Abstract
The meteoric rise in stem-cell-derived organoid technologies has ushered in a new era of “organoid medicine.” Here we discuss how an organoid center can accelerate the translation of laboratory proof-of-principle experiments into clinical practice by developing and utilizing shared platforms for commercial and medical applications.
Challenges for Organoid Medicine Realization
Organoids hold great promise to revolutionize 21st century healthcare through transforming drug development, precision medicine, and ultimately, transplantation-based therapies for end-stage diseases (Lancaster and Knoblich, 2014). However, in order to realize the full potential of “organoid medicine,” a number of major hurdles need to be overcome. The biological complexity of organoids is a strength, but variability between organoids and cost of industry-scale manufacturing represent challenges for translation. The goal of this forum is to discuss these challenges and to highlight the importance of centralized efforts to accelerate the realization of organoid medicine.
State-of-the-Art Efforts for Organoid Transplantation
One of the ultimate goals is to use organoid tissue for transplantation. Successful preclinical efficacy studies with organoids generated from human adult stem cells (ASCs) or pluripotent stem cells (PSCs) have led to several exciting programs aimed at therapeutic uses (Dekkers et al., 2013; Nakamura and Sato, 2017; Takebe et al., 2013; Table 1). For example, Tokyo Medical and Dental University (TMDU) in Japan is planning to conduct a first-in-human trial of intestinal organoids for treatment against inflammatory bowel disease (IBD). Since 2014, TMDU has been elected as a center of excellence (CoE) by the Japanese agency for medical research and development (AMED), with substantial strategic funding ($1 to $4 million per year per institution over 10 years) to accelerate translation of epithelial-organoid-based therapeutics. Work over the last 4 years has established clinical-grade protocols that include crypt isolation, organoid generation and expansion, and quality-control methodologies. Pending institutional review board (IRB) approval, TMDU plans to initiate a clinical study as early as this year.
Table 1.
Comparison of State-of-the-Art Organoid Centers
| TMDU | The HUB | INTENS | YCU | CuSTOM | |
|---|---|---|---|---|---|
| Mission | Organoid tx | Organoid biobank | Organoid tx | Organoid tx | Organoid medicine |
| Organ | Intestine | Multiple | Intestine | Liver | Multiple |
| Indication | Inflammatory bowel disease | NA | Short gut syndrome | Metabolic liver disease | NA |
| Country | Japan | EU | EU | Japan | US |
| Stem Cell Source | ASC | ASC | ASC | PSC | PSC/ ASC |
| Funding source | AMED | ND | Horizon2020 | AMED | CCHMC/ Philanthropy |
| First in Human | 2018- | ND | ND | 2020- | ND |
| Core facility | − | ND | ND | − | + |
| Industry partners | + | ND | + | + | + |
| Accelerator infrastructure | − | − | − | − | + |
| cGMP facility | + | ND | ND | + | + |
There are similar consortium-based efforts in the EU to clinically translate organoid-based technologies. One effort being led by the INTENS team, an EU-based consortium, is aimed at developing a transplantable/anatomizable adult-stem-cell-derived intestinal construct for treating short bowel syndrome (SBS) (http://www.intens.info/project/workplan/). INTENS is a cross-institutional collaboration including over 9 commercial and academic partners. A second effort is led by Hubrecht Organoid Technology (the HUB), founded by the Hubrecht Institute, KNAW, and University Medical Center Utrecht, in the Netherlands. The HUB has developed a robust diagnostic tool that links patient-specific genetic and phenotypic information to preclinical and clinical drug responsiveness (Sachs et al., 2018). Thus, adult-stem-cell-derived epithelial organoid medicine will soon become a reality.
At the moment, there is only one institution geared toward PSC-derived organoid transplantation. The vascularized liver bud organoid transplantation initiative at the Yokohama City University (YCU) Advanced Medical Research Center was established for the treatment of pediatric metabolic liver disease. Production of vascularized liver bud organoids requires cGMP parallel production and assembly of multiple cell-fate lineages (Takebe et al., 2017), and preclinical animal experiments must address miscellaneous safety issues including carcinogenesis after transplantation. To facilitate this, the center relies on the collaborative efforts of over 8 companies and 3 academic institutions for developing semi-automated manufacturing systems. Each group brings a distinct expertise, including cGMP protocols, robotics, culture media, matrices, culture plates, microscopy systems, logistics, delivery devices, cryopreservation, or animal models. As product components, manufacturing strategy, know-how, and IP portfolio can be re-used for many other organoids, such integral efforts will eventually benefit each partner working in a different manufacturing space. This is, however, a challenging model, as individual components are heavily interdependent.
As a USA-based example tackling organoid medicine translation, Cincinnati Children’s Hospital Medical Center has established a Center for Stem Cell and Organoid Medicine (CuSTOM). CuSTOM integrates over 15 collaborative research faculty groups using a diversity of organoid platforms in collaboration with a number of industrial and philanthropic partners. Based on its strong endoderm development expertise, the initial aims of CuSTOM include the development of a gastrointestinal and liver organoid system for drug testing and transplantation. One major distinction between CuSTOM and other initiatives is an organoid-specialized infrastructure that includes high-end scientific cores, a blanket IRB, streamlined processes for generating patient-derived-iPSCs, a cGMP facility, and an accelerator laboratory focused on clinical and commercial translation with a designated space to host industry collaborators. The mission is to accelerate the pace of the organoid-based discovery-translation cycle.
How We Get There: Promise of the Organoid Center Concept
Distinct from the traditional pharmaceutical model, organoid-based diagnostics and therapeutics face a number of significant challenges prior to successful translation, particularly due to the multi-functional and structurally complex nature of organoids. However, the diverse skill sets required to address these key challenges are by far broader than any single academic lab or single collaborative company. Thus, organoid medicine can be realized through an effective acceleration strategy that integrates these diverse skill sets. We herein discuss the challenges associated with acceleration and highlight the importance of targeting four different phases of organoid transformation cycles (Figure 1) that can potentially be united by establishing an organoid center.
Figure 1. Major Acceleration Targets for Organoid Medicine Realization.
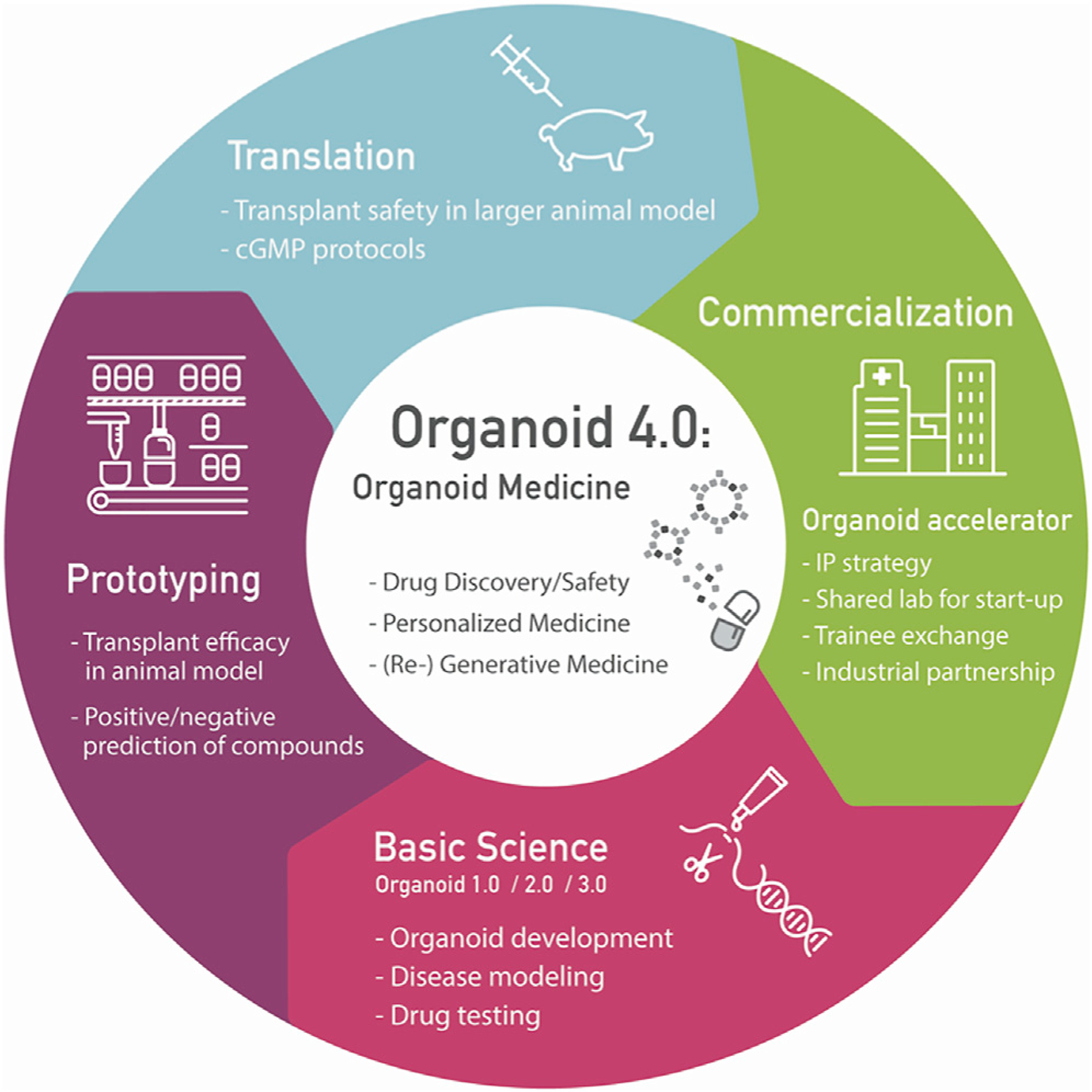
To help launch a new age of organoid medicine applications, including drug safety and discovery, personalized medicine, and (re-)generative medicine, systematic acceleration strategies targeting four main phases of the transformation cycle will be critical.
Focus Area 1: Organoid Science
Decades of research using model organisms have provided a foundational knowledge of embryonic organ development that has informed directed differentiation strategies of PSCs toward multi-lineage 3D organoids. The close proximity of strong developmental and regenerative biology programs will be essential for engineering next-generation organoids with organ-level complexity. Recently, developmental paradigms have informed generation of vascularized (Takebe et al., 2013) and innervated organoids (Workman et al., 2017), and it is anticipated that similar efforts could be used for incorporating other essential components such as immune cells and lymphatics. Perhaps more critically, while human PSC-derived organoids have an impressive array of organ functions, they still do not have the full, broad-spectrum functionality of their adult counterparts. One reason for this could be the speed at which human organoids develop relative to human fetal gestation. Thus, generating more functional and complex tissues is essential for modeling major diseases and ultimately for transplantation-based therapies.
To accelerate the advancement of organoid development, evolving scientific tools including clonal genome editing, high content imaging, and single cell genomics are critical to the study of cell behaviors such as migration, cell-cell interactions, and tissue organization during embryogenesis (Sasai et al., 2013). Institutions that invest in research cores with capabilities for stem cell genomic manipulations, multi-omics, and the supporting bioinformatics, 4D imaging, mechano-characterization, in vivo modeling, and in silico modeling are at a distinct advantage.
Obtaining reference sample data to benchmark organoids with in vivo counterparts will be important for the eventual reverse engineering that will be needed to discover factors that will promote organoid maturation. In humans, considerable metabolic and physiological maturation occurs during the third trimester and shortly after birth; however, little is known about the molecular pathways that drive this process. Given the challenges associated with ethical approval, limited sampling access, and high-volume data management, broader cross-institutional collaborative networks with clinicians will be ideal. These efforts will be potentially facilitated by exciting ongoing initiatives such as the Human Cell Atlas project (Rozenblatt-Rosen et al., 2017).
Focus Area 2: Organoid Prototyping
Personalized medicine is one of the tremendous translational opportunities provided by stem-cell-derived organoids, and it depends on the ability to study patient-specific clinical characteristics. One of the most successful personalized prototypes was reported by teams from the Netherlands, led by the HUB. Precision testing was performed on patient-derived organoids to select effective drugs for treating cystic fibrosis (Dekkers et al., 2013). As another example of personalized therapy, a cancer organoid bank was also recently established for comprehensive drug screening (Sachs et al., 2018). Development of therapeutic prototypes with organoids is thus a critical process for harnessing translation. However, most of the academic laboratories face challenges in defining the specific target area of their prototype, and this challenge may eventually benefit individual industry partners working in various therapeutic spaces toward different commercialization goals. Thus, early functional collaboration with industry partners can help academic labs narrow down their organoid prototyping focus.
Organoid prototyping requires insight from bioengineering and material sciences to optimize reproducibility, standardize processes for organoid manufacturing, and design platforms for screening or transplantation. For example, INTENS organizes a consortium-type collaboration to deliver an organoid-based autologous tissue engineering construct to SBS patients for functional bowel reconstruction, overcoming organ shortage and avoiding the need for immunosuppression. Recent advances in organoid research have come from synergistic interactions between biologists and bioengineers controlling fluid flow and the mechanical properties of the organoids. Encapsulation of engineered tissues is becoming a standard for PSC-based therapy applications in order to ensure safety, which also would be an important consideration for organoid transplantation.
One potential mechanism to promote this collaboration will be through “centralization,” where industry and academic scientists work side-by-side on projects of common interest and benefit by residing physically in the same space. For example, in the cell therapy arena, a new successful partnership between CiRA (the Center for iPS Research and Application, Kyoto University) and Takeda Pharmaceuticals, known as T-CiRA, was initiated at the end of 2015. This particular collaboration brings together Takeda’s pharmaceutical expertise with the expertise of academic stem cell researchers to clinically translate induced pluripotent stem cell (iPSC) technology. This partnership is also distinct from other prevailing biomedical collaborations, as academic investigators can physically work inside the Takeda research center and interact with Takeda research and development scientists, including bioengineering specialists (Karagiannis et al., 2017). To maximize the impact of organoid prototyping, we envision that similar efforts—namely, an “accelerator laboratory” recently installed in CuSTOM—will be an effective strategy to organically catalyze organoid-centered collaboration between groups with complementary expertise. This accelerator laboratory will likely host academics with interests in prototyping and company collaborators, as well as shared personnel and infrastructure, all facilitating organoid-centered prototype product development.
Focus Area 3: Organoid Translation
A major step toward translation includes establishing biobank libraries of clinical-grade organoids from stem cells of healthy and diseased controls. We propose that this would be facilitated by streamlined institutional stem cell cores and broad centralized IRBs with databases containing both clinical and genetics data. The databases would be coordinated by a centralized oversight group that manages the storage and shipping of specific tissues and maintains patient data in the registry. This system can provide tissues and de-identified data to researchers developing disease-specific organoid prototypes to study population level variability. We advocate for broad cross-institutional consent for the collection of tissues to optimize the collection of rare condition samples.
Establishment of in vivo transplantation assays requires animal models, traditionally immunocompromised mice (non-obese, diabetic, severe combined immuno-deficiency interleukin-2Rɣnull [NSG or NOG]). More recently, the field has moved to humanized murine models, wherein a human immune system is reconstituted in the mice. However, larger animal models, such as pigs, need to be established before organoid transplantation in humans can occur. This will require teams of clinicians, engineers, and basic scientists working together to establish reproducible procedures and assays that mimic known human responses to therapies in healthy and diseased states. Current challenges include access to immunosuppressed animals and housing facilities following transplantation, all within in a single institution, as currently available methods for organoid shipment to a distant animal facility limit potency when organoids are transplanted.
Ultimately, all tissue for transplantation will need to be generated using scalable cGMP methodology that addresses the challenges related to reproducibility and quality control. Pioneering attempts in cell-based therapy, such as hematopoietic or mesenchymal stem cell therapy, have highlighted key manufacturing and regulatory challenges; however, many of these principles are simply not applicable to organoids. Therefore, early access to cGMP facilities is crucial—trying to adapt organoid protocols optimized with gel and culture media containing animal products could result in going back to the drawing board and delaying clinical translation by years. Teams at TMDU and YCU tackled this by supporting non-pharma collaborators as they developed a manufacturing pipeline of cGMP-level multi-cellular organoid products. Thus, academic-industry-medical partnerships are important at the early research and development stages so that research prototypes can be efficiently translated into FDA-compliant products and procedures.
Focus Area 4: Organoid Commercialization
Commercialization for both current and novel medical therapeutic approaches will be a key step in making organoid medicine a reality, starting with drug development. The pharmaceutical industry loses billions of dollars annually due to the failures of drug candidates identified in initial screens; this is followed by nearly one-third of drugs being withdrawn from the market, often because of toxicity and inadequate efficacy. This low success rate is due in large part to the reliance of preclinical screens on mouse models and transformed cell lines that do not replicate human physiology. Organoid-based drug assays offer a potential solution to improve predictive accuracy of safety and efficacy in preclinical studies. The total addressable market (TAM) for toxicity screening is estimated to be $9.8 billion with a 14.7% compound annual growth rate (CAGR) (according to a recent BCC research report), whereas drug discovery has a $60B TAM with 11.3% CAGR. Importantly, the organoid platforms being developed for drug screening can also be relevant in the research tool market (TAM: $8.4B, 17.6% CAGR) and the organoid therapeutic market (TAM: $18B, 35% CAGR). Current market estimates for organoid therapy are limited but could potentially expand after organoid transplant therapy demonstrates clear efficacy in first-in-human trials. Organoid centers should provide legal and commercial advisors to support process validation studies that are essential for commercialization but are not funded by typical research grants, facilitating the establishment of new companies or industrial collaborations.
Future Outlook
In order to effectively translate the promising potential of organoid medicine, the academic proof-of-principle is rapidly evolving. Although numerous questions and challenges remain, it is anticipated that bridging current gaps is achievable in a timely manner if approached using a shared-accelerator model. The promise of effectively treating patient-specific diseases by organoid medicine is within reach. The goal is to arrive at this point in the most efficient manner.
ACKNOWLEDGMENTS
We apologize to the many centers whose work could not be cited owing to article restrictions. We thank Drs. Mamoru Watanabe and Ryuichi Okamoto for providing us TMDU’s efforts for organoid transplantation and Asuka Kodaka for creating illustration materials. This work is jointly supported by the Cincinnati Children’s Research Foundation, AMED, NIH U01DK103117, Intestinal Stem Cell Consortium, and the Farmer Foundation. T.T. is a New York Stem Cell Foundation - Robertson Investigator.
Footnotes
DECLARATION OF INTERESTS
The authors declare no interests related to this article.
REFERENCES
- Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, et al. (2013). A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med 19, 939–945. [DOI] [PubMed] [Google Scholar]
- Karagiannis P, Onodera A, and Yamanaka S (2017). New models for therapeutic innovation from Japan. EBioMedicine 18, 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, and Knoblich JA (2014). Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125. [DOI] [PubMed] [Google Scholar]
- Nakamura T, and Sato T (2017). Advancing intestinal organoid technology toward regenerative medicine. Cell Mol Gastroenterol Hepatol 5, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Stubbington MJT, Regev A, and Teichmann SA (2017). The Human Cell Atlas: from vision to reality. Nature 550, 451–453. [DOI] [PubMed] [Google Scholar]
- Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, et al. (2018). A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172, 373–386.e310. [DOI] [PubMed] [Google Scholar]
- Sasai Y (2013). Cytosystems dynamics in self-organization of tissue architecture. Nature 493, 318–326. [DOI] [PubMed] [Google Scholar]
- Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, et al. (2013). Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484. [DOI] [PubMed] [Google Scholar]
- Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, Funayama S, Nakanishi N, Hisai T, Kobayashi T, et al. (2017). Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 21, 2661–2670. [DOI] [PubMed] [Google Scholar]
- Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang CF, Schiesser J, Aubert P, Stanley EG, et al. (2017). Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med 23, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]


