Abstract
Hydroxyurea (HU) is the first-ever approved drug by USFDA for sickle cell anemia (SCA). However, its treatment is associated with severe side effects like myelosuppression. Current studies are focused on the supplementation therapy for symptomatic management of SCA. In the present study, we aimed to explore rutin’s and gallic acid’s potential individually, for concomitant therapy with HU using pharmacokinetic and pharmacodynamic approaches since there is no such precedent till date. In vivo pharmacokinetic studies of HU in rats showed that rutin could be safely co-administered with HU, while gallic acid significantly raised the plasma concentration of HU. Both the phytochemicals did not have any marked inhibitory effect on urease but have considerable effects on horseradish peroxidase enzyme. The experimental phytoconstituents displayed a very low propensity to cause in vitro hemolysis. Gallic acid markedly enhanced the HU-induced decrease in lymphocyte proliferation. A substantial improvement by rutin or gallic acid was observed in HU-induced reduction of the main hematological parameters in rats. Combined treatment of HU with rutin and gallic acid reduced serum levels of both IL-6 and IL-17A. Overall, both rutin and gallic acid are found to have promising phytotherapy potential with HU. Further exploration needs to be done on both candidates for use as phytotherapeutics for SCA.
1. Introduction
Sickle cell anemia (SCA) is an inherited disorder of blood caused by the substitution of glutamic acid by valine at the 6th position in the β-globin chain of hemoglobin (Hb) that leads to severe pathological complications such as hemolysis, vaso-occlusion, organ damage, and death.1,2 Hydroxyurea (HU) is the first-ever approved drug by the United States Food and Drug Administration (USFDA) for the treatment of SCA.1 HU mainly enhances fetal hemoglobin production to decrease the polymerization of sickle hemoglobin and hemolysis.3 Despite the beneficial HU effects, there are potential dose-dependent adverse effects such as myelosuppression.4,5 Gene therapy can provide an ultimate solution for SCA, but this is not available till date. The lack of hemoderivatives in blood bank centers for transfusion therapy and transfusion-associated complications also constitutes a serious problem for the management of SCA. Therefore, current studies are focused on the symptomatic management of patients, mainly pain crisis, and decrease the frequency of hospitalization period.6 Recently, l-glutamine has been approved by the USFDA for reducing the severe complications in SCA patients via reduction of oxidative stress.6,7 Several phytoconstituents/amino acids/botanical drugs have been investigated using in vitro and in vivo preclinical/clinical models for their effectiveness by targeting improvement in the pathophysiology of SCA.6,8 The examples are as follows: curcumin, phenylalanine, cajaminose, limonoid, lunularic acid, epigallocatechin gallate, vanillin, SCD-101, NIPRISAN, resveratrol, angelicin, cucurbitacin, dimethyl butyrate, l-arginine, gum arabic, docosahexaenoic acid, and so forth.6,8−10 Under these circumstances, the present study deals with the individual effects of two important phytoconstituents, namely, rutin and gallic acid (Figure 1), which have several useful pharmacological properties.11−18 Rutin and its aglycone (quercetin) are reported for their function as antisickling agents.19,20Newboludia laevis and Combretum glutinosum are reported to have an antisickling activity where gallic acid is the most active phytoconstituent.21,22 Hence, it would be beneficial to elucidate if any alteration in the pharmacokinetics of HU in the presence of rutin or gallic acid occurred. This investigation can generate information on precipitation of drug-induced toxicities, therapeutic failure, or safe co-administration depending upon the type of pharmacokinetic interaction, namely, positive, negative, or no interaction.23,24 On the other hand, it would be useful to elucidate their pharmacological actions along with HU in counteracting HU-induced myelosuppression.
Figure 1.
Chemical structure of the experimental drug and phytoconstituents.
Therefore, the objective of the present investigation was to explore the individual effects of rutin and gallic acid on oral pharmacokinetics of HU and main hematological parameters in HU-induced myelosuppression using in vivo models. Moreover, mechanistic investigations were also performed to assess the individual effects of rutin and gallic acid on the inhibition of urease, inhibition of horseradish peroxidase (HRP) activity, hemolysis, splenic lymphocyte proliferation, and serum cytokines level using in vitro models.
2. Results and Discussion
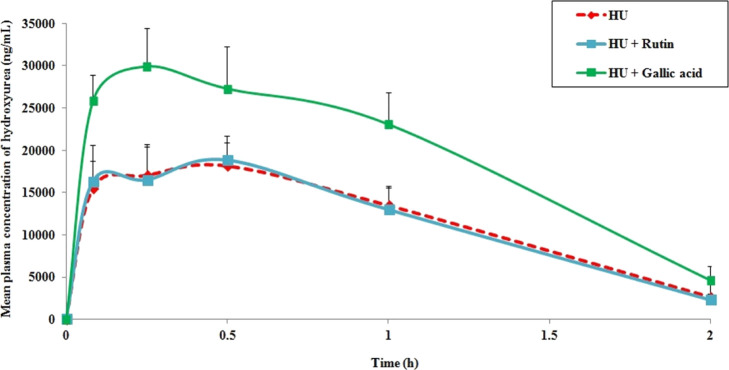
In the quest to explore phytotherapeutics toward HU-induced myelosuppression for SCA treatment, the potential of rutin and gallic acid was investigated. In this direction, it is first and foremost to evaluate if there is any chance of pharmacokinetic interaction between them. We explored the individual effects of rutin and gallic acid on the pharmacokinetics of HU upon oral co-administration in rats. The mean plasma concentrations versus time profiles of HU are represented in Figure 2. The calculated pharmacokinetic parameters are represented in Table 1. The results showed that co-administration of rutin did not alter any HU pharmacokinetic parameters to a significant extent when compared to HU alone. On the contrary, gallic acid upon co-administration significantly raised the overall oral exposure of HU (AUC) by around 1.7-fold (AUC0–t: 39327 ± 5733 vs 23741 ± 3036 ng·h/mL and AUC0–∞: 42677 ± 5870 vs 25441 ± 3037 ng·h/mL) as compared to HU alone. However, the remaining pharmacokinetic parameters of HU were unaffected upon gallic acid treatment. The clearance of HU was slowed down by gallic acid, but it lacks statistical significance. Therefore, the results indicate that rutin can be used safely along with HU based on these pharmacokinetic studies, but gallic acid is likely to cause augmented oral exposure of HU through positive pharmacokinetic interaction upon co-administration.
Figure 2.
Mean plasma concentration versus time profile of HU as alone as well as in combination with rutin or gallic acid after oral administration to rats. The data are presented as mean ± SEM (n = 5).
Table 1. Pharmacokinetic Parameters of HU after Oral Administration as Alone and in Combination with Rutin or Gallic Acid in Ratsa.
| pharmacokinetic parameters | HU | HU + rutin | HU + gallic acid |
|---|---|---|---|
| T1/2 (h) | 0.44 ± 0.04 | 0.36 ± 0.12 | 0.45 ± 0.09 |
| Cmax (ng/mL) | 21112 ± 1221 | 19520 ± 3355 | 29934 ± 4486 |
| Tmax (h) | 0.30 ± 0.08 | 0.42 ± 0.08 | 0.25 ± 0.00 |
| AUC0–t (ng·h/mL) | 23741 ± 3036 | 23443 ± 5169 | 39327 ± 5733* |
| AUC0–∞ (ng·h/mL) | 25441 ± 3037 | 25302 ± 6734 | 42677 ± 5870* |
| Vd/F (L/kg) | 1.36 ± 0.34 | 1.02 ± 0.15 | 0.81 ± 0.24 |
| CL/F (L/h/kg) | 2.17 ± 0.44 | 2.26 ± 0.55 | 1.56 ± 0.26 |
Data are presented as mean ± SEM (n = 5). Statistical significance was evaluated by comparing treatment with HU alone versus treatment with HU in the presence of rutin or gallic acid. p < 0.05 denotes statistically significant (*).
In the literature, it is reported that enzymes such as urease and HRP are involved in the metabolism of HU.25,26 Therefore, in the present study, we evaluated the effects of rutin and gallic acid on urease and HRP activity (Figure 3 and Figure 4). Thiourea was found to have an IC50 of 30 μM against urease activity, corroborating a previous study, which also reported similar IC50.27 Rutin and gallic acid demonstrated IC50 values of 780 and 635 μM, respectively, toward inhibition of urease activity. On the other hand, sodium azide as a standard inhibitor for HRP activity was found to have 97% inhibition at a concentration of 1000 μM (Figure 4). Rutin and gallic acid exhibited 40–64 % and 53–70% inhibition of HRP at the concentration range of 250–1000 μM, respectively. Based on the results of the present study, there is no significant effect by both of these candidates for the inhibition of urease activity. In contrast, gallic acid showed a more pronounced effect than rutin toward HRP enzymatic activity at the experimental concentration range. The inhibitory concentration of rutin or gallic acid against HRP activity was found to be too high to achieve practical blood/liver concentration levels, so that pharmacokinetics of HU becomes affected. HU is a substrate of organic anion transporting polypeptide (OATP) 1A2 and OATP1B2 (rodent)/OATP1B1 and OATP1B3 (human).28 Li et al. reported that gallic acid inhibited the OATP1B3-mediated transport of CCK-8 with an IC50 value of 1.60 μM. At the same time, rutin is found to be a weak inhibitor as it could not attain an IC50 at the highest concentration of 150 μM.29,30 Basu et al. reported a similar result, where the administration of gallic acid augmented the oral exposure of rosuvastatin (substrate of OATP1B3, human) in the rat.31 It is also reported that administration of gallic acid significantly reduced the expression of CYP2E1, but rutin, notably, enhanced its expression32,33 The observed effect of gallic acid, in the present study, may be linked to the major contribution from the inhibition of OATP transporters, which have to be explored to find out the actual reason behind the enhanced oral exposure of HU in the presence of gallic acid. As the dose of HU is high enough and is in the range of 5–35 mg/kg/day,34 there is an yet another important aspect for the co-administration of gallic acid with HU. Here, the intended drug interaction may reduce the dose level of HU so that it can be beneficial to lessen the dose-dependent adverse effects of HU.
Figure 3.
Effect of (a) thiourea as a standard, (b) rutin as test compound, and (c) gallic acid as test compound in the inhibition of urease activity (in vitro). The data are presented as mean ± SEM (n = 3).
Figure 4.
Effect of rutin and gallic acid on the inhibition of HRP activity (in vitro). Data are presented as mean ± SEM (n = 3).
Worldwide, current studies are focused on the supplementation therapy for SCA. Therefore, it is always desired that a particular candidate should aid efficacy and combat toxicities of the co-administered effective drug. Despite the unprecedented advances in modern medicine, HU is the most effective drug for SCA till date. It is an anticancer drug and, therefore, there are liabilities as well, like myelosuppression. Hence, for any candidates that are to be used as phytotherapeutics, like rutin (native form as well as its aglycone)19,20 or gallic acid (enriched in extracts)21,22 which are reported to have an antisickling effect, it is prudent to investigate their potential to combat HU-induced toxicities. In this direction, we evaluated the individual effects of rutin and gallic acid along with HU against the myelosuppressive pitfall of HU.
Hemolysis is one of the major pathophysiological characteristics of SCA, and experimental candidates should not have their own effect on hemolysis. Therefore, we evaluated for any deleterious effects of rutin or gallic acid on erythrocyte membranes that may cause hemolysis. Therefore, the individual influence of rutin and gallic acid toward the hemolysis effect was evaluated and expressed as % Hb released relative to the positive and negative controls. Representative images of the present study are depicted in Figure 5. The results displayed no noteworthy effect (≤5%) of rutin or gallic acid to exhibit RBCs lysis up to the experimental concentration of as high as 1 mg/mL. Hence, the results demonstrated the nonhemolytic nature of these phytochemicals and that they can be used safely.
Figure 5.

Representative images for the effect of rutin and gallic acid on red blood cells, where PBS and 1% Triton X-100 in PBS (v/v) were negative and positive control, respectively (in vitro).
Treatment with HU affects the proliferation of T-lymphocytes, which is linked to its myelosuppressive toxic effect. Any immunomodulatory action of the experimental compounds on the proliferation of T-cells in the presence of HU can be useful for the concomitant treatment with HU. In the present study, Con-A was used only to proliferate the splenic lymphocytes significantly and the concentration of HU was optimized to elucidate the proliferative activity of the test compounds in the presence of HU. The results of the present investigation is presented in Figure 6. There was no considerable influence of rutin on the proliferation of lymphocytes. However, gallic acid significantly elevated the proliferation of lymphocytes, which was decreased by HU treatment. Therefore, treatment with rutin has no negative impact on aggravating the HU-induced reduction in lymphocyte proliferation, whereas gallic acid can restore and even improve HU’s harmful effect.
Figure 6.
Influence of rutin and gallic acid in combination with HU on rat splenic lymphocyte proliferation (in vitro). p < 0.05 denotes statistically significant (#/$/*). Control vs HU is presented as #, Con-A vs HU + Con-A is presented as $, HU + Con-A vs HU + Con-A + Rutin/Gallic acid is presented as *, NS denotes not statistically significant.
Impact of rutin and gallic acid was investigated upon repeated co-administration individually with HU, where the dose of HU caused a significant reduction in three important hematological parameters levels, namely, RBCs, Hb, and platelet count (Figure 7). Both rutin and gallic acid exhibited a substantial effect for the improvement in the RBCs as well as the Hb level. Additionally, the effect on HU-mediated lessening of the platelet count was better in the treatment with both rutin and gallic acid, where the effect of rutin was statistically significant compared to HU. Hence, both the compounds have the potential to restrict the HU treatment-mediated drop in key hematological parameters.
Figure 7.
Effect of rutin and gallic acid in combination with HU on the level of (a) RBCs (×106/μL), (b) Hb (g/dL), and (c) platelet count (×103/μL) in rats. Data are presented as mean ± SEM (n = 5). p < 0.05 denotes statistically significant (#/*), p < 0.01 denotes highly statistically significant (##/**), and p < 0.001 denotes extremely statistically significant (###/***) in comparing control vs HU (#) or HU vs HU in combination with rutin/gallic acid (*), NS denotes not statistically significant.
Two cytokines, namely, IL-6 and IL-17A, have a crucial role in the pathophysiology of SCA.35 We monitored these two cytokine levels in the serum of the above-mentioned study animals just to assess the impact of HU in the presence or absence of rutin or gallic acid. The individual effects of rutin and gallic acid in combination with HU on the above-mentioned serum cytokines are depicted in Figure 8. Both the compounds were found to have a substantial effect on reducing the above-mentioned cytokine's levels compared to HU alone. Therefore, these effects of rutin or gallic acid can be useful against pain crises during SCA.
Figure 8.
Impact of rutin and gallic acid in combination with HU on the level of (a) IL-6 and (b) IL-17A. Data are presented as mean ± SEM (n = 5). p < 0.05 denotes statistically significant (*), p < 0.01 denotes highly statistically significant (**), and p < 0.001 denotes extremely statistically significant (***) in comparing HU vs HU in combination with rutin/gallic acid, NS denotes not statistically significant.
The overall results indicate that both rutin and gallic acid are devoid of hemolytic activity on their own; can restrict HU-mediated reduction in cell proliferation; can improve the dropped levels of RBCs, Hb, and platelet linked to HU therapy; and can lower the levels of two inflammatory cytokines associated with pathophysiological conditions of SCA. Although the same dose was used for both compounds in all the experiments, the results showed more pronounced effects with gallic acid when compared to rutin.
Both rutin and gallic acid showed the potential to be used as phytotherapeutics under the present scope of investigations. It is noteworthy to mention that these phytoconstituents are safe for human use as nutraceuticals and are included in the FSSAI guidelines.36 Rutin is present mainly in Ruta graveolens and Rutacalepensis(37,38) and is mentioned in the GRAS substances list by USFDA.39 Gallic acid is also present in the same list in the form of tannic acid.39 Further studies are needed to explore the effects of gallic acid and rutin on other targets for increasing the evidence of their safe and effective use. Finally, clinical relevance must be established for adjuvant therapy with HU to manage its dose-dependent side effects in SCA patients symptomatically.
3. Conclusions
The present study deals with the elucidation of rutin’s and gallic acid’s potential as supplement therapy using pharmacokinetic and pharmacodynamic approaches to counteract the HU-induced myelosuppression. Preclinical pharmacokinetic interaction results indicate that HU can be safely co-administered with rutin but not with gallic acid, which can cause positive pharmacokinetic interactions with HU. However, gallic acid may be helpful to reduce the dose of HU through intended pharmacokinetic interactions. Both rutin and gallic acid have several pharmacological attributes to minimize HU-induced myelosuppression by improving hematological parameters and inflammatory mediators’ profiles. However, gallic acid has more pronounced effects in combating toxic liabilities of HU than rutin. The present study is the first-time report of rutin and gallic acid on the pharmacokinetic and pharmacodynamic interactions with HU under the purview of adjuvant therapy in SCA. Further studies are warranted on these promising phytoconstituents which are to be developed as phytotherapeutics for the symptomatic management of SCA under HU therapy.
4. Materials and Methods
4.1. Chemicals and Reagents
HU (purity ≥ 98%), gallic acid (purity ≥ 97.5%), thiourea (purity ≥ 99%), urease from Canavalin ensiformis (jack bean), triton X-100, concanavalin-A (Con-A), and Dulbecco’s Modified Eagle’s medium were purchased from Sigma-Aldrich. Rutin (purity ≥ 97%) was procured from Alfa-Aesar. K2HPO4, sodium hydroxide, and ammonium chloride were acquired from Loba Chemie. Phosphate buffer saline, pH 7.4 (PBS), sodium azide, and sodium carbonate were acquired from Himedia. Urea, phenol, 3,3′,5,5′-tetramethylbenzidine (TMB), and HRP were acquired from Ranbaxy lab, Qualigens, Millipore, and Invitrogen, respectively. Acetonitrile and formic acid of LC–MS grade were purchased from Thermo-Fisher Scientific. ELISA kits for cytokine estimation were acquired from Invitrogen. All other materials used were of analytical grade or above. Ultrapure water (Direct-Q3, Merck-Millipore) was used during analysis.
4.2. Animal Husbandry, Maintenance, and Ethical Prerequisite
All the animal experimentations were performed using male Wistar rats having bodyweight between 140 and 160 g. The animals were housed in polypropylene cages and maintained under standard environmental conditions such as a light/dark cycle of 12 h, with a temperature of 25 ± 2 °C, and a relative humidity of 50 ± 20%. Standard pellet diet (M/s Ashirwad Industries, Chandigarh, India) was provided to the animals with water ad libitum. The experimentations were carried out according to the protocol as approved by the Institutional Animal Ethics Committee (IAEC) of our institute (IAEC approval no: 73/141/8/2018).
4.3. Effect on Pharmacokinetics of HU (In vivo)
In vivo pharmacokinetic studies were carried out in the rats to investigate the individual influence of rutin and gallic acid on the pharmacokinetics of HU. The recommended dose of HU is 15 mg/kg/day initially (rounded up to nearest 500 mg), and thereafter, it can be increased by 2.5–5 mg/kg/day, every 4–8 weeks depending on the level of myelosuppression in the treated SCA patients.34,40 The maximum recommended dose of HU is 35 mg/kg/day. However, the initial dose of 5–10 mg/kg/day is recommended if the patient has any chronic kidney disease.41,42 The present experimental dose of HU for single-dose pharmacokinetic studies of HU in rats was 50 mg/kg, which was based on the following aspects: (a) available strength of the HU dosage form (500 mg capsule) to treat SCA patients and (b) the selected dose of HU should be on the lower side of the human recommended dose to achieve sufficient oral exposure of HU, which could be achieved by avoiding any saturated plasma level in normal circumstances as well as in the case of positive or negative pharmacokinetic interactions. The present investigation involved three treatment groups containing five animals/group: HU as alone, HU in the presence of rutin at 10 mg/kg, and HU in the presence of gallic acid at 10 mg/kg. All the dose formulations were prepared freshly on the day of the experimentation as aqueous suspensions containing sodium carboxymethylcellulose (0.5%, w/v). To attain the maximum effect, rutin and gallic acid were orally administrated 0.5 h prior to the HU dose. After the HU treatment, blood samples (∼120 μL each) were collected at each time point, that is, 0 h (predose), 0.083, 0.25, 0.5, 1, and 2 h in the microcentrifuge tubes containing aqueous ethylenediaminetetraacetic acid (EDTA) solution. The blood samples were then centrifuged at 8000 rpm for 10 min to separate 50 μL of plasma. The samples were processed using a simple plasma protein precipitation technique using acetonitrile.43 The estimation of HU in rat plasma samples was carried out by an earlier reported and validated LC–MS/MS-based bioanalytical method.43 The method parameters are presented in Tables S1 and S2 (Supporting Information). Plasma concentration profiles of HU with respect to time were obtained from the measured concentration data by LC–MS/MS and further calculated for pharmacokinetic parameters such as maximum plasma concentration (Cmax), time to reach Cmax (Tmax), area under the curve for plasma concentration from zero to the last measurable plasma sample time (AUC0–t), area under the curve for plasma concentration from zero to infinity (AUC0–∞), elimination half-life (T1/2), volume of distribution after oral administration (Vd/F), and clearance after oral administration (Cl/F) by a noncompartmental method using PK solution software (Summit Research Services, USA).
4.4. Effect on Urease Activity (In vitro)
The effect of rutin and gallic acid on the inhibition of urease activity was determined with some modifications in the earlier reported protocol.44 In brief, urease enzyme (8 U/mL) was preincubated with rutin or gallic acid (10 to 1000 μM) in urease assay buffer (100 mM urea, 0.01 M K2HPO4, 1 mM EDTA, and 0.01 M LiCl2, pH 8.2) in 96-well plates at room temperature for 10 min. Then, 50 μL of phenol reagent [1% phenol (w/v) and 0.005% sodium nitroprusside (w/v)] and 50 μL of alkali reagent [0.5% sodium hydroxide (w/v) and 0.1% sodium hypochlorite (v/v)] were added. The mixture was again incubated for 30 min. Then, absorbance was measured at 625 nm using a plate reader (Make: Tecan; Model: InfiniteM200 Pro). The percentage of inhibition was calculated using the following formula
The concentration–response curves were fitted, and IC50 values were calculated using log (inhibitor) versus response-variable slope using GraphPad PRISM 5.0 software (GraphPad, California, USA). The study was carried out using thiourea as the standard (1–500 μM). All the experiments were performed in triplicate.27,44
4.5. Effect on HRP Activity (In vitro)
The effect of rutin or gallic acid on the inhibition of HRP activity was evaluated by some modification in the earlier reported method.45 The reaction mixture consists of 10 μL of HRP and 10 μL of the test compound (250–1000 μM) with 100 μL of TMB in 96-well plates. The reaction was initiated by the addition of 0.03% (v/v) hydrogen peroxide. The TMB-converted product in blue color was measured at 655 nm using a plate reader (Make: Tecan; Model: InfiniteM200 Pro). The percentage of inhibition was calculated using the formula mentioned in Section 4.4. The activity was also evaluated using sodium azide as the standard (1000 μM). All the experiments were performed in triplicate.45,46
4.6. Effect on Hemolysis (In vitro)
The effect of both the candidates to induce hemolysis was evaluated. Briefly, a fresh blood sample was collected from the control rats in an Eppendorf-containing anticoagulant (aqueous EDTA solution) and subsequently centrifuged at 8000 rpm for 10 min to obtain settled RBCs. The RBCs were washed thrice with PBS and diluted with PBS (1:10, v/v). Then, 100 μL of the diluted RBCs were mixed with 900 μL of PBS containing rutin or gallic acid in the concentration range of 0.1 to 1 mg/mL. The blank PBS and 1% Triton X-100 in PBS (v/v) were used as negative and positive controls, respectively. The samples were incubated in a water bath shaker (Make: Remi Elektrotechnik; Model: RSB-12) for 30 min at 37 °C followed by centrifugation at 8000 rpm for 10 min at 4 °C. The supernatant was analyzed by the plate reader (Make: Tecan; Model: InfiniteM200 Pro) at 540 nm.47 The % hemolysis was calculated using the following formula, where ABS0 and ABS100 were the absorbance of the solution at 0% hemolysis (PBS) and 100% hemolysis (1% Triton X-100 in PBS), respectively
4.7. Effect on Splenic Lymphocyte Proliferation (In vitro)
The effect of the experimental compounds on the proliferation of T-cells in the presence of HU was carried out using rat splenocytes obtained from control rats. Preliminary experimentation was carried out to fix the concentration of HU in the presence of Con-A, where an appropriate concentration of HU was chosen such that it caused a considerable reduction in the number of T-cells in the presence of Con-A.48,49 The impact of phytoconstituents was evaluated based on the improvement in the level of T-lymphocytes that decline during HU treatment. The concentration of HU was fixed to 50 μM, whereas the concentration of rutin or gallic acid was assessed at 10 μM each. A brief study protocol is described below.50 The rat spleen was collected under aseptic conditions in incomplete RPMI media and was minced on a cell strainer to obtain a homogeneous cell suspension. The erythrocytes were lysed with a lysis buffer containing 155 mM ammonium chloride, 12 mM sodium bicarbonate, 0.1 mM EDTA, and then, the cells were centrifuged at 1500 rpm at 4 °C for 10 min. The cell pellet was washed thrice and suspended in complete RPMI media. The cell number was counted using a hemocytometer (Make: Rohem; Model: IS10269/BS749). The spleen lymphocytes (2.5 × 104 cells) were seeded in 96-well plates followed by pre-treatment of rutin or gallic acid along with HU. The plate was incubated at 37 °C for 1 h followed by the treatment of Con-A (5 μg/mL) to stimulate T-cells. After incubation for 48 h, 20 μL of 2.5 mg/mL MTT dye [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added to each well followed by incubation for 4 h. The absorbance was measured by the ELISA plate reader (Make: Tecan; Model: Infinite M200 Pro) at 570 nm.
4.8. Effect on HU-Induced Myelosuppression (In vivo)
The individual effect of rutin and gallic acid on the improvement in HU-induced myelosuppression was assessed by determining the main hematological parameters which alter during oral therapy of HU. The present investigation involved four treatment groups containing five animals/group — control group which was treated with the vehicle; disease control group which was given only HU; rutin-treated group which was given HU in the presence of rutin; and gallic acid-treated group which was given HU in the presence of gallic acid. The dose of HU was chosen at 300 mg/kg orally based on the preliminary experimentation, so as to cause a significant change in the hematological parameters.43 The oral dose of both rutin and gallic acid was 100 mg/kg. The duration of the treatment was 15 days.43 The dose formulations were the same as mentioned above. At the end of the experiment, the blood sample was collected from the overnight-fasted animals for hematological evaluation using an automated hematology analyzer (Make: Sysmex; Model: XT1800i).
4.9. Effect on the Production of Serum Cytokines (In vitro)
The blood samples collected at the end of the study were centrifuged at 8,000 rpm to separate the serum for the analysis of IL-6 and IL-17A using commercially available ELISA kits according to the manufacturer’s protocol (Invitrogen).
4.10. Statistical Analysis
Statistical significance was calculated by Student’s t-test using QuickCalcs online software (GraphPad Prism). The data are presented as mean ± standard error mean (SEM) for each group of animals. p-values of less than 0.05, 0.01, and 0.001 were considered as statistically significant, highly statistically significant, and extremely statistically significant, respectively.
Acknowledgments
A.G., D.K., and A.D. are thankful to DST/CSIR/UGC (New Delhi, India) for providing them with research fellowships. IIIM publication number: CSIR-IIIM/IPR/00274.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01518.
LC and MS conditions for quantification of HU in rat plasma (PDF)
Author Contributions
A.G. and A.D. performed animal experimentation and sample analysis; A.G. performed urease inhibition assay, HRP inhibition assay, hemolysis assay, and MS preparation; D.K. and A.K. performed lymphocyte cell proliferation assay; G.S. performed acquisition of chemicals and MS proof reading; U.N. performed the overall study plan and execution, including MS correction.
This research was supported by the Council of Scientific and Industrial Research, New Delhi, India (HCP0008 & GAP3104).
The authors declare no competing financial interest.
Supplementary Material
References
- Segal J. B.; Strouse J. J.; Beach M. C.; Haywood C.; Witkop C.; Park H.; Wilson R. F.; Bass E. B.; Lanzkron S. Hydroxyurea for the treatment of sickle cell disease. Evid. Rep. Technol. Assess. 2008, 165, 1–95. [PMC free article] [PubMed] [Google Scholar]
- Yuan C.; Quinn E.; Kucukal E.; Kapoor S.; Gurkan U. A.; Little J. A. Priapism, hemoglobin desaturation, and red blood cell adhesion in men with sickle cell anemia. Blood Cells Mol. Dis. 2019, 79, 102350. 10.1016/j.bcmd.2019.102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma H. K.; Lakkakula S.; Lakkakula B. V. K. S. Retrospection of the effect of hydroxyurea treatment in patients with sickle cell disease. Acta Haematol. Pol. 2018, 49, 1–8. 10.2478/ahp-2018-0001. [DOI] [Google Scholar]
- Agrawal R. K.; Patel R. K.; shah V.; Nainiwal L.; Trivedi B. Hydroxyurea in sickle cell disease: drug review. Indian J. Hematol. Blood Transfus. 2014, 30, 91–96. 10.1007/s12288-013-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigano P.; De Franceschi L.; Sainati L.; Piga A.; Piel F. B.; Cappellini M. D.; Fidone C.; Masera N.; Palazzi G.; Gianesin B.; Forni G. L. Real-life experience with hydroxyurea in sickle cell disease: A multicenter study in a cohort of patients with heterogeneous descent. Blood Cells Mol. Dis. 2018, 69, 82–89. 10.1016/j.bcmd.2017.08.017. [DOI] [PubMed] [Google Scholar]
- Kapoor S.; Little J. A.; Pecker L. H. Advances in the treatment of sickle cell disease. Mayo Clin. Proc 2018, 93, 1810–1824. 10.1016/j.mayocp.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Niihara Y.; Miller S. T.; Kanter J.; Lanzkron S.; Smith W. R.; Hsu L. L.; Gordeuk V. R.; Viswanathan K.; Sarnaik S.; Osunkwo I.; Guillaume E.; Sadanandan S.; Sieger L.; Lasky J. L.; Panosyan E. H.; Blake O. A.; New T. N.; Bellevue R.; Tran L. T.; Razon R. L.; Stark C. W.; Neumayr L. D.; Vichinsky E. P. A Phase 3 Trial ofl-Glutamine in Sickle Cell Disease. N. Engl. J. Med. 2018, 379, 226–235. 10.1056/nejmoa1715971. [DOI] [PubMed] [Google Scholar]
- Gour A.; Dogra A.; Bhatt S.; Nandi U.. Effect of Natural Products on Improvement of Blood Pathophysiology for Management of Sickle Cell Anemia. In Botanical Leads for Drug Discovery; Springer, 2020, pp 51–65. 10.1007/978-981-15-5917-4_3 [DOI] [Google Scholar]
- Wandersee N. J.; Maciaszek J. L.; Giger K. M.; Hanson M. S.; Zheng S.; Guo Y.; Mickelson B.; Hillery C. A.; Lykotrafitis G.; Low P. S.; Hogg N. Dietary supplementation with docosahexanoic acid (DHA) increases red blood cell membrane flexibility in mice with sickle cell disease. Blood Cells Mol. Dis. 2015, 54, 183–188. 10.1016/j.bcmd.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badria F. A.; Ibrahim A. S.; Badria A. F.; Elmarakby A. A. Curcumin attenuates iron accumulation and oxidative stress in the liver and spleen of chronic iron-overloaded rats. PLoS One 2015, 10, e0134156 10.1371/journal.pone.0134156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.-Q.; Liu C.-M.; Yang W. Protective effect of rutin against carbon tetrachloride-induced oxidative stress, inflammation and apoptosis in mouse kidney associated with the ceramide, MAPKs, p53 and calpain activities. Chem. Biol. Interact. 2018, 286, 26–33. 10.1016/j.cbi.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Chandra H. K.; Mishra G.; Sahu N.; Nirala S. K.; Bhadauria M. Effect of rutin against high-fat diet and alcohol-induced alterations in hematological variables of rats. Asian J. Pharm. Clin. Res. 2018, 11, 186–189. 10.22159/ajpcr.2018.v11i11.27588. [DOI] [Google Scholar]
- Abdel-Ghaffar O.; Mahmoud S. T.; Said A. A.; Sanad F. A.-A. Y. Ameliorative effect of rutin against isoniazid-induced alterations in certain hematological and biochemical parameters of albino rats. Int. J. Pharmacol. 2018, 14, 39–51. 10.3923/ijp.2018.39.51. [DOI] [Google Scholar]
- Ganeshpurkar A.; Saluja A. K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. 10.1016/j.jsps.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahkeshani N.; Farzaei F.; Fotouhi M.; Alavi S. S.; Bahramsoltani R.; Naseri R.; Momtaz S.; Abbasabadi Z.; Rahimi R.; Farzaei M. H.; Bishayee A. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225. 10.22038/ijbms.2019.32806.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S. M.; Avneet G.; Siddhraj S. S. Gallic acid: Pharmacogical promising lead molecule: A review. Int. J. Pharm. Pharm. Res. 2018, 10, 132–138. 10.25258/phyto.10.4.2. [DOI] [Google Scholar]
- Kunnel S. G.; Subramanya S.; Satapathy P.; Sahoo I.; Zameer F. Acrylamide induced toxicity and the propensity of phytochemicals in amelioration: a review. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 100–113. 10.2174/1871524919666190207160236. [DOI] [PubMed] [Google Scholar]
- Henneberg R.; Otuki M. F.; Furman A. E. F.; Hermann P.; Nascimento A. J. d.; Leonart M. S. S. Protective effect of flavonoids against reactive oxygen species production in sickle cell anemia patients treated with hydroxyurea. Rev. Bras. Hematol. Hemoter. 2013, 35, 52–55. 10.5581/1516-8484.20130015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A.; Waziri A. D.; Forcados G. E.; Sanusi B.; Sani H.; Malami I.; Abubakar I. B.; Oluwatoyin H. Y.; Adinoyi O. A.; Mohammed H. A. Sickling-preventive effects of rutin is associated with modulation of deoxygenated haemoglobin, 2,3-bisphosphoglycerate mutase, redox status and alteration of functional chemistry in sickle erythrocytes. Heliyon 2019, 5, e01905 10.1016/j.heliyon.2019.e01905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A.; Waziri A. D.; Forcados G. E.; Sanusi B.; Sani H.; Malami I.; Abubakar I. B.; Abbah M. F.; Nelson A. T.; Musa B.; Mohammed H. A. Antisickling Effects of Quercetin may be Associated with Modulation of Deoxyhaemoglobin, 2, 3-bisphosphoglycerate mutase, Redox Homeostasis and Alteration of Functional Chemistry in Human Sickle Erythrocytes. Ann.Sci. Technol. 2019, 4, 38–47. 10.2478/ast-2019-0005. [DOI] [Google Scholar]
- Dermane A.; Kpegba K.; Metowogo K.; JOPPA M. K.; Aklikokou A. K. Antisickling activity evaluation of fractions obtained from whole extracts of Newbouldia Laevis P. BEAUV (BIgnoniaceae). Int. J Pharma. Pharma. Sci. 2019, 11, 42–46. 10.22159/ijpps.2019v11i2.30112. [DOI] [Google Scholar]
- Sall C.; Ndoye S.; Dioum M.; Seck I.; Gueye R.; Faye B.; Thiam C.; Seck M.; Gueye P.; Fall D.; Fall M.; Dieye T. Contribution of Three (3) Medicinal Plants of Senegalese Flora in the Management of Sickle Cell. Circulation 2017, 19, 1–11. 10.9734/bjast/2017/31563. [DOI] [Google Scholar]
- Dogra A.; Bhatt S.; Magotra A.; Sharma A.; Kotwal P.; Gour A.; Wazir P.; Singh G.; Nandi U. Intervention of curcumin on oral pharmacokinetics of daclatasvir in rat: A possible risk for long-term use. Phytother Res. 2018, 32, 1967–1974. 10.1002/ptr.6123. [DOI] [PubMed] [Google Scholar]
- Kotwal P.; Dogra A.; Sharma A.; Bhatt S.; Gour A.; Sharma S.; Wazir P.; Singh P. P.; Kumar A.; Nandi U. Effect of natural phenolics on pharmacokinetic modulation of bedaquiline in rat to assess the likelihood of potential food-drug interaction. J. Agric. Food Chem. 2020, 68, 1257–1265. 10.1021/acs.jafc.9b06529. [DOI] [PubMed] [Google Scholar]
- Yahouédéhou S. C. M. A.; Adorno E. V.; da Guarda C. C.; Ndidi U. S.; Carvalho S. P.; Santiago R. P.; Aleluia M. M.; de Oliveira R. M.; Gonçalves M. d. S. Hydroxyurea in the management of sickle cell disease: pharmacogenomics and enzymatic metabolism. Pharmacogenomics J. 2018, 18, 730–739. 10.1038/s41397-018-0045-1. [DOI] [PubMed] [Google Scholar]
- Yahouédéhou S. C. M. A.; Carvalho M. O. S.; Oliveira R. M.; Santiago R. P.; da Guarda C. C.; Carvalho S. P.; Ferreira J. R. D.; Aleluia M. M.; Adorno E. V.; Gonçalves M. d. S. Sickle cell anemia patients in use of hydroxyurea: association between polymorphisms in genes encoding metabolizing drug enzymes and laboratory parameters. Dis. Markers 2018, 2018, 1–11. 10.1155/2018/6105691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanif M.; Kanwal F.; Rafiq M.; Hassan M.; Mustaqeem M.; Seo S.-Y.; Zhang Y.; Lu C.; Chen T.; Saleem M. Symmetrical Heterocyclic Cage Skeleton: Synthesis, Urease Inhibition Activity, Kinetic Mechanistic Insight, and Molecular Docking Analyses. Molecules 2019, 24, 312. 10.3390/molecules24020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. L.; Lancaster C. S.; Finkelstein D.; Ware R. E.; Sparreboom A. Organic anion transporting polypeptide 1B transporters modulate hydroxyurea pharmacokinetics. Am. J. Physiol.: Cell Physiol. 2013, 305, C1223–C1229. 10.1152/ajpcell.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Wang K.; Zheng J.; Cheung F. S. G.; Chan T.; Zhu L.; Zhou F. Interactions of the active components ofPunica granatum(pomegranate) with the essential renal and hepatic human Solute Carrier transporters. Pharm. Biol. 2014, 52, 1510–1517. 10.3109/13880209.2014.900809. [DOI] [PubMed] [Google Scholar]
- Mandery K.; Balk B.; Bujok K.; Schmidt I.; Fromm M. F.; Glaeser H. Inhibition of hepatic uptake transporters by flavonoids. Eur. J. Pharm. Sci. 2012, 46, 79–85. 10.1016/j.ejps.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Basu S.; Jana S.; Patel V. B.; Patel H. Effects of piperine, cinnamic acid and gallic acid on rosuvastatin pharmacokinetics in rats. Phytother Res. 2013, 27, 1548–1556. 10.1002/ptr.4894. [DOI] [PubMed] [Google Scholar]
- Tung Y.-T.; Wu J.-H.; Huang C.-C.; Peng H.-C.; Chen Y.-L.; Yang S.-C.; Chang S.-T. Protective effect of Acacia confusa bark extract and its active compound gallic acid against carbon tetrachloride-induced chronic liver injury in rats. Food Chem. Toxicol. 2009, 47, 1385–1392. 10.1016/j.fct.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Khan R. A.; Khan M. R.; Sahreen S. CCl 4-induced hepatotoxicity: protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complementary Altern. Med. 2012, 12, 178. 10.1186/1472-6882-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. P.; Hillery C. A.; Brown E. R.; Misiewicz V.; Labotka R. J. Hydroxyurea therapy in children severely affected with sickle cell disease. J. Pediatr. 1996, 128, 820–828. 10.1016/s0022-3476(96)70335-9. [DOI] [PubMed] [Google Scholar]
- Keikhaei B.; Mohseni A. R.; Norouzirad R.; Alinejadi M.; Ghanbari S.; Shiravi F.; Solgi G. Altered levels of pro-inflammatory cytokines in sickle cell disease patients during vaso-occlusive crises and the steady state condition. Eur. Cytokine Network 2013, 24, 45–52. 10.1684/ecn.2013.0328. [DOI] [PubMed] [Google Scholar]
- FSSAI Food Safety and Standards Authority of India, Ministry of Health and Family Welfare, 2016. http://www.fssai.gov.
- Asgharian S.; Hojjati M. R.; Ahrari M.; Bijad E.; Deris F.; Lorigooini Z. Ruta graveolens and rutin, as its major compound: investigating their effect on spatial memory and passive avoidance memory in rats. Pharm. Biol. 2020, 58, 447–453. 10.1080/13880209.2020.1762669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Majmaie S.; Nahar L.; Sharples G. P.; Wadi K.; Sarker S. D. Isolation and antimicrobial activity of rutin and its derivatives from Ruta chalepensis (Rutaceae) growing in Iraq. Rec. Nat. Prod. 2019, 13, 64–70. 10.25135/rnp.74.18.03.250. [DOI] [Google Scholar]
- GRAS, Direct food substances affirmed as generally recognized as safe. http://www.archives.gov/federal__register/code__of__federal__regulations/ibr__locations.html (1995). (Accessed on December 17, 2020.
- Dong M.; McGann P. T.; Mizuno T.; Ware R. E.; Vinks A. A. Development of a pharmacokinetic-guided dose individualization strategy for hydroxyurea treatment in children with sickle cell anaemia. Br. J. Clin. Pharmacol. 2015, 81, 742–752. 10.1111/bcp.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute Evidence-Based Management of Sickle Cell Disease: Expert Panel Report, 2014; National Heart, Lung, and Blood Institute, US Department of Health and Human Services: Bethesda, MD, 2014.
- Creary S. E.; Strouse J. J.. A pocket Guide for the Clinician, Hydroxyurea and Transfusion Therapy for the Treatment of Sickle Cell Disease; American Society of Hematology: Washington, DC, 2021, p 20036.
- Gour A.; Dogra A.; Wazir P.; Singh G.; Nandi U. A highly sensitive UPLC-MS/MS method for hydroxyurea to assess pharmacokinetic intervention by phytotherapeutics in rats. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2020, 1154, 122283. 10.1016/j.jchromb.2020.122283. [DOI] [PubMed] [Google Scholar]
- Channar P. A.; Saeed A.; Afzal S.; Hussain D.; Kalesse M.; Shehzadi S. A.; Iqbal J. Hydrazine clubbed 1, 3-thiazoles as potent urease inhibitors: design, synthesis and molecular docking studies. Mol. Diversity 2020, 25, 1–13. 10.1007/s11030-020-10057-7. [DOI] [PubMed] [Google Scholar]
- Yu F.; Huang Y.; Cole A. J.; Yang V. C. The artificial peroxidase activity of magnetic iron oxide nanoparticles and its application to glucose detection. Biomaterials 2009, 30, 4716–4722. 10.1016/j.biomaterials.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanachayangkul P.; Tolleson W. H. Inhibition of heme peroxidases by melamine. Enzyme Res. 2012, 2012, 416062. 10.1155/2012/416062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneja A.; Sharma L.; Dubey R. D.; Mintoo M. J.; Singh A.; Kumar A.; Sangwan P. L.; Tasaduq S. A.; Singh G.; Mondhe D. M.; Gupta P. N. Synthesis, characterization and augmented anticancer potential of PEG-betulinic acid conjugate. Mater. Sci. Eng., C 2017, 73, 616–626. 10.1016/j.msec.2016.12.109. [DOI] [PubMed] [Google Scholar]
- Rocha B.; Larsson E.-L.; Freitas A. A. Effects of Hydroxyurea on Concanavalin-A-Induced T-Cell Proliferation. Scand. J. Immunol. 1984, 19, 315–321. 10.1111/j.1365-3083.1984.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Brindha P.Role of phytochemicals as immunomodulatory agents: A review. Int. J. Green Pharm. 2016, 101−18. 10.22377/ijgp.v10i1.600 [DOI] [Google Scholar]
- Singh J.; Qayum A.; Singh R. D.; Koul M.; Kaul A.; Satti N. K.; Dutt P.; Hamid A.; Singh S. Immunostimulatory activity of plumieride an iridoid in augmenting immune system by targeting Th-1 pathway in balb/c mice. Int. Immunopharmacol. 2017, 48, 203–210. 10.1016/j.intimp.2017.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.