Abstract
Low dose ionizing radiation (LDIR) is known to have a protective effect on atherosclerosis in rodent studies, but how it impacts different cells types involved in lesion formation remains incompletely understood. We investigated the immunomodulatory response of different doses and dose-rates of irradiation in ApoE-/- mice. Mice were exposed to external γ rays at very low (1.4 mGy.h-1) or low (50 mGy.h-1) dose-rates, with cumulative doses spanning 50 to 1000 mGy. Flow cytometry of circulating cells revealed a significant decrease in pro-inflammatory Ly6CHi monocytes at all cumulative doses at low dose-rate, but more disparate effects at very low dose-rate with reductions in Ly6CHi cells at doses of 50, 100 and 750 mGy only. In contrast, Ly6CLo monocytes were not affected by LDIR. Similarly, proportions of CD4+ T cell subsets in the spleen did not differ between irradiated mice and non-irradiated controls, whether assessing CD25+FoxP3+ regulatory or CD69+ activated lymphocytes. In the aorta, gene expression of cytokines such as IL-1 and TGF-ß and adhesion molecules such as E-Selectin, ICAM-1, and VCAM-1 were reduced at the intermediate dose of 200 mGy. These results suggest that LDIR may reduce atherosclerotic plaque formation by selectively reducing blood pro-inflammatory monocytes and by impairing adhesion molecule expression and inflammatory processes in the vessel wall. In contrast, splenic T lymphocytes were not affected by LDIR. Furthermore, some responses to irradiation were nonlinear; reductions in aortic gene expression were significant at intermediate doses, but not at either highest or lowest doses. This work furthers our understanding of the impact of LDIR with different dose-rates on immune system response in the context of atherosclerosis.
Keywords: Low dose, dose-rate, immune system, atherosclerosis
Introduction
Despite multiple studies investigating the health impacts of ionizing radiation, questions remain regarding the effects of exposure on cardiovascular diseases. Both epidemiological 1 -4 and experimental studies 5 -11 revealed that exposure to high doses of ionizing radiation has detrimental cardiovascular outcomes. However, when it comes to low dose ionizing radiation (LDIR), epidemiological studies have not provided a clear message. For instance Schöllnberger et al 12 did not resolve whether or not there is a risk of heart disease for individuals exposed to doses below 2.6 Gy, and Azizova et al 13 showed an increased risk of developing ischemic heart disease for cumulative external doses above 1 Gy. There remains a lack of epidemiological data on the impact of radiation on cardiovascular diseases after exposure to low to moderate doses, especially under 500 mGy. 14
Differing from the paucity of epidemiological information linking LDIR and cardiovascular diseases, several experimental studies have investigated this matter, revealing the importance of considering the dose-rate of radiation exposure. Such studies have been conducted mainly on mouse models of atherosclerosis, using genetically modified ApoE-/- mice which become hyperlipidemic and develop atherosclerotic lesions similar to those found in humans when fed a chow diet. 15 Atherosclerosis is a disease characterized by chronic and exacerbated inflammation in the wall of medium and large arteries, due to lipoprotein influx. Excessive growth or rupture of atherosclerotic plaques underlies most cardiovascular diseases. Mitchel et al 16 demonstrated that exposure to LDIR, especially at low dose-rate, slowed plaque progression in mice. Mancuso et al 17 indicated that acute irradiation at moderate doses (300 mGy) can have detrimental effects on atherosclerosis, but that chronic exposure to the same dose has less impact. Finally, both Le Gallic et al 18 and Ebrahimian et al 19 found that exposure to chronic internal LDIR enhances plaque stability in ApoE-/- mice. These latter 2 studies even showed a decrease in inflammatory parameters after exposure to LDIR, including diminished plaque content of CD68+ foam cells and a shift in aortic mRNA expression favoring anti- rather than pro-inflammatory cytokines. 18,19 These data corroborate the notion that ionizing radiation has an impact in the immune system, with high doses promoting inflammation 20 and proinflammatory macrophages, 21 whereas lower doses lead to decreased inflammation. 22,23
Nevertheless, there is little information available regarding the impact of LDIR on monocytes and lymphocytes that contribute to atherosclerosis lesion formation in the first place. One study reported that in vitro irradiation of RAW264.7 monocytes/macrophages altered the binding of these cells to vascular cell adhesion molecule-1 (VCAM-1), 24 confirming a previous work demonstrating that LDIR reduced adhesion of monocytes to the endothelium. 25 It was also determined that LDIR affects cytokine gene expression by different T cells. 26 Only 1 study explored the effects of LDIR on white and red blood cell populations in the context of atherosclerosis, 19 but specific monocyte and lymphocyte subgroups were not investigated. This represents a significant knowledge gap, since monocytes and T cells can polarize into distinct classes that are considered to be either pro-inflammatory or anti-inflammatory, with potentially opposing effects on atherosclerosis. 27,28
Hence, identifying how low doses of irradiation can modulate the inflammatory response in atherosclerosis is clearly essential. In order to investigate this process, ApoE-/- mice were irradiated at 2 different dose-rates (50 mGy.h-1 and 1.4 mGy.h-1) and a wide range of cumulative doses of irradiation ranging from 50 mGy to 1000 mGy. Immunophenotyping of immune T cells in the spleen and monocyte subsets in circulating blood were explored, and aortic mRNA expression of pro-inflammatory cytokines and adhesion molecules were evaluated.
Methods
Animals
All experiments and procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as published by the French regulations for animal experiments (Ministry of Agriculture Order No. B92-032-01, 2006) with European Directives (86/609/CEE), and approved by the local ethical committee of the Institute for Radiological Protection and Nuclear Safety (permit number P10-11, thematic number T29) and the Swedish Board of Agriculture (permit number N 134/16). Six to 8 week-old ApoE-/- male mice on a C57BL/6 J background were obtained from Charles River Laboratory. Groups consisted of 6 to 8 mice. Animals were maintained in a specific-pathogen-free environment and monitored daily. Mice were fed a regular chow diet ad libitum and maintained in a 12 h light/dark cycle environment.
Irradiation
Mice were distributed into 6 groups according to total cumulative doses of γ rays (137Cs): 50, 100, 200, 500, 750, or 1000 mGy (n = 6 each), and a control non-irradiated group (n = 8). Two dose rates were applied: very low dose rate (1.4 mGy.h-1) for chronic exposure and low dose rate (50 mGy.h-1) for acute exposure. Hence, it was possible to compare not only the impact of absolute doses to one another, but also compare equivalent doses administered at different dose-rates. Animals were irradiated at the Department of Molecular Biosciences, Experimental Animal Core Facility, Stockholm University, Sweden and then sent to IRSN. Application of correct dosimetry was assured by daily calibration using PTW UNIDOS E Universal Dosimeter equipped with a Farmer Ionization Chamber Type 30010 in the 9 different positions inside the cage thus limiting dose uncertainty to approximately +/- 7% Mouse groups exposed to the same dose were sacrificed at the same age (Supplementary Figure 1).
Blood Sampling and Analyses
Mice were terminally anesthetized by intraperitoneal injection of ketamine/xylazine (Ketamine 500 Virbac, Rompun 2% Bayer). Blood was collected by cardiac puncture with a heparinized syringe. Blood was centrifuged for 10 minutes at 1000 g. Spleens were collected in Facs buffer (PBS1X-FBS 2%), whereas aortas were collected in Trizol (Sigma Aldrich LLC).
Flow Cytometry
Total blood and spleens were collected for flow cytometry experiments. Spleens were mashed, filtered and incubated with Fc blocker (#130-059-901 Mylteni) followed with a second incubation in a cocktail of cell surface antibodies: CD3 (AF700 #56-0032-80 Invitrogen), CD4 (FITC #11-0041-82 Invitrogen), CD8 (APC #47-0081-80 Thermo Fisher), CD69 (PE #553237 Biolegend), and CD25 (Efluor 450 #45-0251-80 Thermo Fisher). FoxP3 (APC #17-5773-80 Invitrogen) labeling was performed upon permeabilization with a 30% Fixperm Solution. Blood was incubated first with FcR blocking reagent (# 130-059-901 Mylteni) followed by cell surface antibodies (Invitrogen) identifying monocytes: Ly6G (Gr-1) (FITC, clone 1A8-Ly6 g #11-0112), Ly6C (APC, clone RB6-8C5, # 17-5931-82 Thermo Fisher), CD11b (Efluor450 Clone M1/70 # 48-9668-80 Thermo Fisher), and F4/80 (PE #12-4801-82 Thermo Fisher). Flow cytometry was performed on a FACS CANTO II (BD Bioscience). After forward and side scatter gating, doublets were eliminated. CD11b+ monocytes were selected after exclusion of Ly6G positive cells, for identification of Ly6CHi and Ly6CLo monocytes (Supplementary Figure 2).
Real-time Polymerase Chain Reaction
Total RNA was extracted from aortas using Tri Reagent solution (Sigma Aldrich LLC,). RNA quality (260/280 nm) was determined using a Nanodrop ND 1000 spectrophotometer. One microgram of total RNA was synthesized to 20 µL complementary DNA (cDNA) using the high-capacity cDNA Reverse Transcription Kit from Applied Biosystems (Life Technologies) according to the manufacturer’s protocol. Quantitative polymerase chain reaction analysis was performed with a QuantStudio 12 K Flex Real-Time PCR System (Life Technologies) using TaqMan 6 carboxyfluorescein-labeled probes and a standard thermal cycler protocol (50°C for 2 minutes before the first cycle, 95°C for 15 seconds, and 60°C for 1 minute repeated 45 times). Samples were run in duplicates and normalized with gapdh and hprt using geometric mean using the 2-ΔΔCT method, control samples serving as a reference value of 1. We quantified the mRNA expression of intracellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1), E-Selectin (E-Sel), tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and transforming growth factor beta (TGFβ). The following assays were used: TGFβ-Mm01178820_m1, ICAM-1-Mm00516023_m1, TNFα-Mm00443258_m1, E-Sel-Mm0441278_m1, IL-1rap-Mm00492638_m1, VCAM-1-Mm01320970-m1, GAPDH-Mm99999915_g1, and HPRT-Mm01545399_m1.
Statistics
Results are presented as mean ± SEM. Data were compared with 2-way analysis of variance (ANOVA) or 2-way ANOVA for repeated measures, with all ANOVA tests followed by a Student–Newman–Keuls posthoc test, or with an unpaired t-test, as appropriate. P < 0.05 was considered statistically significant.
Results
Low Doses of Ionizing Radiation Administered at Low or Very low Dose-Rates Decrease Blood pro-Inflammatory Ly6CHi Monocytes
Flow cytometry analysis of blood samples provided an overview of circulating monocyte response to irradiation. Ly6CHi monocytes are dominant in an inflammatory response and are often named “pro-inflammatory monocytes”. They accumulate preferentially in atherosclerotic plaques, compared with Ly6CLo monocytes. 29 We thus distinguished 2 circulating populations of monocytes according to expression of Ly6C, as depicted in Supplementary Figure 2. We observed that exposure to low doses of external gamma rays induced a significant reduction in the proportion of Ly6CHi pro-inflammatory monocytes in the blood (Figure 1, Supplementary Figure 3). This was especially evident when irradiation was administered at the 50 mGy.h-1 dose-rate, which led to a 45%-78% decrease in circulating Ly6CHi cells at every dose tested (P < 0.05), compared with non-irradiated controls. At the 1.4 mGy.h-1 dose-rate, significant reductions in Ly6CHi monocytes were also observed, although significance was only reached at doses of 50, 100 and 750 mGy (Figure 1, Supplementary Figure 3). In contrast, proportions of Ly6CLo “patrolling” monocytes in the blood were not affected by irradiation, whatever the dose or dose-rate applied. These results indicate that low doses of ionizing irradiation have a selective effect on circulating monocyte abundance, resulting in a reduced relative abundance of Ly6CHi, pro-inflammatory populations.
Figure 1.
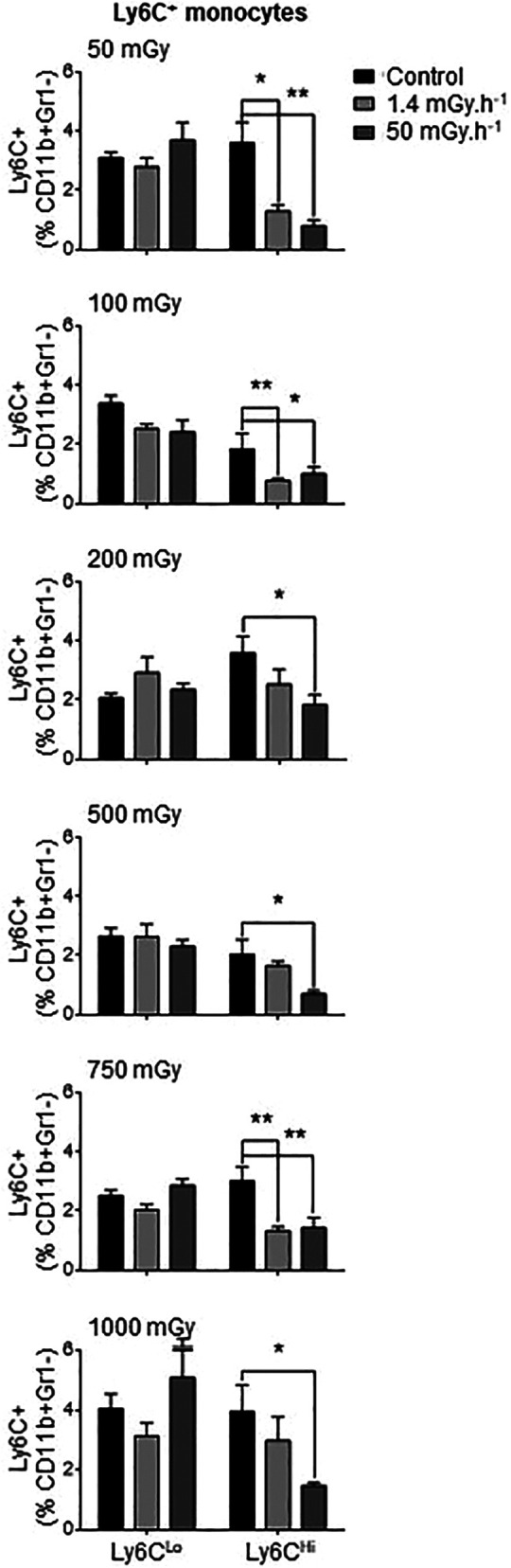
Exposure to low doses of irradiation decreases Ly6CHi pro-inflammatory circulating monocytes but does not affect anti-inflammatory Ly6C Lo cells. ApoE-/- mice were exposed to low doses of y ionizing radiation at dose-rates of 1.4 and 50 mGy.h-1. Mice were sacrificed at day 12-50 post-irradiation and blood monocytes analyzed by flow cytometry. No effects of irradiation were noted in Ly6C Lo cells. However, proportions of Ly6CHI monocytes (among CD11b+Gr1- populations) were reduced at all doses of irradiation, most consistently in mice exposed to the dose-rate of 50 mGy.h-1. Data are mean ± SEM of n = 5-8. *P < 0.05, **P < 0.01 vs non-irradiated, age-matched control.
Low Doses of Ionizing Radiation Have Little Impact on T Cell Populations in the Spleen
The balance between regulatory (Treg) and effector (Teff) T cells can have a profound impact on atherosclerotic plaque formation and progression. Treg reduce the activation and proliferation of effector T cells and are important modulators of atherosclerotic lesion formation. 30 We compared the effects of different irradiation regimens on CD4+ T cells, either regulatory (CD25+FoxP3+) or effector (CD25+FoxP3-). The sorting strategy used to distinguish these populations are depicted in Supplementary Figure 4. Treg cells were not impacted by irradiation at all, whatever the dose or dose-rate tested (Figure 2, Supplementary Figures 4 and 5). However, we found that exposure to LDIR at very low dose and at low dose-rate induced a decrease in activated effector T cells in ApoE-/- mice. Proportions of CD25 FoxP3-CD69+ cells were reduced in mice exposed to 100 mGy, to 3.4%±0.6% of CD4+ cells, compared with 5.8%±0.7% in control mice (P < 0.01). In mice irradiated at the lowest dose (50 mGy), reductions in CD25+FoxP3-CD69+ cells approached but did not reach significance. Hence, LDIR has little impact on splenic T cell populations, affecting only effector T cells at very low doses.
Figure 2.
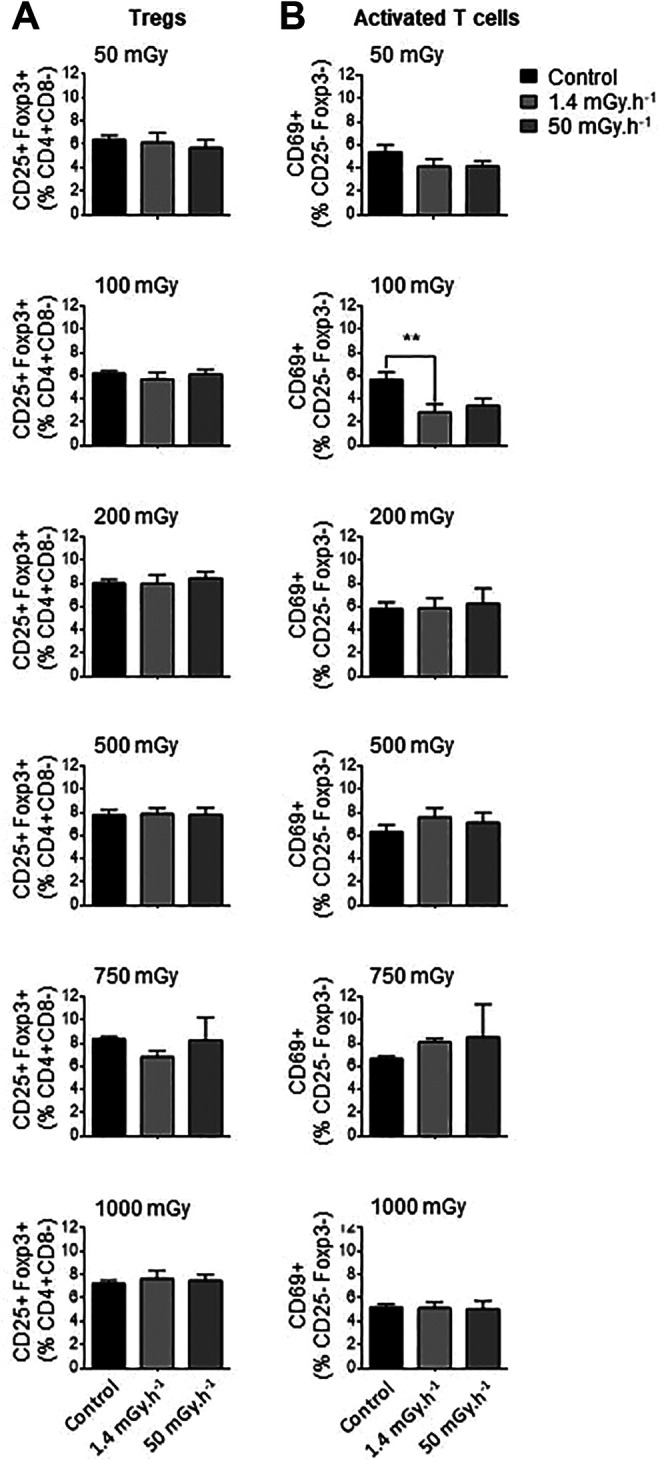
Exposure to low doses of ionizing radiation has little impact on spleen T cells. ApoE- - mice were exposed to low doses of y ionizing radiation at dose-rates of 1.4 and 50 mGy.h-1. Mice were sacrificed at day 12-50 post-irradiation and spleen T cells analyzed by flow cytometry. (A) Proportions of regulatory T cells (CD25+Foxp3+ Tregs) quantified among CD4+CD8- lymphocytes did not vary at any dose or dose-rate. (B) A reduced proportion of activated CD69+ T cells (among CD4+CD8-CD25-Foxp3- lymphocytes) was observed at the single dose of 100 mGy administered at very low dose-rate. Data are mean ± SEM of n = 5-8. **P < 0.01 vs non-irradiated, age-matched control.
Low Doses of Ionizing Radiation Reduced the mRNA Expression of Cytokines and Adhesion Molecules in Aortas of ApoE-/- Mice
Adhesion molecules, chemokines and cytokines have a direct impact in the ability of monocytes to be recruited to the vessel wall, to adhere and transmigrate through the endothelial layer, and to differentiate in inflammatory type macrophages. Their relative expression was investigated in aortas of ApoE-/- mice. No effects were detected in animals exposed to the lowest and highest doses of LDIR, 50 and 1000 mGy (Figure 3). However, at the intermediate dose of 200 mGy, a significant impact on gene expression was noted. Among the adhesion molecules, VCAM-1 was significantly decreased at dose-rates of both 1.4 and 50 mGy.h-1, by 51% and 54% respectively (P < 0.05). E-selectin was only reduced in aortas of mice exposed to at 50 mGy.h-1. As for cytokine response, we observed a significant downregulation of TGFβ at both very low (51%) and low dose-rates (34%) compared with control. IL-1 expression was also reduced by half at the 50 mGy.h-1 dose-rate (P < 0.05). Hence, changes in the vasculature induced by low-dose irradiation point to a reduction in chemokine and adhesion molecule expression.
Figure 3.
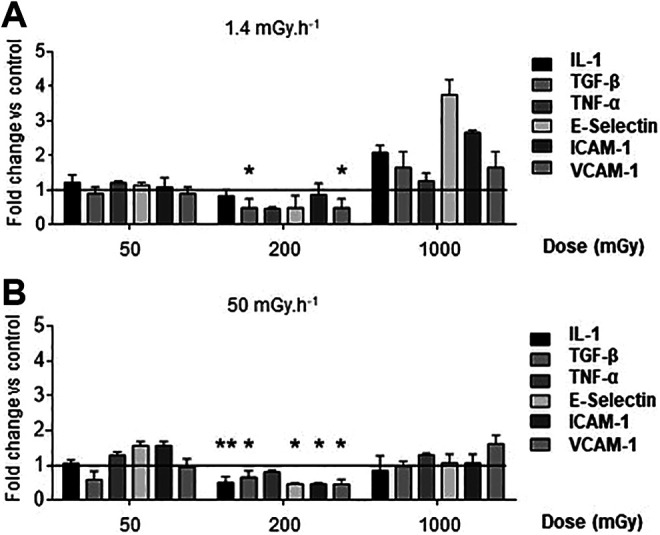
Exposure to low doses of ionizing radiation triggers a non-linear reduction in adhesion molecule and cytokine mRNA expression in the aorta. ApoE-/- mice were exposed to low of y ionizing radiation at dose-rates of 1.4 mGy.h-1 (A) and 50 mGy.h-1 (B). Mice were sacrificed at day 12-50 post-irradiation and aortic mRNA expression levels evaluated by Q-PCR. Samples were run in duplicates and normalized with gapdh and hprt using the 2-ΔΔCT method. Results are expressed as fold change vs non-irradiated, age-matched control mice. Data are mean ± SEM of n = 5-8 animals. *P < 0.05 vs control.
Discussion
In this study we show that very low and low doses of irradiation produce diverse responses in the different cell types we investigated, which are implicated in atherosclerosis plaque formation and progression. In general, we found that the low dose-rate (50 mGy.h-1) had more impact than the very low dose-rate (1.4 mGy.h-1) for a given total dose administered. Interestingly, LDIR mostly affected pro-inflammatory monocytes and aortic cells, resulting in reduced monocyte numbers and lowered expression of adhesion molecules and cytokines that would allow these cells to enter the plaque.
Cell components of both innate and adaptive immunity are involved in atherosclerosis. These cells originate in the bone marrow and are released in the circulation, from which they will migrate into tissues following chemotactic gradients, or transfer into lymphoid tissue such as the spleen for further maturation. Following entry and oxidation of low-density lipoproteins in the intimal layer of arteries, endothelial expression of adhesion molecules including ICAM-1, VCAM-1, and E-Sel, as well as chemokine release, stimulate monocyte recruitment. As monocytes accumulate, they differentiate into macrophages that take up the oxidized lipids and produce pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β, and interferon gamma (IFN-γ). 31 This results in an alteration of the artery wall structure and enhanced recruitment of additional inflammatory cells including T lymphocytes. 32 -35
In mice, circulating blood monocytes can be separated in 2 subsets based on expression levels of Ly6C. ApoE-/- mice have greater amounts of circulating monocytes than their wild-type counterparts, 29 and hypercholesterolemia enhances the proportion of the pro-inflammatory Ly6CHi monocytes in blood. 29,36 Furthermore, Ly6CHi monocytes enter atherosclerotic plaques more readily than their Ly6CLo counterparts. 29 Our results establish that low-dose irradiation prompted a clear decrease in the levels of circulating Ly6CHi monocytes in the ApoE-/- mice, at all applied doses. In contrast, no changes in Ly6CLo cells were observed. Our data support earlier reports that in vitro exposure of monocytes to LDIR favors an anti-inflammatory phenotype, 37 -39 whereas high doses of ionizing radiation have the opposite effect. 40 In addition, Sharma et al 41 demonstrated that LDIR decreased the monocytes count in blood of rats. However, to the best of our knowledge, we are the first to report a specific suppressive effect of LDIR on the Ly6CHi monocyte subtype.
A second set of major actors in atherosclerosis is T lymphocytes, from the adaptive immune system. Specific CD4+ effector T cells can recognize oxidized LDL in the atherosclerotic intima and aggravate the inflammatory response by producing cytokines including IFN-γ. 42 Inversely, regulatory T lymphocytes defined as CD4+CD25+Foxp3+ have the ability to modulate atherosclerosis 30 by producing the anti-inflammatory cytokines IL-10 and transforming growth factor beta (TGFβ). 43 Thus, the balance between inflammatory effector T cells and anti-inflammatory regulatory T cells will influence atherosclerosis progression or regression. 44 LDIR is broadly reported to alter spleen CD4+ T cell proliferation and numbers. 45 -49 Nevertheless, our data demonstrate that in ApoE-/- mice, low dose irradiation had no impact in spleen Treg numbers, and only reduced activated CD69+ cells at a single low dose applied at very low dose-rate. In fact, irradiation appears to produce divergent effects in Tregs. In some studies, regulatory T cells were decreased after exposure of rodents to low dose of ionizing radiation, leading to a rather pro-inflammatory response. 50,51 In contrast, Tregs were significantly increased following irradiation in mice affected with arthritis, 52 asthma, 53 or prostate cancer. 54 Likewise, many, 46,55 but not all 56 low dose radiation studies report enhanced CD69+ activated T cells. Regardless, our findings do not support a predominant role for regulatory or CD69+ T cells in the response to LDIR in the context of atherosclerosis.
Finally, we surveyed the chemokine and adhesion molecule profile of aortas in mice submitted to LDIR. Our experiments showed diminished expression levels of IL-1, TGFβ, E-Sel, ICAM-1, and VCAM-1 at a cumulative dose of 200 mGy. Most effects were observed at the most elevated dose-rate, although reductions were also observed at 1.4 mGy.h-1. Shin et al 57 found a variable serum cytokine profile in C57BL/6 mice irradiated at 200 mGy, noting that some interleukins were elevated but others reduced. Our study most closely resembles that of Mathias et al 58 who also irradiated ApoE-/- mice at different doses and assessed cardiovascular outcomes after 3 and 6 months. They found that expression of some adhesion molecules was reduced in myocardial cross-sections of mice exposed to 25-500 mGy irradiation, but that VCAM-1 and E-Sel were enhanced after 2000 mGy. Variations in levels of circulating pro-inflammatory factors were also noted. 58 VCAM-1, ICAM-1, and E-selectin allow firm adhesion of monocytes to endothelial cells, which precedes their transmigration, 59 which contributes to atherosclerosis. 60,61 Combined with reduced expression of adhesion molecules, the lowered IL-1 expression observed in our study would further favor atherosclerosis abatement, since it is pro-inflammatory and is associated with atherosclerosis progression. 62 -64 IL-1 is even involved in the upregulation of adhesion molecule expression on the endothelial cell surface, 63 suggesting that its decrease could explain the loss of VCAM-1, ICAM-1, and E-Sel we observed. However, the downregulation of TGFβ, an anti-inflammatory and anti-atherogenic cytokine, 65 is a contradictory result that warrants further investigation.
Interestingly, the exposure of ApoE-/- mice to low dose ionizing radiation resulted in nonlinear responses. At both dose rates, cytokine and adhesion molecule expression were reduced in mice exposed to an intermediate dose of 200 mGy, whereas neither the highest nor the lowest doses of irradiation affected mRNA expression. These nonlinear responses are in agreement with previous in vitro studies on gene expression in endothelial cells exposed to LDIR. 66 Also of interest, the dose-rate at which LDIR was applied influenced monocyte subset abundance and aortic gene expression. Specifically, most significant effects were observed at 50 mGy.h-1, suggesting that an accelerated dose-rate enhances the effectiveness of low dose irradiation. Previous in vitro results demonstrated that LDIR decreased inflammation when applied at an even higher dose rate of 1.15 Gy.min-1. 67 To the best of our knowledge the dose-rate used in our study has been used in atherosclerosis models only in the works of Mancuso et al and Mitchel et al. 16,67 However, those studies focused on phenotypical changes that occurred in plaque such as macrophage accumulation and lesion-associated macrophage lipids. They also occurred at a more prolonged time scale post-irradiation, spanning many months rather than days to weeks.
In conclusion, our observations are in line with the reported atheroprotective effect of low dose ionizing radiation observed in previous studies. 16,18,19,67 This study provides new information pertaining to the impact on monocyte populations and aortic gene expression that further explain how LDIR may mitigate lesion formation. We used a wide range of doses and 2 dose-rates to demonstrate how diverse the responses to these stimuli can be. Low dose ionizing radiation clearly has modulating effects on different cell populations. Further testing of monocyte and lymphocyte subsets regulated by radiation could bring a better understanding of immune processes in the context of atherosclerosis.
Supplemental Material
Supplemental Material, sj-jpg-1-dos-10.1177_15593258211016237 for Exposure to Low to Moderate Doses of Ionizing Radiation Induces A Reduction of Pro-Inflammatory Ly6chigh Monocytes and a U-Curved Response of T Cells in APOE -/- Mice by N. Rey, T. Ebrahimian, C. Gloaguen, D. Kereselidze, V. Magneron, C. A. Bontemps, C. Demarquay, G. Olsson, S. Haghdoost, S. Lehoux and Teni G. Ebrahimian in Dose-Response
Supplemental Material, sj-jpg-2-dos-10.1177_15593258211016237 for Exposure to Low to Moderate Doses of Ionizing Radiation Induces A Reduction of Pro-Inflammatory Ly6chigh Monocytes and a U-Curved Response of T Cells in APOE -/- Mice by N. Rey, T. Ebrahimian, C. Gloaguen, D. Kereselidze, V. Magneron, C. A. Bontemps, C. Demarquay, G. Olsson, S. Haghdoost, S. Lehoux and Teni G. Ebrahimian in Dose-Response
Supplemental Material, sj-jpg-3-dos-10.1177_15593258211016237 for Exposure to Low to Moderate Doses of Ionizing Radiation Induces A Reduction of Pro-Inflammatory Ly6chigh Monocytes and a U-Curved Response of T Cells in APOE -/- Mice by N. Rey, T. Ebrahimian, C. Gloaguen, D. Kereselidze, V. Magneron, C. A. Bontemps, C. Demarquay, G. Olsson, S. Haghdoost, S. Lehoux and Teni G. Ebrahimian in Dose-Response
Supplemental Material, sj-jpg-4-dos-10.1177_15593258211016237 for Exposure to Low to Moderate Doses of Ionizing Radiation Induces A Reduction of Pro-Inflammatory Ly6chigh Monocytes and a U-Curved Response of T Cells in APOE -/- Mice by N. Rey, T. Ebrahimian, C. Gloaguen, D. Kereselidze, V. Magneron, C. A. Bontemps, C. Demarquay, G. Olsson, S. Haghdoost, S. Lehoux and Teni G. Ebrahimian in Dose-Response
Supplemental Material, sj-jpg-5-dos-10.1177_15593258211016237 for Exposure to Low to Moderate Doses of Ionizing Radiation Induces A Reduction of Pro-Inflammatory Ly6chigh Monocytes and a U-Curved Response of T Cells in APOE -/- Mice by N. Rey, T. Ebrahimian, C. Gloaguen, D. Kereselidze, V. Magneron, C. A. Bontemps, C. Demarquay, G. Olsson, S. Haghdoost, S. Lehoux and Teni G. Ebrahimian in Dose-Response
Acknowledgments
The authors wish to thank K.Tack and D.Klokov for their support as a laboratory head and also thank D.Denais, M.Sebastien, R.Granger, S.Sache for experimental care.
Author Contributions: Lehoux and T.G. Ebrahimian are shared senior authors on this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Electricité de France (EDF) to B.Leguen, from the Canadian Institutes of Health Research (CIHR) to S.Lehoux, and from the Swedish Radiation Protection Authority.
ORCID iD: T.G. Ebrahimian  https://orcid.org/0000-0001-8413-4679
https://orcid.org/0000-0001-8413-4679
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Mulrooney DA, Armstrong GT, Huang S, et al. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: a cross-sectional study. Ann Int Med. 2016;164(2):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boivin J-F, Hutchison GB, Lubin JH, Mauch P. Coronary artery disease mortality in patients treated for Hodgkin’s disease. Cancer. 1992;69(5):1241–1247. [DOI] [PubMed] [Google Scholar]
- 3. McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100(2):167–175. [DOI] [PubMed] [Google Scholar]
- 4. Shimizu Y, Kodama K, Nishi N, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ. 2010;340:b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuan R, Sun Z, Cai J, et al. A novel anticancer therapeutic strategy to target autophagy accelerates radiation-associated atherosclerosis. Int J Radiat Oncol Biol Phys. 2021;109(2):540–552. doi:10.1016/j.ijrobp.2020.09.007 [DOI] [PubMed] [Google Scholar]
- 6. Lee C-L, Lee JW, Daniel AR, et al. Characterization of cardiovascular injury in mice following partial-heart irradiation with clinically relevant dose and fractionation. Radiother Oncol. 2021;157:155–162. doi:10.1016/j.radonc.2021.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamada N, Kawano K-I, Yusoff FM, et al. Ionizing irradiation induces vascular damage in the aorta of wild-type mice. Cancers (Basel). 2020;12(10):3030. doi:10.3390/cancers12103030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stewart FA, Heeneman S, Te Poele J, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE-/- mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol. 2006;168(2):649–658. doi:10.2353/ajpath.2006.050409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newman AA, Baylis RA, Hess DL, et al. Irradiation abolishes smooth muscle investment into vascular lesions in specific vascular beds. JCI Insight. 2018;3(15):e121017. doi:10.1172/jci.insight.121017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gabriels K, Hoving S, Seemann I, et al. Local heart irradiation of ApoE(-/-) mice induces microvascular and endocardial damage and accelerates coronary atherosclerosis. Radiother Oncol. 2012;105(3):358–364. doi:10.1016/j.radonc.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 11. Hoving S, Heeneman S, Gijbels MJJ, et al. Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE(-/-) mice. Int J Radiat Oncol Biol Phys. 2008;71(3):848–857. doi:10.1016/j.ijrobp.2008.02.031 [DOI] [PubMed] [Google Scholar]
- 12. Schöllnberger H, Eidemüller M, Cullings HM, Simonetto C, Neff F, Kaiser JC. Dose-responses for mortality from cerebrovascular and heart diseases in atomic bomb survivors: 1950–2003. Radiat Environ Biophys. 2018;57(1):17–29. doi:10.1007/s00411-017-0722-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Azizova TV, Muirhead CR, Druzhinina MB, et al. Cardiovascular diseases in the cohort of workers first employed at mayak PA in 1948–1958. Radiat Res. 2010;174(2):155–168. doi:10.1667/RR1789.1 [DOI] [PubMed] [Google Scholar]
- 14. Baselet B, Rombouts C, Benotmane AM, Baatout S, Aerts A. Cardiovascular diseases related to ionizing radiation: the risk of low-dose exposure (review). Int J Molecul Med. 2016;38(6):1623–1641. doi:10.3892/ijmm.2016.2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joven J, Rull A, Ferré N, et al. The results in rodent models of atherosclerosis are not interchangeable: the influence of diet and strain. Atherosclerosis. 2007;195(2):e85–e92. [DOI] [PubMed] [Google Scholar]
- 16. Mitchel REJ, Hasu M, Bugden M, et al. Low-dose radiation exposure and atherosclerosis in ApoE−/− mice. Radiat Res. 2011;175(5):665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mancuso M, Pasquali E, Braga-Tanaka I, et al. Acceleration of atherogenesis in ApoE−/− mice exposed to acute or low-dose-rate ionizing radiation. Oncotarget. 2015;6(31):31263–31271. doi:10.18632/oncotarget.5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le Gallic C, Phalente Y, Manens L, et al. Chronic Internal Exposure to Low Dose 137Cs Induces Positive Impact on the Stability of Atherosclerotic Plaques by Reducing Inflammation in ApoE-/- Mice. PLoS One. 2015;10(6):e0128539. doi:10.1371/journal.pone.0128539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ebrahimian TG, Beugnies L, Surette J, et al. Chronic exposure to external low-dose gamma radiation induces an increase in anti-inflammatory and anti-oxidative parameters resulting in atherosclerotic plaque size reduction in ApoE-/- Mice. Radiat Res. 2018;189(2):187–196. doi:10.1667/RR14823.1 [DOI] [PubMed] [Google Scholar]
- 20. Williams J, Chen Y, Rubin P, Finkelstein J, Okunieff P. The biological basis of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in Radiat Oncol. 2003;13(3):182–188. doi:10.1016/S1053-4296(03)00045-6 [DOI] [PubMed] [Google Scholar]
- 21. Gabriels K, Hoving S, Gijbels MJ, et al. Irradiation of existing atherosclerotic lesions increased inflammation by favoring pro-inflammatory macrophages. Radiother Oncol. 2014;110(3):455–460. doi:10.1016/j.radonc.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 22. Seegenschmiedt MH, Makoski H-B, Trott K-R, Brady LW, eds. Radiotherapy for Non-Malignant Disorders. Springer; 2008. doi:10.1007/978-3-540-68943-0 [Google Scholar]
- 23. Rodel F, Frey B, Gaipl U, et al. Modulation of inflammatory immune reactions by low-dose ionizing radiation: molecular mechanisms and clinical application. CMC. 2012;19(12):1741–1750. doi:10.2174/092986712800099866 [DOI] [PubMed] [Google Scholar]
- 24. Yuan Y, Lee SH, Wu S. The role of ROS in ionizing radiation-induced VLA-4 mediated adhesion of RAW264.7 cells to VCAM-1 under flow conditions. Radiat Res. 2013;179(1):62–68. doi:10.1667/RR3119.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kern PM, Keilholz L, Forster C, Hallmann R, Herrmann M, Seegenschmiedt MH. Low-dose radiotherapy selectively reduces adhesion of peripheral blood mononuclear cells to endothelium in vitro. Radiother Oncol. 2000;54(3):273–282. doi:10.1016/s0167-8140(00)00141-9 [DOI] [PubMed] [Google Scholar]
- 26. Cho S-J, Kang H, Hong E-H, Kim JY, Nam SY. Transcriptome analysis of low-dose ionizing radiation-impacted genes in CD4+ T-cells undergoing activation and regulation of their expression of select cytokines. J Immunotoxicol. 2018;15(1):137–146. doi:10.1080/1547691X.2018.1521484 [DOI] [PubMed] [Google Scholar]
- 27. Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17(7):387–401. doi:10.1038/s41569-020-0352-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Narasimhan PB, Marcovecchio P, Hamers AAJ, Hedrick CC. Nonclassical Monocytes in Health and Disease. Annu Rev Immunol. 2019;37:439–456. doi:10.1146/annurev-immunol-042617-053119 [DOI] [PubMed] [Google Scholar]
- 29. Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117(1):185–194. doi:10.1172/JCI28549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klingenberg R, Gerdes N, Badeau RM, et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123(3):1323–1334. doi:10.1172/JCI63891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rahman MS, Woollard K. Atherosclerosis. Adv Exp Med Biol. 2017;1003:121–144. doi:10.1007/978-3-319-57613-8_7 [DOI] [PubMed] [Google Scholar]
- 32. Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb vasc Biol. 2005;25(10):2054–2061. [DOI] [PubMed] [Google Scholar]
- 33. Moulton KS, Vakili K, Zurakowski D, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Nati Acad Sci. 2003;100(8):4736–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85(1):1–31. [DOI] [PubMed] [Google Scholar]
- 35. Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46(6):937–954. [DOI] [PubMed] [Google Scholar]
- 36. Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117(1):195–205. doi:10.1172/JCI29950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. El-Saghire H, Michaux A, Thierens H, Baatout S. Low doses of ionizing radiation induce immune-stimulatory responses in isolated human primary monocytes. Int J Mol Med. 2013;32(6):1407–1414. doi:10.3892/ijmm.2013.1514 [DOI] [PubMed] [Google Scholar]
- 38. Frischholz B, Wunderlich R, Rühle P-F, et al. Reduced secretion of the inflammatory cytokine IL-1β by stimulated peritoneal macrophages of radiosensitive Balb/c mice after exposure to 0.5 or 0.7 Gy of ionizing radiation. Autoimmunity. 2013;46(5):323–328. doi:10.3109/08916934.2012.747522 [DOI] [PubMed] [Google Scholar]
- 39. Tsukimoto M, Homma T, Mutou Y, Kojima S. 0.5 Gy gamma radiation suppresses production of TNF-alpha through up-regulation of MKP-1 in mouse macrophage RAW264.7 cells. Radiat Res. 2009;171(2):219–224. doi:10.1667/RR1351.1 [DOI] [PubMed] [Google Scholar]
- 40. Teresa Pinto A, Laranjeiro Pinto M, Patrícia Cardoso A, et al. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci Rep. 2016;6:18765. doi:10.1038/srep18765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharma S, Singla N, Chadha VD, Dhawan D. A concept of radiation hormesis: stimulation of antioxidant machinery in rats by low dose ionizing radiation. Hell J Nucl Med. 2019;22(1):43–48. [DOI] [PubMed] [Google Scholar]
- 42. Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein e knockout mice. Circulation. 2000;102(24):2919–2922. doi:10.1161/01.cir.102.24.2919 [DOI] [PubMed] [Google Scholar]
- 43. Robertson A-KL, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112(9):1342–1350. doi:10.1172/JCI18607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tabas I, Lichtman AH. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity. 2017;47(4):621–634. doi:10.1016/j.immuni.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu S, Zhang Y, Zhao Y. Signal transduction in lymphocytes after low dose radiation. Chin Med J. 1994;107(6):431–436. [PubMed] [Google Scholar]
- 46. Pandey R, Shankar BS, Sharma D, Sainis KB. Low dose radiation induced immunomodulation: effect on macrophages and CD8+ T cells. Int J Radiat Biol. 2005;81(11):801–812. [DOI] [PubMed] [Google Scholar]
- 47. Ishii K, Yamaoka K, Hosoi Y, Ono T, Sakamoto K. Enhanced mitogen-induced proliferation of rat splenocytes by low-dose whole-body X-irradiation. Physiol Chem phys Med NMR. 1995;27(1):17–23. [PubMed] [Google Scholar]
- 48. Liu S, Han Z, Liu W. Changes in lymphocyte reactivity to modulatory factors following low dose ionizing radiation. Biomed Environ Sci: BES. 1994;7(2):130–135. [PubMed] [Google Scholar]
- 49. Song K-H, Kim M-H, Kang S-M, et al. Analysis of immune cell populations and cytokine profiles in murine splenocytes exposed to whole-body low-dose irradiation. Int J Radiat Biol. 2015;91(10):795–803. [DOI] [PubMed] [Google Scholar]
- 50. Wang B, Li B, Dai Z, et al. Low-dose splenic radiation inhibits liver tumor development of rats through functional changes in CD4+ CD25+ Treg cells. Int J Biochem Cell Biol. 2014;55:98–108. [DOI] [PubMed] [Google Scholar]
- 51. Liu R, Xiong S, Zhang L, Chu Y. Enhancement of antitumor immunity by low-dose total body irradiationis associated with selectively decreasing the proportion and number of T regulatorycells. Cell Mol Immunol. 2010;7(2):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nakatsukasa H, Tsukimoto M, Tokunaga A, Kojima S. Repeated gamma irradiation attenuates collagen-induced arthritis via up-regulation of regulatory T cells but not by damaging lymphocytes directly. Radiat Res. 2010;174(3):313–324. [DOI] [PubMed] [Google Scholar]
- 53. Park BS, Hong GU, Ro JY. Foxp3(+)-Treg cells enhanced by repeated low-dose gamma-irradiation attenuate ovalbumin-induced allergic asthma in mice. Radiat Res. 2013;179(5):570–583. doi:10.1667/RR3082.1 [DOI] [PubMed] [Google Scholar]
- 54. Kachikwu EL, Iwamoto KS, Liao Y-P, et al. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81(4):1128–1135. doi:10.1016/j.ijrobp.2010.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen JC, Davis BH, Leon MA, Leong LC. Gamma radiation induces CD69 expression on lymphocytes. Cytometry. 1997;30(6):304–312. [PubMed] [Google Scholar]
- 56. Song K-H, Jung S-Y, Park J-I, et al. Evaluation of anti-tumor effects of whole-body low-dose irradiation in metastatic mouse models. Cancers (Basel). 2020;12(5):1126. doi:10.3390/cancers12051126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shin SC, Lee K-M, Kang YM, et al. Alteration of cytokine profiles in mice exposed to chronic low-dose ionizing radiation. Biochem Biophys Res Commun. 2010;397(4):644–649. [DOI] [PubMed] [Google Scholar]
- 58. Mathias D, Mitchel REJ, Barclay M, et al. Low-dose irradiation affects expression of inflammatory markers in the heart of ApoE -/- mice. PLoS One. 2015;10(3):e0119661. doi:10.1371/journal.pone.0119661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. [DOI] [PubMed] [Google Scholar]
- 60. Ting HJ, Stice JP, Schaff UY, et al. Triglyceride-rich lipoproteins prime aortic endothelium for an enhanced inflammatory response to tumor necrosis factor-α. Circ Res. 2007;100(3):381–390. [DOI] [PubMed] [Google Scholar]
- 61. Nageh MF, Sandberg ET, Marotti KR, et al. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1997;17(8):1517–1520. [DOI] [PubMed] [Google Scholar]
- 62. Kamari Y, Shaish A, Shemesh S, et al. Reduced atherosclerosis and inflammatory cytokines in apolipoprotein-E-deficient mice lacking bone marrow-derived interleukin-1α. Biochem Biophys Res Commun. 2011;405(2):197–203. [DOI] [PubMed] [Google Scholar]
- 63. Kirii H, Niwa T, Yamada Y, et al. Lack of interleukin-1β decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23(4):656–660. [DOI] [PubMed] [Google Scholar]
- 64. Freigang S, Ampenberger F, Weiss A, et al. Fatty acid–induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat Immun. 2013;14(10):1045–1053. [DOI] [PubMed] [Google Scholar]
- 65. Lutgens E, Daemen MJ. Transforming Growth Factor-β: A Local or Systemic Mediator of Plaque Stability? Am Heart Assoc; 2001. [PubMed] [Google Scholar]
- 66. Schröder S, Juerß D, Kriesen S, Manda K, Hildebrandt G. Immunomodulatory properties of low-dose ionizing radiation on human endothelial cells. Int J Radiat Biol. 2019;95(1):23–32. [DOI] [PubMed] [Google Scholar]
- 67. Roedel F, Kley N, Beuscher HU, et al. Anti-inflammatory effect of low-dose X-irradiation and the involvement of a TGF-beta1-induced down-regulation of leukocyte/endothelial cell adhesion. Int J Radiat Biol. 2002;78(8):711–719. doi:10.1080/09553000210137671 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-jpg-1-dos-10.1177_15593258211016237 for Exposure to Low to Moderate Doses of Ionizing Radiation Induces A Reduction of Pro-Inflammatory Ly6chigh Monocytes and a U-Curved Response of T Cells in APOE -/- Mice by N. Rey, T. Ebrahimian, C. Gloaguen, D. Kereselidze, V. Magneron, C. A. Bontemps, C. Demarquay, G. Olsson, S. Haghdoost, S. Lehoux and Teni G. Ebrahimian in Dose-Response
Supplemental Material, sj-jpg-2-dos-10.1177_15593258211016237 for Exposure to Low to Moderate Doses of Ionizing Radiation Induces A Reduction of Pro-Inflammatory Ly6chigh Monocytes and a U-Curved Response of T Cells in APOE -/- Mice by N. Rey, T. Ebrahimian, C. Gloaguen, D. Kereselidze, V. Magneron, C. A. Bontemps, C. Demarquay, G. Olsson, S. Haghdoost, S. Lehoux and Teni G. Ebrahimian in Dose-Response
Supplemental Material, sj-jpg-3-dos-10.1177_15593258211016237 for Exposure to Low to Moderate Doses of Ionizing Radiation Induces A Reduction of Pro-Inflammatory Ly6chigh Monocytes and a U-Curved Response of T Cells in APOE -/- Mice by N. Rey, T. Ebrahimian, C. Gloaguen, D. Kereselidze, V. Magneron, C. A. Bontemps, C. Demarquay, G. Olsson, S. Haghdoost, S. Lehoux and Teni G. Ebrahimian in Dose-Response
Supplemental Material, sj-jpg-4-dos-10.1177_15593258211016237 for Exposure to Low to Moderate Doses of Ionizing Radiation Induces A Reduction of Pro-Inflammatory Ly6chigh Monocytes and a U-Curved Response of T Cells in APOE -/- Mice by N. Rey, T. Ebrahimian, C. Gloaguen, D. Kereselidze, V. Magneron, C. A. Bontemps, C. Demarquay, G. Olsson, S. Haghdoost, S. Lehoux and Teni G. Ebrahimian in Dose-Response
Supplemental Material, sj-jpg-5-dos-10.1177_15593258211016237 for Exposure to Low to Moderate Doses of Ionizing Radiation Induces A Reduction of Pro-Inflammatory Ly6chigh Monocytes and a U-Curved Response of T Cells in APOE -/- Mice by N. Rey, T. Ebrahimian, C. Gloaguen, D. Kereselidze, V. Magneron, C. A. Bontemps, C. Demarquay, G. Olsson, S. Haghdoost, S. Lehoux and Teni G. Ebrahimian in Dose-Response


