Abstract
Background and purpose
The aim of this study was to investigate whether morphological evidence of intracranial vascular injury can be found in the occluding thrombi of patients with ischemic stroke.
Methods
From 2015 until 2018 specimens of thrombi from patients with large vessel occlusion treated either by stent-assisted aspiration thrombectomy (TE) or by aspiration thrombectomy were prospectively collected. Thrombus specimens were formalin-fixed, paraffin-embedded and stained. Architectural features, presence of parts of vessel wall or atheromatous material, organisation of the thrombi, technique and number of thrombectomy manoeuvers were evaluated.
Results
Thrombus specimens from 302 patients were analyzed. 238 (80%) patients were treated with stent-assisted aspiration TE and 64 (20%) patients with aspiration TE only. 286 (95%) had fresh thrombi, 14 (4.6%) showed initial/complete thrombus organisation and multi-staged thrombi were found in 2 (<1%) patients. In 8 patients (2.6%) we found atypical thrombus content after a time interval longer 6 hours after onset and final thrombectomy manoeuvre: 4 with atheromatous material and 4 with parts of a vessel wall. In 1 patient with parts of vessel wall angiographically a dissection was detected. No parts of the vessel wall were found after sole aspiration thrombectomy.
Conclusions
The overall risk of arterial vessel wall injury aspiration thrombectomy was low in our study. Aspiration thrombectomy and the procedure related with a retrieving device together with an ischemia time longer than 6 hours may increase the risk of vessel injury through the thrombectomy procedure. Further investigations are necessary to elucidate the influence of the mentioned aspects.
Keywords: Stroke, intracranial thrombosis, thrombectomy, pathology
Background and purpose
Aspiration thrombectomy exerts force to the affected vessel due to negative pressure in addition to shearing stress if a retrieving device is used. The aim of this retrospective study was to look for morphological correlates of injured intracranial vessels in the retrieved thrombi of ischemic stroke patients.
Introduction
Mechanical Thrombectomy (TE) is the procedure of choice in the case of large vessel occlusions (LVO) detected by radiological imaging of brain supplying arteries. TE is regarded as a quick and safe procedure for up to 24 hours following an ischemic event 1 and constitutes an essential therapeutic part within AHA Guideline Acute Ischemic Stroke. 2 Different methods of mechanical TE exist which consists of mono techniques to retrieve the thrombus e.g. sole aspiration with an aspiration catheter or sole approach with a stentriever with use of a guiding catheter which can be supplementary armed with an occlusion balloon to reduce antegrade flow or a combination of these techniques. In addition to these techniques various designs of stentriever were in clinical use. 3 With increasing numbers of treated patients subarachnoid bleeding, intraventricular or parenchymal hemorrhage, air embolism, vessel ruptures and other device related complications have been reported. 4 Concerning the vasculature, endothelial denudation, medial layer edema and mural thrombi were reported in a large animal model. 5 These injuries of the intracranial arteries are suspected as a cause of symptomatic intracranial hemorrhage. 6 However, direct evidence of injuries of the targeted vessel has scarcely been addressed in human. To this end, we retrospectively examined TE specimens retrieved during interventions in ischemic stroke patients either with sole aspiration TE or the combined approach with aspiration and a stentriever.
Material and methods
Patients
From 2015 until 2018, TE samples of 302 patients with ischemic stroke were prospectively collected. All patients with stroke symptoms related to the site of the vessel occlusion were eligible for mechanical TE. A cranial computed tomography (CT) was performed in all patients with known onset of ischemic cerebral symptoms and the site of the affected vessel was verified by CT angiography. Magnetic resonance imaging (MRI) was performed in cases with unknown time of onset of stroke symptoms. According to the ASPECT score, 7 patients with an abnormally low attenuation of brain structures relative to attenuation of other parts of the same structures or of the contralateral hemisphere in the complete vascular territory of the main trunk of the occluded vessel when examined with CT were excluded. The same criteria as above described for CT were used if a visible complete match of brain tissue with edema in fluid attenuated inversion recovery (FLAIR) and consecutive restriction in diffusion weighted imaging (DWI) were apparent in MRI 8 in the complete territory of the main trunk of the occluded vessel and the patients was consecutively excluded. Consent to participate in this clinicopathological study was obtained using an opt-out approach. The ethics committee of our university clinic approved the retrospective analysis of the thrombi under internal vote no.:8374_BO_K_2019
Treatment
Patients without contraindications for systemic thrombolysis received body weight adapted recombinant tissue plasmin activator (rTPA) intravenously before TE. Thrombus specimens were obtained either by stent-assisted aspiration TE or by sole aspiration TE, either in conscious sedation or in general anesthesia. For the procedure, a guiding catheter (Neuron MAX 6 F 088, Penumbra, Inc., Alameda, California) was inserted in the cervical part of the internal carotid artery. For the thrombectomy of the posterior circulation the aspiration catheter was navigated with or without support of a guiding catheter and continuous saline flush to the occlusion site. Sole aspiration procedure was performed using a Sofia 5/6 F 125 cm aspiration catheter (MicroVention Terumo, Tustin, California, USA). After wedging the aspiration catheter to the proximal part of the occlusion, a 20 ml syringe was connected via a stop-cock to the aspiration catheter. After applying vacuum to the connected syringe, the aspiration catheter was pulled back under fluoroscopy. In case the aspiration catheter showed no retrograde flow the aspiration catheter was removed entirely. With retrograde flow the connected syringe was flushed gently through a pad and isolable tissue and clots were transferred to a formalin-filled container. If complete removal of the aspiration catheter was necessary the aspiration catheter was flushed the same way. For stent-assisted TE, the aspiration catheter was navigated as near as possible to the occlusion side and a microwire equipped microcatheter was navigated beyond the occlusion. Intravascular catheter position aside the thrombus was verified by contrast media injection; then the stentriever was unfolded inside or behind the thrombus and pulled inside or next to the aspiration catheter which was pulled back under continuous vacuum into the guiding catheter. Detection of thrombus was done by flushing the aspiration devices as described above. For thrombus aspiration 2 different aspiration catheter were used, the choice of the stentriever was up to the discretion of interventionalists (Supplement 1). Thrombus specimens were collected as outlined above. The number and kind of these procedures (e.g. TE with/without stentriever) was reported. Angiographic results after the final thrombectomy manoeuver were classified according to the modified thrombolysis in cerebral infarction grading system (mTICI) grading system. 9
Histopathology
All thrombus specimens were immediately formalin-fixed before being paraffin-embedded (FFPE) 10 to prevent post-procedure degeneration. Thrombus fractions from multiple manoeuvres of the same patient were analyzed as a whole if they were visually identical. In case a difference was recognized the fractions were analyzed separately. The entire thrombus specimens were then cut to 1 µm thick sections and stained by hematoxylin-eosin and van Gieson’s stain. Slides were evaluated via conventional histopathology assessing cellularity, fibrin, and erythrocyte content, as well as the presence of parts of the vascular wall, atherosclerotic plaques, and thrombus organisation in the form of granulation tissue for temporal classification of the thrombi. Immunohistochemistry was not used, as the morphological features we are interested in can be readily identified using basic histology. The applied established classification of thrombi was used: “red” thrombus: thrombus consists predominantly of erythrocytes with scarce fibrin (≤10% of the thrombus volume), “white” thrombus: thrombus consists mostly of fibrin with erythrocytes making up the minority of the thrombus volume (≤10% of the thrombus volume),“mixed” thrombus: combination of the above; typically consists of a “head” (white thrombus), “tail” (red thrombus). Organized thrombi were defined as older thrombi, organized in part (“initial”; ≤10%) or total by granulation tissue and extracellular matrix deposition/fibrosis, and multi-staged thrombi were composed of a mixture thereof. Thrombi which had not undergone organisation and showed a varying composition of layered platelets, fibrin, and erythrocytes but only showed minimal lytic parts were classified as fresh (less than 1 day old).11,12
Results
Patients
167 male (mean age: 69.7, range from 26.5 to 94.5 y) and 135 female (mean age: 74.5 y, range from 11.2 y to 95 y) patients were included in the present study. All patients suffered from an occlusion in a single intracranial artery. 217 out of 302 (72%) patients had a time interval between onset of stroke symptoms or time of last seen well and final thrombectomy manoeuvre not longer than 6 hours. 73 out of 302 (24%) had a time interval between onset of stroke symptoms or time of last seen well and final thrombectomy manoeuvre between 6 hour and 12 hours and 7 (2.3%) longer than 12 hours, respectively. Finally, in 5 (1.7%) patients the onset of stroke could not be determined (Table 1).
Table 1.
Patients characteristics.
| Female: n (%) | 135 (44,7) |
| Age on Admission in years median (range) | 75,2 (11,2–95) |
| OtlM no longer than 6 h n (%) | 217 (72) |
| OtlM between 6 h and 12 h n (%) | 73 (24) |
| OtlM longer than 12 h n (%) | 7 (2.3) |
| Last seen well unknown n (%) | 5 (1.7) |
OtlM: Stroke onset or time of last seen well to last manoeuver.
Location of vessel occlusions
We included patients with occlusions in the anterior and posterior cerebral circulation. One- third of the patients suffered from an occlusion of the carotid artery, 48% of the M1-segment and 9% in the M2 segment. Finally, 10% of occlusions were located in the basilar artery (Table 2).
Table 2.
Site of occlusion.
| CCA/ICA occlusion n(%)) | 70 (23) |
| T-occlusion n (%) | 30 (10) |
| M1 occlusion n (%) | 145 (48) |
| M1/M2 occlusion n (%) | 5 (2) |
| M2 occlusion n (%) | 22 (7) |
| ACA occlusion n (%) | 1 (0,3) |
| BA occlusion n (%) | 21 (7) |
| Head of BA n (%) | 8 (3) |
CCA: common carotid arter; ICA: internal carotid artery; T: carotid-T; Mx: segment of middle cerebral artery; ACA: anterior cerebral artery; BA: basilar artery.
Number and technique of thrombectomy manoeuvre
80% (n = 238) of the TE were performed as a combination of aspiration TE with stent assisted retrieving and 20% (n = 64) with aspiration only. Atypical thrombus content was detected after 1 to 3 manoeuvres. Concerning the specimen with vessel wall, in 1 out of 4 patient parts of vessel wall were detected after a single stentriever assisted aspiration TE. In 3 out of 4 patients after a previous single attempt of sole thrombus aspiration vessel wall was found in the final stentriever assisted aspiration manoeuvre after a total of 2 and 3 manoeuvers, respectively.
Thrombus specimens
In 286 out of 302 (95%) patients the major portion of thrombus specimens extracted with both methods consisted of fresh mixed thrombi with or without interspersed neutrophils (n = 225 with stentriever assisted aspiration TE: n = 61 with sole aspiration TE; 4.6% of specimens showed initial or complete organisation (aspiration n = 3, combined n = 11). Multi-staged thrombi were found in 2 (<1%) patients of the combined stent-assisted aspiration group and none in the case of sole aspiration (Table 3)
Table 3.
Kind and number of thrombectomies and detected thrombus tissue.
| Manoeuvers |
Pat (n) ∑=302 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Thrombus | 1 | 2 | 3 | 4 | 5 | more than 5 | ||
| Aspiration+Stentriever | Fresh/Fresh+Neurophils | 73/1 | 60/1 | 46/1 | 24 | 12 | 5 | 223 |
| Fresh with atheroma | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Fresh with vessel wall | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| In organization: initial/full | 0/3 | 2/3 | 0 | 0/1 | 0/0 | 0/0 | 9 | |
| In organization with vessel wall | 1 | 0 | 1 | 0 | 0 | 0 | 2 | |
| Multi-staged | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Multi-staged with vessel wall | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Aspiration only | Fresh/+Neurophils | 42/3 | 7/0 | 4/0 | 1/0 | 1/0 | 0/0 | 58 |
| Fresh with atheroma | 3 | 0 | 0 | 0 | 0 | 0 | 3 | |
| Fresh with vessel wall | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| In organization: initial/full | 0/3 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3 | |
| In organization with vessel wall | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Multi-staged | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Multi-staged with vessel wall | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Manoeuvers: Total number (n) per patient of thrombectomy procedures; Thrombus: Histomorphological classification of thrombus specimen.
Atypical thrombus content
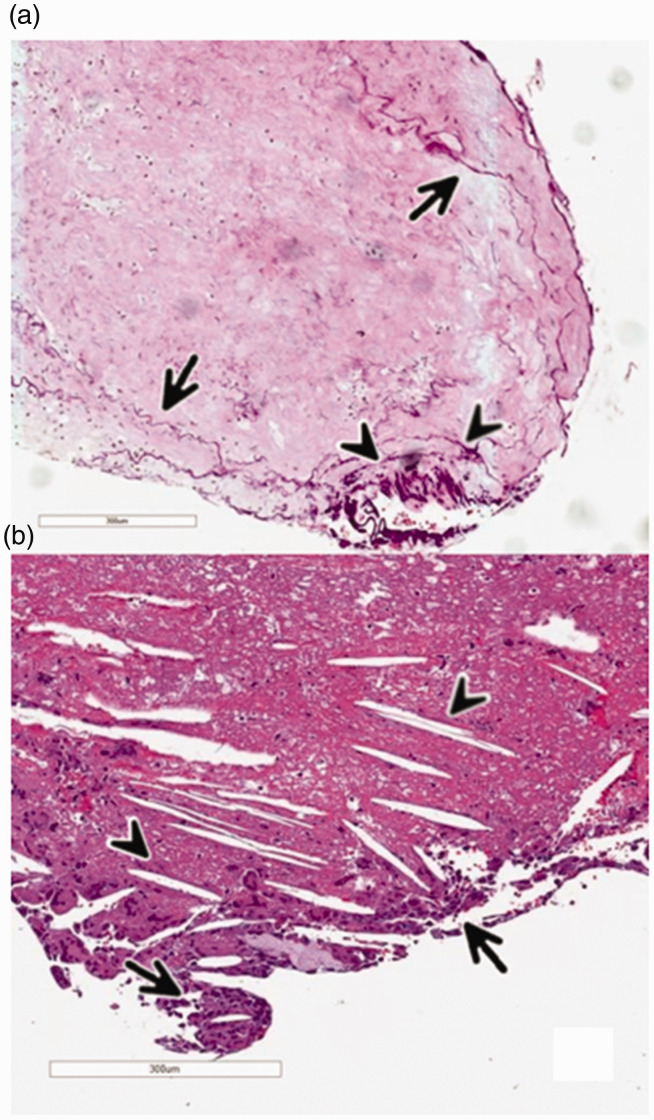
In 8 out of 302 (2.6%) patients there was atypical content in the materials making up the thrombi (Table 4): in 4 patients, we found atheromatous content without parts of vascular wall and in 4 patients there were parts of the vessel wall (Figure 1). In the specimen with atheromatous content only, singular cholesterol crystals and foam cells were detected, a finding in line with previous reports on thrombus formation due to ruptured atheromatous plaques. 13 All 8 patients had at least one cardiovascular risk factor (CVR) with or without specific pre-stroke medication, such as acetylsalicylic acid. 2 out of 4 patients with parts of vascular walls in the thrombus specimens underwent systemic thrombolysis with rTPA; in specimens with atheromatous content, 2 out of 4 patients received systemic thrombolysis. Aspiration was performed in 7 out of 8 patients with the 5/6 F Sofia 125 cm aspiration catheter, in one patient with vascular wall components with the Penumbra 5 F 125 cm Reperfusion catheter. In the 5 patients with atypical thrombus content, different types of stentriever were used (Supplement 1). All atheroma components were found in thrombi histomorphologically classified as fresh, but only 1 out of 4 specimen with parts of the vessel wall inside the thrombus was classified as fresh (Table 4).
Table 4.
Characteristics of patients and methods with atypical thrombus content.
| Specimen with vessel wall | ID | 2 | 90 | 218 | 296 |
| OtlM (h) | 16 | 7 | 8,5 | 6 | |
| Site | M2 | M1 | M1 | ACI | |
| Retriever | yes | yes | yes | yes | |
| Manoeuvre | 2 | 1 | 3 | 3 | |
| Stentriever in manoeuvre | 2 | 1 | 2,3 | 3 | |
| Thrombus in manoeuvre | 2a | 1 | 2,3a | 3a | |
| Vessel wall in manoeuvre | 2 | 1 | 3 | 3 | |
| mTICI | 3 | 2b | 3 | 3 | |
| Dissection: location | no | no | M1 | no | |
| Thrombus composition | fresh | In organisation | multi-staged | In organisation | |
| Specimen with atheroma | ID | 138 | 155 | 179 | 291 |
| OtlM (h) | 4,75 | 8,25 | 7h | 4 | |
| Site | M1 | BA | HB | BA | |
| Retriever | no | yes | no | no | |
| Manoeuvers | 1 | 3 | 1 | 1 | |
| Stentriever in manoeuver | 0 | 1,2,3 | 0 | 0 | |
| Thrombus in manoeuver | 1 | 1,2,3 | 1 | 1 | |
| Atheroma in manoeuvre | 1 | ||||
| mTICI | 3 | 2b | 3 | 3 | |
| Dissection: location | None | None | None | None | |
| Thrombus composition | Fresh | Fresh | Fresh | Fresh |
OtlM: Stroke onset or time of last seen well to last manoeuver in hours (h); Site: M(n): segment of ACM, BA: Basilar artery; HB: Head of BA, Manoeuvers: count of aspiration thrombectomy procedures without/with stentriever; Stentriever in manoeuvre: Ordinal number of manoeuvre in which stentriever was used; Thrombus from manoeuvre: Ordinal number of manoeuvre in which thrombus was sampled; Vessel wall in Manoeuvre: Ordinal number of manoeuvre in which vessel wall was detected.
aProcedure change from sole aspiration to stentriver assisted aspiration TE.
Figure 1.
a: Specimen of a multi-staged thrombus containing single fibers [fractured intimal collagen] (Arrows) and cohesive parts of the vascular wall (Arrowheads). b: Specimen of a fresh thrombus-containing atheromatous material with negative impressions of cholesterol crystals (Arrowheads) and adjacent accumulations of foam cells (Arrow).
Discussion
The aim of the present study was to find evidence of vessel injury in thrombi received from mechanical thrombectomy. Therefore, thrombus specimens were examined beyond conventional histopathology for the presence of atypical thrombus content. Marder et al. found atheroma with adjacent intimal flaps in a thrombus retrieved from the intracranial occluded vessel with the MERCI™ device. 14 Singh examined thrombi of 48 patients treated with stentriever assisted thrombectomy for signs of intimal damage by means for CD24 positive cells but concluded that mechanical thrombectomy with stent-retrievers does not cause relevant intimal damage in acute ischemic stroke treatment. 15 In a more recent study, thrombus specimens with vascular wall components were seen after 1 to 3 stent assisted retrieval maneuvers, confirming that even after only one TE manoeuver, parts of the vessel wall can be found in the retrieved thrombus specimen. 16 Rupture of atheromatous plaques in coronary arteries were a frequent finding proximal to thrombi causing myocardial infarction 17 but were rarely seen in specimen of coronary aspiration thrombectomy. 18 In the cohort of 145 stroke patients, Boeckh-Behrens et al. did neither find any atheromatous tissue nor intimal tissue in the specimens of the retrieved thrombi. 19 In a more recent study in 2018 Krajíčková found atheromatous tissue in 2 out of 44 patients treated with mechanical thrombectomy but no intimal tissue. 20
Pathophysiological consideration
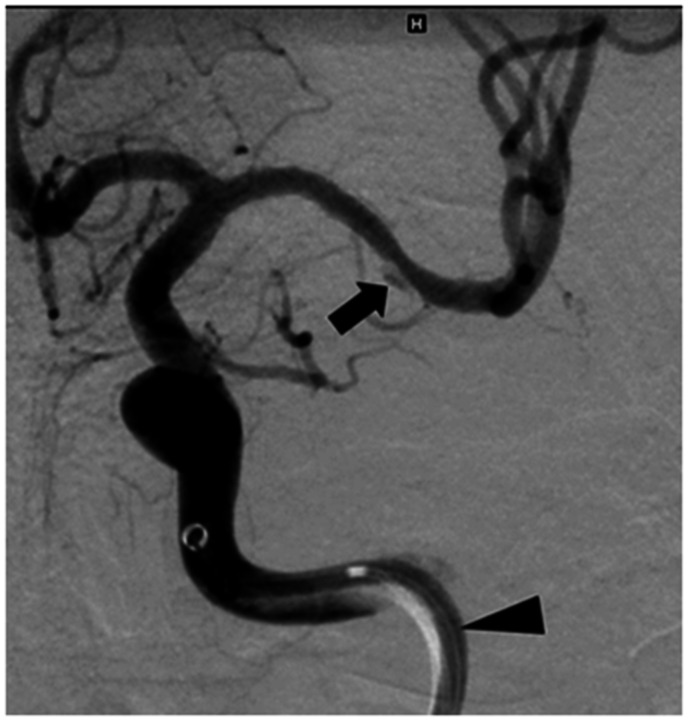
The suction through the aspiration catheter exerts force through negative pressure to the endothelium. Additional shearing stress on the vessel wall, especially the intima, occurs when stent retriever and aspiration catheter were withdrawn. In the present study all 4 patients with parts of the vascular wall had undergone TE with a stentriever, while 1 patient suffered from a dissection angiographically as a complication of the procedure (Figure 2). Vascular wall parts were found after the use of different retrieving devices and interventionalists. Due to the small amount of positive specimens, no accumulation with one specific stentriever used during the study period was found; this was also true in regard to the interventionalists (Supplement 1). After TE, 7 out of 8 patients with parts of vessel wall or atheroma in the retrieved specimens angiographically showed no lesions in the formerly occluded artery, which might indicate a source of the vessel wall other than the occluded vessel. In the patient with the arterial dissection, the dissection was apparent in the second TE manoeuvre which was the first passage with the stentriever and yielded no thrombus. In the third and last stentriever manoeuvre parts of vessel wall were detected in the thrombus specimen, so we accordingly postulate that in this case the finding of vessel components was a result of the thrombectomy procedure itself. The direct interaction from the microwire, the microcatheter, the aspiration catheter or retrieving device with the occluded vessel might have caused an injury of the vessel.
Figure 2.
Pat 218: Dissection of the M1-segment of the middle cerebral artery (black arrow). Aspiration catheter with intraluminal microcatheter in the C2-segment of the internal carotid artery (black arrow head).
Effect of ischemia and mechanical stress
All patients with parts of vessel wall had a time interval of 6 hours or longer between onset of symptoms and final thrombectomy manoeuvre. However, no specimen after sole aspiration TE showed signs of vessel injury by means of histomorphologically detectable parts of the vessel wall, even after a time interval of more than 6 hours between symptom onset and procedure. In the study of Marder et al., the atheromatous content in one thrombus was detected 7 hour after stroke onset, in study of Boeckh-Behrens et al. with a median recanalization time of 231 min after onset - even after 7 hours - no atheromatous or intimal tissue was found.14,19 The different findings may be due to different retrieving devices used in both studies. The finding of atypical thrombus content in the study of Marder et al. and in the present study even after a recanalization time of greater 6 hours may indicate that a prolonged time of ischemia might not only affect the brain, but also the endothelium of the artery, if not all layers of the occluded vessel. A long exposure time of the occluding thrombus with the vessel wall could induce a higher vulnerability for mechanical forces (e.g. suction and shear stress). As we did not find any parts of vessel wall in sole aspiration, we postulate that sole aspiration thrombectomy might be less traumatic to the ischemic vessel than the more complex TE procedure with the combination of an aspiration catheter, a microcatheter, a microwire and a stentriever. The interaction of these devices with the endothelium together with a prolonged ischemia time may cause a vessel wall injury as a result of the thrombectomy procedure, even in one single manoeuver. Nevertheless, our study has some limitations. First, the number of patients treated with stentriever aspiration was greater than those treated with aspiration alone. Second, stentrievers from different manufacturers were used. Third, we regularly did not differentiate the thrombus specimen per every single thrombectomy manoeuvre. However, thrombus containing parts of vessel wall were only detected after the application of a stentriever as well when a stentriever as second line device was used. In spite of one arterial dissection detected, the final result of the TE in this patient was even a TICI 3 (Table 4). This may qualify the importance of atypical thrombus content for the final angiographic result of the thrombectomy. Finally, as we found no parts of vascular wall in the thrombi with atheromatous tissue, the origin of the atheromatous tissue may be apart from the occlusion side, e.g. from the carotid bifurcation, the aortic arc or the heart valves.
Conclusion
The overall risk of arterial vessel wall injury aspiration thrombectomy was low in our study. The combination of aspiration thrombectomy and the procedure related with the use of a retrieving device together with an ischemia time longer than 6 hours may increase the risk of vessel injury through the thrombectomy procedure. Further investigations are necessary to elucidate the influence of the mentioned aspects.
Supplemental Material
Supplemental material, sj-pdf-1-ine-10.1177_1591019920976673 for Interventional stroke treatment – Is it also safe for arteries? Looking at thrombectomy wall damage through clot histology by Frank Donnerstag, Friedrich Götz, Mete Dadak, Peter Raab, Enrico Calvino Iglesias, Christopher Werlein, Heinrich Lanfermann and Danny Jonigk in Interventional Neuroradiology
Footnotes
Authors’ contribution: Frank Donnerstag: Project development, data collection, manuscript drafting and revision. Friedrich Götz: Data collection, manuscript revision. Mete Dadak: Data collection, manuscript revision. Peter Raab: Data collection, manuscript revision. Enrico Calvino Iglesias: Manuscript drafting. Christopher Werlein: Data collection, manuscript drafting and revision. Heinrich Lanfermann: Manuscript revision. Danny Jonigk: Project development, data collection, manuscript drafting and revision.
Acknowledgements: The authors would like to thank Emily Brouwer for editing the manuscript and the KFO311 for funding the work of Danny Jonigk.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Frank Donnerstag received grants from Penumbra and Stryker. Friedrich Götz received grants from Covidien, Microvention and Stryker. Mete Dadak received grants from Stryker. Peter Raab, Enrico Calvino Iglesias, Christopher Werlein, Heinrich Lanfermann und Danny Jonigk reported no conflict of interests with the subject matter.
Ethical approval: The Ethic Committee of Hannover Medical School has approved the study under internal vote no.:8374_BO_K_2019.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Frank Donnerstag https://orcid.org/0000-0002-6798-1929
Supplemental material: Supplemental material for this article is available online.
References
- 1.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al.; American Heart Association Stroke Council. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 3.Samaniego EA, Roa JA, Limaye K, et al. Mechanical thrombectomy: emerging technologies and techniques. J Stroke Cerebrovasc Dis 2018; 27: 2555–2571. [DOI] [PubMed] [Google Scholar]
- 4.Koh JS, Lee SJ, Ryu C-W, et al. Safety and efficacy of mechanical thrombectomy with solitaire stent retrieval for acute ischemic stroke: a systematic review. Neurointervention 2012; 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gory B, Bresson D, Kessler I, et al. Histopathologic evaluation of arterial wall response to 5 neurovascular mechanical thrombectomy devices in a swine model. AJNR Am J Neuroradiol 2013; 34: 2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado Almandoz JE, Kayan Y, Young ML, et al. Comparison of clinical outcomes in patients with acute ischemic strokes treated with mechanical thrombectomy using either solumbra or ADAPT techniques. J Neurointerv Surg 2016; 8: 1123–1128. [DOI] [PubMed] [Google Scholar]
- 7.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000; 355: 1670–1674. [DOI] [PubMed] [Google Scholar]
- 8.Thomalla G, Cheng B, Ebinger M, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol 2011; 10: 978–986. [DOI] [PubMed] [Google Scholar]
- 9.Higashida RT, Furlan AJ. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 10.Fox CH, Johnson FB, Whiting J, et al. Formaldehyde fixation. J Histochem Cytochem 1985; 33: 845–853. [DOI] [PubMed] [Google Scholar]
- 11.Niesten JM, van der Schaaf IC, van Dam L, et al. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One 2014; 9: e88882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laridan E, Denorme F, Desender L, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol 2017; 82: 223–232. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto T, Hayakawa M, Funatsu N, et al. Histopathologic analysis of retrieved thrombi associated with successful reperfusion after acute stroke thrombectomy. Stroke 2016; 47: 3035–3037. [DOI] [PubMed] [Google Scholar]
- 14.Marder VJ, Chute DJ, Starkman S, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke 2006; 37: 2086–2093. [DOI] [PubMed] [Google Scholar]
- 15.Singh P, Doostkam S, Reinhard M, et al. Immunohistochemical analysis of thrombi retrieved during treatment of acute ischemic stroke. Stroke 2013; 44: 1720–1722. [DOI] [PubMed] [Google Scholar]
- 16.Funatsu N, Hayakawa M, Hashimoto T, et al. Vascular wall components in thrombi obtained by acute stroke thrombectomy: clinical significance and related factors. J Neurointerv Surg 2019; 11: 232–236. [DOI] [PubMed] [Google Scholar]
- 17.Horie T, Sekiguchi M, Hirosawa K. Coronary thrombosis in pathogenesis of acute myocardial infarction. Histopathological study of coronary arteries in 108 necropsied cases using serial section. Br Heart J 1978; 40: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami T, Mizuno S, Takahashi Y, et al. Intracoronary aspiration thrombectomy for acute myocardial infarction. Am J Cardiol 1998; 82: 839–844. [DOI] [PubMed] [Google Scholar]
- 19.Boeckh-Behrens T, Schubert M, Förschler A, et al. The impact of histological clot composition in embolic stroke. Clin Neuroradiol 2016; 26: 189–197. [DOI] [PubMed] [Google Scholar]
- 20.Krajickova D, et al. Fibrin clot architecture in acute ischemic stroke treated with mechanical thrombectomy with stent-retrievers – cohort study. Circ J 2018; 82: 866–873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ine-10.1177_1591019920976673 for Interventional stroke treatment – Is it also safe for arteries? Looking at thrombectomy wall damage through clot histology by Frank Donnerstag, Friedrich Götz, Mete Dadak, Peter Raab, Enrico Calvino Iglesias, Christopher Werlein, Heinrich Lanfermann and Danny Jonigk in Interventional Neuroradiology