS (version 9.4; SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
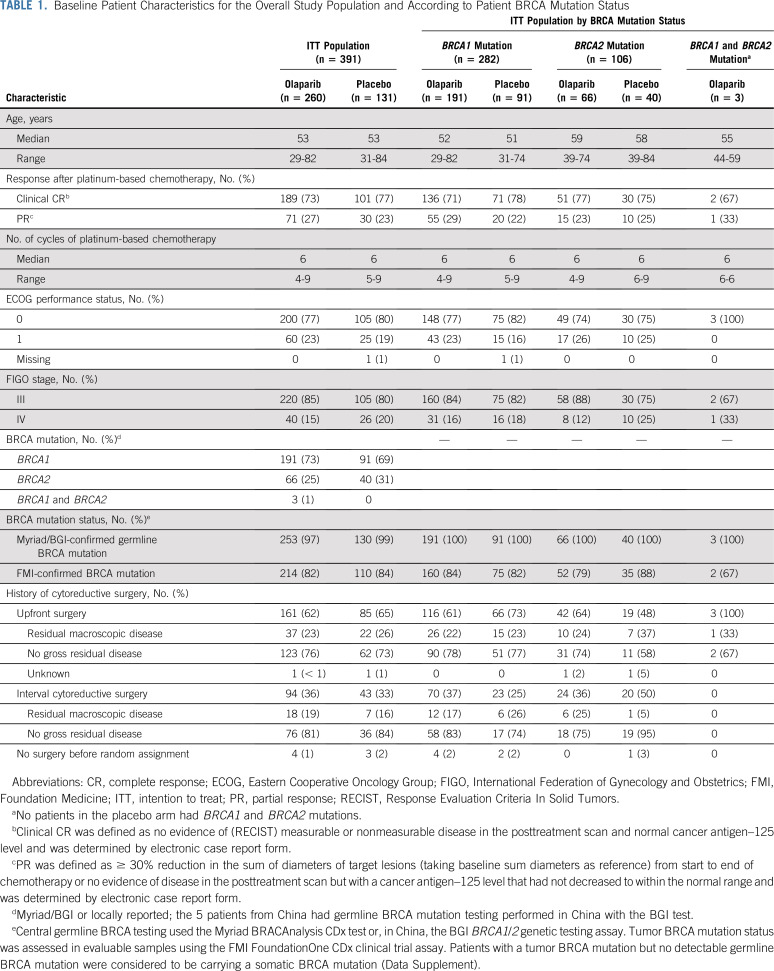
Patient characteristics were generally well balanced between treatment groups (Table 1).10 Overall, 282 patients (72%) had a BRCA1 mutation, 106 (27%) had a BRCA2 mutation, and 3 (1%) had both. Patient baseline characteristics for BRCA mutation (Table 1) and other subgroups (Data Supplement) were generally balanced.
TABLE 1.
Baseline Patient Characteristics for the Overall Study Population and According to Patient BRCA Mutation Status
PFS According to Subgroup Analysis
At DCO, median follow-up was approximately 41 months in both arms. In the olaparib arm, median treatment duration was 24.6 months, consistent with the 2-year prespecified treatment duration; for placebo, this was 13.9 months, consistent with the median PFS reported.
Surgical status.
In total, 63% and 35% of patients underwent upfront and interval surgery, respectively; 21% and 76% had residual and no gross residual disease, respectively.
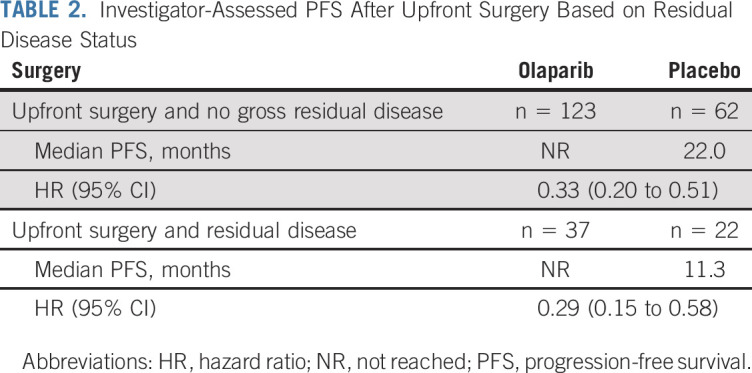
The risk of disease progression or death was reduced with olaparib compared with placebo by 69% (median PFS, not reached [NR] v 15.3 months, respectively; HR, 0.31; 95% CI, 0.21 to 0.46) and 63% (33.6 v 9.8 months; HR, 0.37; 95% CI, 0.24 to 0.58) in patients undergoing upfront and interval surgery, respectively (Fig 2A), and by 56% (29.4 v 11.3 months; HR, 0.44; 95% CI, 0.25 to 0.77) and 67% (NR v 15.3 months; HR, 0.33; 95% CI, 0.23 to 0.46) in patients with residual and no gross residual disease after surgery, respectively (Fig 2B). Similar results were observed for patients with or without residual disease after upfront surgery (Table 2).
FIG 2.
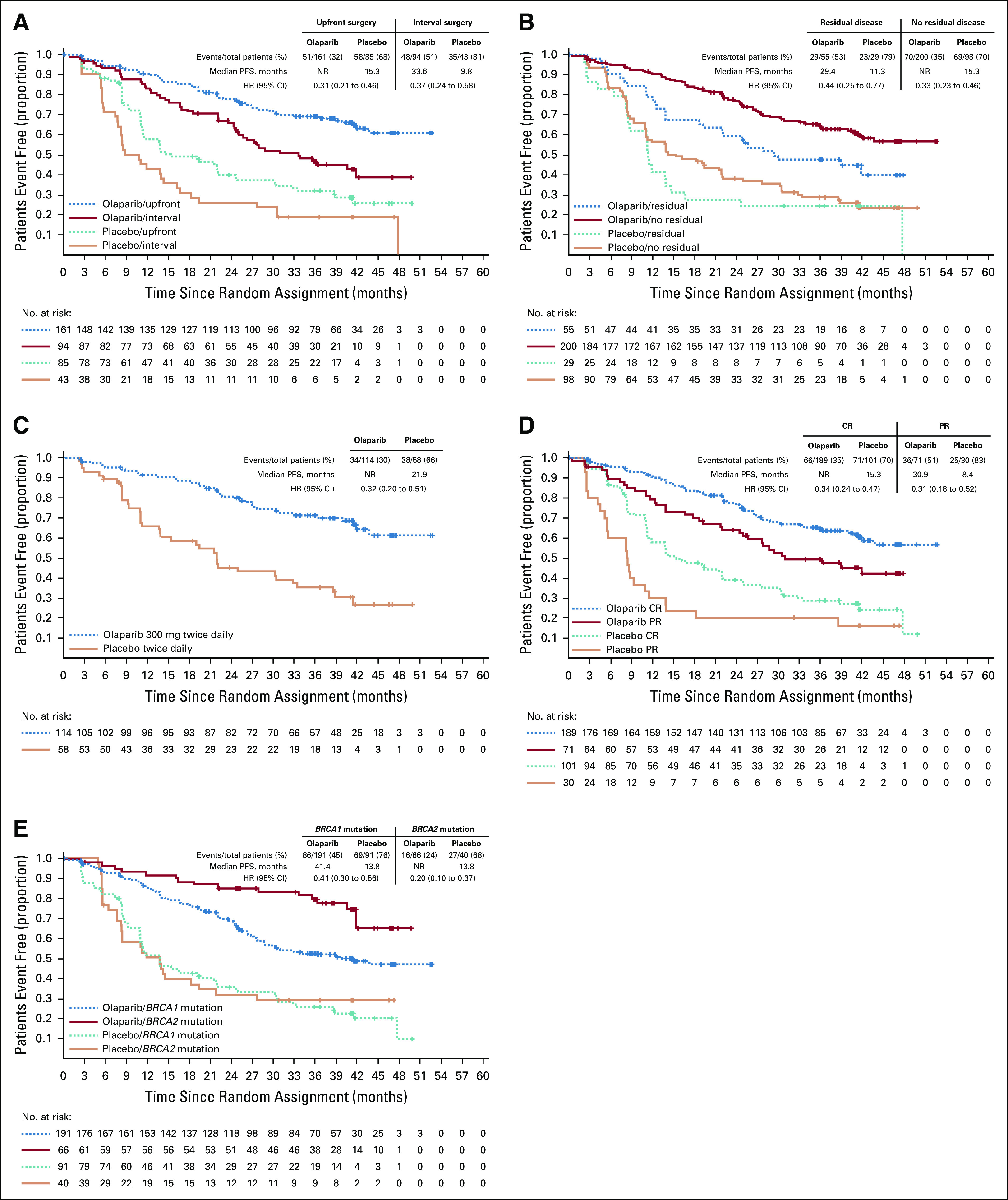
Kaplan-Meier estimates of investigator-assessed progression-free survival (PFS) for subgroup analyses based on (A) surgery timing, (B) residual macroscopic disease status, (C) patients with stage III disease who underwent upfront surgery and had no gross residual disease, (D) response after platinum-based chemotherapy at baseline, and (E) BRCA mutation status. CR, complete response; HR, hazard ratio; NR, not reached; PR, partial response.
TABLE 2.
Investigator-Assessed PFS After Upfront Surgery Based on Residual Disease Status
Kaplan-Meier estimates of the percentage of patients who had undergone upfront surgery, received olaparib, and were progression free at 1, 2, and 3 years were 91%, 78%, and 69% (v 58%, 40%, and 32% receiving placebo), respectively; for those who underwent interval surgery, estimates were 83%, 66%, and 47% (v 43%, 26%, and 19%), respectively (Fig 3A). For patients who had residual macroscopic disease after cytoreductive surgery before entry into the study, 79%, 60%, and 48% of patients who received olaparib were progression free at 1, 2, and 3 years (v 41%, 28%, and 24% who received placebo), respectively; for patients who had no gross residual disease at study entry, the percentages for olaparib-treated patients were 90%, 77%, and 65% (v 57%, 38%, and 29% who received placebo; Fig 3B), respectively.
FIG 3.
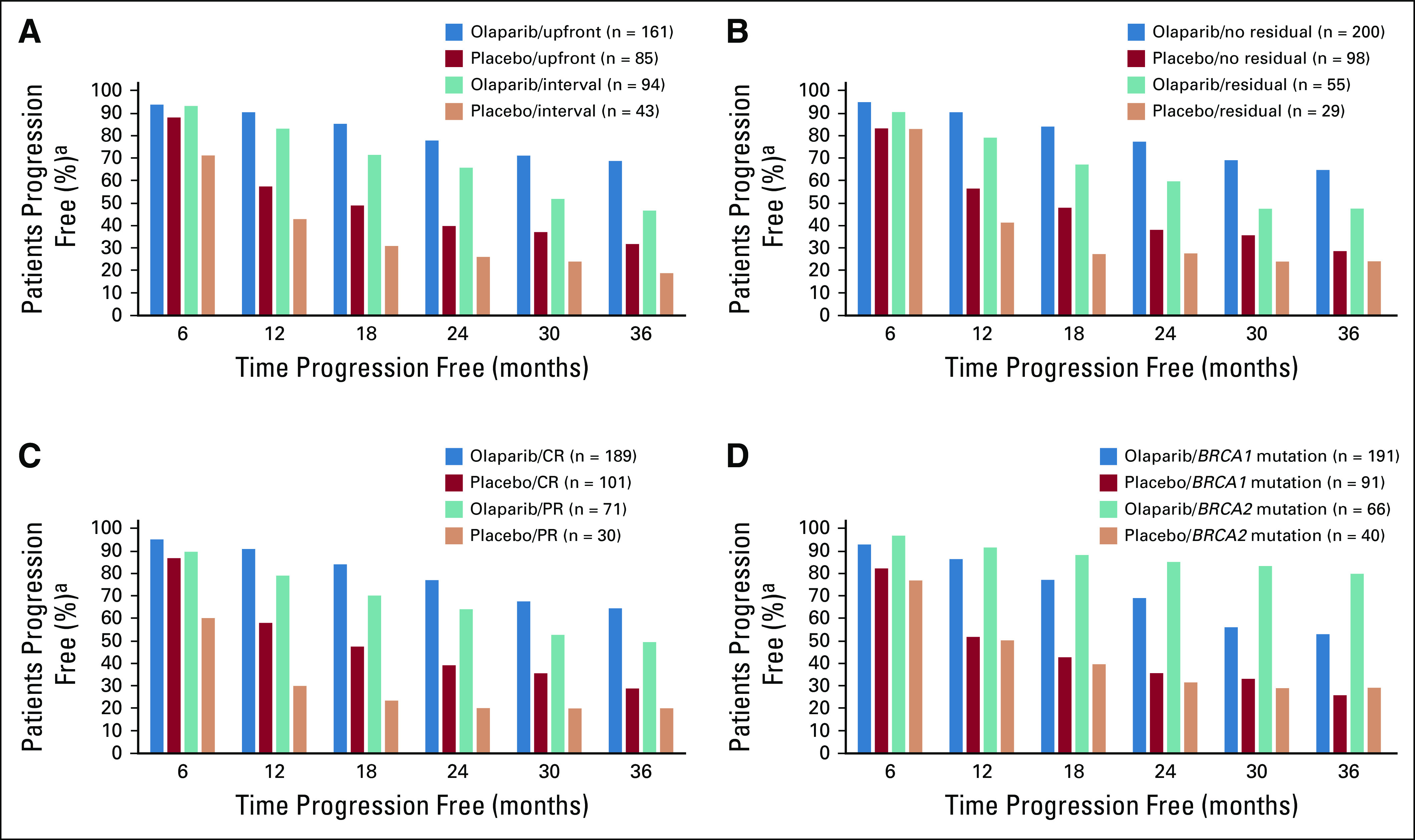
Proportion of patients free of progression or death over time for subgroup analysis–based Kaplan-Meier estimates for (A) surgery timing (8 patients had no surgery or were missing timing data [olaparib arm, n = 5; placebo arm, n = 3]), (B) residual macroscopic disease status, (C) response after platinum-based chemotherapy at baseline, and (D) BRCA mutation status (3 patients [all in olaparib arm] had both BRCA1 and BRCA2 mutations and were progression free up to 42 months). CR, complete response; PR, partial response. (a) Based on Kaplan-Meier estimates.
Forty-four percent of patients with stage III disease (mostly stage IIIC) underwent upfront surgery and had no gross residual macroscopic disease after surgery. For these patients, the risk of disease progression or death was reduced by 68% in patients receiving olaparib compared with placebo (median PFS, NR v 21.9 months; HR, 0.32; 95% CI, 0.20 to 0.51; Fig 2C). Of those receiving olaparib, 92%, 81%, and 71% were progression free at 1, 2, and 3 years (v 66%, 45%, and 35% who received placebo), respectively. Additional data for patients with stage III disease are provided in the Data Supplement.
Response after platinum-based chemotherapy.
On the basis of eCRF data, 74% of women entered the study with no target or nontarget lesions and normal CA-125 (clinical CR), and 26% had a ≥ 30% reduction in the sum of diameters of target lesions, taking as reference the baseline sum diameters from start to end of chemotherapy, or NED in the posttreatment scan but with a CA-125 level that had not decreased to within the normal range (PR; 35% of patients in PR had status determined by elevated CA-125 level). Risk of disease progression or death was reduced for patients receiving olaparib compared with placebo by 66% in women in clinical CR (median PFS, NR v 15.3 months; HR, 0.34; 95% CI, 0.24 to 0.47) and by 69% in women with a PR at baseline (30.9 v 8.4 months; HR, 0.31; 95% CI, 0.18 to 0.52; Fig 2D). On the basis of Kaplan-Meier estimates, the percentages of patients with a baseline CR who received olaparib and were progression free at 1, 2, and 3 years were 91%, 77%, and 65% (v 58%, 39%, and 29% receiving placebo), respectively, and those of patients with a baseline PR were 79%, 64%, and 50% (v 30%, 20%, and 20% Fig 3C), respectively.
BRCA mutation status.
At the primary DCO, 155 patients in the BRCA1-mutated group (55%), 43 in the BRCA2-mutated group (41%), and none in the BRCA1- and BRCA2-mutated group (n = 3) experienced disease progression. Patients receiving placebo who had a BRCA1 mutation or BRCA2 mutation had a median PFS of 13.8 months; this was substantially increased for patients who received olaparib, with a greater PFS benefit observed for those with a BRCA2 mutation (median PFS, NR) relative to a BRCA1 mutation (41.4 months; Fig 2E). Kaplan-Meier estimates of the percentage of BRCA1-mutated patients who received olaparib and were progression free at 1, 2, and 3 years were 86%, 69%, and 53% (v 52%, 36%, and 26% receiving placebo), respectively, and those of BRCA2-mutated patients were 92%, 85%, and 80% (v 50%, 32%, and 29%; Fig 3D), respectively. The risk of disease progression or death was reduced for olaparib-treated patients versus those receiving placebo by 59% (HR, 0.41; 95% CI, 0.30 to 0.56) for BRCA1-mutated patients and by 80% (HR, 0.20; 95% CI, 0.10 to 0.37) for BRCA2-mutated patients (Fig 2E).
ORR
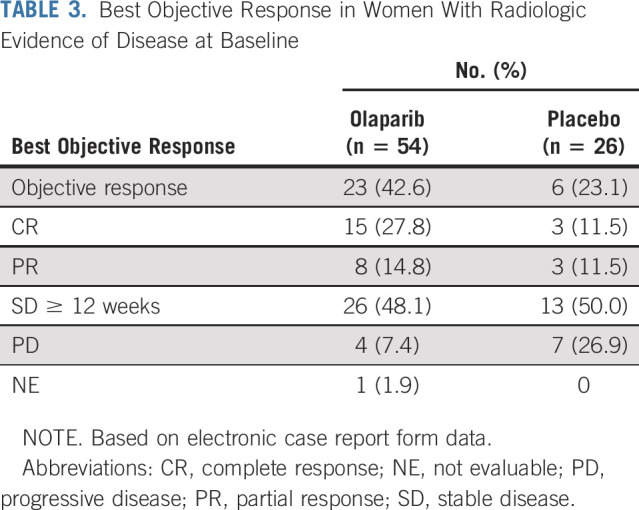
Among women with radiologic evidence of disease at baseline (target and nontarget lesions; RECIST), ORR was 43% (n = 23) in the olaparib arm and 23% (n = 6) in the placebo arm (Table 3). CRs were reported for 28% (n = 15) of olaparib-treated patients compared with 12% (n = 3) of patients receiving placebo, and PRs were reported for 15% (n = 8) and 12% (n = 3) olaparib- and placebo-treated of patients, respectively. In patients with an objective response, median time from random assignment to onset of response and median duration of response were 10.8 and 28.2 months for olaparib and 5.4 and 8.6 months for placebo, respectively.
TABLE 3.
Best Objective Response in Women With Radiologic Evidence of Disease at Baseline
DISCUSSION
The SOLO1 subgroup analyses of PFS reported here were consistent with those in the overall study population,10 demonstrating that olaparib maintenance therapy was substantially beneficial in all reported preselected patient subgroups.
Olaparib demonstrated considerable benefit in the 44% of women in SOLO1 with stage III disease who had undergone upfront surgery and had no gross residual disease, a population ineligible for several recent first-line trials. Despite optimal surgical results, these patients are still at substantial risk of disease recurrence and should be offered olaparib maintenance treatment. In addition, although they had no evidence of gross residual disease, micrometastatic disease probably remains in almost all cases.18 SOLO1 was designed to reflect clinical practice by including all patients with advanced OC regardless of surgical outcome. Our data demonstrate that all BRCA-mutated patients with advanced OC should be considered at high risk of progression and receive appropriate treatment, such as olaparib maintenance, to provide the best chance of delaying disease progression. Additionally, we found that maintenance olaparib improved outcomes compared with placebo, regardless of whether patients had radiologic evidence of disease at baseline. In these patients, maintenance olaparib induced CR in 28% of women, more than double that observed with placebo (12%).
The overall proportion of patients with no gross residual disease after surgery in SOLO1 was slightly higher (77% and 75% of patients in the olaparib and placebo groups, respectively) than may be expected (rate of complete resection in unselected patients with advanced-stage OC ranged between 50% and 70% in surgically specialized gynecologic cancer centers)23; our study results may reflect expertise of surgeons at clinical study sites or different characteristics of BRCA mutation versus sporadic high-grade serous cancers24 rather than patient selection bias. As noted, the SOLO1 population reflects clinical practice and represents 1 of the largest phase III studies in advanced BRCA-mutated OC surgical patients. Furthermore, baseline characteristics were balanced between arms within subgroups analyzed; therefore, outcomes related to timing of surgery or residual disease status after surgery are unlikely to be influenced by baseline differences.
Efficacy results observed in the placebo arm of this study demonstrate that all patients with advanced high-grade OC should be considered at high risk of progression. Despite a large proportion of patients having optimal surgical outcomes and being in CR after chemotherapy, outcomes after placebo treatment were poor, further supporting the use of maintenance olaparib for all patients regardless of baseline characteristics. Although the differential effect of PARP inhibitors in maintenance and treatment settings has not been formally evaluated, olaparib reduced risk of disease progression and death for patients with CR or PR at baseline. There may be different prognostic factors for patients who enter the study in CR compared with PR, and we cannot compare the magnitude of benefit between the 2 subgroups based on an exploratory analysis. However, we can conclude that both subgroups of patients derived meaningful benefit from olaparib treatment, with 30% (olaparib, 50% v placebo, 20%) and 36% (olaparib, 65% v placebo, 29%) more patients being progression free at 3 years in the PR and CR groups, respectively. Similar results were observed in the relapsed setting.21 For patients who initiate olaparib in CR, the goal of treatment is to delay their disease relapse, and for patients with PR, it is to potentially induce CR and/or delay relapse and the need for subsequent chemotherapy.
Among women with evidence of disease at baseline, nearly twice as many had an objective response while receiving olaparib maintenance (43%) compared with placebo (23%). Reasons for patients receiving placebo (ie, not active treatment) experiencing a response may include a carryover effect from platinum-based chemotherapy, timing of patient scans (baseline followed by scans once every 3 months), or variability in measuring RECIST.25 Of note, the ORR analysis reported classified patients as being in clinical CR or PR based on eCRF data, whereas the primary analysis used the randomization code.10
Consistent with previous prevalence studies, in SOLO1, BRCA1 mutation was more frequent in patients with newly diagnosed advanced OC than BRCA2 mutation.26 A significant PFS benefit with olaparib versus placebo was demonstrated for all patients, regardless of mutation type; medium PFS in the placebo arm was consistent for both BRCA1- and BRCA2-mutated patients. Statistical tests were not used to compare BRCA1- and BRCA2-mutated patients; however, those with a BRCA2 mutation (PFS HR, 0.20) seemed to receive greater benefit from maintenance olaparib than those with a BRCA1 mutation (HR, 0.41), although the small size of the BRCA2-mutated subgroup and potential imbalances in baseline characteristics (ie, more adverse prognostic factors in the placebo v olaparib arm) should be noted (baseline characteristics were generally balanced for BRCA1- and BRCA2-mutated patients combined). By 2 years, only 12 BRCA2-mutated patients remained at risk for progression in the placebo arm. It therefore seems a BRCA2 mutation may be a marker of response to olaparib rather than a prognostic indicator in SOLO1. This trend for differential benefit between BRCA1- and BRCA2-mutated patients was not reported with olaparib maintenance therapy for platinum-sensitive relapsed OC in SOLO26 (data on file, AstraZeneca, Cambridge, UK; ClinicalTrials.gov identifier: NCT01874353), although enrichment of BRCA2 mutation was observed among long-term responders to olaparib in Study 19.27 One explanation for this could be resistance mechanisms associated with BRCA1. One mechanism is the production of functional hypomorphic isoforms of BRCA1 protein from alternative messenger RNA splicing, which has been reported to contribute to resistance to platinum-based chemotherapy and PARP inhibition.28 In a subanalysis of GOG-0218, PFS was increased in patients with a BRCA2 versus BRCA1 mutation (median, 21.6 v 15.7 months) regardless of treatment received.29 However, in SOLO1, median PFS and Kaplan-Meier estimates at 1, 2, and 3 years were similar for BRCA1- and BRCA2-mutated patients who received placebo.
A limitation of our analyses is the relatively small patient numbers in some subgroups, including those receiving interval debulking surgery (n = 94 and 43 for olaparib and placebo, respectively), those with residual disease after surgery (n = 55 and 29, respectively), and those in clinical PR after platinum-based chemotherapy (n = 71 and 30, respectively). Although relatively small subgroups, patients receiving olaparib maintenance treatment benefited from treatment.
In conclusion, maintenance therapy with olaparib provided a substantial PFS benefit among women with newly diagnosed advanced OC and a BRCA mutation. This PFS benefit with olaparib was achieved in all subgroups irrespective of surgery timing, residual disease status after surgery, response after platinum-based chemotherapy (CR or PR), or type of BRCA mutation. Continued follow-up of these patients is important to provide information on which subsets of patients will remain progression free and have NED long-term. These data demonstrate that regardless of patient baseline outcomes from surgery and chemotherapy or BRCA mutation type, patients with newly diagnosed advanced OC are at high risk of disease progression and benefit from maintenance olaparib treatment.
ACKNOWLEDGMENT
We thank the patients, their families, and all investigators and study personnel involved. Medical writing assistance was provided by Claire Routley, PhD, from Mudskipper Business Ltd, funded by AstraZeneca and Merck Sharp & Dohme.
SUPPORT
Supported by AstraZeneca and part of an alliance between AstraZeneca and Merck Sharp & Dohme (MSD), a subsidiary of Merck & Co, Kenilworth, NJ. Medical writing support was funded by AstraZeneca and MSD. Supported in part by the MSK Cancer Center Support Grant (P30 CA008748) (C.A.A).
AstraZeneca was involved in the study design, data collection, data analysis, and data interpretation. Merck Sharp & Dohme also provided input in data interpretation.
AUTHOR CONTRIBUTIONS
Conception and design: Charlie Gourley, Amit Oza, Elizabeth S. Lowe, Kathleen N. Moore
Provision of study material or patients: Paul DiSilvestro, Nicoletta Colombo, Byoung-Gie Kim, Ana Oaknin, Michael Friedlander, Alla Lisyanskaya, Alexandra Leary, Gabe S. Sonke, Charlie Gourley, Amit Oza, Carol A. Aghajanian, Cara A. Mathews, Joyce Liu, Kathleen N. Moore
Collection and assembly of data: Nicoletta Colombo, Giovanni Scambia, Byoung-Gie Kim, Ana Oaknin, Michael Friedlander, Alla Lisyanskaya, Charlie Gourley, Amit Oza, William H. Bradley, Cara A. Mathews, Joyce Liu, Elizabeth S. Lowe, Kathleen N. Moore
Data analysis and interpretation: Paul DiSilvestro, Nicoletta Colombo, Byoung-Gie Kim, Ana Oaknin, Anne Floquet, Alexandra Leary, Gabe S. Sonke, Susana Banerjee, Amit Oza, Antonio González-Martín, Carol A. Aghajanian, William H. Bradley, Cara A. Mathews, Elizabeth S. Lowe, Ralph Bloomfield, Kathleen N. Moore
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Efficacy of Maintenance Olaparib for Patients With Newly Diagnosed Advanced Ovarian Cancer With a BRCA Mutation: Subgroup Analysis Findings From the SOLO1 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Paul DiSilvestro
Consulting or Advisory Role: AstraZeneca
Research Funding: Janssen Oncology (Inst), Tesaro (Inst), AstraZeneca (Inst), Genentech (Inst), AbbVie (Inst)
Nicoletta Colombo
Honoraria: Roche/Genentech, AstraZeneca, Tesaro, PharmaMar
Consulting or Advisory Role: Roche/Genentech, PharmaMar, AstraZeneca, Clovis Oncology, Pfizer, MSD Oncology, Takeda, Tesaro, BioCad, GlaxoSmithKline
Giovanni Scambia
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, PharmaMar, Roche, Tesaro
Speakers’ Bureau: Clovis Oncology Italy, Merck Sharp & Dohme Italy
Ana Oaknin
Consulting or Advisory Role: Roche, AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Immunogen, Genmab
Research Funding: AbbVie Deutschland (Inst), Ability Pharma (Inst), Advaxis (Inst), Aeterna Zentaris (Inst), Amgen (Inst), Aprea Therapeutics (Inst), Clovis Oncology (Inst), Eisai (Inst), F. Hoffmann-La Roche (Inst), Regeneron Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche
Michael Friedlander
Honoraria: AstraZeneca, Merck Sharp & Dohme, Lilly, Takeda, Novartis
Consulting or Advisory Role: AstraZeneca, Merck Sharp & Dohme, AbbVie, Lilly, Takeda, Novartis
Speakers’ Bureau: AstraZeneca, ACT Genomics
Research Funding: BeiGene (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Alla Lisyanskaya
Honoraria: Incuron (Inst), Merck Sharp & Dohme (Inst), AstraZeneca (Inst), Regeneron (Inst), Roche (Inst)
Research Funding: Incuron, Roche, AstraZeneca, Regeneron, Merck Sharp & Dohme
Anne Floquet
Consulting or Advisory Role: Roche, MSD Oncology, GlaxoSmithKline, Tesaro, Clovis Oncology, AstraZeneca
Travel, Accommodations, Expenses: Roche, AstraZeneca, Clovis Oncology, GlaxoSmithKline, Tesaro, MSD Oncology
Alexandra Leary
Honoraria: AstraZeneca, Clovis Oncology
Consulting or Advisory Role: Clovis Oncology (Inst), AstraZeneca (Inst), Tesaro (Inst), BioCad, Gritstone Oncology, Seattle Genetics, Ability Pharma (Inst), Merck Sharp & Dohme (Inst), GlaxoSmithKline (Inst), Merck Serono (Inst)
Research Funding: Merus (Inst), GamaMabs Pharma (Inst), Inivata (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Tesaro
Gabe S. Sonke
Consulting or Advisory Role: Novartis (Inst)
Research Funding: Roche (Inst), AstraZeneca (Inst), Novartis (Inst), Merck Sharp & Dohme (Inst)
Charlie Gourley
Research Funding: AstraZeneca
Honoraria: Tesaro, Cor2Ed, GlaxoSmithKline, MSD Oncology, Clovis Oncology
Consulting or Advisory Role: AstraZeneca, Nucana (Inst), Tesaro (Inst), Cor2Ed, Sierra Oncology, GlaxoSmithKline, MSD Oncology
Research Funding: AstraZeneca (Inst), Novartis (Inst), Aprea (Inst), Nucana (Inst), Tesaro (Inst), GlaxoSmithKline (Inst), MSD Oncology (Inst)
Patents, Royalties, Other Intellectual Property: One patent issued and 4 pending for a gene expression signature to predict cancer sensitivity to antiangiogenic therapy (Inst)
Susana Banerjee
Honoraria: Roche
Consulting or Advisory Role: AstraZeneca/MedImmune, Tesaro, Clovis Oncology, Merck, Seattle Genetics, Genmab, Carrick Therapeutics (Inst), Amgen, Roche, GlaxoSmithKline, MSD Oncology
Research Funding: AstraZeneca (Inst), Janssen-Cilag (Inst), GlaxoSmithKline (Inst), NuCana BioMed
Travel, Accommodations, Expenses: AstraZeneca
Amit Oza
Uncompensated Relationships: Ozmosis Research
Antonio González-Martín
Consulting or Advisory Role: Roche, Tesaro/GlaxoSmithKline, Clovis Oncology, AstraZeneca, Merck Sharp & Dohme, Genmab, Immunogen, Oncoinvent, Pfizer/EMD Serono, Amgen
Speakers’ Bureau: Roche, AstraZeneca, Tesaro/GlaxoSmithKline, PharmaMar, Roche (Inst), Tesaro/GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: Roche, AstraZeneca, PharmaMar, Tesaro/GlaxoSmithKline
Carol A. Aghajanian
Consulting or Advisory Role: Tesaro, Mersana, Eisai, Roche
Research Funding: Genentech/Roche (Inst), AbbVie (Inst), Clovis Oncology (Inst), AstraZeneca (Inst)
William H. Bradley
Consulting or Advisory Role: Celsion, Inovio Pharmaceuticals
Travel, Accommodations, Expenses: Inovio, Clovis Oncology
Cara A. Mathews
Research Funding: AstraZeneca (Inst), Tesaro/GlaxoSmithKline (Inst), Syros (Inst), Astellas Pharma (Inst), Seattle Genetics (Inst), Deciphera (Inst)
Joyce Liu
Consulting or Advisory Role: Tesaro, Mersana, Clovis Oncology, Genentech/Roche, GlaxoSmithKline, Regeneron
Research Funding: Genentech/Roche (Inst), AstraZeneca (Inst), Boston Biomedical (Inst), Atara Biotherapeutics (Inst), Acetylon (Inst), Bristol Myers Squibb (Inst), Agenus (Inst), CytomX Therapeutics (Inst), Regeneron (Inst), Tesaro (Inst), Clovis Oncology (Inst), Surface Oncology (Inst), 2X Oncology (Inst), Vigeo Therapeutics (Inst), Aravive (Inst), Arch Oncology (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Merck
Uncompensated Relationships: Merck, AstraZeneca
Elizabeth S. Lowe
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Ralph Bloomfield
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Kathleen N. Moore
Honoraria: Research To Practice, Prime Oncology
Consulting or Advisory Role: Genentech/Roche, Immunogen, AstraZeneca (Inst), Clovis Oncology, Tesaro (Inst), VBL Therapeutics, Janssen Oncology, Merck, Aravive, Samumed, OncoMed, Pfizer/EMD Serono, Eisai, AbbVie, Vavotar, Mersana (Inst)
Research Funding: PTC Therapeutics (Inst), Lilly (Inst), Merck (Inst), Tesaro (Inst), Genentech (Inst), Clovis Oncology (Inst), Lilly Foundation (Inst), Regeneron (Inst), Advaxis (Inst), Bristol Myers Squibb (Inst), Verastem (Inst), Novartis Pharmaceuticals UK (Inst), AstraZeneca (Inst), Agenus (Inst), Takeda (Inst), Forty Seven (Inst), Stem CentRx (Inst), Immunogen (Inst), Bayer (Inst), Novogen (Inst), AbbVie/Stemcentrx (Inst)
REFERENCES
- 1. doi: 10.6004/jnccn.2018.0078. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: Ovarian cancer version 2, 2018. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. [DOI] [PMC free article] [PubMed]
- 2.Ledermann JA, Raja FA, Fotopoulou C, et al. : Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24:vi24-vi32, 2013. (suppl 6) [DOI] [PubMed] [Google Scholar]
- 3. Golan T, Hammel P, Reni M, et al: Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 381:317-327, 2019. [DOI] [PMC free article] [PubMed]
- 4. Mateo J, Porta N, McGovern UB, et al: TOPARP-B: A phase II randomized trial of the poly(ADP)-ribose polymerase (PARP) inhibitor olaparib for metastatic castration resistant prostate cancers (mCRPC) with DNA damage repair (DDR) alterations. J Clin Oncol 37, 2019 (suppl; abstr 5005)
- 5. Penson RT, Villalobos Valencia R, Cibula D, et al: Olaparib monotherapy versus (vs) chemotherapy for germline BRCA-mutated (gBRCAm) platinum-sensitive relapsed ovarian cancer (PSR OC) patients (pts): Phase III SOLO3 trial. J Clin Oncol 37, 2019 (suppl; abstr 5506)
- 6.Pujade-Lauraine E, Ledermann JA, Selle F, et al. : Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 18:1274-1284, 2017 [DOI] [PubMed] [Google Scholar]
- 7. doi: 10.1056/NEJMoa1706450. Robson M, Im SA, Senkus E, et al: Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377:523-533, 2017 [Erratum: N Engl J Med 377:1700, 2017] [DOI] [PubMed] [Google Scholar]
- 8.European Medicines Agency : Lynparza summary of product characteristics. http://ec.europa.eu/health/documents/community-register/2018/20180508140545/anx_140545_en.pdf
- 9. US Food and Drug Administration: Lynparza prescribing information (revised September 2018). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208558s006lbl.pdf.
- 10.Moore K, Colombo N, Scambia G, et al. : Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Burger RA, Brady MF, Bookman MA, et al. : Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473-2483, 2011 [DOI] [PubMed] [Google Scholar]
- 12.González-Martín A, Pothuri B, Vergote I, et al. : Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019 [DOI] [PubMed] [Google Scholar]
- 13. Moore KN, Pignata S: Trials in progress: IMagyn050/GOG 3015/ENGOT-OV39. A phase III, multicenter, randomized study of atezolizumab versus placebo administered in combination with paclitaxel, carboplatin, and bevacizumab to patients with newly-diagnosed stage III or stage IV ovarian, fallopian tube, or primary peritoneal cancer. Int J Gynecol Cancer 29:430-433, 2019. [DOI] [PubMed]
- 14.du Bois A, Floquet A, Kim JW, et al. : Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol 32:3374-3382, 2014 [DOI] [PubMed] [Google Scholar]
- 15.George A, Kristeleit R, Rafii S, et al. : Clinical factors of response in patients with advanced ovarian cancer participating in early phase clinical trials. Eur J Cancer 76:52-59, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Wimberger P, Lehmann N, Kimmig R, et al. : Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival: An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR). Gynecol Oncol 106:69-74, 2007 [DOI] [PubMed] [Google Scholar]
- 17.du Bois A, Reuss A, Pujade-Lauraine E, et al. : Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials—By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 115:1234-1244, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Chan JK, Urban R, Hu JM, et al. : The potential therapeutic role of lymph node resection in epithelial ovarian cancer: A study of 13918 patients. Br J Cancer 96:1817-1822, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ledermann J, Harter P, Gourley C, et al. : Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 366:1382-1392, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Mirza MR, Monk BJ, Herrstedt J, et al. : Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 375:2154-2164, 2016 [DOI] [PubMed] [Google Scholar]
- 21. Oza AM, Combe P, Ledermann J, et al: Evaluation of tumour responses and olaparib efficacy in platinum-sensitive relapsed ovarian cancer (PSROC) patients (pts) with or without measurable disease in the SOLO2 trial (ENGOT Ov-21). Ann Oncol 28:344, 2017 (suppl 5; abstr 9656P)
- 22.Venzon DJ, Moolgavkar SH: A method for computing profile-likelihood-based intervals. Appl Stat 37:87-94, 1988 [Google Scholar]
- 23.Reuss A, du Bois A, Harter P, et al. : TRUST: Trial of Radical Upfront Surgical Therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7). Int J Gynecol Cancer 29:1327-1331, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Madariaga A, Lheureux S, Oza AM: Tailoring ovarian cancer treatment: Implications of BRCA1/2 mutations. Cancers (Basel) 11:416, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson MK, Friedlander ML, Lheureux S, et al. : Resisting RECIST-uniformity versus clinical validity. Int J Gynecol Cancer 27:1619-1627, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Ramus SJ, Gayther SA: The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol Oncol 3:138-150, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lheureux S, Lai Z, Dougherty BA, et al. : Long-term responders on olaparib maintenance in high-grade serous ovarian cancer: Clinical and molecular characterization. Clin Cancer Res 23:4086-4094, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Bernhardy AJ, Cruz C, et al. : The BRCA1-Δ11q alternative splice isoform bypasses germline mutations and promotes therapeutic resistance to PARP inhibition and cisplatin. Cancer Res 76:2778-2790, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norquist BM, Brady MF, Harrell MI, et al. : Mutations in homologous recombination genes and outcomes in ovarian carcinoma patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group study. Clin Cancer Res 24:777-783, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]