Summary
The superior colliculus (SC), called tectum in non-mammalian vertebrates, registers events in the surrounding space often through vision and hearing, but also through electrosensation, infrared detection, and other sensory modalities in diverse vertebrate lineages. This information is used to form maps of the surrounding space and the positions of different salient stimuli in relation to the individual. The sensory maps are arranged in layers with visual input in the uppermost layer, other senses in deeper positions, and a spatially aligned motor map in the deepest layer. Here, we will review the organization and intrinsic function of the tectum/SC and the information that is processed within tectal circuits. We will also discuss tectal/SC outputs that are conveyed directly to downstream motor circuits or via the thalamus to cortical areas to control various aspects of behavior. The tectum/SC is evolutionarily conserved among all vertebrates, but tailored to the sensory specialties of each lineage, and its roles have shifted with the emergence of the cerebral cortex in mammals. We will illustrate both the conserved and divergent properties of the tectum/SC through vertebrate evolution by comparing tectal processing in lampreys belonging to the oldest group of extant vertebrates, larval zebrafish, rodents, and other vertebrates including primates.
In Brief:
Isa et al review recent advances in understanding the sensory inputs, the integral circuitry and computations, and sensorimotor contributions of the tectum/superior colliculus. They highlight both the properties that are conserved and those that have become specialized in different vertebrate lineages.
Introduction
The survival and evolutionary success of all animals depend on their ability to sense their surroundings and to mount appropriate behavioral responses to external stimuli. Depending on the stimulus and context, an animal may choose to approach, avoid, or simply ignore something that is occurring around it. In everyday life as we walk along the street we need to avoid bumping into other pedestrians, but also stop to look at interesting items. The superior colliculus (SC), also called the tectum, is critical for eliciting such orienting or evasive movements 1-6, and through interactions with the basal ganglia and cortex, it contributes importantly to this decision-making 7-11. The tectum/SC contains a retinotopic map of the surrounding space to which other senses may add 12-15. Salient stimuli originating from visual input, but also from sounds and other senses, can activate the tectum/SC with a minimal delay. The sensory input carries information about the stimulus’ position in the surrounding space, allowing the tectum/SC to rapidly guide movements of various body parts towards the object and determine whether to elicit an orienting or an evasive response. It appears that tectum/SC is mostly concerned with co-registration of stimulus location regardless of sensory sources, but tectal/SC circuitry also shows selectivity for specific stimulus features in some contexts. The tectum/SC responds to a wide range of inputs important to the animal’s ethological context, not only vision but also the lateral line in fish, electroreception in lampreys, heat signatures in some snakes, and auditory inputs in most animals.
The intrinsic neural circuitry of tectum/SC, including its visual and other sensory inputs and its output connectivity, is conserved throughout vertebrate phylogeny, but the details of the sensory processing have shifted through evolution and with the varying demands of its diverse owners 12-15. In the oldest group of extant vertebrates, the lampreys, the tectum represents a much larger structure than the entire forebrain, although the latter still contains a visual and a motor representation 7, 16. However, the relative size of the forebrain increases gradually to become many times larger in mammals and particularly primates (Figure 1). With a gross oversimplification, one can say that the tectum/SC contributes with a rapid response to salient stimuli from different locations in the surrounding space, while the forebrain is involved in the nuanced appraisal of these stimuli and in the cognitive control of action 17.
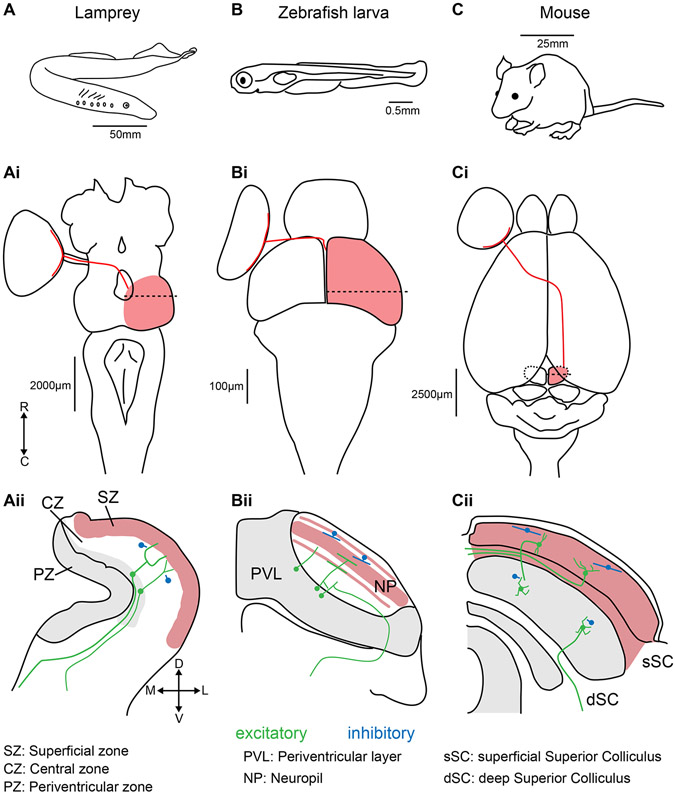
Figure 1: Positions, sizes, and retinal inputs to the tectum/SC in lampreys, zebrafish larvae, and mice.
(A) The lamprey (Lampetra fluviatilis). (Ai) Dorsal view of a lamprey brain. Retinal ganglion cell projections (red line) from the left eye cross the optic chiasm to enter the right tectum (red shading). The dashed line represents the position of the coronal section displayed in (Aii). Major divisions are based on Butler and Hodos, 2005 19. The red shaded region represents the layers that receive retinal input. The grey shaded area represents regions with a high density of neuronal somae. Local inhibitory interneurons are represented in blue. Ipsilateral and contralateral glutamatergic projecting neurons are represented in green. The same features are shown for larval zebrafish (B-Bii) and mouse (C-Cii). R: rostral, C: caudal, D: dorsal, V: ventral, M: medial, L: lateral.
In this review, we will compare the roles of the tectum/SC across the vertebrate lineage, including the different specializations that have occurred in various vertebrate groups. We will focus in greater depth on models in which particular progress has been made in understanding the tectum/SC’s functional contributions and circuitry: the lamprey, larval zebrafish, mouse, and primates. In “The tectum/SC’s position in brain-wide networks”, we will start with a description of the tectum’s gross structure and connectivity, including the contributions that it makes to different ascending sensory pathways in various vertebrates. We will then turn, in “The tectum/SC’s intrinsic networks, and the computations that they perform”, to the sensory and sensorimotor integration that take place in tectum/SC, and will discuss recent developments in describing the functional microcircuits that carry out these calculations. Next, we will discuss the diversity of the sensory modalities processed in the tectum/SC in “Multisensory integration of spatial information in tectum/SC”. While different lineages process different combinations of sensory modalities in the tectum/SC, its conserved role is nonetheless to integrate these sensory inputs and register them in space to permit directed behavior. The ultimate goal of the tectum/SC (and the brain as a whole) is to deliver adaptive behavior, so in “Sensorimotor transformation, premotor outputs, and behavior selection”, we will cover the tectum/SC’s contributions to sensorimotor gating and to the directed selection of appropriate behaviors. Finally, we will conclude with perspectives on the key outstanding questions about tectal/SC function, and possible avenues for addressing them. We aim to provide a broad evolutionary perspective on the tectum/SC, its intrinsic circuitry, its interactions with other brain regions, and its contributions to the processing of diverse sensory stimuli, and we refer readers to a very recent and complementary review by Basso and colleagues 18, which is more focused on the SC’s role in vision, especially in mammals including primates.
The tectum/SC’s position in brain-wide networks
The retinotectal pathway
Retinal ganglion cells, the output neurons of the retina, provide a major input to the tectum/SC in most vertebrates (see Figure 1). Importantly, they terminate in the tectum/SC in a retinotopic manner, forming a retinotectal map in which spatial positions in the retina (and therefore the visual world) are preserved in the tectum/SC. Specifically, retinal ganglion cells in the temporal retina (reporting on the anterior visual field) terminate in the rostral tectum, while nasal retinal ganglion cells (posterior visual field) terminate in the caudal tectum. Similarly, retinal ganglion cells in the dorsal and ventral retina target the lateral and medial tectum/SC, respectively. This means that the visual world is already spatially represented in the tectum/SC, even before any tectal processing takes place, and this scheme is conserved across all vertebrates studied (Figure 2), including fish, frogs, birds and, mammals 12-14, 20-22. As we will discuss in our descriptions of tectal circuits and computations, this has important implications for the overall encoding of sensory space in the tectum/SC.
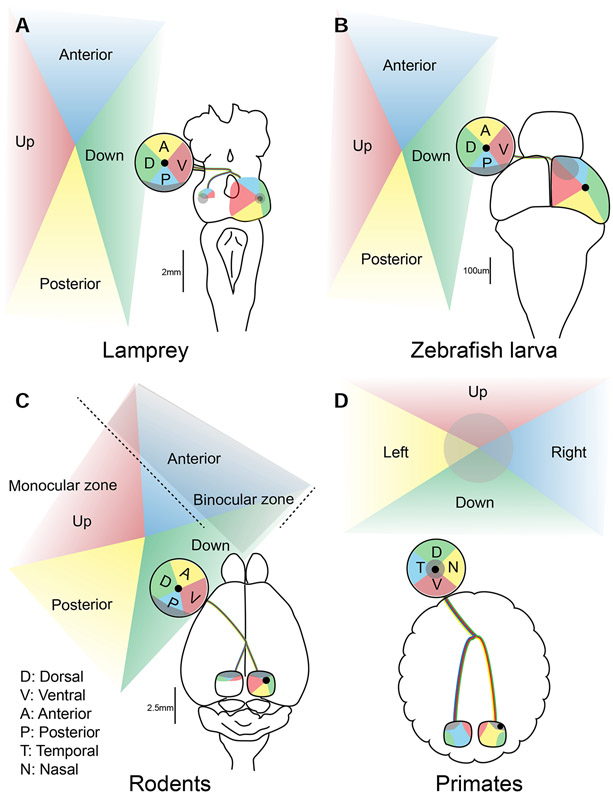
Figure 2: Eye position and retinotectal space across lineages.
Visual topography of the retinotectal projections in the lamprey (A), zebrafish larva (B), rodents (C) and primates (D). Projections from various aspects of the visual field (Up, Down, Anterior and Posterior in A-C, and Right and Left in D) to the retina and tectum/SC are shown in corresponding colors. The center of retina is represented with black dots. The binocular zone for rodents (C) and the foveal region for primates (D) are shaded in gray.
These retinal ganglion cells are not a uniform population, however, and recent studies have made enormous progress in defining and mapping the various subtypes of retinal ganglion cell and their associated neural networks using genetics, viral circuit tracing, and behavioral psychophysics, especially in mice. Based on the soma size, dendritic stratification pattern, and visual response patterns, 20-30 morphologically and physiologically distinct retinal ganglion cell types are estimated to exist in the mouse and primates 23-25. Retinal ganglion cells can also be categorized based on molecular markers, and while some categories of retinal ganglion cells have characteristic sets of markers, there is as yet no clear understanding of whether these markers identify specific structural and functional subtypes of retinal ganglion cells 26-29 (Although see Kölsch et al 2020 30). In rodents, more than 90% of retinal ganglion cells project to the SC 31, 32, and different retinal ganglion cell subtypes target distinct laminar depths in the superficial SC (sSC) 33. Thus, the sSC is subdivided into multiple distinct sublaminar maps, each with input from a set of retinal ganglion cell subtypes. It is also worth noting that different subtypes of retinal ganglion cell are not uniformly distributed across the retina, suggesting that their functional contributions vary across spatial positions in the tectum/SC 15.
A great deal of work has addressed how individual retinal ganglion cell types are connected to different types of sSC neuron, how their inputs contribute to the sSC neurons’ visual response properties, and how this eventually shapes behavioral responses, but many details of these links remain unclear. The visual response properties of sSC neurons may depend on selective retinal ganglion cell or cortical inputs, or on processing in local SC circuits, and this balance appears to vary across different animal species 34. In the mouse, ablation or silencing the cortico-tectal pathway modulates the receptive field and tuning properties of SC neurons, but these inputs do not solely determine SC neurons’ selectivity for features such as the looming speed or the response time course 35-38, as contributions from retinal ganglion cell inputs have also been identified. This arrangement contrasts with that in the cats and hamsters, in which certain visual response features of the SC neurons have been reported to depend on the cortical inputs 39. In contrast, in zebrafish, response properties of some tectal neurons have been shown to arise from the processing in the local tectal circuits 33, 40-42. In primates, inactivation of magnocellular laminae of the lateral geniculate nucleus (LGN) disrupts the visual responses of most of dSC, but not sSC, cells. Meanwhile, the inactivation of the parvocellular lamina of the LGN has no effect on visual responses in SC neurons 43. Generalizing across vertebrates, therefore, tectal outputs are shaped by a combination of retinal ganglion cell inputs, inputs from other brain regions, and integral tectal circuitry.
The retino-thalamic and retino-colliculo-thalamic visual pathways
In the lamprey, a prototypical vertebrate, the shortest visuomotor circuit is trisynaptic. Retinal ganglion cells make direct connections to the output neurons of the tectum, which in turn project to the lower brainstem. Here, they synapse onto reticulospinal neurons that are directly connected to spinal motoneurons, allowing for rapid approach or escape responses to visual stimuli 1, 2. As the tectum/SC has elaborated during the course of evolution, intra-tectal processing has further evolved in this eye-tectum-motor pathway. In mammals, anatomically distinct superficial and deep areas have developed (the sSC and dSC), with information flowing from the eye through the sSC, to the dSC, and on to downstream premotor areas. Although the details of this circuit vary across lineages, this conserved pathway represents the tectum/SC’s simplest role as a relay for fast visuomotor transmission.
However, the tectum/SC is much more than a simple visuomotor relay. In addition to such direct transformation of sensory signals to behavioral responses, the sSC implements intrinsic processing of visual signals to extract their critical features and sends outputs to the posterior thalamus and then to the telencephalon (pallium or extrastriate cortex). This is the retino-colliculo-thalamic pathway. This pathway appears to be involved in the integrated processing of visual information about the location and motion of targets 44. In parallel, visual inputs from the retina also project to the dorsal thalamus (dLGN) and further to the telencephalon. This is the retino-thalamic pathway, which has been implicated in the detection and recognition of objects 45.
These different anatomical paths reflect the distinct visual processing that occurs in the retino-thalamic and retino-colliculo-thalamic/retinotectal pathways, and these differences shed light on the tectum/SC’s contributions to sensory processing as a whole. The curious phenomenon known as “blindsight” (see Box 1) illustrates the functions of retino-thalamic and retinocolliuclar visual pathways, and the ways in which some of these functions are plastically changeable. Blindsight refers to human patients with damage to their visual cortex who lack conscious vision but are nonetheless capable of visually-guided eye movements, obstacle avoidance, and other coarse visuo-motor tasks 46, 47. Long ago, these residual visual behaviors in apparently blind people provided an indication that there are multiple distinct visual pathways, and subsequent studies soon suggested a role for the retino-colliculo-thalamic pathway in blindsight 48-50. Thus, the retino-thalamic pathway is generally regarded as necessary for conscious and high acuity vision, while the retino-colliculo-thalamic pathway has been shown to regulate non-conscious and reflexive visuo-motor processing (see Box for further comments on the thalamic relay for blindsight).
Box: Blindsight.
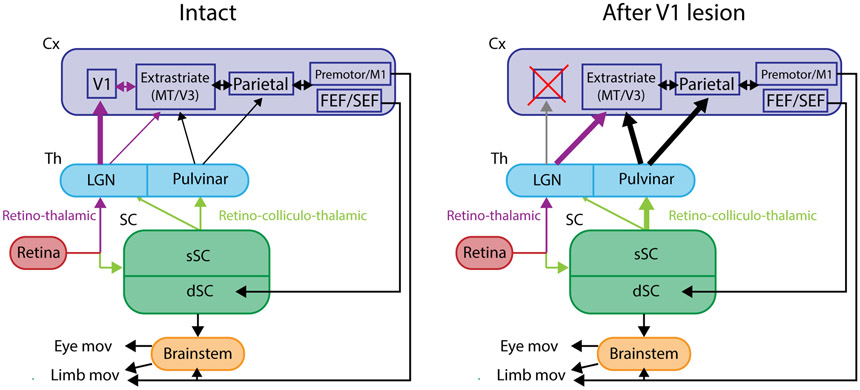
Blindsight is an interesting phenomenon that occurs in some patients with damage to the primary visual cortex. These patients lose visual awareness of objects in the blind visual field but retain a certain level of visuo-motor behavior towards these objects when they are forced to do so 46, 47. The neural mechanisms of blindsight have been intensively studied in the macaque monkey model. It has been shown that visual awareness is impaired in monkeys with V1 lesions, as judged by their behavior in a Yes-No choice task in which the animals are required to register their awareness of the visual cue 69, 70. There is a consensus that the SC is critical as the relay of visual inputs because lesion/inactivation of SC has been shown to impair the visually guided behaviors or visual responses in the extrastriate cortex 48-50. The role of the thalamic relay has been less clear, with some researchers suggesting a major role for the SC-pulvinar and extrastriate cortical pathway 71, 72, and others favoring the SC-dLGN (koniocellular layer)-extrastriate cortex pathway 73-76. A very recent study has addressed this uncertainty by inactivating the dLGN and pulvinar in the same animal on the ipsilesional and contralesional sides of macaque monkeys with unilateral V1 lesions. It was found that inactivation of dLGN (but not of the pulvinar) on the contralesional (intact) side impaired visually guided saccade performance, while both impaired the visually guided saccades on the ipsilesional (affected) side 77. These results suggest the SC-pulvinar pathway partially compensates for the dLGN-striate cortical pathway after the V1 lesion.
Box legend: Schematic of visual pathways before and after V1 damage.
Visuo-motor pathway for the control of eye or limb movements with the intact V1 (left) and following damage to the V1 (right). The thickness of the arrows indicates the strength of connectivity.
Illustrating this, inactivation of the SC in nonhuman primates prolongs reaction times for visually-guided eye saccades and eliminates ultra-short latency “express saccades” 51. However, recent studies have shown that the SC is not only mediating the quick processing of simple visual signals, but is also involved in more complex visual processing in its local circuits. For example, inactivation of the SC impairs “saccadic suppression” in extrastriate cortical neurons (shutting down of visual processing during saccades to reduce the image blur) 52, target selection from a visual field with multiple distractors 53, detection of subtle changes in the properties of visual cues in the periphery 54, and alters perceptual decision making 11. On the sensory side, single unit recordings suggest that sSC neurons are involved in the detection of salient visual stimuli in the visual scene 55 and spatial frequency, contrast, orientation, and temporal frequency 56, 57. In combination with the retinotopic map, these and many other observations show the retino-colliculo-thalamic pathway, and the tectum/SC specifically, to be involved in the coding of visual space, the detection of salient stimuli, the control of rapid or reflexive visual behavior, and decisions related to action.
In addition to the retino-thalamic pathway and the canonical retino-colliculo-thalamic pathway, recent studies have revealed non-canonical ascending pathways from the tectum/SC that are involved in a range of cognitive processes across a variety of animal species. The pathways from the sSC to the amygdala via the lateral posterior nucleus of the thalamus or the nucleus parabigeminalis play a role in encoding or processing stimuli that induce fear in mice 58, 59. This possibility is reinforced by recent human literature describing a relationship between the SC and fear 60. On the other hand, a direct connection of the tectal output fibers with midbrain dopamine neurons has been found in lampreys 61, rodents 62, cats 63, and monkeys 64 and is considered to be involved in visually triggered reinforcement learning. In terms of the functions of the tectum/SC in cognition, more recent studies have shown that blindsight monkeys, which rely on the SC in visual processing 50, can perform a variety of cognitive tasks such as short term memory 65 and associative learning 66. Moreover, blindsight humans show fear responses to visual cues presented in the V1 lesion-affected visual field 67. These results suggest that the tectum/SC and retino-colliculo-thalamic pathway are not just involved in control of rapid reflexive visual behavior and low-level filtering, but can also process more complex images 68 and may contribute to higher cognitive functions such as top-down attention and decision making, as well as emotional processing.
Evolutionary conservation of the tectum/SC’s position in visual pathways
So when in natural history did these separate visual pathways emerge? Lampreys, among our most distant existing vertebrate relatives, have thalamic nuclei that receive retinal and tectal inputs, and that project to the pallium. Some of the thalamic projection neurons produce a retinotopic (and also a somatosensory) arborization field in the pallium 16, while others project to the striatum 78, 79. A subset of the tectal projection neurons targeting the thalamus also send descending axonal branches to the brainstem to drive behavior 80. Among primitive fish, the longnose gar (Lepisosteus osseusv) has a retinal projection to the nucleus anterior (possibly corresponding to the dLGN and retino-thalamic pathway), as well as retinotectal and tecto-thalamic projections terminating in the dorsal posterior nuclei (corresponding to the pulvinar and canonical retino-colliculo-thalamic pathway) 81. The telencephalic (pallial) targets of the thalamic projection neurons have not been clearly identified. Combined, these observations provide clear evidence for the retino-thalamic and retino-colliculo-thalamic pathways in distant vertebrate lineages (Figure 3).
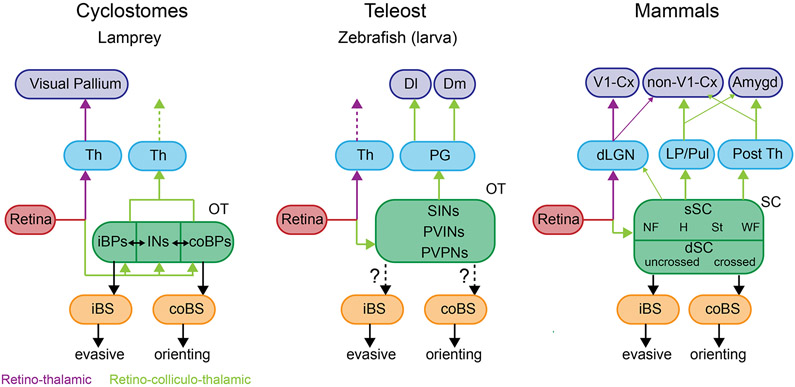
Figure 3: Phylogenetic conservation of brain-wide visual pathways.
Retino-thalamic (violet lines) and retino-collicullo-thalamic (green) lines in the lamprey, zebrafish larva and mammals. Abbreviations: Amygd, amygdala; iBPs, ipsilateral brainstem projecting neurons; coBPs, contralateral brainstem projecting neurons; Cx, cortex ; Dl, dorsolateral subdivision of pallium; Dm, dorsomedial subdivision of pallium; iBS, ipsilateral brainstem; coBS, contralateral brainstem; INs, inhibitory interneurons; Th, thalamus; SINs, GABAergic superficial inhibitory neurons; PVINs, periventricular interneurons; PVPNs, periventricular projection neurons; NF, narrow field vertical cells, H, horizontal cells ; St, stellate cells; WF, wide field vertical cells; OT, optic tract; PG, periglomerular nucleus.
Evidence for these pathways has been inconsistent in the more recently evolved cyprinid fish. Adult goldfish and carp have retino-thalamic projections, but the retinorecipient thalamic nuclei do not contain neurons projecting to the pallium. Furthermore, tecto-thalamic projections have rarely been identified, the one exception being a study by Yamamoto and Ito 82 showing that the ventromedial nucleus (preglomerular nucleus) receives retinal and tectal projections, and sends axons to the dorsomedial (Dm) subdivision of pallium. While studies of adult cyprinids have been equivocal, detailed descriptions of individual neurons in larval zebrafish have recently found stronger correlates of the retino-thalamic and retino-tectal pathways. These include retinorecipient thalamic neurons that project to the pallium 83. A new brain-wide atlas of cellular morphologies across the larval zebrafish brain 84 has revealed neurons in both the ventral and dorsal thalamus projecting to the pallium, and previous work has shown that tectal neurons send projections to both these thalamic areas 85. The differences between the descriptions in adults and larvae could be a function of the species studied, the life stage, or the different methods used. For instance, adult cyprinids almost exclusively use the preglomerular nucleus as a visual relay station, but this nucleus is undifferentiated in larvae. It is also possible that the larval connections are lost during development. Perhaps most likely, the ease with which intact neurons can be observed in small, transparent larvae has allowed a more comprehensive accounting of the connections that exist among the eye, tectum, thalamus, and pallium.
The above results suggest that the fundamentals of the retino-thalamic and retino-colliculo-thalamic pathways are shared across 450 million years of the vertebrate history, spanning from cyclostomes to mammals among modern species. From basic teleosts like the gar, vertebrate evolution has proceeded over many stages to amphibians and higher vertebrates, while the cyprinids and other teleosts were subjected to an additional genome duplication that may have facilitated evolutionary change by allowing for the addition of novel neural circuits 86, 87. Of course, different vertebrate lineages have different ethological landscapes and sensory demands, and the changing structures and roles of the tectum/SC through vertebrate history reflect this. One manifestation of this can be found in the position of the eyes in the head and the corresponding changes in the structure of retinotectal space (Figure 2). Another is in the relative weighting placed on rapid reactions versus cognitive processes. In older lineages and in most modern fish, there is a reliance on the quick reactions provided by the retino-tecto-motor circuit and spatial awareness that comes from the retino-colliculo-thalamic pathway, and a relative de-emphasis of the cognitive sensory functions associated with the retino-thalamic pathway. The extraordinary expansion of the telencephalon in mammals, and primates in particular, may have provided a greater emphasis on cognition in this lineage, and a corresponding weighting toward the retino-thalamic pathway.
The tectum/SC’s intrinsic networks, and the computations that they perform
The tectum/SC’s conserved laminar structure
Tectum/SC receives information regarding the visual world in a spatially arranged retinotopic map. In all vertebrates, there is a similar organization with a superficial visual layer and an intermediate layer containing spatially aligned input from other senses (covered in detail in the next section). A deeper layer contains output neurons that forward motor commands for eye, orienting, or evasive movements 19, 88. This general organization is present in all vertebrates, although the different layers carry different names and have been variably subdivided in different model systems. In addition to the inputs from the different senses, there is input from cortex/pallium both from the frontal eye fields and the visual areas 89, and prominent inhibitory control from the basal ganglia affecting the tectal output layer 79, 90-92. There are also inputs from other midbrain areas and from dopamine, 5-HT, and cholinergic afferents 61, 93-96. These diverse inputs, each with a different role, allow the tectum to receive, process, and integrate sensory information; register it spatially; and direct appropriate behaviors based on the animal’s context. In this section, we will focus on the early steps in the cascade: the processing of visual inputs in the tectum/SC.
The lamprey tectum’s sensory and motor maps and circuit mechanisms
In the lamprey, the retinal ganglion cells projecting into the tectum (Fig 2A) are of four morphologically distinct subtypes that presumably carry different types of information 15. The density of neurons varies across different areas in tectum, with the highest density in the area representing the anterior visual field. In this area, both orienting and evasive responses can be elicited, while in the posterior visual field mostly evasive movements are produced. Importantly, a motor map aligned with the visual map is present at a deeper level among the output neurons. Stimulation of the tectum at the lowest stimulation strength elicits eye-movements, in which the two eyes are coordinated. Small amplitude movements are elicited in the rostral part of tectum, while larger amplitudes are produced more caudally 97. At a somewhat higher strength of stimulation, orienting or evasive movements are elicited, and locomotor movements can be elicited from the caudal region. As discussed in “Sensorimotor transformation, premotor outputs, and behavior selection” below, this regional organization of the tectum is critical for generating the appropriate movements in response to various visual stimuli.
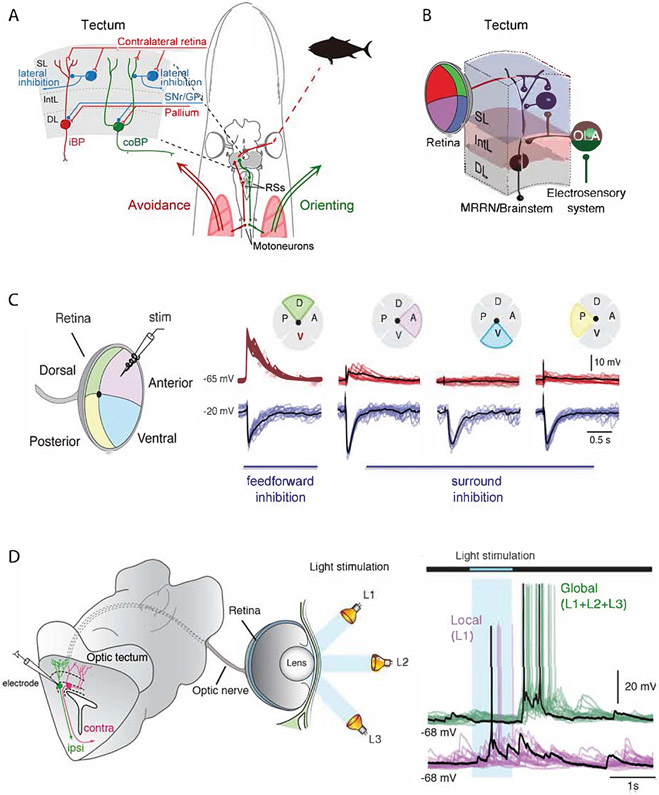
The output neurons in the deep layer are glutamatergic neurons of two kinds. One type, neurons with one stem dendrite extending all the way to the visual layer with an axon projecting to the ipsilateral brainstem’s reticulospinal neurons (iBP in Figure 4A), is involved in evasive movements. The second type instead has two stem dendrites extending to the visual layer and an axon instead projecting to the contralateral brainstem (coBP in Figure 4A) and these are involved in orienting movement as illustrated in Figure 4A 2. Slowly expanding stimuli are sufficient to activate the latter type of neurons (coBP), and an orienting movement will result. On the other hand, rapidly expanding visual stimuli (thus more intense per unit time) will instead elicit an avoidance reaction 3. Thus, the same type of stimulus applied slowly elicits orienting response, but when applied faster instead an avoidance response. One mechanism that can account for this finding is the different membrane properties of these two types of neurons. coBPs have a lower threshold for activation and will start spiking with a stimulus that only gives a subthreshold activation of the iBP neurons 2. Only with a more intense stimulus are the iBPs recruited and fire action potentials that then elicit an avoidance movement. Orienting movements are activated only from the anterior visual field and coBPs are only present in this location, whereas the iBPs that elicit avoidance are present throughout the tectal visual layer, which appears purposeful since threats can originate from any direction. Orienting and evasive movements depend on the tectum and are blocked if processing in tectum is incapacitated by local pharmacological intervention 3, but can of course be gated by signals from the basal ganglia, pallium/cortex and other sources (Figure 4A).
Figure 4: Structure and circuitry of the lamprey tectum.
(A) Drawing of the lamprey head – brain with the circuitry leading to orienting and avoidance responses. In the upper left the tectal circuitry is represented, with the ipsilateral projecting neurons (iBP) underlying avoidance reaction and the contra-laterally projecting neurons(coBP) orienting responses. These neurons have monosynaptic input from retinal afferents and GABAergic interneurons. The output layer has input from both the pallium (cortex) and the substantia nigra pars reticulata (SNr). (B) Diagram illustrating convergence between the retinal input and the electro-sensory input activated from the same point in space. At the distal dendrites the retinal afferents form synapses, while the electro-sensory afferents target the same dendrite but closer to the cell soma. The tectal output neuron targets middle rhombencephalic reticular nucleus (MRRN), located in the brainstem. Abbreviations: SL, superficial layer; IntL, intermediate layer; DL, deep layer; OLA, octavolateral area. (C) Stimulation of retina in one quadrant leads to excitation in tectal neurons in the specific retinotopic projection area in tectum, while stimulation in all other parts of retina instead leads to a strong inhibition mediated by the tectal GABAergic interneurons. The red traces are recorded with a holding potential of −65 mV, and the blue traces at −20 mV, when the inhibition can be seen clearly. There is thus a powerful lateral inhibition. (D) Shows recordings from a lamprey eye-brain preparation in which the tectal output neurons can be patched, while the eye is illuminated with local brief light pulses (blue area) or globally with light on in all parts of retina. The neuron illustrated is excited by the local light (L1), while there is no response when the entire retina is illuminated (global). This illustrates the strong surround inhibition also shown in B. There is, however, a post-inhibitory rebound.
What is the upstream process leading to the activation of the output neurons? A local brief stimulation of the retina with electrodes provides a local discrete activation of the tectum according to the retinotopic map (Figure 4C) and provides monosynaptic activation by the local retinal afferents through synapses onto the dendrites of iBP and coBP 2. The stimulus-evoked EPSPs from the retina are followed by a disynaptic local inhibition elicited by local GABAergic interneurons (Figure 4A, C). Moreover, stimulation of any part of the retina outside the area in tectum receiving monosynaptic retinal input causes only a potent GABAergic inhibition. This shows that there is a marked lateral or surround inhibition in the tectal network. In the visual layer, there is a rich supply of GABAergic interneurons with short and longer axons (Figure 4A) that are responsible for this lateral inhibition. In addition, there are glutamatergic interneurons that presumably contribute to the coordination of the eyes.
What if the retina is activated by light stimuli? If a brief local (1s) light stimulus is applied to the eye while recording from output neurons in the local retinotopic area of the tectum (Figure 4D), there will be a net excitation of the cells in that location, although there is some concurrent inhibition 2. However, if global illumination of the eye occurs instead, the inhibition from surrounding areas will dominate and no excitatory activity is actually produced. After the global illumination has ended, there is, however, a postinhibitory rebound activation. This also means that if two salient stimuli occur simultaneously in different locations on retina, there will be a rivalry between the two, and the most prominent will win and then induce a gaze-shift towards the direction controlled by the particular output neurons that become activated. This is thus a mechanism for selection of motor action at the level of the tectum. Another interesting role for this circuit is the combined processing of multisensory input (Figure 4B), which we will cover in more detail in the next section.
Tectal processing in zebrafish
In larval zebrafish, retinal ganglion cells dominate the inputs to the superficial layers, with at least 20 discrete categories of retinal ganglion cells delivering information on visual stimuli’s motion, orientation, and (through the retinotectal map) position 84, 98. These retinal ganglion cells have distinct molecular profiles and morphologies, and they target specific laminae and are sensitive to particular visual features 30. Deeper retinorecipient layers in the central regions of the neuropil receive luminance information from retinal ganglion cells. Deep laminae of the neuropil receive a smaller number of afferents from diverse sources, including the hypothalamus, thalamus, cerebellum, torus semicircularis (homologous to the inferior colliculus in mammals), serotonergic raphe, the nucleus isthmi, and the contralateral tectum 84, 85, 99-104. These inputs likely carry information from other modalities (auditory and potentially water flow and vestibular), information on satiety levels and other motivational states, and proprioceptive feedback.
With a single continuous neuropil region, the fish tectum has no clear structural separation between its superficial and deep areas, as is seen in mammals. There are, however, numerous superficial-to-deep laminae and sublaminae through which specific types of information flow. The tectal neurons contributing to this presumed information flow include superficial interneurons with broad sharply laminated dendritic structures, interneurons with dendrites spanning multiple superficial laminae and with axons in the deep neuropil, and tectal projection neurons with dendrites restricted to deep layers of the neuropil 105, 106 (Figure 5).
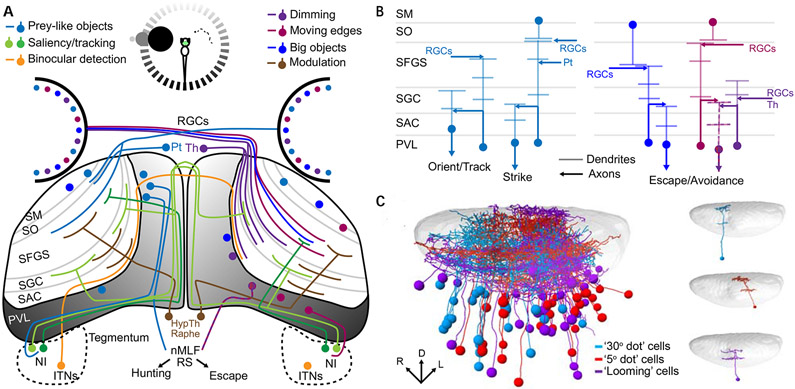
Figure 5: Integral circuitry of the larval zebrafish tectum.
A schematic representation of the larval zebrafish’s tectal inputs and outputs (A) illustrates its role in receiving and integrating information from diverse sources. The inputs are strongly spatial (shading of circle at the top of (A) and of the PVL in the tectum), and differ depending on whether a small stimulus (prey item seen by the right eye) or a large looming stimulus (left eye) is presented. The processing of prey-like stimuli (left tectum, since all retinal ganglion cell axons cross the midline) results in hunting behavior, while looming stimuli (right tectum) trigger escape responses. Inputs from various cell types and brain regions are color coded to indicate the stimulus properties that they encode (legend at the top of A). Different types of information are delivered selectively to different laminae of the tectal neuropil (B), and this dictates the response properties of PVL neurons with dendrites in a specific lamima or laminae. The spatial registration (left, C) of many individual PVL neurons’ morphologies (right, C), taking into account their visual response profiles (colors), reveals the spatial and functional architecture of visual processing in the tectum (Panel C is adapted from Förster et al, 2020 42).
Abbreviations: HypTh: Hypothalamus; ITNs: Intertectal neurons; NI: Nucleus Isthmi; nMLF: nucleus of the medial longitudinal fasciculus; Pt: Pretectum; PVL: Periventricular cell layer; RGCs: Retinal ganglion cells; RS: Reticulospinal neurons; SAC: stratum album centrale; SFGS: stratum fibrosum et griseum superficiale; SGC: stratum griseum centrale; SM: stratum marginale; SO: stratum opticum; Th: Thalamus
The tectum/SC’s inputs, laminar structure, and cellular composition provide a framework by which visual information can be interpreted and contextualized to drive appropriate behaviors. This general layout provides an appealing system in which to characterize the functional microcircuits that perform visual computations in this structure. While these computations have been studied in a range of model systems, the larval zebrafish, with its relatively simple tectum and suitability for microanatomical studies, calcium imaging, and optogenetics, has been an especially effective platform for characterizing the details of information flow through the tectum 42, 85, 107, 108.
In the simple world of the larval zebrafish, survival largely depends on attacking suitable prey items while escaping from predators, and as we have seen for lampreys, the tectum is central to both of these behaviors. Appropriate approach/escape decisions rely on specific information about visual stimuli, such as their size, position, movement, and luminance, and tectal circuits therefore must perform behavioral calculations based on this basic information. Given the visual properties of small prey items, predation involves high-acuity vision and motion detection, and requires the larva to perform extended visual tracking. In contrast to prey items, predators are large and “loom” toward the larva as an expanding object, typically produce a drop in luminance in the relevant portion of the visual field, and require a rapid and dramatic escape response that, while loosely directional, does not have to be tightly spatially targeted.
Both of these behaviors depend on detecting a visual stimulus’ position, motion, and direction. Because retinal ganglion cells axons navigate to particular tectal positions to form a retinotectal map, locations on the retina (and therefore in visual space) are preserved across the rostrocaudal and mediolateral axes of the tectal neuropil 109, 110. As we will describe in subsequent sections, this is an important property of the tectum/SC, as it provides a basis for registering visual space against the spatial properties of other sensory modalities in the deeper layers of the tectum and the dSC. The foundations of motion and direction sensitivity come from the retinal ganglion cells, which deliver information about movement in particular directions to specific laminae in the superficial tectal neuropil 111. These inputs’ laminar specificity allows periventricular layer (PVL) neurons with dendrites in a specific lamina or laminae to respond to motion in particular directions 112, 113. Although the circuit-level details have not been elucidated, these PVL neurons increase direction selectivity from three cardinal axes delivered by retinal ganglion cells to four axes, roughly corresponding to forward, backward, up, and down, represented among the tectal PVL neurons 40. Combined with the positional information that springs from the retinotectal map, this motion and direction sensitivity provides a basis for identifying where an object is and how it is moving 114.
Predatory behavior begins with the identification of a prey item, the gauging of its position and speed, and the decision to pursue it. In larval zebrafish, this perception of prey starts with retinal ganglion cells that respond selectively to objects of a particular size (in visual space on the retina) and speed (in movement across retinal space). Retinal ganglion cells with particular size and speed tuning innervate a subpopulation of pretectal neurons and specific layers of the tectal neuropil, effectively allowing small and large stimuli to activate different tectal layers 115, 116. The size and speed to which these retinal ganglion cells are tuned matches the apparent size and speed of natural prey, and artificial stimuli with these properties are effective at producing hunting routines 117, 118. Presumably by restricting their dendrites to specific laminae, a subset of tectal PVL neurons use this organized input to respond selectively to particular features or combinations of features 41, facilitating the selective response to small or large objects 115, 119. GABAergic superficial inhibitory neurons (SINs), located in the stratum opticum (SO) of the tectal neuropil, have tightly laminated dendritic arbors in specific sublaminae of the neuropil, and are therefore in a position to filter information flow on the basis of object size. Indeed, Preuss and colleagues 115 have shown that SINs are active in response to visual objects of various sizes, but that a given SIN is size-selective, and that this selectivity is tightly coupled to the size selectivity of the retinal ganglion cell axons present in the tectal sublamina containing that SIN’s dendrites. Consistent with this, ablating large-object SINs leads to increased responses to large objects in the deeper non-retinorecipient layers of the tectal neuropil, with a resulting drop in the efficacy of predation 120. Selective inputs from small and large objects, received and processed by PVL neurons, and gated by lamina-specific SINs, therefore, provide a computational framework for the size-selective responses of non-retinorecipient PVL neurons 115, and specifically for the increased emphasis placed on prey-sized objects in the neuropil’s deepest, non-retinorecipient, layers 120. The clear representation of these objects is a prerequisite for coordinating the eye movements required for tracking prey items and the orchestration of pursuit and strike maneuvers. Recent findings suggest that the tectum of larval zebrafish can sample small parts of the visual field and even suppress activity surrounding a preferred portion of it 121.
A recent study combined multiple approaches to elucidate the processing of prey-like stimuli in zebrafish larvae. Förster and colleagues 42 first characterized the profile of responses to multiple visual stimuli in both tectal cells and retinal ganglion cell axons. They found that most tectal cells respond to multiple types of visual stimuli and that they are highly sensitive to object motion. These tectal responses seemed to be produced mainly by multiple types of retinal ganglion cells’ activity profiles (two on average). However, up to 36% of tectal responses could be explained by a single retinal ganglion cell input. Furthermore, there were tectal responses that were poorly explained by the retinal information. These results suggest that tectal cells both combine information already present in the retinal ganglion cells and also perform their own computations. By combining functional imaging and single cell labeling of tectal cells, Förster and colleagues 42 then showed that cells tuned to prey-like objects have distinct positions depending on the size of the object and that their dendrites sample from layers targeted by correspondingly tuned retinal ganglion cells. Finally, they described a group of PVL neurons tuned to large objects (presumably close prey), located in the anterior tectum, which represents the frontal striking-zone of the visual field. Their contributions to predatory behavior were corroborated by ablation experiments, in which the loss of these neurons affected hunting performance.
Once the target prey is located and a hunting sequence is initiated, an interaction between the tectum and the nucleus isthmi facilitates the maintenance of the tracking, probably by selecting the target stimulus based on its saliency relative to other stimuli 103, 104. Finally, another group of GABAergic tegmental neurons that projects to deep layers of the tectal neuropil combine binocular information with commissural connections and are important to identify when the prey is in the striking zone 122. As the tectum grows and becomes more efficient at decoding and transmitting spatial information, a gradual developmental improvement in hunting performance occurs 123. The processing of all of these visual features in the tectum, the interaction between the pretectum and the anterior-ventral tectum, and the tectum’s further connections to premotor areas, therefore, combine to coordinate the hunting behavior 124-126. These premotor and motor elements will be discussed below in “Sensorimotor transformation, premotor outputs, and behavior selection”.
Across a wide range of species, dark looming objects elicit visual escape behavior 127-130. The stimulus’ key properties include its size and motion, involving circuits described above, but also a drop in luminance across the visual field 131. As such, the tectum/SC needs to calculate the combined presence of motion and luminance in order to identify a loom. In the case of larval zebrafish, some of this information is already encoded in retinal ganglion cells as they arrive in the tectal neuropil, including motion- and size-specific retinal ganglion cells that terminate in the superficial and intermediate layers of the neuropil 127, 129, and retinal ganglion cells encoding a drop in luminance (dim-specific retinal ganglion cells) in deeper layers 42, 129. These “off” retinal ganglion cells present different sensitivity profiles and segregate this information in the neuropil, which in turn is received by OFF sensitive tectal cells 132. Additionally, dim-sensitive thalamic neurons project into the deep neuropil layers 102. Since none of these inputs, individually, encodes the presence of a loom, computations must be taking place in the tectum to produce loom-specific responses in PVL neurons (Dunn et al. 2016 127; our unpublished observations). Again, the SINs appear to play an important role in these computations, responding to visual objects growing at particular speeds, and sculpting the activity among PVL neurons to facilitate transmission of loom-relevant information 127. Such information about edges and movement could then be incorporated with luminance information in the deeper layers of the neuropil. Dim-specific projections from the thalamus terminate in the deep neuropil layers and have been shown to contribute to the probability of startle behavior and to be necessary for directional escape 102. This suggests that filtered information on stimulus size and motion from retinorecipient PVLs, combined with dimming information from thalamic projection neurons and retinal ganglion cells, is synthesized in the deeper layers of the neuropil, leading to loom-specific responses in subsequent PVL neurons and providing a basis for downstream behavioral decisions.
The superior colliculus in mammals – neurons, networks, and behavior
As we have seen, the tectum of lampreys and zebrafish larvae differ in their specific inputs and in the fine details of the circuits that carry out visual computations. They nonetheless fill the same role in processing visual information and pivoting this information toward appropriate behavioral responses. As we move to the mammalian superior colliculus, we will see another step up in complexity, reflecting greater communication with the expanded mammalian telencephalon and differences in the sensory landscape. Despite these differences, we will nonetheless find important conservation of the tectum/SC’s structure, sensory processing, and behavioral contributions.
A prerequisite for navigating the visual world is the ability to track objects as they move or to stabilize vision as strong visual flow takes place, and the tectum/SC plays an important role in producing the eye saccades and head movements that allow this tracking and stabilization. The selective delivery of specific types of information to each lamina of the sSC involves visual information from several locations, including the retina, thalamus, and visual cortex. As in the zebrafish tectum, inputs from different visual origins overlap in their laminar distributions within the sSC of mammals, but each shows a unique profile of the laminae innervated, suggesting an as-yet poorly understood logic for the processing of visual information as a whole. These inputs are received by sSC neurons with one of a few basic morphologies, including narrow field vertical (NF), horizontal (H), stellate (St), and wide field vertical (WF) cells (Figure 6). The computations performed by these circuits culminate in the delivery of processed visual information, carried by sSC projection neurons, principally to the dSC and in parallel to the visual thalamus including the lateral posterior nucleus (LP) or pulvinar, and LGN. In earlier studies 133, despite the similar representation of the spatial locations, the direct projection from the sSC to the dSC had been called into question, and neuronal recording studies in monkeys showed that visual bursts in the sSC do not always induce saccade related activity in the dSC. However, from the late 1980’s to 1990’s, direct projections from the sSC to dSC were first demonstrated anatomically 134-136 and then demonstrated physiologically in acute slice preparations from rodents (Fig. 6A1) 137-139. Explaining earlier observations, this sSC-dSC pathway is under suppression of GABAergic inhibition, and removal of the inhibition is necessary for the prominent activation of dSC neurons to the sSC stimulation 138 (Fig. 6A1). The dSC neurons relay the visual inputs from the sSC to the saccade generator circuits in the pontomedullary reticular formation (PMRF) to control saccades.
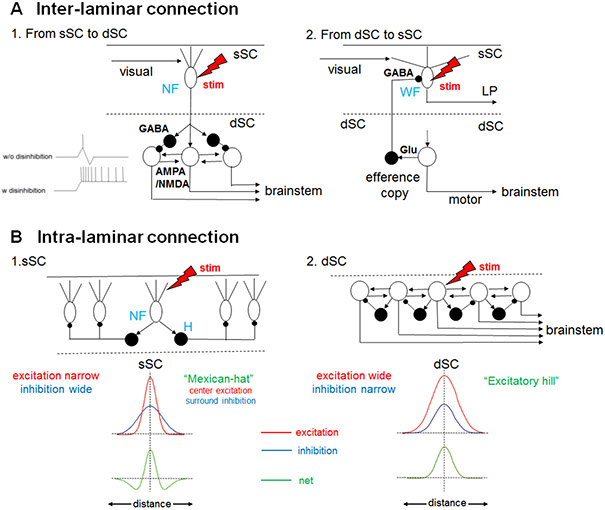
Figure 6: Canonical SC circuits in mammals.
(A) Interlaminar connection. (A1) From the sSC to the dSC. When an electrical stimulus is delivered to narrow field vertical (NF) cells in the sSC (red lightning bolt), excitation is transmitted to brainstem-projecting neurons in the dSC (open circles) through excitatory synapses with AMPA and NMDA receptors. In parallel, GABAergic feedforward inhibition (black circle cells) curtails the excitation as shown in the inset (see the upper inset trace showing intracellular electrophysiological recordings, “w/o disinhibition”) and prevents successive spiking responses upon stimulation of the NF cells. However, if the feedforward GABAergic inhibition is removed, long lasting excitatory responses are induced in the dSC neurons 138 (lower inset trace, “w disinhibition”) through bidirectional reverberating excitatory circuits existing in the dSC (see B2) 145. (A2) Interlaminar connection from the dSC to sSC. The brainstem projection neurons in the dSC (open circle) send collaterals to the GABAergic dSC neurons (black circle), which send axons to the sSC and inhibit WF cells that project to the LP. (Adapted from Phongphanphanee et al. 2014151). (B) Intralaminar connections of the sSC and dSC. (B1) In the sSC, when an electrical stimulus is delivered to the NF cells (red lightening bolt), they excite nearby horizontal cells (H, black circles) which are GABAergic and send horizontal neurites to inhibit remote NF cells. Here, the extent of horizontal excitatory connections (red trace in the upper inset) is narrower than the inhibitory connections (blue trace in the upper inset) and the net effect (green trace in the lower inset) becomes ”Mexican-hat”-like center excitation and surround inhibition in NF cells . Here, “distance” in the inset indicates the medial-lateral distance of the NF cells relative to the stimulated cell. (B2) In the dSC, when an electrical stimulus is delivered to the brainstem-projecting neurons (open circle) the lateral excitation (red trace in the upper inset) is wider than the lateral inhibition in other brainstem-projecting neurons (blue trace in the upper inset) mediated by local GABAergic neurons (black circles), and the net effect (green trace in the lower inset) is an “Excitatory hill”. (Adapted from Phongphanphanee et al. 2014 151).
Thus, the flow of the signals from the sSC to the dSC is gated depending on the level of GABAergic inhibition. The opening or closing of this gate might be reflected in the reaction time of visuomotor responses, and such effects are observed in the distribution of reaction times of visually guided saccades. In the ordinary saccade task in which the target is presented simultaneously with the fixation point offset, the saccadic reaction times are distributed at 150-250 ms in macaques, which are called “regular saccades”. In contrast, when a short time gap (e.g. 200 ms) is inserted between the fixation offset and target onset (gap saccade task), the reaction times are markedly shortened and form a distinct peak around 80 - 120 ms 140. These ultra-short latency saccades are called “express saccades” and are thought to be caused by disengagement of attention toward the fixation point and the predictability of the target location 141. The sSC to dSC pathway is proposed to be involved in the induction of express saccades 142.
On the other hand, dSC neurons, in parallel to sending the output commands to the brainstem and spinal cord to induce saccades or orienting head/body movements, send collateral projections to the GABAergic inhibitory neurons in the same layer. Some of these neurons are known to project to the superficial layer and inhibit the neurons projecting to the visual thalamus (Fig. 6A2). This pathway is thought to suppress the visual inputs during saccades, which prevents blurring of the visual image that saccades would otherwise cause 143. In addition to this inhibitory dSC to sSC pathway, excitatory projections have been reported, which are proposed to play a role in seeing the visual images seamlessly during saccades 144.
The dSC also receives projections from the non-V1 cortex, the SNr, the mesencephalic reticular formation, and the cerebellum, as well as sensory information including proprioceptive feedback and cutaneous and vestibular inputs 4. In order for the dSC neurons to produce their characteristic strong bursting responses to drive saccades or orienting head/body movements, they have to be released from inhibition by GABAergic local interneurons and the outputs of the basal ganglia 90, 145. Relieved from this inhibition, they produce a horizontal excitation which overcomes lateral inhibition to recruit surrounding cells and generate an “excitatory hill” on the saccade vector map in the dSC 146 (Figure 6B2). This may underlie the population coding of saccade vectors by dSC neurons, in which the saccade vector is determined by center of gravity of a large population of dSC neurons that exhibit high frequency presaccadic activity 147.
Several studies during the last decade have revealed the ways in which computations for the detection of salient stimuli are carried out in the mammalian sSC. The wide field (WF) vertical neurons are tuned to small slow-moving objects 148. This selectivity is modulated by horizontal GABAergic neurons that are sensitive to broader visual fields and that inhibit local parts of the dendritic trees of WF neurons 149. These circuits involving the WF neurons appear to be essential for detecting prey 150. The lateral interaction within the sSC was studied in horizontal slice preparations of the sSC, which were obtained by cutting the SC along a horizontal plane containing all the intact visual topology 151. These horizontal slices were placed on multielectrode arrays and electrical stimuli were applied while intracellular potentials were measured by whole cell recordings. In this preparation, NF neurons in the sSC were excited by stimulation of nearby locations while strongly inhibited by the surrounding locations, thus forming a “Mexican hat”-like center excitation-surround inhibition structure that may enhance visual responses in a specific part of the visual field 151. The analysis of population activity using calcium imaging also showed the Mexican-hat like organization in the sSC 152. This modulation is likely due to long range inhibition probably produced by the horizontal cells (“H” in Fig 6B1). In order to orient vision to the prey and then follow and maintain the target while it moves within the visual field, the narrow field (NF) vertical cells and their bidirectional interaction with the parabigeminalis (PBg) nucleus are key in enhancing the contrast through facilitation of lateral inhibition in the sSC 150. These cells have small receptive fields and are particularly tuned to small objects and their directional information 148, 150. Furthermore, they project to the PBg nucleus and intermediate layers of the SC, where premotor neurons driving saccades are located 137, 138, 142, 148.
In mammals, parvalbumin-positive (PV+) cells in the sSC are sensitive to looming stimuli and appear to be involved in visual escape 59, 150. Interestingly, these PV+ neurons are glutamatergic and send projections to the PBg and the lateral posterior nucleus (LP) in the thalamus, but they belong to two distinct populations with different distributions in the sSC layers 153. These distributions indicate that these two categories of PV+ neuron contribute to two parallel pathways, a possibility that is reinforced by their facilitating two opposing types of anti-predator responses. When the PBg pathway is stimulated, the likelihood of an escape response is increased while when the LP pathway is activated, a freezing response is more likely. Both pathways send projections to the amygdala, making it likely that this fear-encoding structure plays a role in both types of behavioral response 58, 59. Further details related to the circuits that involve these PV+ cells are needed to better understand how they process the loom visual information coming from the retina and also a possible role of the projecting horizontal GABAergic neurons of the sSC, as they also seem to be sensitive to large fast objects and project to the thalamus and PBg 148.
In addition to this canonical pathway, the sSC receives neuromodulatory inputs, especially cholinergic inputs from the PBg in rodents 154 and nucleus isthmi in other vertebrate species. Traditionally, the function of the nucleus isthmi has been studied in frogs 155 and birds 156. Lesion in the nucleus ithmi in birds results in impairment of orientation discrimination. In rodents, it has been reported that the sSC and PBg are reciprocally connected in a topographical manner. Cholinergic PBg inputs to the sSC output neurons (NF cells) induce direct excitation in the target neurons, accompanying strong inhibition presumably mediated by nearby GABAergic neurons in the sSC 157-159. These results suggest that the inputs from the nucleus isthmi or PBg enhance the center-excitation and surround-inhibition in the SC circuits for detection of salient stimuli.
As described in this section, the tectum/SC has the necessary microcircuitry to identify both prey items and predators, and elements of these circuits are necessary for the relevant downstream behaviors. At the same time, the products of these computations contain little novel information not already encoded in the retina’s outputs (which include visual objects’ positions, sizes, and movement). So, what value does the tectum add? The answer lies in the tectum/SC’s position in the brain’s broader sensorimotor network. The retinal ganglion cells carry visual information without context, and it is only within the tectum/SC that vision can be registered against the position of the body and eyes, spatial inputs from other sensory modalities, and ethologically important information on the animal’s brain state, motivational state, and recent experience. In the next section, we will discuss how visual information is merged with a diverse array of other inputs in the tectum/SC to produce an integrated and coherent spatial representation of the sensory world.
Multisensory integration of spatial information in tectum/SC
The tectum/SC is at the center of one of the brain’s two major visual pathways, and sensory input to the superficial parts of the tectum/SC is dominated by vision. As a result, this structure is so firmly associated with vision that the unduly specific term “optic tectum” is sometimes still used to describe it. This term, however, belies one of the tectum/SC’s most fundamental contributions: the registration of multiple sensory modalities into a coherent representation of space and movement. In addition to the retinotopic map, other senses provide their own spatial maps, and these maps overlap in the tectum/SC to provide rich information about objects in sensory space. Furthermore, this spatial map is aligned with a motor map that can trigger saccadic eye movements to different points in space or orienting or evasive movements. This integrative role for the tectum/SC is shared among all vertebrates, but it is flexible, and has been adapted to different sensory landscapes and specializations. Below, we will provide examples from diverse vertebrate lineages, each illustrating the integration of a different sensory modality with vision in the tectum/SC.
Spatial integration of vision and electrosensation in cyclostomes
The lamprey, representing the oldest group of extant vertebrates, has a classic layered tectum, with vision represented in the most superficial layer together with predominantly GABAergic, but also glutamatergic interneurons. These visual inputs are represented retinotopically 15, 97. In the next layer, there is input from the octavolateral nerve that conveys electrosensory input from different parts of the animal’s surrounding space 1, 2 (see Figure 4B). In a deeper layer are the output neurons, which have dendrites extending to the superficial visual layer. As we have described above, there are two types of output neuron: those that extend their axons on the ipsilateral side and convey evasive responses and those with contralateral axons that convey orienting responses. The visual input from a given point in space projects to a given location in the retinotopical map in the tectum and activates the tectal output neurons with a monosynaptic linkage, while at the same time activating GABAergic interneurons that inhibit surrounding neurons. Interestingly, the same output neurons that become activated by visual stimuli from a given part in the surrounding space are also activated by the electrosensory input from the same part of surrounding space. The visual and electrosensory stimuli converge to the same neurons and provide mutual facilitation. Conversely, visual and an electrosensory stimuli originating from different parts of the surrounding space will instead inhibit each other. Individual output neurons are thus concerned with a stimulus’ position in space, rather than whether the stimulus originates from vision or electrosensation. As described above for visual inputs in lamprey, the circuitry for deciding whether an orienting or evasive response will result depends on the nature of the stimulus, with rapidly expanding looming stimuli driving evasive movements through ipsilateral projection neurons and slowly expanding stimuli preferentially activating contralaterally projecting neurons to elicit orienting movements 3.
Tectal integration of vision and thermosensation in the rattlesnake
Rattlesnakes survive by catching warm-blooded creatures, and they have evolved a heat-sensing pit organ to assist them with this. Their thermo-sensation provides spatial information about prey, which is integrated with a retinotopic representation in the tectum to provide a coherent representation of the snake’s surroundings 160, 161. The pit organ is innervated by heat-sensitive terminals from the trigeminal nerve, which relays thermal information to the nucleus caloris, which further projects to the contralateral tectum, terminating in a layer ventral to the superficial visual layer 162. Recordings from tectal neurons show that many that were activated by visual stimuli were also activated by heat signatures in the same part of the sensory space, and that copresentation of these stimuli led to facilitation in the tectal neurons. Even in the cases of tectal neurons that only responded to vision or thermo-sensation, copresentation of the off-target stimulus facilitated responses to the detected stimulus. The tectum’s responses to thermal stimuli were spatially selective, being most effective in the anterior visual field where the snake would position them prior to striking. Because the maps for both modalities are registered in the tectum, this arrangement permits the snake to combine visual and thermal information to identify prey items in a complex sensory landscape and under dim light conditions.
Auditory spatial maps allow the barn owl to hunt when it is dark
The barn owl has the astounding ability to hunt down field mice even when it is pitch dark, homing in on its prey purely with auditory information. Mark Konishi and Eric Knudsen have shown that in the tectum of the barn owl, there is a very accurate 3D auditory map of the surrounding space, built on the separate inputs from the two ears. This bi-aural input can create a 3D map since the ears are placed in asymmetric positions. This allows the tectum to calculate the origin of a sound based on the small time difference between when the sound hits the two ears 163-166. Separate neurons in the barn owl tectum respond to different points in 3D space and form a spatial auditory map. As for electro-sensation in lamprey and thermosensation in rattlesnakes, the visual and auditory maps of the surrounding space are aligned in the barn owls, allowing for both modalities to contribute to hunting when light is present 164.
Integration between visual and auditory processing in the mammalian superior colliculus
The integration between vision and auditory stimuli has been studied in considerable detail in the cat 167 and in primates including humans 168, 169. As in other vertebrates, visual and auditory stimuli originating from the same point in space facilitate each other, and conversely, if they originate from different areas in space, they will instead inhibit each other 170. An added complication is that the retinotopic map’s relationship with space is affected by eye movements. As a result, the position of the eye within the orbit has to be taken into account when aligning the auditory spatial map to the retinotectal map 171, 172. This can be handled by utilizing information regarding the efferent commands to the eye muscles, often referred to as an efference copy or corollary discharge. It is debated to what extent afferents from the eye muscles may take part, which may differ between species.
In the previous two sections, we have discussed how visual information is processed within the tectum/SC, and how different sensory modalities are registered to one another spatially to create a single, combined map of the sensory world. The goal of this sensory processing and integration is to provide the necessary sensory information for making behavioral decisions. In the next section, we will discuss how tectal calculations, based on the integrated inputs from multiple sensory modalities, are converted into adaptive behavioral responses.
Sensorimotor transformation, premotor outputs, and behavior selection
As described in previous sections, a variety of bottom-up sensory inputs converge in the tectum/SC, especially in its deeper layers (dSC and deep tectal neuropil). Further top-down attentional or motor commands project to the SC directly from the frontal and parietal cortex, or indirectly through the basal ganglia via the substantia nigra pars reticulata (SNr). In addition, neuromodulator systems such as dopaminergic, cholinergic, noradrenergic, and serotonergic fibers innervate the dSC in mammals. Although they are less well characterized, similar inputs and forms of modulation converge on the deep layers of the tectal neuropil in fish. Thus, the tectum/SC plays a central role in the sensorimotor transformation whereby the nature and position of the stimuli elicit appropriate and spatially targeted responses. Furthermore, the deep tectum and dSC are well positioned to integrate bottom-up, top-down, and neuromodulatory signals to inform behavioral decisions based on the animal’s brain state, risk tolerance, or recent experiences.
Motor control of orienting, approach, and predation
In pioneering studies by Hess and colleagues in 1940-50’s 173, a variety of brain regions in the diencephalon and mesencephalon of the cat were electrically stimulated and behavioral effects were observed. They found that “electrical stimulation of the tectum opticum (superior colliculus) produces a typical and highly specific motor effect consisting of turning of head and eyes to the contralateral side”. The topographical organization of the tectum/SC was first found by Apter 174 by local chemical disinhibition of various locations in the SC combined with global light flashes. The current views on SC function, linking sensory space to movement-related activity, emerged out of a series of studies published in 1972 on behaving monkeys by Robinson, Schiller and Stryker, and by Wurtz and Goldberg. They found that electrical stimulation of a point in the deeper layer of SC drove saccades with a particular vector above a certain stimulus threshold 175, 176. Mapping various locations in SC revealed topographic organization of the saccade vector map in the SC, in which the rostral and caudal SC represent foveal and peripheral visual field, respectively, and medial and lateral SC represent upper and lower visual field, respectively. Furthermore, neurons in the deeper layer were found to exhibit high frequency activity preceding the onset of saccades (presaccadic bursts) 176, 177. Later, it was found that individual SC neurons exhibit presaccadic bursts towards targets in a wide range of directions and eccentricities, which in turn indicates that a saccade of a particular amplitude and direction is generated by the activity of a large number of neurons across the SC map. These observations suggest the population coding of the saccade vector by SC neurons 147. The most rostral part of the SC representing the foveal region were proposed to control either fixation of gaze 178, 179 or microsaccades 180.
Downstream of the SC, sets of saccade-related neurons such as excitatory and inhibitory burst neurons (EBN, IBN), omnipause (OPN), tonic neurons (TN), and neural integrator circuits compose the saccade generator circuits 181, 182 (Figure 7A, reviewed by Sparks 183). One set in the ponto-medullary reticular formation (PMRF) drives the horizontal component and the other in the meso-diencephalic junction (MDJ) (rostral interstitial nucleus of MLF (riMLF) in primates or Forel’s field H (FFH) in cats and the interstitial nucleus of Cajal (INC)) controls the vertical component 184, 185(Figure 7B). It has been shown that the individual SC output neurons have one axon that branches caudally to the PMRF and another that targets the MDJ 186, which may coordinate the horizontal and vertical components of eye movements. On the other hand, a place-coded command signal in the SC is transformed to temporally coded signals carried by the downstream premotor and motoneurons; signals from the rostral SC are transformed to saccade signals with short duration and small amplitude, while those from the caudal SC generates saccades with long duration and large amplitude. This is called the spatio-temporal transformation problem (STTP). Whether STTP is implemented in an intracollicular circuit or in one of the regions downstream of the SC is still unknown. Moschovakis and colleagues have shown that the number of terminal buttons in the PMRF is larger for the caudal SC neurons (coding larger saccades) than the rostral SC neurons (coding smaller saccades), which supports the latter possibility 187.
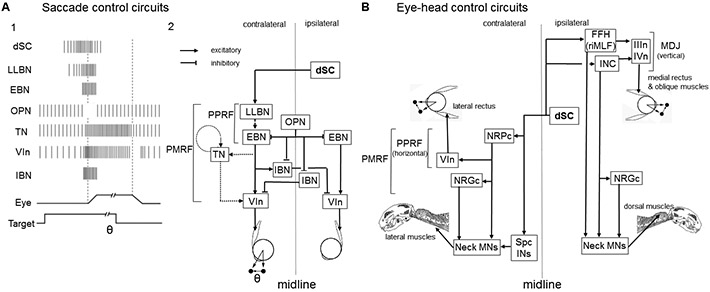
Figure 7: Output pathways from the dSC.
(A) SC output circuits controlling horizontal saccades. (A1) Firing patterns of individual neuron types belonging to the circuits involved in the onset of saccades. The presence of the visual target and movements of the eye are indicated (bottom). (A2) dSC neurons are connected to long-lead burst neurons (LLBNs) and medium-lead excitatory burst neurons (EBNs) in the paramedian pontine reticular formation (PPRF), which transmit the velocity-related high frequency firing activity to abducens motoneuron (VIn). EBN signals are integrated by the tonic neurons (TNs), which relay eye position-related tonic activity to VIn. EBNs are connected to inhibitory burst neurons (IBNs) which further inhibit the VIn on the contralateral side. Panel A2 is adapted from Sparks, 2002 183 and Takahashi and Shinoda, 2018 195. (B) dSC output circuits controlling eye and head movements. dSC neurons send bifurcating axons to the ipsilateral mesodiencephalic junction (MDJ) and contralateral pontomedullary reticular formation (PMRF). The former includes Forel’s field H (FFH) (or rostral interstitial nucleus of MLF (riMLF)) and interstitial nucleus of Cajal (INC). Both of these structures are connected to the oculomotor (IIIn) and trochlear motor (IVn) nuclei to control eye movements and dorsal neck motoneurons (MNs) either directly or indirectly via the nucleus reticularis gigantocellularis (NRGc) to control movements of the head. The latter descending axons are connected to the nucleus reticularis pontis caudalis (NRPc) which is connected to VIn and lateral neck MNs either directly or indirectly via NRGc (horizontal system). Some dSC neurons descend to the spinal cord and connected to MNs via spinal cord interneurons (Spc INs). Panel B is adapted from Isa and Sasaki, 2002 190.
In addition to eye movements, the SC controls head movements during orienting behavior 188. These movements are supposed to be mainly controlled by the reticulospinal neurons in the nucleus reticularis pontis caudalis (NRPc) (horizontal component) and FFH (vertical component) 189, 190 directly or via the reticulospinal neurons in the nucleus reticularis gigantocellularis (NRGc)(Figure 7B). The SC also affects limb movements through reticulospinal or propriospinal neurons, which may underlie the posture during the orienting eye-head turn 191, 192 or the quick adaptation of forearm reaching trajectories 193. Therefore, while the SC’s roles in driving saccades and other eye movements are the best understood mechanistically, the SC appears also to make contributions to the broader orchestration of orienting movements 188, 194.
In zebrafish larvae, the detection of a peripheral prey item can trigger a hunting routine that begins with the convergence of the eyes, which creates a binocular visual field in front of the larva 41, 117. Several lines of evidence suggest that the tectum is involved in these movements. First, optical stimulation of the tectum can produce eye orienting responses 126. Second, there are patterns of activity across assemblies of tectal neurons that do not reflect visual stimuli, but that specifically occur prior to and during convergent saccades, regardless of whether those saccades are visually evoked or spontaneous 41. Finally, there is a group of tectal neurons tuned to prey-like objects in the frontal visual field, and ablation of these neurons reduces the occurrence of predatory pursuit and tracking 42. The circuits responsible for the eye movements themselves have been described 196, but the ways in which tectal information is relayed to the nuclei involved in ocular movements have yet to be fully elucidated.
Beyond the eye convergence that begins the hunting sequence, larval zebrafish display a set of characteristic maneuvers to orient toward, pursue, and eventually strike the prey. These movements follow a sequence that has been described both manually and more recently using unsupervised computational methods 197-201. These maneuvers are coordinated by midbrain and hindbrain premotor neurons that project to the spinal cord 202-204. Among these, the MeLr and the MeLc neurons in the nucleus of the medial longitudinal fasciculus (nMLF) have dendrites in the deep layers of the tectum, and have been specifically implicated in the generation of the orienting tail movements during hunting, likely providing a mechanistic link between tectal processing and eventual behaviors 124.
Distinct motor pathways for approach and avoidance
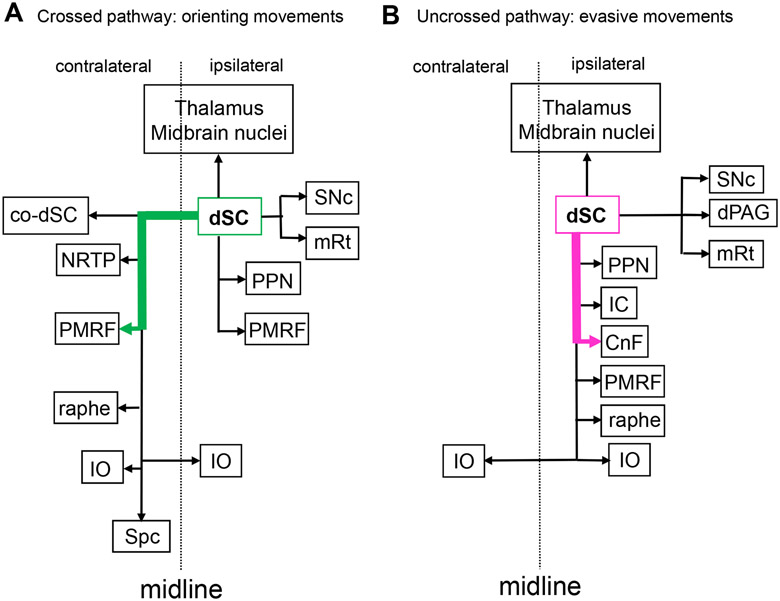
How can animals quickly decide between approach and escape behaviour? In some cases, like the lamprey approach/escape circuits described above, the tectum’s integral circuits, and the physiology of the neurons composing them, are sufficient to guide this decision. In this instance, they permit rapidly expanding visual stimuli to trigger ipsilateral projections to produce escape responses and respond to slowly expanding stimuli with contralateral projections that guide approach.
The arrangement is somewhat more complicated in larval zebrafish, where processing occurs across multiple synapses within the tectum, but behavioral decisions are nonetheless dictated by the downstream structures selectively activated by tectal projection neurons in response to different stimuli. In zebrafish larvae, these projection neurons target the nMLF and reticulospinal neurons 85, 124, 205, 206, which are involved in predatory and escape behavior. These premotor relay neurons then project to the spinal cord, where their outputs are involved in the control of visually triggered motor behaviors 124, 203, 207. A recent study mapping tectal projections to these premotor areas showed different patterns of projecting neurons, including some that project to contralateral reticulospinal neurons and the nMLF, while others innervate ipsilateral reticulospinal neurons 85. This study also found that in the ipsilateral pathway, there is a segregation of the visual information in which looming/threatening visual information seems to be preferentially sent through medial tectobulbar axons. The axons of tectal projection neurons that are sensitive to prey-like stimuli terminate laterally while maintaining the retinotopy, with information from stimuli presented in the posterior visual field traveling more laterally and information from stimuli presented in the anterior visual field going through more medially located axons 85.
Despite the added anatomical complexity found in mammals, the essential ipsilateral/contralateral contributions to escape and approach behavior are maintained. In the 1980’s, Redgrave and colleagues studied these motor pathways by combining electrical stimulation of the SC with ablations in downstream structures 5, 6. They showed that the output pathway from the SC to the contralateral PMRF (crossed pathway) controls orienting head/body turn, while that to the ipsilateral cuneiform nucleus (CnF)(uncrossed pathway) controls defense-like responses such as escape and freezing. More recently, channelrhodopsin-2 (ChR2) was selectively expressed in uncrossed and crossed neurons in mice by combining two viral vectors, one for anterograde transport into the SC, and the second for retrograde transport into the brainstem ipsilateral to the SC injection for ChR2 expression in the uncrossed pathway and in the brainstem contralateral to the SC injection for expression in the crossed pathway 208. Photostimulation of the SC elicited defensive responses in the case of ChR2 expression in the uncrossed pathway, and contraversive orienting head/body turns in the case of the expression in the crossed pathway. As summarized in Figure 8, these two pathways are directed to different target regions in the brainstem and orchestrate the various aspects of orienting and evasive movements, respectively.
Figure 8. Circuits controlling innate visual responses in the mouse.
(A) Projections of the crossed pathway, principally from the dSC to the contralateral PMRF (green), drive orienting movements. In addition to the PMRF, descending collaterals are projected to the contralateral dSC (co-dSC), the precerebellar nuclei such as the nucleus reticularis tegmenti pontis (NRTP) and inferior olive (IO), the raphe, and further down to the spinal cord (Spc). In addition, collaterals are also projected ipsilaterally to the pedunculopontine tegmental nucleus (PPN) and partly to the PRMF in the pons, mesencephalic reticular formation (mRt) and substantia nigra pars compacta (SNc) in the midbrain. Furthermore, ascending axons are projected to several thalamic and midbrain nuclei on the ipsilateral side, some of which might function an efference copy signal. (B) Projections forming the uncrossed pathway (magenta), from the dSC to the ipsilateral cuneiform nucleus (CnF), control evasive movements. Here, collaterals of the descending branch are projected to the PPN, inferior colliculus (IC), PMRF, raphe and IO. In the midbrain, collaterals are projected to the dorsal periaqueductal gray matter (dPAG), SNc and mRt, some of which might be related to the emotional and reward-related aspects of the behavior. Furthermore, ascending axons are projected to several thalamic and midbrain nuclei on the ipsilateral side. Adapted from Dean et al, 1989 5; Sahibzada et al, 1986 6 and Isa et al, 2020 208.
Across diverse vertebrate lineages, additional important information is derived from the region of the tectum/SC (and therefore, of visual space) receiving the information, and on the fine details of the stimulus. In primates, for example, representation of upper visual field in the SC is particularly magnified and saccades are faster and more accurate to this part visual space 209, 210. This reflects the primate’s need to scan the remote environment, which is contained with the upper visual field. In rodents, the orienting (contralateral) pathway mainly originates from the lateral SC encoding the lower visual field, while the escape (ipsilateral) pathway originates from the medial SC, encoding the upper visual field. This is consistent with the ethology of rodents, where predators are expected to come from the above (as a looming stimulus), while the foods or pups are on the ground 211. Such dichotomy of origin of orienting and escape pathway in the SC map has been shown to exist in lamprey in which the orienting stimuli mainly originate from the frontal visual field, while threatening stimuli can be elicited from any part of the visual field 2. In larval zebrafish, the approach/escape decision also hinges on ethologically critical elements of stimuli, including their size (where small visual objects are approached and large objects avoided), their dynamics (where threatening looming stimuli drive escape behavior), and their position in space (like lamprey, with a preference for approaching objects in the frontal visual field and often escaping from objects coming from behind) 42, 85, 121, 126, 127.
In summary, approach/escape decisions hinge on the selective delivery of sensory information to particular layers of the tectum/SC, the intrinsic circuits that process this information within the tectum/SC, the specialization of different tectal/SC regions (corresponding to different sensory spaces), and the selective activation of projection neurons innervating premotor areas responsible for one behavior or the other. Next, we will explore how this sensorimotor network’s computations can be tuned to provide appropriate behavioral outcomes in different situations.
Context-dependent control and modulation of the tectum/SC
Outputs from the tectum/SC must be tightly temporally controlled, and different behaviors are called for in different contexts. For this reason, inputs from additional brain regions, carrying diverse information about sensory and motor context, impinge on tecal/SC processing and on the tectum/SC’s outputs. One of the major inputs that regulates inhibition in the dSC of mice is the GABAergic projection from the substantia nigra pars reticulata (SNr). In the classical studies by Hikosaka and Wurtz 90, SNr inputs to the dSC were found to tonically inhibit the excitatory output neurons from the dSC and inhibition of the SNr neurons preceding the onset of saccades served to disinhibit the dSC circuits, allowing the generation of presaccadic bursts in the dSC. A more recent study by Kaneda and colleagues showed that the SNr also inhibits GABAergic interneurons in the dSC 212, suggesting that the SNr inputs to the dSC may also exert control over SC interneurons. SNr inputs to the dSC may therefore regulate the E/I balance in the dSC network.
In addition to the control by the basal ganglia, several neuromodulator systems innervate the dSC and control its intrinsic processing and generation of motor command signals. First, cholinergic inputs from the peduncolopontine tegmental nucleus and laterodorsal tegmental nucleus project to the intermediate layer and form cholinergic patches. Their primary action on the dSC projection neurons is through excitatory receptors 213, 214, a conclusion supported by the observation that injection of nicotine into the monkey SC shortened the saccadic latency and increased the frequency of express saccades 215. In rodents as well as lamprey, there is a dopaminergic innervation from the SNc to the tectum/SC of the same neurons that innervate the striatum 61, 93. Activation of this dopaminergic projection produces a net enhancement of visual reflexes to oculomotor nuclei and to downstream brainstem structures innervated by the tectum/SC. The locus coeruleus also provides noradrenergic projections to the SC, which enhance looming defensive reactions 94. In contrast, 5-HT projections from the dorsal raphe nucleus to the SC decrease responses to looming stimuli 95.
Neuromodulator systems, like the serotoninergic raphe, can trigger changes in brain states that influence exploratory or hunting behaviours 216. Apart from global modulations, these latter inputs may provide mechanisms whereby the animal’s context influences approach/escape decisions, and a recent study by Filosa and colleagues provides a particularly satisfying example of this in larval zebrafish 99. They found that periods of increased approach/predation and reduced escape behavior correlated with low cortisol levels and relatively little activity in the hypothalamic-pituitary-interrenal (HPI) axis. They then found that starvation led to increased activity in raphe serotonergic neurons that project to the tectum, and that artificially driving activity in these neurons increased approach behavior regardless of hunger levels. Zebrafish larvae generally approach small (prey-like) visual objects and avoid large (predator-like) ones. Filosa and colleagues showed that activity in the HPI axis and in the serotonergic raphe neurons tune the responses of tectal neurons, thereby adjusting the threshold on which this approach/escape decision rests. These artificial modulations match the natural changes in tectal tuning that take place when larvae are starved or satiated, suggesting that it is through this mechanism that hunger state intersects with visual processing. This study, therefore, illustrates how modulation of the core tectal circuit can permit a larva to be appropriately daring when in danger of starvation but risk-averse when satiated.
However, these alone may not be sufficient to make appropriate decision. The top-down signals from the cerebral cortex/pallium may convey the context-dependent information about the environment, and the neuromodulator systems or inputs from the hypothalamus may transmit the intrinsic signals related to emotion or physical status such as hunger, satiety, or fatigue. While many of the important modulatory inputs have been described and partially characterized, much work remains in exploring how animals’ internal states and recent experience mesh with integral tectal/SC circuitry to produce flexible and adaptive behavior.
Conclusions and Future Directions
In this review, we have summarized results from varied experimental approaches and diverse lineages of vertebrates. In total, these studies converge to reveal a conserved role for the tectum/SC in receiving information from multiple senses, in filtering this information to identify salient features, in registering this multisensory information to produce a coherent spatial map of the sensory world, and in driving appropriate downstream premotor circuitry responsible for adaptive behavioral responses. We have highlighted examples of studies that have revealed some of the tectal/SC neurons, connections, and physiology that underlie these calculations, but a great many of the tectum/SC’s circuit-level processes remain enigmatic. For instance, the role of the retino-colliculo-thalamic pathway in the control of visuomotor behavior is critical, and the information conveyed by thalamic neurons to motor structures in the cortex and basal ganglia should be investigated.
Recent years have seen a clear trend suggesting roles for the tectum/SC beyond the simple detection and localization of sensory stimuli and the induction of downstream orienting and evasive responses. Recent studies have shown that cells with similar orientation preferences form large patches that span the vertical thickness of the sSC. Thus, orientation column-like structure have been identified in the SC of rodents 36, 37, although these have not been evident in all studies 217. Furthermore, face-selective SC neurons have been found in primates 218, 219, and the SC has been implicated in processing aversive image and face recognition in primates, including humans 219-222. It has also been reported that some blindsight patients can detect the facial expressions, a phenomenon called “affective blindsight” 67, further suggesting that roles traditionally viewed as cortical can also be performed in the tectum/SC. On the behavioral side, both tectal/SC neuronal activity and downstream behavioral responses have been shown to vary dramatically depending on the cognitive state of the subject 141, the environmental context 208, or the subject’s recent experience 99. Recent rodent studies have shown the induction of fear responses through the SC’s ascending pathways to the amygdala via relay nuclei in the thalamus and midbrain 58, 59, 150, 153. Studies in primates have shown that the SC is involved in complex cognitive processes such as target selection 53, decision making 11, and attentional control 223, presumably involving its ascending projection to the thalamus and cortex. These observations have been corroborated by studies on blindsight monkeys, where the monkeys can conduct a variety of cognitive tasks as long as the stimuli are large and bright 65, 66. Collectively, these new studies challenge the notion of a strict and exclusive dichotomy between the retino-thalamic and retino-colliculo-thalamic pathways, where the former is responsible for cognitive control and the latter for the control of motion. It rather appears that the retino-colliculo-thalamic pathway, and the tectum/SC specifically, are capable of a diverse range of calculations, both overlapping with and distinct from those performed in the cortex/telencephalon.
These two pathways are present in our most distant extant vertebrate relatives, the lampreys, and thus represent a conserved design feature of the vertebrate brain 16. This combination of evolutionary conservation and diversity across the vertebrate lineage presents an opportunity to better understand both the conserved fundamentals of tectal/SC structure and function, and the ways in which its structure and function have changed with diverse sensory landscapes and the emergence of the cortex as an important sensory processing structure in the mammalian lineage. Cross-species comparative studies of the cell types, neural circuit structures, behavioral roles, and cognitive functions of the tectum/SC will help to map out this landscape, and to clarify how the retino-tectal and retino-colliculo-thalamic pathways have interacted and overlapped functionally through evolution. A rapidly accelerating array of methodologies, including single cell RNAseq, CRISPR-mediated targeted transgenesis, a variety of neuroimaging techniques, population-scale calcium imaging, and opto- or chemogenetic manipulations should make such studies increasingly accessible in the coming years. The combination of these emerging methods with electrophysiology and anatomical analysis stands to reveal the microanatomical, microcircuit, and network properties of the tectum/SC, and the ways in which this structure and its function has evolved through time.
Acknowledgements
The helpful comments of Professor Ziad Hafed, Professor Abdel El Manira and Dr Andreas Kardamakis are gratefully acknowledged. We also thank Masatoshi Yoshida, the staff, and the students of Isa lab for helping the literature search. This study was supported by Brain/MINDS grant from AMED/Japan (Nos. 18dm0207020h0005 and 19dm0207093h0001) to T.I. and Grant-in-Aid for Scientific Research by MEXT/Japan (Nos. 22220006, 26112001) to T.I. The support of the Swedish Medical Research Council (VR-M-K2013-62X-03026, VR-M-2015-02816, VR-M-2018-02453 and VR-M-2019-01854 (to J.P.-F)), EU/FP7 Moving Beyond grant ITN-No-316639, European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no.604102 (HBP), EU/Horizon 2020 no.720270 (HBP SGA1), no. 785907 (HBP SGA2) and no. 945539 (HBP SGA3), and the Karolinska Institutet is gratefully acknowledged. E.K.S is supported by an NHMRC Project Grant (APP1066887), a Simons Foundation Research Award (625793), and two ARC Discovery Project Grants (DP140102036 & DP110103612). The research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R01NS118406 to E.K.S. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. E. M-L gratefully acknowledges funding for postgraduate studies provided by the Mexican National Council for Science and Technology (CONACYT) and The University of Queensland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kardamakis AA, Perez-Fernandez J, and Grillner S (2016). Spatiotemporal interplay between multisensory excitation and recruited inhibition in the lamprey optic tectum. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kardamakis AA, Saitoh K, and Grillner S (2015). Tectal microcircuit generating visual selection commands on gaze-controlling neurons. Proc. Natl. Acad. Sci. U. S. A 112, E1956–E1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki DG, Perez-Fernandez J, Wibble T, Kardamakis AA, and Grillner S (2019). The role of the optic tectum for visually evoked orienting and evasive movements. Proc. Natl. Acad. Sci. U. S. A 116, 15272–15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparks DL (1986). Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol. Rev 66, 118–171. [DOI] [PubMed] [Google Scholar]
- 5.Dean P, Redgrave P, and Westby GW (1989). Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 12, 137–147. [DOI] [PubMed] [Google Scholar]
- 6.Sahibzada N, Dean P, and Redgrave P (1986). Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J. Neurosci 6, 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grillner S, and Robertson B (2016). The Basal Ganglia Over 500 Million Years. Curr. Biol 26, R1088–R1100. [DOI] [PubMed] [Google Scholar]
- 8.Hikosaka O, Takikawa Y, and Kawagoe R (2000). Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol. Rev 80, 953–978. [DOI] [PubMed] [Google Scholar]
- 9.Hu F, Kamigaki T, Zhang Z, Zhang S, Dan U, and Dan Y (2019). Prefrontal Corticotectal Neurons Enhance Visual Processing through the Superior Colliculus and Pulvinar Thalamus. Neuron 104, 1141–1152. [DOI] [PubMed] [Google Scholar]
- 10.Distler C, and Hoffmann KP (2015). Direct projections from the dorsal premotor cortex to the superior colliculus in the macaque (macaca mulatta). J. Comp. Neurol 523, 2390–2408. [DOI] [PubMed] [Google Scholar]
- 11.Crapse TB, Lau H, and Basso MA (2018). A Role for the Superior Colliculus in Decision Criteria. Neuron 97, 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drager UC, and Hubel DH (1976). Topography of Visual and Somatosensory Projections to Mouse Superior Colliculus. J. Neurophysiol 39, 91–101. [DOI] [PubMed] [Google Scholar]
- 13.Frost BJ, Wise LZ, Morgan B, and Bird D (1990). Retinotopic Representation of the Bifoveate Eye of the Kestrel (Falco-Sparverius) on the Optic Tectum. Vis. Neurosci 5, 231–239. [DOI] [PubMed] [Google Scholar]
- 14.Cynader M, and Berman N (1972). Receptive-Field Organization of Monkey Superior Colliculus. J. Neurophysiol 35, 187–201. [DOI] [PubMed] [Google Scholar]
- 15.Jones MR, Grillner S, and Robertson B (2009). Selective Projection Patterns from Subtypes of Retinal Ganglion Cells to Tectum and Pretectum: Distribution and Relation to Behavior. J. Comp. Neurol 517, 257–275. [DOI] [PubMed] [Google Scholar]
- 16.Suryanarayana SM, Perez-Fernandez J, Robertson B, and Grillner S (2020). The evolutionary origin of visual and somatosensory representation in the vertebrate pallium. Nature Ecology & Evolution 4, 639–651. [DOI] [PubMed] [Google Scholar]
- 17.Schroder S, and Carandini M (2014). A cortical rein on the tectum's gain. Neuron 84, 6–8. [DOI] [PubMed] [Google Scholar]
- 18.Basso MA, Bickford ME, and Cang J (2021). Unraveling circuits of visual perception and cognition through the superior colliculus. Neuron. 109, 917–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler AB, and Hodos W (2005). Comparative Vertebrate Neuroanatomy: Evolution and Adaptation, 2 Edition, (New Jersey: Wiley; ). [Google Scholar]
- 20.Gaze RM (1958). Binocular Vision in Frogs. Journal of Physiology-London 143, P20–P20. [Google Scholar]
- 21.Jacobson M, and Gaze RM (1964). Types of Visual Response from Single Units in Optic Tectum + Optic Nerve of Goldfish. Q. J. Exp. Physiol. Cogn. Med. Sci 49, 199–209. [DOI] [PubMed] [Google Scholar]
- 22.Diao YC, Wang YK, and Xiao YM (1983). Representation of the binocular visual field in the superior colliculus of the albino rat. Exp. Brain Res 52, 67–72. [DOI] [PubMed] [Google Scholar]
- 23.Coombs J, van der List D, Wang GY, and Chalupa LM (2006). Morphological properties of mouse retinal ganglion cells. Neuroscience 140, 123–136. [DOI] [PubMed] [Google Scholar]
- 24.Völgyi B, Chheda S, and Bloomfield SA (2009). Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J. Comp. Neurol 512, 664–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baden T, Berens P, Franke K, Roman Roson M, Bethge M, and Euler T (2016). The functional diversity of retinal ganglion cells in the mouse. Nature 529, 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badea TC, Cahill H, Ecker J, Hattar S, and Nathans J (2009). Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron 61, 852–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao CA, Li H, Zhang Z, Kiyama T, Panda S, Hattar S, Ribelayga CP, Mills SL, and Wang SW (2014). T-box transcription regulator Tbr2 is essential for the formation and maintenance of Opn4/melanopsin-expressing intrinsically photosensitive retinal ganglion cells. J. Neurosci 34, 13083–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweeney NT, Tierney H, and Feldheim DA (2014). Tbr2 is required to generate a neural circuit mediating the pupillary light reflex. J. Neurosci 34, 5447–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martersteck EM, Hirokawa KE, Evarts M, Bernard A, Duan X, Li Y, Ng L, Oh SW, Ouellette B, Royall JJ, et al. (2017). Diverse Central Projection Patterns of Retinal Ganglion Cells. Cell Rep. 18, 2058–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kölsch Y, Hahn J, Sappington A, Stemmer M, Fernandes AM, Helmbrecht TO, Lele S, Butrus S, Laurell E, Arnold-Ammer I, et al. (2021). Molecular classification of zebrafish retinal ganglion cells links genes to cell types to behavior. Neuron. 109, 645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linden R, and Perry VH (1983). Massive retinotectal projection in rats. Brain Res. 272, 145–149. [DOI] [PubMed] [Google Scholar]
- 32.Hofbauer A, and Dräger UC (1985). Depth segregation of retinal ganglion cells projecting to mouse superior colliculus. J. Comp. Neurol 234, 465–474. [DOI] [PubMed] [Google Scholar]
- 33.Dhande OS, and Huberman AD (2014). Retinal ganglion cell maps in the brain: implications for visual processing. Curr. Opin. Neurobiol 24, 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiller PH, Stryker M, Cynader M, and Berman N (1974). Response characteristics of single cells in the monkey superior colliculus following ablation or cooling of visual cortex. J. Neurophysiol 37, 181–194. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Sarnaik R, Rangarajan K, Liu X, and Cang J (2010). Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J. Neurosci 30, 16573–16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feinberg EH, and Meister M (2015). Orientation columns in the mouse superior colliculus. Nature 519, 229–232. [DOI] [PubMed] [Google Scholar]
- 37.Ahmadlou M, and Heimel JA (2015). Preference for concentric orientations in the mouse superior colliculus. Nat Commun 6, 6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X, Liu M, and Cang J (2014). Visual cortex modulates the magnitude but not the selectivity of looming-evoked responses in the superior colliculus of awake mice. Neuron 84, 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalupa LM, and Rhoades RW (1977). Responses of visual, somatosensory, and auditory neurones in the golden hamster's superior colliculus. J. Physiol 270, 595–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter PR, Lowe AS, Thompson ID, and Meyer MP (2013). Emergent properties of the optic tectum revealed by population analysis of direction and orientation selectivity. J. Neurosci 33, 13940–13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bianco IH, and Engert F (2015). Visuomotor transformations underlying hunting behavior in zebrafish. Curr. Biol 25, 831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forster D, Helmbrecht TO, Mearns DS, Jordan L, Mokayes N, and Baier H (2020). Retinotectal circuitry of larval zebrafish is adapted to detection and pursuit of prey. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiller PH, Malpeli JG, and Schein SJ (1979). Composition of geniculostriate input ot superior colliculus of the rhesus monkey. J. Neurophysiol 42, 1124–1133. [DOI] [PubMed] [Google Scholar]
- 44.Beltramo R, and Scanziani M (2019). A collicular visual cortex: Neocortical space for an ancient midbrain visual structure. Science 363, 64–69. [DOI] [PubMed] [Google Scholar]
- 45.Hubel DH, and Wiesel TN (1959). Receptive fields of single neurones in the cat's striate cortex. J. Physiol 148, 574–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poppel E, Held R, and Frost D (1973). Leter: Residual visual function after brain wounds involving the central visual pathways in man. Nature 243, 295–296. [DOI] [PubMed] [Google Scholar]
- 47.Weiskrantz L, Warrington EK, Sanders MD, and Marshall J (1974). Visual capacity in the hemianopic field following a restricted occipital ablation. Brain 97, 709–728. [DOI] [PubMed] [Google Scholar]
- 48.Mohler CW, and Wurtz RH (1977). Role of striate cortex and superior colliculus in visual guidance of saccadic eye movements in monkeys. J. Neurophysiol 40, 74–94. [DOI] [PubMed] [Google Scholar]
- 49.Rodman HR, Gross CG, and Albright TD (1990). Afferent basis of visual response properties in area MT of the macaque. II. Effects of superior colliculus removal. J. Neurosci 10, 1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kato R, Takaura K, Ikeda T, Yoshida M, and Isa T (2011). Contribution of the retino-tectal pathway to visually guided saccades after lesion of the primary visual cortex in monkeys. Eur. J. Neurosci 33, 1952–1960. [DOI] [PubMed] [Google Scholar]
- 51.Schiller PH, Sandell JH, and Maunsell JH (1987). The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J. Neurophysiol 57, 1033–1049. [DOI] [PubMed] [Google Scholar]
- 52.Berman RA, Cavanaugh J, McAlonan K, and Wurtz RH (2017). A circuit for saccadic suppression in the primate brain. J. Neurophysiol 117, 1720–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McPeek RM, and Keller EL (2004). Deficits in saccade target selection after inactivation of superior colliculus. Nat. Neurosci 7, 757–763. [DOI] [PubMed] [Google Scholar]
- 54.Lovejoy LP, and Krauzlis RJ (2010). Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat. Neurosci 13, 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White BJ, Berg DJ, Kan JY, Marino RA, Itti L, and Munoz DP (2017). Superior colliculus neurons encode a visual saliency map during free viewing of natural dynamic video. Nat Commun 8, 14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen CY, and Hafed ZM (2018). Orientation and Contrast Tuning Properties and Temporal Flicker Fusion Characteristics of Primate Superior Colliculus Neurons. Front Neural Circuits 12, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen CY, Sonnenberg L, Weller S, Witschel T, and Hafed ZM (2018). Spatial frequency sensitivity in macaque midbrain. Nat Commun 9, 2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei P, Liu N, Zhang Z, Liu X, Tang Y, He X, Wu B, Zhou Z, Liu Y, Li J, et al. (2015). Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat Commun 6, 6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shang C, Liu Z, Chen Z, Shi Y, Wang Q, Liu S, Li D, and Cao P (2015). BRAIN CIRCUITS. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science 348, 1472–1477. [DOI] [PubMed] [Google Scholar]
- 60.Almeida I, Soares SC, and Castelo-Branco M (2015). The Distinct Role of the Amygdala, Superior Colliculus and Pulvinar in Processing of Central and Peripheral Snakes. PLoS One 10, e0129949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perez-Fernandez J, Kardamakis AA, Suzuki DG, Robertson B, and Grillner S (2017). Direct Dopaminergic Projections from the SNc Modulate Visuomotor Transformation in the Lamprey Tectum. Neuron 96, 910–924. [DOI] [PubMed] [Google Scholar]
- 62.Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, and Redgrave P (2003). A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat. Neurosci 6, 974–980. [DOI] [PubMed] [Google Scholar]
- 63.McHaffie JG, Jiang H, May PJ, Coizet V, Overton PG, Stein BE, and Redgrave P (2006). A direct projection from superior colliculus to substantia nigra pars compacta in the cat. Neuroscience 138, 221–234. [DOI] [PubMed] [Google Scholar]
- 64.May PJ, McHaffie JG, Stanford TR, Jiang H, Costello MG, Coizet V, Hayes LM, Haber SN, and Redgrave P (2009). Tectonigral projections in the primate: a pathway for pre-attentive sensory input to midbrain dopaminergic neurons. Eur. J. Neurosci 29, 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takaura K, Yoshida M, and Isa T (2011). Neural substrate of spatial memory in the superior colliculus after damage to the primary visual cortex. J. Neurosci 31, 4233–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takakuwa N, Kato R, Redgrave P, and Isa T (2017). Emergence of visually-evoked reward expectation signals in dopamine neurons via the superior colliculus in V1 lesioned monkeys. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morris JS, DeGelder B, Weiskrantz L, and Dolan RJ (2001). Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain 124, 1241–1252. [DOI] [PubMed] [Google Scholar]
- 68.Georgy L, Celeghin A, Marzi CA, Tamietto M, and Ptito A (2016). The superior colliculus is sensitive to gestalt-like stimulus configuration in hemispherectomy patients. Cortex 81, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cowey A, and Stoerig P (1995). Blindsight in monkeys. Nature 373, 247–249. [DOI] [PubMed] [Google Scholar]
- 70.Yoshida M, and Isa T (2015). Signal detection analysis of blindsight in monkeys. Sci. Rep 5, 10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diamond IT, and Hall WC (1969). Evolution of neocortex. Science 164, 251–262. [DOI] [PubMed] [Google Scholar]
- 72.Kinoshita M, Kato R, Isa K, Kobayashi K, Kobayashi K, Onoe H, and Isa T (2019). Dissecting the circuit for blindsight to reveal the critical role of pulvinar and superior colliculus. Nat Commun 10, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmid MC, Mrowka SW, Turchi J, Saunders RC, Wilke M, Peters AJ, Ye FQ, and Leopold DA (2010). Blindsight depends on the lateral geniculate nucleus. Nature 466, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ajina S, and Bridge H (2018). Blindsight relies on a functional connection between hMT+ and the lateral geniculate nucleus, not the pulvinar. PLoS Biol. 16, e2005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ajina S, Pestilli F, Rokem A, Kennard C, and Bridge H (2015). Human blindsight is mediated by an intact geniculo-extrastriate pathway. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu HH, Atapour N, Chaplin TA, Worthy KH, and Rosa MGP (2018). Robust Visual Responses and Normal Retinotopy in Primate Lateral Geniculate Nucleus following Long-term Lesions of Striate Cortex. J. Neurosci 38, 3955–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takakuwa N, Isa K, Onoe H, Takahashi J, and Isa T (2021). Contribution of the Pulvinar and Lateral Geniculate Nucleus to the Control of Visually Guided Saccades in Blindsight Monkeys. J. Neurosci 41, 1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, and Grillner S (2011). Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr. Biol 21, 1081–1091. [DOI] [PubMed] [Google Scholar]
- 79.Stephenson-Jones M, Ericsson J, Robertson B, and Grillner S (2012). Evolution of the basal ganglia: Dual-output pathways conserved throughout vertebrate phylogeny. J. Comp. Neurol 520, 2957–2973. [DOI] [PubMed] [Google Scholar]
- 80.Capantini L, von Twickel A, Robertson B, and Grillner S (2017). The pretectal connectome in lamprey. J. Comp. Neurol 525, 753–772. [DOI] [PubMed] [Google Scholar]
- 81.Northcutt RG, and Butler AB (1980). Projections of the optic tectum in the longnose gar, Lepisosteus osseus. Brain Res. 190, 333–346. [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto N, and Ito H (2008). Visual, lateral line, and auditory ascending pathways to the dorsal telencephalic area through the rostrolateral region of the lateral preglomerular nucleus in cyprinids. J. Comp. Neurol 508, 615–647. [DOI] [PubMed] [Google Scholar]
- 83.Ma M, Ramirez AD, Wang T, Roberts RL, Harmon KE, Schoppik D, Sharma A, Kuang C, Goei SL, Gagnon JA, et al. (2020). Zebrafish dscaml1 Deficiency Impairs Retinal Patterning and Oculomotor Function. J. Neurosci 40, 143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kunst M, Laurell E, Mokayes N, Kramer A, Kubo F, Fernandes AM, Forster D, Dal Maschio M, and Baier H (2019). A Cellular-Resolution Atlas of the Larval Zebrafish Brain. Neuron 103, 21–38. [DOI] [PubMed] [Google Scholar]
- 85.Helmbrecht TO, dal Maschio M, Donovan JC, Koutsouli S, and Baier H (2018). Topography of a Visuomotor Transformation. Neuron 100, 1429–1445. [DOI] [PubMed] [Google Scholar]
- 86.Ito H, Ishikawa Y, Yoshimoto M, and Yamamoto N (2007). Diversity of brain morphology in teleosts: brain and ecological niche. Brain Behav. Evol 69, 76–86. [DOI] [PubMed] [Google Scholar]
- 87.Glasauer SM, and Neuhauss SC (2014). Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. Genomics 289, 1045–1060. [DOI] [PubMed] [Google Scholar]
- 88.Ingle D (1975). Sensorimotor function of the midbrain tectum. II. Classes of visually guided behavior. Neurosci. Res. Program Bull 13, 180–185. [PubMed] [Google Scholar]
- 89.Robinson DL, and Jarvis CD (1974). Superior colliculus neurons studied during head and eye movements of the behaving monkey. J. Neurophysiol 37, 533–540. [DOI] [PubMed] [Google Scholar]
- 90.Hikosaka O, and Wurtz RH (1983). Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J. Neurophysiol 49, 1285–1301. [DOI] [PubMed] [Google Scholar]
- 91.Ocana FM, Suryanarayana SM, Saitoh K, Kardamakis AA, Capantini L, Robertson B, and Grillner S (2015). The Lamprey Pallium Provides a Blueprint of the Mammalian Motor Projections from Cortex. Curr. Biol 25, 413–423. [DOI] [PubMed] [Google Scholar]
- 92.Karabelas AB, and Moschovakis AK (1985). Nigral inhibitory termination on efferent neurons of the superior colliculus: an intracellular horseradish peroxidase study in the cat. J. Comp. Neurol 239, 309–329. [DOI] [PubMed] [Google Scholar]
- 93.Campbell KJ, and Takada M (1989). Bilateral tectal projection of single nigrostriatal dopamine cells in the rat. Neuroscience 33, 311–321. [DOI] [PubMed] [Google Scholar]
- 94.Li L, Feng X, Zhou Z, Zhang H, Shi Q, Lei Z, Shen P, Yang Q, Zhao B, Chen S, et al. (2018). Stress Accelerates Defensive Responses to Looming in Mice and Involves a Locus Coeruleus-Superior Colliculus Projection. Curr. Biol 28, 859–871. [DOI] [PubMed] [Google Scholar]
- 95.Huang L, Yuan T, Tan M, Xi Y, Hu Y, Tao Q, Zhao Z, Zheng J, Han Y, Xu F, et al. (2017). A retinoraphe projection regulates serotonergic activity and looming-evoked defensive behaviour. Nat Commun 8, 14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hall WC, Fitzpatrick D, Klatt LL, and Raczkowski D (1989). Cholinergic innervation of the superior colliculus in the cat. J. Comp. Neurol 287, 495–514. [DOI] [PubMed] [Google Scholar]
- 97.Saitoh K, Menard A, and Grillner S (2007). Tectal control of locomotion, steering, and eye movements in lamprey. J. Neurophysiol 97, 3093–3108. [DOI] [PubMed] [Google Scholar]
- 98.Robles E, Laurell E, and Baier H (2014). The Retinal Projectome Reveals Brain-Area-Specific Visual Representations Generated by Ganglion Cell Diversity. Curr. Biol 24, 2085–2096. [DOI] [PubMed] [Google Scholar]
- 99.Filosa A, Barker AJ, Dal Maschio M, and Baier H (2016). Feeding State Modulates Behavioral Choice and Processing of Prey Stimuli in the Zebrafish Tectum. Neuron 90, 596–608. [DOI] [PubMed] [Google Scholar]
- 100.Heap LA, Goh CC, Kassahn KS, and Scott EK (2013). Cerebellar output in zebrafish: an analysis of spatial patterns and topography in eurydendroid cell projections. Front Neural Circuits 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heap LA, Vanwalleghem GC, Thompson AW, Favre-Bulle I, Rubinsztein-Dunlop H, and Scott EK (2018). Hypothalamic Projections to the Optic Tectum in Larval Zebrafish. Front. Neuroanat 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heap LAL, Vanwalleghem G, Thompson AW, Favre-Bulle IA, and Scott EK (2018). Luminance Changes Drive Directional Startle through a Thalamic Pathway. Neuron 99, 293–301. [DOI] [PubMed] [Google Scholar]
- 103.Henriques PM, Rahman N, Jackson SE, and Bianco IH (2019). Nucleus Isthmi Is Required to Sustain Target Pursuit during Visually Guided Prey-Catching. Curr. Biol 29, 1771–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fernandes AM, Mearns DS, Donovan JC, Larsch J, Helmbrecht TO, Kolsch Y, Laurell E, Kawakami K, Dal Maschio M, and Baier H (2021). Neural circuitry for stimulus selection in the zebrafish visual system. Neuron 109, 805–822. [DOI] [PubMed] [Google Scholar]
- 105.Scott EK, and Baier H (2009). The cellular architecture of the larval zebrafish tectum, as revealed by Gal4 enhancer trap lines. Frontiers in Neural Circuits 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robles E, Smith SJ, and Baier H (2011). Characterization of genetically targeted neuron types in the zebrafish optic tectum. Frontiers in Neural Circuits 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simmich J, Staykov E, and Scott E (2012). Zebrafish as an appealing model for optogenetic studies. Optogenetics: Tools for Controlling and Monitoring Neuronal Activity 196, 145–162. [DOI] [PubMed] [Google Scholar]
- 108.Vanwalleghem GC, Ahrens MB, and Scott EK (2018). Integrative whole-brain neuroscience in larval zebrafish. Curr. Opin. Neurobiol 50, 136–145. [DOI] [PubMed] [Google Scholar]
- 109.Kita EM, Scott EK, and Goodhill GJ (2015). Topographic wiring of the retinotectal connection in zebrafish. Dev. Neurobiol 75, 542–556. [DOI] [PubMed] [Google Scholar]
- 110.Romano SA, Pietri T, Perez-Schuster V, Jouary A, Haudrechy M, and Sumbre G (2015). Spontaneous neuronal network dynamics reveal circuit's functional adaptations for behavior. Neuron 85, 1070–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nikolaou N, Lowe AS, Walker AS, Abbas F, Hunter PR, Thompson ID, and Meyer MP (2012). Parametric functional maps of visual inputs to the tectum. Neuron 76, 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gabriel JP, Trivedi CA, Maurer CM, Ryu S, and Bollmann JH (2012). Layer-Specific Targeting of Direction-Selective Neurons in the Zebrafish Optic Tectum. Neuron 76, 1147–1160. [DOI] [PubMed] [Google Scholar]
- 113.Wang K, Hinz J, Haikala V, Reiff DF, and Arrenberg AB (2019). Selective processing of all rotational and translational optic flow directions in the zebrafish pretectum and tectum. BMC Biol. 17, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Niell CM, and Smith SJ (2005). Functional imaging reveals rapid development of visual response properties in the zebrafish tectum. Neuron 45, 941–951. [DOI] [PubMed] [Google Scholar]
- 115.Preuss Stephanie J., Trivedi Chintan A., vom Berg-Maurer Colette M., Ryu S, and Bollmann Johann H. (2014). Classification of Object Size in Retinotectal Microcircuits. Curr. Biol 24, 2376–2385. [DOI] [PubMed] [Google Scholar]
- 116.Semmelhack JL, Donovan JC, Thiele TR, Kuehn E, Laurell E, and Baier H (2014). A dedicated visual pathway for prey detection in larval zebrafish. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bianco IH, Kampff AR, and Engert F (2011). Prey capture behavior evoked by simple visual stimuli in larval zebrafish. Front. Syst. Neurosci 5, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muto A, Lal P, Ailani D, Abe G, Itoh M, and Kawakami K (2017). Activation of the hypothalamic feeding centre upon visual prey detection. Nat Commun 8, 15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barker AJ, and Baier H (2015). Sensorimotor decision making in the zebrafish tectum. Curr. Biol 25, 2804–2814. [DOI] [PubMed] [Google Scholar]
- 120.Del Bene F, Wyart C, Robles E, Tran A, Looger L, Scott EK, Isacoff EY, and Baier H (2010). Filtering of Visual Information in the Tectum by an Identified Neural Circuit. Science 330, 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang K, Hinz J, Zhang Y, Thiele TR, and Arrenberg AB (2020). Parallel Channels for Motion Feature Extraction in the Pretectum and Tectum of Larval Zebrafish. Cell Rep. 30, 442–453. [DOI] [PubMed] [Google Scholar]
- 122.Gebhardt C, Auer TO, Henriques PM, Rajan G, Duroure K, Bianco IH, and Del Bene F (2019). An interhemispheric neural circuit allowing binocular integration in the optic tectum. Nat Commun 10, 5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Avitan L, Pujic Z, Molter J, McCullough M, Zhu S, Sun B, Myhre AE, and Goodhill GJ (2020). Behavioral Signatures of a Developing Neural Code. Curr. Biol 30, 3352–3363. [DOI] [PubMed] [Google Scholar]
- 124.Gahtan E, Tanger P, and Baier H (2005). Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum. J. Neurosci 25, 9294–9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Antinucci P, Folgueira M, and Bianco IH (2019). Pretectal neurons control hunting behaviour. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fajardo O, Zhu P, and Friedrich RW (2013). Control of a specific motor program by a small brain area in zebrafish. Front Neural Circuits 7, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dunn TW, Gebhardt C, Naumann EA, Riegler C, Ahrens MB, Engert F, and Del Bene F (2016). Neural Circuits Underlying Visually Evoked Escapes in Larval Zebrafish. Neuron 89, 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Preuss T, Osei-Bonsu PE, Weiss SA, Wang C, and Faber DS (2006). Neural representation of object approach in a decision-making motor circuit. J. Neurosci 26, 3454–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Temizer I, Donovan JC, Baier H, and Semmelhack JL (2015). A Visual Pathway for Looming-Evoked Escape in Larval Zebrafish. Curr. Biol 25, 1823–1834. [DOI] [PubMed] [Google Scholar]
- 130.Yilmaz M, and Meister M (2013). Rapid Innate Defensive Responses of Mice to Looming Visual Stimuli. Curr. Biol 23, 2011–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marquez-Legorreta E, Piber M, and Scott EK (2019). Visual Escape in Larval Zebrafish: Stimuli, Circuits, and Behavior. in The behaviour genetics of zebrafish (Danio rerio). (Academic Press; ). [Google Scholar]
- 132.Robles E, Fields NP, and Baier H (2021). The zebrafish visual system transmits dimming information via multiple segregated pathways. J. Comp. Neurol 529, 539–552. [DOI] [PubMed] [Google Scholar]
- 133.Edwards SB (1980). The deep cell layers of the superior colliculus : their reticular characteristics and structural organization. The Reticular Formation Revisited., 193–209. [Google Scholar]
- 134.Behan M, and Appell PP (1992). Intrinsic circuitry in the cat superior colliculus: projections from the superficial layers. J. Comp. Neurol 315, 230–243. [DOI] [PubMed] [Google Scholar]
- 135.Lee P, and Hall WC (1995). Interlaminar connections of the superior colliculus in the tree shrew. II: Projections from the superficial gray to the optic layer. Vis. Neurosci 12, 573–588. [DOI] [PubMed] [Google Scholar]
- 136.Mooney RD, Nikoletseas MM, Hess PR, Allen Z, Lewin AC, and Rhoades RW (1988). The projection from the superficial to the deep layers of the superior colliculus: an intracellular horseradish peroxidase injection study in the hamster. J. Neurosci 8, 1384–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee PH, Helms MC, Augustine GJ, and Hall WC (1997). Role of intrinsic synaptic circuitry in collicular sensorimotor integration. Proc. Natl. Acad. Sci. U. S. A 94, 13299–13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Isa T, Endo T, and Saito Y (1998). The visuo-motor pathway in the local circuit of the rat superior colliculus. J. Neurosci 18, 8496–8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Helms MC, Ozen G, and Hall WC (2004). Organization of the intermediate gray layer of the superior colliculus. I. Intrinsic vertical connections. J. Neurophysiol 91, 1706–1715. [DOI] [PubMed] [Google Scholar]
- 140.Fischer B, and Boch R (1983). Saccadic eye movements after extremely short reaction times in the monkey. Brain Res. 260, 21–26. [DOI] [PubMed] [Google Scholar]
- 141.Dorris MC, Paré M, and Munoz DP (1997). Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J. Neurosci 17, 8566–8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Isa T, and Hall WC (2009). Exploring the superior colliculus in vitro. J. Neurophysiol 102, 2581–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Phongphanphanee P, Mizuno F, Lee PH, Yanagawa Y, Isa T, and Hall WC (2011). A circuit model for saccadic suppression in the superior colliculus. J. Neurosci 31, 1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ghitani N, Bayguinov PO, Vokoun CR, McMahon S, Jackson MB, and Basso MA (2014). Excitatory synaptic feedback from the motor layer to the sensory layers of the superior colliculus. J. Neurosci 34, 6822–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saito Y, and Isa T (2003). Local excitatory network and NMDA receptor activation generate a synchronous and bursting command from the superior colliculus. J. Neurosci 23, 5854–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saito Y, and Isa T (2004). Laminar specific distribution of lateral excitatory connections in the rat superior colliculus. J. Neurophysiol 92, 3500–3510. [DOI] [PubMed] [Google Scholar]
- 147.Lee C, Rohrer WH, and Sparks DL (1988). Population coding of saccadic eye movements by neurons in the superior colliculus. Nature 332, 357–360. [DOI] [PubMed] [Google Scholar]
- 148.Gale SD, and Murphy GJ (2014). Distinct representation and distribution of visual information by specific cell types in mouse superficial superior colliculus. J. Neurosci 34, 13458–13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gale SD, and Murphy GJ (2016). Active Dendritic Properties and Local Inhibitory Input Enable Selectivity for Object Motion in Mouse Superior Colliculus Neurons. J. Neurosci 36, 9111–9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hoy JL, Bishop HI, and Niell CM (2019). Defined Cell Types in Superior Colliculus Make Distinct Contributions to Prey Capture Behavior in the Mouse. Curr. Biol 29, 4130–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Phongphanphanee P, Marino RA, Kaneda K, Yanagawa Y, Munoz DP, and Isa T (2014). Distinct local circuit properties of the superficial and intermediate layers of the rodent superior colliculus. Eur. J. Neurosci 40, 2329–2343. [DOI] [PubMed] [Google Scholar]
- 152.Kasai M, and Isa T (2016). Imaging population dynamics of surround suppression in the superior colliculus. Eur. J. Neurosci 44, 2543–2556. [DOI] [PubMed] [Google Scholar]
- 153.Shang C, Chen Z, Liu A, Li Y, Zhang J, Qu B, Yan F, Zhang Y, Liu W, Liu Z, et al. (2018). Divergent midbrain circuits orchestrate escape and freezing responses to looming stimuli in mice. Nat Commun 9, 1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Graybiel AM (1978). A satellite system of the superior colliculus: the parabigeminal nucleus and its projections to the superficial collicular layers. Brain Res. 145, 365–374. [DOI] [PubMed] [Google Scholar]
- 155.Caine HS, and Gruberg ER (1985). Ablation of nucleus isthmi leads to loss of specific visually elicited behaviors in the frog Rana pipiens. Neurosci. Lett 54, 307–312. [DOI] [PubMed] [Google Scholar]
- 156.Knudsen EI (2018). Neural Circuits That Mediate Selective Attention: A Comparative Perspective. Trends Neurosci. 41, 789–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee PH, Schmidt M, and Hall WC (2001). Excitatory and inhibitory circuitry in the superficial gray layer of the superior colliculus. J. Neurosci 21, 8145–8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Endo T, Yanagawa Y, Obata K, and Isa T (2005). Nicotinic acetylcholine receptor subtypes involved in facilitation of GABAergic inhibition in mouse superficial superior colliculus. J. Neurophysiol 94, 3893–3902. [DOI] [PubMed] [Google Scholar]
- 159.Tokuoka K, Kasai M, Kobayashi K, and Isa T (2020). Anatomical and electrophysiological analysis of cholinergic inputs from the parabigeminal nucleus to the superficial superior colliculus. J. Neurophysiol 124, 1968–1985. [DOI] [PubMed] [Google Scholar]
- 160.Hartline PH, Kass L, and Loop MS (1978). Merging of Modalities in Optic Tectum - Infrared and Visual Integration in Rattlesnakes. Science 199, 1225–1229. [DOI] [PubMed] [Google Scholar]
- 161.Newman EA, and Hartline PH (1981). Integration of Visual and Infrared Information in Biomodal Neurons of the Rattlesnake Optic Tectum. Science 213, 789–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stanford LR, and Hartline PH (1984). Spatial and Temporal Integration in Primary Trigeminal Nucleus of Rattlesnake Infrared System. J. Neurophysiol 51, 1077–1090. [DOI] [PubMed] [Google Scholar]
- 163.Knudsen EI, and Konishi M (1978). Neural Map of Auditory Space in Owl. Science 200, 795–797. [DOI] [PubMed] [Google Scholar]
- 164.Knudsen EI (1982). Auditory and Visual Maps of Space in the Optic Tectum of the Owl. J. Neurosci 2, 1177–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Konishi M (1993). Listening with 2 Ears. Sci. Am 268, 66–73. [DOI] [PubMed] [Google Scholar]
- 166.Carr CE, and Konishi M (1990). A Circuit for Detection of Interaural Time Differences in the Brain-Stem of the Barn Owl. J. Neurosci 10, 3227–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stein BE, and Stanford TR (2008). Multisensory integration: current issues from the perspective of the single neuron. Nature Reviews Neuroscience 9, 255–266. [DOI] [PubMed] [Google Scholar]
- 168.Wang ZY, Yu LP, Xu JH, Stein BE, and Rowland BA (2020). Experience Creates the Multisensory Transform in the Superior Colliculus. Front. Integr. Neurosci 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.White BJ, and Munoz DP (2011). Separate Visual Signals for Saccade Initiation during Target Selection in the Primate Superior Colliculus. J. Neurosci 31, 1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Meredith MA, and Stein BE (1986). Visual, Auditory, and Somatosensory Convergence on Cells in Superior Colliculus Results in Multisensory Integration. J. Neurophysiol 56, 640–662. [DOI] [PubMed] [Google Scholar]
- 171.Jay MF, and Sparks DL (1984). Auditory Receptive-Fields in Primate Superior Colliculus Shift with Changes in Eye Position. Nature 309, 345–347. [DOI] [PubMed] [Google Scholar]
- 172.Peck CK, Baro JA, and Warder SM (1995). Effects of Eye Position on Saccadic Eye-Movements and on the Neuronal Responses to Auditory and Visual-Stimuli in Cat Superior Colliculus. Exp. Brain Res 103, 227–242. [DOI] [PubMed] [Google Scholar]
- 173.Hess WR (1954). Diencephalon: autonomic and extrapyramidal functions, (New York: Grune & Stratton; ). [Google Scholar]
- 174.Apter JT (1946). Eye movements following strychninization of the superior colliculus of cats. J. Neurophysiol 9, 73–86. [DOI] [PubMed] [Google Scholar]
- 175.Robinson DA (1972). Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 12, 1795–1808. [DOI] [PubMed] [Google Scholar]
- 176.Schiller PH, and Stryker M (1972). Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J. Neurophysiol 35, 915–924. [DOI] [PubMed] [Google Scholar]
- 177.Wurtz RH, and Goldberg ME (1972). Activity of superior colliculus in behaving monkey. 3. Cells discharging before eye movements. J. Neurophysiol 35, 575–586. [DOI] [PubMed] [Google Scholar]
- 178.Munoz DP, and Wurtz RH (1993). Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J. Neurophysiol 70, 576–589. [DOI] [PubMed] [Google Scholar]
- 179.Munoz DP, and Wurtz RH (1993). Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J. Neurophysiol 70, 559–575. [DOI] [PubMed] [Google Scholar]
- 180.Hafed ZM, Goffart L, and Krauzlis RJ (2009). A neural mechanism for microsaccade generation in the primate superior colliculus. Science 323, 940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Luschei ES, and Fuchs AF (1972). Activity of brain stem neurons during eye movements of alert monkeys. J. Neurophysiol 35, 445–461. [DOI] [PubMed] [Google Scholar]
- 182.Raybourn MS, and Keller EL (1977). Colliculoreticular organization in primate oculomotor system. J. Neurophysiol 40, 861–878. [DOI] [PubMed] [Google Scholar]
- 183.Sparks DL (2002). The brainstem control of saccadic eye movements. Nat. Rev. Neurosci 3, 952–964. [DOI] [PubMed] [Google Scholar]
- 184.Büttner U, Büttner-Ennever JA, and Henn V (1977). Vertical eye movement related unit activity in the rostral mesencephalic reticular formation of the alert monkey. Brain Res. 130, 239–252. [DOI] [PubMed] [Google Scholar]
- 185.Moschovakis AK, Scudder CA, and Highstein SM (1991). Structure of the primate oculomotor burst generator. I. Medium-lead burst neurons with upward on-directions. J. Neurophysiol 65, 203–217. [DOI] [PubMed] [Google Scholar]
- 186.Grantyn A, and Grantyn R (1982). Axonal patterns and sites of termination of cat superior colliculus neurons projecting in the tecto-bulbo-spinal tract. Exp. Brain Res 46, 243–256. [DOI] [PubMed] [Google Scholar]
- 187.Moschovakis AK, Kitama T, Dalezios Y, Petit J, Brandi AM, and Grantyn AA (1998). An anatomical substrate for the spatiotemporal transformation. J. Neurosci 18, 10219–10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Masullo L, Mariotti L, Alexandre N, Freire-Pritchett P, Boulanger J, and Tripodi M (2019). Genetically Defined Functional Modules for Spatial Orienting in the Mouse Superior Colliculus. Curr. Biol 29, 2892–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Grantyn A, Ong-Meang Jacques V, and Berthoz A (1987). Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat. II. Morphological properties as revealed by intra-axonal injections of horseradish peroxidase. Exp. Brain Res 66, 355–377. [DOI] [PubMed] [Google Scholar]
- 190.Isa T, and Sasaki S (2002). Brainstem control of head movements during orienting; organization of the premotor circuits. Prog. Neurobiol 66, 205–241. [DOI] [PubMed] [Google Scholar]
- 191.Alstermark B, Lundberg A, Pinter M, and Sasaki S (1987). Long C3-C5 propriospinal neurones in the cat. Brain Res. 404, 382–388. [DOI] [PubMed] [Google Scholar]
- 192.Iwamoto Y (1990). Disynaptic tectal and pyramidal excitation of hindlimb motoneurons mediated by pontine reticulospinal neurons in the cat. Exp. Brain Res 79, 175–186. [DOI] [PubMed] [Google Scholar]
- 193.Courjon JH, Olivier E, and Pélisson D (2004). Direct evidence for the contribution of the superior colliculus in the control of visually guided reaching movements in the cat. J. Physiol 556, 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wilson JJ, Alexandre N, Trentin C, and Tripodi M (2018). Three-Dimensional Representation of Motor Space in the Mouse Superior Colliculus. Curr. Biol 28, 1744–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Takahashi M, and Shinoda Y (2018). Brain Stem Neural Circuits of Horizontal and Vertical Saccade Systems and their Frame of Reference. Neuroscience 392, 281–328. [DOI] [PubMed] [Google Scholar]
- 196.Brysch C, Leyden C, and Arrenberg AB (2019). Functional architecture underlying binocular coordination of eye position and velocity in the larval zebrafish hindbrain. BMC Biol. 17, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Budick SA, and O'Malley DM (2000). Locomotor repertoire of the larval zebrafish: Swimming, turning and prey capture. J. Exp. Biol 203, 2565–2579. [DOI] [PubMed] [Google Scholar]
- 198.McClenahan P, Troup M, and Scott EK (2012). Fin-Tail Coordination during Escape and Predatory Behavior in Larval Zebrafish. PLoS One 7, e32295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Marques JC, Lackner S, Felix R, and Orger MB (2018). Structure of the Zebrafish Locomotor Repertoire Revealed with Unsupervised Behavioral Clustering. Curr. Biol 28, 181–195. [DOI] [PubMed] [Google Scholar]
- 200.Johnson RE, Linderman S, Panier T, Wee CL, Song E, Herrera KJ, Miller A, and Engert F (2020). Probabilistic Models of Larval Zebrafish Behavior Reveal Structure on Many Scales. Curr. Biol 30, 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mearns DS, Donovan JC, Fernandes AM, Semmelhack JL, and Baier H (2020). Deconstructing Hunting Behavior Reveals a Tightly Coupled Stimulus-Response Loop. Curr. Biol 30, 54–69. [DOI] [PubMed] [Google Scholar]
- 202.Kimmel CB, Powell SL, and Metcalfe WK (1982). Brain Neurons Which Project to the Spinal-Cord in Young Larvae of the Zebrafish. J. Comp. Neurol 205, 112–127. [DOI] [PubMed] [Google Scholar]
- 203.Orger MB, Kampff AR, Severi KE, Bollmann JH, and Engert F (2008). Control of visually guided behavior by distinct populations of spinal projection neurons. Nat. Neurosci 11, 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Thiele TR, Donovan JC, and Baier H (2014). Descending Control of Swim Posture by a Midbrain Nucleus in Zebrafish. Neuron 83, 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sato T, Hamaoka T, Aizawa H, Hosoya T, and Okamoto H (2007). Genetic single-cell mosaic analysis implicates ephrinB2 reverse signaling in projections from the posterior tectum to the hindbrain in zebrafish. J. Neurosci 27, 5271–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yao Y, Li X, Zhang B, Yin C, Liu Y, Chen W, Zeng S, and Du J (2016). Visual Cue-Discriminative Dopaminergic Control of Visuomotor Transformation and Behavior Selection. Neuron 89, 598–612. [DOI] [PubMed] [Google Scholar]
- 207.Severi KE, Portugues R, Marques JC, O'Malley DM, Orger MB, and Engert F (2014). Neural Control and Modulation of Swimming Speed in the Larval Zebrafish. Neuron 83, 692–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Isa K, Sooksawate T, Kobayashi K, Kobayashi K, Redgrave P, and Isa T (2020). Dissecting the Tectal Output Channels for Orienting and Defense Responses. eNeuro 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hafed ZM, and Chen CY (2016). Sharper, Stronger, Faster Upper Visual Field Representation in Primate Superior Colliculus. Curr. Biol 26, 1647–1658. [DOI] [PubMed] [Google Scholar]
- 210.Hafed ZM, and Goffart L (2020). Gaze direction as equilibrium: more evidence from spatial and temporal aspects of small-saccade triggering in the rhesus macaque monkey. J. Neurophysiol 123, 308–322. [DOI] [PubMed] [Google Scholar]
- 211.Comoli E, Das Neves Favaro P, Vautrelle N, Leriche M, Overton PG, and Redgrave P (2012). Segregated anatomical input to sub-regions of the rodent superior colliculus associated with approach and defense. Front. Neuroanat 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kaneda K, Isa K, Yanagawa Y, and Isa T (2008). Nigral inhibition of GABAergic neurons in mouse superior colliculus. J. Neurosci 28, 11071–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sooksawate T, and Isa T (2006). Properties of cholinergic responses in neurons in the intermediate grey layer of rat superior colliculus. Eur. J. Neurosci 24, 3096–3108. [DOI] [PubMed] [Google Scholar]
- 214.Stubblefield EA, Thompson JA, and Felsen G (2015). Optogenetic cholinergic modulation of the mouse superior colliculus in vivo. J. Neurophysiol 114, 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Aizawa H, Kobayashi Y, Yamamoto M, and Isa T (1999). Injection of nicotine into the superior colliculus facilitates occurrence of express saccades in monkeys. J. Neurophysiol 82, 1642–1646. [DOI] [PubMed] [Google Scholar]
- 216.Marques JC, Li M, Schaak D, Robson DN, and Li JM (2020). Internal state dynamics shape brainwide activity and foraging behaviour. Nature 577, 239–243. [DOI] [PubMed] [Google Scholar]
- 217.Chen H, Savier EL, DePiero VJ, and Cang J (2021). Lack of Evidence for Stereotypical Direction Columns in the Mouse Superior Colliculus. J. Neurosci 41, 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nguyen MN, Nishimaru H, Matsumoto J, Van Le Q, Hori E, Maior RS, Tomaz C, Ono T, and Nishijo H (2016). Population Coding of Facial Information in the Monkey Superior Colliculus and Pulvinar. Front. Neurosci 10, 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Le QV, Le QV, Nishimaru H, Matsumoto J, Takamura Y, Hori E, Maior RS, Tomaz C, Ono T, and Nishijo H (2020). A Prototypical Template for Rapid Face Detection Is Embedded in the Monkey Superior Colliculus. Front. Syst. Neurosci 14, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.McFadyen J, Mattingley JB, and Garrido MI (2019). An afferent white matter pathway from the pulvinar to the amygdala facilitates fear recognition. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.McFadyen J, Dolan RJ, and Garrido MI (2020). The influence of subcortical shortcuts on disordered sensory and cognitive processing. Nat. Rev. Neurosci 21, 264–276. [DOI] [PubMed] [Google Scholar]
- 222.Wang YC, Bianciardi M, Chanes L, and Satpute AB (2020). Ultra High Field fMRI of Human Superior Colliculi Activity during Affective Visual Processing. Sci. Rep 10, 1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zénon A, and Krauzlis RJ (2012). Attention deficits without cortical neuronal deficits. Nature 489, 434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]