Abstract
Background
Although the National Comprehensive Cancer Network guidelines recommend routine lymph node dissection (LND) in intrahepatic cholangiocarcinoma (ICC), the role of LND remains controversial, and the node (N) stage is oversimplified.
Methods
Patients were identified from the Surveillance, Epidemiology, and End Results research data 18 (SEER 18). Propensity score matching (PSM) was used to reduce bias, and Kaplan–Meier curves and Cox proportional hazards models were used to compare overall survival (OS). The best cutoff values were found using X-tile software.
Results
Of 2037 patients included in SEER 18, 1147 underwent LND (56.3%); 389 (34.3%) had pathologically confirmed lymph node metastasis (LNM), and 316 (27.6%) had at least 6 LNDs. The median OS was worse for LND patients (34 months vs. 40 months, respectively), and this result remained after PSM. Male sex, age ≥60 years, tumor size > 5 cm, and LNM were independent prognostic risk factors for ICC. LNM ≥3 was associated with worse OS.
Conclusions
Only a few LNDs met the requirements per the guidelines. LND does not improve OS in ICC, and the best approach to LND and a better N staging method should be explored further.
Keywords: Lymph node dissection, intrahepatic cholangiocarcinoma, Surveillance Epidemiology and End Results, N-staging system, lymph node, metastasis
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a malignant tumor with a poor prognosis, and surgery is the only effective treatment. 1 The 5-year survival rate is approximately 30%,2,3 and the postoperative recurrence rate is high, with a median disease-free survival (DFS) of only 20 months. Several factors can worsen survival, such as pathogenic factors, tumor size, tumor number, lymph node metastasis (LNM), vascular infiltration, degree of differentiation, and carbohydrate antigen (CA)19-9 concentration, all of which are related to the ICC prognosis.4–9 LNM is a clear risk factor and is particularly important.4–6,10
Lymph node dissection (LND) is a component of ICC radical surgery. The American Joint Committee on Cancer (AJCC) 7th Staging system, released in 2010, was the first to conduct independent staging of ICC, and subsequently, the updated AJCC 8th edition recommended that surgery for ICC should include the removal of at least six lymph nodes, for accurate N staging. 11 LND plays an important role in determining the status of ICC LNM and assessing the disease prognosis more accurately, and many studies have focused on the relationship between prognosis and the number of LNMs and their locations.12,13 However, as it is based on whether there is LNM, the existing N stage is not specific enough, and whether LND can improve the survival of patients with ICC remains controversial. The main purpose of this study was to explore the effect of LND and LNM on the prognosis of ICC patients using data from the Surveillance, Epidemiology, and End Results (SEER) database.
Methods
Data collection
SEER research data 18 (SEER 18) covers approximately 27.8% of the US population (based on the 2010 census) between 2000 and 2017. Per the International Classification of Diseases for Oncology (ICD-O-3) topography codes, ICC is classified as C22.0 (liver) and C22.1 (intrahepatic bile duct), and the morphology codes are 8160, 8161, 8032, 8033, 8070, 8071, 8140, 8141, 8160, 8161, 8260, 8480, 8481, 8490, and 8560. 14
Statistical analysis
SPSS version 25.0 for Windows® (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The chi-squared test was used to compare the distribution differences for categorical variables, and propensity score matching (PSM) (1:1) was used to eliminate differences between groups, using SPSS. The best cutoff values were found using X-tile software (Yale School of Medicine, New Haven, CT, USA) with the minimum P value method. The Kaplan–Meier method was used for survival analysis, and the log-rank test was used for verification. Multivariate analysis was performed using the Cox proportional hazards regression model.
Research variables
Regarding SEER 18, which has many nonspecific data, our study included only a few variables, namely, sex, age, tumor size, LND, number of LNDs, and LNM. The cutoff of age was > 60 years, the cutoff LND number was ≥6, and the LNM number was classified as 1–3 or > 3. Tumor size was classified as > 5 cm (in accordance with the AJCC 8th edition staging system). The cutoff number of LNMs was 3 according to the results from the X-tile software.
Ethics
This study granted an exemption by the Cancer Hospital Institutional Review Board because it was a retrospective study. The need to obtain patient consent was waived for the same reason.
Results
Patient characteristics
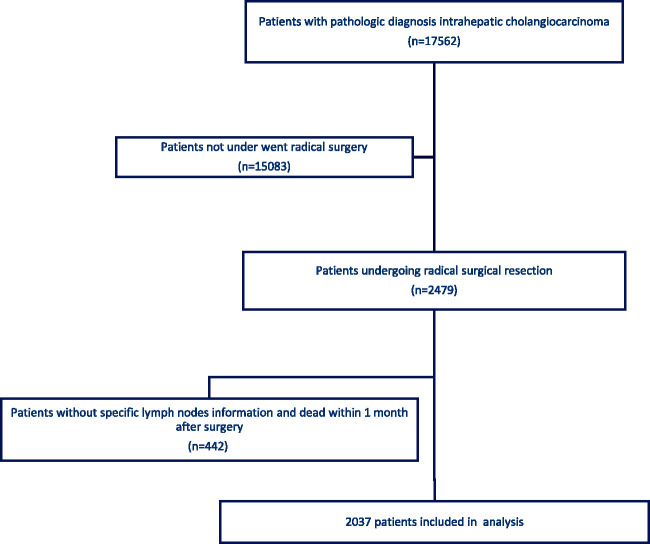
There were 17,562 patients with a pathological diagnosis of ICC in SEER 18, and 2479 underwent surgical treatment. Patients with an unclear LND status and those who died within 1 month (overall survival [OS] = 0–1 month) were excluded. In total, 2037 patients were included in this study (Figure 1).
Figure 1.
Kaplan–Meier curve for overall survival (OS) in the lymph node dissection (LND) group and no lymph node dissection (nLND) group before propensity score matching (PSM).
Among the 2037 patients, 1147 underwent LND (56.3%); 389 (34.3%) patients were pathologically confirmed to have at least one positive LN after surgery. Of these, 316 (27.6%) had ≥6 LNs removed. There were statistically significant differences for age, sex, and tumor size distribution between the LND and no LND (did not undergo LND; nLND) groups (Table 1).
Table 1.
Characteristics of patients with intrahepatic cholangiocarcinoma who underwent surgery.
|
Unadjusted |
PSM |
|||||
|---|---|---|---|---|---|---|
| LND (n = 1147, 56.3%) | nLND (n = 890, 43.7%) | P value | LND (n = 859, 50%) | nLND (n = 859, 50%) | P value | |
| Age (years) | ||||||
| ≥60 | 705 (61.5) | 632 (71.0) | <0.01 | 578 (67.3) | 601 (70.0) | 0.23 |
| >60 | 442 (38.5) | 258 (29.0) | 281 (32.7) | 258 (30.0) | ||
| Sex | ||||||
| Male | 515 (44.9) | 461 (51.8) | <0.01 | 453 (52.7) | 430 (50.1) | 0.27 |
| Female | 632 (55.1) | 429 (48.2) | 406 (47.3) | 429 (49.9) | ||
| At least 6 lymph nodes dissected | ||||||
| ≥6 | 316 (27.6) | NA | 233 (27.1) | NA | ||
| <6 | 831 (72.4) | 626 (72.9) | ||||
| LNM | ||||||
| Yes | 391 (34.4) | NA | 298 (35.0) | NA | ||
| No | 744 (65.6) | 553 (65.0) | ||||
| LNM number | ||||||
| >3 | 64 (16.3) | NA | 51 (17.1) | NA | ||
| 1–3 | 327 (83.7) | 248 (82.9) | ||||
| Tumor size (cm) | ||||||
| >5 | 609 (53.1) | 428 (48.1) | 0.03 | 391 (45.5) | 414 (48.2) | 0.27 |
| ≤5 | 538 (46.9) | 462 (51.9) | 468 (54.5) | 445 (51.8) | ||
LND, lymph node dissection; nLND, did not undergo LND; LNM, lymph node metastasis; PSM, propensity score matching; NA, not available.
Prognostic factors analysis
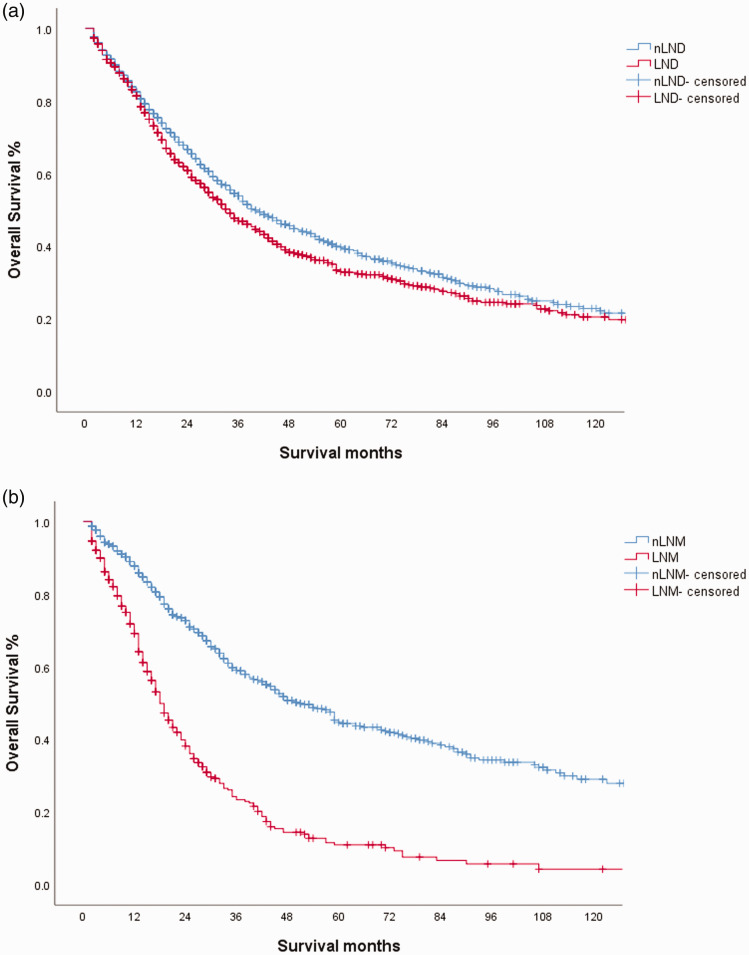
The median follow-up time was 23 months, the median OS was 37 months, and the 5-year OS was 36.1%. The median OS times in the LND and nLND groups were 34 months and 40 months, respectively, and the 5-year OS rates were 32.9% and 38.7%, respectively (Figure 2a).
Figure 2.
Kaplan–Meier curve for overall survival (OS) in the lymph node metastasis (LNM) group and no lymph node metastasis (nLNM) group
Univariate analysis showed that age > 60 years, male sex, tumor size > 5 cm, and having undergone LND were risk factors for worse OS. Multivariate analysis showed that all four factors were independent risk factors (Table 2).
Table 2.
Univariate and multivariate analyses of the factors related to overall survival.
|
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| HR | P value | HR | P value | |
| LND | ||||
| No | 1 | 0.03 | 1 | 0.024 |
| Yes | 1.14 (1.01–1.28) | 1.15 (1.02–1.30) | ||
| Age (years) | ||||
| <60 | 1 | 0.01 | 1 | <0.01 |
| ≥60 | 1.16 (1.04–1.33) | 1.2 (1.06–1.36) | ||
| Sex | ||||
| Female | 1 | <0.01 | 1 | <0.01 |
| Male | 1.17 (1.04–1.32) | 1.22 (1.07–1.35) | ||
| Tumor size (cm) | ||||
| ≤5 | 1 | <0.01 | 1 | <0.01 |
| >5 | 1.33 (1.18–1.50) | 1.35 (1.08–1.37) | ||
| At least six lymph nodes dissected | (Analysis only in the LND group) | |||
| <6 | 1 | 0.26 | 1 | 0.09 |
| ≥6 | 1.11 (0.93–1.33) | 0.85 (0.71–1.02) | ||
| LNM | ||||
| Negative | 1 | <0.01 | 1 | |
| Positive | 2.80 (2.38–3.30) | 2.92 (2.47–3.45) | <0.01 | |
HR, hazard ratio; LNM, lymph node metastasis; LND, lymph node dissection.
95% confidence intervals are shown in brackets.
A subgroup analysis of LND showed no significant difference in survival between LND < 6 and LND ≥6, and the survival of patients with LNM was significantly worse than that for those without LNM (Figure 2b).
PSM analysis of LND
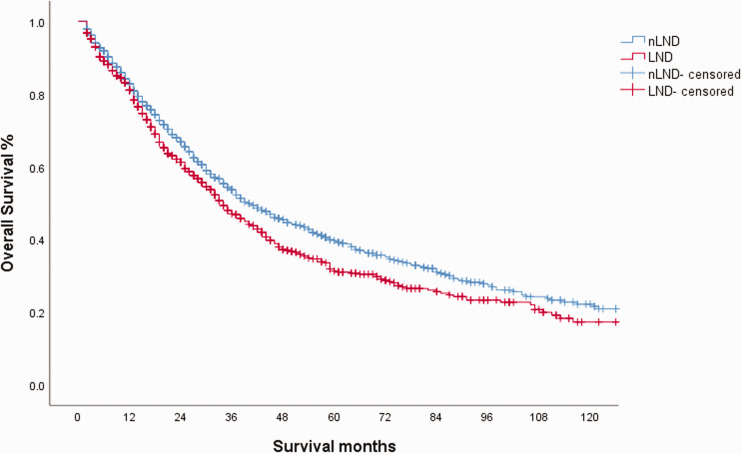
In a previous analysis, we analyzed the effect of LND on prognosis, and we also found differences in the patients’ characteristics at baseline. Therefore, we performed 1:1 propensity score matching (PSM), in this study. It is important to note that in the nLND group, LNM status was unclear. Age, sex, and tumor size underwent PSM, and the results were satisfactory after inspection (Table 1). Survival analysis showed that the median OS of the LND group was still worse than that of the nLND group (Figure 3). The median OS of the LND group was 34 months and that of the nLND group was 40 months (95% confidence interval (CI): 30.4–37.6 months vs. 34.7–45.3 months, respectively; P=0.01).
Figure 3.
Kaplan–Meier curve for overall survival (OS) in the lymph node dissection (LND) group and no lymph node dissection (nLND) group after propensity score matching (PSM).
The value of LNM number
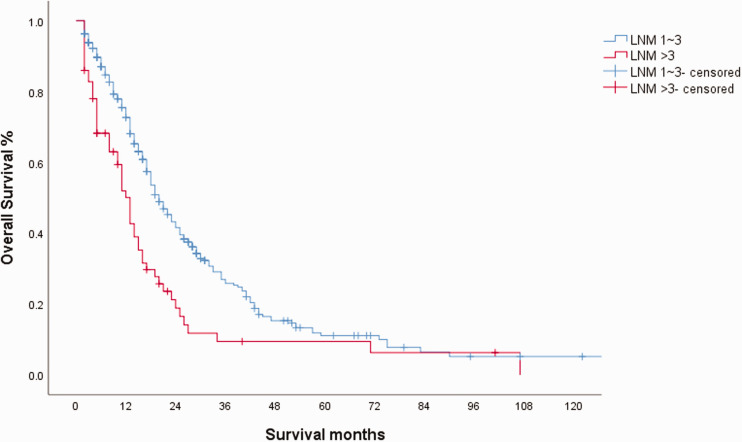
To determine a better N staging method, we studied the number of LNMs in the LNM group. The distribution of sex, age, and tumor size was not statistically significantly different between the LNM 1–3 and LNM > 3 groups (Table 3). The number of LNDs was not balanced, but this was not related to survival (as studied above). OS was significantly worse in the > 3 LNM group (Figure 4). The median OS of the LND and nLND groups was 20 months and 13 months, respectively (95% CI: 17.5–22.5 and 10.7–15.3, respectively; P < 0.01), and the 5-year OS rates were 11.1% and 9.4%, respectively. In the Cox regression model, the hazard ratio (HR) of LNM > 3 was 1.792 (95% CI: 1.32–2.43; P < 0.01).
Table 3.
Characteristics of patients with lymph node-positive status
| LNM 1–3 (n = 325, 83.5%) | LNM >3 (n = 64, 16.5%) | P value | |
|---|---|---|---|
| Age (years) | |||
| ≥60 | 183 (56.3) | 39 (60.9) | 0.49 |
| <60 | 142 (43.7) | 25 (39.1) | |
| Sex | |||
| Male | 151 (46.5) | 33 (51.6) | 0.46 |
| Female | 174 (53.5) | 31 (48.4) | |
| At least six lymph nodes dissected | |||
| ≥6 | 111 (34.2) | 16 (25.0) | <0.01 |
| <6 | 214 (65.8) | 48 (75.0) | |
| Tumor size (cm) | |||
| <5 | 177 (54.5) | 26 (40.6) | 0.47 |
| ≤5 | 148 (45.5) | 38 (59.4) |
LNM, lymph node metastasis.
Figure 4.
Kaplan–Meier curve for overall survival (OS) in the lymph node metastasis (LNM) 1–3 and LNM > 3 groups
Discussion
ICC is a malignant disease with a poor prognosis, and the goal of surgery is complete (R0) resection. The idea that LND is only a staging operation and has little effect on prognosis is supported by some researchers.3,15 Although there were differences in survival between the two groups in our study, and these results persisted after PSM, the LND group had worse OS. The authors do not believe that this suggests that LND worsens the prognosis, but it does suggest that the contribution to survival by LND may be limited (i.e., does not improve survival). The truth may be that the LND group had a higher degree of malignancy, despite our use of PSM. We must note that we could not completely balance the differences between the two groups, and some factors, such as LNM status, may have had a significant impact on the results; i.e., we could not completely balance the LNM statuses of the two groups.
Yo et al. compared the effect of LND on prognosis in patients suspected of having no LNM before surgery. 16 The results indicated that LND improved both DFS and OS in the LND “negative” group; however, only 112 patients were included, and some deviations in preoperative imaging assessment may have existed. Ma et al. 17 found that expanded LND was associated with a better prognosis within the R0 resection group and the group without distant metastases, even after PSM. However, there was no difference for the whole cohort. Kim et al. obtained positive results in patients with an LND number ≥6 and in those without LND. 18 However, the sample size was only 68 patients, and the OS time in the study was much longer than that in other studies. These studies all have drawbacks. Generally, our research supported the mainstream view that there is no prognostic benefit with LND.
ICC has a high rate of LNM, at approximately 31.9% to 58.0%.19,20 Because LNM is a strong indicator of a poor prognosis in ICC, LND still ought to be performed to determine the specific LN state. However, the best approach for LNM is another unsolved problem. The AJCC 8th edition guidelines suggest routine LND and removal of at least 6 LNs. The guidelines also clearly define regional LNs. 21 In addition to the hilar LNs (common bile duct, hepatic artery, portal vein, and cystic duct), regional LNs include the inferior phrenic and gastrohepatic LNs in the left lobe. The right lobe covers periduodenal and peripancreatic LN areas. The consensus among the guidelines was that extraregional LNM, such as distant metastases, was considered a contraindication to surgery. 22 Although the National Comprehensive Cancer Network (NCCN) guidelines require only dissection of the hilar area, 23 the percentage of LND is too low. In SEER 18, the rate of LND was 57.3%, and only 27.6% met the requirement of recovering 6 LNs. This indicates that the N stage of more than half of patients may not be exact, and some patients with LNM are incorrectly assessed because they have not undergone LND or have unqualified LND.
Another question is the best number and area of LND. A multicenter, large sample study was published in 2021 by Zhang et al. The authors found that six was the best number for obtaining better OS. 12 This was not confirmed in our data. In fact, we did not find a significant cutoff LND number for survival using the X-tile software. This conclusion needs further study. Because LND does not improve prognosis, we should pay more attention to the relationship between LND and the LNM detection rate. To obtain the most accurate result, LND should be routine and standardized, not just involve removing the LNs that were probed during the operation.
The next issue is that the current N stage is not specific. LNM positivity and negativity, which are defined as N0 and N1, have been proven to effectively differentiate the prognosis of ICC.17,24–27 The N stages for other bile duct cancers, namely extrahepatic cholangiocarcinoma, perihilar cholangiocarcinoma, and gallbladder carcinoma, are all graded with LNM numbers. 21 Nakagawa et al. proposed a new N stage system: N0 (LNM 0), N1 (LNM 1–2), and N2 (LNM > 2), in 2005, in which survival of patients with N2 status was worse than that of patients with N1 status. 15 Zhang et al.’s research confirmed this conclusion and found that this staging method performed better for patients whose LND number was ≥6. The SEER data were used for external validation in the study. 12 In our study, we came to the same conclusion in our previous analysis, but because the P value was smaller when 3 was used as the cutoff value, we classified patients into the LNM 1–3 and LNM ≥3 groups. Although our LNM numbers were slightly different, our results suggest that the ICC N staging system needs to be reformulated.
There were limitations in this study. First, SEER 18 had little information on specific relevant factors. Additionally, there were many missing values, and many important factors, such as tumor markers and LN location, were not recorded. Second, the data were collected by numerous people, which may have produced incorrect results, and LND was recorded only by the surgeon who performed the operation. Finally, the analysis method in this study (PSM) could not eliminate differences between groups. Despite these limitations, we are still able to provide researchers with some references for ICC surgery, prognostic models, and staging systems.
Conclusions
The LND group did not have better OS (i.e., LND did not improve survival), regardless of whether PSM was performed. Male sex, age ≥60 years, tumor size > 5 cm, and LNM were independent prognostic risk factors for ICC. Whether the LND number was ≥6 was unrelated to survival. The survival of the LNM > 3 group was significantly worse than that of the LNM 1–3 group. The best approach to LND and a better N staging method should be explored further.
Footnotes
Declaration of conflicting interest: The authors report no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the State Key Project on Infection Diseases of China (2017ZX10201021-007-003); the National Capital Health Research and Development of Special (2018-1-4021); the National Natural Science Foundation of China (81672461); the National Natural Science Foundation of China (81972311); and the Sanming Project of Medicine in Shenzhen (No. SZSM202011010).
ORCID iD: Hanjie Hu https://orcid.org/0000-0003-3417-1839
References
- 1.Amini N, Ejaz A, Spolverato G, et al. Temporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: a population-based analysis. J Surg Oncol 2014; 110: 163–170. [DOI] [PubMed] [Google Scholar]
- 2.Groot Koerkamp B, Fong Y. Outcomes in biliary malignancy. J Surg Oncol 2014; 110: 585–591. [DOI] [PubMed] [Google Scholar]
- 3.Cillo U, Fondevila C, Donadon M, et al. Surgery for cholangiocarcinoma. Liver Int 2019; 39: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg 2014; 149: 565–574. [DOI] [PubMed] [Google Scholar]
- 5.Raoof M, Dumitra S, Ituarte P, et al. Development and validation of a prognostic score for intrahepatic cholangiocarcinoma. JAMA Surg 2017; 152: e170117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma CH, Hwang DW, Song KB, et al. Prognostic factors predicting survival rate over 10 years of patients with intrahepatic cholangiocarcinoma after hepatic resection. Ann Surg Treat Res 2020; 98: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohta S, Morine Y, Imura S, et al. Carbohydrate antigen 19-9 is a prognostic factor which correlates with HDAC1 and HIF-1α for intrahepatic cholangiocarcinoma. Anticancer Res 2019; 39: 6025–6033. [DOI] [PubMed] [Google Scholar]
- 8.Spolverato G, Kim Y, Alexandrescu S, et al. Management and outcomes of patients with recurrent intrahepatic cholangiocarcinoma following previous curative-intent surgical resection. Ann Surg Oncol 2016; 23: 235–243. [DOI] [PubMed] [Google Scholar]
- 9.Sahara K, Tsilimigras DI, Mehta R, et al. A novel online prognostic tool to predict long-term survival after liver resection for intrahepatic cholangiocarcinoma: the “metro-ticket” paradigm. J Surg Oncol 2019; 120: 223–230. [DOI] [PubMed] [Google Scholar]
- 10.Spolverato G, Kim Y, Ejaz A, et al. Conditional probability of long-term survival after liver resection for intrahepatic cholangiocarcinoma: a multi-institutional analysis of 535 patients. JAMA Surg 2015; 150: 538–545. [DOI] [PubMed] [Google Scholar]
- 11.Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol 2018; 7: 52. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XF, Xue F, Dong DH, et al. Number and station of lymph node metastasis after curative-intent resection of intrahepatic cholangiocarcinoma impact prognosis. Ann Surg 2020. doi: 10.1097/SLA.0000000000003788. [DOI] [PubMed] [Google Scholar]
- 13.Bagante F, Spolverato G, Weiss M, et al. Assessment of the lymph node status in patients undergoing liver resection for intrahepatic cholangiocarcinoma: the New Eighth Edition AJCC Staging System. J Gastrointest Surg 2018; 22: 52–59. [DOI] [PubMed] [Google Scholar]
- 14.Altekruse SF, Devesa SS, Dickie LA, et al. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J Registry Manag 2011; 38: 201–205. [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa T, Kamiyama T, Kurauchi N, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 2005; 29: 728–733. [DOI] [PubMed] [Google Scholar]
- 16.Yoh T, Cauchy F, Le Roy B, et al. Prognostic value of lymphadenectomy for long-term outcomes in node-negative intrahepatic cholangiocarcinoma: a multicenter study. Surgery 2019; 166: 975–982. [DOI] [PubMed] [Google Scholar]
- 17.Ma WJ, Wu ZR, Hu HJ, et al. Extended lymphadenectomy versus regional lymphadenectomy in resectable hilar cholangiocarcinoma. J Gastrointest Surg 2019. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Han DH, Choi GH, et al. Oncologic impact of lymph node dissection for intrahepatic cholangiocarcinoma: a propensity score-matched study. J Gastrointest Surg 2019; 23: 538–544. [DOI] [PubMed] [Google Scholar]
- 19.Zhang XF, Chakedis J, Bagante F, et al. Trends in use of lymphadenectomy in surgery with curative intent for intrahepatic cholangiocarcinoma. Br J Surg 2018; 105: 857–866. [DOI] [PubMed] [Google Scholar]
- 20.De Jong MC, Nathan H, Sotiropoulos GC, et al . Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011; 29: 3140–3145. [DOI] [PubMed] [Google Scholar]
- 21.Amin MB, Edge S, Greene F, et al. (Eds.). AJCC Cancer Staging Manual 8th ed. Switzerland: Springer Nature. 2017. [Google Scholar]
- 22.Weber SM, Ribero D, O'Reilly EM, et al. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015; 17: 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers Version 5. 2020, https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1438 (2020, accessed 8th October 2020).
- 24.Navarro JG, Lee JH, Kang I, et al. Prognostic significance of and risk prediction model for lymph node metastasis in resectable intrahepatic cholangiocarcinoma: do all require lymph node dissection. HPB (Oxford) 2020; 22: 1411–1419. [DOI] [PubMed] [Google Scholar]
- 25.Sahara K, Tsilimigras DI, Merath K, et al. Therapeutic index associated with lymphadenectomy among patients with intrahepatic cholangiocarcinoma: which patients benefit the most from nodal evaluation. Ann Surg Oncol 2019; 26: 2959–2968. [DOI] [PubMed] [Google Scholar]
- 26.Lurje G, Bednarsch J, Czigany Z, et al. The prognostic role of lymphovascular invasion and lymph node metastasis in perihilar and intrahepatic cholangiocarcinoma. Eur J Surg Oncol 2019; 45: 1468–1478. [DOI] [PubMed] [Google Scholar]
- 27.Ruzzenente A, Conci S, Viganò L, et al. Role of lymph node dissection in small (≤ 3 cm) intrahepatic cholangiocarcinoma. J Gastrointest Surg 2019; 23: 1122–1129. [DOI] [PubMed] [Google Scholar]