Abstract
Introduction
Carotid artery stenting (CAS) has increasingly emerged as an alternative strategy to carotid endarterectomy in the treatment of patients with symptomatic carotid stenosis. Optimal timing for CAS after symptoms onset remains unclear. We aimed to evaluate the safety and efficacy of CAS when performed in an emergency setting.
Patients and methods
We performed a retrospective analysis of CAS patients admitted to our CSC with symptomatic extracranial carotid occlusion or significant stenosis from January 2014-September 2019. Emergency CAS was defined as CAS performed during the same hospitalization from TIA/stroke onset, whereas elective CAS as CAS performed on a subsequent admission. The primary outcome was defined as the occurrence of any stroke, myocardial infarction, or death related to the procedure at 3 months of follow-up. Secondary outcomes included periprocedural complications and the rate of restenosis/occlusion at follow-up. Logistic regression and survival analyses were used to compare outcomes and restenosis at follow-up.
Results
We identified 75 emergency and 104 elective CAS patients. Emergency CAS patients had significantly higher rates of ipsilateral carotid occlusion (17% vs. 2%, p < 0.001) and use of general anesthesia (19% vs. 4%, p = 0.001) than elective CAS. There were no significant differences between emergency and elective CAS in the primary (5.7% vs. 1%, p = 0.161) and secondary (9% vs. 4.8%, p = 0.232) outcomes. We did not find differences in the rate of restenosis/occlusion (7% vs. 11.6%; log-rank test p = 0.3) at a median of 13 months follow-up.
Conclusion
In our study, emergency CAS in symptomatic patients might have a similar safety and efficacy profile to elective CAS at 3 months and long-term follow-up.
Keywords: Symptomatic carotid artery stenosis, mechanical thrombectomy, carotid artery stenting.
Introduction
Carotid artery stenting (CAS) has increasingly emerged as an alternative strategy to carotid endarterectomy (CEA) in the treatment of patients with symptomatic carotid stenosis.1 The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) showed that patients with carotid artery stenosis (symptomatic or asymptomatic) who underwent CAS or CEA had similar outcomes of periprocedural stroke, death, and myocardial infarction (MI).2 Also, there were no significant differences in the rates of periprocedural stroke, MI, death, and restenosis over 10 years of follow-up.3
The Secondary Data Analysis of the German Statutory Quality Assurance Database found that a short time interval between the neurologic index event and CAS of up to 7 days is associated with an increased risk for stroke or death under routine conditions.4 In the contrary, Song et al. found that there were no differences in late events (ipsilateral stroke or any death from 31 days to 1 year from CAS) between early (≤2 weeks) and delay (>2 weeks) CAS.5 Studies supporting early revascularization reported promptly timing carried no additional risks compared with delayed CAS up to 90 days.6,7
Currently, with improved technical modifications in stent design and alternative means of embolic protection, CAS might represent a reasonable and safe option for acute revascularization to minimize the risk of periprocedural complications. However, the timing of a carotid intervention in symptomatic patients after the onset of transient ischemic attack (TIA) or stroke remains controversial. We aimed to evaluate the safety and efficacy of CAS when performed emergently during hospitalization versus electively in a subsequent admission.
Methods
Study design
We performed a retrospective analysis of the prospectively collected database at our Comprehensive Stroke Center from January 2014 to September 2019. We classified our cohort as ‘emergency’ or ‘elective’ CAS, based on the timing of the procedure. Emergency CAS included patients who underwent CAS during the same hospitalization from TIA/stroke onset, whereas, the elective group was composed of patients who had the procedure performed on a subsequent admission. We defined TIA as an acute brief episode of neurological dysfunction lasting less than 24 hours with negative diffusion-weighted imaging. The timing of the procedure was decided by the treating neuro interventionalist. The study was approved by our institutional review board.
Inclusion and exclusion criteria
We included all the patients ≥18 years with symptomatic extracranial carotid artery occlusion or stenosis ≥50% on angiography, ≥70% on ultrasonography, or ≥70% on computed tomography (CT) angiography or magnetic resonance (MR) angiography if the stenosis on ultrasonography was 50% to 69% as determined by CREST.2 Patients with tandem occlusions who underwent concomitant mechanical thrombectomy (MT) and CAS were also included. Our study patients met at least one of the following contraindication criteria for CEA: previous carotid surgery, contralateral carotid occlusion, prior neck radiation, or deemed unfit for the procedure (due to cardiac and/or renal insufficiency). Individual patient consent was waived because of the study’s retrospective design.
Antiplatelet regimens and endovascular procedure
In the emergency CAS group, patients without tandem occlusion received a load of dual antiplatelets aspirin (325 mg followed by 81 mg daily) and clopidogrel (300 mg followed by 75 mg daily) before the procedure. Patients with tandem occlusion were loaded with similar loaded doses at the table and received Tirofiban infusion (maintenance dose of 0.1 mcg/kg/min) without bolus (Aggrastat, Medicure, Manitoba, Canada and West Point, Pennsylvania) during the procedure for a total of four hours.8,9 Patients in the elective group received aspirin (81 mg) and clopidogrel (75 mg) 5–7 days before the procedure. All patients were continued on aspirin (81 mg) and clopidogrel (75 mg) for at least three months after CAS.
Tandem occlusions were approached using a triaxial system without balloon-guide occlusion catheter. The intracranial lesion was treated using stent retriever plus aspiration or aspiration alone, and the extracranial lesion with stenting +/− angioplasty. The treating neuro interventionalist decided the appropriate approach for tandem occlusion, retrograde (intracranial first followed by CAS of the extracranial lesion) or anterograde (CAS of the extracranial lesion first followed by intracranial lesion treatment), and the dosage of systemic heparinization during the procedure. Retrograde approaches were preceded by angioplasty plus aspiration of the extracranial lesion.
CAS was performed using either Xact® Carotid Stent System or RX Acculink® Carotid Stent System (Abbott Vascular Inc, California, USA), both with the Emboshield Embolic Protection System (Abbott Vascular Inc, California, USA). All the procedures were performed by neuroendovascular attendings (SOG, ES, SD) qualified by the CREST-2 volume parameters.10 Conscious sedation was the preferred anesthesia method.
Imaging and clinical evaluation
Patient characteristics including gender, race, age, comorbidities, the National Institutes of Health Stroke Scale (NIHSS), stenosis/occlusion location, pre and post stenosis % based on NASCET criteria,11 presence of ulcerative plaque, length of the lesion, anesthesia method, medications, and imaging were extracted from the electronic medical records. Non-invasive imaging modalities, including ultrasound, MR angiography (MRA), or CT angiography (CTA), were used for the initial diagnosis of stenosis, while the degree of stenosis was confirmed by digital subtraction angiography (DSA) at the time of the procedure. All DSA images were assessed by an independent vascular and interventional neurologist (SOG) to confirm the stenosis degree. Follow-up visits were performed at 90 days and every year for 3 years to obtain neurological examination including mRS and carotid duplex ultrasound (CDUS) or CTA of the neck as part of our institutional clinical protocol.
Outcomes
We defined the primary outcome as any stroke (ischemic or hemorrhagic), MI, or death due to procedure within the first three months after CAS. Stroke was defined as any new neurological deficit after CAS with an increase of ≥ 4 points from baseline NIHSS and was confirmed using head CT and/or MR. MI was defined as a 2-fold increase in troponin of the upper limit of normal.
Secondary outcomes were; (i) periprocedural complications (safety outcome) including seizures, TIA, bradycardia, hypotension, groin or retroperitoneal hematoma requiring transfusion or surgery, aspiration pneumonia, or new onset of atrial fibrillation at discharge (patients with more than one periprocedural complication were considered as a single safety outcome to maintain the principle of independence); and (ii) the rate of restenosis or occlusion at any follow-up visits, defined by a) Peak Systolic Velocity >300 cm/s or no flow signal by CDUS in the affected artery, or b) occlusion or stenosis of >70% on DSA or CTA.12
Statistical analysis
We calculated descriptive statistics to describe the characteristics of the patients. We reported categorical variables as counts and percentages; continuous variables as means (standard deviation, SD) or medians (interquartile range, IQR) as appropriate. We used Chi-square or Fisher's exact test for categorical variables, and t-test or Wilcoxon test for continuous variables. We performed simple logistic regression analyses to compare both groups on the dependent variable (primary and secondary outcomes); subsequently, a sensitivity analysis after excluding all the patients who underwent MT during hospitalization.
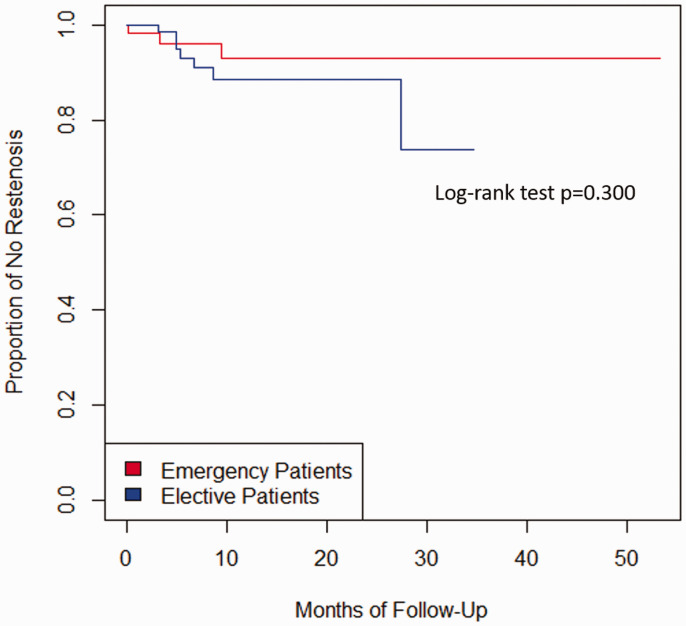
We executed multivariable logistic regression models to assess the risk factors associated with the composite of primary and secondary outcomes. We built our multivariable model using previously well-characterized predictors such as sex and age4,5 in combination with significant variables found to be associated with CAS in our univariate analysis (p < 0.05). Adjusted odds ratios (OR’s) and 95% confidence intervals were calculated as the measure of association. Finally, Cox proportional hazard was used for estimating time-to-event (cumulative risks) among the two groups expressed as Kaplan-Meier estimates. Log-rank test p-value was added to Figure 2. All the analyses were performed using R version 3.3.0 and an alpha level of 0.05 was used for the statistical significance. Data are available from the corresponding author upon reasonable request.
Figure 2.
Kaplan-Meier estimates of restenosis or carotid occlusion in emergency and elective CAS patients. Log-rank test p-value was added to the figure.
Results
Patient characteristics
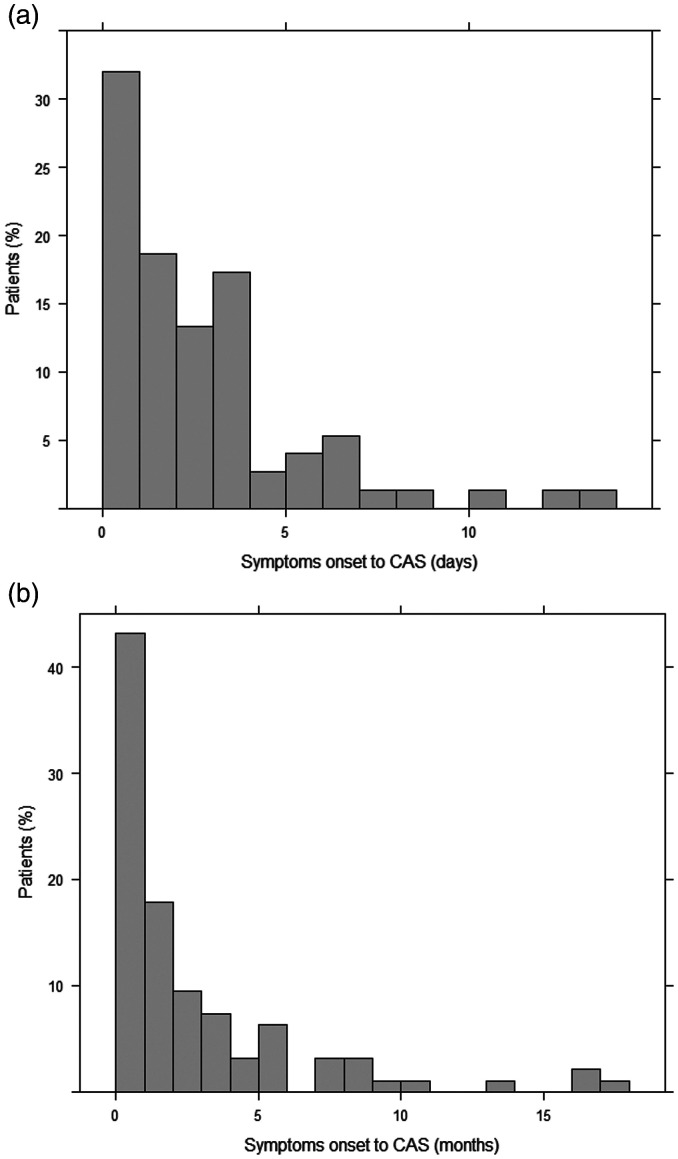
Emergency CAS was performed in 75 patients, whereas elective CAS, in 104. Demographic and clinical characteristics are presented in Table 1. There were no differences in the patients’ age means (68.3 ± 11.7 vs. 68.2 ± 10.2, p = 0.972) and the proportion of males (68% vs. 78%, p = 0.138) among the emergency CAS and the elective CAS groups, respectively. The median time from symptoms onset to CAS was 1.9 days (IQR 0.64–3.65) in the emergency group, and 51% of them were done in the first two days (Figure 1(a)). In the elective group, the median time from symptoms onset to CAS was 44.2 days (IQR 18.3-152), where 56% of them were performed in the first two months and 80% within the first 6 months after their stroke or TIA (Figure 1(b)). Of note, 173 CEAs were performed in patients with symptomatic carotid artery disease in our center during the study period.
Table 1.
Baseline characteristics.
| Emergency CAS, n = 75 | Elective CAS, n = 104 | P value | |
|---|---|---|---|
| Demographics | |||
| Age, mean ± SDa | 68.3 ± 11.7 | 68.2 ± 10.2 | 0.972 |
| Age ≥ 75, n (%) | 24 (32) | 26 (25) | 0.303 |
| Male sex, n (%) | 51 (68) | 81 (78) | 0.138 |
| Ethnicity – white, n (%) | 71 (95) | 102 (98) | 0.211 |
| Medical history, n (%) | |||
| Hypertension | 59 (79) | 83 (80) | 0.852 |
| Diabetes | 22 (29) | 36 (35) | 0.456 |
| Hyperlipidemia | 33 (44) | 69 (66) | 0.003 |
| Prior CVD or CABG | 22 (29) | 29 (28) | 0.832 |
| Cervical radiation exposure | 0 | 5 (5) | – |
| Atrial fibrillation | 5 (7) | 15 (14) | 0.104 |
| Clinical characteristics | |||
| TIA at presentation, n (%) | 12 (16) | 27 (26) | 0.111 |
| Time from symptoms onset to CAS (days), median (IQR)b | 1.9 (0.64–3.65) | 44.2 (18.3–152) | <0.001 |
| NIHSS at admission, median (IQR)b | 6 (1–11) | 3.5 (2–7.8) | 0.439 |
| Pre-procedure SBP (mmHg), mean ± SDa | 141 ± 21.3 | 139 ± 18.7 | 0.362 |
| Pre-procedure DBP (mmHg), mean ± SDa | 75 ± 15.4 | 74 ± 11.6 | 0.819 |
| Length of stay (days), median (IQR)b | 4.9 (3.2–8.3) | 1.2 (1–2.1) | 0.002 |
| Procedural characteristics | |||
| Thrombectomy, n (%) | 18 (24) | 0 | – |
| Ipsilateral carotid near-occlusion, n (%) | 13 (17) | 2 (2) | <0.001 |
| Contralateral carotid occlusion, n (%) | 6 (8) | 9 (9) | 0.876 |
| Ipsilateral dissection, n (%) | 4 (5) | 3 (3) | 0.404 |
| Prior ipsilateral CAS/CEA, n (%) | 3 (4) | 5 (5) | 0.796 |
| Carotid stenosis pre-CAS,c median (IQR)b | 90 (75–99) | 85 (70–90) | 0.024 |
| Target-lesion length (mm), median (IQR)b | 19 (14–22) | 18 (13–25) | 0.888 |
| Ulcerated carotid plaque, n (%) | 30 (48) | 32 (32) | 0.200 |
| Total length of stented segment (mm) (IQR)b | 34 (27–38) | 31.5 (27–37) | 0.926 |
| General anesthesia, n (%) | 14 (19) | 4 (4) | 0.001 |
| Balloon angioplasty before stenting, n (%) | 49 (65) | 81 (78) | 0.063 |
| Balloon angioplasty after stenting, n (%) | 52 (69) | 66 (63) | 0.413 |
| Residual carotid stenosis after CASc (%) (IQR)b | 20 (6.5–26.3) | 18 (7.5–33) | 0.520 |
| Medical treatment, n (%) | |||
| Dual antiplatelet before CAS | 72 (96) | 102 (98) | 0.405 |
| Dual antiplatelet after CAS | 71 (95) | 94 (98.9) | 0.292 |
| Anticoagulation after CAS | 4 (5.3) | 11 (10.5) | 0.212 |
| Statins after CAS | 71 (98.6) | 99 (95) | 0.874 |
CABG: coronary artery bypass grafting; CAS: carotid artery stenting; CEA: carotid endarterectomy; CVD: cardiovascular disease; DBP: diastolic blood pressure; IQR: interquartile range; MI: myocardial infarction; SBP: systolic blood pressure; NIHSS: National Institutes of Health Stroke Scale; TIA: transient ischemic attack.
aStudent’s T-test. For the rest of categorical variables was used Chi-square and Fisher exact test as appropriate.
bWilcoxon test.
c% stenosis calculated by NASCET: North American Symptomatic Carotid Endarterectomy Trial criteria.
Figure 1.
(a) Frequency distribution of emergency CAS patients. CAS indicates Carotid artery stenting. (b) Two-year frequency distribution of elective CAS patients. CAS indicates Carotid artery stenting. Note: Nine patients underwent CAS between two to ten years after symptoms onset, which are not shown.
Emergency CAS patients had significantly higher rates of ipsilateral carotid occlusion (17% vs. 2%, p < 0.001) and general anesthesia (19% vs. 4%, p = 0.001), higher NASCET score pre-procedure median (90, IQR 75–99 vs. 85, IQR 70-90, p = 0.024), and lesser rates of hyperlipidemia (44% vs. 66%, p = 0.003), respectively. Thrombectomy for tandem occlusion was only performed in the emergency CAS group (24%) (Table 1) and represented 5.4% of the 333 thrombectomies of the anterior circulation performed during our period of study. The intracranial occlusion locations were MCA M1 (78%), proximal M2 (11%), and ICA-terminus (11%), and a retrograde approach was done in 12 (66%) tandem occlusion patients.
Primary and secondary outcomes
Three-months follow-up data were available for 168 patients (70 emergency and 98 elective CAS patients). Overall, the primary outcome of any stroke, MI, or death related to the procedure at three months in the entire cohort was 2.9% (5/168). The primary outcome occurred in 4/70 (5.7%) emergency CAS patients and 1/98 (1%) elective CAS patients (p = 0.161). The safety outcome (periprocedural complications) at discharge was found in 7 (9%) patients from the emergency CAS group and 5 (4.8%) patients from the elective CAS group (p = 0.232) (Table 2).
Table 2.
Primary and secondary outcomes.
| Emergency CAS n = 75 | Elective CAS n = 104 | P valuea | |
|---|---|---|---|
| Primary outcome – composite, n (%) | 4/70 (5.7) | 1/98 (1) | 0.161 |
| Deaths | 2 (2.8) | 0 | 1 |
| Stroke | 2 (2.8) | 1 (1) | 0.571 |
| Myocardial infarction | 0 | 0 | 1 |
| Safety outcome – composite, n (%) | 7 (9) | 5 (4.8) | 0.232 |
| Seizures | 2 (2.6) | 0 | 1 |
| Retroperitoneal hematoma | 1 (1.3) | 0 | 1 |
| Atrial fibrillation | 2 (2.6) | 0 | 1 |
| Aspiration pneumonia | 2 (2.6) | 0 | 1 |
| Bradycardia | 1 (1.3) | 4 (3.8) | 0.401 |
| Hypotension | 0 | 2 (1) | 1 |
Note: One emergency CAS patient presented both seizures and pneumonia, and one elective CAS patient presented both bradycardia and hypotension.
CAS: carotid artery stenting.
aFisher’s exact test.
Two deaths occurred in the emergency CAS group. One death was related to a retroperitoneal hemorrhage and the second one to a post-procedure in-stent thrombosis. Of the three strokes, a disabling (mRS 4) and a non-disabling (mRS 1) strokes occurred in the emergency CAS group (66.6%) and a non-disabling stroke in the elective (mRS 2) CAS group (33.3%) at three months of follow-up. Periprocedural complications were due to seizures, which were successfully controlled, retroperitoneal hematoma requiring blood transfusion, aspiration pneumonia after undergoing MT under general anesthesia, atrial fibrillation controlled adequately, and bradycardia and hypotension successfully managed with atropine and vasopressors, respectively (Table 2).
The sensitivity analysis showed similar results in the primary (emergency CAS 5.6% vs. elective CAS 1%, p = 0.124) and secondary (emergency CAS 3.5% vs. elective CAS 4.8%, p = 1) outcomes. However, there was a reduction in periprocedural complications from 9% to 3.5% in the emergency CAS group after excluding MT patients (Supplementary Table 1).
Predictors of perioperative outcomes
Univariate analysis found a higher odds of the composite primary and secondary outcomes in female gender (OR 4.91, 95% CI: 1.75-14.4, p = 0.002), emergency CAS (OR 2.85, 95% CI: 1.03–8.68, p = 0.049) and ipsilateral carotid occlusion (OR 5.87, 95% CI: 1.62-19.6, p = 0.004). The final model built from a stepwise regression showed that female gender (OR 5.11, 95% CI: 1.69–16.6, p = 0.004) and ipsilateral carotid occlusion (OR 8.13, 95% CI: 1.97–33.7, p = 0.003) were independent risk factors associated with the composite of primary and secondary outcomes (Table 3).
Table 3.
Univariate and multivariate logistic regression for the composite of primary and secondary outcomes.
| OR (95% CI) | P value | OR (95% CI) | P value | |
|---|---|---|---|---|
| Age ≥75 | 1.62 (0.52–4.57) | 0.371 | 1.66 (0.47–5.59) | 0.406 |
| Female gender | 4.91 (1.75–14.4) | 0.002 | 5.11 (1.69–16.6) | 0.004 |
| Diabetes Mellitus | 0.84 (0.25–2.40) | 0.758 | – | – |
| Emergency CAS | 2.85 (1.03–8.68) | 0.049 | – | – |
| Hyperlipidemia | 0.67 (0.23–1.84) | 0.438 | – | – |
| Ipsilateral carotid occlusion | 5.87 (1.62–19.6) | 0.004 | 8.13 (1.97–33.7) | 0.003 |
CAS: carotid artery stenting.
Follow-up assessments
The follow-up period was calculated from pooled times of emergency and elective CAS patients. The median time of follow-up was 13 months. The composite secondary outcome of restenosis or occlusion occurred in 10 patients (three emergency CAS patients and seven elective CAS patients). There were no differences in the rate of occlusion or restenosis between groups (emergency CAS 7% vs. elective CAS 11.6%; log-rank test p = 0.3) (Figure 2). The Cox regression model estimated a hazard ratio of 0.64 (95% CI 0.16–2.60; p = 0.537, adjusted for age, and sex). In the emergency CAS group, one patient developed carotid artery occlusion, one had carotid restenosis requiring angioplasty plus stenting, and one developed carotid restenosis requiring angioplasty only. In the elective CAS group, two patients had carotid restenosis requiring angioplasty plus stenting, four patients developed carotid restenosis requiring angioplasty only, and one patient had restenosis, but chose to undergo medical therapy only.
Discussion
This study provides three key findings. First, carotid stenting in symptomatic emergency patients and elective patients showed similar rates of any stroke, MI, or death related to the procedure at 90 days of follow-up. Second, rates of minor complications at discharge were similar in both groups. Finally, CAS in emergency and elective patients is associated with similar rates of restenosis at 13 months of follow-up.
AHA guidelines state that CAS is an alternative to CEA in symptomatic patients with 50% to 99% stenoses who were at average or low risk of suffering complications associated with the endovascular procedure and that intervening ≤2 weeks is “reasonable” unless contraindicated.13 This study supports that performing CAS in an emergency setting may be as safe and feasible as CAS in an elective setting.
Ijas et al. found that early CEAs (within 48 hours from IV thrombolysis) did not differ from CEAs performed later regarding peri/postoperative strokes, neck hematomas, MI, or 3-month modified Rankin Scale.14 Similarly, despite our emergency CAS group included patients with tandem occlusions during thrombectomy (24%) and had a significantly higher number of ipsilateral carotid occlusions (17% vs. 2%) and patients undergoing general anesthesia (19% vs. 4%), there were no differences in the primary and secondary outcomes with the elective CAS group. These findings provide additional evidence that early carotid revascularization (CAS or CEA) in high-risk groups such as thrombectomy patients might be safe.
An overall 2.9% rate of any stroke, MI, or death related to the procedure at three months of follow-up in our entire cohort is lower than the 30-day incidence of any stroke, MI, or death reported in CREST and the CAPTURE registry (5.2% and 12%, respectively), and lower than the 30-day incidence of any stroke or death reported in EVA-3S (3.9%).2,15,16 Our low rate might be due to our high-volume center and well-trained proceduralists.
Regarding CAS timing, Song et al. and Tsantilas et al. found that early symptomatic CAS (within two weeks and one week, respectively) had more events (stroke or death) within 30 days post-procedure and during hospitalization than the delayed group (>2 weeks and 8 to 180 days, respectively).4,5 In contrast, other studies supported early CAS within their 7 or 15 days cut-off, showing no significant differences in stroke or deaths at 30 days.6,7,17,18 A recent prospective study compared CAS to CEA when performed within the first 48 hours and found similar rates of stroke or any cause of death within 30 days (5.1% vs. 4.9%, respectively).19 Those findings are comparable to the primary outcome rate in our emergency CAS group (5.7%).
Local and systemic complications were found in 9.2% in the CAS arm in EVA-3S trial.15 Tsantilas et al. found a complication rate of 4.65% in the patients treated with CAS within the first week and a 3.9% rate in patients treated between 8 to 180 days.4 These results are comparable to the rate of minor complications found in our elective CAS group (4.8%), and lower than our emergency CAS group (9%). This higher proportion in our emergency CAS group might be due to the higher rates of tandem occlusions and ipsilateral carotid occlusions, which demonstrated to be an independent risk factor for the composite of primary and secondary outcomes in the multivariate analysis. However, we decided to include patients with ipsilateral carotid artery occlusion and tandem occlusions in the analysis as that would reflect a real-world scenario, and we could stratify them as high-risk CAS. These results suggest that early carotid revascularization might be safe and effective even in this high-risk group and might add additional evidence of a possible benefit of acute cervical treatment in patients with tandem occlusions when compared to delayed CAS after MT.20,21
Our study population had available follow-up data with CTA or CDUS for both groups. The rates of restenosis or occlusion at 13 months of follow-up (7% in emergency CAS patients vs. 11.6% in elective CAS patients) were comparable to the CAS arm of the clinical trials (CREST 6% at two years, SPACE 10.7% at two years, ICSS 6.9% at one year) and lower than CAVATAS trial (23% at 1 year).12,22–24
Limitations
This retrospective study are the modest sample size and single-center data where the timing of the procedure was self-adjudicated by the interventionalist. Given our small sample size, the absence of a significant difference in our primary and secondary outcomes might be expected. More importantly, we do not account for potential confounders such as stroke infarct volume, hyperglycemia, and other factors that might increase the risk of hemorrhagic conversion after carotid revascularization. On the other hand, some strengths of this study are that we have a follow-up in most patients, the proceduralists have vast endovascular experience, and that emergent CAS was safe despite including a subgroup of a high-risk population, such as tandem occlusions and ipsilateral carotid occlusions when compared to CAS performed in an elective period.
Conclusions
In the present study, the safety of CAS appears to be acceptable when performed in an elective or emergent basis. Moreover, emergency and elective CAS had similar rates of restenosis or occlusion at 13 months of follow-up. At this point, there is no data regarding appropriate carotid stenting timing to suggest a change in clinical practice. Specific time interval from symptom onset, e.g. 0–48 hours25 or 0–7 days4 might warrant further investigation to identify high risk timing to pursue CAS during acute hospitalization. Ultimately, randomized trials will be required to evaluate the optimal timing of revascularization and the safety of carotid stenting in an early period.
Supplemental Material
Supplemental material, sj-pdf-1-ine-10.1177_1591019920977552 for Safety and efficacy of symptomatic carotid artery stenting performed in an emergency setting by Darko Quispe-Orozco, Kaustubh Limaye, Cynthia B Zevallos, Mudassir Farooqui, Alan Mendez-Ruiz, Sameer Ansari, Andres Dajles, Edgar A Samaniego, Colin Derdeyn and Santiago Ortega-Gutierrez in Interventional Neuroradiology
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SOG is consultant for Medtronic and Stryker Neurovascular: modest. EAS is consultant for Medtronic and Microvention. CD is consultant for Penumbra, Nono, Genae (all DSMB for trials), Pulse Therapeutics, Equity (stock options).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Darko Quispe-Orozco https://orcid.org/0000-0003-3627-5439
Cynthia B Zevallos https://orcid.org/0000-0001-8715-240X
Supplemental material: Supplemental material for this article is available online.
References
- 1.Li Y, Yang JJ, Zhu SH, et al. Long-term efficacy and safety of carotid artery stenting versus endarterectomy: a meta-analysis of randomized controlled trials. PloS One 2017; 12: e0180804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brott TG, Hobson RW, Howard G, et al.; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brott TG, Howard G, Roubin GS, et al.; CREST Investigators. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med 2016; 374: 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsantilas P, Kuehnl A, Kallmayer M, et al. Risk of stroke or death is associated with the timing of carotid artery stenting for symptomatic carotid stenosis: a secondary data analysis of the German statutory quality assurance database. JAHA 2018; 7: e007983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song KS, Kwon OK, Hwang G, et al. Early carotid artery stenting for symptomatic carotid artery stenosis. Acta Neurochir (Wien) 2015; 157: 1873–1878. [DOI] [PubMed] [Google Scholar]
- 6.Meschia JF, Hopkins LN, Altafullah I, et al. Time from symptoms to carotid endarterectomy or stenting and perioperative risk. Stroke 2015; 46: 3540–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wach MM, Dumont TM, Mokin M, et al. Early carotid angioplasty and stenting may offer non-inferior treatment for symptomatic cases of carotid artery stenosis. J Neurointerv Surg 2014; 6: 276–280. [DOI] [PubMed] [Google Scholar]
- 8.Lee JI, Gliem M, Gerdes G, et al. Safety of bridging antiplatelet therapy with the gpIIb-IIIa inhibitor tirofiban after emergency stenting in stroke. PloS One 2017; 12: e0190218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruber P, Hlavica M, Berberat J, et al. Acute administration of tirofiban versus aspirin in emergent carotid artery stenting. Interv Neuroradiol 2019; 25: 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lal BK, Roubin GS, Rosenfield K, et al. Quality assurance for carotid stenting in the CREST-2 registry. J Am Coll Cardiol 2019; 74: 3071–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnett HJM, Taylor DW, Haynes RB, et al.; North American Symptomatic Carotid Endarterectomy Trial C. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 12.Lal BK, Beach KW, Roubin GS, et al.; CREST Investigators. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. Lancet Neurol 2012; 11: 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kernan WN, Ovbiagele B, Black HR, et al.; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
- 14.Ijas P, Aro E, Eriksson H, et al. Prior intravenous stroke thrombolysis does not increase complications of carotid endarterectomy. Stroke 2018; 49: 1843–1849. [DOI] [PubMed] [Google Scholar]
- 15.Mas JL, Chatellier G, Beyssen B, et al.; EVA-3S Investigators. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med 2006; 355: 1660–1671. [DOI] [PubMed] [Google Scholar]
- 16.Gray WA, Yadav JS, Verta P, et al.; CAPTURE Trial Collaborators. The CAPTURE registry: predictors of outcomes in carotid artery stenting with embolic protection for high surgical risk patients in the early post-approval setting. Catheter Cardiovasc Interv 2007; 70: 1025–1033. [DOI] [PubMed] [Google Scholar]
- 17.Rantner B, Kollerits B, Roubin GS, et al.; Carotid Stenosis Trialists’ Collaboration. Early endarterectomy carries a lower procedural risk than early stenting in patients with symptomatic stenosis of the internal carotid artery: results from 4 randomized controlled trials. Stroke 2017; 48: 1580–1587. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Chu J, Zhang L, et al. Clinical comparison of outcomes of early versus delayed carotid artery stenting for symptomatic cerebral watershed infarction due to stenosis of the proximal internal carotid artery. BioMed Res Int 2016; 2016: 6241546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankowitz BT, Tonetti DA, Kenmuir C, et al. Urgent treatment for symptomatic carotid stenosis: The Pittsburgh Revascularization and Treatment Emergently After Stroke (PIRATES) Protocol. Neurosurgery 2020; 87: 811–815. [DOI] [PubMed]
- 20.Jadhav AP, Zaidat OO, Liebeskind DS, et al. Emergent management of tandem lesions in acute ischemic stroke. Stroke 2019; 50: 428–433. [DOI] [PubMed] [Google Scholar]
- 21.Papanagiotou P, Haussen DC, Turjman F, et al.; TITAN Investigators. Carotid stenting with antithrombotic agents and intracranial thrombectomy leads to the highest recanalization rate in patients with acute stroke with tandem lesions. JACC Cardiovasc Interv 2018; 11: 1290–1299. [DOI] [PubMed] [Google Scholar]
- 22.Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the Stent-Protected angioplasty versus carotid endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol 2008; 7: 893–902. [DOI] [PubMed] [Google Scholar]
- 23.Bonati LH, Ederle J, McCabe DJH, et al. Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the carotid and vertebral artery transluminal angioplasty study (CAVATAS): long-term follow-up of a randomised trial. The Lancet Neurology 2009; 8: 908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonati LH, Dobson J, Featherstone RL, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the international carotid stenting study (ICSS) randomised trial. Lancet 2015; 385: 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powers WJ, Rabinstein AA, Ackerson T, et al.; American Heart Association Stroke Council. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ine-10.1177_1591019920977552 for Safety and efficacy of symptomatic carotid artery stenting performed in an emergency setting by Darko Quispe-Orozco, Kaustubh Limaye, Cynthia B Zevallos, Mudassir Farooqui, Alan Mendez-Ruiz, Sameer Ansari, Andres Dajles, Edgar A Samaniego, Colin Derdeyn and Santiago Ortega-Gutierrez in Interventional Neuroradiology