Abstract
In this issue of Cell Metabolism, Asadi Shahmirzadi et al. (2020) demonstrate that late-onset dietary supplementation with calcium alpha-ketoglutarate results in increased survival, compressed morbidity, and reduced frailty in mice. The study provides further evidence for critical links between metabolism, inflammation, and aging.
Alpha-ketoglutarate (aKG) is at the nexus of carbon and nitrogen metabolism. aKG is a key metabolite in the tricarboxylic acid (TCA) cycle, a series of reactions that is vital for oxygen-dependent energy derivation. As the alpha-keto acid of glutamate, it is an important component of amino acid metabolism and is an obligate partner in aminotransferase reactions. It directly or indirectly contributes to a host of catabolic and anabolic pathways (Figure 1). Given its breadth of influence, aKG might be viewed as a fundamental metabolic intermediate. Previous work in D. melanogaster and C. elegans demonstrated that aKG could regulate longevity in short-lived species (Chin et al., 2014; Su et al., 2019). In this issue of Cell Metabolism, Asadi Shahmirzadi et al. (2020) take it to the next level, showing that aKG extends healthspan and lifespan in mice. This important work is an excellent example of an aging regulatory pathway that is conserved among species, and places renewed emphasis on the role of metabolism in aging biology.
Figure 1. More Than Meets the Eye with the TCA Cycle.
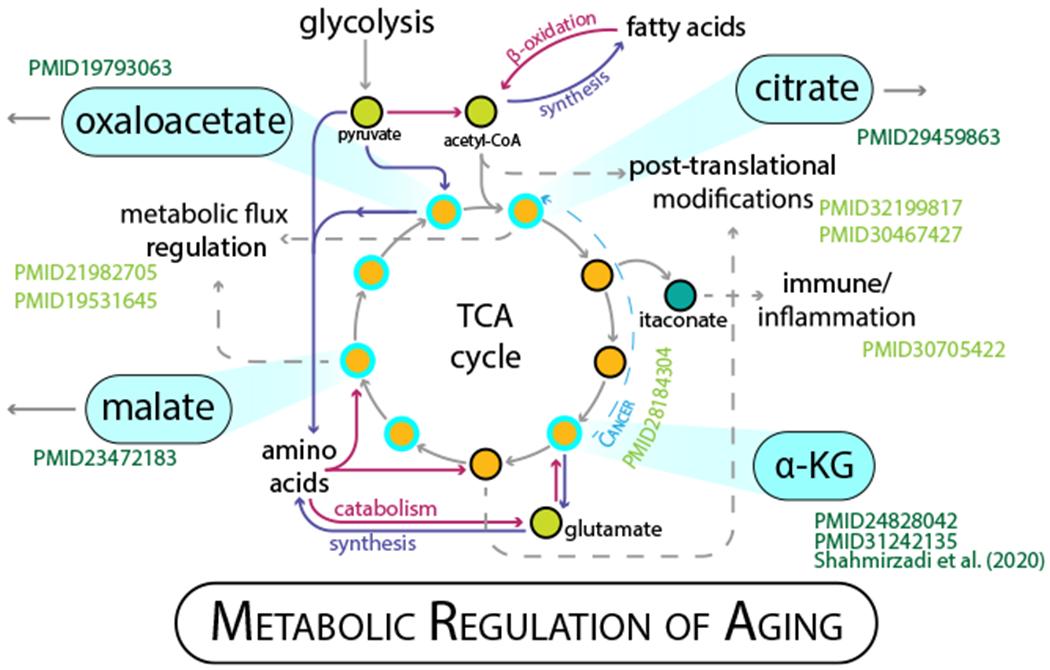
The TCA cycle is a metabolic hub, processing inputs from glucose, fatty acid, and amino acid catabolism; generating reducing equivalents for the respiratory chain; and providing precursors for biosynthesis. What happens in the mitochondria influences cell function in different cellular compartments, including diverse regulatory mechanisms (allostery, post-translational modification, epigenetic regulation of gene expression) and diverse cellular processes (immune and inflammatory pathways, and growth regulation). The main inputs are depicted as circles in neon green, with canonical pathways and functions highlighted by solid arrows and main TCA intermediates in orange. A few of the many signaling roles that metabolites of the TCA participate in are indicated by dashed arrows. Metabolites that have been linked specifically to longevity are highlighted by cyan outlines. Key players including alpha-ketoglutarate, the subject of Asadi Shahmirzadi et al. (2020), are shown in the cyan flyouts. Aging references shown in dark green.
The TCA cycle is a hub for cellular metabolism, taking inputs from multiple sources and using them to derive energy by generating reducing equivalents for the electron transport chain. It has become clear in recent years that many intermediates of the TCA cycle play roles in the cell beyond just serving as spokes of the cycle (Martínez-Reyes and Chandel, 2020). aKG, succinate, fumarate, and citrate (via itaconate) have all been linked to various roles in immune and inflammatory signaling, while aKG and succinate both participate in reactions involved in chromatin modification. The TCA substrate acetyl-CoA is engaged in diverse reactions in multiple cellular compartments (Pietrocola et al., 2015); serving as a substrate for lysine acetylation, it regulates chromatin dynamics and enzyme function and protein stability, exerting unparalleled influence on cellular processes. Given the cardinal position of these intermediates in metabolism and the diverse regulatory roles they are involved in, it is perhaps not surprising that many of them have been linked to longevity. Malate, fumarate, and oxaloacetate have all been shown to extend lifespan in the model organism C. elegans.
The Asadi Shahmirzadi study not only shows that aKG compresses morbidity and delays the onset of aging phenotypes in mice, but what is even more impressive is that these outcomes were observed when treatment was initiated in middle-age. Starting at 18 months of age, male and female mice were fed regular mouse chow supplemented with calcium aKG. The authors report a significant survival benefit in the aKG-treated mice that was somewhat sexually dimorphic. In females, aKG treatment resulted in significant increases in median and maximal (90th percentile) lifespan (16.6% and 19.7%, respectively), while in males median and maximal lifespan were numerically greater, but not significant (9.6% and 12.8%, respectively). Independent of sex, aKG treatment decreased the proportion of life in which mice were frail, with reduction in frailty scores of 46% for females and 41% for males on the aKG diet. Not surprisingly, the authors showed that that higher frailty scores correlated negatively with life expectancy. Increases in healthspan and longevity prompted an investigation of inflammation, a major causal factor in age-related disease vulnerability that is linked to metabolism (Hotamisligil, 2017). Here, too, there was sexual dimorphism: only aKG-fed females were protected against age-dependent increases in circulating inflammatory cytokines. Ex vivo experiments confirmed the ability of aKG to directly impact cytokine production in splenic T cells from treated mice and identified a potential mechanism in the production of significantly higher levels of IL-10, a potent anti-inflammatory cytokine, in cells from aKG mice.
In addition to identifying aKG as a regulator of longevity, several other aspects of the work are important to the aging field overall. A central role of metabolism in aging regulation is also hinted at in studies of caloric restriction (CR): intermediary metabolic pathways are recruited by a variety of mechanisms including transcript abundance, RNA processing, protein abundance, and lysine acetylation (Rhoads et al., 2018). A number of the parameters measured revealed a partial or complete sexual dimorphism; females were more responsive than males to aKG in terms of survival and were alone in exhibiting suppression of the age-related increase in inflammatory tone. This adds to an important growing body of aging research that shows a remarkable effect of sex in aging biology and in longevity regulation (Sampathkumar et al., 2020). Although in the context of diet-induced obesity aKG treatment attenuates weight gain and improves glucose tolerance (Tian et al., 2020), the delayed aging of the aKG mice in this study was not associated with lower body weight or lower adiposity. It remains possible that there may be functional changes in adipose tissue independent of differences in adiposity.
The assessment of healthspan through the use of the frailty index is an important component of the Asadi Shahmirzadi study. The aging field has shifted in recent years to place greater emphasis on measuring health during aging, with consensus frameworks for these assessments available (Huffman et al., 2016). Without the limitation of an insistence of differences in median and maximal lifespan, the focus of genetic, pharmacological, and nutrition studies can now be placed on translational potential. As perfectly illustrated in the Asadi Shahmirzadi study, it is possible to leverage insights from the study of aging in shorter-lived animals to identify agents such as aKG and apply them in rodents (or maybe even nonhuman primates) to demonstrate efficacy in boosting health and longevity.
ACKNOWLEDGMENTS
The authors are supported by federal grants AG040178, AG057408, AG067330, and BX003846, in addition to support from the William S. Middleton Memorial Veterans Hospital.
REFERENCES
- Asadi Shahmirzadi A, Edgar D, Liao C-Y, Hsu Y-M, Lucanic M, Asadi Shahmirzadi A, Wiley CD, Gan G, Kim DE, Kasler HG, et al. (2020). Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab. 32, this issue, 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, et al. (2014). The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 510, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS (2017). Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185. [DOI] [PubMed] [Google Scholar]
- Huffman DM, Justice JN, Stout MB, Kirkland JL, Barzilai N, and Austad SN (2016). Evaluating health span in preclinical models of aging and disease: guidelines, challenges, and opportunities for geroscience. J. Gerontol. A Biol. Sci. Med. Sci. 71, 1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martίnez-Reyes I, and Chandel NS (2020). Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrocola F, Galluzzi L, Bravo-San Pedro JM, Madeo F, and Kroemer G (2015). Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 21, 805–821 [DOI] [PubMed] [Google Scholar]
- Rhoads TW, Burhans MS, Chen VB, Hutchins PD, Rush MJP, Clark JP, Stark JL, Mcllwain SJ, Eghbalnia HR, Pavelec DM, et al. (2018). Caloric restriction engages hepatic RNA processing mechanisms in rhesus monkeys. Cell Metab. 27, 677–688.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar NK, Bravo JI, Chen Y, Danthi PS, Donahue EK, Lai RW, Lu R, Randall LT, Vinson N, and Benayoun BA (2020). Widespread sex dimorphism in aging and age-related diseases. Hum. Genet. 139, 333–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Wang T, Wu N, Li D, Fan X, Xu Z, Mishra SK, and Yang M (2019). Alpha-ketoglutarate extends Drosophila lifespan by inhibiting mTOR and activating AMPK. Aging (Albany N.Y.) 11,4183–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Zhao J, Yang Q, Wang B, Deavila JM, Zhu M-J, and Du M (2020). Dietary alpha-ketoglutarate promotes beige adipogenesis and prevents obesity in middle-aged mice. Aging Cell 19,e13059. [DOI] [PMC free article] [PubMed] [Google Scholar]


