Abstract
Objective:
To evaluate the feasibility of a multi-centre randomised controlled trial to compare the clinical and cost-effectiveness of early patient-directed rehabilitation versus standard rehabilitation following surgical repair of the rotator cuff of the shoulder.
Design:
Two-arm, multi-centre pilot and feasibility randomised controlled trial.
Setting:
Five National Health Service hospitals in England.
Participants:
Adults (n = 73) with non-traumatic rotator cuff tears scheduled for repair were recruited and randomly allocated remotely prior to surgery.
Interventions:
Early patient-directed rehabilitation (n = 37); advised to remove their sling as soon as able and move as symptoms allow. Standard rehabilitation (n = 36); sling immobilisation for four weeks.
Measures:
(1) Randomisation of 20% or more eligible patients. (2) Difference in time out of sling of 40% or more between groups. (3) Follow-up greater than 70%.
Results:
73/185 (39%) potentially eligible patients were randomised. Twenty participants were withdrawn, 11 due to not receiving rotator cuff repair. The between-group difference in proportions of participants who exceeded the cut-off of 222.6 hours out of the sling was 50% (80% CI = 29%, 72%), with the early patient-directed rehabilitation group reporting greater time out of sling. 52/73 (71%) and 52/53 (98%) participants were followed-up at 12 weeks when withdrawals were included and excluded respectively. Eighteen full-thickness re-tears were reported (early patient-directed rehabilitation = 7, standard rehabilitation = 11). Five serious adverse events were reported.
Conclusion:
A main randomised controlled trial is feasible but would require allocation of participants following surgery to counter the issue of withdrawal due to not receiving surgery.
Keywords: Rehabilitation interventions, randomized controlled trial, physiotherapy, shoulder pain
Introduction
Shoulder pain is one of the most common musculoskeletal pain presentations with disorders of the rotator cuff, the muscles and tendons surrounding the shoulder, widely regarded as the most common contributing factor. 1 The prevalence of rotator cuff abnormalities, including rotator cuff tears, increases with age. 1
First-line treatment for people with shoulder pain and a torn rotator cuff includes advice to modify activity, analgesics, corticosteroid injection, and exercise supported by a physiotherapist.2,3 If insufficient, then surgical repair might be considered. 3 Following surgery, rehabilitation is regarded as important.4,5 However, as surgical technique has progressed and the number of operations has increased, our understanding of the optimal approach to post-operative rehabilitation remains limited and opinions of surgeons and physiotherapists conflicting.6,7 Currently, following rotator cuff repair surgery, most patients are immobilised in a sling for up to six weeks5,8 despite research suggesting that early mobilisation by discarding the sling might speed up recovery without long-term consequence.9–11
We conducted a multi-centre pilot and feasibility randomised controlled trial to evaluate the feasibility of a larger, fully powered, multi-centre randomised controlled trial to compare the clinical and cost-effectiveness of early patient-directed rehabilitation with standard rehabilitation incorporating sling immobilisation for four weeks.
Methods
We conducted a two-arm, multi-centre pilot and feasibility randomised controlled trial (Figure 1). This study was funded by the National Institute for Health Research (NIHR) Research for Patient Benefit programme (PB-PG-0816-20009). This study was sponsored by Keele University (RG-0038-16-PCHS). A favourable ethical review was granted by the Wales Research Ethics Committee 5 Bangor on 31st July 2018 (18/WA/0242). The protocol was registered on the ISRCTN Registry (18357968) on 10 August 2018 and is available via https://doi.org/10.1186/ISRCTN18357968. The comprehensive protocol has also been published including detail on methods, sample size calculation, data analysis and interventions. 12 Recruitment took place between November 2018 and November 2019.
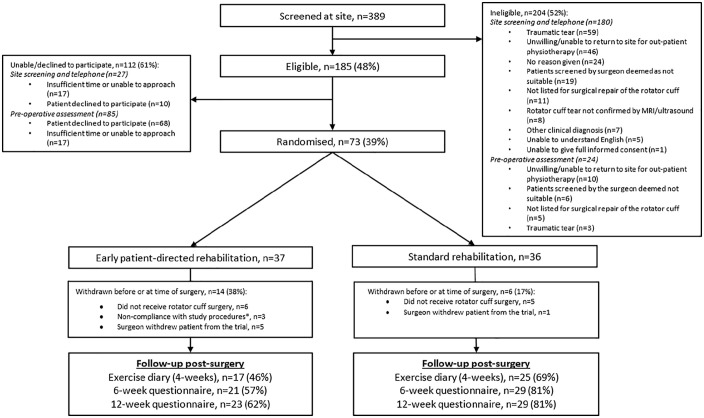
Figure 1.
CONSORT flow diagram.
*Missed early patient-directed rehabilitation intervention = 1; non-NHS surgery = 1; unwilling to return to main or affiliated NHS site for post-operative rehabilitation = 1.
We worked with patients to develop study processes including recruitment, and develop patient-facing materials including participant information sheets. Additionally patient representatives contributed to study management through the Trial Management Group, study oversight through membership of the Trial Steering Committee, and decisions about next steps.
Patients for the randomised controlled trial were identified, invited, screened and recruited from the orthopaedic departments of five National Health Service hospitals in England according to the following criteria:
(1) Patients diagnosed with a non-traumatic symptomatic tear of the rotator cuff and listed for surgical repair
(2) Rotator cuff tear confirmed by ultrasound or MRI
(3) Aged ⩾18 years
(4) Patients screened by the surgeon as suitable to participate
(5) Able to return to the recruiting centre or affiliated site for the initial out-patient follow-up physiotherapy appointment
(6) Access to a mobile phone and willing and able to receive and respond to text messages
(7) Able to understand English
Patients were excluded if it was deemed that they were unable to give full informed consent.
After providing written informed consent, participants were randomly allocated to early patient-directed rehabilitation or standard rehabilitation at variable time points but prior to surgery. Stratified block (block sizes of two and four by recruiting site) randomisation on a 1:1 ratio was undertaken remotely using web-based randomisation, developed and supported by Keele Clinical Trials Unit, to ensure allocation concealment and parity in numbers per treatment group per recruitment site.
Early patient-directed rehabilitation included advice from a physiotherapist to remove the post-operative sling as soon as possible and gradually begin actively using their arm as soon as able within acceptable limits of their pain. Discomfort with movement was permissible providing it was acceptable to the individual participant. Sling removal and movement was progressed by the individual participant over time and according to agreed goals within the context of their own pain experience and tolerance.
In contrast, standard rehabilitation included advice from a physiotherapist to maintain the sling in situ at all times for four weeks and only to remove it while eating, washing, dressing and performing daily exercises prescribed by the physiotherapist.
The key difference between early patient-directed rehabilitation and standard rehabilitation was the advice and support to remove the sling and move the arm, or not. From four weeks post-surgery, both groups continued a similar programme of rehabilitation supported by a physiotherapist. The physiotherapists received study specific training and were able to treat patients in both groups.
The main measures for determining feasibility were:
(1) Number of potentially eligible patients recruited
(2) Number of participants found not to have a rotator cuff tear during surgery (false positive scan)
(3) Feasibility of recruiting participating centres
(4) Rate of retention and response to questionnaires and text messages
(5) Intervention fidelity with regard to time out of sling
(6) Comparison of the sensitivity to change of the potential primary outcome measures for the main trial (Oxford Shoulder Score and Shoulder Pain and Disability Index)
(7) Treatment satisfaction (using 5-point ordinal scales from very satisfied to very dissatisfied)
(8) Patient and clinician views about treatment acceptability (qualitative study – reported separately).
Clinical status and outcomes were collected at baseline, 6-weeks and 12-weeks post-surgery by postal questionnaire. The Oxford Shoulder Score is a 12-item shoulder-specific self-report measure which is reliable, valid, responsive and acceptable to patients.13–15 The Shoulder Pain and Disability Index is also a shoulder-specific self-report measure which is reliable, valid, responsive and is the most commonly used outcome measure in randomised controlled trials of non-surgical interventions.16–18 The EQ-5D-5L is a generic measure of health related quality of life that provides a single index value for health status. 19 Other clinical outcomes included global rating of change on a 6-point ordinal scale, time taken to return to driving, and days lost from work. A patient-completed diary detailing time spent out of sling and adherence with exercise was also completed for four weeks post-surgery. Two text messages were sent each week for 12 weeks; the first asking about pain in the last week and the second asking about work or activity interference, both on a 5-point ordered categorical scale derived from the Oxford Shoulder Score. At 12-weeks post-surgery, a diagnostic ultrasound scan was undertaken to determine the number of rotator cuff tendon re-tears. Number of adverse events post-surgery were collected up to 12-weeks post-surgery via clinician and patient self-report questionnaire.
The following pre-specified success criteria were agreed:
(1) Consent and randomisation of 20% or more of eligible patients
(2) Difference in time with arm out of the sling of 40% or more, that is, the early patient-directed rehabilitation group would report 40% more time out of the sling than the standard rehabilitation group in the first four weeks after surgery
(3) Follow up rates for potential main outcome measure(s) >70% for questionnaires and text messages.
Surgeons were blinded to group allocation until after the surgery to minimise the likelihood of withdrawal through knowledge of the allocated intervention. Ultrasound scan findings were withheld from patient participants and clinicians. No further measures to blind participants, clinicians, research team or oversight committees were implemented.
The sample size was based on the ability to detect a difference in time spent out of sling between the two randomised groups. Allowing 20% for missing adherence-related data, 76 participants would provide 90% power to detect at least a minimum 40% difference in sling use between the two groups, given 1-sided 5% significance level.
As this was a pilot and feasibility randomised controlled trial, the analysis focuses mainly on description of feasibility outcomes. Mean values and confidence intervals of clinical outcomes are calculated, as well as sensitivity to change of the Oxford Shoulder Score and Shoulder Pain and Disability Index by examining the effect size statistic, standardised response mean and Guyatt responsiveness index. To evaluate the difference in time spent out of sling between groups, a cut-off of 222.6 hours over four weeks (7.95 hours per day) was identified as the time whereby 30% of the standard rehabilitation group exceeded that cut-off. The detailed statistical analysis plan was agreed with the independent Trial Steering Committee before the end of recruitment and prior to commencing analysis.
Results
The number of patients screened and participants recruited and followed-up post-surgery is presented in Figure 1. 73/185 eligible patients (39%) were recruited. Eight surgeons treated at least one participant in the randomised controlled trial. Thirty-one physiotherapists with a mean of 16 years of clinical experience supported delivery of the rehabilitation interventions. The baseline characteristics of the patient participants are described in Table 1.
Table 1.
Baseline characteristics of the patient participants.
| All (n = 73) | Early patient-directed rehabilitation (n = 37) | Standard rehabilitation (n = 36) | Number of participants completing the item via questionnaire | |
|---|---|---|---|---|
| Age (years), mean (SD) | 63.0 (10.2) | 60.6 (9.9) | 65.4 (10.0) | 73 |
| Males, n | 42 | 19 | 23 | 73 |
| Current employment status, n | 73 | |||
| Employed | 28 | 14 | 14 | |
| Unemployed/seeking work | 2 | 1 | 1 | |
| Housewife/husband | 4 | 2 | 2 | |
| Retired | 32 | 14 | 18 | |
| Not working because of health | 5 | 4 | 1 | |
| Other | 2 | 2 | 0 | |
| Weight (kg), mean (SD) | 89.3 (26.3) | 84.2 (18.0) | 94.5 (32.1) | 73 |
| Height (cm), mean (SD) | 168.7 (10.3) | 168.6 (8.5) | 168.7 (12.1) | 73 |
| Diabetic | 72 | |||
| Yes, n | 10 | 4 | 6 | |
| No, n | 62 | 32 | 30 | |
| Smoking status | 73 | |||
| Current tobacco smoker | 9 | 8 | 1 | |
| Past tobacco smoker | 31 | 14 | 17 | |
| Current e-cig vaper | 1 | 1 | 0 | |
| Past e-cig vaper | 1 | 0 | 1 | |
| Never smoked or vaped | 31 | 14 | 17 | |
| How long have you had shoulder pain? (months), median (IQR) | 18 (7–24) | 18 (7–24) | 14.5 (7–36) | 72 |
| Preference for treatment, n | 71 | |||
| Early movement | 31 | 14 | 17 | |
| Using a sling | 3 | 1 | 2 | |
| No preference | 37 | 20 | 17 | |
| Shoulder to be operated on, n | 72 | |||
| Left | 27 | 15 | 12 | |
| Right | 45 | 22 | 23 | |
| Size of tear (cm), mean (SD)* | 2.7 | 2.96 | 2.5 | 44 |
| Location of tear, n [tear can be in multiple locations] | ||||
| Supraspinatus | 58 | 28 | 30 | 73 |
| Infraspinatus | 13 | 7 | 6 | 73 |
| Subscapularis | 7 | 1 | 6 | 73 |
| Teres Minor | 0 | 0 | 0 | 73 |
| Health related quality of life (EQ-5D-5L), mean (SD) | 0.53 (0.25) | 0.53 (0.27) | 0.53 (0.23) | 71 |
| Shoulder pain and function (OSS), mean (SD)** | 25.1 (9.2) | 24.8 (9.5) | 25.4 (9.0) | 73 |
| Shoulder pain and function (SPADI), mean (SD) | 62.0 (19.9) | 63.3 (19.6) | 60.7 (20.5) | 73 |
EQ-5D-5L (Crosswalk), −0.59–1.00 (−0.59 = Worst health utility, 1.00 = best health utility); Oxford Shoulder Score (OSS), 0–48 (0 = most severe symptoms, 48 = least symptoms); Shoulder Pain and Disability Index, 0–100 (0 = best, 100 = worst).
Information taken from the post-operative case report form.
One item on the scale was missing for one participant, however, the scale allows for two missing items and the appropriate rules were applied, so scores were still able to be calculated.
Of the 73 participants recruited, 20 were subsequently withdrawn before or at the time of surgery (Figure 1).
Fifty-three post-operative case report forms detailed completeness of the surgical repair. 47/53 were complete repairs (early patient-directed rehabilitation = 17, standard rehabilitation = 30) and six were partial repairs (early patient-directed rehabilitation = 6). There were no reports of inability to repair in the completed case report forms.
At six weeks, follow-up defined by return of questionnaire including the Oxford Shoulder Score, Shoulder Pain and Disability Index, EQ-5D-5L, and healthcare resource was 68% (50/73) (Table 2). Excluding withdrawals, follow-up was 94% (50/53). At 12 weeks, follow-up was 71% (52/73) (Table 2). Excluding withdrawals, follow-up was (98%) (52/53).
Table 2.
Number of questionnaires returned at 6 and 12 weeks and the clinical outcome scores for the Oxford shoulder ccore and shoulder pain and disability index.
| Overall |
Early patient-directed rehabilitation |
Standard rehabilitation |
||||
|---|---|---|---|---|---|---|
| OSS | SPADI | OSS | SPADI | OSS | SPADI | |
| Six weeks | ||||||
| Number of questionnaires returned | 50 | 50 | 21 | 21 | 29 | 29 |
| Mean (SD) | 25.7 (10.9) | 49.8 (25.2) | 26.6 (11.1) | 47 (26.1) | 25.1 (10.9) | 51.8 (24.8) |
| 12 weeks | ||||||
| Number of questionnaires returned | 52 | 52 | 23 | 23 | 29 | 29 |
| Mean (SD) | 34.3 (9.3) | 34.7 (26.2) | 35.6 (8.4) | 31.6 (25) | 33.2 (9.9) | 37.1 (27.3) |
Oxford Shoulder Score (OSS), 0–48 (0 = most severe symptoms, 48 = least symptoms); Shoulder Pain and Disability Index, 0–100 (0 = best, 100 = worst).
At 6- and 12-weeks, the mean difference in Oxford Shoulder Score (positive value favours early patient-directed rehabilitation) was 1.5 (80% CI −2.6, 5.6) and 2.4 (80% CI −1.0, 5.7) respectively. At 6- and 12-weeks, the mean between-group difference in Shoulder Pain and Disability Index score (negative value favours early patient-directed rehabilitation) was −4.8 (80% CI −14.3, 4.6) and −5.5 (80% CI −15.0, 4.1) respectively.
Of the 852 text messages sent, 524 (62%) ‘pain’ responses, and 495 (58%) ‘function’ responses were returned. Excluding withdrawals, of the 612 text messages sent, 524 (86%) ‘pain’ responses, and 495 (81%) ‘function’ responses were returned.
At four weeks, excluding withdrawals, diary data on time spent out of sling were available for 42/53 participants (early patient-directed rehabilitation = 17/23, standard rehabilitation = 25/30). In the early patient-directed rehabilitation group, 14 of 17 participants who returned diaries recorded time spent out of sling greater than 222.6 hours over the 4-week period. Comparing the proportion of participants in the early patient-directed rehabilitation group (n = 14) who exceeded the cut-off compared to those in the standard rehabilitation group (n = 8) produced a statistically significant difference between groups (P = 0.0038) {χ2 = 8.37}. The difference in proportions between the two groups was 50% (80% CI = 29%, 72%; 90% CI = 24%, 77%).
Self-report adherence to the prescribed exercise is reported in Table 3.
Table 3.
Participant self-report of exercise adherence rates based on the question ‘To what extent do you agree with the following statement? ‘I have been doing my exercises as often as prescribed over the last week.’
| Time-point post-surgery | Early patient-directed rehabilitation | Standard rehabilitation |
|---|---|---|
| Week 1, n agree/strongly agree | 13/17 | 18/23 |
| Week 2, n agree/strongly agree | 13/17 | 21/24 |
| Week 3, n agree/strongly agree | 14/16 | 21/24 |
| Week 4, n agree/strongly agree | 12/14 | 20/22 |
Agree/strongly agree.
To inform a conservative sample size calculation for the future fully powered randomised controlled trial, the upper 80% confidence limit for the standard deviation of average follow-up score for the Oxford Shoulder Score and Shoulder Pain and Disability Index are 12 and 30, respectively. Over 12 weeks, the sensitivity to change for both the Oxford Shoulder Score and Shoulder Pain and Disability Index according to the effect size statistic were 0.89 and 1.29, the standardised response mean were 0.71 and 0.86, and the Guyatt responsiveness index based on effect size were 1.18 and 1.21, respectively. This indicates that the Shoulder Pain and Disability Index is the most responsive of the two measures.
Table 4 reports the secondary outcomes.
Table 4.
Secondary outcomes.
| Six weeks | Overall (n = 50) | Early patient-directed rehabilitation (n = 21) | Standard rehabilitation (n = 29) |
|---|---|---|---|
| EQ-5D-5L, mean (SD) [n = 49] | 0.62 (0.21) | 0.58 (0.24) | 0.64 (0.19) |
| EQ-VAS, mean (SD) [n = 48] | 73.2 (18.3) | 71.5 (20.7) | 74.4 (16.7) |
| Global change [n = 50] | |||
| Completely recovered, n | 1 | 0 | 1 |
| Much better, n | 13 | 6 | 7 |
| Better, n | 24 | 10 | 14 |
| No change, n | 6 | 2 | 4 |
| Worse, n | 5 | 3 | 2 |
| Much worse, n | 1 | 0 | 1 |
| Return to driving | |||
| Number not returning to driving [n = 49] | 15 | 6 | 9 |
| Time to return to driving (days), mean (SD) [n = 29] | 27.8 (14.5) | 21.4 (11.5) | 31.8 (14.9) |
| Time to return to driving (days), median (IQR) [n = 29] | 28 (20–42) | 21 (14–27) | 38.5 (23–42) |
| Days lost from work, mean (SD) [n = 21] | 36.3 (21.4) | 37.8 (22.2) | 35.2 (21.8) |
| Twelve weeks | (n = 52) | (n = 23) | (n = 29) |
| EQ-5D-5L, mean (SD)* [n = 48] | 0.68 (0.19) | 0.67 (0.16) | 0.69 (0.21) |
| EQ-VAS, mean (SD) | 74 (18.1) | 76.2 (19.6) | 72.2 (17.1) |
| Global change [n = 51] | |||
| Completely recovered, n | 5 | 2 | 3 |
| Much better, n | 22 | 10 | 12 |
| Better, n | 12 | 4 | 8 |
| No change, n | 6 | 3 | 3 |
| Worse, n | 4 | 3 | 1 |
| Much worse, n | 2 | 0 | 2 |
| Return to driving | |||
| Number not returning to driving [n = 49] | 8 | 3 | 5 |
| Time to return to driving (days), mean (SD) [n = 36] | 33.3 (19.5) | 25.6 (17.0) | 39.6 (19.4) |
| Time to return to driving (days), median (IQR) [n = 36] | 30 (20–42) | 24 (13–30) | 42 (30–45) |
| Days lost from work, mean (SD) [n = 20] | 46.1 (27.5) | 43.9 (25.3) | 47.8 (30.3) |
One participant in the early patient-directed rehabilitation group did not complete the scale completely, therefore a score could not be calculated.
At 12 weeks, 19/22 of participants in the early patient-directed rehabilitation group and 24/27 of participants in the standard rehabilitation group reported being very satisfied or satisfied with their treatment.
Fifty case report forms describing the findings of the 12-week ultrasound scan were available. Eighteen full-thickness re-tears were reported (early patient-directed rehabilitation = 7/23, standard rehabilitation = 11/27). Of the six partial repairs, three had full-thickness re-tears. Three case report forms (all in the standard rehabilitation group) were not completed due to two participants needing clinical intervention before the 12-week time point and thus not receiving an ultrasound scan, and one participant cancelled their ultrasound scan.
Twenty-one non-serious adverse events (early patient-directed rehabilitation = 7, standard rehabilitation = 14) and five serious adverse events (early patient-directed rehabilitation = 2, standard rehabilitation = 3) were reported. Non-serious adverse events were: increased pain post-surgery (early patient-directed rehabilitation = 2, standard rehabilitation = 5), unconfirmed deep vein thombosis (standard rehabilitation = 1), treatment for non-shoulder related conditions (standard rehabilitation = 5), further non-surgical shoulder treatment (early patient-directed rehabilitation = 1, standard rehabilitation = 3), feeling generally unwell (early patient-directed rehabilitation = 1), fall (early patient-directed rehabilitation = 1), suspected infection (early patient-directed rehabilitation = 1), anaemia (early patient-directed rehabilitation = 1).
Unrelated serious adverse events were; detached biceps tendon (standard rehabilitation = 2), pulmonary embolism (early patient-directed rehabilitation = 1). Related serious adverse events were: symptomatic rotator cuff re-tear requiring further surgery (early patient-directed rehabilitation = 1, standard rehabilitation = 1).
Discussion
Based on these findings, a fully powered randomised controlled trial comparing the clinical and cost-effectiveness of early patient-directed rehabilitation versus standard rehabilitation would be feasible with minor amendment to the research design. With reference to our predefined success criteria, 73/189 (39%) eligible patients were recruited, exceeding the target of 20%. A 50% proportional difference was identified between the groups for time spent out of sling (14/17 in the early patient-directed rehabilitation group, 8/25 in the standard rehabilitation group), exceeding the target of 40%. When withdrawals are included, the target of over 70% follow-up rates for the potential primary outcome measures at six weeks (50/73; 68%) was not met. This was met at 12 weeks (52/73; 71%) but would be regarded as insufficient in the context of a future randomised controlled trial with longer term follow-up.
The findings suggest that recruitment of eligible patients is feasible and that advice and support to remove the sling, and vice versa, enables a valid comparison between early and standard rehabilitation, incorporating four weeks of sling immobilisation. The principal issue faced was withdrawal due to participants not receiving rotator cuff repair surgery or not receiving surgery at all (11/73), an issue reported in other rotator cuff repair randomised controlled trials. 15 When withdrawals are not included, the follow-up rates at 6 and 12 weeks were 94% and 98% suggesting that the randomised controlled trial follow-up processes were robust. We randomly allocated participants at variable time-points before surgery which risks the withdrawals we observed. In a future randomised controlled trial, consenting participants before surgery but randomly allocating once rotator cuff repair surgery has been undertaken would address this. We also observed six withdrawals requested by surgeons due to perceived risk of early patient-directed rehabilitation. This reason for withdrawal was more common in the early patient-directed rehabilitation group (n = 5) compared to the standard rehabilitation group (n = 1). Therefore in a future randomised controlled trial, working with surgeons who are in equipoise with regard to the comparison of early patient-directed rehabilitation and standard rehabilitation will be paramount.
In a fully powered randomised controlled trial, the target sample size would be 658 randomised participants (329 in each treatment arm) if the following assumptions were adopted; 90% power and 5% two-sided statistical significance to detect a minimally clinically important difference of eight points 17 between the two treatment groups at 12 weeks on the Shoulder Pain and Disability Index, assuming a standard deviation of 30 (conservative estimate from this pilot RCT) and accounting for an expected 10% loss to follow-up.
Other recent randomised controlled trials have been reported.11,20,21 Mazzocca et al. 20 (n = 73) compared early movement (starting two to three days after surgery) versus delayed movement (starting 28 days after surgery), similar to standard rehabilitation reported here. There was no statistically significant difference in Western Ontario Rotator Cuff scores at six months, but the early movement group reported lower scores (better clinical status) throughout the post-operative period. After six months, there was no difference in re-tear rates (31% vs 34%). Sheps et al. 11 (n = 206) compared sling weaning and early pain-free mobilisation versus standard rehabilitation, including immobilisation, following arthroscopic rotator cuff repair (all tear sizes included). They reported statistically significant improvement in range of movement at six weeks for the early mobilisation group, but no difference in other clinical outcomes. After one year there was no difference in re-tear rates (30% vs 33%) and after two years there was no difference in clinical outcomes. Tirefort et al. 21 (n = 80) compared no sling versus the use of a sling for the first four post-operative weeks following arthroscopic rotator cuff repair (small to medium tears included). There was a statistically significant improvement in range of movement at six and 12 weeks for the no sling group. At six months there was no difference in clinical outcomes or repair integrity.
The findings of these randomised controlled trials reflect the results of our previous systematic review 4 suggesting that early mobilisation facilitates a more rapid recovery of uncertain clinical importance without long-term consequence. Despite this, most patients continue to be immobilised in a sling for up to six weeks with increasing tendency to immobilise as the size of the tear increases.7,8 In contrast to previous interventions evaluated in these randomised controlled trials, the RaCeR early patient-directed rehabilitation intervention is more progressive in nature in terms of encouragement to discard the sling immediately, permitting active movement, and enabling participants to use their acceptable symptom response as a guide. The clinical outcomes reported here provide a signal of effectiveness of early patient-directed rehabilitation given the upper bounds of the 80% confidence intervals for the Shoulder Pain and Disability Index and Oxford Shoulder Score include the upper limit of the range of minimal clinical important differences at 6 and 12 weeks.
In this pilot and feasibility randomised controlled trial we experienced a higher than expected number of withdrawals due to participants not receiving rotator cuff repair surgery. Despite this we were able to address our feasibility objectives and inform the development of a future main randomised controlled trial.
Hence, in the context of the findings that a future main RaCeR randomised controlled trial is feasible, previous randomised controlled trials and the clinical outcomes reported here provide a signal of effectiveness and platform from which to conduct a high-quality fully powered randomised controlled trial comparing early patient-directed rehabilitation with standard rehabilitation. The implications of this study include the need to randomly allocate participants following surgery and to carefully consider clinician equipoise to minimise withdrawal.
From this RaCeR pilot and feasibility randomised controlled trial, we conclude that a fully powered randomised controlled trial is feasible but would require a minor change to randomly allocate participants following surgery, rather than pre-surgery, to counter the issue of withdrawal due to participants not receiving rotator cuff repair surgery. Additionally, consideration of equipoise of the participating clinicians with regard to comparison of the randomised controlled trial interventions will be paramount to further minimise withdrawals.
Clinical messages.
A main randomised controlled trial to compare early patient-directed rehabilitation with standard rehabilitation is feasible and would require 658 participants based on the specified assumptions;
Random allocation before surgery contributed to study withdrawal due to participants not receiving rotator cuff repair surgery.
Acknowledgments
We thank Linda Chesterton and staff within Keele Clinical Trials Unit including Kendra Cooke, Susie Hennings, Sarah Lawton, David Whittaker, Jane Mason, Alicia Bratt, Steven Harper, Jo Smith, Tracy Whitehurst for their contribution to study set-up, delivery and data cleaning. Further acknowledgement is due to Tina Cookson as a patient representative and the Research User Group from the Primary Care Centre Versus Arthritis, School of Primary, Community and Social Care, Keele University who reviewed the developing protocol and offered advice from the patients’ perspective.
Footnotes
Authors’ contributions: CL, MB, TC, LF, LD, LC and NEF conceived of the study and were involved, alongside KB and ML in developing the design and protocol. CL, MB, TC, LF, LD, LC, ML and NEF secured funding for the study. CL, MB, NEF, SBW, SB, JD, MM, RW, SM and GS managed the study and were involved in data collection. CL drafted the manuscript and all other authors reviewed and provided feedback on drafts. All authors read and approved the final version of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Institute for Health Research (NIHR) Research for Patient Benefit programme (PB-PG-0816-20009). CL is supported by a NIHR Post-Doctoral Fellowship, (PDF-2018-11-ST2-005). NEF was supported through an NIHR Research Professorship (NIHR-RP-011-015) and is an NIHR Senior Investigator. KB was supported by an NIHR Research Methods Fellowship (NIHR-RM-FI-2017-08-006). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
ORCID iDs: Chris Littlewood  https://orcid.org/0000-0002-7703-727X
https://orcid.org/0000-0002-7703-727X
Gareth Stephens  https://orcid.org/0000-0001-9373-0026
https://orcid.org/0000-0001-9373-0026
Availability of data and materials: We have established data sharing arrangements to support joint publications and other research collaborations. Applications for access to anonymised data from our research databases are reviewed by the Data Custodian and Academic Proposal Committee and a decision regarding access to the data is made subject to the ethical approval first provided for the study and to new analysis being proposed. Further information on our data sharing procedures can be found by emailing our data manager (primarycare.datasharing@keele.ac.uk).
References
- 1. Littlewood C, May S, Walters S. Epidemiology of rotator cuff tendinopathy: a systematic review. Shoulder Elb 2013; 5(4): 256–265. [Google Scholar]
- 2. American Academy of Orthopaedic Surgeons. Management of rotator cuff injuries clinical practice guideline, https://www.aaos.org/globalassets/quality-and-practice-resources/rotator-cuff/rotator-cuff-cpg-final-12-20-19.pdf (2019, accessed March 11 2019). [DOI] [PubMed]
- 3. Kulkarni R, Gibson J, Brownson P, et al. BESS/BOA patient care pathways: subacromial shoulder pain. Shoulder Elb 2015; 7(2): 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Littlewood C, Bateman M, Clark D, et al. Rehabilitation following rotator cuff repair: a systematic review. Shoulder Elb 2015; 7: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Littlewood C, Bateman M. Rehabilitation following rotator cuff repair: a survey of current UK practice. Shoulder Elb 2015; 7: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Funk L. Arthroscopic shoulder surgery has progressed, has the rehabilitation? Int Musculoskelet Med 2012; 34(4): 141–145. [Google Scholar]
- 7. Kane LT, Lazarus MD, Namdari S, et al. Comparing expert opinion within the care team regarding postoperative rehabilitation protocol following rotator cuff repair. J Shoulder Elb Surg. 2020; 29(9): E330–E337. [DOI] [PubMed] [Google Scholar]
- 8. Littlewood C, Mazuquin B, Bateman M. Rehabilitation following rotator cuff repair: a survey of current practice. Musculoskeletal Care 2020, 10.1002/msc.1514 (2020, accessed 1 December, 2020). [DOI] [PubMed] [Google Scholar]
- 9. Mazuquin BF, Wright AC, Russell S, et al. Effectiveness of early compared with conservative rehabilitation for patients having rotator cuff repair surgery: an overview of systematic reviews. Br J Sports Med 2018; 52: 111–121. [DOI] [PubMed] [Google Scholar]
- 10. Sheps D, Bouliane M, Styles-Tripp F, et al. Early mobilisation following mini-open rotator cuff repair: a randomised control trial. Bone Joint J. 2015; 97(9): 1257–1263. [DOI] [PubMed] [Google Scholar]
- 11. Sheps DM, Silveira A, Beaupre L, et al. Early active motion versus sling immobilization after arthroscopic rotator cuff repair: a randomized controlled trial. Arthroscopy 2019; 35(3): 749–760.e2. [DOI] [PubMed] [Google Scholar]
- 12. Littlewood C, Bateman M, Cooke K, et al. Protocol for a multi-centre pilot and feasibility randomised controlled trial with a nested qualitative study: rehabilitation following rotator cuff repair (the RaCeR study). Trials 2019; 20(1): 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dawson J, Fitzpatrick R, Carr A. Questionnaire on the perceptions of patients about shoulder surgery. J Bone Joint Surg 1996; 78: 593–600. [PubMed] [Google Scholar]
- 14. Dawson J, Rogers K, Fitzpatrick R, et al. The Oxford shoulder score revisited. Arch Orthop Trauma Surg 2009; 129: 119–123. [DOI] [PubMed] [Google Scholar]
- 15. Carr AJ, Cooper CD, Campbell MK, et al. Clinical effectiveness and cost-effectiveness of open and arthroscopic rotator cuff repair [the UK rotator cuff surgery (UKUFF) randomised trial]. Health Technol Assess (Rockv) 2015; 19(80): 1–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. MacDermid J, Solomon P, Prkachin K. The shoulder pain and disability index demonstrates factor, construct and longitudinal validity. BMC Musculoskelet Disord 2006; 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roy J, MacDermid J, Woodhouse L. Measuring shoulder function: a systematic review of four questionnaires. Arthritis Rheum 2009; 61(5): 623–632. [DOI] [PubMed] [Google Scholar]
- 18. Littlewood C, Malliaras P, Chance-Larsen K. Therapeutic exercise for rotator cuff tendinopathy: a systematic review of contextual factors and prescription parameters. Int J Rehabil Res 2015; 38(2): 95–106. [DOI] [PubMed] [Google Scholar]
- 19. van Reenen M, Janssen B. EQ-5D-5L user guide basic information on how to use the EQ-5D-5L instrument. 2015. https://euroqol.org/publications/user-guides/
- 20. Mazzocca AD, Arciero RA, Shea KP, et al. The effect of early range of motion on quality of life, clinical outcome, and repair integrity after arthroscopic rotator cuff repair. Arthrosc J Arthrosc Relat Surg 2017; 33(6): 1138–1148. [DOI] [PubMed] [Google Scholar]
- 21. Tirefort J, Schwitzguebel AJ, Collin P, et al. Postoperative mobilization after superior rotator cuff repair: sling versus no sling: a randomized prospective study. J Bone Joint Surg Am 2019; 101(6): 494–503. [DOI] [PubMed] [Google Scholar]