Abstract
Biased, or non-Mendelian, segregation is frequently observed but not well understood. Two recent studies on a specific type of biased segregation in mammalian meiosis suggest that it arises from centromeric satellite expansion and asymmetric modification of microtubules in the oocyte spindle.
Life is a game and every player is cheating
— from The Winter Palace, by Eva Stachniak
In 1957, Sandler and Novitski proposed the concept of ‘meiotic drive’ to explain biased inheritance of specific alleles in Drosophila species, corn, and mice [1]. In subsequent years, a number of mechanisms by which loci bias their transmission into the next generation have been described, most of which act after completion of meiosis (for a fascinating example reported recently, see [2]). In 2001, Pardo-Manuel de Villena and Sapienza pointed out that the asymmetry of female meiosis (where only one of four meiotic products becomes the egg) and variations in centromere number/structure constitute a perfect scenario to drive preferential segregation during meiosis [3,4]. Malik and Henikoff extended this notion by proposing the ‘centromere drive’ hypothesis, in which centromeric DNA repeats rapidly evolve to win the battle of female meiosis [5,6]. This hypothesis was supported by evidence for positive selection in centromeric DNA-associated proteins, suggesting that they too rapidly evolve, potentially in conflict with the DNA. While much discussed since its initial proposal, concrete evidence for centromere drive has been limited because of the highly repetitive structure of centromeres and their essential role in cell division, which makes traditional genetic approaches untenable. In addition, for segregation to be biased, not only the centromeres on the chromosomal cargo but the meiotic spindle itself must have an asymmetry that distinguishes the inherited egg-side of the spindle from the discarded polar body side. Asymmetry in microtubule density of meiotic spindles has been observed in certain species, such as grasshoppers, where supernumerary B chromosomes exploit it to bias their transmission into the egg [7]. However, in most species, including mammals, asymmetry in spindle microtubule density is not evident during oocyte meiosis.
To identify the mechanisms underlying preferential meiotic segregation, Lampson and colleagues previously exploited natural variation in mice to identify strains with differences in centromere ‘strength’ [8]. Centromere strength is defined in a hybrid state by likelihood of transmission to the egg, and not to the polar body, during female meiosis. In a meiotic bivalent with ‘weak’ and ‘strong’ centromere-bearing homologous chromosomes (obtained from an inter-strain cross), the stronger centromere delivers its chromosome into the egg at >50% frequency (typically ~60%; Figure 1). The establishment of this experimentally tractable system finally enabled direct cell biological and genomic analysis of the mechanisms underlying asymmetric segregation in meiosis.
Figure 1. Mechanisms underlying asymmetric segregation in mammalian meiosis.
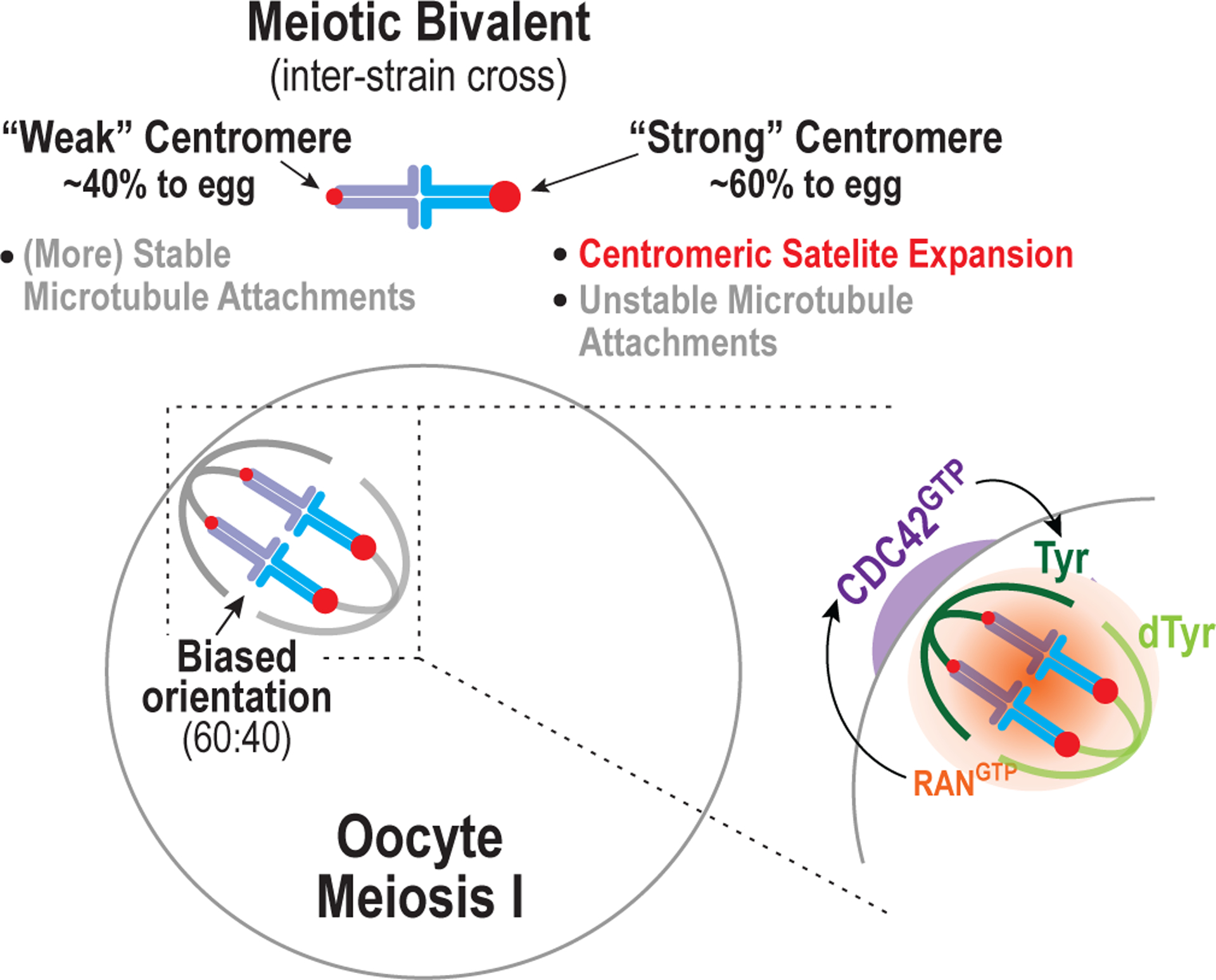
The figure depicts the biased orientation of bivalent chromosomes on the meiosis I spindle of oocytes after an inter-strain cross. One strain has ‘strong’ centromeres that have expanded centromeric minor satellite repeats and recruit more of a key microtubule attachment factor relative to the other strain with ‘weak’ centromeres. Surprisingly, strong centromeres make less stable microtubule attachments than weak centromeres. In oocytes of females derived from crossing the two strains, the chromosome with the strong centromere ends up in the egg more frequently than by chance (60% versus 50%). The spindle itself is asymmetric, in that its proximity to the cortex leads to elevated tyrosination of the microtubules of the half-spindle close to the cortex relative to the half-spindle facing the egg cytoplasm. The tyrosination asymmetry requires chromosome-derived Ran GTP that locally activates the Rho family GTPase Cdc42 on the oocyte cortex. The mechanism by which active Cdc42 promotes local tyrosination of microtubules is not yet known.
As reported in a recent issue of Current Biology, the first question addressed using this system by Iwata-Otsubo, Dawicki-McKenna et al. [9], in a collaboration between the Lampson and Black groups, was what determines centromere strength. Prior work correlated centromere strength with increased amounts of the centromere-localized protein Hec1/Ndc80 that binds to spindle microtubules [8]. The localization of Hec1/Ndc80 depends on the centromeric histone H3 variant CENP-A, which is also mildly elevated at stronger centromeres. However, the difference in strength could arise from a subtle difference in CENP-A or other centromeric proteins between the mouse strains, or in the centromeric DNA satellite repeats that recruit CENP-A. Iwata-Otsubo, Dawicki-McKenna et al. [9] show that the CENP-A polypeptide is identical in the mouse strains with different centromere strengths. Using native CENP-A chromatin immunoprecipitation and sequencing, they find that CENP-A is bound in a highly phased manner to centromeric minor satellite DNA of identical sequence in both strains. However, the stronger centromeres contained significantly more (6–10 fold) minor satellite DNA than the weak centromeres, indicating expansion of the minor satellite domain at centromeres. Chromatin fiber stretching showed that CENP-A occupied nearly the entire minor satellite domain in the weak centromere strain but only a limited region of the much larger minor satellite domain in the strong centromere strain. What limits the amount of CENP-A that is loaded within the expanded minor satellite domain of the strong centromere is currently unclear. Using the minor satellite binding protein CENP-B as a reporter in living oocytes, the authors directly showed that in meiotic bivalent chromosomes derived from an inter-strain cross, the chromosome with higher CENP-B signal, i.e. the chromosome with the strong centromere, oriented preferentially towards the egg on the meiosis I spindle (Figure 1). These results suggest that expansion of centromeric minor satellite repeats biases segregation during oocyte meiosis, which is consistent with the notion proposed in the centromere drive hypothesis that these repeats act as selfish elements. Satellite expansion is correlated with modest elevation of CENP-A chromatin and increased amounts of Hec1/Ndc80, the major microtubule-binding component at centromeres, although the precise relationship between these increases and the biased segregation remains to be defined.
In a second paper published by the Lampson group in Science, Akera et al. [10] address the flip side of the question — what is the oocyte meiotic spindle asymmetry that is read by the strong centromere to bias its orientation? While tubulin density and a number of tested tubulin post-translational modifications were symmetric, Akera et al. observed a striking asymmetry in the tyrosination status of α-tubulin: the half-spindle close to the cortex was enriched for tyrosination relative to the egg-side half-spindle (Figure 1). α-tubulin is translated with a terminal tyrosine that is removed by an unknown protease, revealing a glutamate that becomes the substrate for tubulin tyrosine ligase. The detyrosination/tyrosination cycle of microtubules, which affects interactions with motor and non-motor microtubule-associated proteins, has been implicated in a number of cellular functions [11–13]. Notably, Akera et al. found that the tyrosination asymmetry was linked to location — it was not observed when the spindle was in the center of the oocyte but was evident when the spindle was positioned close to the cortex, where its orientation leads to ejection of half the genome into the polar body (Figure 1). The authors followed up this observation by perturbing the chromatin-based gradient of Ran GTPase that is required to polarize the cortex and eject a polar body [14]. Perturbing Ran eliminated the asymmetry even when the spindle was positioned adjacent to the cortex. Downstream of Ran the small GTPase Cdc42 is required, independently of its well-studied control of actin assembly, for driving the asymmetric tyrosination (Figure 1). In a clever optogenetic experiment, the authors concentrated a constitutively activated Cdc42 mutant near one spindle pole and showed that it weakly stimulated tyrosination even when the spindle was in the center of the cell. These observations suggest that Cdc42 activation downstream of Ran on the cell cortex stimulates tubulin tyrosination of the spindle microtubules close to the cortex (Figure 1); the molecular mechanisms by which Cdc42 controls tyrosination await future work. This result has implications beyond oocyte meiosis, as Cdc42 is widely implicated in cell polarization.
Having discovered and explained the origin of an asymmetry in the oocyte meiotic spindle, Akera et al. next tested whether this asymmetry was indeed important for the biased orientation on the spindle of the strong centromere-bearing homolog. As a first step, they showed that perturbing Ran or Cdc42 eliminated the biased orientation of the strong centromere towards the egg in an inter-strain cross. In addition, overexpression or downregulation of tubulin tyrosine ligase was associated with microtubule destabilization and stabilization, respectively, following cold treatment (which preserves centromere-anchored microtubule fibers). When the authors directly imaged asymmetric bivalents, using a CENP-B fusion to discriminate between the minor satellite-expanded strong versus weak centromere-bearing homologs, they found a surprise. The strong centromeres, despite their increase in CENP-A and microtubule-binding Hec1/Ndc80 complex, made less stable attachments and were observed to detach, leading the entire bivalent to flip until the strong centromere was biased to connect to the detyrosination-enriched egg-side half- spindle. Thus, paradoxically, the strong centromere, despite having more microtubule attachment machinery, makes less stable attachments and the weak centromere more stable attachments. The difference in tyrosination in the spindle biases the less stable attachment-forming centromere towards the egg-side half spindle. While the underlying mechanisms that make the attachments less stable at the strong centromere remain to be defined, the data demonstrate that the tyrosination asymmetry in the spindle is biasing orientation of strong versus weak centromeres.
One intriguing issue raised by Akera et al.’s findings is the function of the spindle asymmetry that is generated by its positioning adjacent to the cortex. It is unlikely that this asymmetry exists solely to promote cheating in oocyte meiosis. It is much more likely that the asymmetry serves a function that is being exploited to beat the even odds of segregation. We suggest that a potential function of this asymmetry is centromere ‘quality control’, with the meiotic spindle selecting and sending into the gamete centromeres that generate sufficiently dynamic attachments to ensure error correction and accurate segregation. Such a mechanism would also protect against centromere aberrations that may arise due to their highly repetitive structure during the complex events of pre-meiotic replication and meiotic prophase. Alternatively, the observed spindle asymmetry may be important for the highly asymmetric cytokinesis-related event of polar body ejection. Conditional genetic ablation of tubulin tyrosine ligase in oocytes may help test these ideas. In addition to defining the function of the cortex-induced spindle asymmetry, a number of mechanistic questions are raised by the work described in the two papers. These include defining the relationship between satellite expansion and centromere strength, the basis for the counter-intuitive difference in microtubule attachment stability between strong and weak centromeres, and the mechanism by which activated Cdc42 controls tyrosination of microtubules.
By providing the first picture of the centromeric and spindle asymmetries that enable chromosomes to beat the even odds during meiosis, the papers by Iwata-Otsubo, Dawicki-McKenna et al. and Akera et al. constitute a quantum leap in understanding this specific type of biased inheritance. They also provide an outstanding example of the power of natural variation in a species to help tackle fascinating and challenging biological questions.
References
- 1.Sandler L, and Novitski E (1957). Meiotic drive as an evolutionary force. Am. Nat 91, 105–110. [Google Scholar]
- 2.Nuckolls NL, Bravo Núñez MA, Eickbush MT, Young JM, Lange JJ, Yu JS, Smith GR, Jaspersen SL, Malik HS, and Zanders SE (2017). wtf genes are prolific dual poison-antidote meiotic drivers. Elife 6, pii: e26033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Villena F, and Sapienza C (2001). Nonrandom segregation during meiosis: the unfairness of females. Mamm. Genome 12, 331–339. [DOI] [PubMed] [Google Scholar]
- 4.de Villena FPM, and Sapienza C (2001). Female meiosis drives karyotypic evolution in mammals. Genetics 159, 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henikoff S, and Malik HS (2002). Centromeres: selfish drivers. Nature 417, 227. [DOI] [PubMed] [Google Scholar]
- 6.Malik HS, and Henikoff S (2002). Conflict begets complexity: the evolution of centromeres. Curr. Opin. Genet. Dev 12, 711–718. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt GM (1976). Meiotic drive for B-chromosomes in the primary oocytes of Myrmeleotettix maculatus (Orthopera: Acrididae). Chromosoma 56, 381–391. [DOI] [PubMed] [Google Scholar]
- 8.Chmatal L, Gabriel SI, Mitsainas GP, Martinez-Vargas J, Ventura J, Searle JB, Schultz RM, and Lampson MA (2014). Centromere Strength Provides the Cell Biological Basis for Meiotic Drive and Karyotype Evolution in Mice. Curr. Biol 24, 2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwata-Otsubo A, Dawicki-McKenna JM, Akera T, Falk SJ, Chmatal L, Yang K, Sullivan BA, Schultz RM, Lampson MA, and Black BE (2017). Expanded Satellite Repeats Amplify a Discrete CENP-A Nucleosome Assembly Site on Chromosomes that Drive in Female Meiosis. Curr. Biol 27, 2365–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akera T, Chmatal L, Trimm E, Yang K, Aonbangkhen C, Chenoweth DM, Janke C, Schultz RM, and Lampson MA Spindle asymmetry drives non-Mendelian chromosome segregation. (in press) [DOI] [PMC free article] [PubMed]
- 11.Yu I, Garnham CP, and Roll-Mecak A (2015). Writing and reading the tubulin code. J. Biol. Chem 290, 17163–17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song Y, and Brady ST (2015). Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol. 25, 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond JW, Cai D, and Verhey KJ (2008). Tubulin modifications and their cellular functions. Curr. Opin. Cell Biol 20, 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng M, Suraneni P, Schultz RM, and Li R (2007). The ran GTPase mediates chromatin signaling to control cortical polarity during polar body extrusion in mouse oocytes. Dev. Cell 12, 301–308. [DOI] [PubMed] [Google Scholar]


