Abstract
Large vessel occlusion in patients on ECMO is challenging to appreciate clinically secondary to sedation or induced paralysis, thus placing more emphasis on neurovascular imaging. However, emergent CTA and CTP are both inaccurate and unreliable in ECMO patients due to altered circuitry and interference with normal physiologic hemodynamics. In this review, the utility of DSA is discussed in evaluating the altered hemodynamics of VA-ECMO circuits and patency of major vasculature. In addition, the potential use of TCD in ECMO patients is discussed.
Keywords: Stroke, angiography, ECMO, neuroimaging
Background
Extracorporeal membrane oxygenation (ECMO) is used in cases of severe cardiac and/or respiratory failure. Neurological complications in patients on ECMO are common and include acute ischemic stroke (AIS), intracerebral hemorrhage (ICH), seizures and anoxic brain injury. 1 Most patients on ECMO are paralyzed and intubated, limiting assessment of neurological function and therefore forcing the reliance on advanced neuroimaging, such as non-contrast computed tomography (NCCT), computed tomography angiography (CTA) and CT perfusion (CTP). Assessment of the intracranial vessels and cerebral perfusion using CTA and CTP, both of which involve the injection of intravenous contrast, can be misleading in the setting of ECMO as it interferes with the normal physiologic flow of blood to the brain.
There are two main types of ECMO circuitry: veno-venous (VV-ECMO) for cases of refractory hypoxia, and veno-arterial (VA-ECMO) in cases of severe cardiac function compromise. Each modality can be further configured differently through various central and peripheral cannulations. 1 Central cannulation consists of the placement of the drainage cannula within the right atrium and the return cannula within the aorta. Peripheral cannulation, the main focus of this report, refers to the position of the drainage cannula in the distal inferior or the superior vena cava before the cavo-atrial junction and the return cannula in the proximal femoral, axillary or subclavian arteries. 2
High pressure inflow from the right axillary (AA)/subclavian (SCA) ECMO port leads to the reversal of blood flow in the brachiocephalic artery (BCA) and can preferentially irrigate the right carotid and vertebral artery (VA). As a result, the imaging appearance of the arterial supply to the head and neck on CTA, as well as the processed CTP images, are significantly altered if contrast administration is via the routine systemic venous access. Hence, diagnosis of large vessel occlusion (LVO), which relies on abrupt cutoff of vessel enhancement on CTA, is significantly limited. However, more than just an incidental imaging artifact, this altered blood flow pattern on CTA and CTP may have significant hemodynamic and perfusion consequences on the brain, with specific pathophysiologic consequences dependent on an individual’s arterial anatomy and collateral flow.
In this review, we describe two cases of patients on VA-ECMO presenting with acute neurological deterioration where CTA and CTP incorrectly suggested the presence of a large vessel occlusion. Digital subtraction angiography (DSA) in these patients was able to characterize the altered hemodynamics of the ECMO circuit and rule out an LVO.
Case description
Case 1
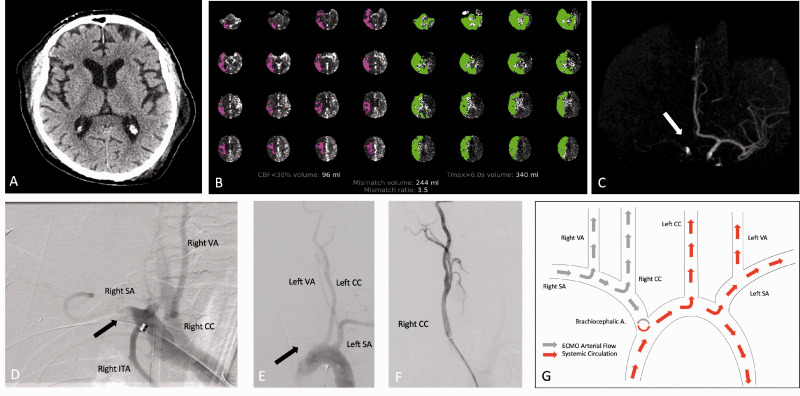
A patient in their seventies was undergoing coronary artery bypass grafting, which was complicated by post-operative cardio-respiratory arrest. Patient was treated with VA-ECMO with peripheral right (AA) return cannula. When sedation was weaned 14 days after ECMO placement, the patient remained comatose. NCCT head was unremarkable (Figure 1(a)). CTP with RAPID™ analysis (Figure 1(b)) demonstrated marked decreased cerebral blood flow (CBF) and volume (CBV) with prolonged Tmax and mean transit time (MTT) involving the right cerebral hemisphere and the posterior fossa with a mismatch volume of 244 ml. CTA (Figure 1(c)) showed non-opacification of the innominate, right common (CCA), right internal carotid (ICA), bilateral VA and basilar arteries with only enhancement of the left carotid artery. The patient was taken emergently for intervention with intention to perform mechanical embolectomy. DSA demonstrated flow reversal in the BCA and SCA artery related to the ECMO inflow (Figure 1(d) and (e)). There was normal patency of the right CCA (Figure 1(f)), which was not opacified on injection of the BCA similar to the CTA findings. Injection of the left VA demonstrated reversal of flow in the right V4 segment. No intervention was performed. The patient remained comatose and a follow-up CT, 6 days later, demonstrated development of right cerebellar and thalamic hemorrhage with extension into the 3rd ventricle (Figure 2(b)). However, there were no ischemic or hemorrhagic changes within the right hemisphere which was thought to be the primarily affected area on initial imaging. The patient’s clinical course worsened and ultimately died after the family decided to withdraw care.
Figure 1.
Case 1. NCCT (a) demonstrates no acute hemorrhage or acute ischemic changes. CT Perfusion (b) demonstrates apparent decreased CBF with prolonged Tmax involving the right cerebral hemisphere and posterior fossa. Volume rendered maximal intensity projection image of the cerebral vasculature (c) demonstrate non-opacification of the right anterior circulation (white arrow). DSA image following right subclavian artery (SA) injection (d) demonstrates non-opacification of the distal right SA (black arrow) with retrograde flow of contrast into the right common carotid (CC) and right vertebral arteries (VA). DSA image following aortic arch injection (e) demonstrates non-opacification of the right SA (black arrow) and patency of the left CC and left SA. DSA image following right CC artery injection (f) demonstrates patent vasculature. Diagram of VA-ECMO hemodynamics (g) depicts ECMO arterial flow (gray arrows) carrying non-opacified blood and systemic flow from the heart (red arrows) carrying contrast enhanced blood.
Figure 2.
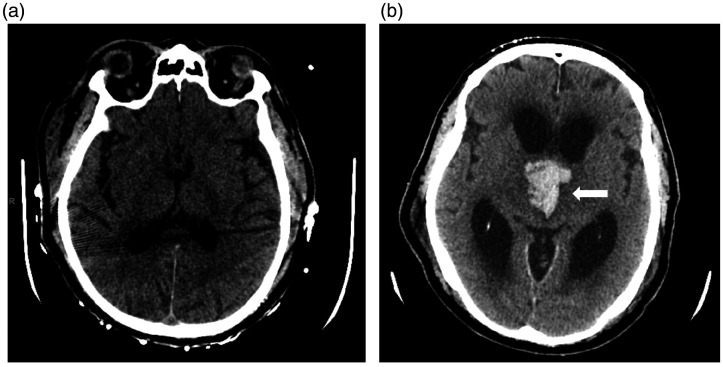
Case 1. NCCT 2 days following cerebral angiography (a) demonstrates no ICH. NCCT head 6 days following DSA (b) demonstrates acute ICH involving bilateral medial thalami (arrow) with extension into the third ventricle and secondary obstructive hydrocephalus of the lateral ventricles.
Case 2
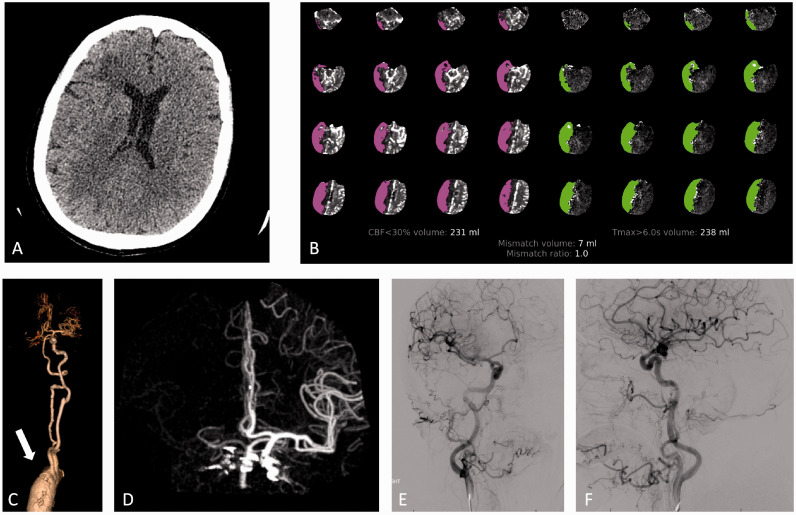
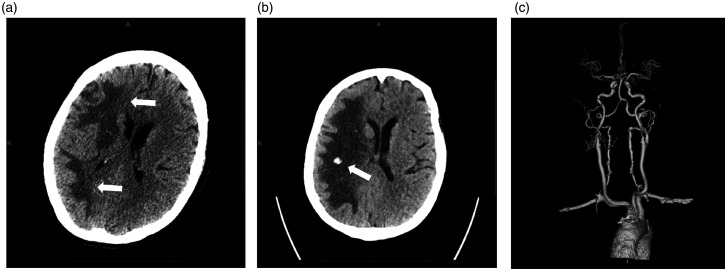
A patient in their seventies who was admitted for aortic valve replacement complicated by cardiogenic shock requiring VA-ECMO with the arterial access in the right AA. Five days after ECMO placement, the patient developed a severe right middle cerebral artery (MCA) syndrome with a National Institutes of Health Stroke Scale (NIHSS) of 20. NCCT of the brain showed subcortical hypoattenuation in the right frontal lobe (Figure 3(a)) however CTA (Figure 3(c) and (d)) showed non opacification of the right BCA, right CCA, and right SCA. CTP (Figure 3(b)) demonstrated a large area of MTT and Tmax prolongation with a matched CBF defect within the right MCA territory and right posterior fossa. Given the patient’s right hemispheric syndrome, DSA was pursued to rule out intracranial occlusion and to perform mechanical embolectomy if indicated. DSA did not reveal any LVO within the neck or intracranial vessels (Figure 3(e) and (f)). Repeat NCCT the following day demonstrated evolving subcortical right hemispheric hypodensities with cortical sparing that continued to progress over the next 7 days with development of multiple small foci of ICH (Figure 4). Repeat CTA failed to show any LVO even after ECMO was decannulated.
Figure 3.
Case 2. NCCT (a) demonstrates subcortical hypoattenuation in the right frontal lobe. CTP (b) demonstrates large area of decreased CBF and prolonged TMax within the right MCA territory and right posterior fossa. CTA 3D reconstruction of the great vessels (c) and volume rendered reconstruction of the cerebral vasculature (d) demonstrate non-opacification of the brachiocephalic artery (arrow), right CC artery, right SA, right VA and right MCA. Anteroposterior (e) and lateral (f) angiography images following right carotid artery injection demonstrate opacification of the right carotid, right MCA and right ACAs without large vessel occlusion.
Figure 4.
Case 2. Follow up NCCT 1 day (a) and 7 days (b) following initial neuroimaging. Figure a demonstrates a large area of hypoattenuation involving the subcortical white matter of the right cerebral hemisphere (arrows). Figure b demonstrates focal ICH within a confluent area of hypoattenuation (arrow). CTA 3D reconstruction of the great vessels and cerebral vasculature (c) following decannulation of the ECMO circuit demonstrates uniform opacification of the major vasculature.
Discussion
Acute neurologic deterioration in patients on VA-ECMO can be related to multiple causes, including AIS or ICH, seizures, toxic, metabolic or electrolyte disturbance, hypoxia, global or regional hypo- or hyperperfusion.1–3 Emergent neuroimaging with CT, CTA head and neck and CTP poses significant interpretation challenges as illustrated in our two patients. In both cases, the presence of VA-ECMO with arterial cannulation of the right axillary artery caused significant contrast opacification artifacts on head and neck CTA and CTP mimicking acute LVO. The presumed mechanism for the imaging findings was the high-pressure arterial inflow from the ECMO (carrying unopacified blood) resulting in retrograde flow into the right SCA and innominate arteries competing with the systemic contrast-opacified arterial blood. As the left carotid system continues to receive contrast-opacified blood directly from the aorta, the result is non-opacification of the right carotid and VAs and their branches on CTA and perfusion asymmetry on CTP.
The CTP maps in both cases were severely abnormal suggesting large territory of hypoperfusion and core infarct. These CTP changes along with the absence of opacification of the ipsilateral carotid system on CTA raised concern for LVO. The apparent prolongation in MTT and Tmax, as well as the marked decrease in CBF and CBV is artifactual, related to the non-enhanced arterial inflow. The CTP information in those cases is totally unreliable. In fact, the territory supplied mainly by the ECMO inflow might be hyper-perfused, as we hypothesize occurred in the second case which went on to develop ipsilateral vasogenic edema and patchy corona radiata white matter hemorrhage.
In a similar fashion, the CTA findings in both cases were also unsubstantiated. The lack of opacification of the right carotid artery would typically raise the concern of an embolic occlusion. However, in the presence of a VA-ECMO circuit, pressure inflow from the ECMO cannula prevents contrast from opacifying the BCA mimicking an LVO. Furthermore, if there were a true embolic occlusion, CTA would be unable to depict the occlusion due to lack of contrast.
There are no large patient studies describing how the various VA-ECMO configurations impact the imaging quality and interpretation of CTA studies. However, recognition and understanding of the potential CT imaging pitfalls in patients receiving VA-ECMO is important. These patients have significant hemodynamic instability and there are risks with transferring these very ill patients for CT imaging or catheter angiography. Also, these patients frequently are not candidates for MRI due to the ECMO machine. Alternative imaging techniques such as Transcranial Doppler (TCD) ultrasound and carotid and subclavian duplex may be reasonable alternatives and/or adjunctive options. Both of these modalities can detect flow in the ICA and VAs without the need for contrast injection. TCD can also detect intracranial occlusions (sensitivity of 50% and a specificity of 100% to detect complete occlusion of the MCA). 4 In a systematic review of 9 studies, TCD and Transcranial Color Doppler (TCCD) showed a pooled estimate of sensitivity of 95% and specificity of 95% in detecting intracranial large vessel occlusions. 5 Additionally, carotid duplex ultrasound has been shown to have a sensitivity of 91% and specificity of 99% for detecting total carotid occlusions. 6 , 7
TCD in particular may have additional utility in patients on ECMO; specifically the ability to show differential mean flow velocities (MFV) and pulsatility indices (PI) between the two MCAs. 8 , 9 Demchuk et al. showed a contralateral increase of flow velocities in 84.6%, 88.9% and 62.5% of patients with proximal ICA, distal ICA, and MCA occlusion, respectively. 10 Another study showed that patients cannulated through the AA had higher MFV and lower PI than those cannulated through the femoral artery. 9 Elevated MFV combined with low PI might also be associated with increased risk of ICH similar to cerebral hyperperfusion syndrome (HPS). 11 In pediatric patients on ECMO elevated MFV were associated with an increased risk of ICH. 12 In another study, it was shown that global changes in velocities had no statistical association with neurologic injury but isolated regional velocity increases were associated with neurologic injury, suggesting that regional changes are more clinically relevant than global changes. 13
In our patients, DSA proved to be extremely valuable in both ruling out embolic LVO as the cause of the acute neurologic deterioration and also correctly identifying the hemodynamic consequences of the axillary VA-ECMO. DSA can identify patency of the carotid and VA and study the effect of the competing inflow from the ECMO cannula. Selective injection in the right carotid and VA demonstrates vessel patency and antegrade flow. Selective injection in the right SCA demonstrates reversal of blood flow in the AA and SCA with antegrade opacification of the carotid and VA and retrograde filling of the BCA with reflux into the aortic arch.
This unique hemodynamic configuration with high flow arterial input supplied by the right axillary inflow can have significant perfusion consequences on regional CBF, related to the individual’s arterial anatomy. Our first case had a dominant large right VA which routed the ECMO inflow to the vertebro-basilar circulation in addition to the right hemisphere via the right carotid artery. In the second case, the ECMO inflow was exclusively perfusing the right hemisphere via the right carotid artery and partially perfused the posterior circulation via the right VA in competition with the slightly larger left VA. As a result, the right cerebral hemisphere likely received a differential blood supply with differences in perfusion pressure and oxygen saturation, which may have contributed to the neurological deficits and imaging findings.
The hospital course in our patients was highly suggestive of regional HPS and hypertensive injury. Case 1 developed AIS and ICH involving the posterior circulation. Case 2 developed diffuse subcortical hypodensities with cortical sparing ipsilateral to the ECMO circuit very suggestive for right hemispheric Posterior Reversible Encephalopathy Syndrome (PRES) with late hemorrhagic conversion. It is postulated that decreased CBF secondary to cardiogenic shock accompanied by reperfusion injury at initiation of ECMO may be responsible for the hemorrhagic injury. 14 , 15 TCD imaging might have been able to document a relative increase in ipsilateral cerebral blood flow responsible for regional HPS. 11
In conclusion, axillary VA-ECMO may result in artifactual findings of LVO and perfusion deficits on CTA and CTP, respectively. This specific hemodynamic environment may be complicated by regional hyperperfusion with encephalopathy, cerebral edema and ICH. Catheter angiography is very useful in understanding the blood flow pattern and evaluating for LVO. Alternative, flow-based imaging modalities such as TCD and carotid Doppler ultrasonography can also be considered in these patients.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Hassan Aboul Nour https://orcid.org/0000-0002-6698-5848
Horia Marin https://orcid.org/0000-0003-1567-959X
References
- 1.Bowling SM, Gomes J and Firstenberg MS. Neurologic Issues in Patients Receiving Extracorporeal Membrane Oxygenation Support. In: Firstenberg MS (ed) Extracorporeal Membrane Oxygenation – Advances in Therapy. IntechOpen, 2016, pp. 321–337. DOI: 10.5772/64269.
- 2.Lee S, Chaturvedi A. Imaging adults on extracorporeal membrane oxygenation (ECMO). Insights Imaging 2014; 5: 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya J, Rajamohan AG, Skalski MR, et al. CT angiography of the head in extracorporeal membrane oxygenation. AJNR Am J Neuroradiol 2017; 38: 773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott Burgin W, Malkoff M, Felberg RA, et al. Transcranial Doppler ultrasound criteria for recanalization after thrombolysis for middle cerebral artery stroke. Stroke 2000; 31: 1128–1132. [DOI] [PubMed] [Google Scholar]
- 5.Mattioni A, Cenciarelli S, Eusebi P, et al. Transcranial Doppler sonography for detecting stenosis or occlusion of intracranial arteries in people with acute ischaemic stroke. 2020;2:CD010722. [DOI] [PMC free article] [PubMed]
- 6.AbuRahma AF, Pollack JA, Robinson PA, et al. The reliability of color duplex ultrasound in diagnosing total carotid artery occlusion. Am J Surg 1997; 174: 185–187. [DOI] [PubMed] [Google Scholar]
- 7.Henry JC, Kiser D, Satiani B. A critical evaluation of carotid duplex scanning in the diagnosis of significant carotid artery occlusive disease. Adv Vasc Med 2015; 2015: 1–6. [Google Scholar]
- 8.Zbornikova V. Clinical investigative studies. J Neuroimaging 2003; 13: 17–27.12593127 [Google Scholar]
- 9.Salna M, Ikegami H, Willey JZ, et al. Transcranial Doppler is an effective method in assessing cerebral blood flow patterns during peripheral venoarterial extracorporeal membrane oxygenation. J Card Surg 2019; 34: 447–452. [DOI] [PubMed] [Google Scholar]
- 10.Demchuk AM, Christou I, Wein TH, et al. Specific transcranial Doppler flow findings related to the presence and site of arterial occlusion. Stroke 2000; 31: 140–146. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto S, Toyoda K, Inoue T, et al. Diagnostic impact of transcranial color-coded real-time sonography with echo contrast agents for hyperperfusion syndrome after carotid endarterectomy. Stroke 2004; 35: 1852–1856. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien NF, Hall MW. Extracorporeal membrane oxygenation and cerebral blood flow velocity in children. Pediatr Crit Care Med 2013; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rilinger JF, Smith CM, deRegnier RAO, et al. Transcranial Doppler identification of neurologic injury during pediatric extracorporeal membrane oxygenation therapy. J Stroke Cerebrovasc Dis 2017; 26: 2336–2345. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher-Sandersjöö A, Thelin EP, Bartek J, et al. Incidence, outcome, and predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: a systematic and narrative review. Front Neurol 2018; 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mateen FJ, Muralidharan R, Shinohara RT, et al. Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol 2011; 68: 1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]