Abstract
Background:
Neurocognitive investigations suggest that conscious sensory perception depends on recurrent neuronal interactions among sensory, parietal and frontal cortical regions, which are suppressed by general anesthetics. The purpose of this work was to investigate if local interactions in sensory cortex are also altered by anesthetics. We hypothesized that desflurane would reduce recurrent neuronal interactions in cortical layer-specific manner consistent with the anatomical disposition of feedforward and feedback pathways.
Methods:
Single-unit neuronal activity was measured in freely moving adult male rats (268 units, 10 animals) using microelectrode arrays chronically implanted in primary and secondary visual cortex. Layer-specific directional interactions were estimated by mutual information and transfer entropy of multineuron spike patterns within and between cortical layers 3 and 5. The effect of incrementally increasing and decreasing steady-state concentrations of desflurane (0% to 8% to 0%) was tested for statistically significant quadratic trend across the successive anesthetic states.
Results:
Desflurane produced robust, state-dependent reduction (p=0.001) of neuronal interactions between primary and secondary visual areas and between layers 3 and 5 as indicated by mutual information (37% and 41% decrease at 8% desflurane from wakeful baseline at 0.52±0.51(SD) and 0.53±0.51(SD) bits, respectively) and transfer entropy (77% and 78% decrease at 8% desflurane from wakeful baseline at 1.86±1.56(SD) bits and 1.87±1.67(SD) bits, respectively). In addition, a preferential suppression of feedback between secondary and primary visual cortex was suggested by the reduction of directional index of transfer entropy overall (p=0.001; 89% decrease at 8% desflurane from 0.11±0.18(SD) bits at baseline) and specifically, in layer 5 (p=0.001; 108% decrease at 8% desflurane from 0.12±0.19(SD) bits at baseline).
Conclusions:
Desflurane anesthesia reduces neuronal interactions in visual cortex with a preferential effect on feedback. The findings suggest that neuronal disconnection occurs locally, among hierarchical sensory regions, which may contribute to global functional disconnection underlying anesthetic-induced unconsciousness.
Summary Statement:
Desflurane disrupts cortical layer-specific neuronal interactions with preferential effect on visual cortical feedback suggesting that neuronal connectivity changes in local circuits may contribute to large-scale cortical functional disintegration as a mechanism of anesthetic-induced unconsciousness.
INTRODUCTION
Pharmacological state manipulation is a rational approach to investigate the neuronal basis of human and animal consciousness. How general anesthetics alter neuronal activity in the central nervous system has been a target of scientific investigations for decades1–6.
Former investigations in humans and primates suggested that conscious perception depends on recurrent neuronal interactions along the hierarchy of early sensory to parietal-frontal cortical regions7–9. Specifically, sensory stimuli reach awareness only if their feedforward processing along hierarchical regions of the cortex is reciprocated by corresponding top-down messages from higher centers. Consistent with this view, preclinical and clinical studies using various methodologies confirmed that pharmacologically diverse anesthetic agents interfere with corticocortical recurrent activity5,10–12. Specifically, most studies suggest that anesthetics exert a preferential reduction of anterioposterior or frontoparietal directional connectivity upon the onset of unconsciousness12–15, notwithstanding a few exceptions possibly due to methodological differences16,17.
Nevertheless, the necessity of prefrontal cortex for consciousness has also been debated18,19. Thus, it is also unclear if anesthetic-induced unconsciousness is causally related to the observed disruption of long-range connectivity or to a local disconnection within a posterior site such as the “posterior cortical hot zone”20 although conscious report of visual stimuli does seem to require the involvement of frontal cortex9.
How do sensory cortical regions participate in the anesthetic disruption of recurrent connectivity? Recent investigations in cortical slice preparations suggest that anesthetics preferentially decrease feedback from higher- to lower-order sensory cortex5. On the other hand, experiments using electrocorticographic measurements find decreased feedforward vs. feedback effect in the visual cortex of monkeys17. However, this has not been tested by a direct measurement of neuronal interactions in the intact animal.
To determine if directional communication in local circuits is suppressed by anesthesia in vivo, we investigated neuronal interactions between primary and secondary visual cortex (V1 and V2, respectively) in a chronically instrumented freely moving rat model. We hypothesized that desflurane would reduce recurrent neuronal interactions especially in the feedback direction. To test our hypothesis, we took advantage of the known layer-specific anatomical segregation of corticocortical feedforward and feedback pathways21. From the simultaneous extracellular recordings of single-unit activity in different depths of two cortical regions, we then calculated mutual information and transfer entropy (primary outcome variables) as measures of layer-specific neuronal interactions between V1 and V2.
MATERIALS AND METHODS
Animals and Experimental procedures
The study was approved by the Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals of the Governing Board of the National Research Council (National Academy Press, Washington, D.C., 2011).
Ten adult male Sprague-Dawley rats of 260–330 g weight (mean age 9 weeks, Charles River Breeding Labs) were housed in a reverse light-dark cycle room for 5–7 days prior to surgical implantation, and remained there for the duration of the experiment. Food and water access was ad libitum. The surgical procedures were previously described in detail22. In this work, a custom-designed electrode array consisting of 32 microwires (wire diameter: 33 μm, electrode spacing: 250 μm, row separation: 375 μm, tip angle: 45°; Tucker-Davis Technologies, Alachua, FL) was chronically implanted in the visual cortex of each rat. Neighboring, microwires within each array had two alternating lengths in order to record from both supragranular and infragranular cortical layers, targeting layer 3 and layer 5; from here on, designated as L3 and L5, respectively. The array was positioned so that 16 of the microwires were in primary visual cortex (V1) and 16 were in secondary visual cortex (V2). In V1, the shanks resided in a cortical area representing the lateral superior quadrant of the left visual field. The representations in V2 mirror image those of V1, thus they should correspond to those in V1. As a result, 8 electrodes targeted each of the subregions designated as V1L3, V1L5, V2L3, and V2L5. The center of the microwire array was −6mm anterioposterior and 3mm lateral from Bregma23. In a subset of animals, the penetration depth of the microwires was histologically verified. The microwire array and its positioning are illustrated in Figure 1A.
Figure 1.
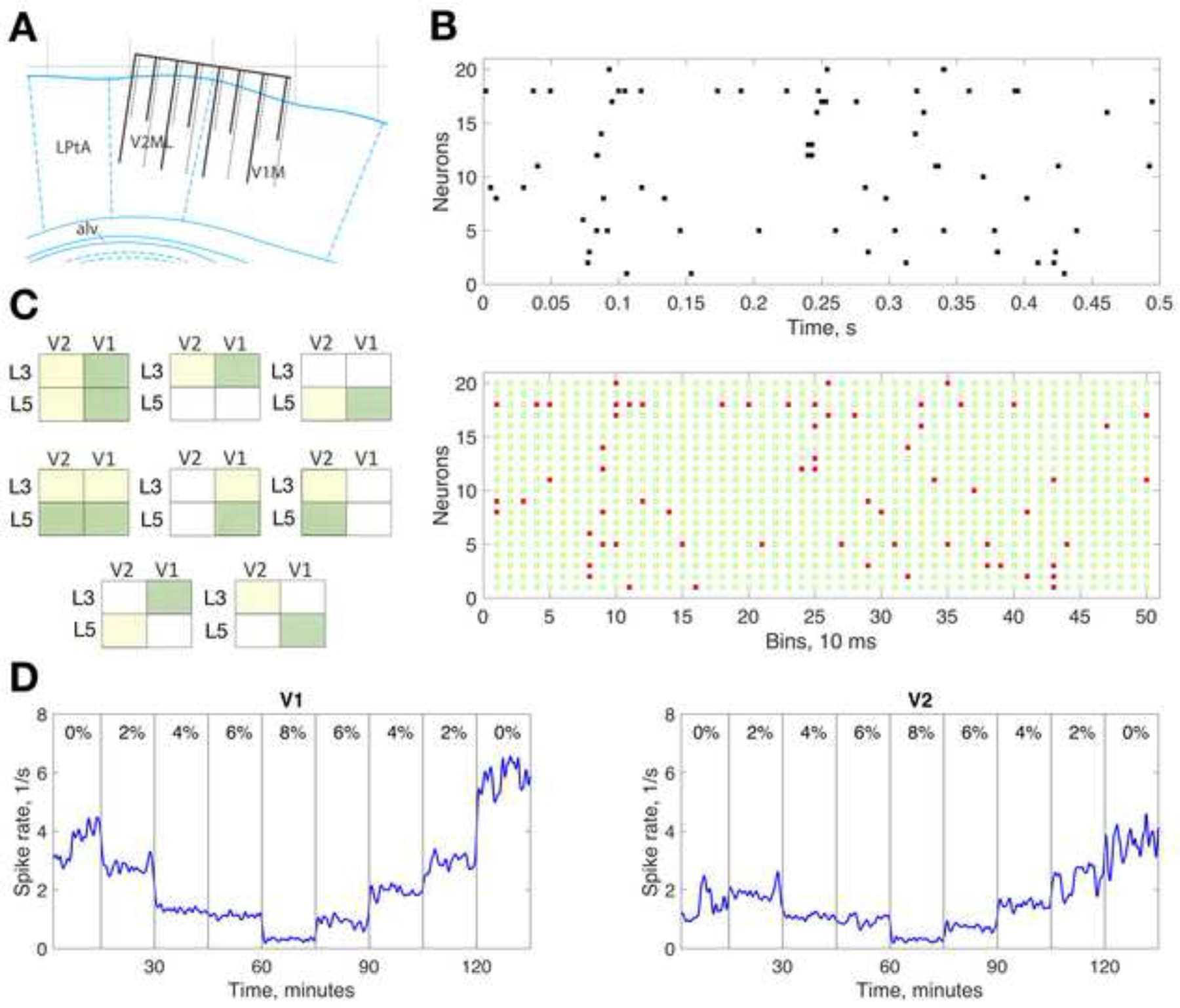
Microwire array and data analysis. (A) Location of the microwire array in rat visual cortex. Eight wires target primary visual cortex monocular area (V1M) and 8 wires target secondary visual cortex mediolateral area (V2ML). The wires have alternating lengths with wire tips residing approximately in cortical layer 3 and layer 5. Only two rows of wires are illustrated; the second row is shown with dotted lines. Scale above is the distance in mm from Bregma at 3.4 mm lateral position. LPtA: lateral parietal association area. Drawing is based on Paxinos and Watson atlas43. (B) Spike raster plot for 20 recorded neurons in one experiments (top) and binned binary representation of spiking (bottom). Red dots indicate at least one spike present in each 10ms bin, green open circles indicate no spike. Each configuration of coincident red dots across neurons represents a population activity pattern. (C) Pairwise cortical regions (yellow and green areas) for which mutual information and transfer entropy were calculated. (D) Time course of average spike rate from all units within V1 and V2 in one experiment. Spike rate was calculated for 10-second time bins and 10-point Gaussian smoothing was applied. Data from consecutive anesthetic states were concatenated, thus the time axis shows cumulative recording time, not real time. Vertical gray lines demarcate anesthetic conditions with percent inhaled concentration of desflurane indicated.
One to eight days after surgery, usually around the middle of the day, the animal was placed in a closed, ventilated anesthesia chamber for continuous recording of extracellular potentials in dark environment. The experimental protocol consisted of consecutively increasing and decreasing desflurane levels at 0%, 2%, 4%, 6%, 8%, 6% 4%, 2%, 0% inhaled concentration (balance 30% O2) for 30 minutes at level. The first 15 minutes was used for equilibration and the second 15 minutes for recording. Although it was not tested in this study, prior experiments in the same species revealed loss and return of the righting reflex between 4% and 6% desflurane and absent nociceptive response at 8% desflurane10,24,25. Anesthetic concentration in the holding chamber was continuously monitored (POET IQ2 monitor; Criticare Systems, Inc., Waukesha, WI). Core body temperature was maintained at 37°C by subfloor radiant heat. Extracellular potentials were recorded with the Cerebus system (Blackrock Microsystems, Salt Lake City, UT) with 250–7500 Hz analog band-pass filter and 30 kHz sampling rate. Animals were euthanized with CO2 inhalation followed by pneumothorax.
Data analysis and Statistics
Extracellular spikes were isolated into single units using the clustering software SpyKING CIRCUS26. This efficient, template-based method allows simultaneous clustering of units recorded on all channels. When animals were tested on more than one day, the experiment yielding more units was used for further analysis. We classified neurons based on their spike waveform and autocorrelogram. Single units with relatively small half-peak width, short trough-to-peak duration, and high overall autocorrelogram were identified as putative fast-spiking interneurons. Neurons with the opposite waveform properties and either bursty or regular spiking pattern were identified as putative excitatory neurons. Overall, 37/268 (14%) units were classified as interneurons. Because of the relatively small sample size, all neurons were included in further calculations.
After clustering, a binary representation of the recorded spike trains was obtained by binning the spike times at 10ms increments. This time bin was used to account for the time delay associated with polysynaptic interaction of neurons between V1 and V227. It also conforms to the average dendritic integration time of principal neurons28. As illustrated in Figure 1B, the binned spike train consisted of zeros (no spike present within 10ms bin) and ones (one or more spike present within 10 ms bin). Each distinct arrangement of zeros and ones in a set of coincident time bins represents the momentary activity pattern of the recorded neuron population.
To quantify neuronal interaction between select cortical regions, we first used mutual information, MI. For example, to quantify the interaction between region x and region y, we first calculated unnormalized mutual information (uMI),
where X and Y are the possible coincident spike patterns in region x and region y, respectively, and H(X) is the Shannon information entropy
calculated from the probability distribution p(X) of coincident binary spike patterns in a region x. H(X, Y) is the joint entropy of X and Y. As calculated, H is a configuration entropy of the coincident spike patterns. It measures the uncertainty, and thereby the potential information content, of neuronal spike patterns of the units sampled in each region over time. Mutual information then measures the symbolic information associated with the interaction of the sampled neuron populations between two regions. These calculations were done independently for each animal in each anesthetic condition.
In order to avoid bias by experiments due to different overall unit count and entropy, all mutual information data were normalized to their mean from the nine consecutive anesthetic conditions j within each experiment:
(Normalizing to the mean was chosen because it does not bias the data at any particular condition, e.g., at awake baseline.) In order to estimate directional interactions, we calculated transfer entropy, TE29. For two regions x and y, TEy→x can be written as a difference of conditional Shannon entropies:
where Xt and Xt+1 are the possible coincident spike patterns of region x at time t and t+1, respectively; and Yt and Yt+1 are the same in the other region. TEy→x is the reduced amount of uncertainty in future of X by knowing the past of Y given past of X. Thus, TEy→x measures the statistical influence of region y on region x and TEy→x measures the reverse. The two quantities are not necessarily equal.
To compare with mutual information, we calculated the sum of transfer entropies, TEs in the forward and reverse directions, which also gives a symmetric measure. As with mutual information, we normalized each value to the experiment mean:
where j is an index of the anesthetic condition from induction to emergence.
Finally, to assess the asymmetry of transfer entropy, we calculated the directionality index:
The pairs of cortical regions for which mutual information, transfer entropy sum and were calculated are graphically shown in Figure 1C. Regions with less than four active units were omitted from the analysis; as a result, 6/40 (15%) regions were excluded. Because of the applied normalization across the anesthetic conditions, all reported measures are dimensionless.
Statistical testing of the effect of desflurane on mutual information, transfer entropy and directionality index was performed using two-factor ANOVA (general linear method) with the 9 consecutive anesthetic states as fixed level and the rat as random level factor variables followed by testing for statistically significant quadratic trend as a function of state. Because 8 tests were performed for each of 3 outcome variables yielding a total of 24 tests, using Bonferroni correction, significance was accepted at p=0.002 level. No statistical power calculation was conducted prior to the study. There were no outliers noted or rejected. Randomization methods and blinding was not used. Calculations were performed using Matlab version 2018b (MathWorks Inc., Natick, MA) and NCSS (NCSS, LLC, Kaysville, UT). All data are reported as mean and standard deviation unless indicated otherwise.
RESULTS
From all experiments, the relative number of units with a minimum of one spike/s activity per all recording sites was close to 70 percent. At a few contact sites, more than one unit could be isolated but this was generally rare; thus, most units were from different recording sites. The number of units found in different subregions of V1 and V2 varied from experiment to experiment but on average, they were similar in the major regions, i.e., V1, V2, L3 and L5. Obviously, the subregions containing fewer recording sites yielded proportionally fewer units. Table 1 summarizes the number of active units in each rat and region. Figure 1D illustrates the time course of average spike rate from all units in V1 and V2 in one experiment as an example.
Table 1.
Number of active units in cortical regions
| Experiment | All | V1 | V2 | L3 | L5 | V1L3 | V2L5 | V2L3 | V2L5 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 23 | 13 | 10 | 7 | 16 | 3 | 10 | 4 | 6 |
| 2 | 19 | 12 | 7 | 7 | 12 | 4 | 8 | 3 | 4 |
| 3 | 27 | 14 | 13 | 8 | 19 | 3 | 11 | 5 | 8 |
| 4 | 17 | 5 | 12 | 9 | 8 | 1 | 4 | 8 | 4 |
| 5 | 27 | 19 | 8 | 12 | 15 | 9 | 10 | 3 | 5 |
| 6 | 31 | 13 | 18 | 17 | 14 | 7 | 6 | 10 | 8 |
| 7 | 36 | 16 | 20 | 16 | 20 | 5 | 11 | 11 | 9 |
| 8 | 42 | 22 | 20 | 34 | 8 | 15 | 7 | 19 | 1 |
| 9 | 24 | 11 | 13 | 10 | 14 | 5 | 6 | 5 | 8 |
| 10 | 22 | 13 | 9 | 11 | 11 | 7 | 6 | 4 | 5 |
| Mean | 27 | 14 | 13 | 13 | 14 | 6 | 8 | 7 | 6 |
| SD | 8 | 5 | 5 | 8 | 4 | 4 | 2 | 5 | 2 |
V1: primary visual cortex, V2: secondary visual cortex. L3: cortical layer 3, L5: cortical layer 5.
To quantify neuronal interactions, we first calculated mutual information between V1 and V2 within the L3 and L5 and in those combined, between the L3 and L5 within V1 and V2 and in the two regions combined, as well as between the crossed subregions. The group average results of mutual information are shown in Figure 2. With the applied sequence of stepwise increasing and decreasing desflurane concentrations, a U-shape change in mutual information was evident as confirmed by the statistically significant quadratic trend (Table 2). The unnormalized mutual information values for V1-V2 and L3-L5 were 0.52±0.33 and 0.53±0.31 bits at 0% desflurane, which were reduced to 0.33±0.34 and 0.31±0.34 bits, respectively, at 8% desflurane. The proportion change of mutual information from baseline to 8% desflurane concentration was −37% for V1-V2 and −41% for L3-L5.
Figure 2.
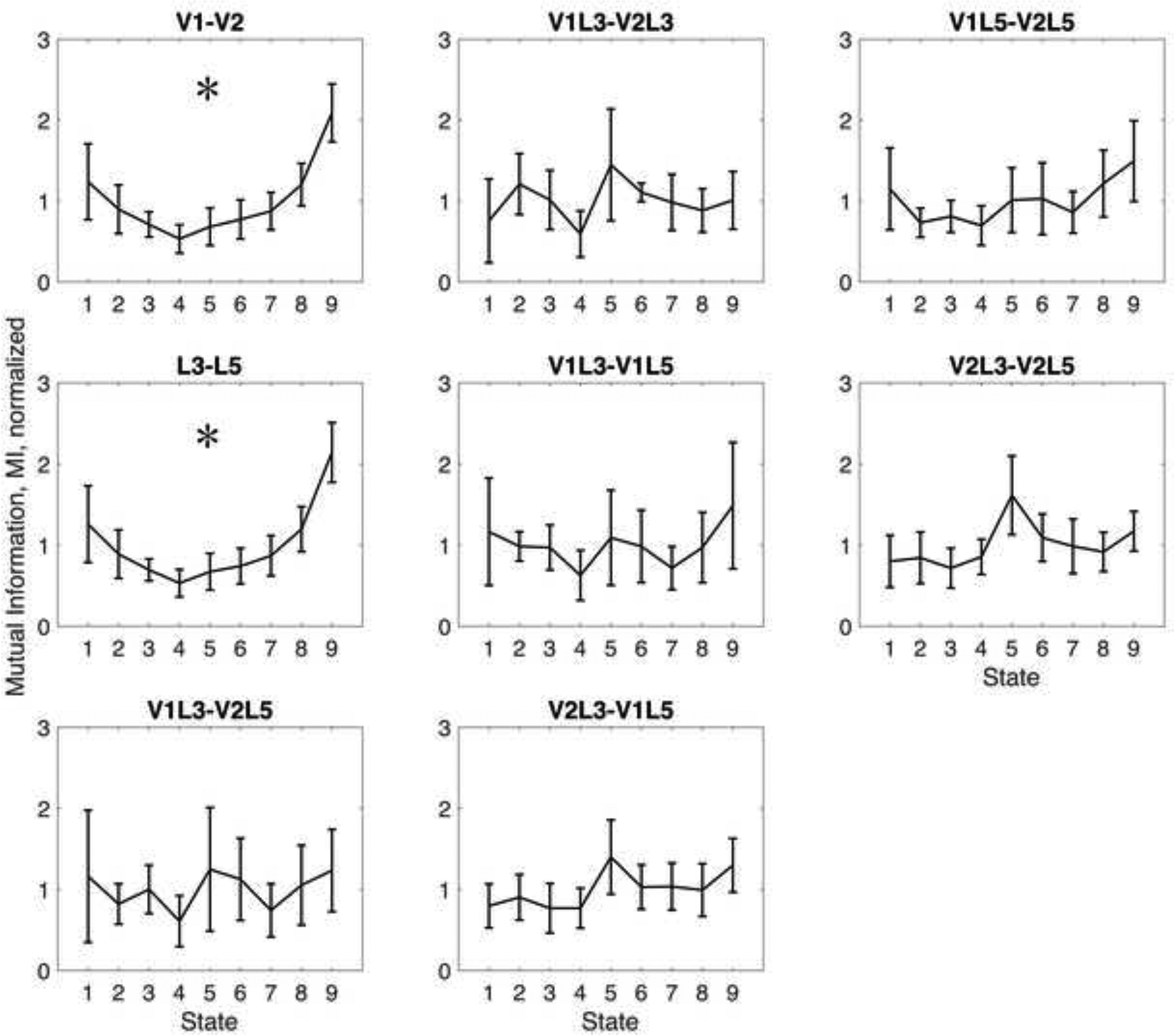
Effect of desflurane on mutual information between neurons recorded in primary and secondary visual cortex (V1, V2), cortical layers 3 and 5 (L3, L5), and their subregions. Anesthetic states 1 to 9 correspond to consecutive desflurane concentrations of 0, 2, 4, 6, 8, 6, 4, 2, 0 percent, respectively. Mutual information is in relative units, plotted as mean and 95% confidence interval. *: statistically significant quadratic trend with consecutive anesthetic states.
Table 2.
Exact p values for quadratic trend vs. desflurane
| Region-pair | MI | TEs | DI |
|---|---|---|---|
| V1-V2 | 0.001* | 0.001* | 0.001* |
| S-D | 0.001* | 0.001* | 0.001* |
| V1S-V1D | 0.273 | 0.001* | 0.003 |
| V2S-V2D | 0.314 | 0.007 | 0.142 |
| V1S-V2S | 0.333 | 0.009 | 0.520 |
| V1D-V2D | 0.126 | 0.001* | 0.001* |
| V1S-V2D | 0.642 | 0.001* | 0.528 |
| V2S-V1D | 0.507 | 0.001* | 0.120 |
significant at p=0.002 level
In addition, upon recovery at 0% desflurane, mutual information was statistically significantly higher than during baseline (by 66% for V1-V2 and by 61% for L3-L5, p=0.002, two-tailed test). The corresponding unnormalized mutual information values were 0.52±0.51 and 0.53±0.51 bits, respectively. In fact, by occasional observations, recovery was often accompanied by a period of behavioral hyperarousal, later followed by relaxed wakefulness or occasionally spontaneous sleep. There was no statistically significant quadratic trend in mutual information among any of the subregions.
By definition, mutual information is a non-directional measure of the interdependence of symbolic information from two sources. In order to assess the effect of desflurane on directional interactions of neurons, we calculated the alternative information-theoretic quantity, transfer entropy and its directionality index that quantifies the imbalance between feedforward and feedback communication.
First, to compare with mutual information, we examined the sum of transfer entropies in forward and reverse directions. Because transfer entropy sum is non-directional, it was expected to behave similarly to mutual information. Figure 3 shows that desflurane statistically significantly decreased transfer entropy sum for all region-pairs suggesting a general decrease in both interregional and interlaminar interactions in visual cortex. Because many of these effects were not seen with mutual information, transfer entropy sum appears to be a more sensitive measure of change than mutual information. The changes from 0% to 8% desflurane were also larger with transfer entropy sum, reaching 77% for V1-V2 and 78% for L3-L5, with subregions combined as done for mutual information. The corresponding unnormalized transfer entropy values were 1.86±1.56 and 1.87±1.67 bits at baseline 0% desflurane, and 0.43±0.37 and 0.41±0.37 bits at 8% desflurane.
Figure 3.
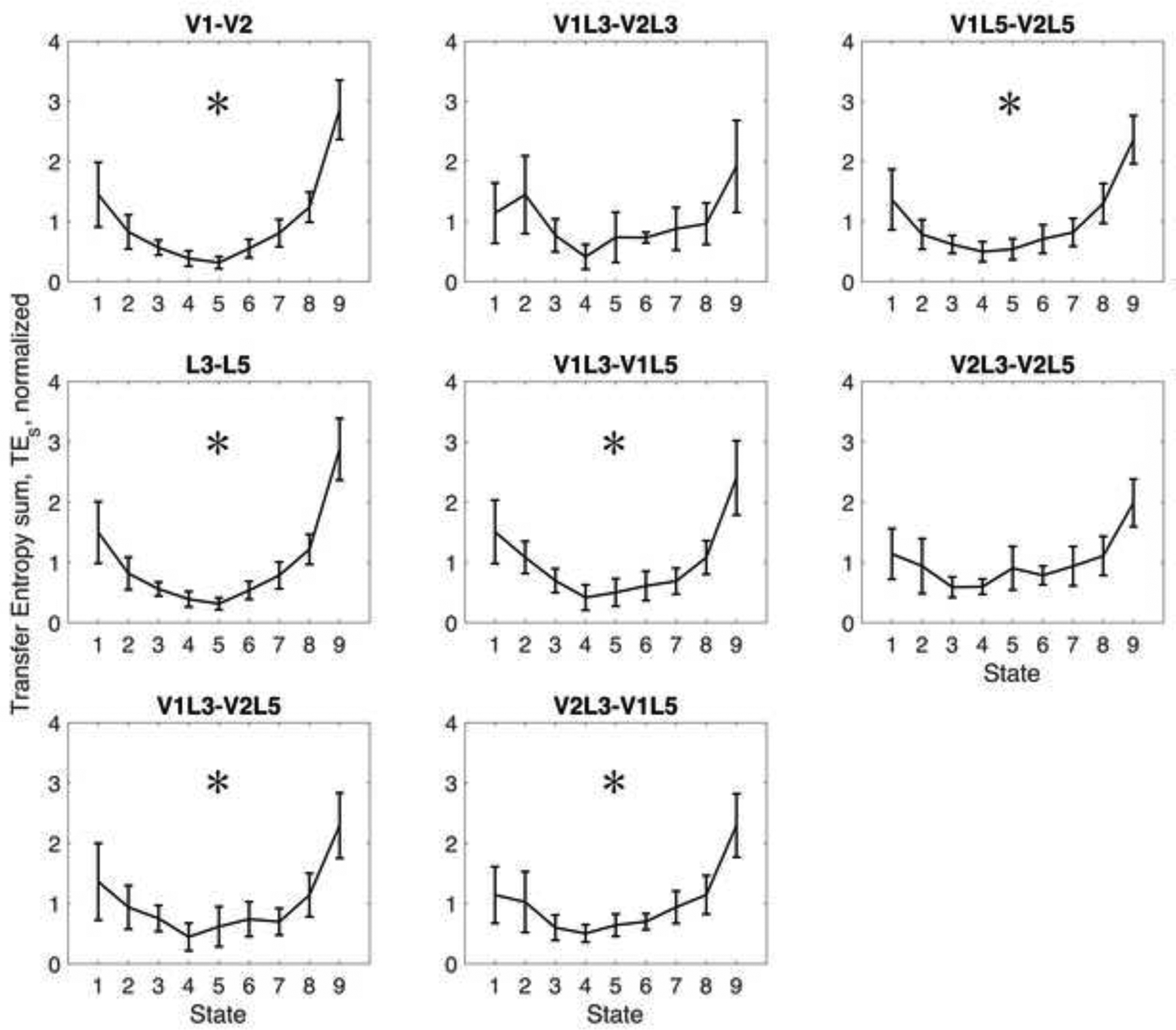
Robust concentration-dependent effect of desflurane on the sum of transfer entropies calculated between primary and secondary visual cortex (V1-V2), between cortical layers 3 and 5 (L3, L5), and between various subregions. Anesthetic states 1 to 9 correspond to consecutive desflurane concentrations of 0, 2, 4, 6, 8, 6, 4, 2, 0 percent, respectively. Transfer entropy sum is in relative units, plotted as mean and 95% confidence interval. *: statistically significant quadratic trend with consecutive anesthetic states.
We then examined the effect of desflurane on the directionality index (Figure 4). As with mutual information and transfer entropy sum, we found statistically significant quadratic trend of the directionality index between V1 and V2 and between L3 and L5. The positive sign of the directionality index at 0% desflurane shows that in this state V2→V1 and L3→L5 directional effects were dominant (0.11±0.18 and 0.12±0.20 for V2→V1 and L3→L5, respectively), but this dominance was reduced during anesthesia by 89% for V2→V1 and 97% for L3→L5 at 8% desflurane to 0.01±0.04 and −0.01±0.05 for V1→V2 and L3→L5, respectively. Moreover, the reduction of directionality index in layer 5 (V2L5→V1L5) implied a larger reduction of feedback than feedforward connectivity (108% decrease at 8% desflurane vs. 0% baseline; higher than 100% indicates a reversal in direction), consistent with the anatomical disposition of V2→V1 feedback between the higher-order and lower-order infragranular regions. The relatively large (97%) reduction in overall L3→L5 directionality could not be partialled into subregions although the quadratic trend of the directionality index for V1L3→V1L5 was close to statistical significance. Individual mutual information, transfer entropy sum and directionality index data from each animal for the three most relevant interactions are provided in Figure S1 as Supplemental Digital Content.
Figure 4.
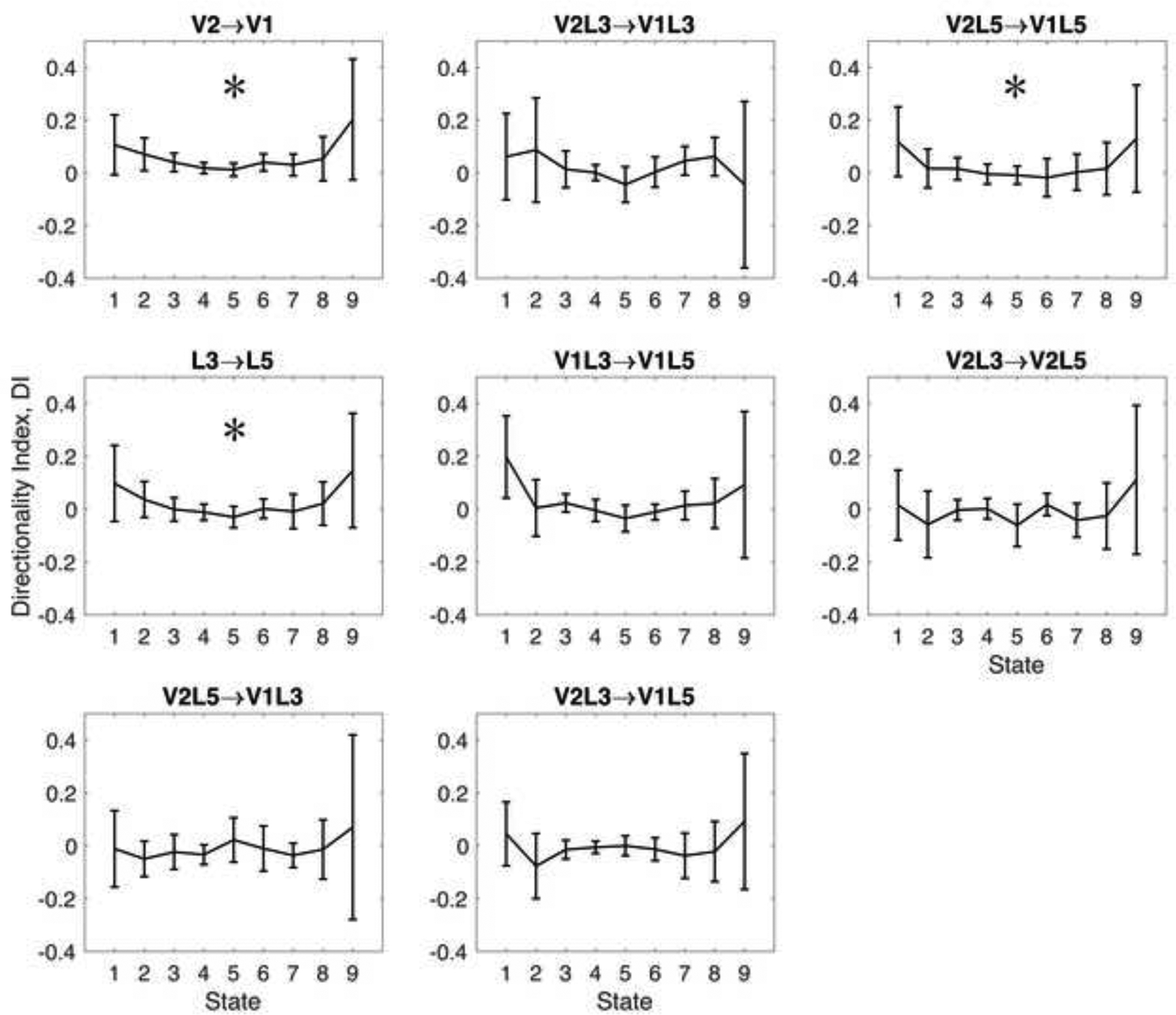
Effect of desflurane on the directionality index. Statistically significant change occurred in layer 5 secondary visual to layer 5 primary visual cortex (V2L5-V1L5) implying a reduction of feedback connectivity at higher desflurane concentration. Anesthetic states 1 to 9 correspond to consecutive desflurane concentrations of 0, 2, 4, 6, 8, 6, 4, 2, 0 percent, respectively. Directionality index is in relative units, plotted as mean and 95% confidence interval. *: statistically significant quadratic trend with consecutive anesthetic states.
DISCUSSION
The goal of this study was to determine if a common, clinically used anesthetic, desflurane would alter neuronal interactions locally, between hierarchical stages of early cortical sensory processing regions in an intact animal in vivo. From the simultaneous recording of many neurons sampled at two depths of primary and secondary visual cortex we found both overall and layer-specific modifications of interregional neuronal interactions by desflurane. Specifically, changes in both mutual information and transfer entropy revealed robust, concentration-dependent reductions of neuronal interactions between V1 and V2 and between layer 3 and layer 5. Transfer entropy suggested a more general suppression of neuronal interactions among all sampled regions of visual cortex. Moreover, the transfer entropy directionality index predicted a greater reduction of V2-V1 feedback than V1-V2 feedforward, specifically within L5 during anesthesia.
The latter results are consistent with the anatomical disposition of feedback pathways that originate mainly in higher order infragranular layers and target both lower order supragranular and lower order infragranular layers5,30. It also agrees with the frequently observed anesthetic reduction of large-scale anterioposterior or frontoparietal directional connectivity10–15,31,32. Here we find that this is also true locally, within visual cortex. Layer-specific modulations of local neuronal firing responses to visual stimulation by isoflurane were also found in the ferret visual cortex31. Although the magnitude of the relevant changes of directionality index were relatively modest, one could speculate that a cumulative effect of local feedback reductions along several hierarchical cortical areas could result in a more robust shift in the feedback/feedforward balance in large-scale cortical connectivity.
Two recent studies challenge the finding of preferential feedback modulation by anesthesia. First, Sanders et al found that the primary effect of propofol on TMS-induced EEG cross-frequency coupling was in the feedforward direction although feedback coupling in the beta frequency band was also reduced16. Clearly, TMS-induced EEG coupling may be different from resting-state EEG33 or sensory-evoked local field potential10 and local unit correlations25. Second, Ma et al reported that bolus injection of propofol in monkeys increased electroencephalographic low-frequency phase-beta amplitude coupling in frontoparietal and V2-V1 regions such that the phase of higher order areas modulated the band power of lower-order areas17. However, such effects of propofol may not apply to those of volatile anesthetics. Previous studies found reduced gamma coherence and transfer entropy10,34 and reduced wide-band phase synchrony31 between frontal and visual cortex during isoflurane anesthesia. Both corticocortical coherence and frontoparietal symbolic transfer entropy in the high gamma band were decreased during sevoflurane anesthesia32.
The fact that transfer entropy sum revealed additional, statistically significant effects of desflurane over those predicted by mutual information suggests that transfer entropy in general may be a more sensitive measure of neuronal interactions. Mutual information is a probabilistic measure of shared symbolic information of two signal sources; in our case, the binary configuration of coincident neuronal spikes in 10ms time bins consistent with the hypothesis of population coding of neuronal information35. A limitation of mutual information is that it does not necessarily measure direct exchange of information between two neuron populations; it may in part indicate the action of an independent common synchronizing input. Under anesthesia, both synchronized spontaneous oscillations and common synchronizing input can contribute to shared information as measured by MI. Moreover, deep levels of anesthesia are associated with burst suppression – a visible alternation of brief high activity bursts and intervening silent periods. The bursts themselves are highly synchronized, which counters the decrease in true neuronal interactions. Neuron bursts develop gradually with increasing anesthetic concentration with the duration of bursts (or UP-states) becoming shorter and the silent periods becoming longer25. Thus, in general, mutual information reveals “functional” but not necessarily direct neuronal interaction. The exact nature of neuronal information is also in question; therefore, the word information in the current context should be understood somewhat metaphorically.
Transfer entropy attempts to circumvent the limitation due to common synchronizing input by estimating the probabilistic causal influence of one signal source on another. Specifically, transfer entropy measures the reduction of uncertainty in future values of Y by knowing the past values of X given the past values of Y. If the spike train Y becomes more synchronized and predictable, such as during slow oscillation or burst suppression, knowing the past of spike train X does not reduce the uncertainty of Y (and vice versa). This leads to a decrease in transfer entropy rather than an increase and may explain the difference in behavior between transfer entropy and mutual information. The validity of transfer entropy as a pure measure of causal influence has been questioned36, as it may be overly influenced by a decrease in source entropy37. This could also explain why more transfer entropy sum interactions were reduced in anesthesia. Alternatives such as normalized transfer entropy have been introduced38 but not applied here due the resulting ambiguity in interpretation. Regardless of these considerations, transfer entropy appears to be a superior estimator of the direct influence of one signal on another compared to mutual information.
In order to evaluate a graded, dose-dependent response, desflurane was administered incrementally from wakefulness to deep anesthesia and back, ending with full emergence. All statistically significant effects of desflurane were reversible upon termination of anesthesia. Moreover, the values of both mutual information and transfer entropy sum after emergence often exceeded those in wakeful baseline. The mechanism of this increase is currently unclear but may reflect persistent post-inhibitory rebound of neuronal activity39. Accordingly, there was no indication of neural inertia, a phenomenon thought to delay the recovery from anesthesia40. This was probably due to the relatively long time course of our experiments (270 minutes), which presumably facilitated the equilibration of state at each anesthesia level41.
What may be the role of anesthetic modulation of local modality-specific circuits in the overall disruption of cortical connectivity for the loss of consciousness? It is generally believed that under anesthesia, a generalized failure of bidirectional information flow across the entire cortical mantle would prevent any kind of information reaching the level of awareness, presumably equating this condition with complete loss of consciousness. Because the anesthetic modulation of local interactions is statistically significant and robust24,42, these may in effect produce loss of conscious perception in a specific modality. Moreover, the cumulative effect of local circuit disruptions across higher levels of the cortical hierarchy may be amplified to the degree that coerces the generalized loss of consciousness. Thus, our current observations using anesthesia as a model of unconsciousness line up well with the prevailing view derived from cognitive neuroscience investigations stating that conscious perception depends critically on recurrent communication along the cortical hierarchy7,8.
In addition to the analytical considerations above, technical limitations of the present study include the relatively sparse sampling of neurons in four cortical subregions and the possible individual variation in V1-V2 region boundaries as defined by the Paxinos atlas. In some experiments, there were an insufficient number of neurons to estimate the entropies in all four regions that resulted in missing data and limited the statistical power. Also, we recorded from only two depths of cortex although specific information from all cortical layers may be desirable. The recording sites were presumed to reside in layer 3 and layer 5, but the exact location of the electrode positions could not be determined. In some cases, histological verification of the electrode sites was attempted; however, this was hampered by the presence of 32 microwires and the tissue damage their removal caused. Future work could examine neuronal interactions among more precisely defined cortical layers and subregions using high-density microelectrode arrays both within and beyond the visual system. The rats were tested in dark environment during their active diurnal cycle to minimize the chance for spontaneous sleep. Given the relatively long duration of anesthetic exposure using multiple doses, the amount of data collected did not allow us to segment the baseline and recovery conditions to specific behavioral states, such as exploration, quiet wakefulness, and sleep. Therefore, these states may not be strictly equivalent to normal attentive wakefulness and should be best designated as anestheticfree. In addition, in order to limit the duration of anesthetic exposure, we did not apply any visual stimuli, thus our results apply to spontaneous ongoing neuronal activity in the visual system. Finally, all experiments were performed on adult male rats of a single sex due to statistical considerations. Possible age and sex differences in the results should be investigated in the future.
We conclude that desflurane disrupts global and cortical layer-specific inter-areal neuronal interactions in rat visual cortex with a preferential effect on V2-V1 feedback. The findings suggest that anesthesia produces neuronal connectivity changes in local circuits, which may cumulatively lead to large-scale cortical functional disintegration as a postulated mechanism of anesthetic-induced unconsciousness.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01-GM056398 and the Department of Anesthesiology, Center for Consciousness Science, University of Michigan Medical School, Ann Arbor, Michigan, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Kathy Zelenock, MS for her assistance in general laboratory operations and manuscript editing.
Funding statement:
This study was supported by the National Institute of General Medical Sciences of the National Institutes of Health, Bethesda, Maryland, USA, under award number R01-GM056398 (to AGH) and by the Department of Anesthesiology, Center for Consciousness Science, University of Michigan Medical School, Ann Arbor, Michigan, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Prior Presentations: Annual Meeting of the American Society of Anesthesiologists, Best Abstract Basic Science Session, San Francisco, CA, 10/19/2018 and Annual Meeting of the Association of University Anesthesiologists, Chicago, IL, 04/27/2018.
Conflicts of Interest: None
REFERENCES
- 1.John ER, Prichep LS, Kox W, Valdes-Sosa P, Bosch-Bayard J, Aubert E, Tom M, diMichele F, Gugino LD: Invariant reversible QEEG effects of anesthetics. Conscious Cogn 2001; 10: 165–83. [DOI] [PubMed] [Google Scholar]
- 2.Alkire MT, Hudetz AG, Tononi G: Consciousness and anesthesia. Science 2008; 322: 876–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonhomme V, Boveroux P, Brichant JF, Laureys S, Boly M: Neural correlates of consciousness during general anesthesia using functional magnetic resonance imaging (fMRI). Arch Ital Biol 2012; 150: 155–63 [DOI] [PubMed] [Google Scholar]
- 4.Kelz MB, Garcia PS, Mashour GA, Solt K: Escape From Oblivion: Neural Mechanisms of Emergence From General Anesthesia. Anesth Analg 2019; 128: 726–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voss LJ, Garcia PS, Hentschke H, Banks MI: Understanding the Effects of General Anesthetics on Cortical Network Activity Using Ex Vivo Preparations. Anesthesiology 2019; 130: 1049–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemmings HC Jr., Riegelhaupt PM, Kelz MB, Solt K, Eckenhoff RG, Orser BA, Goldstein PA: Towards a Comprehensive Understanding of Anesthetic Mechanisms of Action: A Decade of Discovery. Trends Pharmacol Sci 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamme VA, Roelfsema PR: The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci 2000; 23: 571–9 [DOI] [PubMed] [Google Scholar]
- 8.Dehaene S, Changeux JP: Experimental and theoretical approaches to conscious processing. Neuron 2011; 70: 200–27 [DOI] [PubMed] [Google Scholar]
- 9.van Vugt B, Dagnino B, Vartak D, Safaai H, Panzeri S, Dehaene S, Roelfsema PR: The threshold for conscious report: Signal loss and response bias in visual and frontal cortex. Science 2018; 360: 537–542 [DOI] [PubMed] [Google Scholar]
- 10.Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG: Volatile anesthetics disrupt frontal-posterior recurrent information transfer at gamma frequencies in rat. Neurosci Lett 2005; 387: 145–50 [DOI] [PubMed] [Google Scholar]
- 11.Hudetz AG, Mashour GA: Disconnecting Consciousness: Is There a Common Anesthetic End Point? Anesth Analg 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mashour GA, Hudetz AG: Neural Correlates of Unconsciousness in Large-Scale Brain Networks. Trends Neurosci 2018; 41: 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boveroux P, Vanhaudenhuyse A, Bruno MA, Noirhomme Q, Lauwick S, Luxen A, Degueldre C, Plenevaux A, Schnakers C, Phillips C, Brichant JF, Bonhomme V, Maquet P, Greicius MD, Laureys S, Boly M: Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology 2010; 113: 1038–53 [DOI] [PubMed] [Google Scholar]
- 14.Jordan D, Ilg R, Riedl V, Schorer A, Grimberg S, Neufang S, Omerovic A, Berger S, Untergehrer G, Preibisch C, Schulz E, Schuster T, Schroter M, Spoormaker V, Zimmer C, Hemmer B, Wohlschlager A, Kochs EF, Schneider G: Simultaneous electroencephalographic and functional magnetic resonance imaging indicate impaired cortical top-down processing in association with anesthetic-induced unconsciousness. Anesthesiology 2013; 119: 103142. [DOI] [PubMed] [Google Scholar]
- 15.Mashour GA, Hudetz AG: Bottom-Up and Top-Down Mechanisms of General Anesthetics Modulate Different Dimensions of Consciousness. Front Neural Circuits 2017; 11: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders RD, Banks MI, Darracq M, Moran R, Sleigh J, Gosseries O, Bonhomme V, Brichant JF, Rosanova M, Raz A, Tononi G, Massimini M, Laureys S, Boly M: Propofolinduced unresponsiveness is associated with impaired feedforward connectivity in cortical hierarchy. Br J Anaesth 2018; 121: 1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L, Liu W, Hudson AE: Propofol Anesthesia Increases Long-range Frontoparietal Corticocortical Interaction in the Oculomotor Circuit in Macaque Monkeys. Anesthesiology 2019; 130: 560–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boly M, Massimini M, Tsuchiya N, Postle BR, Koch C, Tononi G: Are the Neural Correlates of Consciousness in the Front or in the Back of the Cerebral Cortex? Clinical and Neuroimaging Evidence. J Neurosci 2017; 37: 9603–9613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odegaard B, Knight RT, Lau H: Should a Few Null Findings Falsify Prefrontal Theories of Conscious Perception? J Neurosci 2017; 37: 9593–9602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch C, Massimini M, Boly M, Tononi G: Neural correlates of consciousness: progress and problems. Nat Rev Neurosci 2016; 17: 307–21 [DOI] [PubMed] [Google Scholar]
- 21.Coogan TA, Burkhalter A: Conserved patterns of cortico-cortical connections define areal hierarchy in rat visual cortex. Exp Brain Res 1990; 80: 49–53 [DOI] [PubMed] [Google Scholar]
- 22.Pillay S, Vizuete J, Liu X, Juhasz G, Hudetz AG: Brainstem stimulation augments information integration in the cerebral cortex of desflurane-anesthetized rats. Front Integr Neurosci 2014; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C: The Rat Brain in Stereotaxic Coordinates, 4th edition. San Diego, Academic Press, 1998 [Google Scholar]
- 24.Vizuete JA, Pillay S, Diba K, Ropella KM, Hudetz AG: Monosynaptic functional connectivity in cerebral cortex during wakefulness and under graded levels of anesthesia. Frontiers in Integrative Neuroscience 2012; 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vizuete JA, Pillay S, Ropella KM, Hudetz AG: Graded defragmentation of cortical neuronal firing during recovery of consciousness in rats. Neuroscience 2014; 275: 340–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yger P, Spampinato GL, Esposito E, Lefebvre B, Deny S, Gardella C, Stimberg M, Jetter F, Zeck G, Picaud S, Duebel J, Marre O: A spike sorting toolbox for up to thousands of electrodes validated with ground truth recordings in vitro and in vivo. Elife 2018; 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowak LG, Munk MH, Girard P, Bullier J: Visual latencies in areas V1 and V2 of the macaque monkey. Vis Neurosci 1995; 12: 371–84 [DOI] [PubMed] [Google Scholar]
- 28.Sakmann B: From single cells and single columns to cortical networks: dendritic excitability, coincidence detection and synaptic transmission in brain slices and brains. Exp Physiol 2017; 102: 489–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiber T: Measuring information transfer. Phys Rev Lett 2000; 85: 461–4 [DOI] [PubMed] [Google Scholar]
- 30.Domenici L, Harding GW, Burkhalter A: Patterns of synaptic activity in forward and feedback pathways within rat visual cortex. J Neurophysiol 1995; 74: 2649–64 [DOI] [PubMed] [Google Scholar]
- 31.Sellers KK, Bennett DV, Hutt A, Williams JH, Frohlich F: Awake versus Anesthetized: Layer-Specific Sensory Processing in Visual Cortex and Functional Connectivity between Cortical Areas. J Neurophysiol 2015: jn 00923 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pal D, Silverstein BH, Lee H, Mashour GA: Neural Correlates of Wakefulness, Sleep, and General Anesthesia: An Experimental Study in Rat. Anesthesiology 2016; 125: 929–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee U, Ku S, Noh G, Baek S, Choi B, Mashour GA: Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology 2013; 118: 126475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imas OA, Ropella KM, Wood JD, Hudetz AG: Isoflurane disrupts anterio-posterior phase synchronization of flash-induced field potentials in the rat. Neurosci Lett 2006; 402: 216–21 [DOI] [PubMed] [Google Scholar]
- 35.Mendoza-Halliday D, Martinez-Trujillo JC: Neuronal population coding of perceived and memorized visual features in the lateral prefrontal cortex. Nat Commun 2017; 8: 15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stokes PA, Purdon PL: A study of problems encountered in Granger causality analysis from a neuroscience perspective. Proc Natl Acad Sci U S A 2017; 114: E7063E7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wollstadt P, Sellers KK, Rudelt L, Priesemann V, Hutt A, Frohlich F, Wibral M: Breakdown of local information processing may underlie isoflurane anesthesia effects. PLoS Comput Biol 2017; 13: e1005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gourevitch B, Eggermont JJ: Evaluating information transfer between auditory cortical neurons. J Neurophysiol 2007; 97: 2533–43 [DOI] [PubMed] [Google Scholar]
- 39.Goaillard JM, Taylor AL, Pulver SR, Marder E: Slow and persistent postinhibitory rebound acts as an intrinsic short-term memory mechanism. J Neurosci 2010; 30: 4687–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman EB, Sun Y, Moore JT, Hung HT, Meng QC, Perera P, Joiner WJ, Thomas SA, Eckenhoff RG, Sehgal A, Kelz MB: A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One 2010; 5: e11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Proekt A, Hudson AE: A stochastic basis for neural inertia in emergence from general anaesthesia. Br J Anaesth 2018; 121: 86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzel M, Han S, Smith EH, Hoel E, Greger B, House PA, Yuste R: Reduced Repertoire of Cortical Microstates and Neuronal Ensembles in Medically Induced Loss of Consciousness. Cell Syst 2019; 8: 467–474 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C: The rat brain in stereotaxic coordinates, 6th edition. Amsterdam/Boston, Academic Press/Elsevier, 2007 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


