To the editor:
We read with interest recent reports of minimal change disease and glomerulonephritis following receipt of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine, including 1 case of anti–glomerular basement membrane (anti-GBM) antibody disease.1
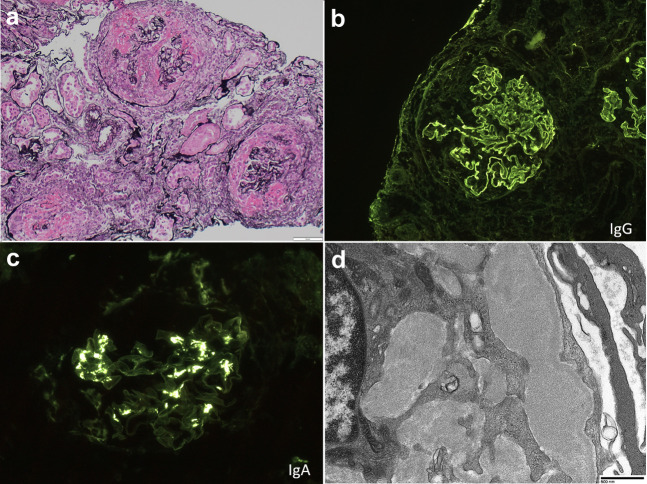
We would like to report another case of anti-GBM disease, which had coexistent mesangial IgA deposits. The patient is an older woman with previously normal renal function and no significant past medical history, prior coronavirus disease 2019 (COVID-19) infection, or medication use, who developed fevers, anorexia, nausea, and gross hematuria 2 weeks after receiving the second dose of the Moderna SARS-CoV-2 vaccine. Symptoms lasted 2 weeks, and she presented with acute kidney injury (peak creatinine, 7.8 mg/dl), a urine protein-to-creatinine ratio of 1.9 g/g, and active urinary sentiment. Serologic evaluation revealed a positive anti-GBM; anti-neutrophil cytoplasmic autoantibody (ANCA), anti-nuclear antibody (ANA), anti–double-stranded DNA, complements, serum and urine protein electrophoresis, hepatitis C virus, hepatitis B virus, and HIV were negative. SARS-CoV-2 was negative by polymerase chain reaction, and blood and urine cultures were negative. Testing for anti–SARS-CoV-2 antibodies was not performed. Kidney biopsy (Figure 1 ) revealed a diffusely crescentic glomerulonephritis, with 100% active cellular crescents and no significant chronic injury. Immunofluorescence showed linear staining of GBMs for IgG (3+), and granular mesangial staining for IgA (2–3+), with associated rare mesangial deposits by electron microscopy. There was no clinical evidence of pulmonary involvement. She was treated with methylprednisolone, Cytoxan, plasmapheresis, and hemodialysis, and she remains dialysis-dependent.
Figure 1.
Anti–glomerular basement membrane (GBM) antibody disease nephritis after severe acute respiratory syndrome coronavirus 2 vaccination. (a) Diffusely crescentic glomerulonephritis, with necrosis, cellular crescents, and destruction of the glomerular tuft and Bowman’s capsule (Jones stain, original magnification ×200). (b) Linear staining of GBMs for IgG (original magnification ×200). (c) Mesangial deposition of IgA by immunofluorescence (original magnification ×400). (d) Rare mesangial deposits by electron microscopy. Direct magnification ×6800. Bar = 800 nm. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Spatial and temporal clustering of anti-GBM2 suggests an environmental trigger, and regionally increased incidence of anti-GBM during the COVID-19 pandemic has been documented.3 In the latter investigation, although all 5 of the 8 tested patients presenting with anti-GBM were negative for SARS-CoV-2 infection by polymerase chain reaction, 4 had IgM antibodies (1 with concurrent IgG) to the SARS-CoV-2 spike protein, raising the possibility that the immune response to SARS-CoV-2 could be related to the development of anti-GBM in some patients. In the setting of widespread infection or vaccination, true disease associations require time to emerge. Whether current cases can be attributed to COVID-19 vaccine–related immune response is speculative but intriguing, and warrants investigation.
References
- 1.Tan H.Z., Tan R.Y., Choo J.C.J. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int. 2021;100:469–471. doi: 10.1016/j.kint.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canney M., O'Hara P.V., McEvoy C.M. Spatial and temporal clustering of anti-glomerular basement membrane disease. Clin J Am Soc Nephrol. 2016;11:1392–1399. doi: 10.2215/CJN.13591215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prendecki M., Clarke C., Cairns T. Anti–glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int. 2020;98:780–781. doi: 10.1016/j.kint.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]