Dear editor,
The disease caused by the coronavirus SARS-CoV-2 (COVID-19), which emerged in China in December 2019 [1], has become a global pandemic in just a few months. The concomitant presentation of COVID-19 and some autoimmune diseases has also been reported, among which is Guillain Barré Syndrome (GBS) [2]. GBS is considered an immune-mediated neuropathy preceded 1 to 6 weeks in 70% of cases by a bacterial or a viral infection. In many cases associated with Campylobacter jejuni (the predominant pathogen) the presence of antiganglioside antibodies is observed. This supports a post-infectious mechanism, with molecular mimicry and antibody cross-response [3]. However, in GBS associated with Zika virus infection, an earlier onset is seen, and associated antiganglioside antibodies are rarely present, suggesting a para-infectious pathogenetic mechanism [4]. There is contradictory information on whether GB associated with COVID-19 has also characteristics that may indicate a para-infectious pathogenetic process [[5], [6], [7]]. To review the accumulated evidence about the pathogenic mechanism of this association, we carried out a review of the literature with a selection of the clinical cases reported until February 1st 2021, adding one own case. We selected studies reporting adult patients with all: Guillain-Barré syndrome, according to diagnostic criteria of the GBS Classification Group [8]; SARS-CoV-2 infection confirmed by nasopharyngeal reverse transcription polymerase chain reaction, antigen-detecting rapid diagnostic tests or serum antibody test; Detailed individual clinical description; A minimum of 6/8 points using the Joanna Briggs Institute Critical Appraisal Checklist for Case Reports and for Case Series studies [,][106], [107]. Finally, we selected 82 full text access articles with information about 104 clinical cases (Table 1 ) to which we added our own case (Patient 32). We searched suggestive features of the three pathogenic pathways proposed to neurologic damage in COVID-19 so far [11,12]: direct damage, dysregulated inflammatory response and antibody-mediated injury (Fig. 1 ). Direct damage: As seen in some viral infections such as poliovirus, enterovirus D68, cytomegalovirus, or other human coronaviruses, SARS-Cov-2 has neuroinvasive capacity [12,13]. The proposed access routes have been through circulation, the blood-brain barrier, or retrograde axonal transport, through the olfactory nerve or the enteric nervous system [12]. Endothelium, glial cells, and neurons express angiotensin-converting enzyme receptor 2 (ACE2) and type II transmembrane serine protease (TMPRSS2), both necessary for the virus to get into the cells [14]. A post mortem study found SARS-CoV-2 RNA in neuroanatomical areas receiving olfactory tract projections [15]. However, PCR in CSF for COVID -19 virus was negative in all reported cases of GBS (Table 1), suggesting no intrathecal viral replication. Furthermore, a recent systematic review and meta-analysis showed that no study detected live SARS-COV-2 in various body fluids beyond day 9 of illness [16] and yet the median days of infection until the debut of GBS in the actual review has been 11 days. Dysregulated inflammatory response: In the ”inflammatory phase” of COVID-19 infection, which characteristically begins throughout the second week of infection, elevated IL-2, IL-2R, IL-6, IL-10, IFN-γ, TNF-α, CCL2, procalcitonin, CRP, erythrocyte sedimentation rate and white blood cell, are characteristic [17]. In 2005, brain autopsy studies demonstrate the infiltration of monocytes, macrophages, and T-lymphocytes into gliocytes and brain mesenchyme of SARS-CoV patients [19]. Pilotto et al. has also described the presence of elevated neuroinflammatory parameters (IL-6, IL-8, β2M and TNF-α) in the CSF of 13 patients with encephalitis and COVID-19 [20]. On the other hand, marked increase of cytokines has previously been reported in GBS and its variants, as well as in experimental autoimmune neuritis, the animal model of GBS [21]. Cell-mediated immunity seems to play a crucial role in immunopathology of all types of GBS, especially the AIDP subtype [22]. Of note, AIDP subtype is the predominant in the current systematic revision (73%, counting with mixed forms) (Table 1). Also, in the present work the medium time between the onset of COVID-19 and the neurological symptoms was 11 days, that is, in the stages of the infection in which inflammatory processes predominate over antibody-mediated. In addition, serum inflammatory parameters were elevated at the beginning of the neurological symptoms in 39/53 patients (73%). Antibody-mediated injury. Anti-GM1 IgG are present in a high proportion of patients with classic GBS, mostly those with AMAN or AMSAN. Also, anti-GQ1b IgG antibodies are present in in 80–95% of patients with Miller-Fisher syndrome (MFS), the most common clinical variant of GBS [23]. Nevertheless, Keddie et al. found no significant similarity between SARS CoV-2 and human genome [24] and only 6/58 cases (10%) in our review had positive antiganglioside antibodies, interestingly only 3 of the 17 patients with Miller-Fisher syndrome (20%) (Table 1). Patient number 92 was seropositive for IgM antibodies against panneurofascin without posterior seroconversion to IgG [25]. However, anti-neurofascin antibodies may also have been triggered by tissue damage related to GBS.
Table 1.
Clinical cases obtained in the systematic review of the literature of patients with Guillen Barre Syndrome and a proven history of SARS-Cov-2 infection. Demographic and clinical characteristics, complementary examinations and evaluation of the quality of the case report.
| First author (Ref.) | Age | Sex | Severity COVID19 1 | Latency2 | GBS Clinical variant3 | EMG | SARS-COV-2 CSF | Antiganglioside antibodies | Biomarkers | Treatment COVID-19 | Treatment GBS | Evolution at day 30 | Study quality [[106], [107]] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbaslou [26] | Patient 1 | 55 | F | 3 | 32 | paraparetic GBS | AMSAN | ‐– | ‐– | ‐– | LPV/r | Ig iv | dead (ARDS) | 7/8 |
| Abolmaali [27] | Patient 2 | 88 | F | 3 | ‐−3 | classic SGB | AMSAN | ‐– | ‐– | ‐– | DEXA, LPV/r, HCQ | PPH | poor | 7/8 |
| Patient 3 | 58 | M | 4 | 9 | classic SGB | AMSAN | ‐– | ‐– | ‐– | Remdensivir, Favipiravir, LPV/r, HCQ | Ig iv + PPH | dead (multi-organ failure) | 7/8 | |
| Abrams [28] | Patient 4 | 67 | F | 2 | 10 | classic SGB | ‐– | PCR Neg | Neg | elevated DD, CRP, IgM | ‐– | PPH | partial improvement | 7/8 |
| Agosti [29] | Patient 5 | 68 | M | 2 | 5 | classic SGB | AIDP | ‐– | ‐– | thrombocythaemia, lymphopenia | antiviral | Ig iv | partial improvement | 7/8 |
| Alberti [30] | Patient 6 | 71 | M | 2 | 4 | classic SGB | AIDP | PCR Neg | ‐– | ‐– | LPV/r, HCQ | Ig iv | dead (ARDS) | 7/8 |
| Ameer [31] | Patient 7 | 30 | M | 1 | 4 | classic SGB | AMAN | PCR Neg | Neg | lymphopenia | ‐– | Ig iv | complet recovery | 8/8 |
| Arnaud [32] | Patient 8 | 64 | M | 2 | 21 | classic SGB | AIDP | PCR Neg | Neg | ‐– | CXM, AZM, HCQ | Ig iv | complet recovery | 8/8 |
| Assini [33] | Patient 9 | 55 | M | 3 | ‐– | Miller-Fisher | AIDP | PCR Neg | Neg | lymphopenia, elevated ferritine, CRP, LDH, oligoclonal bands | HCQ, LPV/r Arbidol | Ig iv | complet recovery | 7/8 |
| Patient 10 | 60 | M | 3 | ‐– | classic SGB | AMSAN | ‐– | Neg | lymphopenia, elevated LDH y GGT, oligoclonal bands | HCQ, LPV/r, TCZ | Ig iv | partial improvement | 6/8 | |
| Atakla [34] | Patient 11 | 40 | M | 3 | 11 | classic SGB | AIDP | PCR Neg | ‐– | neutropenia, elevated ESR, CRP | AZM | Ig iv | partial improvement | 7/8 |
| Barranchina-Esteve [35] | Patient 12 | 54 | F | 3 | 0 | classic SGB | AMSAN | PCR Neg | Neg | elevated DD, ferritine, LDH | CXM, AZM, HCQ, LPV/r, MP, TCZ | Ig iv | complet recovery | 8/8 |
| Bigaut [36] | Patient 13 | 43 | M | 2 | 21 | classic SGB | AIDP | PCR Neg | Neg | ‐– | ‐– | Ig iv | partial improvement | 8/8 |
| Patient 14 | 70 | F | 3 | 7 | classic SGB | AIDP | PCR Neg | Neg | elevated CRP | ‐– | Ig iv | partial improvement | 8/8 | |
| Boostani [37] | Patient 15 | 37 | M | 3 | 15 | classic SGB | AIDP | ‐– | ‐– | elevated ESR, CRP | ‐– | Ig iv | partial improvement | 7/8 |
| Bracaglia [38] | Patient 16 | 66 | F | 1 | ‐– | classic SGB | AIDP | ‐– | Neg | lymphopenia, elevated CRP, CK, LDH, TGO, TGP, IL-6 | LPV/r, HCQ, | Ig iv | partial improvement | 7/8 |
| Bueso [39] | Patient 17 | 60 | F | 2 | 22 | classic SGB | ‐– | ‐– | ‐– | ‐– | AZM, HCQ | Ig iv | partial improvement | 7/8 |
| Caamaño [40] | Patient 18 | 61 | M | 2 | 10 | BWDP | ‐– | PCR Neg | ‐– | ‐– | HCQ, LPV/r | PRED low dose | partial improvement | 8/8 |
| Camdessanche [41] | Patient 19 | 64 | M | 2 | 11 | classic SGB | AIDP | ‐– | Neg | ‐– | LPV/r | Ig iv | ‐– | 6/8 |
| Chan [42] | Patient 20 | 58 | M | 2 | ‐– | BWDP | AIDP | PCR Neg | ‐– | thrombocythaemia, elevated DD | CXM, AZM, | Ig iv | partial improvement | 7/8 |
| Civardi [43] | Patient 21 | 72 | F | 1 | 10 | classic SGB | AIDP | PCR Neg | anti-GM1, anti-GD1a and anti-GD1b | elevated fibrinogen, CRP | HCQ, DOX, | Ig iv | partial improvement | 8/8 |
| Coen [44] | Patient 22 | 70 | M | 1 | 10 | classic SGB | AIDP | PCR Neg | Neg | ‐– | ‐– | Ig iv | partial improvement | 8/8 |
| Colonna [45] | Patient 23 | 62 | M | 3 | 21 | classic SGB | AIDP | ‐– | ‐– | elevated CRP | LPV/r, MP (60 mg/24 h) | Ig iv | partial improvement | 7/8 |
| Defabio [46] | Patient 24 | 70 | F | 1 | 90 | classic SGB | ‐– | ‐– | ‐– | ND | ND | Ig iv | complet recovery | 7/8 |
| Diez-Porras [47] | Patient 25 | 54 | M | 1 | 5 | classic SGB | AIDP | ‐– | IgM for GM2 and GD3 and a weak IgG for GT1b | elevated CRP, LDH y CK | AZM, HCQ, LPV/r | Ig iv | partial improvement | 7/8 |
| El Otmani [48] | Patient 26 | 70 | F | 2 | 3 | classic SGB | AMSAN | PCR Neg | ‐– | lymphopenia | HCQ, AZM | Ig iv | poor | 7/8 |
| Elkhouly [49] | Patient 27 | 75 | M | ‐– | ‐– | classic SGB | ‐– | ‐– | ‐– | ‐– | MP | Ig iv | partial improvement | 6/8 |
| Esteban [50] | Patient 28 | 55 | F | 2 | 14 | classic SGB | AIDP | ‐– | ‐– | elevated CRP | HCQ, CXM, AZM | Ig iv | partial improvement | 7/8 |
| Farzi [51] | Patient 29 | 41 | M | 2 | 10 | classic SGB | AIDP | ‐– | ‐– | lymphopenia, elevated CRP | LPV/r, HCQ | Ig iv | partial improvement | 7/8 |
| Fernandez-Dominguez [52] | Patient 30 | 74 | F | 2 | 15 | Miller-Fisher | AIDP | ‐– | Neg | ‐– | HCQ, LPV/r | Ig iv | partial improvement | 7/8 |
| Ferraris [53] | Patient 31 | 65 | F | 4 | 23 | classic SGB | AIDP | ‐– | ‐– | elevated IL-6 | HCQ, HBPM, AZM, TCZ, LPV/r, MP | Ig iv | partial improvement | 7/8 |
| Freire | Patient 32 | 71 | M | 2 | 9 | classic SGB | AIDP | ‐– | Neg | Elevated CRP, DD, LDH, ferritin, IL-6 | MP | Ig iv | partial improvement | 7/8 |
| Gale [54] | Patient 33 | 58 | M | 2 | ‐– | classic SGB | AIDP | ‐– | ‐– | lymphopenia, elevated CRP | ‐– | Ig iv | partial improvement | 6/8 |
| Garcia-Manzanedo [55] | Patient 34 | 77 | M | 2 | 21 | PCBW | Mixed | ‐– | ‐– | ‐– | LPV/r, HCQ | Ig iv | partial improvement | 7/8 |
| Garnero [56] | Patient 35 | 65 | M | 2 | ‐– | classic SGB | AIDP | ‐– | Neg | ‐– | ‐– | Ig iv | ‐– | 6/8 |
| Patient 36 | 73 | M | 2 | 0 | classic SGB | ‐– | PCR Neg | Neg | ‐– | ‐– | Ig iv | ‐– | 7/8 | |
| Patient 37 | 55 | M | 2 | 20 | Miller-Fisher-GBS overlap | ‐– | PCR Neg | Neg | ‐– | ‐– | Ig iv | ‐– | 7/8 | |
| Patient 38 | 46 | F | 1 | 3 | classic SGB | ‐– | PCR Neg | Neg | ‐– | ‐– | Ig iv | ‐– | 7/8 | |
| Patient 39 | 60 | M | 2 | 20 | classic SGB | AMSAN | PCR Neg | Neg | ‐– | ‐– | Ig iv | ‐– | 7/8 | |
| Patient 40 | 63 | F | 2 | 15 | classic SGB | AMSAN | Neg | ‐– | ‐– | Ig iv | ‐– | 7/8 | ||
| Ghosh [57] | Patient 41 | 20 | M | 1 | 8 | classic SGB | AMAN | ‐– | Neg | lymphopenia | ‐– | Ig iv | partial improvement | 7/8 |
| First author (Ref.) | Age | Sex | Severity COVID191 | Latency2 | GBS Clinical variant3 | EMG | SARS-COV-2 CSF | Antiganglioside antibodies | Biomarkers | Treatment COVID-19 | Treatment GBS | Evolution at day 30 | Study quality4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gigli [58] | Patient 42 | 53 | M | 2 | ‐ – | paraparetic SGB | AIDP | ‐– | Neg | elevated IL-8, IL-6 | ‐– | Ig iv | partial improvement | 7/8 |
| Granjer [59] | Patient 43 | 48 | M | 1 | 25 | classic SGB | AIDP | ‐– | ‐– | ‐– | ‐– | PPH | partial improvement | 7/8 |
| Guijarro-Castro [60] | Patient 44 | 70 | M | 2 | 21 | classic SGB | Mixed | ‐– | Neg | lymphopenia thrombocythaemia, | HCQ, CXM, AZM, DXM | Ig iv | partial improvement | 7/8 |
| Gutierrez-Ortiz [61] | Patient 45 | 50 | M | 1 | 5 | Miller-Fisher | ‐– | PCR Neg | anti-GD1b | lymphopenia, elevated CRP | ‐– | Ig iv | complet recovery | 7/8 |
| Helbok [62] | Patient 46 | 68 | M | 2 | 14 | classic SGB | AIDP | PCR Neg | Neg | elevated CRP, ESR, fibrinogen | ‐– | Ig iv + PPH | partial improvement | 8/8 |
| Hirayama [63] | Patient 47 | 54 | F | 2 | 20 | classic SGB | AMAN | ‐– | Neg | normal | ‐– | partial improvement | 8/8 | |
| Hutchins [64] | Patient 48 | 21 | M | 2 | 16 | BWDP | Mixed | ‐– | Neg | lymphopenia | PPH | partial improvement | 7/8 | |
| Kajani [65] | Patient 49 | 50 | M | 1 | ‐– | Miller-Fisher | ‐– | PCR Neg | Neg | normal | ‐– | Ig iv | dead (ventricular arrhythmia) | 6/8 |
| Khaja [66] | Patient 50 | 44 | M | 1 | 0 | Bifacial weakness | ‐– | PCR Neg | Neg | normal | ‐– | Ig iv | complet recovery | 8/8 |
| Kopscick [67] | Patient 51 | 31 | M | 1 | 0 | Miller-Fisher | ‐– | ‐– | anti-GQ1b | ‐– | convalescent plasma, TCZ | Ig iv | partial improvement | 7/8 |
| Korem [68] | Patient 52 | 58 | F | 1 | 14 | classic SGB | ‐– | ‐– | ‐– | normal | AZM | Ig iv | partial improvement | 7/8 |
| Lampe [69] | Patient 53 | 65 | M | 1 | 1 | classic SGB | AIDP | ‐– | Neg | leucopenia, elevated CRP | ‐– | Ig iv | partial improvement | 7/8 |
| Lantos [70] | Patient 54 | 36 | M | 1 | 4 | Miller-Fisher | ‐– | ‐– | Neg | ‐– | HCQ | Ig iv | complet recovery | 7/8 |
| Lascano [71] | Patient 55 | 52 | F | 1 | 15 | classic SGB | AIDP | PCR Neg | Neg | normal | ‐– | Ig iv | complet recovery | 8/8 |
| Patient 56 | 63 | F | 1 | 7 | classic SGB | AIDP | PCR Neg | ‐– | lymphopenia, elevated transaminases | ‐– | Ig iv | poor | 7/8 | |
| Patient 57 | 61 | F | 1 | 22 | classic SGB | AIDP | PCR Neg | ‐– | lymphopenia | ‐– | Ig iv | partial improvement | 7/8 | |
| Lowery [72] | Patient 58 | 45 | M | 2 | 14 | Overlap Miller Fisher + SGB | ‐– | ‐– | anti-GQ1b | ‐– | HCQ | Ig iv | partial improvement | 8/8 |
| Liberatore [73] | Patient 59 | 49 | M | 2 | 12 | PCBW | AMAN | PCR Neg | Neg | lymphopenia, thrombocythaemia. eElevated CRP | HCQ, LPV/r, CXM | partial improvement | 8/8 | |
| MacDonell [74] | Patient 60 | 54 | M | 2 | 3 | classic SGB | ‐– | Neg | normal | HCQ | Ig iv | complet recovery | 8/8 | |
| Maideniuc [75] | Patient 61 | 61 | F | 1 | 28 | classic SGB | AMAN | PCR Neg | normal | PPH | partial improvement | 8/8 | ||
| Manganotti [76] | Patient 62 | 50 | F | 2 | 16 | Miller-Fisher | ‐– | Neg | ‐– | LPV/r, HCQ | Ig iv | complet recovery | 7/8 | |
| Manganotti [77] | Patient 63 | 72 | M | 2 | 18 | classic SGB | AIDP | PCR Neg | Neg | Elevated IL6 | HCQ, Oseltamivir, darunavir, MP, TCZ | Ig iv | partial improvement | 8/8 |
| Patient 64 | 72 | M | 2 | 30 | classic SGB | Mixed | PCR Neg | Neg | Normal | HCQ, LPV/r, MP | Ig iv | partial improvement | 8/8 | |
| Patient 65 | 49 | F | 2 | 14 | Miller-Fisher | AIDP | PCR Neg | Neg | Normal | HCQ, LPV/r, MP | Ig iv | partial improvement | 8/8 | |
| Patient 66 | 94 | M | 2 | 33 | classic SGB | AIDP | ‐– | ‐– | ‐– | MP | MP | poor | 7/8 | |
| Patient 67 | 76 | M | 2 | 22 | classic SGB | AIDP | PCR Neg | Neg | Elevated IL6 | HCQ, Oseltamivir, darunavir, MP, TCZ. | Ig iv | partial improvement | 8/8 | |
| Marta-Enguita [78] | Patient 68 | 78 | F | 2 | 8 | classic SGB | ‐– | ‐– | ‐– | thrombocythaemia, Elevated DD | ‐– | – | dead | 7/8 |
| Naddaf [79] | Patient 69 | 58 | F | 2 | 17 | classic SGB | AIDP | PCR Neg | Neg | Elevated DD, ferritine | HCQ, MP | PPH | partial improvement | 8/8 |
| Nanda [80] | Patient 70 | 55 | F | 1 | 10 | classic SGB | AMAN | ‐– | ‐– | elevated CRP, Ferritine, IL6, DD, LDH | ‐– | Ig iv | complet recovery | 7/8 |
| Patient 71 | 72 | M | 2 | 6 | classic SGB | AIDP | ‐– | ‐– | elevated CRP, Ferritine, IL6, DD, LDH | ‐– | Ig iv | dead | 7/8 | |
| Patient 72 | 55 | M | 1 | 7 | classic SGB | AMSAN | ‐– | ‐– | Elevated CRP, Ferritine, IL6, DD, LDH | ‐– | Ig iv | complet recovery | 7/8 | |
| Patient 73 | 49 | M | 2 | 10 | classic SGB | AMAN | ‐– | ‐– | Elevated ferritin, LDH | ‐– | Ig iv | complet recovery | 7/8 | |
| Oguz-Akarsu [81] | Patient 74 | 53 | F | 2 | 0 | classic SGB | AIDP | PCR Neg | ‐– | lymphopenia, elevated CRP | HCQ, AZM | PPH | complet recovery | 7/8 |
| Ottavani [5] | Patient 75 | 66 | F | 3 | 7 | classic SGB | Mixed | PCR Neg | Neg | lymphopenia, elevated CRP, DD. | LPV/r, HCQ, | Ig iv | ‐– | 7/8 |
| Paybast [82] | Patient 76 | 38 | M | 1 | 21 | classic SGB | Mixed | ‐– | ‐– | normal | HCQ | PPH | partial improvement | 7/8 |
| Pelea [83] | Patient 77 | 56 | F | 1 | 7 | classic SGB | Mixed | PCR Neg | Neg | normal | ‐– | PPH + Ig iv | partial improvement | 8/8 |
| Petrelli [84] | Patient 78 | 57 | M | 1 | 17 | classic SGB | AMAN | PCR Neg | anti-GM1, anti-GD1a | ‐– | ‐– | Ig iv, DM | partial improvement | 8/8 |
| Padroni [85] | Patient 79 | 70 | F | 1 | 24 | classic SGB | AIDP | ‐– | ‐– | linfocitosis | ‐– | Ig iv | poor | 7/8 |
| First author (Ref.) | Age | Sex | Severity COVID191 | Latency2 | GBS Clinical variant3 | EMG | SARS-COV-2 CSF | Antiganglioside antibodies | Biomarkers | Treatment COVID-19 | Treatment GBS | Evolution at day 30 | Study quality4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raahimi [86] | Patient 80 | 46 | M | 3 | 45 | classic SGB | AIDP | ‐– | ‐– | Normal | ‐– | Ig iv | partial improvement | 7/8 |
| Rajdev [87] | Patient 81 | 36 | M | 3 | 18 | classic SGB | AIDP | ‐– | ‐– | ‐– | remdesivir | Ig iv, PPH | partial improvement | 7/8 |
| Rana [88] | Patient 82 | 54 | M | 1 | 14 | overlap Miller Fisher - classic SGB | AIDP | ‐– | ‐– | ‐– | amoxicilin, short course steroids, HCQ, AZM | Ig iv, PPH | 7/8 | |
| Ray [89] | Patient 83 | 63 | M | 1 | 1 | Miller Fisher | ‐– | ‐– | ‐– | Elevated CRP, lymphopenia, neutropenia | ‐– | ‐– | partial improvement | 6/8 |
| Redondo [90] | Patient 84 | 54 | F | 2 | 15 | classic SGB | AIDP | PCR Neg | ‐– | Normales | ‐– | Ig iv | partial improvement | 7/8 |
| Reyes-Bueno [91] | Patient 85 | 51 | F | 1 | 15 | overlap Miller Fisher - classic SGB | AIDP | Neg | ‐– | ‐– | Ig iv, gabapentina | partial improvement | 7/8 | |
| Riva [92] | Patient 86 | sixties | M | 2 | 20 | classic SGB | AIDP | PCR Neg | Neg | Elevated IL-6, ferritina, LDH, fibrinógeno | ‐– | Ig iv | ‐– | 7/8 |
| Sancho-Saldaña [93] | Patient 87 | 56 | F | 2 | 15 | classic SGB | AIDP | Neg | ‐– | HCQ, AZM | Ig iv | ‐– | 7/8 | |
| Scheidl [94] | Patient 88 | 54 | F | 1 | 21 | classic SGB | AIDP | ‐– | ‐– | CRP normal | ‐– | Ig iv | complet recovery | 7/8 |
| Sedaghat [95] | Patient 89 | 65 | M | 2 | 14 | classic SGB | AMAN | Elevated ESR, CRP | HCQ, LPV/r, AZM | Ig iv | 7/8 | |||
| Senel [96] | Patient 90 | 61 | M | 1 | 20 | Miller Fisher | AIDP | PCRNeg Ac Neg | Neg | Neurofilament light chain (NfL) protein elevated | ‐– | Ig iv | complet recovery | 8/8 |
| Su [97] | Patient 91 | 72 | M | 1 | 6 | classic SGB | AIDP | PCR Neg | Neg | Ig iv | poor | 7/8 | ||
| Tard [25] | Patient 92 | 76 | M | 1 | 7 | overlap Miller Fisher - classic SGB | AIDP | PCR Neg | Neg, Anti-NF155 and anti-NF186 IgM, no IgG seroconversion |
‐– | ‐– | Ig iv, PPH, MP | partial improvement | 7/8 |
| Tiet [98] | Patient 93 | 49 | M | 1 | 21 | classic SGB | AIDP | PCR Neg | ‐– | Elevated CRP, LDH, CK | ‐– | Ig iv, gabapentina | partial improvement | 7/8 |
| Toscano [99] | Patient 94 | 77 | F | 2 | 7 | classic SGB | AMAN | PCR Neg | Neg | lymphopenia, Elevated CRP, LDH | ‐– | Ig iv | poor | 8/8 |
| Patient 95 | 23 | M | 1 | 10 | overlap Miller Fisher - classic SGB | AMAN | PCR Neg | ‐– | lymphopenia, Elevated ferritine, CRP, LDH, AST | ‐– | Ig iv | partial improvement | 7/8 | |
| Patient 96 | 55 | M | 2 | 10 | classic SGB | AMAN | PCR Neg | Neg | lymphopenia, Elevated CRP, LDH, AST, GGT | ‐– | Ig iv | poor | 8/8 | |
| Patient 97 | 76 | M | 1 | 5 | overlap Miller Fisher - classic SGB | AIDP | PCR Neg | ‐– | lymphopenia Raised CRP, | ‐– | Ig iv | partial improvement | 7/8 | |
| Patient 98 | 61 | M | 2 | 7 | classic SGB | AIDP | PCR Neg | Neg | Lymphocytopenia Elevated CRP, LDH, AST | ‐– | Ig iv, PPH | poor | 8/8 | |
| Velayos [100] | Patient 99 | 43 | M | 2 | 10 | classic SGB | AIDP | ‐– | ‐– | ‐– | HCQ, LPV/r, corticoids (NE) | Ig iv | satisfactory | 7/8 |
| Virani [101] | Patient 100 | 54 | M | 2 | 10 | classic SGB | ‐– | ‐– | ‐– | ‐– | ‐– | Ig iv | partial improvement | 7/8 |
| Webb [102] | Patient 101 | 57 | M | 2 | 7 | classic SGB | AIDP | PCR Neg | Neg | lymphopenia, thrombocythaemia, raised CRP | Ig iv | partial improvement | 8/8 | |
| Zhao [103] | Patient 102 | 61 | F | 2 | 7 | classic SGB | AIDP | lymphopenia thrombocytopenia | arbidol, LPV/r | Ig iv | complet recovery | 8/8 | ||
| Zito [104] | Patient 103 | 57 | M | 1 | 12 | classic SGB | AMAN | Neg | elevated CRP | Ig iv | complet recovery | 8/8 | ||
| Zubair [105] | Patient 104 | 32 | M | 4 | 60 | classic SGB | AMSAN | ‐– | Neg | ‐– | TCZ, HCQ, remdesivir | Ig iv | partial improvement | 7/8 |
| Patient 105 | 61 | M | 4 | 60 | classic SGB | AMSAN | ‐– | Neg | ‐– | TCZ | Ig iv | partial improvement | 7/8 |
-: information not available; 1 1: uncomplicated disease, 2: mild pneumonia, 3: respiratory distress, 4: septic shock; 2 Days from onset of COVID-19 symptoms to onset of GB symptoms; 3 According to diagnostic criteria for GBS, MFS and their subtypes of the GBS Classification Group [8].; 4 JBI (Joanna Briggs Institute) Critical Appraisal Checklist for Case Reports and for Case Series studies [,]; F: female; M: male; BWDP: bifacial weaknees whit distal parestesias; PCBW: pharyngeal-cervical-brachial weakness; AMSAN: acute motor-sensory axonal neuropathy; AIDP: Acute inflammatory demyelinating polyneuropathy; AMAN: acute motor axonal neuropathy; Neg: negative; Pos: positive; PCR SARS-COV-2 CSF: Polymerase chain reaction detection of SARS-Cov-2 in cerebrospinal fluid; DD: D-dimer; CRP: c-reactive protein; ESR: erythrocyte sedimentation rate; LPV/r: Lopinavir/ritonavir; NE: not specified; HCQ: Hydroxychloroquine; CXM: ceftriaxone; AZM: azithromycin; MP: methylprednisolone; TCZ: tocilizumab; DOX: doxycycline; DXM: dexamethasone; Ig iv: intravenous immunoglobulins; PPH: plasmapheresis; PRED: prednisone; ARDS: acute respiratory distress syndrome.
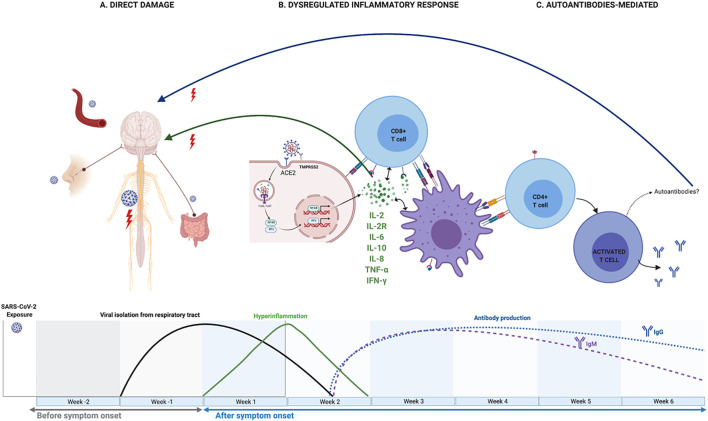
Fig. 1.
Existing hypotheses about pathogenic pathways for neurologic damage associated with COVID-19. A. Direct damage. SARS-COV-2 could reach the central nervous system through circulation or retrograde axonal transport, through the olfactory nerve or the enteric nervous system. B. Dysregulated inflammatory response. IL-2, IL-2R, IL-6, IL-10, IFN-γ and TNF-α, are elevated in the”inflammatory phase” of COVID-19 infection. These molecules can stimulate macrophages, dendritic cells, Schwann cells, and epithelial cells that would damage the nervous system. ACE2: Angiotensin-converting enzyme 2; TMPRSS2: Transmembrane protease, serine 2. C. Autoantibody-mediated injury. The existence of a cross-reactivity between epitopes of the SARS-CoV-2 spike and the glycolipids of the peripheral nerve would be probable. This figure was created using BioRender (https://biorender.com/).
In conclusion, the absence of autoantibodies in most GBS cases associated with SARS-CoV2 infection, would force us to think about pathogenic mechanisms other than molecular mimicry. Both the short of the interval of days between the onset of COVID-19 and the neurological symptoms, and the high proportion of patients with serum elevation of inflammation markers at the beginning of neurological symptoms, support the hypothesis that cell-mediated immunity could play a role, as previously proposed for GBS related to Zika.
Funding
The study had no specific funding.
Declaration of Competing Interest
The authors declare that no conflict of interest exists.
References
- 1.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dotan A., Muller S., Kanduc D., David P., Halpert G., Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;Vol. 20:102792. doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hao Y., Wang W., Jacobs B.C., Qiao B., Chen M., Liu D., et al. Antecedent infections in Guillain-Barré syndrome: a single-center, prospective study. Ann Clin Transl Neurol. 2019;6(12):2510–2517. doi: 10.1002/acn3.50946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parra B., Lizarazo J., Jiménez-Arango J.A., Zea-Vera A.F., González-Manrique G., Vargas J., et al. Guillain–Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375(16):1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- 5.Ottaviani D., Boso F., Tranquillini E., Gapeni I., Pedrotti G., Cozzio S., et al. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. 2020;41(6):1351–1354. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-Rumeileh S., Abdelhak A., Foschi M., Tumani H., Otto M. Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2020;95:1–38. doi: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasan I., Saif-Ur-Rahman K., Hayat S., Papri N., Jahan I., Azam R., et al. Guillain-Barré syndrome associated with SARS-CoV -2 infection: a systematic review and individual participant data meta-analysis. J Peripher Nerv Syst. 2020;25(4):335–343. doi: 10.1111/jns.12419. [DOI] [PubMed] [Google Scholar]
- 8.Wakerley B.R., Uncini A., Yuki N. Guillain-Barré and miller fisher syndromes - new diagnostic classification. Nat Rev Neurol. 2014;10(9):537–544. doi: 10.1038/nrneurol.2014.138. [DOI] [PubMed] [Google Scholar]
- 11.Guadarrama-Ortiz P., Choreño-Parra J., Sánchez-Martínez C., Al E. Neurological aspects of SARS-CoV-2 infection: mechanisms and manifestations. Front Neurol. 2020;11:1039. doi: 10.3389/fneur.2020.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammadi S., Moosaie F., Aarabi M.H. Understanding the immunologic characteristics of neurologic manifestations of SARS-CoV-2 and potential immunological mechanisms. Mol Neurobiol. 2020;57(12):5263–5275. doi: 10.1007/s12035-020-02094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabi F.A., Al Zoubi M.S., Al-Nasser A.D., Kasasbeh G.A., Salameh D.M. Sars-cov-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9(3):231. doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meinhardt J., Radke J., Dittmayer C., Mothes R., Franz J., Laue M., et al. Olfactory transmucosal SARS-CoV-2 invasion as port of central nervous system entry in COVID-19 patients. Nat Neurosci. 2020;24(2):168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 16.Cevik M., Tate M., Lloyd O., Maraolo A.E., Schafers J., Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López-Collazo E., Avendaño-Ortiz J., Martín-Quirós A., Aguirre L.A. Immune response and COVID-19: a mirror image of sepsis. Int J Biol Sci. 2020;16(14):2479–2489. doi: 10.7150/ijbs.48400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J., Zhong S., Liu J., Li L., Li Y., Wu X., et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005;41(8):1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilotto A., Masciocchi S., Volonghi I., De Giuli V., Caprioli F., Mariotto S., et al. SARS-CoV-2 encephalitis is a cytokine release syndrome: evidences from cerebrospinal fluid analyses. Clin Infect Dis. 2021;Jan 4:ciaa1933. doi: 10.1093/cid/ciaa1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X., Wang J., Liu K., Zhu J., Zhang H.L. Are Th17 cells and their cytokines a therapeutic target in Guillain-Barré syndrome? Expert Opin Ther Targets. 2016;20(2):209–222. doi: 10.1517/14728222.2016.1086751. [DOI] [PubMed] [Google Scholar]
- 22.Ebrahim Soltani Z., Rahmani F., Rezaei N. Autoimmunity and cytokines in Guillain-Barré syndrome revisited: review of pathomechanisms with an eye on therapeutic options. Eur Cytokine Netw. 2019;30(1):1–14. doi: 10.1684/ecn.2019.0424. [DOI] [PubMed] [Google Scholar]
- 23.Goodfellow J.A., Willison H.J. Guillain-Barré syndrome: a century of progress. Nat Rev Neurol. 2016;12(12):723–731. doi: 10.1038/nrneurol.2016.172. [DOI] [PubMed] [Google Scholar]
- 24.Keddie S., Pakpoor J., Mousele C., Pipis M., Machado P.M., Foster M., et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021 Mar;144(2):682–693. doi: 10.1093/brain/awaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tard C., Maurage C.A., de Paula A.M., Cassim F., Delval A., Kuchcinski G., et al. Anti-pan-neurofascin IgM in COVID-19-related Guillain-Barré syndrome: evidence for a nodo-paranodopathy. Neurophysiol Clin. 2020;50(5):397–399. doi: 10.1016/j.neucli.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbaslou M.A., Karbasi M., Mozhdehipanah H. A rare axonal variant of guillain-barré syndrome as a neurological complication of covid-19 infection. Arch Iran Med. 2020;23(10):718–721. doi: 10.34172/aim.2020.93. [DOI] [PubMed] [Google Scholar]
- 27.Abolmaali M., Heidari M., Zeinali M., Moghaddam P., Ghamsari M.R., Makiani M.J., et al. Guillain-Barre syndrome as a parainfectious manifestation of SARS-CoV-2 infection: a case series. J Clin Neurosci. 2021 Jan;83:119–122. doi: 10.1016/j.jocn.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrams R.M.C., Kim B.D., Markantone D.M., Reilly K., Paniz-Mondolfi A.E., Gitman M.R., et al. Severe rapidly progressive Guillain-Barré syndrome in the setting of acute COVID-19 disease. J Neuro-Oncol. 2020;26(5):797–799. doi: 10.1007/s13365-020-00884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agosti E., Giorgianni A., D’Amore F., Vinacci G., Balbi S., Locatelli D. Is Guillain-Barrè syndrome triggered by SARS-CoV-2? Case report and literature review. Neurol Sci. 2020;42(2):607–612. doi: 10.1007/s10072-020-04553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alberti P., Beretta S., Piatti M., Karantzoulis A., Piatti M.L., Santoro P., et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol NeuroInflam. 2020;7(4):1–3. doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ameer N., Shekhda K.M., Cheesman A. Guillain-Barré syndrome presenting with COVID-19 infection. BMJ Case Rep. 2020;13(9):3–5. doi: 10.1136/bcr-2020-236978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnaud S., Budowski C., Ng Wing Tin S., Degos B. Post SARS-CoV-2 Guillain-Barré syndrome. Clin Neurophysiol. 2020;131(7):1652–1654. doi: 10.1016/j.clinph.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assini A., Benedetti L., Di Maio S., Schirinzi E., Del Sette M. Correction to: New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases (Neurological Sciences, (2020), 41, 7, (1657–1658)) Neurol Sci. 2020;41(8):2307. doi: 10.1007/s10072-020-04484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atakla H.G., Noudohounsi M.M.U.D., Sacca H., Tassiou N.R.A., Noudohounsi W.C., Houinato D.S. Acute guillain-barré polyradiculoneuritis indicative of covid-19 infection: a case report. Pan Afr Med J. 2020;35(Supp 2):1–6. doi: 10.11604/pamj.supp.2020.35.150.25745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrachina-Esteve O., Palau-Domínguez A., Hidalgo-Torrico I., Viguera Martínez M. Guillain-Barré syndrome as the first manifestation of SARS-CoV-2 infection. Neurología. 2020;35(9):710–712. doi: 10.1016/j.nrleng.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bigaut K., Mallaret M., Baloglu S., Nemoz B., Morand P., Baicry F., et al. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflam. 2020;7(5):4–6. doi: 10.1212/NXI.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boostani R., Talab F.R., Meibodi N.T., Zemorshidi F. COVID-19 associated with sensorimotor polyradiculoneuropathy and skin lesions: a case report. J Neuroimmunol. 2021;350:577434. doi: 10.1016/j.jneuroim.2020.577434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bracaglia M., Naldi I., Govoni A., Brillanti Ventura D., De Massis P. Acute inflammatory demyelinating polyneuritis in association with an asymptomatic infection by SARS-CoV-2. J Neurol. 2020;267(11):3166–3168. doi: 10.1007/s00415-020-10014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bueso T., Montalvan V., Lee J., Gomez J., Ball S., Shoustari A., et al. Guillain-Barre syndrome and COVID-19: a case report. Clin Neurol Neurosurg. 2021;200:106413. doi: 10.1016/j.clineuro.2020.106413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juliao Caamaño D.S., Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain-Barré syndrome as a rare neurological complication of SARS-CoV-2. J Clin Neurosci. 2020;77:230–232. doi: 10.1016/j.jocn.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camdessanche J.P., Morel J., Pozzetto B., Paul S., Tholance Y., Botelho-Nevers E. COVID-19 may induce Guillain–Barré syndrome. Rev Neurol (Paris) 2020;176(6):516–518. doi: 10.1016/j.neurol.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan J.L., Ebadi H., Sarna J.R. Guillain-Barré syndrome with facial Diplegia related to SARS-CoV-2 infection. Can J Neurol Sci. 2020;47(6):852–854. doi: 10.1017/cjn.2020.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Civardi C., Collini A., Geda D.J., Geda C. Antiganglioside antibodies in Guillain-Barré syndrome associated with SARS-CoV-2 infection. J Neurol Neurosurg Psychiatry. 2020;91(12):1361–1362. doi: 10.1136/jnnp-2020-324279. [DOI] [PubMed] [Google Scholar]
- 44.Coena M., Jeansonc G., Culebras Almeida L.A., Hübersd A., Stierlina F., Najjara I., et al. Guillain-Barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav Immun J. 2020;87:111–112. doi: 10.1016/j.bbi.2020.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colonna S., Sciume L., Giarda F., Innocenti A., Beretta G., Dalla Costa D. Case report: Postacute rehabilitation of Guillain-Barre syndrome and cerebral Vasculitis-like pattern accompanied by SARS-CoV-2 infection. Front Neurol. 2021;11:602554. doi: 10.3389/fneur.2020.602554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Defabio A.C., Scott T.R., Stenberg R.T., Simon E.L. Guillain-Barré syndrome in a patient previously diagnosed with COVID-19. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.07.074. Epub:S0735–6757(20)30669–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diez-Porras L., Vergés E., Gil F., Vidal M.J., Massons J., Arboix A. Guillain-Barré-Strohl syndrome and COVID-19: case report and literature review. Neuromuscul Disord. 2020;30(10):859–861. doi: 10.1016/j.nmd.2020.08.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El Otmani H., El Moutawakil B., Rafai M.A., El Benna N., El Kettani C., Soussi M., et al. Covid-19 and Guillain-Barré syndrome: more than a coincidence! Rev Neurol (Paris) 2020;176(6):518–519. doi: 10.1016/j.neurol.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elkhouly A., Kaplan A.C. Noteworthy neurological manifestations associated with COVID-19 infection. Cureus. 2020;12(7):3–7. doi: 10.7759/cureus.8992. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Esteban A., Mata M., Sánchez P., Carrillo A., ISancho I., Sanjuan T. Guillain-Barré syndrome associated with SARS-CoV-2 infection. Med Int. 2020;44(8):513–519. doi: 10.1016/j.medin.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farzi M.A., Ayromlou H., Jahanbakhsh N., Bavil P.H., Janzadeh A., Shayan F.K. Guillain-Barré syndrome in a patient infected with SARS-CoV-2, a case report. J Neuroimmunol. 2020;346:577294. doi: 10.1016/j.jneuroim.2020.577294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernández-Domínguez J., Ameijide-Sanluis E., García-Cabo C., García-Rodríguez R., Mateos V. Miller–fisher-like syndrome related to SARS-CoV-2 infection (COVID 19) J Neurol. 2020;267(9):2495–2496. doi: 10.1007/s00415-020-09912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferraris L.E., Sala G., Casalino S., Losurdo L., De Filippis V. Mesenteric artery thrombosis, microvascular intestinal Endothelitiis, and Guillain-Barrè syndrome in the same SARS-CoV-2 patient. Cureus. 2020;12(11):4–9. doi: 10.7759/cureus.11326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gale A., Sabaretnam S., Lewinsohn A. Guillain-Barré syndrome and COVID-19: association or coincidence. BMJ Case Rep. 2020;13(11) doi: 10.1136/bcr-2020-239241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.García-Manzanedo S., López de la Oliva Calvo L., Ruiz Álvarez L. Guillain-Barré syndrome after covid-19 infection. Med Clin (Barc) 2020;155(8):366. doi: 10.1016/j.medcle.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garnero M., Del Sette M., Assini A., Beronio A., Capello E., Cabona C., et al. COVID-19-related and not related Guillain-Barré syndromes share the same management pitfalls during lock down: The experience of Liguria region in Italy. J Neurol Sci. 2020;418:117114. doi: 10.1016/j.jns.2020.117114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosh R., Roy D., Sengupta S., Benito-León J. Autonomic dysfunction heralding acute motor axonal neuropathy in COVID-19. J Neuro-Oncol. 2020:964–966. doi: 10.1007/s13365-020-00908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gigli G.L., Vogrig A., Nilo A., Fabris M., Biasotto A., Curcio F., et al. HLA and immunological features of SARS-CoV-2-induced Guillain-Barré syndrome. Neurol Sci. 2020;41(12):3391–3394. doi: 10.1007/s10072-020-04787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Granger A., Omari M., Jakubowska-Sadowska K., Boffa M., Zakin E. SARS-CoV-2-associated Guillain-Barre syndrome with good response to plasmapheresis. J Clin Neuromuscul Dis. 2020;22(1):58–59. doi: 10.1097/CND.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 60.Guijarro-Castro C., Rosón-González M., Abreu A., García-Arratibel A., Ochoa-Mulas M. Guillain-Barré syndrome associated with SARS-CoV-2 infection. Comments after 16 published cases. Neurologia. 2020;35(6):412–415. doi: 10.1016/j.nrl.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gutiérrez-Ortiz C., Méndez-Guerrero A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Mañas R., et al. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 62.Helbok R., Beer R., Löscher W., Boesch S., Reindl M., Hornung R., et al. Guillain-Barré syndrome in a patient with antibodies against SARS-COV-2. Eur J Neurol. 2020;27(9):1754–1756. doi: 10.1111/ene.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirayama T., Hongo Y., Kaida K., Kano O. Guillain-Barré syndrome after COVID-19 in Japan. BMJ Case Rep. 2020;13(10):1–4. doi: 10.1136/bcr-2020-239218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hutchins K.L., Jansen J.H., Comer A.D., Scheer R.V., Zahn G.S., Capps A.E., et al. COVID-19-associated bifacial weakness with paresthesia subtype of guillain-barré syndrome. Am J Neuroradiol. 2020;41(9):1707–1711. doi: 10.3174/ajnr.A6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kajani S., Kajani R., Huang C.-W., Tran T., Liu A.K. Miller fisher syndrome in the COVID-19 era - a novel target antigen calls for novel treatment. Cureus. 2021;13(1) doi: 10.7759/cureus.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khaja M., Roa Gomez G.P., Santana Y., Hernandez N., Haider A., Lara J.L.P., et al. A 44-year-old hispanic man with loss of taste and bilateral facial weakness diagnosed with Guillain-Barré syndrome and Bell’s palsy associated with SARS-CoV-2 infection treated with intravenous immunoglobulin. Am J Case Rep. 2020;21:1–6. doi: 10.12659/AJCR.927956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kopscik M., Giourgas B., Presley B. A case report of acute motor and sensory polyneuropathy as the presenting symptom of SARS-CoV-2. Clin Pract Cases Emerg Med. 2020;4(3):352–354. doi: 10.5811/cpcem.2020.6.48683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korem S., Gandhi H., Dayag D.B. Guillain-Barré syndrome associated with COVID-19 disease. BMJ Case Rep. 2020;13(9) doi: 10.1136/bcr-2020-237215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lampe A., Winschel A., Lang C., Steiner T. Vol. 0. 2020. Guillain-Barré Syndrome and SARS-CoV-2. 10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lantos J., Strauss S., Lin E. 2020. COVID-19 – Associated Miller Fisher Syndrome: MRI Findings; pp. 1184–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lascano A.M., Epiney J.B., Coen M., Serratrice J., Bernard-Valnet R., Lalive P.H., et al. SARS-CoV-2 and Guillain–Barré syndrome: AIDP variant with a favourable outcome. Eur J Neurol. 2020;27(9):1751–1753. doi: 10.1111/ene.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lowery M.M., Taimur Malik M., Seemiller J., Tsai C.S. Atypical variant of Guillain Barre syndrome in a patient with COVID-19. J Crit Care Med. 2020;6(4):231–236. doi: 10.2478/jccm-2020-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liberatore G., De Santis T., Doneddu P.E., Gentile F., Albanese A., Nobile-Orazio E. Clinical reasoning: a case of COVID-19-associated pharyngeal-cervical-brachial variant of Guillain-Barré syndrome. Neurology. 2020;95(21):978–983. doi: 10.1212/WNL.0000000000010817. [DOI] [PubMed] [Google Scholar]
- 74.McDonnell E.P., Altomare N.J., Parekh Y.H., Gowda R.C., Parikh P.D., Lazar M.H., et al. COVID-19 as a trigger of recurrent Guillain–Barré syndrome. Pathogens. 2020;9(11):1–9. doi: 10.3390/pathogens9110965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maideniuc C., Memon A.B. Acute necrotizing myelitis and acute motor axonal neuropathy in a COVID-19 patient. J Neurol. 2020;268(2):739. doi: 10.1007/s00415-020-10145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manganotti P., Pesavento V., Buoite Stella A., Bonzi L., Campagnolo E., Bellavita G., et al. Miller fisher syndrome diagnosis and treatment in a patient with SARS-CoV-2. J Neuro-Oncol. 2020;26(4):605–606. doi: 10.1007/s13365-020-00858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manganotti P., Bellavita G., D’Acunto L., Tommasini V., Fabris M., Sartori A., et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J Med Virol. 2020;93(2):766–774. doi: 10.1002/jmv.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marta-Enguita J., Rubio-Baines I., Gastón-Zubimendi I. Síndrome de Guillain-Barré fatal tras infección por el virus SARS-CoV-2. Neurología. 2020;35(4):265–267. doi: 10.1016/j.nrl.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naddaf E., Laughlin R.S., Klein C.J., Toledano M., Theel E.S., Binnicker M.J., et al. Guillain-Barré syndrome in a patient with evidence of recent SARS-CoV-2 infection. Mayo Clin Proc. 2020;95(8):1799–1801. doi: 10.1016/j.mayocp.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nanda S., Handa R., Prasad A., Anand R., Zutshi D., Dass S.K., et al. Covid-19 associated Guillain-Barre syndrome: contrasting tale of four patients from a tertiary care Centre in India. Am J Emerg Med. 2021 Jan;39:125–128. doi: 10.1016/j.ajem.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oguz-Akarsu E., Ozpar R., Mirzayev H., Acet-Ozturk N.A., Hakyemez B., Ediger D., et al. Guillain-Barré syndrome in a patient with minimal symptoms of COVID-19 infection. Muscle Nerve. 2020;62(3):E54–E57. doi: 10.1002/mus.26992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paybast S., Gorji R., Mavandadi S. Guillain-Barré syndrome as a neurological complication of novel COVID-19 infection: a case report and review of the literature. Neurologist. 2020;25(4):101–103. doi: 10.1097/NRL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pelea T., Reuter U., Schmidt C., Laubinger R., Siegmund R., Walther B.W. SARS-CoV-2 associated Guillain–Barré syndrome. J Neurol. 2020;268(4):1191–1194. doi: 10.1007/s00415-020-10133-w. Epub(Aug 8):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petrelli C., Scendoni R., Paglioriti M., Logullo F.O. Acute motor axonal neuropathy related to COVID-19 infection: a new diagnostic overview. J Clin Neuromuscul Dis. 2020;22(2):120–121. doi: 10.1097/CND.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Padroni M., Mastrangelo V., Asioli G.M., Pavolucci L., Abu-Rumeileh S., Piscaglia M.G., et al. Guillain-Barré syndrome following COVID-19: new infection, old complication? J Neurol. 2020;267(7):1877–1879. doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raahimi M.M., Kane A., Moore C.E., Alareed A.W. Late onset of Guillain-Barré syndrome following SARS-CoV-2 infection: part of “long COVID-19 syndrome”? BMJ Case Rep. 2021;14(1) doi: 10.1136/bcr-2020-240178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rajdev K., Victor N., Buckholtz E.S., Hariharan P., Saeed M.A., Hershberger D.M., et al. A case of Guillain-Barré syndrome associated with COVID-19. J Investig Med. 2020;8 doi: 10.1177/2324709620961198. (high impact case reports). 2324709620961198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rana S., Lima A.A., Chandra R., Valeriano J., Desai T., Freiberg W., et al. Novel coronavirus (COVID-19)-associated Guillain-Barré syndrome: case report. J Clin Neuromuscul Dis. 2020 Jun;21(4):240–242. doi: 10.1097/CND.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ray A. Miller fisher syndrome and COVID-19: is there a link. BMJ Case Rep. 2020;13(8):19–22. doi: 10.1136/bcr-2020-236419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Redondo-Urda M.J., Rodríguez-Peguero F.J., Pérez-Gil O., Del Valle-Sánchez M., Carrera-Izquierdo M. SARS-CoV-2, nuevo agente causal del síndrome de Guillain-Barré. Rev Neurol. 2020;71(7):275–276. doi: 10.33588/rn.7107.2020264. [DOI] [PubMed] [Google Scholar]
- 91.Reyes-Bueno J.A., García-Trujillo L., Urbaneja P., Ciano-Petersen N.L., Postigo-Pozo M.J., Martínez-Tomás C., et al. Miller-fisher syndrome after SARS-CoV-2 infection. Eur J Neurol. 2020;27(9):1759–1761. doi: 10.1111/ene.14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riva N., Russo T., Falzone Y.M., Strollo M., Amadio S., Del Carro U., et al. Post-infectious Guillain–Barré syndrome related to SARS-CoV-2 infection: a case report. J Neurol. 2020;267(9):2492–2494. doi: 10.1007/s00415-020-09907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sancho-Saldaña A., Lambea-Gil Á., Capablo Liesa J.L., Barrena Caballo M.R., Garay M.H., Celada D.R., et al. Guillain-Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin Med J R Coll Physicians London. 2020;20(4):E93–E94. doi: 10.7861/clinmed.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scheidl E., Canseco D.D., Hadji-Naumov A., Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. 2020;25(2):204–207. doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sedaghat Z., Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Senel M., Abu-Rumeileh S., Michel D., Garibashvili T., Althaus K., Kassubek J., et al. Miller-fisher syndrome after COVID-19: neurochemical markers as an early sign of nervous system involvement. Eur J Neurol. 2020;27(11):2378–2380. doi: 10.1111/ene.14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Su X.W., Palka S.V., Rao R.R., Chen F.S., Brackney C.R., Cambi F. SARS-CoV-2–associated Guillain-Barré syndrome with dysautonomia. Muscle Nerve. 2020;62(2):E48–E49. doi: 10.1002/mus.26988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tiet M.Y., Alshaikh N. Guillain-Barré syndrome associated with COVID-19 infection: a case from the UK. BMJ Case Rep. 2020;13(7):1–4. doi: 10.1136/bcr-2020-236536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Velayos Galán A., del Saz Saucedo P., Peinado Postigo F., Botia Paniagua E. Síndrome de Guillain-Barré asociado a infección por SARS-CoV-2. Neurología. 2020;35(4):268–269. doi: 10.1016/j.nrl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Virani A., Rabold E., Hanson T., Haag A., Elrufay R., Cheema T., et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Webb S., Wallace V.C.J., Martin-Lopez D., Yogarajah M. Guillain-Barré syndrome following COVID-19: a newly emerging post-infectious complication. BMJ Case Rep. 2020;13(6):1–4. doi: 10.1136/bcr-2020-236182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zito A., Alfonsi E., Franciotta D., Todisco M., Gastaldi M., Cotta Ramusino M., et al. COVID-19 and Guillain–Barré syndrome: a case report and review of literature. Front Neurol. 2020;11:909. doi: 10.3389/fneur.2020.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zubair A.S., Zubair A.S., Desai K., Abulaban A., Roy B. Guillain-Barre syndrome as a complication of COVID-19. Cureus. 2021 Jan;13(1) doi: 10.7759/cureus.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.2017. Joanna Briggs Institute Checklist for Case Reports
- 107.2017. Joanna Briggs Institute Checklist for Case Series